Abstract
Background
The incentive sensitization theory posits that in the transition from sporadic to problematic alcohol use, the incentive value of alcohol increases (wanting) while its hedonic effects (liking) do not change or decreases. The effect of the OPRM1 c.118A>G polymorphism, associated with liking and wanting, and the DRD4-VNTR polymorphism, related to wanting, on the relation between attentional bias and alcohol use was investigated.
Methods
A total of 195 young adolescents (Study 1) and 86 young adult male heavy drinkers (Study 2) completed a visual probe test. Saliva samples were collected to test both polymorphisms.
Results
In Study 1, attentional bias was positively associated with adolescent alcohol use only for OPRM1 G-allele carriers. In Study 2, attentional bias was positively associated with problem drinking for carriers of a DRD4 long allele.
Discussion
It is tentatively proposed that an attentional bias for alcohol is related most strongly to liking and wanting in early adolescents, while in young adults, an attentional bias may reflect wanting. In addition, individual differences associated with two both genetic markers should be taken into account when examining the relation between attentional bias and alcohol use.
Keywords: Adolescence, Alcohol use, Cognitive processes, Genetic polymorphisms, Individual differences, Young adulthood
1. Introduction
In general, people tend to pursue rewards that produce a considerable amount of subjective pleasure. Yet in the case of drug addiction the transition from incidental to compulsive use can be accompanied by decrease in enjoyment of the substance, while consumption is undyingly continued. It appears that in the course of the development of addiction, the motivation to obtain drugs (“wanting”) prevails over the decreased subjective pleasure (“liking”) that is generated from its repeated use (Robinson and Berridge, 1993, Robinson and Berridge, 2003). Support for the notion that “liking” and “wanting” are psychologically dissociable reward components stems primarily from animal studies, but some attempts have been made to dissociate wanting from liking in humans, using self-reports (Hobbs et al., 2005), reaction time measures of associations (Thush and Wiers, 2007, Wiers et al., 2002), and measures of attentional bias (Field et al., 2009), including eye movements (Schoenmakers et al., 2008) and brain responses (Luijten et al., 2010). For example, Hobbs et al. (2005) found that adding a bad taste (Tween) reduces consumption of fruit juice in both light and heavy drinkers. However, it did not decrease beer consumption in heavy drinkers, which was the case for light drinkers. In addition to drinking behavior, indirect measures have been used to assess potential markers of an incentive salient reaction to alcohol cues in humans. An incentive salient reaction has been related to arousal, to a tendency to approach, and to an attentional bias for cues signaling alcohol (Robinson and Berridge, 1993, Robinson and Berridge, 2003). Indeed, heavy drinkers demonstrated stronger automatic associations between alcohol and arousal than light drinkers (Wiers et al., 2002), and alcohol–arousal associations in early adolescent boys who had just started to drink predicted escalation of drinking a year later (Thush and Wiers, 2007). Recent studies have also demonstrated an approach-bias for alcohol in heavy but not in light drinkers (Field et al., 2008, Wiers et al., 2009). Many studies have demonstrated an attentional bias for alcohol in heavy drinkers (Field et al., 2009), including eye movements (Schoenmakers et al., 2008) and brain responses (Luijten et al., 2010). Note that these findings are in line with incentive salience theory, but are no direct proof and some controversy remains (Bradberry, 2008, Leyton, 2007, Robinson and Berridge, 2008).
Animal research has suggested that different neural substrates are involved in “liking” and “wanting”. Whereas “liking” is related to opioid forebrain systems, endocannabinoid and GABA-benzodiazepine neurotransmitter systems (Berridge, 1996, Berridge et al., 2009, Nesse and Berridge, 1997, Robinson and Berridge, 2003), “wanting” has been related to dopamine projections from the ventral tegmental area to the nucleus accumbens (NA), cortical areas and the amygdala (e.g. Le Foll et al., 2009, Breiter et al., 1997). Although the mesocorticolimbic dopamine (DA) system is activated by numerous natural incentives, such as food or sex, drugs also have the unique ability to render this system enduringly hyper responsive, a process entitled sensitization. The incentive sensitization theory (IST) posits that the DA circuit that is targeted by drugs mediates the attribution of incentive salience to drug-related stimuli and actions. As a result, representations of these cues become increasingly “wanted” and engage attentional resources (Robinson and Berridge, 1993, Robinson and Berridge, 2003).
The neuro-adaptations related to emerging alcohol or drug use should be viewed from the more general perspective of adolescent brain development, as most alcohol and drug use starts during adolescence, a period in which emotional–motivational brain systems rapidly develop. The normal (not drug-related) brain changes in adolescence are directly related to the hormonal changes in this period (Forbes and Dahl, 2010) and coincide with the peak in sensation seeking during adolescence (Steinberg et al., 2008). This developmental perspective is useful to distinguish two often confounded systems which both contribute to risk behaviors (Steinberg, 2010), including substance use (Casey and Jones, 2010, Wiers et al., 2010): a social-emotional or associative impulsive system and a cognitive control or reflective system. In adolescence, a rapid increase in dopaminergic activity takes place within the socio-emotional system, which is assumed to lead to increases in reward-seeking. Whereas during the course of adolescence, the cognitive control system gradually matures resulting in more advanced self regulation and impulse control (Steinberg, 2010). Thus, the quick rise in sensation seeking in puberty will make alcohol and drug use more attractive, while the relatively underdeveloped reflective processes will have difficulty in controlling the resulting appetitive impulse, especially when there is social pressure (Casey and Jones, 2010, Steinberg, 2010, Wiers et al., 2007). As a result of the substance-induced neuro-adaptations, continued substance use will increase the appetitive drive and is likely to further delay the development of self-control.
An attentional bias for drugs, the observation that drug cues relatively automatically or reflexively capture attentional processes at the expense of other stimuli in the environment, is thought to play a role in the etiology and maintenance of addiction (e.g. Field and Cox, 2008). The exact mechanism behind the relation between cognitive biases and prolonged drug use remains unclear: while some scholars argue for a subsequent neural transfer, from ventral to dorsal striatal structures, associated with habit formation (Everitt and Robbins, 2005) others stress that, although the rituals accompanying drug taking can be automatized, the key element of compulsive drug use is still motivational instead of habitual (Robinson and Berridge, 2003). Nevertheless, most theorists agree on the fact that with repeated drug use, drug cues not only acquire perceptual salience, but also motivational salience, fueling further drug use.
The main aim of our study was to examine individual differences in the relationship between attentional bias and alcohol use. The focus is on alcohol, as it is one of the most commonly used drugs of abuse worldwide with an age of onset typically occurring in adolescence (Grant and Dawson, 1997, Van Der Vorst et al., 2009). Studies have indicated an attentional bias in alcohol dependent inpatients (Stetter et al., 2006), non treatment seeking heavy drinking adults (Townshend and Duka, 2001) and adolescents (Field et al., 2006). To our knowledge, no studies have yet examined whether a genetic predisposition may identify individuals who are susceptible to the motivational properties of drug cues.
We examined the moderating role of two genetic markers: the c.118A>G single nucleotide polymorphism of the mu-opioid receptor gene (OPRM1) and the dopamine D4 receptor gene (DRD4). The OPRM1 polymorphism has been linked to increases in affective reactions following alcohol consumption (Ray and Hutchison, 2004). Opioid activity has also been implicated in appetitive motivational processes, such as cue-induced alcohol craving (Van Den Wildenberg et al., 2007a), approach biases (Wiers et al., 2009) and a stronger haemodynamic signal in mesocorticolimbic areas in response to a prime dose of alcohol (Filbey et al., 2008). The opioid system moderates the mesolimbic dopaminergic function, which is probably related to the fact that under normal circumstances, and in beginning alcohol and drug use “liking” and “wanting” go hand in hand (Berridge et al., 2009). However, in problematic alcohol and drug use, “liking” and “wanting” can dissociate, which could be related to individual differences in the other genetic marker we investigated: the DRD4 polymorphism. Carriers of the DRD4 long allele demonstrate higher levels of subjective craving after having a few alcoholic drinks, but the DRD4 gene does not seem to be associated with “liking” processes (Hutchison et al., 2002). These findings are in line with research showing that dopamine agonists only influence “wanting” processes, while “liking” processes are left unaffected (Hutchison et al., 2001).
In the current study, we investigated the moderating effect of OPRM1 and DRD4 on the association between attentional bias and alcohol use in two independent samples who differed in their alcohol-involvement. In the first sample of young adolescents, a positive relation between attentional bias and alcohol use was anticipated in risk-allele carriers of the OPRM1 G-allele, since in this stage of drinking, both “liking” and “wanting” processes play a role in the development of alcohol use. To the extent that the DRD4 polymorphism is more specifically related to “wanting”, we hypothesized that DRD4 would interact with attentional bias in explaining alcohol use, in the second sample of young adult heavy drinkers, since “wanting” should have become more relevant in the escalation of alcohol use to abuse. Hence, even though the studies are cross-sectional, the two samples differ in their alcohol-involvement: young adolescent beginning drinkers where liking and wanting should still be strongly related, and young adult heavy drinkers, who begin to experience problems with drinking, which could indicate “wanting” despite adverse consequences.
2. Study 1
2.1. Method
2.1.1. Participants and procedure
The sample included 195 adolescents (56% female) between 12 and 16 years of age (M = 13.69, SD = .89). Almost all adolescents (94.1%) lived in households with both parents being Caucasian, the remainder had a least one non-Caucasian parent. Participants were in first, second and third grade of three Dutch high schools. Roughly 42% were in college-preparatory education, 33% were in intermediate education, 4% were in vocational training, and 21% had not yet decided (adolescents in the Netherlands are allowed to choose an ultimate educational track in the first or second grade).
The sample was drawn from a larger longitudinal project examining risk factors associated with adolescent alcohol use (see Pieters et al., 2010). A total of 1215 adolescents attending four schools were contacted for participation. Of these, 725 agreed to participate by returning a consent form signed by their parents and themselves. Approximately 50% of these parents (n = 378) also provided additional consent for the donation of a saliva sample from their offspring for analysis of genetic polymorphisms. Data collection was completed at schools during 1 h sessions on two separate days. On the first day, students individually completed computer tasks assessing cognitive risk factors under supervision of trained research assistants, and for students whose parents provided additional consent, salvia samples were collected. On the second day, questionnaires were administered to students by trained research assistants. For the current study, we targeted adolescents between 12 and 16 years of age (n = 502), who also provided a saliva sample (n = 232). A total of 37 students were excluded because they did not complete the survey or computer task. This resulted in a total of 195 participants. Independent samples t-tests indicated that the 195 participants reported less frequent alcohol use than the 270 non-participants without genetic data, t(463) = 2.38, p = .005, and lower quantity of alcohol use, t(463) = 2.43, p = .015.
2.1.2. Measures
2.1.2.1. Frequency of alcohol use
Adolescents were asked about the average frequency of their alcohol consumption (Pieters et al., 2010). Answers were given on an 8-point scale: (1) “never”, (2) “once a year”, (3) “once every 6 months”, (4) “once every 3 months”, (5) “once a month”, (6) “twice every month”, (7) “once a week”, and (8) “multiple times a week.” The average frequency of alcohol use score was 2.34 (SD = 1.64)
2.1.2.2. Weekly alcohol use
In four items, adolescents disclosed the amount of glasses of alcohol that they had consumed in the week preceding the study, at home, outside home, on weekdays and in weekends (Engels et al., 1999). Answers on items were summed up to form a measure of the quantity of alcohol use in the past week. Most participants reported not drinking any glasses of alcohol the previous week (M = .39, Mdn = 0, SD = 1.34). A logarithmic transformation was applied to this score, which reduced the degree of skewness and kurtosis in the distribution of this measure. We performed separate analyses using the raw and transformed measures. The pattern of statistically significant results was identical in both analyses, so we report the results using the raw scores for ease of interpretation purposes. The frequency and quantity of alcohol use were moderately correlated (r = .49, p < .001).
2.1.2.3. Visual probe test
Participants completed the visual probe (VP) test, in which they had to respond to a probe (arrow pointing up or down) that appeared on a computer screen shortly after one alcohol and one soft drink picture were presented side by side for 1500 ms (one picture in each hemifield). The probe replaced an alcohol or a soft drink picture randomly over 112 trials and an index of attentional bias was calculated by subtracting mean reaction times to probes replacing alcohol from those replacing soft drink pictures. Positive numbers reflected a relative attentional bias for alcohol: faster reaction times to probes replacing an alcohol picture compared to a soft drink picture. We calculated an estimate of internal consistency by calculating the bias score for each quarter of the trials, and estimate an internal consistency measure (Cronbach's alpha). The internal consistency was low (α = .19), which is in line with other research using this measure (e.g. Schmukle, 2005).
2.1.2.4. Genotyping
For the determination of the OPRM1 A118G (rs 1799971) genotype we refer to previous work by Van Den Wildenberg et al. (2007b). DRD4 VNTR alleles were amplified by PCR using the PCR enzyme kit Phusion High-Fidelity DNA polymerase (Finnzymes) with forward primer 5′-TCTTCCTACCCTGCCCGCTCAT-3′ and reversed primer 5′-GCCTTGCGGAAGACGTTGCGGAACT-3′. The reaction mixture contained 0.5 mM of each primer, approximately 10–50 ng DNA, 200 mM dNTPs, 5× Phusion GC buffer and 3% DMSO in a final volume of 25 μl. After an initial denaturation step at 94 °C for 3 min, PCR consisted of 30 cycles of 30 seconds at 94 °C, 35 s at 60 °C, and 2 min at 72 °C. A final extension step of 5 min at 72 °C was followed by cooling of the samples to room temperature. PCR products were separated on a 2% agarose TAE gel at 100 V for 1.5 h next to a O’rangeruler 200 bp ladder mix and visualized using ethidium bromide. The allele frequencies for the OPRM1 A118G (rs 1799971) genotype in this sample were in conformity with Hardy–Weinberg equilibrium expectations (p > .05). However, this was not the case for the DRD4 (p < .05). Therefore, we did not include the DRD4 in the analyses. A total of 151 participants had a “no risk” profile for OPRM1 (AA genotype), and 44 participants had a “risk” profile for the OPRM1 genotype (at least one G-allele)
2.2. Results
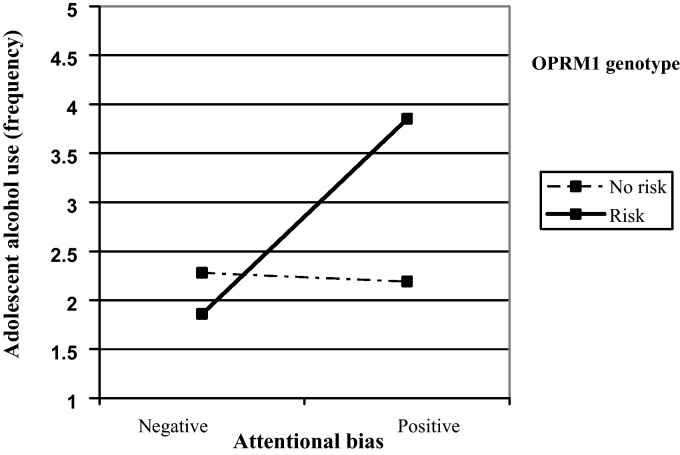
Two hierarchical linear regressions were performed to examine whether links between attentional bias and alcohol use were limited to adolescents with the OPRM1 risk genotype. In both analyses age, gender, OPRM1 genotype, attentional bias, and the interaction between OPRM1 and attentional bias were entered as predictors. In the first analysis, frequency of alcohol use was the dependent variable; quantity of alcohol use was the dependent variable in the second analysis. Table 1 presents the results of the regression involving frequency of alcohol use. The predictors explained a total of 17% of the variance on alcohol use frequency, F(5,189) = 7.86, p < .001. The main effect of age emerged as statistically significant, with older students reporting more frequent alcohol use than younger participants. The main effect for OPRM1 genotype was significant, but was qualified by an interaction with attentional bias. Fig. 1 presents the simple slopes describing this interaction. Specifically, attentional bias was positively associated with frequency of alcohol use for G-allele carriers (b = .99, se = .34, p = .004) and not significantly related to frequency of alcohol use for non G-allele carriers (b = −.04, se = .11, p = .71). So, attentional bias predicted more frequent alcohol use only for those in the OPRM1 risk group.
Table 1.
Linear regression analysis predicting the frequency of adolescent alcohol use (Study 1).
| Predictors | b | se | β | p |
|---|---|---|---|---|
| Gender (female = 1) | −.30 | .22 | −.09 | .173 |
| Age | .61 | .12 | .33 | <0.001 |
| OPRM1 genotype (risk = 1) | .53 | .26 | .14 | .045 |
| Attentional bias | −.04 | .12 | −.03 | .711 |
| OPRM1 genotype × attentional bias | 1.03 | .36 | .20 | .004 |
Note:N = 195.
Fig. 1.
Two-way interaction between OPRM1 genotype and attentional bias on frequency of alcohol use.
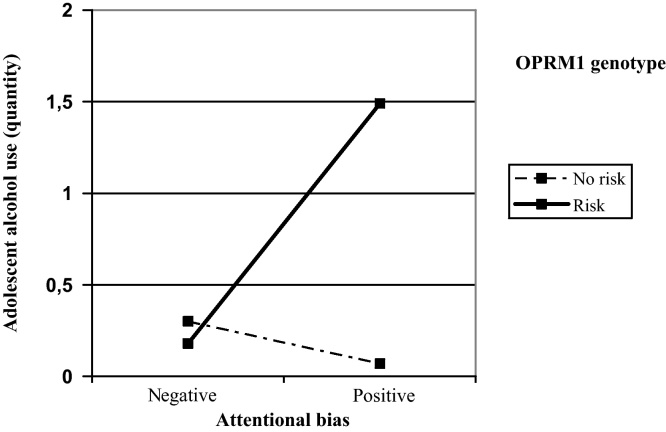
Table 2 presents the results of the regression involving alcohol use quantity. The predictors explained a total of 15% of the variance on alcohol use quantity, F(5,189) = 6.56, p < .001. The main effect of age emerged as statistically significant, with older students reporting more intense alcohol use than younger participants. The main effect for OPRM1 genotype was significant, but was qualified by an interaction with attentional bias. Fig. 2 presents the simple slopes describing this interaction. Specifically, attentional bias was positively associated with quantity of alcohol use for G-allele carriers (b = .54, se = .18, p = .003) and not for non G-allele carriers (b = −.09, se = .08, p = .25). So, attentional bias also predicted more intense alcohol use only for those in the OPRM1 risk group.
Table 2.
Linear regression analysis predicting the quantity of adolescent alcohol use (Study 1).
| Predictors | b | se | β | p |
|---|---|---|---|---|
| Gender (female = 1) | −.04 | .12 | −.02 | .756 |
| Age | .13 | .07 | .13 | .050 |
| OPRM1 genotype (risk = 1) | .65 | .14 | .32 | <.001 |
| Attentional bias | −.05 | .06 | −.06 | .438 |
| OPRM1 genotype × attentional bias | .59 | .19 | .22 | .003 |
Note: N = 195.
Fig. 2.
Two-way interaction between OPRM1 genotype and attentional bias on quantity of alcohol use.
In sum, the findings show that only for the OPRM1 G-allele carriers, attentional bias predicted more frequent alcohol use and higher levels of alcohol use in adolescents, indicating that “liking” and “wanting” both play a role in the development of alcohol use. It is expected that in later stages of alcohol use “wanting” plays becomes more important in the motivation to drink than “liking” when use has progressed to problematic use. We, therefore, concentrated on indices of problems associated with alcohol use as dependent variable in Study 2. In this study, only male heavy drinkers were included for two reasons: first, heavy drinking is far more prevalent in male late adolescents and young adults, and second, some evidence points to a more pronounced role in the development of problem drinking in males (Barr et al., 2007).
3. Study 2
3.1. Method
3.1.1. Participants and procedure
Participants were 86 heavy drinking male undergraduate students ranging in age from 18 to 26 years (M = 21.4, SD = 2.15). Participants were recruited by emails, posters and flyers that were posted throughout the university campus. Screening (e.g. of alcohol use) took place during a telephone interview. The inclusion criteria were as follows: male gender, age range between 18 and 28 years; a minimum of 20 standard alcoholic consumptions per week (containing approximately 10 g of pure alcohol, in the Netherlands, which is less than in the US and more than in the UK); at least 1 binge episode (6 or more standard drinks) in the past 2 weeks; and the participant as well as his parents were required to be from Dutch origin to prevent population admixture. The exclusion criteria were as follows: dyslexia and color blindness (related to another study in which the participants took part). Participants gave informed consent when they arrived at the lab, after which they completed the attentional bias task on a computer in a cubicle and completed short surveys. These included alcohol and drug use (based on time-line follow back method, Sobell and Sobell, 1990, Wiers et al., 2007), the affect-grid to assess mood (Russell et al., 1989), and craving for alcohol using a Visual Analogue Scale (Kozlowski et al., 1996; c.f. Schoenmakers et al., 2007). Before commencing the task, the experienced drinkers were given a small sip of beer, in order to prime alcohol-related attentional bias (Cox et al., 2003, Duka and Townshend, 2004, Jones and Schulze, 2000). For a detailed description of the procedure we refer to prior publications on this study (Schoenmakers et al., 2007, Van Den Wildenberg et al., 2007a, Van Den Wildenberg et al., 2007b). A total of 109 students participated in the study. Of these, 22 participants were excluded due to missing genetic data and one participant was excluded because he did not complete the items describing alcohol use. In total, this resulted in 86 participants.
3.1.2. Measures
3.1.2.1. 4.2.1 Problem drinking
Problem drinking was measured using seven items from the alcohol use disorders identification test (AUDIT; Saunders et al., 1993). The AUDIT is a screening instrument for alcohol use and problems, with the first three items assessing quantity and frequency of use, and items 4–10 assessing problems related to alcohol (ab)use. These latter items were used because they indicate drinking heavily drinking despite negative consequences. Response categories range from 0 (never or not at all) to 4 (daily or almost daily). Reliability was sufficient (α = .73). The average number of problems identified by participants was 5.02 (SD = 3.11).
3.1.2.2. Visual probe test
Data were used from the pre-test visual probe task that is described in detail elsewhere (Schoenmakers et al., 2007).1 In short, the task description resembled the one described in Study 1, although the picture-pairs in Study 2 were presented for 500 instead of 1500 ms and in Study 2, 48 trials were administered instead of 112. As in other studies (e.g. Schmukle, 2005), the internal consistency was low (α = .18).
3.1.2.3. Genotyping
OPRM1 and DRD4 were tested using the same method as in Study 1. A total of 49 participants had a “no risk” profile for both OPRM1 (c.118-AA genotype) and DRD4 (both alleles < 7 repeats) genotype, 13 participants had a “risk” profile only for the OPRM1 genotype (at least one G-allele), 20 participants had a “risk” profile only for the DRD4 genotype (7+ repeats), and 4 participants had a “risk” profile on both genotypes (OPRM1 G-allele and 7+ repeats for DRD4). Due to the small size of this dual risk group, these individuals were excluded from the analyses resulting in n = 82. The allele frequencies for both the OPRM1 and the DRD4 were in conformity with Hardy–Weinberg equilibrium expectations (p > .05) in this sample (see also Van Den Wildenberg et al., 2007a, Van Den Wildenberg et al., 2007b).
3.2. Results
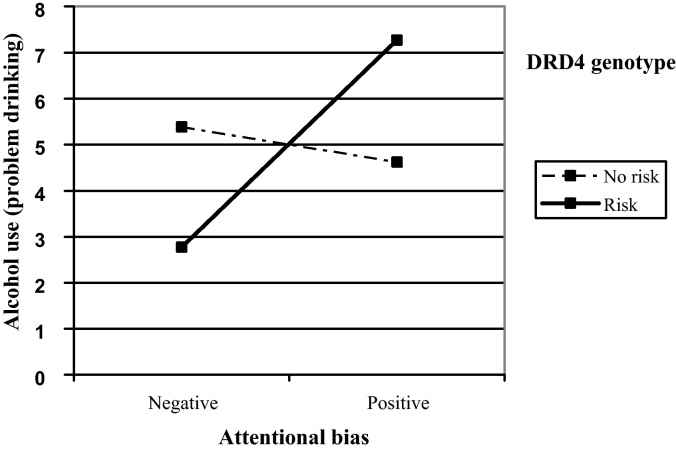
A hierarchical linear regression was performed to test whether the link between attentional bias and problem drinking was moderated by DRD4 and OPRM1 risk genotypes. Main effects of age, OPRM1 genotype, DRD4 genotype, attentional bias (centered), and two interactions (OPRM1 × attentional bias and DRD4 × attentional bias) were included as predictors of problem drinking. The predictors explained a total of 15% of the variance on problem drinking, F(6,75) = 2.28, p = .045. The DRD4 by attentional bias interaction emerged as the only statistically significant predictor of problem drinking (see Table 3). Fig. 3 presents the simple slopes describing the association between attentional bias and alcohol problems calculated separately for participants with and without the DRD4 risk profile. Attentional bias was positively associated with problem drinking for those with the DRD4 risk profile (b = 1.41, se = .63, p = .028) and was unrelated to problem drinking for those with a non-risk DRD4 profile (b = −.24, se = .40, p = .552). So, attentional bias predicted more problem drinking only for those in the DRD4 risk group.
Table 3.
Linear regression analysis predicting problem drinking of young adults (Study 2).
| Predictors | b | se | β | p |
|---|---|---|---|---|
| Age | −.16 | .16 | −.11 | .312 |
| OPRM1 genotype (risk = 1) | −.51 | 1.02 | −.06 | .618 |
| DRD4 genotype (risk = 1) | −.04 | .80 | −.01 | .964 |
| Attentional bias | −.24 | .40 | −.08 | .552 |
| OPRM1 genotype × attentional bias | −3.10 | 1.64 | −.23 | .062 |
| DRD4 genotype × attentional bias | 1.64 | .74 | .28 | .030 |
Note: N = 82.
Fig. 3.
Two-way interaction between DRD4 genotype and attentional bias on problem drinking.
4. General discussion
The main aim of this project was to explore the moderating role of the OPRM1 and DRD4 polymorphisms on the association between attentional bias and alcohol consumption in two independent samples. The interaction between OPRM1 genotype and attentional bias on alcohol use in the early adolescent sample corresponds with the literature stating that in the initial phases of drug use, “liking” and “wanting” are both important predictors of drug use and are still closely linked (Robinson and Berridge, 1993). For both frequency and quantity of alcohol consumption, results indicated a positive relation between attentional bias and alcohol consumption only in G-allele carriers compared to subjects homozygous for the A allele. In the young adult sample of heavy drinkers, it was found that DRD4 moderated the relation between attentional bias and alcohol problems. Only in the young adults with 7+ repeats on the polymorphism, attentional bias positively predicted problematic alcohol consumption. This suggests that in young adult heavy drinkers, individual differences related to “wanting” are more important concerning the relation between attentional bias and alcohol use than differences related to “liking”. The IST also points out that the development of addiction is associated with an increasing dissociation between “wanting” and “liking”, where the incentive properties of drugs gradually increase and incrementally dominate over the subjective pleasure generated by drugs, which steadily decreases (Robinson and Berridge, 1993). The present findings could be related to the previous research demonstrating that adult heavy drinkers do not decrease alcohol intake when liking is reduced, which does occur in light drinkers (Hobbs et al., 2005), and to research showing that attentional bias for alcohol increases in heavy drinkers after a prime dose of alcohol (Schoenmakers et al., 2008). Hence, these findings could indicate that an attentional bias for alcohol is related primarily to “wanting” in young heavy drinkers (where “wanting” and “liking” start to dissociate), while in early adolescents who begin to drink, “wanting” and “liking” still go hand in hand.
Although the findings are promising, the underlying mechanism is still unclear. Prior research has already examined direct effects of a genetic polymorphism on the processing of emotional stimuli (e.g. Beevers et al., 2007, Luscher et al., 2009, Munafò et al., 2005, Wiers et al., 2009). In these studies, a relation can be suggested between the presence of a certain risk allele that codes for functional differences at a neural level that impinge attention. This reasoning does not hold for the current study. The current study showed interactions between performance on the tasks and genetic polymorphisms on actual alcohol use. We thus demonstrated that the combination of having a certain genotype and an attentional bias for alcohol is relevant in explaining alcohol use.
Accordingly, this implies that a bias in the processing of alcohol cues is only partly affected by ones genotype. Recent dual process models of addiction (e.g. Wiers et al., 2007) put forward that drug addiction is the result of an imbalance in the relation between automatic and controlled processes. Related to alcohol use, it is stated that relatively automatic processes (e.g. attentional bias) strengthen due to repeated alcohol use, resulting in higher levels of alcohol use. However, controlled processes, which are compromised by alcohol, could inhibit the effect of automatic processes. It has been demonstrated that the effect of automatic processes is larger in individuals with poor working memory capacity (Thush et al., 2008). Regarding the current study, one could argue that the polymorphisms function as similar moderators. For instance, DRD4 risk alleles have been related to stronger risk taking and weaker behavioral inhibition (e.g. Congdon et al., 2007). This suggests that individuals without this risk are better at inhibiting automatic processes. Future research should examine how inhibitory processes are interfering with the effects we found.
Several caveats must be acknowledged. First, results are based on cross-sectional data in both studies, so no statements can be made regarding the causal order of the attentional bias and alcohol use. Future longitudinal research is needed to assess intra-individual differences over time. Second, the two samples were selective, thus limiting the generalizability of the results. Replication of these results in similar and more normative samples is warranted. Third, the attentional bias measure demonstrated a modest internal consistency in both studies (a known problem of this measure, c.f. Schmukle, 2005, Tull et al., in press), and the attentional bias and alcohol use measures were not identical in the two studies. The attentional bias measure demonstrated a modest internal consistency in both studies (a known problem of this measure, c.f. Schmukle, 2005, Tull et al., in press). Attentional bias was assessed in Study 2 after participants were given a small dose of alcohol before completing the attentional bias task, to prime the effect. This was not done in Study 1 because of ethical considerations. Frequency and quantity of alcohol use were assessed in Study 1, and problem drinking was assessed in Study 2. While these measures are age appropriate they are not directly comparable. Fourth, the allele frequencies of the DRD4 polymorphism in Study 1 were not in conformity with Hardy–Weinberg equilibrium expectations, and were subsequently excluded. Fifth, the inclusion of additional variables could impact these results. For instance, pubertal timing has been found to impact on the development of appetitive processes of adolescents (Casey and Jones, 2010, Steinberg, 2010, Wiers et al., 2007). Finally, “wanting” and “liking” were not directly assessed in this study. It would be of interest to relate the genotypes studied here to other indices of “liking” vs. “wanting” (e.g. the moderating effect of these genotypes on the differential effects between light and heavy drinkers on consumption of alcohol after an experimental decrease in liking, c.f. Hobbs et al., 2005). A similar manipulation could also be used to test genetic effects of other indirect measures proposed to reflect a sensitized reaction to alcohol-related cues (alcohol–arousal associations, attentional bias, alcohol-approach tendencies), and their relationship with alcohol-related problems.
In conclusion, this study has shown that individual differences related to two genetic polymorphisms previously related to alcohol use, might be important in explaining the developmental interplay between attentional bias and alcohol use. It is tentatively proposed that these polymorphisms are related to distinct psychological processes involved in adolescents’ and young adults’ appraisal of alcohol stimuli, “liking” and “wanting”, although future longitudinal research is required to critically test this hypothesis.
Footnotes
In fact, the order of the studies was so that the young adult study was run first, and assessment was optimized there for that sample (including an alcohol prime). The procedure in the younger sample was then adjusted for suitability in young adolescent group: no alcohol prime and longer presentation time, as has been done before in adolescents (e.g. Dalgeish et al., 2001).
Contributor Information
Sara Pieters, Email: Sarapieters@gmail.com.
Haske Van Der Vorst, Email: H.vandervorst@pwo.ru.nl.
William J. Burk, Email: W.burk@psych.ru.nl.
Tim M. Schoenmakers, Email: Schoenmakers@ivo.nl.
Esther Van Den Wildenberg, Email: Evandenwildenberg@stap.nl.
Hubert J. Smeets, Email: Bert.smeets@maastrichtuniversity.nl.
Ellen Lambrichs, Email: Ellen.lambrichs@maastrichtuniversity.nl.
Matt Field, Email: M.field@liverpool.ac.uk.
Rutger C.M.E. Engels, Email: R.engels@pwo.ru.nl.
Reinout W. Wiers, Email: R.W.H.J.Wiers@uva.nl.
References
- Barr C.S., Schwandt M., Lindell S.G., Chen S.A., Goldman D., Suomi S.J., Higley J.D., Heilig M. Association of a functional polymorphism in the mu-opioid receptor gene with alcohol response and consumption in male rhesus macaques. Arch. Gen. Psychiatry. 2007;64:369–376. doi: 10.1001/archpsyc.64.3.369. [DOI] [PubMed] [Google Scholar]
- Beevers C.G., Gibb B.E., McGeary J.E., Miller I.W. Serotonin transporter genetic variation and biased attention for emotional word stimuli among psychiatric inpatients. J. Abnorm. Psychol. 2007;116:208–212. doi: 10.1037/0021-843X.116.1.208. [DOI] [PubMed] [Google Scholar]
- Berridge K.C. Food reward: brain substrates of wanting and liking. Neurosci. Biobehav. Rev. 1996;20:1–25. doi: 10.1016/0149-7634(95)00033-b. [DOI] [PubMed] [Google Scholar]
- Berridge K.C., Robinson T.E., Aldridge J.W. Dissecting components of reward: ‘liking’ ‘wanting’, and learning. Curr. Opin. Pharmacol. 2009;9:65–73. doi: 10.1016/j.coph.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradberry C.W. Comparison of acute and chronic neurochemical effects of cocaine and cocaine cues in rhesus monkeys and rodents: focus on striatal and cortical dopamine systems. Rev. Neurosci. 2008;19:113–128. doi: 10.1515/revneuro.2008.19.2-3.113. [DOI] [PubMed] [Google Scholar]
- Breiter H.C., Gollub R.J., Weisskoff R.M., Kennedy D.N., Makris N., Berke J.D., Goodman J.M., Kantor H.L., Gastfriend D.R., Riorden J.P., Mathew R.T., Rosen B.R., Hyman S.E. Acute effects of cocaine on human brain activity and emotion. Neuron. 1997;19:591–611. doi: 10.1016/s0896-6273(00)80374-8. [DOI] [PubMed] [Google Scholar]
- Casey B.J., Jones R.M. Neurobiology of the adolescent brain and behavior: implications for substance use disorders. J. Am. Acad. Child Adolesc. Psychiatry. 2010;49:1189–1201. doi: 10.1016/j.jaac.2010.08.017. (quiz 1285) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Congdon E.M.A., Lesch K.P., Canli T. Analysis of DRD4 and DAT polymorphisms and behavioral inhibition in healthy adults: implications for impulsivity. Am. J. Med. Genet. 2007;147B:27–32. doi: 10.1002/ajmg.b.30557. [DOI] [PubMed] [Google Scholar]
- Cox W.M., Brown M.A., Rowlands L.J. The effects of alcohol cue exposure on non-dependent drinkers’ attentional bias for alcohol-related stimuli. Alcohol Alcohol. 2003;38:45–49. doi: 10.1093/alcalc/agg010. [DOI] [PubMed] [Google Scholar]
- Dalgeish T., Moradi A.R., Taghavi M.R., Neshat-Doos H.T., Yule W. An experimental investigation of hypervigilance for threat in children and adolescents with post-traumatic stress disorder. Psychol. Med. 2001;31:541–547. doi: 10.1017/s0033291701003567. [DOI] [PubMed] [Google Scholar]
- Duka T., Townshend J.M. The priming effect of alcohol pre-load on attentional bias to alcohol-related stimuli. Psychopharmacology. 2004;176:353–361. doi: 10.1007/s00213-004-1906-7. [DOI] [PubMed] [Google Scholar]
- Engels R.C.M.E., Knibbe R.A., Drop M.J. Visiting public drinking places: an explorative study into the functions of pub-going for late adolescents. Addiction. 1999;94:115–124. doi: 10.3109/10826089909039408. [DOI] [PubMed] [Google Scholar]
- Everitt B.J., Robbins T.W. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat. Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Field M., Christiansen P., Cole J., Goudie A. Delay discounting and the alcohol stroop in heavy drinking adolescents. Addiction. 2006;102:579–586. doi: 10.1111/j.1360-0443.2007.01743.x. [DOI] [PubMed] [Google Scholar]
- Field M., Cox W.M. Attentional bias in addictive behaviors: a review of its development, causes, and consequences. Drug Alcohol Depend. 2008;97:1–20. doi: 10.1016/j.drugalcdep.2008.03.030. [DOI] [PubMed] [Google Scholar]
- Field M., Kiernan A., Eastwood B., Child R. Rapid approach responses to alcohol cues in heavy drinkers. J. Behav. Therap. Exp. Psychiatry. 2008;39:209–218. doi: 10.1016/j.jbtep.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Field M., Munafo M.R., Franken I.H. A meta-analytic investigation of the relationship between attentional bias and subjective craving in substance abuse. Psychol. Bull. 2009;135:589–607. doi: 10.1037/a0015843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filbey F.M., Ray L., Smolen A., Claus E.D., Audette A., Hutchison K.E. Differential neural response to alcohol priming and alcohol taste cues is associated with DRD4 VNTR and OPRM1 genotypes. Alcohol Clin. Exp. Res. 2008;32:1113–1123. doi: 10.1111/j.1530-0277.2008.00692.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes E.E., Dahl R.E. Pubertal development and behavior: hormonal activation of social and motivational tendencies. Brain Cogn. 2010;72:66–72. doi: 10.1016/j.bandc.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant B.F., Dawson D.A. Age at onset of alcohol use and its association with DSM-IV alcohol abuse and dependence: results from the national longitudinal alcohol epidemiologic survey. J. Subst. Abuse Treat. 1997;9:103–110. doi: 10.1016/s0899-3289(97)90009-2. [DOI] [PubMed] [Google Scholar]
- Hobbs M., Remington B., Glautier S. Dissociation of wanting and liking for alcohol in humans: a test of the incentive-sensitization theory. Psychopharmacology. 2005;178:493–499. doi: 10.1007/s00213-004-2026-0. [DOI] [PubMed] [Google Scholar]
- Hutchison K.E., Swift R., Rohsenow D.J., Monti P.M., Davidson D., Almeida A. Olanzapine reduces urge to drink after drinking cues and a priming dose of alcohol. Psychopharmacology. 2001;155:27–34. doi: 10.1007/s002130000629. [DOI] [PubMed] [Google Scholar]
- Hutchison K.E., McGeary J., Smolen A., Bryan A., Swift R.M. The DRD4 VNTR polymorphism moderates craving after alcohol consumption. J. Health Psychol. 2002;21:139–146. [PubMed] [Google Scholar]
- Jones B.T., Schulze D. Alcohol-related words of positive affect are more accessible in social drinkers’ memory than are other words when sip-primed by alcohol. Addict. Res. Theory. 2000;8:221–232. [Google Scholar]
- Kozlowski L.T., Pillitteri J.L., Sweeney C.T., Whitfield K.E. Asking questions about urges or cravings for cigarettes. Psychol. Addict. Behav. 1996;10:248–260. [Google Scholar]
- Le Foll B., Gallo A., Le Strat Y., Lu L., Gorwood P. Genetics of dopamine receptors and drug addiction: a comprehensive review. Behav. Pharmacol. 2009;20:1–17. doi: 10.1097/FBP.0b013e3283242f05. [DOI] [PubMed] [Google Scholar]
- Leyton M. Conditioned and sensitized responses to stimulant drugs in humans. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2007;31(8):1601–1613. doi: 10.1016/j.pnpbp.2007.08.027. [DOI] [PubMed] [Google Scholar]
- Luijten M., Veltman D.J., van den Brink W., Hester R., Field M., Smits M. Neurobiological substrate of smoking-related attentional bias. Neuroimage. 2010;54:2374–2381. doi: 10.1016/j.neuroimage.2010.09.064. [DOI] [PubMed] [Google Scholar]
- Luscher J., Chandler C., Ball D. The dopamine D4 receptor gene (DRD4) is associated with attentional bias in heroin abusers and cigarette smokers. Open Addict. J. 2009;2:6–11. [Google Scholar]
- Munafò M.R., Johnstone E.C., Mackintosh B. Association of serotonin transporter genotype with selective processing of smoking-related stimuli in current smokers and ex-smokers. Nicotine Tob. Res. 2005;7:773–778. doi: 10.1080/14622200500259861. [DOI] [PubMed] [Google Scholar]
- Nesse R.M., Berridge K.C. Psychoactive drugs in evolutionary perspective. Science. 1997;278:63–66. doi: 10.1126/science.278.5335.63. [DOI] [PubMed] [Google Scholar]
- Pieters S., Van Der Vorst H., Burk W.J., Wiers R.W., Engels R.C.M.E. Puberty-dependent sleep regulation and alcohol use in early adolescents. Alcohol Clin. Exp. Res. 2010;34:1–7. doi: 10.1111/j.1530-0277.2010.01235.x. [DOI] [PubMed] [Google Scholar]
- Ray L.A., Hutchison K.E. Polymorphisms in the mu-opioid receptor gene (OPRM1) and the implications for alcohol dependence in humans. Alcohol Clin. Exp. Res. 2004;28:1789–1795. doi: 10.1097/01.alc.0000148114.34000.b9. [DOI] [PubMed] [Google Scholar]
- Robinson T.E., Berridge K.C. The neural basis of drug craving an incentive-sensitization theory of addiction. Brain Res. Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Robinson T.E., Berridge K.C. Addiction. Annu. Rev. Psychol. 2003;54:25–53. doi: 10.1146/annurev.psych.54.101601.145237. [DOI] [PubMed] [Google Scholar]
- Robinson T.E., Berridge K.C. Review. The incentive sensitization theory of addiction: some current issues. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2008;363:3137–3146. doi: 10.1098/rstb.2008.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell J.A., Weiss A., Mendelsohn G.A. Affect grid: a single-item scale of pleasure and arousal. J. Pers. Soc. Psychol. 1989;57:493–502. [Google Scholar]
- Saunders J.B., Aasland O.G., Babor T.F., De La Fuente J.R., Grant M. Development of the alcohol use disorders identification test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption. II. Addiction. 1993;88:791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- Schoenmakers T., Wiers R.W., Jones B.T., Bruce G., Jansen A.T.M. Attentional re-training decreases attentional bias in heavy drinkers without generalization. Addiction. 2007;102:399–405. doi: 10.1111/j.1360-0443.2006.01718.x. [DOI] [PubMed] [Google Scholar]
- Schoenmakers T., Wiers R.W., Field M. Effects of a low dose of alcohol on cognitive biases and craving in heavy drinkers. Psychopharmacology. 2008;197:169–178. doi: 10.1007/s00213-007-1023-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmukle S.C. Unreliability of the dot probe task. Eur. J. Pers. 2005;19:595–605. [Google Scholar]
- Sobell L.C., Sobell M.B. Self-report issues in alcohol abuse: state of the art and future directions. Behav. Assess. 1990;12:77–90. [Google Scholar]
- Steinberg L. A dual systems model of adolescent risk-taking. Dev. Psychobiol. 2010;52:216–224. doi: 10.1002/dev.20445. [DOI] [PubMed] [Google Scholar]
- Steinberg L., Albert D., Cauffman E., Banich M., Graham S., Woolard J. Age differences in sensation seeking and impulsivity as indexed by behavior and self-report: evidence for a dual systems model. Dev. Psychol. 2008;44:1764–1778. doi: 10.1037/a0012955. [DOI] [PubMed] [Google Scholar]
- Stetter F., Ackermann K., Bizer A., Straube E.R., Mann K. Effects of disease-related cues in alcoholic inpatients: results of a controlled “Alcohol Stroop” Study. Alcohol Clin. Exp. Res. 2006;19:593–599. doi: 10.1111/j.1530-0277.1995.tb01553.x. [DOI] [PubMed] [Google Scholar]
- Thush C., Wiers R.W. Explicit and implicit alcohol-related cognitions and the prediction of current and future drinking in adolescents. Addict. Behav. 2007;32:1367–1383. doi: 10.1016/j.addbeh.2006.09.011. [DOI] [PubMed] [Google Scholar]
- Thush C., Wiers R.W., Ames S.L., Grenard J.L., Sussman S., Stacy A.W. Interactions between implicit and explicit cognition and working memory capacity in the prediction of alcohol use in at-risk adolescents. Drug Alcohol Depend. 2008;94:116–124. doi: 10.1016/j.drugalcdep.2007.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townshend J.M., Duka T. Attentional bias associated with alcohol cues: differences between heavy and occasional social drinkers. Psychopharmacology. 2001;157:67–74. doi: 10.1007/s002130100764. [DOI] [PubMed] [Google Scholar]
- Tull, M.T., McDermott, M.J., Gratz, K.L., Coffey, S.F., Lejuez, C.W. Cocaine-related attentional bias following trauma cue exposure among cocaine. Addiction, in press. [DOI] [PMC free article] [PubMed]
- Van Der Vorst H., Vermulst A.A., Meeus W., Deković M., Engels R.C.M.E. Identification and prediction of drinking trajectories in early and mid-adolescence. J. Clin. Child Adoles. Psychol. 2009;38:329–341. doi: 10.1080/15374410902851648. [DOI] [PubMed] [Google Scholar]
- Van Den Wildenberg E., Wiers R.W., Dessers J., Janssen R.G.J.H., Lambrichs E.H., Smeets H.J.M., Van Breukelen G.J.P. A functional polymorphism of the μ-opioid receptor gene (OPRM1) influences cue-induced craving for alcohol in male heavy drinkers. Alcohol Clin. Exp. Res. 2007;31:1–10. doi: 10.1111/j.1530-0277.2006.00258.x. [DOI] [PubMed] [Google Scholar]
- Van Den Wildenberg E., Janssen R.G.J.H., Hutchison K.E., Van Breukelen G.J.P., Wiers R.W. Polymorphisms of the dopamine D4 receptor gene (DRD4 VNTR) and cannabinoid CB1 receptor gene (CNR1) are not strongly related to cue-reactivity after alcohol exposure. Addict. Biol. 2007;12:210–220. doi: 10.1111/j.1369-1600.2007.00064.x. [DOI] [PubMed] [Google Scholar]
- Wiers R.W., Ames S.L., Hofmann W., Krank M., Stacy A.W. Impulsivity, impulsive and reflective processes and the development of alcohol use and misuse in adolescents and young adults. Front Psychol. 2010;1(144):1–12. doi: 10.3389/fpsyg.2010.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiers R.W., Bartholow B.D., Van Den Wildenberg E., Thush C., Engels R.C.M.E., Sher K.J., Grenard J., Ames S.L., Stacy A.W. Automatic and controlled processes and the development of addictive behaviors in adolescence: a review and a model. Pharmacol. Biochem. Behav. 2007;86:263–283. doi: 10.1016/j.pbb.2006.09.021. [DOI] [PubMed] [Google Scholar]
- Wiers R.W., Rinck M., Dictus M., Van Den Wildenberg E. Relatively strong automatic appetitive action-tendencies in male carriers of the OPRM1 G-allele. Genes Brain Behav. 2009;8:101–106. doi: 10.1111/j.1601-183X.2008.00454.x. [DOI] [PubMed] [Google Scholar]
- Wiers R.W., van Woerden N., Smulders F.T.Y., de Jong P.J. Implicit and explicit alcohol-related cognitions in heavy and light drinkers. J. Abnorm. Psychol. 2002;111:648–658. doi: 10.1037/0021-843X.111.4.648. [DOI] [PubMed] [Google Scholar]