Abstract
Background
The molecular mechanism of quercetin in the prevention and treatment of AS has been widely reported. However, the microbial and metabolic characteristics of quercetin in AS treatment are still poorly understood. In this study, we aimed to explore the gut microbial and metabolic signatures of quercetin in AS treatment and conduct an integrative analysis on its biomechanism.
Methods
An atherosclerosis mouse model was induced by a high cholesterol diet (HCD). The duration of the quercetin treatment was 12 weeks. We measured TC, TG, HDL and LDL for plasma biochemical analysis and TNF-α and IL-6 for plasma inflammatory analysis. Haematoxylin-eosin (HE) staining was conducted to evaluate the aortic structure and atherosclerosis. Bacterial DNA, which was extracted from mouse faeces, was identified by the V3−V4 regions of the 16S rRNA for microbiological analysis. The HeatMap package of BTtools was applied to visualize the data of the microbial difference matrix according to the OTU results. Fecal metabolites were assessed through LC-MS. Multivariate data analysis was conducted on the normalized data with SIMCA-P+. Significantly different metabolites were extracted based on the Pearson correlation coefficients at the level of P<0.05. Key significantly changed metabolites were screened from the intersection between metabolic signatures of the normal-model and model-quercetin groups. To investigate the biological function of quercetin on AS, we identified the differential metabolic signatures of the model vs. quercetin groups and performed KEGG analyses via MBROLE, MetaboAnalyst database.
Results
Quercetin treatment for 12 weeks significantly reduced the levels of TC (P<0.001), TG (P<0.05), HDL (P<0.001), LDL (P<0.001), TNF-α (P<0.001) and IL-6 (P<0.001) compared with the model group. HE staining indicated that quercetin could protect damaged vessels caused by HFD. Bacteroidetes, Firmicutes and Proteobacteria were dominant microbial groups in the samples. There was no significant difference between the three groups (P>0.05) at the phylum level, and the genera Phascolarctobacterium and Anaerovibrio can be regarded as the key microbiota signatures of quercetin treatment. PLS-DA results further showed that these 18 faecal metabolites (clustered in 3 groups) had significant differences between the control, model and quercetin groups throughout the 12-day treatment. According to the quantitative analysis results, 32 key metabolic signatures were screened for quercetin treatment. The main pathway in quercetin treatment is primary bile acid biosynthesis, as 3α,7α,12α,26-tetrahydroxy-5β-cholestane (C27H48O4) was defined as the most important key metabolic signature.
Conclusions
We explored the gut microbial and metabolic involvement of quercetin in AS treatment and suggest the association between AS and gut metabolic regulation.
Keywords: Microbiome, metabolomics, quercetin, atherosclerosis
Introduction
Cardiovascular disease (CVD), caused by atherosclerosis (AS), has become the top cause of death (1) worldwide and has become a dominant public health problem. According to Martin’s research (2), from 2005 to 2015, there was a marked rising trend of atherosclerosis-related problems, particularly in Eastern Asia, which increase the total deaths and disability-adjusted life years by 117.2% and 115.3%, respectively. Recent years have brought a significant amount of new results in the field of atherosclerosis (3). Various medicines, such as HMG-CoA reductase inhibitors (statins) (4), Cholesteryl ester transfer protein (CETP) inhibitors (anacetrapib) (5), and cholesterol absorption inhibitors (ezetimibe) (6) have been clinically proven in atherosclerosis treatment.
In addition to clinical drugs, natural products such as flavonoids are considered important resources in AS treatment and are widely distributed in nature. There are abundant studies on the bio-function of flavonoids in the clinical intervention of CVD (7). As an important flavonoid, the effects of quercetin in the prevention and treatment of AS have been widely reported. Some of the bio-targets of quercetin include nicotinamide-adenine dinucleotide phosphate (NADPH) oxidase (8), which can be blocked by quercetin, inhibiting the formation of reactive oxygen species (ROS); nitric oxide synthetase (NOS) (9), which can be upregulated by quercetin, limiting the formation of atherosclerotic plaque in the aorta; and matrix metalloproteinase-1 (MMP-1) (10), which can be downregulated by quercetin, stabilizing endothelial atherosclerotic plaque. Metabolic factors have been identified as an important reason for the occurrence and development of AS; however, the metabolic mechanism and the biomarkers related to quercetin treatment are still poorly understood. Therefore, it is essential to confirm the relationship between the metabolic activity of quercetin and AS intervention. This could supply the theoretical basis for quercetin in the treatment of AS and provide novel approaches for relative studies on AS.
Most prior studies support a dominant role of the gut microbiota in hypercholesterolemia and the formation of AS plaques, and some researchers even attributed gut microbiota disorder as an independent risk factor of AS (11). Gut microbiota participate in the digestive system, as the intestinal immune system is deeply affected by the primary and secondary metabolites, especially short-chain fatty acids (SCFs) (12), of gut microbiota (13). Considering that the inflammatory response caused by disturbances in the gut immune system could lead to the occurrence of AS (14), the community composition of gut microbiota is strongly associated with the metabolic characteristics of AS cases (15). Therefore, the identifying the metabolites of the gut microbiota can serve as an approach for understanding the mechanism of AS. Studies have shown the correlation of gut microbiota and metabolites during AS processes (16); therefore, the combination of microbiology and metabolomics in quercetin research could provide sufficient and accurate data efficiently and find a series robust microbial and metabolic targets for AS treatment.
In this study, we measured TC, TG, HDL and LDL for plasma biochemical analysis and TNF-α and IL-6 for plasma inflammatory analysis. Haematoxylin-eosin (HE) staining was conducted to evaluate the aortic structure and atherosclerosis. For microbiological analysis, bacterial DNA was extracted from mouse faeces and identified by sequencing the V3-V4 regions of the 16S rRNA. To find the significantly differentiated metabolites, an LC-MS system was utilized to analyse the metabolomics of the faecal samples in positive and negative ion modes. Finally, we identified the differential metabolic signatures during quercetin treatment and confirmed the bio-function and bio-pathway of these signatures based on the KEGG, MBROLE and MetaboAnalyst databases.
Methods
Animal and samples collection
Eighteen mice (aged 28–56 d) were obtained from Nanjing University-Nanjing Institute of Biomedicine (Nanjing, China), including twelve ApoE−/− mice (SCXK Su 202015-0001) and six C57BL/6J mice (SCXK Su 202015-0001). All mice were raised in the Animal Laboratory Center of Liaoning University of Traditional Chinese Medicine (LNTCM) in accordance with the national rodent feeding standards, and the relevant researchers strictly followed the animal ethics regulations during the experiment. The protocol of the study was approved by the Ethics Committee of LNTCM.
After one week of adaptation, the ApoE−/− mice were randomly separated into 2 groups (n=6) according to the body weights and the level of plasma total cholesterol. The quercetin group (n=6) was treated with a high cholesterol diet (HCD) (standard mouse chow supplemented with 0.25% w/w cholesterol and 15% w/w animal oil) and perfused with quercetin alcohol solution at a dosage of 100 mg/kg/d. The model group (n=6) was treated with an HCD, and the C57BL/6J mice (control group) received a diet of standard mouse chow. The model group and control group were supplied with the same volume of saline as the quercetin group. The duration of the treatment was 12 weeks. Diet and water were available ad libitum during the experimental period.
Blood samples were collected into Na-heparin tubes, and plasma was obtained after centrifugation (3,000 ×g, 4 °C for 10 min) (5804 R, Eppendorf, Germany). The plasma samples were frozen at −80 °C before analysis. At the end of the experiment, all mice were euthanized and subjected to autopsy. The aorta tissues were removed and fixed in 10% formalin for histopathology examination. The metabolites and microbiome samples were collected from the colon contents of mice, and faecal samples, respectively, were frozen at −80 °C before analysis.
Clinical chemical analysis and histopathology
TC, TG, HDL and LDL were measured by an automatic biochemical analyser (AU680, Beckman Coulter, USA). An ELISA kit was used for the quantitative analysis of TNF-α (LT-E21016, LanTubio, China) and IL-6 (LT-E21018, LanTubio, China). The mouse ventricular (heart) samples (fixed in 10% formalin) were embedded in paraffin and cut into sections. The sections were dyed with haematoxylin-eosin (HE) to evaluate the aortic structure and atherosclerosis.
Microbiological analysis
Microbiological analysis was conducted by Hangzhou Lian-Chuan Biotechnology Co., Ltd. Bacterial DNA was extracted from approximately 100 mg mouse faeces (colon contents) using the E.Z.N.A.® Stool DNA Kit (Omega Bio-tek HiBind Technology, Norcross, GA) and amplified with 806R (5'- GGACTACHVGGGTWTCTAAT -3') as the forward primer and 338F (5'- ACTCCTACGGGAGGCAGCAG -3') as the reverse primer, which are specific for the V3–V4 region of the 16S rRNA. PCR-amplified products were detected by 2% agarose-gel electrophoresis, and the target fragments were recovered. The recovery was conducted with the AxyPrep PCR Cleanup Kit. The inserted segments were in the range of 200 to 450 bp. The purified PCR products were quantified by the Quant-iT Pico Green dsDNA Assay Kit in the Promega QuantiFluor Fluorescence Assay Kit system, thus confirming that the sample concentrations were above 2 nM. After gradient dilution of all eligible sequencing libraries (index sequences were not repeatable), the libraries were mixed in a certain proportion according to the required sequencing amount and then transformed into a single chain by NaOH aq. with the MiSeq Reagent Kit V3 (600 cycles) for 2×300 bp double end sequencing. After the completion of MiSeq sequencing, the original data were obtained. Overlaps were applied to combine the double-end data, and quality control and chimaera filtering were conducted to obtain the microbiome data. The HeatMap package of BTtools was applied to visualize the data of the microbial difference matrix.
Metabolomics analysis
Metabolomics analysis was conducted by Hangzhou Lian-Chuan Biotechnology Co., Ltd. An LC-MS system (TripleTOF 5600, AB SCIEX, USA) was utilized to analyse the metabolomics of the faecal samples in positive and negative ion modes. Multivariate data analysis was conducted on the normalized data with SIMCA-P+ (V14.0, Umetrics, Sweden). To obtain the correlation of the samples, principal component analysis (PCA) was performed with the standardized data to generate an overview of group clustering and to detect possible outliers. The projection to latent structure discriminant analysis (PLS-DA) was conducted with unit-variance scaling to obtain positive-ion and negative-ion metabolites with significant inter-group differences. To avoid overfitting, we conducted 200 permutations times and confirmed a Q2 value under 0. Qualities of the PLS-DA models were further assessed with ANOVA with P<0.05 as significant. The significantly differentiated metabolites were extracted based on Pearson correlation coefficients at the level of P<0.05.
Based on the PLS-DA results, we generated a volcano plot using the ggplot2 package to visualize the significantly changed metabolites. Then, we obtained the intersecting metabolic signatures of the normal-model group and model-quercetin group by constructing Venn diagrams, and a heatmap was generated to visualize the hubs of significantly changed metabolites as a function of quercetin treatment duration.
To investigate the biological function of quercetin in AS, we identified the differential metabolic signatures of the model vs. quercetin groups and performed KEGG analyses with the MBROLE and MetaboAnalyst databases.
Statistical analysis
All values were expressed as the mean ± SD. The significance of the differences between the means of the groups was compared by ANOVA using the Statistical Package for PASW Statistics (version 18.0, SPSS Inc., USA). The significance threshold was set at P<0.05 for this test. Please mention in statistical part For the Identification of metabolic signature in the control and AS groups, the Q value (t test P value corrected by the BH method) was used.
Results
Blood lipid and inflammatory indexes
The plasma biochemical parameters and experimental animals are summarized in Table 1. The concentrations of TC, TG, HDL and LDL in the AS model ApoE-/- mice were apparently higher than those in control mice. Quercetin treatment for 12 weeks significantly reduced the TC (P<0.001), TG (P<0.05), HDL (P<0.001) and LDL (P<0.001) levels compared with the model group. These results indicated that quercetin might decelerate the increase in serum lipid levels caused by a high-fat diet (HFD). In addition, as shown in Table 1, quercetin treatment also significantly reduced inflammatory factors, such as TNF-α (P<0.001) and IL-6 (P<0.001), compared with the model group. Which means, quercetin could block the inflammatory reaction and thus prevent the occurrence of vascular injury.
Table 1. Plasma biochemical and inflammatory parameters in control and treatment groups.
| Group | TG/mmol·L−1 | TC/mmol·L−1 | LDL-C/mmol·L−1 | HDL-C/mmol·L−1 | TNF-α/μg·L−1 | IL-6/μg·L−1 |
|---|---|---|---|---|---|---|
| Control | 1.03±0.27** | 2.51±0.15** | 0.34±0.19** | 0.78±0.11** | 35.89±3.37** | 266.44±17.67** |
| Model | 3.61±0.97 | 22.11±1.97 | 3.26±0.28 | 6.87±1.07 | 200.85±17.84 | 685.72±30.51 |
| Quercetin | 2.46±0.14* | 9.59±0.82** | 1.88±0.40** | 2.84±0.35** | 120.54±9.97** | 431.39±27.62** |
Student t-test was conducted for statistic analysis. *P<0.05 compared with model group. **P<0.001 compared with model group. TG, triglyceride; TC, total cholesterol; LDL-C, low density lipoprotein; HDL-C, high density lipoprotein; TNF-α, tumor necrosis factor-α; IL-6, interleukin-6.
HE staining
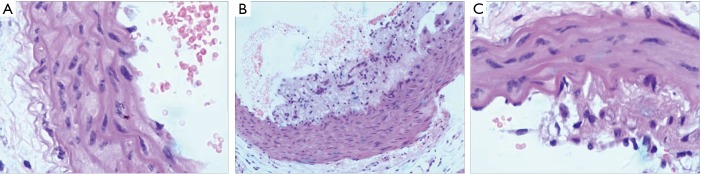
As shown in Figure 1, the arteries of the control group were evenly spaced, and the arterial endothelial cells were arranged neatly, where the inner surface of the arterial vessels was smooth without plaque formation or inflammatory cell infiltration. The arterial samples in the model group were uneven and restructured by a series of visible plaques. Among the endothelial cells, a large number of inflammatory cells were infiltrated, which caused the inflammatory reactions followed by intimal thickening, with the loss of muscular tissue, and some of the myofilaments were ruptured. Similar to the model group, the arterial samples in the quercetin group were uneven; however, there was no plaque formed in the artery. On the other hand, less inflammatory cells were infiltrated among the endothelial cells compared with the model group; although the intima tissues were thickening as a result of the inflammatory reaction, the group exhibited a thinner intima hyperplasia compared with the model group. In addition, there was no myofilament rupturing phenomenon in the group. From the vascular anatomy results, it is clear that quercetin could protect damaged vessels caused by HFD.
Figure 1.
HE stain results of the control and AS groups at 12 weeks (HE, ×200). (A) Control samples. (B) Model samples. (C) Quercetin samples.
Microbiome in mouse faecal extract
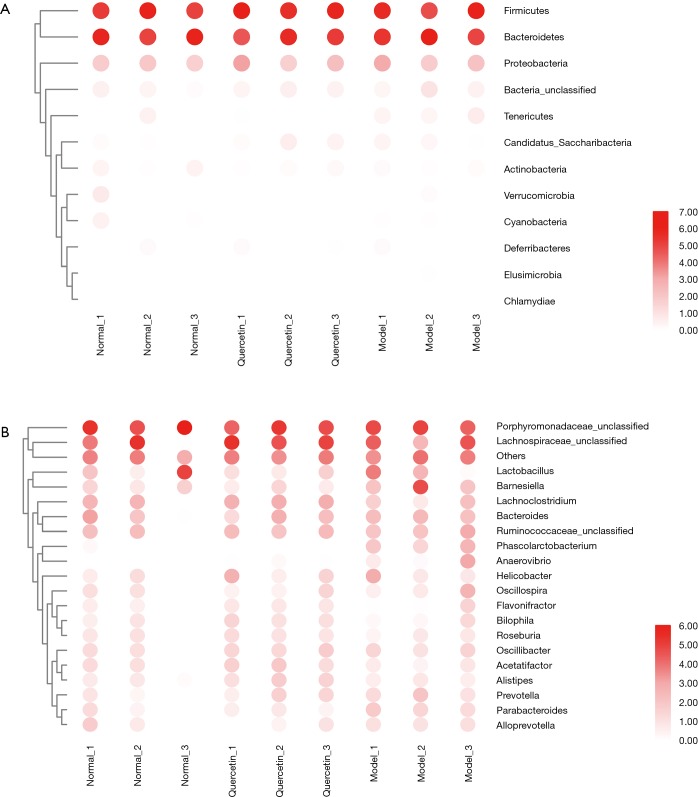
To reveal the effects of quercetin on the gut microbiota structure of a HFD model, the richness of the gut microbiota was reflected as the total OTUs. We analysed the microbial composition of faecal samples before and after 12 weeks of treatment. As shown in Figure 2A, there was no significant difference between the three groups (P>0.05) at the phylum levels, and Bacteroidetes, Firmicutes and Proteobacteria were dominant microbial groups in the groups. At the gene level, the top 20 abundance-specific genes are listed in Figure 2B. Based on the genes-OTU dataset, we discovered 11 significantly different genera (P<0.05), including Streptophyta, Enterobacter, Mobilitalea, Clostridium, Phascolarctobacterium, Candidatus Stoquefichus, Faecalimonas, Faecalibaculum, Anaerovibrio, Deltaproteobacteria Unclassified and Acutalibacter. The Phascolarctobacterium and Anaerovibrio genera are listed in Figure 2B, and two genera can be regarded as the key microbiota signatures of quercetin treatment. Considering that limited gut microbiota structure changes were observed, to obtain the mechanism of quercetin in AS treatment, it is necessary to further analyse the metabonomic changes in the groups.
Figure 2.
Identification of the microbiome in mouse faecal extract. (A) A heatmap plot was drawn to show the differential microbial community at the phylum level. (B) A heatmap plot was drawn to show the differential microbial community at the genus level.
Multivariate analysis of LC-MS data
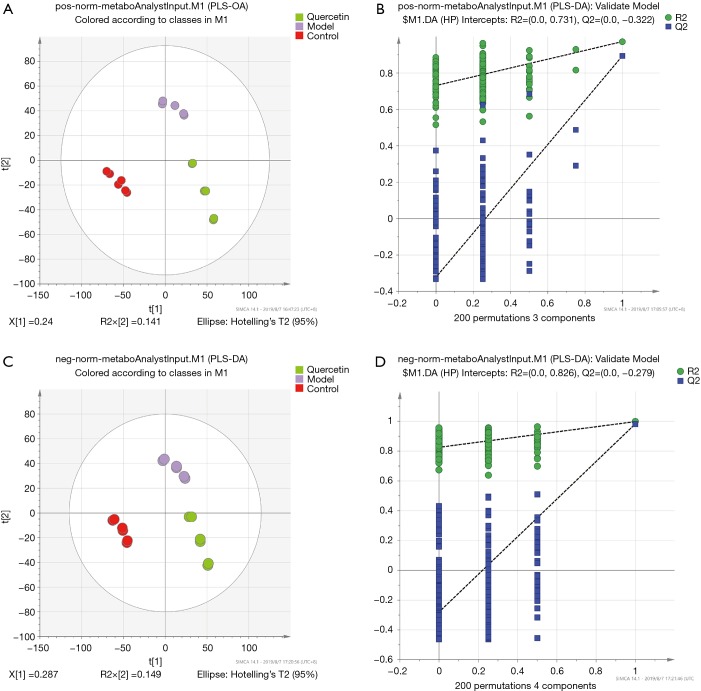
The corresponding PLS-DA (partial least squares discriminant analysis) models all showed good qualities (see Figure 3A,B). This further confirmed the presence of significant faecal metabonomic differences (including positive and negative ion metabolites) among the control, model and quercetin groups at all sampling points. In the present study, 200 permutations were conducted to validate the fitting degree of this model. Three components were selected for positive-ion metabonomic signatures (see Figure 3B), and the R2 value and Q2 value were 0.731 and −0.322, respectively. On the other hand, four components were selected for negative-ion metabonomic signatures (see Figure 3D), and the R2 value and Q2 value were 0.826 and −0.279, respectively. According to the results, the model was stable without over-fitting. The PLS-DA results further showed that these 18 faecal metabolites (clustered in 3 groups) had significant differences between the control, model and quercetin groups throughout the 12-day treatment. To screen out the metabonomic signatures and key metabonomic signatures during the quercetin treatment, we analysed the metabonomic dataset by statistical methods.
Figure 3.
PLS-DA score plot of the control and AS groups based on LC-MS technology. (A) PLS-DA score plot (R2X=0.141) and permutation plot (200 times, R2=0.731, Q2=−0.322) of positive ions. (B) PLS-DA score plot (R2X=0.149) and permutation plot (200 times, R2=0.826, Q2=−0.279) of negative ions.
Identification of key metabolic signature in the control and AS groups
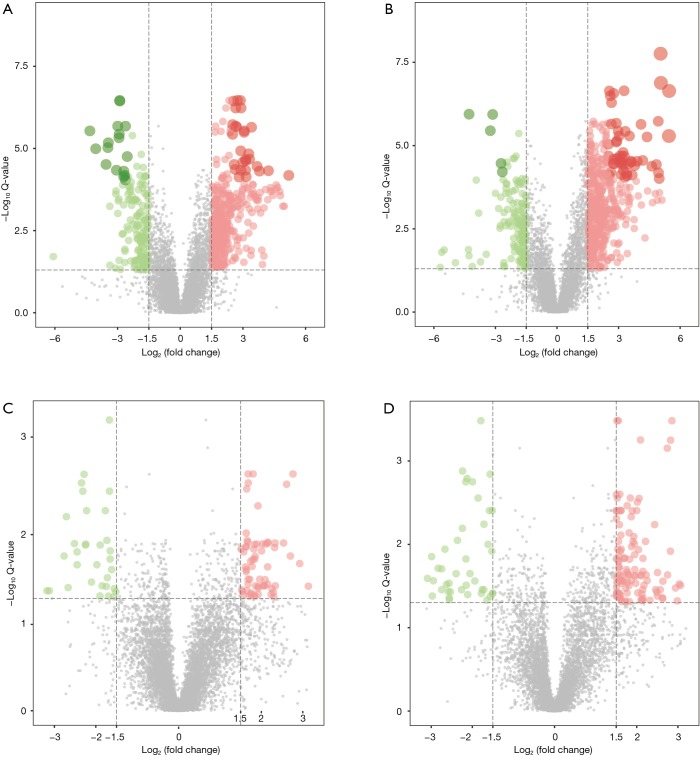
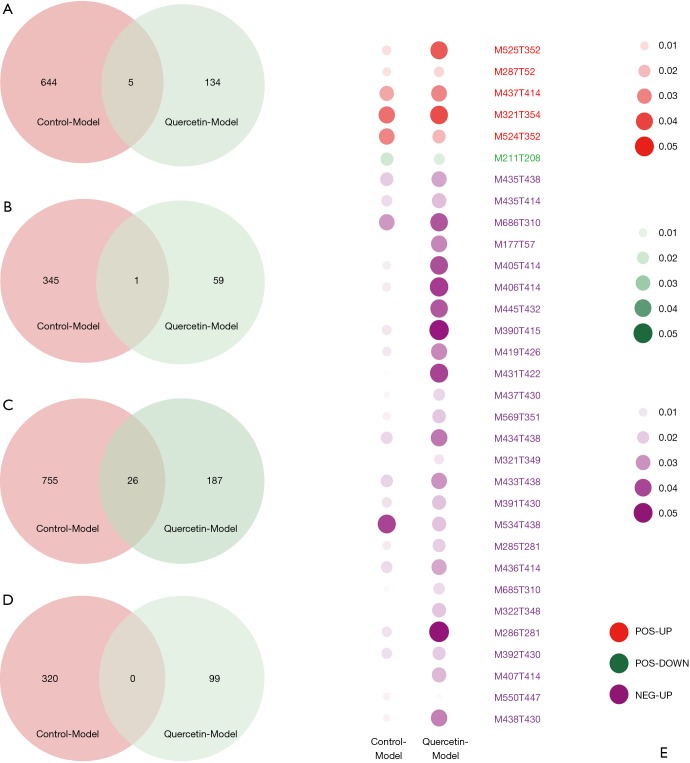
According to the quantitative analysis results, the Q value (t-test P value corrected by the BH method) was utilized to screen significantly different metabolites. The volcano plot to exhibit the upregulated or downregulated metabolites is shown in Figure 4. We identified 649 upregulated and 346 downregulated significantly different positive-ion metabolites and 781 upregulated and 320 downregulated significantly different negative-ion metabolites in the control group vs. model group, which can be regarded as the pathway background network of AS occurrence. We identified 139 upregulated and 60 downregulated significantly different positive-ion metabolites and 213 upregulated and 99 downregulated significantly different negative-ion metabolites in the quercetin group vs. model group, which can be regarded as the significantly different metabolites in response to the quercetin treatment. Given the metabolites that these metabolic signatures showed close correlation to AS occurrence and treatment, we wanted to further screen the key metabolic signatures among them. We identified 32 key metabolic signatures, which included 5 upregulated and 1 downregulated significantly different positive-ion metabolic signatures and 26 upregulated and 0 downregulated significantly different negative-ion metabolic signatures, as shown in the Venn diagrams (Figure 5A,B,C,D). The regulative level of these key metabolic signatures was shown in a heatmap (Figure 5E) generated by the Q value, and we observed that most of the key metabolic signatures in the quercetin vs. model groups exhibited a higher Q value compared with the control vs. model groups. The results suggest that although quercetin could effectively relieve the symptoms of AS by these key metabolic signatures, quercetin group could not obtain a similar signatures level compare with that of the control group.
Figure 4.
Identification of metabolic signatures in the control and AS groups (Q<0.05). The red point indicates upregulation, and the green point indicates downregulation. (A) A heatmap plot was drawn to show the differential positive-ion metabolites in control samples versus model samples. (B) A heatmap plot was drawn to show the differential negative-ion metabolites in the control samples versus the model samples. (C) A heatmap plot was drawn to show the differential positive-ion metabolites in the quercetin samples versus the model samples. (D) A heatmap plot was drawn to show the differential negative-ion metabolites in the quercetin samples versus the model samples.
Figure 5.
The hub metabolic signature of quercetin in the treatment of AS. The intersecting metabolic signatures were associated with the differential (A) positive-ion upregulation, (B) positive-ion downregulation, (C) negative-ion upregulation and (D) negative-ion downregulation of metabolite signatures of the control samples versus the model samples, where the list of metabolic signatures on the right was obtained from the quercetin samples versus the model samples. (E) A heatmap plot was drawn to show the difference (Q value) between the control-model and quercetin-model samples.
With the KEGG functional enrichment analysis, we obtained 10 reported/identified signatures in the database, including spergualin, promazine, N1,N12-diacetylspermine, isolithocholate, gymnodimine, diisononyl phthalate, cassaidine, 6-ethyl-chenodeoxycholic acid, 3-oxo-5β-cholanate, 3α,7α,12α,26-tetrahydroxy-5β-cholestane and paspaline.
In addition, the quantitative analysis between the quercetin and model groups revealed that, in addition to the metabolite pathways relative to these 32 signatures, an additional metabolite pathway also participated in the AS treatment. Therefore, we conducted a metabolic function enrichment for the signatures selected in the quercetin group vs. model group to identify these metabolite pathways and uncover the mechanism of quercetin treatment.
The potential metabolic mechanism of quercetin in AS
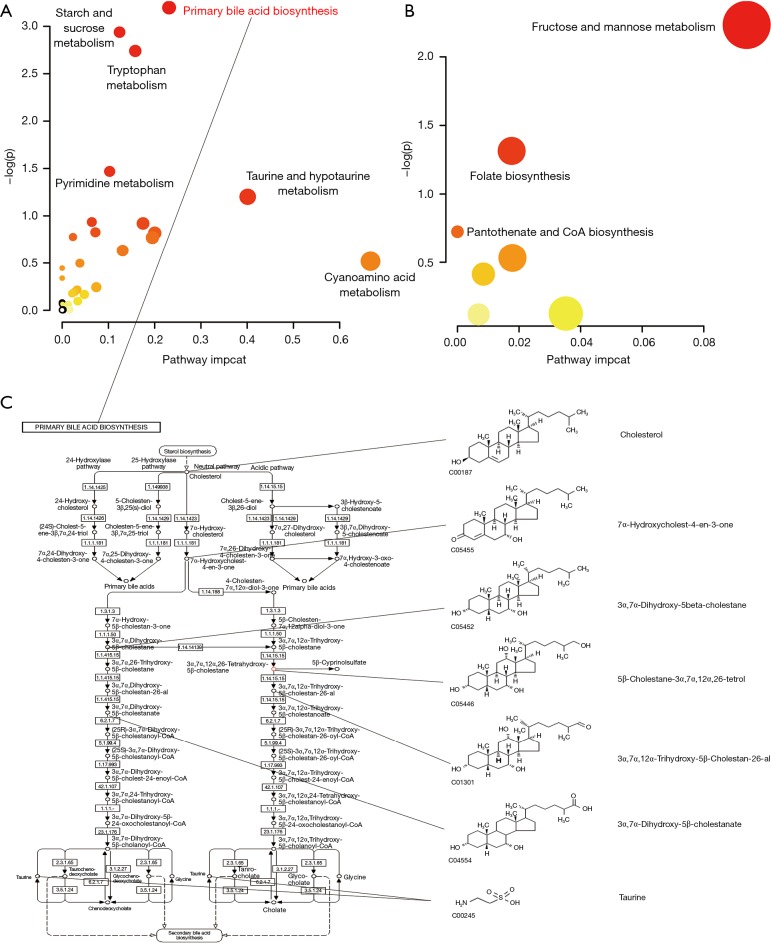
Based on the MBROLE database, 511 metabolic signatures from the quercetin group vs. model group were selected, and we discovered 38 potential metabolite pathways in quercetin treatment (see Table 2), including 31 positive-ion metabolic pathways and 7 negative-ion metabolic pathways. However, in addition to the primary bile acid biosynthesis pathway, the P values of the other metabolic pathways were beyond 0.05. Additionally, the sucrose metabolism (P=0.05), tryptophan metabolism (P=0.06) pathways can also be regarded as important metabolic pathways, too. This is consistent with the MetaboAnalyst database analysis results. As shown in Figure 6, among the metabolite pathways screened, the primary bile acid biosynthesis pathway was the single pathway with a P value under 0.05. The results suggest that the main pathway in quercetin treatment is primary bile acid biosynthesis.
Table 2. The potential metabolites PATHWAY of quercetin to AS.
| Type | No. | KEGG pathway | Total | P value | Expected | Impact |
|---|---|---|---|---|---|---|
| Positive-ion | 1 | Primary bile acid biosynthesis | 47 | 0.040875 | 2.5775 | 0.23115 |
| 2 | Starch and sucrose metabolism | 50 | 0.052959 | 2.742 | 0.12408 | |
| 3 | Tryptophan metabolism | 79 | 0.064557 | 4.3324 | 0.15805 | |
| 4 | Pyrimidine metabolism | 60 | 0.23011 | 3.2904 | 0.10265 | |
| 5 | Taurine and hypotaurine metabolism | 20 | 0.30079 | 1.0968 | 0.40108 | |
| 6 | Galactose metabolism | 41 | 0.39253 | 2.2484 | 0.06409 | |
| 7 | One carbon pool by folate | 9 | 0.39859 | 0.49356 | 0.17486 | |
| 8 | Histidine metabolism | 44 | 0.43758 | 2.413 | 0.07185 | |
| 9 | Pantothenate and CoA biosynthesis | 27 | 0.44108 | 1.4807 | 0.20016 | |
| 10 | beta-Alanine metabolism | 28 | 0.45994 | 1.5355 | 0.02328 | |
| 11 | Biotin metabolism | 11 | 0.46299 | 0.60324 | 0.19512 | |
| 12 | Vitamin B6 metabolism | 32 | 0.53153 | 1.7549 | 0.13073 | |
| 13 | Cyanoamino acid metabolism | 16 | 0.59559 | 0.87744 | 0.66667 | |
| 14 | Tyrosine metabolism | 76 | 0.60822 | 4.1678 | 0.03828 | |
| 15 | Nitrogen metabolism | 39 | 0.64062 | 2.1388 | 0 | |
| 16 | Retinol metabolism | 22 | 0.71247 | 1.2065 | 0 | |
| 17 | Phenylalanine, tyrosine and tryptophan biosynthesis | 27 | 0.78376 | 1.4807 | 0.0738 | |
| 18 | Arginine and proline metabolism | 77 | 0.80551 | 4.2227 | 0.03245 | |
| 19 | Cysteine and methionine metabolism | 56 | 0.82284 | 3.071 | 0.02707 | |
| 20 | Lysine biosynthesis | 32 | 0.83747 | 1.7549 | 0.02161 | |
| 21 | Terpenoid backbone biosynthesis | 33 | 0.8465 | 1.8097 | 0.04799 | |
| 22 | Folate biosynthesis | 42 | 0.90836 | 2.3033 | 0.03372 | |
| 23 | Nicotinate and nicotinamide metabolism | 44 | 0.9183 | 2.413 | 0 | |
| 24 | Phenylalanine metabolism | 45 | 0.92287 | 2.4678 | 0 | |
| 25 | Lysine degradation | 47 | 0.93125 | 2.5775 | 0 | |
| 26 | Glycine, serine and threonine metabolism | 48 | 0.93509 | 2.6323 | 0.00071 | |
| 27 | Glyoxylate and dicarboxylate metabolism | 50 | 0.94216 | 2.742 | 0.01267 | |
| 28 | Steroid hormone biosynthesis | 99 | 0.97666 | 5.4292 | 0.00391 | |
| 29 | Drug metabolism-cytochrome P450 | 86 | 0.99285 | 4.7162 | 0.01613 | |
| 30 | Purine metabolism | 92 | 0.99497 | 5.0453 | 0.00158 | |
| 31 | Porphyrin and chlorophyll metabolism | 104 | 0.99752 | 5.7034 | 0 | |
| Negative-ion | 32 | Fructose and mannose metabolism | 48 | 0.10721 | 1.1566 | 0.09393 |
| 33 | Folate biosynthesis | 42 | 0.26866 | 1.012 | 0.0176 | |
| 34 | Pantothenate and CoA biosynthesis | 27 | 0.48428 | 0.6506 | 0 | |
| 35 | Ubiquinone and other terpenoid-quinone biosynthesis | 36 | 0.58713 | 0.86747 | 0.01786 | |
| 36 | Nicotinate and nicotinamide metabolism | 44 | 0.66144 | 1.0602 | 0.00843 | |
| 37 | Drug metabolism-cytochrome P450 | 86 | 0.88188 | 2.0723 | 0.03519 | |
| 38 | Amino sugar and nucleotide sugar metabolism | 88 | 0.88771 | 2.1205 | 0.00686 |
Figure 6.
The network of the potential signature for the effect of quercetin on AS according to the KEGG PATHWAY database. (A) Integrative analysis between the pathway impact and P value of positive-ion metabolite signatures in quercetin-model. (B) Integrative analysis between pathway impact and the P value of negative-ion metabolite signatures in quercetin model. (C) Primary bile acid biosynthesis pathway (P<0.05) with the potential signature.
According to the primary bile acid biosynthesis pathway map obtained from the KEGG database, we identified 7 metabolic signatures (see Figure 6C). We observed that 3α,7α,12α,26-tetrahydroxy-5β-cholestane (C27H48O4) was a potential key metabolic signature in section 2.5. We inferred that this signature implicated the significant therapeutic value of quercetin to AS.
Discussion
Although the pathogenesis of AS (17) and the relationship between a series of external factors, such as diet, environment, susceptibility genes, etc., and AS (18) have been well studied, the establishment of an AS evaluation system is still lacking. Taking into account the importance of metabolic factors and gut microbiota in the pathogenesis and development of AS (19), the finding of novel AS-related biomarkers in the present evaluation approaches may provide systemic AS data in clinical evaluation and treatment. The present study established a robust microbial/metabolic related risk signature for AS using microbiome and metabonomic analyses. We could evaluate the function of compounds, such as quercetin, in the AS treatment with the model.
To determine the effect of quercetin in the treatment of AS, the pathology, gut microbiota and intestinal metabolite composition were evaluated. According to the results, quercetin could protect the anatomy of the artery and reconstruct the micro-environment of the gut. The microbial analysis results indicated that at the phylum level, there was no significant difference among the groups after 12 weeks of feeding. However, at the genus level, significantly different microorganisms can be screened and can be regarded as potential therapeutic targets for AS in the future. In addition, from the metabolic dataset obtained, 32 metabolic signatures were selected as the key metabolic signatures in the quercetin treatment. According to the MBROLE and MetaboAnalyst databases, the primary bile acid biosynthesis pathway was selected as the main pathway that our signatures take part in, where 3α,7α,12α,26-tetrahydroxy-5β-cholestane (C27H48O4) is the critical signature in the pathway. In addition to the primary bile acid biosynthesis pathway, starch and sucrose metabolism and tryptophan metabolism were also considered important pathways. This can further explain the effect of these signatures on metabolite regulation in the pathogenesis of AS. Taken together, the microbial and metabolic regulation of quercetin plays an important role in the treatment of AS.
Our investigation was the first to uncover the effects of quercetin on gut microecology and metabolic pathways. The result obtained indicated that, compared with gut microbiota, metabolic regulation was the dominant factor of quercetin in the AS treatment. Therefore, our methods can also be regarded as an efficient approach in therapeutic mechanism research and biomarker screening of AS.
Previous studies have shown that the cardiovascular protective function of quercetin includes the following: (I) inhibiting thrombosis (20), (II) releasing vascular endothelial dysfunction and (21) and (III) limiting endothelial apoptosis (22). Quercetin not only inhibits platelet aggregation and the activation of glycoprotein IIb/IIA (GPIIb/IIA) caused by collagen, adenosine diphosphate and arachidonic acid (20) but also inhibits the extracellular secretion of platelet granules. With the continuous aggregation of platelets and the thickening of the blood vessel wall, the clinical risk of AS will be greatly increased (23). The present study indicated that quercetin can limit the trend of intimal thickening. Similar to Xiao’s research (24), quercetin can reduce the area of AS plaque, which can be attributed to the blocking of NADPH oxidase. Considering that vascular endothelial dysfunction is one of the important mechanisms of AS (23), quercetin increased the activity of nitric oxide synthase and the expression of haem oxygenase-1 (HO-1) in endothelial cells, thus regulating vascular endothelial dysfunction by inhibiting oxidants (21). Except for platelet aggregation and vascular endothelial dysfunction, endothelial vascular apoptosis is also one of the important reasons for the induction/aggravation of AS (25). The biomarker indicating that quercetin inhibits the inflammatory response and apoptosis is reactive oxygen species (ROS), which regulate the PI3K/AKT pathway downstream, thus causing the atherosclerosis symptom (22). The inflammatory response is considered an important pathway in the occurrence and prognosis of AS (26). In our study, TNF-α and IL-6 were selected as the identified inflammatory factors; quercetin downregulated the expression of these two biomarkers, thus protecting the injured arterioles. Additionally, there have been studies showing that quercetin could decrease the levels of LDL (27), TG (28) and TC (29), which was also confirmed in our research. Therefore, we suggest that quercetin can also be used as an important positive drug for evaluating AS biomarkers, in addition to being a promising drug in clinical practice.
As described above, although the biological function of quercetin has been well studied, research focusing on the effect of quercetin on gut microbiota in the treatment of AS is lacking. Taking into account the importance of gut microbiota in the occurrence and development of AS (19), the finding that AS-related microorganisms change may prove useful for quercetin treatment. Diet was considered one risk factor for AS (30), and with the administration of diet, compared with the control group, the AS model mouse exhibited a significantly different gut microbiota composition at the phylum and genus levels. Annika summarized the effect of gut microbiota on the pathogenesis of AS, which included (19) that the local or distal infection could stimulate inflammation and accelerate the enlargement or rupture of AS plaques; gut microbiota significantly correlated with cholesterol and lipid metabolism, which accelerate the formation of AS plaques with an HFD. The special metabolites of gut microecology will directly affect the formation and aggravation of AS. In the present study, the limitation of available biological duplicate samples resulted in little microbial change among the groups at the phylum level (P>0.05). However, at the genus level, Streptophyta, Enterobacter, Mobilitalea, Clostridium, Phascolarctobacterium, Candidatus Stoquefichus, Faecalimonas, Faecalibaculum, Anaerovibrio, Deltaproteobacteria is unclassified, and Acutalibacter were screened as significantly different microorganisms. Phascolarctobacterium and Anaerovibrio, both of which are Firmicutes, were the genera that were selected in the TOP-20 most abundant genera according to the OTU results. The results indicate that Firmicutes is the most sensitive microbiota to quercetin compared with the other phyla. In Emoto’s research (31), Lactobacilli increased significantly in coronary heart disease patients, Bacteroidetes (Bacteroides + Platella) decreased significantly, and the proportion of Firmicutes/Bacteroidetes increased significantly. Thus, the fluctuation of Firmicutes is one of the important factors affecting the incidence of AS.
Considering the limited qualified biological samples of the microbial community obtained in this study, it is inevitable that the mechanism of action of quercetin on AS is too weak to explain from this perspective. On the other hand, previous studies support the importance of microbial metabolites in the occurrence and improvement of AS (32). Therefore, we investigated the correlation of metabolites and AS, thus supporting the outcomes of gut microbiota, and inferred the therapeutic mechanism of quercetin to AS.
Compared with microbiology results, metabonomics is the dominant function of quercetin in the treatment of AS. As shown in Figure 6A, in all metabolic pathways selected from functional enrichment, primary bile acid biosynthesis is the only screened pathway based on the statistical analysis results (P<0.05), which can be regarded as the main pathway in quercetin treatment. Cholic acid metabolism is correlated with gut microenvironment remodelling (33) and cholesterol metabolism, as cholesterol metabolic abnormalities were considered risk factors for AS. Primary bile acid is synthesized by cholesterol oxidation in the liver and secreted into the gut for lipid dissolution and absorption. Nearly 95% of bile acid is reabsorbed in the gut for metabolic circulation, and 5% is in faeces, which is the main mechanism of excreting cholesterol (34). In our study, in the model vs. quercetin group, cholesterol was selected as an up-regulated signature (see Figure 6C), which means that the quercetin group consumed significantly more cholesterol by primary bile acid biosynthesis. Regarding the metabolic cholesterol function of the pathway, quercetin could accelerate the excretion of cholesterol and decrease the clinical risk of AS caused by HFD. In addition to cholesterol metabolism, quercetin could promote extra bile acid yield by primary bile acid biosynthesis in AS model mice (35). With the cooperation of specific gut microbes, bile acid could increase the consumption of brown adipose tissue (BAT) and insulin sensitivity by farnesoid X receptor (FXR) and bile acid G protein-coupled receptor-5 (TGR5) 23, 24 receptors (36), therefore inhibiting AS inflammation.
Moreover, in addition to primary bile acid biosynthesis (see Table 2), starch and sucrose metabolism (P=0.05), tryptophan metabolism (P=0.06) and fructose and mannose metabolism (P=0.11) can also be regarded as important metabolic pathways. The results indicated that, in addition to cholesterol metabolism, carbon metabolism plays an important role in quercetin treatment. With the digestion of bacteria, carbohydrates decompose into monosaccharides, which are the main precursors of short-chain fatty acids (SCFAs), including acetate, butyrate, propionate, etc. (37). Quercetin stimulated the starch and sucrose metabolism and fructose and mannose metabolism pathways, thus increasing the SCFA concentration in the gut. As previous reported (19), SCFAs were defined as an important metabolite to systemic inflammation, and systemic inflammation was closely correlated to the enlargement and rupture of AS plaque. In addition, as the upstream pathways, tryptophan metabolism regulates the kynurenine pathway, inducing immune response, thus leading to the rise in AS plaque rupture (38). These results suggest that the bio-function of quercetin in AS treatment includes the acceleration of cholesterol metabolism, systemic inflammation and immune response inhibition. However, the present study focused only on the metabolic pathways screened by the enrichment results, and the other important AS metabolites, such as trimethylamine nitrogen oxides (TMAO), were ignored. Therefore, further research is needed to investigate the effects of quercetin on other metabolites.
Taken together, our study analyses microbiome and metabolome in response to quercetin for the treatment of AS and to identify and validate two microbial genes, 1 metabolic signature and one metabolic pathway according to the results. Our results can further prove the connection of AS and gut metabolic regulation, which could provide a novel approach in the development of medicine for AS.
Compared with traditional studies of AS, our study analysed the effects of natural products on the gut microbiome and metabolomics in the treatment of AS. With the omics results, the relationships between the regulation of gut metabolism and the incidence and therapies of AS were investigated in depth. The use of omics improved the efficiency of research and avoided heavy experimental processes. However, considering the sample quantity enrolled in the study, its accuracy needs to be further assessed in verified studies. In addition, the signatures obtained in this study need to be combined with clinical studies to determine their functions and provide a basis for future clinical research.
With the method proposed in this study, we can utilize omics results for AS-related medicine studies without the understanding of specific microbial and metabolic signatures. The method proposed in this study enables us to fully examine the main metabolic mechanism to assess the specific changes in corresponding biomarkers and to discover new biological relationships among the biomarkers. The method proposed in this study provides a new therapeutic target for clinical treatment. The feasibility of this study is high and provides a feasible method for future AS research.
Acknowledgments
The authors sincerely thank for the help from Xiao Ya and Hangzhou Lianchuan Biotechnology co. LTD.
Funding: The Project was supported by the Open fund of Key Laboratory of Ministry of Education for TCM Viscera-State Theory and Applications, Liaoning University of Traditional Chinese Medicine; The Project was supported by the Shenyang science and technology project (18-013-0-44); The Project was supported by Research Administration Office of Liaoning Economy Vocational and Technical College (Ljz2018-qn-07).
Ethical Statement: Our experimental study was approved by the School Animal Experimental Ethics Committee. The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Barquera S, Pedroza-Tobías A, Medina C, et al. Global Overview of the Epidemiology of Atherosclerotic Cardiovascular Disease. Arch Med Res 2015;46:328-38. 10.1016/j.arcmed.2015.06.006 [DOI] [PubMed] [Google Scholar]
- 2.Wong MCS, De xing Z, Wang HHX. Rapid Emergence of Atherosclerosis in Asia: a Systematic Review of Coronary Atherosclerotic Heart Disease Epidemiology and Implications for Prevention and Control Strategies. Curr Opin Lipidol 2015;26:257. 10.1097/MOL.0000000000000191 [DOI] [PubMed] [Google Scholar]
- 3.Bergheanu SC, Bodde M, Jukema J. Pathophysiology and Treatment of Atherosclerosis. Neth Heart J 2017;25:231-42. 10.1007/s12471-017-0959-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reiner Ž, Catapano AL, De Backer G, et al. ESC/EAS Guidelines for the management of dyslipidaemias: the Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS). Eur Heart J 2011;32:1769-818. 10.1093/eurheartj/ehr158 [DOI] [PubMed] [Google Scholar]
- 5.van der Tuin SJ, Kühnast S, Berbée JF, et al. Anacetrapib reduces (V)LDL cholesterol by inhibition of CETP activity and reduction of plasma PCSK9. J Lipid Res 2015;56:2085-93. 10.1194/jlr.M057794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsujita K, Sugiyama S, Sumida H, et al. Impact of Dual Lipid-lowering Strategy with Ezetimibe and Atorvastatin on Coronary Plaque Regression in Patients with Percutaneous Coronary Intervention: the Multicenter Randomized Controlled Precise-ivus Trial. J Am Coll Cardiol 2015;66:495-507. 10.1016/j.jacc.2015.05.065 [DOI] [PubMed] [Google Scholar]
- 7.Zeka K, Ruparelia K, Arroo R, et al. Flavonoids and Their Metabolites: Prevention in Cardiovascular Diseases and Diabetes. Diseases 2017;5:19 10.3390/diseases5030019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xiao L, Liu L, Guo X, et al. Quercetin Attenuates High Fat Diet-induced Atherosclerosis in Apolipoprotein E Knockout Mice: a Critical Role of Nadph Oxidase. Food Chem Toxicol 2017;105:22-33. 10.1016/j.fct.2017.03.048 [DOI] [PubMed] [Google Scholar]
- 9.Loke WM, Proudfoot JM, Hodgson JM, et al. Specific Dietary Polyphenols Attenuate Atherosclerosis in Apolipoprotein E-knockout Mice By Alleviating Inflammation and Endothelial Dysfunction. Arterioscler Thromb Vasc Biol 2010;30:749-57. 10.1161/ATVBAHA.109.199687 [DOI] [PubMed] [Google Scholar]
- 10.Song L, Xu M, Lopes-Virella MF, et al. Quercetin Inhibits Matrix Metalloproteinase-1 Expression in Human Vascular Endothelial Cells Through Extracellular Signal-regulated Kinase. Arch Biochem Biophys 2001;391:72-8. 10.1006/abbi.2001.2402 [DOI] [PubMed] [Google Scholar]
- 11.Drosos I, Tavridou A, Kolios G. New Aspects on the Metabolic Role of Intestinal Microbiota in the Development of Atherosclerosis. Metabolism 2015;64:476-81. 10.1016/j.metabol.2015.01.007 [DOI] [PubMed] [Google Scholar]
- 12.Rooks MG, Garrett WS. Gut Microbiota, Metabolites and Host Immunity. Nat Rev Immunol 2016;16:341. 10.1038/nri.2016.42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thaiss CA, Zmora N, Levy M, et al. The Microbiome and Innate Immunity. Nature 2016;535:65. 10.1038/nature18847 [DOI] [PubMed] [Google Scholar]
- 14.Gominak SC, Vitamin D. Deficiency Changes the Intestinal Microbiome Reducing B Vitamin Production in the Gut. the Resulting Lack of Pantothenic Acid Adversely Affects the Immune System, Producing a “pro-inflammatory” State Associated with Atherosclerosis and Autoimmunity. Med Hypotheses 2016;94:103-7. 10.1016/j.mehy.2016.07.007 [DOI] [PubMed] [Google Scholar]
- 15.Org E, Mehrabian M, Lusis AJ. Unraveling the Environmental and Genetic Interactions in Atherosclerosis: Central Role of the Gut Microbiota. Atherosclerosis 2015;241:387-99. 10.1016/j.atherosclerosis.2015.05.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin H, An Y, Hao F, et al. Correlations of Fecal Metabonomic and Microbiomic Changes Induced By High-fat Diet in the Pre-obesity State. Sci Rep 2016;6:21618. 10.1038/srep21618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang X, Li Y, Li Y, et al. Oxidative Stress-mediated Atherosclerosis: Mechanisms and Therapies. Front Physiol 2017;8:600. 10.3389/fphys.2017.00600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frohlich J, Dobiasova M, Lear S, et al. The Role of Risk Factors in the Development of Atherosclerosis. Crit Rev Clin Lab Sci 2001;38:401-40. 10.1080/20014091084245 [DOI] [PubMed] [Google Scholar]
- 19.Barrington WT, Lusis AJ. Association Between the Gut Microbiome and Atherosclerosis. Nat Rev Cardiol 2017;14:699. 10.1038/nrcardio.2017.169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mosawy S, Jackson DE, Woodman OL, et al. The Flavonols Quercetin and 3',4'-dihydroxyflavonol Reduce Platelet Function and Delay Thrombus Formation in a Model of Type 1 Diabetes. Diab Vasc Dis Res 2014;11:174-81. 10.1177/1479164114524234 [DOI] [PubMed] [Google Scholar]
- 21.Shen Y, Ward NC, Hodgson JM, et al. Dietary Quercetin Attenuates Oxidant-induced Endothelial Dysfunction and Atherosclerosis in Apolipoprotein E Knockout Mice Fed a High-fat Diet: a Critical Role for Heme Oxygenase-1. Free Radic Biol Med 2013;65:908-15. 10.1016/j.freeradbiomed.2013.08.185 [DOI] [PubMed] [Google Scholar]
- 22.Bense RD, Sotiriou C, Piccart-Gebhart MJ, et al. Relevance of Tumor-infiltrating Immune Cell Composition and Functionality for Disease Outcome in Breast Cancer. J Natl Cancer Inst 2016;109. doi: . 10.1093/jnci/djw192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ellulu MS, Patimah I, Khaza’ai H, et al. Atherosclerotic Cardiovascular Disease: a Review of Initiators and Protective Factors. Inflammopharmacology 2016;24:1-10. 10.1007/s10787-015-0255-y [DOI] [PubMed] [Google Scholar]
- 24.Xiao L, Liu L, Guo X, et al. Quercetin Attenuates High Fat Diet-induced Atherosclerosis in Apolipoprotein E Knockout Mice: a Critical Role of Nadph Oxidase. Food Chem Toxicol 2017;105:22. 10.1016/j.fct.2017.03.048 [DOI] [PubMed] [Google Scholar]
- 25.Qin M, Luo Y, Meng XB, et al. Myricitrin Attenuates Endothelial Cell Apoptosis to Prevent Atherosclerosis: an Insight Into Pi3k/akt Activation and Stat3 Signaling Pathways. Vascul Pharmacol 2015;70:23-34. 10.1016/j.vph.2015.03.002 [DOI] [PubMed] [Google Scholar]
- 26.Bäck M, Hansson GK. Anti-inflammatory Therapies for Atherosclerosis. Nat Rev Cardiol 2015;12:199. 10.1038/nrcardio.2015.5 [DOI] [PubMed] [Google Scholar]
- 27.Padma VV, Lalitha G, Shirony NP, et al. Effect of Quercetin Against Lindane Induced Alterations in the Serum and Hepatic Tissue Lipids in Wistar Rats. Asian Pac J Trop Biomed 2012;2:910-5. 10.1016/S2221-1691(12)60252-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vidyashankar S, Sandeep Varma R, Patki PS. Quercetin Ameliorate Insulin Resistance and Up-regulates Cellular Antioxidants During Oleic Acid Induced Hepatic Steatosis in Hepg2 Cells. Toxicol In Vitro 2013;27:945-53. 10.1016/j.tiv.2013.01.014 [DOI] [PubMed] [Google Scholar]
- 29.Duchnowicz P, Broncel M, Podsędek A, et al. Hypolipidemic and Antioxidant Effects of Hydroxycinnamic Acids, Quercetin, and Cyanidin 3-glucoside in Hypercholesterolemic Erythrocytes (in Vitro Study). Eur J Nutr 2012;51:435-43. 10.1007/s00394-011-0227-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lindskog Jonsson A, Caesar R, Akrami R, et al. Impact of Gut Microbiota and Diet on the Development of Atherosclerosis in Apoe−/− Mice. Arterioscler Thromb Vasc Biol 2018;38:2318-26. 10.1161/ATVBAHA.118.311233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Emoto T, Yamashita T, Kobayashi T, et al. Characterization of Gut Microbiota Profiles in Coronary Artery Disease Patients Using Data Mining Analysis of Terminal Restriction Fragment Length Polymorphism: Gut Microbiota Could Be a Diagnostic Marker of Coronary Artery Disease. Heart Vessels 2017;32:39-46. 10.1007/s00380-016-0841-y [DOI] [PubMed] [Google Scholar]
- 32.Brown JM, Hazen SL. The Gut Microbial Endocrine Organ: Bacterially Derived Signals Driving Cardiometabolic Diseases. Annu Rev Med 2015;66:343-59. 10.1146/annurev-med-060513-093205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tremaroli V, Bäckhed F. Functional Interactions Between the Gut Microbiota and Host Metabolism. Nature 2012;489:242. 10.1038/nature11552 [DOI] [PubMed] [Google Scholar]
- 34.Fiorucci S, Distrutti E. Bile Acid-activated Receptors, Intestinal Microbiota, and the Treatment of Metabolic Disorders. Trends Mol Med 2015;21:702-14. 10.1016/j.molmed.2015.09.001 [DOI] [PubMed] [Google Scholar]
- 35.Wahlström A, Sayin SI, Marschall H, et al. Intestinal Crosstalk Between Bile Acids and Microbiota and Its Impact on Host Metabolism. Cell Metab 2016;24:41-50. 10.1016/j.cmet.2016.05.005 [DOI] [PubMed] [Google Scholar]
- 36.Miyazaki-Anzai S, Masuda M, Kohno S, et al. Simultaneous Inhibition of Fxr and Tgr5 Exacerbates Atherosclerotic Formation. J Lipid Res 2018;59:1709-13. 10.1194/jlr.M087239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ohira H, Tsutsui W, Fujioka Y. Are Short Chain Fatty Acids in Gut Microbiota Defensive Players for Inflammation and Atherosclerosis. J Atheroscler Thromb 2017;24:660-72. 10.5551/jat.RV17006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu G, Chen S, Zhong J, et al. Crosstalk Between Tryptophan Metabolism and Cardiovascular Disease, Mechanisms, and Therapeutic Implications. Oxid Med Cell Longev 2017;2017:1602074. 10.1155/2017/1602074 [DOI] [PMC free article] [PubMed] [Google Scholar]