Research highlights
▸ An extensive review of near-infrared spectroscopy for novice and experienced users. ▸ A summary of a decade of experience from five developmental laboratories. ▸ Guidelines for experimental design, methodological practice and data analysis. ▸ Novel empirical data on key methodological issues.
Keywords: Near-infrared spectroscopy, Optical imaging, Newborns, Infants
Abstract
Near-infrared spectroscopy (NIRS) is a new and increasingly widespread brain imaging technique, particularly suitable for young infants. The laboratories of the McDonnell Consortium have contributed to the technological development and research applications of this technique for nearly a decade. The present paper provides a general introduction to the technique as well as a detailed report of the methodological innovations developed by the Consortium. The basic principles of NIRS and some of the existing developmental studies are reviewed. Issues concerning technological improvements, parameter optimization, possible experimental designs and data analysis techniques are discussed and illustrated by novel empirical data.
1. Introduction
One of the greatest advances in the recent history of cognitive neuroscience has been the refinement and increasingly more versatile application of brain imaging techniques. Different methods measuring the electrophysiological (e.g. electroencephalography (EEG), magnetic encephalography (MEG)) or metabolic (e.g. magnetic resonance imaging (MRI), positron emission tomography (PET)) aspects of neural activity have been used with a wide range of healthy and clinical populations to explore brain organization and function non-invasively in behaving participants.
As a result of this progress, in the last 10–15 years it has become possible to use brain imaging techniques with developmental populations. However, not all imaging tools are equally well suited for infants and children for a number of different reasons, including safety concerns, the necessity to stay motionless for extended time periods, and for infants in particular, the need to initiate a motor response and understand verbal instructions. The present paper describes a brain imaging technique known as near-infrared spectroscopy (NIRS) or optical topography (OT), which has proven particularly useful in studying the brain mechanisms of the youngest developmental populations from birth to the toddler years. We provide a general introduction to the NIRS technique as well as a synopsis of nearly a decade of research and technological development conducted in five developmental laboratories supported by the J.S. McDonnell Foundation.1
Why is NIRS of particular interest for the cognitive developmental neuroscientist or psychologist? Beyond its practical advantages like low operating costs, ease of application, or tolerance of movement, NIRS has proven its usefulness through the already significant empirical contributions it has made to our understanding of cognitive and neural development from birth through infancy and early childhood.
NIRS has allowed us to clarify the origins of the left lateralization of language processing in the brain, revealing lateralization to the native language at birth (Peña et al., 2003, Gervain et al., 2008) and a readiness of the left hemisphere to preferentially process stimuli in the 25–160 ms range, corresponding roughly to the length of a syllable (Telkemeyer et al., 2009). At the same time, NIRS has also shown that this initial lateralization to speech notwithstanding, the processing of some language-specific cues and patterns take several months to lateralize (Minagawa-Kawai et al., 2007, Sato et al., 2009), uncovering the neural basis of the process of perceptual attunement to the native language. In the domain of social cognition, the development of face perception has been documented in great detail and its neural correlates have been identified. At 4 months, infants already respond differently to faces compared to visual noise stimuli (Csibra et al., 2004, Blasi et al., 2007). Between 5 and 8 months, they start to show increased activation to upright vs. inverted faces in the right temporal areas (Otsuka et al., 2007), and at 8 months they start recognizing the same faces seen from different angles (Nakato et al., 2009). In parallel with this development, starting at around 4 months, preferential responses to dynamic social cues such as eye gaze, eye, and mouth movement, has also been found in the bilateral temporal and inferior frontal regions of the brain (Lloyd-Fox et al., 2009, Grossmann et al., 2008). NIRS also holds promise as a measure of multisensory processing. Several studies have successfully documented the combined as well as the modality-specific effects of joint auditory and visual stimulation in infants (Bortfeld et al., 2007, Bortfeld et al., 2009, Taga and Asakawa, 2007, Shukla et al., 2009). NIRS is also sensitive to pathological brain activity in infants (Chen et al., 2002). Since perinatal complications often affect the blood flow and oxygenation of the brain, exploring brain function in premature newborns and in infants with hypoxia will greatly advance our understanding of how these early traumas impact cognitive development.
Given this brief background, we first describe the basic principles and different technological implementations of near-infrared optical imaging (Section 1). We then provide a brief comparison with other brain imaging modalities, as well as an overview of the NIRS literature, focusing mostly on developmental work (Section 2). Next, technical issues relating to the practical application of NIRS will be discussed and some of the main points will be illustrated using data collected in our laboratories (Section 3). This will be followed by a discussion of possible experimental designs that can be used for NIRS studies (Section 4), the basic principles and most important algorithms for analyzing NIRS data (Section 5), and empirical data from other imaging and behavioral techniques that validate and complement NIRS (Section 6). Finally, we conclude by outlining some of the remaining challenges and possible directions for future research using this promising technique (Section 7).
1.1. Optical imaging: a general introduction
Optical changes accompanying physiological states and functions in the body are well known: increased blood circulation during exercise renders the cheeks rosy, and decreased circulation from low temperatures can turn the fingernails bluish. Neural activity is similarly accompanied by changes in blood oxygenation, which can be detected by near-infrared light. Optical imaging is thus an indirect measure of neural activity, like fMRI, but unlike more direct electrophysiological measures such as EEG and MEG. In addition to the optical imaging technique that detects the relatively slow changes in oxygenated (oxyHb) and deoxygenated (deoxyHb) hemoglobin concentrations related to neural activity, another optical imaging technique measures the transient optical properties of the neurons themselves as they discharge (see Section 1.1.4).
The physical and physiological principles underlying optical imaging have been introduced and discussed in great detail in different studies (Aslin and Mehler, 2005, Chance et al., 1992, Ferrari et al., 2004, Hiraoka et al., 1993, Jobsis, 1977, Meek, 2002, Minagawa-Kawai et al., 2008, Okada and Delpy, 2003a, Okada and Delpy, 2003b, Villringer and Chance, 1997). Below, we provide a brief overview of the basic principles behind the most commonly used techniques.
1.1.1. Continuous light optical imaging
The most common technique in developmental research uses continuous wave (CW), monochromatic near-infrared light to monitor in vivo the changes in the concentration of certain chromophores such as oxyHb, deoxyHb or cytochrome c oxidase in biological tissue, related to neural activity.
When monochromatic light travels through a medium, some of it is absorbed in the medium, some of it is scattered and some of it is transmitted, i.e. continues its trajectory unaffected by the medium. Exactly what proportions of the light are absorbed, scattered and transmitted depend on the properties of the medium (e.g. absorption coefficient, concentration etc.) and the light (wavelength etc.). In ideal cases, the scatter is negligible, so most of the light is absorbed or transmitted. This situation can be described by the Beer–Lambert law:
| (1) |
where A is the absorbance, I is the intensity of the transmitted light, i.e. the light after the medium, I0 is the intensity of the incident light, i.e. the light before the medium, c is the concentration or density of the medium, ɛλ is the molar extinction coefficient characteristic of the medium for a light of wavelength λ, and l is the distance that the light travels in the medium, which, in this ideal case, is equal to the length of the medium in the relevant dimension.
From this equation, the concentration of the medium can be obtained by measuring the intensity of the light that leaves the medium (assuming that the original light intensity, the length of the medium and the molar extinction coefficient are known).
Since the concentrations of oxyHb and deoxyHb in brain tissue are indicators of neural activity, we can use the above relationship to calculate these concentrations by shining light of an appropriate wavelength on the head and measuring the intensity of the exiting light. However, biological tissues like the skin, the skull and the brain are highly scattering media, so the above equation needs to be modified to take into account the scatter and the fact that light does not travel through these media in a straight line. The modified Beer–Lambert law thus states the following:
| (2) |
where DPF is the differential pathlength factor that accounts for the non-linear trajectory of light in biological media and G is the scatter. Using CW techniques, these two factors cannot be measured directly (although satisfactory estimates of the DPF exist). As a consequence, absolute values for the concentrations of oxyHb and deoxyHb cannot be obtained. However, the scatter is constant and can thus be eliminated when changes in the concentration of oxyHb and deoxyHb are calculated:
| (3) |
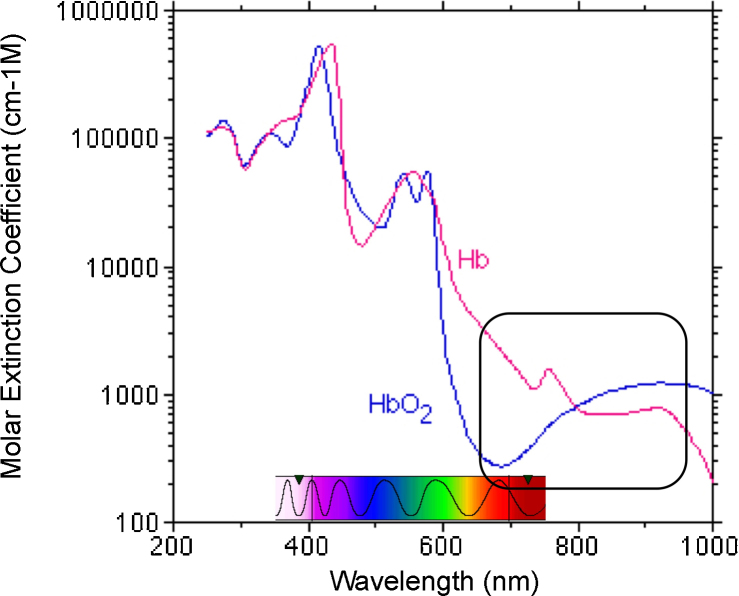
If two wavelengths are used, yielding two equations, then the relative concentrations of oxyHb and deoxyHb (Δcoxy and Δcdeoxy, respectively) can be calculated from the change in absorbance (ΔA). The molar extinction coefficients of oxyHb and deoxyHb are shown in Fig. 1.
Fig. 1.
The molar extinction coefficients of oxyHb and deoxyHb for different wavelength. The spectrum of visible light is overlaid on the x-axis. The black square indicates the region of the spectrum that is relevant for NIRS with the isosbestic point of oxyHb and deoxyHb.
The red and near-infrared range of the spectrum, encircled in Fig. 1, contains the most adequate wavelengths for the optical imaging of biological media, because intervening tissues (e.g. skin, bone) are transparent to light at these wavelengths. This can easily be observed when light shines through one's fingertips or earlobes: the tissue becomes transparent, with an orange glow, and the larger blood vessels are clearly visible. At lower wavelengths, hemoglobin, and at higher wavelengths, water, absorb too much light for tissues to be transparent. However, finding the two wavelengths that provide the strongest signal is challenging even within the near-infrared (NIR) range. We discuss some related technical issues and the search for optimal wavelengths conducted at various McDonnell labs in Section 3.
Another important factor in the equation for relative change (3) is the distance that the light travels in the medium. As mentioned before, the CW technique cannot directly measure the DPF, although estimates exist for the newborn and infant brain (Duncan et al., 1996, Wyatt et al., 1990). In addition, the distance that the light travels in the brain depends on l, which, in the case of NIRS applied to the human brain, corresponds to the separation between the light source and the light detector locations on the surface of the scalp. Before the 1990s, NIRS systems usually applied one source and one detector, or optode, pair constituting one measurement channel. Since then, multichannel systems have appeared using several sources and detectors, which are positioned at equal distances in some systems and at different distances in others.
The availability of multiple channels has given rise to two distinct types of techniques: optical topography and optical tomography. The former provides a two-dimensional sampling from the surface of the cortex, whereas the latter allows a three-dimensional reconstruction of the hemodynamic signals from the brain.
In topography systems, the coupled sources and detectors forming channels are located at a distance of a few centimeters from one another and the light travels through a banana-shaped trajectory from the source to the detector (Fig. 2), penetrating the surface of the cortex. The source–detector separation is an important parameter of NIRS systems, as it determines the depth of penetration, as well as the spatial resolution. Larger separations sample from areas deeper in the cortex (the arc of the banana-shape samples from is larger), providing more information about neural activity, but at the same time, they decrease the spatial resolution of the measurements.2 In newborns, whose surface tissues are thin, NIR light typically penetrates about 30 mm deep into the head (measured from the scalp surface), thus 10–15 mm into the cortex at a source–detector separation of 3 cm, whereas in adults, the penetration into the cortex is only about 3–5 mm deep with the same separation (see our Consortium's extensive empirical research on the optimal source–detector separation in Section 3).
Fig. 2.
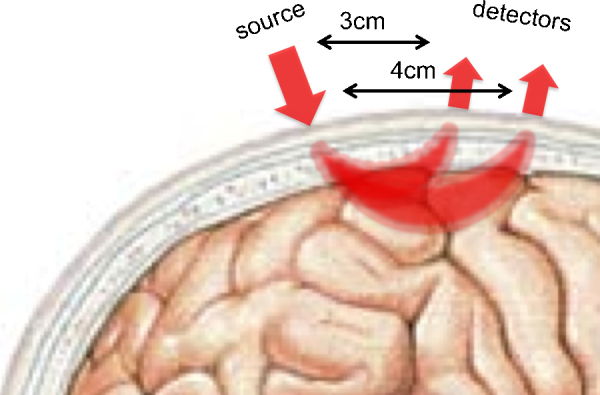
The trajectory of NIR light in the human brain at different source–detector separations in optical topography systems.
In tomography systems, a dense array of sources and detectors is placed around the whole head in order to generate a cross-sectional, 3D reconstruction of the brain using complex mathematical algorithms. This technique is mostly used in clinical applications with newborns (Hebden et al., 2002, Hebden et al., 2004), as head size and tissue thickness attenuate light too much in children and adults. Other practical issues that prevent the routine use of optical tomography in empirical research include long data acquisition and image reconstruction times, high cost and low spatial resolution.
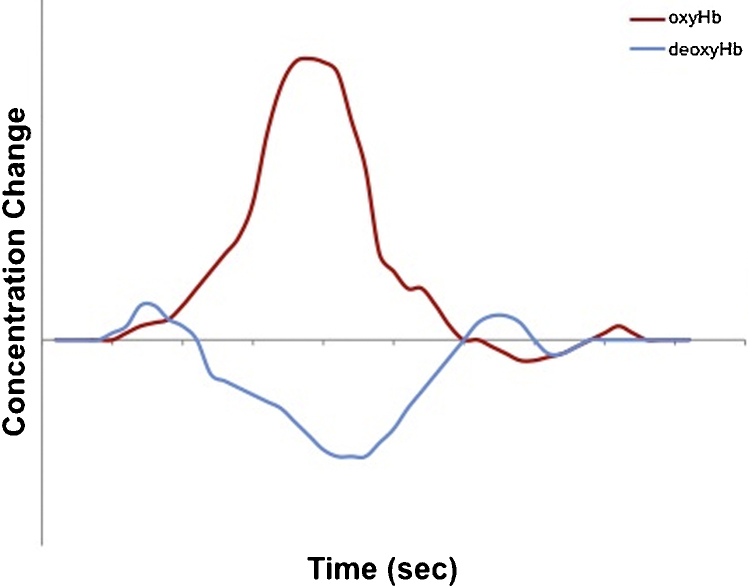
CW systems use changes in oxyHb and deoxyHb concentrations as an indirect measure of brain activity. This is possible because there is a relationship between focal brain activity and regional cerebral blood volume and flow. This neurovascular coupling means that increased activity, requiring additional metabolic supplies, e.g. oxygen and glucose, is accompanied by local vasodilation, increased blood flow and oxygenation. Crucially, the increase in blood flow and oxygen exceeds the demands, resulting in a local and transient excess of oxygen. This metabolic response, known as the hemodynamic response function (HRF), follows local neural activity by several seconds and the exact physiological mechanisms relating the HRF to neural firing are not fully understood (Logothetis et al., 2001). While the shape of the HRF for adults is well described (Boden et al., 2007, Fox and Raichle, 1986, Obrig et al., 2000, Roy and Sherrington, 1890; Fig. 3), more research is required to characterize it in developmental populations. Existing results suggest that the infant HRF might be delayed, slower to peak and/or slower to return to baseline in infants than in adults (for a summary, see Meek, 2002).
Fig. 3.
A typical hemodynamic response function (HRF) in adults. Stimulation is delivered at time zero. The response often starts with a small increase in deoxyHb, followed by an increase in oxyHb and a decrease in deoxyHb concentrations (measured here in arbitrary units). The signals peak several seconds after stimulus onset and then slowly return to baseline, with possible under- and overshoots.
Just as with fMRI, the low temporal frequency of the HRF needs to be taken into account when designing NIRS experiments. Some mathematical algorithms to analyze faster, event-related NIRS signals have recently been proposed (Plichta et al., 2006, Plichta et al., 2007, Schroeter et al., 2004), mostly on the basis of analysis methods used in fMRI, but longer stimulation periods and block designs are more typically employed in the existing developmental literature. We will discuss experimental design considerations in Section 3.
Measuring relative oxyHb and deoxyHb concentrations is usually sufficient for research-related applications of NIRS. However, in some cases, for instance in clinical practice, it might be relevant to obtain absolute concentration values. The CW technique, as we have seen, cannot provide this, but several methods have been developed to estimate the path length and/or the scatter, allowing absolute concentrations to be calculated from the modified Beer–Lambert law (2). We now briefly summarize two of these NIRS methods: the intensity-modulated or frequency-resolved technique and the time-of-flight or time-resolved technique.
1.1.2. Frequency-resolved optical imaging
In frequency-resolved NIRS, the intensity of the light is modulated at a certain frequency. While travelling through tissues, the intensity is attenuated and the phase of the modulation is shifted due to scattering. By measuring the attenuation and the phase-shift, the pathlength and the scatter can be determined, allowing the calculation of absolute oxyHb and deoxyHb concentration values. This technique was first introduced and experimentally tested by Chance et al. (1990) and now there are commercially available systems based on this approach (for an overview of commercially available systems, see Lloyd-Fox et al., 2010, Wolf et al., 2007). However, they typically have lower sampling rates and higher costs than the CW systems, so they are less commonly used in developmental research.
1.1.3. Time-resolved optical imaging
Another approach for determining the pathlength of light in biological tissue is to use single light pulses instead of continuous light. When a very short pulse of light is sent through tissues, the time distribution of individual photons that leave the head can be registered by a detector or photon-camera. The average time-of-flight of the photons is then multiplied by the speed of light to determine the mean pathlength. This technique was proposed by Delpy et al. (1988) and several systems have been developed since then (Lloyd-Fox et al., 2010, Torricelli et al., 2008, Wolf et al., 2007). The slow acquisition time makes this technique less well suited for developmental populations than CW systems.
1.1.4. Fast optical imaging
Unlike the previous three techniques, which measure the metabolic correlates of brain activity, fast optical imaging (also known as event-related optical signal, EROS) detects the changes in the optical properties of neurons and surrounding tissue during firing (Gratton et al., 1995, Gratton and Fabiani, 1998, Gratton and Fabiani, 2003). As this measure is a correlate of the electrophysiological activity of neurons and not of metabolism, it is a much faster signal, with a latency in the millisecond range, comparable to that of electrophysiological techniques. This fast signal and the slow, metabolic response can be detected using the same equipment, which could provide an ideal method to characterize neural activity with high temporal and spatial resolution at the same time. However, the reliability and strength of this fast optical signal as measured non-invasively in behaving adult subjects may be limited, at least with the currently available technologies (Steinbrink et al., 2005). Nevertheless, EROS has been validated against fMRI and ERP in adults (Tse et al., 2007). No systematic methodological study has yet evaluated this technique with developmental populations.
1.1.5. The NIRS systems used by the laboratories of the McDonnell Consortium
The laboratories of the McDonnell Consortium, with the exception of the Birkbeck-UCL lab (see below), use the Hitachi ETG-4000 system. This CW machine uses light at 690 nm and 830 nm wavelengths and samples each channel at 10 Hz. It has 20 frequency-modulated light sources3 and 16 detectors, which can be configured to form up to 52 channels, although studies with the youngest age groups, e.g. newborns, often use fewer channels (typically 24). The optical fibers are arranged into probes or caps with different channel configurations, typically using 3 cm source–detector separations. Probes with different shapes and spatial configurations exist for different age ranges. Adults and older infants are tested using square-shaped probe holder matrices (3 × 3 arrays) or cap-shaped probe sets covering the whole head, whereas chevron- or square-shaped silicone pads are used with newborns and younger infants (Gervain et al., 2008, Peña et al., 2003). Some of the technical issues concerning probes as well as the most recent probe development projects will be discussed in Section 3.
Unlike the other four labs of the McDonnell Consortium, the Birkbeck-UCL laboratory uses an in-house system developed at UCL called NTS2 (Everdell et al., 2005). This CW optical topography system uses multiple light sources and detectors, up to a maximum of 32 laser diode sources (16 at 770 nm and 16 at 850 nm; for a discussion of the different wavelength pairs used by the two systems, see Section 3.2) and 16 avalanche photodiode detectors. The multiple source signals, which are each modulated at a different frequency in the range 2–4 kHz, are decoded using a Fourier transform, with a data acquisition rate of approximately 10 Hz. The source diodes, along with the detectors, are coupled to the array with optical fibers and configured to form up to 45 channels. Each detector records the amount of light coming from a subset of neighboring sources, which can be arranged into different configurations according to the research question and age of participants. The configurations can be changed to measure whichever source–detector separation is required, which for infants ranges from 2 cm to 4.5 cm. The probes typically house multiple source–detector separations so that the hemodynamic response from the brain can be investigated at several depths simultaneously. A range of headgear has been developed to hold these probes, which are used with infants at different ages (Blasi et al., 2007, Lloyd-Fox et al., 2009).
Using two different systems within the Consortium allowed us to explore different options for optimizing the NIRS technique. Necessarily, the choices made were often specific to each system. However, and more importantly, the optimization process very often resulted in converging solutions across machines and setups at Birkbeck-UCL and the other four laboratories, strengthening our methodological choices and theoretical conclusions. In our review, we thus report results from both systems, highlighting differences and similarities, to allow for a more robust comparison.
2. Optical imaging in developmental populations
NIRS is ideally suited to perform brain imaging in developmental populations as it presents several advantages over other methods. We first discuss these advantages and then go on to review the developmental NIRS literature to illustrate the kinds of theoretical and methodological questions that can be addressed in infants using NIRS.
2.1. The advantages and disadvantages of NIRS over other techniques
When the hemodynamic response of the brain is measured non-invasively through the head, light has to traverse several layers of biological tissue (skin, skull, cerebrospinal fluid etc.) before reaching the cortex. Therefore, tissue thickness is an important parameter in determining the depth of penetration into the cortex, the brain areas that can be reached, and the magnitude of the obtained signal. Newborns and young infants have significantly thinner skin and skull than adults, resulting in a threefold increase in penetration from 3–5 mm to 10–15 mm into the cortex. (This results from a light penetration of about 25–30 mm into the head, measured from the surface of the scalp in both populations.) Young infants also have less hair than adults, which reduces the noise and the artifacts in the signal by allowing better contact between the head and the optodes and by reducing light scatter.
When compared to other techniques, NIRS has several clear advantages for use with infants. Unlike the magnetic gradients used in MRI, NIRS is completely silent, providing a non-intrusive environment and allowing for an easy presentation of auditory stimuli. No strong magnetic field or radio frequency (RF) pulses are involved, alleviating safety concerns. In terms of the obtained signal, NIRS has the advantage of measuring both oxyHb and deoxyHb changes, providing a physiologically plausible measure of blood flow and volume, while the BOLD response in fMRI is related to deoxyHb only, thereby creating potential confounds with blood flow. The costs of NIRS are also considerably lower than those of MRI.
As compared to EEG, NIRS provides better spatial localization, as most topographic implementations of NIRS are not subject to the inverse problem associated with source localization of electrical potentials from the scalp. NIRS is also less sensitive to motion artifacts than both MRI and EEG, requiring less rigid stabilization of the head and body. While the time resolution of NIRS is lower than that of EEG, the sampling rate of most CW machines is around 10 Hz compared to 0.5 Hz in fMRI, allowing for an improvement in temporal resolution, once mathematical algorithms are in place to adequately analyze event-related data and the infant HRF. PET, SPECT and other nuclear imaging techniques are rarely used with infants for research purposes due to safety issues. NIRS, by contrast, does not require a tracer or carrier substance to be injected into the blood stream.
The key limitation of NIRS is that it only probes the surface layers of the cortex. Consequently, brain structures that lie deeper in the cortex or below it are not visible by CW topography NIRS techniques. In addition, the temporal resolution of NIRS is lower than that of EEG, rendering the detection of fast responses to single events more challenging (see the relevant discussion about experimental designs in Section 4). Similarly, the spatial resolution of NIRS is inferior to that of MRI.
2.2. A brief overview of developmental NIRS research
Given the abovementioned advantages, it is not surprising that the last decade has witnessed a considerable increase in the use of NIRS with infants since the first published studies (Meek et al., 1998). We now briefly summarize some of the existing developmental research.
2.2.1. Broad localization of perceptual abilities
The first studies used NIRS as a new brain imaging method for developmental populations to establish broad spatial localizations for simple perceptual stimulation. In one of the earliest studies with newborns, Sakatani et al. (1999) registered increased activation in the bilateral frontal lobes while participants were listening to music. This response manifested itself in increased oxyHb and totalHb concentrations in almost all participants, but, interestingly, deoxyHb concentrations increased in two-thirds of the infants and decreased in one-third. This paper constitutes one of the first reports on the variability of the NIRS signal in young infants, an issue we will take up in Section 3.
NIRS has also proven useful to register orbitofrontal brain activation in newborns during olfactory stimulation (Bartocci et al., 2000). Newborns between 6 h and 8 days old showed increased response to a vanilla scent as well as to the odor of their mother's colostrum as compared to water. The intensity of the response to colostrum attenuated with postnatal age, demonstrating the sensitivity of NIRS to detect a graded response.
Probing visual perception, Kusaka et al. (2004) tested infants’ response to photostimulation during sleep in the visual cortex, and obtained an ‘inverted’ response, i.e. a decrease in oxyHb and totalHb concentrations and an increase in deoxyHb concentrations. The authors attributed the inversion to maturational factors, e.g. the immaturity of the retina in young infants. Sleep might be another possible explanation.
Looking at awake visual perception, Taga et al. (2003) found an increased response in the occipital areas of 2–4-month old infants while the infants were watching checkerboard pattern reversals.
When measuring infants’ responses to multiple sensory stimulation, Bortfeld et al. (2007) and subsequently Taga and Asakawa (2007) found that NIRS is sensitive enough to detect brain responses specific to auditory stimulation while a simultaneous visual stimulus is also present, suggesting that NIRS has sufficient selectivity to localize neural activity triggered by different perceptual modalities. Similar results were later obtained by Bortfeld et al. (2009), who showed that the response to speech, even when presented together with visual stimulation, was left lateralized in 6–9-month old infants.
2.2.2. Early lateralization and functional specialization for language
The origin and developmental trajectory of the left lateralization of language, found in most right-handed adults (Kimura, 1967), is another functional-localizational question that has been addressed using NIRS. Peña et al. (2003) compared newborns’ responses to three blocks of stimuli: (i) normal, forward-going, infant-directed speech in the native language, (ii) the same stimuli played backwards and (iii) silence. Newborns showed increased activation in the left temporal area in response to forward speech, but not to backward speech or silence. Similar results were obtained in an fMRI study with 3-month old infants, using the same design (Dehaene-Lambertz et al., 2002). This suggests that the native language is left lateralized very early on, at least when it is presented in its full acoustic complexity.
Individual acoustic/phonological properties, language-specific contrasts and other single features, however, might take longer to lateralize, mirroring the behavioral transition in language perception during the first year of life from general, broad-based abilities towards enhanced sensitivity to the specifics of the native language (perceptual attunement). A recent study by Minagawa-Kawai et al. (2007) illustrates this attunement using the short–long vowel contrast in Japanese. Infants show a U-shaped discrimination curve, distinguishing the two categories at around 6–7 months and after 13–14 months, but not at around 10–11 months. That is, an increased totalHb activation was obtained in the young and the old ages, but not in the middle age group, to blocks containing stimuli whose difference in duration crossed the category boundary as compared to stimulation blocks containing within-category durational differences. Importantly, left lateralization in response to these stimuli, which is the typical adult pattern (Minagawa-Kawai et al., 2002), appears only at around 13–14 months and approximates the exact adult spatial distribution after 25–28 months. These results suggest that left lateralization for the short–long vowel contrast emerges as the perception of the contrast is fine-tuned, shifting from a simple acoustic difference to a language-relevant phonological property.
Another example of lateralization comes from work by Sato et al. (2009), who investigated the development of the behavioral and neural response to pitch accent patterns that are used by speakers of Japanese and their pure tone equivalents that served as a non-linguistic control. Behaviorally, both 4- and 10-month old infants could discriminate the different pitch accent patterns (High–Low vs. Low–High). However, at 4 months, infants did not show different NIRS responses to real Japanese words and their pure tone equivalents, suggesting that processing is acoustic/non-linguistic at this age. At 10 months, by contrast, infants showed greater activation to a change in word pitch accent patterns than to a change in the corresponding pure tone patterns, and this differential response was localized in the left hemisphere, indicating that processing is linguistic in nature.
A similar trend was found for the perception of prosody (the melodic property of speech that includes variations in pitch and duration). Results with newborns (Saito et al., 2007a) indicate that they show increased frontal response to infant-directed speech as compared to adult-directed speech (both were produced by each infant's mother), allowing infants to tune into speech addressed to them early on. A key difference between the two speech registers is the highly variable, exaggerated prosody in infant-directed speech, which might explain the more prominent activation found for this stimulus. Indeed, the same authors (Saito et al., 2007b) showed that newborns can discriminate between normally intonated prosody and monotone speech with flat prosody, as they show increased activation in the bilateral frontal areas to the former, but not to the latter. While the frontal areas do not show lateralization at this early stage, Homae et al., 2006, Homae et al., 2007 found that at 3 months, infants show greater activation in the right temporoparietal areas to normally intonated speech as compared to speech with flattened prosody. At 10 months, infants showed a more complex pattern of differential activation, involving the right temporal and temporoparietal regions, as well as the bilateral prefrontal regions. Interestingly, these regions exhibited greater activation to flattened than to normal speech. The authors took the results to indicate that prosody is initially processed acoustically, hence the increased activation to the more informative normal prosody at 3 months. Over development, prosodic processing becomes linguistic in nature and needs to be integrated with other aspects of speech processing. Since flat prosody is atypical in natural language, attempts to integrate it into the linguistic system are effortful, giving rise to increased activation in different language-related areas at 10 months. Wartenburger et al. (2007) further elucidated the development of prosodic processing and provided converging evidence for a hemispheric specialization to different aspects of speech processing. Testing 4-year old children, they found that when prosody is presented devoid of linguistic content, triggering non-linguistic processing, the right frontotemporal areas are involved, whereas presenting the same prosody together with the corresponding linguistic content engages the left hemisphere. Interestingly, the authors also recorded EEG together with the NIRS data, although they did not report the results of the former measure. For co-recording, they used an Omniat ISS NIRS system, the optical fibers of which were embedded in a commercially available EasyCap EEG cap. More recently, the same group of authors used this headgear to co-register EEGs and NIRS in newborn infants (Telkemeyer et al., 2009), and obtained converging EEG and NIRS results indicating that the left lateralization of speech perception might originate from the preferential processing of auditory stimuli modulated within the 25–160 ms range (i.e. typical phoneme and syllable durations) by the left hemisphere.
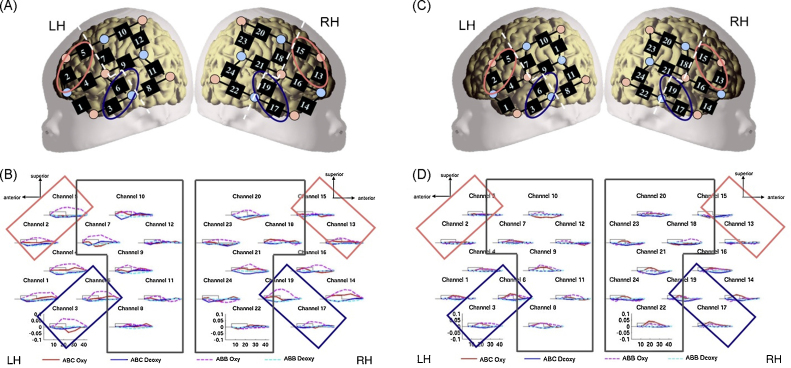
Investigating when the ability to process the structural properties of language begin, Gervain et al. (2008) found increased neural activity in the bilateral temporal and left frontal areas of newborns (Fig. 4B) when they listened to speech that followed a structural rule (ABB: e.g. “mubaba”) as compared to unstructured speech (ABC: “mubage”). The activation was more pronounced in the left than in the right temporal area, reproducing previous lateralization results. In addition, the results suggest that newborns are already capable of extracting simple regularities from speech, based on immediate repetitions. The same differential response was not found when non-adjacent repetitions (ABA) were compared to the random ABC sequences. These results have been expanded by Gervain et al. (in preparation), who investigated the perception of sequence-initial vs. sequence-final immediate repetitions (AAB vs. ABB) and found that they were discriminated by the newborn brain.
Fig. 4.
(A) Probe placement and regions of interest from Gervain et al. (2008). (B) The results of Experiment 1 from Gervain et al. (2008). (C) Probe placement and regions of interest from the follow-up experiment using 2.0 cm optode separations. (D) The results of the follow-up experiment.
2.2.3. Cognitive development
Neural signatures for non-linguistic cognitive functions have also been explored using NIRS. Two main directions have been explored: how infants represent the physical world and how they process the social world.
One of the first studies (Baird et al., 2002) investigated the neural correlates of object permanence in a longitudinal study with 5–12-month old infants. Infants were tested behaviorally for the presence of object permanence while neural activity in their frontal cortex was recorded using NIRS. NIRS data from the session where the infant first showed object permanence behaviorally was compared to NIRS data from the session preceding this developmental onset. Increased activation was observed in the object permanence sessions as compared to the pre-permanence ones. Object processing was also assessed by NIRS (Wilcox et al., 2005, Wilcox et al., 2008, Wilcox et al., 2009). Infants were presented with physically possible and impossible events involving object movement and identity. In Wilcox et al. (2005), infants showed significantly greater activation in two brain areas known to be involved in object processing, the primary visual and inferior temporal areas, in response to the possible event as compared to a zero baseline. The possible event involved two different objects emerging from either side of a wide occluder, whereas the impossible event involved the same two objects emerging from behind an occluder that was too narrow to hide both objects. In Wilcox et al., 2008, Wilcox et al., 2009, a similar paradigm was used, but now the featural differences between the objects were systematically manipulated. The objects either differed in multiple features, or only shape or color, or they were identical. In the occipital areas, all four conditions gave rise to similar activations, while in the inferior temporal areas, only the multi-featural and shape change conditions evoked increased responses.
In the social domain, one of the first steps was to explore the neural correlates of face perception in infants. In an initial study, Csibra et al. (2004) found a difference between neural activation in the occipital cortex in response to faces and visual noise stimuli with adults and 4-month old infants. Interestingly, though, while adults showed an increase in oxyHb concentration and a concomitant decrease in deoxyHb concentration when watching the face stimuli as compared to the visual noise, infants exhibited a decrease in oxyHb concentration for the faces and an increase for the visual noise stimuli. The authors provided two possible explanations for this difference. First, skull locations where data were obtained from might have been slightly different in the two populations due to possible anatomical changes in development (see Section 3.6 for a discussion). Second, similar inverted responses to face stimuli have also been observed in fMRI studies with infants, suggesting that this pattern of response might be related to the development of face perception and/or the occipital cortex. A second study (Blasi et al., 2007), which repeated this paradigm, but used a multiple channel array (rather than a dual channel array), allowed a wider cortical region to be investigated. This study replicated the results of the adult study, finding significant increases in oxyHb concentration to the face stimuli and not to visual noise, and did not find significant decreases. Therefore, perhaps the single recording channel used in the previous study may have not been positioned over the optimal cortical region for face processing, an issue which reinforces the importance of multiple channel arrays. A later study by Otsuka et al. (2007) found a brain signature for the face inversion effect, i.e. the fact that while upright faces are special, preferred stimuli, processed by a dedicated brain area from a very young age, inverted faces are not privileged. The authors compared activation in the left and right temporal areas while 5–8-month old infants were watching upright faces, inverted faces or objects. They found increased activation in the right temporal areas in response to upright, but not to inverted faces. The same group of authors (Nakato et al., 2009) also explored the neural mechanisms underlying the development of view-invariance in face perception. They found that 5-month olds showed increased activation in the previously identified right temporal areas for front views of faces only, not for profile views, but by 8 months, increased activation emerges for the latter type of stimuli as well, indicating the emergence of view-invariance between these two ages.
In another line of research within the social domain, Shimada and Hiraki (2006) used NIRS to show that 6–7-month old infants respond differently to live and televised action. The authors found significantly larger activation in infants’ motor areas when the infants were observing a real person manipulating an object as compared to the same object moving freely. They interpreted these results as suggestive of a mechanism similar to the mirror neuron system that has been observed in monkey cortex. This difference was not observed for televised actions.
Recently, another component of social perception, namely the perception of dynamic visual social cues, has been investigated using NIRS. Lloyd-Fox et al. (2009) found that 5-month olds showed increased cortical activation in response to dynamic social stimuli (such as ‘Peek-a-boo’, eye and mouth movements) in the bilateral superior temporal and inferior frontal regions of the cortex. Further this response was not found to dynamic non-social stimuli (such as mechanical toys, cogs and pistons), suggesting that the response was to the social component and not simply the dynamic nature of these cues. Further, a study investigating eye gaze perception with an accompanying smile cue in 4-month olds found larger activation in infants’ prefrontal cortex and right superior temporal region in response to a gaze shift toward (but not away from) the infant (Grossmann et al., 2008).
2.2.4. Clinical applications
NIRS is also commonly used in clinical pediatric practice, providing a useful tool for the measurement of cerebral oxygenation and the functional assessment of babies in the perinatal period. An early example of such work comes from Chen et al.'s (2002) study, who compared healthy newborns’ brain response to auditory stimulation with that of newborns suffering from hypoxic–ischemic encephalopathy (HIE). Normal newborns showed increased oxyHb and totalHb concentrations in the frontal areas during stimulation, whereas about two-thirds of the newborns with HIE exhibited a decrease in oxyHb and totalHb concentrations, the extent of which negatively correlated with the severity of HIE. These results indicate that regional cerebral blood flow is decreased in newborns with HIE during functional activation.
2.2.5. Other general reviews
The interested reader may wish to consult other reviews describing the general principles and the use of NIRS with developmental populations. Greisen (2006) and Meek (2002) provide a good introduction to the clinical use of optical imaging, e.g. in premature newborns or for assessing perinatal brain trauma etc. Aslin and Mehler (2005) focus on some of the methodological aspects of NIRS, comparing it to other techniques. Minagawa-Kawai et al. (2008) provide an overview of the developmental literature with a particular emphasis on language and speech perception studies. Lloyd-Fox et al. (2010) give a detailed comparison of existing developmental NIRS studies, reporting several methodological aspects of each study, such as number of infants tested, attrition rate, general procedure, NIRS system, statistical analysis used etc. They also review the technological and methodological advances that have been made in study design, optical probe development, and interpretation and analyses of the hemodynamic response in NIRS work with infants. Wolf et al. (2007) provides a systematic comparison of commercially available NIRS systems.
3. Optimizing the technical parameters of NIRS measurements
As the review of the developmental literature suggests, NIRS is a valuable tool for the investigation of cognitive functions and brain organization in infants. However, some methodological and technical challenges remain. In this section, we review the most important technical issues that affect the quality of the NIRS signal. Our labs have explored different NIRS setups and parameters in order to optimize the sensitivity and robustness of this technique for use with infants.
3.1. Source–detector separation
The distance between emitters (sources) and detectors has a considerable impact on the depth of penetration into the cortex. A detector further away from the source gathers light that has traveled a greater (vertical) distance in the cortex, thus it samples from deeper cortical areas. Deeper penetration means that the neural response contributes more to the signal (as compared to blood flow changes in the skin etc.) and more of the structures that lie deeper under the surface of the cortex can be explored. Source–detector separations large enough to ensure sufficient penetration are thus necessary. However, increasing the separation decreases the spatial resolution of the measurement, allowing fewer channels to be placed on the head, and decreases the signal-to-noise ratio, which could mask the effect of the experimental stimulus. Further, it must be noted that the optimal separation may vary depending on the intensity of the source lights (which must fall within safety guidelines), the age of the infant, and the area of the cortex under investigation. The trade-off between depth of penetration and spatial resolution thus requires the optimal separation to be determined as a function of factors like the age group being tested, the brain areas involved etc.
Our laboratories conducted extensive testing to determine the optimal source–detector separations at different ages using different optode configurations. Some of these studies have been reported elsewhere in detail (Blasi et al., 2007, Maki et al., 2000); others are published here for the first time.
3.1.1. Early Hitachi results
The collaboration leading to the creation of the current consortium was initiated by the pioneering work of Jacques Mehler, Atsushi Maki and Marcela Peña at the Laboratoire de Sciences Cognitives et Psycholinguistique (CNRS-EHESS-ENS, Paris, France). The Mehler laboratory (in Paris between 1998 and 2000 and later in Trieste from 2001) initially used a Hitachi ETG-100 system. Optimal source–detector separations for measuring NIRS responses in newborns to auditory stimuli were explored using custom-built probes with a single light source coupled with detectors at distances of 2.0 cm, 2.5 cm, and 3.0 cm. Reliable signals were obtained only with the two larger distances, but not with the 2.0 cm separation (Maki et al., 2000).
Other technical parameters were also optimized as a result of these initial explorations. The ETG-100 is a CW system with light sources at 780 nm and 830 nm. These wavelengths proved to be suboptimal for the measurement of deoxyHb. As a result, in subsequent Hitachi systems such as the ETG-4000, the lower wavelength was replaced by 690 nm to remedy the problem (see Section 3.2).
Because the Maki et al. (2000) study used one set of stimulus conditions and an array of optodes over a limited part of the cortex, two follow-up studies were conducted to further examine the issue of source–detector separations.
3.1.2. Comparing the 2 cm and 3 cm newborn probe sets at UBC
The experiment by Gervain et al. (2008), summarized in Section 2.2.2, used the Hitachi ETG-4000 system with 3 cm source–detector separations and was conducted at SISSA's newborn lab (Santa Maria della Misericordia Hospital, Udine, Italy). Here we provide previously unpublished data from UBC's newborn lab (BC Women's Hospital, Vancouver, Canada) using a smaller, 2 cm separation probe set to determine whether this smaller separation is nevertheless sufficient to replicate the main findings of Gervain et al.'s (2008) Experiment 1. The study procedures, the stimuli and the NIRS machines used were identical across the two studies, providing for an ideal comparison between the two different separations.
Twenty-nine healthy, full term newborns (16 females; mean age: 1.5 days; age range: 0–3 days) were tested. Nine (4 females) were excluded from data analysis because they were fussy, had movement artifacts, or their hair interfered with the NIRS signals.
To summarize the procedure briefly, newborns were exposed to trisyllabic sequences (‘words’) generated by two artificial grammars: a repetition-grammar ABB (“mubaba”, “penana” etc.) and a random control grammar ABC (“mubage”, “penaku” etc.). The A and B categories were implemented using 20 consonant–vowel syllables that appeared in each position with equal frequency. The sounds were synthesized with a monotonous pitch of 200 Hz and a syllable duration of 270 s. The two artificial languages were presented in 28 randomly intermixed blocks (14 blocks per condition), separated by silences of variable duration (25–35 s). A block consisted of 10 words from the same grammar. All 280 words were different and appeared in the experiment only once. The optical probes were placed on newborns’ head as indicated in Fig. 4C.
The light attenuation values were converted into relative concentrations of oxyHb and deoxyHb using the modified Beer–Lambert law. The data were filtered and detrended using a 0.01–0.7 Hz band pass filter, and movement artifacts were removed. OxyHb and deoxyHb concentrations were averaged across trials for each condition in each channel. Two areas of interest (AOI) were defined (Fig. 4C): temporal (channels 3, 6, 17, 19) and frontal (channels 2, 5, 13, 15).
The results are shown in Fig. 4D, using the same plotting conventions as in the original experiment (Fig. 4B). The general pattern of results is, in part, comparable across the two studies. Both find increased activation in response to the stimuli in the auditory areas (channels 3, 6, 17, 19 and, to a lesser extent, the neighboring channels) as compared to the rest of the brain, suggesting that the babies perceived the stimuli. Indeed, in a three-way ANOVA with factors Grammar (ABB/ABC), Hemisphere (LH/RH) and Area of Interest (AOI; temporal/frontal), we obtained a significant main effect of AOI using oxyHb as the dependent variable (F(1,19) = 4.802, p = 0.041).4 This was due to greater activation in the temporal than in the frontal AOI in both hemispheres.
However, the 2 cm replication failed to reproduce two crucial aspects of the original study. First, no difference was observed between the two conditions (in the above ANOVA, the main effect of Grammar was not significant, F(1,19) = 0.708, ns). Second, no response was recorded in the frontal channels (2, 5, 13, 15), as the significant main effect of AOI indicates. While the latter null result might be due to the fact that the 2 cm probes are smaller and thus cover a smaller region of the head, possibly missing some of the frontal areas, the absence of any differences between the two conditions is not attributable to the size of the probes. It is also important to note that using a 3 cm separation probe set, all the original effects have been replicated at UBC. The absence of results in the 2 cm replication cannot, therefore, be attributed to simple equipment failure or minor differences between the original and the replication setups.
The 2 cm separation probes thus seem to capture the most robust overall effect, i.e. the general auditory activation, but fail to register the more subtle results. This is problematic for studies that focus precisely on subtle discriminations, e.g. a minimal difference between two artificial grammars, two phonemic categories etc. Interestingly, adult studies investigating subtle phonological differences also often use 3 cm source–distance separations (e.g. Chen et al., 2008).
3.1.3. Comparing channel separations in the Birkbeck-UCL probes
One advantage of the NTS2 system at Birkbeck-UCL is that the optical fibers can be rearranged to form whichever probe configuration and channel separation is required. The work of the Birkbeck-UCL laboratory focuses on the investigation of social perception in young infants from the age of 4–7 months. Over the last 5 years many different source and detector configurations have been used over the frontal, temporal and occipital cortices, with channel separations varying from 1.47 cm to 4.5 cm.
The first infant study with the NTS2 system utilized an 8 cm2 occipital probe containing 8 source pairs and 8 detectors in a 30-channel configuration (Blasi et al., 2007). The channels overlaid one another with three separations (1.43 cm, 1.78 cm and 2.2 cm) to allow interrogation at three different depths within the infant head.5 The results revealed activation to face stimuli at all three depths, but the conclusions that we could draw were limited by the early design of the probe and accompanying level of data rejection.
In light of the continuing debate about optimal source–detector distances for infant NIRS studies, the Birkbeck-UCL lab undertook an investigation of optimal channel separation over the frontal cortex of 5-month old infants. The frontal cortex is of particular concern for developmental researchers as often there is a greater distance between the skin surface and underlying cortex here than over the temporal cortex in young infants, which may affect measurements of underlying cortical activity. The methods and results of this previously unpublished study are described below.
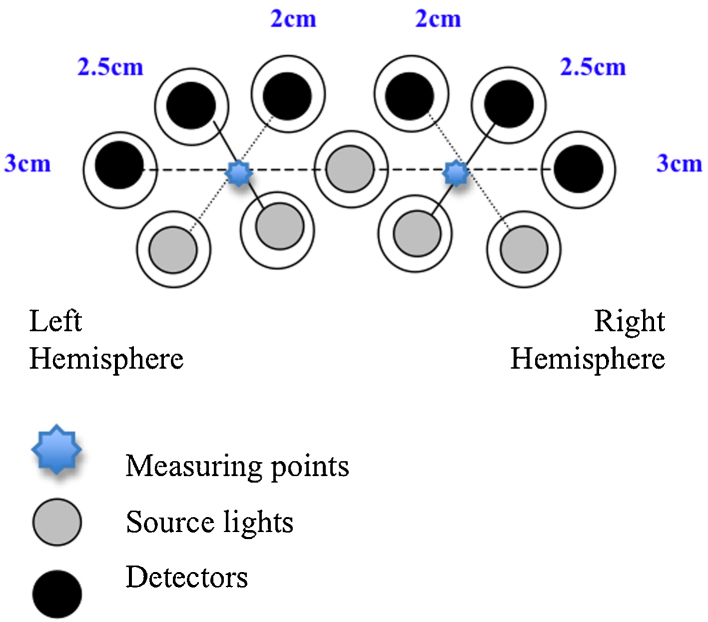
Eleven healthy infants (6 female; mean age: 151 days; age range: 140–167 days) were tested. A further seven infants were excluded from data analysis as they did not provide a sufficient number of frontal channels with valid data following artifact detection.
As shown in Fig. 5, a multi-separation probe measured cortical responses from two locations. Three channels measured data over each of these locations, with separations of 2 cm, 2.5 cm and 3 cm, thereby providing a direct comparison of activations at three depths over the same scalp locations. We repeated a previously published protocol of eye gaze and social communication (Grossmann et al., 2008). To summarize the procedure briefly, the infants wore a NIRS headgear with two temporal arrays and a customized frontal array. While they sat on their parent's lap, the infants watched videos of computer-animated humans, on a 117 cm plasma screen with a viewing distance of approximately 100 cm, who acted out socially communicative scenes. The figure either moved their eye gaze to provide mutual eye contact with the infant and smiled (experimental condition 1), or to provide no eye contact (experimental condition 2). A baseline condition consisting of swirling patterns of animated cars was presented between each experimental trial. This allowed us to compare the effects from the published study with the new findings to determine whether an optimal separation could be identified, and to ensure replication of the previous findings.
Fig. 5.
The multi-separation probes developed at Birkbeck-UCL to be used with the UCL in-house machine.
The recorded NIR attenuation measurements were converted into relative changes in oxyHb and deoxyHb using the modified Beer–Lambert law, and low-pass filtered at 1.8 Hz. Trials and channels were assessed using movement artifact detection algorithms.
For each infant, we compared significant changes in oxyHb and deoxyHb concentration within each channel separation over each measuring point. The results from the bilateral temporal arrays were in line with the results from the previous study (Grossmann et al., 2008). For the multi-separation frontal array, there was no overall effect of channel separation, but rather a high level of individual differences. While the 3 cm channel yielded the highest significant effects and replicated the findings of the previous study in some infants, for other infants the effects were strongest in the 2 cm or 2.5 cm channels. These results suggest that all of these channel separations may be appropriate for infants of this age group when recording from the frontal cortex. Moreover, the use of a multi-distance array could help to accommodate individual differences in physiology and anatomy.
More recent work from the Birkbeck-UCL lab has used a pair of temporal probes to investigate the superior temporal and inferior frontal region of the cortex. The temporal probes house 2 cm and 4.5 cm channels. The first two studies with these probes (Grossmann et al., 2008, Lloyd-Fox et al., 2009) only used data from the 2 cm channels, as the signal intensity picked up by the detectors at the 4.5 cm separation was too low (and therefore noisy) to reveal any stimulus effects. However, when the NTS2 system was upgraded to use glass optical fibers (which do not attenuate as much light as the original plastic fibers), recordings in the 4.5 cm channels were achievable. Two recently completed studies using these glass fibers (Lloyd-Fox et al., submitted for publication, Lloyd-Fox et al., in preparation) have obtained robust effects in the 4.5 cm channels. The effects were particularly strong in a study using auditory stimuli, where interrogation of a greater depth into the brain to reach the auditory cortex may be more appropriate. Further, there was great individual variability in the 4.5 cm data – for some infants, the 4.5 cm channels did not yield any significant effects, and were often excluded after the use of artifact detection algorithms. This suggests that anatomical (i.e. amount of hair, melanin in the skin, skull thickness and CSF) and physiological differences (i.e. differing vasculature) or differences in the location and pattern of brain responses between individuals hinder the definition of an optimal channel separation.
In summary, the results using the Hitachi system suggest an optimal source–detector separation of 2.5–3 cm, whereas the Birkbeck-UCL findings support the use of 2–3 cm separations. These findings may show system-specific differences or they may be the result of factors such as the age of interest, individual anatomical differences, differences in brain function, and region of cortical interest. In some cases smaller or larger separations may be effective, suggesting that systems employing two or more separations in the same probe set could be more efficient.
3.2. Wavelength and laser power
In addition to the source–detector separation, wavelength and laser power also contribute to the quality of the NIRS signals. An optimal pair of wavelengths should take into account cross-talk (contamination of oxyHb and deoxyHb signals by one another) and separability (differential systemic and physical noise effects on the signal at different wavelengths) for assessing the deoxyHb and oxyHb signals (for more discussion, see Lloyd-Fox et al., 2010).
The early Hitachi systems (ETG-100) used CW at 780 nm and 830 nm. Initial work in the Mehler laboratory revealed that while 830 nm was optimal for oxyHb, 780 nm provided a relatively noisy signal for detecting deoxyHb concentrations. The Hitachi Medical Corporation (HMC) thus undertook experiments to determine the most suitable lower wavelength for their system. The lower wavelengths tested were chosen to be below the isosbestic point of oxyHb and deoxyHb (i.e. the point where the extinction coefficients of the two chromophores are equal, see Fig. 1). This practice, common to most CW NIRS systems, ensures that one wavelength is sensitive to deoxyHb, the other to oxyHb. Accordingly, the HMC study (Sato et al., 2004) tested four different lower wavelengths together with 830 nm: 678 nm, 692 nm, 750 nm and 782 nm. Measurement points were placed over all four lobes. The authors observed a less noisy signal with the three lower wavelengths than with 782 nm. The highest signal-to-noise ratio was obtained for 692 nm, which is the wavelength that subsequent Hitachi machines adopted. Other NIRS systems (Boas et al., 2004) also converged on wavelengths around or below 760–770 nm for a more optimal measurement of the deoxyHb signal. A theoretical study by Uludağ et al. (2004) used model-based estimates of cross-talk and separability to assess all combinations of two wavelengths between 610 nm and 920 nm. They concluded that cross-talk is low and separability is high if one wavelength is between 650 nm and 720 nm or 750 nm and 770 nm and the other between 730 nm and 920 nm.
In this regard, it is important to mention that the two systems used by the McDonnell Consortium have two different wavelength pairs. The NTS2 system at Birkbeck-UCL uses 770 nm and 850 nm, while the Hitachi ETG-4000 system, used in the other four labs, employs 690 nm and 830 nm. There is thus a difference in the lower wavelengths chosen. Given the previous empirical findings (Sato et al., 2004, Boas et al., 2004) as well as the modeling results (Uludağ et al., 2004), both wavelength pairs nevertheless seem optimal choices to minimize cross-talk and maximize separability.
Laser power is another parameter contributing to the signal-to-noise ratio of NIRS measurements. Light intensities up to 2–5 mW (even up to 10 mW in adults) are considered safe (Koizumi et al., 2003). It is, therefore, tempting to increase power to obtain a stronger signal. However, noise also increases as a function of light intensity. The best signal-to-noise ratio thus results from a trade-off between the increase in signal strength and noise at different light intensities. In infants, whose tissues are thin, good signal quality can already be obtained at low intensities (most cited studies use laser power between 0.5 mW and 1.5 mW), ensuring maximal safety even for the youngest babies.
3.3. OxyHb and deoxyHb: two sides of the same coin?
One advantage of NIRS over fMRI is that it measures both oxyHb and deoxyHb concentration changes, providing physiologically more relevant data about the metabolic correlates of brain activity, as the sum of oxyHb and deoxyHb corresponds to the regional cerebral blood volume (rCBV).
However, the richness of the NIRS signal raises certain challenges. Different machines and experimental setups do not always provide equally good assessments of the two hemoglobin species. Wavelength and the material of the optical fibers are two of the factors that can affect the quality of the NIRS signal and produce different signal-to-noise ratios for oxyHb and deoxyHb. Depending on the technical parameters of the systems used, different studies variably report oxyHb, deoxyHb and/or totalHb. This inconsistency may be problematic, because it renders comparisons between studies difficult. The best practice is, therefore, to report and conduct statistical analyses on both species of hemoglobin.
Setting technical difficulties aside, many studies obtain a significant result only for one of the hemoglobin species. Indeed, infant studies often find more significant or more robust effects with oxyHb than with deoxyHb (Hoshi et al., 2001, Meek, 2002, Shimada and Hiraki, 2006), although stronger deoxyHb results are also sometimes reported (Schroeter et al., 2004). In typical, healthy adult and infant participants, oxyHb and deoxyHb are usually correlated and show the same effects (with an increase in oxyHb and a decrease in deoxyHb). However, in the youngest age groups as well as in clinical populations, oxyHb and deoxyHb sometimes appear uncoupled and/or the direction of the change is inverted (i.e. decrease in oxyHb and increase in deoxyHb; Chen et al., 2002, Meek, 2002, Sakatani et al., 1999). Currently, no physiological explanation exists to account for such patterns of results. According to one hypothesis, infants might show atypical hemodynamic responses under certain circumstances because their vasculature, and consequently the neurovascular coupling, is not fully mature. More work is needed to understand the possible physiological significance, if any, of these atypical responses.
In this respect, comparison with other imaging modalities, especially fMRI, can be particularly instructive. The fMRI BOLD signal has been proposed to originate from the paramagnetic properties of deoxyHb (Buxton et al., 1998, Ogawa et al., 1993). Accordingly, it is assumed to correlate with the deoxyHb signal of NIRS. However, the findings of existing NIRS–fMRI co-registration studies are not always completely clear (for an excellent review, see Steinbrink et al., 2006). While most studies do find a temporal and/or spatial correlation between deoxyHb and the BOLD signal (e.g. Boas et al., 2003, Kleinschmidt et al., 1996, Toronov et al., 2001), others observe equal correlation between the two hemoglobin species and BOLD (e.g. Okamoto et al., 2004) or find the strongest correlation between totalHb and the BOLD signal (e.g. Hess et al., 2000, Strangman et al., 2002, Vignal et al., 2008). As these studies differ in the experimental tasks used (visual, motor etc.), the participants tested (animal models, human subjects etc.), as well as the technical specifications of the NIRS machines utilized, such divergence is not surprising and indicates that the relationship between fMRI and NIRS measures might be complex. Further research is necessary, therefore, to elucidate these issues.
3.4. Probe designs and placement
In optical imaging systems for measuring cerebral hemodynamics, NIR light is delivered to and collected from the participant through fiber optic bundles. The ends of each bundle are embedded into plastic probes (optodes), which are then secured to the scalp of the participant. These probes have to be designed to balance the need for comfort against the demand to keep them from moving against the scalp while making good contact with the scalp and preventing channel cross-talk.
Designing NIRS probes provides significant challenges due to different constraints and demands in different subject populations. For example, while soft, flat probes might be ideal for newborns, who typically have very little hair, they do not allow much light to penetrate through the denser mat of hair typically encountered with adults. In contrast, the narrow plastic tips at the ends of the optical fibers in the adult probes, while allowing the researcher some maneuverability past the hair on the adult scalp, are ill-suited to the infant's scalp. Further, motion artifacts play a different role in different populations. While adults can be instructed to keep still, infant probes must be designed to maintain their positions relative to the scalp despite small head movements, while still being comfortable for the infants. Studies of sleeping newborns are largely immune from motion artifacts that can be substantial in older awake infants.
Given the increased mobility of infants older than 2–3 months of age, probe design has proven to be particularly challenging for awake infants in the 2–12-month old age range, which comprises the vast majority of all infant studies. In experimenting with various designs, our labs have discovered two key features that contribute to the stability of the optodes and the quality of the NIRS signal. The first is to make the optodes as flat as possible, so the fiberglass bundles lay as close to the scalp as possible. This can be achieved by having short, squat optode tips that are perpendicular to the optic fibers, for example by using a prism to bend the light through 90°. The mass of fibers from all the optodes, lying along the scalp and traveling to the back of the infant's head, helps to maintain the optodes close to the scalp, and the weight of these fibers is counterbalanced with chin straps attached to the matrix in which the optodes are embedded. The second feature, developed at the Birkbeck-UCL lab, is to have the probe tips slightly recessed into silicone. The silicone provides a safe and fairly non-slippery surface that gently adheres to the scalp of infants and significantly reduces lateral movement. The recessed optode tip helps to localize the NIR light and prevents channel cross-contamination.
In contrast to the fMRI scanner bore or the MEG dewar, NIRS probes can be moved to various locations on the scalp, similar to EEG/ERP electrodes. However, as noted in Section 1.1.1, the distance between the optodes is crucial. Therefore, NIRS probes cannot be placed using a proportional (variable inter-optode distance) placement system like the 10/20 system used in placing EEG/ERP electrodes. This raises several difficulties. The first is that, in order to maintain fixed inter-optode distances, the optodes must themselves be in a rigid plane or mesh, and this rigid structure must then be embedded in a suitable, stretchy matrix that can accommodate variations in head sizes. If the optodes are fixed into a rigid pad, they cannot be re-configured to suit different experiments. Indeed, the Hitachi ETG system is limited in that the software cannot handle arbitrary source–detector configurations, but can only handle rigid deformations of a few basic layouts. For example, a 3 × 3 array of sources and detectors (where the corners and the central position are sources) is limited to the same relative configuration, e.g. as a square or as a chevron, and cannot be re-configured (e.g. into two rows of alternating sources and detectors, as in the Birkbeck-UCL system). In more recent versions, developed in collaboration with the Rochester lab, Hitachi Medical Corporation has modified the probes so that the individual fibers can snap into a light grid of probe holders, similar to the probes used with the TechEN system (http://www.nirsoptix.com/).
A second, potentially more serious problem with the requirement of fixed inter-optode distances is that, for heads of different sizes, the individual channels will sample slightly different anatomical areas. However, given the poorer spatial resolution of current NIRS systems (estimated voxel dimension is 15 mm compared to 4 mm in fMRI, for instance), the effect of sampling from slightly different anatomical areas in across participant averages may not be a serious problem, although it remains an empirical issue. Analytic techniques that can be used with individual participants (like pattern classification, see Section 5.2) or aggregating responses across multiple channels may provide more robust between-participant results.
Finally, because optode placement is typically guided solely by external, skull landmarks, their relation to underlying cortex is not validated for individual infants. In order to understand the relation between the spatial layout of optodes on the scalp and where in cortex the hemodynamic signals arise, the Rochester lab has gathered data from a set of six adult participants who saw identical visual stimuli while cortical activity was monitored by NIRS and fMRI (in separate sessions). The visual stimuli consisted of flickering checkerboards that either rotated or alternated between left and right hemifields. These stimuli are known to activate the occipital cortex with a periodicity matching the periodicity of rotations or alternations (see related work by White and Culver, 2010). Crucially, the placement of the optodes on the scalp of each participant and the alignment of the fMRI runs were both registered to the participant's own (T1-weighted) structural images. In order to align the probe locations with the structural images, a commercial system called Brainsight (www.rogue-research.com) was used. Brainsight is a “frameless stereotaxy” system that uses stereo cameras to detect the location of custom “pointers” in 3D space. These pointers are used to mark external landmarks on the participant's skull that are also clearly visible on the structural MR image, like the nose tip or the tragus of the ear. The software then computes the correspondence between the location of the pointer in 3D space and the underlying brain tissue. By “pointing” to each of the optodes, their precise location can be specified in MR space prior to gathering NIRS data from each participant. Of course, co-registration of NIRS would require a structural MRI for each infant, which is not feasible for most studies. However, average structural MRIs may prove useful if external landmarks are also gathered across infant age groups (see Section 3.6).
3.5. States of alertness and motion artifacts
One central issue for NIRS data acquisition is the reduction of movement artifacts. Motion artifacts are usually characterized by abrupt changes in the signal occurring simultaneously in several channels, which are quite distinctive from the usual slow and smooth hemodynamic response. NIRS is less sensitive to such movement-induced distortions of the signal than EEG or MRI measurements, but it is not entirely exempt from this problem. The issue is particularly relevant for awake infants who cannot follow verbal instructions and have a tendency to move during testing.
Several solutions have been developed to reduce movement artifacts in these age groups. The design of the headgear (see Section 3.4) and the engaging quality of the stimuli (e.g. interesting visual stimuli, see Section 4) play a critical role. Movement artifacts can also be captured and eliminated a posteriori during data analysis (see Section 5).
An alternative paradigm is to test newborns and very young infants in a state of sleep or quiet rest (e.g. Gervain et al., 2008, Peña et al., 2003). This method considerably reduces or even completely eliminates movement artifacts. Additionally, it allows for extended measurement periods (up to 30 min or even longer), which increases the number of trials and thus the quality of the averaged signal. It, nevertheless, has certain disadvantages. First, it only suits certain stimulus modalities. Auditory processing is robust even during sleep in young infants, but visual stimuli, for instance, cannot be presented to sleeping participants (other than very simple light–darkness contrasts that may be perceived even with the eyes closed).
Another question that arises is whether brain responses to the same stimuli are similar in different states of alertness. No systematic NIRS study has yet explored this question and the answer will probably vary as a function of the stimuli, task types, brain areas, and age ranges involved. No differences between awake (but quietly resting) and sleeping newborns have been observed in the studies our laboratories have conducted with this age group (Gervain et al., 2008, Peña et al., 2003). It needs to be noted, however, that these studies were not specifically designed to test for such a difference, as sleep states were not monitored by EEG and typically too few infants were unambiguously awake to allow for a statistical comparison. In older infants, one fMRI study did find a statistically significant difference between awake and sleeping babies’ responses to speech stimuli (Dehaene-Lambertz et al., 2002). In this study, awake 3-month olds showed activation to normal, forward (but not to backwards, reversed speech) in the right dorsolateral prefrontal cortex, whereas sleeping babies did not. Nevertheless, both groups showed activation in response to forward speech in left lateral brain areas. These findings suggest that auditory brain responses might be relatively unaffected by sleep, while other, possibly more attention-related areas, e.g. the prefrontal cortex, are only activated in wakeful states. Again, however, the primary aim of this study was not to evaluate the effects of sleep states on auditory perception, and as a result, only a small number of infants were included in the statistical analyses to evaluate the effects of sleep. Clearly, more methodological studies are needed to gain a full understanding of the effects of different states of alertness on the NIRS signal.
3.6. Brain anatomy and function across development
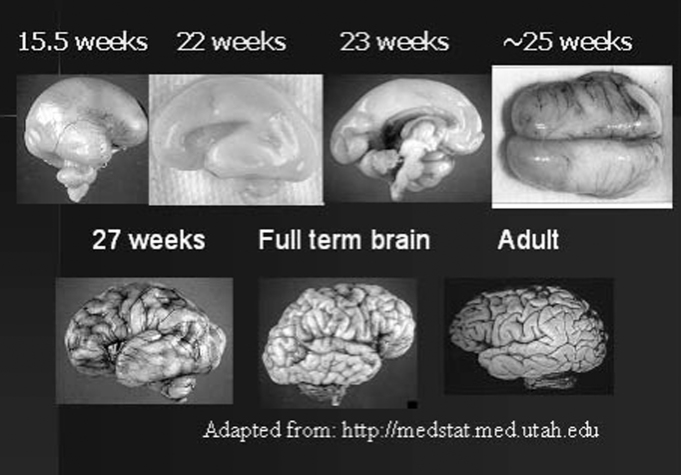
As discussed earlier, NIRS is ideally suited to the study of infants and young children in part because the skull is thinner, thereby permitting a more transparent view of the cortical surface. As children age and the skull thickens, there is a corresponding reduction in the field of view of the cortex. However, these are minor considerations compared to underlying changes in brain development across the first years of life. For example, the overall gyral pattern that exists in the adult is nearly in place in full term newborns, although sulci are shallower. In the preterm infant, however, the gyral pattern is far from developed, particularly as one moves to the study of infants born 2–3 months early. This is illustrated in Fig. 6. Because of the depth NIRS can penetrate, it is possible to examine activity generated in a sulcus early in life, but not later in life, as this same sulcus deepens and the skull thickens (thereby moving the sulcus further away from the optode).
Fig. 6.
Changes in brain anatomy across development.
Currently, a multi-site, structural MRI project, funded by the National Institutes of Health (“NIH MRI Study of Normal Brain Development”) is under way, providing an MRI scan library of typically developing brains across development from a few days after birth up to 18 years of age. Qualified researchers may obtain brain scans from the Consortium's web site (http://www.bic.mni.mcgill.ca/nihpd/info/) after registration. A list of related publications (e.g. Almli et al., 2007, Sanchez et al., in press) can also be found at the website. John Richards’ laboratory also provides structural brain scans of the developing brain for professional researchers following authorization (http://jerlab.psych.sc.edu/NeurodevelopmentalMRI/index.html, Richards, 2010). A recent publication by Hill et al. (2010) has quantified the individual anatomic variation of the cortex in full term newborns using structural brain scans, and found that the amount of variability found in these infants is comparable to that of adults.
Other developmental considerations include changes in the vascular system and the tendency of a tesselated surface structure to stretch such that certain gyri begin to shift in location, moving them out of the line of sight of nearby optodes. An example here might be the difficulty in imaging the fusiform gyrus, which over time moves to a more dorsal position, making it impossible to image. In contrast, most gyri sitting on the lateral cortical surface probably remain viable candidates for imaging.
A last consideration has more to do with the functional neuroanatomy of the brain. Given the constraint that NIRS can only visualize the cortical surface, it is not a method that lends itself to asking questions about structures that lie deep in the cortex or out of the line of sight of the optodes. Thus, studies of memory and emotion (subserved by structures that lie in the medial temporal areas) are impossible with NIRS. Similarly, complex, higher-level phenomena that depend on elaborate cortical circuitry may be difficult to image because a distributed network is involved, with some nodes lying deep in the brain (e.g. projections from the amygdala to the orbitofrontal cortex). In this context it is imperative for investigators to generate well-defined and biologically plausible hypotheses. An example of the latter are investigators who argue that their EEG measures reflect amygdala function, when in fact EEG currents are generated by pyramidal cells and the amygdala does not contain pyramidal cells. So it is with NIRS: unless the function being targeted is subserved by regions that lie in close proximity to the optodes, NIRS is not an appropriate measure.
4. Experimental designs
The signal measured by NIRS, the hemodynamic response function (HRF), is a metabolic, and thus indirect and slow correlate of brain activity. As discussed before, peak response latencies are in the order of several seconds following stimulus onset, with a plateau of several seconds (depending on stimulus duration), and a slow return to baseline over 5–10 s or longer. The relatively slow temporal dynamics of the HRF needs to be taken into account when designing NIRS experiments.
4.1. Block designs
The classical and most commonly used type of experimental design in NIRS studies is the block design. In this experimental design, the different stimulus types, i.e. different experimental conditions, are presented separately, in relatively long epochs (blocks) of stimulation. Within a block, tokens of the same stimulus type are presented repeatedly. An experiment might contain a single, relatively long block of each experimental condition (e.g. Peña et al., 2003) or several blocks per condition, in which case the different blocks may be randomly intermixed (e.g. Gervain et al., 2008) or may alternate regularly (e.g. Sato et al., 2009). Blocks are separated by suitably long periods of rest or silence to allow for the HRF to return to baseline. These periods are usually filled with silence (Gervain et al., 2008, Peña et al., 2003, Wilcox et al., 2005), especially when participants are asleep or at rest. However, with older infants, who are typically awake during test, it is often useful to maintain some kind of stimulation even during the rest period (e.g. a silent video etc.) to reduce movement artifacts and keep infants engaged in the task (e.g. Otsuka et al., 2007, Shimada and Hiraki, 2006). The rest stimulus needs to be chosen carefully in order to draw appropriate conclusions about which aspect of the experimental stimuli is reflected in the differential activation above the resting period baseline. In addition, the inter-block interval should be varied to reduce expectation (entrainment) effects or phase-locked brain oscillations unrelated to the experimental stimulus.
One major advantage of the block design is the robustness and strength of the obtained signal. As each block consists of repeated presentations of a given stimulus type, the HRF measured for the entire block is obtained as a superposition of the individual HRFs to each stimulus. This increases the strength of the signal. Averaging across blocks in the same condition further enhances signal reliability.
Nevertheless, this design is not without its flaws. Because the response is measured for entire blocks of stimulation, the effects of individual stimulus tokens cannot be captured. In addition, responses to entire blocks might include sustained attention- or task-related effects, which do not, strictly speaking, result from the processing of the stimuli.
4.2. Event-related paradigms
As mathematical techniques for data analysis advance and methods are borrowed from the fMRI literature, event-related designs, exempt from some of the inconveniences of block designs, are gaining popularity. In an event-related design, single, short stimuli are presented at randomly distributed time intervals. If interstimulus intervals (ISIs) are chosen to be long enough to allow the HRF to return to baseline (or at least to partially return so the response is not saturated), the total number of data acquisition events can be substantially greater than in a block design. To avoid extended data acquisition time, which is not ideal for infant populations, ISIs can be shortened to a few seconds, and data analysis methods can be used to deconvolve the HRFs (given certain assumptions about the linear superposition of HRFs) from the stimulus event, like in fMRI studies (e.g. see Amaro and Barker, 2006 for a review).
The event-related design has the advantage of capturing single hemodynamic responses to individual stimuli, divorcing task- or attention-related, sustained responses from activation triggered by the different stimuli. In infant research, few studies have used this technique so far (see Plichta et al., 2006, Plichta et al., 2007, Chen et al., 2008 for a discussion and illustration of event-related NIRS in adults), mostly due to the relative weakness of the obtained signal. However, the use of event-related paradigms will not only provide valuable information about cognitive functions, but will also help to characterize the HRF in infants.
4.3. Mixed designs
In an attempt to combine the advantages of event-related and block designs, mixed stimulus presentation designs can also be used. In these studies, individual stimuli are presented repeatedly, with relatively short ISIs. These stimulation periods are intermixed with the presentation of the control condition. One application of this design is to study what in the fMRI literature is called repetition suppression (Grill-Spector et al., 2006), similar to response habituation in the infant looking-time literature (see review and comparison by Turk-Browne et al., 2008). Pilot studies from the Rochester lab have documented robust repetition suppression effects from temporal cortex in adults listening to musical stimuli, and repetition suppression as well as recovery to novelty effects have been reported in 3-month old infants with speech stimuli (Nakano et al., 2009). By contrast, repetition enhancement effects were found in newborns for linguistic stimuli (Gervain et al., 2008), but not for piano tone equivalents (Gervain et al., in preparation). More infant studies are needed to explore repetition effects and the use of mixed designs in infants.
5. Data analysis and interpretation
Different stimulus presentation designs require different analysis techniques. In this section, we will review the major approaches to the analysis of NIRS data, introducing the main steps and techniques, reviewing some of the available software packages, and drawing attention to some of the pitfalls that researchers might face when interpreting NIRS findings.
5.1. Analysis: towards a standard method
Since NIRS is a relatively new brain imaging technique, analyzing NIRS data is only now beginning to be standardized. Analysis methods reported in the literature range from time series averaging techniques (e.g. Gervain et al., 2008, Otsuka et al., 2007, Peña et al., 2003, Shibata et al., 2007) through statistical parametric mapping borrowed from fMRI research (e.g. Shimada and Hiraki, 2006, Telkemeyer et al., 2009, Wartenburger et al., 2007) to more sophisticated data mining and pattern recognition techniques (see Section 5.2).
In time series analysis techniques, the NIRS signal is typically averaged over the trials (blocks/epochs) of the same condition to obtain a mean time course for each condition. For statistical purposes, the average (or peak) concentration change within a relevant window of the time series is calculated. To compare the significance of the change to a pre-stimulation baseline, t-tests are used, whereas analyses of variance are conducted to compare signal change across conditions, hemispheres, channels or groups of participants. The time window within which the concentration change is averaged varies across studies, but it typically includes the stimulation period and sometimes, especially for short stimuli, a period of a few seconds after stimulation (before the HRF begins to return to baseline). This analysis is particularly well suited for studies using block designs and/or long ISIs, allowing the HRF to return to baseline before the presentation of a new stimulus.
Inspired by fMRI research (Friston et al., 1999, Worsley et al., 1995, Worsley et al., 1997, Zarahn et al., 1997), an increasing number of studies use the general linear model (GLM) and statistical parametric mapping (SPM) approaches (Plichta et al., 2007, Schroeter et al., 2004). When using these techniques, the measured NIRS data are correlated with a predictor, obtained by convolving a boxcar function for the stimulus design with the typical HRF (and with other regressors, if necessary). As mentioned before, the exact shape of the HRFs for infants of different ages (and for different brain areas, tasks etc.) has not been fully described. Consequently, many developmental studies use the adult HRF or some variant thereof. It would be desirable to have better estimates of infant HRFs in the future. According to the GLM, NIRS data (Y) can be modeled as Y = β1X1 + β2X2 + β3X3 + … + ɛ, where β1…n are coefficients measuring the contribution of each predictor to explaining Y, X1…n are the predictors and ɛ is an error term. The values of β can be used for statistical purposes (t-tests, ANOVAs etc.). These techniques offer an appropriate way to analyze event-related designs or studies where ISIs between stimuli are short, as they are able to take into account the superposition of consecutive HRFs.
Different software packages are now available on-line (as freeware), implementing the different analysis methods. The most commonly used ones include HomER (Huppert et al., 2009), fOSA (Koh et al., 2007) and NIRS-SPM (Ye et al., 2009).
NIRS data are often pre-processed before analysis to improve the quality of the signal. Data processing typically includes denoising/filtering, detrending and movement artifact removal (Huppert et al., 2009). High frequency noise in the data results from instrumental or other noise as well as from physiological signals not related to stimulation, such as heart beat, breathing, sucking on a pacifier etc. To reduce these sources of noise, some form of filtering, typically a low-pass filter at a value between 0.5 Hz and 1 Hz, is used. Low-frequency oscillations originate from slower changes in systemic cardiovascular properties, e.g. blood pressure. These are usually eliminated using high-pass filters at values around 0.01–0.05 Hz. As the frequency of these oscillations can fall within the frequency range of the targeted brain response itself, care has to be taken when choosing the filter value to avoid removing the signal. To eliminate systematic, but stimulus-unrelated noise (of both low and high frequency), auto-regression analyses can also be used, as this type of noise is typically present in several channels.
One option to retrospectively deal with movement artifact is to use thresholding algorithms to remove sections of the data containing these abrupt changes. However, thresholds must be defined carefully in order to preserve the changes that truly result from activation. Steps in motion artifact rejection include: the detection of unreasonably large changes in concentration within individual trials (Lloyd-Fox et al., 2009), the detection of channels which contain data with a high level of variation outside of the median range (Blasi et al., 2007), and filtering techniques such as principal components analysis (PCA; Wilcox et al., 2005).
5.2. Multi-voxel (channel) pattern analysis
Traditionally, fMRI analyses have looked for significant changes in the BOLD signal, derived from an ‘expected’ hemodynamic response, over a contiguous set of voxels (a region-of-interest, ROI). The rationale for averaging across clusters of voxels is to increase statistical power over noisy voxels. However, this method assumes that the voxels relevant to a particular cognitive process are spatially clustered rather than spatially distributed. There are clear cases where this assumption is incorrect (e.g. orientation columns in primary visual cortex). The rationale for so-called pattern classification methods is that the pattern of activation may carry information about the stimulus type, even if the overall activation within an ROI cannot discriminate between them. Accordingly, several recent fMRI studies have examined how information about the stimulus might be carried by the activity pattern across a set of voxels (see Norman et al., 2006 for a review).
This same idea, called multi-voxel pattern classification, can be applied to the analysis of NIRS signals from an array of channels. That is, while activity in a single channel, or aggregated across multiple channels, might not have the spatial resolution to differentiate between two sets of stimuli, the pattern of activity across all the channels might uncover differences. Moreover, because different channels might show different patterns of activation, a variety of pattern recognition algorithms provide an alternative way of analyzing differential brain activation to different stimuli. The basic analysis paradigm is to present two types of stimuli to a single participant across a series of trials, to train a computational model with half of the data to determine which weights to assign to each voxel so that the pattern of voxels best fits that training set, and then to test that model fit with the other half of the data on a trial-by-trial basis.
The Rochester lab has collected pilot data from adults using a block design during which the participant either heard speech or viewed a series of faces. NIRS data were collected with a 24-channel ETG-4000 system with 12 channels placed over the occipital cortex along the midline and 12 channels placed over the left temporal areas. Importantly, reliable results were obtained from single adult participants. First, a traditional ROI analysis showed that occipital channels had a greater increase in oxyHb concentration for faces, while left temporal channels had a greater increase for speech.
Second, we used the Sparse Logistic Regression Toolbox (v. 1.2.1alpha, Yamashita et al., 2008) for Matlab to analyze the data across all 24 channels. We trained the data on half the trials for the two conditions (faces vs. speech) and tested the resultant pattern classifier on the remaining half of the trials. We used a bootstrap procedure to estimate the reliability of finding a classifier that could correctly classify the withheld half of the trials, by repeating the train–test procedure 1000 times. On average, the pattern classifier obtained with the training set was able to correctly assign the remaining trials as face or speech trials with an accuracy of 70.6% (99% limits: 69.4–71.7%) on the test set.
In summary, pattern classification techniques can be fruitful complements to more traditional “waveform” or ROI analyses, which are based on simple comparisons between stimulus conditions for each channel.
5.3. The dos and don'ts of interpreting optical data
Perhaps the greatest challenge facing a researcher who has conducted a NIRS study is not the myriad methodological pitfalls, but rather how to draw inferences about underlying cognitive processes from NIRS data. Given optode arrays with 12–48 channels, covering multiple cortical areas, the prior probability of obtaining statistically significant differences across channels for two-stimulus conditions is rather high. But what do these differences mean? As in BOLD imaging with fMRI, where a 4 mm3 voxel samples activity from 100,000 or more neurons, a NIRS channel samples an even larger pool of neurons (perhaps 3–5 times larger), and those neurons come from multiple cortical depths, in contrast to fMRI slices whose 3D coordinates are well specified. Moreover, optodes are almost always positioned on the scalp using external landmarks rather than brain anatomy, an ambiguity that is not present in fMRI because of co-registration with structural MRI. Thus, there is considerable heterogeneity in the NIRS signal from any channel, including (a) differences in the relationship between neural and hemodynamic responses, (b) the relative proportion of excitatory and inhibitory pools of neurons, (c) the distance of cortical sulci and gyri from the surface of the scalp, and (d) the precise positioning of the optodes over relevant brain anatomy.
5.3.1. Linking hypotheses
Although there are ambiguities about how neural, hemodynamic, and anatomical variables contribute to NIRS signals, these signals must nevertheless be linked to an underlying cognitive process, if they are to contribute to our understanding of brain function and organization in infants. Unfortunately, most linking hypotheses are either unstated or rather naïve. For example, it is often assumed that greater activation, as indexed by larger NIRS signal amplitudes in a particular cortical area (e.g. the frontal cortex), implies that this cortical area is “the” area mediating a cognitive process (e.g. recognition memory). But of course any stimulus condition leads to the activation of a diverse set of brain areas, many of which cannot be detected by NIRS because it is limited to surface cortical areas. Moreover, NIRS signals are neural correlates of a stimulus condition. These NIRS signals can only be confirmed as a causal mechanism if they are shown to be necessary for the cognitive process. It is quite possible, especially if only a limited number of channels (and cortical areas) are sampled by the optodes, that some unsampled brain area (a so-called “third” variable) is the causal agent in mediating the neural activation to the stimulus of interest. That is, a large number of cortical areas may be activated by a given stimulus, but only one of these areas may be necessary (i.e. causal) in a network of neural areas.
As an example, given the known anatomy of the visual system, we would expect the primary visual cortex to be activated by any suprathreshold visual stimulus, with information from this initial analysis of the visual stimulus passed on to higher-level visual processing areas (e.g. dorsal and ventral streams). If optodes are located over temporal or frontal cortical areas, then activations from these NIRS channels could indicate (a) further analysis of the visual information (e.g. extraction of more complex features), (b) activations that “compare” the current visual information with stored visual or auditory information based on past experience (e.g. memories of what sounds were present with a visual object), or (c) expectations about what stimulus is most likely to come next (e.g. habituation or sensitization effects), as well as many other possible interpretations. In the domain of learning, it is typically assumed that the growth of activation over time (or exposure) is indicative of greater learning, but it is equally plausible that greater learning leads to decreasing activation because the pool of neurons required to learn (or recognize) the stimulus is smaller. Moreover, large pools of neurons may be involved in the attempt to learn, even though no learning has taken place (e.g. if the learning task is too difficult). The foregoing interpretive challenges are merely a sampling of the complexities involved in precisely specifying the linking hypothesis between neural activations and underlying cognitive processes.
5.3.2. Two-stimulus vs. continuous-dimension designs
Another interpretive dilemma faced by neuroimaging studies, including NIRS, is the over-reliance on two-stimulus designs. The logic of such designs is that greater activation to stimulus A than to stimulus B, when these stimuli differ in their category membership (e.g. only stimulus A is a member of category X), implies that category X is “encoded” by any NIRS channel that shows a statistically significant difference in activation to stimulus A vs. stimulus B (e.g. faces vs. non-faces). But this same pattern of results could be obtained if there is a featural difference between stimulus A and stimulus B that is correlated with category X (e.g. presence vs. absence of two eyes) but fails to meet the defining characteristics of category X (e.g. having no more than 2 eyes). And the absence of a difference in activation to stimulus A vs. stimulus B could occur if activation in the pool of neurons sampled by a given NIRS channel suffers from either a floor or a ceiling effect to both stimuli (i.e. preventing the activation from going any lower or higher because of a compressive or a saturating non-linearity).
A design that mitigates these interpretive errors involves the rather simple addition of a third stimulus that varies along a dimension that defines category X (see discussion in Aslin and Fiser, 2005). If this third stimulus extends the range of stimulus A (no eyes) and stimulus B (two eyes) to stimulus C (three eyes), then the pool of neurons that responds to category X should be greater to stimulus B than to stimulus A and greater to stimulus B than to stimulus C (an inverted U-shaped function along the dimension “number of eyes”). This is a much more convincing demonstration that NIRS channels are responding selectively to category X than any pair of stimulus conditions. The same logic applies to the problem of a compressive or saturating non-linearity. If a NIRS channel is interpreted as responding to dimension Y (e.g. the number of elements in a visual array), and there is no difference in activation to stimulus A vs. stimulus B (e.g. 6 vs. 12 elements), then it is seductive to conclude that the absence of a difference indicates dimension Y (e.g. numerosity) is not encoded in that NIRS channel. But this interpretation presumes that the pool of neurons triggering NIRS activation in that channel has sufficient gain to reveal activation differences. By adding stimulus C (e.g. 18 elements) one can determine if increasing the stimulus difference (from 2:1 to 3:1) reveals sensitivity to dimension Y (numerosity).
6. Comparison of NIRS with other measures
Because NIRS is a relatively novel method in developmental brain imaging, it is important to evaluate the strengths and weaknesses of NIRS measurements. One way to achieve this is to compare NIRS data with behavioral and other neurophysiological measures in an attempt to obtain converging findings. It is important to note, however, that dissociations between different measures may indicate that they each assess slightly different aspects of neural processing, so the absence of convergence across measures is not necessarily a cause for concern.
6.1. Comparison with a behavioral measure in newborns at UBC
Exploring newborns’ speech perception and language learning abilities has been one of the most prolific areas of application for NIRS. It is, therefore, important to show the convergence between optical imaging measures and more traditional, well-established behavioral techniques in this domain.
The laboratory at UBC used the high amplitude sucking (HAS; Floccia et al., 1997, Moon et al., 1993, Nazzi et al., 1998, Shi et al., 1999, Byers-Heinlein et al., 2010) procedure to replicate Gervain et al.'s (2008) results, providing a behavioral comparison. Ten newborns participated in the ABB condition (4 females; average age: 2.1 days; range: 1–3 days;) and 10 different newborns participated in the ABA condition (4 females; average age: 2 days; range: 1–3 days). An additional 10 babies were tested, but failed to complete the experiment due to crying, fussiness or falling asleep, a standard attrition rate for the HAS procedure (Floccia et al., 1997, Moon et al., 1993, Nazzi et al., 1998).
In the habituation phase, newborns were exposed to a stimulus whenever they made a high amplitude suck on a pacifier linked to a pressure transducer. Half of the infants were habituated to the adjacent repetition (ABB) grammar, the other half to the non-adjacent repetition (ABA) grammar. At the beginning of this first habituation phase, infants typically produced a large number of high amplitude sucks, because the stimuli were novel. Over a period of several minutes, as the stimuli gradually became familiar, they decreased their sucking rate. When the sucking rate decreased by 25% as compared to the sucking rate of the previous two (consecutive) minutes, infants were considered to be habituated (Floccia et al., 1997, Moon et al., 1993, Nazzi et al., 1998). After habituation, new stimuli were presented in the test phase: both groups were switched over to the random (ABC) grammar. An increase in sucking rate after the switch indicates that infants can discriminate the pre- and post-switch stimuli.
Results showed that only the infants in the ABB group noticed the switch and increased their sucking rate (mean number of high amplitude sucks 2 min pre-switch: 13.15 and 2 min post-switch: 23.4). Infants in the ABA group continued to decrease sucking after the switch (mean number of high amplitude sucks 2 min pre-switch: 17.95 and 2 min post-switch: 13.05). Using the number of high amplitude sucks in the 2 min pre- and post-switch as the dependent variable, as is customary for HAS (Floccia et al., 1997, Moon et al., 1993, Nazzi et al., 1998), an analysis of variance with Grammar (ABB/ABA) as a between-subject factor and Stimulus Type (pre-switch/post-switch) as a within-subject factor revealed a significant Grammar × Stimulus Type interaction (F(1,18) = 4.569, p = 0.046). This result was carried by a significant increase in the number of HA sucks in the ABB group (t(9) = 2.515, p = 0.033), but not in the ABA group (t(9) = 1.692, ns). No main effects were significant. The numerical difference between the number of high amplitude sucks in the pre-switch phases of the two groups (ABB: 13.15 and ABA: 17.95) was not significant (t(18) = 1.121, ns).
These results are convergent with the NIRS results obtained by Gervain et al. (2008) and provide a strong behavioral validation for the latter.
6.2. TOBII eye tracker and optical imaging
Measures of looking behavior have emerged as potent tools in studying human cognition. Looking behavior is largely automatic, and has been shown to reflect cognitive processing at various levels and at different developmental stages (see reviews by Aslin, 2007, Tanenhaus et al., 1995). Eye-tracking is particularly suited to infants, who have a limited behavioral repertoire, and gaze data can reveal different cognitive states on a trial-by-trial basis. Therefore, integrating looking behavior with NIRS allows us to examine the relation between the hemodynamic signal correlated with neural activity and the presented stimuli, modulo the cognitive state of the participant, as revealed by the gaze data. Indeed, comparisons with eye gaze data can corroborate the canonical interpretation of NIRS measurements in terms of the metabolic cost and effort of processing, neural commitment etc. In addition, gaze data can itself be used to validate and improve the quality of the NIRS signal. For example, in looking studies, imaging trials can be accepted or rejected based on how much visual stimulation was accessed, as revealed by the eye tracker. In addition, gaze data can be used to ensure that differences in the hemodynamic response to two sets of visual stimuli, as estimated by the NIRS data, are not due to different looking behavior to the two sets.
In the Rochester and Harvard labs, we have integrated the ETG-4000 with the commercially available Tobii system (www.tobii.com), in which the eye-tracking system is embedded into the frame of a video monitor. This system is particularly well suited to infants, as it does not require the infant to wear any headgear, has a large field of view, can be quickly calibrated, and can automatically re-acquire the gaze position if the infant turns away from and back to the display monitor.
The ETG-4000 accepts a predefined set of commands over the serial port. These commands start and stop data acquisition, and can also send timing “marks” that are incorporated into the acquired NIRS data. Therefore, any system that measures behavior and is capable of addressing the serial port on the ETG-4000 system can be used to synchronize the NIRS data with behavior. In our case, we either use Psyscope (www.psy.ck.sissa.it) or SMART-T (Shukla et al., submitted for publication). Psyscope is a general-purpose experimental software that can interact with Tobii over the Ethernet port to trigger the collection of eye gaze data, and communicate over the serial port to trigger the ETG-4000. SMART-T is a system designed primarily for anticipatory eye-tracking paradigms (see McMurray and Aslin, 2004), written in the Matlab environment, using the Psychtoolbox suite, which includes a serial port interface.
6.3. EEG/ERP and optical imaging
All neuroimaging tools have advantages and disadvantages; thus, fMRI has excellent spatial resolution but relatively poor temporal resolution; it is also expensive and cannot easily be used in children younger than 5 years. In contrast, ERPs have excellent temporal resolution, can be used across the entire life span, and are relatively inexpensive. However, ERPs have relatively poor spatial resolution.
To compensate for these pros and cons, some investigators have moved to multi-modal imaging. In some cases this means testing the same participant using the same experimental design but using different imaging modalities and during different sessions; for others, it may mean adopting more than one imaging modality simultaneously (e.g. recording EEG at the same time as performing fMRI). The Harvard lab has explored the possibility of recording ERPs (using high density arrays) while simultaneously recording NIRS. Standard EGI electrode nets have been modified to accommodate optodes from the Hitachi ETG-4000 system. This has the advantage of being able to record electrophysiological data that is spatially coupled to NIRS data. Thus, for example, if our optodes sit over the occipital “face area” of the brain, we can also record ERPs from that same region. Indeed, given the superior spatial resolution of NIRS relative to ERPs, we can target a region-of-interest with NIRS and then record ERPs from that region. If we then do source modeling of our ERP data, we can also constrain the number of dipoles using the NIRS information.
7. Open questions and future directions
Different neuroimaging techniques allow researchers to address different questions. NIRS is particularly well suited for identifying the brain mechanisms involved in cognitive and social processes in pediatric populations not only because infants have thinner skulls that allow for deeper penetration of light, but also because NIRS is quiet, non-invasive and because it allows for detection of meaningful information even with movement. Moreover, unlike EEG which is also relatively easy to use with infants and young children, there is no inverse problem in the interpretation of NIRS signals. As described throughout this paper, enormous progress has been made in adapting NIRS for use with infant populations. NIRS is now being used to address questions in many domains from language processing and visual perception through social understanding, and being implemented in increasingly sophisticated ways. In this final section, we review the challenges that still remain along with the promise for, and new directions in which, the technique can be used in the future.
7.1. Continuing challenges
One of the most significant remaining challenges for NIRS concerns standardization. This challenge is not unique to NIRS – it still exists for fMRI, EEG, and other techniques for which different hardware, software, and data reduction algorithms are used across laboratories. There are, however, also unique challenges to NIRS. The majority of NIRS systems in use for cognitive science research in developmental populations employ continuous wavelengths (CW) of light, and focus on the slow response in optical topography (OT). Differences remain across CW-OT-NIRS systems in the wavelengths of light used and in the separation between light sources and detectors. Following direct empirical testing, consensus is being reached as to the optimal range of wavelengths that is most revealing for simultaneously measuring both deoxyHb and oxyHB, but the most effective wavelengths still seem to differ across NIRS systems, in part as a function of the fiber optic cable design. Differences also remain concerning the separation between sources and detectors used. The research reviewed in this paper suggests that a separation between 2 cm and 3 cm is ideal, but there is still disagreement about whether other separations may be adequate and whether deeper brain structures can be sampled. To add to the complexity, as noted in our review, it appears that the source/detector separation that is optimal for imaging one neural system in infants may be different than that required for imaging another neural system.
There are specific challenges to using a neuroimaging technique with pediatric populations. Most laboratories currently use head landmarks for determining appropriate placement of sources and detectors. The relation between external markers and internal structures varies among individuals, a problem that is exacerbated in a pediatric population in which the head and brain are rapidly maturing. The ongoing studies that include direct measures of structural anatomy along with NIRS will be helpful in guiding the appropriate use of head landmarks for determining probe placement.
Unanswered questions also remain concerning the dynamics of the HRF in developmental populations of different ages. Progress is being made to determine more precisely when the HRF peaks, but individual differences, developmental differences, and differences across brain systems will remain. Similarly, although research has begun, further work is still required to determine precisely what changes in oxyHB vs. deoxyHb indicate about neural functioning, and what it means when one laboratory reports NIRS results using one of the variables while another laboratory (or even another study from the same laboratory) reports another. Studies comparing NIRS to fMRI are helpful in this regard, but given the continuing lack of a full understanding of the BOLD response in fMRI, and the paucity of infant fMRI studies, caution is necessary for making strong claims about the meaning of different components of the NIRS HRF.
Although far easier to use with infants and special populations than fMRI, there is still less precision in localization with NIRS than with fMRI. The use of new metrics, such as the multi-voxel pattern classification technique being tested by the Rochester lab, show considerable promise for improving the spatial resolution of NIRS. Moreover, continuing studies comparing NIRS with fMRI are essential for shedding light on the overlap vs. specificity of the knowledge that can be obtained from each technique.
Different laboratories use different methods of data reduction and data analysis. Some laboratories use software packages that are built into the equipment, whereas others use customized routines programmed into a common language, such as Matlab. In addition to the wide variety of software packages available, the amount of preprocessing has varied in the past across groups and across studies, with different techniques for detrending, for removing motion artifacts etc. It is essential to continue to move toward standardization of these issues and/or a greater empirically based understanding of the consequences of different solutions.
7.2. Looking to the future
To date, NIRS has been used primarily in pediatric populations to record a summary response to estimate the underlying brain systems that are activated in various tasks, rather than for assessing on-line processing or revealing the sequence of neural structures involved. As more laboratories begin to use NIRS in combination with other techniques, such as ERPs and eye-tracking, the field will be able to make more progress in combining measurement of the brain systems involved with tracking the time course of on-line processing. Moreover, several groups are working on developing more reliable event-related NIRS methodologies.
To date, the vast majority of NIRS studies have involved infants or toddlers of only a single age. This is, in part, a consequence of our collective understanding that with a changing brain and a thickening skull, comparisons across ages have an added layer of interpretive complexity. Nonetheless, one of the most exciting aspects of NIRS is its potential to be used across the life span, to reveal whether and how the underlying brain systems involved in any particular task stay constant or change with maturation, experience, and learning. As more laboratories gain the ability to implement NIRS with different aged infants and toddlers, and as the technique becomes more standardized across labs, we can expect more studies that vary the age and experience of the participant.
NIRS also shows considerable promise as a technique to probe not just the static relation between events and the underlying brain systems involved, but also how representations may change across experience and learning. Only a few studies to date (e.g. Gervain et al., 2008) have used NIRS to probe learning, but this is an area with tremendous promise. Are all kinds of information learned equally well? Is there a change in the brain systems utilized when content is being acquired vs. after more expertise is obtained? Does this change with development? NIRS is particularly well suited to address this collection of questions.
NIRS is known to be less susceptible to movement artifacts than are many other imaging tools. The most obvious difficulties from movement ensue when the probes are displaced on the head. To address this issue, the new advances in cap design and probe holder configurations have considerable promise. Nonetheless, interpretive problems still remain for other types of movement. New routines are being developed to remove these movement artifacts (Blasi et al., 2007, Lloyd-Fox et al., 2009). As algorithms continue to be perfected for distinguishing the neural signature of muscle movement from that of cognitive activity and as the design of headgear advances, it may become possible to use NIRS to record neural activity in infants who are undergoing gross motor movement (e.g. locomotion). This is a potential that exists for NIRS more than for any other imaging device.
Many of the NIRS platforms have been developed for estimating group rather than individual differences in hemodynamic changes. But it is possible that with the increased precision of NIRS, there may come a time when it can be applied to clinical populations, for example infants who have experienced cerebral infarcts, to begin to estimate how damaged the brain is and where, and whether there is recovery or redistribution of function following injury. NIRS is much less expensive and much less invasive to administer than are other neuroimaging tools. As such, it is more amenable to repeated use. NIRS has been used by clinical neonatologists and neurologists, but to date they have tended to use only single channel NIRS systems, and used them to address only very basic questions about whether or not there is activity in a particular area of the brain. It would also be very valuable and instructive to determine how compensation occurs across time, as has been done in some fMRI studies with brain damaged infants. Future studies using NIRS in infants with brain injury (in conjunction with some structural imaging task) will be revealing as to whether NIRS can also be used as an adjunct to current standard clinical practice.
One of the most exciting aspects of NIRS to date is that new knowledge has been obtained through its use. A common criticism of neuroimaging tools is that they have often been used in the past to confirm, in the brain, what we have already learned from behavioral tasks. Of course such validation studies are a critical part of methodology development, and many such studies have been done with NIRS in order to ensure that it is a sensitive technique. However, the true promise of neuroimaging tasks is to address questions that cannot be addressed behaviorally, and to probe these questions more deeply to obtain a more mechanistic explanation. NIRS research has been used to address new questions. Ultimately, the value of any instrument lies in the hands of the scientist who is using it. The final challenge and promise for the future entails not only gaining more information about the function of localized and interconnected neural systems, but also becoming increasingly creative in the questions we ask, and increasingly precise in specifying the “linking hypotheses”, in articulating what activation of different brain areas can tell us about the theoretical questions of interest.
Footnotes
Consortium grant #220020096 entitled “Program grant to develop near-infrared spectroscopy in combination with ERPs and fMRI to assess cognitive development in human infants and young children” to Richard N. Aslin as PI and Jacques Mehler as co-PI. The participating laboratories are the following: Language, Cognition and Development Lab (SISSA, Trieste, Italy), the Rochester Baby Lab (University of Rochester, USA), the Laboratories of Cognitive Neuroscience (Children's Hospital/Harvard Medical School, Boston, USA), the Centre for Brain and Cognitive Development (Birkbeck, University of London, UK) and the Infant Studies Centre (University of British Columbia, Vancouver, Canada).
In principle, one could interleave pairs of optodes to increase spatial resolution, but in practice this is not feasible with CW systems because the emitter whose light is intended for a detector 3 cm away would saturate the nearby detector that was paired with another more distant emitter.
Each emitter is frequency-modulated at a slightly different rate so that each detector can identify from which emitter the light originates, thereby eliminating cross-talk between channels.
The same result was obtained when totalHb was used as the dependant variable (F(1,19) = 4.539, p = 0.046).
One advantage of using a probe with several depths is that we can reconstruct a linear 3D tomographic image of the oxyHb and deoxyHb concentration data using a software package known as TOAST (Arridge et al., 2000). This approach has advantages over 2D mapping as it incorporates a full light transport model for tissue and shows a 3D section of the tissue.
References
- Almli C.R., Rivkin M.J., McKinstry R.C., Brain Development Cooperative Group The NIH MRI study of normal brain development (objective-2): newborns, infants, toddlers, and preschoolers. NeuroImage. 2007;35(1):308–325. doi: 10.1016/j.neuroimage.2006.08.058. [DOI] [PubMed] [Google Scholar]
- Amaro E., Jr., Barker G.J. Study design in fMRI: basic principles. Brain and Cognition. 2006;60(3):220–232. doi: 10.1016/j.bandc.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Arridge S.R., Dehghani H., Schweiger M., Okada E. The finite element model for the propagation of light in scattering media: a direct method for domains with nonscattering regions. Medical Physics. 2000;27(1):252–264. doi: 10.1118/1.598868. [DOI] [PubMed] [Google Scholar]
- Aslin R.N. What's in a look? Developmental Science. 2007;10(1):48–53. doi: 10.1111/J.1467-7687.2007.00563.X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aslin R.N., Fiser J. Methodological challenges for understanding cognitive development in infants. Trends in Cognitive Sciences. 2005;9(3):92–98. doi: 10.1016/j.tics.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Aslin R.N., Mehler J. Near-infrared spectroscopy for functional studies of brain activity in human infants: promise, prospects, and challenges. Journal of Biomedical Optics. 2005;10(1):11009. doi: 10.1117/1.1854672. [DOI] [PubMed] [Google Scholar]
- Baird A.A., Kagan J., Gaudette T., Walz K.A., Hershlag N., Boas D.A. Frontal lobe activation during object permanence: data from near-infrared spectroscopy. NeuroImage. 2002;16(4):1120–1125. doi: 10.1006/nimg.2002.1170. [DOI] [PubMed] [Google Scholar]
- Bartocci M., Winberg J., Ruggiero C., Bergqvist L.L., Serra G., Lagercrantz H. Activation of olfactory cortex in newborn infants after odor stimulation: a functional near-infrared spectroscopy study. Pediatric Research. 2000;48(1):18–23. doi: 10.1203/00006450-200007000-00006. [DOI] [PubMed] [Google Scholar]
- Blasi A., Fox S., Everdell N., Volein A., Tucker L., Csibra G., Gibson A.P., Hebden J.C., Johnson M.H., Elwell C.E. Investigation of depth dependent changes in cerebral haemodynamics during face perception in infants. Physics in Medicine and Biology. 2007;52(23):6849–6864. doi: 10.1088/0031-9155/52/23/005. [DOI] [PubMed] [Google Scholar]
- Boas D.A., Dale A.M., Franceschini M.A. Diffuse optical imaging of brain activation: approaches to optimizing image sensitivity, resolution, and accuracy. NeuroImage. 2004;23(Suppl. 1):S275–S288. doi: 10.1016/j.neuroimage.2004.07.011. [DOI] [PubMed] [Google Scholar]
- Boas D.A., Strangman G., Culver J.P., Hoge R.D., Jasdzewski G., Poldrack R.A., Rosen B.R., Mandeville J.B. Can the cerebral metabolic rate of oxygen be estimated with near-infrared spectroscopy? Physics in Medicine and Biology. 2003;48(15):2405–2418. doi: 10.1088/0031-9155/48/15/311. [DOI] [PubMed] [Google Scholar]
- Boden S., Obrig H., Kohncke C., Benav H., Koch S.P., Steinbrink J. The oxygenation response to functional stimulation: is there a physiological meaning to the lag between parameters? NeuroImage. 2007;36(1):100–107. doi: 10.1016/j.neuroimage.2007.01.045. [DOI] [PubMed] [Google Scholar]
- Bortfeld H., Wruck E., Boas D.A. Assessing infants’ cortical response to speech using near-infrared spectroscopy. NeuroImage. 2007;34(1):407–415. doi: 10.1016/j.neuroimage.2006.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortfeld H., Fava E., Boas D.A. Identifying cortical lateralization of speech processing in infants using near-infrared spectroscopy. Developmental Neuropsychology. 2009;34(1):52–65. doi: 10.1080/87565640802564481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxton R.B., Wong E.C., Frank L.R. Dynamics of blood flow and oxygenation changes during brain activation: the balloon model. Magnetic Resonance in Medicine: Official Journal of the Society of Magnetic Resonance in Medicine/Society of Magnetic Resonance in Medicine. 1998;39(6):855–864. doi: 10.1002/mrm.1910390602. [DOI] [PubMed] [Google Scholar]
- Byers-Heinlein K., Burns T.C., Werker J.F. The roots of bilingualism in newborns. Psychological Science. 2010;21(3):343–348. doi: 10.1177/0956797609360758. [DOI] [PubMed] [Google Scholar]
- Chance B., Maris M., Sorge J., Zhang M.Z. A phase modulation system for dual wavelength difference spectroscopy of hemoglobin deoxygenation in tissues. Proceedings of SPIE. 1990;1204:481–491. [Google Scholar]
- Chance B., Villringer A., Dirnagl V., Einhaupl K.M. Optical imaging of brain function and metabolism. Journal of Neurology. 1992;239(7):359–360. doi: 10.1007/BF00812149. [DOI] [PubMed] [Google Scholar]
- Chen S., Sakatani K., Lichty W., Ning P., Zhao S., Zuo H. Auditory-evoked cerebral oxygenation changes in hypoxic–ischemic encephalopathy of newborn infants monitored by near infrared spectroscopy. Early Human Development. 2002;67(1–2):113–121. doi: 10.1016/s0378-3782(02)00004-x. [DOI] [PubMed] [Google Scholar]
- Chen H.C., Vaid J., Bortfeld H., Boas D.A. Optical imaging of phonological processing in two distinct orthographies. Experimental Brain Research. 2008;184(3):427–433. doi: 10.1007/s00221-007-1200-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csibra G., Henty J., Volein A., Elwell C., Tucker L., Meek J., Johnson M.H. Near infrared spectroscopy reveals neural activation during face perception in infants and adults. Journal of Pediatric Neurology. 2004;2:85–89. [Google Scholar]
- Dehaene-Lambertz G., Dehaene S., Hertz-Pannier L. Functional neuroimaging of speech perception in infants. Science. 2002;298(5600):2013–2015. doi: 10.1126/science.1077066. [DOI] [PubMed] [Google Scholar]
- Delpy D.T., Cope M., van der Zee P., Arridge S., Wray S., Wyatt J. Estimation of optical pathlength through tissue from direct time of flight measurement. Physics in Medicine and Biology. 1988;33(12):1433–1442. doi: 10.1088/0031-9155/33/12/008. [DOI] [PubMed] [Google Scholar]
- Duncan A., Meek J.H., Clemence M., Elwell C.E., Fallon P., Tyszczuk L., Cope M., Delpy D.T. Measurement of cranial optical path length as a function of age using phase resolved near infrared spectroscopy. Pediatric Research. 1996;39(5):889–894. doi: 10.1203/00006450-199605000-00025. [DOI] [PubMed] [Google Scholar]
- Everdell N.L., Coulthard M.G., Crosier J., Keir M.J. A machine for haemodialysing very small infants. Pediatric Nephrology. 2005;20(5):636–643. doi: 10.1007/s00467-004-1785-5. [DOI] [PubMed] [Google Scholar]
- Ferrari M., Mottola L., Quaresima V. Principles, techniques, and limitations of near infrared spectroscopy. Revue Canadienne De Physiologie AppliqueeCanadian Journal of Applied Physiology. 2004;29(4):463–487. doi: 10.1139/h04-031. [DOI] [PubMed] [Google Scholar]
- Floccia C., Christophe A., Bertoncini J. High-amplitude sucking and newborns: the quest for underlying mechanisms. Journal of Experimental Child Psychology. 1997:64. doi: 10.1006/jecp.1996.2349. [DOI] [PubMed] [Google Scholar]
- Fox P.T., Raichle M.E. Focal physiological uncoupling of cerebral blood flow and oxidative metabolism during somatosensory stimulation in human subjects. Proceedings of the National Academy of Sciences of the United States of America. 1986;83(4):1140–1144. doi: 10.1073/pnas.83.4.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K.J., Holmes A.P., Price C.J., Buchel C., Worsley K.J. Multisubject fMRI studies and conjunction analyses. NeuroImage. 1999;10(4):385–396. doi: 10.1006/nimg.1999.0484. [DOI] [PubMed] [Google Scholar]
- Gervain, J., Berent, I., Werker, J.F., in preparation. Binding at birth: newborns detect identity relations and sequential position in speech. [DOI] [PMC free article] [PubMed]
- Gervain J., Macagno F., Cogoi S., Peña M., Mehler J. The neonate brain detects speech structure. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(37):14222–14227. doi: 10.1073/pnas.0806530105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton G., Fabiani M. Dynamic brain imaging: event-related optical signal (EROS) measures of the time course and localization of cognitive-related activity. Psychonomic Bulletin and Review. 1998;5(4):535–563. [Google Scholar]
- Gratton G., Fabiani M. The event-related optical signal (EROS) in visual cortex: replicability, consistency, localization, and resolution. Psychophysiology. 2003;40(4):561–571. doi: 10.1111/1469-8986.00058. [DOI] [PubMed] [Google Scholar]
- Gratton G., Corballis P.M., Cho E., Fabiani M., Hood D.C. Shades of gray matter: noninvasive optical images of human brain responses during visual stimulation. Psychophysiology. 1995;32(5):505–509. doi: 10.1111/j.1469-8986.1995.tb02102.x. [DOI] [PubMed] [Google Scholar]
- Greisen G. Is near-infrared spectroscopy living up to its promises? Seminars in Fetal and Neonatal Medicine. 2006;11(6):498–502. doi: 10.1016/j.siny.2006.07.010. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K., Henson R., Martin A. Repetition and the brain: neural models of stimulus-specific effects. Trends in Cognitive Sciences. 2006;10(1):14–23. doi: 10.1016/j.tics.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Grossmann T., Johnson M.H., Lloyd-Fox S., Blasi A., Deligianni F., Elwell C., Csibra G. Early cortical specialization for face-to-face communication in human infants. Proceedings of the Biological Sciences. The Royal Society. 2008;275(1653):2803–2811. doi: 10.1098/rspb.2008.0986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebden J.C., Gibson A., Austin T., Yusof R.M., Everdell N., Delpy D.T., Arridge S.R., Meek J.H., Wyatt J.S. Imaging changes in blood volume and oxygenation in the newborn infant brain using three-dimensional optical tomography. Physics in Medicine and Biology. 2004;49(7):1117–1130. doi: 10.1088/0031-9155/49/7/003. [DOI] [PubMed] [Google Scholar]
- Hebden J.C., Gibson A., Yusof R.M., Everdell N., Hillman E.M., Delpy D.T., Arridge S.R., Austin T., Meek J.H., Wyatt J.S. Three-dimensional optical tomography of the premature infant brain. Physics in Medicine and Biology. 2002;47(23):4155–4166. doi: 10.1088/0031-9155/47/23/303. [DOI] [PubMed] [Google Scholar]
- Hess A., Stiller D., Kaulisch T., Heil P., Scheich H. New insights into the hemodynamic blood oxygenation level-dependent response through combination of functional magnetic resonance imaging and optical recording in gerbil barrel cortex. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2000;20(9):3328–3338. doi: 10.1523/JNEUROSCI.20-09-03328.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill J., Dierker D., Neil J., Inder T., Knutsen A., Harwell J., Coalson T., Van Essen D. A surface-based analysis of hemispheric asymmetries and folding of cerebral cortex in term-born human infants. Journal of Neuroscience. 2010;30(6):2268–2276. doi: 10.1523/JNEUROSCI.4682-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraoka M., Firbank M., Essenpreis M., Cope M., Arridge S.R., van der Zee P., Delpy D.T. A monte carlo investigation of optical pathlength in inhomogeneous tissue and its application to near-infrared spectroscopy. Physics in Medicine and Biology. 1993;38(12):1859–1876. doi: 10.1088/0031-9155/38/12/011. [DOI] [PubMed] [Google Scholar]
- Homae F., Watanabe H., Nakano T., Taga G. Prosodic processing in the developing brain. Neuroscience Research. 2007;59(1):29–39. doi: 10.1016/j.neures.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Homae F., Watanabe H., Nakano T., Asakawa K., Taga G. The right hemisphere of sleeping infant perceives sentential prosody. Neuroscience Research. 2006;54(4):276–280. doi: 10.1016/j.neures.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Hoshi Y., Kobayashi N., Tamura M. Interpretation of near-infrared spectroscopy signals: a study with a newly developed perfused rat brain model. Journal of Applied Physiology (Bethesda, Md., 1985) 2001;90(5):1657–1662. doi: 10.1152/jappl.2001.90.5.1657. [DOI] [PubMed] [Google Scholar]
- Huppert T.J., Diamond S.G., Franceschini M.A., Boas D.A. HomER: a review of time-series analysis methods for near-infrared spectroscopy of the brain. Applied Optics. 2009;48(10):D280–D298. doi: 10.1364/ao.48.00d280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobsis F.F. Noninvasive, infrared monitoring of cerebral and myocardial oxygen sufficiency and circulatory parameters. Science (New York, NY) 1977;198(4323):1264–1267. doi: 10.1126/science.929199. [DOI] [PubMed] [Google Scholar]
- Kimura D. Functional asymmetry of the brain in dichotic listening. Cortex. 1967;3:163–178. [Google Scholar]
- Kleinschmidt A., Obrig H., Requardt M., Merboldt K.D., Dirnagl U., Villringer A., Frahm J. Simultaneous recording of cerebral blood oxygenation changes during human brain activation by magnetic resonance imaging and near-infrared spectroscopy. Journal of Cerebral Blood Flow and Metabolism: Official Journal of the International Society of Cerebral Blood Flow and Metabolism. 1996;16(5):817–826. doi: 10.1097/00004647-199609000-00006. [DOI] [PubMed] [Google Scholar]
- Koh P.H., Glaser D.E., Flandin G., Kiebel S., Butterworth B., Maki A., Delpy D.T., Elwell C.E. Functional optical signal analysis: a software tool for near-infrared spectroscopy data processing incorporating statistical parametric mapping. Journal of Biomedical Optics. 2007;12(6):064010. doi: 10.1117/1.2804092. [DOI] [PubMed] [Google Scholar]
- Koizumi H., Yamamoto T., Maki A., Yamashita Y., Sato H., Kawaguchi H., Ichikawa N. Optical topography: practical problems and new applications. Applied Optics. 2003;42(16):3054–3062. doi: 10.1364/ao.42.003054. [DOI] [PubMed] [Google Scholar]
- Kusaka T., Kawada K., Okubo K., Nagano K., Namba M., Okada H., Imai T., Isobe K., Itoh S. Noninvasive optical imaging in the visual cortex in young infants. Human Brain Mapping. 2004;22(2):122–132. doi: 10.1002/hbm.20020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd-Fox S., Blasi A., Elwell C.E. Illuminating the developing brain: the past, present and future of functional near infrared spectroscopy. Neuroscience and Biobehavioral Reviews. 2010;34(3):269–284. doi: 10.1016/j.neubiorev.2009.07.008. [DOI] [PubMed] [Google Scholar]
- Lloyd-Fox, S., Blasi, A., Elwell, C.E., Johnson, M.H., submitted for publication. Selective cortical mapping of biological motion perception in young infants. [DOI] [PubMed]
- Lloyd-Fox, S., Blasi, A., Mercure, A., Brammer, M., Murphy, D., Johnson, M.H., in preparation. Peek-a-boo, yawns and giggles: studying social perception in young infants with fNIRS and fMRI.
- Lloyd-Fox S., Blasi A., Volein A., Everdell N., Elwell C.E., Johnson M.H. Social perception in infancy: a near infrared spectroscopy study. Child Development. 2009;80(4):986–999. doi: 10.1111/j.1467-8624.2009.01312.x. [DOI] [PubMed] [Google Scholar]
- Logothetis N.K., Pauls J., Augath M., Trinath T., Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001;412(6843):150–157. doi: 10.1038/35084005. [DOI] [PubMed] [Google Scholar]
- Maki, A., Dehaene-Lambertz, G., Peña, M., Fujiwara, M., Mehler, J., Koizumi, H., 2000. Hemodynamic fluctuation in the neonate brain evaluated using optical topography. The Frontier of Mind-Brain Science and its Practical Applications. Tokyo, Japan. 116–117.
- McMurray B., Aslin R.N. Anticipatory eye movements reveal infants’ auditory and visual categories. Infancy. 2004;6(2):203–229. doi: 10.1207/s15327078in0602_4. [DOI] [PubMed] [Google Scholar]
- Meek J. Basic principles of optical imaging and application to the study of infant development. Developmental Science. 2002;5(3):371–380. [Google Scholar]
- Meek J.H., Firbank M., Elwell C.E., Atkinson J., Braddick O., Wyatt J.S. Regional hemodynamic responses to visual stimulation in awake infants. Pediatric Research. 1998;43(6):840–843. doi: 10.1203/00006450-199806000-00019. [DOI] [PubMed] [Google Scholar]
- Minagawa-Kawai Y., Mori K., Furuya I., Hayashi R., Sato Y. Assessing cerebral representations of short and long vowel categories by NIRS. Neuroreport. 2002;13(5):581–584. doi: 10.1097/00001756-200204160-00009. [DOI] [PubMed] [Google Scholar]
- Minagawa-Kawai Y., Mori K., Hebden J.C., Dupoux E. Optical imaging of infants’ neurocognitive development: recent advances and perspectives. Developmental Neurobiology. 2008;68(6):712–728. doi: 10.1002/dneu.20618. [DOI] [PubMed] [Google Scholar]
- Minagawa-Kawai Y., Mori K., Naoi N., Kojima S. Neural attunement processes in infants during the acquisition of a language-specific phonemic contrast. Journal of Neuroscience. 2007;27(2):315–321. doi: 10.1523/JNEUROSCI.1984-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon C., Cooper R.P., Fifer W.P. Two-day-olds prefer their native language. Infant Behavior and Development. 1993;16(4):495–500. [Google Scholar]
- Nakano T., Watanabe H., Homae F., Taga G. Prefrontal cortical involvement in young infants’ analysis of novelty. Cerebral Cortex. 2009;19:455–463. doi: 10.1093/cercor/bhn096. [DOI] [PubMed] [Google Scholar]
- Nakato E., Otsuka Y., Kanazawa S., Yamaguchi M.K., Watanabe S., Kakigi R. When do infants differentiate profile face from frontal face? A near-infrared spectroscopic study. Human Brain Mapping. 2009;30(2):462–472. doi: 10.1002/hbm.20516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazzi T., Bertoncini J., Mehler J. Language discrimination by newborns: toward an understanding of the role of rhythm. Journal of Experimental Psychology: Human Perception and Performance. 1998;24(3):756–766. doi: 10.1037//0096-1523.24.3.756. [DOI] [PubMed] [Google Scholar]
- Norman K.A., Polyn S.M., Detre G.J., Haxby J.V. Beyond mind-reading: multi-voxel pattern analysis of fMRI data. Trends in Cognitive Sciences. 2006;10(9):424–430. doi: 10.1016/j.tics.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Obrig H., Wenzel R., Kohl M., Horst S., Wobst P., Steinbrink J., Thomas F., Villringer A. Near-infrared spectroscopy: does it function in functional activation studies of the adult brain? International Journal of Psychophysiology: Official Journal of the International Organization of Psychophysiology. 2000;35(2–3):125–142. doi: 10.1016/s0167-8760(99)00048-3. [DOI] [PubMed] [Google Scholar]
- Ogawa S., Menon R.S., Tank D.W., Kim S.G., Merkle H., Ellermann J.M., Ugurbil K. Functional brain mapping by blood oxygenation level-dependent contrast magnetic resonance imaging. A comparison of signal characteristics with a biophysical model. Biophysical Journal. 1993;64(3):803–812. doi: 10.1016/S0006-3495(93)81441-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada E., Delpy D.T. Near-infrared light propagation in an adult head model. I. Modeling of low-level scattering in the cerebrospinal fluid layer. Applied Optics. 2003;42(16):2906–2914. doi: 10.1364/ao.42.002906. [DOI] [PubMed] [Google Scholar]
- Okada E., Delpy D.T. Near-infrared light propagation in an adult head model. II. Effect of superficial tissue thickness on the sensitivity of the near-infrared spectroscopy signal. Applied Optics. 2003;42(16):2915–2922. doi: 10.1364/ao.42.002915. [DOI] [PubMed] [Google Scholar]
- Okamoto M., Dan H., Shimizu K., Takeo K., Amita T., Oda I., Konishi I., Sakamoto K., Isobe S., Suzuki T., Kohyama K., Dan I. Multimodal assessment of cortical activation during apple peeling by NIRS and fMRI. NeuroImage. 2004;21(4):1275–1288. doi: 10.1016/j.neuroimage.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Otsuka Y., Nakato E., Kanazawa S., Yamaguchi M.K., Watanabe S., Kakigi R. Neural activation to upright and inverted faces in infants measured by near infrared spectroscopy. NeuroImage. 2007;34(1):399–406. doi: 10.1016/j.neuroimage.2006.08.013. [DOI] [PubMed] [Google Scholar]
- Peña M., Maki A., Kovacic D., Dehaene-Lambertz G., Koizumi H., Bouquet F., Mehler J. Sounds and silence: an optical topography study of language recognition at birth. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(20):11702–11705. doi: 10.1073/pnas.1934290100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plichta M.M., Heinzel S., Ehlis A.C., Pauli P., Fallgatter A.J. Model-based analysis of rapid event-related functional near-infrared spectroscopy (NIRS) data: a parametric validation study. NeuroImage. 2007;35(2):625–634. doi: 10.1016/j.neuroimage.2006.11.028. [DOI] [PubMed] [Google Scholar]
- Plichta M.M., Herrmann M.J., Baehne C.G., Ehlis A.C., Richter M.M., Pauli P., Fallgatter A.J. Event-related functional near-infrared spectroscopy (fNIRS): are the measurements reliable? NeuroImage. 2006;31(1):116–124. doi: 10.1016/j.neuroimage.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Richards J.E. Attention in the brain and early infancy. In: Johnson S., editor. Neoconstructivist Views on Infant Development. Oxford University Press; New York: 2010. pp. 3–37. [Google Scholar]
- Roy C.S., Sherrington C.S. On the regulation of the blood-supply of the brain. The Journal of Physiology. 1890;11(1–2) doi: 10.1113/jphysiol.1890.sp000321. 85–158.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito Y., Aoyama S., Kondo T., Fukumoto R., Konishi N., Nakamura K., Kobayashi M., Toshima T. Frontal cerebral blood flow change associated with infant-directed speech. Archives of Disease in Childhood. Fetal and Neonatal Edition. 2007;92(2):F113–F116. doi: 10.1136/adc.2006.097949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito Y., Kondo T., Aoyama S., Fukumoto R., Konishi N., Nakamura K., Kobayashi M., Toshima T. The function of the frontal lobe in neonates for response to a prosodic voice. Early Human Development. 2007;83(4):225–230. doi: 10.1016/j.earlhumdev.2006.05.017. [DOI] [PubMed] [Google Scholar]
- Sakatani K., Chen S., Lichty W., Zuo H., Wang Y.P. Cerebral blood oxygenation changes induced by auditory stimulation in newborn infants measured by near infrared spectroscopy. Early Human Development. 1999;55(3):229–236. doi: 10.1016/s0378-3782(99)00019-5. [DOI] [PubMed] [Google Scholar]
- Sanchez, C., Richards, J.E., Almli, C.R., in press. Age-specific MRI brain templates for healthy brain development from 4 to 24 years.
- Sato H., Kiguchi M., Kawaguchi F., Maki A. Practicality of wavelength selection to improve signal-to-noise ratio in near-infrared spectroscopy. NeuroImage. 2004;21(4):1554–1562. doi: 10.1016/j.neuroimage.2003.12.017. [DOI] [PubMed] [Google Scholar]
- Sato Y., Sogabe Y., Mazuka R. Development of hemispheric specialization for lexical pitch–accent in Japanese infants. Journal of Cognitive Neuroscience. 2009 doi: 10.1162/jocn.2009.21377. [DOI] [PubMed] [Google Scholar]
- Schroeter M.L., Bucheler M.M., Muller K., Uludag K., Obrig H., Lohmann G., Tittgemeyer M., Villringer A., von Cramon D.Y. Towards a standard analysis for functional near-infrared imaging. NeuroImage. 2004;21(1):283–290. doi: 10.1016/j.neuroimage.2003.09.054. [DOI] [PubMed] [Google Scholar]
- Shi R., Werker J.F., Morgan J.L. Newborn infants’ sensitivity to perceptual cues to lexical and grammatical words. Cognition. 1999;72(2):B11–B21. doi: 10.1016/s0010-0277(99)00047-5. [DOI] [PubMed] [Google Scholar]
- Shibata H., Suzuki M., Gyoba J. Cortical activity during the recognition of cooperative actions. Neuroreport. 2007;18(7):697–701. doi: 10.1097/WNR.0b013e3280d94375. [DOI] [PubMed] [Google Scholar]
- Shimada S., Hiraki K. Infant's brain responses to live and televised action. NeuroImage. 2006;32(2):930–939. doi: 10.1016/j.neuroimage.2006.03.044. [DOI] [PubMed] [Google Scholar]
- Shukla M., Gebhart A.L., Aslin R.N. Optical imaging of multimodal sensory processing in infants. Talk presented at the SRCD Biennial Meeting; 2–4 April, Denver, USA ; 2009. [Google Scholar]
- Shukla, M., Wen, J., White, K.S., Aslin, R.N., submitted for publication. Smart-T: a system for fully automated anticipatory eye-tracking paradigms. [DOI] [PMC free article] [PubMed]
- Steinbrink J., Kempf F.C., Villringer A., Obrig H. The fast optical signal – robust or elusive when non-invasively measured in the human adult? NeuroImage. 2005;26(4):996–1008. doi: 10.1016/j.neuroimage.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Steinbrink J., Villringer A., Kempf F., Haux D., Boden S., Obrig H. Illuminating the BOLD signal: combined fMRI–fNIRS studies. Magnetic Resonance Imaging. 2006;24(4):495–505. doi: 10.1016/j.mri.2005.12.034. [DOI] [PubMed] [Google Scholar]
- Strangman G., Culver J.P., Thompson J.H., Boas D.A. A quantitative comparison of simultaneous BOLD fMRI and NIRS recordings during functional brain activation. NeuroImage. 2002;17(2):719–731. [PubMed] [Google Scholar]
- Taga G., Asakawa K. Selectivity and localization of cortical response to auditory and visual stimulation in awake infants aged 2 to 4 months. NeuroImage. 2007;36(4):1246–1252. doi: 10.1016/j.neuroimage.2007.04.037. [DOI] [PubMed] [Google Scholar]
- Taga G., Asakawa K., Maki A., Konishi Y., Koizumi H. Brain imaging in awake infants by near-infrared optical topography. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(19):10722–10727. doi: 10.1073/pnas.1932552100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanenhaus M.K., Spivey-Knowlton M.J., Eberhard K.M., Sedivy J.E. Integration of visual and linguistic information in spoken language comprehension. Science. 1995;268:1632–1634. doi: 10.1126/science.7777863. [DOI] [PubMed] [Google Scholar]
- Telkemeyer S., Rossi S., Koch S.P., Nierhaus T., Steinbrink J., Poeppel D., Obrig H., Wartenburger I. Sensitivity of newborn auditory cortex to the temporal structure of sounds. The Journal of Neuroscience. 2009;29(47):14726–14733. doi: 10.1523/JNEUROSCI.1246-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toronov V., Webb A., Choi J.H., Wolf M., Michalos A., Gratton E., Hueber D. Investigation of human brain hemodynamics by simultaneous near-infrared spectroscopy and functional magnetic resonance imaging. Medical Physics. 2001;28(4):521–527. doi: 10.1118/1.1354627. [DOI] [PubMed] [Google Scholar]
- Torricelli A., Contini D., Pifferi A., Spinelli L., Cubeddu R. Functional brain imaging by multi-wavelength time-resolved near infrared spectroscopy. Opto-Elecetronics Review. 2008;16(2):131–135. [Google Scholar]
- Tse C.Y., Lee C.L., Sullivan J., Garnsey S.M., Dell G.S., Fabiani M., Gratton G. Imaging cortical dynamics of language processing with the event-related optical signal. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(43):17157–17162. doi: 10.1073/pnas.0707901104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turk-Browne N.B., Scholl B.J., Chun M.M. Babies and brains: habituation in infant cognition and functional neuroimaging. Frontiers in Human Neuroscience. 2008;2:16. doi: 10.3389/neuro.09.016.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uludağ K., Steinbrink J., Villringer A., Obrig H. Separability and cross talk: optimizing dual wavelength combinations for near-infrared spectroscopy of the adult head. Neuroimage. 2004;22(2):583–589. doi: 10.1016/j.neuroimage.2004.02.023. [DOI] [PubMed] [Google Scholar]
- Vignal C., Boumans T., Montcel B., Ramstein S., Verhoye M., Van Audekerke J., Mathevon N., Van der Linden A., Mottin S. Measuring brain hemodynamic changes in a songbird: responses to hypercapnia measured with functional MRI and near-infrared spectroscopy. Physics in Medicine and Biology. 2008;53(10):2457–2470. doi: 10.1088/0031-9155/53/10/001. [DOI] [PubMed] [Google Scholar]
- Villringer A., Chance B. Non-invasive optical spectroscopy and imaging of human brain function. Trends in Neurosciences. 1997;20(10):435–442. doi: 10.1016/s0166-2236(97)01132-6. [DOI] [PubMed] [Google Scholar]
- Wartenburger I., Steinbrink J., Telkemeyer S., Friedrich M., Friederici A.D., Obrig H. The processing of prosody: evidence of interhemispheric specialization at the age of four. NeuroImage. 2007;34(1):416–425. doi: 10.1016/j.neuroimage.2006.09.009. [DOI] [PubMed] [Google Scholar]
- White B.R., Culver J.P. Phase-encoded retinotopy as an evaluation of diffuse optical neuroimaging. NeuroImage. 2010;49(1):568–577. doi: 10.1016/j.neuroimage.2009.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox T., Bortfeld H., Woods R., Wruck E., Boas D.A. Using near-infrared spectroscopy to assess neural activation during object processing in infants. Journal of Biomedical Optics. 2005;10(1):11010. doi: 10.1117/1.1852551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox T., Bortfeld H., Woods R., Wruck E., Boas D.A. Hemodynamic response to featural changes in the occipital and inferior temporal cortex in infants: a preliminary methodological exploration. Developmental Science. 2008;11(3):361–370. doi: 10.1111/j.1467-7687.2008.00681.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox T., Bortfeld H., Woods R., Wruck E., Armstrong J., Boas D. Hemodynamic changes in the infant cortex during the processing of featural and spatiotemporal information. Neuropsychologia. 2009;47(3):657–662. doi: 10.1016/j.neuropsychologia.2008.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf M., Ferrari M., Quaresima V. Progress of near-infrared spectroscopy and topography for brain and muscle clinical applications. Journal of Biomedical Optics. 2007;12(6):062104. doi: 10.1117/1.2804899. [DOI] [PubMed] [Google Scholar]
- Worsley K.J., Poline J.B., Friston K.J., Evans A.C. Characterizing the response of PET and fMRI data using multivariate linear models. NeuroImage. 1997;6(4):305–319. doi: 10.1006/nimg.1997.0294. [DOI] [PubMed] [Google Scholar]
- Worsley K.J., Poline J.B., Vandal A.C., Friston K.J. Tests for distributed, nonfocal brain activations. NeuroImage. 1995;2(3):183–194. doi: 10.1006/nimg.1995.1024. [DOI] [PubMed] [Google Scholar]
- Wyatt J.S., Cope M., Delpy D.T., van der Zee P., Arridge S., Edwards A.D., Reynolds E.O. Measurement of optical path length for cerebral near-infrared spectroscopy in newborn infants. Developmental Neuroscience. 1990;12(2):140–144. doi: 10.1159/000111843. [DOI] [PubMed] [Google Scholar]
- Yamashita O., Sato M.A., Yoshioka T., Tong F., Kamitani Y. Sparse estimation automatically selects voxels relevant for the decoding of fMRI activity patterns. NeuroImage. 2008;42(4):1414–1429. doi: 10.1016/j.neuroimage.2008.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye J.C., Tak S., Jang K.E., Jung J., Jang J. NIRS-SPM: statistical parametric mapping for near-infrared spectroscopy. NeuroImage. 2009;44(2):428–447. doi: 10.1016/j.neuroimage.2008.08.036. [DOI] [PubMed] [Google Scholar]
- Zarahn E., Aguirre G.K., D’Esposito M. Empirical analyses of BOLD fMRI statistics. I. Spatially unsmoothed data collected under null-hypothesis conditions. NeuroImage. 1997;5(3):179–197. doi: 10.1006/nimg.1997.0263. [DOI] [PubMed] [Google Scholar]