Abstract
Background
Left ventricular (LV) extracellular volume fraction (ECV) provides prognostic information in patients with variety of cardiomyopathies. However, data on the clinical significance of LV ECV in patients with atrial fibrillation (AF), especially in patients without replacement fibrosis are sparse. This study sought to investigate whether the presence of LV fibrosis identified by cardiac magnetic resonance (CMR) ECV quantification would independently predict the recurrence of AF after first catheter ablation (CA) in patients with AF.
Methods
A total of 130 consecutive patients who were referred for CA of AF underwent CMR examination prior to ablation. LV function, T1 mapping derived LV ECV, LV late gadolinium enhancement (LGE) were assessed. Patients were followed for arrhythmia recurrence after the CA procedure.
Results
Of 130 AF patients, 65 patients had paroxysmal AF, and 65 patients had persistent AF. There were 50 AF recurrences over a median follow-up period of 13 months. LV ECV were significantly higher in patients with recurrent AF compared to those with no recurrence (30.4%±3.3% vs. 27.4%±2.9%, P<0.001). In multivariable model, gender (HR: 0.348, 95% CI: 0.174–0.697, P=0.003), body mass index (BMI) (HR: 1.159, 95% CI: 1.050–1.279, P=0.003), AF duration (HR: 1.006, 95% CI: 1.001–1.011, P=0.017), and LV ECV (HR: 1.158, 95% CI: 1.071–1.251, P=0.000) were significantly associated with AF recurrence. In subgroup of patients without LGE, gender, BMI, AF duration and LV ECV were still the independent predictors of AF recurrence.
Conclusions
LV ECV expansion is associated with AF recurrence after CA and is a strong independent predictor of AF recurrence.
Keywords: Atrial fibrillation (AF), recurrence, left ventricular fibrosis (LV fibrosis), extracellular volume fraction (ECV)
Introduction
Atrial fibrillation (AF) is one of the most common cardiac rhythm disturbance (1). The presence of AF, both persistent and paroxysmal, is associated with an increase in both cardiovascular and all-cause mortality (2,3). Catheter ablation (CA) has been shown to be useful in restoring sinus rhythm and improving patient symptoms in both symptomatic paroxysmal and persistent AF refractory to antiarrhythmic medication (3,4). Despite this, 50% of patients with persistent AF and 30% of patients with paroxysmal AF have a recurrence of AF within the first year (5). Although, factors such as atrial size, age, and gender are known to be associated with increased risk of AF recurrence (6,7), the myocardial substrate underpinning the risk of AF recurrence is incompletely understood.
AF is known to be associated with fibrotic processes in both atria and ventricles. Although atrial fibrosis is considered one of the key substrates for AF perpetuation, the implication of left ventricular (LV) fibrosis in AF remains unclear (8). The systematic evaluation of LV fibrosis in AF is limited by poor access to human tissue samples (9). In recent years due to advances in cardiovascular magnetic resonance (CMR) techniques development, LV replacement and diffuse fibrosis can be qualitatively and quantitatively identified by late gadolinium enhancement (LGE) and T1 mapping (10,11). Furthermore, T1 mapping derived extracellular volume fraction (ECV) dichotomizes the myocardium into its cellular and interstitial components and measures the size of the extracellular space. Although, the value of LGE and T1 mapping assessed LV fibrosis in predicting AF recurrence has been previously investigated in hypertensive AF patients (12), it is not clear whether this extends to unselected patients with persistent and paroxysmal AF. In addition, patients with lone AF have impaired LV myocardial energetics (13), whether LV fibrosis is associated with AF recurrence in this subgroup of patients remained unclear. We, therefore, set out to investigate, in a prospective observational study, whether the LV diffuse fibrosis identified by CMR ECV would independently predict the AF recurrence after CA in unselected patients with persistent or paroxysmal AF and without overt structural heart disease.
Methods
Study population
We prospectively recruited consecutive patients with symptomatic persistent or paroxysmal AF refractory to at least one antiarrhythmic medication from January 2016 through December 2017. We excluded patients with prior ablation, prior implantable cardiac devices, prior known left or right ventricular cardiomyopathy, significant coronary artery disease (87 patients diagnosed by invasive coronary angiography, 43 patients diagnosed by coronary CT angiography), valvular heart disease, severe claustrophobia, or severe impairment of renal function (glomerular filtration rate <30 mL/min/1.73 m2). Paroxysmal AF was defined as AF episodes were self-terminating within 7 days, while persistent AF was defined as that those episodes beyond 7 days. Baseline characteristics including gender, age, body mass index (BMI), current medications were recorded. All CMR exams were performed before CA, the time interval of these two procedures within one week. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by our Institutional Review Board, written informed consent was obtained from all patients.
CMR protocol
All studies were performed using a 3T MR system (MAGNETOM Verio, Siemens Healthcare, Erlangen, Germany). Steady-state free-precession cine images were obtained for cardiac structure and function assessment in two long axes (horizontal and vertical) and in a stack of short axes covering the LV. Imaging parameters were: repetition time (TR), 3.1 ms; echo time (TE), 1.3 ms; flip angle (FA), 45°; field of view (FOV), 276×340 mm2; matrix, 156×192; slice thickness, 6 mm; gap, 2 mm; receiver bandwidth (BW), 704 Hz/px. In the case of the AF episode during the exam, a 2-dimension real-time true fast imaging with steady precession sequence was performed for the LV during a single breath-hold. LGE imaging was performed using a phase-sensitive inversion-recovery sequence approximately 10 minutes after administration of 0.1 mmol/kg gadopentetate dimeglumine (Magnevist, Bayer Healthcare). Imaging parameters were: TR, 10.5 ms; TE, 5.4 ms; FA, 30°; FOV, 350×262 mm2; matrix, 256×162; slice thickness, 6 mm; inversion time, 200 to 300 ms, GRAPPA acceleration factor 2. T1 mapping was performed with an ECG gated modified look-locker inversion recovery sequence (MOLLI) as previously described (14). Heart rate-dependent pulse sequence sampling scheme was performed. Data were acquired in three short-axis slices including basal, mid-ventricular, and apical short-axis planes before and 15 min after the injection of gadolinium. Imaging parameters were: TR, 2.6–2.7 ms; TE, 1.0–1.1 ms; FA, 35°; FOV, 270×360 mm2; matrix, 256 for heart rate <90 bpm, 192 for heart rate >90 bpm; slice thickness, 6 mm; linear phase-encoding ordering, minimum TI of 120 ms. The blood sample was taken before the study to measure hematocrit for ECV calculation.
Data were analyzed using a commercial workstation (Siemens Healthcare, Erlangen, Germany). The following LV functional indices were measured: ejection fraction (EF), end-diastolic volume (EDV), end-systolic volume (ESV), stroke volume (SV), cardiac output (CO), and LV mass. Left atrium (LA) volume was measured at end-systole using the biplane area-length method (13). Visual assessment was performed to determine whether LGE lesions presence or not. It was considered present only if confirmed on all corresponding locations on short-axis and long-axis images. T1 time of the myocardium and blood were measured in the LV myocardium of T1 maps and LV cavity of T1* map, respectively. The average T1 time of each layer was obtained by sketching the myocardial borders of the basal, mid-ventricular and apical levels. The per-patient overall LV myocardial pre- and post-contrast T1 time, overall LV blood pre- and post-contrast T1 time were mean of respective T1 times of basal, mid-ventricular and apical levels. Then according to the per-patient overall LV myocardial pre- and post-contrast T1 time, overall LV blood pre- and post-contrast T1 time and formula, the per-patient LV ECV was calculated (14).
CA
All patients were stopped using antiarrhythmic drugs before CA. The comprehensive details of the ablation procedure have been described previously (15). The pulmonary vein venography was performed. A 3.5 mm open-irrigation ablation catheter (NAVISTAR THERMOCOOL, Biosense-Webster, CA, USA) was advanced into the LA for mapping and ablation with 3D electro anatomical mapping system (CARTO XP or CARTO 3, Biosense-Webster, CA, USA). Pulmonary vein electrical isolation was achieved by continuous circumferential pulmonary vein ablation. For paroxysmal AF patients combined with typical right atrial flutter, the cavotricuspid isthmus was ablated. For persistent AF patients, PV isolation and linear ablation across mitral annulus, LA roof, and tricuspid isthmus were ablated (15). The procedural endpoint was pulmonary vein isolation and blocked all ablated lines which were achieved in all the cases.
Clinical follow-up
Procedural success was defined as freedom from any atrial arrhythmia after an initial 3 months blanking period. All patients were followed up via office visits or telephone interviews after discharge. The endpoint of interest was the recurrence of AF identified clinically or on repeat Holter monitoring. Recurrence was defined as any documented sustained atrial arrhythmia lasting longer than 30 seconds without using antiarrhythmic drugs after the first 3 months blanking period. Clinical assessments, 12-lead ECG and 24-hour Holter monitoring were obtained at 3, 6, 12 months after the initial ablation. Patients were asked to report all episodes of any symptoms suggestive of arrhythmias such as palpitations, dizziness, or shortness of breath. In such cases, an immediate ECG during a symptomatic period and a 24-hour Holter monitoring were suggested.
Statistical analysis
Continuous data are presented as mean ± SD. Variables lacking a normal distribution and evaluated with non-parametric tests are summarized with medians and quartiles. Continuous data were compared using an unpaired Student t-test or Mann-Whitney non-parametric test as appropriate. Nominal data are presented as numbers and percentages and were compared with a Fisher exact test or a Chi-squared test, whichever was appropriate. The interobserver and intraobserver characteristics of the LV ECV were compared using Bland-Altman analysis and determination of the 95% limits of agreement (LOA). The hazard ratio (HR) was calculated for the AF recurrence using Cox Regression analysis in univariate and multivariate models. We sought the multivariable models for AF recurrence by stepwise-forward selection. The significance level for a predictor to enter the model was 0.05, the limit to stay in the model was 0.10. Event-free survival curves were determined according to the Kaplan-Meier method using the median LV ECV value, and comparison of AF recurrence rate was performed using the log-rank test (12). A two-sided P value <0.05 was considered statistically significant. All statistical analysis was performed using SPSS software (version 21, IBM, NY, USA).
Results
Baseline characteristics
We included 130 consecutive AF patients who underwent CMR and first CA (Figure 1). There were 27 women (21%) with a mean age of 55±12 years and a median AF duration of 30 months (Q1–Q3: 9 to 84 months). Sixty-five patients had paroxysmal AF, and 65 patients had persistent AF. There were 26 patients (20%) with diabetes, 51 patients (39%) with hypertension. The LV ECV was 28.8%±3.5%. The LOA and mean difference were −5.3% to 4.7%, −0.3% for interobserver agreement, and −3.9% to 4.7%, 0.4% for intraobserver agreement. There were 47 patients (36%) with LV LGE and 83 (64%) without LV LGE. The LGE pattern was ischemic in 10 (21%, subendocardial in 10) patients and non-ischemic in 37 (79%, mid-myocardial in 14, epicardial in 3, ventricle insertional in 17, and mid-to epicardial in 3) patients. In a subgroup of 44 AF patients without hypertension, diabetes, obstructive sleep apnea, or LGE lesion, and CHA2DS2-VASc ranged from 0 to 1, the LV functional indices as follows, LV EF: 53%±9%, LV SV index: 28±8 mL/m2, LV mass index: 49±8 g/m2.
Figure 1.
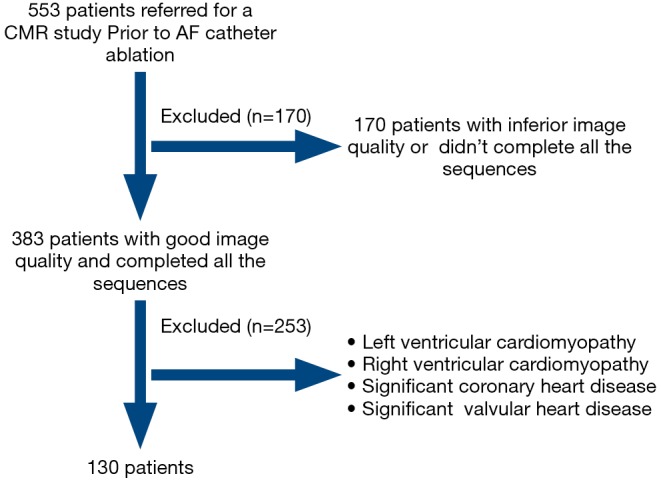
Flow diagram of study protocol. A total of 553 patients underwent CMR before the ablation of atrial fibrillation. We excluded patients with inferior image quality (images with severe artifacts which cannot be assessed) and patients who had known structural heart disease.
AF recurrence
No patient was lost to follow-up. The clinical demographics and imaging characteristics between the patients with and without AF recurrence are represented in Table 1. In all, there were 50 AF recurrences (38%) following CA. This occurred in 18 of 65 (28%) patients with paroxysmal AF and 32 of 65 (49%) patients with persistent AF over a median follow-up period of 13 months (Q1–Q3: 12 to 16 months). The median time to recurrence in this cohort was 8 months. In the AF recurrence group, there were more women and longer AF duration time (all P<0.05). Patients with recurrent AF had larger left atrial volume and lower LVEF (all P<0.01). LV ECV were significantly higher in patients with recurrent AF post-procedure compared to those with no recurrence (30.4%±3.3% vs. 27.4%±2.9%, P<0.001). The recurrence rate was 38.3% (18/47) in patients with LV LGE compared with 38.6% (32/83) in patients without LV LGE (P=0.977). In the subgroup of AF patients without LGE, LV ECV was significantly higher in patients with recurrent AF compared to those with no recurrence (30.6%±2.4% vs. 26.9%±2.5%, P<0.001). In the subgroup of AF patients without cardiovascular disease risk factor and LGE, 16 AF recurrences (36.4%) were recorded, and LV ECV was significantly higher in patients with recurrent AF compared to those with no recurrence (30.0%±2.0% vs. 26.7%±2.3%, P<0.001).
Table 1. Baseline demographic data and imaging characteristics between patients with and without AF recurrence.
| Variables | Patients with AF recurrence (n=50) | Patients without AF recurrence (n=80) | P value |
|---|---|---|---|
| Age (years) | 55±10 | 56±13 | 0.606 |
| Female | 16 | 11 | 0.013 |
| Body mass index (kg/m2) | 26.6±3.5 | 26.2±3.2 | 0.507 |
| Duration of AF (months) | 67±58 | 44±55 | 0.026 |
| Paroxysmal AF | 18 | 47 | 0.012 |
| Persistent AF | 32 | 33 | 0.012 |
| Longstanding AF | 42 | 54 | 0.037 |
| Diabetes | 15 | 11 | 0.024 |
| Hypertension | 20 | 31 | 0.887 |
| Dyslipidemia | 8 | 14 | 0.824 |
| Alcohol excess | 6 | 10 | 0.933 |
| Smoking | 8 | 13 | 0.970 |
| OSAHS | 1 | 0 | 0.204 |
| CHA2DS2-VaSc score | 1.0±1.0 | 1.1±1.3 | 0.452 |
| Medications | |||
| ACE inhibitor or ARB | 8 | 6 | 0.128 |
| Beta-blocker | 29 | 18 | 0.000 |
| Calcium-channel blocker | 15 | 10 | 0.014 |
| Warfarin | 14 | 50 | 0.014 |
| NOAC | 36 | 25 | 0.000 |
| Class I anti-arrhythmic | 35 | 22 | 0.000 |
| Class III anti-arrhythmic | 15 | 21 | 0.642 |
| Statin | 28 | 18 | 0.000 |
| Cardiac magnetic resonance | |||
| LA volume (mL) | 174±45 | 148±54 | 0.004 |
| LV EF (%) | 43±12 | 54±12 | 0.000 |
| LV EDV index (mL/m2) | 55±18 | 50±16 | 0.097 |
| LV ESV index (mL/m2) | 32±15 | 24±11 | 0.001 |
| LV SV index (mL/m2) | 23±8 | 27±9 | 0.026 |
| LV CI (L/min⸱m2) | 1.9±0.6 | 2.0±1.0 | 0.595 |
| LV mass index (g/m2) | 56±14 | 51±12 | 0.017 |
| LV LGE, n (%) | 18 (36.0) | 29 (36.3) | 0.977 |
| LV ECVaverage (%) | 31.2±3.5 | 27.2±2.4 | 0.000 |
Values are means ± standard deviation or n (%). Longstanding AF, AF lasting for >1 year; OSAHS, obstructive sleep apnea-hypopnea syndrome; CHA2DS2-VaSc, congestive heart failure, hypertension, age >75 years, diabetes, stroke, vascular disease, age 65 to <75 years, sex category (female) (1 point each; 2 points for age >75 years and previous stroke). AF, atrial fibrillation; ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; NOAC, novel oral anticoagulants for atrial fibrillation; LA, left atrium; LV, left ventricle; EF, ejection fraction; EDV, end-diastolic volume; ESV, end-systolic volume; SV, stroke volume; CI, cardiac index; LGE, late gadolinium.
Univariable and multivariable associations with AF recurrence
First, in all patients, on univariate analysis to test the association between variables and the AF recurrence, gender (male, HR: 0.442, 95% CI: 0.243 to 0.805, P=0.008), diabetes (HR: 1.900, 95% CI: 1.035 to 3.490, P=0.038), AF pattern (persistent AF, HR: 1.912, 95% CI: 1.073 to 3.406, P=0.028), AF duration (HR: 1.006, 95% CI: 1.001 to 1.010, P=0.010), LV EF (HR: 0.961, 95% CI: 0.942 to 0.981, P=0.000), LA volume (HR: 1.006, 95% CI: 1.001 to 1.011, P=0.019), and LV ECV (HR: 1.150, 95% CI: 1.086 to 1.218, P=0.000) were associated with the recurrence of AF (Table 2). In the multivariate model, gender (male, HR: 0.348, 95% CI: 0.174 to 0.697, P=0.003), BMI (HR: 1.159, 95% CI: 1.050 to 1.279, P=0.003), AF duration (HR: 1.006, 95% CI: 1.001 to 1.011, P=0.017), LV ECV (HR: 1.158, 95% CI: 1.071 to 1.251, P=0.000) were the independent predictors of arrhythmia recurrence after CA (Table 3). Kaplan-Meier curves were generated for comparison of event-free survival from recurrent AF according to the median of LV ECV (Figure 2). Second, when excluded patients with LGE lesion, in the multivariate model, gender, BMI, AF duration, and LV ECV still provided the strongest association with arrhythmia recurrence after CA (LV ECV, HR: 1.112, 95% CI: 1.005 to 1.231, P=0.040). Finally, in AF patients without cardiovascular disease risk factor and LGE, on univariate analysis, LV EF (HR: 0.923, 95% CI: 0.852 to 0.999, P=0.048) and LV ECV (HR: 1.312, 95% CI: 1.097 to 1.570, P=0.003) were associated with AF recurrence (Table S1); in the multivariate model, only LV ECV (HR: 1.712, 95% CI: 1.260 to 2.325, P=0.001) was the independent predictor of arrhythmia recurrence after CA.
Table 2. Univariate analyses for associations with AF recurrence in all patients.
| Variables | Univariate analyses | ||
|---|---|---|---|
| Hazard ratio | 95% CI | P value | |
| Age | 0.995 | 0.972–1.019 | 0.692 |
| Gender | 0.442 | 0.243–0.805 | 0.008 |
| BMI | 1.045 | 0.960–1.137 | 0.311 |
| Diabetes | 1.900 | 1.035–3.490 | 0.038 |
| Hypertension | 1.003 | 0.570–1.768 | 0.990 |
| AF pattern | 1.912 | 1.073–3.406 | 0.028 |
| Duration of AF | 1.006 | 1.001–1.010 | 0.010 |
| CHA2DS2VASc | 0.971 | 0.768–1.229 | 0.808 |
| LA volume | 1.006 | 1.001–1.011 | 0.019 |
| LV EF | 0.961 | 0.942–0.981 | 0.000 |
| LV LGE | 0.916 | 0.514–1.634 | 0.767 |
| LV ECVa | 1.150 | 1.086–1.218 | 0.000 |
For categorical data entered into the univariate and multivariate logistic regression models, indicator =1, as follows: gender, male =1, female =0; diabetes, positive =1, negative =0; hypertension, positive =1, negative =0; AF pattern, persistent =1, paroxysmal =0; LGE, positive =1, negative =0. a, LV ECV increased by 1%. AF, atrial fibrillation; BMI, body mass index; LA, left atrium; LV, left ventricle; EF, ejection fraction; LGE, late gadolinium; ECV, extracellular volume fraction.
Table 3. Multivariate analyses for associations with AF recurrence in all patients.
| Variables | Multivariate analyses | ||
|---|---|---|---|
| Hazard ratio | 95% CI | P value | |
| Gender | 0.348 | 0.174–0.697 | 0.003 |
| BMI | 1.159 | 1.050–1.279 | 0.003 |
| Duration of AF | 1.006 | 1.001–1.011 | 0.017 |
| LV ECVa | 1.158 | 1.071–1.251 | 0.000 |
a, LV ECV increased by 1%. AF, atrial fibrillation; BMI, body mass index; LV, left ventricle; ECV, extracellular volume fraction.
Figure 2.
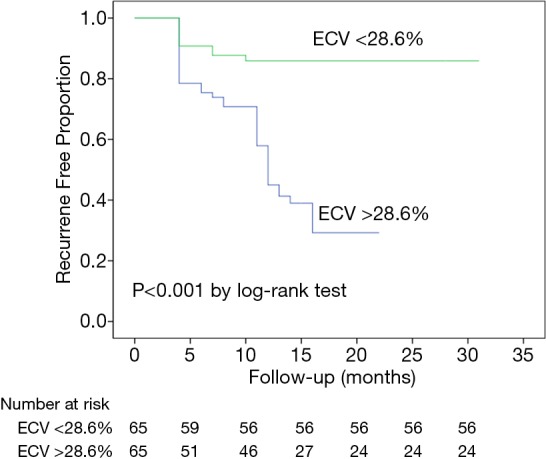
Kaplan-Meier curve showing survival free of AF recurrence according to ECV. AF, atrial fibrillation; ECV, extracellular volume fraction.
Discussion
We performed a comprehensive CMR study including cine, LV LGE and T1 mapping imaging in consecutive symptomatic patients with AF. The principal findings of this study were: (I) LV ECV was significantly elevated in patients with AF recurrence post-procedure compared to those with no recurrence; (II) LV ECV, female gender, BMI, AF duration are associated with AF recurrence after CA in AF patients with or without LGE lesion; (III) in AF patients without cardiovascular disease risk factor and LGE, LV ECV was the only independent predictor of arrhythmia recurrence after CA.
More than an atrial disease, AF is associated with systemic inflammation, endothelial dysfunction, and impairs the structure and function of the LV myocardium (9). Atrial and ventricular fibrosis in AF is likely to share many common mechanisms, the cardiac profibrotic microenvironment in AF affects both atrial and ventricular myocardium (8). In a post-mortem study, Mitrofanova et al. found that patients with a history of AF consistently had two- to three-fold more extensive interstitial fibrosis in the ventricular myocardium than patients without AF (16). Previous studies had demonstrated that diffuse myocardial fibrosis is a key pathologic feature of many heart diseases and LV diffuse fibrosis identified using CMR T1 mapping has been shown to be a predictor of adverse outcomes in a broad spectrum of disease (17). Although pulmonary vein reconnection is considered the major electrophysiological mechanism of AF recurrence after CA (6), there are a number of myocardial and systemic factors that determine AF recurrence after CA, including coronary artery disease, valvular heart disease, congestive heart failure, and obesity (18). Hereby, we assumed that AF recurrence, as part of disease progression, can be predicted by T1 mapping derived ECV. Our findings are in agreement with prior work by Neilan et al. that found that in patients with AF and hypertension, ECV was the only predictor of AF recurrence (12). Different from the previous study, we have now extended this to a more typical AF population. In the present study, among the entire cohort, our results showed that LV ECV can be used as an independent predictor of AF recurrence. Elevated LV T1 mapping indices can be explained by myocardial fibrosis in patients with AF (11,12). The presence of fibrosis can render LV less compliant, impaired relaxation with increased LV filling pressure, resulting in increased left atrium pressures and structural remodeling, the latter is the substrate for AF (19). This might be the potential explanation of LV ECV predicting AF recurrence. In addition, our results are consistent with prior studies that female gender, BMI, and AF duration are independent predictors of AF recurrence (7,19-21).
Clinically, hypertension, diabetes, and myocardial infarction are the common concomitant diseases of AF, which are the risk factors of developing AF, AF recurrence and AF related complications (5). These concomitant cardiovascular diseases are contributed as the confounder of LV fibrosis. When excluding these interacting factors, recent clinical study shows that patients with lone AF have impaired LV myocardial energetics and do not normalize after ablation (13). When retrospectively reviewed our data, there were 44 AF patients conformed to apparently lone AF (without cardiovascular disease risk factor and LGE), and LV ECV was the only independent predictor of AF recurrence. This finding supports the further investigation on the impairment of LV myocardium in lone AF and determination whether AF is the consequence of underlying cardiomyopathy.
There is heterogeneity in published work relates to the association between LV LGE and recurrence of AF. McLellan et al. reported a 9% of LV LGE and it has an insignificant association with recurrence of AF (11). The rate of LGE positive patients in our AF cohort was at 36% (ischemic vs. nonischemic: 2:8). There was no significant difference in the recurrence rate of AF between patients with and without LV LGE, and LGE was not significantly associated with AF recurrence. However, Suksaranjit et al. reported a 6.5% of LV LGE and 40% of AF recurrence, LGE pattern was ischemic in 46% of these LGE positive patients, their results suggested that LV LGE is a predictor of AF recurrence (19). We only did qualitative assessment of LGE images, lack of quantification may have affected the results. Although the incidence of LGE was high in our cohort, the small extent of LGE may partially explain the insignificant impact on the recurrence of AF in our study. Acknowledging that the diversity of enrolled subjects reflected by the incidence of LGE and patterns of LGE between different studies, the association with LV LGE and recurrence of AF requires further elucidation.
Our study has several limitations. First, patients were followed up for a median of 13 months, the ECV performance in predicting AF recurrence in long term follow-up remained unclear. Second, we did not perform endomyocardial biopsies in our population to verify the presence of myocardial fibrosis and correlate the extent of pathological fibrosis with ECV. Third, we did not perform LA LGE due to technical limitations of the thinness of the LA wall, however, LA fibrosis has also been identified by other authors as an independent predictor for recurrence of AF after CA.
Conclusions
LV ECV expansion is associated with AF recurrence after CA. In AF patients conformed to apparently lone AF, LV ECV was the only independent predictor of AF recurrence. T1 mapping derived ECV may assist clinicians to individualize therapeutic approaches for patients with AF.
Table S1. Univariate analyses for associations with AF recurrence in AF patients without cardiovascular disease risk factor and LGE (n=44).
| Variables | Univariate analyses | ||
|---|---|---|---|
| Hazard ratio | 95% CI | P value | |
| Age | 1.018 | 0.978–1.061 | 0.385 |
| Gender | 0.460 | 0.100–2.111 | 0.318 |
| BMI | 1.015 | 0.863–1.193 | 0.857 |
| AF pattern | 1.369 | 0.494–3.799 | 0.546 |
| Duration of AF | 1.002 | 0.992–1.011 | 0.731 |
| CHA2DS2VASc | 1.407 | 0.600–3.300 | 0.432 |
| LA volume | 1.005 | 0.996–1.014 | 0.303 |
| LV EF | 0.923 | 0.852–0.999 | 0.048 |
| LV ECVa | 1.312 | 1.097–1.570 | 0.003 |
a, LV ECV increased by 1%. AF, atrial fibrillation; BMI, body mass index; LA, left atrium; LV, left ventricle; EF, ejection fraction; LGE, late gadolinium; ECV, extracellular volume fraction.
Acknowledgments
Funding: This work was supported by the National Natural Science Foundation of China (grant number 81671647, 81770322, and 81771787).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by our Institutional Review Board, written informed consent was obtained from all patients.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Chugh SS, Havmoeller R, Narayanan K, et al. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation 2014;129:837-47. 10.1161/CIRCULATIONAHA.113.005119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Conen D, Chae CU, Glynn RJ, et al. Risk of death and cardiovascular events in initially healthy women with new-onset atrial fibrillation. JAMA 2011;305:2080-87. 10.1001/jama.2011.659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marrouche NF, Brachmann J, Andresen D, et al. Catheter Ablation for Atrial Fibrillation with Heart Failure. N Engl J Med 2018;378:417-27. 10.1056/NEJMoa1707855 [DOI] [PubMed] [Google Scholar]
- 4.January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2014;64:e1-76. 10.1016/j.jacc.2014.03.022 [DOI] [PubMed] [Google Scholar]
- 5.Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS: The Task Force for the management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC Endorsed by the European Stroke Organisation (ESO). Europace 2016;18:1609-78. 10.1093/europace/euw295 [DOI] [PubMed] [Google Scholar]
- 6.Takigawa M, Takahashi A, Kuwahara T, et al. Long-term follow-up after catheter ablation of paroxysmal atrial fibrillation: the incidence of recurrence and progression of atrial fibrillation. Circ Arrhythm Electrophysiol 2014;7:267-73. 10.1161/CIRCEP.113.000471 [DOI] [PubMed] [Google Scholar]
- 7.Kuck KH, Brugada J, Fürnkranz A, et al. Impact of Female Sex on Clinical Outcomes in the FIRE AND ICE Trial of Catheter Ablation for Atrial Fibrillation. Circ Arrhythm Electrophysiol 2018;11:e006204. 10.1161/CIRCEP.118.006204 [DOI] [PubMed] [Google Scholar]
- 8.Dzeshka MS, Lip GY, Snezhitskiy V, et al. Cardiac Fibrosis in Patients With Atrial Fibrillation: Mechanisms and Clinical Implications. J Am Coll Cardiol 2015;66:943-59. 10.1016/j.jacc.2015.06.1313 [DOI] [PubMed] [Google Scholar]
- 9.Wijesurendra RS, Casadei B. Atrial fibrillation: effects beyond the atrium? Cardiovasc Res 2015;105:238-47. 10.1093/cvr/cvv001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neilan TG, Shah RV, Abbasi SA, et al. The incidence, pattern, and prognostic value of left ventricular myocardial scar by late gadolinium enhancement in patients with atrial fibrillation. J Am Coll Cardiol 2013;62:2205-14. 10.1016/j.jacc.2013.07.067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McLellan AJ, Ling LH, Azzopardi S, et al. Diffuse ventricular fibrosis measured by T1 mapping on cardiac MRI predicts success of catheter ablation for atrial fibrillation. Circ Arrhythm Electrophysiol 2014;7:834-40. 10.1161/CIRCEP.114.001479 [DOI] [PubMed] [Google Scholar]
- 12.Neilan TG, Mongeon FP, Shah RV, et al. Myocardial extracellular volume expansion and the risk of recurrent atrial fibrillation after pulmonary vein isolation. JACC Cardiovasc Imaging 2014;7:1-11. 10.1016/j.jcmg.2013.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wijesurendra RS, Liu A, Eichhorn C, et al. Lone Atrial Fibrillation Is Associated With Impaired Left Ventricular Energetics That Persists Despite Successful Catheter Ablation. Circulation 2016;134:1068-81. 10.1161/CIRCULATIONAHA.116.022931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao L, Li S, Ma X, et al. Systolic MOLLI T1 mapping with heart-rate-dependent pulse sequence sampling scheme is feasible in patients with atrial fibrillation. J Cardiovasc Magn Reson 2016;18:13. 10.1186/s12968-016-0232-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dong JZ, Sang CH, Yu RH, et al. Prospective randomized comparison between a fixed '2C3L' approach vs. stepwise approach for catheter ablation of persistent atrial fibrillation. Europace 2015;17:1798-806. 10.1093/europace/euv067 [DOI] [PubMed] [Google Scholar]
- 16.Mitrofanova LB, Orshanskaya V, Ho SY, et al. Histological evidence of inflammatory reaction associated with fibrosis in the atrial and ventricular walls in a case-control study of patients with history of atrial fibrillation. Europace 2016;18:iv156-62. [DOI] [PubMed] [Google Scholar]
- 17.Schelbert EB, Messroghli DR. State of the Art: Clinical Applications of Cardiac T1 Mapping. Radiology 2016;278:658-76. 10.1148/radiol.2016141802 [DOI] [PubMed] [Google Scholar]
- 18.Lau DH, Nattel S, Kalman JM, et al. Modifiable Risk Factors and Atrial Fibrillation. Circulation 2017;136:583-96. 10.1161/CIRCULATIONAHA.116.023163 [DOI] [PubMed] [Google Scholar]
- 19.Suksaranjit P, Akoum N, Kholmovski EG, et al. Incidental LV LGE on CMR Imaging in Atrial Fibrillation Predicts Recurrence After Ablation Therapy. JACC Cardiovasc Imaging 2015;8:793-800. 10.1016/j.jcmg.2015.03.008 [DOI] [PubMed] [Google Scholar]
- 20.Pathak RK, Elliott A, Middeldorp ME, et al. Impact of CARDIO respiratory FITness on Arrhythmia Recurrence in Obese Individuals With Atrial Fibrillation: The CARDIO-FIT Study. J Am Coll Cardiol 2015;66:985-96. 10.1016/j.jacc.2015.06.488 [DOI] [PubMed] [Google Scholar]
- 21.Fornengo C, Antolini M, Frea S, et al. Prediction of atrial fibrillation recurrence after cardioversion in patients with left-atrial dilation. Eur Heart J Cardiovasc Imaging 2015;16:335-41. 10.1093/ehjci/jeu193 [DOI] [PubMed] [Google Scholar]


