Highlights
▸ BOLD fMRI signals change with brain development. ▸ Some of these changes may not reflect altered neural information processing. ▸ Altered neurovascular coupling or neural O2 use will also change BOLD signals. ▸ We review how neurovascular coupling and O2 use change with development. ▸ Understanding neurovascular coupling is essential to interpret BOLD signal changes.
Keywords: BOLD fMRI, Blood flow, Glutamate, Neurovascular coupling, Development, Energy
Abstract
BOLD fMRI (blood oxygenation level dependent functional magnetic resonance imaging) is increasingly used to detect developmental changes of human brain function that are hypothesized to underlie the maturation of cognitive processes. BOLD signals depend on neuronal activity increasing cerebral blood flow, and are reduced by neural oxygen consumption. Thus, developmental changes of BOLD signals may not reflect altered information processing if there are concomitant changes in neurovascular coupling (the mechanism by which neuronal activity increases blood flow) or neural energy use (and hence oxygen consumption). We review how BOLD signals are generated, and explain the signalling pathways which convert neuronal activity into increased blood flow. We then summarize in broad terms the developmental changes that the brain's neural circuitry undergoes during growth from childhood through adolescence to adulthood, and present the changes in neurovascular coupling mechanisms and energy use which occur over the same period. This information provides a framework for assessing whether the BOLD changes observed during human development reflect altered cognitive processing or changes in neurovascular coupling and energy use.
1. Introduction
By way of the BOLD (blood oxygenation level dependent) signal, functional magnetic resonance imaging (fMRI) provides an effective means of measuring brain activity in humans. Critically, the technique is non-invasive and thus safe for use across all age groups, including children. This makes fMRI potentially the most powerful tool available today for studying the neural underpinnings of the vast cognitive and social development that occurs during the first years of life. Over the past twenty years, fMRI has been vital in providing insight into the normal development of the neural strategies involved in human language acquisition, visual recognition, and memory (Baird et al., 1999, Casey et al., 1995, Gaillard et al., 2000, Thomas et al., 1999). fMRI is also well suited to studying abnormal neural development in diseases such as epilepsy and schizophrenia, and in disorders such as dyslexia and ADHD (Gaillard, 2000, Gur and Gur, 2010, Pugh et al., 2000, Vaidya et al., 1998).
It is important to note, however, that the conclusions drawn from developmental fMRI studies often rely on the assumption that the BOLD signal reflects the same set of processes in the developing brain as in the adult brain (despite neuronal signalling changes as dramatic as GABA switching from being an excitatory transmitter in early development to being inhibitory later: see Section 7.3). This assumption is worth assessing critically because the BOLD signal is not a direct measure of any one component of neural activity, but in fact reflects the behaviour of several different brain processes, which all undergo massive developmental changes in the early years of life. For example, regional blood flow, the levels of neuronal and astrocytic enzymes that regulate blood vessel diameter, astrocyte morphology, and hence the coupling of neuronal activity to blood flow changes (neurovascular coupling), may all change alongside neural activity. These changes in neurovascular coupling mechanisms (and also in cellular energy use) may compromise the power of BOLD imaging to detect “real” developmental changes in neuronal information processing, possibly producing changes of BOLD signal with development when there is no change in neuronal processing (false positives), or obscuring BOLD signal changes that would otherwise occur as neuronal signalling changes with age (false negatives). An extreme example of such interpretional problems, discussed in detail later (Section 6), is that sensory stimulation evokes a “normal” positive BOLD signal in neonates and adults, but a negative BOLD signal in infants and young children, implying that there are two transitional ages at which no BOLD signal is produced by neural activity. For robust interpretation of developmental fMRI data, therefore, it is crucially important to understand in as much detail as possible the developmental changes of brain processes other than neuronal activity which may influence the BOLD signal.
Here, we review the physiological changes observed in neuronal and vascular networks in the developing brain, with a particular focus on those features which may alter the BOLD signal with age. We then discuss which factors are primarily responsible for changes in the BOLD signal during development, and outline the main considerations that should be taken into account when interpreting the results of developmental fMRI studies.
2. How is the BOLD signal generated?
To understand the factors that may affect the BOLD signal during development, we must first understand how it is generated. Essentially, by monitoring local increases in cerebral blood flow (CBF) that are associated with neural activity, the BOLD signal provides an indirect measure of brain activity with reasonably high spatial precision (1.5–3 mm). As outlined in Fig. 1, the method depends critically on the relationship between neural activity and CBF, which was first noted over 100 years ago, but is now much better characterized (Mosso, 1880, Roy and Sherrington, 1890; early studies are reviewed by Iadecola, 2004). Neural activity leads to ATP expenditure, largely on the pumping out from neurons of ions which enter to generate synaptic and action potentials (Attwell and Iadecola, 2002). This evokes an increase in oxygen consumption to regenerate ATP which, within a few seconds, is followed by a disproportionate increase of blood flow to the active area. Associated with these local changes in oxygen consumption and blood supply, there are changes in the local oxygen concentration (which initially falls due to consumption, and then rises above its baseline value when the increased blood flow brings in more oxygen (Offenhauser et al., 2005, Enager et al., 2009)) and, concomitantly, changes in the levels of oxygenated and deoxygenated haemoglobin.
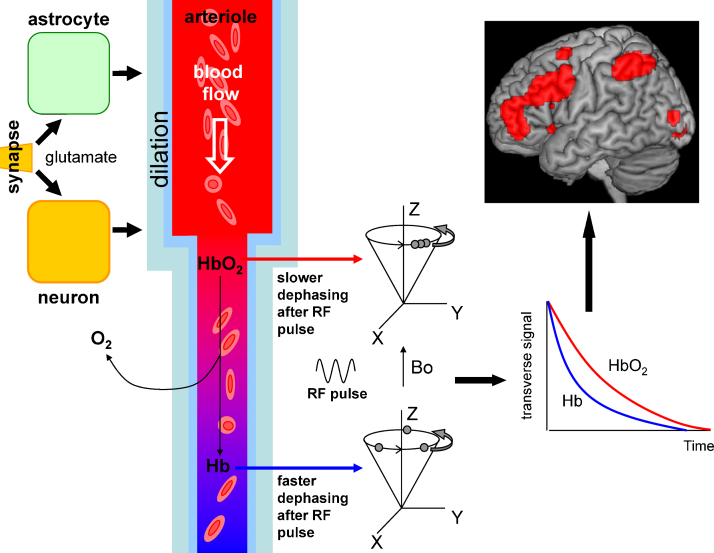
Fig. 1.
Schematic diagram showing the different stages of how BOLD signals are generated, from neurobiology through physics to data analysis. On the left, neuronal activity releases transmitters (glutamate) which act via neuronal and astrocytic signalling systems to trigger an increase of local blood flow. Neuronal activity also leads to O2 consumption and generation of paramagnetic deoxygenated haemoglobin (Hb) from diamagnetic oxygenated haemoglobin (HbO2). The blood flow increase brings in fresh oxygenated blood which (in adults) lowers the local concentration of Hb. This decreases the non-homogenizing effect that Hb has on the local magnetic field which protons in H2O experience. As a result, after a radiofrequency (RF) pulse is applied transverse to the magnetic field used to align the proton spins (Bo), the synchronised spin precession in the transverse plane dephases more slowly (graph on right). The difference in decay time between the red (HbO2) and blue (Hb) curves in the graph generates the increased MRI signal from protons in areas where neurons are active, which is represented as the red spots superimposed on a structural image of the brain at the top right.
Through their different magnetic properties (Pauling and Coryell, 1936), these molecules provide the basis of the BOLD signal (Ogawa et al., 1990). During the initial increase in oxygen consumption, oxygen is taken from diamagnetic oxyhaemoglobin, leaving behind paramagnetic deoxyhaemoglobin, which makes the magnetic field experienced by protons in surrounding water molecules less homogenous and thus decreases the magnetic resonance signal from these protons. During the subsequent increase of blood flow (which in fact partly overlaps with the increase of O2 consumption), the initial tendency to an increase in deoxyhaemoglobin level is converted to a decrease, by the delivery of fresh oxygenated blood, thus increasing the magnetic resonance signal (Fig. 1). It is this delayed decrease of deoxyhaemoglobin level, reflecting a local increase in blood flow, that dominates the (positive) fMRI signal detected in most BOLD imaging experiments (there is also a contribution to the signal from a local increase in blood volume which we ignore for simplicity as it is probably driven passively (Hoge et al., 1999) by the increase in flow). Thus, the size of the BOLD fMRI signal is determined by the difference between the amount of blood flow increase, which increases the signal, and the use of O2 by neurons, which reduces the signal.
It is important to note that the BOLD signal cannot be viewed as a direct read-out of the metabolic demand associated with activity in the tissue (indeed, as noted above, O2 consumption decreases the BOLD signal). In particular, BOLD signals need not directly report spiking activity in the imaged area, but instead reflect the many factors associated with neural activity which lead to an increase in blood flow. Most importantly, neurotransmitters released during synaptic activation are now known to directly influence local blood flow and it is thought that the BOLD signal may most closely reflect the excitatory synaptic component, rather than the action potential component, of neural activity (reviewed by Attwell and Iadecola, 2002, Logothetis, 2008, Attwell et al., 2010).
Critically for developmental studies, the synaptic architecture and signalling pathways that control blood flow change with age, implying that the relationship between neural activity and blood flow is likely to change as well. In addition, the O2 use associated with neural activity, which decreases the positive BOLD signal, is also likely to change with development. Finally, vascular networks themselves change with age, directly altering the way in which blood flow responds to neural activity, and potentially altering the signal to noise ratio of the BOLD signal, which increases with the fraction of the tissue that is occupied by blood (Ogawa et al., 1993, Bandettini and Wong, 1997, Mandeville and Marota, 1999, Buxton et al., 2004). Thus, in addition to developmental changes in neural information processing, these changes of neurovascular coupling will alter the BOLD signal at different ages.
3. Neurovascular coupling
Neurovascular coupling is the mechanism by which neuronal activity produces an increase of local blood flow, termed functional hyperaemia. Roy and Sherrington (1890) postulated that chemical products of metabolism affected the vessel wall, altering the vessel calibre, and it is often assumed that functional hyperaemia serves to adjust the blood flow to meet energy needs. Accumulation of the metabolic products adenosine and lactate does contribute to activity-induced blood flow increases (Gordon et al., 2008, Ko et al., 1990), and metabolic CO2 generation may promote blood flow after conversion to H+ by carbonic anhydrase (Colonnese et al., 2008). However, the idea that a metabolic need signal, such as a fall of oxygen or glucose concentration, is the main trigger of increased blood flow in response to neuronal activity has been superseded with the discovery that neurotransmitter mediated pathways alter blood flow (Akgören et al., 1994, Li and Iadecola, 1994, Zonta et al., 2003; reviewed by Attwell et al., 2010), and that block of these pathways greatly reduces functional hyperaemia without affecting energy use by the tissue (Leithner et al., 2010, Offenhauser et al., 2005, St Lawrence et al., 2003).
The supply of oxygen and glucose to neurons occurs mainly across the walls of capillaries in the brain parenchyma (but with some oxygen passing across arteriolar walls: Yaseen et al., 2011, Kasischke et al., 2011). The amount of blood flow providing these nutrients is determined by the contractile tone of smooth muscle cells around pre-capillary arterioles (Fig. 2), which controls the diameter of the vessels and hence the resistance to blood flow. Although the basal tone setting the resting blood flow is, in part, set by mediators released from endothelial cells lining the blood vessels, changes in blood flow in response to neuronal activity are evoked by events in neurons and astrocytes situated in close proximity to the vasculature. In brief, the release of neurotransmitters from active neurons generates neuron- and astrocyte-derived messengers (Fig. 2) which either modulate ion channel activity in the smooth muscle, leading to a change of intracellular calcium concentration ([Ca2+]i), or change the calcium-sensitivity of the contractile apparatus, both of which lead to altered contraction (reviewed by Attwell et al., 2010, Iadecola, 2004, Koehler et al., 2009). Developmental changes in the strength of these signalling pathways can therefore alter the relationship between neuronal activity and the blood flow response that generates BOLD signals. The remainder of this section reviews in more detail the main signalling pathways underlying neurovascular coupling, as a basis for understanding how developmental changes in components of these pathways may contribute to changes in the BOLD signal during development.
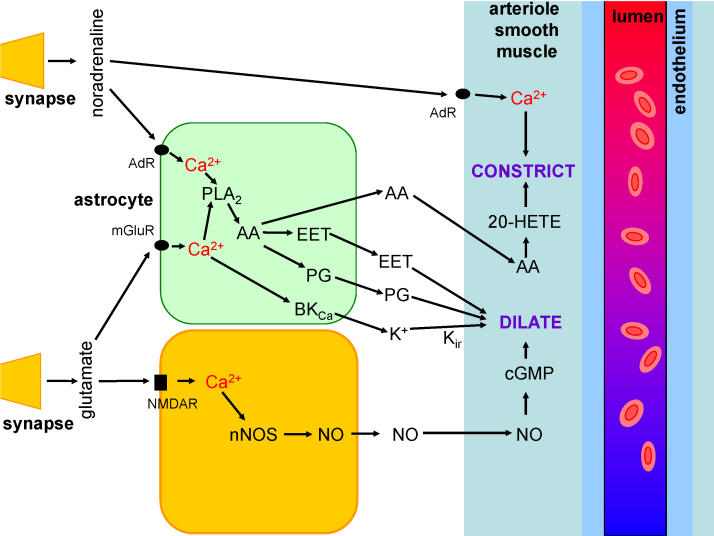
Fig. 2.
Signalling pathways mediating neurovascular coupling. When neurons are active they release glutamate which generates action potentials in interneurons containing NOS and activates metabotropic glutamate receptors (mGluR) in astrocytes. As a result [Ca2+]i rises in both cell types, releasing NO and derivatives of arachidonic acid to dilate local arterioles and increase blood flow. In addition arachidonic acid diffuses to arterioles and is converted into 20-HETE which constricts the vessels. K+ release from astrocytes via large conductance Ca2+-activated K+ channels (BKCa channels) may also dilate vessels by promoting K+ efflux through smooth muscle inward rectifier K+ channels (Kir) and thus generating hyperpolarization. Blood flow changes evoked by release of adenosine, lactate and interneuron peptide transmitters are not shown on this diagram. Vasodilation occurs in the context of constriction of arterioles produced by the amine transmitters noradrenaline, dopamine and 5-HT. Noradrenaline evokes constriction by acting on α-adrenergic receptors on arteriole smooth muscle and on astrocytes. Developmental changes in any of the components will alter the BOLD response.
3.1. Neurovascular coupling via neuronal nitric oxide
Neuronal activity can dilate blood vessels as a result of synaptically released glutamate binding to NMDA (N-methyl-d-aspartate) receptors on neurons, causing a calcium influx which activates neuronal nitric oxide synthase (nNOS) (Garthwaite et al., 1988). Nitric oxide then diffuses to the vasculature where it produces vessel dilation (Fig. 2; Busija et al., 2007). Nitric oxide acts as a direct mediator of neuronally evoked blood flow responses in the cerebellum (Akgören et al., 1996) whereas in the cerebral cortex, although it is necessary for NO to be present in order for neural activity induced dilation to occur, changes of the level of NO are not the primary mediator of vasodilation (Lindauer et al., 1999). In the cortex nNOS is often found in GABAergic inhibitory interneurons near blood vessels (for review see Estrada and DeFelipe, 1998, Hamel, 2006), which can also express other vasoconstrictive mediators such as neuropeptide Y and somatostatin (Cauli et al., 2004). Other interneurons, which lack nNOS (Estrada and DeFelipe, 1998), may release vasoactive intestinal peptide which mediates dilation. In human cortex, and thus potentially relevant to BOLD studies, in addition to being present in interneurons, nNOS is also expressed by a subpopulation of excitatory pyramidal cells (Judas et al., 1999, Wallace et al., 1995), and it has also been reported in excitatory pyramidal cells of the rat hippocampus (Burette et al., 2002).
3.2. Neurovascular coupling via astrocytes
Astrocytes are large glial cells which extend many processes that envelope surrounding synapses. They show a rise of intracellular calcium concentration in response to synaptic activity, as a result of metabotropic glutamate receptors being activated by synaptic glutamate release (Porter and McCarthy, 1996). In humans, an individual astrocyte may be able to sense the activity at ∼2 million synapses (Oberheim et al., 2006). Astrocytes also form perivascular endfeet on blood vessels (Simard et al., 2003), and so have an anatomy suitable for signalling from neurons to arterioles to control blood flow. Calcium concentration rises in astrocytes can lead to arteriole dilation (Gordon et al., 2008, Takano et al., 2006, Zonta et al., 2003). This occurs mainly as a result of calcium activating the enzyme phospholipase A2 (Fig. 2) to generate arachidonic acid and its vasoactive metabolites, which include prostaglandins, epoxyeicosatrienoic acids (EETs) and 20-hydroxyarachidonic acid (20-HETE). Because developmental changes in the levels of the enzymes synthesizing these derivatives, or in their inactivation mechanisms, may contribute to changes in BOLD signals through development, we will briefly review their actions.
Prostaglandins are produced from arachidonic acid by cyclooxygenase (COX) enzymes, both forms of which (COX1 in astrocytes and COX2 in astrocytes and neurons) are involved in blood flow control (Niwa et al., 2000, Takano et al., 2006). COX initially produces prostaglandin H2 which is then converted to the vasodilator prostaglandin E2 by PGE synthase. PGE2 relaxes smooth muscle by activating cyclic AMP production (Davis et al., 2004), ultimately resulting in decreased myosin light chain phosphorylation (Conti and Adelstein, 1980). Prostaglandins also hyperpolarize arteriolar smooth muscle cells by activating calcium-dependent potassium channels (Serebryakov et al., 1994), reducing voltage-gated Ca2+ entry and thus decreasing contraction. The actions of prostaglandins are terminated by re-uptake into cells expressing the prostaglandin transporter.
EETs are produced in astrocytes from arachidonic acid by epoxygenases, a class of cytochrome P450 enzyme (Alkayed et al., 1996, Amruthesh et al., 1993). EETs can hyperpolarize and thus relax smooth muscle by activating a Ca2+ influx through TRPV4 channels which in turn activates large conductance (BKCa) Ca2+-activated K+ channels (Campbell et al., 1996, Earley et al., 2005). EETs may also cause relaxation by inhibiting vasoconstricting thromboxane A2 receptors (Behm et al., 2009). EETs are inactivated by soluble epoxide hydrolase which converts epoxides to their corresponding dihydroxyeicosatrienoic acids (Fang et al., 2001, Iliff et al., 2010, Spector et al., 2004), although EETs can also be inactivated by COX activity or by slow insertion into phospholipid stores (Bernstrom et al., 1992, Ellis et al., 1990).
Arachidonic acid formed in astrocytes can also diffuse to arteriolar smooth muscle and be converted into 20-HETE by the ω-hydroxylase enzyme, CYP4A (Gebremedhin et al., 1998, Metea and Newman, 2006, Mulligan and MacVicar, 2004). 20-HETE constricts smooth muscle by activating protein kinase C, inhibiting potassium channels and thus depolarizing the cell and increasing voltage-gated Ca2+ influx (Lange et al., 1997). Although this system alone constricts arterioles and reduces cerebral blood flow, it provides an extra mechanism for activity-evoked dilation which may contribute to the generation of BOLD signals: NO released by neural activity inhibits CYP4A and thus reduces 20-HETE mediated constriction (Roman, 2002). Activity-evoked [Ca2+]i rises can also increase blood flow by opening calcium-activated potassium channels in astrocytes. This leads to an increase in extracellular potassium concentration ([K+]o) which enhances the conductance of inward-rectifying K+ channels in smooth muscle, causing hyperpolarization and vessel dilation (Filosa et al., 2006).
3.3. Vasoconstriction by noradrenaline, dopamine and 5-HT
For the activity-evoked dilation of arteriolar smooth muscle described above to occur there has to be a tonic constriction of the arterioles, which is partly produced by aminergic input to the vessels. Within the brain parenchyma, arterioles and capillaries (which can also be constricted by isolated cells called pericytes (Peppiatt et al., 2006) although little is known about control of blood flow at the capillary level) are close to noradrenergic terminals of neurons from the locus coeruleus, which appear to release their transmitter close to astrocyte endfeet (Cohen et al., 1997, Paspalas and Papadopoulos, 1996). Noradrenaline constricts brain blood vessels (Raichle et al., 1975). In part this may be because it increases astrocytic [Ca2+]i by acting on α1 receptors (Bekar et al., 2008, Duffy and MacVicar, 1995) leading to vessel constriction, presumably via the 20-HETE pathway described above (Mulligan & MacVicar, 2004). Noradrenergic innervation can also directly affect vascular smooth muscle. Smooth muscle cells express both α1 and α2 receptors (Drew and Whiting, 1979) and activation of these receptors increases [Ca2+]i leading to vessel constriction (Vanhoutte and Rimele, 1982). Dopaminergic fibres from the ventral tegmental area and 5-HT-releasing axons from the raphe nucleus may also contribute to vasoconstriction (Cohen et al., 1996, Krimer et al., 1998). The arteriole tone set by the amount of aminergic input may affect how much blood flow increase is produced by the mainly glutamate-mediated signalling pathways which dilate vessels in response to synaptic activity. For example dilations evoked by raising [K+]o or by raising astrocyte [Ca2+]i pharmacologically are larger when the vessels are more preconstricted by a thromboxane agonist (Blanco et al., 2008). Thus, developmental changes in the amine systems may alter the tone of arterioles and lead to corresponding developmental changes in the blood flow increases, and BOLD signals, generated by a fixed amount of neuronal activity.
3.4. Implications
With this overview in mind of the mechanisms by which glutamate released by neural activity leads to increases of local blood flow, and hence to BOLD fMRI signals, we will now examine how both the brain's neural circuitry and its blood flow control mechanisms alter during brain development.
4. Changes in neural circuitry with development
As the brain develops, its processing capabilities become increasingly sophisticated through changes in several components of the neural circuitry. These include experience-dependent and -independent changes to synaptic architecture, transmitter signalling, cell numbers, temporal response characteristics and myelination. This section follows several of these changes from birth, through childhood and adolescence, to adulthood.
Inevitably, much of our knowledge of developmental changes in neural circuitry and in the systems mediating neurovascular coupling has come from animals (particularly rodents) rather than humans. In what follows, therefore, it is useful to bear in mind the following approximate correspondence between the stages of rodent and human brain development. The main period of corpus callosum myelination in rats and mice occurs from about postnatal day 7 (P7) to P35 (Hamano et al., 1998), corresponding to human ages from birth to ∼18 years (Lenroot and Giedd, 2006, Pujol et al., 1993). Human adolescence has been suggested to map onto rat ages P30–P42 (Spear and Brake, 1983), with the rat brain being regarded as adult by P60. As detailed data for developmental changes were not available for all the species from which data were taken for this review, and developmental comparisons may be complicated by interspecies differences in the order of developmental events, we have kept age classifications to the broad time windows of childhood, adolescence and adulthood.
4.1. Cortical thickness and wiring
One of the most remarkable developmental circuitry changes is apparent in the increase in thickness and then slower thinning of human cortical grey matter which occurs over the period from childhood through adolescence into early adulthood (Ostby et al., 2009, Paus, 2005, Shaw et al., 2008, Tamnes et al., 2010), which at least partly reflects the excess formation and then selective deletion of synapses (this is after the main period of neurogenesis and programmed cell death that occurs earlier in brain development: Chan et al., 2002). The initial phase of expansion–underpinned by synaptogenesis in parallel with dendritic growth (Rakic et al., 1994)—coincides with rapid changes in the functional properties of neurons and cortical circuitry (Khazipov et al., 2001). The slower thinning phase – underpinned by activity-dependent synaptic pruning (Bourgeois and Rakic, 1993, Huttenlocher, 1990) – allows for the deletion of unnecessary synapses, thereby increasing processing efficiency (Mimura et al., 2003).
While this general pattern is followed across cortical areas, the exact time-course of the expansion and thinning phases differs depending on the region, and appears closely related to the developmental timeline of the behaviour that the region subserves. Synaptogenesis in the human primary visual cortex, for example, begins in the foetus reaching a maximum at around 8 months postnatally, after which visual experience-dependent pruning decreases synaptic density to the adult level (about 60% of the maximum) by 11 years (Garey and de Courten, 1983). This timescale correlates with the period of visual plasticity in infant monkeys, and with the establishment of visual acuity, stereopsis and oculomotor function in humans (Garey and de Courten, 1983). In the human prefrontal cortex (PFC), on the other hand, grey matter density increases up until the onset of puberty and then decreases throughout adolescence and into early adulthood (Toga et al., 2006, Sowell et al., 2003). Over the period of grey matter reduction there is a marked behavioural maturation in the social skills that the PFC is considered responsible for, namely, an understanding of other people's intentions, beliefs and desires, and an ability to predict the behaviour of others (Blakemore, 2008).
Over the period of synaptic density changes, specific neuronal connections are developed and maintained. The determinants of which connections become stable are likely to vary depending on the function of the region, but one common principle is the wiring up of similarly tuned cells. This is clearly seen in topographic maps, where the spatial layout of sensory input or motor output varies systematically across a cortical region, and neurons that are tuned to the same spatial field have a higher likelihood of being connected (Alonso and Martinez, 1998, Hubel and Wiesel, 1963). The formation of these maps tends to rely both on an intrinsic organisation mechanism – the retinotectal map, for example, is patterned in the nasotemporal and dorsoventral planes from the earliest stages of tectal innervation (Holt and Harris, 1983) – and a component of experience-dependent development – proper binocular organisation in the visual cortex map, for example, depends on visual input from both eyes (Blakemore, 1979). In addition to sensory and motor regions, topographic maps have recently been found in human cortical areas associated with high order cognition: visual field topography has been observed in the parietal (Sereno et al., 2001) and frontal (Kastner et al., 2007) cortex, and may be important for spatial attention, working memory and decision making (Silver and Kastner, 2009). It is as yet unclear, however, on what time course these higher order maps develop and on what processes their development depends.
4.2. Inhibition
Another important principle of synaptic connectivity that develops early on is lateral inhibition, where the neighbours of excited neurons are inhibited by GABAergic interneurons, thus sharpening the neural response to a stimulus (Sillito, 1975, Crook et al., 1998, Gabernet et al., 2005). This effect requires inhibitory circuitry to develop alongside excitatory circuitry and, indeed, there is evidence that, within days of sensory experience, inhibitory responses to sensory stimulation become increasingly selective and correlated with excitatory responses (in rat auditory cortex: Dorrn et al., 2010; in mouse visual cortex: Gandhi et al., 2008). In addition to shaping receptive fields, the balance between excitation and inhibition becomes critical for regulating firing rates and information flow in the developing network (Akerman and Cline, 2007). To achieve this balance, in most brain regions different GABAergic interneuron classes appear at different times, which are related to critical periods in network development. In monkey visual cortex, for example, calbindin-containing interneurons appear early in development, correlated with the onset of the thalamocortical innervation, while parvalbumin-containing interneurons appear after birth, correlated with the onset of visually driven activity (Hendrickson et al., 1991). In humans the GABAergic system in visual cortex continues to mature well into adulthood (Pinto et al., 2010), undergoing three distinct developmental changes during childhood (when receptor subunits change), adolescence (when proteins mediating GABA synthesis increase in level while vesicular uptake falls) and adulthood (when GABA synthesis decreases and vesicular uptake increases).
The development of interneuron networks also plays a role in shaping the temporal characteristics of circuit-level activity. While early development is characterized by spontaneous bursts of synchronous activity which are thought to be critical for the activity-dependent wiring of similarly tuned cells (Garaschuk et al., 1998, Meister et al., 1991), later development is characterized by more oscillatory network-level activity, particularly in the gamma frequency range (30–100 Hz). Werkle-Bergner et al. (2009), for example, used EEG in humans to show that visually-evoked synchronous gamma oscillations developed by 11 years, but were not maximally sensitive to stimulus changes until adulthood. Gamma oscillations are thought to be generated by an interaction between stimulus-driven excitatory and inhibitory activity (Atallah and Scanziani, 2009, Ray and Maunsell, 2010), and therefore the integration of GABAergic interneurons into the excitatory circuitry may be important in the development of several behavioural functions. For example, gamma oscillations have been suggested as a solution to the binding problem (Singer and Gray, 1995; but see Ray and Maunsell, 2010), promoting integration of distributed information for its coherent processing and possibly contributing to the improving executive function seen throughout adolescence (Blakemore and Choudhury, 2006). Gamma oscillations may also have a direct role in learning and memory, as their synchronisation of synaptic inputs has been shown to directly enhance synaptic strength (König et al., 1995, Salinas and Sejnowski, 2001).
4.3. Myelination
Synaptic synchrony is also increased throughout development by myelination, which is now known to play a role in fine tuning the timing of spike arrival (Salami et al., 2003, Seidl et al., 2010) and thus increasing connection efficiency (Hagmann et al., 2010). The human corpus callosum, for example, undergoes an intricate myelination process throughout childhood and adolescence, with several thickness changes thought to result from varying the degree of myelination (Luders et al., 2010), possibly in order to fine tune input arrival times in cortical areas. A similar process of myelin maturation, involving changes in axonal diameter and myelin wrap number and density, occurs postnatally across the whole brain in a wave from posterior to anterior regions. The progression is slow, with the frontal lobes being the last to myelinate towards the end of adolescence (Fields, 2008) and the white matter not becoming fully mature until well into adulthood (20–30 years, Benes et al., 1994, Yakovlev and Lecours, 1967). One potential benefit of this slow progression is in maintaining white matter plasticity in regions that can be influenced by experience and learning, through activity-dependent effects on myelination (reviewed in Markham and Greenough, 2004, Ullen, 2009). For example, Bengtsson et al. (2005) used diffusion tensor imaging (DTI) to show that myelination in many regions, including the corticospinal tract (a white matter tract involved in voluntary skilled movements of distal limbs), is positively correlated with amount of piano practice in children. They also found a positive correlation between myelination and piano practice in adults, but mainly in corticocortical pathways and not in the now mature corticospinal tract.
4.4. Amine system development
Another relatively slow process is the maturation of amine neurotransmitter systems, which are thought to play key roles in cognitive development. The dopamine, serotonin and noradrenaline networks, which are closely involved in mood and attention, undergo large-scale changes at several developmental stages, from the infant to the ageing brain. Catecholamines and serotonin, for example, show early postnatal surges of development on different timescales (Lambe et al., 2000, Murrin et al., 2007), influencing the functional development of different cortical systems (Levitt et al., 1997). Broadly speaking, noradrenaline synthesis and endogenous concentration increases steadily throughout the brain until adulthood (Goldman-Rakic and Brown, 1982). Serotonergic development occurs more quickly, reaching mature levels of synthesis and concentration by childhood, with the largest percentage increases occurring in the parietal and occipital cortex (Goldman-Rakic and Brown, 1982). Dopamine synthesis and concentration, on the other hand, tends to peak during adolescence, and gradually decrease towards adulthood, remaining the highest in frontal regions (Goldman-Rakic and Brown, 1982). These changes are accompanied by dramatic changes in those behaviours that the dopamine system is thought to subserve, namely, reward-seeking and incentive-driven action, which also peak during adolescence (Wahlstrom et al., 2010).
4.5. Plasticity in the mature brain
Finally, even after these large-scale neural features have reached maturity, the brain retains a high level of plasticity. Synapses can alter their strength on the basis of activity, allowing us to learn and remember new things, throughout life. Entire cortical regions retain the capacity to reorganise themselves, and may do so in extreme circumstances, such as the loss of part of a limb (Merzenich and Jenkins, 1993).
4.6. Implications
The developmental neural circuitry changes discussed here (and schematized in Fig. 3), which can be closely linked to behavioural changes, are often the very neurophysiological features that we hope to track across ages with fMRI, and their particular effects on the BOLD signal are discussed in Section 7. However, less commonly considered are parallel developmental changes in the microvasculature and neurovascular coupling, which may have confounding age-related effects on the BOLD signal. It is therefore important to have a clear picture of how these components of the vascular system develop alongside the neural circuitry.
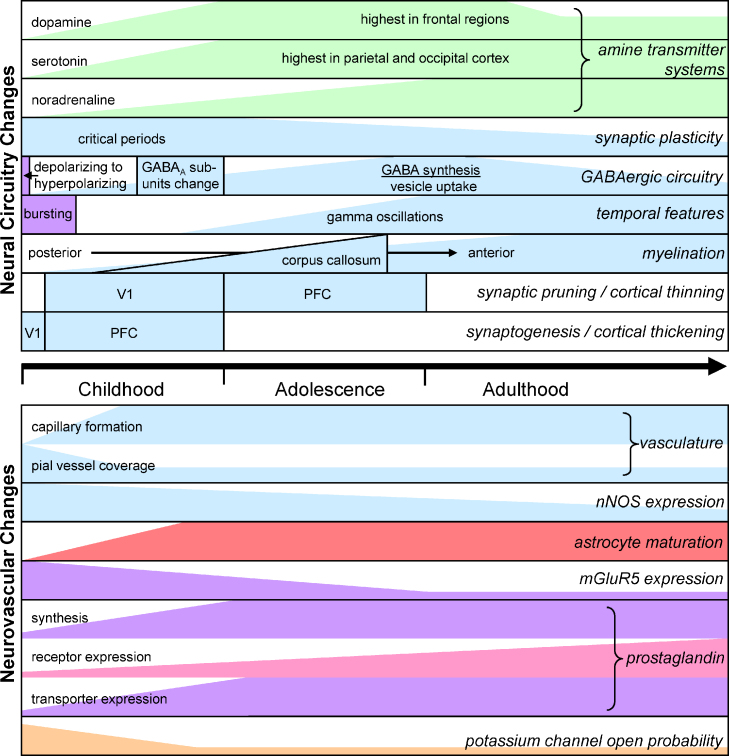
Fig. 3.
Summary of the developmental time courses of changes in neural information processing mechanisms (top) and of components of the signalling pathways regulating blood flow and thus controlling the BOLD response (bottom). Changes shown in blue are from human data; green is from macaque; lilac from rat or mouse; red from cat; pink from pig; light orange from rabbit.
5. Changes in neurovascular coupling with development
The relationship between neuronal activity and blood flow depends critically on the characteristics of the signalling pathways regulating blood flow. It not only varies between brain regions (Sloan et al., 2010), but can depend on which set of afferents to a brain area is activated. For example, even at a fixed developmental stage, Enager et al. (2009) found that the relationship between neural activity and blood flow response differed within the same small cortical area, the barrel field of the primary somatosensory cortex, depending on which set of synaptic inputs was activated. Stimulating at a low frequency evoked a larger blood flow response when stimulation was applied to the thalamocortical afferents than when applied to trans-callosal inputs from the contralateral cortex, whereas at high stimulation frequencies the relative influence of the two pathways was reversed. Thus neurovascular coupling exhibits pathway-specific signalling differences, possibly reflecting differences in the signalling molecules involved (Gotoh et al., 2001) or in the anatomical arrangement of the neurons (and astrocytes) relative to the blood vessels they control. These differences in the dependence of blood flow on neural activity even in the same cortical area and at the same developmental stage show that a constant relationship between BOLD signal and neural activity cannot be taken for granted as neurovascular coupling changes during brain development. This is reinforced by the fact that human regional cerebral blood flow at the age of 5–6 years is 50–85% higher than that in the adult or at birth (Chiron et al., 1992). During development the brain undergoes many changes which have the potential to alter neurovascular coupling, ranging from the development of the vasculature to changes of the expression of enzymes responsible for producing vasoactive mediators. This section will give an overview of the changes that occur in neurovascular coupling during development.
5.1. Vascular development
At birth vascular development is incomplete, and the vasculature matures as the brain develops. From newborn to adult, the primate cerebral vascular vessel volume increases 2.7-fold and the mean distance between any point in the tissue and the nearest vessel decreases by 32% (Risser et al., 2009). Similarly, in rat, the capillary volume fraction increases 3-fold between postnatal days 7 and 20 (corresponding in humans roughly to the period from birth to adolescence) and then decreases slowly to become approximately 30% less in adulthood (Keep & Jones, 1990). These changes in blood volume fraction will, other things being equal, produce proportional changes in BOLD signal with development (Ogawa et al., 1993, Bandettini and Wong, 1997; Mandeville & Marota, 1999; Buxton et al., 2004). Vessel growth results from a mismatch in microvascular supply and metabolic demand, so that areas that use more energy become more highly vascularized than less active areas (Riddle et al., 1993, Weber et al., 2008). In both the cerebral and the cerebellar cortices of rats, the vascular density increases through early development from the inside to the outside of the tissue (Conradi et al., 1980, Yu et al., 1994), while in cat visual cortex the rich capillary supply to the highly active layer IV is not present at birth but appears at 5 weeks postnatally (Tieman et al., 2004). Similarly, human cerebral cortical studies show a rapid increase in blood vessel density between either 26–35 gestational weeks (Mito et al., 1991) or post 36 gestational weeks (Miyawaki et al., 1998). In a study of foetal and juvenile vasculature, radially orientated vessels were seen at 15 gestational weeks, with lateral branching observed at 20–27 weeks (Norman and O’Kusky, 1986). Branching occurs at an earlier stage in the lower half of the cortex in keeping with the inside to outside pattern mentioned above. Capillary formation occurs after birth and is most prominent in vascular layer 3/neuronal laminae IV and Va (starting between term and 3 months postnatal: Norman and O’Kusky, 1986). Other post-birth changes include a decrease in pial vessel coverage of the brain's surface occurring over the first postnatal years (Norman and O’Kusky, 1986). During the developmental increases of blood vessel density in the brain parenchyma, neural activity presumably is not coupled to blood flow increases as efficiently as when the vasculature is fully developed.
5.2. Neuronal NOS development
Interneuron numbers and the vasoactive substances they produce show developmental changes, which will alter the neuronal pathway of neurovascular coupling (Fig. 2). In mice the number of nNOS containing interneurons in the mouse cerebral cortex decreases somewhat with age (Eto et al., 2010), with higher expression for the first two postnatal weeks and a decrease from four to eight weeks (i.e. through adolescence and into adulthood). There is disagreement over whether the number of nNOS expressing neurons then increases or decreases as animals become senile (Reuss et al., 2000, Sánchez-Zuriaga et al., 2007). The total level of nNOS (reflecting number of cells expressing NOS and expression level per cell) has also been studied in the first few weeks of rodent postnatal life (Ogilvie et al., 1995, Riobo et al., 2002). Cytoplasmic nNOS is expressed weakly at embryonic stages and increases in early development. The age at which the peak expression is reached varies between brain regions being, for example, P9 in the neocortex and P20 in the cerebellum. Expression then declines to an adult level, with the time taken for this process varying between regions, occurring between P12-15 in the neocortex and P20-60 in the cerebellum (Ogilvie et al., 1995). Studies of nNOS development in human cortex have focussed on foetal and adult expression, and information on juvenile and adolescent expression is lacking. Expression of nNOS in the human cerebral cortex has a similar distribution to that in the foetal brain but at a lower density: the density of nNOS expressing cells increases from 13 to 32 gestational weeks (GW) and then decreases (Ohyu and Takashima, 1998, Downen et al., 1999). Two types of nNOS positive interneuron are found in developmental studies (Estrada and DeFelipe, 1998, Ohyu and Takashima, 1998). Type 1 cells are large and intensely stained, appear at 15–18 GW (Ohyu and Takashima, 1998, Yan et al., 1996), and reach an adult distribution at 32 GW. Type 2 cells are smaller and more weakly stained, appear at 26–32 GW (Ohyu and Takashima, 1998, Yan et al., 1996), and increase in expression until term. The pattern of expression follows the inside to outside development mentioned above.
5.3. Astrocyte development
Astrocytes also show developmental changes in size and connectivity which may affect the size and spatial extent of blood flow responses caused by neuronal activity. Immunocytochemical study of cat visual cortex found that mature astrocytes only develop in the 3rd postnatal week, reaching adult density at the 4th week and then continuing to mature until 7 weeks after birth (Müller, 1992). Astrocytes increase in number and size during development, reaching their adult number by about P24 in rat hippocampus and P50 in rat cortex (Nixdorf-Bergweiler et al., 1994, Stichel et al., 1991). Changes in density and size are accompanied by changes in morphology, such as branching and orientation. Gap junctional coupling of astrocytes, which may enlarge the area over which neuronal activity can influence blood flow, develops by P11 in rat visual cortex, and remains stable throughout development (Binmöller and Müller, 1992), although before this stage astrocytes appear not to be coupled.
Developmental changes in the expression of the metabotropic glutamate receptors (mGluR5: Romano et al., 1995) which raise astrocyte [Ca2+]i in response to neuronal activity may alter the astrocytic pathway of neurovascular coupling. mGluR5 is highly expressed in the brain during early life (rat postnatal day 1) and decreases within the first postnatal weeks to an adult level between P30 and P60 (Catania et al., 1994). The decrease in expression differs between brain regions: mGluR5 expression in the hypothalamus decreases 6 fold, whereas in the cortex expression decreases by only 3 fold (van den Pol et al., 1995). These measurements include mGluR5 expressed on neurons, but suggest that there may be significant developmental changes in astrocyte signalling downstream of mGluR5 mediated by arachidonic acid derivatives, since mGluR5 expression correlates with the amount of phosphatidylinositol hydrolysis evoked by an mGluR agonist (Casabona et al., 1997). Prostaglandin signalling from astrocytes to blood vessels is probably altered during development. Prostaglandin synthesis in rat brain homogenates or induced by convulsions in in vivo brain is low at postnatal day 1 and increases strongly to adult levels in three weeks (Seregi et al., 1987), suggesting that prostaglandin induced dilations will increase with development. Similarly, expression of prostaglandin receptors is lower in newborn pigs than in adults (Li et al., 1995). However, the signalling consequences of upregulation of prostaglandin synthesis and receptor density with development are opposed, over the first postnatal month in mice, by an increase in expression of the transporter (PGT) which terminates prostaglandin signalling (Scafidi et al., 2007).
Astrocyte-mediated increases in blood flow may also be altered by developmental changes in the expression or properties of potassium channels, including the calcium-activated large conductance (BKCa) K+ channels expressed on astrocyte endfeet, as well as inward rectifying potassium channels on vascular smooth muscle (Filosa et al., 2006). For example, in rabbit Müller cells (astrocyte-like cells found in the retina), the open probability of BKCa channels strongly decreases within the early postnatal period, possibly due to the resting potential becoming more negative (Bringmann et al., 1999). As a result, to activate the channel in older cells requires both a strong depolarization and an increase in intracellular calcium, whereas in early postnatal cells only a small change in membrane potential is needed to activate the channels, suggesting that neurovascular coupling mediated by these channels might become less efficient with age.
5.4. Vasoconstricting pathways
All of these developmental changes in vasodilating pathways may be potentiated or opposed by corresponding effects on constricting pathways, including either the aminergic pathways that help to set the basal tone of the arterioles (Blanco et al., 2008) or the 20-HETE pathway which can constrict vessels in response to astrocyte [Ca2+]i rises (Mulligan and MacVicar, 2004).
5.5. Implications
The complex developmental changes in components of the signalling systems mediating neurovascular coupling that we have outlined above (and schematized in Fig. 3) do not provide a simple prediction for how neuronal activity evoked blood flow changes, and hence the BOLD response, will alter, suggesting that far more research is needed to understand this. However, they highlight the importance of considering how developmental changes in the BOLD signal may in some cases reflect factors other than neuronal activity.
6. Changes in neural energy use with development
In Section 2 we explained that the magnitude of the BOLD signal is affected by the O2 use of the area of brain being studied—the larger is the increase of O2 use evoked by neural activity (relative to the increase of blood flow) the smaller is the positive BOLD response. Although many factors affect the O2 use, most brain energy in primates is predicted to be used on synaptic transmission (Attwell and Laughlin, 2001), and this conclusion has been reinforced by the fact that action potentials are now thought to use 3-fold less energy than was originally believed (Alle et al., 2009). Thus, the neural activity evoked increase of O2 consumption is expected to increase with the increase of synaptic density that occurs over the first ∼10 years of life, and to decrease as synaptic pruning occurs later on. Indeed, O2 usage has been reported to rapidly increase with synapse development in children (Muramoto et al., 2002) and later to slowly decrease by ∼0.5% per year with ageing in the adult (Pantano et al., 1984, Leenders et al., 1990, Takada et al., 1992).
A greater fractional increase of neural activity-evoked energy (O2) use than of blood flow has been postulated to occur in infants and young children to explain why (in contrast to the situation in neonates and adults) over a few years of early development infants and young children show negative BOLD signals in response to sensory stimulation, i.e. a decrease of MRI signal rather than the usual increase (Anderson et al., 2001, Born et al., 1998, Muramoto et al., 2002, Yamada et al., 1997). Of concern for the interpretation of developmental changes in BOLD signals, the transition from a negative BOLD signal (in infants, when the task-evoked increase in O2 use outweighs the increase in blood flow) to a positive BOLD signal (when, as is usual in adults, the increase of blood flow outweighs the increase in O2 consumption) implies that at some intermediate developmental stage it is possible for an active area to show no BOLD response. This would occur if the increase of O2 use and the increase of blood flow cancel out each other's effects on the deoxyhaemoglobin level. For a study starting from this (young) age, the developmental increase in task-evoked blood flow increase (relative to that of O2 use) would result in the appearance of activation in an area that previously did not show it, even if that area was equally involved in processing the information at all ages.
7. How do developmental changes of neural circuitry and of neurovascular coupling combine to produce developmental changes in BOLD signals?
Many of the neural changes that underpin behavioural changes during development are expected to have clear effects on the BOLD signal, making their detection possible with fMRI. It would be ideal if this notion could be reversed, so that changes in BOLD signals could be assumed to reflect a change in the underlying neuronal processing of information. Conclusions based on BOLD data, however, depend critically on the usually unstated assumption that there are no confounding changes in the neurovascular coupling that generates the BOLD signal. Given the vast array of developmental changes in the mechanisms mediating neurovascular coupling, which we have described above, it would be extremely surprising if they did not contribute significantly to at least some of the developmental changes in BOLD signals that have been observed experimentally.
Developmental changes in BOLD signals include those showing the appearance at one developmental stage of a BOLD response in an anatomical region where it does not occur at another developmental stage (e.g. Blakemore et al., 2007, Monk et al., 2003, Passarotti et al., 2003), a change in the amplitude of the BOLD signal at one anatomical location (e.g. Casey et al., 1995, Durston et al., 2006, Keulers et al., 2011, Kwon et al., 2002, Maril et al., 2010, Monk et al., 2003, Turkeltaub et al., 2008, Wang et al., 2006), or an expansion or contraction of the spatial area activated within one anatomical region (e.g. Casey et al., 2002, Durston et al., 2006, Gaillard et al., 2000, Gaillard et al., 2003, Golarai et al., 2007, Kwon et al., 2002, Passarotti et al., 2003). We will now consider the possible problems of interpreting each of these kinds of change.
7.1. Alterations of activation locus
In general, a movement of the locus of BOLD activation is interpreted as the involvement of a new area for processing of information to perform a task, as the neural wiring progresses and regional specialisation develops. For example, tasks taxing social cognition start to activate the right superior temporal sulcus more (and the prefrontal cortex less) as development proceeds (Blakemore et al., 2007), suggesting a change in the neural strategies underpinning social cognition. Similarly, Monk et al. (2003) found a loss of the amygdala response to viewing fearful faces on going from adolescence to adult, and Holland et al. (2001) found increased left hemisperic lateralization of responses with age during a verb generation task. Alteration of the anatomical location of an activated area is the developmental change in BOLD response that is most likely to unequivocally reflect changes occurring in the underlying neuronal information processing (but see Section 6 for a scenario where activity could evoke no BOLD response at a young age and a positive BOLD response in the adult, as a result of a decrease with development of the ratio of O2 use evoked by activity to blood flow increase evoked by activity).
7.2. Alterations of BOLD amplitude at one anatomical location
Increases or decreases in BOLD signal amplitude with development are usually interpreted as indicating more or less involvement of neuronal activity in the activated area in the task being performed. For this conclusion to be secure, however, it is necessary to be sure that the altered amplitude does not simply reflect a change in blood volume fraction (see Section 5.1) or in the strength of neurovascular coupling, for example producing a larger increase of blood flow for the same amount of neural activity and energy use, and thus producing a larger BOLD signal.
In rats, BOLD signals increase in amplitude and decrease in latency with development (Colonnese et al., 2008) and some human studies also report a shorter latency for BOLD signals in adults than in children (Brauer et al., 2008; but see Richter and Richter, 2003). This might be expected from many of the developmental changes in the neural circuitry summarized in Section 4, e.g. the development and refinement of synaptic connections leading to more effective excitation in adults (indeed Colonnese et al. (2008) found that neural excitation occurred with a decreased latency at older ages), increased myelination leading to more synchrony of incoming synaptic activation (Fornari et al., 2007, Olesen et al., 2003), and perhaps a greater influence of attention mediated by amine transmitter systems. However, the many developmental changes in neurovascular coupling noted in Section 5 are also likely to contribute to developmental alterations of BOLD signal amplitude and latency.
First, as noted in Section 6, a developmental increase in the amplitude of the positive BOLD signal can result from the activity-evoked blood flow increasing more with age than does the activity-evoked O2 use, for example as a result of the developmental maturation of the systems mediating neurovascular coupling. Complicating this, however, the extra blood flow and O2 use evoked by neural activity may have different dependencies on the amount of neural activity occurring, as seen in visual cortex where increasing the number of active neurons apparently increases O2 use more than it increases blood flow, resulting in an unchanged or even a smaller BOLD signal (Goodyear and Menon, 1998, Marcar et al., 2004a, Marcar et al., 2004b). This implies that the age-related changes of BOLD signal may depend critically on the amount of neural activity produced by the task.
Second, the increase of vascular volume fraction that develops with age (Keep and Jones, 1990, Risser et al., 2009) is expected to increase the BOLD signal (Ogawa et al., 1993, Bandettini and Wong, 1997; Mandeville & Marota, 1999; Buxton et al., 2004), all other factors being equal. On the other hand, the increase in vascular density may lead to a decrease in the fall of deoxyhaemoglobin level occurring during activity and thus decrease the BOLD signal, as suggested by Marcar et al. (2004b) when comparing the more highly vascularized visual cortical area V1 with V2 (although differences in the information processing occurring in V1 and V2 could also contribute to the differences observed). An increased vascular density, perhaps decreasing the distance that neurovascular messengers must diffuse to increase blood flow, may also explain the decrease of BOLD latency with development (which is too large to be accounted for by alterations of the onset of neural activity: Colonnese et al., 2008).
Third, developmental alterations of the basal tone provided by amine transmitter systems may alter the blood flow response to neural activity, and thus alter the BOLD response, even in the absence of changes of neuronal information processing. However, it is currently unclear exactly how an increased baseline vasoconstriction might alter the BOLD signal. One possibility is that an overall lowering of blood flow and volume would simply reduce the BOLD signal. On the other hand, tighter vasoconstriction might increase the amount by which a vessel can dilate in response to glutamatergic transmission, thereby increasing activity-related blood flow responses and BOLD signals (Blanco et al., 2008). Consistent with this, dilation of blood vessels with CO2 to increase basal blood flow has been shown to decrease the BOLD response (Cohen et al., 2002) and also alters the apparent area of activation by altering the signal to noise ratio (Thomason et al., 2005). Of course developmental changes in amine systems will also produce changes in neuronal function, for example dopamine system development between childhood and adulthood can directly modulate the excitability of interneurons in the prefrontal cortex (Tseng and O’Donnell, 2007). The challenge, as with “pharmacological fMRI” of adults in which amine systems are manipulated with drugs (Attwell and Iadecola, 2002), is to separate amine effects on neuronal function from effects on neurovascular coupling.
7.3. Expansion or contraction of the spatial area activated within one anatomical region
Changes in the area showing BOLD activation within an anatomical region are often used to infer changes in the underlying neuronal processing. An expansion of the activated area of V1 after trace (but not after delay) eyeblink conditioning of rabbits (Miller et al., 2008) was interpreted as showing that more neurons were activated after learning to carry out the more cognitively complex task. In humans, expansion of activated somatosensory and motor areas over a three-week period of motor skill acquisition was considered to reflect the cortical plasticity that underpinned performance improvement (Karni et al., 1998, Hluštík et al., 2004). Importantly, while Miller et al.'s work studied relatively rapid changes in synaptic strength, allowing unequivocal conclusions to be reached, for longer-term learning and developmental studies over greater time durations changes in neurovascular coupling need to be ruled out as a cause of the activated area altering in size. In addition, because changes of the amplitude of the BOLD signal also commonly occur during development, it is essential to consider the possibility that the activated area merely appears to change in size because of an altered signal to noise ratio.
Changes in the spatial scale of neurovascular coupling may come about as a consequence of the developmental changes described in Section 5. Alterations of enzyme levels (see Section 3) will change the spatial area over which levels of neurovascular messengers are produced at a high enough concentration to affect blood vessels, and the development of the astrocyte network (Binmöller and Müller, 1992) may allow neurons to influence more distant vessels. Furthermore, the development of the vascular network itself may either provide more spatially localized control of blood flow (due to local control of individual vessels developing within a network which has increased in vessel density) or allow activity in one area to influence blood flow over a larger area (if vascular dilatory responses propagate back to larger vessels which feed a larger volume of brain: Iadecola et al., 1997). However, whereas the expansion of the activated area reported by Miller et al. (2008) occurred on a millimetre scale, neurovascular coupling changes seem more likely to alter blood flow on a scale of a few hundred microns.
Frequently, brain development is associated with a decrease in the area activated in BOLD studies, presumably correlating with the refinement of cortical wiring being accompanied by a shift from spatially diffuse brain activity to more focal brain activity, as regional specialisation emerges. For example, Casey et al. (2002) in the striatum and hippocampus, and Gaillard et al. (2000) and Passarotti et al. (2003) in the cortex, using stimulus-response compatibility, verbal fluency, and face matching tasks, respectively, found that progression from childhood to adulthood was accompanied by a shift from diffuse to more focal activation. This supports the idea that, before precise synaptic pruning, the less efficient connections that exist result in more widespread activity in response to tasks that later activate only highly specialised brain areas.
Part of the increasing spatial localization of BOLD signals that occurs in such studies may reflect the development of inhibitory circuits. GABAergic signalling is thought to decrease the BOLD signal by inhibiting excitatory cells, thus reducing glutamate release and decreasing the local CBF response (Lauritzen, 2005, Lauritzen and Gold, 2003, Muthukumaraswamy et al., 2009, Donahue et al., 2010). This may enhance the spatial localization of positive BOLD responses by generating an area of decreased neuronal firing and decreased blood flow around a central area of increased cell firing (Devor et al., 2007). This development of inhibitory responses may also contribute to sparser stimulus-evoked activation. In adult monkeys, for example, visual object learning is associated with more selective object representation in the inferior temporal cortex (Baker et al., 2002, Sigala and Logothetis, 2002), and in humans, the fusiform face area (FFA) has been shown to respond to faces with increasing selectivity across development from childhood to adulthood (Peelen et al., 2009). Similarly, the spatial receptive field organisation in topographically mapped regions becomes increasingly refined, and blocking inhibition removes this specificity (Sillito, 1975, Crook et al., 1998). As noted in Section 3, some inhibitory interneurons release nitric oxide. Thus, in addition to indirectly reducing the BOLD signal by inhibiting glutamatergic neurons, interneuron activity can influence the BOLD signal through the release of NO and other vasoactive agents which directly regulate the local blood flow.
In early development, GABA is not inhibitory but produces depolarizing potentials, perhaps providing an excitatory drive for synapse formation before glutamate takes over (Ben-Ari et al., 1989, Ben-Ari, 2002, Cherubini et al., 1991, Tyzio et al., 2011; but see Rheims et al., 2009). This occurs because at young ages the Nernst potential for the Cl− ions that pass through GABAA receptors is more positive than the resting potential. The reduction and spatial sharpening of the BOLD signal by inhibitory activity may, therefore, not be in effect until glutamate and GABA are well established in their mature roles as excitatory and inhibitory neurotransmitters. Once GABA is inhibitory, increases in the strength of GABAergic inhibition may decrease the size of the BOLD signal (Muthukumaraswamy et al., 2009, Donahue et al., 2010), thus decreasing the signal to noise ratio and possibly decreasing the area apparently activated, or even induce negative BOLD signals (Northoff et al., 2007).
8. Can neurovascular coupling changes ever be ruled out as a cause of developmental BOLD changes?
The main message of this review is that developmental changes in neurovascular coupling are likely to contribute to observed developmental changes in BOLD signals. How, then, can the BOLD researcher reliably attribute changes in BOLD signals to a change in neural information processing rather than a change of blood flow control? While there is no simple answer to this question, we believe there are three approaches to the problem.
First, being aware (for example from animal work) of the changes of neurovascular coupling and blood volume fraction that are occurring in the region of interest over the period under consideration, it may be possible to rule them out as a cause of the changes seen. For example, changes in the spatial scale of BOLD responses may be larger than is plausible to be explained by changes in astrocyte morphology or blood vessel branching patterns, as outlined above. Similarly, changes that occur rapidly as a result of learning, at a time after the enzyme systems controlling blood flow have matured, are unlikely to reflect alterations of neurovascular coupling or blood volume fraction. However, it is always possible that studies which document a change during development in the amplitude of the BOLD signal in a particular area may be confounded by developmental changes of neurovascular signalling mechanisms (or neuronal energy use) that have yet to be discovered by researchers working at the cellular level. Measuring how development alters some parameter of the stimulus that evokes a BOLD response may suggest an explanation in terms of changes of neural information processing (e.g. an increase in a parameter describing the specificity of a stimulus may be interpreted in terms of increasing lateral inhibition between cortical columns or between different cortical areas), but it is still necessary to consider whether non-linear changes in the relationship between neuronal activity and blood flow could account for the data. It is not obvious how any kind of subtractive protocol design can get around the need to consider possible confounding by changes of neurovascular coupling.
Second, detailed information on the changes of neurovascular coupling and of neural energy use occurring over the period of a study will often not be available, particularly for humans. In this case a functional approach needs to be taken. Church et al. (2010) have put forward the notion that neurovascular coupling changes cannot be the cause of changes of BOLD signal during development if different tasks produce opposing changes with development in the same area (i.e. one task produces an increase in response with development, while the other task produces a decrease with development). This is a promising approach, but one needs to be sure that the same input connections to the area studied are activated for both tasks. As noted in Section 5, the relationship between blood flow and neuronal activity is different for different inputs to cortical regions (Enager et al., 2009), and so may undergo different developmental changes.
Thirdly, it may be advantageous to document, and try to understand, developmental BOLD changes in simpler “low level” neural circuits, such as the visual, somatosensory and auditory cortices, the wiring and function of which are reasonably well known, in order to have a better framework for interpreting changes of BOLD signals in “higher” areas where the neural basis of the information processing is far more poorly understood. We therefore advocate the construction of a publicly accessible database as a repository in which to deposit atlases of BOLD responses to standard stimuli, recorded in a standardised imaging setting at key developmental stages. The stimuli used could include stationary and drifting gratings or random moving dots for the visual system, vibration to the skin for the somatosensory system, and tone sequences for the auditory system (some stimuli for which BOLD signals in sensory cortices are reasonably well understood in terms of the responses of neurons are collected by Schölvinck et al., 2008).
Ultimately, a full understanding of developmental changes in BOLD responses is likely to require much work characterizing how neurovascular coupling alters, in order to isolate changes produced by the maturation of neural information processing. Being aware of this issue is crucial for the rigorous interpretation of developmental fMRI studies.
Acknowledgements
This work was supported by grants from the Fondation Leducq, European Research Council, Wellcome Trust and Medical Research Council. We thank Geraint Rees for comments on the manuscript, and Iroise Dumontheil, Rachael Houlton, Kalina Christoff & Sarah-Jayne Blakemore for the BOLD image in Fig. 1.
References
- Akerman C.J., Cline H.T. Refining the roles of GABAergic signaling during neural circuit formation. Trends Neurosci. 2007;30:382–389. doi: 10.1016/j.tins.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Akgören N., Fabricius M., Lauritzen M. Importance of nitric oxide for local increases of blood flow in rat cerebellar cortex during electrical stimulation. Proc. Natl. Acad. Sci. USA. 1994;91:5903–5907. doi: 10.1073/pnas.91.13.5903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akgören N., Dalgaard P., Lauritzen M. Cerebral blood flow increases evoked by electrical stimulation of rat cerebellar cortex: relation to excitatory synaptic activity and nitric oxide synthesis. Brain Res. 1996;710:204–214. doi: 10.1016/0006-8993(95)01354-7. [DOI] [PubMed] [Google Scholar]
- Alkayed N.J., Narayanan J., Gebremedhin D., Medhora M., Roman R.J., Harder D.R. Molecular characterization of an arachidonic acid epoxygenase in rat brain astrocytes. Stroke. 1996;27:971–979. doi: 10.1161/01.str.27.5.971. [DOI] [PubMed] [Google Scholar]
- Alle H., Roth A., Geiger J.R. Energy-efficient action potentials in hippocampal mossy fibers. Science. 2009;325:1405–1408. doi: 10.1126/science.1174331. [DOI] [PubMed] [Google Scholar]
- Alonso J.M., Martinez L.M. Functional connectivity between simple cells and complex cells in cat striate cortex. Nat. Neurosci. 1998;1:395–403. doi: 10.1038/1609. [DOI] [PubMed] [Google Scholar]
- Amruthesh S.C., Boerschel M.F., McKinney J.S., Willoughby K.A., Ellis E.F. Metabolism of arachidonic acid to epoxyeicosatrienoic acids, hydroxyeicosatetraenoic acids, and prostaglandins in cultured rat hippocampal astrocytes. J. Neurochem. 1993;61:150–159. doi: 10.1111/j.1471-4159.1993.tb03550.x. [DOI] [PubMed] [Google Scholar]
- Anderson A.W., Marois R., Colson E.R., Peterson B.S., Duncan C.C., Ehrenkranz R.A., Schneider K.C., Gore J.C., Ment L.R. Neonatal auditory activation detected by functional magnetic resonance imaging. Magn. Reson. Imaging. 2001;19:1–5. doi: 10.1016/s0730-725x(00)00231-9. [DOI] [PubMed] [Google Scholar]
- Atallah B.V., Scanziani M. Instantaneous modulation of gamma oscillation frequency by balancing excitation with inhibition. Neuron. 2009;62:566–577. doi: 10.1016/j.neuron.2009.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attwell D., Buchan A.M., Charpak S., Lauritzen M., Macvicar B.A., Newman E.A. Glial and neuronal control of brain blood flow. Nature. 2010;468:232–243. doi: 10.1038/nature09613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attwell D., Iadecola C. The neural basis of functional brain imaging signals. Trends Neurosci. 2002;25:621–625. doi: 10.1016/s0166-2236(02)02264-6. [DOI] [PubMed] [Google Scholar]
- Attwell D., Laughlin S.B. An energy budget for signaling in the grey matter of the brain. J. Cereb. Blood Flow Metab. 2001;21:1133–1145. doi: 10.1097/00004647-200110000-00001. [DOI] [PubMed] [Google Scholar]
- Baird A.A., Gruber S.A., Fein D.A., Maas L.C., Steingard R.J., Renshaw P.F., Cohen B.M., Yurgelun-Todd D.A. Functional magnetic resonance imaging of facial affect recognition in children and adolescents. J. Am. Acad. Child Adolesc. Psychiatry. 1999;38:195–199. doi: 10.1097/00004583-199902000-00019. [DOI] [PubMed] [Google Scholar]
- Baker C.I., Behrmann M., Olson C.R. Impact of learning on representation of parts and wholes in monkey inferotemporal cortex. Nat. Neurosci. 2002;5:1210–1216. doi: 10.1038/nn960. [DOI] [PubMed] [Google Scholar]
- Bandettini P.A., Wong E.C. A hypercapnia-based normalization method for improved spatial localization of human brain activation with fMRI. NMR Biomed. 1997;10:197–203. doi: 10.1002/(sici)1099-1492(199706/08)10:4/5<197::aid-nbm466>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Behm D.J., Ogbonna A., Wu C., Burns-Kurtis C.L., Douglas S.A. Epoxyeicosatrienoic acids function as selective, endogenous antagonists of native thromboxane receptors: identification of a novel mechanism of vasodilation. J. Pharmacol. Exp. Ther. 2009;328:231–239. doi: 10.1124/jpet.108.145102. [DOI] [PubMed] [Google Scholar]
- Bekar L.K., He W., Nedergaard M. Locus coeruleus alpha-adrenergic-mediated activation of cortical astrocytes in vivo. Cereb. Cortex. 2008;18:2789–2795. doi: 10.1093/cercor/bhn040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ari Y. Excitatory actions of gaba during development: the nature of the nurture. Nat. Rev. Neurosci. 2002;3:728–739. doi: 10.1038/nrn920. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y., Cherubini E., Corradetti R., Gaiarsa J.L. Giant synaptic potentials in immature rat CA3 hippocampal neurones. J. Physiol. 1989;416:303–325. doi: 10.1113/jphysiol.1989.sp017762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benes F.M., Turtle M., Khan Y., Farol P. Myelination of a key relay zone in the hippocampal formation occurs in the human brain during childhood, adolescence, and adulthood. Arch. Gen. Psychiatry. 1994;51:477–484. doi: 10.1001/archpsyc.1994.03950060041004. [DOI] [PubMed] [Google Scholar]
- Bengtsson S.L., Nagy Z., Skare S., Forsman L., Forssberg H., Ullen F. Extensive piano practicing has regionally specific effects on white matter development. Nat. Neurosci. 2005;8:1148–1150. doi: 10.1038/nn1516. [DOI] [PubMed] [Google Scholar]
- Bernstrom K., Kayganich K., Murphy R.C., Fitzpatrick F.A. Incorporation and distribution of epoxyeicosatrienoic acids into cellular phospholipids. J. Biol. Chem. 1992;267:3686–3690. [PubMed] [Google Scholar]
- Binmöller F.J., Müller C.M. Postnatal development of dye-coupling among astrocytes in rat visual cortex. Glia. 1992;6:127–137. doi: 10.1002/glia.440060207. [DOI] [PubMed] [Google Scholar]
- Blakemore C. The development of stereoscopic mechanisms in the visual cortex of the cat. Proc. R. Soc. Lond. B: Biol. Sci. 1979;204:477–484. doi: 10.1098/rspb.1979.0041. [DOI] [PubMed] [Google Scholar]
- Blakemore S.J. The social brain in adolescence. Nat. Rev. Neurosci. 2008;9:267–277. doi: 10.1038/nrn2353. [DOI] [PubMed] [Google Scholar]
- Blakemore S.J., Choudhury S. Development of the adolescent brain: implications for executive function and social cognition. J. Child Psychol. Psychiatry. 2006;47:296–312. doi: 10.1111/j.1469-7610.2006.01611.x. [DOI] [PubMed] [Google Scholar]
- Blakemore S.J., den Ouden H., Choudhury S., Frith C. Adolescent development of the neural circuitry for thinking about intentions. Soc. Cogn. Affect Neurosci. 2007;2:130–139. doi: 10.1093/scan/nsm009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco V.M., Stern J.E., Filosa J.A. Tone-dependent vascular responses to astrocyte-derived signals. Am. J. Physiol. Heart Circ. Physiol. 2008;294:H2855–2863. doi: 10.1152/ajpheart.91451.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Born P., Leth H., Miranda M.J., Rostrup E., Stensgaard A., Peitersen B., Larsson H.B., Lou H.C. Visual activation in infants and young children studied by functional magnetic resonance imaging. Pediatr. Res. 1998;44:578–583. doi: 10.1203/00006450-199810000-00018. [DOI] [PubMed] [Google Scholar]
- Bourgeois J.P., Rakic P. Changes of synaptic density in the primary visual cortex of the macaque monkey from fetal to adult stage. J. Neurosci. 1993;13:2801–2820. doi: 10.1523/JNEUROSCI.13-07-02801.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brauer J., Neumann J., Friederici A.D. Temporal dynamics of perisylvian activation during language processing in children and adults. Neuroimage. 2008;41:1484–1492. doi: 10.1016/j.neuroimage.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bringmann A., Biedermann B., Reichenbach A. Expression of potassium channels during postnatal differentiation of rabbit Muller glial cells. Eur. J. Neurosci. 1999;11:2883–2896. doi: 10.1046/j.1460-9568.1999.00706.x. [DOI] [PubMed] [Google Scholar]
- Burette A., Zabel U., Weinberg R.J., Schmidt H.H., Valtschanoff J.G. Synaptic localization of nitric oxide synthase and soluble guanylyl cyclase in the hippocampus. J. Neurosci. 2002;22:8961–8970. doi: 10.1523/JNEUROSCI.22-20-08961.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busija D.W., Bari F., Domoki F., Louis T. Mechanisms involved in the cerebrovascular dilator effects of N-methyl-d-aspartate in cerebral cortex. Brain. Res. Rev. 2007;56:89–100. doi: 10.1016/j.brainresrev.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxton R.B., Uludağ K., Dubowitz D.J., Liu T.T. Modeling the hemodynamic response to brain activation. NeuroImage. 2004;23(Suppl 1):S220–233. doi: 10.1016/j.neuroimage.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Campbell W.B., Gebremedhin D., Pratt P.F., Harder D.R. Identification of epoxyeicosatrienoic acids as endothelium-derived hyperpolarizing factors. Circ. Res. 1996;78:415–423. doi: 10.1161/01.res.78.3.415. [DOI] [PubMed] [Google Scholar]
- Casabona G., Knöpfel T., Kuhn R., Gasparini F., Baumann P., Sortino M.A., Copani A., Nicoletti F. Expression and coupling to polyphosphoinositide hydrolysis of group I metabotropic glutamate receptors in early postnatal and adult rat brain. Eur. J. Neurosci. 1997;9:12–17. doi: 10.1111/j.1460-9568.1997.tb01348.x. [DOI] [PubMed] [Google Scholar]
- Casey B.J., Cohen J.D., Jezzard P., Turner R., Noll D.C., Trainor R.J., Giedd J., Kaysen D., Hertz-Pannier L., Rapoport J.L. Activation of prefrontal cortex in children during a nonspatial working memory task with functional MRI. Neuroimage. 1995;2:221–229. doi: 10.1006/nimg.1995.1029. [DOI] [PubMed] [Google Scholar]
- Casey B.J., Thomas K.M., Davidson M.C., Kunz K., Franzen P.L. Dissociating striatal and hippocampal function developmentally with a stimulus-response compatibility task. J. Neurosci. 2002;22:8647–8652. doi: 10.1523/JNEUROSCI.22-19-08647.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catania M.V., Landwehrmeyer G.B., Testa C.M., Standaert D.G., Penney J.B., Jr., Young A.B. Metabotropic glutamate receptors are differentially regulated during development. Neuroscience. 1994;61:481–495. doi: 10.1016/0306-4522(94)90428-6. [DOI] [PubMed] [Google Scholar]
- Cauli B., Tong X.K., Rancillac A., Serluca N., Lambolez B., Rossier J., Hamel E. Cortical GABA interneurons in neurovascular coupling: relays for subcortical vasoactive pathways. J. Neurosci. 2004;24:8940–8949. doi: 10.1523/JNEUROSCI.3065-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan W.Y., Lorke D.E., Tiu S.C., Yew D.T. Proliferation and apoptosis in the developing human neocortex. Anat. Rec. 2002;267:261–276. doi: 10.1002/ar.10100. [DOI] [PubMed] [Google Scholar]
- Cherubini E., Gaiarsa J.L., Ben-Ari Y. GABA: an excitatory transmitter in early postnatal life. Trends Neurosci. 1991;14:515–519. doi: 10.1016/0166-2236(91)90003-d. [DOI] [PubMed] [Google Scholar]
- Chiron C., Raynaud C., Maziere B., Zilbovicius M., Laflamme L., Masure M.C., Dulac O., Bourguignon M., Syrota A. Changes in regional cerebral blood flow during brain maturation in children and adolescents. J. Nucl. Med. 1992;33:696–703. [PubMed] [Google Scholar]
- Church J.A., Petersen S.E., Schlaggar B.L. The “Task B problem” and other considerations in developmental functional neuroimaging. Hum. Brain Mapp. 2010;31:852–862. doi: 10.1002/hbm.21036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen E.R., Ugurbil K., Kim S.G. Effect of basal conditions on the magnitude and dynamics of the blood oxygenation level-dependent fMRI response. J. Cereb. Blood Flow Metab. 2002;22:1042–1053. doi: 10.1097/00004647-200209000-00002. [DOI] [PubMed] [Google Scholar]
- Cohen Z., Bonvento G., Lacombe P., Hamel E. Serotonin in the regulation of brain microcirculation. Prog. Neurobiol. 1996;50:335–362. doi: 10.1016/s0301-0082(96)00033-0. [DOI] [PubMed] [Google Scholar]
- Cohen Z., Molinatti G., Hamel E. Astroglial and vascular interactions of noradrenaline terminals in the rat cerebral cortex. J. Cereb. Blood Flow Metab. 1997;17:894–904. doi: 10.1097/00004647-199708000-00008. [DOI] [PubMed] [Google Scholar]
- Colonnese M.T., Phillips M.A., Constantine-Paton M., Kaila K., Jasanoff A. Development of hemodynamic responses and functional connectivity in rat somatosensory cortex. Nat. Neurosci. 2008;11:72–79. doi: 10.1038/nn2017. [DOI] [PubMed] [Google Scholar]
- Conradi N.G., Engvall J., Wolff J.R. Angioarchitectonics of rat cerebellar cortex during pre- and postnatal development. Acta Neuropathol. 1980;50:131–138. doi: 10.1007/BF00692863. [DOI] [PubMed] [Google Scholar]
- Conti M.A., Adelstein R.S. Phosphorylation by cyclic adenosine 3′:5′-monophosphate-dependent protein kinase regulates myosin light chain kinase. Fed. Proc. 1980;39:1569–1573. [PubMed] [Google Scholar]
- Crook J.M., Kisvárday Z.F., Eysel U.T. Evidence for a contribution of lateral inhibition to orientation tuning and direction selectivity in cat visual cortex: reversible inactivation of functionally characterized sites combined with neuroanatomical tracing techniques. Eur. J. Neurosci. 1998;10:2056–2075. doi: 10.1046/j.1460-9568.1998.00218.x. [DOI] [PubMed] [Google Scholar]
- Davis R.J., Murdoch C.E., Ali M., Purbrick S., Ravid R., Baxter G.S., Tilford N., Sheldrick R.L., Clark K.L., Coleman R.A. EP4 prostanoid receptor-mediated vasodilatation of human middle cerebral arteries. Br. J. Pharmacol. 2004;141:580–585. doi: 10.1038/sj.bjp.0705645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devor A., Tian P., Nishimura N., Teng I.C., Hillman E.M., Narayanan S.N., Ulbert I., Boas D.A., Kleinfeld D., Dale A.M. Suppressed neuronal activity and concurrent arteriolar vasoconstriction may explain negative blood oxygenation level-dependent signal. J. Neurosci. 2007;27:4452–4459. doi: 10.1523/JNEUROSCI.0134-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahue M.J., Near J., Blicher J.U., Jezzard P. Baseline GABA concentration and fMRI response. Neuroimage. 2010;53:392–398. doi: 10.1016/j.neuroimage.2010.07.017. [DOI] [PubMed] [Google Scholar]
- Dorrn A.L., Yuan K., Barker A.J., Schreiner C.E., Froemke R.C. Developmental sensory experience balances cortical excitation and inhibition. Nature. 2010;465:932–936. doi: 10.1038/nature09119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downen M., Zhao M.L., Lee P., Weidenheim K.M., Dickson D.W., Lee S.C. Neuronal nitric oxide synthase expression in developing and adult human CNS. J. Neuropathol. Exp. Neurol. 1999;58:12–21. doi: 10.1097/00005072-199901000-00002. [DOI] [PubMed] [Google Scholar]
- Drew G.M., Whiting S.B. Evidence for two distinct types of postsynaptic alpha-adrenoceptor in vascular smooth muscle in vivo. Br. J. Pharmacol. 1979;67:207–215. doi: 10.1111/j.1476-5381.1979.tb08668.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy S., MacVicar B.A. Adrenergic calcium signaling in astrocyte networks within the hippocampal slice. J. Neurosci. 1995;15:5535–5550. doi: 10.1523/JNEUROSCI.15-08-05535.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durston S., Davidson M.C., Tottenham N., Galvan A., Spicer J., Fossella J.A., Casey B.J. A shift from diffuse to focal cortical activity with development. Dev. Sci. 2006;9:1–8. doi: 10.1111/j.1467-7687.2005.00454.x. [DOI] [PubMed] [Google Scholar]
- Earley S., Heppner T.J., Nelson M.T., Brayden J.E. TRPV4 forms a novel Ca2+ signaling complex with ryanodine receptors and BKCa channels. Circ. Res. 2005;97:1270–1279. doi: 10.1161/01.RES.0000194321.60300.d6. [DOI] [PubMed] [Google Scholar]
- Ellis E.F., Police R.J., Yancey L., McKinney J.S., Amruthesh S.C. Dilation of cerebral arterioles by cytochrome P-450 metabolites of arachidonic acid. Am. J. Physiol. 1990;259:H1171–1177. doi: 10.1152/ajpheart.1990.259.4.H1171. [DOI] [PubMed] [Google Scholar]
- Enager P., Piilgaard H., Offenhauser N., Kocharyan A., Fernandes P., Hamel E., Lauritzen M. Pathway-specific variations in neurovascular and neurometabolic coupling in rat primary somatosensory cortex. J. Cereb. Blood Flow Metab. 2009;29:976–986. doi: 10.1038/jcbfm.2009.23. [DOI] [PubMed] [Google Scholar]
- Estrada C., DeFelipe J. Nitric oxide-producing neurons in the neocortex: morphological and functional relationship with intraparenchymal microvasculature. Cereb. Cortex. 1998;8:193–203. doi: 10.1093/cercor/8.3.193. [DOI] [PubMed] [Google Scholar]
- Eto R., Abe M., Kimoto H., Imaoka E., Kato H., Kasahara J., Araki T. Alterations of interneurons in the striatum and frontal cortex of mice during postnatal development. Int. J. Dev. Neurosci. 2010;28:359–370. doi: 10.1016/j.ijdevneu.2010.04.004. [DOI] [PubMed] [Google Scholar]
- Fang X., Kaduce T.L., Weintraub N.L., Harmon S., Teesch L.M., Morisseau C., Thompson D.A., Hammock B.D., Spector A.A. Pathways of epoxyeicosatrienoic acid metabolism in endothelial cells. Implications for the vascular effects of soluble epoxide hydrolase inhibition. J. Biol. Chem. 2001;276:14867–14874. doi: 10.1074/jbc.M011761200. [DOI] [PubMed] [Google Scholar]
- Fields R.D. White matter matters. Sci. Am. 2008;298:42–49. [PubMed] [Google Scholar]
- Filosa J.A., Bonev A.D., Straub S.V., Meredith A.L., Wilkerson M.K., Aldrich R.W., Nelson M.T. Local potassium signaling couples neuronal activity to vasodilation in the brain. Nat. Neurosci. 2006;9:1397–1403. doi: 10.1038/nn1779. [DOI] [PubMed] [Google Scholar]
- Fornari E., Knyazeva M.G., Meuli R., Maeder P. Myelination shapes functional activity in the developing brain. Neuroimage. 2007;38:511–518. doi: 10.1016/j.neuroimage.2007.07.010. [DOI] [PubMed] [Google Scholar]
- Gabernet L., Jadhav S.P., Feldman D.E., Carandini M., Scanziani M. Somatosensory integration controlled by dynamic thalamocortical feed-forward inhibition. Neuron. 2005;48:315–327. doi: 10.1016/j.neuron.2005.09.022. [DOI] [PubMed] [Google Scholar]
- Gaillard W.D. Cortical function in epilepsy. Curr. Opin. Neurol. 2000;13:193–200. doi: 10.1097/00019052-200004000-00013. [DOI] [PubMed] [Google Scholar]
- Gaillard W.D., Hertz-Pannier L., Mott S.H., Barnett A.S., LeBihan D., Theodore W.H. Functional anatomy of cognitive development: fMRI of verbal fluency in children and adults. Neurology. 2000;54:180–185. doi: 10.1212/wnl.54.1.180. [DOI] [PubMed] [Google Scholar]
- Gaillard W.D., Sachs B.C., Whitnah J.R., Ahmad Z., Balsamo L.M., Petrella J.R., Braniecki S.H., McKinney C.M., Hunter K., Xu B., Grandin C.B. Developmental aspects of language processing: fMRI of verbal fluency in children and adults. Hum. Brain Mapp. 2003;18:176–185. doi: 10.1002/hbm.10091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi S.P., Yanagawa Y., Stryker M.P. Delayed plasticity of inhibitory neurons in developing visual cortex. Proc. Natl. Acad. Sci. USA. 2008;105:16797–16802. doi: 10.1073/pnas.0806159105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garaschuk O., Hanse E., Konnerth A. Developmental profile and synaptic origin of early network oscillations in the CA1 region of rat neonatal hippocampus. J. Physiol. 1998;507(Pt 1):219–236. doi: 10.1111/j.1469-7793.1998.219bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garey L.J., de Courten C. Structural development of the lateral geniculate nucleus and visual cortex in monkey and man. Behav. Brain Res. 1983;10:3–13. doi: 10.1016/0166-4328(83)90145-6. [DOI] [PubMed] [Google Scholar]
- Garthwaite J., Charles S.L., Chess-Williams R. Endothelium-derived relaxing factor release on activation of NMDA receptors suggests role as intercellular messenger in the brain. Nature. 1988;336:385–388. doi: 10.1038/336385a0. [DOI] [PubMed] [Google Scholar]
- Gebremedhin D., Lange A.R., Narayanan J., Aebly M.R., Jacobs E.R., Harder D.R. Cat cerebral arterial smooth muscle cells express cytochrome P450 4A2 enzyme and produce the vasoconstrictor 20-HETE which enhances L-type Ca2+ current. J. Physiol. 1998;507(Pt 3):771–781. doi: 10.1111/j.1469-7793.1998.771bs.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golarai G., Ghahremani D.G., Whitfield-Gabrieli S., Reiss A., Eberhardt J.L., Gabrieli J.D., Grill-Spector K. Differential development of high-level visual cortex correlates with category-specific recognition memory. Nat. Neurosci. 2007;10:512–522. doi: 10.1038/nn1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic P.S., Brown R.M. Postnatal development of monoamine content and synthesis in the cerebral cortex of rhesus monkeys. Dev. Brain Res. 1982;4:339–349. doi: 10.1016/0165-3806(82)90146-8. [DOI] [PubMed] [Google Scholar]
- Goodyear B.G., Menon R.S. Effect of luminance contrast on BOLD fMRI response in human primary visual areas. J. Neurophysiol. 1998;79:2204–2207. doi: 10.1152/jn.1998.79.4.2204. [DOI] [PubMed] [Google Scholar]
- Gordon G.R., Choi H.B., Rungta R.L., Ellis-Davies G.C., MacVicar B.A. Brain metabolism dictates the polarity of astrocyte control over arterioles. Nature. 2008;456:745–749. doi: 10.1038/nature07525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotoh J., Kuang T.Y., Nakao Y., Cohen D.M., Melzer P., Itoh Y., Pak H., Pettigrew K., Sokoloff L. Regional differences in mechanisms of cerebral circulatory response to neuronal activation. Am. J. Physiol. Heart Circ. Physiol. 2001;280:H821–829. doi: 10.1152/ajpheart.2001.280.2.H821. [DOI] [PubMed] [Google Scholar]
- Gur R.E., Gur R.C. Functional magnetic resonance imaging in schizophrenia. Dialogues Clin. Neurosci. 2010;12:333–343. doi: 10.31887/DCNS.2010.12.3/rgur. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagmann P., Sporns O., Madan N., Cammoun L., Pienaar R., Wedeen V.J., Meuli R., Thiran J.P., Grant P.E. White matter maturation reshapes structural connectivity in the late developing human brain. Proc. Natl. Acad. Sci. USA. 2010;107:19067–19072. doi: 10.1073/pnas.1009073107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamano K., Takeya T., Iwasaki N., Nakayama J., Ohto T., Okada Y. A quantitative study of the progress of myelination in the rat central nervous system, using the immunohistochemical method for proteolipid protein. Brain Res. Dev. Brain Res. 1998;108:287–293. doi: 10.1016/s0165-3806(98)00063-7. [DOI] [PubMed] [Google Scholar]
- Hamel E. Perivascular nerves and the regulation of cerebrovascular tone. J. Appl. Physiol. 2006;100:1059–1064. doi: 10.1152/japplphysiol.00954.2005. [DOI] [PubMed] [Google Scholar]
- Hendrickson A.E., Van Brederode J.F., Mulligan K.A., Celio M.R. Development of the calcium-binding protein parvalbumin and calbindin in monkey striate cortex. J. Comp. Neurol. 1991;307:626–646. doi: 10.1002/cne.903070409. [DOI] [PubMed] [Google Scholar]
- Hluštík P., Solodkin A., Noll D.C., Small S.L. Cortical plasticity during three-week motor skill learning. J. Clin. Neurophysiol. 2004;21:180–191. doi: 10.1097/00004691-200405000-00006. [DOI] [PubMed] [Google Scholar]
- Hoge R.D., Atkinson J., Gill B., Crelier G.R., Marrett S., Pike G.B. Linear coupling between cerebral blood flow and oxygen consumption in activated human cortex. Proc. Natl. Acad. Sci. USA. 1999;96:9403–9408. doi: 10.1073/pnas.96.16.9403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland S.K., Plante E., Weber Byars A., Strawsburg R.H., Schmithorst V.J., Ball W.S., Jr. Normal fMRI brain activation patterns in children performing a verb generation task. Neuroimage. 2001;14:837–843. doi: 10.1006/nimg.2001.0875. [DOI] [PubMed] [Google Scholar]
- Holt C.E., Harris W.A. Order in the initial retinotectal map in Xenopus: a new technique for labelling growing nerve fibres. Nature. 1983;301:150–152. doi: 10.1038/301150a0. [DOI] [PubMed] [Google Scholar]
- Hubel D.H., Wiesel T.N. Shape and arrangement of columns in cat's striate cortex. J. Physiol. 1963;165:559–568. doi: 10.1113/jphysiol.1963.sp007079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttenlocher P.R. Morphometric study of human cerebral cortex development. Neuropsychologia. 1990;28:517–527. doi: 10.1016/0028-3932(90)90031-i. [DOI] [PubMed] [Google Scholar]
- Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer's disease. Nat. Rev. Neurosci. 2004;5:347–360. doi: 10.1038/nrn1387. [DOI] [PubMed] [Google Scholar]
- Iadecola C., Yang G., Ebner T.J., Chen G. Local and propagated vascular responses evoked by focal synaptic activity in cerebellar cortex. J. Neurophysiol. 1997;78:651–659. doi: 10.1152/jn.1997.78.2.651. [DOI] [PubMed] [Google Scholar]
- Iliff J.J., Jia J., Nelson J., Goyagi T., Klaus J., Alkayed N.J. Epoxyeicosanoid signaling in CNS function and disease. Prostaglandins Other Lipid Mediat. 2010;91:68–84. doi: 10.1016/j.prostaglandins.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judas M., Sestan N., Kostovic I. Nitrinergic neurons in the developing and adult human telencephalon: transient and permanent patterns of expression in comparison to other mammals. Microsc. Res. Tech. 1999;45:401–419. doi: 10.1002/(SICI)1097-0029(19990615)45:6<401::AID-JEMT7>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Karni A., Meyer G., Rey-Hipolito C., Jezzard P., Adams M.M., Turner R., Ungerleider L.G. The acquisition of skilled motor performance: fast and slow experience-driven changes in primary motor cortex. Proc. Natl. Acad. Sci. USA. 1998;95:861–868. doi: 10.1073/pnas.95.3.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasischke K.A., Lambert E.M., Panepento B., Sun A., Gelbard H.A., Burgess R.W., Foster T.H., Nedergaard M. Two-photon NADH imaging exposes boundaries of oxygen diffusion in cortical vascular supply regions. J. Cereb. Blood Flow Metab. 2011;31:68–81. doi: 10.1038/jcbfm.2010.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastner S., DeSimone K., Konen C.S., Szczepanski S.M., Weiner K.S., Schneider K.A. Topographic maps in human frontal cortex revealed in memory-guided saccade and spatial working-memory tasks. J. Neurophysiol. 2007;97:3494–3507. doi: 10.1152/jn.00010.2007. [DOI] [PubMed] [Google Scholar]
- Keep R.F., Jones H.C. Cortical microvessels during brain development: a morphometric study in the rat. Microvasc. Res. 1990;40:412–426. doi: 10.1016/0026-2862(90)90036-q. [DOI] [PubMed] [Google Scholar]
- Keulers E.H., Stiers P., Jolles J. Developmental changes between ages 13 and 21 years in the extent and magnitude of the BOLD response during decision making. Neuroimage. 2011;54:1442–1454. doi: 10.1016/j.neuroimage.2010.08.059. [DOI] [PubMed] [Google Scholar]
- Khazipov R., Esclapez M., Caillard O., Bernard C., Khalilov I., Tyzio R., Hirsch J., Dzhala V., Berger B., Ben-Ari Y. Early development of neuronal activity in the primate hippocampus in utero. J. Neurosci. 2001;21:9770–9781. doi: 10.1523/JNEUROSCI.21-24-09770.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko K.R., Ngai A.C., Winn H.R. Role of adenosine in regulation of regional cerebral blood flow in sensory cortex. Am. J. Physiol. 1990;259:H1703–1708. doi: 10.1152/ajpheart.1990.259.6.H1703. [DOI] [PubMed] [Google Scholar]
- Koehler R.C., Roman R.J., Harder D.R. Astrocytes and the regulation of cerebral blood flow. Trends Neurosci. 2009;32:160–169. doi: 10.1016/j.tins.2008.11.005. [DOI] [PubMed] [Google Scholar]
- König P., Engel A.K., Singer W. Relation between oscillatory activity and long-range synchronization in cat visual cortex. Proc. Natl. Acad. Sci. USA. 1995;92:290–294. doi: 10.1073/pnas.92.1.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krimer L.S., Muly E.C., Williams G.V., Goldman-Rakic P.S. Dopaminergic regulation of cerebral cortical microcirculation. Nat. Neurosci. 1998;1:286–289. doi: 10.1038/1099. [DOI] [PubMed] [Google Scholar]
- Kwon H., Reiss A.L., Menon V. Neural basis of protracted developmental changes in visuo-spatial working memory. Proc. Natl. Acad. Sci. USA. 2002;99:13336–13341. doi: 10.1073/pnas.162486399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambe E.K., Krimer L.S., Goldman-Rakic P.S. Differential postnatal development of catecholamine and serotonin inputs to identified neurons in prefrontal cortex of rhesus monkey. J. Neurosci. 2000;20:8780–8787. doi: 10.1523/JNEUROSCI.20-23-08780.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange A., Gebremedhin D., Narayanan J., Harder D. 20-Hydroxyeicosatetraenoic acid-induced vasoconstriction and inhibition of potassium current in cerebral vascular smooth muscle is dependent on activation of protein kinase C. J. Biol. Chem. 1997;272:27345–27352. doi: 10.1074/jbc.272.43.27345. [DOI] [PubMed] [Google Scholar]
- Lauritzen M. Reading vascular changes in brain imaging: is dendritic calcium the key? Nat. Rev. Neurosci. 2005;6:77–85. doi: 10.1038/nrn1589. [DOI] [PubMed] [Google Scholar]
- Lauritzen M., Gold L. Brain function and neurophysiological correlates of signals used in functional neuroimaging. J. Neurosci. 2003;23:3972–3980. doi: 10.1523/JNEUROSCI.23-10-03972.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leenders K.L., Perani D., Lammertsma A.A., Heather J.D., Buckingham P., Healy M.J., Gibbs J.M., Wise R.J., Hatazawa J., Herold S. Cerebral blood flow, blood volume and oxygen utilization. Normal values and effect of age. Brain. 1990;113:27–47. doi: 10.1093/brain/113.1.27. [DOI] [PubMed] [Google Scholar]
- Leithner C., Royl G., Offenhauser N., Fuchtemeier M., Kohl-Bareis M., Villringer A., Dirnagl U., Lindauer U. Pharmacological uncoupling of activation induced increases in CBF and CMRO2. J. Cereb. Blood Flow Metab. 2010;30:311–322. doi: 10.1038/jcbfm.2009.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenroot R.K., Giedd J.N. Brain development in children and adolescents: insights from anatomical magnetic resonance imaging. Neurosci. Biobehav. Rev. 2006;30:718–729. doi: 10.1016/j.neubiorev.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Levitt P., Harvey J.A., Friedman E., Simansky K., Murphy E.H. New evidence for neurotransmitter influences on brain development. Trends Neurosci. 1997;20:269–274. doi: 10.1016/s0166-2236(96)01028-4. [DOI] [PubMed] [Google Scholar]
- Li D.Y., Varma D.R., Chemtob S. Up-regulation of brain PGE2 and PGF2 alpha receptors and receptor-coupled second messengers by cyclooxygenase inhibition in newborn pigs. J. Pharmacol. Exp. Ther. 1995;272:15–19. [PubMed] [Google Scholar]
- Li J., Iadecola C. Nitric oxide and adenosine mediate vasodilation during functional activation in cerebellar cortex. Neuropharmacology. 1994;33:1453–1461. doi: 10.1016/0028-3908(94)90049-3. [DOI] [PubMed] [Google Scholar]
- Lindauer U., Megow D., Matsuda H., Dirnagl U. Nitric oxide: a modulator, but not a mediator, of neurovascular coupling in rat somatosensory cortex. Am. J. Physiol. 1999;277:H799–811. doi: 10.1152/ajpheart.1999.277.2.H799. [DOI] [PubMed] [Google Scholar]
- Logothetis N.K. What we can do and what we cannot do with fMRI. Nature. 2008;453:869–878. doi: 10.1038/nature06976. [DOI] [PubMed] [Google Scholar]
- Luders E., Thompson P.M., Toga A.W. The development of the corpus callosum in the healthy human brain. J. Neurosci. 2010;30:10985–10990. doi: 10.1523/JNEUROSCI.5122-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandeville J.B., Marota J.J.A. Vascular filters of functional MRI: spatial localization using BOLD and CBV Contrast. Mag. Reson. Med. 1999;42:591–598. doi: 10.1002/(sici)1522-2594(199909)42:3<591::aid-mrm23>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Marcar V.L., Loenneker T., Straessle A., Girard F., Martin E. How much luxury is there in ‘luxury perfusion’? An analysis of the BOLD response in the visual areas V1 and V2. Magn. Reson. Imaging. 2004;22:921–928. doi: 10.1016/j.mri.2004.02.013. [DOI] [PubMed] [Google Scholar]
- Marcar V.L., Straessle A., Girard F., Loenneker T., Martin E. When more means less: a paradox BOLD response in human visual cortex. Magn. Reson. Imaging. 2004;22:441–450. doi: 10.1016/j.mri.2004.01.019. [DOI] [PubMed] [Google Scholar]
- Maril A., Davis P.E., Koo J.J., Reggev N., Zuckerman M., Ehrenfeld L., Mulkern R.V., Waber D.P., Rivkin M.J. Developmental fMRI study of episodic verbal memory encoding in children. Neurology. 2010;75:2110–2116. doi: 10.1212/WNL.0b013e318201526e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham J.A., Greenough W.T. Experience-driven brain plasticity: beyond the synapse. Neuron Glia Biol. 2004;1:351–363. doi: 10.1017/s1740925x05000219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister M., Wong R.O., Baylor D.A., Shatz C.J. Synchronous bursts of action potentials in ganglion cells of the developing mammalian retina. Science. 1991;252:939–943. doi: 10.1126/science.2035024. [DOI] [PubMed] [Google Scholar]
- Merzenich M.M., Jenkins W.M. Reorganization of cortical representations of the hand following alterations of skin inputs induced by nerve injury, skin island transfers, and experience. J. Hand Ther. 1993;6:89–104. doi: 10.1016/s0894-1130(12)80290-0. [DOI] [PubMed] [Google Scholar]
- Metea M.R., Newman E.A. Glial cells dilate and constrict blood vessels: a mechanism of neurovascular coupling. J. Neurosci. 2006;26:2862–2870. doi: 10.1523/JNEUROSCI.4048-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M.J., Weiss C., Song X., Iordanescu G., Disterhoft J.F., Wyrwicz A.M. Functional magnetic resonance imaging of delay and trace eyeblink conditioning in the primary visual cortex of the rabbit. J. Neurosci. 2008;28:4974–4981. doi: 10.1523/JNEUROSCI.5622-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimura K., Kimoto T., Okada M. Synapse efficiency diverges due to synaptic pruning following overgrowth. Phys. Rev. E: Stat. Nonlin. Soft Matter Phys. 2003;68:031910. doi: 10.1103/PhysRevE.68.031910. [DOI] [PubMed] [Google Scholar]
- Mito T., Konomi H., Houdou S., Takashima S. Immunohistochemical study of the vasculature in the developing brain. Pediatr. Neurol. 1991;7:18–22. doi: 10.1016/0887-8994(91)90100-y. [DOI] [PubMed] [Google Scholar]
- Miyawaki T., Matsui K., Takashima S. Developmental characteristics of vessel density in the human fetal and infant brains. Early Hum. Dev. 1998;53:65–72. doi: 10.1016/s0378-3782(98)00043-7. [DOI] [PubMed] [Google Scholar]
- Monk C.S., McClure E.B., Nelson E.E., Zarahn E., Bilder R.M., Leibenluft E., Charney D.S., Ernst M., Pine D.S. Adolescent immaturity in attention-related brain engagement to emotional facial expressions. Neuroimage. 2003;20:420–428. doi: 10.1016/s1053-8119(03)00355-0. [DOI] [PubMed] [Google Scholar]
- Mosso A. Sulla circolazione del cervello dell'uomo. Atti. R. Accad. Lincei. 1880;5:237–358. [Google Scholar]
- Müller C.M. Astrocytes in cat visual cortex studied by GFAP and S-100 immunocytochemistry during postnatal development. J. Comp. Neurol. 1992;317:309–323. doi: 10.1002/cne.903170308. [DOI] [PubMed] [Google Scholar]
- Mulligan S.J., MacVicar B.A. Calcium transients in astrocyte endfeet cause cerebrovascular constrictions. Nature. 2004;431:195–199. doi: 10.1038/nature02827. [DOI] [PubMed] [Google Scholar]
- Muramoto S., Yamada H., Sadato N., Kimura H., Konishi Y., Kimura K., Tanaka M., Kochiyama T., Yonekura Y., Ito H. Age-dependent change in metabolic response to photic stimulation of the primary visual cortex in infants: functional magnetic resonance imaging study. J. Comput. Assist. Tomogr. 2002;26:894–901. doi: 10.1097/00004728-200211000-00007. [DOI] [PubMed] [Google Scholar]
- Murrin L.C., Sanders J.D., Bylund D.B. Comparison of the maturation of the adrenergic and serotonergic neurotransmitter systems in the brain: implications for differential drug effects on juveniles and adults. Biochem. Pharmacol. 2007;73:1225–1236. doi: 10.1016/j.bcp.2007.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthukumaraswamy S.D., Edden R.A., Jones D.K., Swettenham J.B., Singh K.D. Resting GABA concentration predicts peak gamma frequency and fMRI amplitude in response to visual stimulation in humans. Proc. Natl. Acad. Sci. USA. 2009;106:8356–8361. doi: 10.1073/pnas.0900728106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa K., Araki E., Morham S.G., Ross M.E., Iadecola C. Cyclooxygenase-2 contributes to functional hyperemia in whisker-barrel cortex. J. Neurosci. 2000;20:763–770. doi: 10.1523/JNEUROSCI.20-02-00763.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixdorf-Bergweiler B.E., Albrecht D., Heinemann U. Developmental changes in the number, size, and orientation of GFAP-positive cells in the CA1 region of rat hippocampus. Glia. 1994;12:180–195. doi: 10.1002/glia.440120304. [DOI] [PubMed] [Google Scholar]
- Norman M.G., O’Kusky J.R. The growth and development of microvasculature in human cerebral cortex. J. Neuropathol. Exp. Neurol. 1986;45:222–232. [PubMed] [Google Scholar]
- Northoff G., Walter M., Schulte R.F., Beck J., Dydak U., Henning A., Boeker H., Grimm S., Boesiger P. GABA concentrations in the human anterior cingulate cortex predict negative BOLD responses in fMRI. Nat. Neurosci. 2007;10:1515–1517. doi: 10.1038/nn2001. [DOI] [PubMed] [Google Scholar]
- Oberheim N.A., Wang X., Goldman S., Nedergaard M. Astrocytic complexity distinguishes the human brain. Trends Neurosci. 2006;29:547–553. doi: 10.1016/j.tins.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Offenhauser N., Thomsen K., Caesar K., Lauritzen M. Activity-induced tissue oxygenation changes in rat cerebellar cortex: interplay of postsynaptic activation and blood flow. J. Physiol. 2005;565:279–294. doi: 10.1113/jphysiol.2005.082776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S., Lee T.M., Kay A.R., Tank D.W. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc. Natl. Acad. Sci. USA. 1990;87:9868–9872. doi: 10.1073/pnas.87.24.9868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S., Lee R.M., Barrere B. The sensitivity of magnetic resonance image signals of a rat brain to changes in the cerebral venous blood oxygenation. Magn. Reson. Med. 1993;29:205–210. doi: 10.1002/mrm.1910290208. [DOI] [PubMed] [Google Scholar]
- Ogilvie P., Schilling K., Billingsley M.L., Schmidt H.H. Induction and variants of neuronal nitric oxide synthase type I during synaptogenesis. FASEB J. 1995;9:799–806. doi: 10.1096/fasebj.9.9.7541381. [DOI] [PubMed] [Google Scholar]
- Ohyu J., Takashima S. Developmental characteristics of neuronal nitric oxide synthase (nNOS) immunoreactive neurons in fetal to adolescent human brains. Brain Res. Dev. Brain Res. 1998;110:193–202. doi: 10.1016/s0165-3806(98)00107-2. [DOI] [PubMed] [Google Scholar]
- Olesen P.J., Nagy Z., Westerberg H., Klingberg T. Combined analysis of DTI and fMRI data reveals a joint maturation of white and grey matter in a fronto-parietal network. Brain Res. Cogn. Brain Res. 2003;18:48–57. doi: 10.1016/j.cogbrainres.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Ostby Y., Tamnes C.K., Fjell A.M., Westlye L.T., Due-Tønnessen P., Walhovd K.B. Heterogeneity in subcortical brain development: A structural magnetic resonance imaging study of brain maturation from 8 to 30 years. J. Neurosci. 2009;29:11772–11782. doi: 10.1523/JNEUROSCI.1242-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantano P., Baron J.C., Lebrun-Grandié P., Duquesnoy N., Bousser M.G., Comar D. Regional cerebral blood flow and oxygen consumption in human aging. Stroke. 1984;15:635–641. doi: 10.1161/01.str.15.4.635. [DOI] [PubMed] [Google Scholar]
- Paspalas C.D., Papadopoulos G.C. Ultrastructural relationships between noradrenergic nerve fibers and non-neuronal elements in the rat cerebral cortex. Glia. 1996;17:133–146. doi: 10.1002/(SICI)1098-1136(199606)17:2<133::AID-GLIA5>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Passarotti A.M., Paul B.M., Bussiere J.R., Buxton R.B., Wong E.C., Stiles J. The development of face and location processing: an fMRI study. Dev. Sci. 2003;6:100–117. [Google Scholar]
- Pauling L., Coryell C.D. The magnetic properties and structure of hemoglobin, oxyhemoglobin and carbonmonoxyhemoglobin. Proc. Natl. Acad. Sci. USA. 1936;22:210–216. doi: 10.1073/pnas.22.4.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T. Mapping brain maturation and cognitive development during adolescence. Trends Cogn. Sci. 2005;9:60–68. doi: 10.1016/j.tics.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Peelen M.V., Glaser B., Vuilleumier P., Eliez S. Differential development of selectivity for faces and bodies in the fusiform gyrus. Dev. Sci. 2009;12:F16–25. doi: 10.1111/j.1467-7687.2009.00916.x. [DOI] [PubMed] [Google Scholar]
- Peppiatt C.M., Howarth C., Mobbs P., Attwell D. Bidirectional control of CNS capillary diameter by pericytes. Nature. 2006;443:700–704. doi: 10.1038/nature05193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto J.G., Hornby K.R., Jones D.G., Murphy K.M. Developmental changes in GABAergic mechanisms in human visual cortex across the lifespan. Front. Cell Neurosci. 2010;4:16. doi: 10.3389/fncel.2010.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter J.T., McCarthy K.D. Hippocampal astrocytes in situ respond to glutamate released from synaptic terminals. J. Neurosci. 1996;16:5073–5081. doi: 10.1523/JNEUROSCI.16-16-05073.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh K.R., Mencl W.E., Jenner A.R., Katz L., Frost S.J., Lee J.R., Shaywitz S.E., Shaywitz B.A. Functional neuroimaging studies of reading and reading disability (developmental dyslexia) Ment. Retard. Dev. Disabil. Res. Rev. 2000;6:207–213. doi: 10.1002/1098-2779(2000)6:3<207::AID-MRDD8>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Pujol J., Vendrell P., Junque C., Marti-Vilalta J.L., Capdevila A. When does human brain development end? Evidence of corpus callosum growth up to adulthood. Ann. Neurol. 1993;34:71–75. doi: 10.1002/ana.410340113. [DOI] [PubMed] [Google Scholar]
- Raichle M.E., Hartman B.K., Eichling J.O., Sharpe L.G. Central noradrenergic regulation of cerebral blood flow and vascular permeability. Proc. Natl. Acad. Sci. USA. 1975;72:3726–3730. doi: 10.1073/pnas.72.9.3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P., Bourgeois J.P., Goldman-Rakic P.S. Synaptic development of the cerebral cortex: implications for learning, memory, and mental illness. Prog. Brain Res. 1994;102:227–243. doi: 10.1016/S0079-6123(08)60543-9. [DOI] [PubMed] [Google Scholar]
- Ray S., Maunsell J.H. Differences in gamma frequencies across visual cortex restrict their possible use in computation. Neuron. 2010;67:885–896. doi: 10.1016/j.neuron.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuss S., Schaeffer D.F., Laages M.H., Riemann R. Evidence for increased nitric oxide production in the auditory brain stem of the aged dwarf hamster (Phodopus sungorus): an NADPH-diaphorase histochemical study. Mech. Ageing Dev. 2000;112:125–134. doi: 10.1016/s0047-6374(99)00082-2. [DOI] [PubMed] [Google Scholar]
- Rheims S., Holmgren C.D., Chazal G., Mulder J., Harkany T., Zilberter T., Zilberter Y. GABA action in immature neocortical neurons directly depends on the availability of ketone bodies. J. Neurochem. 2009;110:1330–1338. doi: 10.1111/j.1471-4159.2009.06230.x. [DOI] [PubMed] [Google Scholar]
- Richter W., Richter M. The shape of the fMRI BOLD response in children and adults changes systematically with age. Neuroimage. 2003;20:1122–1131. doi: 10.1016/S1053-8119(03)00347-1. [DOI] [PubMed] [Google Scholar]
- Riddle D.R., Gutierrez G., Zheng D., White L.E., Richards A., Purves D. Differential metabolic and electrical activity in the somatic sensory cortex of juvenile and adult rats. J. Neurosci. 1993;13:4193–4213. doi: 10.1523/JNEUROSCI.13-10-04193.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riobo N.A., Melani M., Sanjuan N., Fiszman M.L., Gravielle M.C., Carreras M.C., Cadenas E., Poderoso J.J. The modulation of mitochondrial nitric-oxide synthase activity in rat brain development. J. Biol. Chem. 2002;277:42447–42455. doi: 10.1074/jbc.M204580200. [DOI] [PubMed] [Google Scholar]
- Risser L., Plouraboue F., Cloetens P., Fonta C. A 3D-investigation shows that angiogenesis in primate cerebral cortex mainly occurs at capillary level. Int. J. Dev. Neurosci. 2009;27:185–196. doi: 10.1016/j.ijdevneu.2008.10.006. [DOI] [PubMed] [Google Scholar]
- Roman R.J. P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol. Rev. 2002;82:131–185. doi: 10.1152/physrev.00021.2001. [DOI] [PubMed] [Google Scholar]
- Romano C., Sesma M.A., McDonald C.T., O’Malley K., Van den Pol A.N., Olney J.W. Distribution of metabotropic glutamate receptor mGluR5 immunoreactivity in rat brain. J. Comp. Neurol. 1995;355:455–469. doi: 10.1002/cne.903550310. [DOI] [PubMed] [Google Scholar]
- Roy C.S., Sherrington C.S. On the regulation of the blood-supply of the brain. J. Physiol. 1890;11 doi: 10.1113/jphysiol.1890.sp000321. 85–158, 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salami M., Itami C., Tsumoto T., Kimura F. Change of conduction velocity by regional myelination yields constant latency irrespective of distance between thalamus and cortex. Proc. Natl. Acad. Sci. USA. 2003;100:6174–6179. doi: 10.1073/pnas.0937380100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinas E., Sejnowski T.J. Correlated neuronal activity and the flow of neural information. Nat. Rev. Neurosci. 2001;2:539–550. doi: 10.1038/35086012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Zuriaga D., Marti-Gutierrez N., De La Cruz M.A., Peris-Sanchis M.R. Age-related changes of NADPH-diaphorase-positive neurons in the rat inferior colliculus and auditory cortex. Microsc. Res. Tech. 2007;70:1051–1059. doi: 10.1002/jemt.20512. [DOI] [PubMed] [Google Scholar]
- Scafidi S., Douglas R.M., Farahani R., Banasiak K.J., Haddad G.G. Prostaglandin transporter expression in mouse brain during development and in response to hypoxia. Neuroscience. 2007;146:1150–1157. doi: 10.1016/j.neuroscience.2007.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schölvinck M.L., Howarth C., Attwell D. The cortical energy needed for conscious perception. Neuroimage. 2008;40:1460–1468. doi: 10.1016/j.neuroimage.2008.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidl A.H., Rubel E.W., Harris D.M. Mechanisms for adjusting interaural time differences to achieve binaural coincidence detection. J. Neurosci. 2010;30:70–80. doi: 10.1523/JNEUROSCI.3464-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serebryakov V., Zakharenko S., Snetkov V., Takeda K. Effects of prostaglandins E1 and E2 on cultured smooth muscle cells and strips of rat aorta. Prostaglandins. 1994;47:353–365. doi: 10.1016/0090-6980(94)90053-1. [DOI] [PubMed] [Google Scholar]
- Seregi A., Keller M., Hertting G. Are cerebral prostanoids of astroglial origin? Studies on the prostanoid forming system in developing rat brain and primary cultures of rat astrocytes. Brain Res. 1987;404:113–120. doi: 10.1016/0006-8993(87)91361-8. [DOI] [PubMed] [Google Scholar]
- Sereno M.I., Pitzalis S., Martinez A. Mapping of contralateral space in retinotopic coordinates by a parietal cortical area in humans. Science. 2001;294:1350–1354. doi: 10.1126/science.1063695. [DOI] [PubMed] [Google Scholar]
- Shaw P., Kabani N.J., Lerch J.P., Eckstrand K., Lenroot R., Gogtay N., Greenstein D., Clasen L., Evans A., Rapoport J.L., Giedd J.N., Wise S.P. Neurodevelopmental trajectories of the human cerebral cortex. J. Neurosci. 2008;28:3586–3594. doi: 10.1523/JNEUROSCI.5309-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigala N., Logothetis N.K. Visual categorization shapes feature selectivity in the primate temporal cortex. Nature. 2002;415:318–320. doi: 10.1038/415318a. [DOI] [PubMed] [Google Scholar]
- Sillito A.M. The contribution of inhibitory mechanisms to the receptive field properties of neurones in the striate cortex of the cat. J. Physiol. 1975;250:305–329. doi: 10.1113/jphysiol.1975.sp011056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver M.A., Kastner S. Topographic maps in human frontal and parietal cortex. Trends Cogn. Sci. 2009;13:488–495. doi: 10.1016/j.tics.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simard M., Arcuino G., Takano T., Liu Q.S., Nedergaard M. Signaling at the gliovascular interface. J. Neurosci. 2003;23:9254–9262. doi: 10.1523/JNEUROSCI.23-27-09254.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer W., Gray C.M. Visual feature integration and the temporal correlation hypothesis. Annu. Rev. Neurosci. 1995;18:555–586. doi: 10.1146/annurev.ne.18.030195.003011. [DOI] [PubMed] [Google Scholar]
- Sloan H.L., Austin V.C., Blamire A.M., Schnupp J.W., Lowe A.S., Allers K.A., Matthews P.M., Sibson N.R. Regional differences in neurovascular coupling in rat brain as determined by fMRI and electrophysiology. Neuroimage. 2010;53:399–411. doi: 10.1016/j.neuroimage.2010.07.014. [DOI] [PubMed] [Google Scholar]
- Sowell E.R., Peterson B.S., Thompson P.M., Welcome S.E., Henkenius A.L., Toga A.W. Mapping cortical change across the human life span. Nat. Neurosci. 2003;6:309–315. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- Spear L.P., Brake S.C. Periadolescence: age-dependent behavior and psychopharmacological responsivity in rats. Dev. Psychobiol. 1983;16:83–109. doi: 10.1002/dev.420160203. [DOI] [PubMed] [Google Scholar]
- Spector A.A., Fang X., Snyder G.D., Weintraub N.L. Epoxyeicosatrienoic acids (EETs): metabolism and biochemical function. Prog. Lipid Res. 2004;43:55–90. doi: 10.1016/s0163-7827(03)00049-3. [DOI] [PubMed] [Google Scholar]
- St Lawrence K.S., Ye F.Q., Lewis B.K., Frank J.A., McLaughlin A.C. Measuring the effects of indomethacin on changes in cerebral oxidative metabolism and cerebral blood flow during sensorimotor activation. Magn. Reson. Med. 2003;50:99–106. doi: 10.1002/mrm.10502. [DOI] [PubMed] [Google Scholar]
- Stichel C.C., Muller C.M., Zilles K. Distribution of glial fibrillary acidic protein and vimentin immunoreactivity during rat visual cortex development. J. Neurocytol. 1991;20:97–108. doi: 10.1007/BF01279614. [DOI] [PubMed] [Google Scholar]
- Takada H., Nagata K., Hirata Y., Satoh Y., Watahiki Y., Sugawara J., Yokoyama E., Kondoh Y., Shishido F., Inugami A. Age-related decline of cerebral oxygen metabolism in normal population detected with positron emission tomography. Neurol. Res. 1992;14:128–131. doi: 10.1080/01616412.1992.11740031. [DOI] [PubMed] [Google Scholar]
- Takano T., Tian G.F., Peng W., Lou N., Libionka W., Han X., Nedergaard M. Astrocyte-mediated control of cerebral blood flow. Nat. Neurosci. 2006;9:260–267. doi: 10.1038/nn1623. [DOI] [PubMed] [Google Scholar]
- Tamnes C.K., Ostby Y., Fjell A.M., Westlye L.T., Due-Tønnessen P., Walhovd K.B. Brain maturation in adolescence and young adulthood: regional age-related changes in cortical thickness and white matter volume and microstructure. Cereb. Cortex. 2010;20:534–548. doi: 10.1093/cercor/bhp118. [DOI] [PubMed] [Google Scholar]
- Thomas K.M., King S.W., Franzen P.L., Welsh T.F., Berkowitz A.L., Noll D.C., Birmaher V., Casey B.J. A developmental functional MRI study of spatial working memory. Neuroimage. 1999;10:327–338. doi: 10.1006/nimg.1999.0466. [DOI] [PubMed] [Google Scholar]
- Thomason M.E., Burrows B.E., Gabrieli J.D., Glover G.H. Breath holding reveals differences in fMRI BOLD signal in children and adults. Neuroimage. 2005;25:824–837. doi: 10.1016/j.neuroimage.2004.12.026. [DOI] [PubMed] [Google Scholar]
- Tieman S.B., Mollers S., Tieman D.G., White J. The blood supply of the cat's visual cortex and its postnatal development. Brain Res. 2004;998:100–112. doi: 10.1016/j.brainres.2003.11.023. [DOI] [PubMed] [Google Scholar]
- Toga A.W., Thompson P.M., Sowell E.R. Mapping brain maturation. Trends Neurosci. 2006;29:148–159. doi: 10.1016/j.tins.2006.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng K.Y., O’Donnell P. Dopamine modulation of prefrontal cortical interneurons changes during adolescence. Cereb. Cortex. 2007;17:1235–1240. doi: 10.1093/cercor/bhl034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkeltaub P.E., Flowers D.L., Lyon L.G., Eden G.F. Development of ventral stream representations for single letters. Ann. N. Y. Acad. Sci. 2008;1145:13–29. doi: 10.1196/annals.1416.026. [DOI] [PubMed] [Google Scholar]
- Tyzio R., Allene C., Nardou R., Picardo M.A., Yamamoto S., Sivakumaran S., Caiati M.D., Rheims S., Minlebaev M., Milh M., Ferre P., Khazipov R., Romette J.L., Lorquin J., Cossart R., Khalilov I., Nehlig A., Cherubini E., Ben-Ari Y. Depolarizing actions of GABA in immature neurons depend neither on ketone bodies nor on pyruvate. J. Neurosci. 2011;31:34–45. doi: 10.1523/JNEUROSCI.3314-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullen F. Is activity regulation of late myelination a plastic mechanism in the human nervous system? Neuron Glia Biol. 2009;5:29–34. doi: 10.1017/S1740925X09990330. [DOI] [PubMed] [Google Scholar]
- Vaidya C.J., Austin G., Kirkorian G., Ridlehuber H.W., Desmond J.E., Glover G.H., Gabrieli J.D. Selective effects of methylphenidate in attention deficit hyperactivity disorder: a functional magnetic resonance study. Proc. Natl. Acad. Sci. USA. 1998;95:14494–14499. doi: 10.1073/pnas.95.24.14494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Pol A.N., Romano C., Ghosh P. Metabotropic glutamate receptor mGluR5 subcellular distribution and developmental expression in hypothalamus. J. Comp. Neurol. 1995;362:134–150. doi: 10.1002/cne.903620108. [DOI] [PubMed] [Google Scholar]
- Vanhoutte P.M., Rimele T.J. Calcium and alpha-adrenoceptors in activation of vascular smooth muscle. J. Cardiovasc. Pharmacol. 1982;4(Suppl 3):S280–286. [PubMed] [Google Scholar]
- Wahlstrom D., White T., Luciana M. Neurobehavioral evidence for changes in dopamine system activity during adolescence. Neurosci. Biobehav. Rev. 2010;34:631–648. doi: 10.1016/j.neubiorev.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace M.N., Brown I.E., Cox A.T., Harper M.S. Pyramidal neurones in human precentral gyrus contain nitric oxide synthase. Neuroreport. 1995;6:2532–2536. doi: 10.1097/00001756-199512150-00020. [DOI] [PubMed] [Google Scholar]
- Wang A.T., Lee S.S., Sigman M., Dapretto M. Developmental changes in the neural basis of interpreting communicative intent. Soc. Cogn. Affect Neurosci. 2006;1:107–121. doi: 10.1093/scan/nsl018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber B., Keller A.L., Reichold J., Logothetis N.K. The microvascular system of the striate and extrastriate visual cortex of the macaque. Cereb. Cortex. 2008;18:2318–2330. doi: 10.1093/cercor/bhm259. [DOI] [PubMed] [Google Scholar]
- Werkle-Bergner M., Shing Y.L., Muller V., Li S.C., Lindenberger U. EEG gamma-band synchronization in visual coding from childhood to old age: evidence from evoked power and inter-trial phase locking. Clin. Neurophysiol. 2009;120:1291–1302. doi: 10.1016/j.clinph.2009.04.012. [DOI] [PubMed] [Google Scholar]
- Yakovlev P.I., Lecours A.R. Blackwell Scientific Publications; Boston: 1967. Regional Development of the Brain in Early Life. [Google Scholar]
- Yamada H., Sadato N., Konishi Y., Kimura K., Tanaka M., Yonekura Y., Ishii Y. A rapid brain metabolic change in infants detected by fMRI. Neuroreport. 1997;8:3775–3778. doi: 10.1097/00001756-199712010-00024. [DOI] [PubMed] [Google Scholar]
- Yan X.X., Garey L.J., Jen L.S. Prenatal development of NADPH-diaphorase-reactive neurons in human frontal cortex. Cereb. Cortex. 1996;6:737–745. doi: 10.1093/cercor/6.5.737. [DOI] [PubMed] [Google Scholar]
- Yaseen M.A., Srinivasan V.J., Sakadžić S., Radhakrishnan H., Gorczynska I., Wu W., Fujimoto J.G., Boas D.A. Microvascular oxygen tension and flow measurements in rodent cerebral cortex during baseline conditions and functional activation. J. Cereb. Blood Flow Metab. 2011;31:1051–1063. doi: 10.1038/jcbfm.2010.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu B.P., Yu C.C., Robertson R.T. Patterns of capillaries in developing cerebral and cerebellar cortices of rats. Acta Anat. (Basel) 1994;149:128–133. doi: 10.1159/000147567. [DOI] [PubMed] [Google Scholar]
- Zonta M., Angulo M.C., Gobbo S., Rosengarten B., Hossmann K.A., Pozzan T., Carmignoto G. Neuron-to-astrocyte signaling is central to the dynamic control of brain microcirculation. Nat. Neurosci. 2003;6:43–50. doi: 10.1038/nn980. [DOI] [PubMed] [Google Scholar]