Highlights
* Category membership affects 7-month-olds’ processing of single visual stimuli. * Oddball stimuli elicit a large Nc and a positive slow wave (PSW) in infants’ ERPs. * A different-category oddball elicits a prolonged Nc latency and a pronounced PSW. * Infants regard category-relevant information when processing single visual stimuli.
Keywords: Category identification, Infants (age: 7 months), Event-related potentials, Visual stimulus processing, Recognition memory
Abstract
Event-related potentials (ERPs) to single visual stimuli were recorded in 7-month-old infants. In a three-stimulus oddball paradigm, infants watched one frequently occurring standard stimulus (either an animal or a furniture item) and two infrequently occurring oddball stimuli, presenting one exemplar from the same and one from the different superordinate category as compared to the standard stimulus. Additionally, visual attributes of the stimuli were controlled to investigate whether infants focus on category membership or on perceptual similarity when processing the stimuli. Infant ERPs indicated encoding of the standard stimulus and discriminating it from the two oddball stimuli by larger Nc peak amplitude and late-slow-wave activity for the infrequent stimuli. Moreover, larger Nc latency and positive-slow-wave activity indicated increased processing for the different-category as compared to the same-category oddball. Thus, 7-month-olds seem to encode single stimuli not only by surface perceptual features, but they also regard information of category membership, leading to facilitated processing of the oddball that belongs to the same domain as the standard stimulus.
1. Introduction
The ability to discriminate between living and non-living objects is a core component of human cognition. Infants appear to have this ability from very early on (e.g., Gelman and Opfer, 2002, Rakison and Poulin-Dubois, 2001), and different brain areas are involved in processing of stimuli from these two domains (e.g., Mahon and Caramazza, 2007, Wiggett et al., 2009). Further evidence for a deeply rooted animate–inanimate distinction comes from studies on preverbal reasoning (e.g., Luo et al., 2009, Pauen and Träuble, 2009). Likewise, studies on infant categorization indicate a global-to-basic-level shift within the first year of life (e.g., Mandler and McDonough, 1998, Pauen, 2002, Quinn and Johnson, 2000) and demonstrate that superordinate-level categories (Rosch, 1978) crossing the animate-inanimate boundary (e.g., animals vs. furniture) can be discriminated at an earlier age than basic-level categories (e.g., cats vs. dogs, chairs vs. tables). Hence, there is good reason to assume that one of the first categorical distinctions made by infants is that between living and non-living things.
Thus far, infants’ global-level categorization has mainly been assessed by behavioral measures (e.g., Mandler, 1992, Pauen, 2002, Quinn and Eimas, 1996). However, event-related potentials (ERPs) provide an additional measure of infants’ visual attention and recognition memory (see de Haan, 2007, for a review). The oddball paradigm, in which one standard stimulus is presented frequently while the other oddball stimulus is presented infrequently, typically elicits a Nc (negative central) component in infants, a negative deflection between 350 and 750ms after stimulus onset with maximal amplitudes at midline fronto-central electrodes (e.g., Ackles and Cook, 1998, Karrer and Monti, 1995, Nelson and Collins, 1991). Many studies reported a larger Nc amplitude, and some also a larger Nc latency, for the oddball than for the standard stimulus (Courchesne et al., 1981, Hill-Karrer et al., 1998, Hunter and Karrer, 1993). Because the Nc is related to activity in the anterior cingulate and the frontal cortex, it probably reflects the allocation of infants’ visual attention to salient stimuli (Reynolds and Guy, 2012, Reynolds and Richards, 2005).
A second infant ERP component reflecting visual recognition memory is a long-latency slow wave (LSW) occurring between 1000 and 1500ms after stimulus onset over temporal, parietal, frontal, and central leads (de Haan, 2007, Nelson et al., 2000). Oddball stimuli that are repeated in a small number of trials typically elicit a positive slow wave (PSW), which is taken to reflect updating of memory for a partially encoded stimulus (de Haan and Nelson, 1997, Nelson and Collins, 1991). In contrast, the LSW for the standard stimulus typically returns to baseline, indicating complete stimulus encoding.
Recently, the Nc and the PSW have been related to infants’ categorization of visual stimuli. Three of these studies (Grossmann et al., 2009, Quinn et al., 2006, Quinn et al., 2010) presented 6-month-olds with several basic-level exemplars for familiarization, and then with novel stimuli from the familiar or from a contrasting category. Here, the Nc amplitude was related to the detection of a novel category, in that it was greater for the novel unfamiliar stimulus. In contrast, the PSW signified the formation of a new category (Quinn et al.), or the integration of new exemplars into an already existing category (Grossman et al.). In another study, infants were not familiarized with a given category before the start of the test-phase, but rather saw multiple different exemplars from each superordinate category (animals and furniture) in a semi-random sequence. Jeschonek et al. (2010) presented each category on 50% of the time, and Nc amplitude indicated that 7-month-olds discriminated animals from furniture. However, this study does not inform about whether global-category membership affects the processing of single visual stimuli. This question seems of crucial importance if we want to find out whether infants are involved in the process of category identification or category formation.
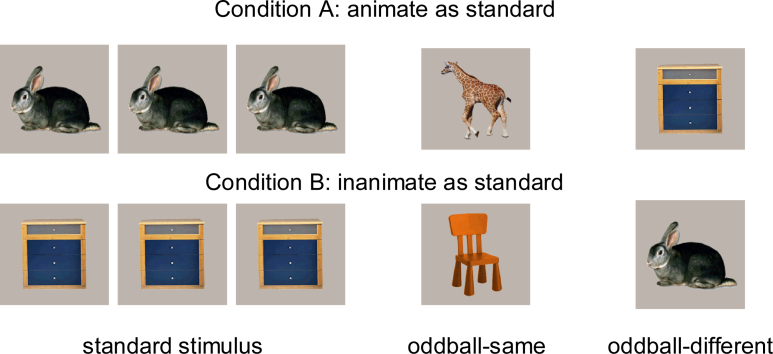
To address this question, we tested 7-month-olds with a three-stimulus oddball paradigm (Fig. 1) in which one picture (standard stimulus; animal or furniture item) appeared in 60% of the trials. Of two pictures appearing infrequently, in 20% of the trials each, one shared the standard's global category (oddball-same), but the other did not (oddball-different). Our reasoning was that if infants recognized the category membership of the stimuli, they should allocate comparably more attention to the oddball stimulus that belonged to a different domain than the standard stimulus. Since animals and furniture items look rather differently from each other, special care needed to be taken in selecting the stimuli. In detail, we chose a restricted number of items, ensuring that the standard stimulus had a high overlap in perceptual features (i.e., shape, color, visibility of legs) with the oddball from the contrasting category (Fig. 1), but a low overlap with the oddball from the same category.
Fig. 1.
Stimulus material of the ERP study. Three pictures (rabbit, giraffe, dresser, or dresser, chair, rabbit) were presented in random order: one stimulus in 60% of the trials (standard stimulus; rabbit or dresser), one stimulus from the same superordinate category in 20% of the trials (oddball-same), and another stimulus from the contrasting category in 20% of the trials (oddball-different). The number of pictures reflects the presentation ratio. The rabbit and the dresser were of bluish-gray color, the giraffe and the chair were of reddish-brown color. Half of the infants were randomly assigned to condition A, the other half to condition B. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
For the ERP components, we expected first, a larger Nc amplitude and/or a larger Nc latency for each of the oddball stimuli as compared to the standard stimulus. This would reflect that 7-month-olds recognized the oddball stimuli as being less frequent or less familiar than the standard stimulus. Second, we predicted differences in the Nc to the two infrequent stimuli. If infants focused mainly on perceptual differences, the oddball-same should elicit the stronger novelty response. However, if infants were involved in a process of category identification and included category-relevant information in their stimulus processing, there should be a larger Nc amplitude and/or Nc latency for the oddball-different as compared to the oddball-same. Third, we expected a larger PSW-activity for both oddball stimuli than for the standard, reflecting a need for memory updating for the partially encoded infrequent stimuli. Moreover, we speculated that noticed similarities between the standard stimulus and the oddball stimuli should facilitate memory encoding for the latter. Thus, a large PSW to the oddball-same would indicate that infants focused on perceptual similarities, whereas a large PSW to the oddball-different would reflect infants’ focusing on category-relevant information.
2. Method
2.1. Participants
Twelve healthy, full term infants (M age: 7 months; 23 days, range: 7;06 to 8;13; 6 girls, 6 boys) participated in the study. Infants’ names came from the residents’ registration office, and their families were contacted via letters and phone calls. Parents were informed about the ERP method and the procedure before the study, and all agreed for their infants to participate. Another 39 infants were tested but were excluded from the final sample due to fussiness during stimulus presentation (n=12), technical problems (n=11), or because of an insufficient number of trials left for ERP averaging after analyses of the EEG data (n=16). This relatively high dropout rate lies in the normal range for infant ERP studies to visual stimuli (Stets et al., 2012) and was due to task demands (e.g., inclusion of a trial only if an infant produced overt gaze to the screen; see below).
2.2. Stimuli and apparatus
The stimulus material and the experimental conditions are depicted in Fig. 1. The stimuli were colored photographs of two rather different looking animals (rabbit, giraffe) and furniture items (dresser, chair), respectively, presented on a homogeneous gray background. The rabbit and the dresser looked quite similar in the sense that they had a stocky shape, bluish-gray color, and no visible legs. In contrast, the giraffe and the chair had a more delicate shape, reddish-brown color, and visible legs. Each stimulus picture occupied an area of 14 visual degrees in width and 17 visual degrees in height on a 19-in. computer monitor, so the covered area was comparable between conditions.
Because we wanted to control for the perceptual similarity of the stimuli, we presented the infants with only a very reduced set of three stimuli in each condition. The perceptual similarity of the stimuli was confirmed by both objective and subjective measures. First, we counted the number of pixels for 256 levels on the RGB scale for each picture. Two analyses of variance (ANOVAs) yielded significant main effects of stimulus for the number of pixels for luminosity (gray) in levels 1–85 for both rabbit–giraffe–dresser, F(2,168)=5.09, p=.007, η2=.06, and dresser–chair–rabbit, F(2,168)=2.74, p=.05, η2=.03. Post hoc t tests confirmed that rabbit (M=284, SE=54.5) and dresser (M=401, SE=123.6) did not differ, p=.21. However, the number of those pixels was significantly higher for rabbit than for giraffe (M=50, SE=10.3), t(84)=4.15, p<.001, and also for dresser than for chair (M=124, SE=42.3), t(84)=2.11, p=.02.
Two other ANOVAs yielded a similar pattern of results for the number of pixels for red in levels 161–192. Again, there were significant main effects of stimulus for both rabbit–giraffe–dresser, F(2,60)=10.7, p<.001, η2=.26, and dresser–chair–rabbit, F(2,60)=6.10, p=.004, η2=.17. Again, rabbit (M=79, SE=32.1) and dresser (M=79, SE=28.6) did not differ, p=.99. However, the number of those pixels was significantly higher for giraffe than for rabbit (M=251, SE=66.7), t(30)=3.80, p=.001, and also for chair than for dresser (M=243, SE=80.1), t(30)=2.38, p=.02.
Second, subjective assessments of category similarity and perceptual similarity were obtained from N=44 adults (mean age=23.5 years; SD=3.44; all female). The adults were presented with pairs of the stimulus pictures (e.g., rabbit and giraffe, dresser and chair) and were asked to indicate how similar they found the two pictures in terms of (a) category membership and (b) visual appearance. Ratings were marked on a 4-point scale (0: very dissimilar, 1: rather dissimilar, 2: rather similar, 3: very similar). For each stimulus triplet, we analyzed the ratings with an ANOVA with the within-subject factors similarity type (category vs. perceptual) and pair (rabbit–dresser, rabbit–giraffe, or rabbit–dresser, dresser–chair). Both ANOVAs yielded significant main effects of similarity type and of pair (all ps<.001), and also a significant interaction, for rabbit–giraffe–dresser, F(1,43)=610.0, p<.001, η2=.93, and for dresser–chair–rabbitt, F(1,43)=780.9, p<.001, η2=.95. The similarity of category membership was rated significantly lower for rabbit and dresser (M=.05, SE=0.02) than for rabbit and giraffe (M=2.57, SE=0.06), t(43)=37.74, p<.001, and also lower for rabbit and dresser than for dresser and chair (M=2.65, SE=0.06), t(43)=38.70, p<.001. However, the similarity of visual appearance was rated significantly higher for rabbit and dresser (M=1.17, SE=0.05) than for rabbit and giraffe (M=0.70, SE=0.10), t(43)=4.28, p<.001, and also higher for rabbit and dresser than for dresser and chair (M=0.83, SE=0.11), t(43)=3.28, p=.002.
2.3. Procedure
Before the start of the study, a research assistant placed the electrode cap on the infant's head. Then, the infant was seated in a highchair, at a viewing distance of 60cm from the monitor. The caretaker sat on a chair behind the infant.
The infants were randomly assigned to one of two experimental conditions (see Fig. 1): half of the boys and girls saw the rabbit in 60% of the trials (standard stimulus), the giraffe in 20% (oddball-same), and the dresser in 20% of the trials (oddball-different). The other half of infants saw the dresser as standard stimulus, the chair as oddball-same, and the rabbit as oddball-different. We used the same pictures as standard stimulus and as oddball-different in the two subject groups, that is, the rabbit and the dresser were presented at a frequency of 60% in one group and of 20% in the other. Thus, any differences in ERPs to the standard stimulus and to the oddball-different are due to presentation frequency, processing demands in the stimulus set, and category membership, but not to stimulus identity.
The experiment consisted of a maximum number of 203 trials. The standard stimulus appeared in the first three trials, and after that, the three stimuli occurred in a presentation order randomized for blocks of five trials (i.e., three standard stimuli, one oddball-same, one oddball-different). The stimuli were presented as long as the infant could be attracted to the screen. Infants watched an average number of 166 trials (range: 108–203 trials).
Each trial started with the presentation of a central attractor (black-and-gray checkerboard; 8×8 visual degrees) for 500ms. Following a 100-ms pause (blank screen), one stimulus picture was presented for 1000ms in the center of the screen. After that, the monitor went blank for a variable intertrial interval of 1100–1900ms. In case that the infant looked away from the screen, the experimenter could interrupt the stimulus presentation by a key press, which started an attention-catcher trial featuring a rotating black spiral (8cm×8cm) in the screen center and a bell sound. As soon as the infant's attention returned to the monitor, the stimulus presentation was continued. EEG was recorded continuously, and the whole session was videotaped for offline coding of looking behavior.
2.4. EEG recording and analysis
The EEG was recorded with a 32-channel BrainAmp amplifier and Ag-AgCl electrodes from 25 scalp locations of the ten-twenty system referenced to the left mastoid (TP10). Bipolar channels of the horizontal and vertical electrooculogram were recorded. The sampling rate was 250Hz. Scalp impedances generally averaged 5kΩ. Electrodes were re-referenced offline to linked mastoids.
For analyses, EEG data were filtered with bandpass filter of 0.1–35Hz and a 50-Hz notch filter. Data were segmented into trials consisting of the 100ms before and the 1500ms after stimulus onset. The EEG segments were inspected for artifacts and poor recordings, and individual channels within trials were eliminated from the analyses if these occurred. When artifacts occurred in channels of interest (F3, Fz, F4, C3, Cz, C4, Pz), or when the infant did not look at the stimulus, the complete trial was excluded. Only data of infants who contributed at least 5 artifact-free trials for each stimulus type were included into the final analyses (for a discussion of the use of low numbers of trials in infant ERP studies, see Stets and Reid, 2011). To equalize the signal-to-noise ratio (Thomas et al., 2004), a comparable number of trials was randomly selected from the available artifact-free trials for each stimulus type, and three separate averages were formed (M=8 trials in each average, range=5–17).
Nc mean amplitude and Nc peak latency were analyzed from 350 to 700ms following stimulus onset at three frontal (F3, Fz, F4) and three central electrodes (C3, Cz, C4). For the late slow waves, the mean activity in the intervals from 1000 to 1500ms following stimulus onset was analyzed at midline central and parietal electrodes (Cz, Pz). To explore possible differences in slow-wave activity also at other scalp sites, additional analyses were performed for frontal (Fz, F3, F4, FC5, FC6) and temporal electrodes (CP5, CP6, T7, T8).
An alpha level of .05 was used for all statistical tests. Violations of sphericity were addressed by the Greenhouse–Geisser correction.
3. Results
3.1. Nc mean amplitude
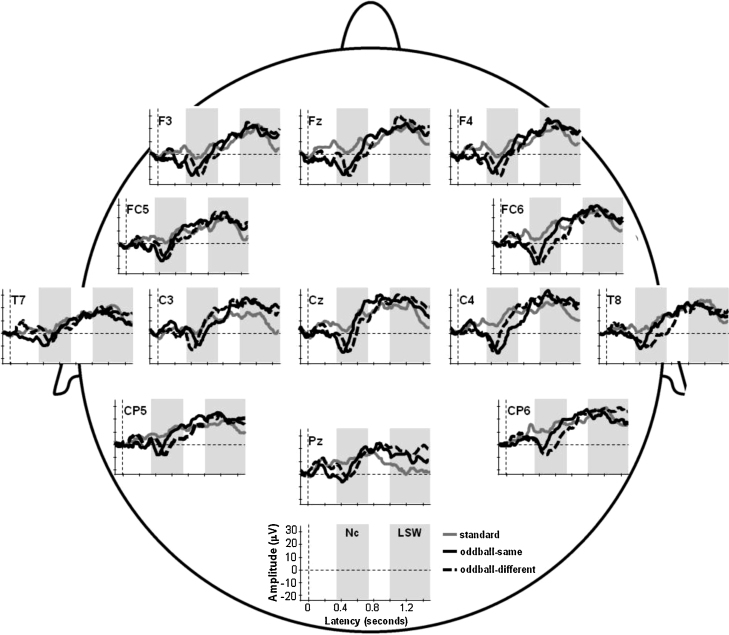
The effects of category membership and electrode position on Nc mean amplitude were analyzed by a 3×6 ANOVA with the within-subject factors stimulus (standard, oddball-same, oddball-different) and location (F3, Fz, F4, C3, Cz, C4). Fig. 2 depicts the Nc mean amplitudes at the six electrodes. The ANOVA yielded a significant main effect of stimulus, F(2,22)=8.22, p=.002, η2=.43, whereas the main effect of location and the interaction were not significant, ps>.10. Post hoc t tests for the main effect of stimulus indicated that the Nc mean amplitude was greater to the two oddball stimuli than to the standard stimulus [oddball-same – standard: t(11)=3.91, p<.02; oddball-different – standard: t(11)=3.72, p<.01], but did not differ for the two oddball stimuli, p=.52. Thus, both oddball stimuli elicited an Nc at fronto-central electrodes that was larger than that for the standard stimulus, but that did not differ between the two oddballs.
Fig. 2.
Grand-average ERPs at the 13 electrode sites that were analyzed in the present study. ERPs are depicted for single electrodes (F3, Fz, F4, FC5, FC6, C3, Cz, C4, T7, T8, CP5, CP6, and Pz) in response to the standard stimulus (solid gray line), the oddball stimulus from the same category (solid black line), and the oddball stimulus from the contrasting category (broken black line). Gray areas depict the time windows during which Nc peak amplitude and Nc peak latency were analyzed (350–700ms) and during which the mean activity of the late slow waves (LSWs) was analyzed (1000–1500ms).
3.2. Nc peak latency
A 3×6 ANOVA on Nc peak latency with the within-subject factors stimulus (standard, oddball-same, oddball-different) and location (F3, Fz, F4, C3, Cz, C4) yielded a significant main effect of stimulus, F(2,22)=3.27, p=.05, η2=.23, and a significant main effect of location, F(2,27)=4.23, p<.02, η2=.28, whereas the interaction was not significant, p=.76. Fig. 2 depicts Nc peak latencies at the six electrodes. To explain the main effect of location, mean Nc peak latencies were calculated for the frontal (F3, Fz, F4) and the central (C3, Cz, C4) electrodes. A t test indicated that the Nc appeared later at the frontal (504ms) than at the central electrodes (475ms), t(11)=2.72, p<.02. For the main effect of stimulus, t tests indicated that Nc peak latency was larger to the oddball-different than to the oddball-same, t(11)=3.06, p<.02, but did not differ between the standard stimulus and the two oddball stimuli, ps>.16. The different-category oddball elicited a larger Nc peak latency than did the same-category oddball.
3.3. Mean activity of late slow waves
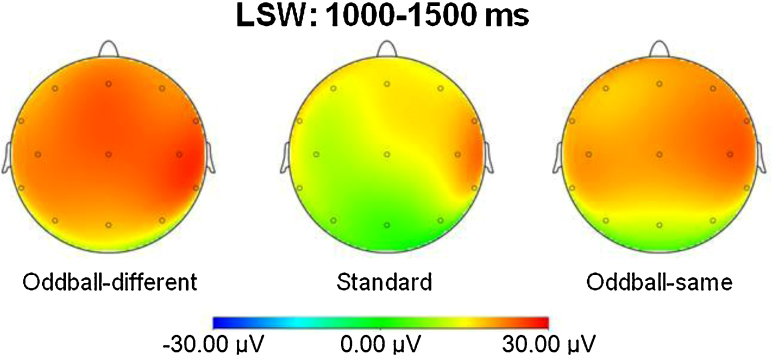
A 3×11 ANOVA on the mean activity of the late slow waves with the within-subject factors stimulus (standard, oddball-same, oddball-different) and location (Fz, F3, F4, FC5, FC6, Cz, CP5, CP6, Pz, T7, T8) did not yield a significant main effect of stimulus, p=.39, but a significant main effect of location, F(10,110)=4.43, p<.01, η2=.29, and a significant interaction, F(20,220)=1.89, p<.02, η2=.15. Fig. 2 depicts LSW activity at the eleven electrodes, and Fig. 3 shows a topographical map of the LSW mean activity. To explore the interaction, separate ANOVAs with the within-subject factor stimulus were calculated for each electrode, which yielded a significant main effect of stimulus at the electrodes Cz, F(2,22)=4.16, p<.03, η2=.27, and Pz, F(2,22)=3.38, p=.05, η2=.24, but not at the other electrodes, all ps>.38. At Pz and Cz, all stimuli elicited positive slow waves (PSWs), except for the standard stimulus at Pz, where the LSW was not different from the prestimulus baseline level, p=.20. Post hoc t tests for the main effect of stimulus indicated a greater PSW mean activity at Cz to the oddball-different than to the standard, t(11)=3.02, p<.02, but no difference for the two other comparisons, ps>.13. The same pattern emerged at Pz, with larger PSW mean activity to the oddball-different than to the standard, t(11)=2.45, p<.04, but no difference for the two other comparisons, ps>.17.
Fig. 3.
Scalp topography of the late-slow-wave activity for the frequently presented stimulus (standard), and for the infrequent stimuli from the same (oddball-same) and from the contrasting category (oddball-different). The scalp topography is an average for a time window from 1000 to 1500ms.
In sum, effects of stimulus type on the LSWs were restricted to the midline central and parietal electrodes, with the LSW to the standard stimulus returning to baseline at Pz. Moreover, both oddball stimuli elicited a PSW at Cz and Pz, with a pronounced PSW to the infrequent stimulus from the different category.
4. Discussion
This experiment explored ERPs related to 7-month-olds’ processing of single visual stimuli from the categories animals and furniture. In a three-stimulus oddball paradigm, the standard stimulus was presented in 60% of the trials, and two oddball stimuli were presented in 20% of the trials, each. One of the infrequent stimuli (oddball-same) belonged to the same superordinate category as did the standard, but was perceptually dissimilar, and the other (oddball-different) belonged to the contrasting category, but was perceptually similar. Each of the two oddball stimuli elicited a greater Nc peak amplitude at frontocentral electrodes than did the standard stimulus. This indicates that 7-month-olds encoded and memorized the frequently presented standard stimulus, and that they detected the infrequently presented oddball stimuli, resulting in an allocation of attention to these stimuli (Reynolds and Richards, 2005). The Nc amplitude did not differ for the two oddball stimuli, indicating that both stimuli elicited a comparable amount of attention. In the same vein, the LSW to the standard stimulus returned to baseline at electrode Pz, which denotes complete encoding of the frequently presented stimulus, whereas both oddball stimuli elicited a PSW, reflecting partial encoding of infrequently presented stimuli and a need for memory updating (de Haan and Nelson, 1997, Nelson and Collins, 1991, Reynolds and Richards, 2005, Reynolds and Guy, 2012). Hence, our findings confirm that 7-month-olds are well able to discriminate between infrequently and frequently presented pictures, and that the processing of infrequent visual stimuli requires more attentional and memory capacities.
In the present study, we were especially interested in differences in infants’ ERPs to the two infrequent stimuli that belonged to either the same or the different category (i.e., animals or furniture) as compared to the standard stimulus. We found that the Nc appeared later for the different-category oddball than for the same-category oddball. Because some studies found a larger Nc latency to an oddball as compared to a standard stimulus (e.g., Courchesne et al., 1981, Hill-Karrer et al., 1998), we take the present Nc latencies to reflect a stronger novelty response for the oddball-different than for the oddball-same. Second, the oddball-different elicited a larger PSW activity than did the standard, which implies that encoding of the former stimulus required more processing capacity (de Haan and Nelson, 1997, Nelson et al., 2000). The strong ERP responses to the oddball-different are very remarkable because this stimulus was rather similar to the standard stimulus on a perceptual level (i.e., in terms of shape, color, absence of legs). If infants had merely focused on perceptual similarity, the oddball-same, which looked dissimilar, should have required more processing than the standard. Therefore, we take the differences in Nc latency and in PSW activity to the two oddball stimuli as evidence that 7-month-olds include information that is indicative of category membership when they process single visual stimuli.
The findings of the present study contribute to research on the early development of cognitive capacities in important aspects, because they provide further information about the significance of ERPs for infants’ processing of visual stimuli as well as for infant categorization, and they may inform us about the type of information that underlies infants’ discrimination of stimuli from superordinate categories, like animals and furniture items.
In the literature, there is an ongoing discussion about whether infants’ ERPs in the oddball paradigm reflect responses to the familiarity vs. novelty, or rather to the frequency of presentation of the stimuli (see de Haan, 2007). When only two stimuli are presented without prior familiarization, these aspects are confounded because the standard is both familiar and frequent, and the oddball is both novel and infrequent. Therefore, some studies (e.g., Nelson and Collins, 1991, Reynolds and Richards, 2005) familiarized infants to stimuli that were used in a subsequent oddball paradigm and then compared ERPs to the frequent familiar standard stimulus, to an infrequent familiar oddball stimulus, and to a class of infrequent and trial-unique novel oddball stimuli. These studies yielded somewhat inconsistent results regarding Nc amplitude, leading some researchers to conclude that a larger Nc to the infrequent novel stimulus may result from a higher task difficulty of the three-stimulus as compared to the two-stimulus oddball task (de Haan, 2007), or may indicate a strong attentional response regardless of familiarity or presentation frequency of the stimuli (Reynolds and Richards, 2005).
Because we did not include a familiarization phase, one may argue that the aspects of familiarity and presentation rate were confounded also in the present study. However, our study differed in one important aspect from the previous three-stimulus oddball studies with prior familiarization: both oddball stimuli were presented repeatedly, at the same low frequency. Thus, we did not have any trial-unique infrequent novel stimuli. As a consequence, both oddball stimuli elicited a PSW, which is typically found for the infrequent familiar stimulus in the oddball-paradigm with prior familiarization (e.g., Nelson et al., 2000). In the present study, the issue of familiarity vs. presentation frequency is only relevant for the comparisons of the ERPs to the oddball stimuli and the standard stimulus. Because all stimuli are novel at the beginning of the experiment, we can conclude that the larger Nc amplitudes and the occurrence of PSWs to the oddball stimuli reflect infants’ detection of differences in presentation frequency as well as processes of the formation of cognitive representations for each of the stimuli.
However, the ERP differences between the two oddball stimuli can neither be related to presentation frequency nor to familiarity vs. novelty, because these aspects were identical for the two oddball stimuli. Thus, ERP differences to the oddball stimuli have to result from differences in visual features or in category membership. Because we counterbalanced the pictures for the stimulus types across infants, we can rule out that our findings reflect simple visual preferences for specific objects. Moreover, because the oddballs differed in an orthogonal fashion from the standard with respect to perceptual similarity and category membership, we take the larger Nc latency and PSW activity to the oddball-different as reflecting the inclusion of category information in infants’ processing of single stimuli.
As to the relevance of ERP components for infant categorization, Quinn et al., 2006, Quinn et al., 2010 and Grossmann et al. (2009) found that Nc peak amplitude was connected to the detection of a novel test stimulus from an unfamiliar category. In the present study, both oddball stimuli elicited a Nc of comparable amplitude. This may result from differences in the study designs. The previous studies presented several trial-unique pictures for familiarization, which may have led infants to habituate to the perceptual differences between the stimuli. Therefore, the perceptual variance of the novel familiar test stimulus may be less arousing than the categorical variance of the novel unfamiliar test stimulus. In contrast, infants in the present study saw the same picture in the majority of the trials, such that both oddball stimuli provided novel information, leading to a comparable allocation of attention that was reflected in the Nc amplitudes to the oddball stimuli (Reynolds and Richards, 2005).
A similar argument holds for the interpretation of the PSW. Quinn et al., 2006, Quinn et al., 2010 related the PSW to the formation of a new category, and Grossman et al. (2009) took this ERP component to reflect the integration of new exemplars into an already existing category. In the present study, the PSW was pronounced to the oddball-different, indicating a strong need for memory updating for this infrequent stimulus. For further research, it may be worthwhile to discuss whether ERP components like the Nc and LSWs reflect specific processes of infant categorization or rather more general processes of visual attention or recognition memory that can be activated by categorical as well as by non-categorical stimulus material.
Because the same stimulus pictures were presented repeatedly in the present study, processes of online-category formation can hardly account for any differences in infants’ responses to the oddball-same and oddball-different. Rather, infants were presumably forming representations of the single stimuli, and this process apparently required different attentional and memory resources for each of the three pictures. Most importantly, infants showed different responses to both oddball stimuli. If infants had relied mainly on overall perceptual similarity when discriminating between the stimuli (as proposed for instance by Quinn, 2002), the oddball-same should have elicited more pronounced ERP responses (in terms of Nc and PSW) because it shared less perceptual features with the standard stimulus than the oddball-different. Rather, infants showed a larger Nc latency and PSW in response to the oddball-different, thus suggesting that infants were involved in the process of category-identification. This raises the question what type of information they may have used to identify category membership. Based on an analysis of the pictures we assume that facial features, general shape, or shape configuration must have played a crucial role in this context (Gelman and Opfer, 2002, Rakison and Poulin-Dubois, 2001, Wiggett et al., 2009).
In combination with previous findings of Jeschonek et al. (2010) who also studied infants’ brain responses to animal and furniture stimuli but presented multiple different exemplars of each category without prior familiarization, our results suggest that 7-month-olds regard category-relevant information during stimulus processing, even when online-category formation is unlikely or even impossible. Based on what they saw on previous trials, infants may have formed an expectation regarding the category identity of the next stimulus. If the next stimulus bears similar category-relevant features as the previous stimuli, stimulus processing is facilitated. If the next stimulus is perceptually different, increased attention is required. If it is different in both perceptual and category-relevant features, special cognitive effort is required for stimulus encoding.
Taken together, the results reported here corroborate that category identification is a fundamental ability of the human cognitive system, probably influencing stimulus processing on a very basal level. It is up to future research to investigate whether infants activate different brain areas when processing stimuli of animals or of non-living objects, like this has been suggested for adults (e.g., Mahon and Caramazza, 2007, Wiggett et al., 2009). Moreover, further studies should aim at expanding our knowledge on the significance of ERP components as indicators for infants’ visual attention and recognition memory as well as for infant categorization on different hierarchical levels.
Conflict of interest statement
None declared.
Acknowledgements
Much appreciation to the infants and parents who participated in this research. Thanks to the student research assistants for running the experiments and scoring the video tapes.
Contributor Information
Birgit Elsner, Email: birgit.elsner@uni-potsdam.de.
Susanna Jeschonek, Email: susanna.jeschonek@haw-hamburg.de.
Sabina Pauen, Email: sabina.pauen@psychologie.uni-heidelberg.de.
References
- Ackles P., Cook K. Stimulus probability and event-related potentials of the brain in 6-month-old human infants. A parametric study. International Journal of Psychophysiology. 1998;29:115–143. doi: 10.1016/s0167-8760(98)00013-0. [DOI] [PubMed] [Google Scholar]
- Courchesne E., Ganz L., Norcia A.M. Event-related brain potentials to human faces in infants. Child Development. 1981;52:804–811. [PubMed] [Google Scholar]
- de Haan M. Psychology Press; New York: 2007. Infant EEG and Event-related Potentials. [Google Scholar]
- de Haan M., Nelson C.A. Recognition of the mother's face by six-month-old infants. Child Development. 1997;68:187–210. [PubMed] [Google Scholar]
- Gelman S.A., Opfer J.E. Development of the animate–inanimate distinction. In: Goswami U., editor. Blackwell Handbook of Childhood Cognitive Development. Blackwell Publishing; Malden: 2002. pp. 151–166. [Google Scholar]
- Grossmann T., Gliga T., Johnson M.H., Mareschal D. The neural basis of perceptual category learning in human infants. Journal of Cognitive Neuroscience. 2009;21:2276–2286. doi: 10.1162/jocn.2009.21188. [DOI] [PubMed] [Google Scholar]
- Hill-Karrer J., Karrer R., Bloom D., Chaney L., Davis R. Event-related brain potentials during an extended visual recognition memory task depict delayed development of cerebral inhibitory processes among 6-month-old infants with Down syndrome. International Journal of Psychophysiology. 1998;29:167–200. doi: 10.1016/s0167-8760(98)00015-4. [DOI] [PubMed] [Google Scholar]
- Hunter S., Karrer R. ERPs indicate event duration effects on infants’ visual attention and recognition memory. Electroencephalography and Clinical Neurophysiology. 1993;87:S38. [Google Scholar]
- Jeschonek S., Marinovic V., Hoehl S., Elsner B., Pauen S. Do animals and furniture items elicit different brain responses in human infants? Brain & Development. 2010;32:863–871. doi: 10.1016/j.braindev.2009.11.010. [DOI] [PubMed] [Google Scholar]
- Karrer R., Monti L.A. Event-related potentials of 4–7-week-old infants in a visual recognition memory task. Electroencephalography and Clinical Neurophysiology. 1995;94:414–424. doi: 10.1016/0013-4694(94)00313-a. [DOI] [PubMed] [Google Scholar]
- Luo Y., Kaufman L., Baillargeon R. Young infants’ reasoning about physical events involving inert and self-propelled objects. Cognitive Psychology. 2009;58:441–486. doi: 10.1016/j.cogpsych.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahon B.Z., Caramazza A. The organization and representation of conceptual knowledge in the brain: living kinds and artifacts. In: Margolis E., Laurence S., editors. Creations of the Mind: Theories of Artifacts and Their Representation. Oxford University Press; New York: 2007. pp. 157–187. [Google Scholar]
- Mandler J.M. How to build a baby. II. Conceptual primitives. Psychological Review. 1992;99:587–604. doi: 10.1037/0033-295x.99.4.587. [DOI] [PubMed] [Google Scholar]
- Mandler J.M., McDonough L. On developing a knowledge base in infancy. Developmental Psychology. 1998;34:1274–1288. doi: 10.1037//0012-1649.34.6.1274. [DOI] [PubMed] [Google Scholar]
- Nelson C.A., Collins P.F. Event-related potential and looking-time analysis of infants’ responses to familiar and novel events: implications for visual recognition memory. Developmental Psychology. 1991;27:50–58. [Google Scholar]
- Nelson C.A., Wewerka S., Thomas K.M., deRegnier R.-A., Tribbey-Walbridge S., Georgieff M. Neurocognitive sequelae of infants of diabetic mothers. Behavioral Neuroscience. 2000;114:950–956. [PubMed] [Google Scholar]
- Pauen S. The global-to-basic level shift in infants’ categorical thinking: first evidence from a longitudinal study. International Journal of Behavioral Development. 2002;26:492–499. [Google Scholar]
- Pauen S., Träuble B. How 7-month-olds interpret ambiguous motion events: category-based reasoning in infancy. Cognitive Psychology. 2009;59:275–295. doi: 10.1016/j.cogpsych.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Quinn P.C. Early categorization: a new synthesis. In: Goswami U., editor. Blackwell Handbook of Childhood Cognitive Development. Blackwell; Malden: 2002. pp. 84–101. [Google Scholar]
- Quinn P.C., Doran M.M., Reiss J.E., Hoffman J.E. Neural markers of subordinate-level categorization in 6- to 7-month-old infants. Developmental Science. 2010;13:499–507. doi: 10.1111/j.1467-7687.2009.00903.x. [DOI] [PubMed] [Google Scholar]
- Quinn P.C., Eimas P.D. Perceptual cues that permit categorical differentiation of animal species by infants. Journal of Experimental Child Psychology. 1996;63:189–211. doi: 10.1006/jecp.1996.0047. [DOI] [PubMed] [Google Scholar]
- Quinn P.C., Johnson M.H. Global-before-basic object categorization in connectionist networks and 2-month-old infants. Infancy. 2000;1:31–46. doi: 10.1207/S15327078IN0101_04. [DOI] [PubMed] [Google Scholar]
- Quinn P.C., Westerlund A., Nelson C.A. Neural markers of categorization in 6-month-old infants. Psychological Science. 2006;17:59–66. doi: 10.1111/j.1467-9280.2005.01665.x. [DOI] [PubMed] [Google Scholar]
- Rakison D.H., Poulin-Dubois D. Developmental origin of the animate–inanimate distinction. Psychological Bulletin. 2001;127:209–228. doi: 10.1037/0033-2909.127.2.209. [DOI] [PubMed] [Google Scholar]
- Reynolds G.D., Guy M.W. Brain–behavior relations in infancy: integrative approaches to examining infant looking behavior and event-related potentials. Developmental Neuropsychology. 2012;37:210–225. doi: 10.1080/87565641.2011.629703. [DOI] [PubMed] [Google Scholar]
- Reynolds G.D., Richards J.E. Familiarization, attention and recognition memory in infancy: an ERP and cortical source localisation study. Developmental Psychology. 2005;41:598–615. doi: 10.1037/0012-1649.41.4.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosch E. Principles of categorization. In: Rosch E., Lloyd B., editors. Cognition and Categorization. Erlbaum; Hillsdale: 1978. pp. 27–48. [Google Scholar]
- Stets M., Reid V.M. Infant ERP amplitudes change over the course of an experimental session: implications for cognitive processes and methodology. Brain & Development. 2011;33:558–568. doi: 10.1016/j.braindev.2010.10.008. [DOI] [PubMed] [Google Scholar]
- Stets M., Stahl D., Reid V.M. A meta-analysis investigating factors underlying attrition rates in infant ERP studies. Developmental Neuropsychology. 2012;37:226–252. doi: 10.1080/87565641.2012.654867. [DOI] [PubMed] [Google Scholar]
- Thomas D.G., Grice J.W., Najm-Briscoe R.G., Miller J.W. The influence of unequal numbers of trials on comparisons of average event-related potentials. Developmental Neuropsychology. 2004;26:753–774. doi: 10.1207/s15326942dn2603_6. [DOI] [PubMed] [Google Scholar]
- Wiggett A.J., Pritchard I.C., Downing P.E. Animate and inanimate objects in human visual cortex: evidence for task-independent category effects. Neuropsychologia. 2009;47:3111–3117. doi: 10.1016/j.neuropsychologia.2009.07.008. [DOI] [PubMed] [Google Scholar]