Abstract
Background:
The latest biomechanical studies on some form of internal bracing have shown improved stabilization for anterior cruciate ligament (ACL) repair, but gap formation and load-sharing function have not yet been reported.
Hypothesis:
Internal bracing of an adjustable ACL repair construct provides improved stabilization with reduced gap formation and higher residual loading on the ACL.
Study Design:
Controlled laboratory study.
Methods:
Internally braced ACL repair constructs with single– and double–cinch loop (CL) cortical buttons, a knotless suture anchor, and a single-CL cortical button with adjustable loop fixation (CLS-ALD) were tested (n = 20 each) in a porcine model at 4 different loads (n = 5 each) over 4000 cycles at 0.75 Hz (n = 80 total). The CLS-ALD technique allowed for additional preconditioning (10 cycles at 0.5 Hz). Test results of the isolated internal brace groups served as a baseline for comparison. Lastly, specimens were pulled to failure (50 mm/min) with a cut internal brace. Final loading and gap formation on the ACL repair construct as well as ultimate strength were analyzed.
Results:
A statistical significance for peak loads over peak elongation was found between the CLS-ALD and all other reinforced groups (analysis of covariance, P < .001). Accordingly, the adjustable repair technique showed improved load-bearing capability with the internal brace compared with all other fixed repair groups and revealed significantly higher loads than the knotted single-CL group. Also, significantly reduced gap formation was found for the CLS-ALD compared with all other groups (P < .001), with no gap formation up to 150 N with a final gap of 0.85 ± 0.31 mm at 350 N. A significantly higher ultimate failure load (866.2 ± 104.0 N; P < .001) was found for the button-fixed internal brace group compared with all other groups.
Conclusion:
Internal bracing had a crucial role in improving the stabilization potential of ACL repair at loads occurring during normal daily activity. The added strength of the internal brace allowed for reducing peak loads on the ACL repair construct as well as restricting gap formation to below 3 mm at loads up to 350 N.
Clinical Relevance:
Improvements in the mechanical characteristics of current ACL repair techniques that enable reduced gap formation and allow for early range of motion and accelerated rehabilitation may strengthen the self-healing response with the formation of stable scar tissue.
Keywords: ACL repair, adjustable loop, suspensory fixation, gap formation, biomechanical testing
An anterior cruciate ligament (ACL) rupture is one of the most common knee injuries, with about 150,000 orthopaedic surgical procedures performed annually in the United States.26 Previous attempts to surgically repair proximal ACL tears may have had inconsistent results because of the lack of mechanical protection of the ligament.19,38,39 Insufficient postoperative knee stability can lead to increased anteroposterior (AP) translation and compromised healing capability, without the formation of stable scar tissue.3,13 Although no exact threshold for AP laxity to allow scarring of the native tissue back to the bone has been reported, the nonoperative treatment of acute ACL ruptures with mild laxity (Lachman grade ≤1) can confer functional joint stability restoration.3
Improved patient selection of only treating proximal tears, along with novel arthroscopic approaches with biological enhancement of the healing environment and some kind of internal bracing, have sparked renewed interest in primary ACL repair.5,15,42,43 Recent preclinical studies using nonabsorbable high-strength sutures for internal bracing of the ACL repair construct were able to restore normal knee laxity and allow healing.33,44 In addition, several short-term clinical follow-up studies on internal bracing of repaired ACLs have shown promising outcomes with radiographic and arthroscopic proof of healing as well as good function of the knee.10,16,18,28,36,46 These data suggest that additional internal bracing might reduce the failure rate after primary repair of proximal ACL tears.
Biomechanical studies including some kind of internal bracing for ACL repair have revealed improved AP laxity in the range of the ACL-intact state.13,17,21 However, the loading protocols used in these studies with AP translation measured were mainly based on an anterior drawer test, which may not account for the effects of dynamic loading in the range of daily activity. The role of internal bracing on the biomechanical stability of ACL repair techniques dynamically tested in the whole range of early and late rehabilitation loads is currently unknown. With modern rehabilitation principles emphasizing early range of motion and accelerated return to activity, knowing whether augmented ACL repair can withstand the rigors of a more aggressive protocol would be useful. Our previous study6 analyzed the biomechanical properties of an adjustable technique and 3 different fixed ACL repair techniques without internal brace augmentation.
The purpose of this study was to evaluate and compare the stabilization and gap formation behavior of the 4 previously tested repair techniques, including internal brace augmentation with a final load on the ACL repair construct as well as ultimate failure strength, in a biomechanical in vitro study using a porcine model. It was hypothesized that internal bracing of an adjustable ACL repair technique would provide for improved stabilization with reduced gap formation and higher residual loads on the ACL compared with fixed repair techniques. Furthermore, it was assumed that internal brace augmentation would significantly improve the biomechanical characteristics of the different ACL repair techniques to be more in line with the functional data of the native ACL.
Methods
Testing Groups
ACL repair constructs with single– and double–cinch loop (CL) cortical button fixation as well as knotless suture anchor fixation (group 1; anchor) were biomechanically tested with the addition of an internal brace (Figure 1). Button groups consisted of single-CL adjustable fixation (group 2; CLS-ALD) and 2 fixed techniques with single-CL (group 3; CLS) and double-CL fixation (group 4; CLD) of the ACL. Each group was cyclically tested at 4 different load levels (n = 5 each), resulting in 20 test samples for each technique (n = 80 total). Test results of isolated internal brace groups (IB-Anchor, IB-Button) served as a reference and baseline for comparison to the internally braced ACL repair groups. Overall, 24 tests were performed for the isolated internal brace groups at the same 4 load levels (n = 3 each).
Figure 1.
(A) Schematic illustration of the bone tunnel and graft-related definitions of the internal brace anterior cruciate ligament repair groups with the (B) final experimental setup.
Specimen Preparation
Porcine tibias with preserved ACLs and femurs were utilized for testing because of previously reported morphometric and mechanical similarity to young adult humans.20,31 A total of 104 fresh porcine tibias and femurs (aged 12 months) were collected from a local slaughterhouse and removed of all soft tissue. The ACL was released from the femoral footprint, measured with a digital caliper from the center of the tibial footprint along the longitudinal axis, and cut to a constant length of 30 mm. Embedding was carried out in line with the anatomic ACL long axis using RenCast (Huntsman Advanced Materials), a bicomponent embedding material. A custom-made rectangular fixture was used to embed 2 cm distal to the predetermined exit of the tibial tunnel axis to allow sufficient space for knotting the internal brace and secure fixation of the tibias.
A tibial aiming device was used to create a 4-mm tibial tunnel along the anatomic ACL long axis through the midportion of the ACL footprint. An ACL guide was used to pass a 2.4-mm pin through the lateral wall of the notch within the center of the native ACL footprint and out the proximal lateral cortex at a distance of 15 mm proximal and 5 mm anterior to the lateral epicondyle.14 A bone block of 32 mm in diameter and 35 mm in length was extracted along the guide pin by using a cylinder drill and sawing off the medial bone portion. The cylindrical bone block was docked in a custom-made steel fixture and prepared with a continuous 4 mm–diameter tunnel for cortical button specimens and an anchor-specific drill hole of 3.7 mm in diameter (Figure 1). Prepared bones were stored at –20°C and thawed at room temperature overnight before biomechanical testing.
The embedded tibia and femoral-sided bone block were secured to the base plate and actuator of a dynamic testing machine (ElectroPuls E10000; Instron) using custom clamps. The initial distance from the femoral cortex to the ACL footprint was set to 65 mm to allow reattachment of the ACL stump to the femoral block. All tests were performed at room temperature, and soft tissue was kept moist with physiological saline solution during preparation and testing.
Repair Techniques With Internal Brace
Ultimately, 4 different ACL repair techniques as previously reported (see part 16) were internally braced with a nonabsorbable high-strength suture tape (2-mm FiberTape; Arthrex) and tested in this study. These suture repair techniques are based on CL and multiple cross-stitch fixation and were utilized in a similar fashion to the aforementioned study to reattach the ACL to the femoral bone. Single- and double-CL fixation for fixed cortical button groups (CLS, CLD) was performed by passing the suture (FiberSnare; Arthrex) at a distance of 10 mm from the cut end of the ligament through and around the ACL for closing the CL by transferring the suture through the looped end. For single-CL adjustable fixation (CLS-ALD), the loop portion of the repair suture was passed through the ACL to shuttle the attached adjustable loop device (ALD) through the loop of the repair suture for tightening the CL. Finally, the suture portion was cut close to the repair suture loop. For single- and double-CL fixation, only the anteromedial bundle and both major bundles were reattached, respectively.
For knotless suture anchor fixation, we utilized 3 cross-type Bunnell stitches for each No. 2 suture limb connecting both ACL bundles with final suture locking passes through the ACL below the most proximal Bunnell stitches.
Adding the internal brace to the cortical button specimens was done before passing the button (TightRope RT; Arthrex) through the tibial tunnel. In addition to available ACL repair sutures, the internal brace was transferred through the central button holes, creating a loop with the 2 free ends on the tibial side. The button was then shuttled through the femoral tunnel and flipped on the cortex. For CLS specimens, the No. 2 flipping suture remained in position for later femoral knot tying; otherwise, this flipping suture was removed. The internal brace for knotless suture anchor fixation with cross-type Bunnell stitches was looped over the anchor eyelet with the 2 free ends on the tibial side. The isolated internal brace groups (IB-Anchor, IB-Button) were configured in the same way as for the cortical button and suture anchor repair groups without additional repair sutures.
Construct Fixation
Once the sutures were fixed to the ACL, a manual 50-N pull over 5 seconds (Figure 2, point a ) was performed using a spring-loaded tensiometer to simulate intraoperative single-hand tensioning.2 Preconditioning of the ACL repair sutures ensured proper fixation strength with more homogeneous engagement and reduced settling effects. Fixed constructs were secured on the femoral side by suture knot tying over the button with 4 half-hitch knots using an arthroscopic knot pusher or knotless suture anchor fixation within the femoral bone. The single suture of the CLS specimens was knotted to the externally positioned No. 2 button flipping suture (Figure 1A). During femoral knotting or anchoring, the test machine’s actuator was locked in position. Because no restriction of the primary tension was considered for the adjustable group, specimens were manually tensioned to a defined ACL time-zero preload (60 N) by alternating tensioning of the loop shortening strands and were kept knotless thereafter for later preconditioning (Figure 2, point b ). The CLS-ALD time-zero preload was chosen as the representative load-carrying capacity to protect the ACL during the first few weeks after repair.34 Knot tying of the ALD shortening strands was performed after preconditioning (point c). Finally, tibial-sided fixation of the internal brace was performed by tying 4 half-hitch knots over a button (TightRope ABS; Arthrex) before dynamic testing. Tightening handles were utilized for knot tying to adjust the tension on the internal brace for all groups to approximately 50 N measured by the test machine (point d). The initial position for ACL repair and internal brace fixation (point b) served as a reference for later dynamic elongation analysis and simulated a knee in full extension (joint space of 30 mm).
Figure 2.
Schematic testing protocol for a peak load of 250 N with anterior cruciate ligament repair and internal brace fixation and points of data analysis (a-g). Metrics for comparisons included final peak elongation (sp, Δbe), gap formation (sGap, Δfg), and residual load (FR, Δfg) as well as ultimate load and stiffness during pull to failure (Δfg).
Biomechanical Testing
Load was applied in line with the ACL and tunnel axis to simulate a “worst-case” loading situation at a frequency of 0.75 Hz over 4000 cycles. Specimens in the adjustable group underwent additional precycling by actuator translation between the time-zero position and –3 mm of slackening for a total of 10 cycles at 0.5 Hz (see part 16), simulating intraoperative knee flexion activity between full extension and 90° of flexion.24 Thereafter, retensioning to 60 N was manually performed in the time-zero position (simulating a knee in full extension) before ALD knotting.
Augmentation of weaker ACL repair fixation mechanically enhances the overall construct; thus, force-controlled cycling was utilized in this study. Constructs were dynamically tested at 4 peak load levels representing typical in vivo ACL loads during early and late rehabilitation phases and a constant valley load of 10 N.35,41 Peak load levels ranged from 50 N and increased in 100-N increments to 350 N, which allowed for gaining information about gap formation, load sharing, and stability for each construct and load level. Each construct was loaded at a constant peak load level over 4000 cycles. Finally, test samples were displaced starting from the time-zero position during a pull-to-failure test at 50 mm/min. For ACL repair constructs, the internal brace was cut before the failure test to acquire isolated ACL repair data. Load-displacement data during cycling and pull to failure were recorded using WaveMatrix software (Instron) with a sampling rate of 500 Hz.
Outcome Data
Metrics for comparisons included peak elongation (sp) for all groups at the end of cycling (4000th cycle). “Final peak elongation” refers to the specific residual load-bearing capability of each test group at the applied peak load and served as the stabilization potential for the groups.
Gap formation (sGap) as well as residual load (FR) at final peak elongation (Figure 2, point e ) were determined for the ACL repair samples with a cut internal brace during pull to failure. “Gap formation” represents plastic deformation (laxity) with no load (<1 N) on the repair construct. According to the principle of superposition, the residual load (FR) at final peak elongation (with an internal brace) represents the load portion on the repair construct after cycling in a synergistic load-sharing configuration. Ultimate load and stiffness were determined for all constructs in the linear portion of the load-elongation curve.
Statistical Analysis
In this study, the repair techniques were independent variables. All metrics for comparisons were dependent variables. Final peak elongation (sp), gap formation (sGap), and residual load (FR) as well as ultimate load and stiffness were defined as primary outcome variables. Statistical analysis was performed using SigmaPlot software for Windows (version 13.0; Systat Software).
Statistical analysis included a 1-way analysis of variance (ANOVA) with the Tukey post hoc test performed for significant pairwise analysis of primary outcome variables. Significance was defined as P ≤ .05, and the desired power level was set at 0.8. The Shapiro-Wilk test was used to confirm that each data set followed a normal distribution. A nonparametric test, the Kruskal-Wallis test, was used for data sets that failed this test. For Kruskal-Wallis tests that found significance, the Tukey post hoc test was conducted to further analyze the differences. The observed post hoc average power values of all 1-way ANOVAs were much higher than the desired power level of 0.8, leading us to conclude that our sample size was sufficient.
A 1-way analysis of covariance (ANCOVA) for regression analysis of peak elongation (sp) and gap formation as a function of peak loads was performed by comparing all groups over the whole load spectrum with each other. The Shapiro-Wilk test was used to confirm that each data set followed a normal distribution. For ANCOVAs that were considered significant, the Holm-Sidak post hoc test was performed for pairwise analysis. Significance was defined as P ≤ .05, and the desired power level was set at 0.8. Data analysis was performed with MATLAB (version R2018a; MathWorks).
Results
The results of peak elongation and gap formation as well as regression analysis for mean outcome data as a function of applied peak loads with R 2 values are shown in Table 1. Linear regression curves of the peak loads in dependence on peak elongation provided an accuracy in the order of at least R 2 = 0.97 for all groups. The ANCOVA between the CLS-ALD and all other groups showed a statistical difference (P < .001). No significance was found between the other groups (P > .05).
Table 1.
Peak Elongation and Gap Formation With Corresponding Regression Curves and R 2 Valuesa
| 50 N | 150 N | 250 N | 350 N | Regression Curve | R 2 Value | |
|---|---|---|---|---|---|---|
| Peak elongation (sp), mm | ||||||
| IB-Button | 0.38 ± 0.18 | 1.18 ± 0.21 | 2.34 ± 0.44 | 4.00 ± 0.25 | y(x) = 80.9x + 39.6 | 0.97 |
| IB-Anchor | 0.15 ± 0.03 | 1.17 ± 0.22 | 2.49 ± 0.58 | 3.82 ± 0.33 | y(x) = 80.7x + 46.1 | 0.99 |
| CLS | 0.31 ± 0.14 | 1.28 ± 0.45 | 2.57 ± 0.37 | 3.60 ± 0.21 | y(x) = 85.5x + 37.6 | 0.99 |
| CLD | 0.43 ± 0.24 | 1.62 ± 0.38 | 2.87 ± 0.26 | 3.60 ± 0.46 | y(x) = 91.7x + 4.5 | 0.98 |
| Anchor | 0.10 ± 0.08 | 1.08 ± 0.18 | 2.48 ± 0.30 | 4.21 ± 0.37 | y(x) = 71.8x + 58.7 | 0.97 |
| CLS-ALD | –0.32 ± 0.07 | 0.64 ± 0.21 | 1.72 ± 0.21 | 2.62 ± 0.24 | y(x) = 101.0x + 82.0 | 0.99 |
| Gap formation (sGap), mm | ||||||
| CLS | 0.41 ± 0.48 (0.0%) | 1.00 ± 0.38 (22.1%) | 1.38 ± 0.20 (46.4%) | 2.38 ± 0.29 (34.0%) | y(x) = 0.0063x + 0.36 | 0.99 |
| CLD | 0.04 ± 0.02 (90.2%) | 0.50 ± 0.20 (69.2%) | 1.16 ± 0.21 (59.8%) | 1.96 ± 0.30 (45.5%) | y(x) = 0.0064x – 0.37 | 0.98 |
| Anchor | 0.02 ± 0.01 (87.0%) | 0.10 ± 0.20 (91.0%) | 1.36 ± 0.29 (45.0%) | 2.45 ± 0.37 (41.9%) | y(x) = 0.0086x – 0.73 | 0.88 |
| CLS-ALD | 0.00 ± 0.00 (100.0%) | 0.00 ± 0.00 (100.0%) | 0.52 ± 0.23 (69.9%) | 0.85 ± 0.31 (67.7%) | y(x) = 0.004x – 0.60 | 0.97 |
aData are shown as mean ± SD unless otherwise indicated. Values in parentheses indicate the percentile displacement with anterior cruciate ligament repair under loads in relation to peak elongation. Groups are defined in the text.
Thus, the peak elongation behavior between fixed internally braced ACL repair and isolated internal brace groups at different load levels did not significantly differ, combined functional zones were established (Figure 3) compared with the CLS-ALD technique as well as available ACL repair data (see part 16) and the native ACL.7 The native ACL zone was based on available literature data4,9,24,29,40,45,47 and was established to quantify and qualify the stabilization potential of ACL reconstruction.7 The model correlates the functional ACL lengthening behavior at daily activity loads. Each ACL repair functional zone covers the relevant range of mean and standard deviation values of peak elongation and indicates the ultimate stabilization potential at different load levels. Functional zones containing the internal brace are almost completely within the native ACL functional zone for all load levels, whereas ACL repair without an internal brace reveals a complete loose state with considerable lengthening at lower loads.
Figure 3.
Functional zones with peak elongation for distinct loads (including mean and standard deviation data) as indicators for the stabilization potential of anterior cruciate ligament (ACL) repair with internal brace augmentation and the isolated internal brace groups (shaded in blue) in reference to the native ACL functional zone7 as well as ACL repair without an internal brace (see part 16).
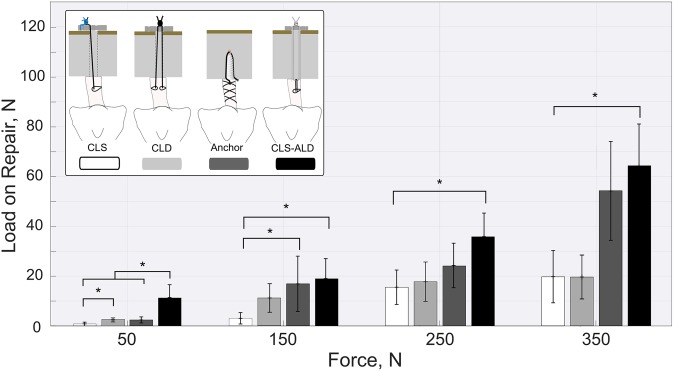
The adjustable repair technique showed at all applied load levels an increased load-bearing capability compared with the fixed techniques (Figure 4). Compared with both single-CL ACL suspension groups, the adjustable technique revealed significantly higher loads at each load level. For all repair techniques and load levels, the internal brace serves as a primary stabilizer at peak loads, leading to load reduction on the ACL repair construct and a decrease in gap formation.
Figure 4.
Residual loading on the anterior cruciate ligament repair construct for different peak loads with statistical analysis. Error bars indicate standard deviations. *Statistically significant difference: P < .05 (test power = 0.87).
Linear regression curves of the mean gap formation in dependence on applied peak loads provided an accuracy in the order of at least R 2 = 0.88. The ANCOVA between the CLS-ALD and all other groups (P < .001) as well as the CLS and CLD groups (P = .025) showed significance. A combined zone and isolated gap formation zone of fixed (shaded in dark gray) and adjustable techniques (shaded in light gray) were established by connecting the utmost standard deviation values between load levels (Figure 5).
Figure 5.
Gap formation zones (including mean and standard deviation data) over peak loads with linear regression curves for adjustable and merged fixed anterior cruciate ligament (ACL) repair techniques in reference to ACL repair without an internal brace (see part 16).
The fixed groups already experienced gap formation at the initial load level (50 N) but remained below a 3-mm gap at 350 N. The CLS-ALD technique showed no gap formation up to 150 N and revealed a mean gap of 0.85 ± 0.31 mm at 350 N. Previously shown gap formation behaviors of the same ACL repair techniques without an internal brace (see part 16) served as a reference for the test results and exhibited substantial gap formation at lower loads.
Pull to Failure
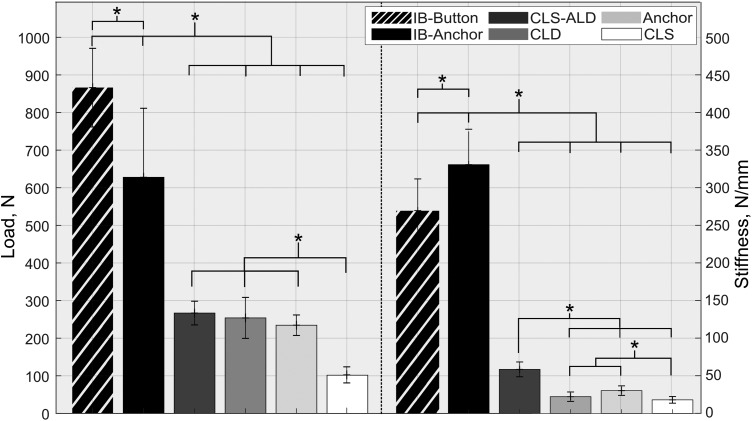
None of the specimens failed during cyclic testing; thus, all constructs were pulled to failure. A significantly higher failure load and stiffness (P < .001) were found for the button and anchor groups compared with all other ACL repair groups. The common mode of failure for internal brace specimens with cortical button and suture anchor fixation was a tibial knot-sided suture rupture and anchor pullout, respectively.
The CLS-ALD group revealed the highest ultimate failure and stiffness, with 266.8 N and 58.2 N/mm, respectively (Figure 6). For all repair groups, a significant difference was found for the ultimate failure load compared with the CLS group (P < .001). The most common mode of failure for the CLD and CLS-ALD groups was femoral knot-sided suture rupture and breakage of the CL suture, respectively. The CLS and anchor groups failed because of knot slippage and suture slippage at the anchor fixation site.
Figure 6.
Ultimate failure data of isolated anterior cruciate ligament repair and internal brace groups with statistical analysis. Error bars indicate standard deviations. *Statistically significant difference: P < .001 (test power ≥ .99).
Discussion
The most important finding of this study is that internal bracing of ACL repair provided for sufficient stabilization to protect the healing ACL at loads occurring during normal daily activity for an intact knee. Possessing a synergistic load-sharing function with the ACL repair, the internal brace served as a protective primary stabilizer and provided for reduced peak loads on the ACL repair as well as for gap formation of less than 3 mm, commensurate with the simulation of loads experienced during late rehabilitation (350 N). The internal brace showed a significantly higher ultimate failure load and stiffness compared with all ACL repair techniques (P < .001).
Augmentation for ACL repair is historically not a new concept. Back in 1980, the ligament augmentation device, a band-like braid of polypropylene, was applied using either a transfemoral procedure parallel to the reattached ACL23 or an over-the-top transfer procedure divergent to it.30 The decline in the use of the aforementioned transfer procedure combined with ligament augmentation device–related synovitis32 has resulted in the device no longer being used in the clinic. The latest clinical10,16,18,28,36,46 and biomechanical studies13,17,21 including some form of internal bracing for stabilization of the repaired ligament revealed acceptable results for ACL repair. To the best of our knowledge, this study is the first to evaluate the stabilization effect with the load-sharing function of internal bracing on primary proximal ACL repair with either adjustable-length loop cortical button fixation in comparison with conventional cortical button or suture anchor fixation. Internal bracing of ACL repair constructs allowed for the functional restoration of normal knee stability at loads occurring during normal daily activity. Dynamic testing over 4000 load cycles within the whole range of early (50 N) and late rehabilitation loads (350 N) should protect the current approach of ACL repair to allow for early range of motion and accelerated rehabilitation. Loads applied longitudinally to the ACL should comply with requirements of worst-case testing for ACL repair and are in line with most commonly used in vitro loading scenarios for ACL reconstruction.7,27
The stabilization potential of previously established non–internally braced ACL repair constructs (see part 16), isolated internal brace groups, and the native ACL functional zone7 served as references and baseline for the obtained internally braced repair results. ACL repair with internal brace augmentation almost completely restored the native ACL function, whereas the same ACL repair techniques without an internal brace showed considerable lengthening at low loads. Although different test methodologies were used in both parts, the test results should be consistent and comparable. The isolated groups with the internal brace fixed in a simulated full-extension position revealed similar stabilization compared with internally braced ACL repair constructs, so the internal brace can be assumed to function as the primary stabilizer. Primary fixation in simulated full extension with the native ACL at its greatest length24 allows for optimizing ACL repair tension without initial gap formation and the creation of a protective safety belt function of the internal brace. The added strength of the internal brace allows for reducing peak loads on the ACL repair construct. This peak “stress shielding” with permanent loads on the repair construct might impede ACL healing. Although the internal brace increases ACL repair strength at time zero, only in vivo testing can accurately show the effect of the internal brace on healing of an ACL repair construct. While the optimal timing and magnitude of loading are still unclear, it is well accepted that healing tissue should be loaded in an adequate manner to promote favorable revascularization, structural remodeling, and functional outcomes.8,11
The added strength of the internal brace to the ACL repair construct allowed for managing intermittent peak loads during the time of early and accelerated rehabilitation and protected the ACL repair construct from overstretching. The adjustable repair technique revealed at all loads reduced gap formation with improved load-bearing capability compared with fixed techniques. Higher adjustable time-zero tension with improved load-carrying capacity, combined with an increased time-zero gap formation after suture knot tying or knotless anchoring due to settling effects, may explain the superior stabilization potential seen with the adjustable repair technique.
Insufficient postoperative knee stability with increased AP translation results in an increased gap formation between the ACL and femoral bone as well as a compromised self-healing response without the formation of stable scar tissue.3,13 Improving the stabilization potential of ACL repair with internal bracing provided for reduced gap formation even at high rehabilitation loads (350 N) in a range below 3 mm, independent of the technique. It is not currently known how small the gap formation is needed to be for adequate healing. Previous ACL repair data (see part 16) without an internal brace already showed substantial gap formation (>5 mm) at loads less than 60 N, especially for fixed techniques.
The ultimate failure strength and stiffness of the isolated internal brace groups covered the range of needed tensile strength for daily activity of a normal ACL, which has been estimated to not be more than 20% of the native ACL’s ultimate load to failure.25 However, button fixation shows some advantages over single-anchor fixation, with significantly higher failure loads with suture failure instead of anchor pullout and a construct stiffness in the range of the normal ACL.47 Clinically, anchor pullout in the joint could cause third-body wear damage and might be more problematic than a loose internal brace. Slipping effects of the suture material within the bone anchor may explain increasing elongation at higher loads compared with the button group.
Recent biomechanical studies have also investigated the influence of augmentation on ACL repair.13,17,21 Fleming et al13 showed in a porcine model that isolated augmentation (suture repair between bony fixation points) results in knee laxity values that are within 0.5 mm of the intact ACL joint. Another study using human tissue supports these findings, with AP laxity restoration and augmented ACL repair in the range of an intact knee.21 In both studies, primary suture repair showed significant increased AP laxity compared with normal knee laxity. The AP laxity values of both studies were assessed after cyclic shear loading according to an anterior drawer test. Another attempt to compare anterior tibial translation across the arc of flexion for different repair techniques showed that only ACL repair with augmentation was able to restore laxity to values similar to the ACL-intact state directly postoperatively and after applying cyclic loads.17
A direct comparison between these findings and the results of the current study is difficult because of the test protocol as well as setup differences. In the aforementioned studies, AP laxity was defined as relative motion between the tibia and femur at defined AP shear load limits (<100 N), whereas in this study, the final peak elongation along the native ACL axis at different load levels was utilized for the evaluation of the stabilization potential. Studies based on a loading protocol according to an anterior drawer test may not account for the effects of dynamic loading in the range of daily activity but rather rely on a clinical examination protocol with AP translation measured.
Suture tape for internal bracing has been used for several intra- and extra-articular indications within different joints, with good mechanical, biocompatible, and nonreactive characteristics.22,36,37,48 No severe inflammatory or immune responses, bony erosion, or premature osteoarthritis were noted in an in vivo canine study even with a transected internal brace in the knee.37 Another canine study showed that internal bracing of ACL reconstruction with a quadriceps tendon allograft allowed for intra-articular and 4-zone graft-to-bone healing without inflammatory responses or foreign body reactions.12 These studies support the clinical strategy of mechanical time-zero internal brace protection of the ACL repair construct with a gradual load transition along progressively greater frequencies and magnitudes toward a strengthened ACL.
Limitations
We recognize that this study has some limitations. Porcine tissue was utilized as a substitute material for human tissue to allow for better comparability within the testing groups as well as to ensure more consistent mechanical properties. Porcine tibias with preserved ACLs were chosen because of previously reported morphometric and mechanical similarity to young adult human bones and tendons.1,20,31 The load vector of the test machine actuator was in line with the native ACL and internal brace long axis, which does not correspond to the common in vivo loading situation but represents a worst-case loading scenario for biomechanical testing. Finally, this is an in vitro, time-zero biomechanical study, evaluating the effect of internal bracing of different ACL repair techniques on the mechanical stabilization potential; thus, further short- and long-term clinical follow-up studies including information about rehabilitation and postsurgical care are needed to confirm the obtained biomechanical outcome data and their effect on clinical outcomes.
Conclusion
Internal bracing improved the stabilization potential of ACL repair at loads occurring during normal daily activity in a porcine in vitro study. The added strength of the internal brace reduced loads on the ACL repair construct as well as restricted gap formation below 3 mm at loads up to 350 N.
Acknowledgment
The authors acknowledge Professor Gordon MacKay, who provided the initial description of the internal brace.
Footnotes
Final revision submitted September 2, 2019; accepted September 18, 2019.
One or more of the authors declared the following potential conflict of interest or source of funding: Research support for this study was provided by Arthrex. S.B., D.R., and C.A.W. are employed by Arthrex; W.A.D. and L.J.P. have received speaking fees from Arthrex; and G.S.D., P.A.S., and D.R. have received consulting fees from Arthrex. G.S.D. has received educational support from Gemini Mountain Medical and hospitality payments from DePuy. W.A.D. has received educational support from Supreme Orthopedic Systems and hospitality payments from DePuy. P.A.S. has received speaking fees from Alpha Orthopedic Systems and hospitality payments from DePuy and Elite Orthopedics. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
Ethical approval was not sought for the present study.
References
- 1. Aerssens J, Boonen S, Lowet G, Dequeker J. Interspecies differences in bone composition, density, and quality: potential implications for in vivo bone research. Endocrinology. 1998;139(2):663–670. [DOI] [PubMed] [Google Scholar]
- 2. Aga C, Rasmussen MT, Smith SD, et al. Biomechanical comparison of interference screws and combination screw and sheath devices for soft tissue anterior cruciate ligament reconstruction on the tibial side. Am J Sports Med. 2013;41(4):841–848. [DOI] [PubMed] [Google Scholar]
- 3. Ahn JH, Chang MJ, Lee YS, Koh KH, Park YS, Eun SS. Non-operative treatment of ACL rupture with mild instability. Arch Orthop Trauma Surg. 2010;130(8):1001–1006. [DOI] [PubMed] [Google Scholar]
- 4. Arnold MP, Verdonschot N, van Kampen A. ACL graft can replicate the normal ligament’s tension curve. Knee Surg Sports Traumatol Arthrosc. 2005;13(8):625–631. [DOI] [PubMed] [Google Scholar]
- 5. Ateschrang A, Schreiner AJ, Ahmad SS, et al. Improved results of ACL primary repair in one-part tears with intact synovial coverage. Knee Surg Sports Traumatol Arthrosc. 2019;27(1):37–43. [DOI] [PubMed] [Google Scholar]
- 6. Bachmaier S, DiFelice GS, Sonnery-Cottet B, et al. Treatment of acute proximl anterior cruciate ligament tears-part 1. Gap formation and stabilization potential of repair techniques. Orthop J Sports Med. 2020;8(1):2325967119897421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bachmaier S, Smith PA, Bley J, Wijdicks CA. Independent suture tape reinforcement of small and standard diameter grafts for anterior cruciate ligament reconstruction: a biomechanical full construct model. Arthroscopy. 2018;34(2):490–499. [DOI] [PubMed] [Google Scholar]
- 8. Bedi A, Kovacevic D, Fox AJ, et al. Effect of early and delayed mechanical loading on tendon-to-bone healing after anterior cruciate ligament reconstruction. J Bone Joint Surg Am. 2010;92(14):2387–2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Beynnon BD, Fleming BC. Anterior cruciate ligament strain in-vivo: a review of previous work. J Biomech. 1998;31(6):519–525. [DOI] [PubMed] [Google Scholar]
- 10. Caborn DN, Nyland J, Wheeldon B, Kalloub A. ACL femoral avulsion reapproximation with internal bracing and PRP augmentation: excellent return to sports outcomes and low re-injury rates at 3 year follow-up. Presented at: Annual Meeting of the European Society of Sports Traumatology, Knee Surgery and Arthroscopy; Glasgow, UK: ESSKA Academy 2018;209475:P06–248. [Google Scholar]
- 11. Clancy WG, Ray JM. Anterior cruciate ligament autografts In: Jackson DW, Drez DJ, eds. The Anterior Cruciate Deficient Knee. St Louis: Mosby; 1987:139–270. [Google Scholar]
- 12. Cook JL, Smith P, Stannard JP, et al. A canine arthroscopic anterior cruciate ligament reconstruction model for study of synthetic augmentation of tendon allografts. J Knee Surg. 2017;30(7):704–711. [DOI] [PubMed] [Google Scholar]
- 13. Fleming BC, Carey JL, Spindler KP, Murray MM. Can suture repair of ACL transection restore normal anteroposterior laxity of the knee? An ex vivo study. J Orthop Res. 2008;26(11):1500–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gadikota HR, Sim JA, Hosseini A, Gill TJ, Li G. The relationship between femoral tunnels created by the transtibial, anteromedial porportal, and outside-in techniques and the anterior cruciate ligament footprint. Am J Sports Med. 2012;40(4):882–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Henle P, Roder C, Perler G, Heitkemper S, Eggli S. Dynamic intraligamentary stabilization (DIS) for treatment of acute anterior cruciate ligament ruptures: case series experience of the first three years. BMC Musculoskelet Disord. 2015;16:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Heusdens CHW, Hopper GP, Dossche L, Roelant E, Mackay GM. Anterior cruciate ligament repair with independent suture tape reinforcement: a case series with 2-year follow-up. Knee Surg Sports Traumatol Arthrosc. 2018;27(1):60–67. [DOI] [PubMed] [Google Scholar]
- 17. Hoogeslag RAG, Brouwer RW, Huis In ‘t Veld R, Stephen JM, Amis AA. Dynamic augmentation restores anterior tibial translation in ACL suture repair: a biomechanical comparison of non-, static and dynamic augmentation techniques. Knee Surg Sports Traumatol Arthrosc. 2018;26(10):2986–2996. [DOI] [PubMed] [Google Scholar]
- 18. Jonkergouw A, van der List JP, DiFelice GS. Arthroscopic primary repair of proximal anterior cruciate ligament tears: outcomes of the first 56 consecutive patients and the role of additional internal bracing. Knee Surg Sports Traumatol Arthrosc. 2019;27(1):21–28. [DOI] [PubMed] [Google Scholar]
- 19. Jorjani J, Altmann D, Auen R, Koopmann C, Lyutenski B, Wirtz DC. Medium- to long-term follow-up after anterior cruciate ligament rupture and repair in healing response technique. Z Orthop Unfall. 2013;151(6):570–579. [DOI] [PubMed] [Google Scholar]
- 20. Kato Y, Ingham SJ, Linde-Rosen M, Smolinski P, Horaguchi T, Fu FH. Biomechanics of the porcine triple bundle anterior cruciate ligament. Knee Surg Sports Traumatol Arthrosc. 2010;18(1):20–25. [DOI] [PubMed] [Google Scholar]
- 21. Kohl S, Evangelopoulos DS, Ahmad SS, et al. A novel technique, dynamic intraligamentary stabilization creates optimal conditions for primary ACL healing: a preliminary biomechanical study. Knee. 2014;21(2):477–480. [DOI] [PubMed] [Google Scholar]
- 22. LaPrade RF, Matheny LM, Moulton SG, James EW, Dean CS. Posterior meniscal root repairs: outcomes of an anatomic transtibial pull-out technique. Am J Sports Med. 2017;45(4):884–891. [DOI] [PubMed] [Google Scholar]
- 23. Letsch R, Stürmer KM, Kock HJ, Schmid-Neuerburg KP. Primary repair of acute tears of the anterior cruciate ligament and protection by synthetic augmentation (Trevira@ hochfest): indication, technique and results of a five-year-study. Unfallchirurg. 1993;96:499–507. [PubMed] [Google Scholar]
- 24. Li G, DeFrate LE, Rubash HE, Gill TJ. In vivo kinematics of the ACL during weight-bearing knee flexion. J Orthop Res. 2005;23(2):340–344. [DOI] [PubMed] [Google Scholar]
- 25. Magen HE, Howell SM, Hull ML. Structural properties of six tibial fixation methods for anterior cruciate ligament soft tissue grafts. Am J Sports Med. 1999;27:35–43. [DOI] [PubMed] [Google Scholar]
- 26. Mall NA, Chalmers PN, Moric M, et al. Incidence and trends of anterior cruciate ligament reconstruction in the United States. Am J Sports Med. 2014;42(10):2363–2370. [DOI] [PubMed] [Google Scholar]
- 27. Monaco E, Bachmaier S, Fabbri M, Lanzetti RM, Wijdicks CA, Ferretti A. Intraoperative workflow for all-inside anterior cruciate ligament reconstruction: an in vitro biomechanical evaluation of preconditioning and knot tying. Arthroscopy. 2018;34(2):538–545. [DOI] [PubMed] [Google Scholar]
- 28. Murray MM, Kalish LA, Fleming BC, et al. Bridge-enhanced anterior cruciate ligament repair: two-year results of a first-in-human study. Orthop J Sports Med. 2019;7(3):2325967118824356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Noyes FR, Butler D, Grood E, Zernicke R, Hefzy M. Biomechanical analysis of human ligament grafts used in knee-ligament repairs and reconstructions. J Bone Joint Surg Am. 1984;66(3):344–352. [PubMed] [Google Scholar]
- 30. Paessler HH, Deneke J, Dahners LE. Augmented repair and early mobilization of acute anterior cruciate ligament injuries. Am J Sports Med. 1992;20(6):667–674. [DOI] [PubMed] [Google Scholar]
- 31. Proffen BL, McElfresh M, Fleming BC, Murray MM. A comparative anatomical study of the human knee and six animal species. Knee. 2012;19(4):493–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Roth JH, Shkrum MJ, Bray RC. Synovial reaction associated with disruption of polypropylene braid augmented intraarticular anterior cruciate ligament reconstruction: a case report. Am J Sports Med. 1988;16:301–305. [DOI] [PubMed] [Google Scholar]
- 33. Seitz H, Pichl W, Matzi V, Nau T. Biomechanical evaluation of augmented and nonaugmented primary repair of the anterior cruciate ligament: an in vivo animal study. Int Orthop. 2013;37(11):2305–2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Seitz H, Wielke B, Schlenz I, Pichl W, Vecsei V. Load sharing in augmented anterior cruciate ligament repair: a mathematical analysis based on in vitro measurements. Clin Biomech (Bristol, Avon). 1996;11(8):431–438. [DOI] [PubMed] [Google Scholar]
- 35. Shelbourne KB, Pandy MG, Anderson FC, Torry MR. Pattern of anterior cruciate ligament force in normal walking. J Biomech. 2004;37(6):797–805. [DOI] [PubMed] [Google Scholar]
- 36. Smith JO, Yasen SK, Palmer HC, Lord BR, Britton EM, Wilson AJ. Paediatric ACL repair reinforced with temporary internal bracing. Knee Surg Sports Traumatol Arthrosc. 2016;24(6):1845–1851. [DOI] [PubMed] [Google Scholar]
- 37. Smith PA, Bozynski CC, Kuroki K, Henrich SM, Wijdicks CA, Cook JL. Intra-articular biocompatibility of multistranded, long-chain polyethylene suture tape in a canine ACL model. J Knee Surg. 2019;32:525–531. [DOI] [PubMed] [Google Scholar]
- 38. Steadman JR, Cameron-Donaldson ML, Briggs KK, Rodkey WG. A minimally invasive technique (“healing response”) to treat proximal ACL injuries in skeletally immature athletes. J Knee Surg. 2006;19(1):8–13. [DOI] [PubMed] [Google Scholar]
- 39. Steadman JR, Matheny LM, Briggs KK, Rodkey WG, Carreira DS. Outcomes following healing response in older, active patients: a primary anterior cruciate ligament repair technique. J Knee Surg. 2012;25(3):255–260. [DOI] [PubMed] [Google Scholar]
- 40. Taylor KA, Cutcliffe HC, Queen RM, et al. In vivo measurement of ACL length and relative strain during walking. J Biomech. 2013;46(3):478–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Toutoungi DE, Lu TW, Leardini A, Catani F, O’Connor JJ. Cruciate ligament forces in the human knee during rehabilitation exercises. Clin Biomech (Bristol, Avon). 2000;15(3):176–187. [DOI] [PubMed] [Google Scholar]
- 42. van der List JP, DiFelice GS. Role of tear location on outcomes of open primary repair of the anterior cruciate ligament: a systematic review of historical studies. Knee. 2017;24(5):898–908. [DOI] [PubMed] [Google Scholar]
- 43. van Eck CF, Limpisvasti O, ElAttrache NS. Is there a role for internal bracing and repair of the anterior cruciate ligament? A systematic literature review. Am J Sports Med. 2017;46(9):2291–2298. [DOI] [PubMed] [Google Scholar]
- 44. Vavken P, Proffen B, Peterson C, Fleming BC, Machan JT, Murray MM. Effects of suture choice on biomechanics and physeal status after bioenhanced anterior cruciate ligament repair in skeletally immature patients: a large-animal study. Arthroscopy. 2013;29(1):122–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wascher DC, Markolf KL, Shapiro MS, Finerman GA. Direct in vitro measurement of forces in the cruciate ligaments, part I: the effect of multiplane loading in the intact knee. J Bone Joint Surg Am. 1993;75(3):377–386. [DOI] [PubMed] [Google Scholar]
- 46. Wilson WT, Hopper GP, Byrne PA, Mackay GM. Anterior cruciate ligament repair with internal brace ligament augmentation. Surg Technol Int. 2016;29:273–278. [PubMed] [Google Scholar]
- 47. Woo SL-Y, Hollis JM, Adams DJ, Lyon RM, Takai S. Tensile properties of the human femur-anterior cruciate ligament-tibia complex. Am J Sports Med. 1991;19(3):217–225. [DOI] [PubMed] [Google Scholar]
- 48. Yasen SK, Borton ZM, Eyre-Brook AI, et al. Clinical outcomes of anatomic, all-inside, anterior cruciate ligament (ACL) reconstruction. Knee. 2017;24(1):55–62. [DOI] [PubMed] [Google Scholar]