Abstract
Positive social interactions are essential for emotional well-being, healthy development, establishment and maintenance of adequate social structures and reproductive success of humans and animals. Here, we review the studies that have investigated whether forms of social interaction that occur in different phases of the lifespan of animals, i.e., maternal behavior, social play and sexual interaction are rewarding in rodents and non-human primates. We show that these three forms of social interaction can be used as incentive for place conditioning, lever pressing and maze learning, three setups that have been extensively used to study the rewarding properties of food and drugs of abuse and their neural underpinnings. The experience of positive social interactions during key developmental ages has profound and long-lasting effects on brain function and behavior in emotional, motivational and cognitive domains. For instance, pup interaction is more rewarding than cocaine for early postpartum dams and rats deprived of the opportunity to play during adolescence show social and cognitive impairments at adulthood. Furthermore, sexual behavior is only overtly rewarding when animals can control the rate at which the sexual interaction occurs. Last, we discuss how animal models contributed to our understanding of social reward mechanisms and its psychological components throughout development.
Keywords: Social play, Sexual behavior, Maternal behavior, Place conditioning, Operant behavior, Reward
1. Introduction
The pleasure arising from positive social interactions is a powerful driving force of human and animal behavior. Positive social interactions are beneficial for all aspects of life. For instance, maternal care is essential for offspring survival, as well as for proper neural and behavioral development. After weaning, playful interactions with peers facilitate the acquisition of social and cognitive competence, while courtship and mating serve reproduction which, in turn, is essential for the survival of the species. Maternal contact, social play behavior and sexual behavior are among the best described examples of positive social interactions in mammals. These forms of social interaction can be labelled as highly rewarding by humans and animals, for at least three main reasons. First, they are capable of instilling a sense of well-being and pleasure. Second, they motivate approach behaviors towards a specific social stimulus. Third, they elicit associative learning to predict that certain social stimuli are positive on the basis of past experience, and to attribute salience to social-related cues. Thus, the dissociation of rewards into hedonic (“liking”), motivational (“wanting”), and cognitive (“learning”) components (Berridge and Kringelbach, 2008, Berridge and Robinson, 2003, Berridge et al., 2009) applies not only to food and drug rewards, but to social reward as well.
During the last decades, extensive research has been performed in the neuroscience field to understand brain reward mechanisms, in health and disease states. Multiple experimental approaches, including studies of behavior, pharmacology, neuroanatomy, neurophysiology, genetics and molecular biology, in different species have begun to reveal the precise neural circuits that coordinate reward-related behaviors (Cardinal et al., 2002, Kelley et al., 2005, Berridge and Kringelbach, 2008). The result is that we now know in more detail how brain reward circuits work, how these circuits drive appropriate behavioral responses, and how changes in these brain circuits result in different forms of psychopathology. In particular, studies aimed at unravelling the neural mechanisms of social reward started to shed light on social brain function. This has laid an empirical foundation to understand and develop innovative treatments for psychiatric diseases characterized by social impairments, including the inability to attribute positive value to social stimuli, including autism, schizophrenia, depression and personality disorders (American Psychiatric Association, 2000).
Here, we provide an overview of the studies that have specifically investigated the rewarding aspects of maternal behavior, social play behavior and sexual interaction in rodents (Table 1) and non-human primates. We focus on three paradigms commonly used to study natural and drug rewards: place conditioning, operant lever-pressing and T-maze discrimination tasks. We discuss the extent to which these paradigms contributed to our understanding of social reward mechanisms in different phases of development, ranging from infancy and adolescence until adulthood.
Table 1.
Rodent developmental milestones.
| Developmental age | Postnatal day (PND) | Behavior |
|---|---|---|
| Infancy | 0–21 | Suckling, ontogeny of motor behavior, ontogeny of sensory capabilities; |
| • 2–17 | Ultrasonic vocalization to elicit maternal care | |
| Adolescence | 21–60 | Social play behavior |
| • Early adolescence (juvenile period) | • 21–34 | |
| • Mid-adolescence | • 34–46 | |
| • Late adolescence | • 46–60 | |
| Adulthood | 60+ | Sexual and maternal behavior |
| • Young adulthood | • 60–90 | |
| • Adulthood | • 90+ | |
2. Place conditioning
Among the experimental approaches that have been used to study natural and drug rewards in laboratory animals, place conditioning is one of the most popular (Bardo and Bevins, 2000, Tzschentke, 1998, Tzschentke, 2007). Place conditioning experiments follow the principles of classical (Pavlovian) conditioning: neutral environmental cues can gain the capacity of evoking approach behaviors after being repeatedly paired with a rewarding stimulus. Thus, the rewarding properties of a certain drug or event serve as unconditioned stimulus (US), while neutral environmental cues can function as a conditioned stimulus (CS). Through their association with the US, the CS may acquire motivational value and induce approach behaviors.
By and large, most place conditioning studies use a test apparatus consisting of at least two compartments with distinct visual, olfactory, or tactile cues, so that an animal will be able to distinguish between these compartments, and preferably will have no innate preference for either of them. Most often, a third, neutral compartment serves as a connection between the two conditioning compartments and is used as a “start box” into which the animal is introduced during the test session (Fig. 1). Although methodological details differ among laboratories, a standard place conditioning experiment involves several conditioning sessions where the animal is presented with a certain US (i.e., the subjective effects of a drug of abuse, palatable food or a social stimulus) in one distinct compartment of the apparatus. Intermixed with these compartment-US pairings is similar exposure to the other compartment without the US or with a control treatment. Through repeated association, the environment paired with the US acquires motivational value. Following conditioning, the animal receives a test session where it can freely move around the whole apparatus in the absence of the US. The amount of time spent in the compartment previously associated with the US serves as an indicator of conditioned place preference (CPP).
Fig. 1.
A standard CPP apparatus consists of two conditioning compartments with distinct visual, olfactory, or tactile cues, separated by a third, neutral compartment used as a “start box” into which the animal is introduced during the test session. During conditioning, closed dividers between the compartments are used to confine the animals to the conditioning compartment. During habituation and testing, the closed dividers are replaced by dividers that contained an arched gateway, allowing the animals access to all compartments.
In the last 20 years, the number of studies that used place conditioning experiments to study brain reward mechanisms has enormously increased. CPP can be induced by a wide variety of psychoactive drugs and food, and by positive social interactions like maternal behavior, social play behavior and sexual behavior (Bardo and Bevins, 2000, Tzschentke, 1998, Tzschentke, 2007).
2.1. CPP induced by maternal behavior
Pup interaction is one of the most highly motivating behaviors in maternal mammals. In rats, maternal responsiveness develops during late pregnancy, peaks at parturition, is sustained after delivery and then slowly declines. Maternal motivation in the postpartum period is highly adaptive, since the strong drive to seek out and interact with pups stimulates the expression of maternal behaviors essential for the development and survival of the offspring, and promotes vigorous pup-seeking behavior if pups are out of the nest.
Place conditioning experiments have provided empirical support to the notion that pups are powerful rewarding stimuli to maternal animals. Fleming and colleagues were the first to perform a parametric analysis of pup-induced CPP in rats (Fleming et al., 1994). They used a two-compartment CPP apparatus and compared pup- and food-induced CPP in either postpartum or virgin female rats. Postpartum and virgin females were separated from pups or food-deprived for either 10 min or 23 h, and re-exposed to either pups or food in one compartment of the CPP apparatus on 1, 2 or 4 alternate days for 15, 30 or 60 min. On the other days, the animals were exposed to the second compartment of the CPP apparatus without pups or food. During testing, the animals were allowed to move around the two compartments of the CPP apparatus in the absence of pups or food. The results showed that pup interaction produced CPP only in postpartum dams that received at least two 60-min conditioning sessions (Fleming et al., 1994). Although 10 min of pup-deprivation before conditioning resulted in pup-associated CPP, more dams had stronger preference after 23 h of pup-deprivation prior to conditioning. Conversely, pup interaction did not induce CPP in virgin rats (Fleming et al., 1994). The opposite pattern was observed when food rather than pups was used as the rewarding stimulus: 73% of virgin rats developed a strong preference for the food-associated compartment, while 50% of the postpartum dams did (Fleming et al., 1994). These results suggest that postpartum and nulliparous rats differ in the salience attributed to environments associated with food and pups, and that the rewarding properties of pups are exclusively related to the value they have to the dam (Fleming et al., 1994). Interestingly, maternal behavior and preference for the pup-associated compartment could be induced in nulliparous animals by either hormonal manipulation that mimicked the physiological hormonal changes occurring in the dam at the end of pregnancy and after parturition (Fleming et al., 1994), or by prolonged pup exposure (Fleming et al., 1994, Seip and Morrell, 2008). In a follow-up study, the same group showed that physical interaction with pups during conditioning, and in particular access to pups’ olfactory and somatosensory cues, was essential in establishing pup-associated CPP (Magnusson and Fleming, 1995).
In the early postpartum period, the rewarding properties of pups are so strong that they can compete with drug reward. This principle has been investigated by Mattson and colleagues, who used place conditioning experiments to compare the conditioned response of rat dams given a choice between cocaine- and offspring-associated compartments at different postpartum time points (Mattson et al., 2001, Mattson et al., 2003). During the early postpartum period (postpartum day 8), the majority of dams showed preference for the pup-associated compartment. However, the preference of the dams dramatically changed during the postpartum period, with the majority of them showing preference for the cocaine-associated compartment at postpartum day 16. In these studies, cocaine was administered subcutaneously (Mattson et al., 2001, Mattson et al., 2003). When the incentive value of cocaine was increased by administering the drug via intraperitoneal injections, which leads to a more rapid increase in plasma and brain cocaine levels compared to subcutaneous administration, a considerable proportion of dams in the early postpartum period still showed preference for the pup-associated compartment (Seip and Morrell, 2007). Consistent with the previous studies, the preference for the pup-associated compartment was higher in the early versus late postpartum period (Seip and Morrell, 2007). Together, these results show that early postpartum dams may be in a unique state of reward responsivity during which pups are more rewarding than cocaine. As the postpartum period progresses, the rewarding value of pups decreases, at least as compared to cocaine. Two factors could be responsible of this decline in pup reward: changes in the dynamics of mother–pup interaction due to hormonal fluctuations in the dam during the postpartum period, or changes in the needs of the pups due to their development, that might alter their salience to the dam. To address this issue, the same group investigated the preference for the pup-associated compartment in early and late postpartum dams that were deprived of the pups for 15 min, 2, 6, 12 or 22 h prior to conditioning and testing, and were conditioned with either young (4–7-day old) or older (12–15-day old) pups (Wansaw et al., 2008). The magnitude of pup-induced CPP in early postpartum dams did not depend on the duration of pup-deprivation prior to conditioning and testing. Furthermore, most early postpartum dams showed preference for the pup-associated compartment regardless of the age of pups used for conditioning. In contrast, late postpartum dams only exhibited robust pup-associated place preference when they were conditioned with young (4–7-day old) pups or after a 22 h period of deprivation from pups (Wansaw et al., 2008). Together, these results suggest that both factors are involved in mother–pup interaction, i.e., changes in the pups as they develop and changes in the physiological and hormonal state of the dam throughout the postpartum period.
Functional magnetic resonance studies have provided additional support for the notion that pup interaction activates brain reward circuits and that during early lactation, these circuits are more sensitive to pups than to cocaine. Thus, pup suckling in lactating dams activated the same reward-related brain areas that are activated by cocaine in virgin females, i.e., dorsal and ventral striatum and prefrontal cortex. However, lactating dams exposed to cocaine instead of pups showed a suppression of activity in these brain regions (Ferris et al., 2005). Thus, the ability of early postpartum rats to choose against competing hedonic stimuli may have evolved to ensure the engagement in interaction with pups at the expense of other rewarding activities. Interestingly, place conditioning experiments have also been used to study pup motivation towards mother-associated cues (Cromwell, 2011). Nelson and Panksepp (1996) performed an experiment in which pups were isolated from the dam for 3 h prior to a reunion during which the ventral surface of the dam's body was coated with a lemon extract solution. Twenty-four hours after a set of three pairings between the dam and the odor in a smaller version of the CPP apparatus, pups were tested for odor preference and showed a strong preference for the compartment previously associated with dam's odor (Nelson and Panksepp, 1996). Using the same conditioned odor paradigm developed by Nelson and Panksepp, Cromwell showed that variations in maternal care and environmental stressors alter pup preference for dam-related cues (Cromwell, 2011). These results are important, since they clearly show that social motivation appears early in ontogeny.
2.2. CPP induced by social play behavior
Social play, also known as “rough-and-tumble” play, is the most characteristic form of social interaction displayed by young mammals (Panksepp et al., 1984, Vanderschuren et al., 1997, Pellis and Pellis, 2009, Trezza et al., 2010). It is almost intuitive that social play is a pleasurable social activity. Its proximal functions include the development and maintenance of social relationships, i.e., to maintain group cohesion, as well as stress reduction. In the long-term, social play is of major importance for proper social and cognitive development. During social play, mammals develop and master certain skills necessary for their adult lives. They learn how to deal with fellow group members and practice behavioral flexibility, i.e., how to behave appropriately in certain situations, and how to alter behavior when their changeable environment demands it (Špinka et al., 2001, Pellis and Pellis, 2009). Support for this notion comes from studies that showed that play deprivation in rats during two weeks in adolescence when social play is most abundant leads to altered patterns of mating behavior, agonistic and social behavior during adulthood (Hol et al., 1999, Potegal and Einon, 1989, Van den Berg et al., 1999a, Van den Berg et al., 1999b). These results suggest that the opportunity to engage in social play during adolescence is essential for the development of normal socio-affective responses and to acquire social skills. Consistent with its important functions for survival, social play is a highly rewarding activity. It can be used as incentive for place conditioning, lever pressing and maze learning in laboratory animals (Calcagnetti and Schechter, 1992, Crowder and Hutto, 1992, Douglas et al., 2004, Humphreys and Einon, 1981, Ikemoto and Panksepp, 1992, Mason et al., 1963, Normansell and Panksepp, 1990, Thiel et al., 2008, Thiel et al., 2009, Trezza et al., 2009, Trezza et al., 2010, Vanderschuren, 2010).
Calcagnetti and Schechter were the first to study the rewarding properties of social play using a place conditioning paradigm (Calcagnetti and Schechter, 1992). Rats were conditioned twice daily over four days in the place conditioning apparatus. In the first conditioning session, they were placed in one compartment of the apparatus with a partner that had been rendered unable to respond to play solicitation by treatment with scopolamine. During the second conditioning session, rats were placed in the other compartment with an undrugged, playful partner. Twenty-four hours after the fourth and final day of conditioning, each rat was placed into the middle compartment and allowed free access to whole apparatus for 15 min to assess CPP. The authors showed that rats significantly preferred the compartment previously paired with a playful social partner, thus showing that social play can be used to induce CPP. Following up on this study, the rewarding properties of social play have been repeatedly investigated in adolescent rats using place conditioning experiments (Crowder and Hutto, 1992, Douglas et al., 2004, Thiel et al., 2008, Thiel et al., 2009, Trezza et al., 2009, Van den Berg et al., 1999b). Despite slight differences in the protocols used, these studies have provided consistent evidence that it is indeed social play, rather than social interaction in general, that is rewarding. Thus, if adolescent rats were paired with a partner that had been rendered nonplayful by treatment with either scopolamine or methylphenidate, CPP did not develop (Calcagnetti and Schechter, 1992, Trezza et al., 2009). The doses of scopolamine and methylphenidate used in these experiments reduced both play solicitation and responsiveness to play solicitation, without affecting social exploratory behavior or locomotor activity during social interaction (Pellis and McKenna, 1995, Vanderschuren et al., 2008). These data therefore suggest that mere social interaction, without the opportunity to engage in reciprocal rough-and-tumble play, is not rewarding for adolescent rats. Interestingly, comparable to drug-induced CPP, social play-induced CPP extinguished with repeated exposure to the conditioning environment in the absence of the social partner. After extinction, CPP could be reinstated by a single re-conditioning session (Trezza et al., 2009). These data demonstrate that the acquired positive value of neutral environmental stimuli by association with social play is quite persistent, since a single re-exposure to the social partner in the social-paired compartment after extinction reinstated CPP.
Place conditioning experiments have also been used to study the interaction between social and drug reward in adolescent rats. Thiel and colleagues showed that subeffective doses of cocaine or nicotine, given in combination with sub-threshold levels of social experience (i.e., a level of social activity insufficient to induce place conditioning), produced CPP, whereas either stimulus alone did not (Thiel et al., 2008, Thiel et al., 2009). This means that the positive subjective properties of cocaine or nicotine can sum up to the positive effects of social interaction in adolescent rats. These findings have important implications for understanding the influence of social context on drug reward during adolescence, by showing that social context influences the affective valence of drugs of abuse, and vice versa. Interestingly, in these studies both cocaine and nicotine interacted synergistically with the rewarding effects of social interaction, although both drugs reduced social play (Thiel et al., 2008, Thiel et al., 2009). These results seem at odds with the idea that the degree of reward derived from a social interaction is related to the amount of play behavior experienced during conditioning, as emerged from studies that used scopolamine- or methylphenidate-treated nonplayful partners (Calcagnetti and Schechter, 1992, Trezza et al., 2009). Apparently, the rewarding properties of low doses of cocaine and nicotine can add up to the rewarding properties of small amounts of play to produce CPP, although it cannot be excluded that when under the influence of cocaine or nicotine, other aspects of the social encounter also contribute to social reward. It should be noted, however, that Thiel and colleagues measured social play only during the last conditioning session. It could therefore also be that the effects of cocaine and nicotine on social play differ between earlier and later conditioning sessions, leaving open the possibility that higher levels of play during early conditioning sessions significantly contributed to the development of CPP.
In contrast to rats, adolescent mice rarely engage in forms of social play that can be readily distinguished from nonplayful social activities (Pellis and Pasztor, 1999). This is not surprising, given that the social repertoire of mice is less complex than that of rats. However, place conditioning experiments have demonstrated that social proximity is rewarding for early-adolescent mice from three different inbred strains. Early-adolescent (25–30-day old) A/J, C57BL/6J and DBA/2J mice approached and explored environments previously associated with social contact to a greater extent than environments associated with social isolation, whereas BALB/cJ mice did not (Panksepp and Lahvis, 2007). Early-adolescent C57BL/6J mice also emitted vocalizations during social contact at a higher rate than BALB/cJ mice (Panksepp and Lahvis, 2007). Importantly, these strain differences were not related to variability in exploratory behavior or contextual learning, nor influenced by between-strain variations in maternal care (Panksepp and Lahvis, 2007). Thus, genetic variation has a direct impact on the expression of social approach and the emission of ultrasonic vocalizations by early-adolescent mice, as well as on the positive value they assign to the opportunity for social contact. Altogether, these studies show that social play in adolescent rats and social proximity in adolescent mice can be used as incentive for place conditioning experiments, supporting the notion that these forms of social interaction are rewarding for adolescent rodents. The development of mouse models to study social reward, particularly at early developmental ages, is an important challenge of social neuroscience research, since advances in the fields of genetics and molecular biology have led to the creation of mouse models with genetic and phenotypic aberrations characteristic of human developmental disorders, such as autism and schizophrenia (Kellendonk et al., 2006, Peça et al., 2011), and gene expression profiles for mouse models characterized by aberrant social behavior could suggest new genetic targets for studies in human populations.
2.3. CPP induced by sexual behavior
Place conditioning paradigms have been extensively used to study the rewarding properties of sexual behavior, in both male and female rats and mice. In early studies, subjects were usually allowed to interact with sexually receptive partners in one distinct compartment of the place conditioning apparatus, and spent the same time alone in the alternative compartment. After several pairings in each compartment, a test session evaluated whether the experience of copulation would support the acquisition of preference for the compartment where sexual interaction took place. Using this experimental approach, it has been repeatedly shown that male rats and mice developed preference for the compartment previously paired with sexual interaction, whereas control animals that did not mate did not develop CPP (Paredes, 2009). However, the first study that investigated whether sexual behavior could support place conditioning in female rats provided controversial results, since a change of preference was only observed in the third 5-min interval of a 15 min test session, and not in the preceding intervals (Oldenburger et al., 1992). Furthermore, compared to the studies performed in male rats, where sexual interaction with a receptive female (Everitt, 1990, Hughes et al., 1990, Mehrara and Baum, 1990, Miller and Baum, 1987) or ejaculation in a separate neutral cage immediately followed by place conditioning (Agmo and Berenfeld, 1990) induced large and enduring CPP, the magnitude of the preferences shown by the females in this study was small and of short duration (Oldenburger et al., 1992). The authors performed an additional experiment where female rats were exposed to a male with which copulation occurred in one compartment of the CPP apparatus and to a sexually active, but caged, male in the other compartment. Surprisingly, the females tended to prefer the compartment paired with the caged male, and not the one where copulation had been experienced (Oldenburger et al., 1992). A likely explanation for these results is that copulation can have aversive properties for female animals when they are unable to voluntarily regulate (“pace”) the rate at which copulation occurs (Paredes and Vazquez, 1999). In the study by Oldenburger and co-workers, the females were mated in the conditioning cage and were not able to pace their sexual interaction. Therefore, potentially aversive aspects of unpaced mating could reduce any pleasurable aspects of the sexual interaction, leading females to prefer the compartment paired with a caged male to the compartment where copulation actually occurred. In later studies, Paredes and co-workers combined pacing and place conditioning methods to reduce the possible aversive aspects of unpaced mating and increase the likelihood of detecting the rewarding effects of sexual interaction in female rats. In their experiments, the females were mated in an adjacent mating cage and put in one distinct compartment of the CPP apparatus immediately after receiving an ejaculation. The mating cage consisted of a chamber equally divided by a removable wood partition with a small hole through which the female was able to enter or exit the other half of the cage in which the male was confined. The hole was too small for the male to go through, allowing only the female to pace the sexual interaction. The authors showed that only the females that were allowed to pace their sexual interactions developed a clear-cut CPP (Paredes and Alonso, 1997, Paredes and Vazquez, 1999). Interestingly, two follow-up studies showed that pacing is important for male rats as well: when males had control over the sexual interaction, they developed clear-cut CPP, while males that were mated with females that paced their coital contacts did not, even after 10 tests in which males consistently displayed sexual behavior (Camacho et al., 2004, Martinez and Paredes, 2001). The absence of CPP despite males actively and consistently engaging in sexual interaction during the 10 weeks of testing suggests that either estrous females are powerful incentives for males, even in situations where the rewarding value of sex is reduced, or that males find sexual behavior rewarding even if females control the interaction, but the CPP paradigm is not sensitive enough to detect such a reward state. In either case, these findings indicate that sexual interactions in males, as well as in females, can induce CPP only when animals are able to control the sexual interaction. From a survival point of view, controlling the rate of sexual behavior might be a strategy developed by both sexes to ensure the best physical conditions for reproduction. Furthermore, the fact that rats developed CPP when transferred to one conditioning compartment after experiencing the actual sexual interaction in a separate mating chamber shows that sexual interaction induces a positive subjective state that outlasts the mere execution of the behavior.
Besides laboratory rats and mice, CPP has been shown to be produced by sexual interaction both in female (Kohlert and Olexa, 2005, Meisel and Joppa, 1994, Meisel et al., 1996) and male (Bell et al., 2010) Syrian hamsters. Interestingly, among the rodents species for which sexual behavior induces CPP, both male and female mice (Agustin-Pavon et al., 2007, Martinez-Ricos et al., 2007, Pankevich et al., 2006, Pierman et al., 2006) and male Syrian hamsters (Bell et al., 2010) are known to show CPP for chemosensory stimuli from the opposite sex. Thus, in both these species, not only chemosensory stimuli influence different aspects of social behavior (Bruce, 1959, Coppola and O’Connell, 1988), but they are also intrinsically rewarding, since they can induce CPP even in animals that were sexually naïve and had never before associated these stimuli with sexual behavior (Agustin-Pavon et al., 2007, Bell et al., 2010).
3. Operant lever-pressing tasks
In contrast to classical conditioning experiments, where rewards are delivered independently of any action taken by the animal, in operant (or instrumental) conditioning tasks animals are trained to perform an arbitrary act to obtain a reward, such as pressing a lever, poking the nose into a hole, or pulling a chain. For instance, in a typical operant conditioning experiment, an animal is placed in a computer-controlled box (a so-called “Skinner box”) with one or more levers that protrude from a wall and some cue lights (Fig. 2). After pressing the lever, the animal obtains a reward, like release of food from a dispenser or infusion of a drug of abuse. The animal learns the contingency between lever pressing and reward delivery and, when placed again in the box, it is likely to press the lever again to obtain the reward. Responses are reinforced, or not, according to some programmed schedules, that are rules that govern the contingency between responses and outcomes, such as rewards or cues that are associated with a reward. Two basic types of manipulations of schedules of reinforcement are used: one in which the contingency depends on the number of responses given by the animal, and one in which the contingency depends on their timing. Schedules that depend on the number of responses made are called ratio schedules, where ratio of the schedule refers to the number of responses required for each reinforcement. If the contingency between responses and reinforcement depends on time, the schedule is called an interval schedule, in which responses are reinforced only if a pre-determined time interval has elapsed. The ratios and intervals may be either fixed or variable, thus resulting in four main schedules: (a) fixed-ratio (FR), in which a fixed number of responses must be made before the reinforcement occurs; (b) fixed-interval (FI) in which reinforcement becomes available upon the first response made after a given time interval; (c) variable-ratio (VR) in which the number of responses required varies between reinforcements; and (d) variable-interval (VI), in which interval requirements vary between reinforcements around a specified average value. A widely used schedule to study the motivation for rewards is the progressive-ratio (PR) schedule (Richardson and Roberts, 1996), in which the response requirement is increased after every reward obtained by the animal, until the animal stops responding. The maximal number of responses performed to obtain one single reward, called breakpoint, is used as a measure for motivation. A schedule often used to study instrumental behavior maintained by conditioned stimuli is the second-order schedule, where animals learn to respond for the presentation of an originally neutral cue (such as a light), which has been previously associated with reward delivery, and signifies its final presentation after a protracted period of instrumental responding. One of the advantages of second-order schedule paradigms is that animals will perform a high rate of responding and extended sequences of behavior before any reward delivery. This allows for the dissociation of reinforcing properties (and their neural substrates) of primary and secondary rewards. Operant conditioning tasks have given a major contribution to the understanding of brain reward mechanisms: indeed, operant responding can be maintained by most if not all drugs of abuse, food, and social rewards (Everitt and Robbins, 2000, Panlilio and Goldberg, 2007, O’Connor et al., 2011).
Fig. 2.
An operant conditioning chamber is a computer-controlled box (a so-called “Skinner box”) with one or more levers that protrude from a wall and some cue lights. After pressing the lever, the animal obtains a reward, like release of food from a dispenser, infusion of a drug of abuse, or opportunity for social contact (for instance, by opening of a sliding door). The animal learns the contingency between lever pressing and reward delivery and, when placed again in the box, it is likely to press the lever again to obtain the reward.
3.1. Operant responding for pup reinforcement
In 1969, Wilsoncroft made the first successful attempt to use an operant task to study the rewarding properties of pups in female rats (Wilsoncroft, 1969). He trained pregnant rats to press a bar in order to obtain food rewards. One day after delivery, the dams were removed from their nest and placed in the test box for a 3-h session. Their first six bar-presses were rewarded with food pellets, all subsequent bar-presses were rewarded first with their own pups, and then with foster pups of equal age. The dams repetitively bar-pressed, picked up each pup and retrieved it into the adjacent nest box. Bar pressing and retrieval of the pup to the nest continued throughout the whole session, reaching a maximum of more than 600 responses within 3 h (Wilsoncroft, 1969). Subsequently, operant procedures have been successfully used to study the rewarding properties of pups in female mice (Hauser and Gandelman, 1985, Van Hemel, 1973), rats (Lee et al., 1999), and marmosets (Pryce et al., 1993). Van Hemel (1973) showed that nulliparous female mice can be trained to press a bar reinforced by the opportunity to retrieve pups to the nest (Van Hemel, 1973). After training, the mice were allowed to choose between two different bars: pressing one bar gave the opportunity to retrieve pups, while pressing the other bars produced sensory but not physical contact with pups presented behind a screen door. All mice made more responses on the bar yielding retrievable pups and, when the bar–pup contingency was reversed, learned to press the opposite bar in order to gain access and retrieve the pups (Van Hemel, 1973). This study showed that virgin mice could perform operant behavior to gain access to pups as long as they could physically interact with the pups and retrieve them to the nest. Hauser and Gandelman (1985) extended these findings by showing that the operant response rate for pups varies systematically with reproductive and hormonal state. Thus, they showed that the rate of responding was lower in virgin mice compared to lactating postpartum females, and that ovarian hormones play a critical role in the facilitation of pup-reinforced lever pressing that occurs in the postpartum period (Hauser and Gandelman, 1985). A study performed in female marmosets confirmed the importance of hormonal influence on maternal operant responding (Pryce et al., 1993). Female marmosets could be trained to perform operant behavior maintained by visual and auditory stimuli elicited by infant marmosets. The response rate of pregnant females increased significantly as pregnancy advanced, and was maximal just before delivery. Increased operant responding for infant stimuli could be reproduced in non-pregnant marmosets treated with progesterone and estradiol to mimic the hormonal status reached at the end of pregnancy (Pryce et al., 1993). These results are in line with place conditioning experiments showing that rats that became maternal through hormonal stimulation developed pup-induced CPP (Fleming et al., 1994).
3.2. Operant responding for play reinforcement
In 1962, Mason et al. showed that social play can be used as incentive to acquire and maintain operant responding in young chimpanzees (Mason et al., 1962). They used an apparatus consisting of a wooden cage containing a retractable window that could be opened by pressing a horizontal lever that protruded from the front wall inside the cage. Opening of the window gave access to various forms of social interaction presented by the experimenter: play, that had a pattern similar to the play-fighting of young chimpanzees, with the experimenter vigorously tickling, pulling and pushing the chimpanzee; petting, with the experimenter holding and gently stroking the hands or face of the chimpanzee; grooming, corresponding closely to chimpanzee grooming; and soliciting grooming, with the experimenter presenting his forearm to be groomed by the chimpanzee. A lamp was located on the ceiling, right over the lever. At the beginning of each trial, the lamp was lighted for 10 s, and a lever press during this period resulted in the opening of the window for 30 s. The number of window openings was recorded automatically. Results showed that play was the social activity that induced the highest rate of responding, followed by petting and being groomed by the experimenter. Grooming the experimenter was the least-preferred social activity. Interestingly, grooming the experimenter was more likely to occur following play than after being groomed by the experimenter (Mason et al., 1962). This study shows that specific forms of social interaction have different rewarding properties and that in young chimpanzees play may facilitate the emergence of other forms of social interactions, like grooming or sexual behavior. The authors designed a follow-up study to determine whether young chimpanzees would equally respond for food or social rewards (Mason et al., 1963). Now chimpanzees could press two levers: pressing one lever was rewarded by either play, the most highly preferred social activity in the previous experiment, or petting, that induced intermediate levels of responding in the previous experiment, whereas pressing the other lever was rewarded with food. In the first experiment, the animals could choose between social interaction and a highly preferred food (grape or apple). The animals were tested when deprived of food or immediately after feeding. As in the previous study, play consistently elicited more responses than petting, confirming that various kinds of social activities differ in their rewarding properties, with play being the most preferred social activity in young chimpanzees. Sated chimpanzees chose play reliably more often than food. Food-deprived chimpanzees chose more often food than social activity; interestingly, however, they still chose play on almost half of the occasions. In the second experiment, the animals were tested sated, and could choose between social activity (play or petting) or food of high, moderate or low preference (fruit, dried apricot or chow). Play again induced more responding than petting, and the preference for social activity increased with decreasing attractiveness of the food alternative. Remarkably, the animals still chose play on half the occasions when the food reinforcer was highly palatable, and this preference increased to 80% when the choice was between play and low-preferred food (Mason et al., 1963). Together, these experiments demonstrate that play is a strong reinforcer in chimpanzees and that, depending on the experimental conditions, the rewarding value of play may similar, or superior, to that of food. To our knowledge, these are the only two studies that used social play as a reinforcer for operant responding.
3.3. Operant responding for sexual reinforcement
Males and females of several species have demonstrated a strong willingness to perform operant tasks in order to have access to a sex partner. Typically, these experiments involve the subject learning to press a lever in a Skinner box in order to gain access to a mate for sexual interaction. Thus, early studies showed that male rats learn to respond on a lever to receive access to a receptive female (Beck, 1971, Beck, 1978, Beck and Chmielewska, 1976), as do female rats for a sexually active male (Beck, 1971, Beck, 1974, Beck, 1978). Both male (Michael and Keverne, 1968) and female rhesus monkeys can also learn to press a lever for mates (Keverne, 1976). However, the operant tasks used in these early studies had the limitation that they did not allow to clearly separate changes in incentive motivation from changes in consummatory competence, because presentation of the sexual reward interfered with the operant response being measured, making it difficult to parse the effects of hormonal or pharmacological manipulations on motivational versus consummatory aspects of the sexual repertoire of the animals. To resolve this issue, Everitt and colleagues developed an alternative lever-pressing task based on a second-order schedule of reinforcement (Everitt, 1990, Everitt et al., 1987, Everitt and Stacey, 1987). In their experiments, male rats were allowed to copulate with a receptive female in the presence of a light that served as CS. Then, when tested in the operant box in which they had previously copulated, they learned to respond at high rates on the lever just to earn the presentation of the CS, which preceded the ultimate presentation of the female at the end of the session. Thus, in this particular task, the instrumental behavior of the male was maintained by a secondary, or conditioned reinforcer, which had gained its motivational significance through its prior association with copulation. The main advantage of this schedule is that it generated high baseline levels of responding for the CS, before the sexual interaction actually occurred, and thus allowed to measure incentive motivation for sex-related cues without interference of the physiological changes induced by copulation. Furthermore, this schedule maintained high levels of responding for an estrous female even without prior training of the animals to lever press for food reward (Everitt et al., 1987). This is an important issue, since responding for food during training could interfere with subsequent responding for an estrous female. When the authors compared the operant response of male rats that were lever pressing to gain access to either a receptive female or food, they found that, in terms of time, acquisition of the task was comparable with both rewards. However, the overall rate of responding was higher in rats that were working for food compared to rats that were working for sexual reward (Everitt et al., 1987). Both procedural and quantitative differences between food and sexual reward might be responsible of this different rate of responding. For instance, in operant tasks that use sex as a reinforcer, there is usually a variable delay between the correct response and the actual intromission and ejaculation, which may weaken the effectiveness of the reinforcer. The authors also showed that following copulation, a refractory period occurred, in which responding for an estrous female was markedly reduced but, interestingly, responding for food was unaffected. Conversely, prior free feeding reduced responding for food but did not affect responding for an estrous female (Everitt et al., 1987). Altogether, these results suggest that both operant responding and the satiety state induced by reward consumption are specific for either food or sex reward. This behavioral specificity has important implications for the interpretation of experiments involving pharmacological or hormonal manipulations. The development of second-order schedules of reinforcement to study sexual reward has given an important contribution to our knowledge of the neural underpinnings of sexual reward. Using this procedure, it has indeed been possible to dissociate the neurochemical mechanisms underlying appetitive and consummatory aspects of the sexual repertoire of the male rat (Everitt, 1990, Everitt et al., 1989, Everitt and Stacey, 1987).
It is worth mentioning that anticipatory and consummatory patterns of the rat sexual behavior, and their neural underpinnings, have also been investigated using bilevel chambers, that allow the females to pace mating by having access to another level of the experimental chamber to escape from the male. In this setup, anticipatory level changes, preceding the actual sexual interaction, are used as a measure for sexual motivation in male rats (Mendelson and Pfaus, 1989, Van Furth and Van Ree, 1996, Pfaus et al., 1999). Interestingly, bilevel chamber studies (Pfaus and Phillips, 1991, Graham and Pfaus, 2010, Afonso et al., 2009, Pfaus and Pfaff, 1992, Van Furth et al., 1995) and second-order schedules of reinforcement experiments (Everitt, 1990, Everitt et al., 1989, Everitt and Stacey, 1987) indicate that similar neurotransmitter systems mediate the motivational and consummatory aspects of sexual behavior.
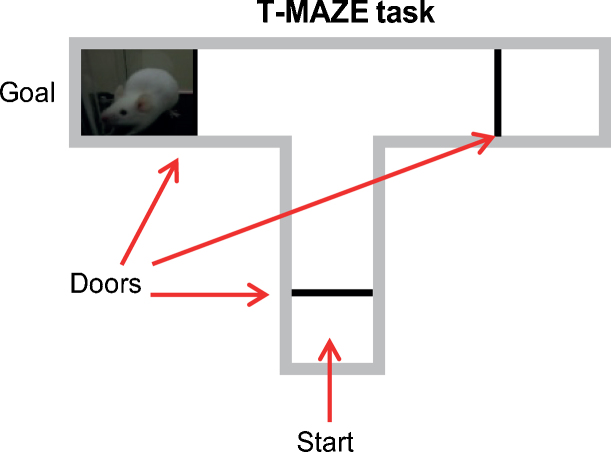
4. T-maze paradigms
The T-maze task has been conceived on the basis of the innate tendency of animals to explore their environment and obtain natural rewards, like food, with a minimum effort. The T-maze is a choice task and, as the name indicates, it is shaped like a T (Fig. 3). The experimental animal is placed at the base of the T and, following a short delay, it is allowed to explore the maze and choose to enter either the right or left arm. A reward may be placed in one arm of the maze, or different rewards may be placed in each arm. Thus, according to the protocol used, the T-maze can be used to study learning and memory, preference for different rewards placed in each arm of the maze, or spontaneous alternation behavior, that is, the tendency of animals tested in two consecutive trials to choose, on the second trial, the arm not visited before.
Fig. 3.
The T-maze is shaped like a T. The experimental animal is placed in a start box at the base of the T and, following a short delay, it is allowed to explore the maze and choose to enter either the right or left arm. A reward may be placed in one arm of the maze, or different rewards may be placed in each arm.
Rodents tested in a T-maze can adopt three possible strategies to get rewarded (Wenk, 2001). The first is a place strategy in which they use remote sensory cues to explore the maze and find the reward. The second is a cue strategy in which local cues in the goal arm help the animals to find the reward. The third is a response strategy in which the animals use a specific sequence of motor acts that are independent of the available sensory cues. In order to solve the task, the animal may or may not use these strategies independently of one another. In addition, different components of these strategies may dominate during different stages of training. Therefore, in the T-maze task, the rewarded arm can be distinguished from the non-rewarded arm by its spatial location, by local cues associated with the maze itself, or by the direction in which the animal turns to approach the goal arm (Wenk, 2001).
Different T-maze protocols have been used in a wide variety of experiments. Salamone and his colleagues, for instance, trained rats to go to one arm of a T-maze to get four food pellets, while the other arm was rewarded with two food pellets. After training, barriers were placed in the way of the four food pellets to test the willingness of rats to expend effort in order to obtain the larger reward, and to determine the involvement of nucleus accumbens dopaminergic neurotransmission on choice behavior (Cousins et al., 1996). Using this task, the authors showed that accumbens dopamine depletions cause animals to change their instrumental response selection based upon the response requirements of the task, and select lower effort alternatives for obtaining rewards. Thus, when the arm with the barrier contained four pellets, but the other arm contained no pellets, rats with dopamine depletions in the nucleus accumbens still chose the high-density arm, climbed the barrier, and consumed the pellets (Cousins et al., 1996). However, accumbens dopamine depletions altered choice behavior when the high-density arm (four pellets) had the barrier present and the arm without the barrier contained an alternative food source (two pellets). Similarly, different T-maze protocols have been used to study social reward and its neural underpinnings. Some T-maze tasks allow experimental subjects to interact with a social stimulus located in one goal box, while the other goal box is empty. Alternatively, different social stimuli can be located in each goal box. For instance, if the subject is a sexually experienced male, the stimulus animal could be a sexually receptive female on one side and a non-receptive female on the other. When the subject reaches the goal box it can interact with the stimulus animal or, in a test of incentive motivation, it may be separated from the stimulus animal by a wire mesh to enable the measurement of approach behavior before any consummatory response takes place. Parameters measured in these tasks include the percentage of choices of the experimental subject for each social stimulus or its time to reach the goal box (Wenk, 2001).
4.1. Pup-rewarded T-maze
The first studies that used T-maze tasks to study maternal responsiveness towards pups date back to the early seventies. Bridges and colleagues reported selective retrieval of pups over other objects by maternal rats tested in a T-maze (Bridges et al., 1972), and comparable results were obtained in mice (Gandelman et al., 1971). In both studies, the authors also showed differences in maternal responsiveness between lactating dams and virgin females that had been rendered maternal by pup exposure. Thus, compared to virgin females, both lactating rats (Bridges et al., 1972) and mice (Gandelman et al., 1971) showed a better pup retrieval performance in the T-maze. Interestingly, although the home-cage maternal behavior of virgin animals that become maternal after daily pup exposure is almost indistinguishable from that of lactating mothers (Fleming and Rosenblatt, 1974), the two groups differ in their willingness to retrieve pups in the T-maze. Stern and Mackinnon investigated whether hormonal factors might underlie this difference between lactating dams and animals rendered maternal by pup exposure (Stern and Mackinnon, 1976). They showed that postpartum dams that could not suckle due to prior nipple removal were able to efficiently retrieve pups from the T-maze, similar to control lactating dams and virgin females that were rendered maternal by hormonal exposure. On the contrary, only a small percentage of the virgin animals rendered maternal by pup exposure retrieved pups from the maze (Stern and Mackinnon, 1976). These results show that hormonal factors associated with pregnancy and/or parturition, but not suckling stimulation, facilitate pup retrieval in the T-maze, in line with place conditioning and operant lever-pressing experiments.
Interestingly, not only has the T-maze task successfully been used to study maternal responsiveness of females towards pups, it has recently been used to study the rewarding properties of maternal contact in rat pups as well (Panagiotaropoulos et al., 2009). In this study, at the end of one arm of the T-maze there was a small sliding door connected to a cage containing the mother, while at the other end of the maze there was another cage, without access from inside the T-maze, containing a virgin female rat (Panagiotaropoulos et al., 2009). The pups were divided in two groups. Pups from the first group were allowed to interact with the dam during training by opening of the sliding door once they reached the right end of maze, while pups from the second group were denied access to the mother-containing cage by keeping the sliding door closed. During training, pups that were given the opportunity to interact with the dam showed a clear preference for the right arm of the T-maze from the third day of training. When tested 2 h later, in the absence of the mother, these pups still showed a clear preference for the arm of the T-maze leading to the position of the mother during training. Pups denied maternal contact during training also learned to make the correct choice in the T-maze, although less efficiently than pups rewarded with maternal contact. More importantly, in these pups the expression of the learned information was contingent upon the presence of the mother in the maze. Thus, in the absence of the mother, these pups did not show seeking behavior but rather displayed behavioral inhibition, expressed as preference for the start compartment, and inhibition or avoidance for the arm of the T-maze which during training led to the mother-containing cage (Panagiotaropoulos et al., 2009). Thus, only pups that experienced maternal contact during training efficiently acquired the T-maze task, which did not require the presence of the reinforcing stimulus for its successful expression. The absence of preference for the correct arm of the T-maze in pup denied maternal contact during training may indicate impaired memory consolidation. However, against this possibility, the authors showed that when pups denied maternal contact were tested with contextual cues identical to those experienced during training, behavioral inhibition was relieved, and they moved towards the target arm. Conversely, when the mother-containing cage was in the arm opposite from the one it was at during training, pups remained in the start compartment and inhibition was maximal (Panagiotaropoulos et al., 2009). Thus, the less efficient learning in pups denied maternal contact during training was interpreted by the authors as indicative of a frustration state induced by lack of reward experience following reward expectation (Panagiotaropoulos et al., 2009).
4.2. Social play-rewarded T-maze
In 1981, Humphreys and Einon showed for the first time that social play could serve as a reinforcer for T-maze learning in adolescent rats (Humphreys and Einon, 1981). In the first experiment, they tested the learning abilities of adolescent rats in the T-maze by offering them the choice between an empty arm and a food-reinforced arm, following a period of food deprivation. Rats learned to discriminate between the two arms easily, choosing the food side in over 90% of the trials. In the second experiment, rats were given the choice between two social partners at the opposite arms of the maze: a social partner they could freely interact with, and another one that was confined under a wire mesh container throughout the interaction period, and who was therefore unable to play. Both male and female adolescent rats developed a preference for the free partner and maintained this preference after reversal by learning to run to the opposite side. In two last experiments, the rats were offered the choice between a normal social partner or one that had been treated with amphetamine or chlorpromazine, two drugs that selectively inhibit social play, but not general social exploration. In both cases, rats developed a preference for the arm where they could interact with a playful partner (Humphreys and Einon, 1981). In line with place conditioning experiments (Calcagnetti and Schechter, 1992, Trezza et al., 2009), these results show that social play, and not mere social interaction, is the most rewarding component of the social repertoire of adolescent rats.
Another study by Normansell and Panksepp confirmed that adolescent rats could readily learn a T-maze task rewarded with the opportunity to play with a conspecific (Normansell and Panksepp, 1990). In this study, the rats chose the side of the maze where they could find a playful partner between 40 and 50% of the time on the first day of training. Their performance improved over days, with rats choosing the play-associated side around 95% of the time after seven days of training. Over this time course, the running time of the animals decreased from over 40 s on the first day to an average of about 10 s on the seventh day (Normansell and Panksepp, 1990).
Ikemoto and Panksepp compared the level of social motivation between food-deprived adolescent rats that had been housed either with mother and siblings, or in isolation since they were 15 days old (Ikemoto and Panksepp, 1992). The animals were tested in a T-maze in which they could choose between either food or social interaction. The animals raised in isolation made reliably more choices for social interaction reward over food reward than animals that had been raised in groups, suggesting that prolonged social isolation makes social partners even more reinforcing than food (Ikemoto and Panksepp, 1992). This finding is in line with the results obtained by Mason and co-workers in chimpanzees offered the choice to work for either food or play reward (Mason et al., 1963), and show that play can have a rewarding value stronger than food.
4.3. Sex-rewarded T-maze
It has been shown that rats (Kagan, 1955, Whalen, 1961), mice (Imwalle et al., 2002) and ferrets (Kindon et al., 1996, Paredes and Baum, 1995) are all able to quickly run a T-maze in order to locate a mate. Some early studies investigated whether different degrees of sexual stimulation would have different reward value as reflected by the rate of learning in the T-maze. In 1955, Kagan showed that rat males that were allowed mounting, intromission and ejaculation in the goal box of the T-maze made more correct choices and developed faster running speeds compared to males that were allowed to mount and intromit only (Kagan, 1955). Interestingly, the former also showed an increase in the tendency to engage in copulatory behavior in the goal box over the course of training. On the contrary, despite an overall increase in correct choices over time, males that were not allowed to ejaculate in the goal box of the T-maze showed a significant decrease in the tendency to engage in coital behavior as training progressed (Kagan, 1955). Altogether, these results suggest that only ejaculation is able to maintain both the instrumental behavior of maze learning and the consummatory response of copulation. The relative contribution of mounting, intromission and ejaculation to T-maze learning was further investigated by Whalen (1961). He showed that males allowed one or four intromissions as rewards, without ejaculation, developed fast preference for the goal box containing the female over an empty goal box. Males given four intromissions ran faster than males allowed only one intromission. The sexual behavior performed in the goal box, however, was similar in both groups, with copulation occurring on approximately 95% of the occasions. Rats that were offered the choice between mounting a female as the reward or an empty box, showed a slight preference for the female box, exhibited a moderate increase in speed from the start box to choice point, but a decline in speed from the choice point to the female box. Males that were trained to choose between mounting without intromission in one goal box, and intromission in the other goal box showed a poorer performance in terms of choice, speed, and sexual behavior compared to males that were offered intromission only as reward (Whalen, 1961). These results suggest that intromission without ejaculation can induce T-maze learning, and are therefore in contrast with Kagan's (Kagan, 1955) previous finding that intromission may not be rewarding. The discrepancy between the two studies might be explained by the fact that in Kagan's study, males could choose between a normal receptive female in the “correct” goal box of the T-maze and either a nonreceptive female or another male in the “incorrect” goal box (Kagan, 1955). Thus, it is possible that such a protocol masked the “pure” effects of mounting without intromission, and copulation without ejaculation. From either study, it is clear that sexual behavior can induce T-maze learning, although different patterns of sexual stimulation may have different reward value.
5. Conclusions
Positive social interactions, like maternal behavior, social play behavior and sexual interaction, are an essential part of the behavioral repertoire of mammals and have a high reward value. The experience of these forms of social reward is crucial throughout development, since it influences the emotional well-being, healthy development, establishment and maintenance of adequate social structures and reproductive success of the organism. In mammals, mother–infant interactions are the primary source of social stimulation and result in long-term changes in offspring phenotypes. Indeed, it has been suggested that social experiences early in life epigenetically shape brain development and adult behavior (Champagne and Curley, 2005). After weaning, the opportunity to engage in social play with peers helps to develop communicative skills, to acquire cognitive and social competence, and it contributes to the development of behavioral and mental flexibility (Panksepp et al., 1984, Špinka et al., 2001, Pellis and Pellis, 2009, Vanderschuren et al., 1997). During adulthood, sexual behavior is associated with reproduction which, in turn, is essential for the survival of the species. Not only are positive social interactions crucial for survival and reproduction, they also provide a sense of security and well-being, and reduce feelings of stress or anxiety. The inability to attribute positive value to social stimuli is frequently observed in psychiatric disorders such as schizophrenia, depression, personality disorders and autism, and the inability to form normal social bonds is responsible for a considerable degree of the disability and poor prognosis in these disorders. Therefore, an important challenge of social neuroscience research is to shed light on the brain mechanisms that mediate positive social interactions, both in health and disease states. Animal studies are instrumental to address this issue. Although it is possible to study human brain activation in different social settings, a precise pharmacological and anatomical mapping of the brain areas and neurotransmitter systems mediating positive social interactions in humans is difficult, for both practical and ethical reasons. By allowing selective neural and pharmacological manipulations, animal studies provide the unique opportunity to unravel the relationships between brain function and behavior. Since the neural underpinnings of complex behaviors are highly comparable in mammals of different species, the information obtained from animal research can be readily translated to humans. Furthermore, animal paradigms allow precise manipulation of experimental conditions, without interference from the confounding variables often present in human studies.
The studies reviewed here provide strong evidence that maternal behavior, social play and sexual interaction and can be used as incentives in three different behavioral paradigms that have been widely used to study the rewarding properties of drug and food rewards in laboratory animals: place conditioning, lever pressing and maze learning. This demonstrates that maternal behavior, social play and sexual interaction are rewarding. Two more lines of evidence further support this notion. First, pharmacological studies have consistently shown that neurotransmitter systems that are closely implicated in the motivational and pleasurable properties of food and drug rewards also mediate the positive properties of maternal behavior, social play and sexual interaction. Second, this modulation occurs in brain regions involved in positive emotions and motivation (for neuroanatomical and pharmacological analyses of maternal behavior, social play and sexual behavior, see Everitt, 1990, Everitt and Stacey, 1987, Fleming et al., 1999, Grimm and Bridges, 1983, Kristal, 2009, Nelson and Panksepp, 1998, Panksepp et al., 1980, Panksepp et al., 1984, Pfaus et al., 2001, Pfaus et al., 2003, Trezza et al., 2010, Vanderschuren, 2010, Vanderschuren et al., 1997, Van Furth et al., 1995).
Natural and drug rewards can be dissociated in terms of their hedonic (“liking”), motivational (“wanting”), and cognitive (“learning”) psychological components (Berridge and Kringelbach, 2008, Berridge and Robinson, 2003, Berridge et al., 2009). “Liking” refers to the actual feelings of pleasure induced by reward consumption; in other words, it is the hedonic impact of a reward. Rewards that are liked are usually also wanted. “Wanting” is motivation for a reward, which promotes approach behaviors and makes subjects work to get a certain reward. “Learning” is an important link between wanting and liking, because it allows subjects to choose what they really like, and to make associations, representations, and predictions about future rewards based on past experiences and to adapt their behavior accordingly. The motivational, cognitive and hedonic properties of rewards are closely related to each other and are all necessary for rewards to be fully reinforcing. The behavioral procedures most commonly used to study natural and drug rewards always involve a combination of motivation, learning and affective responses, although certain behavioral tasks can be more sensitive to detect each of the different psychological components of reward. In addition, brain manipulations experiments in laboratory animals can help to dissociate these processes and to reveal their psychological and neural substrates. For instance, ‘taste reactivity’ experiments in rats allowed to identify the brain substrates for food “liking” (Peciña et al., 2006, Smith and Berridge, 2007, Mahler et al., 2007). The same holds true when social reward is investigated. Thus, the studies reviewed here show that second-order schedules of reinforcement can measure operant lever pressing for a social partner without interference with the physiological changes induced by the actual social interaction. Studies with responsive or unresponsive social partners allow to investigate which aspect of the social interaction is actually rewarding. Furthermore, although CPP and T-maze paradigms involve hedonic, motivational and cognitive responses, measuring the amount of social activity exhibited during CPP conditioning or in the goal box of a T-maze gives an indication of the hedonic impact of the social stimulus. Last, both Pavlovian and operant conditioning experiments allow for the study of associative learning of contextual cues paired with social reward. Anatomical and pharmacological manipulations can be used to further dissociate the different neural components of rewards. Therefore, using the behavioral paradigms described in this paper, future studies should be directed at dissociating the neural substrates mediating the motivational, hedonic and cognitive components of social reward. Investigating the brain mechanisms involved in social reward in laboratory animals will lead, in turn, to a better understanding and treatment of psychiatric diseases characterized by reduced motivation to engage in social interactions and inability to experience their rewarding character.
Acknowledgements
Supported by National Institute on Drug Abuse Grant R01 DA022628-01 (L.J.M.J.V.), the Dutch Top Institute Pharma (project T5-107) (L.J.M.J.V.), and Veni Grant 91611052 from ZonMW (The Netherlands Organisation for Health Research and Development) to V.T. We thank Marijke Achterberg and Cathy Fernandes for making the pictures in Fig. 1, Fig. 2, Fig. 3.
Contributor Information
Viviana Trezza, Email: vtrezza@uniroma3.it.
Louk J.M.J. Vanderschuren, Email: l.j.m.j.vanderschuren@umcutrecht.nl.
References
- Afonso V.M., Mueller D., Stewart J., Pfaus J.G. Amphetamine pretreatment facilitates appetitive sexual behaviors in the female rat. Psychopharmacology (Berl.) 2009;205:35–43. doi: 10.1007/s00213-009-1511-x. [DOI] [PubMed] [Google Scholar]
- Agmo A., Berenfeld R. Reinforcing properties of ejaculation in the male rat: role of opioids and dopamine. Behav. Neurosci. 1990;104:177–182. doi: 10.1037//0735-7044.104.1.177. [DOI] [PubMed] [Google Scholar]
- Agustin-Pavon C., Martinez-Ricos J., Martinez-Garcia F., Lanuza E. Effects of dopaminergic drugs on innate pheromone-mediated reward in female mice: a new case of dopamine-independent “liking”. Behav. Neurosci. 2007;121:920–932. doi: 10.1037/0735-7044.121.5.920. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . American Psychiatric Association; Washington, DC: 2000. Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
- Bardo M.T., Bevins R.A. Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacology (Berl.) 2000;153:31–43. doi: 10.1007/s002130000569. [DOI] [PubMed] [Google Scholar]
- Beck J. Instrumental conditioned reflexes with sexual reinforcement in rats. Acta Neurobiol. Exp. (Wars) 1971;31:251–252. [PubMed] [Google Scholar]
- Beck J. Contact with male or female conspecifics as a reward for instrumental responses in estrus and anestrus female rats. Acta Neurobiol. Exp. (Wars) 1974;34:615–620. [PubMed] [Google Scholar]
- Beck J. A positive correlation between male and female response latencies in the mutually reinforced instrumental sexual responses in rats. Acta Neurobiol. Exp. (Wars) 1978;38:153–156. [PubMed] [Google Scholar]
- Beck J., Chmielewska J. Contact with estrus female as a reward for instrumental response in a growing male rat from the 3rd up to the 14th week of life. Acta Neurobiol. Exp. (Wars) 1976;36:535–543. [PubMed] [Google Scholar]
- Bell M.R., Meerts S.H., Sisk C.L. Male Syrian hamsters demonstrate a conditioned place preference for sexual behavior and female chemosensory stimuli. Horm. Behav. 2010;58:410–414. doi: 10.1016/j.yhbeh.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge K.C., Kringelbach M.L. Affective neuroscience of pleasure: reward in humans and animals. Psychopharmacology (Berl.) 2008;199:457–480. doi: 10.1007/s00213-008-1099-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge K.C., Robinson T.E. Parsing reward. Trends Neurosci. 2003;26:507–513. doi: 10.1016/S0166-2236(03)00233-9. [DOI] [PubMed] [Google Scholar]
- Berridge K.C., Robinson T.E., Aldridge J.W. Dissecting components of reward: ‘liking’, ‘wanting’, and learning. Curr. Opin. Pharmacol. 2009;9:65–73. doi: 10.1016/j.coph.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges R.S., Zarrow M.X., Gandehnan R., Denenberg V.H. Differences in maternal responsiveness between lactating and sensitized rats. Dev. Psychobiol. 1972:123–127. doi: 10.1002/dev.420050205. [DOI] [PubMed] [Google Scholar]
- Bruce H.M. An exteroceptive block to pregnancy in the mouse. Nature. 1959;184:105. doi: 10.1038/184105a0. [DOI] [PubMed] [Google Scholar]
- Calcagnetti D.J., Schechter M.D. Place conditioning reveals the rewarding aspect of social interaction in juvenile rats. Physiol. Behav. 1992;51:667–672. doi: 10.1016/0031-9384(92)90101-7. [DOI] [PubMed] [Google Scholar]
- Camacho F., Sandoval C., Paredes R.G. Sexual experience and conditioned place preference in male rats. Pharmacol. Biochem. Behav. 2004;78:419–425. doi: 10.1016/j.pbb.2004.04.015. [DOI] [PubMed] [Google Scholar]
- Cardinal R.N., Parkinson J.A., Hall J., Everitt B.J. Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci. Biobehav. Rev. 2002;26:321–352. doi: 10.1016/s0149-7634(02)00007-6. [DOI] [PubMed] [Google Scholar]
- Champagne F.A., Curley J.P. How social experiences influence the brain. Curr. Opin. Neurobiol. 2005;15:704–709. doi: 10.1016/j.conb.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Coppola D.M., O’Connell R.J. Are pheromones their own reward? Physiol. Behav. 1988;44:811–816. doi: 10.1016/0031-9384(88)90067-4. [DOI] [PubMed] [Google Scholar]
- Cousins M.S., Atherton A., Turner L., Salamone J.D. Nucleus accumbens dopamine depletions alter relative response allocation in a T-maze cost/benefit task. Behav. Brain Res. 1996;74:189–197. doi: 10.1016/0166-4328(95)00151-4. [DOI] [PubMed] [Google Scholar]
- Cromwell H.C. Rat pup social motivation: a critical component of early psychological development. Neurosci. Biobehav. Rev. 2011;35:1284–1290. doi: 10.1016/j.neubiorev.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowder W.F., Hutto C.W., Jr. Operant place conditioning measures examined using two nondrug reinforcers. Pharmacol. Biochem. Behav. 1992;41:817–824. doi: 10.1016/0091-3057(92)90233-6. [DOI] [PubMed] [Google Scholar]
- Douglas L.A., Varlinskaya E.I., Spear L.P. Rewarding properties of social interactions in adolescent and adult male and female rats: impact of social versus isolate housing of subjects and partners. Dev. Psychobiol. 2004;45:153–162. doi: 10.1002/dev.20025. [DOI] [PubMed] [Google Scholar]
- Everitt B.J. Sexual motivation: a neural and behavioural analysis of the mechanisms underlying appetitive and copulatory responses of male rats. Neurosci. Biobehav. Rev. 1990;14:217–232. doi: 10.1016/s0149-7634(05)80222-2. [DOI] [PubMed] [Google Scholar]
- Everitt B.J., Cador M., Robbins T.W. Interactions between the amygdala and ventral striatum in stimulus-reward associations: studies using a second-order schedule of sexual reinforcement. Neuroscience. 1989;30:63–75. doi: 10.1016/0306-4522(89)90353-9. [DOI] [PubMed] [Google Scholar]
- Everitt B.J., Fray P., Kostarczyk E., Taylor S., Stacey P. Studies of instrumental behavior with sexual reinforcement in male rats (Rattus norvegicus): I. Control by brief visual stimuli paired with a receptive female. J. Comp. Psychol. 1987;101:395–406. [PubMed] [Google Scholar]
- Everitt B.J., Robbins T.W. Second-order schedules of drug reinforcement in rats and monkeys: measurement of reinforcing efficacy and drug-seeking behaviour. Psychopharmacology (Berl.) 2000;153:17–30. doi: 10.1007/s002130000566. [DOI] [PubMed] [Google Scholar]
- Everitt B.J., Stacey P. Studies of instrumental behavior with sexual reinforcement in male rats (Rattus norvegicus): II. Effects of preoptic area lesions, castration, and testosterone. J. Comp. Psychol. 1987;101:407–419. [PubMed] [Google Scholar]
- Ferris C.F., Kulkarni P., Sullivan J.M., Jr., Harder J.A., Messenger T.L., Febo M. Pup suckling is more rewarding than cocaine: evidence from functional magnetic resonance imaging and three-dimensional computational analysis. J. Neurosci. 2005;25:149–156. doi: 10.1523/JNEUROSCI.3156-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming A.S., Korsmit M., Deller M. Rut pups are potent reinforcers to the maternal animal: effects of experience, parity, hormones, and dopamine function. Psychobiology. 1994;22:44–53. [Google Scholar]
- Fleming A.S., O’Day D.H., Kraemer G.W. Neurobiology of mother-infant interactions: experience and central nervous system plasticity across development and generations. Neurosci. Biobehav. Rev. 1999;23:673–685. doi: 10.1016/s0149-7634(99)00011-1. [DOI] [PubMed] [Google Scholar]
- Fleming A.S., Rosenblatt J.S. Maternal behavior in the virgin and lactating rat. J. Comp. Physiol. Psychol. 1974;86:957–972. doi: 10.1037/h0036414. [DOI] [PubMed] [Google Scholar]
- Gandelman R., Zarrow M.X., Denenberg V.H. Maternal behavior: differences between mother and virgin mice as a function of the testing procedure. Dev. Psychobiol. 1971;3:207–214. doi: 10.1002/dev.420030308. [DOI] [PubMed] [Google Scholar]
- Graham M.D., Pfaus J.G. Differential regulation of female sexual behaviour by dopamine agonists in the medial preoptic area. Pharmacol. Biochem. Behav. 2010;97:284–292. doi: 10.1016/j.pbb.2010.08.012. [DOI] [PubMed] [Google Scholar]
- Grimm C.T., Bridges R.S. Opiate regulation of maternal behavior in the rat. Pharmacol. Biochem. Behav. 1983;19:609–616. doi: 10.1016/0091-3057(83)90336-2. [DOI] [PubMed] [Google Scholar]
- Hauser H., Gandelman R. Lever pressing for pups: evidence for hormonal influence upon maternal behavior of mice. Horm. Behav. 1985;19:454–468. doi: 10.1016/0018-506x(85)90041-8. [DOI] [PubMed] [Google Scholar]
- Hol T., Van den Berg C.L., Van Ree J.M., Spruijt B.M. Isolation during the play period in infancy decreases adult social interactions in rats. Behav. Brain Res. 1999;100:91–97. doi: 10.1016/s0166-4328(98)00116-8. [DOI] [PubMed] [Google Scholar]
- Hughes A.M., Everitt B.J., Herbert J. Comparative effects of preoptic area infusions of opioid peptides, lesions and castration on sexual behaviour in male rats: studies of instrumental behaviour, conditioned place preference and partner preference. Psychopharmacology (Berl.) 1990;102:243–256. doi: 10.1007/BF02245929. [DOI] [PubMed] [Google Scholar]
- Humphreys A.P., Einon D.F. Play as a reinforcer for maze-learning in juvenile rats. Anim. Behav. 1981;29:259–270. [Google Scholar]
- Ikemoto S., Panksepp J. The effects of early social isolation on the motivation for social play in juvenile rats. Dev. Psychobiol. 1992;25:261–274. doi: 10.1002/dev.420250404. [DOI] [PubMed] [Google Scholar]
- Imwalle D.B., Scordalakes E.M., Rissman E.F. Estrogen receptor alpha influences socially motivated behaviors. Horm. Behav. 2002;42:484–491. doi: 10.1006/hbeh.2002.1837. [DOI] [PubMed] [Google Scholar]
- Kagan J. Differential reward value of incomplete and complete sexual behavior. J. Comp. Physiol. Psychol. 1955;48:59–64. doi: 10.1037/h0043461. [DOI] [PubMed] [Google Scholar]
- Kellendonk C., Simpson E.H., Polan H.J., Malleret G., Vronskaya S., Winiger V., Moore H., Kandel E.R. Transient and selective overexpression of dopamine D2 receptors in the striatum causes persistent abnormalities in prefrontal cortex functioning. Neuron. 2006;49:603–615. doi: 10.1016/j.neuron.2006.01.023. [DOI] [PubMed] [Google Scholar]
- Kelley A.E., Baldo B.A., Pratt W.E., Will M.J. Corticostriatal-hypothalamic circuitry and food motivation: integration of energy, action and reward. Physiol. Behav. 2005;86:773–795. doi: 10.1016/j.physbeh.2005.08.066. [DOI] [PubMed] [Google Scholar]
- Keverne E.B. Sexual receptivity and attractiveness in the female rhesus monkey. Adv. Study Behav. 1976;7:155–200. [Google Scholar]
- Kindon H.A., Baum M.J., Paredes R.J. Medial preoptic/anterior hypothalamic lesions induce a female-typical profile of sexual partner preference in male ferrets. Horm. Behav. 1996;30:514–527. doi: 10.1006/hbeh.1996.0055. [DOI] [PubMed] [Google Scholar]
- Kohlert J.G., Olexa N. The role of vaginal stimulation for the acquisition of conditioned place preference in female Syrian hamsters. Physiol. Behav. 2005;84:135–139. doi: 10.1016/j.physbeh.2004.10.020. [DOI] [PubMed] [Google Scholar]
- Kristal M.B. The biopsychology of maternal behavior in nonhuman mammals. ILAR J. 2009;50:51–63. doi: 10.1093/ilar.50.1.51. [DOI] [PubMed] [Google Scholar]
- Lee A., Clancy S., Fleming A.S. Mother rats bar-press for pups: effects of lesions of the mpoa and limbic sites on maternal behavior and operant responding for pup-reinforcement. Behav. Brain Res. 1999;100:15–31. doi: 10.1016/s0166-4328(98)00109-0. [DOI] [PubMed] [Google Scholar]
- Magnusson J.E., Fleming A.S. Rat pups are reinforcing to the maternal rat: role of sensory cues. Psychobiology. 1995;23:69–75. [Google Scholar]
- Mahler S.V., Smith K.S., Berridge K.C. Endocannabinoid hedonic hotspot for sensory pleasure: anandamide in nucleus accumbens shell enhances ‘liking’ of a sweet reward. Neuropsychopharmacology. 2007;32:2267–2278. doi: 10.1038/sj.npp.1301376. [DOI] [PubMed] [Google Scholar]
- Martinez-Ricos J., Agustin-Pavon C., Lanuza E., Martinez-Garcia F. Intraspecific communication through chemical signals in female mice: reinforcing properties of involatile male sexual pheromones. Chem. Senses. 2007;32:139–148. doi: 10.1093/chemse/bjl039. [DOI] [PubMed] [Google Scholar]
- Martinez I., Paredes R.G. Only self-paced mating is rewarding in rats of both sexes. Horm. Behav. 2001;40:510–517. doi: 10.1006/hbeh.2001.1712. [DOI] [PubMed] [Google Scholar]
- Mason W.A., Hollis J.H., Sharpe L.G. Differential responses of chimpanzees to social stimulation. J. Comp. Physiol. Psychol. 1962;55:1105–1110. [Google Scholar]
- Mason W.A., Saxon S.V., Sharpe L.G. Preferential responses of young chimpanzees to food and social rewards. Psychol. Rec. 1963;13:341–345. [Google Scholar]
- Mattson B.J., Williams S., Rosenblatt J.S., Morrell J.I. Comparison of two positive reinforcing stimuli: pups and cocaine throughout the postpartum period. Behav. Neurosci. 2001;115:683–694. doi: 10.1037//0735-7044.115.3.683. [DOI] [PubMed] [Google Scholar]
- Mattson B.J., Williams S.E., Rosenblatt J.S., Morrell J.I. Preferences for cocaine- or pup-associated chambers differentiates otherwise behaviorally identical postpartum maternal rats. Psychopharmacology (Berl.) 2003;167:1–8. doi: 10.1007/s00213-002-1351-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehrara B.J., Baum M.J. Naloxone disrupts the expression but not the acquisition by male rats of a conditioned place preference response for an oestrous female. Psychopharmacology (Berl.) 1990;101:118–125. doi: 10.1007/BF02253728. [DOI] [PubMed] [Google Scholar]
- Meisel R.L., Joppa M.A. Conditioned place preference in female hamsters following aggressive or sexual encounters. Physiol. Behav. 1994;56:1115–1118. doi: 10.1016/0031-9384(94)90352-2. [DOI] [PubMed] [Google Scholar]
- Meisel R.L., Joppa M.A., Rowe R.K. Dopamine receptor antagonists attenuate conditioned place preference following sexual behavior in female Syrian hamsters. Eur. J. Pharmacol. 1996;309:21–24. doi: 10.1016/0014-2999(96)00389-5. [DOI] [PubMed] [Google Scholar]
- Mendelson S.D., Pfaus J.G. Level searching: a new assay of sexual motivation in the male rat. Physiol. Behav. 1989;45:337–341. doi: 10.1016/0031-9384(89)90136-4. [DOI] [PubMed] [Google Scholar]
- Michael R.P., Keverne E.B. Pheromones in the communication of sexual status in primates. Nature. 1968;218:746–749. doi: 10.1038/218746a0. [DOI] [PubMed] [Google Scholar]
- Miller R.L., Baum M.J. Naloxone inhibits mating and conditioned place preference for an estrous female in male rats soon after castration. Pharmacol. Biochem. Behav. 1987;26:781–789. doi: 10.1016/0091-3057(87)90611-3. [DOI] [PubMed] [Google Scholar]
- Nelson E.E., Panksepp J. Brain substrates of infant-mother attachment: contributions of opioids, oxytocin, and norepinephrine. Neurosci. Biobehav. Rev. 1998;22:437–452. doi: 10.1016/s0149-7634(97)00052-3. [DOI] [PubMed] [Google Scholar]
- Nelson E.E., Panksepp J. Oxytocin mediates acquisition of maternally associated odor preferences in preweanling rat pups. Behav. Neurosci. 1996;110:583–592. doi: 10.1037//0735-7044.110.3.583. [DOI] [PubMed] [Google Scholar]
- Normansell L., Panksepp J. Effects of morphine and naloxone on play-rewarded spatial discrimination in juvenile rats. Dev. Psychobiol. 1990;23:75–83. doi: 10.1002/dev.420230108. [DOI] [PubMed] [Google Scholar]
- O’Connor E.C., Chapman K., Butler P., Mead A.N. The predictive validity of the rat self-administration model for abuse liability. Neurosci. Biobehav. Rev. 2011;35:912–938. doi: 10.1016/j.neubiorev.2010.10.012. [DOI] [PubMed] [Google Scholar]
- Oldenburger W.P., Everitt B.J., de Jonge F.H. Conditioned place preference induced by sexual interaction in female rats. Horm. Behav. 1992;26:214–228. doi: 10.1016/0018-506x(92)90043-u. [DOI] [PubMed] [Google Scholar]
- Panagiotaropoulos T., Diamantopoulou A., Stamatakis A., Dimitropoulou M., Stylianopoulou F. Learning of a T-maze by rat pups when contact with the mother is either permitted or denied. Neurobiol. Learn. Mem. 2009;91:2–12. doi: 10.1016/j.nlm.2008.09.007. [DOI] [PubMed] [Google Scholar]
- Pankevich D.E., Cherry J.A., Baum M.J. Accessory olfactory neural Fos responses to a conditioned environment are blocked in male mice by vomeronasal organ removal. Physiol. Behav. 2006;87:781–788. doi: 10.1016/j.physbeh.2006.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panksepp J., Herman B.H., Vilberg T., Bishop P., DeEskinazi F.G. Endogenous opioids and social behavior. Neurosci. Biobehav. Rev. 1980;4:473–487. doi: 10.1016/0149-7634(80)90036-6. [DOI] [PubMed] [Google Scholar]
- Panksepp J., Siviy S., Normansell L. The psychobiology of play: theoretical and methodological perspectives. Neurosci. Biobehav. Rev. 1984;8:465–492. doi: 10.1016/0149-7634(84)90005-8. [DOI] [PubMed] [Google Scholar]
- Panksepp J.B., Lahvis G.P. Social reward among juvenile mice. Genes Brain Behav. 2007;6:661–671. doi: 10.1111/j.1601-183X.2006.00295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panlilio L.V., Goldberg S.R. Self-administration of drugs in animals and humans as a model and an investigative tool. Addiction. 2007;102:1863–1870. doi: 10.1111/j.1360-0443.2007.02011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paredes R.G. Evaluating the neurobiology of sexual reward. ILAR J. 2009;50:15–27. doi: 10.1093/ilar.50.1.15. [DOI] [PubMed] [Google Scholar]
- Paredes R.G., Alonso A. Sexual behavior regulated (paced) by the female induces conditioned place preference. Behav. Neurosci. 1997;111:123–128. doi: 10.1037//0735-7044.111.1.123. [DOI] [PubMed] [Google Scholar]
- Paredes R.G., Baum M.J. Altered sexual partner preference in male ferrets given excitotoxic lesions of the preoptic area/anterior hypothalamus. J. Neurosci. 1995;15:6619–6630. doi: 10.1523/JNEUROSCI.15-10-06619.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paredes R.G., Vazquez B. What do female rats like about sex? Paced mating. Behav. Brain Res. 1999;105:117–127. doi: 10.1016/s0166-4328(99)00087-x. [DOI] [PubMed] [Google Scholar]
- Peça J., Feliciano C., Ting J.T., Wang W., Wells M.F., Venkatraman T.N., Lascola C.D., Fu Z., Feng G. Shank3 mutant mice display autistic-like behaviours and striatal dysfunction. Nature. 2011;472:437–442. doi: 10.1038/nature09965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peciña S., Smith K.S., Berridge K.C. Hedonic hot spots in the brain. Neuroscientist. 2006;12:500–511. doi: 10.1177/1073858406293154. [DOI] [PubMed] [Google Scholar]
- Pellis S.M., McKenna M. What do rats find rewarding in play fighting?—an analysis using drug-induced non-playful partners. Behav. Brain Res. 1995;68:65–73. doi: 10.1016/0166-4328(94)00161-8. [DOI] [PubMed] [Google Scholar]
- Pellis S.M., Pasztor T.J. The developmental onset of a rudimentary form of play fighting in C57 mice. Dev. Psychobiol. 1999;34:175–182. [PubMed] [Google Scholar]
- Pellis S.M., Pellis V. Oneworld Publications; Oxford, UK: 2009. The Playful Brain: Venturing to the Limits of Neuroscience. [Google Scholar]
- Pfaus J.G., Phillips A.G. Role of dopamine in anticipatory and consummatory aspects of sexual behavior in the male rat. Behav. Neurosci. 1991;105:727–743. doi: 10.1037//0735-7044.105.5.727. [DOI] [PubMed] [Google Scholar]
- Pfaus J.G., Pfaff D.W. Mu-, delta-, and kappa-opioid receptor agonists selectively modulate sexual behaviors in the female rat: differential dependence on progesterone. Horm. Behav. 1992;26:457–473. doi: 10.1016/0018-506x(92)90014-m. [DOI] [PubMed] [Google Scholar]
- Pfaus J.G., Smith W.J., Coopersmith C.B. Appetitive and consummatory sexual behaviors of female rats in bilevel chambers. I. A correlational and factor analysis and the effects of ovarian hormones. Horm. Behav. 1999;35:224–240. doi: 10.1006/hbeh.1999.1516. [DOI] [PubMed] [Google Scholar]
- Pfaus J.G., Kippin T.E., Centeno S. Conditioning and sexual behavior: a review. Horm. Behav. 2001;40:291–321. doi: 10.1006/hbeh.2001.1686. [DOI] [PubMed] [Google Scholar]
- Pfaus J.G., Kippin T.E., Coria-Avila G. What can animal models tell us about human sexual response? Annu. Rev. Sex Res. 2003;14:1–63. [PubMed] [Google Scholar]
- Pierman S., Tirelli E., Douhard Q., Baum M.J., Bakker J. Male aromatase knockout mice acquire a conditioned place preference for cocaine but not for contact with an estrous female. Behav. Brain Res. 2006;174:64–69. doi: 10.1016/j.bbr.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Potegal M., Einon D. Aggressive behaviors in adult rats deprived of playfighting experience as juveniles. Dev. Psychobiol. 1989;22:159–172. doi: 10.1002/dev.420220206. [DOI] [PubMed] [Google Scholar]
- Pryce C.R., Döbeli M., Martin R.D. Effects of sex steroids on maternal motivation in the common marmoset (Callithrix jacchus): development and application of an operant system with maternal reinforcement. J. Comp. Psychol. 1993;107:99–115. doi: 10.1037/0735-7036.107.1.99. [DOI] [PubMed] [Google Scholar]
- Richardson N.R., Roberts D.C.S. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J. Neurosci. Methods. 1996;1:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- Seip K.M., Morrell J.I. Increasing the incentive salience of cocaine challenges preference for pup- over cocaine-associated stimuli during early postpartum: place preference and locomotor analyses in the lactating female rat. Psychopharmacology (Berl.) 2007;194:309–319. doi: 10.1007/s00213-007-0841-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seip K.M., Morrell J.I. Exposure to pups influences the strength of maternal motivation in virgin female rats. Physiol. Behav. 2008;95:599–608. doi: 10.1016/j.physbeh.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K.S., Berridge K.C. Opioid limbic circuit for reward: interaction between hedonic hotspots of nucleus accumbens and ventral pallidum. J. Neurosci. 2007;27:1594–1605. doi: 10.1523/JNEUROSCI.4205-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Špinka M., Newberry R.C., Bekoff M. Mammalian play: training for the unexpected. Q. Rev. Biol. 2001;76:141–168. doi: 10.1086/393866. [DOI] [PubMed] [Google Scholar]
- Stern J.M., Mackinnon D.A. Postpartum, hormonal, and nonhormonal induction of maternal behavior in rats: effects on T-maze retrieval of pups. Horm. Behav. 1976;7:305–316. doi: 10.1016/0018-506x(76)90036-2. [DOI] [PubMed] [Google Scholar]
- Thiel K.J., Okun A.C., Neisewander J.L. Social reward-conditioned place preference: a model revealing an interaction between cocaine and social context rewards in rats. Drug Alcohol Depend. 2008;96:202–212. doi: 10.1016/j.drugalcdep.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel K.J., Sanabria F., Neisewander J.L. Synergistic interaction between nicotine and social rewards in adolescent male rats. Psychopharmacology (Berl.) 2009;204:391–402. doi: 10.1007/s00213-009-1470-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trezza V., Baarendse P.J.J., Vanderschuren L.J.M.J. The pleasures of play: pharmacological insights into social reward mechanisms. Trends Pharmacol. Sci. 2010;31:463–469. doi: 10.1016/j.tips.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trezza V., Damsteegt R., Vanderschuren L.J.M.J. Conditioned place preference induced by social play behavior: parametrics, extinction, reinstatement and disruption by methylphenidate. Eur. Neuropsychopharmacol. 2009;19:659–669. doi: 10.1016/j.euroneuro.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzschentke T.M. Measuring reward with the conditioned place preference paradigm: a comprehensive review of drug effects, recent progress and new issues. Prog. Neurobiol. 1998;56:613–672. doi: 10.1016/s0301-0082(98)00060-4. [DOI] [PubMed] [Google Scholar]
- Tzschentke T.M. Measuring reward with the conditioned place preference (CPP) paradigm: update of the last decade. Addict. Biol. 2007;12:227–462. doi: 10.1111/j.1369-1600.2007.00070.x. [DOI] [PubMed] [Google Scholar]
- Van den Berg C.L., Hol T., Van Ree J.M., Spruijt B.M., Everts H., Koolhaas J.M. Play is indispensable for an adequate development of coping with social challenges in the rat. Dev. Psychobiol. 1999;34:129–138. [PubMed] [Google Scholar]
- Van den Berg C.L., Pijlman F.T., Koning H.A., Diergaarde L., Van Ree J.M., Spruijt B.M. Isolation changes the incentive value of sucrose and social behaviour in juvenile and adult rats. Behav. Brain Res. 1999;106:133–142. doi: 10.1016/s0166-4328(99)00099-6. [DOI] [PubMed] [Google Scholar]
- Van Hemel S.B. Pup retrieving as a reinforcer in nulliparous mice. J. Exp. Anal. Behav. 1973;19:233–238. doi: 10.1901/jeab.1973.19-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderschuren L.J.M.J. How the brain makes play fun. Am. J. Play. 2010;2:315–337. [Google Scholar]
- Vanderschuren L.J.M.J., Niesink R.J.M., Van Ree J.M. The neurobiology of social play behavior in rats. Neurosci. Biobehav. Rev. 1997;21:309–326. doi: 10.1016/s0149-7634(96)00020-6. [DOI] [PubMed] [Google Scholar]
- Vanderschuren L.J.M.J., Trezza V., Griffioen-Roose S., Schiepers O.J.G., Van Leeuwen N., De Vries T.J., Schoffelmeer A.N.M. Methylphenidate disrupts social play behavior in adolescent rats. Neuropsychopharmacology. 2008;33:2946–2956. doi: 10.1038/npp.2008.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Furth W.R., Wolterink G., van Ree J.M. Regulation of masculine sexual behavior: involvement of brain opioids and dopamine. Brain Res. Rev. 1995;21:162–184. doi: 10.1016/0165-0173(96)82985-7. [DOI] [PubMed] [Google Scholar]
- Van Furth W.R., Van Ree J.M. Appetitive sexual behavior in male rats: 2. Sexual reward and level-changing behavior. Physiol. Behav. 1996;60:1007–1012. doi: 10.1016/0031-9384(96)00009-1. [DOI] [PubMed] [Google Scholar]
- Wansaw M.P., Pereira M., Morrell J.I. Characterization of maternal motivation in the lactating rat: contrasts between early and late postpartum responses. Horm. Behav. 2008;54:294–301. doi: 10.1016/j.yhbeh.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenk G.L. Assessment of spatial memory using the T maze. Curr. Protoc. Neurosci. 2001 doi: 10.1002/0471142301.ns0805bs04. (Chapter 8, Unit 8 5B) [DOI] [PubMed] [Google Scholar]
- Whalen R.E. Effects of mounting without intromission and intromission without ejaculation on sexual behavior and maze learning. J. Comp. Physiol. Psychol. 1961;54:409–415. doi: 10.1037/h0046385. [DOI] [PubMed] [Google Scholar]
- Wilsoncroft W.E. Babies by bar-press: maternal behavior in the rat. Behav. Res. Methods. 1969;1:229–230. [Google Scholar]