Research highlights
▸ A description of neuroscience findings and classic theories of cognitive development. ▸ A review of working memory research as an illustrative point from two perspectives. ▸ Shortcomings of prior research in mergence of neuroscience and developmental theory. ▸ Suggestions and starting points for crosstalk in future research, which can be applied to other domains as well.
Keywords: Brain maturation, Neuroimaging, Cognitive development, Neuroconstructivism, Working memory
Abstract
Surprisingly little headway has been made towards understanding how brain growth maps onto mental growth during child development. This review aims at bridging and integrating recent human neuroscientific brain maturation findings with the conceptual thinking of theorists in the behavioural tradition of studying cognitive development. Developmental research in the field of internal control and self-regulation serves as a reference point for understanding the relation between brain maturation and mental growth. Using several recent neuroimaging findings as points in case, we show how a deeper appreciation of structural and functional neural development can be obtained from considering the traditional conceptual frameworks, and vice versa. We conclude that paradigmatic progress in developmental neuroscience can rely more on knowledge from developmental experimental psychology, and that developmental models of cognitive development can be constrained and articulated with more precision on the basis of knowledge of differential structural and functional brain maturation.
1. Introduction
Developmental neuroimaging studies have had a great impact on thinking about developmental changes in behaviour. Textbooks on cognitive development are now incorporating brain development as further explanations for developmental improvements in a wide area of skills (e.g., Blakemore and Frith, 2005, Goswami, 2008), and neuroscientists are speculating about how brain development results in changes in cognitive function (e.g., Shaw et al., 2006). Despite this mutual interest, the two research areas (developmental psychology and neuroscience) are still segregated and a gap remains between our knowledge of brain development and cognitive development. Developmental neuroimaging studies tend to be data-driven rather than theory-driven; that is, these studies tend to be inspired more by the prospect of finding differential maturational trajectories of specific structures and functions than by predictions derived from theoretical perspectives on mental growth (see also Johnson, 2001, Johnson, 2010). It is not uncommon that developmental changes in cognitive function are interpreted as the simple maturation of a single brain area or circuit, ignoring the long history of cognitive theorizing about the development of thought and behaviour. Likewise, it is not uncommon that developmental changes in domains such as working memory are explained in terms of classic stage-wise development while ignoring the significant improvements that have been made in relating these functions to brain maturation, which could constrain the currently available developmental theories.
The goal of this review is to identify initial steps towards understanding recent neuroscientific brain development findings in the context of classic developmental theories of cognitive development. We identify several problems which occur when neuroimaging studies are designed without taking into account the prior findings from classic developmental theories.
Before the advent of developmental neuroimaging, several endeavors have been undertaken towards integrating mental development and brain maturation (e.g., Crnic and Pennington, 1987, Dawson and Fischer, 1994, Schore, 1994, Segalowitz and Rose-Krasnor, 1992; for an extensive review see van der Molen and Ridderinkhof, 1998). Initial attempts focused on measures of brain size. In a series of studies, Epstein, 1974a, Epstein, 1974b derived his phrenoblysis hypothesis (the Greek word “phreno” stands for skull or mind while “blysis” refers to welling-up of matter), suggesting that the brain grows in spurts with peaks in growth rate occurring between 6 and 8, 10 and 12, and 14 and 17 years of age. The correlated patterns of peaks and troughs in brain growth and mental growth (as indexed by intelligence tests) suggested to Epstein (1978) a link with Piagetian theory. The spurts in brain growth would precede and prepare stage transitions in cognitive development. The phrenoblysis hypothesis has met with considerable methodological criticism, however.
Other attempts focused on the spectral analysis of brain electrical activity, as measured using the electro-encephalogram (EEG). Matousek and Petersén (1973) reported developmental changes in spectral power that showed periods of rapid growth alternating with periods of slow growth, taken to support stage theories of brain maturation (Hudspeth and Pribram, 1992), echoing Epstein's hypothesis formulated almost 20 years earlier. Thatcher (1994) suggested that EEG coherence, a spectral measure that provides an index of the functional coupling of neural generators, revealed growth spurts in cortical organization are repeated during three major developmental cycles, with transitions at approximately 6 and 10 years. These findings were interpreted to provide support for neo-Piagetian views of cognitive development. The hypothesis of stage-wise cognitive development driven by iterative and sequential brain-growth cycles remains to be confirmed, however, for instance by longitudinal studies showing consistent relations between individual differences in brain growth and cognitive development.
Here, we set out to revive and rejuvenate this general approach by relating recent neuroimaging findings using cognitive paradigms to the theoretical bases which have shaped the thinking of developmental scientists. Given that functional developmental neuroimaging studies to date have focused mainly childhood and adolescence (ages 6–7 and older), we will restrict the review to this age period. This focus allows us to make more solid inferences on the relation between brain developmental and cognitive development. We further restrict our review to the functional domain of internal control and working memory development, because this domain is well described in both the information-processing theories of cognitive development and in recent neuroimaging studies. Many of the recommendations we present following this review, however, are also applicable to other domains of cognitive development where links are made with brain development, such as inhibition, reasoning, self-regulation, and mentalizing functions (e.g., theory of mind).
2. Brain maturation
Biological models of brain development have made great progress in understanding the development of brain structure, especially since the use of magnetic resonance imaging (MRI). In the early days of developmental neuroscience, our knowledge of brain structure and development were mostly based on post-mortem studies, which demonstrated that the size of the brain increases until age 9–10 and by that time the size and weight of the brain does not change that much anymore until senescence (Huttenlocher, 1979, Huttenlocher et al., 1983). These studies reported on two main changes which occur in the developing brain. First, expansion of the layer of myelin around the axon of developing neurons continues into adolescence, especially for the frontal regions of the brain. Second, synaptic density, or the number of synapses in a certain volume of brain tissue, increases dramatically in early in post-natal development, followed by pruning during maturation.
The rise of in vivo brain scanning methods in the last 20–30 years, including MRI, allowed researchers to examine changes in brain structure on a much broader scale and in much more detail. Brain structure changes could now be studied across time within the same individuals, and several reports have confirmed that there are important changes in grey and white matter structure until late adolescence. The development of white-matter tracks has recently been studied by the use of diffusion tensor imaging (DTI), which provides sensitive measures of the changes in microstructure of white matter in the brain that occurs across childhood and adolescence. These studies have reported a steady increase in white-matter density and myelination across childhood, and despite some reports showing region-specific changes, the majority of studies propose that these changes are wide-spread across brain regions (Pfeffenbaum et al., 1994, Paus et al., 1999, Giorgio et al., 2008). In contrast to white-matter development, grey-matter development seems to follow an inverted U-shaped pattern of region-specific developmental changes. Thus, whereas white matter develops steadily and linearly across brain areas, grey-matter density follows progressive and regressive changes, which follow a different time course depending on the specific brain region. The latest changes are observed in the prefrontal cortex, parietal cortex and superior temporal cortex, with grey-matter peaks around puberty. In prefrontal and parietal cortex grey matter peaks around age 12 for males and around age 11 for females, whereas in temporal cortex grey matter peaks around age 17 (Gogtay et al., 2004, Sowell et al., 2004).
Despite the large body of evidence about structural brain development, much less is known about how these changes map onto the development of cognitive functions which are observed across childhood and adolescence. A handful of studies have examined how structural brain development is predictive of cognitive functioning, as expressed in for instance IQ, and these studies show that region-specific changes in synaptogenesis in prefrontal cortex can predict whether children are low, middle or high in IQ (Shaw et al., 2006). It is also believed that critical or sensitive periods in learning, which are time points in development during which children learn new skills that cannot be learned with the same precision and success during other periods of life, are the result of synaptic changes which allow a great speed of assimilation to currently changing environmental demands (Blakemore and Choudhury, 2006, Blakemore, 2008, Johnson, 2001, Johnson, 2010). The exact relation between structural brain development and cognitive development, however, is still largely unknown.
A method which maps cognitive functioning to brain function more directly is functional MRI (fMRI). Using this approach, it is possible to examine brain functioning in vivo in developing populations while they perform a certain cognitive task (Casey et al., 2005). Despite the limitations that co-occur with this technique, such as the correlative nature of the data or the commonly applied use of reversed inferences (Poldrack, 2005), the cumulating number of developmental MRI studies is most likely the most promising approach for integrating knowledge of brain development with classic developmental theories of cognitive development. The promising steps in this direction are indicative of interpretable changes in brain function and behaviour, although it should be noted at the outset that the developmental imaging studies to date do not yet allow for a direct test of cognitive developmental theories. These shortcomings are mostly related to the use of wide age ranges and broad experimental manipulations, and these limitations will also be highlighted during this review.
3. Cognitive development theories vis-à-vis brain development
3.1. Developmental theories
The work of Jean Piaget has probably been most influential in our thinking about cognitive development, as it describes the periods of major change in cognitive processes that support abstract thinking (Piaget, 1952a, Piaget, 1952b, Piaget, 1965, Piaget and Inhelder, 1974). Before he started to describe his observations of developing children, cognitive developmental psychology was hardly if at all established as a discipline in its own right. His thinking about development was based on questions which have inspired philosophers for centuries, such as ‘Where does knowledge come from?’ and ‘How does intelligence develop?’ In addition, Piaget's early interest in cognitive development grew out of his interest in biology. Inspired by the likes of Darwin, Piaget was interested in questions such as ‘How do people and knowledge evolve?’ In this sense, the theory of Piaget had already a strong link with brain development, because it was based on assumptions of interaction between preprogrammed biological systems and changing environmental demands, which together produce rapid changes in development. Piaget's theory does nowadays no longer accommodate for the full range of findings derived from developmental experimental psychology, particularly with respect to its description of mental changes in infancy. Much research in recent years with young infants and toddlers has shown that Piaget's approach to studying cognitive development may have underestimated cognitive abilities in younger children by using complicated designs and methods. For example, there is evidence that shows that preoperational toddlers will pass a false-belief task, if it is designed appropriately (e.g., Southgate et al., 2010), or that 9-month-old infants exhibit predictive motor activation when perceiving grasping movements (Southgate et al., 2009). Despite the controversial nature of his detailed theoretical claims, Piaget's theory can provides an example to illustrate the kinds of ideas and concepts that originated from psychology and that developmental neuroscience needs to tackle.
Perhaps the most influential of Piaget's ideas are his developmental stages, propelled by dynamic processes of assimilation and accommodation. These ideas could be said to be reminiscent of sensitive periods in brain development, as indicated by synaptogenesis and synaptic pruning. For example, Piaget suggested that a child cannot reach a new stage before mastering the old one (Brainerd, 1978, Flavell, 1963, Flavell, 1971), which has similarities with the idea of a hierarchical development of conscious control levels (Zelazo, 2004). Interestingly, synaptic density studies suggest that changes in grey matter develop at different rates for different brain regions (Huttenlocher et al., 1983; see also Gogtay et al., 2004), and the change in grey matter in a higher-order brain region would not contribute to cognitive function if the grey-matter changes in a supporting brain region were not yet completed (Casey et al., 2005). Whether the changes occur suddenly or through slow accumulation of knowledge (which is suddenly observable) has remained unclear (Fisher and Bidell, 1991, Siegler, 1981), but theories of brain maturation and of stage-wise mental development agree that changes in cognitive skills occur through an interplay between biological programs and accumulating environmental input (see also Karmiloff-Smith, 2006).
Information-processing theories, which often build upon the classic Piagetian framework, have emphasized how biologically based growth of internal control, self-regulation, working memory and automatization allows children to progressively increase processing limits (Demetriou et al., 1993, Fisher, 1980, Halford, 1993, Halford, 1995). For example, the development of working memory capacity has been explained as an age-related increase in processing space, in which the absolute capacity of working memory does not change, but the capacity functions more efficiently with advancing age. Early in development, children are thought to rely more on working memory storage space, but the processes are less efficient. In contrast, across development, children increase in processing ability, and consequently, there is a decrease in the necessary storage space (Case, 1992). These changes in capacity may be influenced by a general increase in processing speed which could underlie performance enhancements in a wide variety of domains, including working memory (Kail, 1991, Kail, 2007).
More recently, and influenced by findings from patients with prefrontal damage, it is assumed that internal control and working memory capacity are associated with the emergence of executive functions (Dempster, 1993, Diamond, 2002). Executive functions refer to the ability to control our thoughts and actions with the purpose of achieving future goals. Changes in executive functions could account for developmental improvements in a variety of higher-order processing domains, although it is still debated whether these are co-occurring but separate developments (Huizinga et al., 2006) or whether a general reflective level of processing underlies these improvements (Zelazo, 2004). For example, an information-processing theory referred to as the levels-of-consciousness theory, postulates that the development of the levels of consciousness goes via hierarchical functional system changes, where young children may master one level of processing (e.g., keeping rules active in mind) but not another level of processing (e.g., flexibly switching between competing rules). Once the highest level of consciousness is reached, children can solve the most complex problems, which can explain the observed age-related improvements in executive functions (Zelazo, 2004).
Based on these behavioural models, it is generally predicted that two or more brain systems work closely together when performing complex tasks, but the way in which they work together is predicted to be different. For example, according to Case's conceptualization of working memory development, there should be one system which is important for storage of information, and a second system which is important for processing information (or executive control of stored information, Baddeley, 1992, Baddeley, 2003), and the relative contribution of these systems changes during development. In contrast, the levels of conscious processing account suggests that executive function and reflective consciousness is dependent on the maturation of additional brain regions in prefrontal cortex (see also Bunge and Zelazo, 2006). The challenge is now to use neuroimaging methods to directly test the developmental theories of working memory and executive function. For this purpose, we will summarize neuroimaging research which focused on different types of working memory and control functions.
3.2. Brain development supporting changes in working memory and control
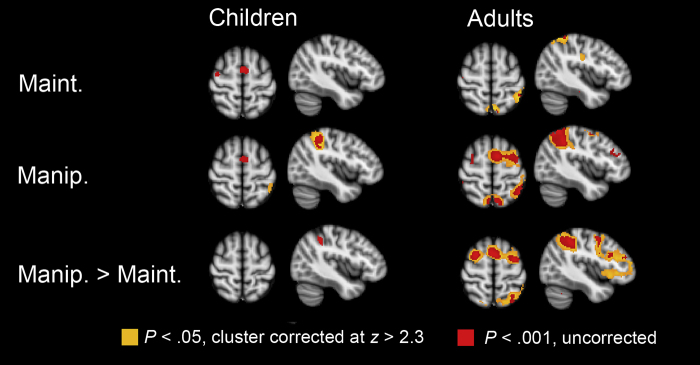
Functional neuroimaging studies have built upon the idea that working memory development increases across this specific age period, by comparing brain activation in 8–17-year-old children relative to adults. Neuroimaging studies in adults have shown that regions within prefrontal cortex (PFC, in particular lateral PFC) and parietal cortex are important for the maintenance and manipulations of information in working memory (Smith and Jonides, 1999, Passingham et al., 2000, Bunge et al., 2003, Olesen et al., 2004, see also Fig. 1). The first developmental neuroimaging studies have focused on the pure maintenance of information in memory, by instructing participants to maintain verbal or spatial information online over the course of several seconds, followed by a probe demanding a button press. These studies have shown that the increased ability to maintain information online between ages 8 and 12 and young adulthood coincides with increased activation in lateral PFC and parietal cortex (Klingberg et al., 2002, Luna et al., 2001, Kwon et al., 2002, O’Hare et al., 2008, Thomason et al., 2009). A comparison of fMRI data and DTI data revealed that increased fractional anisotropy in fronto-parietal white matter – suggestive of increased strength of anatomical connectivity between these regions – is positively correlated with BOLD activation in the lateral PFC and parietal cortex and with visuospatial working memory capacity (Olesen et al., 2003). Thus, these findings seem to be in favour of the hypothesis that processing capacity increases over the course of child development.
Fig. 1.
Working memory related activation in children and adults. In the working memory task, participants were required to remember a sequence of objects in forward order (maintenance condition) or backward order (manipulation condition) during a delay of 6 s. After the delay period, one object was presented and participants had to indicate the position of the object in the forward or backward sequence. This figure shows delay-period activation when three objects had to be remembered. The images are overlaid on axial and sagittal slices (z = 60 and x = −42) of a standard anatomical image. The left side of the image is the right side of the brain. Results are thresholded at p < .05, cluster corrected, using clusters determined by z > 2.3 (orange) and at p < .001, uncorrected (red). Maint. = maintenance; Manip = manipulation (based on Jolles et al., 2010; Developmental Science). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
Prior behavioural research, however, postulated that working memory depends on the interplay between storage capacity and processing efficiency (Case, 1992). In this sense, the developmental studies which have focused on pure maintenance may only have tapped improvements in storage capacity with stable processing demands. Subsequent studies have examined these processing demands in more detail by asking children to manipulate information in working memory (Crone et al., 2006a, Crone et al., 2006b, Jolles et al., in press; Fig. 1). These studies differentiated between pure maintenance and manipulation and found evidence for different neural systems which support these functions. That is, ventrolateral PFC was active only for maintenance trials, whereas dorsolateral PFC was additionally recruited for manipulation trials. The finding of slower development of dorsolateral PFC could be taken as evidence for delayed processing development. According to Case's terminology, especially working memory manipulation should result in increased demands on processing capacity, whereas working memory maintenance should rely more on storage capacity. Therefore, the brain imaging results could be taken to suggest that storage capacity can remain stable (as indicated by stable levels of ventrolateral PFC activation), and processing capacity may independently increase with advancing age (as indicated by increased levels of dorsolateral PFC activation when processing demands increase).
Whereas the neuroimaging studies described above have mainly reported change in terms of increases and decreases of task-relevant areas, qualitative developmental differences have been reported as well. For example, Scherf et al. (2006) demonstrated that 8–12-year-old children and 13–17 years old show a qualitatively different activation pattern when performing a working memory maintenance task. This study showed that 8–12-year-old children failed to recruit the working memory network that is core to working memory performance in adults (lateral PFC and parietal cortex), but instead rely on a different region within PFC to perform the task (ventromedial PFC). In contrast, adolescents aged 13–17 years recruited lateral PFC, just like adults did, but failed to activate this region to a similar extent. The results by Scherf et al. (2006) could be interpreted as a shift in storage or processing capacity between ages 8–12 and 13–17 (see also Ciesielski et al., 2006 for a similar shift between children and adults, although in a different network). The results also indicate that adult levels of working memory are not yet reached in adolescence, and that there is a refinement of control abilities that cannot always be observed on the basis of behaviour only (see also Kwon et al., 2002, Klingberg et al., 2002). In this sense, the neuroimaging findings extend the results from information-processing theories by showing that the underlying network which is important for abstract thought is still improving across adolescent development.
Taken together, prior studies which have used working memory paradigms have reported that the brain regions that are important for these functions in adults are differentially engaged in childhood and adolescence. However, the paradigms are often relatively simple and do not allow for a direct test of changes in storage capacity, the processing of information in working memory, and the relation with competing information in working memory. Therefore, a challenge for future research is to develop experimental paradigms based on developmental theory which allows for the test of different neural development accounts.
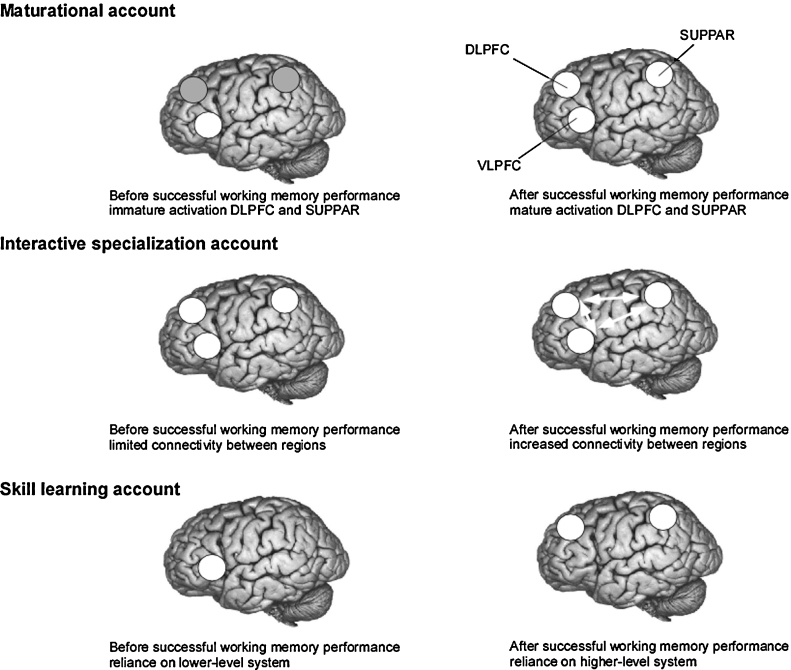
What theoretical framework can we use to interpret the neuroimaging findings? There is probably not one theory available which serves this goal, but there are theories available which allow for a direct mapping between cognitive theories and brain development trajectories. The functional development of the human brain could, for example, be related to developmental theories in a testable way using the neural maturation accounts put forward by Johnson, 2001, Johnson, 2010 and Johnson et al. (2009). This theory was developed to provide a biological framework for the development of early attention systems. According to Johnson, 2001, Johnson, 2010, cognitive abilities emerge through three possible routes of neuroanatomical development: (1) maturational progress (the maturation of additional brain areas), (2) interactive specialization (changes in interactions between brain areas that were already partially active) and (3) skill learning (the patterns of activation of cortical regions change during the acquisition of new skills). Even though this model was developed to account for postnatal and experience-dependent changes in the first two years of life, these trajectories of neuroanatomical development could also be used as a starting point for studying more advanced levels of cognitive development, such as working memory. Fig. 2 provides an example of how these maturation accounts can be translated in activation patterns observed in ventrolateral PFC, dorsolateral PFC and superior parietal cortex. For example, the additional recruitment of dorsolateral PFC in working memory manipulation studies could be interpreted in terms of the maturational account (as predicted by theories of executive function development), but also in terms of the interactive specialization account (as predicted by classic working memory information-processing theories). In contrast, the qualitative differences in brain activation between children and adults support the skill learning account. Judicious task manipulations should allow for the test of these different accounts. Even though all three theoretical possibilities can account for observed changes in brain activation, Johnson (2010) argues in favour of the interactive specialization. Future studies should test whether this developmental trajectory accounts for developmental data better than the maturational and skill learning account.
Fig. 2.
Neural basis of development accounts, based on Johnson (2001), applied to development of working memory. Brain regions implicated in working memory function are ventrolateral prefrontal cortex (VLPFC), dorsolateral prefrontal cortex (DLPFC) and superior parietal cortex (SUPPAR). The maturational account poses that children become better at performing manipulation trials because of the maturation of additional brain areas, such as DLPFC and SUPPAR. The interactive specialization account suggests that the performance increases are associated with a refinement of connectivity between brain regions (VLPFC, DLPFC and SUPPAR). The skill learning account suggests that children may first perform a working memory task based on reliance on a lower-level system (such as VLPFC) but, when older, they rely on different system (such as DLPFC and SUPPAR).
4. Merging developmental theory with developmental imaging
In the prior sections we showed that the interpretation of brain imaging results is limited by the relative absence of developmental theories. Despite the advances which have been accomplished in the field of developmental neuroimaging, it is now time to also consider the limitations of the approaches that have been used so far, and to think about ways of improving our experimental designs based on theoretical knowledge of cognitive development. Here, we summarize several problems and possible solutions for achieving the integration of these fields (see also Table 1).
Table 1.
Guidelines for neuroimaging research based on developmental theory.
| Suggested guidelines for neuroimaging research | |
|---|---|
| 1 | Selection of age groups should be theory-driven |
| 2 | Compute brain-behaviour correlations and be aware of differences in effect sizes |
| 3 | Maturation and change can only be examined using longitudinal designs |
| 4 | Training and intervention studies can demonstrate the malleability of cognitive functions and underlying neural mechanisms |
| 5 | Compensatory brain activation should be interpreted with caution |
| 6 | Differential brain activation can be caused by strategy differences |
One of the main problems with current neuroimaging evidence which could support or falsify developmental theories lies in the selection of age groups. Whereas information-processing theories argue that the most prominent changes in problem solving and working memory are seen between ages 7 and 12, almost all developmental neuroimaging studies have collapsed across 7–12 years old. Even though the studies report differences in behaviour between children and adults, it remains to be determined whether these changes are predominantly driven by the youngest children within the selected age group or occur across the whole child group. In future studies, it will be important to carefully select age groups based on theoretical predictions about when the changes are expected to occur.
A second problem concerns the differences in brain and behaviour effect sizes. The correlations between performance and brain activation are usually modest across all participants, but poor when the analysis only includes one age group. This difficulty with interpreting brain-behaviour correlations across age groups is associated with the difficulty in distinguishing between effects of maturation versus individual differences in performance. It is possible that these processes cannot be dissociated at all, that is, performance is usually correlated with maturational changes. In addition, the poor correlations with performance within age groups are usually associated with smaller effect sizes for either the brain or the behavioural measures. Again, a straightforward way to address these problems involves using a theoretically based selection of participants for age-related comparisons, and by using tasks which vary the construct under study at different levels. A combination between functional development and structural development indices (such as developmental DTI studies) is necessary to further expose the extent to which mental growth corresponds to growth in (white-matter) brain connectivity.
A third problem is that little is known about test–retest effects or longitudinal changes. There is much variation within individuals and the time at which spurts in development occur may change between individuals and depend on external factors. In order to track developmental changes over time, it is important to use multi-level models of change in longitudinal designs (see also Ferrer et al., 2009). A recently completed 3-year longitudinal study tested 26 participants between ages 8 and 28 on a performance monitoring task using fMRI (Koolschijn et al., submitted for publication). This study showed that, even though the network of brain regions which was activated was highly similar at both measurements, age was a poor predictor of test–retest effects. Instead, performance was a very good predictor for change; those individuals who improved most from measurement 1 to measurement 2, also showed the largest change in brain activation. These findings indicate that using only age as a predictor for change and ignoring performance changes can result in false or incomplete conclusions.
Based on this stable brain-behaviour relation, we reasoned that performance can increase with training, and that brain activation should change accordingly. To test this hypothesis, we performed a developmental training study and demonstrated that reduced activation in lateral PFC in children aged 11–12 years can be improved by extensive working memory training (Jolles et al., submitted for publication). These results indicate that the developmental differences in brain activation are not fixed and can be modified by instruction or training. These preliminary findings do not fit with a purely maturational account and concomitant structural brain development. Instead they fit in with a skill learning model. More work is necessary to understand how brain function is sensitive to training or interventions.
One pervasive problem in studies of brain development is that the different control functions have often been studied in isolation. Even though consistent findings are reported across studies, these are often limited to the observation that a certain brain area which is important for behaviour in adults is not yet activated to the same level in children. Most of these studies, however, report that children activate several other brain areas in the critical contrast that are not seen in adults. What do these activation patterns indicate? Currently, we can only interpret these post hoc, based on reverse inference about the function of these unexpectedly activated brain areas in adults. For example, prior studies have suggested that when children activate the left rather then right hemisphere more in a certain cognitive task, this can possibly be interpreted to reflect that they used a more verbally guided approach, such as rehearsal, because in adults language functions tend to be left-lateralized (Bunge et al., 2002). Along a similar line of reasoning, it has been suggested that 15-year-old adolescents may have larger compensatory skills because besides PFC, they additionally recruit the hippocampus when they perform a memory task (Finn et al., 2010).
Finally, the differential activation patterns hint towards another important explanation, that is, children may use different strategies when performing a task. Here, we may again learn from classic developmental theories, which have differentiated between different types of memory function, including storage of verbal information, storage of spatial information, and an executive system which works with information in working memory. Consider, for example, the differentiation of verbal versus spatial working memory and the inhibition of irrelevant information. In adults, presenting distracting spatial information impairs their spatial working memory capacity, but not their verbal working memory capacity. In contrast, presenting distracting verbal information impairs their verbal working memory capacity, but not their spatial working memory capacity. These results suggest a strong interplay between working memory and inhibition, which is modality specific. In 8-year-old children, distracting spatial information also impairs their verbal working memory performance and distracting verbal information also impairs their spatial working memory performance (Hale et al., 1997). How to interpret these changes? Apparently, in early childhood the differentiation between functions and modalities is much less segregated than in adults, and this theoretical knowledge should be taken into account when designing neuroimaging experiments, such that the developmental comparisons are meaningful for restricting current models of cognitive development (see also Johnson et al., 2009).
5. Conclusions
In this review, we presented a critical view on the parallels between brain maturation and developmental theories. We proposed that the selection of paradigms and age groups can be more readily based on knowledge from decades of research in developmental psychology, and we argued that to date conclusions of brain-imaging findings often rely on post hoc interpretation. We presented the example of a major developmental theory, Piaget's concepts of stage-like development, and saw that it can be related to current data-based approaches in structural and functional brain imaging
We are enthusiastic about the use of psychophysiological measures, including brain-imaging analysis, which can provide a more solid basis for relating developmental changes in performance on tasks to actual brain maturation, and the results from prior fMRI studies provide the building blocks for starting this new direction. One example of a how these building blocks can be implemented is by using the neural basis of development accounts proposed by Johnson, 2001, Johnson, 2010. This theory provides a principled division of possible processes in brain development. Even though this theory was originally developed to account for changes observed in young children (ages 0–2 years), the concepts of maturation, interactive specialization and skill learning can readily be applied to functional neuroimaging studies in children and adolescents. The accounts provide direct starting points for understanding differences in brain recruitment and to relate these to developmental theory.
Taken together, we suggest that paradigmatic progress in developmental neuroscience can rely more on knowledge from developmental experimental psychology, and that developmental models of cognitive development can be articulated further on the basis of anatomical and functional differentiation of target brain regions. Much progress is currently made in the technological possibilities of brain imaging techniques, which allows, for example, for the estimation of age based on brain connectivity indices (Dosenbach et al., 2010). This is a very important development for a better understanding of changes in brain function. The task for the future, however, is to gain converging evidence from developmental theory, task performance and brain activity to account for developmental changes and individual differences in various aspects of internal control and self-regulation.
Acknowledgements
The research by both authors was supported by NWO VIDI (E.A.C.) and VICI (K.R.R.) grants.
References
- Baddeley A. Working memory. Science. 1992;255:556–559. doi: 10.1126/science.1736359. [DOI] [PubMed] [Google Scholar]
- Baddeley A. Working memory: looking back and looking forward. Nature Review Neuroscience. 2003;4:829–839. doi: 10.1038/nrn1201. [DOI] [PubMed] [Google Scholar]
- Blakemore S.J., Frith U. Blackwell Publishing; Oxford: 2005. The Learning Brain: Lessons for Education. [DOI] [PubMed] [Google Scholar]
- Blakemore S.J. Development of the social brain during adolescence. The Quarterly Journal of Experimental Psychology. 2008;61:40–49. doi: 10.1080/17470210701508715. [DOI] [PubMed] [Google Scholar]
- Blakemore S.J., Choudhury S. Development of the adolescent brain: implications for executive function and social cognition. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2006;47:296–312. doi: 10.1111/j.1469-7610.2006.01611.x. [DOI] [PubMed] [Google Scholar]
- Brainerd C.J. The stage question in cognitive developmental theory. Behavioral and Brain Sciences. 1978;1:173–213. [Google Scholar]
- Bunge S.A., Dudukovic N.M. Immature frontal lobe contributions to cognitive control in children: evidence from fMRI. Neuron. 2002;33(2):301–311. doi: 10.1016/s0896-6273(01)00583-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunge S.A., Kahn I. Neural circuits subserving the retrieval and maintenance of abstract rules. Journal of Neurophysiology. 2003;90(5):3419–3428. doi: 10.1152/jn.00910.2002. [DOI] [PubMed] [Google Scholar]
- Bunge S.A., Zelazo P.D. A brain-based account of the development of rule use in childhood. Current Directions in Psychological Science. 2006;15:118–121. [Google Scholar]
- Case R. Erlbaum; Hillsdale, NY: 1992. The Mind's Staircase: Exploring the Conceptual Underpinnings of Children's? Thought and Knowledge. [Google Scholar]
- Casey B.J., Tottenham N. Imaging the developing brain: what have we learned about cognitive development? Trends in Cognitive Sciences. 2005;9(3):104–110. doi: 10.1016/j.tics.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Ciesielski K.T., Lesnik P.G., Savoy R.A., Grant E.P., Ahlfors S.P. Developmental neural networks in children performing a categorical n-back task. Neuroimage. 2006;33:980–990. doi: 10.1016/j.neuroimage.2006.07.028. [DOI] [PubMed] [Google Scholar]
- Crnic L.S., Pennington B.F. Developmental psychology and the neurosciences: an introduction. Child Development. 1987;58:533–553. [PubMed] [Google Scholar]
- Crone E.A., Donohue S.E. Brain regions mediating flexible rule use during development. Journal of Neuroscience. 2006;26(43):11239–11247. doi: 10.1523/JNEUROSCI.2165-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone E.A., Wendelken C. Neurocognitive development of the ability to manipulate information in working memory. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(24):9315–9320. doi: 10.1073/pnas.0510088103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson G., Fischer K.W., editors. Human Behavior and the Developing Brain. Guilford Press; New York: 1994. [Google Scholar]
- Demetriou A., Efklides A., Platsidou M. The architecture and dynamics of the developing mind. Monographs of the Society for Research in Child Development. 1993;58(5/6) Serial No. 234. [PubMed] [Google Scholar]
- Dempster F.N. Resistance to interference: developmental changes in a basic processing mechanism. In: Howe M.L., Pasnak R., editors. vol. 1. Springer; New York: 1993. pp. 3–27. (Emerging Themes in Cognitive Development: Foundations). [Google Scholar]
- Diamond A. Principles of Frontal Lobe Function. Oxford University Press; S.A. Knight, London: 2002. Normal development of prefrontal cortex from birth to young adulthood: cognitive functions, anatomy and biochemistry. pp. 466–503. [Google Scholar]
- Dosenbach N.U.F. Predicting individual brain maturity using fMRI. Science. 2010;329:1358–1361. doi: 10.1126/science.1194144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein H.T. Phrenoblysis: special brain and mind growth periods. I. Human brain and skull development. Developmental Psychobiology. 1974;7:207–216. doi: 10.1002/dev.420070304. [DOI] [PubMed] [Google Scholar]
- Epstein H.T. Phrenoblysis: special brain and mind growth periods. II. Human brain and skull development. Developmental Psychobiology. 1974;7:217–224. doi: 10.1002/dev.420070304. [DOI] [PubMed] [Google Scholar]
- Epstein H.T. Growth spurts during brain development: implications for educational policy and practice. In: Chall J.S., Mikky A.F., editors. Education and the Brain: The Seventy-seventh Yearbook of the National Society for the Study of Education, Part II. Chicago University Press; Chicago: 1978. pp. 343–370. [Google Scholar]
- Ferrer E., O’Hare E.D., Bunge S.A. Fluid reasoning and the developing brain. Frontiers in Neuroscience. 2009;3(1):46–51. doi: 10.3389/neuro.01.003.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn A.S., Sheridan M.A., Hudson Kam C.L., Hinshaw S., D’Esposito M. Longitudinal evidence for functional specialization of the neural circuit supporting working memory in the human brain. Journal of Neuroscience. 2010;30:11062–11067. doi: 10.1523/JNEUROSCI.6266-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher K.W., Bidell T.R. Constraining nativist inferences about cognitive capacities. In: Carey S., Gelman R., editors. The Epigenesis of Mind: Essays on Biology and Cognition. Erlbaum; Hillsdale, NJ: 1991. [Google Scholar]
- Fisher K.W. A theory of cognitive development: the control and construction of hierarchies of skills. Psychological Review. 1980;87:477–531. [Google Scholar]
- Flavell J.H. Stage-related properties of cognitive development. Cognitive Psychology. 1971;2:421–453. [Google Scholar]
- Flavell J.H. Van Nostrand; New York: 1963. The Developmental Psychology of Jean Piaget. [Google Scholar]
- Giorgio A., Watkins K.E. Changes in white matter microstructure during adolescence. Neuroimage. 2008;39:52–61. doi: 10.1016/j.neuroimage.2007.07.043. [DOI] [PubMed] [Google Scholar]
- Gogtay N., Giedd J.N. Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(21):8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goswami U. Psychology Press: Taylor & Francis; Hove and New York: 2008. Cognitive Development: The Learning Brain. [Google Scholar]
- Hale S., Bronik M.D., Fry A.F. Verbal and spatial working memory in school-age children: developmental differences in susceptibility to interference. Developmental Psychology. 1997;33:364–371. doi: 10.1037//0012-1649.33.2.364. [DOI] [PubMed] [Google Scholar]
- Halford G.S. Erlbaum; Hillsdale: 1993. Children's Understanding: The Development of Mental Models. [Google Scholar]
- Halford G.S. Learning processes in cognitive development: a reassessment with some unexpected implications. Child Development. 1995;38:295–301. [Google Scholar]
- Hudspeth W.J., Pribram K.H. Psychophysiological indices of cognitive functioning. International Journal of Psychophysiology. 1992;12:19–29. doi: 10.1016/0167-8760(92)90039-e. [DOI] [PubMed] [Google Scholar]
- Huizinga M., Dolan C.V., Van der Molen M.W. Age-related change in executive function: developmental trends and a latent variable analysis. Neuropsychologia. 2006;44:2017–2036. doi: 10.1016/j.neuropsychologia.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Huttenlocher P.R. Synaptic density in human frontal cortex-developmental changes and effects of aging. Brain Research. 1979;163:195–205. doi: 10.1016/0006-8993(79)90349-4. [DOI] [PubMed] [Google Scholar]
- Huttenlocher P.R., De Courten C. Synaptic development in human cerebral cortex. International Journal of Neurology. 1983;16–17:144–154. [PubMed] [Google Scholar]
- Johnson M.H. Functional brain development in humans. Nature Reviews Neuroscience. 2001;2:472–483. doi: 10.1038/35081509. [DOI] [PubMed] [Google Scholar]
- Johnson M.H., Grossmann T., Cohen Kadosh K. Mapping functional brain development: building a social brain through interactive specialization. Developmental Psychology. 2009;45:151–159. doi: 10.1037/a0014548. [DOI] [PubMed] [Google Scholar]
- Johnson M.H. Interactive specialization: a domain-general framework for human functional brain development? Developmental Cognitive Neuroscience. 2010;1:7–21. doi: 10.1016/j.dcn.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolles, D.D., Kleibeuker S.W., Rombouts, S.A.R.B. & Crone, E.A. (in press). Developmental Differences in Prefrontal Activation During Working Memory Maintenance and Manipulation for Different Memory Loads. Developmental Science. [DOI] [PubMed]
- Jolles D.D., Van Buchem, M. A., Rombouts, S. A. & Crone, E. A. (submitted for publication). The potential of the developing brain: Practice reduces developmental differences in brain activation.
- Jolles D.D., Grol M.J., Van Buchem M.A., Rombouts S.A.R.B., Crone E.A. Practice effects in the brain: Changes in cerebral activation after working memory practice depend on task demands. Neuroimage. 2010;15(2):658–668. doi: 10.1016/j.neuroimage.2010.04.028. [DOI] [PubMed] [Google Scholar]
- Kail R. Developmental change in speed of processing during childhood and adolescence. Psychological Bulletin. 1991;109:490–501. doi: 10.1037/0033-2909.109.3.490. [DOI] [PubMed] [Google Scholar]
- Kail R.V. Longitudinal evidence that increases in processing speed and working memory enhances children's reasoning. Psychological Science. 2007;18:312–313. doi: 10.1111/j.1467-9280.2007.01895.x. [DOI] [PubMed] [Google Scholar]
- Karmiloff-Smith A. The torturous route from genes to behavior: a neuroconstructivism approach. Cognitive, Affective and Behavioral Neuroscience. 2006;6(1):9–17. doi: 10.3758/cabn.6.1.9. [DOI] [PubMed] [Google Scholar]
- Klingberg T., Forssberg H. Increased brain activity in frontal and parietal cortex underlies the development of visuospatial working memory capacity during childhood. Journal of Cognitive Neuroscience. 2002;14(1):1–10. doi: 10.1162/089892902317205276. [DOI] [PubMed] [Google Scholar]
- Koolschijn, P. C. M. P., Schel, M. A., de Rooij, M., Rombouts, S. A. R. B. & Crone, E. A. (submitted for publication). A 3-year longitudinal fMRI study of performance monitoring and test-retest reliability from childhood to early adulthood. [DOI] [PMC free article] [PubMed]
- Kwon H., Reiss A.L. Neural basis of protracted developmental changes in visuo-spatial working memory. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(20):13336–13341. doi: 10.1073/pnas.162486399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna B., Thulborn K.R. Maturation of widely distributed brain function subserves cognitive development. Neuroimage. 2001;13(5):786–793. doi: 10.1006/nimg.2000.0743. [DOI] [PubMed] [Google Scholar]
- Matousek M., Petersén I. Frequency analysis of the EEG in normal children and adolescents. In: Kelleway P., Petersén I., editors. Automation of Clinical Electroencephalography. Raven Press; New York: 1973. pp. 75–102. [Google Scholar]
- O’Hare E.D., Lu L.H., Houston S.M., Bookheimer S.Y., Sowell E.R. Neurodevelopmental changes in verbal working memory load-dependency: an fMRI investigation. Neuroimage. 2008;42:1678–1685. doi: 10.1016/j.neuroimage.2008.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olesen P.J., Nagy Z. Combined analysis of DTI and fMRI data reveals a joint maturation of white and grey matter in a fronto-parietal network. Brain Research. Cognitive Brain Research. 2003;18(1):48–57. doi: 10.1016/j.cogbrainres.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Olesen P.J., Westerberg H. Increased prefrontal and parietal activity after training of working memory. Nature Neuroscience. 2004;7(1):75–79. doi: 10.1038/nn1165. [DOI] [PubMed] [Google Scholar]
- Passingham R.E., Toni I. Specialisation within the prefrontal cortex: the ventral prefrontal cortex and associative learning. Experimental Brain Research. 2000;133(1):103–113. doi: 10.1007/s002210000405. [DOI] [PubMed] [Google Scholar]
- Paus T., Zijdenbos A. Structural maturation of neural pathways in children and adolescents: in vivo study. Science. 1999;283:1908–1911. doi: 10.1126/science.283.5409.1908. [DOI] [PubMed] [Google Scholar]
- Pfeffenbaum A., Mathalon D.H. A quantitative magnetic response imaging study of changes in brain morphology from infancy to late adulthood. Archives of Neurology. 1994;51:874–887. doi: 10.1001/archneur.1994.00540210046012. [DOI] [PubMed] [Google Scholar]
- Piaget J. W.W. Norton; New York: 1952. The Child's Concept of Number. [Google Scholar]
- Piaget J. International Universities Press; New York: 1952. The Origin of Intelligence in Children. [Google Scholar]
- Piaget J., Inhelder B. Routledge; London: 1974. The Child's Construction of Quantities. [Google Scholar]
- Piaget J. New York; The Free Press: 1965. The Moral Judgment of the Child. [Google Scholar]
- Poldrack R.A. Can cognitive processes be inferred from neuroimaging data? Trends in Cognitive Sciences. 2005;10:59–63. doi: 10.1016/j.tics.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Scherf K.S., Sweeney J.A. Brain basis of developmental change in visusospatial working memory. Journal of Cognitive Neuroscience. 2006;18:1045–1058. doi: 10.1162/jocn.2006.18.7.1045. [DOI] [PubMed] [Google Scholar]
- Schore A.N. Erlbaum; New York: 1994. Affect Regulation and the Origin of Self: The Neurobiology of Emotional Development. [Google Scholar]
- Segalowitz S.J., Rose-Krasnor L. The construct of brain maturation in theories of child development. Brain & Cognition. 1992;20(1):1–7. doi: 10.1016/0278-2626(92)90058-t. [DOI] [PubMed] [Google Scholar]
- Siegler R.S. Developmental sequences within and between concepts. Monographs of the Society for Research in Child Development. 1981;46(2) (Whole No. 189) [Google Scholar]
- Shaw P., Greenstein D. Intellectual ability and cortical development in children and adolescents. Nature. 2006;440(7084):676–679. doi: 10.1038/nature04513. [DOI] [PubMed] [Google Scholar]
- Smith E.E., Jonides J. Storage and executive processes in the frontal lobes. Science. 1999;283(5408):1657–1661. doi: 10.1126/science.283.5408.1657. [DOI] [PubMed] [Google Scholar]
- Southgate V., Johnson M.H., Osborne T., Csibra G. Predictive motor activation during action observation in human infants. Biology Letters. 2009;5:769–777. doi: 10.1098/rsbl.2009.0474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southgate V., Chevallier C., Csibra G. Seventeen-month-olds appeal to false beliefs to interpret others’ referential communication. Developmental Science. 2010;13(6):907–912. doi: 10.1111/j.1467-7687.2009.00946.x. [DOI] [PubMed] [Google Scholar]
- Sowell E.R., Thompson P.M. Longitudinal mapping of cortical thickness and brain growth in normal children. Journal of Neuroscience. 2004;24(38):8223–8231. doi: 10.1523/JNEUROSCI.1798-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thatcher R.W. Cyclic cortical reorganization: origens of cognitive development. In: Dawson G., Fischer K.W., editors. Human Behavior and the Developing Brain. Guilford; New York: 1994. pp. 232–266. [Google Scholar]
- Thomason M.E., Race E., Burrows B., Whitfield-Gabrieli S., Glover G.H., Gabrieli J.D. Development of spatial and verbal working memory capacity in the human brain. Journal of Cognitive Neuroscience. 2009;21:316–332. doi: 10.1162/jocn.2008.21028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Molen M.W., Ridderinkhof K.R. The growing and aging brain: life-span changes in brain and cognitive functioning. In: Demetriou A., Doise W., van Lieshout C.F.M., editors. Life-Span Developmental Psychology: A European Perspective. Wiley; New York: 1998. pp. 35–100. [Google Scholar]
- Zelazo P.D. The development of conscious control in childhood. Trends in Cognitive Sciences. 2004;8(1):12–17. doi: 10.1016/j.tics.2003.11.001. [DOI] [PubMed] [Google Scholar]