Dear Editor,
Neurodegeneration with brain iron accumulation (NBIA) refers to a group of rare diseases that share clinical characteristics associated with excessive iron accumulation in the basal ganglia as well as progressive neurological deficits, such as chorea, dystonia, parkinsonism and cognitive impairment. Neuroferritinopathy is a form of NBIA caused by mutations in the ferritin light chain (FTL1) gene on chromosome 19q13.3. The most common initial clinical manifestations of neuroferritinopathy are chorea and dystonia, and only six patients have been described who initially presented with tremor [1]. Here, we report a neuroferritinopathy patient who exhibited early prominent voice tremor and carried an insertional mutation in the FTL1 gene. This study was approved by the institutional review board of Gangnam Severance Hospital (3-2019-0057).
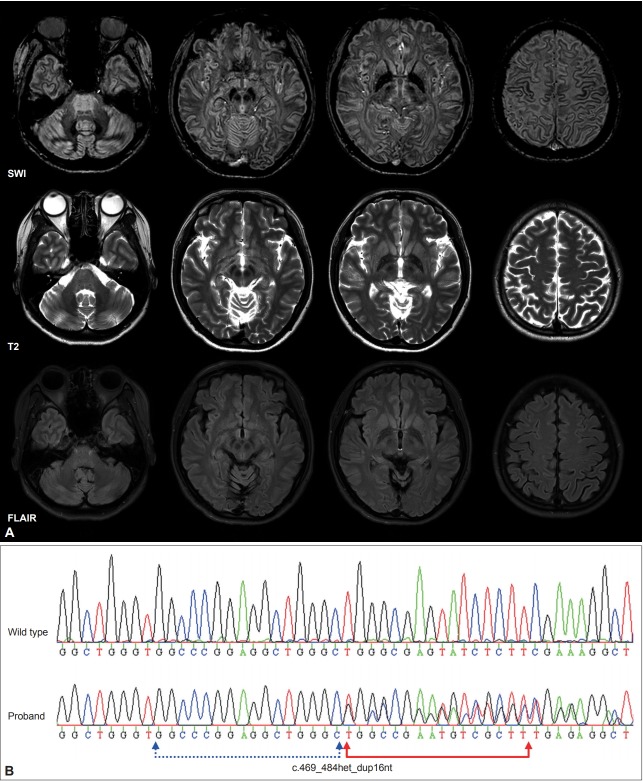
A 27-year-old woman visited our movement disorders clinic for severe voice tremor and mild hand tremor. She reported first experiencing a mild voice tremor at 13 years of age. Her voice tremor began to progressively worsen in her early 20s, and she had since experienced hand tremors most commonly when holding a cup or spoon. She was the second child of two siblings, and her birth, perinatal, and developmental histories were not remarkable. Her father also experienced severe hand tremors that began in his 30s and died of suspected parkinsonism in his mid-40s. Her mother and elder brother showed no abnormalities on neurological examination. Although her speech volume was almost normal, she spoke with a severe voice tremor without breaks in her voice. When sitting on a chair, she exhibited mild cervical dystonia that caused a slight turning of her head leftward and a continuous mild head tremor. When her arms and hands were extended or positioned under the nose, she showed mild dystonic hyperextension of both fingers and fine postural tremor with her left fingers. She also showed mild kinetic tremor in both hands. Rigidity was absent. Her hand-rolling and finger-tapping movement was normal, and she was able to walk with a normal gait. She had normal cognition, and her thyroid function tests and ceruloplasmin levels were normal. However, her serum iron (23 μg/dL, reference 60–180 μg/dL) and ferritin (3.8 ng/mL, reference 10–122 ng/mL) levels were lower than the normal range. A susceptibility-weighted brain magnetic resonance (MR) imaging study showed hypointense lesions in the bilateral globus pallidus, thalamus, substantia nigra, red nucleus, and dentate nucleus without cavitary lesions. In addition, there were thin hypointense lines along the junctions between the diffuse cortical gray and white matter, suggesting a pencil lining sign (Figure 1A). Whole-genome sequencing and subsequent confirmatory Sanger sequencing of the FTL1 gene revealed a 16-bp insertional mutation in exon 4 (c.469_484het_dup16nt, NM_000146.3), that caused a frameshift and early termination of transcription after the insertion of 5 amino acids (p. G156GGPEAGX, NP_000137.2) (Figure 1B). This mutation was not present in her mother or elder brother. Her voice tremor did not respond to several trials of injection of botulinum toxin into the adductor muscles of the vocal cord. Propranolol and trihexyphenidyl were also not effective at reducing voice tremors and hand tremors. Her tremor was slightly reduced by small doses of zonisamide (50 mg twice a day).
Figure 1.
Brain MRI and DNA sequence of the neuroferritinopathy patient. (A) Susceptibility-weighted magnetic resonance (MR) images (SWI) showing low signal intensity lesions in the bilateral globus pallidus, thalamus, substantia nigra, red nucleus, and dentate nucleus. Pencil lining signs were observed around the junction between the cortical gray and white matter. T2-weighted image MR images showing slightly increased hypointense lesions in the same subcortical nuclei, while fluid-attenuated inversion recovery (FLAIR) images showed no remarkable change. (B) Sanger sequencing result obtained in the proband showed a frameshift mutation (c.469_484het_dup16nt) in the FTL1 gene.
Eleven mutations in the FTL1 gene have been reported in more than 90 patients with neuroferritinopathy [1-3]. In these patients, six patients in two families presented with tremor as an initial clinical symptom. Four French patients harboring the c.497_498dupTC mutation initially presented with hand tremors beginning in their 20s or 40s and later developed various other motor symptoms, including cerebellar dysfunction, chorea, dystonia, or parkinsonism [4]. Two Japanese patients with the same FTL1 mutation (c.469_484het_dup16nt) found in our patient presented with hand tremor as an initial symptom at ages 10 and 15 years old and later developed parkinsonism and cognitive impairment in their mid-30s [5]. Unlike these patients, our patient showed an early prominent voice tremor rather than hand tremor. In addition to these Japanese patients, one Italian patient with the same 469_484het_dup16nt mutation presented with reports of frequent falls and behavioral changes in his 20s, and that patient later developed dystonia, parkinsonism, cerebellar ataxia and hand tremor [6]. Unlike these Japanese and Italian patients, who commonly showed cavitary lesions in the pallidum and striatum, our patient exhibited iron accumulation in the subcortical nuclei and a pencil lining sign without cavitary lesions, possibly due to a shorter disease duration and milder clinical symptoms. However, it is worth noting that all patients with the c.469_484het_dup16nt mutation, which causes a conformational change in the helix-E of the ferritin light chain protein, commonly showed their initial symptoms early in life (in their teens or 20s) and then progressed to show parkinsonism and cognitive impairment, while patients with other types of FTL1 mutations that cause conformational changes in the helix-D tend to become symptomatic in their 30s to 50s and to develop a relatively milder phenotype [1,7]. Our patient might be too young to show additional signs of parkinsonism and cognitive impairment, considering the later development of parkinsonism observed in her father. Long-term follow-up is required to monitor additional symptoms and the appearance of cavitary lesions on MR imaging.
To our knowledge, this is the first report of a neuroferritinopathy patient who presented with voice tremor as an initial and prominent clinical symptom. Neuroferritinopathy may be considered as a differential diagnosis, particularly in patients presenting with juvenile-onset voice tremor.
Acknowledgments
This study was financially supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIP) (No. 2015 R1C1A2A01054507) and a Basic Science Research Program through the NRF funded by the Ministry of Science, ICT & Future Planning (2017R1A2B 2006694).
Footnotes
Conflicts of Interest
The authors have no financial conflicts of interest.
REFERENCES
- 1.Kumar N, Rizek P, Jog M. Neuroferritinopathy: pathophysiology, presentation, differential diagnoses and management. Tremor Other Hyperkinet Mov (N Y) 2016;6:355. doi: 10.7916/D8KK9BHF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ni W, Li HF, Zheng YC, Wu ZY. FTL mutation in a Chinese pedigree with neuroferritinopathy. Neurol Genet. 2016;2:e74. doi: 10.1212/NXG.0000000000000074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yoon SH, Kim NY, Kim YJ, Lyoo CH. Novel ferritin light chain gene mutation in a Korean patient with neuroferritinopathy. J Mov Disord. 2019;12:63–65. doi: 10.14802/jmd.18062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ory-Magne F, Brefel-Courbon C, Payoux P, Debruxelles S, Sibon I, Goizet C, et al. Clinical phenotype and neuroimaging findings in a French family with hereditary ferritinopathy (FTL498-499InsTC) Mov Disord. 2009;24:1676–1683. doi: 10.1002/mds.22669. [DOI] [PubMed] [Google Scholar]
- 5.Ohta E, Nagasaka T, Shindo K, Toma S, Nagasaka K, Ohta K, et al. Neuroferritinopathy in a Japanese family with a duplication in the ferritin light chain gene. Neurology. 2008;70(16 Pt 2):1493–1494. doi: 10.1212/01.wnl.0000310428.74624.95. [DOI] [PubMed] [Google Scholar]
- 6.Storti E, Cortese F, Di Fabio R, Fiorillo C, Pierallini A, Tessa A, et al. De novo FTL mutation: a clinical, neuroimaging, and molecular study. Mov Disord. 2013;28:252–253. doi: 10.1002/mds.25275. [DOI] [PubMed] [Google Scholar]
- 7.Kubota A, Hida A, Ichikawa Y, Momose Y, Goto J, Igeta Y, et al. A novel ferritin light chain gene mutation in a Japanese family with neuroferritinopathy: description of clinical features and implications for genotypephenotype correlations. Mov Disord. 2009;24:441–445. doi: 10.1002/mds.22435. [DOI] [PubMed] [Google Scholar]