Abstract
The brain continues to develop during adolescence, and exposure to exogenous substances such as nicotine can exert long-lasting adaptations during this vulnerable period. In order to fully understand how nicotine affects the adolescent brain it is important to understand normal adolescent brain development. This review summarizes human and animal data on brain development, with emphasis on the prefrontal cortex, for its important function in executive control over behavior. Moreover, we discuss how nicotine exposure during adolescence can disrupt brain development bearing long-term consequences on executive cognitive function in adulthood.
Keywords: Adolescence, Prefrontal cortex, Nicotine, Development
1. Rationale
Adolescence is the developmental period during which individuals undergo the mental transition from childhood to adulthood, and an individual's adult personality is being formed. During adolescence, most individuals will for the first time engage in close relationships outside the borders of their family. Peers become increasingly important, thus making adolescents more independent from their family members. Besides these increments in peer-directed social interaction, both human and other mammalian adolescents show increased rates of impulsivity and risk-taking (Spear, 2000). This seemingly maladaptive impulsive behavior might be very useful since it may increase the chances to find a partner and to reproduce, and is probably due to ongoing development of the brain.
Brain development does not stop at birth, but continues in childhood and throughout adolescence. Initially synaptic connections of neuronal cells are overproduced and receptor levels increased, and subsequently activity-dependent processes are engaged to fine-tune adult levels of connectivity and signaling toward the end of adolescence. Also, myelination of axons increases during adolescence, thereby increasing processing speed. In particular long-range connections between different brain regions have not yet completely matured, and the crosstalk between different brain areas still has to reach a final balance. As a consequence, developing adolescent brains are functionally different from adult brains (Ernst et al., 2005, Eshel et al., 2007). Therefore, it is conceivable that abnormal brain development during adolescence that arises from the interplay of genetic and environmental factors such as drugs of abuse, might result in a partial maintenance of the adolescent state, thereby leaving adult individuals with features of adolescence.
High rates of impulsivity and risk-taking behaviors that are evident in adolescents are thought to result in the experimental use of alcohol, tobacco (nicotine) and illicit drugs (Chassin et al., 1996, Spear, 2000). Substance use disorders identified in adults most commonly have their onset in adolescence or in young adulthood (Chambers et al., 2003). Early onset substance use is a risk factor to develop substance use disorder or addiction, and leads to greater addiction severity and morbidity (Anthony and Petronis, 1995, Kandel et al., 1992, Taioli and Wynder, 1991). In fact, most adult smokers have started their habit long before the age of 19 and several retrospective and prospective studies have suggested that early onset of smoking may predict cigarette smoking and nicotine dependence in adulthood (Brown et al., 1996, Chassin et al., 1996, Dappen et al., 1996). Specifically, even smoking a few cigarettes during adolescence was shown to increase the probability of developing dependence for nicotine, and was shown to lead to a 16-fold increase in the risk of adult smoking (Chassin et al., 1990, Russell, 1990). In the Netherlands, 71% of adolescents have tried a cigarette at least once (Van Andel et al., 2003), and approximately 40% smoke at least monthly at the age of 19 years. These figures are comparable to other European countries (WHO-Europe, 2004), and are even higher compared with the USA, where 25% of adolescents in 12th grade (age 17–18 years) report cigarette use in the past 30 days (Johnson et al., 2006). Despite all epidemiological data, the causative relationship of human adolescent brain development and drug-induced aberrations thereof, as well as its consequence for later life are hard to establish empirically. Both individual genetic differences and complex gene by environment interactions are confounding factors. Importantly, adolescence and adolescent brain development is well conserved between mammalian species. Therefore, causal relationships of adolescent drug exposure and the consequences in adult life can be studied uniquely in laboratory animals. These also allow for investigation of molecular and cellular synaptic mechanisms, and drug-induced adaptations over time (Box 1 and Fig. 1).
Box 1. Adolescence in rodents.
Adolescence is a developmental period that is conserved between mammalian species, and serves an important purpose, since this is the period between childhood and adulthood when animals leave their protected nest-environment. Similar to human adolescents, their rodent counterparts show incremented social behavior compared to juveniles and adults, such as increased peer-directed interactions, which can be observed as an elevation of social play behavior. Also, adolescents show elevated levels of impulsivity (for review, Spear, 2000). Another characteristic of adolescence that is shared between mammalian species is the increased sensitivity to the rewarding effects of drugs of abuse, such as nicotine, and a decreased sensitivity to withdrawal (for review, O’Dell, 2009) (see Fig. 1).
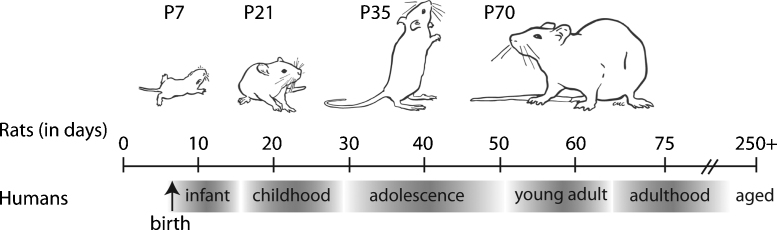
Fig. 1.
A relative comparison between the age of a rat and the developmental stages of human brain development (adapted with permission from (McCutcheon and Marinelli, 2009), age comparison from (Andersen, 2003)).
In this review, we focus on the effects of tobacco smoking, and intake of its active substance nicotine. Tobacco smoking creates the largest number of drug dependent users worldwide and has tremendous impact on their health. Here, we assess the effects of nicotine on adolescent brain development and, through this, how it may cause long-lasting cognitive disturbances. We will discuss the process of adolescent brain development, the age-dependent differences in sensitivity toward nicotine, and the long-lasting effects of nicotine on the prefrontal cortex (PFC) in more detail. The PFC develops strongly during adolescence, allowing nicotine to impact on its executive control function. An important feature of this review is that we are discussing and comparing literature on both human and animal research.
2. Adolescent brain development
In humans, brain development continues throughout adolescence into early adulthood (Galvan et al., 2006). Structural and functional neuroimaging approaches have shown that cortical association areas, such as the PFC, develop later than primary sensorimotor areas (Durston et al., 2006, Gogtay et al., 2004). This developmental program parallels cognitive milestones in human brain development (for review, Casey et al., 2005). Regions necessary for primary survival functions, such as motor and sensory systems, develop well before temporal and parietal association areas, which are involved in basic language and attentional skills. In particular, higher-order association areas such as the PFC, which are involved in integration of sensorimotor processes and executive tasks, develop last. Because higher-order association areas depend on ‘lower’ motor and sensory areas for their input, the cross-talk between these systems changes during development, consequently impacting on behavior. It is thought that the relatively early presence of sensorimotor output provides a stable input on which the higher cortical areas can build (Guillery, 2005). Thus, adolescent brain development is a dynamic combination of regressive and progressive changes, of which the most important processes are described in more detail below.
For reasons of its late adolescent development, we focus here on the PFC. In terms of cortical connectivity, pyramidal neurons in the PFC communicate using glutamate as the primary excitatory neurotransmitter. In turn, GABA-secreting interneurons inhibit the activity of the pyramidal cells. Tracer studies have shown that many brain areas have afferent projections to the PFC (mostly to layer II/III), including thalamic nuclei, the amygdala, areas in the basal forebrain, and the ventral tegmental area (VTA) (Hoover and Vertes, 2007, Van Eden et al., 1992). Moreover, local PFC circuitry is modulated by a variety of neurotransmitters such as dopamine, serotonin and acetylcholine, which are produced by small groups of neurons located in the mid- and hindbrain. The efferent projections of the PFC, originating mostly from layer V pyramidal neurons, innervate many brain areas, among which the striatum, amygdala, thalamus, and the VTA comprise important projection areas (Gabbott et al., 2005).
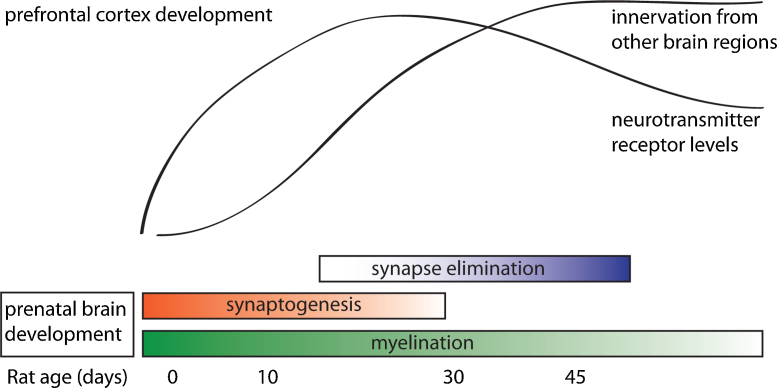
As shown in Fig. 2, and discussed in more detail below, the development of the PFC during adolescence is characterized by continuous innervation (Section 2.1) of fibers with modulatory neurotransmitters and elimination (Section 2.2) of overproduced glutamatergic synapses (so-called synaptic pruning). Also, myelination (Section 2.3) of long-range connections continues throughout adolescence. In addition, the levels of various neurotransmitter receptors are adapted (Section 2.4) to reach adult levels.
Fig. 2.
A summary of the processes occurring during development of the rat prefrontal cortex (adapted with permission from (Casey et al., 2005)). Adolescence in the rat is considered to occur between postnatal day 25 and 50.
2.1. Innervation
During adolescence, the input of modulatory neurotransmitters and glutamatergic projections from other areas into the PFC increases (Cunningham et al., 2002, Cunningham et al., 2008, Gould et al., 1991, Kalsbeek et al., 1988, Rosenberg and Lewis, 1995). In the rat and macaque brain it has been shown that dopamine neurons from the VTA increasingly innervate the PFC throughout adolescence (Kalsbeek et al., 1988, Rosenberg and Lewis, 1995). In addition, glutamatergic fibers projecting from the amygdala increasingly innervate GABAergic interneurons in the PFC during adolescence (Cunningham et al., 2002, Cunningham et al., 2008). Although cholinergic innervation of the PFC increases to mature levels (Gould et al., 1991), this maturation is already completed before adolescence, as there are no differences in density of acetylcholinesterase/ChAT positive fibers between P28 and adulthood in rat (Kiss and Patel, 1992). Despite the sparsely available evidence for this increased innervation of the PFC during adolescence, altogether these data might suggest that the incremented input enables the PFC to sustain more efficient integration of information from different brain regions. In addition to this notion, there is reason to think that the presynaptic release machinery matures during adolescence (Counotte et al., 2010), suggesting that also at synapses within the PFC signal processing may proceed in a more efficient way.
2.2. Synapse elimination
A prominent hallmark of the developing central nervous system is the overproduction of synapses (see above) that are subsequently eliminated in an activity-dependent manner. This synaptic pruning may allow for a more efficient synaptic neuronal network communication (Laughlin and Sejnowski, 2003). In particular, these processes have been studied in detail for the neuromuscular junction and the developing visual system (for review, Buffelli et al., 2004, Huberman, 2007). Synaptic pruning during adolescence is activity-dependent, which means that connections that are often used are retained and strengthened, while those that are used less are eliminated (Lichtman and Colman, 2000). This type of synapse elimination, specifically in higher-order association cortices like the PFC, is believed to underlie the improvement in working memory that occurs during adolescence (Paus, 2005). Evidence for synapse elimination in the PFC during adolescence is sparse. It has been demonstrated in non-human primates that cortical synapses are overproduced during childhood, and subsequently eliminated (Elston et al., 2009, Rakic et al., 1994), but most of this occurs before 18 months of age, whereas adolescence in the rhesus monkey is broadly considered to occur between 2 and 5 years of age. In work performed in macaque monkeys, Bourgeois and colleagues (Bourgeois et al., 1994) described that about half of the synapses per cortical neuron that were present at the time of the peak of synaptogenesis are eliminated, but they also described that during early adolescence (from 2 months to 3 years of age) there is a plateau phase during which synapse density remains unchanged. A complicating factor in the interpretation of synapse elimination is that differences may reside in different brain regions (Elston et al., 2009). In the rhesus monkey PFC, electrophysiological data show that before synapse elimination occurs, most synapses are already mature (Gonzalez-Burgos et al., 2008), suggesting that in the PFC synapse elimination is not directed at immature synapses.
In the human cortex, synapse elimination during adolescence has also been described (Huttenlocher, 1979, Huttenlocher, 1990). Nonetheless, the limited number of adolescent brains used in these studies prevents firm conclusions about synapse elimination or a decrease in synapse production. Moreover, in these studies, spines are counted and regarded as synapses, whereas this is in fact only the postsynaptic element. The use of such measures cannot provide a full answer, due to potential absence of a presynaptic element and thus a full functional synapse. Studies of synapse elimination in the developing visual cortex (Huberman, 2007) or the neuromuscular junction (Buffelli et al., 2004) have taken the presynaptic terminal into account, and show that development of circuits in the nervous system involves the elimination of synapses.
Neuroimaging studies have been used as an alternative and non-invasive method to study brain development during human adolescence. Structural magnetic resonance imaging (MRI) studies have shown that grey matter density increases during childhood, with subsequent grey matter density loss during adolescence (Giedd et al., 1999, Jernigan et al., 1991, Sowell et al., 2001). The latter has often been interpreted as synapse elimination. However, MRI resolution is not adequate to study changes at the sub-cellular level, and only reflects use of neuronal circuitry at a higher level of function. Loss of grey matter may also reflect other developmental changes, such as a change in the number of glial cells or blood vessels, or changes in neuronal activity other than elimination of synapses, e.g. neuronal cell loss. In rats, it has been shown that in the PFC, neuronal numbers decrease specifically in ventral regions (Markham et al., 2007). Although this process has not been investigated for human cortical development, it may explain the decrease observed in grey matter during adolescence.
In conclusion, as yet there is only sparse data available in favor of synapse elimination in the (m)PFC during adolescence, and the underlying mechanisms remain to be solved.
2.3. Myelination
Postmortem research of human brain development has shown that myelination begins toward the end of the second trimester of gestation, and extends until approximately 30 years of age (Yakovlev, 1967). Similar to developmental changes in grey matter density, primary motor and sensory areas show myelination at an early stage (during childhood), whereas higher-order association areas like the PFC are myelinated during adolescence (Giedd, 2004). As myelin increases the speed of signal transduction along axons, increased myelination is thought to improve communication between distant brain areas. The increased myelination of axons that occurs during adolescence was shown using immunohistochemical staining techniques (Benes, 1989), T1-weighted MRI (Benes, 1989, Paus et al., 1999), or using diffusion tensor imaging (DTI) (Snook et al., 2005) (for review see, Klingberg, 2006).
In the rat hippocampus, myelin staining revealed that myelination stops before adolescence (P25), after which it remains stable (Meier et al., 2004). However, Bockhorst and coworkers showed (using DTI) that myelination is still ongoing during adolescence, with an increase in myelin in the body of the corpus callosum (but not the splenium and genu) from P21 up to P56 (Bockhorst et al., 2008). They also showed that some brain regions, including the external capsule, internal capsule and anterior commisure are already fully myelinated at P28 (Bockhorst et al., 2008). These observations argue for distinct temporal differences in myelination in specific brain regions during adolescence.
2.4. Receptor level adaptations
During adolescent brain development, the expression of various types of receptors changes, in order to reach adult levels at the end of adolescence. In this section, we will focus on glutamate receptors, as most PFC synapses are glutamatergic, and on dopamine and acetylcholine receptors given their importance in modulating PFC circuitry and mediating (nicotine) reward signals.
2.4.1. Glutamate receptors
Glutamate receptors can be classified as ionotropic (ion channels) or metabotropic (G-protein coupled receptors). Ionotropic receptors, and specifically the GluN2A and GluN2B subunits of the N-methyl-D-aspartic acid (NMDA) receptor, are known to be involved in synaptic plasticity, and their expression is dependent on synaptic activity (Hoffmann et al., 2000). Although it has not been studied in the PFC specifically, newly formed synapses express more GluN2B-containing NMDA receptors than GluN2A-containing ones (for review, Carpenter-Hyland and Chandler, 2007). Brain expression of both GluN2A and GluN2B follows an inverted U-shape (Jin et al., 1997) (Barria and Malinow, 2002) during development. The level of GluN2B expression is low before birth, and subsequently increases and peaks around the start of adolescence (P30 in the rat; see Fig. 1 in Box 1). Subsequently, it decreases to reach the adult level of expression. GluN2A receptors are not expressed until shortly after birth, and also increase during development to peak around P30 (Jin et al., 1997). This differential expression pattern is probably important for experience-dependent plasticity, and is also observed in brain regions important for learning and memory (Carpenter-Hyland and Chandler, 2007). Although GluN2A receptors have higher peak currents and a greater open channel probability (Chen et al., 1999), GluN2B-containing receptors have higher affinity for agonists and confer greater calcium influx than GluN2A-containing receptors due to their slower inactivation kinetics (Krupp et al., 1996). These characteristics have implicated GluN2B as being particularly important in experience-based plasticity. From our own proteomics study on rat medial PFC (mPFC) synapses, it appears that there is no change in the expression of GluN2A and GluN2B between early adolescence and adulthood (Counotte et al., 2010). This might indicate that the differential developmental expression of GluN2A and GluN2B occurs in only a few synapses and is therefore not observed in homogenate of mPFC synaptic membranes. Neither did we observe the developmental regulation of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors, the other subclass of ionotropic glutamate receptors (Counotte et al., 2010). Since developmental changes of both AMPA and NMDA receptors in the mPFC during adolescence have been shown to be cell-specific (Wang and Gao, 2009), we may not have observed these cell-specific alterations in an mPFC cell homogenate.
Metabotropic glutamate receptors (mGluRs 1–5) are coupled to G-proteins and modulate glutamatergic transmission through various signal-transduction pathways. mGluRs are differentially expressed during development, with gradients of expression. For example, mGluR1 is expressed earlier in superficial layers of the cortex than in the deeper layers, reaching an adult pattern of expression around P35 (Defagot et al., 2002). For mGluR2/3 a similar gradient was observed in the striatum, where a gradient in intensity from dorsal to ventral and from medial to lateral was observed (Jokel et al., 2001). This differential pattern becomes homogenous already in the second and third postnatal weeks. In addition, the developmental pattern of mGluR4 is again different, with peak expression at P4, after which the labeling of neuronal cell bodies decreased throughout development to adult levels (Defagot et al., 2002).
2.4.2. Dopamine receptors
Dopamine receptors (DRD) also belong to the class of G-protein coupled receptors, and they fall into two categories. DRD1 and DRD5 belong to the D1-like family, and are coupled to activating G-proteins, causing an increase of 2nd messengers, whereas DRD2, DRD3 and DRD4 belong to the D2-like family of receptors, which are coupled to an inhibitory G-protein, and hence their activation leads to a decrease of cyclic AMP (Seamans and Yang, 2004).
For the development of both families of dopamine receptors, an inverted U-shaped developmental expression pattern has been demonstrated. For instance, in adolescent rats, the level of dopamine DRD1 receptors on PFC output neurons projecting to the nucleus accumbens is higher than in younger or adult rats (Brenhouse et al., 2008). This seems to be a cell specific regulation, since the level of DRD1 receptors on GABAergic interneurons in the PFC does not change during adolescence. Also, DRD2 receptors were found to attenuate local excitatory synaptic transmission in the PFC in adulthood, but not yet in adolescence (Tseng and O’Donnell, 2007), indicating subtle developmental differences in dopamine modulation of PFC circuitry that occur during adolescence.
2.4.3. Nicotinic acetylcholine receptors
Given the focus of this review on differential effects of nicotine on adolescent versus adult brain development and behavior, it is of interest to investigate the developmental regulation of nicotinic acetylcholine receptors (nAChRs). Nicotinic receptors are pentameric ligand-gated ion channels that can be either homomeric (of which the α7-type is the most common) or heteromeric (of which the high-affinity α4β2-type is most common) (Gotti et al., 2007). In rats, higher expression of α4β2 and α7 nAChRs in adolescents versus adults has been reported in the majority of brain regions, including the nucleus accumbens and the lateral septum (Doura et al., 2008, Trauth et al., 1999). Specifically, in adolescence binding to α4β2 receptors in slices is higher in most brain regions, such as the nucleus accumbens, frontal cortex and the visual cortex, whereas binding to α7 receptors is higher most notably in the substantia nigra. Binding to α6-containing receptors is similar between adolescent and adult rats (Doura et al., 2008). In mice, the developmental pattern of α4β2 nAChRs was studied at multiple time points, and follows a similar inverted U-shape pattern observed with other types of receptors (e.g. dopamine receptors), with peak expression levels at P21 (age of weaning, earlier than adolescence), and following this period declining to adult levels (Yu et al., 2007). In humans, similar expression patterns were observed in a PET imaging study; binding to β2-containing nAChRs declines with age from 18 years on (Mitsis et al., 2007). Regarding the downregulation of different types of receptors between adolescence and adulthood, it is difficult to determine whether these receptors are already expressed to a lesser extent or, alternatively, whether the postsynaptic cells or presynaptic terminals expressing these receptors are eliminated altogether.
In summary, many developmental processes are ongoing during (and maybe even following) adolescence. Both the number of synapses and the levels of various receptor types follow a similar pattern with high expression levels during childhood, leading to peak levels in adolescence, followed by a decline to adult levels. In addition, the myelination and innervation of several brain regions increases during adolescence. For some of these processes it has been shown that they occur early during development in ‘evolutionary older’ regions, and occur late in development of association areas, such as the prefrontal cortex. Altogether, different developmental stages make the adolescent brain different from the mature adult brain, which in turn might lead to differences in behavior and a differential sensitivity to drugs of abuse, such as nicotine.
3. Differential sensitivity to nicotine during adolescence
Next, we will discuss whether adolescents, perhaps because their brain is still developing and/or because they show a different nicotine-induced regulation of nicotinic receptors, are differentially sensitive to nicotine's effects. These differences can be attributed to the rewarding properties of the drug, but also in the severity of withdrawal symptoms that adolescents experience when they discontinue nicotine (e.g. tobacco) use. Obviously, there are numerous external factors that are unique to human adolescents, such as sensitivity to peer pressure that will make them more prone to initiate smoking than adults.
3.1. Rewarding properties
The addictive properties of drugs of abuse such as nicotine can be measured in laboratory animals using various behavioral paradigms (e.g., conditioned place preference (CPP) and self-administration of drugs), either making use of forced or voluntary intake of drugs (O’Dell and Khroyan, 2009). Many studies have demonstrated that adolescent animals are more sensitive to display CPP for nicotine than adult animals, and that nicotine produces place preference at lower doses in adolescents compared to adults (Belluzzi et al., 2004, Brielmaier et al., 2007, Shram et al., 2006, Torres et al., 2008, Vastola et al., 2002). Although this difference in reward sensitivity may be due to an immaturely developed and more sensitive reward system, the study by Torres et al. showed that the findings with nicotine could not be generalized to different drugs of abuse, such as the psychostimulant amphetamine (Torres et al., 2008). However, others showed that differences in CPP for psychostimulants between adolescents and adults are dependent on the dose that is used, since adolescents show CPP at lower doses than adults (Brenhouse and Andersen, 2008, Zakharova et al., 2009).
A better way of evaluating whether drugs are more rewarding is to have animals self-administer nicotine. Despite some experimental difficulties, adolescent rats learn to self-administer nicotine intravenously in instrumental self-administration paradigms (Chen et al., 2007, Levin et al., 2003). Interestingly, there are some reports describing that adolescent animals self-administer more nicotine compared to adults (Chen et al., 2007, Levin et al., 2003, Levin et al., 2007). However, the contrary has also been reported. In particular, with lower doses of nicotine or higher response requirements to obtain an infusion of nicotine, adolescent rats were less willing to self-administer nicotine compared to adults (Shram et al., 2008a, Shram et al., 2008b). In both behavioral paradigms it seems that the dose of nicotine used determines whether adolescents are shown to be more sensitive to nicotine, suggesting that it is not a straightforward relationship, and research into this aspect is required.
In humans it is difficult to recruit adolescents and adults who have smoked at a given age for a similar time span and have experienced a comparable amount of nicotine intake, since most adult smokers have started their habit already during adolescence. In addition, adults who only started smoking after they had reached adulthood may form a different population (i.e. genetically) than adolescents who have started smoking early, and may as a result differ in terms of vulnerability to start smoking, as well as in relapse to smoking. Possibly for these reasons, studies comparing adolescent and adult smokers are sparse. In contrast, a vast amount of literature describes the progression of adolescent smokers from recreational to compulsive use and nicotine dependence. These studies conclude that compared with adult smokers, adolescents tend to smoke with less regularity, and they smoke less cigarettes per day (for review, Colby et al., 2000). Regardless of how dependence is measured (usually using a questionnaire), adolescents are typically classified as dependent at only half of the intake-rate of adults (Prokhorov et al., 1996). Also, compared with adults, adolescents at the same level of self-reported intake, were more likely to be diagnosed as dependent (Kandel and Chen, 2000). Moreover, adolescents consider themselves to be addicted at much lower levels of use and adolescents who start smoking regularly continue to smoke well into adulthood. Taken together, even though adolescents smoke fewer cigarettes than adults, they are considered to become more easily dependent. This is in line with most of the animal literature stating that adolescents are sensitive to the rewarding effects of nicotine at lower doses than adults. When adolescents start to smoke, the sensation of their first smoking experience is associated to later dependence, in a manner that those who experience dizziness, nausea and relaxation in response to their first cigarette are more likely to develop symptoms of nicotine dependence (Audrain-McGovern et al., 2007, DiFranza et al., 2004).
3.2. Withdrawal symptoms
Another potential difference between adolescent and adult rats regarding their sensitivity to nicotine is diminished withdrawal from repeated nicotine administration. When adolescent and adult rats were treated with nicotine, and subsequently given the nicotinic acetylcholine receptor antagonist mecamylamine to precipitate withdrawal, adults showed more somatic signs of withdrawal (O’Dell et al., 2004), and a higher conditioned place aversion for mecamylamine (O’Dell et al., 2006, O’Dell et al., 2007, Shram et al., 2008c). Spontaneous withdrawal from nicotine, however, did not produce differences in physical withdrawal signs between adolescent and adult animals (Shram et al., 2008c). In conclusion, although adolescent animals might be less sensitive to the negative withdrawal effects – to our knowledge – it is unknown how this would translate into nicotine dependence.
In support of the view that adolescents are less sensitive to nicotine withdrawal effects (O’Dell et al., 2004, O’Dell et al., 2006, O’Dell et al., 2007, Shram et al., 2008c), human adolescents who quit smoking with the help of a nicotine patch report fewer withdrawal symptoms than adults. In the first few days withdrawal experiences were mild in both adults and adolescents, but after this initial period adolescents’ withdrawal reports declined whereas adult levels of withdrawal signs remained relatively steady over a 6-week period (Smith et al., 1996). A confounding factor in this latter study is that many adolescents reported smoking in the period during which they were supposed to have quit smoking, whereas adults were in a controlled smoke-free setting (Colby et al., 2000). In another study, no differences in severity of nicotine withdrawal symptoms were found between adolescent and adult smokers, although there were clear age and age by gender effects in the type of withdrawal symptoms perceived (Pergadia et al., 2010). Although self-report measures of smoking and withdrawal symptoms make it difficult to draw definitive conclusions, current human data are in line with preclinical data showing that adolescents are less sensitive to specific withdrawal symptoms. However, in light of the above-discussed increased likelihood to initiate and maintain smoking in adolescents, it is unlikely that diminished or altered withdrawal symptoms play a crucial factor in becoming nicotine-dependent. The increased liability to become dependent on nicotine is merely due to the enhanced rewarding effects of nicotine during adolescence (for review, O’Dell, 2009).
3.3. Is differential sensitivity due to regulation of nicotinic receptors?
One of the explanations for a different sensitivity in nicotine in adolescents may be that nicotinic receptors are differentially regulated by nicotine during development. Nicotine acts on nicotinic acetylcholine receptors. Repeated or prolonged nicotine exposure increases the number of high-affinity (mainly α4β2) nicotine binding sites, in heterologous systems (Kuryatov et al., 2008, Tumkosit et al., 2006), nicotine-treated animals (Marks et al., 1983, Schwartz and Kellar, 1983, Yates et al., 1995), and in postmortem brains of smokers (Benwell et al., 1988, Breese et al., 1997, Perry et al., 1999) (for review, Govind et al., 2009). The nicotine-induced up-regulation of nAChRs in the brain is both subtype and region specific (Lai et al., 2005, Moretti et al., 2010, Mugnaini et al., 2006, Nashmi et al., 2007, Perry et al., 2007, Xiao et al., 2009). Interestingly, adolescent nicotine exposure has been shown to lead to larger and longer-lasting changes in nAChR binding (Abreu-Villaca et al., 2003, Doura et al., 2008) and function (Kota et al., 2009) in brain regions such as cortex and striatum, which may (in part) underlie the enhanced sensitivity of adolescents to nicotine.
3.4. Other factors that influence adolescent smoking in humans
In addition to the physical effects and enhanced sensitivity to the rewarding effects of nicotine, it must be noted that smoking is more than just inhaling nicotine. Not only because cigarettes contain a wealth of substances other than nicotine, for example acetaldehyde, which in adolescent but not adult rats facilitates the rate of self-administration of nicotine (Belluzzi et al., 2005), but also because smoking in vulnerable individuals quickly becomes a complex addictive habit. Smoking in human adolescents is determined by various factors, such as peer pressure and individual differences in impulsivity and risk-taking. Especially, when their best friend smokes, adolescents between 12 and 15 years are at a higher risk to initiate smoking (a relative risk of 10 for boys and 15 for girls between the age of 12–15 years) (Vink et al., 2003). This relative risk is much lower at the age of 21–40 years, as it decreases by half with age, demonstrating that adolescents are specifically vulnerable for peer-pressure. This environmental risk is even further increased in those individuals who have a higher degree of impulsivity and risk-taking behavior, such as individuals with ADHD (for review, McClernon and Kollins, 2008). From a biological point of view one might argue that the adolescent brain, and in particular association areas like the PFC, has not yet fully developed, causing adolescents to show increased impulsive and risk-taking behavior, and do not oversee the long-term consequences of their behavior. As a result, many adolescents believe there are no health risks in the first few years of nicotine exposure, and they believe that they will be able to stop before the damage is done (Arnett, 2000). In particular, because nicotine-related cues act on the same cortical (o.a. PFC) brain areas in adolescent moderate smokers as in adults (Lee et al., 2005, Rubinstein et al., 2011), adolescents apparently exhibit heightened reactivity to smoking cues that in turn could impact on initiation and maintenance of smoking.
In conclusion, both human and rat adolescents seem to be more sensitive to some of the effects of nicotine compared to their adult counterparts. Adolescents become dependent after having smoked fewer cigarettes, and are much less aware of the risks of smoking, collectively causing many adolescents to start and continue smoking far into adulthood. In terms of human health, this leads to the important question what the long-term effects of adolescent exposure to nicotine are.
4. Long-term effects of nicotine during adolescence
Addictive drugs such as nicotine and alcohol are legal in our society, and therefore, these substances are often the first type of drugs of abuse people experience in their lives. Regarding nicotine, 71% of Dutch adolescents have tried a cigarette at least once, often leading to long-term use. These numbers have led to efforts to better understand what the long-term effects of early nicotine use are, both in prospective and longitudinal human studies, as well as in preclinical animal studies.
4.1. Adolescent nicotine and its effects on cognitive functioning
The brain's cholinergic signaling system is important for a variety of cognitive functions, such as attention, inhibitory control and decision-making mechanisms, the latter two subserving impulse behavior (Everitt and Robbins, 1997, Pattij and Vanderschuren, 2008). Nicotine, similar to the endogenous ligand acetylcholine, acts by modulating synaptic signaling through increased neurotransmitter release in the brain (for review, Mansvelder et al., 2009, Mansvelder et al., 2006). Nicotine has often been shown to enhance specific aspects of cognitive functioning, such as selective attention, that is the capacity to maintain a behavioral or cognitive set in the face of distracting or competing stimuli, in both humans and rodents (Day et al., 2007, Grottick and Higgins, 2000, Pattij et al., 2007, Rycroft et al., 2005). Oftentimes, this nicotine-induced enhancement of cognition is stronger, or even only present, in smokers compared with non-smokers (or in chronically exposed rats compared with nicotine-naïve rats). Non-smokers or drug-naïve rats are sometimes reported not to cognitively benefit from nicotine at all (van Gaalen et al., 2006), especially when baseline levels of performance are already high in the absence of nicotine (Poltavski and Petros, 2006).
4.1.1. Attention
In adolescent smokers disturbances in working memory and attention have been reported (Jacobsen et al., 2005), as well as reduced attention-associated prefrontal cortical blood-oxygen level dependent (BOLD)-responses (Musso et al., 2007). Thus, these studies suggest some detrimental effects of nicotine on cognitive functioning during adolescence. Nonetheless, it is important to note that these studies only focused on the short-term effects of adolescent smoking on cognition, whereas the levels of smoking were determined by self-report measures and were not controlled for pre-smoking levels of cognitive performance. Despite these limitations, they suggest that adolescent exposure to nicotine may impair cognitive performance on the long-term, yet this has never been examined in humans in more detail. In rats, we recently observed that adolescent nicotine exposure decreases visual attention into adulthood, even after a relatively long nicotine-free period (Counotte et al., 2009). Furthermore, we discovered a neurobiological mechanism revealing that these changes were caused by decreased synaptic expression of mGluR2 in the mPFC that led to disruptions in short-term plasticity (Counotte et al., 2011). Restoring mGluR2 tone by local infusion of a group II mGluR agonist increased performance in these animals. These data not only show that adolescent nicotine exposure has long-lasting consequences for mPFC synapse function and cognition, but also shows the importance of mPFC mGluR2 in mediating attention.
4.1.2. Impulsive behavior
Smoking appears to be closely related to several forms of impulsivity in humans. Impulsivity includes categories of behaviors that result from problems to inhibit behavioral responses, often referred to as impulsive action, and behaviors that reflect impulsive decision making, for example, delay aversion, which is exemplified by increased preference for immediate reward over more beneficial delayed reward (Pattij and Vanderschuren, 2008). In humans, adult smoking has been associated with both impulsive choice (Bickel et al., 1999, Mitchell, 1999) as well as deficits in inhibitory control, when compared with non-smokers (Mitchell, 1999, Skinner et al., 2004, Spinella, 2002). However, whether impulsivity in adult smokers results from nicotine exposure, or alternatively, is a pre-existing vulnerability trait predisposing individuals to initiate and maintain smoking is as yet unclear, since none of these studies controlled for pre-smoking levels of impulsivity. Recent preclinical data would support either view; trait impulsivity in the form of impulsive action may predict the vulnerability to initiate and maintain nicotine seeking (Diergaarde et al., 2008), and we recently found that adolescent nicotine exposure increases impulsive action (Counotte et al., 2009). In this respect, nicotine itself likely exerts a much larger effect on impulsivity, as a main nicotine effect was found in a general population of rats (Counotte et al., 2009, Counotte et al., 2011). Hence, differences in impulsivity might render an individual more vulnerable to initiate smoking, and depending on the age of intake, this might create these individuals to become even more impulsive and less attentive, which could result in increased intake of nicotine. However, impulsive choice, that was found to predict a higher vulnerability to relapse to nicotine seeking (Diergaarde et al., 2008), was unchanged following adolescent nicotine exposure (Counotte et al., 2009). Thus, although this form of impulsivity does not seem to play a role in the age-dependent effect of nicotine, it suggests that once individuals with increase levels of impulsive choice have initiated nicotine use, efforts to quit might become more difficult (Diergaarde et al., 2008).
To date, human evidence describing the long-term effects (in absence of nicotine) of adolescent smoking on impulsivity in adulthood is limited. It has been demonstrated recently though, that the inability to abstain from smoking in adolescents is associated with elevated levels of impulsive action in the continuous performance task (Krishnan-Sarin et al., 2007) as well as increased impulsive choice in a real-time delay discounting paradigm (Dallery and Raiff, 2007, Krishnan-Sarin et al., 2007), which is comparable to the rat delayed reward paradigm. In a different study, young adult smokers show higher rates of delay discounting than both adolescent smokers and young adult non-smokers (Reynolds, 2004). The number of cigarettes smoked correlated with delay discounting, but reported length of smoking history did not correlate with delay discounting. This suggests that despite the clear relationship between smoking and impulsivity, as yet no conclusion can be drawn on the long-term adverse effects of adolescent nicotine exposure on delay discounting because the long-lasting effects of smoking on measures of impulsivity in both abstinent as well as non-abstinent adolescent smokers and non-smoking adolescents have not been evaluated (Reynolds, 2004).
Collectively, these findings from human and animal research clearly show that there is a relationship between nicotine exposure and impulsivity, but the exact nature of this relationship and empirical evidence whether nicotine exposure causes impulsivity in humans, or pre-existing impulsivity increases sensitivity to nicotine, or perhaps both, remains to be determined.
4.2. Mental health: adolescent nicotine and affective disorders
There are many reciprocal interactions reported between psychiatric disorders and nicotine dependence. On the one hand, psychiatric patients are more likely to smoke than the general population; it has been estimated that 60% of patients with depression and post-traumatic stress disorder are smokers, and in schizophrenic patients the prevalence of smoking can be as high as 90% (Fagerstrom and Aubin, 2009). On the other hand, there are also associations reported between adolescent smoking and the development of mental health problems (for review, Mathers et al., 2006). In summary, several studies have found that adolescent tobacco use or dependence is associated with the onset of psychiatric disorders, such as antisocial personality disorder, major depressive disorder, anxiety disorder and panic disorder, even after correction for confounding factors, such as the occurrence of previous psychiatric disorders (Brook et al., 1998, Brook et al., 2002, Brown et al., 1996, Johnson et al., 2000, McGee et al., 2000, Pedersen and von Soest, 2009). Similar to what was observed for adults, in adolescent patients with schizophrenia rates of nicotine dependence are high (Hakko et al., 2006). However, there are no studies that have investigated whether adolescent smoking increases the relative risk to develop psychosis or schizophrenia. Although (early onset of) psychiatric disorders are predictors of nicotine dependence, sometimes the start of adolescent nicotine dependence is preceded by the onset of psychiatric disorders (Griesler et al., 2008).
It is well established that smoking is significantly related to depression (for review, Park and Romer, 2007), however it is unclear what the direction of this relationship is. There is evidence that adolescent smoking is a determinant of developing depressive symptoms (Choi et al., 1997a, Goodman and Capitman, 2000), and it has also been shown that adolescents with depressive symptoms are more likely to start smoking (Escobedo et al., 1998, Kenney and Holahan, 2008, Prinstein and La Greca, 2009). Also, individuals with depressive symptoms who start to smoke during adolescence increase their risk for subsequent depressive symptoms (Choi et al., 1997b), and depressed smokers are less likely to quit smoking (Glassman et al., 1988).
To circumvent the difficulty to assess the direction of the relationship of long-term effects of adolescent nicotine use on mental health in humans, one can try to model this in preclinical animal studies. In particular, animal studies allow for controlling various environmental conditions including housing, food intake and social interactions. Although it may be difficult to model human psychiatric disorders in rodents, it is possible to measure various distinct parameters related to particular endophenotypes, such as measures of anxiety, as well as executive cognitive functions such as attention and impulsive behaviors described earlier in this review. In terms of modeling aspects of affective disorders, indeed, exposing adolescent, but not adult rats to nicotine resulted in an increase in anxiety-related behavior in adulthood compared with their control counterparts, observed as less time spent in the center of an open field (Slawecki et al., 2003, Smith et al., 2006), or on the open arms of an elevated plus maze (Slawecki et al., 2005). Also, animals exposed to a low dose of nicotine during adolescence failed to extinguish fear-related memories in a fear-conditioning task (Smith et al., 2006). However, in the forced swim task, a behavioral task that measures depressive-like symptoms such as learned helplessness, adolescent nicotine exposure decreases these behavioral symptoms, in conjunction with changes in the CRF and NPY systems (Slawecki et al., 2005). We should note however, that the two studies from Slawecki et al. did not include an adult group exposed to nicotine, which makes it harder to draw firm conclusions.
Taken together, there is a strong relationship between smoking and mental health disorders, in particular depression. However, the relationship between these two needs further study using longitudinal studies in humans, and preclinical animal studies to investigate the cellular mechanisms by which adolescent nicotine exposure causes mental health problems.
4.3. Mental health: adolescent nicotine and drug dependence
Adolescent use of tobacco has been described to precede initiation and subsequent use of other (illicit) drugs of abuse, a theory generally known as the gateway hypothesis (Kandel, 1975). When looking at the age of first use, both alcohol and nicotine use precede use of illicit drugs of abuse, such as cocaine and heroin (Arria et al., 2008), but it is unclear as to whether these observations are causally related. A recent review elegantly summarized all prospective and longitudinal studies that investigated the effects of adolescent smoking on subsequent use of other drugs of abuse (Mathers et al., 2006). In particular, adolescent tobacco smoking is associated with subsequent alcohol abuse and dependence, even after controlling for several potential confounding factors, such as psychiatric disorders, demographic factors and parental education (Brook et al., 2002, Jackson et al., 2002, Lewinsohn et al., 1999). Although several studies reported that tobacco use predicts or is related to later use of cannabis (Ellickson et al., 2001, Kandel et al., 1986, McGee et al., 2000), confounding factors, such as demographic factors and psychiatric disorders, have not been adjusted for. Thus, although the gateway hypothesis seems to apply to most studies examining smoking as prediction for illicit drug use, corrections for confounding factors have oftentimes not been performed (Ellickson et al., 2001, Johnson et al., 1995, Kandel et al., 1986, Lewinsohn et al., 1999). In this regard, only a single study reports a clear association between adolescent smoking and subsequent use of illicit drugs after controlling for confounding factors (Brown et al., 1996). Nonetheless, recent work examining the relationship between adolescent tobacco use and illicit drug use contrasts this observation and reports that there is no significant association after controlling for demographic factors, psychiatric disorders and substance use disorders (Brook et al., 2002).
The difficulty to control gene by environment interactions in the human population hampers a straightforward interpretation of adolescent nicotine exposure on subsequent behavioral changes. Animal models can therefore greatly contribute to our understanding of the adolescent brain and can thus be used to explore putative causal relationships between early drug exposure during adolescence and vulnerability to drugs later in life. Adolescent, but not adult nicotine exposure has been shown to increase the locomotor response to amphetamine as long as 30 days after nicotine was given (Collins et al., 2004). Also, exposing adolescent rats to nicotine increases intravenous nicotine self-administration when rats have reached adulthood (Adriani et al., 2003), and increases intravenous cocaine self-administration in late adolescence (McQuown et al., 2007). In addition, nicotine exposure during early adulthood seems to reduce subsequent sensitivity to nicotine. When rats are only exposed to nicotine following adolescence, this causes a reduction in the rewarding properties of nicotine, observed as a rightward shift in the conditioned place preference dose-response curve, suggesting that lower doses of nicotine were no longer rewarding to them (Adriani et al., 2006). However, when alcohol-preferring rats were exposed to nicotine during adolescence, this did not increase subsequent alcohol intake, compared with their saline controls (Kemppainen et al., 2009). In conclusion, these data argue for an increased sensitivity to drugs when exposed during adolescence, and a reduced risk to develop dependence when exposed following adolescence.
5. Overall conclusion
In conclusion, the brain and specifically the prefrontal cortex continue to develop during adolescence, making the adolescent brain uniquely different from the adult brain. One of the differences is that adolescents are more sensitive to the rewarding effects of nicotine, which may be a reason that many people start to smoke during adolescence. Both prospective and longitudinal human studies suggest that adolescent exposure to nicotine has long-term effects, among which are 1) the risk to develop substance use disorder and 2) various mental health problems, the most prevalent ones relating to affective disorders such as anxiety and depression. In addition, inasmuch our animal studies can be extrapolated to humans, adolescent exposure to nicotine may lead to decreased attention performance and increased impulsivity on the long-term. The latter observation in turn might promote the maintenance of smoking behavior. Based on studies in human subjects, it is difficult to determine whether adolescent smoking underlies these problems, or whether smoking and mental health disorders have a common origin that predisposes an enhanced risk to the development thereof. In order to understand the effects of drugs of abuse on motivational systems, it is important to gain a better understanding of their development in the adolescent brain. We believe this should be done both through longitudinal human studies, and by using preclinical animal approaches to understand the precise mechanistic molecular synaptic changes as well as changes in neuronal circuitry that take place during adolescent development.
Acknowledgements
The authors would like to thank Dr. Patricio O’Donnell for helpful comments on the manuscript. DSC received funding from NWO (ZonMW TOP grant 91206148). ABS received funding from the European Union Seventh Framework Programme under grant agreement no. HEALTH-F2-2007-202088 (“NeuroCypres” project).
References
- Abreu-Villaca Y., Seidler F.J., Qiao D., Tate C.A., Cousins M.M., Thillai I. Short-term adolescent nicotine exposure has immediate and persistent effects on cholinergic systems: critical periods, patterns of exposure, dose thresholds. Neuropsychopharmacology. 2003;28(11):1935–1949. doi: 10.1038/sj.npp.1300221. [DOI] [PubMed] [Google Scholar]
- Adriani W., Deroche-Gamonet V., Le Moal M., Laviola G., Piazza P.V. Preexposure during or following adolescence differently affects nicotine-rewarding properties in adult rats. Psychopharmacology (Berl) 2006;184(3–4):382–390. doi: 10.1007/s00213-005-0125-1. [DOI] [PubMed] [Google Scholar]
- Adriani W., Spijker S., Deroche-Gamonet V., Laviola G., Le Moal M., Smit A.B. Evidence for enhanced neurobehavioral vulnerability to nicotine during periadolescence in rats. J. Neurosci. 2003;23(11):4712–4716. doi: 10.1523/JNEUROSCI.23-11-04712.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen S.L. Trajectories of brain development: point of vulnerability or window of opportunity? Neurosci. Biobehav. Rev. 2003;27(1–2):3–18. doi: 10.1016/s0149-7634(03)00005-8. [DOI] [PubMed] [Google Scholar]
- Anthony J.C., Petronis K.R. Early-onset drug use and risk of later drug problems. Drug Alcohol Depend. 1995;40(1):9–15. doi: 10.1016/0376-8716(95)01194-3. [DOI] [PubMed] [Google Scholar]
- Arnett J.J. Optimistic bias in adolescent and adult smokers and nonsmokers. Addict. Behav. 2000;25(4):625–632. doi: 10.1016/s0306-4603(99)00072-6. [DOI] [PubMed] [Google Scholar]
- Arria A.M., Caldeira K.M., O’Grady K.E., Vincent K.B., Fitzelle D.B., Johnson E.P. Drug exposure opportunities and use patterns among college students: results of a longitudinal prospective cohort study. Subst. Abus. 2008;29(4):19–38. doi: 10.1080/08897070802418451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audrain-McGovern J., Al Koudsi N., Rodriguez D., Wileyto E.P., Shields P.G., Tyndale R.F. The role of CYP2A6 in the emergence of nicotine dependence in adolescents. Pediatrics. 2007;119(1):e264–274. doi: 10.1542/peds.2006-1583. [DOI] [PubMed] [Google Scholar]
- Barria A., Malinow R. Subunit-specific NMDA receptor trafficking to synapses. Neuron. 2002;35(2):345–353. doi: 10.1016/s0896-6273(02)00776-6. [DOI] [PubMed] [Google Scholar]
- Belluzzi J.D., Lee A.G., Oliff H.S., Leslie F.M. Age-dependent effects of nicotine on locomotor activity and conditioned place preference in rats. Psychopharmacology (Berl) 2004;174(3):389–395. doi: 10.1007/s00213-003-1758-6. [DOI] [PubMed] [Google Scholar]
- Belluzzi J.D., Wang R., Leslie F.M. Acetaldehyde enhances acquisition of nicotine self-administration in adolescent rats. Neuropsychopharmacology. 2005;30(4):705–712. doi: 10.1038/sj.npp.1300586. [DOI] [PubMed] [Google Scholar]
- Benes F.M. Myelination of cortical-hippocampal relays during late adolescence. Schizophr. Bull. 1989;15(4):585–593. doi: 10.1093/schbul/15.4.585. [DOI] [PubMed] [Google Scholar]
- Benwell M.E., Balfour D.J., Anderson J.M. Evidence that tobacco smoking increases the density of (-)-[3H]nicotine binding sites in human brain. J. Neurochem. 1988;50(4):1243–1247. doi: 10.1111/j.1471-4159.1988.tb10600.x. [DOI] [PubMed] [Google Scholar]
- Bickel W.K., Odum A.L., Madden G.J. Impulsivity and cigarette smoking: delay discounting in current, never, and ex-smokers. Psychopharmacology (Berl) 1999;146(4):447–454. doi: 10.1007/pl00005490. [DOI] [PubMed] [Google Scholar]
- Bockhorst K.H., Narayana P.A., Liu R., Ahobila-Vijjula P., Ramu J., Kamel M. Early postnatal development of rat brain: in vivo diffusion tensor imaging. J. Neurosci. Res. 2008;86(7):1520–1528. doi: 10.1002/jnr.21607. [DOI] [PubMed] [Google Scholar]
- Bourgeois J.P., Goldman-Rakic P.S., Rakic P. Synaptogenesis in the prefrontal cortex of rhesus monkeys. Cereb. Cortex. 1994;4(1):78–96. doi: 10.1093/cercor/4.1.78. [DOI] [PubMed] [Google Scholar]
- Breese C.R., Marks M.J., Logel J., Adams C.E., Sullivan B., Collins A.C. Effect of smoking history on [3H]nicotine binding in human postmortem brain. J. Pharmacol. Exp. Ther. 1997;282(1):7–13. [PubMed] [Google Scholar]
- Brenhouse H.C., Andersen S.L. Delayed extinction and stronger reinstatement of cocaine conditioned place preference in adolescent rats, compared to adults. Behav. Neurosci. 2008;122(2):460–465. doi: 10.1037/0735-7044.122.2.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenhouse H.C., Sonntag K.C., Andersen S.L. Transient D1 dopamine receptor expression on prefrontal cortex projection neurons: relationship to enhanced motivational salience of drug cues in adolescence. J. Neurosci. 2008;28(10):2375–2382. doi: 10.1523/JNEUROSCI.5064-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brielmaier J.M., McDonald C.G., Smith R.F. Immediate and long-term behavioral effects of a single nicotine injection in adolescent and adult rats. Neurotoxicol. Teratol. 2007;29(1):74–80. doi: 10.1016/j.ntt.2006.09.023. [DOI] [PubMed] [Google Scholar]
- Brook D.W., Brook J.S., Zhang C., Cohen P., Whiteman M. Drug use and the risk of major depressive disorder, alcohol dependence, and substance use disorders. Arch. Gen. Psychiatry. 2002;59(11):1039–1044. doi: 10.1001/archpsyc.59.11.1039. [DOI] [PubMed] [Google Scholar]
- Brook J.S., Cohen P., Brook D.W. Longitudinal study of co-occurring psychiatric disorders and substance use. J. Am. Acad. Child Adolesc. Psychiatry. 1998;37(3):322–330. doi: 10.1097/00004583-199803000-00018. [DOI] [PubMed] [Google Scholar]
- Brown R.A., Lewinsohn P.M., Seeley J.R., Wagner E.F. Cigarette smoking, major depression, and other psychiatric disorders among adolescents. J. Am. Acad. Child Adolesc. Psychiatry. 1996;35(12):1602–1610. doi: 10.1097/00004583-199612000-00011. [DOI] [PubMed] [Google Scholar]
- Buffelli M., Busetto G., Bidoia C., Favero M., Cangiano A. Activity-dependent synaptic competition at mammalian neuromuscular junctions. News Physiol. Sci. 2004;19:85–91. doi: 10.1152/nips.01464.2003. [DOI] [PubMed] [Google Scholar]
- Carpenter-Hyland E.P., Chandler L.J. Adaptive plasticity of NMDA receptors and dendritic spines: implications for enhanced vulnerability of the adolescent brain to alcohol addiction. Pharmacol. Biochem. Behav. 2007;86(2):200–208. doi: 10.1016/j.pbb.2007.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey B.J., Tottenham N., Liston C., Durston S. Imaging the developing brain: what have we learned about cognitive development? Trends Cogn. Sci. 2005;9(3):104–110. doi: 10.1016/j.tics.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Chambers R.A., Taylor J.R., Potenza M.N. Developmental neurocircuitry of motivation in adolescence: a critical period of addiction vulnerability. Am. J. Psychiatry. 2003;160(6):1041–1052. doi: 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chassin L., Presson C.C., Rose J.S., Sherman S.J. The natural history of cigarette smoking from adolescence to adulthood: demographic predictors of continuity and change. Health Psychol. 1996;15(6):478–484. doi: 10.1037//0278-6133.15.6.478. [DOI] [PubMed] [Google Scholar]
- Chassin L., Presson C.C., Sherman S.J., Edwards D.A. The natural history of cigarette smoking: predicting young-adult smoking outcomes from adolescent smoking patterns. Health Psychol. 1990;9(6):701–716. doi: 10.1037//0278-6133.9.6.701. [DOI] [PubMed] [Google Scholar]
- Chen H., Matta S.G., Sharp B.M. Acquisition of nicotine self-administration in adolescent rats given prolonged access to the drug. Neuropsychopharmacology. 2007;32(3):700–709. doi: 10.1038/sj.npp.1301135. [DOI] [PubMed] [Google Scholar]
- Chen X., Moore-Nichols D., Nguyen H., Michaelis E.K. Calcium influx through NMDA receptors, chronic receptor inhibition by ethanol and 2-amino-5-phosponopentanoic acid, and receptor protein expression. J. Neurochem. 1999;72(5):1969–1980. doi: 10.1046/j.1471-4159.1999.0721969.x. [DOI] [PubMed] [Google Scholar]
- Choi W.S., Patten C.A., Gillin J.C., Kaplan R.M., Pierce J.P. Cigarette smoking predicts development of depressive symptoms among U.S. adolescents. Ann. Behav. Med. 1997;19(1):42–50. doi: 10.1007/BF02883426. [DOI] [PubMed] [Google Scholar]
- Choi W.S., Pierce J.P., Gilpin E.A., Farkas A.J., Berry C.C. Which adolescent experimenters progress to established smoking in the United States. Am. J. Prev. Med. 1997;13(5):385–391. [PubMed] [Google Scholar]
- Colby S.M., Tiffany S.T., Shiffman S., Niaura R.S. Are adolescent smokers dependent on nicotine? A review of the evidence. Drug Alcohol Depend. 2000;59(Suppl. 1):S83–95. doi: 10.1016/s0376-8716(99)00166-0. [DOI] [PubMed] [Google Scholar]
- Collins S.L., Montano R., Izenwasser S. Nicotine treatment produces persistent increases in amphetamine-stimulated locomotor activity in periadolescent male but not female or adult male rats. Brain Res. Dev. Brain Res. 2004;153(2):175–187. doi: 10.1016/j.devbrainres.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Counotte D.S., Goriounova N.A., Li K.W., Loos M., van der Schors R.C., Schetters D. Lasting synaptic changes underlie attention deficits caused by nicotine exposure during adolescence. Nat. Neurosci. 2011;14(4):417–419. doi: 10.1038/nn.2770. [DOI] [PubMed] [Google Scholar]
- Counotte D.S., Li K.W., Wortel J., Gouwenberg Y., Van Der Schors R.C., Smit A.B. Changes in molecular composition of rat medial prefrontal cortex synapses during adolescent development. Eur. J. Neurosci. 2010;32(9):1452–1460. doi: 10.1111/j.1460-9568.2010.07404.x. [DOI] [PubMed] [Google Scholar]
- Counotte D.S., Spijker S., Van de Burgwal L.H., Hogenboom F., Schoffelmeer A.N., De Vries T.J. Long-lasting cognitive deficits resulting from adolescent nicotine exposure in rats. Neuropsychopharmacology. 2009;34(2):299–306. doi: 10.1038/npp.2008.96. [DOI] [PubMed] [Google Scholar]
- Cunningham M.G., Bhattacharyya S., Benes F.M. Amygdalo-cortical sprouting continues into early adulthood: implications for the development of normal and abnormal function during adolescence. J. Comp. Neurol. 2002;453(2):116–130. doi: 10.1002/cne.10376. [DOI] [PubMed] [Google Scholar]
- Cunningham M.G., Bhattacharyya S., Benes F.M. Increasing Interaction of amygdalar afferents with GABAergic interneurons between birth and adulthood. Cereb. Cortex. 2008;18(7):1529–1535. doi: 10.1093/cercor/bhm183. [DOI] [PubMed] [Google Scholar]
- Dallery J., Raiff B.R. Delay discounting predicts cigarette smoking in a laboratory model of abstinence reinforcement. Psychopharmacology (Berl) 2007;190(4):485–496. doi: 10.1007/s00213-006-0627-5. [DOI] [PubMed] [Google Scholar]
- Dappen A., Schwartz R.H., O’Donnell R. A survey of adolescent smoking patterns. J. Am. Board Fam. Pract. 1996;9(1):7–13. [PubMed] [Google Scholar]
- Day M., Pan J.B., Buckley M.J., Cronin E., Hollingsworth P.R., Hirst W.D. Differential effects of ciproxifan and nicotine on impulsivity and attention measures in the 5-choice serial reaction time test. Biochem. Pharmacol. 2007;73(8):1123–1134. doi: 10.1016/j.bcp.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Defagot M.C., Villar M.J., Antonelli M.C. Differential localization of metabotropic glutamate receptors during postnatal development. Dev. Neurosci. 2002;24(4):272–282. doi: 10.1159/000066741. [DOI] [PubMed] [Google Scholar]
- Diergaarde L., Pattij T., Poortvliet I., Hogenboom F., de Vries W., Schoffelmeer A.N. Impulsive choice and impulsive action predict vulnerability to distinct stages of nicotine seeking in rats. Biol. Psychiatry. 2008;63(3):301–308. doi: 10.1016/j.biopsych.2007.07.011. [DOI] [PubMed] [Google Scholar]
- DiFranza J.R., Savageau J.A., Fletcher K., Ockene J.K., Rigotti N.A., McNeill A.D. Recollections and repercussions of the first inhaled cigarette. Addict. Behav. 2004;29(2):261–272. doi: 10.1016/j.addbeh.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Doura M.B., Gold A.B., Keller A.B., Perry D.C. Adult and periadolescent rats differ in expression of nicotinic cholinergic receptor subtypes and in the response of these subtypes to chronic nicotine exposure. Brain Res. 2008;1215:40–52. doi: 10.1016/j.brainres.2008.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durston S., Davidson M.C., Tottenham N., Galvan A., Spicer J., Fossella J.A. A shift from diffuse to focal cortical activity with development. Dev. Sci. 2006;9(1):1–8. doi: 10.1111/j.1467-7687.2005.00454.x. [DOI] [PubMed] [Google Scholar]
- Ellickson P.L., Tucker J.S., Klein D.J. High-risk behaviors associated with early smoking: results from a 5-year follow-up. J. Adolesc. Health. 2001;28(6):465–473. doi: 10.1016/s1054-139x(00)00202-0. [DOI] [PubMed] [Google Scholar]
- Elston G.N., Oga T., Fujita I. Spinogenesis and pruning scales across functional hierarchies. J. Neurosci. 2009;29(10):3271–3275. doi: 10.1523/JNEUROSCI.5216-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M., Nelson E.E., Jazbec S., McClure E.B., Monk C.S., Leibenluft E. Amygdala and nucleus accumbens in responses to receipt and omission of gains in adults and adolescents. Neuroimage. 2005;25(4):1279–1291. doi: 10.1016/j.neuroimage.2004.12.038. [DOI] [PubMed] [Google Scholar]
- Escobedo L.G., Reddy M., Giovino G.A. The relationship between depressive symptoms and cigarette smoking in US adolescents. Addiction. 1998;93(3):433–440. doi: 10.1046/j.1360-0443.1998.93343311.x. [DOI] [PubMed] [Google Scholar]
- Eshel N., Nelson E.E., Blair R.J., Pine D.S., Ernst M. Neural substrates of choice selection in adults and adolescents: development of the ventrolateral prefrontal and anterior cingulate cortices. Neuropsychologia. 2007;45(6):1270–1279. doi: 10.1016/j.neuropsychologia.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt B.J., Robbins T.W. Central cholinergic systems and cognition. Annu. Rev. Psychol. 1997;48:649–684. doi: 10.1146/annurev.psych.48.1.649. [DOI] [PubMed] [Google Scholar]
- Fagerstrom K., Aubin H.J. Management of smoking cessation in patients with psychiatric disorders. Curr. Med. Res. Opin. 2009;25(2):511–518. doi: 10.1185/03007990802707568. [DOI] [PubMed] [Google Scholar]
- Gabbott P.L., Warner T.A., Jays P.R., Salway P., Busby S.J. Prefrontal cortex in the rat: projections to subcortical autonomic, motor, and limbic centers. J. Comp. Neurol. 2005;492(2):145–177. doi: 10.1002/cne.20738. [DOI] [PubMed] [Google Scholar]
- Galvan A., Hare T.A., Parra C.E., Penn J., Voss H., Glover G. Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. J. Neurosci. 2006;26(25):6885–6892. doi: 10.1523/JNEUROSCI.1062-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd J.N. Structural magnetic resonance imaging of the adolescent brain. Ann. N.Y. Acad. Sci. 2004;1021:77–85. doi: 10.1196/annals.1308.009. [DOI] [PubMed] [Google Scholar]
- Giedd J.N., Blumenthal J., Jeffries N.O., Castellanos F.X., Liu H., Zijdenbos A. Brain development during childhood and adolescence: a longitudinal MRI study. Nat. Neurosci. 1999;2(10):861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Glassman A.H., Stetner F., Walsh B.T., Raizman P.S., Fleiss J.L., Cooper T.B. Heavy smokers, smoking cessation, and clonidine Results of a double-blind, randomized trial. JAMA. 1988;259(19):2863–2866. [PubMed] [Google Scholar]
- Gogtay N., Giedd J.N., Lusk L., Hayashi K.M., Greenstein D., Vaituzis A.C. Dynamic mapping of human cortical development during childhood through early adulthood. Proc. Natl. Acad. Sci. U.S.A. 2004;101(21):8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Burgos G., Kroener S., Zaitsev A.V., Povysheva N.V., Krimer L.S., Barrionuevo G. Functional maturation of excitatory synapses in layer 3 pyramidal neurons during postnatal development of the primate prefrontal cortex. Cereb. Cortex. 2008;18(3):626–637. doi: 10.1093/cercor/bhm095. [DOI] [PubMed] [Google Scholar]
- Goodman E., Capitman J. Depressive symptoms and cigarette smoking among teens. Pediatrics. 2000;106(4):748–755. doi: 10.1542/peds.106.4.748. [DOI] [PubMed] [Google Scholar]
- Gotti C., Moretti M., Gaimarri A., Zanardi A., Clementi F., Zoli M. Heterogeneity and complexity of native brain nicotinic receptors. Biochem. Pharmacol. 2007;74(8):1102–1111. doi: 10.1016/j.bcp.2007.05.023. [DOI] [PubMed] [Google Scholar]
- Gould E., Woolf N.J., Butcher L.L. Postnatal development of cholinergic neurons in the rat: I. Forebrain. Brain Res. Bull. 1991;27(6):767–789. doi: 10.1016/0361-9230(91)90209-3. [DOI] [PubMed] [Google Scholar]
- Govind A.P., Vezina P., Green W.N. Nicotine-induced upregulation of nicotinic receptors: underlying mechanisms and relevance to nicotine addiction. Biochem. Pharmacol. 2009 doi: 10.1016/j.bcp.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griesler P.C., Hu M.C., Schaffran C., Kandel D.B. Comorbidity of psychiatric disorders and nicotine dependence among adolescents: findings from a prospective, longitudinal study. J. Am. Acad. Child Adolesc. Psychiatry. 2008;47(11):1340–1350. doi: 10.1097/CHI.0b013e318185d2ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grottick A.J., Higgins G.A. Effect of subtype selective nicotinic compounds on attention as assessed by the five-choice serial reaction time task. Behav. Brain Res. 2000;117(1–2):197–208. doi: 10.1016/s0166-4328(00)00305-3. [DOI] [PubMed] [Google Scholar]
- Guillery R.W. Is postnatal neocortical maturation hierarchical? Trends Neurosci. 2005;28(10):512–517. doi: 10.1016/j.tins.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Hakko H., Lintunen J., Lappalainen J., Makikyro T., Rasanen P., Timonen M. Nicotine use and dependence and their association to psychiatric disorders in a large sample of adolescent psychiatric inpatients. Addict. Behav. 2006;31(10):1873–1880. doi: 10.1016/j.addbeh.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Hoffmann H., Gremme T., Hatt H., Gottmann K. Synaptic activity-dependent developmental regulation of NMDA receptor subunit expression in cultured neocortical neurons. J. Neurochem. 2000;75(4):1590–1599. doi: 10.1046/j.1471-4159.2000.0751590.x. [DOI] [PubMed] [Google Scholar]
- Hoover W.B., Vertes R.P. Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Struct. Funct. 2007;212(2):149–179. doi: 10.1007/s00429-007-0150-4. [DOI] [PubMed] [Google Scholar]
- Huberman A.D. Mechanisms of eye-specific visual circuit development. Curr. Opin. Neurobiol. 2007;17(1):73–80. doi: 10.1016/j.conb.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Huttenlocher P.R. Synaptic density in human frontal cortex - developmental changes and effects of aging. Brain Res. 1979;163(2):195–205. doi: 10.1016/0006-8993(79)90349-4. [DOI] [PubMed] [Google Scholar]
- Huttenlocher P.R. Morphometric study of human cerebral cortex development. Neuropsychologia. 1990;28(6):517–527. doi: 10.1016/0028-3932(90)90031-i. [DOI] [PubMed] [Google Scholar]
- Jackson K.M., Sher K.J., Cooper M.L., Wood P.K. Adolescent alcohol and tobacco use: onset, persistence and trajectories of use across two samples. Addiction. 2002;97(5):517–531. doi: 10.1046/j.1360-0443.2002.00082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen L.K., Krystal J.H., Mencl W.E., Westerveld M., Frost S.J., Pugh K.R. Effects of smoking and smoking abstinence on cognition in adolescent tobacco smokers. Biol. Psychiatry. 2005;57(1):56–66. doi: 10.1016/j.biopsych.2004.10.022. [DOI] [PubMed] [Google Scholar]
- Jernigan T.L., Trauner D.A., Hesselink J.R., Tallal P.A. Maturation of human cerebrum observed in vivo during adolescence. Brain. 1991;114(Pt 5):2037–2049. doi: 10.1093/brain/114.5.2037. [DOI] [PubMed] [Google Scholar]
- Jin D.H., Jung Y.W., Ham S.H., Ko B.H., Moon I.S. Developmental expression, subcellular localization, and tyrosine phosphorylation of NR2A and NR2B in the rat brain. Mol. Cells. 1997;7(1):64–71. [PubMed] [Google Scholar]
- Johnson E.O., Schutz C.G., Anthony J.C., Ensminger M.E. Inhalants to heroin: a prospective analysis from adolescence to adulthood. Drug Alcohol Depend. 1995;40(2):159–164. doi: 10.1016/0376-8716(95)01201-x. [DOI] [PubMed] [Google Scholar]
- Johnson J.G., Cohen P., Pine D.S., Klein D.F., Kasen S., Brook J.S. Association between cigarette smoking and anxiety disorders during adolescence and early adulthood. JAMA. 2000;284(18):2348–2351. doi: 10.1001/jama.284.18.2348. [DOI] [PubMed] [Google Scholar]
- Johnson L.D., O’Malley P.M., Bachman J.G., Schulenberg J.E. NIH Publication. National Institute on Drug Abuse (NIDA); 2006. Monitoring the future: national results on adolescent drug use. Overview of key findings 2005. [Google Scholar]
- Jokel E.S., Garduno E.R., Ariano M.A., Levine M.S. Metabotropic glutamate receptors mGluR1alpha and mGluR2/3 display dynamic expression patterns in developing rat striatum. Dev. Neurosci. 2001;23(1):1–6. doi: 10.1159/000048690. [DOI] [PubMed] [Google Scholar]
- Kalsbeek A., Voorn P., Buijs R.M., Pool C.W., Uylings H.B. Development of the dopaminergic innervation in the prefrontal cortex of the rat. J. Comp. Neurol. 1988;269(1):58–72. doi: 10.1002/cne.902690105. [DOI] [PubMed] [Google Scholar]
- Kandel D. Stages in adolescent involvement in drug use. Science. 1975;190(4217):912–914. doi: 10.1126/science.1188374. [DOI] [PubMed] [Google Scholar]
- Kandel D.B., Chen K. Extent of smoking and nicotine dependence in the United States: 1991–1993. Nicotine Tob. Res. 2000;2(3):263–274. doi: 10.1080/14622200050147538. [DOI] [PubMed] [Google Scholar]
- Kandel D.B., Davies M., Karus D., Yamaguchi K. The consequences in young adulthood of adolescent drug involvement. An overview. Arch. Gen. Psychiatry. 1986;43(8):746–754. doi: 10.1001/archpsyc.1986.01800080032005. [DOI] [PubMed] [Google Scholar]
- Kandel D.B., Yamaguchi K., Chen K. Stages of progression in drug involvement from adolescence to adulthood: further evidence for the gateway theory. J. Stud. Alcohol. 1992;53(5):447–457. doi: 10.15288/jsa.1992.53.447. [DOI] [PubMed] [Google Scholar]
- Kemppainen H., Hyytia P., Kiianmaa K. Behavioral consequences of repeated nicotine during adolescence in alcohol-preferring AA and alcohol-avoiding ANA rats. Alcohol Clin. Exp. Res. 2009;33(2):340–349. doi: 10.1111/j.1530-0277.2008.00838.x. [DOI] [PubMed] [Google Scholar]
- Kenney B.A., Holahan C.J. Depressive symptoms and cigarette smoking in a college sample. J. Am. Coll. Health. 2008;56(4):409–414. doi: 10.3200/JACH.56.44.409-414. [DOI] [PubMed] [Google Scholar]
- Kiss J., Patel A.J. Development of the cholinergic fibres innervating the cerebral cortex of the rat. Int. J. Dev. Neurosci. 1992;10(2):153–170. doi: 10.1016/0736-5748(92)90043-y. [DOI] [PubMed] [Google Scholar]
- Klingberg T. Development of a superior frontal-intraparietal network for visuo-spatial working memory. Neuropsychologia. 2006;44(11):2171–2177. doi: 10.1016/j.neuropsychologia.2005.11.019. [DOI] [PubMed] [Google Scholar]
- Kota D., Robinson S.E., Imad Damaj M. Enhanced nicotine reward in adulthood after exposure to nicotine during early adolescence in mice. Biochem. Pharmacol. 2009;78(7):873–879. doi: 10.1016/j.bcp.2009.06.099. [DOI] [PubMed] [Google Scholar]
- Krishnan-Sarin S., Reynolds B., Duhig A.M., Smith A., Liss T., McFetridge A. Behavioral impulsivity predicts treatment outcome in a smoking cessation program for adolescent smokers. Drug Alcohol Depend. 2007;88(1):79–82. doi: 10.1016/j.drugalcdep.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupp J.J., Vissel B., Heinemann S.F., Westbrook G.L. Calcium-dependent inactivation of recombinant N-methyl-D-aspartate receptors is NR2 subunit specific. Mol. Pharmacol. 1996;50(6):1680–1688. [PubMed] [Google Scholar]
- Kuryatov A., Onksen J., Lindstrom J. Roles of accessory subunits in alpha4beta2(*) nicotinic receptors. Mol. Pharmacol. 2008;74(1):132–143. doi: 10.1124/mol.108.046789. [DOI] [PubMed] [Google Scholar]
- Lai A., Parameswaran N., Khwaja M., Whiteaker P., Lindstrom J.M., Fan H. Long-term nicotine treatment decreases striatal alpha 6* nicotinic acetylcholine receptor sites and function in mice. Mol. Pharmacol. 2005;67(5):1639–1647. doi: 10.1124/mol.104.006429. [DOI] [PubMed] [Google Scholar]
- Laughlin S.B., Sejnowski T.J. Communication in neuronal networks. Science. 2003;301(5641):1870–1874. doi: 10.1126/science.1089662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.H., Lim Y., Wiederhold B.K., Graham S.J. A functional magnetic resonance imaging (FMRI) study of cue-induced smoking craving in virtual environments. Appl. Psychophysiol. Biofeedback. 2005;30(3):195–204. doi: 10.1007/s10484-005-6377-z. [DOI] [PubMed] [Google Scholar]
- Levin E.D., Lawrence S.S., Petro A., Horton K., Rezvani A.H., Seidler F.J. Adolescent vs. adult-onset nicotine self-administration in male rats: duration of effect and differential nicotinic receptor correlates. Neurotoxicol. Teratol. 2007;29(4):458–465. doi: 10.1016/j.ntt.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin E.D., Rezvani A.H., Montoya D., Rose J.E., Swartzwelder H.S. Adolescent-onset nicotine self-administration modeled in female rats. Psychopharmacology (Berl) 2003;169(2):141–149. doi: 10.1007/s00213-003-1486-y. [DOI] [PubMed] [Google Scholar]
- Lewinsohn P.M., Rohde P., Brown R.A. Level of current and past adolescent cigarette smoking as predictors of future substance use disorders in young adulthood. Addiction. 1999;94(6):913–921. doi: 10.1046/j.1360-0443.1999.94691313.x. [DOI] [PubMed] [Google Scholar]
- Lichtman J.W., Colman H. Synapse elimination and indelible memory. Neuron. 2000;25(2):269–278. doi: 10.1016/s0896-6273(00)80893-4. [DOI] [PubMed] [Google Scholar]
- Mansvelder H.D., Mertz M., Role L.W. Nicotinic modulation of synaptic transmission and plasticity in cortico-limbic circuits. Semin. Cell Dev. Biol. 2009;20(4):432–440. doi: 10.1016/j.semcdb.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansvelder H.D., van Aerde K.I., Couey J.J., Brussaard A.B. Nicotinic modulation of neuronal networks: from receptors to cognition. Psychopharmacology (Berl) 2006;184(3–4):292–305. doi: 10.1007/s00213-005-0070-z. [DOI] [PubMed] [Google Scholar]
- Markham J.A., Morris J.R., Juraska J.M. Neuron number decreases in the rat ventral, but not dorsal, medial prefrontal cortex between adolescence and adulthood. Neuroscience. 2007;144(3):961–968. doi: 10.1016/j.neuroscience.2006.10.015. [DOI] [PubMed] [Google Scholar]
- Marks M.J., Burch J.B., Collins A.C. Effects of chronic nicotine infusion on tolerance development and nicotinic receptors. J. Pharmacol. Exp. Ther. 1983;226(3):817–825. [PubMed] [Google Scholar]
- Mathers M., Toumbourou J.W., Catalano R.F., Williams J., Patton G.C. Consequences of youth tobacco use: a review of prospective behavioural studies. Addiction. 2006;101(7):948–958. doi: 10.1111/j.1360-0443.2006.01438.x. [DOI] [PubMed] [Google Scholar]
- McClernon F.J., Kollins S.H. ADHD and smoking: from genes to brain to behavior. Ann. N.Y. Acad. Sci. 2008;1141:131–147. doi: 10.1196/annals.1441.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutcheon J.E., Marinelli M. Age matters. Eur. J. Neurosci. 2009;29(5):997–1014. doi: 10.1111/j.1460-9568.2009.06648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee R., Williams S., Poulton R., Moffitt T. A longitudinal study of cannabis use and mental health from adolescence to early adulthood. Addiction. 2000;95(4):491–503. doi: 10.1046/j.1360-0443.2000.9544912.x. [DOI] [PubMed] [Google Scholar]
- McQuown S.C., Belluzzi J.D., Leslie F.M. Low dose nicotine treatment during early adolescence increases subsequent cocaine reward. Neurotoxicol. Teratol. 2007;29(1):66–73. doi: 10.1016/j.ntt.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier S., Brauer A.U., Heimrich B., Nitsch R., Savaskan N.E. Myelination in the hippocampus during development and following lesion. Cell Mol. Life Sci. 2004;61(9):1082–1094. doi: 10.1007/s00018-004-3469-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell S.H. Measures of impulsivity in cigarette smokers and non-smokers. Psychopharmacology (Berl) 1999;146(4):455–464. doi: 10.1007/pl00005491. [DOI] [PubMed] [Google Scholar]
- Mitsis E.M., Cosgrove K.P., Staley J.K., Frohlich E.B., Bois F., Tamagnan G.D. [123I]5-IA-85380 SPECT imaging of beta2-nicotinic acetylcholine receptor availability in the aging human brain. Ann. N.Y. Acad. Sci. 2007;1097:168–170. doi: 10.1196/annals.1379.015. [DOI] [PubMed] [Google Scholar]
- Moretti M., Mugnaini M., Tessari M., Zoli M., Gaimarri A., Manfredi I. A comparative study of the effects of the intravenous self-administration or subcutaneous minipump infusion of nicotine on the expression of brain neuronal nicotinic receptor subtypes. Mol. Pharmacol. 2010;78(2):287–296. doi: 10.1124/mol.110.064071. [DOI] [PubMed] [Google Scholar]
- Mugnaini M., Garzotti M., Sartori I., Pilla M., Repeto P., Heidbreder C.A. Selective down-regulation of [(125)I]Y0-alpha-conotoxin MII binding in rat mesostriatal dopamine pathway following continuous infusion of nicotine. Neuroscience. 2006;137(2):565–572. doi: 10.1016/j.neuroscience.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Musso F., Bettermann F., Vucurevic G., Stoeter P., Konrad A., Winterer G. Smoking impacts on prefrontal attentional network function in young adult brains. Psychopharmacology (Berl) 2007;191(1):159–169. doi: 10.1007/s00213-006-0499-8. [DOI] [PubMed] [Google Scholar]
- Nashmi R., Xiao C., Deshpande P., McKinney S., Grady S.R., Whiteaker P. Chronic nicotine cell specifically upregulates functional alpha 4* nicotinic receptors: basis for both tolerance in midbrain and enhanced long-term potentiation in perforant path. J. Neurosci. 2007;27(31):8202–8218. doi: 10.1523/JNEUROSCI.2199-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Dell L.E. A psychobiological framework of the substrates that mediate nicotine use during adolescence. Neuropharmacology. 2009;56(Suppl. 1):263–278. doi: 10.1016/j.neuropharm.2008.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Dell L.E., Bruijnzeel A.W., Ghozland S., Markou A., Koob G.F. Nicotine withdrawal in adolescent and adult rats. Ann. N.Y. Acad. Sci. 2004;1021:167–174. doi: 10.1196/annals.1308.022. [DOI] [PubMed] [Google Scholar]
- O’Dell L.E., Bruijnzeel A.W., Smith R.T., Parsons L.H., Merves M.L., Goldberger B.A. Diminished nicotine withdrawal in adolescent rats: implications for vulnerability to addiction. Psychopharmacology (Berl) 2006;186(4):612–619. doi: 10.1007/s00213-006-0383-6. [DOI] [PubMed] [Google Scholar]
- O’Dell L.E., Khroyan T.V. Rodent models of nicotine reward: what do they tell us about tobacco abuse in humans? Pharmacol. Biochem. Behav. 2009;91(4):481–488. doi: 10.1016/j.pbb.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Dell L.E., Torres O.V., Natividad L.A., Tejeda H.A. Adolescent nicotine exposure produces less affective measures of withdrawal relative to adult nicotine exposure in male rats. Neurotoxicol. Teratol. 2007;29(1):17–22. doi: 10.1016/j.ntt.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S., Romer D. Associations between smoking and depression in adolescence: an integrative review. Taehan Kanho Hakhoe Chi. 2007;37(2):227–241. doi: 10.4040/jkan.2007.37.2.227. [DOI] [PubMed] [Google Scholar]
- Pattij T., Janssen M.C., Loos M., Smit A.B., Schoffelmeer A.N., van Gaalen M.M. Strain specificity and cholinergic modulation of visuospatial attention in three inbred mouse strains. Genes Brain Behav. 2007;6(6):579–587. doi: 10.1111/j.1601-183X.2006.00284.x. [DOI] [PubMed] [Google Scholar]
- Pattij T., Vanderschuren L.J. The neuropharmacology of impulsive behaviour. Trends Pharmacol. Sci. 2008;29(4):192–199. doi: 10.1016/j.tips.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Paus T. Mapping brain maturation and cognitive development during adolescence. Trends Cogn. Sci. 2005;9(2):60–68. doi: 10.1016/j.tics.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Paus T., Zijdenbos A., Worsley K., Collins D.L., Blumenthal J., Giedd J.N. Structural maturation of neural pathways in children and adolescents: in vivo study. Science. 1999;283(5409):1908–1911. doi: 10.1126/science.283.5409.1908. [DOI] [PubMed] [Google Scholar]
- Pedersen W., von Soest T. Smoking, nicotine dependence and mental health among young adults: a 13-year population-based longitudinal study. Addiction. 2009;104(1):129–137. doi: 10.1111/j.1360-0443.2008.02395.x. [DOI] [PubMed] [Google Scholar]
- Pergadia M.L., Agrawal A., Heath A.C., Martin N.G., Bucholz K.K., Madden P.A. Nicotine withdrawal symptoms in adolescent and adult twins. Twin Res. Hum. Genet. 2010;13(4):359–369. doi: 10.1375/twin.13.4.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry D.C., Davila-Garcia M.I., Stockmeier C.A., Kellar K.J. Increased nicotinic receptors in brains from smokers: membrane binding and autoradiography studies. J. Pharmacol. Exp. Ther. 1999;289(3):1545–1552. [PubMed] [Google Scholar]
- Perry D.C., Mao D., Gold A.B., McIntosh J.M., Pezzullo J.C., Kellar K.J. Chronic nicotine differentially regulates alpha6- and beta3-containing nicotinic cholinergic receptors in rat brain. J. Pharmacol. Exp. Ther. 2007;322(1):306–315. doi: 10.1124/jpet.107.121228. [DOI] [PubMed] [Google Scholar]
- Poltavski D.V., Petros T. Effects of transdermal nicotine on attention in adult non-smokers with and without attentional deficits. Physiol. Behav. 2006;87(3):614–624. doi: 10.1016/j.physbeh.2005.12.011. [DOI] [PubMed] [Google Scholar]
- Prinstein M.J., La Greca A.M. Childhood depressive symptoms and adolescent cigarette use: a six-year longitudinal study controlling for peer relations correlates. Health Psychol. 2009;28(3):283–291. doi: 10.1037/a0013949. [DOI] [PubMed] [Google Scholar]
- Prokhorov A.V., Pallonen U.E., Fava J.L., Ding L., Niaura R. Measuring nicotine dependence among high-risk adolescent smokers. Addict. Behav. 1996;21(1):117–127. doi: 10.1016/0306-4603(96)00048-2. [DOI] [PubMed] [Google Scholar]
- Rakic P., Bourgeois J.P., Goldman-Rakic P.S. Synaptic development of the cerebral cortex: implications for learning, memory, and mental illness. Prog. Brain Res. 1994;102:227–243. doi: 10.1016/S0079-6123(08)60543-9. [DOI] [PubMed] [Google Scholar]
- Reynolds B. Do high rates of cigarette consumption increase delay discounting? A cross-sectional comparison of adolescent smokers and young-adult smokers and nonsmokers. Behav. Process. 2004;67(3):545–549. doi: 10.1016/j.beproc.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Rosenberg D.R., Lewis D.A. Postnatal maturation of the dopaminergic innervation of monkey prefrontal and motor cortices: a tyrosine hydroxylase immunohistochemical analysis. J. Comp. Neurol. 1995;358(3):383–400. doi: 10.1002/cne.903580306. [DOI] [PubMed] [Google Scholar]
- Rubinstein M.L., Luks T.L., Moscicki A.B., Dryden W., Rait M.A., Simpson G.V. Smoking-related cue-induced brain activation in adolescent light smokers. J. Adolesc. Health. 2011;48(1):7–12. doi: 10.1016/j.jadohealth.2010.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell M.A. The nicotine addiction trap: a 40-year sentence for four cigarettes. Br. J. Addict. 1990;85(2):293–300. doi: 10.1111/j.1360-0443.1990.tb03085.x. [DOI] [PubMed] [Google Scholar]
- Rycroft N., Rusted J.M., Hutton S.B. Acute effects of nicotine on visual search tasks in young adult smokers. Psychopharmacology (Berl) 2005;181(1):160–169. doi: 10.1007/s00213-005-2220-8. [DOI] [PubMed] [Google Scholar]
- Schwartz R.D., Kellar K.J. Nicotinic cholinergic receptor binding sites in the brain: regulation in vivo. Science. 1983;220(4593):214–216. doi: 10.1126/science.6828889. [DOI] [PubMed] [Google Scholar]
- Seamans J.K., Yang C.R. The principal features and mechanisms of dopamine modulation in the prefrontal cortex. Prog. Neurobiol. 2004;74(1):1–58. doi: 10.1016/j.pneurobio.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Shram M.J., Funk D., Li Z., Le A.D. Periadolescent and adult rats respond differently in tests measuring the rewarding and aversive effects of nicotine. Psychopharmacology (Berl) 2006;186(2):201–208. doi: 10.1007/s00213-006-0373-8. [DOI] [PubMed] [Google Scholar]
- Shram M.J., Funk D., Li Z., Le A.D. Nicotine self-administration, extinction responding and reinstatement in adolescent and adult male rats: evidence against a biological vulnerability to nicotine addiction during adolescence. Neuropsychopharmacology. 2008;33(4):739–748. doi: 10.1038/sj.npp.1301454. [DOI] [PubMed] [Google Scholar]
- Shram M.J., Li Z., Le A.D. Age differences in the spontaneous acquisition of nicotine self-administration in male Wistar and Long-Evans rats. Psychopharmacology (Berl) 2008;197(1):45–58. doi: 10.1007/s00213-007-1003-9. [DOI] [PubMed] [Google Scholar]
- Shram M.J., Siu E.C., Li Z., Tyndale R.F., Le A.D. Interactions between age and the aversive effects of nicotine withdrawal under mecamylamine-precipitated and spontaneous conditions in male Wistar rats. Psychopharmacology (Berl) 2008;198(2):181–190. doi: 10.1007/s00213-008-1115-x. [DOI] [PubMed] [Google Scholar]
- Skinner M.D., Aubin H.J., Berlin I. Impulsivity in smoking, nonsmoking, and ex-smoking alcoholics. Addict. Behav. 2004;29(5):973–978. doi: 10.1016/j.addbeh.2004.02.045. [DOI] [PubMed] [Google Scholar]
- Slawecki C.J., Gilder A., Roth J., Ehlers C.L. Increased anxiety-like behavior in adult rats exposed to nicotine as adolescents. Pharmacol. Biochem. Behav. 2003;75(2):355–361. doi: 10.1016/s0091-3057(03)00093-5. [DOI] [PubMed] [Google Scholar]
- Slawecki C.J., Thorsell A.K., El Khoury A., Mathe A.A., Ehlers C.L. Increased CRF-like and NPY-like immunoreactivity in adult rats exposed to nicotine during adolescence: relation to anxiety-like and depressive-like behavior. Neuropeptides. 2005;39(4):369–377. doi: 10.1016/j.npep.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Smith L.N., McDonald C.G., Bergstrom H.C., Brielmaier J.M., Eppolito A.K., Wheeler T.L. Long-term changes in fear conditioning and anxiety-like behavior following nicotine exposure in adult versus adolescent rats. Pharmacol. Biochem. Behav. 2006;85(1):91–97. doi: 10.1016/j.pbb.2006.07.014. [DOI] [PubMed] [Google Scholar]
- Smith T.A., House R.F., Jr., Croghan I.T., Gauvin T.R., Colligan R.C., Offord K.P. Nicotine patch therapy in adolescent smokers. Pediatrics. 1996;98(4 Pt 1):659–667. [PubMed] [Google Scholar]
- Snook L., Paulson L.A., Roy D., Phillips L., Beaulieu C. Diffusion tensor imaging of neurodevelopment in children and young adults. Neuroimage. 2005;26(4):1164–1173. doi: 10.1016/j.neuroimage.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Sowell E.R., Thompson P.M., Tessner K.D., Toga A.W. Mapping continued brain growth and gray matter density reduction in dorsal frontal cortex: inverse relationships during postadolescent brain maturation. J. Neurosci. 2001;21(22):8819–8829. doi: 10.1523/JNEUROSCI.21-22-08819.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear L.P. The adolescent brain and age-related behavioral manifestations. Neurosci. Biobehav. Rev. 2000;24(4):417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spinella M. Correlations between orbitofrontal dysfunction and tobacco smoking. Addict. Biol. 2002;7(4):381–384. doi: 10.1080/1355621021000005964. [DOI] [PubMed] [Google Scholar]
- Taioli E., Wynder E.L. Effect of the age at which smoking begins on frequency of smoking in adulthood. N. Engl. J. Med. 1991;325(13):968–969. doi: 10.1056/NEJM199109263251318. [DOI] [PubMed] [Google Scholar]
- Torres O.V., Tejeda H.A., Natividad L.A., O’Dell L.E. Enhanced vulnerability to the rewarding effects of nicotine during the adolescent period of development. Pharmacol. Biochem. Behav. 2008;90(4):658–663. doi: 10.1016/j.pbb.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trauth J.A., Seidler F.J., McCook E.C., Slotkin T.A. Adolescent nicotine exposure causes persistent upregulation of nicotinic cholinergic receptors in rat brain regions. Brain Res. 1999;851(1–2):9–19. doi: 10.1016/s0006-8993(99)01994-0. [DOI] [PubMed] [Google Scholar]
- Tseng K.Y., O’Donnell P. D2 dopamine receptors recruit a GABA component for their attenuation of excitatory synaptic transmission in the adult rat prefrontal cortex. Synapse. 2007;61(10):843–850. doi: 10.1002/syn.20432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumkosit P., Kuryatov A., Luo J., Lindstrom J. Beta3 subunits promote expression and nicotine-induced up-regulation of human nicotinic alpha6* nicotinic acetylcholine receptors expressed in transfected cell lines. Mol. Pharmacol. 2006;70(4):1358–1368. doi: 10.1124/mol.106.027326. [DOI] [PubMed] [Google Scholar]
- Van Andel I., Rambali B., Van Amsterdam J., Wolterink G., Van Aerts L.A., Vleeming W. RIVM; Bilthoven: 2003. Nicotine Addiction. [Google Scholar]
- Van Eden C.G., Lamme V.A., Uylings H.B. Heterotopic cortical afferents to the medial prefrontal cortex in the rat. A combined retrograde and anterograde tracer study. Eur. J. Neurosci. 1992;4(1):77–97. doi: 10.1111/j.1460-9568.1992.tb00111.x. [DOI] [PubMed] [Google Scholar]
- van Gaalen M.M., Brueggeman R.J., Bronius P.F., Schoffelmeer A.N., Vanderschuren L.J. Behavioral disinhibition requires dopamine receptor activation. Psychopharmacology (Berl) 2006;187(1):73–85. doi: 10.1007/s00213-006-0396-1. [DOI] [PubMed] [Google Scholar]
- Vastola B.J., Douglas L.A., Varlinskaya E.I., Spear L.P. Nicotine-induced conditioned place preference in adolescent and adult rats. Physiol. Behav. 2002;77(1):107–114. doi: 10.1016/s0031-9384(02)00818-1. [DOI] [PubMed] [Google Scholar]
- Vink J.M., Willemsen G., Boomsma D.I. The association of current smoking behavior with the smoking behavior of parents, siblings, friends and spouses. Addiction. 2003;98(7):923–931. doi: 10.1046/j.1360-0443.2003.00405.x. [DOI] [PubMed] [Google Scholar]
- Wang H.X., Gao W.J. Cell type-specific development of NMDA receptors in the interneurons of rat prefrontal cortex. Neuropsychopharmacology. 2009;34(8):2028–2040. doi: 10.1038/npp.2009.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO-Europe, 2004. In: Currie, C., Roberts, C., Morgan, A., Smith, R., Settertobulte, W., Samdal, O., et al. (Eds.), Health Policy for Children and Adolescents, No. 4. Copenhagen, Denmark.
- Xiao C., Nashmi R., McKinney S., Cai H., McIntosh J.M., Lester H.A. Chronic nicotine selectively enhances alpha4beta2* nicotinic acetylcholine receptors in the nigrostriatal dopamine pathway. J. Neurosci. 2009;29(40):12428–12439. doi: 10.1523/JNEUROSCI.2939-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakovlev P.I. The myelogenetic cycles of regional maturation of the brain. In: Minkowski A., editor. Regional Development of the Brain in Early Life. Blackwell; Oxford: 1967. pp. 3–70. [Google Scholar]
- Yates S.L., Bencherif M., Fluhler E.N., Lippiello P.M. Up-regulation of nicotinic acetylcholine receptors following chronic exposure of rats to mainstream cigarette smoke or alpha 4 beta 2 receptors to nicotine. Biochem. Pharmacol. 1995;50(12):2001–2008. doi: 10.1016/0006-2952(95)02100-0. [DOI] [PubMed] [Google Scholar]
- Yu W.F., Guan Z.Z., Nordberg A. Postnatal upregulation of alpha4 and alpha3 nicotinic receptor subunits in the brain of alpha7 nicotinic receptor-deficient mice. Neuroscience. 2007;146(4):1618–1628. doi: 10.1016/j.neuroscience.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Zakharova E., Wade D., Izenwasser S. Sensitivity to cocaine conditioned reward depends on sex and age. Pharmacol. Biochem. Behav. 2009;92(1):131–134. doi: 10.1016/j.pbb.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]