Abstract
The bacterium Kluyvera georgiana MCC 3673 transfers electrons directly to the electrode for bio-electricity generation in microbial fuel cell (MFC). This could be due to the formation of biofilm on the surface of electrode or with through the extracellular appendages, or both. The role of extracellular appendages pili and flagella in exo-electron transfer mechanism was investigated. The expression level of the genes fli P and pil Q for pili and flagella, respectively, in K. georgiana MCC 3673 was compared in MFC and in shake flask. The transcript analysis was done by qRT-PCR at different times and conditions. The expression level of pil Q transcript in K. georgiana MCC 3673 showed over twofold higher expression during bio-electrogenic process, compared to the one inoculated in shake flask. Similarly, fli P had also showed similar kind of expression in MFC compared to that in shake flask. Higher level of pil Q and fli P transcripts were observed throughout bio-electrogenic process. The level of pil Q was found to be nearly fourfold higher in biofilm-forming cells forming compared to the cells in suspension form. The obtained results suggest that flagella have a role in movement of bacterium towards electrode for donating the electron in absence of oxygen, and pili aiding in adhering on the surface of electrode and forming biofilm. The cumulative effect of fli P and pil Q resulted in exo-electron transfer to the electrode and bio-electricity generation process. The open circuit potential (OCV) of + 0.7 V was produced with the maximum power density of 393 ± 14 mW/m2 in MFC.
Electronic supplementary material
The online version of this article (10.1007/s13205-020-2050-8) contains supplementary material, which is available to authorized users.
Keywords: Bio-electricity, Kluyvera georgiana MCC 3673, Microbial fuel cells, Type IV pili, qRT-PCR, Power density
Introduction
The electroactive property in some bacteria during electricity generation has been studied extensively in past decade (Cao et al. 2019). These electroactive bacteria having tendency to transfer electron extracellular are termed as electrogens (electricity generators). In the absence of oxygen, bacteria tend to find terminal electron acceptor to donate the electron and regenerating NAD+ and FAD for cellular metabolism simultaneous to reducing a wide range of organic molecule (Shrestha and Rotaru 2014). When these bacteria are inoculated in MFC, where the sole electron acceptor is an electrode, the terminal electrons are collected and transported through the electric circuit (Logan et al. 2006). The transfer of electrons could be either directly (mediator-less) or by the aid of redox compounds (mediators) in the medium by the bacterium (Schröder 2007).
Carbonaceous materials are common choice of electrode due to high conductivity and less toxic to the bacteria (Sonawane et al. 2017). Apart from carbon materials, use of metals electrodes have also been reported in the literature (Baudler et al. 2015). The metals, silver and copper are regarded as an excellent conductor of electricity but are toxic to bacteria. Baudler et al. (2015) have reported that the electrogenic bacteria are resistance towards metal toxicity. The electrogenic bacteria are believed to have physiologically, metabolically and genetically unique property enabling them to grow under anaerobic condition reducing wide range of compounds in stringent environment conditions (Lovley 2006).
The exo-electron transfer can be due to biofilm formation (Li and Nealson 2015), synthesis of redox mediators by bacterium (Rabaey et al. 2005), secretion system (Richter et al. 2012) or electric conductive pili (Reguera et al. 2005). The bacterium Geobacter and Shewanella are known to be efficient electrogens in bio-electrochemical system apart from their dissimilatory metal reducing property (Richter et al. 2012). The exo-electron transfer mechanism is mainly due to the presence of pili on the bacterial cell. Pili are extracellular thin hair-like appendages in nanometer size commonly observed in eubacteria. They are known to play an important role in transfer of genetic material during conjugation, virulence of the bacteria, biofilm formation, motility and adhesion etc. (Proft and Baker 2009). Among all, the type IV pili is predominantly responsible for motility and secretion of peptides and compounds, and other various functions of the cell (Shi and Sun 2002). Though the type IV pili are present in almost all eubacteria, presence of conductive pili (called nanowire) has been reported in some bacteria making them electroactive in nature (Gorby et al. 2006). Similar to Geobacter and Shewanella, a recent study reports the presence of type IV conductive pili has also been observed in Pseudomonas aeruginosa (Liu et al. 2018). The type IV pili are a cluster of at least 30 genes assembled for twitching motility of the bacteria (Mattick 2002). Pil Q is an important component involved in the biogenesis of type IV pili, playing an important role in growing pilus fiber (Tonjum et al. 2002).
Similar to pili, flagella are extra cellular, hair-like appendages produced by bacteria. They aid in motility and adhesion of the bacteria on the surface (Moens and Vanderleyden 1996). Flagella are composed more than fifty genes, assembled as fliLMNOPQRflhBA gene cluster. Fli P is a transmembrane protein that plays an important role in transportation process during flagella synthesis and assembly for motility of the bacterium (Segura et al. 2001). Barker and co-worker showed the role of fli P in motility of bacterium in type three excretion system (Barker et al. 2014). However, so far, there are no reports on role of flagella during electron transport process in MFC.
Kluyvera georgiana MCC 3673 is a gram negative bacterium, recently reported for its electrogenic property in bio-electrochemical system (Thapa and Chandra 2019). This bacterium is found to generate bio-electricity through direct mode of exo-electron transfer mechanism in MFC. This could be due to the help of pili and flagella during the process. The present study reports the differential level of pili and flagella genes under bio-electrochemical condition and shake-flask condition in K. georgiana MCC 3673.
Materials and methods
MFC condition
Two chambered MFC with working volume of 100 ml each was used for this study as discussed elsewhere (Thapa and Chandra 2019; Thapa et al. 2019). Briefly, carbon cloth with the geometric surface area of 1.5 cm2 as anode and a graphite rod with approx. 15 cm2 as cathode were used. The cathode chamber was filled with 0.1 M potassium ferricyanide prepared in phosphate buffer (0.1 M, pH 7.2). Anode chamber was filled with sterile LB media (Himedia, India) and inoculated with 10 µl of active culture of K. georgiana MCC 3673. Both the compartments were separated by a pre-treated cation exchange membrane (CM 7000). The electrodes were connected to the data logger (Picolog ADC 20/24, UK) with no external resistance for recording voltage at 10 min interval.
RNA isolation and cDNA synthesis
Isolation of RNA was done by Trizol method (Chomczynski and Sacchi 1987). The cells were prepared by spinning at 10,000 rpm for 5 min at 4 °C followed by ice cold phosphate buffer (pH 7.2) wash. Cell lysis was done by adding equal amount of Trizol reagent (Takara, Clontech, Japan), followed by vortex and incubation at room temperature for 5 min each. To this 0.25 ml of chloroform was added to precipitate the RNA. The final extraction was done with 0.5 ml of isopropanol, washed with ice cold 70% ethanol, air dried the pellet and dissolved in 20 µl of RNAse-free water and stored at − 20 °C until further use. The RNA was reverse transcribed as instructed by the manufacturer (TAKARA, Clontech, Japan). The template RNA 1 µl was mixed with 2 µl of RT buffer, 0.5 µl of oligo dT, hexamer and reverse transcriptase enzyme each in a 10 µl reaction incubated for 15 min at 37 °C and 85 °C for 5 s.
Primer design
The primers for the genes pil Q and fli P, with reference genes 16S rDNA and gyr B were synthesized using online tool primer3 plus (https://primer3plus.com). The primers used in this study are given in Table 1.
Table 1.
Table listing the primer sequence for different genes analysed in this study
| Gene | Primer sequence |
|---|---|
| K.gn 16S rDNA | Fwd. primer: ACCGCATAACGTCGCAAGACCA |
| Rev. Primer: TTCATACACGCGGCATGGCT | |
| gyrB | Fwd. primer: ACAGCAGCAGTTCGAGCCGATT |
| Rev. Primer: AACGCTGAATCGCAAGGCCA | |
| fliP | Fwd. primer: ATCAGCGGCGACAGCATCATCA |
| Rev. primer: ACATGCTGGCGCAGACCAACAA | |
| pilQ | Fwd. primer: TCGCATCAATGGTCGGCTGCTT |
| Rev. primer: AACACCGTTGGCGTCACTTCCA |
Semi-quantitative PCR
The amplification was performed in a thermo cycler (Eppendorf, Germany). 0.5 µl of cDNA was mixed with 1 µl of each forward and reverse primer, 2.5 µl of nuclease free water and 5 µl of master mix (Ampliqon, Denmark) containing dNTPs, Mg2+, DNA polymerase and reaction buffer. PCR reaction was done by initial denaturation at 96 °C for 5 min, followed by 35 cycles of denaturation at 96 °C for 30 s, primers annealing at 56 °C for 45 s and elongation at 72 °C for 150 s, and a final elongation for 10 min.
Agarose gel electrophoresis
The amplified products were mixed with 2% of ethidium bromide staining solution and loaded on 2% agarose gel prepared in 1 × Tris acetate buffer. A 100 bp DNA ladder (Genedirex, India) was run as a marker on the gel to analyse the size of the amplified product. Electrophoresis was carried out in tris acetate buffer by applying 80 V potential for a period of 40 min for DNA fragments migration. The bands were observed and analysed under gel documentation (BioRad, USA).
Quantitative PCR
The real time quantitative PCR was performed in Quantstudio 7 (Applied biosystem, USA) in a 96 well plate titter. In RT-PCR, amplification of cDNA was done with SYBR green master mix (Promega, USA) containing SYBR green as active dye, dNTPs, Mg2+, DNA polymerase, and reaction buffer. Prior to the reaction, template DNA was diluted to tenfold. A 0.5 µl of template was added with 1 µl of forward and reverse primer each and 2.5 µl of SYBR Green master mix to a 10 µl reaction with volume nuclease free water. The PCR reaction was performed by setting the cycling conditions as 50 °C and 95 °C each for 5 min for initial activation followed by amplification at 96 °C for 15 s and 60 °C for 1 min, up to 40 cycles and a final melt curve was performed at 96, 60 and 50 °C for 15 s, 75 s, respectively. The amplification plot and melt curve were analysed as per Livak method (Livak and Schmittgen 2001).
Data analysis
The analysis of the genes was done as per Livak method (Livak and Schmittgen 2001). This is a relative and convenient method of analysing the level of gene expression by relating the PCR signal of the target transcript to the reference one. The difference in the level is calculated using the Ct which is the threshold cycle of amplification in PCR to produce the minimum number of copies that is detected for fluorescence. The lower the Ct values higher the accumulation of the product. The change in expression level was calculated using the formula, , where Ct (or dCt) is the difference in Ct of the target gene to the Ct of the reference gene, and (or ddCt) is the difference in Ct of the target gene at different conditions:
Results and discussion
Semi-quantitative analysis
The RNA isolated from the different inoculum at different conditions and times was found to be pure as analysed by the ratio of nucleic acid to protein by UV absorbance. The A260/280 ratio of over 1.8 indicated the high purity of RNA with minimal DNA contamination and no contamination with proteins. The intensity of the gel bands for the genes pil Q and fli P was observed to be higher in cells inoculated in MFC compared to those inoculated in shake flask, on analysis using Quantity One software (Biorad, USA). These indicate the genes pil Q and fli P under electrogenic condition of cells inoculated in MFC were almost twice that of genes expressed under normal growth conditions in shake flask. Similar results were obtained for the gel bands analysed using the image tool, Image J (https://imagej.nih.gov/ij/). This confirmed the expression level of genes for pili and flagella is higher in K. georgiana MCC 3673 under electrogenic condition in MFC. The results suggest that the extra cellular appendages pili and flagella playing a role in electron transfer during electrogenic process.
Quantitative analysis of gene expression level
Similar to the semi quantitative method, optimization of the PCR conditions were done as per MIQE guidelines (Bustin et al. 2009) (supplementary information). Normalization of the reaction is very crucial to know the optimal reaction temperature, number of cycles, primer annealing and elongation condition, template and primer concentrations etc. The optimization performed by standardizing the reaction with the reference gene gyr B and 16S rDNA resulted that the amplification cycles at 60 °C was suitable for the reaction with the primer concentration of 1 µM for 40 cycles. Change in these conditions caused non-specific amplification of the DNA, which was evident by the formation of more than one amplified products from the reaction, observed as multiple peaks on melt curve (supplementary information).
Standard curve analysis for RT-PCR
The 16S rRNA and gyr B genes were used as a reference gene for comparative studies. These genes have equal level of expression at any stage of growth and under any environmental condition. The standard curve performed by amplifying the template DNA with diluted series of template DNA in 100, 50, 25 and 12.5 ng/µl resulted in decreased Ct with increased template concentration. The slope value of the curve was found to be − 3.5, which determines the efficiency of the reaction to be within acceptable range. The values between − 3.1 and − 3.7 (85–110%) are considered to be acceptable and reliable for this study:
The coefficient of determination (or coefficient of correlation), r2 of 0.99 obtained from the standard curve confirms the efficiency of the reaction. The standard curve confirms the efficiency and reliability of the reaction.
Expression of fli P gene in MFC
Fli P in K. georgiana MCC 3673 is one of the important proteins responsible for flagella assembly and function that play an important role in bacterial movement. The filament of the bacterial flagella is composed of over 20 structural genes. Among them, flhA, flhB, fliI, fliP, fliR, and fliQ constitute for export apparatus of the flagella (Liu and Ochman 2007). The fliP is reported to be well-conserved and commonly studied flagella gene (Poggio et al. 2007). The expression level of fli P in K. georgiana MCC 3673 was observed under different conditions, aerobically in shake flask at 35 °C and 150 rpm, and anaerobically in MFC. The Ct for fli P was found to be 28.9 ± 0.21 for shake flask which was higher compared to Ct for cells inoculated in MFC. The Ct was calculated by taking the 16S rDNA gene as reference which has constant expression irrespective of growth conditions. The Ct for the reference gene was found to be 12.78 ± 0.2. The Ct is the change in expression level of genes under different conditions, which is the difference between the Ct of gene of interest and Ct of reference gene:
The lower ∆Ct for fli P in MFC clearly indicates higher level of expression of flagella gene. The expression level of the genes calculated by showed that flagella genes are expressed 2.4-fold higher under bio-electrogenic condition compared to that incubated in shake flask. Flagella are locomotory organelles aiding bacteria in movement towards favourable condition (away from repellent and towards attractant). Over twofold higher level of flagella indicates its role in movement of bacterium towards electrode for transfer of electrons for regenerating NAD+ and FAD to continue the metabolic and cellular activities in absence of oxygen. A time course study was performed on fli P to understand its role in MFC. The level of fli P genes was found to be increasing with the increase in incubation time in MFC. The increasing fold level 2.04 ± 0.056, 2.42 ± 0.084, and 2.58 ± 0.125 was observed after 12, 24 and 48 h, respectively. Similarly, the biofilm-forming bacteria had 2.93 ± 010-fold higher level of expression compared to that incubated in shake flask. The result is shown in Fig. 1.
Fig. 1.

Expression levels of genes fli P and pil Q in suspended cells of K. georgiana MCC 3673 at different times in MFC
Expression of pil Q in MFC
The role of pili in electron transfer and bio-electricity generation is realized to be very important in the past decade (Cao et al. 2019). Pili are the extracellular thin hair-like appendages that help bacteria in attachment to the surface and biofilm formation. In electrogenic bacteria pili are referred as “nanowires” due to their electric conductive property that play an important role in electron conduction from extracellular surface of the bacterium to the electrode. The ∆Ct for pil Q incubated in MFC and shake flask was found to be 13.44 ± 0.13 and 14.48 ± 0.16, respectively. Similar to fli P, a twofold higher amount of pili gene was observed in cells under electrogenic condition in MFC, which indicates the important role during exo-electron transfer mechanism. When the bacterial cells are inoculated in MFC, in absence of oxygen the electrons were transported towards extracellular surface and into the medium via c-type cytochrome and other inter membrane proteins and mediators. These electrons are directly transported to the electrode by the cells adhered on the electrode by pili:
An increase from 1.94 ± 0.04 to 2.59 ± 0.04 fold was observed in the expression level within 48 h of incubation in MFC, as shown in Fig. 1. This could be due to the adaptation of bacterium to the condition.
Since the pili plays an important role in adhesion of bacterial cells on the surface to form biofilm, the quantity of pil Q in biofilm-forming cells was studied in comparison with the cells occurring in free suspended state. A 3.63-fold higher amount of pil Q was observed in biofilm-forming cells.
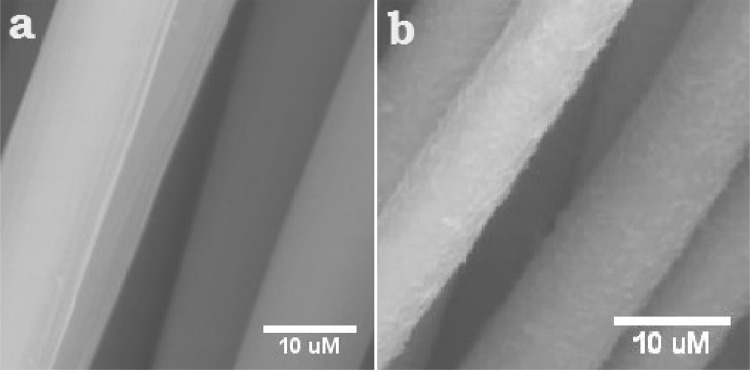
The scanning electron microscopic (SEM) analysis of the electrode revealed the formation of biofilm on the surface of the electrode during electron transfer (Fig. 2). This confirms the formation of biofilm for exo-electron transfer in MFC. The importance of biofilm formation during electron transfer is reported extensively in the literature. This phenomenon is regarded as the direct electron transfer mechanism exhibited mixed culture and by pure cultures of Geobacter, Shewanella and other electrogenic bacteria.
Fig. 2.
SEM images of the electrode before (a) and after (b) MFC. The plain carbon fiber before inoculation is turned into rough and thick fiber with the biofilm formation in MFC
Comparison of fli P and pil Q genes in biofilm and suspended cells
The increased level of expression of fli P and pil Q genes in K. georgiana MCC 3673 during exo-electron transfer process in MFC confirms their importance during bio-electricity generation in bio-electrochemical systems. The flagella and pili are believed to have a role in movement towards the attractant (which is an electrode) and attachment on the surface and forming biofilm, respectively. The amount of the transcripts for these genes was found to increase with time, indicating the increased expression level of these genes. The fold expression of pili and flagella is summarized in supplementary information T1. So far, the role of flagella in bio-electrochemical system is not reported in the literature. However, with the results obtained from this study, it is predicted that flagella is having role in motility of the bacterium towards the attractant into the medium. They are known to mobilise cells, either by towards the attractant or away from the repellent, based on the environmental factors. Here, it is predicted that due to higher reduction potential the electrode acted as an attractant for driving the bacterial cells towards itself. The expression of fli P was found to be nearly equal in suspension culture and biofilm (differ in 0.35-fold), indicating it has no role in biofilm formation (Fig. 3). Whereas, the expression of pil Q was found to be higher in biofilm-forming cells compared to cells suspended in culture medium. This confirms that pili are required for biofilm formation, which in turn required for the transfer of electrons to the electrode. The colonization of bacterial cells on the surface of electrode was also observed by SEM, as shown in Fig. 2. However, the exact mechanism by which the pili show electric conductivity or transfer electrons is yet to be understood in this bacterium.
Fig. 3.

Expression levels of genes fli P and pil Q after 48 h in K. georgiana MCC 3673 in the biofilm and suspended cells in MFC
Performance in MFC
The electrogenic performance of K. georgiana MCC 3673 was found to be active for over 4 days in MFC. The increase in potential was observed immediately after inoculation of bacteria in MFC. The maximum potential of 0.7 V was produced within 12 h of operation and remained for 5 days, after which a steady drop was observed which is due to exhaustion of nutrients and growth source. The polarization curve was drawn understand the electrochemical performance of the cell. The maximum power density of 393 ± 14 mW/m2 was produced with the OCV of 0.75 V, as shown in Fig. 4.
Fig. 4.

Polarization and power density curve showing the performance of K. georgiana MCC 3673 in MFC
The significance of the work is in the finding that selected flagellar fli P and pili genes pil Q are overexpressed during growth under anaerobic condition in the anode of MFC leading to formation of biofilm and transfer of electrons to the electrode. The practical applications are that a better understanding of the regulation of genes in the MFC conditions can enable selective mutations to increase the power density in MFC. The applications of electroactive bacteria in MFCs are well known in the literature with regard to wastewater treatment, dyes reduction, bioremediation, electro-synthesis etc., but their genetic regulation aspects are less known. There is a need for better understanding of this aspect to improve MFC performance as they have emerged as second most powerful microbial systems for alternate energy from recyclable wastes after bio-methanation.
Conclusion
The electrogenic ability of the bacterium K. georgiana MCC 3673 was assumed to be due to the pili, whose expression levels were found to be nearly fourfold higher in MFC compared to shake flask incubation. The role of pili during bio-electricity generation is believed to be playing an important role in forming biofilm, there by exo-electron transfer to the electrode, directly. Hence, resulting in the generation of maximum power density of nearly 400 mW/m2 in a bio-electrochemical system. The flagella has aided in motility of the bacterium towards the electrode, which has higher redox potential.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Authors are thankful to the DST-FIST for providing real time PCR facility in the Department of Biotechnology, IIT Madras.
Author’s contribution
BST and TSC planned the study, BST performed the experiments, BST and TSC analysed the data, and BST drafted the manuscript. TSC corrected the manuscript. Both the authors have agreed for communicating the present work with the journal 3 Biotech.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Contributor Information
Bhim Sen Thapa, Email: bhim.thapa19@gmail.com.
T. S. Chandra, Email: chandra@iitm.ac.in, Email: chandrasainathan@gmail.com
References
- Barker CS, Meshcheryakova IV, Inoue T, Samatey FA. Assembling flagella in Salmonella mutant strains producing a type III export apparatus without FliO. J Bacteriol. 2014;196:4001–4011. doi: 10.1128/JB.02184-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudler A, Schmidt I, Langner M, et al. Does it have to be carbon? Metal anodes in microbial fuel cells and related bioelectrochemical systems. Energy Environ Sci. 2015;8:2048–2055. doi: 10.1039/C5EE00866B. [DOI] [Google Scholar]
- Bustin SA, Benes V, Garson JA, et al. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem. 2009;55:611–622. doi: 10.1373/clinchem.2008.112797. [DOI] [PubMed] [Google Scholar]
- Cao Y, Mu H, Liu W, et al. Electricigens in the anode of microbial fuel cells: pure cultures versus mixed communities. Microb Cell Fact. 2019;18:1–14. doi: 10.1186/s12934-019-1087-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1016/0003-2697(87)90021-2. [DOI] [PubMed] [Google Scholar]
- Gorby YA, Yanina S, McLean JS, et al. Electrically conductive bacterial nanowires produced by Shewanella oneidensis strain MR-1 and other microorganisms. Proc Natl Acad Sci. 2006;103:11358–11363. doi: 10.1073/pnas.0604517103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SL, Nealson KH. Enriching distinctive microbial communities from marine sediments via an electrochemical-sulfide-oxidizing process on carbon electrodes. Front Microbiol. 2015;6:1–8. doi: 10.3389/fmicb.2015.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R, Ochman H. Stepwise formation of the bacterial flagellar system. Proc Natl Acad Sci USA. 2007;104:7116–7121. doi: 10.1073/pnas.0700266104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Liu H, Zhang L, et al. Biological synthesis of high-conductive pili in aerobic bacterium Pseudomonas aeruginosa. Appl Microbiol Biotechnol. 2018;103:1535–1544. doi: 10.1007/s00253-018-9484-5. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Logan BE, Hamelers B, Rozendal R, et al. Microbial fuel cells: methodology and technology. Environ Sci Technol. 2006;40:5181–5192. doi: 10.1021/es0605016. [DOI] [PubMed] [Google Scholar]
- Lovley DR. Microbial fuel cells: novel microbial physiologies and engineering approaches. Curr Opin Biotechnol. 2006;17:327–332. doi: 10.1016/j.copbio.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Mattick JS. Type IV pili and twitching motility. Annu Rev Microbiol. 2002;56:289–314. doi: 10.1146/annurev.micro.56.012302.160938. [DOI] [PubMed] [Google Scholar]
- Moens S, Vanderleyden J. Functions of bacterial flagella. Crit Rev Microbiol. 1996;22:67–100. doi: 10.3109/10408419609106456. [DOI] [PubMed] [Google Scholar]
- Poggio S, Abreu-Goodger C, Fabela S, et al. A complete set of flagellar genes acquired by horizontal transfer coexists with the endogenous flagellar system in Rhodobacter sphaeroides. J Bacteriol. 2007;189:3208–3216. doi: 10.1128/JB.01681-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proft T, Baker EN. Pili in Gram-negative and Gram-positive bacteria—structure, assembly and their role in disease. Cell Mol Life Sci. 2009;66:613–635. doi: 10.1007/s00018-008-8477-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabaey K, Boon N, Höfte M, Verstraete W. Microbial phenazine production enhances electron transfer in biofuel cells. Environ Sci Technol. 2005;39:3401–3408. doi: 10.1021/es048563o. [DOI] [PubMed] [Google Scholar]
- Reguera G, McCarthy KD, Mehta T, et al. Extracellular electron transfer via microbial nanowires. Nature. 2005;435:1098–1101. doi: 10.1038/nature03661. [DOI] [PubMed] [Google Scholar]
- Richter K, Schicklberger M, Gescher J. Dissimilatory reduction of extracellular electron acceptors in anaerobic respiration. Appl Environ Microbiol. 2012;78:913–921. doi: 10.1128/AEM.06803-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröder U. Anodic electron transfer mechanisms in microbial fuel cells and their energy efficiency. Phys Chem Chem Phys. 2007;9:2619–2629. doi: 10.1039/b703627m. [DOI] [PubMed] [Google Scholar]
- Segura A, Duque E, Hurtado A, Ramos JL. Mutations in genes involved in the flagellar export apparatus of the solvent-tolerant Pseudomonas putida DOT-T1E strain impair motility and lead to hypersensitivity to toluene shocks. J Bacteriol. 2001;183:4127–4133. doi: 10.1128/JB.183.14.4127-4133.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi W, Sun H. Type IV pilus-dependent motility and its possible role in bacterial pathogenesis. Infect Immun. 2002;70:1–4. doi: 10.1128/IAI.70.1.1-4.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrestha PM, Rotaru AE. Plugging in or going wireless: strategies for interspecies electron transfer. Front Microbiol. 2014;5:1–8. doi: 10.3389/fmicb.2014.00237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonawane JM, Yadav A, Ghosh PC, Adeloju SB. Recent advances in the development and utilization of modern anode materials for high performance microbial fuel cells. Biosens Bioelectron. 2017;90:558–576. doi: 10.1016/j.bios.2016.10.014. [DOI] [PubMed] [Google Scholar]
- Thapa BS, Chandra TS. Kluyvera georgiana MCC 3673: a novel electrogen enriched in microbial fuel cell fed with oilseed cake. Curr Microbiol. 2019;76(5):650–657. doi: 10.1007/s00284-019-01673-0. [DOI] [PubMed] [Google Scholar]
- Thapa BS, Seetharaman S, Chetty R, Chandra TS. Xerogel based catalyst for improved cathode performance in microbial fuel cells. Enzym Microb Technol. 2019;124:1–8. doi: 10.1016/j.enzmictec.2019.01.007. [DOI] [PubMed] [Google Scholar]
- Tonjum T, Collins RF, Ford RC, et al. Analysis of the PilQ secretin from neisseria meningitidis by transmission electron microscopy reveals a dodecameric quaternary structure. J Bacteriol. 2002;183:3825–3832. doi: 10.1128/jb.183.13.3825-3832.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.