Abstract
Secreted frizzled-related proteins (Sfrps) are a family of secreted proteins that bind extracellularly to Wnt ligands and frizzled receptors. This binding modulates the Wnt signaling cascade, and Sfrps interact with their corresponding receptors. Sfrps are thought to play an important role in the pathological mechanism of cardiac disease such as myocardial infarction, cardiac remodeling, and heart failure. However, the overall role of Sfrps in cardiac disease is unknown. Some members of the Sfrps family modulate cellular apoptosis, angiogenesis, differentiation, the inflammatory process, and cardiac remodeling. In this review, we summarize the evidence of Sfrps association with cardiac disease. We also discuss how multiple mechanisms may underlie Sfrps being involved in such diverse pathologies.
Keywords: cardiac remodeling, cardiovascular disease, secreted frizzled-related protein, Wnt signal transduction pathway, β-catenin
Introduction
Secreted frizzled-related proteins (Sfrps) were initially and independently identified as soluble factors that act as extracellular signaling ligands and as modulators of apoptotic events (prosurvival effects); Sfrps are also implicated in early embryonic development.1,2 The Sfrps family has now been implicated in diverse and complicated cellular processes related to the dose and type of Wnt ligands, cells, and tissue and environmental cues in cardiovascular disease. However, the precise mechanisms of these processes are more complicated than previously thought and remain controversial.
Sfrps were the first Wnt antagonists to be identified that modulate the Wnt signal transduction pathway (Figure 1).3–5 Wnt signaling plays an important role in cell proliferation, differentiation, and death during normal developmental processes.1–5 Most secreted Wnt proteins are bound to extracellular matrix glycosaminoglycans, suggesting that these proteins might act as short-range autocrine and paracrine signaling molecules. Wnt signaling is divided mainly into β-catenin dependent (canonical) and β-catenin independent (noncanonical) pathways. The β-catenin independent pathway comprises noncanonical planar cell polarity and the Wnt/Ca2+ pathways. However, recent evidence has shown that crosstalk and interaction occur among these pathways.5 Wnt antagonists can be divided into two classes on the basis of their mechanisms of action. The first class includes the Sfrps family, Wnt inhibitory factor-1, and Cerberus. Wnt antagonists belonging to this class can bind to Wnt proteins and block all Wnt signaling pathways. The second class consists of members of the Dickkopf family, which bind to Wnt coreceptors and lipoprotein receptor-related protein 5/6, and inhibit only the canonical β-catenin pathway.
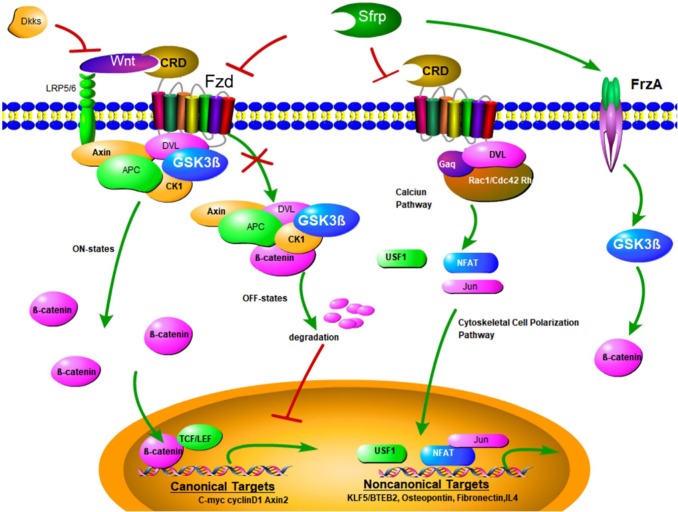
Figure 1.
Overview of Sfrps and Wnt signaling and their corresponding receptors. Binding of secreted Wnt factors to frizzled receptors on the cell membrane transduces a signal to the APC, Axin, and GSK-3β complex, which inhibits phosphorylation and degradation of β-catenin. β-catenin then accumulates in the cytoplasm and can translocate to the nucleus, where it interacts with TCF/LEF and other families of transcription factors to regulate expression of target genes. Sfrp mediates inhibition of Wnt, and binds to Wnt and frizzled receptors. This then causes phosphorylation, followed by ubiquitylation of β-catenin, priming it for proteosomal degradation. Inhibitory proteins of the sclerostin and Dkk families that are bound directly to lipoprotein receptor-related protein 5/6 can also inhibit Wnt signaling. G proteins of the Rho/Rac/cdc42 and Gαq families, and intracellular calcium and cytoskeletal signals use transcription factors of nuclear factor of activated T cells, USF, and Jun/AP1 to convey these noncanonical signals. Dvl platform proteins contribute to canonical and noncanonical pathways. Sfrps interact with their corresponding receptors (e.g. FrzA subsequently increases GSK-3β and β-catenin).
Ap1, activator protein 1; APC, adenomatous polyposis coli; Dkk, Dickkopf; Dvl, Disheveled; GSK-3β, glycogen synthase kinase-3β; Jun, Jun proto-oncogene; Sfrps, secreted frizzled-related proteins; TCF/LEF, T cell factor/lymphoid enhancer factor; USF, upstream stimulatory factor.
On the basis of sequence homology, the Sfrps family can be divided into two groups. Sfrp1, Sfrp2, and Sfrp5 form one group, while Sfrp3 and Sfrp4 form the other.5 The Sfrp gene possesses a cysteine-rich region that is similar to a cysteine-rich region present on the frizzled receptor. These regions share 30–50% sequence homology with the ligand binding cysteine-rich domain (CRD) of the frizzled protein. This shared sequence homology between the frizzled and Sfrps CRDs suggests that binding of Wnt to the Sfrps CRD may be responsible for inhibiting Wnt activity in the extracellular compartment.6 Consistent with this notion, evidence has shown that the CRD of Sfrps is necessary and sufficient for interaction with Wnt proteins or their corresponding receptors.4,7 Sfrps may interact with Wnt ligands through their CRD domain, thus antagonizing Wnt signaling. The CRD of Sfrps might also interact with frizzled receptors to form nonfunctional complexes, thereby interfering with the Wnt signaling pathway. Structurally, other than the CRD, a C-terminal netrin-like domain (NTR) of Sfrps was also reported to interact with Wnt ligands.8,9 It is interesting that different domains of Sfrps have opposing regulatory effects on Wnt signaling.10
Sfrps have also been shown to interact with molecules unrelated to Wnt pathways. For example, Sfrp1 binds to, and inhibits, the activity of the receptor activator of nuclear factor (NF)-κB ligand (RANKL), which is a member of the tumor necrosis factor (TNF) family involved in osteoclast formation.11 Sfrp2 specifically binds to tolloid metalloproteinases and regulates procollagen processing.12
In cardiovascular disease, Sfrps also interact with the Wnt signal transduction pathway and the others such as NF-κB signaling. This interaction promotes myocardial survival and repair, reducing the infract size,13,14 and favoring angiogenesis.15 More importantly, inhibition of Sfrps alleviates inflammation, collagen deposition, and fibrosis in healed cardiac tissue, and predicts cardiovascular outcome in patients with stable coronary artery disease on treatment.16,17
In this review, we summarize evidence of Sfrps in modulating cellular apoptosis, differentiation, the inflammatory process, angiogenesis, and cardiac remodeling in cardiovascular disease. We also discuss multiple mechanisms that may explain how Sfrps involved in such diverse pathologies.
Sfrps improve cardiac function by modulating cellular pathophysiological progress
Apoptosis of cardiomyocytes
Apoptosis of cardiomyocytes plays an important role in pathological progress of cardiovascular disease, especially in myocardial infarction (MI). In such situations, nonregenerative myocardial cells are lost, resulting in nonmyocardial cell activation. This activation then causes cardiac inflammation and fibrosis, which is termed cardiac remodeling. Reducing apoptosis of cardiomyocytes is a promising way to treat MI and heart failure. Multiple proteins of the Sfrps family have been reported to be associated with this process.
Sfrp1 can inhibit the apoptosis of cardiomyocytes in appropriate situations. Glycogen synthase kinase-3β (GSK-3β) is a downregulator of Wnt signaling, which is modulated by Sfrp1 when binding to its receptor FrzA (Figure 2a). Compared with wild-type mice, FrzA transgenic mice with MI show a larger infarct size and worse cardiac function, which is mediated by activation of GSK-3β.18 However, Barandon and colleagues showed that overexpression of FrzA in mouse can reduce infarct size and improve cardiac function.19 These effects were associated with a decrease in β-catenin and attenuation of Wnt signaling.19,20 However, these effects may also be indirect because inflammatory activation [e.g. interleukin (IL)-6 and IL-8] was decreased in the FrzA-Tg mouse.19 Global overexpression of Sfrp1 can improve cardiac function, reduce infarct size and cardiac rupture post-MI.21 Interestingly, a cardiac conditional expressing Sfrp1 (specifically overexpressing in cardiomyocytes) was associated with a larger infarct size and worse cardiac function in transgenic mice during ischemic preconditioning.21 In transverse aortic constriction (TAC)-induced heart failure model, Sfrp1 was also proven to attenuate cardiac dysfunction by inhibiting apoptosis mediated by Wnt signaling pathway activation.22 However, Sfrp1 also potentiates Wnt signaling by binding directly to the frizzled receptor under certain circumstances.23 Another recent study showed that, in a rat model of doxorubicin-induced cardiotoxicity, Sfrp1 has a biphasic effect on cardiomyocyte apoptosis in a cellular location-dependent manner.24 These inconsistent results suggest that the effects of Sfrp1 on cardiomyocyte apoptosis may be different in different pathophysiological processes and cellular circumstances.
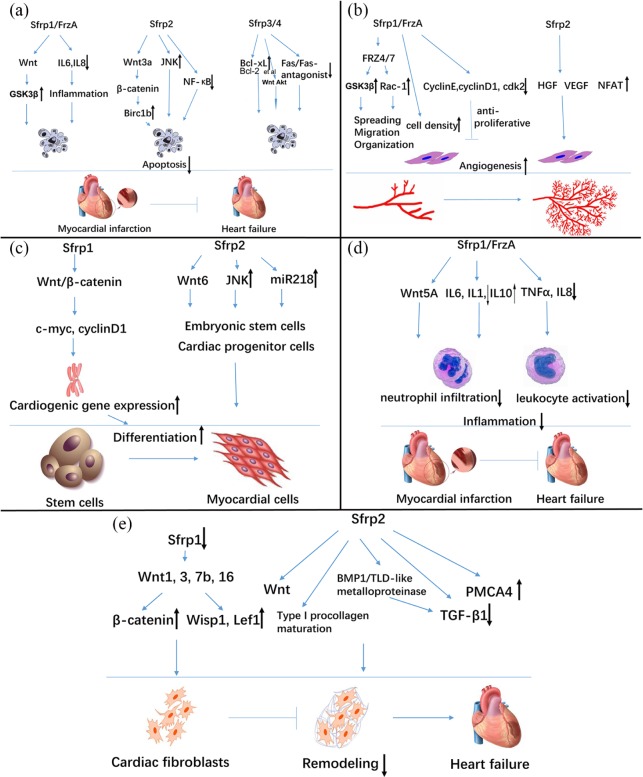
Figure 2.
Potential mechanisms of how Sfrps modulate cellular apoptosis, angiogenesis, differentiation, inflammatory process, and cardiac remodeling. (a) Sfrps inhibit apoptosis via a variety of pathways, including classic Wnt signaling (GSK3β, β-catenin), inflammation-induced apoptosis, such as NF-κB, IL6, and IL8, and interaction with the antiapoptotic protein Bcl-xL, Bcl-2, etc., and the Fas pathway. Sfrp4 might regulate both Wnt signaling and the Akt pathway. (b) Sfrps enhance spreading, migration, and organization of vascular endothelial cells, and increase release of growth factors (VEGF, hepatocyte growth factor) and cell density, thus promoting formation of a capillary network. However, in an antiproliferation role, Sfrps bind to cyclins to impair angiogenesis. (c) When Sfrps block c-myc and cyclin D1, expression of cardiac genes, and, subsequently, cardiogenesis and differentiation of cardiomyocytes, is promoted. Canonical and noncanonical Wnt pathways are indispensable in embryonic cardiogenesis and cardiac rehabilitation. (d, e) Sfrps negatively regulate activation of leukocytes and cardiac fibroblasts, and infiltration of neutrophils. This regulation is achieved by mediating Wnt signaling, tolloid-like metalloproteinase, TGF-β1, and calcium channels (PMCA4). This process reduces overproduction of ECM proteins and ameliorates ventricular remodeling and heart failure.
ECM, extracellular matrix; GSK-3β, glycogen synthase kinase-3β; NF-κB, nuclear factor κB; PMCA4, plasma membrane calcium ATPase 4; Sfrps, secreted frizzled-related proteins; TCF/LEF, T cell factor/lymphoid enhancer factor; TGF-β1, transforming growth factor β1; USF, upstream stimulatory factor; VEGF, vascular endothelial growth factor.
Sfrp2 is also reported to exert an inhibitory effect on cardiomyocyte apoptosis. In Akt-modified mesenchymal stem cells transplantation, Sfrp2 is the key stem cell paracrine factor that promotes myocardial survival and repair after ischemic injury, mediated by modulating Wnt signaling.13,14 Zhang and colleagues also reported that Sfrp2 was released from MSCs, bound to Wnt3a, and then decreased cellular caspase activity in a MI model.25 These studies suggested that the antiapoptosis effect of Sfrp2 was mediated by inhibition of the β-catenin/TCF transcriptional activities induced by Wnt3a. However, in cardiomyocytes treated with Sfrp2, the expression of Birc1b (an antiapoptotic gene) was upregulated, accompanied by an increase in total and nuclear β-catenin, indicating activation of the canonical Wnt/β-catenin pathway13 (Figure 2a). Therefore, it seems that Sfrp2 also has biphasic effect on Wnt signaling pathways in cardiomyocytes. Actually, although Sfrp2 has generally been considered as an antagonist of the canonical Wnt/β-catenin pathway, more and more studies have found that Sfrp2 can also enhance Wnt-mediated signaling in different cell types.26,27 The underlying mechanisms of Sfrp2 in activating Wnt/β-catenin signaling have not been fully elucidated. It was proposed that Sfrp2 can form complexes with both Wnt ligands and frizzled receptor through differential domain binding, or modulate signaling pathways mediated by frizzled receptor independent of Wnt ligands.28 Undoubtedly, further studies are urgently needed to explore the exact mechanisms of Sfrp2 on the Wnt pathway under different cardiovascular pathophysiological conditions.
In addition to the canonical Wnt signal, previous studies have indicated an antiapoptotic role for Sfrp2 in mediating cellular resistance to ultraviolet- and TNF-induced apoptosis in other mammalian cell lines through other signaling pathways, such as NF-κB activation or JNK suppression (Figure 2a).2,29,30
Sfrp3 and Sfrp4 are increased in volume-overloaded human hearts.31 Sfrp3 and Sfrp4 are expressed in cardiomyocytes, and upregulated expression correlates positively with mRNA expression of the pro-apoptotic Fas/Fas-antagonist ratio, but inversely with expression of antiapoptotic genes Bcl-xL and β-catenin. Sfrp3 and Sfrp4 might also bind to frizzled receptors (Figure 2a).31 In a myocardial ischemia/reperfusion injury model, knockdown of Sfrp4 led to a reduction in Bax and caspase 3, and upregulation of Bcl-2 and c-Myc in cardiac tissue via activation of the AKT signal,32 finally decreasing the apoptosis of cardiomyocytes (Figure 2a). However, whether the effects of Sfrp3 and Sfrp4 on cardiomyocytes are associated with the Wnt pathways remains unknown. Recently, Deng and colleagues revealed that serum Sfrp3 levels were higher in aged mice than in young mice,33,34 suggesting that Sfrp3 may be a novel biomarker of aging. Whether the increase in Sfrp3 accompanying ageing plays a role in apoptosis of cardiomyocytes, and further causes of heart failure, remains unknown.
Angiogenesis
Formation of new vessels from a pre-existing vascular network is a critical process in embryonic development and contributes to pathologies involving tissue repair, including MI. Recent evidence indicates that Wnt signaling interacting with Sfrps is important for vessel growth.35,36
Sfrp1 is expressed in all cultured endothelial cell populations. Sfrp1 expression leads to robust vessel formation in different angiogenic models (e.g. tumor assays).37 Sfrp1 and its receptor (FrzA) have been detected at high levels during embryogenesis in the developing heart, adult aortic endothelium and media, in the majority of vessels in the cardiovascular system and in neovascularization after an ischemic event.38 Overexpression of FrzA leads to an increase in capillary density, lumen area, and muscularization in scars (Figure 2b).19 Additionally, FrzA can regulate vascular cell growth by increasing migration, differentiation, and organization of endothelial cells into capillary-like structures.39 Sfrp1 treatment can increase endothelial cells spreading in the extracellular matrix in neovascularization. Moreover, Sfrp1 can interact with the Wnt receptors frizzled 4 and 7 in endothelial cells to activate Rac-1 in cooperation with GSK-3β.40 However, Ezan and colleagues reported that FrzA/Sfrp1 overexpression, which caused impairment of the Wnt-frizzled pathway, could control proliferation and neovascularization during and after muscle ischemia,38 which was achieved by reducing vascular cell proliferation, as shown by decreased expression of cyclin E, cyclin D1, and cdk2 activity (Figure 2b). Another study also showed that overexpression of Sfrp1 in bone marrow stem cells can increase cellular density, but not capillary density in scars.41
The inconsistency among these studies may be explained by differences in host vascular cells (endothelial cells and pericytes), which are recruited in the angiogenesis process in two steps (i.e. endothelial cells migrate and proliferate, and pericytes are recruited), or to differences in cytokine secretion [e.g. vascular endothelial growth factor (VEGF)] attributable to differences in the microenvironment. FrzA is able to exert angiogenic effects on endothelial cells by inducing their migration and organization into capillary-like structures, and protecting them from apoptosis.39
Sfrp2 also appears to be responsible for favoring neovascularization.15,42 Recently, a study showed that Sfrp2 served as a novel angiogenic factor to stimulate nuclear translocation of nuclear factor of activated T cells in angiogenic responses43 (Figure 2b). This pathway is shared with other stimulators of angiogenesis, such as VEGF. Sfrp2 blockade also increases myocardial levels of VEGF and hepatocyte growth factor along with increased angiogenesis. In contrast, Sfrp4 has recently been found to be an angiogenic inhibitor by inhibiting endothelial cell migration and development of sprouts and pseudopodia.44 Sfrp4 also disrupts the stability of endothelial rings in addition to inhibiting proliferation, inhibits angiogenesis by decreasing proliferation, migration, and tube formation of endothelial cells.45 Sfrp4 antagonizes the canonical Wnt/β-catenin pathway and blocks the effect of VEGF on endothelial cells. Furthermore, Sfrp4 also selectively induces apoptotic events by increasing cellular levels of reactive oxygen species (ROS) that harm formation of angiogenesis. Therefore, Sfrp4 may have an opposite effect on angiogenesis to other Sfrps members.
Differentiation of cardiomyocytes
Understanding how to promote differentiation of cardiomyocytes to replace damaged cardiomyocytes and regenerate damaged heart muscle is an exciting prospect and could greatly affect clinical treatment of patients with heart disease. Wnt/β-catenin activation is necessary for differentiation of mesenchymal stem cells to osteocytes, chondrocytes, myoblasts, and adipocytes.46,47 Wnt/β-catenin activation is also an important regulator of heart development, and, particularly, of differentiation of cardiomyocytes through particular target genes, such as c-myc and cyclin D148 (Figure 2c). Notably, blockade of canonical Wnt/β-catenin signaling during cardiac injury reduces infarct size and induces differentiation of adult stem cell antigen-1+α-myosin+ cardiac progenitors.49,50 Endogenous Sfrp1 is expressed in the differentiating myocardium during the later stages, but not the early stages, of organogenesis. Sfrp1 regulates cellular identity by allowing maintenance of cardiogenic gene expression of not only markers for myocardial differentiation, but also cardiogenic transcription factors.51
Similarly, Sfrp2 gene expression plays an indispensable role during embryonic stem cell differentiation into myocardial cells by inhibiting the disruption of a positive transcriptional auto feedback loop of Wnt3a in the late stage52 (Figure 2c). Sfrp2 can also promote differentiation of cardiac progenitor cells (CPCs) after ischemia–reperfusion injury by modulation of canonical and noncanonical Wnt signaling pathways. Sfrp2 inhibits proliferation of CPCs and induces cardiac differentiation by binding to Wnt6 and inhibiting the following canonical pathway. In addition, Sfrp2 activates the noncanonical Wnt pathway by interacting with JNK and inducing expression of cardiac transcription factors and differentiation of CPCs.53 Interestingly, Sfrp2 is a direct target of miR218, which mediates cardiac differentiation through the canonical signaling pathway.54 More evidence has shown that canonical signaling is involved in retaining cardiac precursors in a proliferative and precursor state, and noncanonical signaling is involved in inducing cardiac differentiation.35 This process needs to be studied further in more detail.
Inflammation
The inflammatory response after MI plays a crucial role in the healing process. This response contributes to scar remodeling and deterioration of ventricular function. Cardiac fibrosis has adverse effects on left ventricular function.55,56 Therefore, providing a therapeutic target for antifibrotic events to inhibit or reverse ventricular dysfunction is a promising treatment for heart failure.
The Wnt/frizzled pathway may play a distinct role in inflammation.41,57 Sfrp1 overexpression in stem cells transplanted to the heart reduces postinfarction scar size, and a decrease in neutrophilic infiltration in ischemic tissue, indicating that Sfrp1 might be involved in the inflammatory process.41 Indeed, Sfrp1 impairs activation of cytokine amplification, and decreases activation and recruitment of neutrophils into scars, without altering the properties of neutrophils (Figure 2d). In transgenic mice with overexpressed FrzA, myeloperoxidase-positive cell infiltration in infarcted areas (mainly polymorphonuclear cells) was reduced significantly in the first week after MI.19 Granulocytes adhere and migrate in response to release of several cytokines, such as IL-6 and IL-8 (Figure 2d).58 Another study examined Sfrp1, which was not overexpressed in endothelial cells or cardiomyocytes, but, in the bone marrow, also exerts anti-inflammation ability.59 Sfrp1 affects proliferation and recruitment of leukocytes, but does not play a role in apoptosis of leukocytes, chemotactism, polarization, or integrin expression. However, Sfrp1 significantly impairs activation of leukocytes in response to triggers (Figure 2d). In response to TNF-α, Sfrp1 significantly reduces in vitro expression of TNF-α and IL-8, which are cytokines that contribute to the pro-inflammatory response.60,61
Sfrp1 also significantly reduces IL-6 expression and increases expression of IL-10, which is a potent anti-inflammatory cytokine. Furthermore, Sfrp1 tends to decrease TNF-α and IL-1β expression. In vitro, Sfrp1 dramatically reduces IL-8 mRNA upregulation, specifically in neutrophils.41 However, the role of IL-10 in regulating postinfarction inflammation remains controversial.62
In summary, Sfrp1 can exert an anti-inflammatory effect via targeting the Wnt signaling pathways. Cardiovascular disease and heart failure have been considered as a chronic inflammatory activation. Recently, canakinumab, an interleukin-1β inhibitor, was proved to reduce heart failure hospitalization and heart failure-related mortality in a dose-dependent manner.63,64 Whether Sfrp1 can be used as a novel anti-inflammatory target for cardiovascular disease needs to be further explored.
Remodeling
Fibrosis of the heart, which is caused by overproduction of extracellular matrix proteins by fibroblasts, has diverse negative functional consequences, such as diastolic dysfunction in the heart. Fibrosis of the heart also disrupts electrical conduction, causing arrhythmias and heart failure. There is currently no precise therapy on the market that specifically treats the underlying cause of fibrosis. Therefore, it is of great significance to explore new therapeutic targets for myocardial fibrosis.
Cardiac fibroblasts play major roles in maintaining homeostasis of the extracellular matrix.65 Cardiac fibroblasts that lack endogenous Sfrp1 show increased α-smooth muscle actin expression, cell proliferation rates, and collagen production. These findings are consistent with the cardiac phenotype in aged Sfrp1 knockout mice. Loss of Sfrp1 leads to increased expression of Wnt ligands (Wnt1, 3, 7b, and 16) and Wnt target genes (Wisp1 and Lef1) in aged hearts, and increased β-catenin protein levels21 (Figure 2e). Upregulation of Wisp1 is critical in fibrotic processes in the lungs and the heart. Collagen 1 and 3 production is increased not only in the heart, but also in primary cardiac fibroblasts treated with Wisp1.66,67 These findings suggest that Sfrp1 plays a major role in cardiac fibrosis and remodeling.66,67 FrzA can also activate recruitment and organization of myofibroblasts and collagen deposition after MI. In the FrzA-Tg heart, abundant myofibroblasts and collagen deposition have been found in organized arrays in the epicardium and endocardium, resulting in severe deterioration of cardiac function.19
Sfrp2 is a major fibrotic-related cytokine in the heart (Figure 2e). However, the effect of Sfrp2 in cardiac remodeling is still controversial. Sfrp2-null mice exhibit reduced collagen deposition and significantly improved cardiac function after MI. Sfrp2 expression becomes greatly elevated after onset of the fibrotic phase and remains significantly elevated, which correlates with myocardial fibrosis in the failing heart.12 An antibody-based Sfrp2 blockade strategy reduced myocardial fibrosis, increased angiogenesis, and improved cardiac function in the failing hamster heart.31 In contrast, other studies have shown that exogenous Sfrp2 inhibits type I procollagen maturation in primary cardiac fibroblast culture medium. Injection of Sfrp2 protein into the infarct area of the rat left ventricle inhibited MI-induced fibrosis, prevented anterior wall thinning, and significantly improved cardiac function.68 Sfrp2 at a therapeutic dosage has a strong antifibrotic effect.
As mentioned above, Sfrp2 may promote cardiac fibrosis through procollagen C proteinase activation of tolloid-like metalloproteinases, and then promote collagen maturation and thickening, and fibrocalcification of the extracellular matrix through tissue-nonspecific alkaline phosphatase12,69 (Figure 2e). Bone morphogenic protein 1 (BMP1)/Drosophila proteinase tolloid-like metalloproteinases play major roles in maturation and deposition of collagen in species ranging from Drosophila to humans.70 Sfrp2 binds BMP1 mildly through its frizzled domain, which enhances the interaction between BMP1 and its substrate procollagen, then promotes procollagen processing and collagen deposition in the extracellular matrix. BMP1 can also activate transforming growth factor (TGF)-β1. Sfrp2 and TGF-β1may share a similar role in promoting fibrocalcification of tissue. Sfrp2 activation of Wnt signaling was required for TGF-β1 in cardiac fibroblasts to promote fibrosis in a rat MI model.71 Cardiac fibroblasts that lack plasma membrane calcium ATPase 4 (PMCA4) produce higher Sfrp2 levels, which inhibit the hypertrophic response in cardiomyocytes (Figure 2e). In addition, treatment with anti-Sfrp2 antibody abolishes the antihypertrophic effect of PMCA4 ablation in mice.72
Therefore, Sfrp2 seems to regulate cardiac remodeling through multiple pathways such as TGF-β1 and Wnt signaling, with inconsistent results. These controversial results may be caused by different disease models, and different Sfrp2 concentrations. Mastri and colleagues and Alfaro and colleagues show that high doses Sfrp2 (>6 μg/ml) can effectively produce antifibrotic effects, while at low doses (<1 μg/ml), a profibrotic effect was observed.42,73 More studies are needed to unveil the exact mechanisms of Sfrp2 on cardiac fibrosis.
Clinical application of Sfrps
Because Sfrps are secretory proteins, there is interest in their clinical application as biomarkers for disease detection and risk stratification. Ress and colleagues found that baseline systemic Sfrp1 levels were significantly higher in patients with cardiovascular events, and that this precedes development of symptomatic atherosclerotic disease.74 Circulating Sfrp4 was also increased in patients with coronary artery disease.34,75 However, this did not predict cardiovascular outcomes in patients with coronary artery disease.17 Therefore, whether Sfrp1 and Sfrp4 are risk factors for cardiovascular disease, or biomarkers caused by compensatory mechanisms in patients with cardiovascular disease, remains unknown. On the contrary, serum Sfrp5 was inversely associated with multiple risk factors for cardiovascular diseases and subclinical target organ damage.76,77
In populations with heart failure (HF), the Gruppo Italiano per lo Studio della Sopravvivenza nell’Insufficienza Cardiaca-heart failure(GISSI-HF) trial showed that baseline Sfrp3 concentration was significantly associated with all-cause and cardiovascular mortality.78 However, the Controlled Rosuvastatin Multinational Trial in Heart Failure (CORONA) showed that patients with intermediate serum Sfrp3 levels were associated with better prognosis than those with low or high levels.79 These results again verified the biphasic effects of Sfrps, which may depend on their concentration, and reflect their complex mechanisms of interaction.
Conclusions and perspectives
Current evidence indicates that Sfrps plays a disease stage-specific and dosage-specific role in the pathogenesis of cardiovascular disease. At the early stage of MI, Sfrps are secreted from many cell types to antagonize the Wnt pathway. This attenuates cellular apoptosis and the inflammatory response, thereby contributing to cardiac preservation. During progression of MI and heart failure, Sfrps affect cellular apoptosis, differentiation, angiogenesis, and cardiac remodeling.
Controversy remains regarding the effects of Sfrps, especially on ventricular inflammation and remodeling. Taking into consideration that Sfrps play different roles at different stages, the complex multiple steps of inflammation, and the long-term progress of remodeling, inconsistent outcomes among studies are not unexpected.
We have provided a comprehensive summary of the roles of the Wnt antagonist Sfrps in cardiovascular disease and the potential underlying mechanisms. As discussed in this review, insight into the roles of Sfrps in cardiovascular disease will help to guide development of targeting strategies.
Acknowledgments
We would like to thank the authors’ whose work has been reviewed and cited in this paper, as well the funding organizations that funded the primary research. We thank Ellen Knapp, from Liwen Bianji, Edanz Group China (www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript.
Footnotes
Authors contributions: Anqing Huang conducted literature research and wrote the draft manuscript, Yuli Huang provided editorial input and revision of the final version. Both authors read and approved the final manuscript.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and publication of this article: The project was supported by the National Natural Science Foundation of China (No: 81600239), the Science and Technology Innovation Project from Foshan, Guangdong (FS0AA-KJ218-1301-0006), Medical Science and Technology Research Foundation of Guangdong Province(No: A2018209), and the Clinical Research Startup Program of Shunde Hospital, Southern Medical University (CRSP2019001).
Conflict of interest statement: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
ORCID iD: Yuli Huang  https://orcid.org/0000-0001-5104-567X
https://orcid.org/0000-0001-5104-567X
Contributor Information
Anqing Huang, Department of Cardiology, Shunde Hospital, Southern Medical University, Foshan, China.
Yuli Huang, Department of Cardiology, Shunde Hospital, Southern Medical University, Jiazhi Road, Lunjiao Town, Shunde District, Foshan, Guangdong 528300, China The George Institute for Global Health, NSW 2042, Australia.
References
- 1. Hoang BH, Thomas JT, Abdul-Karim FW, et al. Expression pattern of two frizzled-related genes, Frzb-1 and Sfrp-1, during mouse embryogenesis suggests a role for modulating action of Wnt family members. Dev Dyn 1998; 212: 364–372. [DOI] [PubMed] [Google Scholar]
- 2. Melkonyan HS, Chang WC, Shapiro JP, et al. SARPs: a family of secreted apoptosis-related proteins. Proc Natl Acad Sci U S A 1997; 94: 13636–13641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. van Andel H, Kocemba KA, Spaargaren M, et al. Aberrant Wnt signaling in multiple myeloma: molecular mechanisms and targeting options. Leukemia 2019; 33: 1063–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Surana R, Sikka S, Cai W, et al. Secreted frizzled related proteins: implications in cancers. Biochim Biophys Acta 2014; 1845: 53–65. [DOI] [PubMed] [Google Scholar]
- 5. Gay A, Towler DA. Wnt signaling in cardiovascular disease: opportunities and challenges. Curr Opin Lipidol 2017; 28: 387–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shi Y, He B, You L, et al. Roles of secreted frizzled-related proteins in cancer. Acta Pharmacol Sin 2007; 28: 1499–1504. [DOI] [PubMed] [Google Scholar]
- 7. Vincent KM, Postovit LM. Matricellular proteins in cancer: a focus on secreted frizzled-related proteins. J Cell Commun Signal 2018; 12: 103–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rattner A, Hsieh JC, Smallwood PM, et al. A family of secreted proteins contains homology to the cysteine-rich ligand-binding domain of frizzled receptors. Proc Natl Acad Sci U S A 1997; 94: 2859–2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bukhari SA, Yasmin A, Zahoor MA, et al. Secreted frizzled-related protein 4 and its implication in obesity and type-2 diabetes. IUBMB Life 2019; 71: 1701–1710. [DOI] [PubMed] [Google Scholar]
- 10. Liang CJ, Wang ZW, Chang YW, et al. SFRPs are biphasic modulators of Wnt-signaling-elicited cancer stem cell properties beyond extracellular control. Cell Rep 2019; 28: 1511–1525.e5. [DOI] [PubMed] [Google Scholar]
- 11. Hausler KD, Horwood NJ, Chuman Y, et al. Secreted frizzled-related protein-1 inhibits RANKL-dependent osteoclast formation. J Bone Miner Res 2004; 19: 1873–1881. [DOI] [PubMed] [Google Scholar]
- 12. Kobayashi K, Luo M, Zhang Y, et al. Secreted frizzled-related protein 2 is a procollagen C proteinase enhancer with a role in fibrosis associated with myocardial infarction. Nat Cell Biol 2009; 11: 46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mirotsou M, Zhang Z, Deb A, et al. Secreted frizzled related protein 2 (Sfrp2) is the key Akt-mesenchymal stem cell-released paracrine factor mediating myocardial survival and repair. Proc Natl Acad Sci U S A 2007; 104: 1643–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Alfaro MP, Pagni M, Vincent A, et al. The Wnt modulator sFRP2 enhances mesenchymal stem cell engraftment, granulation tissue formation and myocardial repair. Proc Natl Acad Sci U S A 2008; 105: 18366–18371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Blankesteijn WM, Creemers E, Lutgens E, et al. Dynamics of cardiac wound healing following myocardial infarction: observations in genetically altered mice. Acta Physiol Scand 2001; 173: 75–82. [DOI] [PubMed] [Google Scholar]
- 16. Mahdi T, Hanzelmann S, Salehi A, et al. Secreted frizzled-related protein 4 reduces insulin secretion and is overexpressed in type 2 diabetes. Cell Metab 2012; 16: 625–633. [DOI] [PubMed] [Google Scholar]
- 17. Hoffmann MM, Werner C, Bohm M, et al. Association of secreted frizzled-related protein 4 (SFRP4) with type 2 diabetes in patients with stable coronary artery disease. Cardiovasc Diabetol 2014; 13: 155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Barandon L, Dufourcq P, Costet P, et al. Involvement of FrzA/sFRP-1 and the Wnt/frizzled pathway in ischemic preconditioning. Circ Res 2005; 96: 1299–1306. [DOI] [PubMed] [Google Scholar]
- 19. Barandon L, Couffinhal T, Ezan J, et al. Reduction of infarct size and prevention of cardiac rupture in transgenic mice overexpressing FrzA. Circulation 2003; 108: 2282–2289. [DOI] [PubMed] [Google Scholar]
- 20. Tao J, Abudoukelimu M, Ma YT, et al. Secreted frizzled related protein 1 protects H9C2 cells from hypoxia/re-oxygenation injury by blocking the Wnt signaling pathway. Lipids Health Dis 2016; 15: 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sklepkiewicz P, Shiomi T, Kaur R, et al. Loss of secreted frizzled-related protein-1 leads to deterioration of cardiac function in mice and plays a role in human cardiomyopathy. Circ Heart Fail 2015; 8: 362–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pan S, Zhao X, Wang X, et al. Sfrp1 attenuates TAC-induced cardiac dysfunction by inhibiting Wnt signaling pathway- mediated myocardial apoptosis in mice. Lipids Health Dis 2018; 17: 202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Uren A, Reichsman F, Anest V, et al. Secreted frizzled-related protein-1 binds directly to Wingless and is a biphasic modulator of Wnt signaling. J Biol Chem 2000; 275: 4374–4382. [DOI] [PubMed] [Google Scholar]
- 24. Hu Y, Guo Z, Lu J, et al. sFRP1 has a biphasic effect on doxorubicin-induced cardiotoxicity in a cellular location-dependent manner in NRCMs and Rats. Arch Toxicol 2019; 93: 533–546. [DOI] [PubMed] [Google Scholar]
- 25. Zhang Z, Deb A, Zhang Z, et al. Secreted frizzled related protein 2 protects cells from apoptosis by blocking the effect of canonical Wnt3a. J Mol Cell Cardiol 2009; 46: 370–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hasebe T, Fujimoto K, Kajita M, et al. Thyroid hormone activates Wnt/β-catenin signaling involved in adult epithelial development during intestinal remodeling in Xenopus laevis. Cell Tissue Res 2016; 365: 309–318. [DOI] [PubMed] [Google Scholar]
- 27. Lin H, Angeli M, Chung KJ, et al. sFRP2 activates Wnt/β-catenin signaling in cardiac fibroblasts: differential roles in cell growth, energy metabolism, and extracellular matrix remodeling. Am J Physiol Cell Physiol 2016; 311: C710–C719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Esteve P, Sandonìs A, Ibañez C, et al. Secreted frizzled-related proteins are required for Wnt/β-catenin signalling activation in the vertebrate optic cup. Development 2011; 138: 4179–4184. [DOI] [PubMed] [Google Scholar]
- 29. Lee JL, Lin CT, Chueh LL, et al. Autocrine/paracrine secreted frizzled-related protein 2 induces cellular resistance to apoptosis: a possible mechanism of mammary tumorigenesis. J Biol Chem 2004; 279: 14602–14609. [DOI] [PubMed] [Google Scholar]
- 30. Lee JL, Chang CJ, Chueh LL, et al. Secreted frizzled related protein 2 (sFRP2) decreases susceptibility to UV-induced apoptosis in primary culture of canine mammary gland tumors by NF- κB activation or JNK suppression. Breast Cancer Res Treat 2006; 100: 49–58. [DOI] [PubMed] [Google Scholar]
- 31. Schumann H, Holtz J, Zerkowski HR, et al. Expression of secreted frizzled related proteins 3 and 4 in human ventricular myocardium correlates with apoptosis related gene expression. Cardiovasc Res 2000; 45: 720–728. [DOI] [PubMed] [Google Scholar]
- 32. Zeng W, Cao Y, Jiang W, et al. Knockdown of Sfrp4 attenuates apoptosis to protect against myocardial ischemia/reperfusion injury. J Pharmacol Sci 2019; 140: 14–19. [DOI] [PubMed] [Google Scholar]
- 33. Deng J, Fu R, Li Q, et al. Increased sFRP3 expression correlated to senescence of endothelial cells in the aging process of mice. Am J Transl Res 2019; 11: 1810–1818. [PMC free article] [PubMed] [Google Scholar]
- 34. Ji Q, Zhang J, Du Y, et al. Human epicardial adipose tissue-derived and circulating secreted frizzled-related protein 4 (SFRP4) levels are increased in patients with coronary artery disease. Cardiovasc Diabetol 2017; 16: 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ruiz-Villalba A, Hoppler S, van den Hoff MJ. Wnt signaling in the heart fields: variations on a common theme. Dev Dyn 2016; 245: 294–306. [DOI] [PubMed] [Google Scholar]
- 36. Patten RD, Aronovitz MJ, Deras-Mejia L, et al. Ventricular remodeling in a mouse model of myocardial infarction. Am J Physiol 1998; 274: H1812–H1820. [DOI] [PubMed] [Google Scholar]
- 37. Barandon L, Couffinhal T, Dufourcq P, et al. Exact relevance of bone marrow cells in the healing process after myocardial infarction: analysis with a murine model of bone marrow cell transplantation. Can J Cardiol 2005; 21: 563–568. [PubMed] [Google Scholar]
- 38. Ezan J, Leroux L, Barandon L, et al. FrzA/sFRP-1, a secreted antagonist of the Wnt-frizzled pathway, controls vascular cell proliferation in vitro and in vivo. Cardiovasc Res 2004; 63: 731–738. [DOI] [PubMed] [Google Scholar]
- 39. Dufourcq P, Couffinhal T, Ezan J, et al. FrzA, a secreted frizzled related protein, induced angiogenic response. Circulation 2002; 106: 3097–3103. [DOI] [PubMed] [Google Scholar]
- 40. Dufourcq P, Leroux L, Ezan J, et al. Regulation of endothelial cell cytoskeletal reorganization by a secreted frizzled-related protein-1 and frizzled 4- and frizzled 7-dependent pathway: role in neovessel formation. Am J Pathol 2008; 172: 37–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Barandon L, Casassus F, Leroux L, et al. Secreted frizzled-related protein-1 improves postinfarction scar formation through a modulation of inflammatory response. Arterioscler Thromb Vasc Biol 2011; 31: e80–e87. [DOI] [PubMed] [Google Scholar]
- 42. Mastri M, Shah Z, Hsieh K, et al. Secreted frizzled-related protein 2 as a target in antifibrotic therapeutic intervention. Am J Physiol Cell Physiol 2014; 306: C531–C539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Siamakpour-Reihani S, Caster J, Bandhu Nepal D, et al. The role of calcineurin/NFAT in SFRP2 induced angiogenesis–a rationale for breast cancer treatment with the calcineurin inhibitor tacrolimus. PLoS One 2011; 6: e20412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Muley A, Majumder S, Kolluru GK, et al. Secreted frizzled-related protein 4: an angiogenesis inhibitor. Am J Pathol 2010; 176: 1505–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Longman D, Arfuso F, Viola HM, et al. The role of the cysteine-rich domain and netrin-like domain of secreted frizzled-related protein 4 in angiogenesis inhibition in vitro. Oncol Res 2012; 20: 1–6. [DOI] [PubMed] [Google Scholar]
- 46. Gaur T, Lengner CJ, Hovhannisyan H, et al. Canonical WNT signaling promotes osteogenesis by directly stimulating Runx2 gene expression. J Biol Chem 2005; 280: 33132–33140. [DOI] [PubMed] [Google Scholar]
- 47. Yano F, Kugimiya F, Ohba S, et al. The canonical Wnt signaling pathway promotes chondrocyte differentiation in a Sox9-dependent manner. Biochem Biophys Res Commun 2005; 333: 1300–1308. [DOI] [PubMed] [Google Scholar]
- 48. Gessert S, Kuhl M. The multiple phases and faces of Wnt signaling during cardiac differentiation and development. Circ Res 2010; 107: 186–199. [DOI] [PubMed] [Google Scholar]
- 49. Bergmann MW. WNT signaling in adult cardiac hypertrophy and remodeling: lessons learned from cardiac development. Circ Res 2010; 107: 1198–1208. [DOI] [PubMed] [Google Scholar]
- 50. Zelarayan LC, Noack C, Sekkali B, et al. β-Catenin downregulation attenuates ischemic cardiac remodeling through enhanced resident precursor cell differentiation. Proc Natl Acad Sci U S A 2008; 105: 19762–19767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gibb N, Lavery DL, Hoppler S. sfrp1 promotes cardiomyocyte differentiation in Xenopus via negative-feedback regulation of Wnt signalling. Development 2013; 140: 1537–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Deb A, Davis BH, Guo J, et al. SFRP2 regulates cardiomyogenic differentiation by inhibiting a positive transcriptional autofeedback loop of Wnt3a. Stem cells 2008; 26: 35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Schmeckpeper J, Verma A, Yin L, et al. Inhibition of Wnt6 by Sfrp2 regulates adult cardiac progenitor cell differentiation by differential modulation of Wnt pathways. J Mol Cell Cardiol 2015; 85: 215–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wang Y, Liu J, Cui J, et al. MiR218 modulates Wnt signaling in mouse cardiac stem cells by promoting proliferation and inhibiting differentiation through a positive feedback loop. Sci Rep 2016; 6: 20968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Jugdutt BI. Ventricular remodeling after infarction and the extracellular collagen matrix: when is enough? Circulation 2003; 108: 1395–1403. [DOI] [PubMed] [Google Scholar]
- 56. Sun Y, Weber KT. Infarct scar: a dynamic tissue. Cardiovasc Res 2000; 46: 250–256. [DOI] [PubMed] [Google Scholar]
- 57. Wang Y, Bao DJ, Xu B, et al. Neuroprotection mediated by the Wnt/frizzled signaling pathway in early brain injury induced by subarachnoid hemorrhage. Neural Regen Res 2019; 14: 1013–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Frangogiannis NG, Smith CW, Entman ML. The inflammatory response in myocardial infarction. Cardiovasc Res 2002; 53: 31–47. [DOI] [PubMed] [Google Scholar]
- 59. Sen M, Lauterbach K, El-Gabalawy H, et al. Expression and function of wingless and frizzled homologs in rheumatoid arthritis. Proc Natl Acad Sci U S A 2000; 97: 2791–2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Anguera I, Miranda-Guardiola F, Bosch X, et al. Elevation of serum levels of the anti-inflammatory cytokine interleukin-10 and decreased risk of coronary events in patients with unstable angina. Am Heart J 2002; 144: 811–817. [DOI] [PubMed] [Google Scholar]
- 61. Pannitteri G, Marino B, Campa PP, et al. Interleukins 6 and 8 as mediators of acute phase response in acute myocardial infarction. Am J Cardiol 1997; 80: 622–625. [DOI] [PubMed] [Google Scholar]
- 62. Yang Z, Zingarelli B, Szabo C. Crucial role of endogenous interleukin-10 production in myocardial ischemia/reperfusion injury. Circulation 2000; 101: 1019–1026. [DOI] [PubMed] [Google Scholar]
- 63. Everett BM, Cornel JH, Lainscak M, et al. Anti-inflammatory therapy with canakinumab for the prevention of hospitalization for heart failure. Circulation 2019; 139: 1289–1299. [DOI] [PubMed] [Google Scholar]
- 64. Colombo PC, Onat D, Harxhi A, et al. Peripheral venous congestion causes inflammation, neurohormonal, and endothelial cell activation. Eur Heart J 2014; 35: 448–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Souders CA, Bowers SL, Baudino TA. Cardiac fibroblast: the renaissance cell. Circ Res 2009; 105: 1164–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Konigshoff M, Kramer M, Balsara N, et al. WNT1-inducible signaling protein-1 mediates pulmonary fibrosis in mice and is upregulated in humans with idiopathic pulmonary fibrosis. J Clin Invest 2009; 119: 772–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Yoshida M, Ohkusa T, Nakashima T, et al. Alterations in adhesion junction precede gap junction remodelling during the development of heart failure in cardiomyopathic hamsters. Cardiovasc Res 2011; 92: 95–105. [DOI] [PubMed] [Google Scholar]
- 68. He W, Zhang L, Ni A, et al. Exogenously administered secreted frizzled related protein 2 (Sfrp2) reduces fibrosis and improves cardiac function in a rat model of myocardial infarction. Proc Natl Acad Sci U S A 2010; 107: 21110–21115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Martin S, Lin H, Ejimadu C, et al. Tissue-nonspecific alkaline phosphatase as a target of sFRP2 in cardiac fibroblasts. Am J Physiol Cell Physiol 2015; 309: C139–C147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Hopkins DR, Keles S, Greenspan DS. The bone morphogenetic protein 1/Tolloid-like metalloproteinases. Matrix Biol 2007; 26: 508–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ge G, Greenspan DS. BMP1 controls TGFβ1 activation via cleavage of latent TGFβ-binding protein. J Cell Biol 2006; 175: 111–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Mohamed TM, Abou-Leisa R, Stafford N, et al. The plasma membrane calcium ATPase 4 signalling in cardiac fibroblasts mediates cardiomyocyte hypertrophy. Nat Commun 2016; 7: 11074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Alfaro MP, Vincent A, Saraswati S, et al. sFRP2 suppression of bone morphogenic protein (BMP) and Wnt signaling mediates mesenchymal stem cell (MSC) self-renewal promoting engraftment and myocardial repair. J Biol Chem 2010; 285: 35645–35653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Ress C, Paulweber M, Goebel G, et al. Circulating Wnt inhibitory factor 1 levels are associated with development of cardiovascular disease. Atherosclerosis 2018; 273: 1–7. [DOI] [PubMed] [Google Scholar]
- 75. Senyigit A, Uzun H, Gultepe I, et al. The relationship between carotid intima-media thickness and serum secreted frizzled-related protein-4 and dipeptidyl peptidase-4 in diabetic patients with cardiovascular diseases. Bratislavske lekarske listy 2019; 120: 188–194. [DOI] [PubMed] [Google Scholar]
- 76. Carstensen-Kirberg M, Kannenberg JM, Huth C, et al. Inverse associations between serum levels of secreted frizzled-related protein-5 (SFRP5) and multiple cardiometabolic risk factors: KORA F4 study. Cardiovasc Diabetol 2017; 16: 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Teliewubai J, Bai B, Zhou Y, et al. Association of asymptomatic target organ damage with secreted frizzled related protein 5 in the elderly: the Northern Shanghai study. Clin Interv Aging 2018; 13: 389–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Askevold ET, Aukrust P, Nymo SH, et al. The cardiokine secreted frizzled-related protein 3, a modulator of Wnt signalling, in clinical and experimental heart failure. J Intern Med 2014; 275: 621–630. [DOI] [PubMed] [Google Scholar]
- 79. Askevold ET, Gullestad L, Nymo S, et al. Secreted frizzled related protein 3 in chronic heart failure: analysis from the controlled rosuvastatin multinational trial in heart failure (CORONA). PLoS One 2015; 10: e0133970. [DOI] [PMC free article] [PubMed] [Google Scholar]