Dear Editor,
Orthostatic tremor (OT) is a rare condition, and its prevalence has not been appropriately estimated [1]. OT has been redefined and reclassified according to its frequency [2]. Pseudo-orthostatic tremor, the term given to OT with a lower frequency (13 Hz), was reported to be associated with Parkinson’s disease (PD).
Characteristic tremors in PD start unilaterally and occur exclusively during the resting state. Tremor amplitude is mitigated at movement onset and retains its features during posturing with a latency of a few seconds [2]. However, OT is rarely observed in association with PD [1]. Herein, we report a rare case of PD in a patient whose disease initiated with a unilateral OT that had overlapping features of pseudo-OT and parkinsonism.
A 69-year-old female complained of right leg tremor during standing that began a few months prior to her visit. She reported that the tremor, peculiarly, emerged during prolonged standing while she was manipulating her arms, such as when she was washing dishes or her hair. She also occasionally experienced right hand tremor that was time-locked to the onset of the OT. The patient did not recall any separately induced hand tremors. The tremor was not task-specific, and motionless standing seldomly provoked tremor. She reported that the leg tremor immediately disappeared when she moved her lower limbs, and it did not disturb her gait. Her medical history was unremarkable, and she had not taken any medications known to cause parkinsonism or tremor, such as neuroleptics or prokinetics. In addition, she did not have any family members with movement disorders.
Neurological examination revealed right-sided mild bradykinesia with limb rigidity. Resting tremor in her limbs was not observed during sitting. OT was not provoked during quiescent stance (Supplementary Video 1 in the online-only Data Supplement, segment 1). However, when she was instructed to imitate washing dishes or her hair, her right leg began to tremble at a latency of 5–10 seconds with increasing amplitude until plateau. The frequency of the tremor was approximately 4–6 Hz (Supplementary Video 2 in the online-only Data Supplement, segment 2). The tremor disappeared when the patient moved her legs and only reoccurred when the upper limbs simulated the aforementioned tasks. Occasionally, an ipsilateral coherent hand tremor of 4–6 Hz that was time-locked to the leg tremor was also observed. This tremor transiently subsided at the onset of movement and re-emerged after a similar latency of delay as that in the lower limb. The tremor was restricted to the right side. Neither postural or intentional tremor in the bilateral upper limbs nor titubation were observed. Additionally, neither dystonic posture nor any pyramidal signs were found.
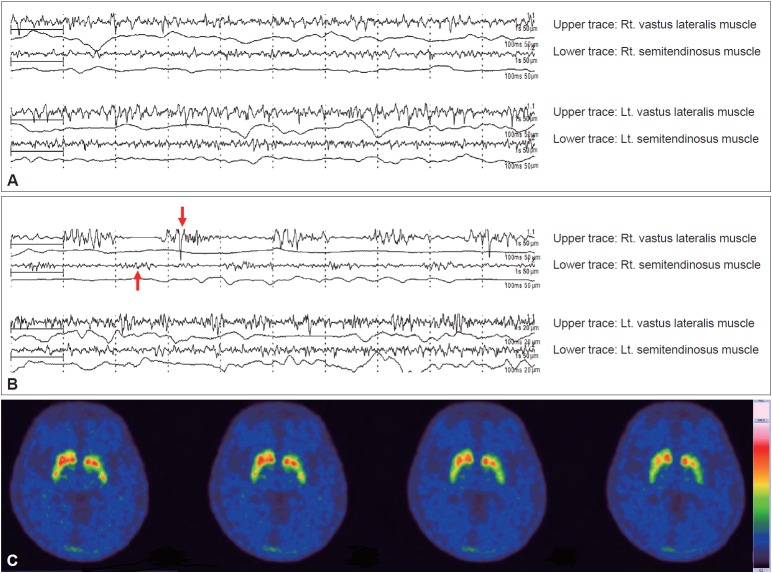
The patient’s blood tests, including thyroid function, did not reveal any abnormalities. Surface electromyography was performed. At motionless standing (Figure 1A), oscillating contractions of antagonistic muscles were not observed. However, after provocation of the upper limbs, only her right leg manifested alternating contractions of antagonistic muscles at 5 Hz, with bursts of activation lasting approximately 100 ms, while her contralateral leg remained static (Figure 1B). Positron emission tomography using 18F-N-(3-fluoropropyl)-2beta-carbon ethoxy-3beta-(4-iodophenyl) nortropane demonstrated asymmetric decreases in dopamine transporter uptake in the posterior putamen, predominantly on the left side (Figure 1C).
Figure 1.
Two-channel surface electromyography was performed on both the vastus lateralis and semitendinosus muscles and showed the following: (A) no alternating contractions of antagonistic muscles in the lower extremities during standing without provocation and (B) sinusoidal oscillating contractions with bursts of motor activity lasting approximately 100 ms (red arrows) in the right leg after provocation with a latency of 5–10 seconds. However, there was no discernible change in the contralateral limb. (C) A presynaptic dopamine transporter imaging study revealed asymmetric decreased uptake in the posterior putamen, predominantly on the left side. Rt.: right, Lt.: left.
Levodopa/carbidopa and pramipexole were prescribed and titrated, and her symptoms improved with a levodopa equivalent dose of 300 mg, but with residual partially responsive tremor. Because overall performance was improved, the current medication dosage was maintained to limit drug side effects.
OT is a progressive disorder that occurs in elderly individuals with a female predominance [3]. Its pathophysiology is unclear, although its clinical characteristics often overlap with those of other hyperkinetic disorders depending on tremor frequency [1]. Primary OT is defined by its high frequency of > 13 Hz and intermuscular coherence [1,2]. Slower OT has been reported in neurodegenerative diseases such as PD [1]. However, unilateral OT in PD provoked by a remote limb is unprecedented.
Our patient demonstrated unilateral OT that shared characteristics with parkinsonian tremor in terms of asymmetry, frequency of 5 Hz with a duration of 100 ms, and extinguishment after voluntary movement [1,2,4]. However, the patient did not display any resting tremor in any limbs, and OT was produced by volitional action in the upper limbs.
In this patient, deliberate upper limb manipulation produced a 5 Hz tremor in the postured static leg that was remote from the original site. Active selection in the motor cortex intrudes on the tremor-related cerebello-thalamo-cortical circuit, reducing hyperkineticism in PD. The basal ganglia are involved in changing movement sets but not in the maintenance of a fixed posture. Thus, parkinsonian tremor may be associated with limb movement in the absence of selection demands [4]. The remote lower extremity tremor observed in our patient could be explained by this mechanism. While upper limb movements demanded active motor selection that interfered with tremorgenic activity within the related somatotopic arrangements of the basal ganglia [5], the uninterrupted circuit linked to the lower limbs became stimulated and prone to tremor by PD pathophysiology [4]. This hypothesis may also explain why the right hand tremor was coherent and time-locked to the leg tremor: it spread due to triggered tremor pathophysiology spreading from the lower limb to the upper limb somatotopy of the basal ganglia. The right hand tremor also ceased at the beginning of upper limb movement and reemerged with similar latency to tremor seen in the leg. The initiation of tremor in a limb without active selection, its temporal spread to the ipsilateral hand and the alleviation of tremor at motion commencement all suggest that these symptoms are rooted in the same dopaminergic deficiency underlying PD [2].
OT with different ranges of frequencies has been previously reported in PD. Slower frequency tremor, as in this case, was shown to be at least partially responsive to dopaminergic treatment, while faster primary OT did not respond well to dopamine supplementation [6,7]. Interestingly, slower frequency tremor in the range close to that of parkinsonian tremor was responsive to treatment. Most pathological tremors are in the range of 4 to 8 Hz and are characteristically resting tremors in PD [2]. Dopamine deficiency activates the pathological basal loop (tremor episode trigger), while this activation, in turn, activates the cerebello-thalamic loop (tremor production and control of amplitude) [4]. Thus, slower OT responded better to dopamine supplementation. Faster OT in PD did not respond to treatment because this type of tremor might have a different origin (independent cerebello-thalamic loop) [6]. In conclusion, the present report is a rare case of unilateral OT in PD that was unusually aroused by remote upper limb movements.
Footnotes
Ethics
The Institutional Review Board at Seoul St. Mary’s Hospital approved this article (IRB No. KC19ZESI0288).
Patient consent
The patients have consented to the submission of this article to the journal.
Conflict of Interest
The authors have no conflicts of interest or financial support to report.
Author Contributions
Conceptualization: Sang-Won Yoo and Kwang-Soo Lee. Data curation: All authors. Formal analysis: Sang-Won Yoo. Writing—original draft: Sang-Won Yoo. Writing—review & editing: All authors.
Supplementary Materials
The online-only Data Supplement is available with this article at https://doi.org/10.14802/jmd.19056.
Video 1. Resting tremor in the limbs was not observed. During stationary stance, there was no visible tremor in the legs.
Video 2. When the patient was instructed to imitate washing dishes, her right leg began to tremble at a latency of 5–10 seconds at approximately 4–6 Hz. Her hands did not display tremor with constant selection demands. When imitating washing her hair, an additional ipsilateral coherent hand tremor of 4–6 Hz was time-locked to the onset of the leg tremor. The hand tremor transiently subsided at the onset of movement and re-emerged after a similar latency of delay as that in the lower limb.
REFERENCES
- 1.Benito-León J, Domingo-Santos Á. Orthostatic tremor: an update on a rare entity. Tremor Other Hyperkinet Mov (N Y) 2016;6:411. doi: 10.7916/D81N81BT. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhatia KP, Bain P, Bajaj N, Elble RJ, Hallett M, Louis ED, et al. Consensus statement on the classification of tremors. From the task force on tremor of the International Parkinson and Movement Disorder Society. Mov Disord. 2018;33:75–87. doi: 10.1002/mds.27121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hassan A, Ahlskog JE, Matsumoto JY, Milber JM, Bower JH, Wilkinson JR. Orthostatic tremor: clinical, electrophysiologic, and treatment findings in 184 patients. Neurology. 2016;86:458–464. doi: 10.1212/WNL.0000000000002328. [DOI] [PubMed] [Google Scholar]
- 4.Helmich RC, Hallett M, Deuschl G, Toni I, Bloem BR. Cerebral causes and consequences of parkinsonian resting tremor: a tale of two circuits? Brain. 2012;135(Pt 11):3206–3226. doi: 10.1093/brain/aws023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rodriguez-Oroz MC, Jahanshahi M, Krack P, Litvan I, Macias R, Bezard E, et al. Initial clinical manifestations of Parkinson’s disease: features and pathophysiological mechanisms. Lancet Neurol. 2009;8:1128–1139. doi: 10.1016/S1474-4422(09)70293-5. [DOI] [PubMed] [Google Scholar]
- 6.Apartis E, Tison F, Arné P, Jedynak CP, Vidailhet M. Fast orthostatic tremor in Parkinson’s disease mimicking primary orthostatic tremor. Mov Disord. 2001;16:1133–1136. doi: 10.1002/mds.1218. [DOI] [PubMed] [Google Scholar]
- 7.Lee SY, Chung EJ, Kim YJ, Kim SJ. Dopa responsive slow orthostatic tremor in Parkinson’s disease. J Mov Disord. 2011;4:82–84. doi: 10.14802/jmd.11019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video 1. Resting tremor in the limbs was not observed. During stationary stance, there was no visible tremor in the legs.
Video 2. When the patient was instructed to imitate washing dishes, her right leg began to tremble at a latency of 5–10 seconds at approximately 4–6 Hz. Her hands did not display tremor with constant selection demands. When imitating washing her hair, an additional ipsilateral coherent hand tremor of 4–6 Hz was time-locked to the onset of the leg tremor. The hand tremor transiently subsided at the onset of movement and re-emerged after a similar latency of delay as that in the lower limb.