Abstract
Mesonephric carcinoma is a rare gynecologic neoplasm commonly mistaken for clear cell carcinoma, due to their overlapping morphologic features. Both tumors are negative for estrogen receptor (ER) and p16, magnifying this diagnostic dilemma. Recently, HNF-1β, a marker for clear cell carcinoma, has also been shown to be positive in mesonephric carcinomas. Other more recent markers for clear cell carcinoma, however, such as Napsin-A and AMACR, have not yet been studied in mesonephric carcinomas. Here, we examine HNF-1β, AMACR, and Napsin-A immunohistochemistry in 18 mesonephric and 55 endometrial/cervical clear cell carcinomas. HNF-1β was considered positive if nuclear staining was present in ≥70% of cells and ≥ moderate intensity; for Napsin-A and AMACR any cytoplasmic staining was considered positive (≥1%). H-scores were determined by multiplying the intensity score by proportion score. HNF-1β was positive in a substantial portion of mesonephric carcinomas (9/18, 50%; H-score 98) and clear cell carcinomas (34/55, 62%; H-score 163) and did not distinguish between the two entities (specificity: 50%; p-value of H-score: 0.08). Napsin-A and AMACR expression was significantly higher in clear cell [43/55 (62%) and 41/55 (75%), respectively] than mesonephric carcinomas [4/18 (22%) and 4/18 (22%) respectively], and helpful in this differential (specificity: 78% and 78%; p-values <0.05 for both). When Napsin-A and AMACR staining were seen in mesonephric carcinomas, staining was focal (≤ 5%), while staining in clear cell carcinomas was patchy/diffuse. In summary, Napsin-A and AMACR are helpful in distinguishing mesonephric carcinomas from clear cell carcinomas of the female genital tract, but HNF-1β is not.
Keywords: mesonephric carcinoma, clear cell carcinoma, Napsin-A, AMACR, HNF-1β
INTRODUCTION
Mesonephric carcinomas are rare and aggressive1,2 gynecologic neoplasms thought to arise from remnants of the embryologic Wolffian (also known as mesonephric) duct. Mesonephric carcinoma most frequently involves the cervix, where mesonephric remnants are common, but it can also develop within any structure that passes along the primitive mesonephric duct, including the ovaries, broad ligament, uterus and vagina1,3.
Mesonephric carcinoma has many histologic patterns, which can vary within the same tumor and between cases, making the diagnosis incredibly difficult. The classic histologic patterns of mesonephric carcinoma are tubular, consisting of small back-to-back tubules lined by cuboidal epithelium and filled with intraluminal eosinophilic secretions, and ductal, consisting of larger glands lined by columnar epithelium and also referred to as the “pseudo-endometrioid” pattern4,5. Mesonephric carcinoma can also exhibit many other architectural patterns, including retiform, papillary, solid, spindled, and sex-cord like, and cytologic features, including hobnail cells and clear cells4,6,7.
In the early stages of discovery, mesonephric carcinomas were not infrequently confused with clear cell carcinomas8,7,9. Between the 1940s and 1970s, tumors thought to represent mesonephric carcinoma were designated a variety of names, including “mesonephroma” and “clear cell adenocarcinoma of mesonephric origin”9,10,11,12. These tumors were made of “clear or hobnail” cells arranged in “cysts, tubules and solid masses”7,9. Due to their resemblance to renal cell carcinoma of the kidney, an organ which is partially derived from the mesonephric duct, many authors believed these tumors to be of mesonephric origin10,9. It was later explicated that a large subset of these tumors actually represented clear cell carcinomas of Mullerian origin, as many of these tumors were associated with endometriosis9,11,12. This period of discovery was also confounded by the use of DES (diethylstilbestrol) in pregnant women at the time, which had the inadvertent effects of increasing the rates of clear cell carcinomas affecting the gynecologic tract13. It is not surprising that clear cell carcinomas were misconstrued for mesonephric carcinomas. Both tumors affect the gynecologic tract (particularly the cervix), both are human papillomavirus (HPV) independent and both tumors can exhibit overlapping histologic features14,15. Similar to mesonephric carcinoma, clear cell carcinoma exhibits papillary, solid, and tubulocystic (small tubular) architecture, predominantly cuboidal cells, and cytologic hobnailing15. Distinction between the two tumors remains, even in present day, very challenging. This problem is further complicated by their shared immunohistochemical profile. Estrogen receptor (ER) and p16, two of the most commonly used immunohistochemical markers in the diagnosis of adenocarcinomas of the cervix, are negative in both tumors1,16–19,20,21,22.
HNF-1β was identified as a useful marker for clear cell carcinoma of the gynecologic tract in 200323, and has been subsequently validated in many studies21,24,20,22,25. HNF-1β is a homeobox transcription factor that plays a role in glucose homeostasis, anti-apoptosis and the embryologic development of urogenital organs23,26. Unexpectedly, HNF-1β was also found to be positive in a subset of mesonephric carcinomas17. Subsequent to HNF-1β, additional markers for clear cell carcinoma have been reported, which include Napsin-A27,24,21,20,28,22 and AMACR (alpha-Methylacyl-CoA racemase, p504S)29,24. Napsin-A is an aspartic protease, better known for its role in processing pulmonary surfactant in the lung and was initially discovered in studies exploring napsin’s ability to separate primary and metastatic tumors in the lung30,31. AMACR (p504s) is an enzyme involved in the oxidation of branched chain fatty acids and is better known for its diagnostic role in separating prostatic adenocarcinoma from normal prostatic tissue, discovered via gene expression profiling (cDNA microarrays)32. Its usefulness in the diagnosis of clear cell carcinomas of the gynecologic tract was sparked by the detection of AMACR in clear cell carcinomas of the bladder/urethra29.
To the best of our knowledge, no studies have compared the expression of HNF-1β, Napsin-A and AMACR in mesonephric carcinomas. Given the common confusion between mesonephric carcinomas and clear cell carcinomas, the goal of our study was to compare the usefulness of HNF-1β, Napsin-A and AMACR in separating clear cell carcinomas and mesonephric carcinomas in the gynecologic tract.
MATERIALS AND METHODS
Study Cases
Cases were acquired from the anatomical pathology archives of Vancouver General Hospital (VGH) and Memorial Sloan Kettering Cancer Center (MSK). All gynecologic tumors were identified by searching the pathology intranet database (1986–2018). All mesonephric neoplasms were reviewed by a gynecologic subspecialty pathologist (LH, KP). Diagnoses made on prior biopsy by the primary pathologists were also recorded if available.
Tissue Microarrays
Hematoxylin and eosin (H&E) stained slides of endocervical and endometrial clear cell carcinomas were reviewed by a gynecologic subspecialty pathologist (LH, BTC). A slide with representative tumor was selected from each case. The area of tumor was circled on the slide and corresponding formalin-fixed paraffin embedded (FFPE) tissue block. Duplicate 0.6 mm cores were taken from each case for tissue microarray (TMA) construction.
Immunohistochemistry
Immunohistochemical stains were performed on 4-μm thickness whole tissue sections for the mesonephric neoplasms and on TMA sections for the clear cell carcinomas. This was done using the Ventana Discovery XT and Ventana Benchmark XT systems (Ventana Medical Systems, Tucson, AZ) following manufacturer recommendations. Sections were cut onto charged glass slides, air dried for 10 minutes and baked at 60°C for 10 minutes. Cell conditioning solution CC1 (Ventana), heat induced antigen retrieval (37°C for 32 minutes) and Ventana XT Optiview DAB detection kit was used for all antibodies.
The following immunohistochemical stains were performed: HNF-1β, Napsin-A, and AMACR. At VGH, the rabbit polyclonal HNF-1β antibody (catalogue number HPA002083) was obtained from Sigma, while at MSK the mouse monoclonal antibody (clone: CLO374; catalogue number: AMAB90733) was obtained from Sigma. At both VGH and MSK the mouse monoclonal Napsin-A antibody (clone: 1p64; catalogue number: NCL-L-NAPSINA) was obtained from Leica. At VGH, the rabbit AMACR antibody (clone 13H4; catalogue number GA060) was obtained from DAKO, while at MSK the AMACR antibody (clone: 13H4; catalogue number: Z2001L) was obtained from Zeta Corp. All immunohistochemical stains were performed at VGH with the exception of the exception of 6 slides (HNF-1β for 4 cases, Napsin-A for 1 case, AMACR for 1 case), which were done at MSK. To assess concordance, 3 unstained slides were available and re-stained at VGH, which showed complete concordance with the results obtained from MSK.
Nuclear staining was considered positive staining for HNF-1β and cytoplasmic staining for Napsin-A and AMACR. For each stain, staining was quantified based on the percentage of tumor cells staining: negative (0%); focal (1% to 25%); patchy (26% to 49%); diffuse (≥50%), as well as the intensity of staining: 0 = none, 1+ = weak, 2+ = moderate, 3+ =strong. Modified histoscores (H-score) were calculated by multiplying the proportion of cells staining and the intensity, yielding H-scores between 0 to 300.
Statistical Analysis
For the calculation of binary classification test performance (sensitivity, specificity, negative predictive value (NPV), positive predictive value (PPV)), the stains were categorized into positive or negative. For Napsin-A and AMACR, any cytoplasmic immunohistochemical staining was considered positive (any tumor cells staining ≥1%). For HNF-1β immunohistochemical stain was considered positive only if staining was seen in ≥70% of cells and at least moderate intensity, as previously described33. Test scores for each marker were determined using an online statistical calculator (https://www.medcalc.org/calc/diagnostic_test.php) and using clear cell carcinoma as the test reference.
RESULTS
Clinical and Histologic Features of Study Cases
A cohort of 73 cases were examined that included 18 mesonephric neoplasms from various sites (8 cervical mesonephric carcinomas, 3 cervical mesonephric carcinosarcomas, 5 endometrial mesonephric-like carcinomas, and 2 pelvic masses) and 55 pure clear cell carcinomas from various sites (5 cervical, 50 endometrial). Thirteen mesonephric neoplasms have been described in a previous study6.
The clinicopathologic features of patients with mesonephric neoplasms are summarized in Table 1. Patients with mesonephric neoplasms ranged in age from 30 to 75 years (mean age: 58 years). Information on stage was available for 16 tumors, and 8 of these (50%) were diagnosed at stage pT2 or above. The most common presenting symptoms were abnormal uterine bleeding and postmenopausal bleeding. The most common histologic patterns were tubular, papillary, and ductal (Figures 1–2). Biopsy diagnosis was available in 13 of 18 cases. The diagnosis of mesonephric carcinoma was not made on any of the prior biopsy sampling (Table 1). Mesonephric carcinomas were confused for endometrioid carcinoma (7 of 13 cases), clear cell carcinoma (1 of 13 cases), mixed endometrioid and serous carcinoma (1 of 13 cases), endometrial hyperplasia (1 of 13 cases), moderate to poorly differentiated cervical adenocarcinoma (2 of 13 cases), and Mullerian carcinosarcoma (1 of 13 cases).
Table 1.
Summary of Clinical Features and immunohistochemical Findings in 18 Mesonephric Neoplasms
| Case | Age | FIGO stage | Biopsy Diagnosis | Immunohistochemistry (Intensity; Proportion) |
||
|---|---|---|---|---|---|---|
| HNF-1β | Napsin-A | AMACR | ||||
| 1 | 65 | IVB | Clear cell CA | Negative | Negative | Negative |
| 2 | 31 | IIIA | Endometrioid CA, grade 1 | Negative | +++; 1% | Negative |
| 3 | 75 | IB | Endometrioid CA, grade 2 | +; 90% | +++; 1% | Negative |
| 4 | 65 | IA | Endometrioid CA, high-grade | Negative | Negative | Negative |
| 5 | 65 | IB | Endometrioid CA | Negative | Negative | Negative |
| Cervical | ||||||
| 6* | 49 | IIB | None** | Negative | Negative | Negative |
| 7 | 78 | IB1 | Mixed endometrioid and serous | Negative | Negative | Negative |
| 8 | 64 | IB1 | Endometrial hyperplasia | +++; 90% | Negative | Negative |
| 9 | 50 | IIIA | Endometrioid CA, grade 2 | +++; 85% | Negative | +++; 1% |
| 10 | 62 | IIA2 | Endometrioid CA, grade 1 | +++; 100% | Negative | Negative |
| 11* | 59 | IIB | Mullerian carcinosarcoma | +; 80% | Negative | Negative |
| 12 | 43 | IIB | Invasive endocervical adenocarcinoma, mod-poorly differentiated | ++; 70% | +++, 20% | ++, 5% |
| 13 | 30 | IB1 | Invasive endocervical adenocarcinoma, poorly differentiated | Negative | Negative | +++, 5% |
| 14 | 75 | IB | Endometrioid CA, grade 3 | Negative | Negative | Negative |
| 15 | ---- | ---- | None | ++, 100% | Negative | Negative |
| 16* | 57 | IB2 | Unknown | ++, 90% | +/++, 5% | ++, 20% |
| Vaginal/Pelvic Mass | ||||||
| 17 | 66 | III | None | +++; 100% | Negative | Negative |
| 18 | 62 | ---- | None | Negative | Negative | Negative |
Weak;
Moderate;
Strong intensity staining
Carcinosarcoma
Thought to be a prolapsed cervical fibroid, therefore no biopsy was done
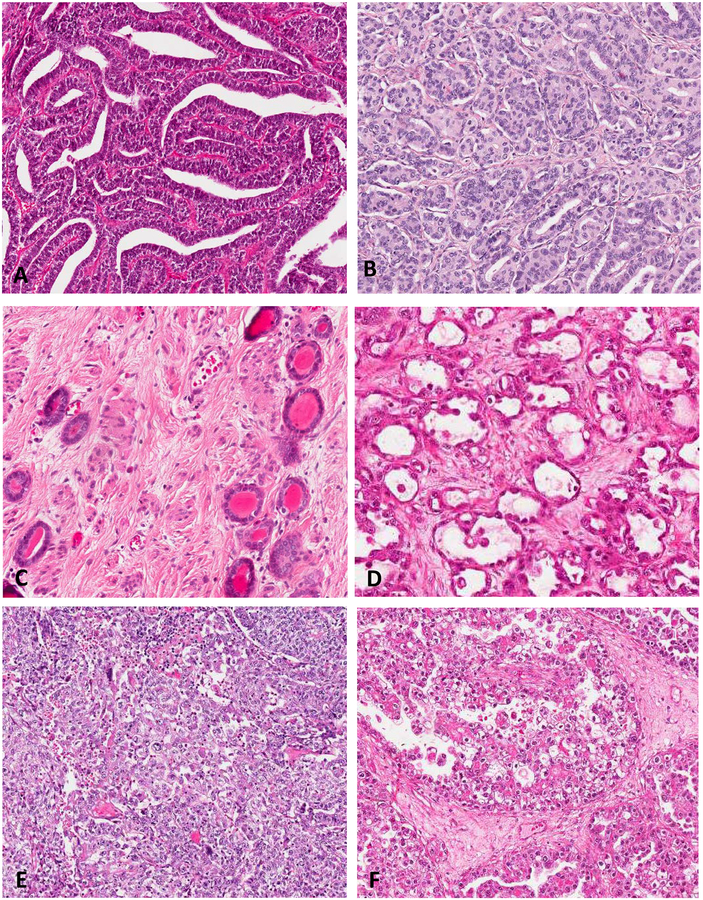
FIGURE 1.
Classic morphologic patterns of mesonephric carcinoma: Ductal (A); Tubular (B); often associated with adjacent mesonephric remnants (C). Classic morphologic patterns of clear cell carcinoma: Tubulocystic (D); Solid (E); and Papillary (F).
FIGURE 2.
Similar to clear cell carcinoma, mesonephric carcinoma can show Tubulocystic (A), Solid (B) and Papillary Areas (C-D) as well as cytologic clearing (E) and hobnailing (F).
The resection specimens in three cases were initially diagnosed as mixed cell carcinomas; two mixed endometrioid and mesonephric carcinomas, and one mixed mesonephric, clear cell and endometrioid carcinoma. Additional immunohistochemical stains were performed (ER and, in some cases, Napsin-A). All tumors were negative for ER, including the endometrioid-like areas. The tumor with a questionable clear cell carcinoma component was negative for Napsin-A. Given these findings, these three tumors were re-classified as pure mesonephric carcinomas.
Immunohistochemical Findings
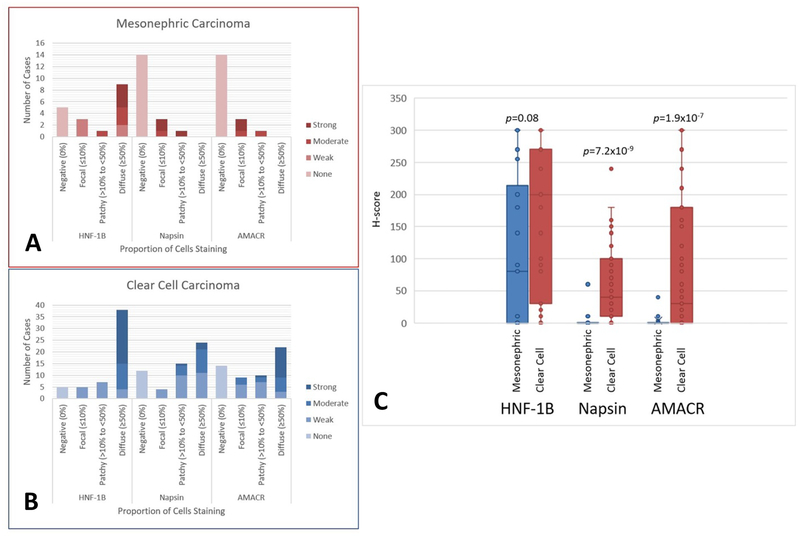
The immunohistochemical findings are summarized in Tables 2-3 and Figures 3–4. HNF-1β showed staining in 9 of 18 cases (50%) of mesonephric carcinoma (1 endometrial, 7 cervical, and 1 pelvic mass) (Table 1). Eight of the 18 had staining in ≥70% of cells and at least moderate intensity. Fifty of 55 (91%) clear cell carcinomas demonstrated staining for HNF-1β, and 34/55 (67%) exhibited staining in ≥70% of cells and at least moderate intensity. The H-score for HNF-1β was higher in clear cell carcinoma compared to mesonephric carcinoma (163 vs 98), but was not statistically significant (p=0.08) (Figure 3). The specificity and PPV of HNF-1β for separating clear cell carcinoma from mesonephric carcinoma was 50% and 79% (Table 2).
Table 2.
Immunohistochemical Staining and Test Performance of HNF-1β, Napsin-A, and AMACR in Mesonephric Carcinoma and Clear Cell Carcinoma*.
| Immunohistochemical stain | Mesonephric CA (n=18) | Clear cell CA (n=55) | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|---|---|
| HNF-1β+** | 9/18 (50%) |
34/55 (62%) |
62% | 50% | 79% | 30% |
| Napsin-A+ | 4/18 (22%) |
43/55 (78%) |
78% | 78% | 92% | 54% |
| AMACR+ | 4/18 (22%) |
41/55 (75%) |
75% | 78% | 91% | 50% |
PPV, Positive predictive value; NPV, Negative predictive value
Statistics were performed using clear cell carcinoma as the reference
For HNF-1β, cases are considered positive if staining ≥70%, and at least moderate intensity
FIGURE 3.
Box plots demonstrating distribution of immunohistochemical staining in mesonephric carcinomas (A) and clear cell carcinomas (B). H-scores comparing mesonephric and clear cell carcinoma for each immunohistochemical marker.
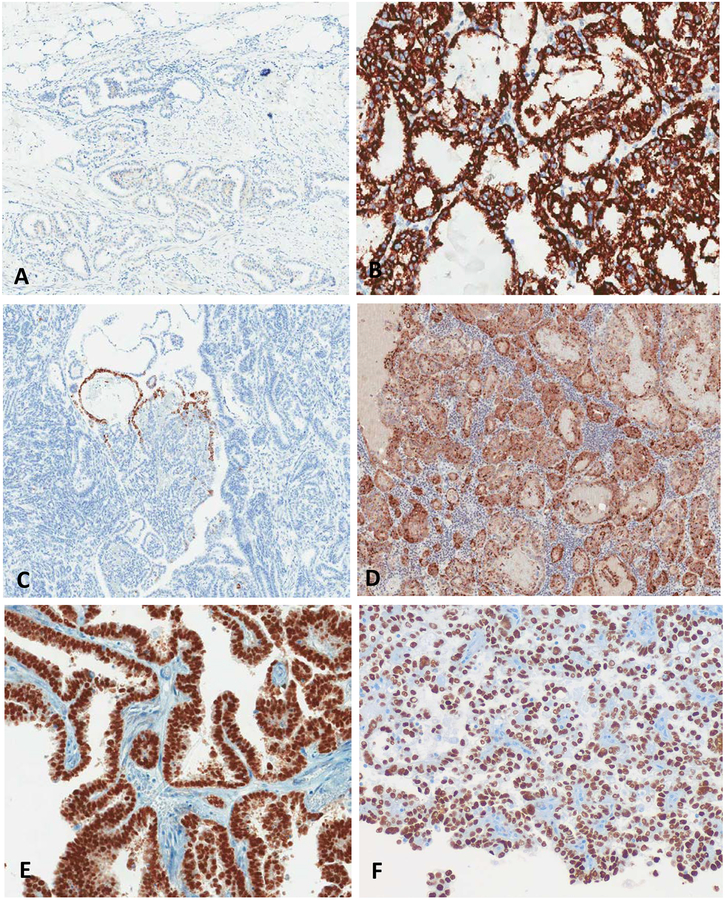
FIGURE 4.
Differential staining patterns in mesonephric carcinoma and clear cell carcinoma. Mesonephric carcinoma typically showed focal AMACR staining (A), focal Napsin A staining (C) and variable HNF-1β staining (E). By contrast, clear cell carcinoma usually showed diffuse AMACR (B) and Napsin A (D) staining, and variable HNF-1β (F) staining.
Napsin-A showed focal positive staining in 4 of 18 (22%) mesonephric cases (2 endometrial, 2 cervical). The staining was intense (3+) in 3 of the 4 cases, but all focal or patchy (1%, 1%, 5% and 20% of tumor cells) in proportion. Napsin-A showed staining in 43 of 55 (78%) cases of clear cell carcinoma (4 focal, 15 patchy, 24 diffuse)(Figure 3). The H-score of Napsin-A was statistically higher in clear cell carcinoma compared to mesonephric carcinoma (59 vs 4) (p<0.001) (Figure 3). The specificity and PPV of Napsin-A for separating clear cell carcinomas from mesonephric carcinomas was higher than that for HNF-1β, 78% and 92% respectively (Table 2). If we set a cut-off of Napsin-A staining at 10%, which has been proposed previously34, the specificity and PPV increases to 95% and 98% respectively.
AMACR showed focal positive staining in 4 of 18 mesonephric cases, all 4 were cervical. Staining intensity was moderate to strong (2+ to 3+) but proportion was focal to patchy (1%, 5%, 5% and 20% of cells) (Table 1). AMACR showed staining in 41 of 55 (75%) cases of clear cell carcinoma (9 focal, 10 patchy, 22 diffuse). The H-score of AMACR was statistically higher in clear cell carcinoma compared to mesonephric carcinoma (81 vs 2, p<0.001, Figure 3). The specificity and PPV for AMACR was very similar to that for Napsin-A (78% vs 78%, and 92% vs 91%, respectively) (Table 2).
There was no correlation between HNF-1β, Napsin-A and AMACR staining and morphologic pattern in the mesonephric carcinomas.
DISCUSSION
In our study, we examined HNF-1β, Napsin-A and AMACR immunohistochemistry in a series of 18 mesonephric carcinomas from various gynecologic sites and 55 endometrial and cervical clear cell carcinomas. Our results show that Napsin-A and AMACR are comparable, and that both are superior to HNF-1β in distinguishing between mesonephric carcinomas and clear cell carcinomas of the female genital tract.
Traditionally, before the advent of positive markers, clear cell carcinoma was diagnosed by its “negative phenotype” including lack of immunoreactivity for ER24. HNF-1β was the first “positive” marker identified for the diagnosis of ovarian clear cell carcinoma, and in the first cardinal study by Tsuchiya et al., it was shown to be very sensitive and specific, with staining in 95% of clear cell carcinoma and only 2% of non-clear cell carcinomas23. Subsequent studies of clear cell carcinomas involving other gynecologic sites reiterated that, with few exceptions, HNF-1β is good marker for distinguishing clear cell carcinoma from serous and endometrioid carcinoma21,22,28,20,19,18,35. In the gynecologic tract, HNF-1β has also been shown to be positive in usual-type endocervical carcinomas, gastric-type endocervical carcinomas, ovarian clear cell tumors and ovarian yolk sac tumor18,19,24,36 thus its usefulness is not global and is limited to context-specific scenarios. In the scenario of clear cell carcinoma versus mesonephric carcinoma, we found that HNF-1β was not useful, as it was positive in 39% of mesonephric carcinomas and 62% of clear cell carcinomas. Our findings align with the observations noted by Kenny et al.17. In their study, 3 of 8 (38%) mesonephric carcinomas were positive for HNF-1β and all 3 cases showed diffuse (≥50% of cells) staining. The only other report of HNF-1β is in a case report of a mesonephric-like carcinoma of the uterine corpus, which was negative for HNF-1β16.
In the past few years, Napsin-A has been identified as a good immunohistochemical marker for clear cell carcinoma of the gynecologic tract, showing expression in 56% to 93% of endometrial27,21,20,22, 82% to 93% of ovarian24,20,22 and 70% to 71% of endocervical22,28 clear cell carcinomas. Napsin-A has been reported to have superior specificity to HNF-1β20,24. Our study showed that Napsin-A was a helpful marker in distinguishing mesonephric and clear cell carcinomas, with a specificity of 78%. Napsin-A staining was very focal in 3 of the 4 mesonephric carcinomas that were positive, and was predominantly patchy/diffuse in clear cell carcinomas. If a diagnostic threshold for Napsin-A is set at ≥10% of cells, which was done previously by Yamashita et al.34, the specificity increases to 95%. To the best of our knowledge, this is the first report of Napsin-A staining in mesonephric carcinomas.
AMACR expression has been reported to occur in up to 7% of endometrial carcinomas37 and up to 7% of ovarian carcinomas38, but the histotypes were not listed. Noske et al.39 examined two cohorts of ovarian carcinoma (n=136 and n=252) and found AMACR staining in 11.8% and 5.4%, respectively. In the first cohort there was no correlation between AMACR staining and histologic subtype, but in the second cohort, AMACR expression was significantly related to the endometrioid and clear cell histotypes39. In 2013, Fadare et al performed a larger study where they evaluated the expression of AMACR in a series of 49 endometrial clear cell carcinomas, 13 endometrial serous carcinomas, and 49 endometrial endometrioid carcinomas and showed AMACR to be highly sensitive (75%) and specific (79%) for the diagnosis of endometrial clear cell carcinoma29. This group also investigated the expression of AMACR in ovarian clear cell carcinomas, and found that, in this subset of tumors, it was highly specific (99%) but relatively less sensitive (82%) compared to Napsin-A and HNF-1β24. In our study, the findings for AMACR were very similar to that of Napsin-A. AMACR staining was seen in 4 out of 14 (22%) of mesonephric carcinoma, but tended to be focal, while staining in clear cell carcinomas was seen in 75% of cases and were more likely to be strong and diffuse. The specificity was the same as Napsin-A, 78%. There has been only one case report, documenting AMACR staining in a mesonephric carcinoma of the cervix40. In this report, the curettage was misdiagnosed as clear cell carcinoma due to the presence of papillary, glandular, tubular, hobnail cells and clear cells. The finding of adjacent mesonephric remnants and a pseudoendometrioid pattern that was ER negative, prompted the diagnosis of mesonephric carcinoma. This tumor was positive for AMACR and HNF-1β, and negative for Napsin-A.
In summary, in their classic forms, mesonephric and clear cell carcinoma are readily distinguished from each other, but present diagnostic challenges when they show overlapping morphology, particularly on small biopsies. In this study, we found that none of the 13 mesonephric carcinomas had an accurate diagnosis of mesonephric carcinoma made on biopsy. Immunohistochemistry is therefore a valuable tool in distinguishing between these two neoplasms. While HNF-1β, Napsin-A and AMACR have all been shown to be expressed in a variety of different tumor types across the body, these markers can be extremely helpful in specific diagnostic situations. In the scenario of mesonephric carcinoma versus clear cell carcinoma, Napsin-A and AMACR are helpful markers but HNF-1β is not.
ACKNOWLEDGEMENTS
We would like to thank Simon Cheung for his technical assistance.
Footnotes
Conflicts of Interest and Source of Funding: None declared
Disclosure: This study has been presented as an abstract at the 2018 European Congress of Pathology (ECP) meeting.
REFERENCES
- 1.Silver SA, Devouassoux-Shisheboran M, Mezzetti TP et al. Mesonephric adenocarcinomas of the uterine cervix: a study of 11 cases with immunohistochemical findings. Am J Surg Pathol 2001;25:379–387. [DOI] [PubMed] [Google Scholar]
- 2.Bagué S, Rodríguez IM & Prat J Malignant mesonephric tumors of the female genital tract: a clinicopathologic study of 9 cases. Am J Surg Pathol 2004;28:601–607. [DOI] [PubMed] [Google Scholar]
- 3.McFarland M, Quick CM & McCluggage WG Hormone receptor-negative, thyroid transcription factor 1-positive uterine and ovarian adenocarcinomas: report of a series of mesonephric-like adenocarcinomas. Histopathology 2016;68:1013–1020. [DOI] [PubMed] [Google Scholar]
- 4.Clement PB, Young RH, Keh P, Ostör, et al. Malignant mesonephric neoplasms of the uterine cervix. A report of eight cases, including four with a malignant spindle cell component. Am J Surg Pathol 1995;19:1158–1171. [DOI] [PubMed] [Google Scholar]
- 5.Mirkovic J, Sholl LM, Garcia E et al. Targeted genomic profiling reveals recurrent KRAS mutations and gain of chromosome 1q in mesonephric carcinomas of the female genital tract. Mod Pathol 2015;28:1504–1514. [DOI] [PubMed] [Google Scholar]
- 6.Pors J, Cheng A, Leo JM et al. A Comparison of GATA3, TTF1, CD10, and Calretinin in Identifying Mesonephric and Mesonephric-like Carcinomas of the Gynecologic Tract. The American Journal of Surgical Pathology 2018;42:1596–1606. [DOI] [PubMed] [Google Scholar]
- 7.Hart WR & Norris HJ Mesonephric adenocarcinomas of the cervix. Cancer 1972;29: 106–113. [DOI] [PubMed] [Google Scholar]
- 8.Valente PT & Susin M Cervical adenocarcinoma arising in florid mesonephric hyperplasia: report of a case with immunocytochemical studies. Gynecol Oncol 1987;27:58–68. [DOI] [PubMed] [Google Scholar]
- 9.Scully RE & Barlow JF “Mesonephroma” of ovary. Tumor of Mullerian nature related to the endometrioid carcinoma. Cancer 1967;20:1405–1417. [DOI] [PubMed] [Google Scholar]
- 10.Schiller W Mesonephroma ovarii. Am J Cancer 1939;1–21. [Google Scholar]
- 11.Herbst AL & Scully RE Adenocarcinoma of the vagina in adolescence. A report of 7 cases including 6 clear-cell carcinomas (so-called mesonephromas). Cancer 1970;25:745–757. [DOI] [PubMed] [Google Scholar]
- 12.McGee CT, Cromer DW & Green RR Mesonephric carcinoma of the cervix – Differentiation from endocervical adenocarcinoma. American Journal of Obstetrics and Gynecology 1962;84:358–366. [Google Scholar]
- 13.Huo D, Anderson D & Herbst AL Follow-up of Patients with Clear-Cell Adenocarcinoma of the Vagina and Cervix. N Engl J Med 2018;378:1746–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pirog EC Prevalence of human papillomavirus DNA in different histological subtypes of cervical adenocarcinoma. Am J Pathol 2000;157:1055–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fadare O, Zheng W, Crispens MA et al. Morphologic and other clinicopathologic features of endometrial clear cell carcinoma: a comprehensive analysis of 50 rigorously classified cases. Am J Cancer Res 2013;3:70–95. [PMC free article] [PubMed] [Google Scholar]
- 16.Wani Y, Notohara K & Tsukayama C Mesonephric adenocarcinoma of the uterine corpus: a case report and review of the literature. Int J Gynecol Pathol 2008;27:346–352. [DOI] [PubMed] [Google Scholar]
- 17.Kenny SL, McBride HA, Jamison J et al. Mesonephric adenocarcinomas of the uterine cervix and corpus: HPV-negative neoplasms that are commonly PAX8, CA125, and HMGA2 positive and that may be immunoreactive with TTF1 and hepatocyte nuclear factor 1-β. Am J Surg Pathol 2012;36:799–807. [DOI] [PubMed] [Google Scholar]
- 18.Stolnicu S, Barsan I, Hoang L et al. Diagnostic Algorithmic Proposal Based on Comprehensive Immunohistochemical Evaluation of 297 Invasive Endocervical Adenocarcinomas. American Journal of Surgical Pathology 2018;42(8):989–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park KJ Kiyokawa T, Soslow RA et al. Unusual endocervical adenocarcinomas: an immunohistochemical analysis with molecular detection of human papillomavirus. American Journal of Surgical Pathology 2011;35:633–646. [DOI] [PubMed] [Google Scholar]
- 20.Lim D, Ip PPC, Cheung ANY, et al. Immunohistochemical comparison of ovarian and uterine endometrioid carcinoma, endometrioid carcinoma with clear cell change, and clear cell carcinoma. American Journal of Surgical Pathology 2015;39:1061–1069. [DOI] [PubMed] [Google Scholar]
- 21.Hoang LN, McConechy MK, Meng B et al. Targeted mutation analysis of endometrial clear cell carcinoma. Histopathology 2015;66:664–674. [DOI] [PubMed] [Google Scholar]
- 22.Ju B, Wang J, Yang B et al. Morphologic and Immunohistochemical Study of Clear Cell Carcinoma of the Uterine Endometrium and Cervix in Comparison to Ovarian Clear Cell Carcinoma. Int J Gynecol Pathol 2018;37:388–396. [DOI] [PubMed] [Google Scholar]
- 23.Tsuchiya A, Sakamoto M, Yasuda J et al. Expression profiling in ovarian clear cell carcinoma: identification of hepatocyte nuclear factor-1β as a molecular marker and a possible molecular target for therapy of ovarian clear cell carcinoma. American Journal of Pathology 2003;163(6)2503–2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fadare O, Zhao C, Khabele D et al. Comparative analysis of Napsin-A, alpha-methylacyl-coenzyme A racemase (AMACR, P504S), and hepatocyte nuclear factor 1 beta as diagnostic markers of ovarian clear cell carcinoma: an immunohistochemical study of 279 ovarian tumours. Pathology 2015;47:105–111. [DOI] [PubMed] [Google Scholar]
- 25.DeLair D, Oliva E, Kobel M et al. Morphologic spectrum of immunohistochemically characterized clear cell carcinoma of the ovary: a study of 155 cases. Am J Surg Pathol 2011;35:36–44. [DOI] [PubMed] [Google Scholar]
- 26.Kato N & Motoyama T Hepatocyte nuclear factor-1beta(HNF-1beta) in human urogenital organs: its expression and role in embryogenesis and tumorigenesis. Histol Histopathol 2009;24:1479–1486. [DOI] [PubMed] [Google Scholar]
- 27.Fadare O, Desouki MM, Gwin K et al. Frequent expression of Napsin-A in clear cell carcinoma of the endometrium: potential diagnostic utility. American journal of surgical pathology 2014;38(2):189–196. [DOI] [PubMed] [Google Scholar]
- 28.Talia KL, Wong RW & McCluggage WG Expression of Markers of Müllerian Clear Cell Carcinoma in Primary Cervical and Vaginal Gastric-type Adenocarcinomas. Int J Gynecol Pathol 2018. [DOI] [PubMed] [Google Scholar]
- 29.Fadare O, Parkash V, Gwin K et al. Utility of α-methylacyl-coenzyme-A racemase (p504s) immunohistochemistry in distinguishing endometrial clear cell carcinomas from serous and endometrioid carcinomas. Hum Pathol 2013;44:2814–2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kadivar M & Boozari B Applications and limitations of immunohistochemical expression of “Napsin-A” in distinguishing lung adenocarcinoma from adenocarcinomas of other organs. Appl Immunohistochem Mol Morphol 2013;21:191–195. [DOI] [PubMed] [Google Scholar]
- 31.Kim MY, Go H, Koh J et al. Napsin-A is a useful marker for metastatic adenocarcinomas of pulmonary origin. Histopathology 2014;65:195–206. [DOI] [PubMed] [Google Scholar]
- 32.Jiang Z, Woda BA, Wu C-L et al. Discovery and Clinical Application of a Novel Prostate Cancer Marker: α-Methylacyl CoA Racemase (P504S). Am J Clin Pathol 2004;122: 275–289. [DOI] [PubMed] [Google Scholar]
- 33.Hoang LN, Han G, McConechy M et al. Immunohistochemical characterization of prototypical endometrial clear cell carcinoma--diagnostic utility of HNF-1β and oestrogen receptor. Histopathology 2014;64:585–596. [DOI] [PubMed] [Google Scholar]
- 34.Yamashita Y, Nagasaka T, Nakiki-Ito A et al. Napsin-A is a specific marker for ovarian clear cell adenocarcinoma. Mod Pathol 2015;28:111–117. [DOI] [PubMed] [Google Scholar]
- 35.Fadare O & Liang SX Diagnostic utility of hepatocyte nuclear factor 1-beta immunoreactivity in endometrial carcinomas: lack of specificity for endometrial clear cell carcinoma. Appl Immunohistochem Mol Morphol 2012;20:580–587). [DOI] [PubMed] [Google Scholar]
- 36.Carleton C, Hoang L, Sah S et al. A Detailed Immunohistochemical Analysis of a Large Series of Cervical and Vaginal Gastric-type Adenocarcinomas. Am J Surg Pathol 2016;40:636–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nassar A, Amin MB, Sexton DG et al. Utility of alpha-methylacyl coenzyme A racemase (p504s antibody) as a diagnostic immunohistochemical marker for cancer. Appl Immunohistochem Mol Morphol 2005;13:252–255. [DOI] [PubMed] [Google Scholar]
- 38.Jiang Z, Fanger GR, Woda BA et al. Expression of α-methylacyl-coa racemase (p504s) in various malignant neoplasms and normal tissues: a study of 761 cases. Human Pathology 2003;34:792–796. [DOI] [PubMed] [Google Scholar]
- 39.Noske A, Zimmermann AK, Caduff R, et al. Alpha-methylacyl-CoA racemase (AMACR) expression in epithelial ovarian cancer. Virchows Arch 2011;459:91–97. [DOI] [PubMed] [Google Scholar]
- 40.Kır G, Seneldir H & Kıran G A case of mesonephric adenocarcinoma of the uterine cervix mimicking an endometrial clear cell carcinoma in the curettage specimen. J Obstet Gynaecol 2016;36:827–829. [DOI] [PubMed] [Google Scholar]