Abstract
The diagnosis of various histological subtypes of pituitary tumors is made using serum based hormone panel test. However, certain subtypes secrete more than one hormone, making the diagnosis ambiguous. Here, we performed 1H-NMR based metabolomic analysis of serum and whole-blood from luteinizing/follicle-stimulating (LH/FSH)-secreting (n = 24), prolactinomas (n = 14), and non-functional (NF) (n = 9) tumors. We found elevated levels of betahydroxybutyrate (BHB) in serum and whole-blood (WB) of prolactinomas (0.481 ± 0.211/0.329 ± 0.228 mM in serum/WB), but it was statistically significant (p ≤ 0.0033, Bonferroni correction) only in serum when compared with LH/FSH-secreting tumor patients (0.269 ± 0.139/0.167 ± 0.113 mM in serum/WB). Phenylalanine in NF tumors was found to be elevated in both serum and WB when compared with prolactinomas but it met the statistical significance criteria (p ≤ 0.0028) only in the serum. Alanine (p ≤ 0.011), tyrosine (p ≤ 0.014) and formate (p ≤ 0.011) were also elevated in NF tumors but none showed statistically significance when compared with prolactinomas. Quantification of BHB and the above amino acids in the circulation may aid in the development of blood-based in vitro diagnostic methods which can supplement the currently used serum hormone panel in the diagnosis of various subtypes of pituitary tumors.
Subject terms: Physiology, Pituitary diseases
Introduction
The pituitary gland secretes a range of hormones including growth (GH), adrenocorticotropic (ACTH), prolactin (PRL), luteinizing (LH), follicle-stimulating (FSH), and thyroid-stimulating (TSH) hormones1. PRL-secreting (prolactinomas) and LH/FSH-secreting are the most common subtypes of pituitary adenomas2,3. Non-functional (NF) pituitary adenomas are another common subtype of pituitary adenomas which do not secrete any hormones4. Although pituitary adenomas are generally benign tumors, they pose a major health challenge in patients through the oversecretion of hormones that may lead to infertility, sexual dysfunction, gigantism and other health issues such as vision problems, depression and osteoporosis3,5. Unlike other human diseases, there has not been any patient-derived pituitary tumor cell lines or mouse models currently available which limit the ability to understand molecular mechanisms behind tumor growth and also in discovering new diagnostic markers of pituitary adenomas. MRI is commonly used in the diagnosis of pituitary adenomas. However, it may be very difficult to detect microadenomas in the MRI scan3. In vivo 1H magnetic resonance spectroscopy (MRS) will be of some help in diagnosing hemorrhagic pituitary macroadenomas6. Currently, the clinical MRI scanners use lower magnetic field strength which makes it difficult to fully characterize pituitary adenomas using in vivo 1H MRS. Whereas, ex vivo high resolution MRS makes use of higher magnetic field strength which provides higher sensitivity and better resolution resulting in superior characterization of pituitary tumors2. The metabolite information obtained from ex vivo 1H MRS on pituitary adenomas will further aid in the definitive characterization of pituitary tumors using in vivo 1H MRS. Recently, we have identified a set of metabolites using1H MRS in surgically resected tumor tissues that distinguish various immunohistochemical (IHC) subtypes of pituitary tumors2. However, very limited accessibility of the pituitary gland complicates the use of tissue biopsies for diagnostics. Currently, diagnosis of pituitary tumors is based on the abnormal serum hormone panel. Certain subtypes of pituitary tumors oversecrete more than one hormone and NF pituitary adenomas do not secrete any hormones, making the hormone panel based diagnostic methods ambiguous. Blood metabolomics has been used to identify cancer biomarkers and in the case of pituitary tumors it can be of great value by supplementing to the current clinical diagnostics7. In addition, whole blood (WB) contains red blood cells (RBCs) and other cellular components of blood such as white blood cells and platelets which can further add to the information derived from serum metabolomics8,9. Also, RBCs are known to contain high concentrations of cofactors involved in redox reactions and cellular energy metabolism. Redox mechanisms are fundamental biochemical reactions that are essential to all living cells and directly involved in the homeostasis of key metabolites such as pyruvate and lactate which play pivotal role in various metabolic pathways10. Here, we studied the metabolome of serum and WB obtained from patients with prolactinomas, LH/FSH-secreting and NF pituitary adenomas using 1H NMR spectroscopy with the aim of quantitative identification of metabolite markers that can be of potential value in the development of in vitro diagnostics to differentiate various pituitary tumor subtypes.
Materials and Methods
Patients and blood samples collection
Blood samples were collected from 47 patients (prolactinoma = 14; LH/FSH-secreting = 24; and NF = 9) undergoing transsphenoidal selective adenomectomy for the surgical excision of pituitary adenomas at the Houston Methodist Hospital. Informed consent was obtained from each patient following an Institutional Review Board protocol approved by the Houston Methodist Hospital and Research Institute. The work described in this article has been carried out in accordance with “The Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans”. The diagnosis of pituitary tumors was made on the basis of clinical, biochemical, imaging and IHC criteria. The patient characteristics along with tumor type (IHC), MIB-1 index, PRL and betahydroxybutyrate (BHB) levels are provided in Table 1. Prolactinoma was predominantly observed in females, and 11 out of 14 prolactinoma patients enrolled in this study were females.
Table 1.
Characteristics of PRL and LH/FSH secreting tumor patients showing their sex, age, tumor type, MIB-1 index (%) along with BHB and PRL levels in the serum and whole blood (WB).
| Patient # | Sex | Age (years) | Tumor type (IHC) | MIB-1 (%) | BHB* (mM/L) | PRL (ng/mL) | Medical Therapy# | |
|---|---|---|---|---|---|---|---|---|
| Serum | WB | |||||||
| 1 | M | 45 | LH | 1–2 | 0.058 | 0.042 | 8.0 | No |
| 2 | F | 26 | LH | 2 | 0.106 | 0.054 | 37.0 | No |
| 3 | F | 77 | LHΨ | 1 | 0.438 | 0.255 | 30.0 | No |
| 4 | F | 64 | FSHΨ | 1 | 0.482 | 0.418 | 37.0 | No |
| 5 | M | 61 | FSH | 1 | 0.373 | 0.072 | Normal | No |
| 6 | M | 46 | FSH | 2–3 | 0.261 | 0.158 | 14.1 | No |
| 7 | F | 57 | FSH | 1 | 0.455 | 0.287 | NA | No |
| 8 | F | 77 | FSHΨ | 1–2 | 0.484 | 0.287 | 37.0 | No |
| 9 | F | 66 | LH/FSH | 2–3 | 0.331 | 0.225 | Normal | No |
| 10 | M | 81 | LH/FSH | 1 | 0.215 | 0.188 | NA | No |
| 11 | F | 25 | LH/FSH | 2 | 0.223 | 0.166 | 148.0 | No |
| 12 | F | 54 | LH/FSH | 1 | 0.135 | 0.086 | 51.2 | No |
| 13 | M | 53 | LH/FSH | 2 | 0.155 | 0.134 | 23.3 | No |
| 14 | M | 61 | LH/FSH | 1–2 | 0.304 | 0.252 | NA | No |
| 15 | M | 47 | LH/FSH | 1 | 0.268 | 0.103 | <1 | No |
| 16 | F | 65 | LH/FSH | 1 | 0.455 | 0.291 | NA | No |
| 17 | M | 59 | LH/FSH | 1–2 | 0.160 | 0.054 | 6.0 | No |
| 18 | M | 55 | LH/FSH | 2 | 0.104 | 0.037 | 23.0 | No |
| 19 | M | 79 | LH/FSH | 1 | 0.282 | 0.161 | 20.4 | No |
| 20 | M | 72 | LH/FSH | 1 | 0.091 | 0.038 | NA | No |
| 21 | F | 65 | LH/FSH | 1 | 0.560 | 0.334 | NA | No |
| 22 | M | 62 | LH/FSH | 1 | 0.210 | 0.092 | 17.4 | No |
| 23 | M | 71 | LH/FSH | 1–2 | 0.215 | 0.085 | 39.4 | No |
| 24 | M | 65 | LH/FSH | 2 | 0.238 | 0.105 | 13.5 | No |
| 25 | F | 49 | PRL | 3–4 | 0.930 | 0.937 | 79.4 | No |
| 26 | F | 34 | PRL | 2 | 0.656 | 0.482 | 274 | Yes |
| 27 | F | 36 | PRL | 3 | 0.640 | 0.416 | 197.2 | No |
| 28 | F | 22 | PRL | NA | 0.232 | 0.122 | Elevated | Yes |
| 29 | F | 33 | PRL | No staining | 0.624 | 0.444 | 114 | Yes |
| 30 | F | 50 | PRL | 1 | 0.614 | 0.513 | NA | No |
| 31 | F | 37 | PRL | 1–2 | 0.549 | 0.380 | 82.5 | No |
| 32 | M | 22 | PRL | 5–7 | 0.179 | 0.133 | 115.3 | Yes |
| 33 | M | 59 | PRL | No staining | 0.429 | 0.175 | 249.0 | Yes |
| 34 | F | 45 | PRL | 2 | 0.268 | 0.104 | 41 | No |
| 35 | F | 33 | PRL | 1 | 0.291 | 0.209 | 82.2 | Yes |
| 36 | F | 27 | PRL | 1 | 0.294 | 0.120 | 64.0 | Yes |
| 37 | M | 24 | PRL | 5–7 | 0.463 | 0.216 | 1029.0 | Yes |
| 38 | F | 53 | PRL | 1 | 0.567 | 0.355 | 10.0 | No |
| 39 | F | 46 | NF | 1 | 0.169 | 0.159 | 9.0 | No |
| 40 | M | 37 | NF | 3 | 0.078 | 0.057 | 7.6 | No |
| 41 | F | 39 | NF | 1 | 0.058 | 0.0416 | NA | No |
| 42 | M | 42 | NF | 1 | 0.470 | 0.313 | 7.4 | No |
| 43 | M | 65 | NF | 2 | 0.188 | 0.153 | NA | No |
| 44 | F | 75 | NF | 1 | 0.669 | 0.459 | NA | No |
| 45 | F | 72 | NF | 2 | 0.439 | 0.213 | NA | No |
| 46 | F | 73 | NF | 1 | 0.318 | 0.164 | NA | No |
| 47 | M | 73 | NF | 1 | 0.849 | No sample | 8.0 | No |
(IHC, Immunohistochemistry; LH, luteinizing hormone; FSH, follicle stimulating hormone; PRL, prolactin; NA, not available; PRL-reference range = 2–23 ng/mL; *, BHB levels measured by 1H NMR spectroscopy; Ψ, IHC was positive for LH/FSH, also showed scattered immunoreactivity for PRL, and these patient also showed elevated levels of PRL and BHB similar to prolactinoma patients; #, patients were treated with Cabergoline, 2–4 mg/week).
Two vials of blood (4.0 mL each) were collected in serum separator tubes (BD Diagnostics, NJ) during surgical resection of pituitary tumors. One of the vials was immediately snap-frozen, while the other vial was kept at room temperature for 20–30 min., and then the serum was separated by centrifugation (RCF = 1,800 g for 15 minutes at 4 °C), transferred to cryovials and all specimens were stored at −80 °C until further analysis.
Chemicals
Methanol, chloroform, chloroform-d (CDCl3), Na2HPO4, and NaH2PO4 were purchased from Millipore Sigma (St. Louis, MO, USA). D2O, DCl, and NaOD were purchased from Cambridge isotope laboratories (Tewksbury, MA, USA). 3-(trimethylsilyl)-1-propane sulfonic acid-d6 sodium salt (DSS-d6) solution was purchased from Chenomx Inc. (Edmonton, Canada).
Preparation of phosphate buffer solution
Phosphate buffer solution (100 mM) was prepared by dissolving 2.24 g of anhydrous Na2HPO4 and 0.5 g of anhydrous NaH2PO4 in 200 g of D2O. A 4.99 mM solution of DSS-d6 was added to the buffer solution to obtain a final DSS-d6 concentration of 0.1 mM. The pH of the resulting phosphate buffer was 7.40.
Methanol-chloroform extraction
To 0.25 ml of the serum or WB, 0.5 mL of methanol and 0.5 mL chloroform (both solvents kept at 4 °C) were added in the ratio 1:2:2 (sample:methanol:chloroform, v/v/v)8. The sample-solvent mixtures were vortexed for 1 minute and kept on ice for 2 minutes. This step was repeated twice and the resultant mixtures were incubated at −20 °C for 20 minutes followed by centrifugation at 10,000 g (RCF) for 15 minutes. Only the upper aqueous methanolic layers were carefully collected and the solvents were dried in a CentriVap® vacuum concentrator (Labconco Corporation, Kansas City, MO). The dried residue from each sample collected from the above step (for each sample) was reconstituted in 600 µL phosphate buffer (pH = 7.40) containing 0.1 mM DSS-d6 (internal standard).
1H NMR experiments
One-dimensional (1D) 1H NMR experiments of serum and WB extracts were performed on a Bruker 600 MHz spectrometer (1H Larmor frequency) equipped with a cryogenically-cooled 1H/13C detection probe (Bruker Biospin, Billerica, MA) at 300 K. The sample solutions prepared above were taken in 5 mm NMR tubes and the 1H NMR spectra were obtained by using 1D NOESY (nuclear Overhauser spectroscopy) and CPMG (Carr-Purcell-Meiboom-Gill) pulse sequences with water suppression by presaturation. Both NOESY and CPMG experiments were performed using 32,768 time domain data points, with a 9615.38 Hz spectral width. A 90° excitation pulse with a pulse-width of 10 µs and an inter-pulse delay of 5 s were used. In 1D NOESY experiments, a mixing time of 100 ms was used while in CPMG experiments a total echo-time of 160 ms was used to attenuate broad signal arising from residual lipid components in the serum extracts. The data were zero-filled (2× time domain points), an exponential window function with line broadening of 0.3 Hz was applied, and Fourier transformed. DSS-d6 (resonating at 0 ppm) was used as an internal chemical shift and concentration reference (0.1 mM). Peak areas of 1H signals were measured by using Bruker TopSpin version 3.5 software program.
Quantification of aqueous metabolites
We have identified water-soluble metabolites (in the aqueous methanol layers of the serum and WB extracts) using 1H chemical shifts data of pituitary tumors from our recent study and other reported data2,8,10,11. Quantification of metabolites was performed as described in our recent publication2. Details of quantified metabolites, and their corresponding 1H NMR chemical shifts (of peaks used for quantification) are given in Supplementary Tables S1 and S2.
Statistical analysis
All statistical analyses were performed using Microsoft Excel software (Microsoft Office 2013). The means and standard deviations of metabolites quantified were compared between LH/FSH-secreting tumors, prolactinomas, and NF tumors. The Bonferroni correction (p-value ≤ 0.0038) was applied to the p-values obtained from Student’s t-test to determine the significance level by taking multiple metabolite testing into consideration (Supplementary Tables S1 and S2)12. The diagnostic accuracy of BHB in the differentiation of prolactinomas from other pituitary adenomas was assessed using receiver operative characteristic (ROC) analysis13,14. The area under curve (AUC) was computed to determine the diagnostic accuracy of the test. ROC curves were generated by plotting the true positive rate and the false positive rate.
Results and Discussion
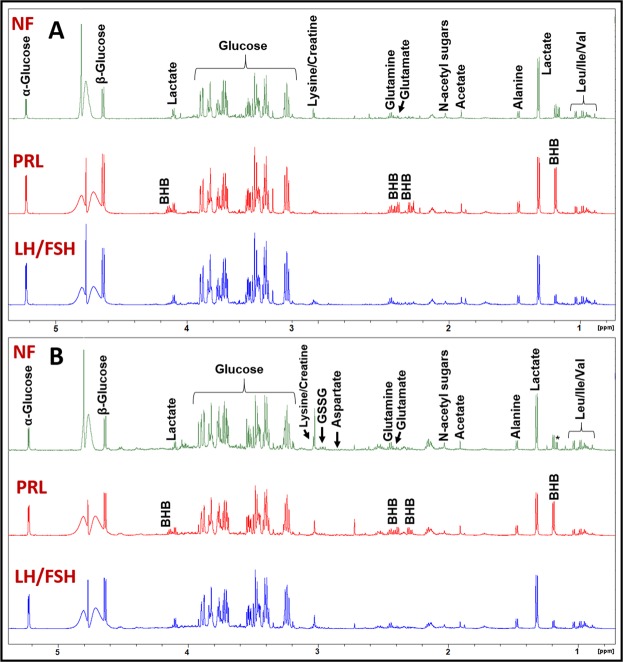
Figure 1A shows the 1H NMR spectral profile of metabolites in the aqueous-methanolic extract of serum from LH/FSH-secreting, prolactinoma, and NF pituitary tumor patients. Similarly, Fig. 1B shows the 1H NMR spectral profile of an aqueous methanolic extract of WB from the same patients. Both serum and WB showed the presence of leucine (Leu), isoleucine (Ile), valine (Val), BHB, lactate, alanine, acetate, N-acetyl sugars, glutamate, glutamine, lysine/creatine, glucose, tyrosine, histidine, phenylalanine, and formate. In addition to the above metabolites, the WB showed the presence of peaks arising from aspartate, glutathione (GSSG, oxidized form) and cofactors involved in the cellular redox reactions – ATP, ADP, AMP, NAD+, and NADP+. The NMR spectral assignments of aromatic amino acids (tyrosine, phenylalanine, and histidine) and cofactors (ATP, ADP, AMP, NAD+, and NADP+) are shown in Fig. 2 for WB extracts of LH/FSH-secreting, prolactinoma, and NF pituitary tumor patients. The mean metabolite concentrations in serum and WB of LH/FSH-secreting, prolactinoma, and NF tumor patients are shown in Fig. 3A,B respectively (also see Supplementary Tables S1 and S2). From Fig. 3A,B, we can see that BHB was significantly elevated only in serum of prolactinoma patients compared to the LH/FSH-secreting patients (p = 0.0033). Although in NF tumors, tyrosine (p ≤ 0.023) and formate (p ≤ 0.011) were elevated in serum, and N-acetyl sugars were found to be elevated in WB, none of them met the statistical significance criteria (p ≤ 0.0038) used in this study to account for multiple metabolite testing while comparing with LH/FSH-secreting tumors. On the other hand, phenylalanine in NF tumors was found to be elevated in both serum and WB when compared with prolactinomas but it met the statistical significance criteria (p ≤ 0.0028) only in the serum. Also, alanine (p ≤ 0.011), tyrosine (p ≤ 0.014) and formate (p ≤ 0.011) showed statistically nonsignificant elevated levels in the serum of NF tumors when comparing with prolactinomas.
Figure 1.
Representative 1H NMR spectra of aqueous methanol extracts of (A) serum and (B) whole blood (WB) showing spectral profiles of LH/FSH-secreting pituitary tumor, prolactinoma (PRL-secreting), and Non-functional (NF) pituitary tumor patients. The BHB is highly elevated in the serum and WB of prolactinoma patient. *Refers to solvent impurity signal.
Figure 2.
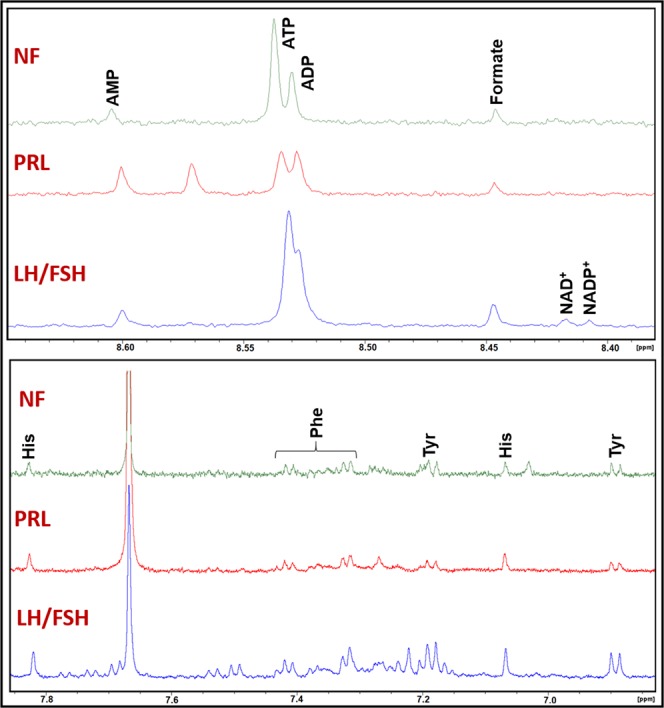
Representative 1H NMR spectra of aqueous methanol extracts of whole blood (WB) showing signals arising from cofactors ATP, ADP, AMP, NAD+, and NADP+ in LH/FSH-secreting pituitary tumor, prolactinoma, and NF pituitary tumor patients. Also shown are the 1H NMR signal assignments for aromatic amino acids tyrosine, phenylalanine, and histidine.
Figure 3.
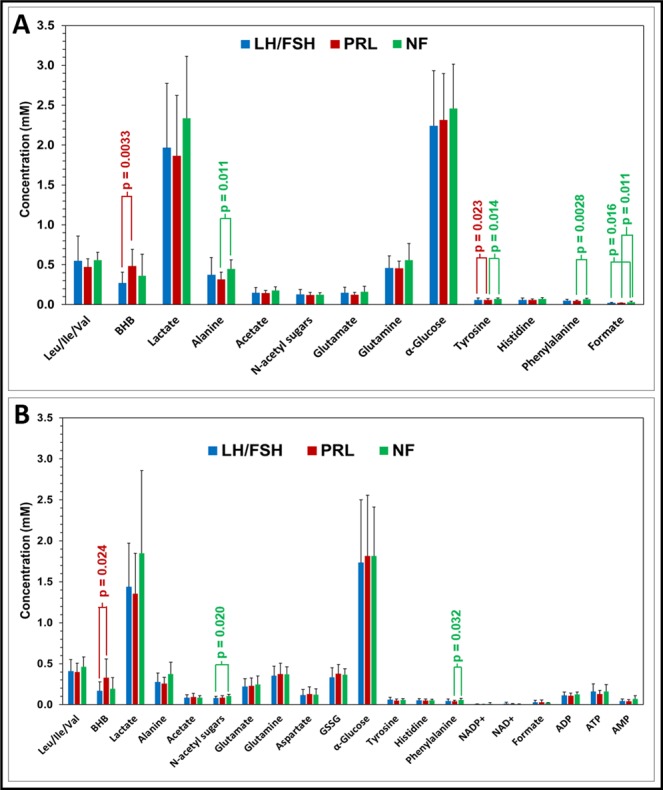
Charts showing the concentrations of various water soluble metabolites quantified in (A) serum and (B) WB of LH/FSH-secreting pituitary tumor, prolactinoma, and NF pituitary tumor patients. BHB was significantly elevated in both serum and WB of prolactinoma patients compared to LH/FSH-secreting pituitary tumors. Total glucose concentration can be determined from α-Glucose (36% anomeric contribution) using the relation, [Total Glucose] = [α-Glucose] × (100/36).
In WB extracts, the levels of ATP and ADP in three types of tumor patients were comparable, but the levels of AMP were slightly elevated in NF tumors (Table S2). The ATP/ADP ratio was lower in prolactinoma patients compared to LH/FSH-secreting and NF tumor patients (Fig. 3B; Table S2). ATP/ADP ratio plays a key role in the cellular metabolism that determines the balance in the relative fluxes between glycolysis and oxidative phosphorylation15. In this study, we observed a slight decrease in the ATP/ADP ratio and the lactate levels in prolactinoma patients along with slight increase in the aspartate levels when compared with LH/FSH-secreting tumors. This suggests that prolactinomas may have slightly downregulated glycolysis than the mitochondrial oxidative metabolism. On the other hand, NF tumors showed elevated levels of lactate when compared with both prolactinomas and LH/FSH-secreting tumors, indicating an upregulated Warburg glycolysis. Moreover, we also observed slightly elevated levels of NADP+ in LH/FSH secreting tumors compared to prolactinomas and NF tumor patients, but NAD+ levels were similar in all three types of tumors (Fig. 3B, Table S2). Together, this may be indicative of enhanced glycolysis in NF pituitary tumors than other subtypes. Pituitary tumors are often small in size and procuring enough tumor tissues for ex vivo metabolomic analysis will be challenging. As described in this study, detection of cofactors and antioxidants in WB using a simple 1H NMR based method may be a valuable alternative approach towards understanding their role in various IHC subtypes of pituitary tumors.
Glucose is the primary energy source for the central nervous system. It is known that under prolonged fasting brain switches to the utilization of ketone body (BHB) as an energy substrate which directly enters the citric acid cycle in the mitochondria. It was assumed that brain tumors lacked the ability to efficiently metabolize ketone body due to dysfunctional mitochondria. Our recent surprising findings demonstrate that malignant brain tumors are fully capable of oxidizing BHB, glutamine and acetate as alternate fuels16,17. In a similar fashion, we hypothesize that elevated levels of circulating BHB in prolactinoma patients may be taken up preferentially and utilized by the tumors to meet their increased bioenergetic requirements.
The BHB levels in fasting individuals generally do not exceed 0.2 mM18. In the current study, we observed that the levels of BHB were elevated in prolactinoma patients (0.481 ± 0.211 mM and 0.329 ± 0.228 mM in serum and WB respectively) compared to the LH/FSH-secreting tumor patients (0.269 ± 0.139 mM and 0.167 ± 0.113 mM in serum and WB respectively) and NF tumors (0.360 ± 0.272 mM and 0.195 ± 0.137 mM in serum and WB respectively). The difference in the levels of BHB in LH/FSH-secreting and prolactinomas was statistically significant only in serum (p = 0.0033) with Bonferroni correction (corrected p ≤ 0.0038). We did not see a statistically significant difference between BHB levels in NF tumors when compared with LH/FSH-secreting tumors or prolactinomas. BHB is synthesized in the liver via the oxidation of fatty acids during starvation or under the conditions of depleted dietary carbohydrates/glycogen in the body19 and is transported to the brain as a fuel. It is also synthesized endogenously in astrocytes and is supplied to other brain cells20. A recent in vitro study carried out in dairy cow anterior pituitary (DCAP) cells has shown a link between BHB and PRL secretion21. The authors have shown that BHB can inhibit PRL gene transcription and secretion via cAMP/protein kinase signalling pathway21. Secretion of PRL was decreased in DCAP cells in a dose dependent manner when treated with BHB. On the contrary, in this study prolactinoma patients showed elevated levels of both BHB and PRL. Although the levels of both PRL and BHB in prolactinoma patients were high, we could not find any correlation between the levels of PRL and BHB. This could be due to the fact that most of prolactinoma patients were on cabergoline therapy (Table 1) that is used to lower PRL levels. We also observed that 7 patients with LH or FSH secreting tumors also showed elevated levels of PRL (patients #2, 3, 4, 8, 11, 12 and 23; Table 1). It is interesting to note that the BHB levels in 4 of these 7 patients (#2, 11, 12 and 23), were in the normal fasting range18. However, in the remaining 3 patients (#3, 4, and 8), BHB levels were found to be elevated. These 3 patients also showed scattered positive immunoreactivity for PRL which may be the reason for the elevation in the BHB levels .
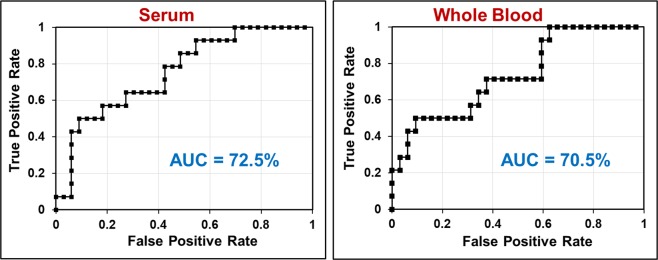
To assess the diagnostic accuracy of BHB in differentiating prolactinomas from LH/FSH-secreting and NF tumors, we performed ROC analysis and generated the ROC curves. Figure 4 shows the ROC plots of BHB levels determined from serum and WB of tumor patients. The area under the curve (AUC) for serum and WB were 72.5% and 70.5% respectively, indicating that the BHB can be a good metabolic marker for the differential diagnosis of prolactinomas from other subtypes of pituitary adenomas. The observation of elevated circulating BHB levels may imply a possible association with PRL secretion in pituitary tumor patients. The reason for the elevated levels of circulating BHB observed only in prolactinoma patients (Table 1) is not fully understood. Further studies are needed to unravel the relationship between BHB and PRL levels in the circulation of prolactinoma patients. It could be possible that prolactinomas may preferentially utilize BHB as an alternate fuel for their growth similar to the malignant brain tumors16,17. Currently, prolactinomas are the only pituitary tumors which can be treated by medical therapy. Cabergoline and bromocriptine are the two drugs prescribed to the patients with PRL-secreting pituitary tumors. Our current observation of elevated levels of circulating BHB along with increased PRL secretion in prolactinoma patients suggest that modulating the levels of BHB in the circulation may have a therapeutic role in treating these patients.
Figure 4.
ROC curves for BHB levels in serum and whole blood (WB) showing diagnostic potential of BHB in differentiating prolactinomas from LH/FSH-secreting and NF pituitary adenomas.
Conclusions
Elevated levels of BHB in the circulation distinguish prolactinomas from LH/FSH-secreting and NF tumors. Quantification of BHB levels in serum/WB may aid in the development of blood based non-invasive diagnostic methods which can augment the currently available serum hormone panel in the unambiguous diagnosis of PRL-secreting tumors. Although exact role of elevated BHB in prolactinoma patients is not fully clear, we hypothesize that higher levels of circulating BHB in prolactinoma patients may be taken up by the tumors to meet their increased energy requirements. In the serum of NF tumors the levels of alanine, phenylalanine and tyrosine were found to be elevated but only phenylalanine showed statistically significant elevation when compared with prolactinomas. Given a small sample size, we were unable to determine a definitive decision threshold for BHB in differentiating the prolactinomas from other pituitary tumor subtypes. However, we obtained an accuracy of ~72% using ROC analysis. We are continuing to enroll more prolactinoma, LH/FSH-secreting and NF pituitary tumor patients to further validate our preliminary findings. Also, we are recruiting other rarely occurring histological subtypes (GH-secreting and ACTH-secreting tumors) to test whether BHB or any other metabolites in the circulation play any physiological role in the diagnostics of these pituitary tumors.
Supplementary information
Acknowledgements
This study was supported by the Donna and Kenneth R. Peak Foundation, The Kenneth R. Peak Brain and Pituitary Tumor Treatment Center at Houston Methodist Hospital, The Houston Methodist Foundation, The Taub Foundation, The Pauline Sterne Wolff Foundation, The Veralan Foundation, The Marilee A. and Gary M. Schwarz Foundation, The John S. Dunn Foundation and The McKone Family Foundation. We thank all the patients who participated in this study and the members of Neurosurgery team of Dr. Baskin at The Houston Methodist Hospital for obtaining blood samples during surgery. We thank NMR and Drug Metabolism Core of Baylor College of Medicine for the use of their facility.
Author contributions
K.P. conceived and designed this study. D.S.B. performed patient surgical procedures and obtained blood samples. O.B.I., C.H., J.H. and K.P. performed N.M.R. experiments, data processing and analysis. M.A.S. helped in the R.O.C. analysis and contributed to the revision of the manuscript. All authors were involved in the manuscript preparation.
Data availability
1H NMR spectroscopic data from prolactinomas, LH/FSH secreting, and NF pituitary tumors generated for this are available in this article and its supporting information file.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-58244-8.
References
- 1.Drouin, J. Pituitary development. (ed. Melmed, S.) The Pituitary. 3–22, 10.1016/C2009-0-61488-4. (Academic Press, 2017).
- 2.Ijare OB, Baskin DS, Pichumani K. Ex Vivo1H NMR study of pituitary adenomas to differentiate various immunohistochemical subtypes. Sci. Rep. 2019;9:3007. doi: 10.1038/s41598-019-38542-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yao A, Balchandani P, Shrivastava RK. Metabolic In Vivo Visualization of Pituitary Adenomas: a Systematic Review of Imaging Modalities. World Neurosurg. 2017;104:489–498. doi: 10.1016/j.wneu.2017.04.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chanson P, et al. French Endocrinology Society non-functioning pituitary adenoma work-group. Management of clinically non-functioning pituitary adenoma. Ann. Endocrinol. 2015;76:239–47. doi: 10.1016/j.ando.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 5.Raverot G, Jouanneau E, Trouillas J. Management of endocrine disease: clinicopathological classification and molecular markers of pituitary tumors for personalized therapeutic strategies. Eur. J. Endocrinol. 2014;70:R121–132. doi: 10.1530/EJE-13-1031. [DOI] [PubMed] [Google Scholar]
- 6.Stadlbauer A1, et al. Proton magnetic resonance spectroscopy in pituitary macroadenomas: preliminary results. J. Neurosurg. 2008;109:306–312. doi: 10.3171/JNS/2008/109/8/0306. [DOI] [PubMed] [Google Scholar]
- 7.Berker Y, et al. Magnetic Resonance Spectroscopy-based Metabolomic Biomarkers for Typing, Staging, and Survival Estimation of Early-Stage Human Lung Cancer. Sci. Rep. 2019;9:10319. doi: 10.1038/s41598-019-46643-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nagana Gowda GA, Raftery D. Whole blood metabolomics by 1H NMR spectroscopy provides a new opportunity to evaluate coenzymes and antioxidants. Anal. Chem. 2017;89:4620–4627. doi: 10.1021/acs.analchem.7b00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stringer KA, et al. Whole blood reveals more metabolic detail of the human metabolome than serum as measured by 1H NMR spectroscopy: Implications for sepsis metabolomics. Shock. 2015;44:200–208. doi: 10.1097/SHK.0000000000000406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nagana Gowda GA, Abell L, Lee CF, Tian R, Raftery D. Simultaneous analysis of major coenzymes of cellular redox reactions and energy using ex vivo1H NMR spectroscopy. Anal. Chem. 2016;88:4817–24. doi: 10.1021/acs.analchem.6b00442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Otvos JD, et al. GlycA: A Composite Nuclear Magnetic Resonance Biomarker of Systemic Inflammation. Clin. Chem. 2015;61:714–23. doi: 10.1373/clinchem.2014.232918. [DOI] [PubMed] [Google Scholar]
- 12.Antonelli J, et al. Statistical Workflow for Feature Selection in Human Metabolomics Data. Metabolites. 2019;9:143. doi: 10.3390/metabo9070143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zweig MH, Campbell G. Receiver-operating characteristic (ROC) plots: a fundamental evaluation tool in clinical medicine. Clin. Chem. 1993;39:561–77. doi: 10.1093/clinchem/39.4.561. [DOI] [PubMed] [Google Scholar]
- 14.Dalaman G, et al. Early detection of peritonitis in continuous ambulatory peritoneal dialysis patients by use of chemiluminescence: evaluation of diagnostic accuracy by receiver-operating characteristic curve analysis. Clin. Chem. 1998;44:1680–1684. [PubMed] [Google Scholar]
- 15.Maldonado EN, Lemasters JJ. ATP/ADP ratio, the missed connection between mitochondria and the Warburg effect. Mitochondrion. 2014;19(Pt A):78–84. doi: 10.1016/j.mito.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ijare OB, Hoppe A, Holan C, Baskin DS, Pichumani K. Oxidation of ketone body in human glioblastoma cell lines using 13C NMR spectroscopy. Neuro. Oncol. 2017;19(Suppl 6):vi129. doi: 10.1093/neuonc/nox168.531. [DOI] [Google Scholar]
- 17.Ijare OB, et al. Human Glioblastoma Cell Lines Co-oxidize [2,4-13C]betahydroxy-butyrate and [U-13C]-glucose: A 13C NMR Spectroscopic Study. Proc. Intl. Soc. Mag. Reson. Med. 2018;26:0450. [Google Scholar]
- 18.Burstal RJ, Reilly JR, Burstal B. Fasting or starving? Measurement of blood ketone levels in 100 fasted elective and emergency adult surgical patients at an Australian tertiary hospital. Anaesth. Intensive Care. 2018;46:463–467. doi: 10.1177/0310057X1804600506.. [DOI] [PubMed] [Google Scholar]
- 19.Newman JC, Verdin E. β-Hydroxybutyrate: A Signaling Metabolite. Annu. Rev. Nutr. 2017;37:51–76. doi: 10.1146/annurev-nutr-071816-064916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Achanta LB, Rae CD. β-Hydroxybutyrate in the Brain: One Molecule, Multiple Mechanisms. Neurochem. Res. 2017;42:35–49. doi: 10.1007/s11064-016-2099-2. [DOI] [PubMed] [Google Scholar]
- 21.Fu SP, et al. β-hydroxybutyrate sodium salt inhibition of growth hormone and prolactin secretion via the cAMP/PKA/CREB and AMPK signaling pathways in dairy cow anterior pituitary cells. Int. J. Mol. Sci. 2015;16:4265–4280. doi: 10.3390/ijms16024265. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
1H NMR spectroscopic data from prolactinomas, LH/FSH secreting, and NF pituitary tumors generated for this are available in this article and its supporting information file.