Abstract
Breast cancer survival has improved with significant progress in treatment and disease management. However, compliance with treatment varies. Treatment guidelines for older patients are unclear. We aim to identify predictors of noncompliance with recommended therapy in a large breast cancer population and assess the impact of noncompliance on survival. Our study included 19,241 non-metastatic female breast cancer patients, of whom 3,158 (16%) died within 10 years post-diagnosis (median survival = 5.8 years). We studied the association between treatment noncompliance and factors with logistic regression, and the impact of treatment noncompliance on survival with a flexible parametric survival model framework. The highest proportion of noncompliance was observed for chemotherapy (18%). Predictors of noncompliance with chemotherapy, radiotherapy and endocrine therapy included age, tumor size, nodal involvement and subtype (except radiotherapy). Factors associated with not receiving surgery included age and subtype. Treatment noncompliance was associated with worse overall survival for surgery (HR: 2.26 [1.80–2.83]), chemotherapy (1.25 [1.11–1.41]), radiotherapy (2.28 [1.94–2.69]) and endocrine therapy (1.70 [1.41–2.04]). Worse survival was similarly observed in older patients for whom guidelines generally do not apply. Our results highlight the importance of following appropriate treatment as recommended by current guidelines. Older patients may benefit from similar recommendations.
Subject terms: Epidemiology, Risk factors
Introduction
Breast cancer is the leading type of cancer among Asian women, with an increasing number of cases diagnosed every year1. Between 2003 and 2008, there were more than 2 million women living with breast cancer in South-East Asia, where a population of over 650 million women resides2. As breast cancer is common, the number of lives claimed by the disease is high. On average ~100,000 deaths from breast cancer were recorded every year in the same region3. With significant progress in treatment and disease management, a growing number of women are surviving breast cancer4. However, breast cancer survival can vary between countries. While 90% of patients in the United States live at least five years after the cancer is found5, the corresponding proportion is lower in South-East Asia. In a report by Bhoo-Pathy et al., five-year overall survival rates were estimated to range from 58.5% to 75.8% in South-East Asia6.
Several professional organizations and consensus groups exist to translate evidence-based medicine into recommendations for best patient care7. Examples of providers of such clinical practice guidelines include the American Society of Clinical Oncology (ASCO), the European Society for Medical Oncology (ESMO), the U.S. National Comprehensive Cancer Network (NCCN) and the St. Gallen International Breast Cancer Consensus Conference7–9. Guideline-based treatment has been shown to improve overall survival in breast cancer patients10–13. For example, breast cancer patients who underwent recommended radiotherapy were up to four times less likely to die from the disease than patients who did not follow the recommended treatment12–14. Similarly, breast cancer patients who complied to recommended endocrine therapy were two times less likely to die from any cause, compared to patients who were noncompliant with recommended treatment12,15.
Despite the established survival benefit, some patients decline evidence-based treatment recommendations offered by their physicians16. Attitudes towards health is known to vary between cultures, which may have a strong impact on the uptake of recommended treatment and survival outcome17,18. Horne et al. defined compliance as “the extent to which the patient’s behavior matches the prescriber’s recommendations”19. In this study, we aim to identify factors associated with compliance with recommended treatment in an Asian breast cancer population and assess the impact of treatment compliance on survival. As treatment guidelines are unclear for older breast cancer patients20, we also examined whether recommended treatment confer a survival benefit to patients over 70 years of age.
Methods
Study population
Hospital-based registry data on 20,999 citizens and permanent residents of Singapore diagnosed with breast cancer (ICD 9: 174*; ICD 10: C50*) between 2005 and 2015 was obtained from six major restructured public hospitals in Singapore (Changi General Hospital [CGH], KK Women’s and Children’s Hospital [KKH], National Cancer Center Singapore [NCCS], National University Hospital [NUH], Singapore General Hospital [SGH], and Tan Tock Seng Hospital [TTSH]). Patients who were diagnosed with metastatic breast cancer (n = 1,644), who were male (n = 26), whose date of birth was not reported (n = 6), or who had missing information on death date (n = 82) were excluded (Fig. S1). Ethical approval for using the de-identified pooled data was obtained from the National Healthcare Group Domain Specific Review Board (NHG DSRB Ref: 2013/01085 and CIRB Ref: 2016/3010). The institutional review boards of the respective hospitals involved in this study had approved the conduct of this study without the need for informed consent.
Compliance with treatment guidelines
The restructured public hospitals in Singapore generally follow the guidelines of the NCCN and the St Gallen 2005 consensus (Table 1)9,20. For each treatment (chemotherapy, radiotherapy and endocrine therapy), patients were classified into three groups: (1) started recommended treatment [compliant], (2) did not start recommended treatment [noncompliant], or (3) treatment not recommended [compliant]. All patients were non-metastatic at diagnosis and should be recommended surgery (i.e. started recommended treatment [compliant] or did not start recommended treatment [noncompliant]).
Table 1.
Recommended treatment for women diagnosed with non-metastatic breast cancer as adapted from the St Gallen 2005 consensus.
| Treatment | Criteria |
|---|---|
| Surgery | Non-metastatic breast cancer patients |
| Chemotherapy |
If a patient meets the following criteria the patient would be considered indicated for chemotherapy: ● Positive nodes ● Tumor size from pathology report > 20 mm or attached to chest wall ● Tumor size > 5 mm with one or more of the following high risk criteria ○ Grade 3 tumor ○ Estrogen receptor status negative ○ HER2-positive ○ Age ≤ 35 years |
| Radiotherapy | Patients who fulfill either or both criteria: ● Positive nodes ● Breast conserving surgery ● Tumor size > 50 mm or attached to chest wall |
| Endocrine therapy | Patients who are estrogen or progesterone receptor status positive |
Demographic and clinical characteristics
Demographic and clinical characteristics were extracted from hospital-based registries, which include age at diagnosis (<50, 50–69, ≥70 years), ethnicity (Chinese, Malay, Indian, other), year of diagnosis (2005–2010, 2011–2015), pre-surgical tumor size according to TNM classification (≤20 mm, 21–50 mm, >50 mm, attached to chest wall, unknown), pathological TNM nodal stage (N0, N1, N2, N3, unknown), pathological tumor stage (in situ, stage I, II, III, unknown), grade (well-differentiated, moderately-differentiated, poorly-differentiated, unknown), estrogen receptor status (ER: positive, negative, unknown), progesterone receptor status (PR: positive, negative, unknown), human epidermal growth factor receptor 2 status (HER2: positive, negative, unknown), proxy tumor subtypes (luminal A [ER-positive/PR-positive, and well- or moderately-differentiated], luminal B (HER2-positive) [ER-positive/PR-positive, HER2-positive, and poorly-differentiated], luminal B (HER2-negative) [ER-positive/PR-positive, HER2-negative, and poorly-differentiated], HER2-enriched [ER-negative, PR-negative, and HER2-positive], basal [ER-negative, PR-negative, and HER2-negative], unknown)9.
Outcome of interest
The primary outcome of interest was overall survival, with time since diagnosis in years as the underlying time scale. Patients were followed up until death, or censored due to either loss of follow-up or end of study period (ten years after date of diagnosis). Information on death was verified by each hospital with the National Registry of Births and Deaths in Singapore through National Registry Disease Office21. Date of last follow-up for breast cancer patients verified as alive for SGH and NCCS was the date of last visit to the clinic. Date of last follow-up for other institutions was defined as the date on which the patient’s vital status was verified with National Registry of Births and Deaths (KKH: 30-June-2017, NUH: 30-April-2017, TTSH: 30-April-2018, CGH: 16-April-2018).
Statistical analysis
Bivariate associations between vital status (dead or alive) and patient-related characteristics (demographic, clinical and treatment) were evaluated using the Chi-square test. To identify patient-related characteristics associated with the compliance with recommended treatment, logistic regression was used to estimate the odds ratios (OR) and corresponding 95% confidence intervals.
To examine the effect of treatment compliance on survival, mortality rate was calculated. This risk estimate was defined as the number of deaths divided by risk time (total person-years [PY] for which the patients were alive), modeled using flexible parametric survival model, with cubic splines function for baseline mortality. The associations between individual treatments and mortality rates (unadjusted) was first modelled assuming proportion hazards throughout the follow-up time. A flexible parametric survival model framework was then used to model time-dependent effects (STATA version 8, command: stpm2), hazard ratios (HR) and corresponding 95% confidence intervals were estimated. The Akaike Information Criterion (AIC) statistic was used to optimize the number of internal knots (i.e. degrees of freedom of the baseline model) and the number of knots for the time varying component knots (i.e. degrees of freedom of the time varying component). Degrees of freedom used in the respective flexible parametric survival models are shown in Supplementary Table 1. Kaplan-Meier survival curves were plotted to graphically represent survival over time (R Version 3.4.3, package: survival).
Results
A total of 19,241 female breast cancer patients with 3,158 (16%) deaths occurring within 10 years of diagnosis were included in this study (Table 2). The median survival time was 5.8 years (interquartile range: 3.3–8.7). The majority were Chinese (n = 15,419, 80%), 2,108 (11%) were Malay, and 1,113 (6%) were Indian. Thirty-five percent (n = 6,699) of our patients were diagnosed before age 50, 52% (n = 10,027) between 50 and 69, and 13% (n = 2,515) at age 70 and above. Fifteen percent (n = 2,884), 27% (n = 5,183), 33% (n = 6,312), and 15% (n = 2,861) of our patients were diagnosed with in situ, stage I to III breast cancer, respectively. The largest proportion of our patients were of luminal A proxy subtype (n = 5,934, 31%), followed by luminal B [HER2-negative] (n = 2,610, 14%), luminal B [HER2-positive] (n = 2,189, 11%), HER2-enriched (n = 1,491, 8%), and basal (n = 1,658, 9%). The highest proportion of noncompliance with treatment was observed for chemotherapy 18% (n = 3,482), followed by radiotherapy (8%, n = 1,614), surgery (8%, n = 1604), and endocrine therapy (8%, n = 1,533) (Table 2).
Table 2.
Demographic, tumor characteristic and treatment variables of 19,241 women diagnosed with breast cancer between 2005 and 2015 by vital status.
| Vital status | P-value | ||
|---|---|---|---|
| Alive | Dead | ||
| n = 16,083 (84%) | n = 3,158 (16%) | ||
| Median survival time (IQR) | 6.3 (3.8–9.1) | 3.2 (1.7–5.4) | <0.001 |
| Demographics | |||
| Ethnicity | |||
| Chinese | 13,101 (85%) | 2,318 (15%) | <0.001 |
| Malay | 1,588 (75%) | 520 (25%) | |
| Indian | 897 (81%) | 216 (19%) | |
| Other | 497 (83%) | 104 (17%) | |
| Median age (IQR) | |||
| Age group, years | 53 (46–62) | 59 (50–72) | <0.001 |
| <50 | 5,941 (89%) | 758 (11%) | <0.001 |
| 50–69 | 8,566 (85%) | 1,461 (15%) | |
| ≥70 | 1,576 (63%) | 939 (37%) | |
| Year of diagnosis | |||
| 2005–2010 | 8,257 (80%) | 2,115 (20%) | <0.001 |
| 2011–2015 | 7,826 (88%) | 1,043 (12%) | |
| Center | |||
| CGH | 995 (79%) | 258 (21%) | <0.001 |
| KKH | 3,659 (89%) | 455 (11%) | |
| NUH | 2,218 (84%) | 425 (16%) | |
| SGH and NCCS | 7,575 (82%) | 1,609 (18%) | |
| TTSH | 1,636 (80%) | 411 (20%) | |
| Tumor characteristics | |||
| Pre-surgical tumor size | |||
| ≤20 mm | 7,518 (93%) | 608 (7%) | <0.001 |
| 21–50 mm | 4,823 (82%) | 1,074 (18%) | |
| >50 mm | 1,684 (79%) | 444 (21%) | |
| Attached to chest wall | 494 (57%) | 379 (43%) | |
| Unknown | 1,564 (71%) | 653 (29%) | |
| TNM nodal stage | |||
| N0 | 10,628 (92%) | 928 (8%) | <0.001 |
| N1 | 2,823 (83%) | 564 (17%) | |
| N2 | 1,083 (74%) | 383 (26%) | |
| N3 | 623 (60%) | 422 (40%) | |
| Unknown | 926 (52%) | 861 (48%) | |
| Tumor stage | |||
| In situ | 2,734 (95%) | 150 (5%) | <0.001 |
| I | 4,857 (94%) | 326 (6%) | |
| II | 5,464 (87%) | 848 (13%) | |
| III | 1,933 (68%) | 928 (32%) | |
| Unknown | 1,095 (55%) | 906 (45%) | |
| Grade | |||
| Well-differentiated | 2,522 (92%) | 218 (8%) | <0.001 |
| Moderately-differentiated | 5,925 (88%) | 788 (12%) | |
| Poorly-differentiated | 6,397 (80%) | 1,552 (20%) | |
| Unknown | 1,239 (67%) | 600 (33%) | |
| Estrogen receptor status | |||
| Positive | 11,069 (86%) | 1,786 (14%) | <0.001 |
| Negative | 3,275 (77%) | 985 (23%) | |
| Unknown | 1,739 (82%) | 387 (18%) | |
| Progesterone receptor status | |||
| Positive | 9,577 (87%) | 1,484 (13%) | <0.001 |
| Negative | 4,631 (79%) | 1,255 (21%) | |
| Unknown | 1,875 (82%) | 419 (18%) | |
| HER2 status | |||
| Positive | 3,020 (82%) | 675 (18%) | 0.001 |
| Negative | 8,975 (84%) | 1,743 (16%) | |
| Unknown | 4,088 (85%) | 740 (15%) | |
| Proxy subtype | |||
| Luminal A | 5,342 (90%) | 592 (10%) | <0.001 |
| Luminal B, HER2-negative | 2,091 (80%) | 519 (20%) | |
| Luminal B, HER2-positive | 1,810 (83%) | 379 (17%) | |
| HER2-enriched | 1,198 (80%) | 293 (20%) | |
| Basal | 1,217 (73%) | 441 (27%) | |
| Unknown | 4,425 (83%) | 934 (17%) | |
| Treatment | |||
| Surgery | |||
| Yes | 15,295 (87%) | 2,342 (13%) | <0.001 |
| No | 788 (49%) | 816 (51%) | |
| Chemotherapy | |||
| Yes | 6,251 (83%) | 1,301 (17%) | <0.001 |
| No | 2,521 (72%) | 961 (28%) | |
| Not recommended | 7,311 (89%) | 896 (11%) | |
| Radiotherapy | |||
| Yes | 7,013 (87%) | 1,044 (13%) | <0.001 |
| No | 1,180 (73%) | 434 (27%) | |
| Not recommended | 6,243 (91%) | 635 (9%) | |
| Unknown | 1,647 (61%) | 1,045 (39%) | |
| Endocrine therapy | |||
| Yes | 9,217 (86%) | 1,501 (14%) | <0.001 |
| No | 1,305 (85%) | 228 (15%) | |
| Not recommended | 2,843 (77%) | 831 (23%) | |
| Unknown | 2,718 (82%) | 598 (18%) | |
TNM nodal stage: post-surgery if surgery is done.
Tumor stage: post-surgery, patients without surgery are classified as unknown.
Factors associated with treatment noncompliance in patients who were recommended treatment
Proxy subtype had the strongest association with not undergoing surgery (luminal B [HER2-positive], HER2-enriched, and basal vs. luminal A, OR: 4.08 [2.72–6.12], 2.50 [1.52–4.12] and 2.03 [1.24–3.35] respectively), followed by age at diagnosis (<50 and ≥70 vs. 50–69 years, OR: 1.57 [1.30–1.89] and 2.94 [2.34–3.68] respectively) and pre-surgical tumor size (21–50 mm and >50 mm vs. <20 mm, OR: 2.23 [1.85–2.68] and 2.54 [1.93–3.34] respectively) (Table 3).
Table 3.
The odds of not receiving treatment with respect to demographic characteristics and treatment was studied using logistic model.
| Surgery*, n = 19,241 | Chemotherapy*, n = 11,034 | Radiotherapy*, n = 9,671 | Endocrine therapy*, n = 12,251 | |||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted | Adjusted^ | Unadjusted | Adjusted^ | Unadjusted | Adjusted^ | Unadjusted | Adjusted^ | |
| OR (95%CI) | OR (95%CI) | OR (95%CI) | OR (95%CI) | OR (95%CI) | OR (95%CI) | OR (95%CI) | OR (95%CI) | |
| Ethnicity | ||||||||
| Chinese | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| Malay | 1.12 (0.87 to 1.43) | 1.25 (0.95 to 1.63) | 0.70 (0.62 to 0.80) | 0.90 (0.78 to 1.04) | 1.23 (1.05 to 1.44) | 1.10 (0.92 to 1.30) | 0.98 (0.83 to 1.17) | 1.07 (0.89 to 1.28) |
| Indian | 1.27 (0.93 to 1.73) | 1.33 (0.95 to 1.85) | 1.08 (0.92 to 1.28) | 1.25 (1.04 to 1.52) | 1.40 (1.14 to 1.72) | 1.26 (1.01 to 1.58) | 0.98 (0.78 to 1.24) | 1.02 (0.79 to 1.30) |
| Other | 1.16 (0.75 to 1.79) | 1.31 (0.82 to 2.09) | 0.90 (0.72 to 1.14) | 0.91 (0.70 to 1.18) | 0.95 (0.69 to 1.30) | 0.80 (0.57 to 1.13) | 1.04 (0.76 to 1.40) | 0.97 (0.70 to 1.34) |
| Age at diagnosis, years | ||||||||
| <50 | 1.54 (1.29 to 1.84) | 1.57 (1.30 to 1.89) | 0.87 (0.79 to 0.95) | 0.77 (0.69 to 0.85) | 0.83 (0.74 to 0.94) | 0.80 (0.70 to 0.91) | 1.25 (1.11 to 1.40) | 1.17 (1.03 to 1.32) |
| 50–69 | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| ≥70 | 2.77 (2.25 to 3.41) | 2.94 (2.34 to 3.68) | 9.92 (8.61 to 11.42) | 13.96 (11.94 to 16.32) | 3.17 (2.72 to 3.69) | 3.27 (2.75 to 3.88) | 1.15 (0.98 to 1.36) | 1.08 (0.91 to 1.29) |
| Year of diagnosis | ||||||||
| 2005–2010 | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| 2011–2015 | 0.75 (0.64 to 0.88) | 1.15 (0.96 to 1.38) | 1.20 (1.11 to 1.31) | 1.15 (1.05 to 1.27) | 1.11 (1.00 to 1.23) | 1.05 (0.93 to 1.19) | 1.05 (0.94 to 1.17) | 1.14 (1.02 to 1.28) |
| Pre-surgical tumor size | ||||||||
| ≤20 mm | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| 21–50 mm | 1.45 (1.23 to 1.72) | 2.23 (1.85 to 2.68) | 0.63 (0.57 to 0.69) | 0.61 (0.54 to 0.68) | 1.21 (1.07 to 1.36) | 1.02 (0.88 to 1.18) | 0.51 (0.45 to 0.58) | 0.67 (0.58 to 0.77) |
| >50 mm | 1.24 (0.97 to 1.58) | 2.54 (1.93 to 3.34) | 0.58 (0.51 to 0.67) | 0.59 (0.50 to 0.70) | 1.25 (1.06 to 1.47) | 0.92 (0.77 to 1.12) | 0.43 (0.35 to 0.54) | 0.46 (0.37 to 0.58) |
| Attached to chest wall | 0.54 (0.44 to 0.65) | 0.31 (0.24 to 0.39) | 1.28 (1.00 to 1.64) | 0.81 (0.61 to 1.07) | 0.52 (0.39 to 0.70) | 0.55 (0.39 to 0.76) | ||
| TNM nodal stage | ||||||||
| N0 | — | — | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| N1 | 0.51 (0.46 to 0.57) | 0.35 (0.31 to 0.40) | 2.55 (2.26 to 2.89) | 2.53 (2.20 to 2.92) | 0.24 (0.19 to 0.29) | 0.30 (0.25 to 0.38) | ||
| N2 | 0.36 (0.31 to 0.42) | 0.26 (0.21 to 0.30) | 0.79 (0.64 to 0.96) | 0.71 (0.57 to 0.89) | 0.25 (0.19 to 0.34) | 0.36 (0.27 to 0.49) | ||
| N3 | 0.51 (0.43 to 0.60) | 0.32 (0.26 to 0.39) | 1.46 (1.21 to 1.78) | 1.22 (0.98 to 1.51) | 0.57 (0.43 to 0.74) | 0.67 (0.50 to 0.90) | ||
| Proxy subtype | ||||||||
| Luminal A | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| Luminal B, HER2-negative | 1.28 (0.77 to 2.13) | 1.22 (0.73 to 2.05) | 1.15 (1.01 to 1.31) | 0.72 (0.62 to 0.85) | 1.10 (0.93 to 1.30) | 1.08 (0.90 to 1.30) | 1.20 (1.01 to 1.42) | 1.49 (1.25 to 1.78) |
| Luminal B, HER2-positive | 4.17 (2.80 to 6.21) | 4.08 (2.72 to 6.12) | 1.05 (0.92 to 1.20) | 0.57 (0.48 to 0.67) | 1.16 (0.97 to 1.39) | 1.16 (0.95 to 1.42) | 1.45 (1.22 to 1.73) | 1.80 (1.50 to 2.16) |
| HER2-enriched | 2.71 (1.66 to 4.44) | 2.50 (1.52 to 4.12) | 0.88 (0.75 to 1.02) | 0.41 (0.34 to 0.50) | 1.08 (0.87 to 1.34) | 1.04 (0.82 to 1.32) | — | — |
| Basal | 2.37 (1.45 to 3.89) | 2.03 (1.24 to 3.35) | 0.93 (0.80 to 1.08) | 0.42 (0.35 to 0.50) | 0.98 (0.80 to 1.20) | 0.94 (0.75 to 1.17) | — | — |
*Women who started treatment was used as the reference category of the outcome, hence an odds ratio (OR) value above 1 implies that the exposure category (e.g. Indian) has higher odds of not starting recommended treatment than the reference level of the exposure factor (e.g. Chinese). CI: Confidence interval. ^Additionally adjusted for site.
Factors associated with chemotherapy noncompliance were ethnicity (Indian vs. Chinese, OR: 1.25 [1.04–1.52]), older age at diagnosis (≥70 vs. 50–59 years, OR: 13.96 [11.94–16.32]), and year of diagnosis (2011–2015 vs. 2005–2010, OR: 1.15 [1.05–1.27]) (Table 3). Pre-surgical tumor size (21–50 mm, >50 mm, or attached to chest wall vs. <20 mm), nodal stage (N1, N2, or N3 vs. N0), and proxy subtype (luminal B [HER2-negative], luminal B [HER2-positive], HER2-enriched or basal vs. luminal A), were associated with compliance with recommended chemotherapy (OR <1.00 and P < 0.001 for all comparison).
Radiotherapy noncompliance was associated with ethnicity (Indian vs. Chinese, OR: 1.26 [1.01–1.58]), age at diagnosis (≥70 vs. 50–59 years, OR: 3.27 [2.75–3.88]), and nodal stage (N1 vs N0, OR: 2.53 [2.20–2.92]) (Table 3). Factors associated with radiotherapy compliance were pre-surgical tumor size (21–50 mm, >50 mm, or attached to chest wall vs. <20 mm: OR <1.00 and P < 0.001 for all comparison) and nodal stage (N2 vs N0, OR: 0.71 [0.57–0.89]).
Younger age at diagnosis (<50 vs. 50–59 years, OR: 1.17 [1.03–1.32]), year of diagnosis (2011–2015 vs. 2005–2010, OR: 1.14 [1.02–1.28]), and proxy subtype (luminal B [HER2-negative] and luminal B [HER2-positive] vs. luminal A, OR: 1.49 [1.25–1.78] and 1.80 [1.50–2.16], respectively) were associated with endocrine therapy noncompliance (Table 3). Pre-surgical tumor size (21–50 mm, >50 mm, or attached to chest wall vs. <20 mm) and nodal stage (N1, N2, or N3 vs. N0) were associated with compliance with recommended endocrine therapy (OR <1.00 and P < 0.001 for all comparisons).
Mortality rates by treatment compliance
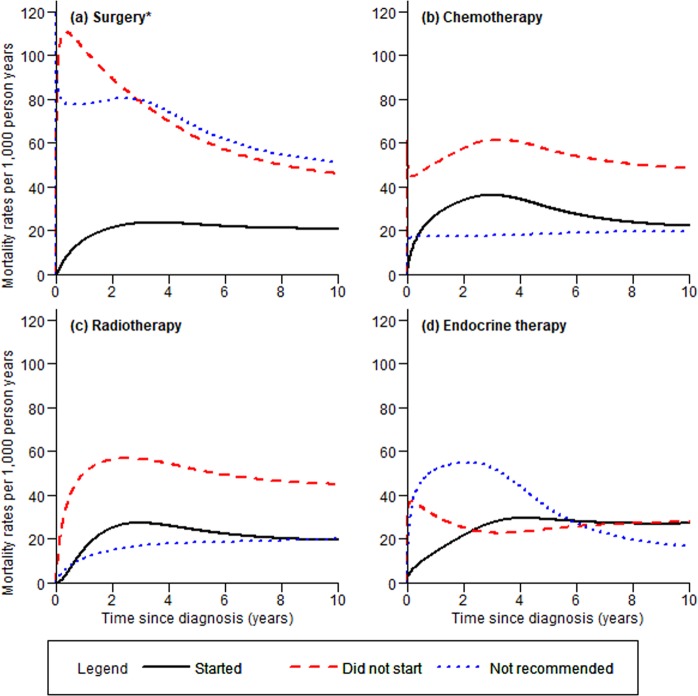
Not undergoing recommended surgery resulted in worse survival, particularly in the first year after breast cancer diagnosis (Fig. 1a). A larger proportion of patients who did not start recommended chemotherapy or radiotherapy died compared to patients who started recommended treatment and patients for whom treatment was not recommended (Fig. 1b,c). While mortality rates for patients who were not recommended treatment (vs. started treatment) were slightly lower for chemotherapy and radiotherapy, the mortality rate for patients who were not recommended endocrine therapy (i.e. patients who are ER- and PR-negative) was higher than the corresponding rate for patients who started endocrine therapy within ~6 years post-diagnosis (Fig. 1d).
Figure 1.
Unadjusted mortality rates of breast cancer patients by treatments; (a) surgery, (b) chemotherapy, (c) radiotherapy, and (d) endocrine therapy. *Patients who had tumors attached to chest wall or whose pre-surgical tumor size were not known were represented by the blue dotted line.
Overall survival in patients aged <70 years
Noncompliance with recommended therapy was associated with worse overall survival for surgery (HR: 2.2 [1.80–2.83]), chemotherapy (HR: 1.25 [1.11–1.41]), radiotherapy (HR: 2.28 [1.94–2.67]) and endocrine therapy (HR: 1.70 [1.41–2.04]) (Table 4). Largest differences (HR ≥ 9.66, P < 0.001) in overall survival was observed in patients who did not undergo surgery across all proxy subtypes. However, the effect was diminished for luminal B [HER2-negative] (HR: 6.43 [2.62–15.80]), basal (HR: 5.46 [2.05–14.54]) and luminal A (HR: 2.85 [1.31–6.18]), and was not significant for luminal B [HER2-positive] and HER2-enriched, upon adjusting for other treatments, tumor characteristics and patient’s demographics.
Table 4.
The associations between demographic, clinical, and treatment variables and overall survival using the flexible parametric survival model, stratified by proxy subtype, in patients aged <70 years.
| All patients aged < 70 years | Luminal A | Luminal B, HER2-negative | ||||
|---|---|---|---|---|---|---|
| Unadjusted | Adjusted^ | Unadjusted | Adjusted^ | Unadjusted | Adjusted^ | |
| HR (95%CI) | HR (95%CI) | HR (95%CI) | HR (95%CI) | HR (95%CI) | HR (95%CI) | |
| Surgery | ||||||
| Yes | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| No | 6.97 (6.28–7.74) | 2.26 (1.80–2.83) | 12.92 (9.30–17.95) | 2.85 (1.31–6.18) | 14.04 (9.78–20.14) | 6.43 (2.62–15.80) |
| Chemotherapy | ||||||
| Yes | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| No | 1.51 (1.35–1.70) | 1.25 (1.11–1.41) | 1.51 (1.04–2.19) | 1.57 (1.03–2.39) | 1.19 (0.96–1.49) | 1.12 (0.87–1.45) |
| Not recommended | 0.47 (0.42–0.52) | 0.69 (0.60–0.80) | 0.64 (0.50–0.82) | 0.60 (0.32–1.12) | 0.14 (0.04–0.58) | 0.35 (0.08–1.43) |
| Radiotherapy | ||||||
| Yes | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| No | 2.50 (2.13–2.94) | 2.28 (1.94–2.67) | 2.12 (1.45–3.12) | 2.36 (1.53–3.64) | 2.25 (1.70–2.98) | 2.32 (1.68–3.21) |
| Not recommended | 0.68 (0.58–0.79) | 1.37 (1.14–1.64) | 1.18 (0.89–1.56) | 2.40 (1.60–3.61) | 0.52 (0.39–0.70) | 1.11 (0.73–1.67) |
| Endocrine therapy | ||||||
| Yes | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| No | 1.41 (1.17–1.69) | 1.70 (1.41–2.04) | 1.86 (1.29–2.69) | 1.71 (1.15–2.53) | 2.12 (1.55–2.90) | 1.81 (1.28–2.55) |
| Not recommended | 2.19 (1.95–2.45) | 1.82 (1.63–2.04) | — | — | — | — |
| Pre-surgical tumor size | ||||||
| ≤20 mm | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| 21–50 mm | 2.89 (2.48–3.35) | 1.78 (1.53–2.06) | 1.92 (1.49–2.48) | 1.40 (1.07–1.84) | 2.84 (1.94–4.16) | 1.83 (1.24–2.71) |
| >50 mm | 4.05 (3.41–4.81) | 2.66 (2.25–3.15) | 2.95 (2.17–4.02) | 2.30 (1.63–3.25) | 4.01 (2.62–6.14) | 2.24 (1.44–3.48) |
| Attached to chest wall | 11.71 (9.83–13.93) | 4.37 (3.63–5.21) | 7.58 (5.38–10.70) | 3.49 (2.37–5.13) | 9.66 (6.14–15.20) | 4.17 (2.59–6.71) |
| TNM nodal stage | ||||||
| N0 | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| N1 | 2.35 (2.00–2.74) | 1.71 (1.43–2.05) | 1.31 (0.94–1.83) | 0.87 (0.43–1.76) | 2.13 (1.51–2.99) | 1.66 (1.07–2.59) |
| N2 | 3.75 (3.14–4.49) | 2.98 (2.47–3.60) | 2.89 (2.02–4.13) | 2.03 (0.99–4.15) | 3.09 (2.12–4.53) | 2.63 (1.66–4.18) |
| N3 | 7.73 (6.60–9.04) | 4.54 (3.76–5.49) | 6.27 (4.39–8.95) | 4.39 (2.13–9.06) | 6.11 (4.38–8.54) | 5.42 (3.52–8.34) |
| Ethnicity | ||||||
| Chinese | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| Malay | 2.08 (1.87–2.31) | 1.56 (1.40–1.74) | 2.65 (2.07–3.39) | 1.78 (1.37–2.31) | 1.88 (1.49–2.38) | 1.59 (1.25–2.03) |
| Indian | 1.47 (1.24–1.73) | 1.15 (0.97–1.36) | 1.39 (0.90–2.12) | 1.28 (0.83–1.97) | 1.18 (0.79–1.78) | 0.87 (0.58–1.32) |
| Other | 1.07 (0.82–1.38) | 1.09 (0.84–1.41) | 1.64 (0.99–2.72) | 1.75 (1.05–2.93) | 0.92 (0.45–1.85) | 1.01 (0.50–2.06) |
| Age at diagnosis, years | ||||||
| <50 | 0.73 (0.67–0.80) | 0.78 (0.72–0.86) | 0.44 (0.33–0.58) | 0.42 (0.31–0.57) | 0.80 (0.66–0.98) | 0.85 (0.69–1.04) |
| 50–69 | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| Year of diagnosis | ||||||
| 2005–2010 | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| 2011–2015 | 0.96 (0.87–1.06) | 0.98 (0.90–1.08) | 0.89 (0.71–1.13) | 0.96 (0.75–1.24) | 0.81 (0.65–1.00) | 0.92 (0.73–1.16) |
| Surgery | ||||||
| Yes | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| No | 10.27 (7.79–13.53) | 2.55 (0.84–7.77) | 11.59 (8.25–16.26) | 1.62 (0.68–3.81) | 9.66 (6.89–13.55) | 5.46 (2.05–14.54) |
| Chemotherapy | ||||||
| Yes | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| No | 1.52 (1.16–2.01) | 1.23 (0.90–1.67) | 1.56 (1.10–2.20) | 1.23 (0.83–1.81) | 1.60 (1.22–2.10) | 1.48 (1.08–2.03) |
| Not recommended | 0.22 (0.07–0.76) | 0.54 (0.16–1.85) | 0.48 (0.23–0.99) | 1.34 (0.60–3.00) | 0.25 (0.05–1.15) | 0.71 (0.15–3.47) |
| Radiotherapy | ||||||
| Yes | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| No | 2.17 (1.51–3.13) | 3.11 (2.05–4.71) | 2.43 (1.52–3.86) | 2.64 (1.57–4.44) | 2.15 (1.52–3.05) | 2.68 (1.80–3.98) |
| Not recommended | 0.58 (0.41–0.81) | 2.79 (1.35–5.76) | 0.56 (0.37–0.85) | 1.91 (0.86–4.29) | 0.42 (0.30–0.60) | 0.64 (0.37–1.11) |
| Endocrine therapy | ||||||
| Yes | 1.00 (Reference) | 1.00 (Reference) | — | — | — | — |
| No | 1.46 (0.96–2.21) | 1.79 (1.13–2.83) | ||||
| Pre-surgical tumor size | ||||||
| ≤20 mm | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| 21–50 mm | 3.39 (2.31–4.96) | 2.06 (1.39–3.08) | 3.46 (1.96–6.09) | 1.95 (1.09–3.48) | 2.29 (1.59–3.31) | 1.72 (1.23–2.41) |
| >50 mm | 3.64 (2.33–5.68) | 2.22 (1.39–3.55) | 4.52 (2.46–8.29) | 2.61 (1.39–4.92) | 4.48 (3.01–6.66) | 3.16 (2.15–4.64) |
| Attached to chest wall | 10.77 (6.96–16.67) | 4.13 (2.54–6.70) | 14.65 (7.92–27.11) | 6.50 (3.39–12.48) | 6.02 (3.81–9.53) | 3.05 (1.90–4.88) |
| TNM nodal stage | ||||||
| N0 | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| N1 | 2.36 (1.64–3.38) | 3.55 (1.73–7.28) | 2.83 (1.79–4.46) | 3.31 (1.49–7.34) | 2.90 (1.95–4.30) | 1.61 (1.10–2.35) |
| N2 | 4.35 (3.02–6.28) | 7.21 (3.53–14.74) | 4.13 (2.60–6.56) | 5.89 (2.65–13.10) | 4.78 (3.09–7.40) | 2.63 (1.72–4.03) |
| N3 | 7.48 (5.01–11.18) | 11.96 (5.71–25.05) | 5.64 (3.58–8.88) | 6.35 (2.84–14.21) | 9.63 (6.50–14.29) | 6.93 (4.69–10.25) |
| Ethnicity | ||||||
| Chinese | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| Malay | 1.76 (1.32–2.34) | 1.16 (0.86–1.56) | 1.82 (1.26–2.62) | 1.74 (1.19–2.54) | 1.80 (1.34–2.42) | 1.44 (1.07–1.95) |
| Indian | 1.78 (1.18–2.70) | 1.11 (0.72–1.71) | 1.67 (0.93–3.01) | 1.32 (0.70–2.49) | 1.05 (0.68–1.62) | 0.76 (0.50–1.16) |
| Other | 0.42 (0.13–1.31) | 0.38 (0.12–1.19) | 1.55 (0.72–3.30) | 1.45 (0.67–3.17) | 0.81 (0.35–1.84) | 0.90 (0.40–2.04) |
| Age at diagnosis, years | ||||||
| <50 | 0.49 (0.38–0.63) | 0.54 (0.41–0.71) | 0.78 (0.57–1.06) | 0.93 (0.67–1.28) | 1.10 (0.88–1.38) | 1.17 (0.93–1.47) |
| 50–69 | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| Year of diagnosis | ||||||
| 2005–2010 | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| 2011–2015 | 0.85 (0.66–1.09) | 0.91 (0.70–1.19) | 0.92 (0.68–1.25) | 1.01 (0.73–1.40) | 1.08 (0.84–1.37) | 1.20 (0.93–1.54) |
HR: Hazards ratio, CI: Confidence interval. ^Adjusted for all variables listed in the table and site.
Chemotherapy noncompliance was associated with higher mortality within 10 years post-diagnosis, in patients with luminal A (HR: 1.57 [1.03–2.39]) and basal (HR: 1.48 [1.08–2.03]) proxy subtypes. Noncompliance with radiotherapy was associated with worse overall survival across all breast cancer proxy subtypes (adjusted P < 0.001). Patients who were noncompliant with recommended endocrine therapy had worse overall survival than compliant patients, in proxy subtypes luminal A (HR: 1.71 [1.15–2.53]), luminal B [HER2-negative] (HR: 1.81 [1.28–2.55]) and luminal B [HER2-positive] (HR: 1.79 [1.13–2.83]) tumors.
Overall survival in older patients aged ≥70 years
Patients aged ≥70 years who did not undergo surgery had a 1.74 [1.33–2.29] times increased risk of death within 10 years post-diagnosis as compared with those who had surgery (Supplementary Table 2). Noncompliance with recommended treatment was associated with worse overall survival (chemotherapy (HR: 1.31 [1.03–1.66]), radiotherapy (HR: 1.64 [1.30–2.07]) and endocrine therapy (HR: 1.57 [1.20–2.07])). In addition, the survival benefit associated with compliance to recommended chemotherapy was observed only when there is also compliance with radiotherapy (No vs Yes [reference], HR: 1.46 [0.98–2.17], log rank P = 0.061) (Fig. 2). Compliance with chemotherapy treatment was associated with worse survival in patients who were noncompliant with recommended radiotherapy (Yes vs No [reference], HR: 2.04 [1.19–3.49], log rank P = 0.008).
Figure 2.
Overall survival of women diagnosed with and had surgery for breast cancer by age and compliance with chemotherapy by compliance with radiotherapy. The average life expectancy of females in Singapore is 85, thus patients aged 86 years and above were excluded from this analysis42. Survival curves for age group 51–69 and patients not recommend chemotherapy are not shown.
Discussion
Treatment compliance, which has an impact on survival, was high for primary surgery, radiotherapy and endocrine therapy but low for chemotherapy. Factors associated with chemotherapy, radiotherapy and endocrine therapy noncompliance included older age, smaller pre-surgical tumor size, and lower nodal stage. Patients with luminal A proxy subtype were more likely to be noncompliant with chemotherapy than other proxy subtypes. Patients with luminal B [HER2-positive] or luminal B [HER2-negative] proxy subtype were more likely to be noncompliant with endocrine therapy than patients with luminal A proxy subtype. Noncompliance with recommended treatment (chemotherapy, radiotherapy, or endocrine therapy) was associated with a ~1.5 to 6-fold increased risk of dying within 10 years post-diagnosis. In patients aged ≥70 years who were recommended chemotherapy, noncompliance with recommended chemotherapy and radiotherapy were associated with worse survival.
Factors associated with treatment noncompliance
In agreement with previous studies, tumor characteristics (pre-surgical tumor size, grade, proxy subtype) and age at diagnosis were associated with initiation of recommended treatment22–24. Many factors can potentially influence a patient’s decision to go along with recommended treatment: previous experiences and personal values17, fear of treatment-related side effects18, financial ability, age, education and health status, among others19,20. Although information on financial status was not available, the subjects in our study were patients at restructured hospitals and were eligible for subsidized treatment. It is thus less likely that any ethnic disparity observed was due to differences in financial resources25.
An ethnic disparity in recommended treatment uptake has also been observed by others; recommended treatment uptake is frequently lower in minority populations26,27. Although higher noncompliance with chemotherapy and radiotherapy were observed in Indian patients as compared with Chinese patients, the observed worse survival was not significant. To the contrary, we observed no disparate treatment uptake among Malay and Chinese ethnic groups where worse survival was observed in Malay patients (HR: 2.08 [1.87–2.31]). This worse survival of Malay patients remained (HR: 1.57 [1.41–1.75]) after accounting for compliance with treatment, pre-surgical tumor size, nodal status, age at diagnosis, year of diagnosis and center. Since Malay patients were just as likely to start recommended treatment as Chinese patients, it is unlikely that the survival difference was due to treatment compliance. Moreover, according to the work of Hill et al. in breast cancer patients in the United States, the disparity in the survival of different ethnicities among the older patients in Asia was not due to deviation from guideline recommended treatment12. Further studies to identify other factors that delay or prevent the timely receipt of recommended treatment would allow efforts to be directed towards their mitigation.
Treatment compliance and overall survival
The high mortality rate associated with not proceeding with recommended surgery is in agreement with results reported in existing literature10,13. Notably, patients who did not follow recommended chemotherapy, radiotherapy or endocrine therapy were ~2 to 3 times more likely to die from breast cancer than patients who do12–15.
Our results are in agreement with previous studies which found increased risk of death in patients who did not start endocrine therapy12,15. Interestingly, while mortality rates of breast cancer patients who followed recommended endocrine therapy were lower than that of patients who did not, the curves converged and crossed at ~2.5 and ~6 years after diagnosis (i.e. mortality rate for treatment compliant patients increased and higher than non-compliant patients). Endocrine therapy is typically recommended for at least five years. While we were not able to study drug adherence in our patients, unsatisfactory adherence to treatment for the entire recommended duration is known to be associated with worse survival, which could potentially explain the observed increasing mortality rate among treatment-compliant patients in our study28. As such, the survival benefit of endocrine therapy compliance would better reach its full potential if common factors contributing to non-adherence, such as patient forgetfulness and concerns about adverse effects, were followed up and addressed29.
Chemotherapy may appear to be less effective among patients with less aggressive breast cancers, where extensive treatment was unnecessarily administered for early stage disease30–32. For example, in patients who have favorable tumor characteristics (ER-positive/PR-positive, HER2-negative, and without nodal involvement) who met established guidelines for the recommendation of chemotherapy, those with favorable gene-expression profile may benefit from endocrine therapy alone33. Herr et al., found that chemotherapy did not improve survival in patients with luminal A subtype, who had tumor size >20 mm and >3 positive nodes34. We observed that the survival benefit of starting recommended chemotherapy was more pronounced in patients with luminal A and basal proxy subtypes and starting recommended radiotherapy has the largest survival benefit in luminal B [HER2-positive] patients. We were not able to study comorbidities in our population which may have contributed to the contradictory findings with Herr et al. in luminal A subtype patients, as patients who are more frail are more likely to not start chemotherapy and also have worse survival.
Age at diagnosis and recommended chemotherapy
The NCCN guidelines restrict their recommendations on chemotherapy to breast cancer patients aged <70 years20. In addition, clinical trials on the use of chemotherapy typically do not recruit patients older than 70 years, providing little empirical support for the use of such treatment in older patients35. Clinicians are less likely to recommend aggressive or invasive treatment such as chemotherapy or surgery for older women due to the increased likelihood of existing comorbidities23,24. Nonetheless, older patients can benefit from optimal anti-cancer therapy. For example, a previous study in stage I and II breast cancer patients found that having surgery reduced the risk of breast cancer specific death in patients aged ≥80 years36.
While chemotherapy compliance is generally associated with better survival, this was not the case for older patients who were noncompliant with radiotherapy. The reason for not starting recommended treatment is not known. Clinicians may at their discretion not advise guideline-recommended treatment for patients who were already in ill health. Patient level information such as comorbidities and performance status was not available as a proxy of health status at diagnosis. Hence, it is unclear whether the worse survival observed in patients who did not receive treatment was due to the lack of treatment or other causes (e.g. patients’ preference, fear of adverse effects, or the lack of support)26,37.
Older patients may also be more prone to side effects from treatment, such as chemotherapy-induced neutropenia, fever and infection38. In older patients who complied with radiotherapy, chemotherapy conferred a substantial survival advantage. While the better survival linked to treatment compliance in the older breast cancer population could be an artifact due to more favorable health status, a similar survival benefit was still seen in a subset of patients who survived at least two years after diagnosis. This suggests that with close monitoring of patient response and the use of prophylactic agents such as granulocyte colony-stimulating factor to prevent adverse side effects, older patients too, may benefit from appropriate chemotherapy treatment39. Nonetheless, this result is noteworthy because women over 70 years of age comprise 13% of all breast cancer patients. Mortality rate is also highest for this age group.
Our study is not without limitations. Our classification of treatment was largely based on international guidelines which changes over time. However, most treatment modality and indication has seen small changes within our study period 2005–201540. Our results on overall survival may not be generalizable to the women diagnosed between 2011 and 2015 due to the shorter follow-up time. However, stratified analysis by year of diagnosis (2005–2010 and 2011–2015, Supplementary Table 3) resulted in estimates of the adjusted hazard ratios that were similar with those of 2005–2015 in Table 4. The introduction of targeted therapy, which is commonly administered to HER2 positive patients on chemotherapy, during this period (2005–2015) could not be accounted for. This may lead to an overestimation of the benefit of chemotherapy in our study. In an attempt to mitigate this influence, we performed stratified analysis by proxy subtype. We were not able to account for potential survival differences due to disparity in treatment intensity and toxicity. However, our result is likely to be biased towards the null, as the premature stopping of treatment, dose reduction and adverse drug reactions will reduce survival in the group of patients who started treatment. In addition, the evaluation of endocrine therapy impact on survival can be improved by studying the 5 year adherence of treatment instead of only the start of treatment. Information on comorbidities was not available, which may have result in contraindication of treatments and worse survival in patients who did not start treatment. Here, we partially accounted for comorbidities in patients aged >70 who would be more affected by comorbidities. There was no appreciable difference in the results (apart from uptake in surgery), when we excluded those who survived >2 years post-diagnosis in patients aged >70. The socioeconomic status and health seeking behavior of patients attending restructured hospitals (patients are eligible for subsidized care) may be different from patients attending private hospitals. However, it is recognized that the majority (~80%) of Singaporean’s attend restructured hospitals and healthcare policies are targeted at these patients. Being geographically small with an extensive public transport system, refusal of recommended treatment due to inaccessibility is less common than in areas where specialized centers may be far from residential districts41.
Conclusion
Noncompliance with recommended treatment was associated with worse survival (HR between 1.5 and 6.4). A survival benefit was observed when older breast cancer patients were treated with chemotherapy otherwise recommended only for younger patients (<70 years), suggesting that older patients may benefit from similar recommendations. Proxy subtype, pre-surgical tumor size, nodal stage, age at diagnosis and year of diagnosis were associated with treatment compliance. Our results highlight the importance of following appropriate treatment as recommended by current guidelines.
Supplementary information
Acknowledgements
The study was supported by the National Research Foundation Singapore (NRF-NRFF2017–02). MH was supported by the National Medical Research Council Clinician Scientist Award (Senior Investigator Category, NMRC/CSA-SI/0015/2017), the National University Cancer Institute Singapore Centre Grant Programme (CGAug16M005), the Saw Swee Hock School of Public Health Programme of Research Seed Funding (SSHSPH-Res-Prog) and the Asian Breast Cancer Research Fund (N-176-000–023-091). We want to thank research staff – Yen Shing Yeoh, Siew Li Tan, Ying Jia Chew, Siok Hoon Yeo, Ting Ting Koh, Amanda Ong, Jin Yee Lee, Michelle Jia Qi Mo, Jing Jing Hong, Ganga Devi D/O Chandrasegran, Nur Khaliesah Binte Mohamed Ri, Hui Wen Loh, Wei Xiang Lian and the co-investigators on the Joint Breast Cancer Registry for their contributions.
Author contributions
Conceptualization, M.H., J. Li., P.J.H. and J. Liu; Methodology, M.H., J. Li, P.J.H. and J. Liu; Formal Analysis, P.J.H.; Resources, M.H.; Data Curation, M.H., F.Y.W., B.K.T.T., E.Y.T., S.H.L. and S.M.T.; Writing – Original Draft Preparation, P.J.H., J. Li and M.H.; Writing – Review & Editing, P.J.H., J. Li, M.H., J. Liu, F.Y.W., S.G.W.O., Y.S., S.H.L., E.Y.T., S.M.T., S.C.L., B.K.T.T., V.K.M.T., Y.S.Y. and W.Y.C.; Supervision, M.H. and J. Li.; Project Administration, P.J.H. and J. Liu; Funding Acquisition, M.H. and J. Li.
Data availability
Data used in this study is de-identified. Data is available upon request from Ms Yen Shing Yeoh (email: yen_shing_yeoh@nuhs.edu.sg).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors jointly supervised this work: Jingmei Li and Mikael Hartman.
Supplementary information
is available for this paper at 10.1038/s41598-020-58007-5.
References
- 1.Youlden DR, Cramb SM, Yip CH, Baade PD. Incidence and mortality of female breast cancer in the Asia-Pacific region. Cancer biology & medicine. 2014;11:101–115. doi: 10.7497/j.issn.2095-3941.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.SingHealth. The landscape of breast cancer screening and treatment in Singapore – how well do we know it, https://www.singhealth.com.sg/DoctorsAndHealthcareProfessionals/Medical-News/2016/Pages/landscape-breast-screening-treatment-singapore.aspx (2016).
- 3.Cancer Fact Sheets: Breast Cancer, https://gco.iarc.fr/today/data/pdf/fact-sheets/cancers/cancer-fact-sheets-15.pdf (2016).
- 4.Narod SA, Iqbal J, Miller AB. Why have breast cancer mortality rates declined? Journal of Cancer Policy. 2015;5:8–17. doi: 10.1016/j.jcpo.2015.03.002. [DOI] [Google Scholar]
- 5.Gloeckler Ries LA, Reichman ME, Lewis DR, Hankey BF, Edwards BK. Cancer survival and incidence from the Surveillance, Epidemiology, and End Results (SEER) program. The oncologist. 2003;8:541–552. doi: 10.1634/theoncologist.8-6-541. [DOI] [PubMed] [Google Scholar]
- 6.Bhoo-Pathy N, et al. Ethnic differences in survival after breast cancer in South East Asia. PloS one. 2012;7:e30995. doi: 10.1371/journal.pone.0030995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tyagi NK, Dhesy-Thind S. Clinical practice guidelines in breast cancer. Current oncology (Toronto, Ont.) 2018;25:S151–s160. doi: 10.3747/co.25.3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Senkus E, et al. Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of oncology: official journal of the European Society for Medical Oncology. 2015;26(Suppl 5):v8–30. doi: 10.1093/annonc/mdv298. [DOI] [PubMed] [Google Scholar]
- 9.Goldhirsch A, et al. Meeting highlights: international expert consensus on the primary therapy of early breast cancer 2005. Annals of oncology: official journal of the European Society for Medical Oncology. 2005;16:1569–1583. doi: 10.1093/annonc/mdi326. [DOI] [PubMed] [Google Scholar]
- 10.Verkooijen HM, et al. Patients’ refusal of surgery strongly impairs breast cancer survival. Annals of surgery. 2005;242:276–280. doi: 10.1097/01.sla.0000171305.31703.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lawrenson R, et al. Treatment and survival disparities by ethnicity in New Zealand women with stage I-III breast cancer tumour subtypes. Cancer causes & control: CCC. 2017;28:1417–1427. doi: 10.1007/s10552-017-0969-9. [DOI] [PubMed] [Google Scholar]
- 12.Hill DA, et al. Breast cancer survival, survival disparities, and guideline-based treatment. Breast cancer research and treatment. 2018;170:405–414. doi: 10.1007/s10549-018-4761-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gaitanidis A, et al. Refusal of Cancer-Directed Surgery by Breast Cancer Patients: Risk Factors and Survival Outcomes. Clinical breast cancer. 2018;18:e469–e476. doi: 10.1016/j.clbc.2017.07.010. [DOI] [PubMed] [Google Scholar]
- 14.Wockel A, et al. The impact of adjuvant radiotherapy on the survival of primary breast cancer patients: a retrospective multicenter cohort study of 8935 subjects. Annals of oncology: official journal of the European Society for Medical Oncology. 2014;25:628–632. doi: 10.1093/annonc/mdt584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wockel A, et al. Effects of guideline adherence in primary breast cancer–a 5-year multi-center cohort study of 3976 patients. Breast (Edinburgh, Scotland) 2010;19:120–127. doi: 10.1016/j.breast.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 16.Joseph K, et al. Outcome analysis of breast cancer patients who declined evidence-based treatment. World journal of surgical oncology. 2012;10:118. doi: 10.1186/1477-7819-10-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chui, P. L., Abdullah, K. L., Wong, L. P. & Taib, N. A. Quality of life in CAM and Non-CAM users among breast cancer patients during chemotherapy in Malaysia. PloS one10 (2015). [DOI] [PMC free article] [PubMed]
- 18.Kang E, et al. Complementary and alternative medicine use and assessment of quality of life in Korean breast cancer patients: A descriptive study. Supportive Care in Cancer. 2012;20:461–473. doi: 10.1007/s00520-011-1094-z. [DOI] [PubMed] [Google Scholar]
- 19.Horne, R., Weinman, J., Barber, N., Elliott, R. & Morgan, M. Concordance, adherence and compliance in medicine taking. 12 (UK: National Co-ordinating Centre for NHS Service Delivery and Organisation R and D, 2005).
- 20.NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) Breast Cancer, https://www.nccn.org/professionals/physician_gls/default.aspx (2018).
- 21.Torres E, Dixon C, Richman AR. Understanding the Breast Cancer Experience of Survivors: a Qualitative Study of African American Women in Rural Eastern North Carolina. Journal of cancer education: the official journal of the American Association for Cancer Education. 2016;31:198–206. doi: 10.1007/s13187-015-0833-0. [DOI] [PubMed] [Google Scholar]
- 22.LeMasters T, et al. Receipt of Guideline-Concordant Care Among Older Women With Stage I-III Breast Cancer: A Population-Based Study. Journal of the National Comprehensive Cancer Network: JNCCN. 2018;16:703–710. doi: 10.6004/jnccn.2018.7004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chereau E, et al. Discordance with local guidelines for adjuvant chemotherapy in breast cancer: reasons and effect on survival. Clinical breast cancer. 2011;11:46–51. doi: 10.3816/CBC.2011.n.008. [DOI] [PubMed] [Google Scholar]
- 24.Minicozzi Pamela, Van Eycken Liesbet, Molinie Florence, Innos Kaire, Guevara Marcela, Marcos-Gragera Rafael, Castro Clara, Rapiti Elisabetta, Katalinic Alexander, Torrella Ana, Žagar Tina, Bielska-Lasota Magdalena, Giorgi Rossi Paolo, Larrañaga Nerea, Bastos Joana, Sánchez Maria José, Sant Milena. Comorbidities, age and period of diagnosis influence treatment and outcomes in early breast cancer. International Journal of Cancer. 2018;144(9):2118–2127. doi: 10.1002/ijc.31974. [DOI] [PubMed] [Google Scholar]
- 25.Biggers A, et al. Medicare D Subsidies and Racial Disparities in Persistence and Adherence With Hormonal Therapy. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2016;34:4398–4404. doi: 10.1200/jco.2016.67.3350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bickell NA, LePar F, Wang JJ, Leventhal H. Lost opportunities: physicians’ reasons and disparities in breast cancer treatment. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2007;25:2516–2521. doi: 10.1200/jco.2006.09.5539. [DOI] [PubMed] [Google Scholar]
- 27.Fang P, et al. Racial disparities in guideline-concordant cancer care and mortality in the United States. Advances in radiation oncology. 2018;3:221–229. doi: 10.1016/j.adro.2018.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Farias AJ, Wu WH, Du XL. Racial differences in long-term adjuvant endocrine therapy adherence and mortality among Medicaid-insured breast cancer patients in Texas: Findings from TCR-Medicaid linked data. BMC cancer. 2018;18:1214. doi: 10.1186/s12885-018-5121-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ali EE, et al. Prevalence and Determinants of Adherence to Oral Adjuvant Endocrine Therapy among Breast Cancer Patients in Singapore. Asia-Pacific journal of oncology nursing. 2017;4:283–289. doi: 10.4103/2347-5625.212864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kurian AW, et al. Recent Trends in Chemotherapy Use and Oncologists’ Treatment Recommendations for Early-Stage Breast Cancer. Journal of the National Cancer Institute. 2018;110:493–500. doi: 10.1093/jnci/djx239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Katz SJ, Jagsi R, Morrow M. Reducing Overtreatment of Cancer With Precision Medicine: Just What the Doctor Ordered. Jama. 2018;319:1091–1092. doi: 10.1001/jama.2018.0018. [DOI] [PubMed] [Google Scholar]
- 32.Mamounas EP, Russell CA, Lau A, Turner MP, Albain KS. Clinical relevance of the 21-gene Recurrence Score((R)) assay in treatment decisions for patients with node-positive breast cancer in the genomic era. NPJ breast cancer. 2018;4:27. doi: 10.1038/s41523-018-0082-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sparano JA, et al. Prospective Validation of a 21-Gene Expression Assay in Breast Cancer. The New England journal of medicine. 2015;373:2005–2014. doi: 10.1056/NEJMoa1510764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Herr D, et al. Does chemotherapy improve survival in patients with nodal positive luminal A breast cancer? A retrospective Multicenter Study. PloS one. 2019;14:e0218434–e0218434. doi: 10.1371/journal.pone.0218434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gray Ewan, Marti Joachim, Brewster David H., Wyatt Jeremy C., Piaget-Rossel Romain, Hall Peter S. Real-world evidence was feasible for estimating effectiveness of chemotherapy in breast cancer: a cohort study. Journal of Clinical Epidemiology. 2019;109:125–132. doi: 10.1016/j.jclinepi.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 36.Lee CM, et al. Surgery for early breast cancer in the extremely elderly leads to improved outcomes - An Asian population study. Breast (Edinburgh, Scotland) 2017;36:44–48. doi: 10.1016/j.breast.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 37.Duric V, Stockler M. Patients’ preferences for adjuvant chemotherapy in early breast cancer: a review of what makes it worthwhile. The Lancet. Oncology. 2001;2:691–697. doi: 10.1016/s1470-2045(01)00559-9. [DOI] [PubMed] [Google Scholar]
- 38.Repetto L, et al. European journal of cancer (Oxford, England: 1990) 2003. EORTC Cancer in the Elderly Task Force guidelines for the use of colony-stimulating factors in elderly patients with cancer; pp. 2264–2272. [DOI] [PubMed] [Google Scholar]
- 39.Ladwa R, et al. Maintaining Dose Intensity of Adjuvant Chemotherapy in Older Patients With Breast Cancer. Clinical breast cancer. 2018;18:e1181–e1187. doi: 10.1016/j.clbc.2018.04.016. [DOI] [PubMed] [Google Scholar]
- 40.Jensen MB, Ejlertsen B, Mouridsen HT, Christiansen P. Improvements in breast cancer survival between 1995 and 2012 in Denmark: The importance of earlier diagnosis and adjuvant treatment. Acta oncologica (Stockholm, Sweden) 2016;55(Suppl 2):24–35. doi: 10.3109/0284186x.2015.1128119. [DOI] [PubMed] [Google Scholar]
- 41.Jones AP, et al. Travel time to hospital and treatment for breast, colon, rectum, lung, ovary and prostate cancer. European journal of cancer (Oxford, England: 1990) 2008;44:992–999. doi: 10.1016/j.ejca.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 42.Death and Life Expectancy, https://www.singstat.gov.sg/find-data/search-by-theme/population/death-and-life-expectancy/latest-data (2019).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data used in this study is de-identified. Data is available upon request from Ms Yen Shing Yeoh (email: yen_shing_yeoh@nuhs.edu.sg).