Abstract
Locally advanced pancreatic cancer (LAPC) has a dismal prognosis even after standard chemotherapy, and local progression contributes to nearly one-third of the deaths of these patients. As a local destructive method, irreversible electroporation (IRE) can feasibly treat LAPC. The aim of this study was to evaluate IRE combined with chemotherapy as a new treatment and compare its efficacy with that of chemotherapy alone in patients with LAPC. The data of LAPC patients who received chemotherapy with or without IRE were extracted from Surveillance, Epidemiology, and End Results (SEER) database and medical records of Sun Yat-sen University Cancer Center (SYSUCC). The efficacy of these two treatments was compared using propensity score matching (PSM) analysis. LAPC patients treated with the combination therapy had better overall survival (OS). Significantly higher cancer-specific survival (CSS) and progression-free survival (PFS) rates were also observed in patients after IRE combined with chemotherapy, compared with chemotherapy alone. IRE combined with chemotherapy was established as a favorable factor for OS, CSS, and PFS in LAPC patients. This combination method may be a more suitable treatment for patients with LAPC.
Keywords: locally advanced pancreatic cancer, irreversible electroporation, chemotherapy, efficacy, SEER
Introduction
Pancreatic adenocarcinoma (PDAC) is the seventh leading cause of death in cancer patients (1), and locally advanced pancreatic cancer (LAPC) accounts for ~40% of all PDAC cases. Despite currently available therapies, the 5-year overall survival (OS) rate of LAPC patients was only 3% and the median OS were only 11 months (2). Palliative chemotherapy remains the recommended therapy for patients with LAPC. However, progression of the primary tumor contributes to more than 30% of deaths and the rate of downstaging is only 4–15%, which suggests the need for novel local control approaches (2, 3).
Irreversible electroporation (IRE) is a novel ablative and non-thermal method (4). IRE was shown to be a suitable treatment for LAPC that encases surrounding vessels because its unique features are not sensitive to the heat sink effect (5, 6). Although the role of concurrent IRE and conventional treatment has been discussed in previous studies, these studies were mostly based on small cohorts of patients and had contradictory results (7, 8). Moreover, prospective data for combination therapy of IRE and chemotherapy in clinical trials is still lacking. It is necessary to fill this gap with new information on the clinical and treatment characteristics of a large cohort of patients with LAPC. In this study, the Surveillance, Epidemiology, and End Results (SEER) database and a study cohort from Sun Yat-sen University Cancer Center (SYSUCC) were adopted to evaluate the efficacy of combination therapy with IRE and chemotherapy in patients with LAPC.
Materials and Methods
Patients
The first cohort of patients with LAPC was from the SEER database (2012–2015) of the United States (US) National Cancer Institute, which provided data on cancer incidence and survival in the US and covered 30% of the population. Patients with the following information were included in this study: International Classification of Diseases for Oncology, Third Edition (ICD-O-3) site codes C25.1, C25.2, C25.3, and C25.8. All included patients had pathologically confirmed pancreatic adenocarcinoma or infiltrating ductal carcinoma (ICD-O-3 histology codes 8140/3 and 8500/3, respectively). Only patients who were without distant metastases and whose tumor were classified as T4 were included. The SYSUCC cohort consisted of patients who were initially treated with chemotherapy alone or induction chemotherapy followed by IRE from August 2015 to August 2017. The inclusion criteria were as follows: (1) pathologically confirmed pancreatic adenocarcinoma and radiologically confirmed LAPC. LAPC was defined per the seventh edition of the AJCC staging system for pancreatic cancer, which describes LAPC as arterial encasement of either the celiac axis or superior mesenteric artery or unreconstructable superior mesenteric or portal vein involvement, with no evidence of metastatic disease from abdominal and thoracic computed tomography (CT) (9, 10); (2) four months of induction chemotherapy [FOLFIRINOX or gemcitabine (GEM)-based chemotherapy] without radiotherapy. The following exclusion criteria were adopted: (1) second primary cancer; (2) distant metastases; (3) other treatments, including surgical resection or radiofrequency ablation (RFA); (4) an Eastern Cooperative Oncology Group performance status (ECOG PS) score larger than 2; and (5) missing or incomplete information.
Data Collection
The SEER database and the medical management system of SYSUCC were used to extract the demographic, clinical, and pathological variables of patients, including age at diagnosis, gender, tumor site, grade and size, tumor-node-metastasis (TNM) stage, ECGO PS score, chemotherapy, radiotherapy, IRE treatment, and cause of death. In addition, other clinical factors, including white blood cell (WBC) count, hemoglobin (HGB), platelet (PLT) count, serum levels of alanine transaminase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), glutamyltranspeptidase (GGT), albumin (ALB), total bilirubin (TBIL), indirect bilirubin (IBIL), C-reactive protein (CRP), carcinoembryonic antigen (CEA), carbohydrate antigen 19-9 (CA19-9), which were the same as those in our previous reports (11), were also investigated in this study. The endpoints were OS, cancer-specific survival (CSS), and progression-free survival (PFS), which were defined as the duration from the date of treatment to death from all causes, cancer-related deaths, and tumor progression, respectively, or last follow-up. September 30, 2018 was the last date of follow-up.
Treatment Procedure
Induction chemotherapy of either FOLFIRINOX or GEM-based chemotherapy was used for a duration of 4 months (totaling 3 cycles of GEM-based chemotherapy or 4–6 cycles of FOLFIRINOX-based chemotherapy), which was in accordance with previous studies (12, 13). The same line of chemotherapy was performed 7–14 days after IRE treatment. Patients who did not receive IRE treatment received FOLFIRINOX or GEM-based chemotherapy as the standard treatment. The same procedure of IRE which was reported in our previous research was adopted in the present study (11). During the procedure of IRE, two to six probes will be used according to the size and location of the tumor to create an electric field around the tumor, which will finally cause nanoscale pore formation in the plasma membrane. The generator unit software is used to analysis the probe configuration data of ultrasound and provides optimal voltage and pulse length delivery. A setting of 1,500 V/cm is often used as initial setting, with a planned delivery of 90 pulses at a pulse length of 70–90 ms. Simply, this procedure was similar with the proposed procedure suggested by Martin et al., which was also adopted in cases of SEER database (14, 15).
Follow-Up
Regular follow-up data was available for each patient: 1 month after IRE for the initial follow-up, and every 2–3 months thereafter. Abdominal CT or MRI, physical examination, and serum CA19-9 and CEA analyses were performed for each follow-up.
Statistical Analysis
Continuous and categorical variables were compared by student's t-test and chi-square test, respectively. The Kaplan-Meier method was used to analyze OS and PFS and the log-rank test was used to compare the survival differences. The cumulative incidence function (CIF) of the variables on cancer- and non-cancer-specific mortality was evaluated by Fine and Gray's model (16, 17). Multivariate analysis was performed using the Cox regression model for variables that were significantly associated with OS, CSS, and PFS in the univariate analysis, to determine the prognostic factors of survival with 95% confidence intervals (CIs) and a significance level of 0.05. Balanced variables were selected by a logistic regression model with propensity score matching (PSM) analysis. One-to-fifteen and one-to-two nearest-neighbor matching algorithms were used in the SEER and SYSUCC databases, respectively (18). PSM results were also reported as effect size: |value| < 0.2 indicated a negligible difference, |value| < 0.5 indicated a small difference, |value| < 0.8 indicated a moderate difference, and any other value indicated a large difference (19, 20). R statistical software (R software version 3.4.2; R Foundation for Statistical Computing, Vienna, Austria) was used to perform all statistical analyses.
Results
Patient Characteristics
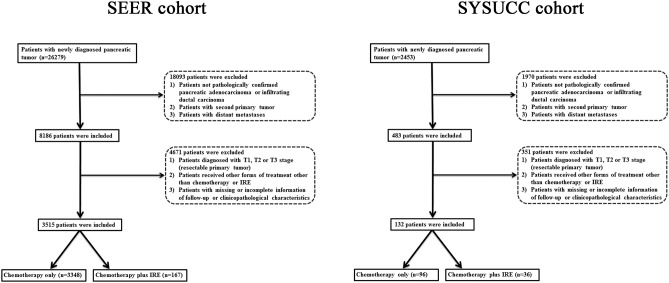
The flow diagram for data selection is shown in Figure 1. In total, 3,515 patients with LAPC from SEER database, including 3,348 patients who received chemotherapy only and 167 patients who received combination therapy with IRE and chemotherapy, were included in this study. The baseline clinical and pathological characteristics were compared between the two groups (Table 1). The median age was 65 years (range, 26–97 years) and 67 years (range, 29–96 years) for patients in the combination therapy group and chemotherapy group, respectively. More than half of the tumors were located in the head of the pancreas in both groups. Compared with patients in the combination therapy group, significantly more patients in the chemotherapy group underwent radiotherapy. To equilibrate significantly different baseline characteristics, 167 patients in the combination therapy group and 2,505 matched patients in the chemotherapy group were selected. All variables were balanced after the PSM analysis.
Figure 1.
Flow diagram of the data selection process.
Table 1.
Comparisons of clinical and imaging characteristics of patients.
| Characteristic | Before PSM | After PSM | Effect size | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
Chemotherapy + IRE |
Chemotherapy | Total number | P value |
Chemotherapy + IRE |
Chemotherapy | Total number | P value | |||
| SEER DATASET | ||||||||||
| Total number | 167 | 3,348 | 3,515 | 167 | 2,505 | 2,672 | ||||
| Age (years) | ≤60 | 51 | 946 | 997 | 0.538 | 51 | 681 | 732 | 0.370 | 0.002 |
| >60 | 116 | 2,402 | 2,518 | 116 | 1,824 | 1,940 | ||||
| Gender | Female | 78 | 1,665 | 1,743 | 0.476 | 78 | 1,251 | 1,329 | 0.425 | 0.001 |
| Male | 89 | 1,683 | 1,772 | 89 | 1,254 | 1,343 | ||||
| Race | Black | 16 | 458 | 474 | 0.088 | 16 | 351 | 387 | 0.084 | 0.005 |
| White | 141 | 2,583 | 2,724 | 141 | 1,929 | 2,070 | ||||
| Others | 10 | 307 | 317 | 10 | 225 | 235 | ||||
| Tumor size (cm) | ≤2 | 7 | 120 | 127 | 0.778 | 7 | 91 | 98 | 0.808 | 0.021 |
| 2~4 | 71 | 1,508 | 1,579 | 71 | 1,124 | 1,195 | ||||
| >4 | 89 | 1,720 | 1,809 | 89 | 1,290 | 1,379 | ||||
| Tumor grade | Well | 15 | 174 | 189 | 0.058 | 15 | 140 | 155 | 0.051 | 0.016 |
| Moderate | 74 | 1,522 | 1,596 | 74 | 1,094 | 1,168 | ||||
| Poor | 78 | 1,652 | 1,730 | 78 | 1,271 | 1,349 | ||||
| LN metastasis | Absent | 119 | 2,189 | 2,308 | 0.133 | 119 | 1,654 | 1,773 | 0.177 | 0.007 |
| Present | 48 | 1,159 | 1,207 | 48 | 851 | 899 | ||||
| Tumor site | Head | 84 | 1,799 | 1,883 | 0.473 | 84 | 1,352 | 1,436 | 0.547 | 0.024 |
| Body | 53 | 1,057 | 1,110 | 53 | 772 | 825 | ||||
| Tail | 30 | 492 | 522 | 30 | 381 | 411 | ||||
| Radiotherapy | No | 127 | 2,119 | 2,246 | 0.001 | 127 | 1,870 | 1,897 | 0.783 | 0.156 |
| Yes | 40 | 1,229 | 1,269 | 40 | 635 | 675 | ||||
| SYSUCC DATASET | ||||||||||
| Total number | 36 | 96 | 132 | 36 | 36 | 72 | ||||
| Age (years) | ≤60 | 20 | 52 | 72 | 0.887 | 20 | 20 | 40 | 1.000 | 0.032 |
| >60 | 16 | 44 | 60 | 16 | 16 | 32 | ||||
| Gender | Female | 17 | 71 | 88 | 0.006 | 17 | 24 | 41 | 0.153 | 0.146 |
| Male | 19 | 25 | 44 | 19 | 12 | 31 | ||||
| Tumor size (cm) | ≤2 | 1 | 9 | 10 | 0.061 | 1 | 1 | 2 | 0.256 | 0.032 |
| 2~4 | 20 | 34 | 54 | 20 | 13 | 33 | ||||
| >4 | 15 | 53 | 68 | 15 | 22 | 37 | ||||
| Tumor grade | Well | 3 | 1 | 4 | 0.092 | 3 | 1 | 4 | 0.588 | 0.047 |
| Moderate | 20 | 56 | 76 | 20 | 21 | 41 | ||||
| Poor | 13 | 39 | 52 | 13 | 14 | 27 | ||||
| LN metastasis | Absent | 29 | 31 | 60 | <0.001 | 29 | 29 | 58 | 1.000 | 0.066 |
| Present | 7 | 65 | 72 | 7 | 7 | 14 | ||||
| Tumor site | Head | 18 | 45 | 63 | 0.001 | 18 | 19 | 37 | 0.015 | 0.014 |
| Body | 15 | 16 | 31 | 15 | 6 | 21 | ||||
| Tail | 3 | 35 | 38 | 3 | 11 | 14 | ||||
| WBC (*109) | ≤10 | 32 | 80 | 112 | 0.588 | 32 | 34 | 66 | 0.674 | 0.037 |
| >10 | 4 | 16 | 20 | 4 | 2 | 6 | ||||
| HGB (g/L) | ≤120 | 10 | 25 | 35 | 0.828 | 10 | 10 | 20 | 1.000 | 0.011 |
| >120 | 26 | 71 | 97 | 26 | 26 | 52 | ||||
| PLT (*109) | ≤300 | 31 | 77 | 108 | 0.613 | 31 | 30 | 61 | 0.743 | 0.087 |
| >300 | 5 | 19 | 24 | 5 | 6 | 11 | ||||
| ALT (U/L) | ≤40 | 26 | 70 | 96 | 0.936 | 26 | 27 | 53 | 0.789 | 0.092 |
| >40 | 10 | 26 | 36 | 10 | 9 | 19 | ||||
| AST (U/L) | ≤40 | 29 | 78 | 107 | 0.928 | 29 | 31 | 60 | 0.753 | 0.073 |
| >40 | 7 | 18 | 25 | 7 | 5 | 12 | ||||
| ALP (U/L) | ≤100 | 18 | 44 | 62 | 0.439 | 19 | 18 | 37 | 0.816 | 0.071 |
| >100 | 18 | 52 | 70 | 17 | 18 | 35 | ||||
| GGT (U/L) | ≤45 | 19 | 37 | 56 | 0.166 | 19 | 19 | 38 | 0.989 | −0.001 |
| >45 | 17 | 59 | 76 | 17 | 17 | 34 | ||||
| ALB (g/L) | ≤40 | 4 | 42 | 46 | <0.001 | 4 | 10 | 14 | 0.135 | 0.110 |
| >40 | 32 | 54 | 86 | 32 | 26 | 58 | ||||
| TBIL (umol/L) | ≤20.5 | 27 | 76 | 103 | 0.640 | 27 | 27 | 54 | 1.000 | 0.013 |
| >20.5 | 9 | 20 | 29 | 9 | 9 | 18 | ||||
| IBIL (umol/L) | ≤15 | 32 | 93 | 125 | 0.099 | 32 | 36 | 68 | 0.115 | −0.182 |
| >15 | 4 | 3 | 7 | 4 | 0 | 4 | ||||
| CRP (ng/L) | ≤3 | 24 | 30 | 54 | <0.001 | 25 | 20 | 45 | 0.216 | 0.072 |
| >3 | 12 | 66 | 78 | 11 | 16 | 27 | ||||
| CEA (ng/mL) | ≤5 | 21 | 40 | 61 | 0.116 | 21 | 15 | 36 | 0.157 | 0.175 |
| >5 | 15 | 56 | 71 | 15 | 21 | 36 | ||||
| CA19-9 (U/ml) | ≤35 | 9 | 13 | 22 | 0.189 | 9 | 5 | 14 | 0.372 | −0.212 |
| >35 | 27 | 83 | 110 | 27 | 30 | 57 | ||||
| HBsAg | Negative | 33 | 88 | 121 | 0.974 | 33 | 33 | 66 | 1.000 | 0.003 |
| Positive | 3 | 8 | 11 | 3 | 3 | 6 | ||||
| ECGO PS score | 0 | 15 | 41 | 56 | 0.809 | 15 | 20 | 35 | 0.468 | 0.165 |
| 1 | 19 | 52 | 71 | 19 | 15 | 34 | ||||
| 2 | 2 | 3 | 5 | 2 | 1 | 3 | ||||
| Chemotherapy | FOLFIRINOX | 21 | 52 | 73 | 0.668 | 22 | 23 | 45 | 0.796 | 0.087 |
| Gem | 15 | 44 | 59 | 14 | 13 | 27 | ||||
LN, lymph node metastasis; TNM, tumor-node-metastasis stage; WBC, white blood cell count; PLT, platelet count; ALT, alanine transaminase; AST, aspartate aminotransferase; ALP, alkaline phosphatase; GGT, glutamyl transpeptidase; ALB, albumin; TBIL, total bilirubin; IBIL, indirect bilirubin; CRP, C-reactive protein; HBsAg, hepatitis B surface antigen; CEA, carcinoembryonic antigen; CA19-9, carbohydrate antigen 19-9; HBsAg, hepatitis B surface antigen; ECOG PS, Eastern Cooperative Oncology Group (ECOG) performance status.
Additionally, 132 patients from the SYSUCC were included. The median age for patients in the combination therapy group and the chemotherapy group was 60 years (range, 39–80 years) and 60 years (range, 45–87 years), respectively. Female patients, patients with tumors larger than 4 cm or those with moderately differentiated tumors were commonly seen in both groups. The two groups of patients had substantially balanced variables after the PSM analysis.
OS Comparison Between the Two Groups
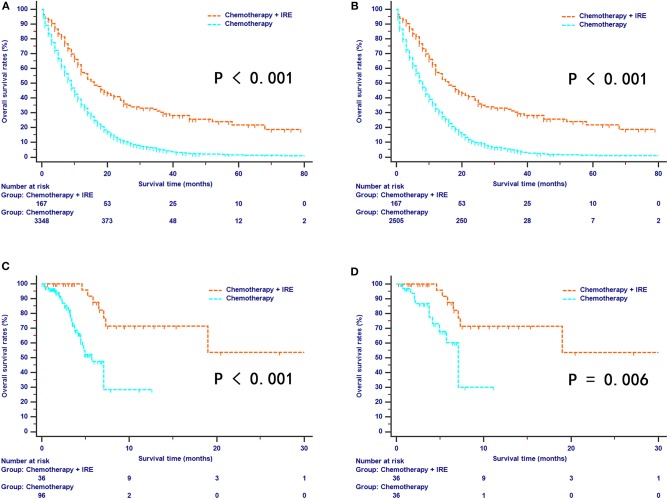
For the SEER series, survival curves for OS were well-separated between the combination therapy and chemotherapy groups. The median OS was 16 months (95% CI, 12–21 months) for the combination therapy group and 9 months (95% CI, not available) for the chemotherapy group (Figure 2A). After the PSM analysis, combination therapy provided a more obvious survival benefit than chemotherapy alone (median OS, 16 months vs. 8 months; 1-year OS rates, 57.1% vs. 32.4%; 3-year OS rates, 30.2% vs. 4.4%; 5-year OS rates, 21.8% vs. 0.9%, P < 0.001, Figure 2B). For the SYSUCC series, survival curves were also well-separated by the different treatments. During the follow-up period, the median OS for the combination therapy group was 21.6 months while the median OS for the chemotherapy group was 7.1 months (Figure 2C). In addition, compared with the chemotherapy group, the combination therapy group experienced significantly higher survival rates (1-year OS rates, 71.4% vs. 30.1%; 2-year OS rates, 53.5% vs. 30.1%, P = 0.006, Figure 2D).
Figure 2.
The survival curves of overall survival stratified by treatment strategies for patients with LAPC from SEER dataset before (A) and after (B) propensity score matching. The survival curves of overall survival stratified by treatment strategies for patients with LAPC from SYSUCC dataset before (C) and after (D) propensity score matching.
CSS and PFS Comparisons Between the Two Groups
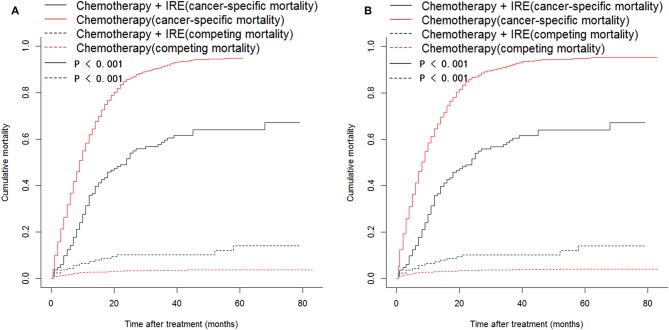
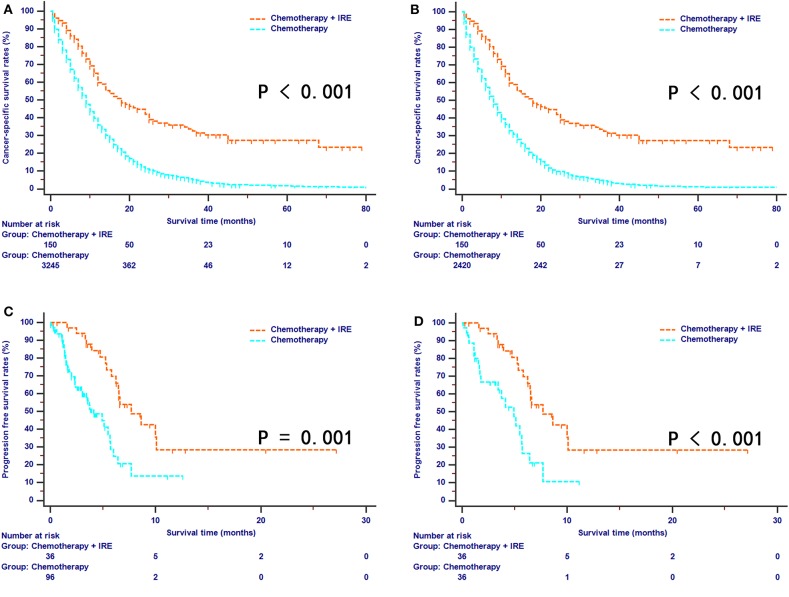
For the SEER series, a total of 2,844 out of 3,348 (84.9%) patients died during the follow-up period, which was more than 80 months. Among these deaths, there were 87 (52.1%) cancer-specific and 17 (10.2%) non-cancer-specific deaths for the combination therapy group and 2,637 (78.8%) cancer-specific and 103 (3.1%) non-cancer-specific deaths for the chemotherapy group. The comparison of cancer-specific mortality and non-cancer-specific mortality is shown in Figure 3. Patients in the chemotherapy group were at higher risk of the cumulative incidence of higher cancer-specific mortality than those in the combination therapy group (Figure 4A). Compared with the chemotherapy group, the combination therapy group experienced significantly higher CSS rates (median CSS, 18 months vs. 8 months; 1-year CSS rates, 59.7% vs. 32.9%; 3-year CSS rates, 32.5% vs. 4.4%; 5-year CSS rates, 27.3% vs. 1.0%, P < 0.001, Figure 4B). For the SYSUCC series, 15 (41.7%) patients experienced disease progression in the combination therapy group, while 42 (43.8%) patients experienced disease progression in the chemotherapy group. After PSM, a higher proportion of patients (19, 52.8%) experienced disease progression in the chemotherapy group compared with that (15, 41.7%) in the combination therapy group. The median PFS for patients was 7.7 months (95% CI, 6.2–10.1 months) in the combination therapy group and 4.1 months (95% CI, 3.1–5.7 months) in the chemotherapy group (P < 0.001, Figure 4C). Similar results were also obtained for patients after PSM (P = 0.001, Figure 4D). Patients in the chemotherapy group were 2.67 times more likely to experience tumor progression compared with those in the combination therapy group.
Figure 3.
Cumulative cancer-specific and competing mortalities stratified by treatment strategies for patients with LAPC from SEER dataset before (A) and after (B) propensity score matching.
Figure 4.
The survival curves of cancer-specific survival stratified by treatment strategies for patients with LAPC from SEER dataset before (A) and after (B) propensity score matching. The survival curves of progression-free survival stratified by treatment strategies for patients with LAPC from SYSUCC dataset before (C) and after (D) propensity score matching.
Prognostic Factors for OS, CSS, and PFS
Univariate and multivariate analyses were conducted for OS, CSS, and PFS in this study. For the SEER database, a univariate analysis illustrated that age, tumor size, tumor grade, radiotherapy, and chemotherapy combined with IRE were associated with OS and CSS for patients with LAPC. Furthermore, a multivariate model illustrated that chemotherapy combined with IRE was associated with increased OS and CSS. Other significant independent unfavorable prognostic factors for OS included age older than 60 years [hazard ration (HR) 1.283, 95% CI 1.166–1.412, P < 0.001], poor tumor differentiation (HR 1.081, 95% CI 1.007–1.160, P = 0.032), tumor size larger than 4 cm (HR 1.138, 95% CI 1.056–1.226, P = 0.001), and history of radiotherapy (HR 0.608, 95% CI 0.552–0.671, P < 0.001) (Table 2). The same factors were identified as significant prognostic factors for CSS in patients with LAPC (Table 3).
Table 2.
Univariate and multivariate analyses of OS in patients.
| Characteristic | Before PSM | After PSM | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | ||||||||||
| HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | ||
| SEER DATASET | |||||||||||||
| Age (years) | ≤60/>60 | 1.295 | 1.193–1.406 | <0.001 | 1.281 | 1.180–1.391 | <0.001 | 1.304 | 1.186–1.435 | <0.001 | 1.283 | 1.166–1.412 | <0.001 |
| Gender | Female/Male | 0.999 | 0.928–1.075 | 0.984 | NI | 0.994 | 0.914–1.082 | 0.895 | NI | ||||
| Race | Black/White/Others | 0.949 | 0.876–1.027 | 0.194 | NI | 0.937 | 0.855–1.026 | 0.159 | |||||
| Tumor size (cm) | ≤2/2~4/>4 | 1.137 | 1.066–1.213 | <0.001 | 1.148 | 1.075–1.225 | <0.001 | 1.135 | 1.054–1.222 | 0.001 | 1.138 | 1.056–1.226 | 0.001 |
| Tumor grade | Well/Moderate/Poor | 1.115 | 1.048–1.186 | 0.001 | 1.077 | 1.012–1.147 | 0.019 | 1.119 | 1.043–1.200 | 0.002 | 1.081 | 1.007–1.160 | 0.032 |
| LN metastasis | Absent/Present | 1.076 | 0.996–1.162 | 0.064 | NI | 1.072 | 0.981–1.172 | 0.123 | NI | ||||
| Tumor site | Head/Body/Tail | 0.956 | 0.909–1.006 | 0.082 | NI | 0.960 | 0.960–1.016 | 0.157 | NI | ||||
| Radiotherapy | No/Yes | 0.640 | 0.592–0.691 | <0.001 | 0.610 | 0.565–0.660 | <0.001 | 0.630 | 0.572–0.694 | <0.001 | 0.608 | 0.552–0.671 | <0.001 |
| Chemotherapy | Without IRE/With IRE | 0.428 | 0.351–0.522 | <0.001 | 0.369 | 0.302–0.451 | <0.001 | 0.403 | 0.329–0.492 | <0.001 | 0.370 | 0.302–0.453 | <0.001 |
| SYSUCC DATASET | |||||||||||||
| Age (years) | ≤60/>60 | 1.154 | 0.600–2.222 | 0.668 | NI | 0.889 | 0.351–0.253 | 0.804 | NI | ||||
| Gender | Female/Male | 2.399 | 1.077–5.343 | 0.052 | NI | 4.630 | 1.317–16.275 | 0.017 | 4.975 | 1.081–22.891 | 0.039 | ||
| Tumor size (cm) | ≤2/2~4/>4 | 1.657 | 0.843–3.257 | 0.143 | NI | 2.863 | 1.021–8.033 | 0.046 | 2.012 | 0.764–5.294 | 0.157 | ||
| Tumor grade | Well/Moderate/Poor | 1.182 | 0.669–2.086 | 0.565 | NI | 1.797 | 0.680–3.293 | 0.316 | NI | ||||
| LN metastasis | Absent/Present | 7.966 | 3.285–19.315 | <0.001 | 4.091 | 1.484–11.278 | 0.006 | 7.264 | 2.220–23.775 | 0.001 | 4.799 | 1.173–19.625 | 0.029 |
| Tumor site | Head/Body/Tail | 1.317 | 0.879–1.973 | 0.182 | NI | 1.310 | 0.700–2.452 | 0.398 | NI | ||||
| WBC (*109) | ≤10/>10 | 1.058 | 0.371–3.019 | 0.916 | NI | 0.463 | 0.061–3.527 | 0.457 | NI | ||||
| HGB (g/L) | ≤120/>120 | 0.852 | 0.419–1.733 | 0.659 | NI | 1.401 | 0.461–4.264 | 0.552 | NI | ||||
| PLT (*109) | ≤300/>300 | 0.513 | 0.181–1.455 | 0.209 | NI | 0.484 | 0.110–2.126 | 0.337 | NI | ||||
| ALT (U/L) | ≤40/>40 | 0.929 | 0.435–1.981 | 0.848 | NI | 1.034 | 0.365–2.929 | 0.950 | NI | ||||
| AST (U/L) | ≤40/>40 | 1.006 | 0.417–2.428 | 0.989 | NI | 0.623 | 0.143–2.719 | 0.529 | NI | ||||
| ALP (U/L) | ≤100/>100 | 1.686 | 0.867–3.277 | 0.124 | NI | 1.395 | 0.549–3.546 | 0.484 | NI | ||||
| GGT (U/L) | ≤45/>45 | 1.646 | 0.840–3.224 | 0.146 | NI | 2.106 | 0.821–5.400 | 0.121 | NI | ||||
| ALB (g/L) | ≤40/>40 | 0.261 | 0.133–0.515 | 0.101 | NI | 0.437 | 0.153–1.244 | 0.121 | NI | ||||
| TBIL (umol/L) | ≤20.5/>20.5 | 0.712 | 0.296–1.715 | 0.449 | NI | 0.360 | 0.083–1.569 | 0.174 | NI | ||||
| IBIL (umol/L) | ≤15/>15 | 0.354 | 0.048–2.589 | 0.306 | NI | 0.043 | 0.001–77.525 | 0.411 | NI | ||||
| CRP (ng/L) | ≤3/>3 | 3.312 | 1.582–6.936 | 0.001 | 1.741 | 0.757–4.005 | 0.192 | 3.094 | 1.136–8.428 | 0.127 | NI | ||
| CEA (ng/mL) | ≤5/>5 | 1.029 | 0.527–2.011 | 0.933 | NI | 1.264 | 0.495–3.232 | 0.624 | NI | ||||
| CA19-9 (U/ml) | ≤35/>35 | 1.745 | 0.676–4.507 | 0.250 | NI | 1.714 | 0.494–5.951 | 0.396 | NI | ||||
| HBsAg | Negative/positive | 0.220 | 0.030–1.610 | 0.136 | NI | 0.264 | 0.094–0.738 | 0.011 | NI | ||||
| Chemotherapy | Without IRE/with IRE | 0.206 | 0.082–0.515 | 0.001 | 0.363 | 0.132–0.998 | 0.050 | 0.264 | 0.094–0.738 | 0.011 | 0.313 | 0.098–0.992 | 0.048 |
| Chemotherapy type | FOLFIRINOX/Gem | 0.910 | 0.648–1.277 | 0.584 | NI | 0.852 | 0.513–1.414 | 0.535 | NI | ||||
OS, overall survival; HR, hazard ratio; NI, not included; Other abbreviations as in Table 1.
Table 3.
Univariate and multivariate analyses of CSS and PFS in patients.
| Characteristic | Before PSM | After PSM | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | ||||||||||
| HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | ||
|
CSS SEER DATASET | |||||||||||||
| Age (years) | ≤60/>60 | 1.282 | 1.179–1.394 | <0.001 | 1.269 | 1.167–1.381 | <0.001 | 1.278 | 1.160–1.408 | <0.001 | 1.258 | 1.142–1.387 | <0.001 |
| Gender | Female/Male | 0.998 | 0.926–1.076 | 0.957 | NI | 0.988 | 0.906–1.077 | 0.784 | NI | ||||
| Race | 0.963 | 0.888–1.045 | 0.368 | NI | 0.955 | 0.870–1.048 | 0.328 | NI | |||||
| Tumor size (cm) | ≤2/2~4/>4 | 1.128 | 1.056–1.204 | <0.001 | 1.161 | 1.086–1.243 | <0.001 | 1.125 | 1.043–1.213 | 0.002 | 1.129 | 1.046–1.219 | 0.002 |
| Tumor grade | Well/Moderate/Poor | 1.114 | 1.046–1.187 | 0.001 | 1.167 | 1.082–1.260 | <0.001 | 1.116 | 1.039–1.200 | 0.003 | 1.079 | 1.004–1.161 | 0.040 |
| LN metastasis | Absent/Present | 1.090 | 1.007–1.179 | 0.032 | 1.069 | 0.988–1.157 | 0.099 | 1.088 | 0.994–1.192 | 0.069 | NI | ||
| Tumor site | Head/Body/Tail | 0.950 | 0.902–1.000 | 0.050 | 0.884 | 0.830–0.941 | <0.001 | 0.951 | 0.897–1.009 | 0.095 | NI | ||
| Radiotherapy | No/Yes | 0.647 | 0.598–0.700 | <0.001 | 0.614 | 0.567–0.664 | NI | 0.635 | 0.575–0.701 | <0.001 | 0.611 | 0.553–0.675 | <0.001 |
| Chemotherapy | Without IRE/With IRE | 0.372 | 0.300–0.462 | <0.001 | 0.332 | 0.267–0.413 | <0.001 | 0.351 | 0.283–0.437 | <0.001 | 0.323 | 0.260–0.403 | <0.001 |
|
PFS SYSUCC DATASET | |||||||||||||
| Age (years) | ≤60/>60 | 0.690 | 0.410–1.163 | 0.164 | NI | 0.728 | 0.379–1.397 | 0.340 | NI | ||||
| Gender | Female/Male | 1.513 | 0.868–2.639 | 0.144 | NI | 1.692 | 0.864–3.315 | 0.125 | NI | ||||
| Tumor size (cm) | ≤2/2~4/>4 | 1.375 | 0.848–2.232 | 0.196 | NI | 1.537 | 0.827–2.856 | 0.174 | NI | ||||
| Tumor grade | Well/Moderate/Poor | 1.271 | 0.814–1.986 | 0.291 | NI | 1.226 | 0.724–2.077 | 0.447 | NI | ||||
| LN metastasis | Absent/Present | 2.867 | 1.588–5.176 | <0.001 | 2.380 | 1.269–4.460 | 0.007 | 3.190 | 1.526–6.672 | 0.002 | 4.170 | 1.898–9.163 | <0.001 |
| Tumor site | Head/Body/Tail | 1.281 | 0.930–1.763 | 0.130 | NI | 1.388 | 0.900–2.142 | 0.138 | NI | ||||
| WBC (*109) | ≤10/>10 | 1.282 | 0.628–2.616 | 0.495 | NI | 0.995 | 0.315–2.823 | 0.993 | NI | ||||
| HGB (g/L) | ≤120/>120 | 1.155 | 0.642–2.077 | 0.631 | NI | 1.311 | 0.620–2.774 | 0.479 | NI | ||||
| PLT (*109) | ≤300/>300 | 0.819 | 0.412–1.628 | 0.570 | NI | 0.512 | 0.198–1.323 | 0.167 | NI | ||||
| ALT (U/L) | ≤40/>40 | 0.753 | 0.413–1.374 | 0.355 | NI | 0.733 | 0.346–1.550 | 0.416 | NI | ||||
| AST (U/L) | ≤40/>40 | 0.775 | 0.381–1.579 | 0.483 | NI | 0.841 | 0.350–2.018 | 0.697 | NI | ||||
| ALP (U/L) | ≤100/>100 | 1.024 | 0.614–1.709 | 0.927 | NI | 0.956 | 0.506–1.808 | 0.890 | NI | ||||
| GGT (U/L) | ≤45/>45 | 0.870 | 0.518–1.459 | 0.597 | NI | 0.729 | 0.376–1.411 | 0.348 | NI | ||||
| ALB (g/L) | ≤40/>40 | 0.775 | 0.445–1.349 | 0.367 | NI | 0.750 | 0.354–1.587 | 0.452 | NI | ||||
| TBIL (umol/L) | ≤20.5/>20.5 | 0.544 | 0.266–1.112 | 0.095 | NI | 0.419 | 0.174–1.008 | 0.052 | NI | ||||
| IBIL (umol/L) | ≤15/>15 | 1.116 | 0.445–2.804 | 0.815 | NI | 0.796 | 0.244–2.600 | 0.705 | NI | ||||
| CRP (ng/L) | ≤3/>3 | 1.605 | 0.922–2.795 | 0.095 | NI | 1.419 | 0.696–2.893 | 0.336 | NI | ||||
| CEA (ng/mL) | ≤5/>5 | 1.123 | 0.668–1.890 | 0.662 | NI | 1.221 | 0.643–2.320 | 0.542 | NI | ||||
| CA19-9 (U/ml) | ≤35/>35 | 1.965 | 0.927–4.167 | 0.078 | NI | 3.258 | 1.148–9.247 | 0.027 | 1.191 | 0.394–3.606 | 0.757 | ||
| HBsAg | Negative/Positive | 0.501 | 0.181–1.393 | 0.185 | NI | 0.805 | 0.246–2.634 | 0.719 | NI | ||||
| Chemotherapy | Without IRE/With IRE | 0.357 | 0.197–0.649 | 0.001 | 0.491 | 0.251–0.960 | 0.038 | 0.350 | 0.180–0.678 | 0.002 | 0.305 | 0.146–0.638 | 0.002 |
| Chemotherapy type | FOLFIRINOX/Gem | 1.006 | 0.781–1.298 | 0.960 | NI | 0.996 | 0.719–1.380 | 0.980 | NI | ||||
CSS, cancer-specific survival; PFS, progression-free survival; HR, hazard ratio; Other abbreviations as in Table 1.
The data from the SYSUCC series were also used to analyze the prognostic factors of OS and PFS. Gender, tumor size, lymph node (LN) metastasis and chemotherapy plus IRE were associated with OS in patients with LAPC. In addition, after a stepwise removal of variables in the multivariate analysis, male gender (HR 4.975, 95% CI 1.081–22.891, P = 0.039), presence of LN metastasis (HR 4.799, 95% CI 1.173–19.625, P = 0.029), and chemotherapy plus IRE (HR 0.313, 95% CI 0.098–0.992, P = 0.048) were identified as independent prognostic factors for OS (Table 2). A multivariate analysis revealed that chemotherapy plus IRE was identified as an independent favorable prognostic factor for PFS in patients in both the entire cohort and the matched cohort (Table 3).
Discussion
Previous studies have shown a survival benefit for IRE plus conventional treatment in patients with LAPC (6, 7, 21). Most of the aforementioned studies were single-arm studies. There was one study that suggested that treatment with IRE combined with chemotherapy increased the median OS of patients with LAPC compared to conventional treatments (22). However, all these studies contained limited patient cohorts or lacked external validation. In contrast, another small cohort and single-center study showed no significant survival benefit from IRE after induction chemotherapy (8). The significance of the lack of rigorous quality assessments in these studies in regard to their negative outcomes is unknown. Multiple smaller studies with varying chemotherapy regimens have provided mixed results (6, 8, 23). Therefore, there has been no strong evidence for the survival benefit of IRE combined with chemotherapy in LAPC patients. The current study compared the efficacy between IRE plus chemotherapy and chemotherapy in patients with LAPC based on the cohorts from the SEER database and the SYSUCC database, a “real-world” dataset. To our knowledge, this is the largest study evaluating the role of IRE combined with chemotherapy in patients with LAPC. Our analysis provided evidence that patients with LAPC who were treated with IRE combined with chemotherapy had a more obvious survival benefit compared with those who were treated with chemotherapy alone.
In this study, all patients in the SYSUCC dataset received standard chemotherapy, including GEM-based chemotherapy or FOLFIRINOX-based chemotherapy. In addition, gemcitabine-based chemotherapy has been the first-line therapy for advanced pancreatic cancer since the positive survival results of gemcitabine-based chemotherapy were revealed in 1997 (24). It can be assumed that the chemotherapy regimen, which was mainly GEM-based chemotherapy for patients from the SEER database, was uniform and recommended for patients with LAPC during this time period (24, 25). The median OS was 16 and 21.6 months for patients after IRE combined with chemotherapy in the SEER and SYSUCC datasets, respectively. The median OS was similar to that from a study conducted by Huang et al. (26) and was significantly higher than that of patients after chemotherapy alone (8.0 months for the SEER patients and 7.1 months for the SYSUCC patients). In addition, the SEER patients had a long follow-up period, and it was shown that combination therapy was superior to chemotherapy alone with respect to not only 5-year OS rates but also 5-year CSS rates for all enrolled patients even though larger proportions of patients in the chemotherapy group received radiotherapy. After the PSM analysis, the advantages of survival benefits in terms of OS and CSS from combination therapy were even more obvious, compared with those from chemotherapy alone in both the SEER and SYSUCC patients. In the present study, 21 patients received FOLFIRINOX-based chemotherapy in the SYSUCC cohort, although patients with LAPC had significantly improved survival from the newest modified FOLFIRINOX-based chemotherapy (27). FOLFIRINOX-based chemotherapy became popular more recently. Only a proportion of patients received FOLFIRINOX-based chemotherapy in this study. The absence of uniform protocols and the intolerance to the full dosages of FOLFIRINOX-based chemotherapy might partially explain the unsatisfactory results for chemotherapy in this study. In addition, cross-study comparisons were also conducted. Studies conducted by Krishnan et al. (28) and Huguet et al. (29) showed that chemotherapy combined with radiotherapy contributed to the median OS, which was nearly 12 months for patients with LAPC, and significantly lower than that of patients who received IRE combined with chemotherapy. These comparisons further consolidated the survival advantages of combination therapy over chemotherapy alone. In addition, there was a disparity in the survival between the SEER and SYSUCC patients, the development of chemotherapy regimens and technology of IRE may ultimately improve the survival of LAPC patients after combination therapy. However, a survival benefit of IRE combined with chemotherapy was observed in patients with LAPC who received any type of chemotherapy. Radiotherapy was also shown as a favorable prognostic factor for both OS and CSS for patients with LAPC after IRE plus chemotherapy therapy. The enhanced local and systemic control of diseases further improved survival outcomes, showing that maybe IRE was an effective supplement to the standard therapy for LAPC patients.
Tumor progression was commonly observed in patients with LAPC even after treatment. Compared with the 19 out of 36 (52.8%) patients who experienced disease progression after chemotherapy, 15 out of 36 (41.7%) patients experienced disease progression after IRE combined with chemotherapy during the follow-up period. Compared with chemotherapy alone, IRE combined with chemotherapy resulted in significantly longer PFS. IRE assists the chemotherapy delivery to the tumor by disrupting the dense stroma of pancreatic cancer (30, 31). Another mechanism of IRE is the formation of nanoscale micropores in the lipid bilayer of cell membranes increases its permeability to macromolecules, including chemical (12). Moreover, in IRE treatment, an electric field surrounds the entire tumor without causing thermal damage to important nearby structures (12). Maybe these mechanisms help to explain the synergism of IRE and chemotherapy compared with chemotherapy alone. In addition, as a local destructive treatment, maybe IRE could only control the local disease, the subsequent chemotherapy was needed to adequately control microscopic disease. IRE combined with chemotherapy created a synergistic effect to decrease the local recurrence rates and to lengthen PFS for patients with LAPC.
Similar to another study with a large cohort conducted by Martin et al. (32), which was a single-arm study showing an encouraging 24.9-month median OS and 12.4-month median PFS, our study provided new data illustrating the survival benefit from IRE combined with chemotherapy. In contrast to our study, 25% of patients in Martin's study underwent resection and IRE margin accentuation. Our study focused on patients which were not suitable for concomitant resection and aimed to provide new insights on this combination therapy for patients with LAPC. In addition, with the large cohort from the SEER database and the external cohort from the SYSUCC database, a survival benefit for IRE combined with chemotherapy was clearly shown, which suggests that IRE combined with chemotherapy was an effective multimodal approach and assisted both local tumor reduction and systemic control of the disease in patients with LAPC.
This study has limitations. First, there was potential selection bias in this retrospective trial. The PSM analysis decreased the selection bias associated with the variables incorporated into the comparisons, but these biases could not be completely removed. The SEER database provided a sufficient sample size to eliminate heavily selected observations without reducing power to robustly evaluate treatment effects. However, despite such matching, residual confounding by indication due to unmeasured variables cannot be ruled out. Second, detailed information on chemotherapy, including the dose and course of chemotherapy, and some important hematological indexes, such as CA19-9 and CEA, are not available in the SEER database. Third, there was no information on the comorbidities or performance status of the patients in this study. Although strict inclusion criteria were followed and only patients who received IRE treatment were included, the sample size of patients from the SYSUCC database was small, and the follow-up period was not long enough to make definitive conclusions. Therefore, the survival benefit of IRE in patients with LAPC needs to be validated in another large cohort.
In conclusion, compared with chemotherapy alone, IRE combined with chemotherapy contributed to better long-term survival in patients with LAPC. A randomized clinical trial comparing the efficacy of IRE and chemotherapy is therefore warranted.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.
Ethics Statement
This study was approved by the Institutional Review Board (IRB) of the Sun Yat-sen University Cancer Center. Each individual participant from SYSUCC database has signed the informed written consent. All procedures performed in studies involving human participants were in accordance with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Author Contributions
SL designed the project, reviewed, and edited the manuscript, respectively. CH, XH, and YZ performed the study selection, data extraction, statistical analyses, and wrote the main manuscript. CH, XH, YZ, ZC, and XL contributed in classification criteria discussion. All authors reviewed the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This work was supported by grants from the National Natural Science Funds (No. 81672390) and the National Key Research and Development Plan (No. 2017YFC0910002).
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2018) 68:394–424. 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2.Loehrer PJ, Sr, Feng Y, Cardenes H, Wagner L, Brell JM, Cella D, et al. Gemcitabine alone versus gemcitabine plus radiotherapy in patients with locally advanced pancreatic cancer: an Eastern Cooperative Oncology Group trial. J Clin Oncol. (2011) 29:4105–12. 10.1200/JCO.2011.34.8904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hammel P, Huguet F, van Laethem JL, Goldstein D, Glimelius B, Artru P, et al. Effect of chemoradiotherapy vs chemotherapy on survival in patients with locally advanced pancreatic cancer controlled after 4 months of gemcitabine with or without erlotinib: the LAP07 randomized clinical trial. JAMA. (2016) 315:1844–53. 10.1001/jama.2016.4324 [DOI] [PubMed] [Google Scholar]
- 4.Martin RC, II, McFarland K, Ellis S, Velanovich V. Irreversible electroporation in locally advanced pancreatic cancer: potential improved overall survival. Ann Surg Oncol. (2013) 20 (Suppl 3):S443–9. 10.1245/s10434-012-2736-1 [DOI] [PubMed] [Google Scholar]
- 5.Paiella S, Butturini G, Frigerio I, Salvia R, Armatura G, Bacchion M, et al. Safety and feasibility of Irreversible Electroporation (IRE) in patients with locally advanced pancreatic cancer: results of a prospective study. Digest Surg. (2015) 32:90–7. 10.1159/000375323 [DOI] [PubMed] [Google Scholar]
- 6.Mansson C, Brahmstaedt R, Nilsson A, Nygren P, Karlson BM. Percutaneous irreversible electroporation for treatment of locally advanced pancreatic cancer following chemotherapy or radiochemotherapy. Eur J Surg Oncol. (2016) 42:1401–6. 10.1016/j.ejso.2016.01.024 [DOI] [PubMed] [Google Scholar]
- 7.de Liguori Carino N, O'Reilly DA, Siriwardena AK, Valle JW, Radhakrishna G, Pihlak R, et al. Irreversible Electroporation in pancreatic ductal adenocarcinoma: is there a role in conjunction with conventional treatment? Eur J Surg Oncol. (2018) 44:1486–93. 10.1016/j.ejso.2018.07.047 [DOI] [PubMed] [Google Scholar]
- 8.Vogel JA, Rombouts SJ, de Rooij T, van Delden OM, Dijkgraaf MG, van Gulik TM, et al. Induction chemotherapy followed by resection or irreversible electroporation in locally advanced pancreatic cancer (IMPALA): a prospective cohort study. Ann Surg Oncol. (2017) 24:2734–43. 10.1245/s10434-017-5900-9 [DOI] [PubMed] [Google Scholar]
- 9.Edge SB, Byrd DR, Comptom CC, Fritz AG, Greene FL, Trotti A. editors. AJCC Cancer Staging Manual. 7th ed New York, NY: Springer; (2010). [Google Scholar]
- 10.Callery MP, Chang KJ, Fishman EK, Talamonti MS, William Traverso L, Linehan DC. Pretreatment assessment of resectable and borderline resectable pancreatic cancer: expert consensus statement. Ann Surg Oncol. (2009) 16:1727–33. 10.1245/s10434-009-0408-6 [DOI] [PubMed] [Google Scholar]
- 11.He C, Wang J, Sun S, Zhang Y, Lin X, Lao X, et al. Irreversible electroporation versus radiotherapy after induction chemotherapy on survival in patients with locally advanced pancreatic cancer: a propensity score analysis. BMC Cancer. (2019) 19:394. 10.1186/s12885-019-5607-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Al Efishat M, Wolfgang CL, Weiss MJ. Stage III pancreatic cancer and the role of irreversible electroporation. BMJ. (2015) 350:h521. 10.1136/bmj.h521 [DOI] [PubMed] [Google Scholar]
- 13.Weiss MJ, Wolfgang CL. Irreversible electroporation: a novel therapy for stage III pancreatic cancer. Adv Surg. (2014) 48:253–8. 10.1016/j.yasu.2014.05.002 [DOI] [PubMed] [Google Scholar]
- 14.Martin RC, II, McFarland K, Ellis S, Velanovich V. Irreversible electroporation therapy in the management of locally advanced pancreatic adenocarcinoma. J Am Coll Surg. (2012) 215:361–9. 10.1016/j.jamcollsurg.2012.05.021 [DOI] [PubMed] [Google Scholar]
- 15.Martin RC, II, Durham AN, Besselink MG, Iannitti D, Weiss MJ, Wolfgang CL, et al. Irreversible electroporation in locally advanced pancreatic cancer: a call for standardization of energy delivery. J Surg Oncol. (2016) 114:865–71. 10.1002/jso.24404 [DOI] [PubMed] [Google Scholar]
- 16.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. (1988) 16:1141–54. 10.1214/aos/1176350951 [DOI] [Google Scholar]
- 17.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. (1999) 94:496–509. 10.1080/01621459.1999.10474144 [DOI] [Google Scholar]
- 18.Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat. (2011) 10:150–61. 10.1002/pst.433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pompili M, Saviano A, de Matthaeis N, Cucchetti A, Ardito F, Federico B, et al. Long-term effectiveness of resection and radiofrequency ablation for single hepatocellular carcinoma </=3 cm. Results of a multicenter Italian survey. J Hepatol. (2013) 59:89–97. 10.1016/j.jhep.2013.03.009 [DOI] [PubMed] [Google Scholar]
- 20.Cohen J. A power primer. Psychol Bull. (1992) 112:155–9. 10.1037/0033-2909.112.1.155 [DOI] [PubMed] [Google Scholar]
- 21.Leen E, Picard J, Stebbing J, Abel M, Dhillon T, Wasan H. Percutaneous irreversible electroporation with systemic treatment for locally advanced pancreatic adenocarcinoma. J Gastrointest Oncol. (2018) 9:275–81. 10.21037/jgo.2018.01.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Belfiore G, Belfiore MP, Reginelli A, Capasso R, Romano F, Ianniello GP, et al. Concurrent chemotherapy alone versus irreversible electroporation followed by chemotherapy on survival in patients with locally advanced pancreatic cancer. Med Oncol. (2017) 34:38. 10.1007/s12032-017-0887-4 [DOI] [PubMed] [Google Scholar]
- 23.Liu S, Qin Z, Xu J, Zeng J, Chen J, Niu L, et al. Irreversible electroporation combined with chemotherapy for unresectable pancreatic carcinoma: a prospective cohort study. Onco Targets Ther. (2019) 12:1341–50. 10.2147/OTT.S186721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burris HA, III, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. (1997) 15:2403–13. 10.1200/JCO.1997.15.6.2403 [DOI] [PubMed] [Google Scholar]
- 25.Sajjad M, Batra S, Hoffe S, Kim R, Springett G, Mahipal A. Use of radiation therapy in locally advanced pancreatic cancer improves survival: a SEER database analysis. Am J Clin Oncol. (2018) 41:236–41. 10.1097/COC.0000000000000261 [DOI] [PubMed] [Google Scholar]
- 26.Huang KW, Yang PC, Pua U, Kim MD, Li SP, Qiu YD, et al. The efficacy of combination of induction chemotherapy and irreversible electroporation ablation for patients with locally advanced pancreatic adenocarcinoma. J Surg Oncol. (2018) 118:31–6. 10.1002/jso.25110 [DOI] [PubMed] [Google Scholar]
- 27.Suker M, Beumer BR, Sadot E, Marthey L, Faris JE, Mellon EA, et al. FOLFIRINOX for locally advanced pancreatic cancer: a systematic review and patient-level meta-analysis. Lancet Oncol. (2016) 17:801–10. 10.1016/S1470-2045(16)00172-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krishnan S, Rana V, Janjan NA, Varadhachary GR, Abbruzzese JL, Das P, et al. Induction chemotherapy selects patients with locally advanced, unresectable pancreatic cancer for optimal benefit from consolidative chemoradiation therapy. Cancer. (2007) 110:47–55. 10.1002/cncr.22735 [DOI] [PubMed] [Google Scholar]
- 29.Huguet F, André T, Hammel P, Artru P, Balosso J, Selle F, et al. Impact of chemoradiotherapy after disease control with chemotherapy in locally advanced pancreatic adenocarcinoma in GERCOR phase II and III studies. J Clin Oncol. (2007) 25:326–31. 10.1200/JCO.2006.07.5663 [DOI] [PubMed] [Google Scholar]
- 30.Moir J, White SA, French JJ, Littler P, Manas DM. Systematic review of irreversible electroporation in the treatment of advanced pancreatic cancer. Eur J Surg Oncol. (2014) 40:1598–604. 10.1016/j.ejso.2014.08.480 [DOI] [PubMed] [Google Scholar]
- 31.Narayanan G, Hosein PJ, Arora G, Barbery KJ, Froud T, Livingstone AS, et al. Percutaneous irreversible electroporation for downstaging and control of unresectable pancreatic adenocarcinoma. J Vasc Intervent Radiol. (2012) 23:1613–21. 10.1016/j.jvir.2012.09.012 [DOI] [PubMed] [Google Scholar]
- 32.Martin RC, II, Kwon D, Chalikonda S, Sellers M, Kotz E, Scoggins C, et al. Treatment of 200 locally advanced (stage III) pancreatic adenocarcinoma patients with irreversible electroporation: safety and efficacy. Ann Surg. (2015) 262:486–94; discussion 92–4. 10.1097/SLA.0000000000001441 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.