Research highlights
▸ Developmental changes in the cortex affect networks of regions. ▸ The emerging networks are shaped by functional and structural brain factors. ▸ Moreover, improving behavioural proficiencies play an important role. ▸ The specific influence and mechanisms of these factors remain to be determined. ▸ Theoretical frameworks can provide testable predictions for future studies.
Keywords: Developmental neuroimaging, Face processing, Neuroconstructivism, Neural re-use theory
Abstract
This opinion paper suggests that developmental neuroimaging studies investigating emerging cortical networks for specific cognitive functions can contribute substantially to our understanding of mature brain organisation. Based on a review of the literature on the neural correlates of face processing abilities, this paper shows how developmental neuroimaging can help resolve outstanding issues, such as whether specific brain regions actually start out by responding to specific stimulus classes, and how this response changes with development. It has been suggested for example, that improving specialisation in a particular brain regions may be the result of increasing connectivity with other network regions supporting the same cognitive function. Developmental neuroimaging studies are particularly well suited to disentangle the interplay between changes at different network levels, such as improving behavioural proficiencies and functional and structural brain development, as well as overall network configuration changes. However, much of the future progress will depend on whether developmental changes are assessed by combining multiple network observations. This paper makes specific suggestions as to how such a multifaceted approach may look like by exploring the suitability of different theoretical frameworks, such as the neural re-use theory or the neuroconstructivist approach for providing guiding principles for future research.
1. Introduction
Until recently, scientists interested in describing the neural bases subserving cognitive functions in the brain had but few choices: they could study mature brain activation in adults or rely on animal models which would allow for a more invasive investigation of the underlying neural substrates (Haug and Whalen, 1999, Johnson et al., 2005). A third approach was to gather insights from atypical brain responses to temporary or permanent brain lesions, such as for example non-invasive transcranial magnetic stimulation (TMS) (Wassermann et al., 2008), or neuropsychological studies with patients (Avidan et al., 2005, Price and Friston, 2002). The three methodological approaches represent valid and important venues, which have done much to advance our understanding of the neural bases for specific cognitive functions. However, with the recent advent of paediatric neuroimaging methods (functional) magnetic resonance imaging (f/MRI), new vistas have been opened to study the implementation of cognitive functions in the developing brain (Blakemore et al., 2004). Indeed, despite the additional difficulties that paediatric neuroimaging poses, such as lower participant compliance, high dropout rates due to movement or lower performance accuracies, the number of publications of developmental neuroimaging studies is ever increasing (Blakemore, 2011). While this recent exponential increase in neuroimaging studies with children is very encouraging, much of the progress that can be made will depend on the theoretical framework that guides our research.
The neural basis of face processing abilities is just one example of a cognitive function that has benefited from this multi-method approach. We are now able to systematically pinpoint the trajectories of emerging face processing abilities at the behavioural and neural level (Cohen Kadosh and Johnson, 2007). As faces are central to social interactions and there is evidence that humans process them extensively and preferentially from birth (Farroni et al., 2005, Johnson et al., 1991), they represent an ideal stimulus category to investigate the interplay of improving cognitive processing abilities and underlying functional and structural development (Cohen Kadosh and Johnson, 2007). With regard to face processing in the brain, a multitude of brain imaging studies has uncovered a cortical core network that responds reliable to different face properties such as facial identity, expression or eye gaze (Allison et al., 1994, Haxby et al., 2000). Moreover, the neural responses in these network regions have been shown to be modulated flexibly by different cognitive processing strategies (Cohen Kadosh et al., 2010, Ganel et al., 2005). Neuropsychological and virtual lesion approaches have yielded somewhat contradictory findings by showing that disruptions to the core network regions can in some cases lead to significantly impaired face processing abilities (Barton et al., 2002, Schiltz et al., 2006, Steeves et al., 2006, Steeves et al., 2009), while other studies found evidence for more selective and face-property-specific impairments (Avidan et al., 2005, Cohen Kadosh et al., 2011, Pourtois et al., 2004). In particular, the report of impaired processing abilities along with inconspicuous brain activation in individuals with developmental prosopagnosia (DP, adults with DP exhibit severe difficulties recognising faces in the absence of specific brain injuries) has been puzzling (Avidan et al., 2005, Hasson et al., 2003, see also the next section). It remains to be determined therefore, how different brain regions come to be specialised for cognitive functions. More important however, and this may help resolve any outstanding issues from the (virtual) lesion studies: further work is needed to help uncover how the different brain regions interact to allow the proficient processing of different face properties. Note that the current paper uses the term network to refer to multiple brain regions that are simultaneously active, subserve the same cognitive function and/or exhibit patterns of functional/effective connectivity.
Research on the developmental trajectories for cognitive functions holds an immense potential for addressing these questions. With regard to face processing abilities for example, it has been shown that both typically developing children until the age of 14, as well as adult participants with DP will use less efficient featural processing strategies for extracting facial information (Cohen Kadosh et al., submitted for publication, DeGutis et al., 2007, Mondloch et al., 2002). In the following, I will consider several aspects that are essential for designing developmental neuroimaging studies. My approach will be based on examples of recent progress made in pinpointing emerging cortical networks supporting face-processing abilities, but it also hopes to guide future research in other social cognitive domains, such as reasoning, perspective taking, theory of mind, as well as more general cognitive abilities, such as attention, cognitive control, numerical cognition, and language.
2. Developmental changes affect networks of regions
Much research on developmental differences has focused on comparing activation profiles in specific brain regions across different age groups. This assumption is reminiscent of the longstanding neuropsychological lesion approach, which proposes that cognitive functions can be mapped onto different brain areas in a mosaic-like fashion. There are several problems with this approach. In recent years, considerable evidence has accumulated that this static one-to-one mapping of brain structure to function is somewhat simplistic, as it does not take into account the dynamics of interacting brain areas and influences of cognitive top-down control strategies (Bressler and Menon, 2010, Friston and Price, 2001, Ishai, 2008, Norman et al., 2006, but see Kanwisher, 2010). While fMRI can be very useful for pinpointing the different brain areas that support a particular cognitive function, its relatively poor temporal resolution poses a significant challenge, as it can mask repeated activations of the same brain region (e.g. as a result of feedback from other brain regions) at different time points. Using complementary neuroimaging techniques with a high-temporal resolution (such as TMS or event-related potentials) can therefore be useful to further differentiate the response characteristics in a specific brain area. A second challenge for the neuropsychological lesion approach arises from the currently available neuroimaging findings of developmental disorders. So far, and for a range of disorders, such as autism, Williams syndrome, attention deficit disorder, there is only weak and inconsistent evidence for functionally localised deficits (Filipek, 1999, Karmiloff-Smith, 1998, Knudsen, 2004). Other, more selective disorders may be the result of a more localised deficit that affects several network regions, as it appears to be case for example in individuals with DP. Behrmann et al. (2007) used structural imaging to show that participants with developmental prosopagnosia had significantly larger anterior and posterior middle temporal gyri and a smaller anterior fusiform gyrus. Moreover, a recent diffusion tensor imaging-based tractography study by Thomas et al. (2009) uncovered a marked reduction in structural integrity in the inferior longitudinal fasciculus and the inferior fronto-occipito fasciculus, the two major tracts that connect the core fusiform region to the anterior temporal and frontal cortices. Last, a training study by DeGutis et al. (2007), reported increased functional connectivity in the core face network in a participant with DP as a function of improving face processing abilities. While the studies reviewed above have yielded important findings that point us towards specific brain regions and white matter tracts that exhibit atypical structural morphologies in individuals with DP, we are still far from understanding how exactly these structural variations lead to DP. Clearly, a more integrative approach, which takes into account the interaction between multiple brain regions, is needed to resolve this issue.
In recent years, groundbreaking research-guiding theories for understanding the implementation of cognition in the human brain have been put forward that take these considerations into account. Specifically, based on the theoretical frameworks of neural re-use or neuroconstructivism, several, not necessarily incompatible, models have been derived (Anderson, 2010, Sirois et al., 2008).
Neural re-use models, such as the massive redeployment hypothesis (Anderson, 2007a, Anderson, 2007b) or the neural recycling hypothesis (Dehaene and Cohen, 2007) suggest that the acquisition of new cognitive abilities will result in the re-use or the recycling of previously established neural circuits. The neural re-use will combine existing components for new tasks, thus resulting in one brain region supporting a range of cognitive functions. The neural recycling hypothesis also proposes that cultural acquisitions, such as calculations or reading and writing must find their “neuronal niche” and will therefore re-use brain areas are functionally close and still sufficiently plastic to allow for such an invasion (Dehaene and Cohen, 2007). Numerical abilities are one example for such a relatively late acquired, culture-dependent skill. There is strong evidence that numerical abilities are supported by brain regions in the parietal lobes (for recent meta-analyses see Arsalidou and Taylor, 2011, Cohen Kadosh et al., 2008b, Houde et al., 2010). One particular region in the number network, the intraparietal sulcus, has been commonly reported for general magnitude processing tasks, such as size, luminance, space, and time judgements (Bueti and Walsh, 2009, Cantlon et al., 2009, Cappeletti et al., 2009, Cohen Kadosh et al., 2007, Cohen Kadosh et al., 2008b). The neural re-use theory predicts that the later a specific cognitive function develops, the more distributed the underlying supporting neural network will be (Anderson, 2010). A more distributed network for a novel, and consequently more complex function would be due to prior neural constraints of the recombined brain areas. In the case of numerical cognition, the underlying brain network encompasses brain regions in the parietal lobe, but also a region in the left and right Fusiform gyri for digit symbol recognition (Cohen and Dehaene, 2000, Pesenti et al., 2000), and the prefrontal cortex (Ansari, 2008). The wide-spread recruitment prediction has received particularly strong support from numerical cognition studies in both non-human species and in young children, both of which exhibit basic and less proficient numerical abilities (Houde et al., 2010, Nieder et al., 2002). In fact, one might speculate that these early activation patterns may very well reflect previous phylogenetic stages, i.e. pre-recycling stages.
In its present form, it is less clear whether neural re-use theories will need to remain restricted to describing mechanisms of phylogenesis, or whether they can also serve as a framework for the investigation of ontogenetic brain development. For example, the suggestion of more distributed neural bases subserving later acquired cognitive functions could be extended to accommodate the diffuse to local hypothesis that has been put forward to describe the results of developmental neuroimaging studies (Brown et al., 2006, Church et al., 2010, Durston et al., 2006, see also Ramsey et al., 2010) The diffuse to local hypothesis refers to the finding that younger children will exhibit more widespread and less focal brain activation for a specific cognitive function, an activation pattern that becomes increasingly localised with development (Cohen Kadosh and Johnson, 2007, Durston et al., 2006).
Neuroconstructivist models (Mareschal et al., 2007a, Mareschal et al., 2007b) have been developed to derive testable hypotheses for emerging cognitive functions in the brain. They combine important aspects of two earlier theoretical approaches to the investigation of functionally direct growth in the brain: constructivism and selection. Constructivism (Quartz, 1999) on the one hand proposes that brain regions that are activated simultaneously will come to build up connections between them, a suggestion based on the Hebbian principle. Selectivism (Changeux and Danchin, 1976) on the other hand stresses the opposite, but not necessarily incompatible principle; namely, it suggests that functionally directed brain development is based on the gradual elimination of redundant neural connections between different brain regions.
Neuroconstructivist models accommodate both principles. The Interactive Specialisation approach (IS) (Johnson, 2001, Johnson, 2011, Johnson et al., 2009), for example proposes that postnatal functional brain development involves a reorganisation process, which establishes systematic connections between cortical areas (Johnson, 2001, Johnson, 2005, Johnson, 2011, Johnson et al., 2009). Most importantly, as a result of the improving connectivity pattern, neural responses will become increasingly localised within the final core regions as well as specialised for specific stimulus categories. The functional specialisation of a particular brain area therefore does not depend only on its position on a pre-determined map, but rather on its connectivity patterns with other brain regions, which shape the specialisation process. Note that this approach does not suggest that virtually any brain area can take on any cognitive function. Rather, it proposes that cortical specialisation is guided by so-called “architectural constraints” (Elman et al., 1996) and it has been suggested that these constraints are expressed in slight differences in the patterns of intrinsic connectivity, the balance of neurotransmitters or the synaptic density (Johnson et al., 2002), as well as differences in gene expression (Lenroot and Giedd, 2008, Shaw et al., 2009).
Based on the theoretical frameworks reviewed above, it seems therefore that substantial progress will only be made if future work can move away from looking at specific brain regions in isolation and instead begin to adopt a larger brain network perspective within a leading theoretical framework, such as those offered by the neuroconstructivist or the neural re-use hypotheses. Within a network approach, activation in a specific region is not only determined by cognitive processing proficiency levels and the underlying structural morphology, but also the excitatory and/or inhibitory input of other brain regions. As will be described in greater detail below, recent studies on the effective connectivity patterns in the face network have shown that cognitive task demands influence neural responses in the core network regions but also exert modulatory influence on the network connections (Cohen Kadosh et al., in press, Fairhall and Ishai, 2007, Rotshtein et al., 2007). A new approach that focuses on connectivity patterns between brain regions, rather than single brain regions may also prove fruitful for work on atypically developing brain. For example, it may very well be that atypical functioning is the result of a lack of or abnormal cortical connectivity network pattern for a specific function.
3. Developmental trajectories affect functional and structural network aspects
Several recent studies have investigated the developmental trajectories of specific network aspects, such as structural and functional network changes (Lu et al., 2009, Olesen et al., 2003, Shaw et al., 2006, Sowell et al., 2004). However, very little is known about the integrative nature or otherwise of these trajectories.
At the structural network level, several MRI studies have uncovered ongoing structural brain development throughout childhood and adolescence (Giedd et al., 1999, Gogtay et al., 2004, Sowell et al., 2004). A study by Shaw et al. (2008) has shown that overall brain volume growth follows different linear and non-linear trajectories (with most of the cortex exhibiting cubic growth changes), depending on the specific cortical lobe and the phylogenetic age of the particular brain structure. In the occipital and temporal lobes for example, which contain the core face network regions, brain volume change follows a cubic trajectory (Shaw et al., 2008). It has been shown that cortical grey matter decreases with age, and that this decrease varies significantly, depending on the specific brain region (Giedd et al., 1999, Gogtay et al., 2004). For example, a longitudinal study that tested grey matter density development in 4–21-year-old participants found that the temporal lobe exhibits protracted development until approximately 16 years of age, while grey matter in the occipital lobe continues to develop beyond 21 years (Giedd et al., 1999). These findings are of central relevance for this review of emerging cortical face networks, as the areas of the core face-processing network are localised in these lobes.
Cortical white matter on the other hand has been shown to increase continually and linearly with age throughout the brain (Giedd et al., 1999, Paus et al., 1999). Insights in underlying structural development can contribute important clues as to whether developmental differences in brain activation in a specific brain region are due to general differences in brain maturity, or rather the result of differential connectivity differences with other network regions supporting the specific cognitive function.
At the functional network level, most research has focused on changes in functional connectivity between brain regions across age in the default resting state network (i.e., in the absence of a cognitive task) (Uddin et al., 2010), or on comparing neural response profiles in specific brain regions of interest across different age groups in a given cognitive task (Cohen Kadosh and Johnson, 2007). The main difference between these two approaches is that only the latter one allows us to draw conclusions about the possible influence of concurrently changing cognitive abilities. In a functional connectivity study, Fair et al. (2008) used resting-state functional connectivity MRI analysis to probe the default resting state network in 7–9-year-old children in comparison to adults. This analysis method can determine whether specific brain regions are functionally connected via cross-correlations of the BOLD signal time series between the regions, while the subject is passively lying in the scanner, doing no specific task. They found a slow increase in correlation strength over age between the regions of the default network. That is, while children activated similar regions in comparison to the adults, these regions were only sparsely connected. A further difference was that the increasing connectivity patterns concerned mostly intrahemispheric connections as opposed to interhemispheric connections, which were present at a comparable level in both age groups. Another study by Superkar et al. supported this finding by showing that the emerging large-scale networks are the result of a systematic weakening of short-range functional connectivity, along with an increase in long-range functional connectivity (Supekar et al., 2009). In a different study, Fair et al. (2007) assessed the network for task control, using 39 putative regions frequently reported in previous studies. When comparing the results for children aged 7–15 years and young adults, they found that while long-range connections increased significantly, short-range connections decreased. These observed changes were attributed at the neurobiological level to changes in myelination (for the long-range connections, Giedd et al., 1999) and synaptic pruning (for the short-range connections, Chugani et al., 1996, Huttenlocher et al., 1982). It is important to note that all developmental differences were assessed with regard to the brain regions commonly reported for adult participants. Therefore, while these studies are important and they can provide some support for the changes in localisation as predicted by the IS approach, future studies should ascertain that all these areas are actually task-relevant for the different developmental groups as well. That is, without making sure that all age groups use the same/or a similarly structured network, any changes in functional connectivity become difficult to assess and only a dual approach can provide the complete picture of changes in task-specific activation patterns over development.
With regard to developmental changes for specific cognitive functions, a recent review of the developmental neuroimaging literature on the emerging neural bases of face processing abilities from age 5 through 17 years showed that cortical regions within the core network show reliable activation to faces from at least mid-childhood (Cohen Kadosh and Johnson, 2007). For example, Scherf et al. (2007) used naturalistic movies of faces, objects, buildings and navigation scenes in a passive viewing task with children (5–8 years), adolescents (11–14 years) and adults. They found that the children exhibited similar activation distribution patterns in the face-processing areas commonly reported in adults (such as the Fusiform face area (FFA) (Kanwisher et al., 1997). However, in children this activation was not selective for the category of face stimuli; the regions were equally strongly activated by objects and landscapes. Moreover, this lack of fine-tuning of core face-processing areas stood in contrast to distinct preferential activation patterns for other object categories (lateral object area (LOC) and the parahippocampal place area (PPA)). In a similar study, Golarai et al. (2007) tested children (7–11 years), adolescents (12–16 years) and adults with static object categories (faces, objects, places and scrambled abstract patterns). They found that right FFA activation volume increased substantially with age and that this increase also correlated significantly with an improved recognition memory for faces. They speculate that during the development, this area expands into the surrounding cortex, a finding that differed for other brain areas, such as the LOC and the STS whose volumes and response levels remained constant through ages and also did not correlate with object-recognition memory. Other studies have reported the task-dependent activation of additional areas that are not typically found in the mature face network, such as the left and right inferior frontal gyrus (Gathers et al., 2004, Passarotti et al., 2003). Recent evidence from two developmental fMRI contrast sharply with the studies reviewed above (Cantlon et al., 2011, Pelphrey et al., 2009). For example, Cantlon et al. (2011) observed a robust FFA response in 4-year-old children for faces in comparison to other categories, such as shoes, letters, numbers or scrambled images. Moreover, they observed a significant decrease for the non-preferred stimulus categories with age. A different study by Pelphrey et al. (2009) compared neural responses to faces, flowers, objects and bodies in the ventral-temporal stream. When contrasting face-specific responses with those to flower stimuli, no developmental changes in face-selectivity were found in the FFA from mid-childhood. It is less clear however, whether similar results would have been obtained in this study upon contrasting face responses with neural responses to the other stimulus categories (i.e., objects, bodies), a question that is particularly relevant as both categories are preferentially processed in adjacent cortical areas in the mature brain. From the review of studies presented above, it becomes clear that the empirical issue of continuous face-specialisation in the core face network regions is far from being settled and will need to be addressed in future studies. One way to solve this would be to conduct a longitudinal developmental fMRI study, which compares an active task (that encourages in-depth stimulus processing) with a passive version of the same task. Such a study could also shed light on the differing developmental trajectories for different social stimuli, such as faces and bodies.
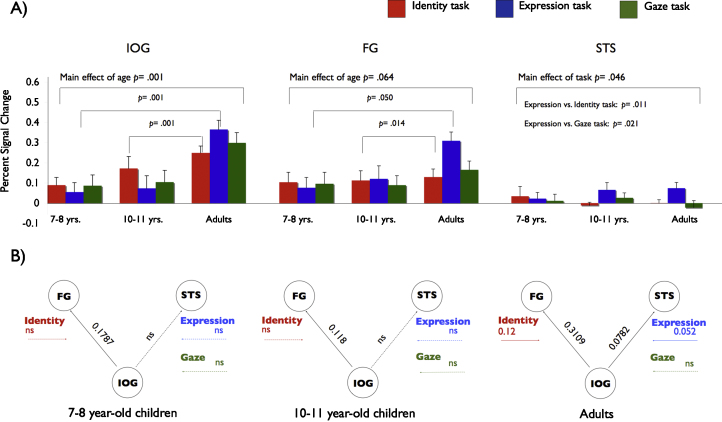
Finally, a recent study implemented dynamic causal modelling analysis (Friston et al., 2003) to examine the influence of task-dependent causal interactions between core face network regions during a target detection task. Dynamic causal modelling (DCM) approaches can be used to assess not only the functional connectivity patterns between different brain regions, but also to determine how experimental input influences these connectivity patterns (Friston et al., 2003). This allows one to test the influence of top-down modulation via different cognitive processes. The main strength of this analysis approach is, that is enables the investigation of age group differences and task demands on effective connectivity between regions, in younger children (7–8 years), older children (10–11 years) and adults (Cohen Kadosh et al., in press, Cohen Kadosh et al., 2011) (Fig. 1). The same basic cortical network, comprising the FFA, STS and inferior occipital gyrus (‘occipital face area’, OFA) was present in all age groups. However, there was an age-related increase in extent of differential top-down modulation of specific network connections depending on the task. These findings were explained by the cumulative effect of exposure and training, such that the cortical network for face-processing becomes increasingly fine-tuned with age. Hence, these functional neuroimaging studies support the notion that developmental changes in the neural network supporting face processing abilities are not restricted to single brain regions but rather affect a network of multiple regions simultaneously that together come to form an efficient, functioning network with time.
Fig. 1.
Developmental changes in cortical response patterns in the core face network in children aged 7–11 years and adults. While the overall network configuration was confirmed for all three age groups, a continuous increase in functional response was observed in the two child groups, along with changing patterns of effective connectivity in the network. Most notably, the two child groups did not exhibit any task-dependent changes in network connectivity, a findings which has been attributed to lower levels of processing proficiency. (A) Percent signal change in brain activation in three core face network regions as a function of age and task (Identity task = red; Expression task = blue, Gaze task = green). (B) Dynamic causal modelling analysis of the developmental changes in the core face network in three age groups. Solid arrows indicate significant changes in effective connectivity between two network regions, dotted arrows indicate nonsignificant effects. Black arrows indicate the intrinsic connection between the areas of interest. Colored arrows indicate modulatory effects of each task on the connection between the areas. Abbreviations: IOG = inferior occipital gyrus; FG = fusiform gyrus; STS = superior temporal sulcus. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
Adapted with permission from Cohen Kadosh et al. (2010).
While the studies reported above have contributed significantly to our understanding of specific developmental trajectories, much of our future progress will depend on work that combines data at multiple levels of network observation. For example, future studies could investigate the respective influence of improving processing proficiencies on functional and structural brain development and vice versa. Recently, a number of developmental neuroimaging studies have been published that combine multiple levels of network observation. For example, Sowell et al. (2004) conducted a longitudinal mapping study looking at the relationship of changing cortical thickness and changing cognitive abilities in children aged 5–11 years. They found that grey matter thinning in the left hemisphere correlated significantly with improving vocabulary, with the exception of the specialised language areas (such as Broca's and Wenicke's area), where the relationship was reversed.
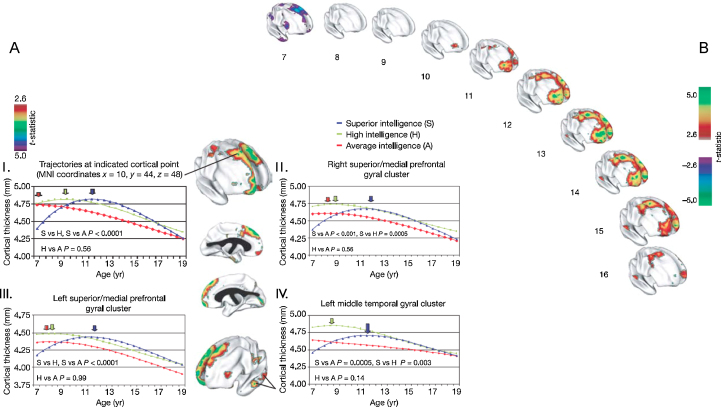
Shaw et al. (2006) conducted a study that probed the relationship between changes in intellectual abilities and cortical thickness in children and adolescents (aged 5–11 years). They established a complex and dynamic relationship between intelligence scores and cortical thickness. That is, children with superior intelligence scores exhibited a relatively thinner cortex in the frontal and temporal brain regions, a relationship, which was significantly reversed during adolescence (Fig. 2). This finding led the authors to suggest that higher intelligence may be related to more dynamic changes in brain morphology.
Fig. 2.
Developing differences in cortical thickness between superior, high and average intelligence level groups in children and adolescents aged 6–16 years. The brain maps illustrate the finding that the superior intelligence group exhibited the most dynamic pattern of changes in cortical thickness: an initially thinner cortex showed a rapid increase in thickness, followed by continuous thinning during adolescence. (A) Centre panel: brain maps showing prominent clusters where children and adolescents with superior and average intelligence differed significantly in the trajectories of cortical development (t-statistic maps show significant interactions between IQ score and the cubic age term). (I) Trajectories of cortical development in the right superior frontal gyrus (see brain map). (II–IV) Developmental changes in cortical thickness in the right and left superior/medial prefrontal gyrus (II, III), and the left middle temporal gyrus (IV). (B) Trajectories of changing cortical thickness between the superior and average intelligence groups (t-statistics, t = 2.6). Abbreviations: MNI, Montreal Neurological Institute.
Adapted with permission from Shaw et al. (2006).
In a different study, Lu and colleagues used cortical pattern matching techniques to investigate changes in functional brain activation with co-occurring changes in grey matter in 6–15-year-old children (Lu et al., 2009). Cortical pattern matching techniques proceed based on the premise that mature cortical thickness patterns correspond to specialised functional activation in this regions, which in turn, will be an indicator of processing proficiency for a specific cognitive function. Using an orthographic matching task, they found that strong neural responses in the fronto-parietal brain network were predictive of advanced structural morphology in fast-responding children. Similarly, Olesen et al. found that in 7–18-year-old children and adolescents, grey and white matter volume correlations with functional brain responses were modulated by age in a working memory task (Olesen et al., 2003). The latter approach of investigating developmental changes across different network levels is particularly promising, as it allows us to disentangle the respective influence of functional, structural and cognitive development on changing cortical response patterns.
4. What can multiple observations of network changes contribute to our understanding of mature brain organisation?
The developmental neuroimaging studies reviewed above reveal some of the potential of multilevel f/MRI analysis for investigating mechanisms of cortical specialisation for cognitive functions. However, much of the future progress will depend on choosing a suitable theoretical framework to integrate the findings from different network levels. The different theoretical perspectives offered by the neuroconstructivist or, to a lesser extent also the neural re-use approach to human brain development may be particularly suitable as it can accommodate dynamic changes in recruitment of brain regions across the developmental trajectory. The network approach does also allows for specific hypotheses of dynamic patterns of network recruitment to be tested. For example, specific network paths could be selectively strengthened or inhibited, depending on the different task conditions (Fairhall and Ishai, 2007). This has also been shown in a recent DCM study (Cohen Kadosh et al., in press, Cohen Kadosh et al., 2011), which found that specific network pathways were selectively strengthened, depending on the task-relevant facial feature. Variable face network responses as a function of top-down task-influences were also shown in a previous fMRI-adaptation study that showed that the same network of regions responds flexibly to different facial features (Cohen Kadosh et al., 2010). The task-dependent network changes offer some insights into the flexibility of mature brain organisation. Namely, they highlight the important influence that cognitive processing strategies exert on neural activation patterns. That is, they seem suggest that human brain organisation may depend less on the specific information that it processes and on establishing stimulus-specific brain regions (e.g., the FFA, Kanwisher et al., 1997) and support the notion instead that the brain may be organised according to the processing mechanisms that deal with the specific input. Developmental neuroimaging studies can therefore provide a context within which to approach ongoing debates in the adult face processing literature, such as the debate on the face-specificity or otherwise of the FFA (Gauthier et al., 1999). For example, they can reveal that the FFA is initially responsive to different object categories, but also faces (Cohen Kadosh and Johnson, 2007, Tzourio-Mazoyer et al., 2002) and that with improving processing proficiencies and brain maturation these regions become more specialised. In fact, the recent study by Cantlon et al. (2011) supports this notion, by showing a significant decrease in neural response to non-preferred stimulus categories in the face network with age. In addition, it may be that while these categories activate the same brain region, they interact differently with other brain areas, which would support the idea of differing levels of specialisation in this region, again, something which could not be detected by focusing on a single brain area (Cohen Kadosh et al., 2008a). Last, it could very well be that these differential interactions with other brain areas are the reason for the observed temporal discrepancies in specialisation for different social stimuli in the ventral-temporal stream (e.g., Peelen et al., 2010).
In addition to task-induced changes, overall network configurations also change with age. For example, while a recent study has shown that young children recruit the same core face regions as older children and adults (Cohen Kadosh et al., in press, Cohen Kadosh et al., 2011), there is evidence that face processing in young children relies on additional brain regions that are not included in the final core face network (Johnson et al., 2009). This may be the result of changing connectivity patterns in the emerging face networks, with some of the initial brain regions that support less proficient processing strategies being excluded from the final network configuration.
These changes in network configurations are particularly relevant for work on neuropsychological patients that experience impairments following brain insults to specific regions. That is, they can help to separate necessary from merely supportive brain regions that subserve specific cognitive functions. More importantly however, they could be used to pinpoint trajectories of atypically developing brain functions and to establish early markers of failures in network specialisation. This would be especially interesting in the case of DP. There is some evidence for changes in structural morphology in these participants (Behrmann et al., 2007, Garrido et al., 2009, Thomas et al., 2009). However, it remains to be determined how these differences in grey and white matter affect brain anatomy and function. Recent work has shown that face processing in adult participants with DP does not so much differ within the core face network regions in the brain (Avidan et al., 2005), but that their brain network comprises some of the network regions recruited in young children (Johnson et al., 2009). Further research is needed that systematically investigates changing network constellations in typically and atypically developing children and adults. One possible way to mimic these changes would be to use transcranial magnetic stimulation methods, which can selectively impair the functioning of a network area in an otherwise healthy brain (Cohen Kadosh et al., in press, Cohen Kadosh et al., 2011, Pourtois et al., 2004, Walsh and Pascual-Leone, 2003, Wassermann et al., 2008).
In sum, the research evidence reviewed above suggests that developmental neuroimaging studies can tell us much about mature brain organisation. They can tell us, whether specific brain regions actually start out by responding to specific stimulus classes. For example, it has been shown that the FFA responds to faces from early on (Cohen Kadosh and Johnson, 2007, Tzourio-Mazoyer et al., 2002), but at the same time, this activation is not sufficient to support proficient processing levels. Developmental neuroimaging studies can also show us how, along with improving behavioural proficiencies, this response becomes increasingly localised and fine-tuned to faces, a finding that differs significantly for other stimulus categories (Peelen et al., 2010). Improving specialisation may be the result of increasing connectivity with other network regions that support this cognitive function (Cohen Kadosh et al., in press, Cohen Kadosh et al., 2011) and developmental neuroimaging studies are particularly well suited to disentangle the interplay between changes at different network levels, such as structural changes (white and grey matter), functional changes (fine-tuning of specialised cortical responses), as well as overall network configuration changes as cognitive strategies improve and become more proficient. This will ultimately allow us to determine what shapes functional brain response patterns across the developmental trajectory, bringing us a step closer towards highlighting markers of atypical brain development and specialisation (Fig. 3).
Fig. 3.
Questions and suggestions for future research.
Acknowledgements
The author would like to thank Mark H. Johnson, Roi Cohen Kadosh and Sarah-Jayne Blakemore for valuable comments and suggestions on an earlier draft of this paper.
References
- Allison T., Ginter H., McCarthy G., Nobre A.C., Puce A., Luby M., Spencer D.D. Face recognition in the human extrastriate cortex. Journal of Neurophysiology. 1994;71(2):821–825. doi: 10.1152/jn.1994.71.2.821. [DOI] [PubMed] [Google Scholar]
- Anderson M.L. Evolution of cognitive function via redeployment of brain areas. The Neuroscientist. 2007;13:13–21. doi: 10.1177/1073858406294706. [DOI] [PubMed] [Google Scholar]
- Anderson M.L. The massive redeployment hypothesis and the functional topography of the brain. Philosophical Psychology. 2007;21(2):143–174. [Google Scholar]
- Anderson M.L. Neural reuse: a fundamental organization principle of the brain. Behavioral Brain Sciences. 2010;33(4):245–266. doi: 10.1017/S0140525X10000853. [DOI] [PubMed] [Google Scholar]
- Ansari D. Effects of development and enculturation on number representation in the brain. Nature Reviews Neuroscience. 2008;9:279–291. doi: 10.1038/nrn2334. [DOI] [PubMed] [Google Scholar]
- Arsalidou M., Taylor M. Is 2 + 2 = 4? Meta-analyses of brain areas needed for numbers and calculations. NeuroImage. 2011;54:2382–2393. doi: 10.1016/j.neuroimage.2010.10.009. [DOI] [PubMed] [Google Scholar]
- Avidan G., Hasson U., Malach R., Behrmann M. Detailed exploration of face-related processing in congenital prosopagnosia. 2. Functional neuroimaging findings. Journal of Cognitive Neuroscience. 2005;17(7):1150–1167. doi: 10.1162/0898929054475145. [DOI] [PubMed] [Google Scholar]
- Barton J.J.S., Press D.Z., Keenan J.P., O’Connor M. Lesions of the fusiform face area impair perception of facial configuration in prosopagnosia. Neurology. 2002;58:71–78. doi: 10.1212/wnl.58.1.71. [DOI] [PubMed] [Google Scholar]
- Behrmann M., Avidan G., Gao F., Black S. Structural imaging reveals anatomical alterations in inferotemporal cortex in congenital prosopagnosia. Cerebral Cortex. 2007;17(10):2354–2363. doi: 10.1093/cercor/bhl144. [DOI] [PubMed] [Google Scholar]
- Blakemore S.-J. Developmental cognitive neuroscience. Developmental Cognitive Neuroscience. 2011;1(1):3–6. doi: 10.1016/j.dcn.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore S.-J., Winston J., Frith U. Social cognitive neuroscience: where are we heading? Trends in Cognitive Sciences. 2004;8(5):216–222. doi: 10.1016/j.tics.2004.03.012. [DOI] [PubMed] [Google Scholar]
- Bressler S.L., Menon V. Large-scale brain networks in cognition: emerging methods and principles. Trends in Cognitive Sciences. 2010;14:277–290. doi: 10.1016/j.tics.2010.04.004. [DOI] [PubMed] [Google Scholar]
- Brown T.T., Petersen S.E., Schlaggar B.L. Does human functional brain organization shift from diffuse to focal with development? Developmental Science. 2006;9(3):9–11. doi: 10.1111/j.1467-7687.2005.00455.x. [DOI] [PubMed] [Google Scholar]
- Bueti D., Walsh V. The parietal cortex and the representation of time, space, number and other magnitudes. Philosophical Transactions of the Royal Society B: Biological Sciences. 2009;364:2369–2380. doi: 10.1098/rstb.2009.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantlon J.F., Pinel P., Dehaene S., Pelphrey K.A. Cortical representations of symbols, objects, and faces are pruned back during early childhood. Cerebral Cortex. 2011;21(1):191–199. doi: 10.1093/cercor/bhq078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantlon J.F., Platt M.L., Brannon E.M. Beyond the number domain. Trends in Cognitive Sciences. 2009;13:83–91. doi: 10.1016/j.tics.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappeletti M., Freeman E.D., Cipolotti L. Dissociations and interactions between time, numerosity and space processing. Journal of Cognitive Neuroscience. 2009;47:2732–2748. doi: 10.1016/j.neuropsychologia.2009.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Changeux J.P., Danchin A. Selective stabilisation of developing synapses as a mechanism for the specification of neuronal networks. Nature. 1976;264:705–712. doi: 10.1038/264705a0. [DOI] [PubMed] [Google Scholar]
- Chugani H.T., Mueller R.-A., Chugani D.C. Functional brain reorganization in children. Brain and Development. 1996;18:347–356. doi: 10.1016/0387-7604(96)00032-0. [DOI] [PubMed] [Google Scholar]
- Church J.A., Petersen S.E., Schlaggar B.L. The “Task B Problem” and other considerations in developmental functional neuroimaging. Human Brain Mapping. 2010;31:852–862. doi: 10.1002/hbm.21036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen Kadosh, K., Cohen Kadosh, R., Dick, F., Johnson, M.H. Developmental changes in effective connectivity in the emerging core face network. Cerebral Cortex, in press. [DOI] [PMC free article] [PubMed]
- Cohen Kadosh, K., Cohen Kadosh, R., Johnson, M.H. Developmental changes of facial feature processing, submitted for publication.
- Cohen Kadosh K., Henson R.N.A., Cohen Kadosh R., Johnson M.H., Dick F. Task-dependent activation of face-sensitive cortex: an fMRI adaptation study. Journal of Cognitive Neuroscience. 2010;22(5):903–917. doi: 10.1162/jocn.2009.21224. [DOI] [PubMed] [Google Scholar]
- Cohen Kadosh K., Johnson M.H. Developing a cortex specialized for face perception. Trends in Cognitive Sciences. 2007;11(9):267–269. doi: 10.1016/j.tics.2007.06.007. [DOI] [PubMed] [Google Scholar]
- Cohen Kadosh K., Walsh V., Cohen Kadosh R. Investigating face-property specific processing in the right OFA. Social Cognitive and Affective Neuroscience. 2011;6(1):58–65. doi: 10.1093/scan/nsq015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen Kadosh R., Bahrami B., Walsh V., Butterworth B., Price C. Numerical specialisation: within and between dimensions. Paper Presented at the Human Brain Mapping; Melbourne, Australia ; 2008. [Google Scholar]
- Cohen Kadosh R., Cohen Kadosh K., Linden D.E.J., Gevers W., Berger A., Henik A. The brain locus of interaction between number and size: a combined functional magnetic resonance imaging and event-related potential study. Journal of Cognitive Neuroscience. 2007;19(6):957–970. doi: 10.1162/jocn.2007.19.6.957. [DOI] [PubMed] [Google Scholar]
- Cohen Kadosh R., Lammertyn J., Izard V. Are numbers special? An overview of chronometric, neuroimaging, developmental and comparative studies of magnitude representation. Progress in Neurobiology. 2008;84:132–147. doi: 10.1016/j.pneurobio.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Cohen L., Dehaene S. Calculating without reading: unsuspected residual abilities in pure alexia. Cognitive Neuropsychology. 2000;17:563–583. doi: 10.1080/02643290050110656. [DOI] [PubMed] [Google Scholar]
- DeGutis J.M., Bentin S., Robertson L.C., D’Esposito M. Functional plasticity in ventral temporal cortex following cognitive rehabilitation of a congenital prosopagnosic. Journal of Cognitive Neuroscience. 2007;19(11):1790–1802. doi: 10.1162/jocn.2007.19.11.1790. [DOI] [PubMed] [Google Scholar]
- Dehaene S., Cohen L. Cultural recycling of cortical maps. Neuron. 2007;56:384–398. doi: 10.1016/j.neuron.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Durston S., Davidson M.C., Tottenham N., Galvan A., Spicer J., Fosella J.A., Casey B.J. A shift from diffuse to focal cortical activity with development. Developmental Science. 2006;9(1):1–20. doi: 10.1111/j.1467-7687.2005.00454.x. [DOI] [PubMed] [Google Scholar]
- Elman J.L., Bates E.A., Johnson M.H., Karmiloff-Smith A., Parisi D., Plunkett K. MIT Press; Cambridge, MA: 1996. Rethinking Innateness: A Connectionists Perspective on Development. [Google Scholar]
- Fair D.A., Cohen A.L., Dosenbach N.U.F., Church J.A., Miezin F.M., Barch D.M., Schlaggar B.L. The maturing architecture of the brain's default network. Procedings of the National Academy of Sciences. 2008;105(10):4028–4032. doi: 10.1073/pnas.0800376105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair D.A., Dosenbach N.U.F., Church J.A., Cohen A.L., Brahmbhatt S., Miezin F.M., Schlaggar B.L. Development of distinct control networks through segregation and integration. Procedings of the National Academy of Sciences. 2007;104(33):13507–13512. [Google Scholar]
- Fairhall S.L., Ishai A. Effective connectivity within the distributed cortical network for face perception. Cerebral Cortex. 2007;17:2400–2406. doi: 10.1093/cercor/bhl148. [DOI] [PubMed] [Google Scholar]
- Farroni T., Johnson M.H., Menon E., Zulian L., Faraguna D., Csibra G. Newborn's preference for face-relevant stimuli: effects of contrast polarity. Proceedings of the National Academy of Sciences. 2005;102(47):17245–17250. doi: 10.1073/pnas.0502205102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipek P.A. Neuroimaging in the developmental disorders: the state of the science. Journal of Child Psychology and Psychiatry. 1999;40(1):113–128. [PubMed] [Google Scholar]
- Friston K.J., Harrison L., Penny W. Dynamic causal modelling. NeuroImage. 2003;19(4):1273–1302. doi: 10.1016/s1053-8119(03)00202-7. [DOI] [PubMed] [Google Scholar]
- Friston K.J., Price C.J. Dynamic representations and generative models of brain function. Brain Research Bulletin. 2001;54(3):275–285. doi: 10.1016/s0361-9230(00)00436-6. [DOI] [PubMed] [Google Scholar]
- Ganel T., Valyear K.F., Goshen-Gottstein Y., Goodale M.A. The involvement of the “fusiform face area” in processing facial expression. Neuropsychologia. 2005;43:1645–1654. doi: 10.1016/j.neuropsychologia.2005.01.012. [DOI] [PubMed] [Google Scholar]
- Garrido L., Furl N., Draganski B., Weiskopf N., Stevens J., Chern-Yee Tan G., Duchaine B. Voxel-based morphometry reveals reduced grey matter volume in the temporal cortex of developmental prosopagnosics. Brain. 2009;132:3443–3455. doi: 10.1093/brain/awp271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gathers A.D., Bhatt R., Corbly C.R., Farley A.B., Joseph J.E. Developmental shifts in cortical loci for face and object recognition. NeuroReport. 2004;15(10):1549–1553. doi: 10.1097/01.wnr.0000133299.84901.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier I., Tarr M.J., Anderson A.W., Skudlarski P., Gore J.C. Activation of the middle fusiform “face area” increases with expertise in recognizing novel objects. Nature Neuroscience. 1999;2(6):568–573. doi: 10.1038/9224. [DOI] [PubMed] [Google Scholar]
- Giedd J.N., Blumenthal J., Jeffries N.O., Castellanos F.X., Liu H., Zijdenbos A., Rapoport J.L. Brain development during childhood and adolescence: a longitudinal MRI study. Nature Neuroscience. 1999;2(10):861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Gogtay N., Giedd J.N., Lusk L., Hayashi K.M., Greenstein D., Vaituzis A.C., Thompson P.M. Dynamic mapping of human cortical development during childhood through early adulthood. Procedings of the National Academy of Sciences. 2004;101(21):8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golarai G., Gharemani D.G., Whitfield-Gabrieli S., Reiss A., Eberhardt J.L., Gabrieli J.D.E., Grill-Spector K. Differential development of high-level visual cortex correlates with category-specific recognition memory. Nature Neuroscience. 2007;10(4):512–522. doi: 10.1038/nn1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasson U., Avidan G., Deouell L.Y., Bentin S., Malach R. Face-selective activation in a congenital prosopagnosic subject. Journal of Cognitive Neuroscience. 2003;15(3):419–431. doi: 10.1162/089892903321593135. [DOI] [PubMed] [Google Scholar]
- Haug M., Whalen R.E. American Psychological Association; Washington, DC: 1999. Animal Models of Human Emotion and Cognition. [Google Scholar]
- Haxby J.V., Hoffman E.A., Gobbini M.I. The distributed human neural system for face perception. Trends in Cognitive Sciences. 2000;4(6):223–233. doi: 10.1016/s1364-6613(00)01482-0. [DOI] [PubMed] [Google Scholar]
- Houde O., Rossi S., Lubin A., Joliot Mapping numerical processing, reading, and executive functions in the developing brain: an fMRI meta-analysis of 52 studies including 842 children. Developmental Science. 2010;13:876–885. doi: 10.1111/j.1467-7687.2009.00938.x. [DOI] [PubMed] [Google Scholar]
- Huttenlocher P.R., De Courten C., Garey L.J., Van der Loos H. Synaptogenesis in human visual cortex – evidence for synapse elimination during normal development. Neuroscience Letters. 1982;33(3):247–252. doi: 10.1016/0304-3940(82)90379-2. [DOI] [PubMed] [Google Scholar]
- Ishai A. Let's face it: it's a cortical network. NeuroImage. 2008;40:415–419. doi: 10.1016/j.neuroimage.2007.10.040. [DOI] [PubMed] [Google Scholar]
- Johnson M.H. Functional brain development in humans. Nature Reviews Neuroscience. 2001;2:475–483. doi: 10.1038/35081509. [DOI] [PubMed] [Google Scholar]
- Johnson M.H. The ontogeny of the social brain. In: Mayr U., Awh E., Keele S.W., editors. Developing Individuality in the Human Brain: A Tribute to Michael Posner. APA Press; Washington, DC: 2005. pp. 125–140. [Google Scholar]
- Johnson M.H. Interactive Specialization: A domain-general framework for human functional brain development? Developmental Cognitive Neuroscience. 2011;1(1):7–21. doi: 10.1016/j.dcn.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M.H., Dziurawiec S., Ellis H., Morton J. Newborn's preferential tracking of face-like stimuli and its subsequent decline. Cognition. 1991;40:1–19. doi: 10.1016/0010-0277(91)90045-6. [DOI] [PubMed] [Google Scholar]
- Johnson M.H., Halit H., Grice S.J., Karmiloff-Smith A. Neuroimaging of typical and atypical development: A perspective from multiple levels of analysis. Development and Pathology. 2002;14(3):521–536. doi: 10.1017/s0954579402003073. [DOI] [PubMed] [Google Scholar]
- Johnson M.H., Griffin R., Csibra G., Halit H., Farroni T., de Haan M., Richards J. The emergence of the social brain network: evidence from typical and atypical development. Development and Psychopathology. 2005;17:599–619. doi: 10.1017/S0954579405050297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M.H., Grossmann T., Cohen Kadosh K. Mapping functional brain development: building a social brain through interactive specialization. Developmental Psychology. 2009;45:151–159. doi: 10.1037/a0014548. [DOI] [PubMed] [Google Scholar]
- Kanwisher N. Functional specificity in the human brain: a window into the functional architecture of the mind. Procedings of the National Academy of Sciences. 2010;107(25):11163–11170. doi: 10.1073/pnas.1005062107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwisher N., McDermott J., Chun M.M. The fusiform face area: a module in human extrastriate cortex specialized for face perception. Journal of Neuroscience. 1997;17(11):4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karmiloff-Smith A. Development itself is the key to understanding developmental disorders. Trends in Cognitive Sciences. 1998;2:389–398. doi: 10.1016/s1364-6613(98)01230-3. [DOI] [PubMed] [Google Scholar]
- Knudsen E.I. Sensitive periods in the development of the brain and behavior. Journal of Cognitive Neuroscience. 2004;16:1412–1425. doi: 10.1162/0898929042304796. [DOI] [PubMed] [Google Scholar]
- Lenroot R.K., Giedd J.N. The changing impact of genes and environment on brain development during childhood and adolescence: initial findings from a neuroimaging study of pediatric twins. Development and Psychopathology. 2008;20:1161–1175. doi: 10.1017/S0954579408000552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L.H., Dapretto M., O’Hare E.D., Kan E., McCourt S.T., Thompson P.M., Sowell E.R. Relationships between brain activation and brain structure in normally developing children. Cerebral Cortex. 2009;19:2595–2604. doi: 10.1093/cercor/bhp011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mareschal D., Johnson M.H., Sirois S., Spratling M.W., Thomas M.S.C., Westermann G. Oxford University Press; Oxford: 2007. Neuroconstructivism, vol. 1: How the Brain Constructs Cognition. [DOI] [PubMed] [Google Scholar]
- Mareschal D., Sirois S., Westermann G. Oxford University Press; Oxford: 2007. Neuroconstructivism, vol. II: Perspectives and Prospects. [Google Scholar]
- Mondloch C.J., Le Grand R., Maurer D. Configural face processing develops more slowly than featural face processing. Perception. 2002;31:553–566. doi: 10.1068/p3339. [DOI] [PubMed] [Google Scholar]
- Nieder A., Freedman D.J., Miller E.K. Representation of the quantity of visual items in the primate prefrontal cortex. Science. 2002;297:1708–1711. doi: 10.1126/science.1072493. [DOI] [PubMed] [Google Scholar]
- Norman K.A., Polyn S.M., Detre G.J., Haxby J.V. Beyond mind-reading: multi-voxel pattern analysis of fMRI data. Trends in Cognitive Science. 2006;10(9):424–430. doi: 10.1016/j.tics.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Olesen P.J., Nagy Z., Westerberg H., Klingberg T. Combined analysis of DTI and fMRI data reveals a joint activation of white and grey matter in a fronto-parietal network. Cognitive Brain Research. 2003;18:48–57. doi: 10.1016/j.cogbrainres.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Passarotti A.M., Paul B.M., Bussiere J.R., Buxton R.B., Wong E.C., Stiles J. The development of face and location processing: an fMRI study. Developmental Science. 2003;6(1):100–117. [Google Scholar]
- Paus T., Zijdenbos A., Worsley K., Collins D.L., Blumenthal J., Giedd J.N., Evans A.C. Structural maturation of neural pathwats in children and adolescents: in vivo study. Science. 1999;283:190–1911. doi: 10.1126/science.283.5409.1908. [DOI] [PubMed] [Google Scholar]
- Peelen M.V., Glaser B., Vuilleumier P., Eliez S. Differential development of selectivity for faces and bodies in the fusiform gyrus. Developmental Science. 2010;12(6):F16–F25. doi: 10.1111/j.1467-7687.2009.00916.x. [DOI] [PubMed] [Google Scholar]
- Pelphrey K.A., Lopez J., Morris J.P. Developmental continuity and change in responses to social and nonsocial categories in human extrastriate visual cortex. Frontiers in human neuroscience. 2009;3 doi: 10.3389/neuro.09.025.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesenti M., Thioux M., Seron X., De Volder A. Neuroanatomical substrates of Arabic number processing, numerical comparison and simple addition: a PET study. Journal of Cognitive Neuroscience. 2000;12:461–479. doi: 10.1162/089892900562273. [DOI] [PubMed] [Google Scholar]
- Pourtois G., Sander D., Andres M., Grandjean D., Reveret L., Olivier E., Vuilleumier P. Dissociable roles of the human somatosensory and superior temporal cortices for processing social face signals. European Journal of Neuroscience. 2004;20:3507–3515. doi: 10.1111/j.1460-9568.2004.03794.x. [DOI] [PubMed] [Google Scholar]
- Price C.J., Friston K.J. Degeneracy and cognitive anatomy. Trends in Cognitive Science. 2002;6(10):416–421. doi: 10.1016/s1364-6613(02)01976-9. [DOI] [PubMed] [Google Scholar]
- Quartz S.R. The constructivist brain. Trends in Cognitive Sciences. 1999;3:48–57. doi: 10.1016/s1364-6613(98)01270-4. [DOI] [PubMed] [Google Scholar]
- Ramsey J.D., Hanson S.J., Hanson C., Halchenko Y.O., Poldrack R.A., Glymour C. Six problems for causal inference from fMRI. NeuroImage. 2010;49(2):1545–1558. doi: 10.1016/j.neuroimage.2009.08.065. [DOI] [PubMed] [Google Scholar]
- Rotshtein P., Vuilleumier P., Winston J., Driver J., Dolan R. Distinct and convergent visual processing of high and low spatial frequency information in faces. Cerebral Cortex. 2007;17:2713–2724. doi: 10.1093/cercor/bhl180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherf K.S., Behrmann M., Humphreys K., Luna B. Visual category-selectivity for faces, places and objects emerges along different developmental trajectories. Developmental Science. 2007;10(4):F15–F31. doi: 10.1111/j.1467-7687.2007.00595.x. doi:10.111/j.1467-7687.2007.00595.x. [DOI] [PubMed] [Google Scholar]
- Schiltz C., Sorger B., Caldara R., Ahmed F., Mayer E., Goebel R., Rossion B. Impaired face discrimination in acquired prosopagnosia is associated with abnormal response to individual faces in the right middle fusiform gyrus. Cerebral Cortex. 2006;16:574–586. doi: 10.1093/cercor/bhj005. [DOI] [PubMed] [Google Scholar]
- Shaw P., Greenstein D., Lerch J.P., Clasen L., Lenroot R., Gogtay N., Giedd J.N. Intellectual ability and cortical development in children and adolescents. Nature. 2006;440:676–679. doi: 10.1038/nature04513. [DOI] [PubMed] [Google Scholar]
- Shaw P., Kabani N.J., Lerch J.P., Eckstrand K., Lenroot R., Gogtay N., Wise S.P. Neurodevelopmental trajectories of the human cerebral cortex. Journal of Neuroscience. 2008;28(14):3586–3594. doi: 10.1523/JNEUROSCI.5309-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P., Wallace G.L., Addington A., Evans A., Rapoport J., Giedd J.N. Effects of the Val158 Met catechol-O-methyltransferase polymorphism on cortical structure in children and adolescents. Molecular Psychiatry. 2009;14:348–355. doi: 10.1038/mp.2008.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirois S., Spratling M.W., Thomas M.S.C., Westermann G., Mareschal D., Johnson M.H. Precis of neuroconstructivism: how the brain constructs cognition. Behavioral Brain Sciences. 2008;31:321–356. doi: 10.1017/S0140525X0800407X. [DOI] [PubMed] [Google Scholar]
- Sowell E.R., Thompson P.M., Leonard C.M., Welcome S.E., Kan E., Toga A.W. Longitudinal mapping of cortical thickness and brain growth in normal children. Journal of Neuroscience. 2004;24(38):8223–8231. doi: 10.1523/JNEUROSCI.1798-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steeves J., Dricot L., Goltz H.C., Sorger B., Peters J., Milner A.D., Rossion B. Abnormal face identity coding in the middle fusiform gyrus of two brain-damaged prosopagnosic patients. Neuropsychologia. 2009;47(12):2584–2593. doi: 10.1016/j.neuropsychologia.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Steeves J.K.E., Culham J.C., Duchaine B.C., Pratesi C.C., Valyear K.F., Schindler I., Goodale M.A. The fusiform face area is not sufficient for face recognition: evidence from a patient with dense prosopagosia and no occipital face area. Neuropsychologia. 2006;44:594–609. doi: 10.1016/j.neuropsychologia.2005.06.013. [DOI] [PubMed] [Google Scholar]
- Supekar K., Mussen M., Menon V. Development of large-scale functional brain networks in children. PLoS Biology. 2009;7(7):e1000157. doi: 10.1371/journal.pbio.1000157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C., Avidan G., Humphreys K., Jung K.-j., Gao F. Reduced structural connectivity in ventral visual cortex in congenital prosopagnosia. Nature Neuroscience. 2009;12(1):29–31. doi: 10.1038/nn.2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N., De Schonen S., Crivello F., Reutter B., Aujard Y., Mazoyer B. Neural correlates of woman face processing by 2-month-old infants. NeuroImage. 2002;15:454–461. doi: 10.1006/nimg.2001.0979. [DOI] [PubMed] [Google Scholar]
- Uddin L.Q., Supekar K., Menon V. Typical and atypical development of functional human brain networks: insights from resting-state fMRI. Frontiers in Systems Neuroscience. 2010;4 doi: 10.3389/fnsys.2010.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh V., Pascual-Leone A. The MIT press; Cambridge, MA: 2003. Transcranial Magnetic Stimulation: A Neurochronometrics of Mind. [Google Scholar]
- Wassermann E., Epstein C., Ziemann U., Walsh V., Paus T., Lisanby S. 1st ed. Oxford University Press; Oxford: 2008. The Oxford Handbook of Transcranial Stimulation. [Google Scholar]