How are leukocyte dynamics controlled and regulated in an intact vertebrate? Deficient recruitment of innate immune cells can permit damage to an organism or allow infection to persist, while overexuberant or lasting inflammation can result in collateral harm to the host. Chemokines and their cognate receptors play central roles in leukocyte recruitment; their regulation affects the trajectory of inflammation, infection, and resolution. In this issue, Sommer et al. uncover a fascinating mechanism by which the CXC chemokine receptor 3 (CXCR3) axis is regulated in zebrafish: a paralogous copy of the cxcr3 gene generates an atypical chemokine receptor on macrophages that influences macrophage migration, the immune response, and the dynamics and distribution of mycobacterial infection [1].
In mammals, the CXCR3 axis plays important roles in recruiting a variety of immune cells, including macrophages. In zebrafish, a close functional and sequence orthologue of CXCR3 is expressed on macrophages and neutrophils, and is responsive to zebrafish CXC Chemokine Ligand 11 (CXCL11) orthologues [2]. Zebrafish larvae represent a powerful system to dissect the role of specific chemokine receptors and signalling events during the host response to injury and infection; the larvae are optically transparent, genetically accessible, and have been widely used to understand conserved vertebrate immune responses during infection and inflammation. Sommer et al. use this platform to examine the role of the zebrafish cxcr3 genes in the context of mycobacterial infection, asking how alterations in macrophage behavior and chemokine responsiveness impact pathogenesis.
Macrophages, the first responders in Mycobacterium tuberculosis (M. tb) infection play an essential role in tuberculosis (TB) disease progression. During early stages of M. tb infections, macrophages serve as a replicative niche and recruit additional macrophages, initiating formation of mycobacterial granulomas, the hallmark structures of TB. Infection of zebrafish with Mycobacterium marinum, a close relative of M. tb and a natural fish pathogen, generates mycobacterial granulomas with conserved features of human TB granulomas. While granulomas may ultimately help to contain infection, initially they also facilitate the spread of infection to newly recruited macrophages or, once fully mature, limit immune access to bacteria within [3, 4]. Importantly, many host genes associated with human TB pathogenesis are conserved in the zebrafish model. Induction of key chemokines and cytokines mirrors that observed in human mycobacterial infections, with important roles in infection trajectory for the C-C motif chemokine ligand 2 (CCL2) and for Vegfa induction by granuloma macrophages [5, 6].
Using the zebrafish infection model, Torraca et. al had previously shown that zebrafish Cxcr3.2, the orthologue of human CXCR3, and its ligands Cxcl11aa and Cxcl11af help mediate macrophage recruitment to sites of mycobacterial infection [2]. The Cxcr3.2 receptor is a class A (rhodopsin‐like) G‐protein‐coupled receptor. Genetic disruption or pharmacological inhibition of this signalling axis caused a reduction in mycobacterial burden as well as decreased dissemination to distal tissues [2]. These findings agree with a number of other studies suggesting that macrophage recruitment and early granuloma formation can serve to promote mycobacterial growth and dissemination [3]
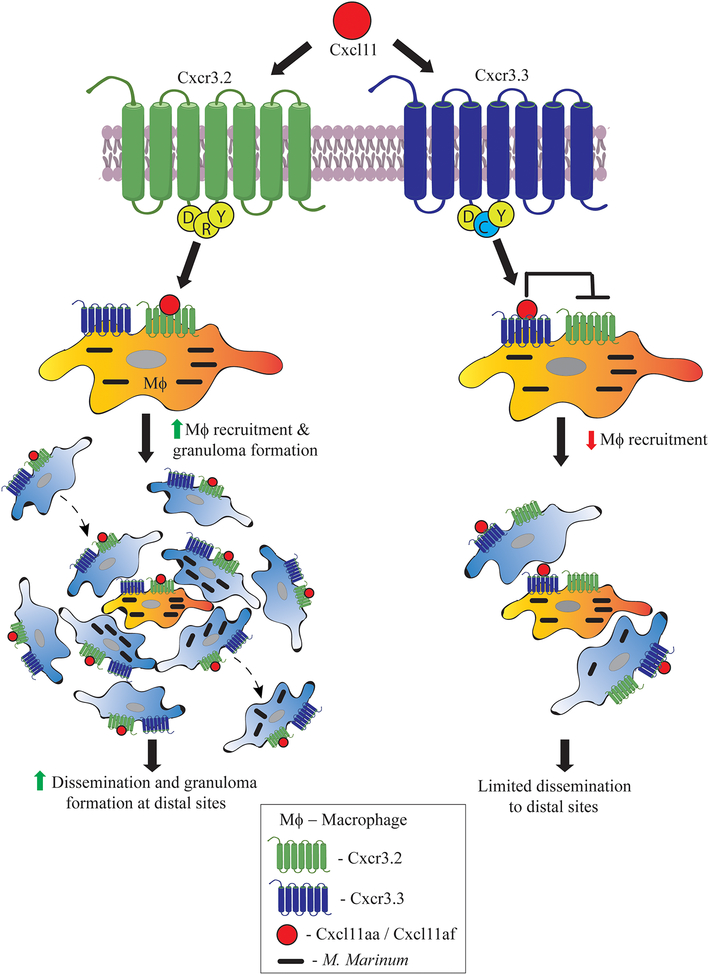
In the featured article, Sommer et. al show that the paralogous gene Cxcr3.3 encodes a receptor with many properties of an atypical chemokine receptor (ACKR) (Figure 1). In mammals, ACKRs are appreciated to play important roles in fine-tuning canonical chemokine responses, through their action as competing decoy or scavenger receptors. Sommer et al. present data suggesting that Cxcr3.3 acts antagonistically to Cxcr3.2 to regulate the CXCR3-CXCL11 signalling axis [1]. The Meijer group had previously found that zebrafish macrophages express Cxcr3.3 along with its paralogue Cxcr3.2 [2]. Here the authors closely analyzed the protein sequences and found that the paralogues share a conserved ligand binding site; however, unlike Cxcr3.2, Cxcr3.3 has an altered E/DRY-motif as well as alterations to predicted microswitches within the transmembrane domains, resembling ACKRs (Figure 1). Mammalian ACKRs lack the ability to signal robustly through G-proteins, but can bind and scavenge chemokines, limiting their availability and fine-tuning the amounts and distribution of bioavailable chemokines. The ACKRs thus aid in regulating inflammation and play a significant role in resolving chronic inflammation [7]. Hence the authors hypothesized that Cxcr3.3 acts similarly to antagonize the function of Cxcr3.2 (Figure 1).
Figure 1. Regulation of the Cxcr3 signalling axis in zebrafish via an antagonistic Cxcr3 receptor pair.
In this issue, Sommer et. al describe a novel regulatory function for zebrafish Cxcr3.3, a paralogue of the previously characterized chemokine receptor Cxcr3.2. In comparison to Cxcr3.2, Cxcr3.3 has an altered E/DRY motif (DCY) that resembles atypical chemokine receptors (ACKRs), potentially limiting signalling through G-proteins. Upon binding to its ligand Cxcl11, Cxcr3.2 has been shown to drive increased macrophage recruitment to M. marinum infection sites and consequently promote dissemination to distal sites. In contrast, Cxcr3.3 is posited to compete with Cxcr3.2 for Cxcl11, thereby acting to antagonize Cxcr3.2 function. This antagonism leads to reduced macrophage migration and ultimately limits dissemination of infection to distal sites. Thus, deficiencies for the antagonistic Cxcr3.3 receptor result in more dissemination and higher bacterial burdens during mycobacterial infections.
A genetic knockout of cxcr3.3 resulted in an increase in the mycobacterial burden, a phenotype roughly opposite to that of the cxcr3.2 knockout phenotype observed earlier. Notably, cxcr3.3 knockout macrophages retained their bactericidal properties and expression of proinflammatory markers, discounting the possibility that reduced activation status of these macrophages might explain the observed increase in mycobacterial burden. Using a tail amputation model, the authors showed that Cxcr3.3-deficient macrophages exhibited enhanced migration toward the wound site. This result suggested that enhanced recruitment of cxcr3.3 knockout macrophages to an infection site or subsequently enhanced efflux might promote dissemination of infection, leading to increased bacterial burden in these mutants. Indeed, mycobacterial infection in cxcr3.3 mutant larvae resulted in more frequent distal granuloma formation when compared to wildtype or cxcr3.2 mutant fish. Thus, the Cxcr3.3 mutants were more likely to promote mycobacterial dissemination (Figure 1). These results suggest functionally antagonistic interactions in vivo between these two zebrafish Cxcr3 paralogues.
Although canonical ACKR-mediated regulation of human CXCR3 has not been described, an alternative mechanism exists to regulate its function. Two of the three CXCR3 splice variants bind to identical ligands but carry out antagonistic functions, suggesting isoform rather than paralogue-based regulation in mammals [8]. CXCR3 has been detected on other immune cells, including T lymphocytes during Mycobacterium tuberculosis infection [9]. Hence, its overall role during infection might depend on its combined effects on diverse immune cell populations. In Salmonella enterica infections in mice, CXCR3+ Th1 cells reside at the periphery of a different, non-epithelioid granuloma type, and play an important role in limiting infection [10]. Thus, expansion of these findings to other cell types and infections will be an interesting future direction.
Thus far, at least four ACKRs have been identified and characterized in humans, of which two -- ACKR2 and ACKR3 -- have been found on leukocytes [7]. The zebrafish genome has four ACKR orthologues annotated as ackr3a, ackr3b, ackr4a and ackr4b. Future work identifying and characterizing such atypical receptors may further complement our understanding of the regulation described here. The work by Sommer et al opens new opportunities to study potential roles for these orthologues, especially ackr3a and ackr3b, as well as yet to be characterized ACKRs during infection and inflammation.
More generally, this work illustrates that chemokine signalling during leukocyte recruitment is regulated at multiple levels, including via antagonistic receptors that likely control the magnitude, spatial, and temporal dynamics of chemokine gradients. Pathogenic microbes that interact with these cell types may have evolved mechanisms to target these pathways and promote their own success.
Acknowledgements
We thank C. Pyle for helpful comments on the manuscript. Funding for the authors’ laboratory is provided by the National Institutes of Health grants AI130236, AI125517, and AI127115.
References
- 1.Sommer F, Torraca V, Kamel SM, Lombardi A, Meijer AH (2019) Frontline Science: Antagonism between regular and atypical Cxcr3 receptors regulates macrophage migration during infection and injury in zebrafish. Journal of leukocyte biology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Torraca V, Cui C, Boland R, Bebelman JP, van der Sar AM, Smit MJ, Siderius M, Spaink HP, Meijer AH (2015) The CXCR3-CXCL11 signaling axis mediates macrophage recruitment and dissemination of mycobacterial infection. Dis Model Mech 8, 253–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davis JM and Ramakrishnan L (2009) The role of the granuloma in expansion and dissemination of early tuberculous infection. Cell 136, 37–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cronan MR, Beerman RW, Rosenberg AF, Saelens JW, Johnson MG, Oehlers SH, Sisk DM, Jurcic Smith KL, Medvitz NA, Miller SE, Trinh LA, Fraser SE, Madden JF, Turner J, Stout JE, Lee S, Tobin DM (2016) Macrophage epithelial reprogramming underlies mycobacterial granuloma formation and promotes infection. Immunity 45, 861–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cambier CJ, Takaki KK, Larson RP, Hernandez RE, Tobin DM, Urdahl KB, Cosma CL, Ramakrishnan L (2014) Mycobacteria manipulate macrophage recruitment through coordinated use of membrane lipids. Nature 505, 218–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oehlers SH, Cronan MR, Scott NR, Thomas MI, Okuda KS, Walton EM, Beerman RW, Crosier PS, Tobin DM (2015) Interception of host angiogenic signalling limits mycobacterial growth. Nature 517, 612–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonecchi R and Graham GJ (2016) Atypical chemokine receptors and their roles in the resolution of the inflammatory response. Front Immunol 7, 224–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lasagni L, Francalanci M, Annunziato F, Lazzeri E, Giannini S, Cosmi L, Sagrinati C, Mazzinghi B, Orlando C, Maggi E, Marra F, Romagnani S, Serio M, Romagnani P (2003) An alternatively spliced variant of CXCR3 mediates the inhibition of endothelial cell growth induced by IP-10, Mig, and I-TAC, and acts as functional receptor for platelet factor 4. The Journal of experimental medicine 197, 1537–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Silveira-Mattos PS, Narendran G, Akrami K, Fukutani KF, Anbalagan S, Nayak K, Subramanyam S, Subramani R, Vinhaes CL, Souza DO, Antonelli LR, Satagopan K, Porter BO, Sher A, Swaminathan S, Sereti I, Andrade BB (2019) Differential expression of CXCR3 and CCR6 on CD4+ T-lymphocytes with distinct memory phenotypes characterizes tuberculosis-associated immune reconstitution inflammatory syndrome. Scientific reports 9, 1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldberg MF, Roeske EK, Ward LN, Pengo T, Dileepan T, Kotov DI, Jenkins MK (2018) Salmonella Persist in Activated Macrophages in T Cell-Sparse Granulomas but Are Contained by Surrounding CXCR3 Ligand-Positioned Th1 Cells. Immunity 49, 1090–1102 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]