Highlights
* Prenatal MeHg exposure induced long-lasting learning and memory impairments * Cipura paludosa may be of interest in neuropathological conditions * Cipura improves behaviour and memory of rats exposed to MeHg during early development
Keywords: Cipura paludosa, Learning and memory, Methylmercury (MeHg), Neurological deficits
Abstract
Previous studies from our group have indicated important biological properties of the ethanolic extract (EE) and isolated compounds from the bulbs of Cipura paludosa (Iridaceae), a native plant widely distributed in northern Brazil. In the present study, the effects of chronic treatment with the EE on the memory of adult rats exposed to methylmercury (MeHg) during early development were assessed. Pregnant rats were treated by gavage with a single dose of MeHg (8mg/kg) on gestational day 15, the developmental stage critical for cortical neuron proliferation. Adult offspring were administered orally with the EE of C. paludosa (1, 10 or 100mg/kg) over 14 consecutive days. EE improved short-term social memory in a specific manner and facilitated the step-down inhibitory avoidance of short- and long-term memory. MeHg exposure induced pronounced long-lasting impairments in social recognition memory that were improved by EE. Moreover, EE significantly increased the step-down latencies specifically during the short-term session in prenatal MeHg-exposed rats. These results demonstrate that EE reduced the long-lasting short-term learning and memory deficits induced by MeHg exposure. These findings may encourage further studies evaluating the cognitive enhancing properties of C. paludosa and its components on neuropathological conditions associated with exposure to environmental contaminants.
1. Introduction
Consumption of methylmercury (MeHg)-contaminated food by pregnant women represents one of the most serious potential hazards for their offspring. While high-dose exposure may result in cerebral palsy, deafness, and severe mental retardation associated with disorganisation of cerebral cortex cyto-architecture and atrophy of the folia of cerebellum hemispheres, lower MeHg doses may produce more subtle neurobehavioural changes (Choi, 1989, Liang et al., 2009, National Research Council, 2000).
Experimental data obtained in rodents show that the consequences of in utero exposure to MeHg include increased intrauterine mortality of offspring, delayed developmental growth, altered cellular brain arrangements, and more subtle effects such as delayed reflexive behaviours, impairment of locomotor activity and motor coordination, cognitive and emotional dysfunctions, depending on the duration and level of MeHg exposure at different developmental stages of offspring (Ferraro et al., 2009, Lucena et al., 2010, Maia et al., 2009). Moreover, as recently reviewed by Farina et al. (2011), experimental studies have been pivotal in elucidating the molecular mechanisms that mediate MeHg-induced neurotoxicity that include the impairment of intracellular calcium homeostasis (Sirois and Atchison, 2000), oxidative stress (Franco et al., 2007, Lucena et al., 2010, Ou et al., 1999), and the alteration of glutamate homeostasis (Aschner et al., 2000, Farina et al., 2003, Manfroi et al., 2004).
Although MeHg-induced neurotoxicity is an extensively reported phenomenon, there are no effective treatments available that completely abolish its toxic effects; thus, supportive care is often needed to maintain vital functions. In addition, chelating agents that aid the body's ability to eliminate mercury from tissues are of limited use because of their adverse side effects (Tchounwou et al., 2003). Interestingly, more recent studies have indicated the potential of some purified phytochemicals or plant extracts to confer protection against excitotoxicity and oxidative stress following co-exposure with MeHg (Lucena et al., 2007a, Farina et al., 2005, Yongjin et al., 2008). Possible mechanisms involved in the mitigation of MeHg effects by phytochemicals may include the reduction of reactive oxygen species, activation of enzymatic antioxidant systems, restoration of the mitochondrial membrane potential, and modulation of cell signalling pathways (Campos-Esparza et al., 2009, Farina et al., 2011, Franco et al., 2010).
In this context, previous findings from our group have demonstrated the protective effects of the ethanolic extract (EE) of Cipura paludosa Aubl. against the MeHg-induced neurotoxicity in adult mice (Lucena et al., 2007a). C. paludosa, a member of the large family Iridaceae, is principally characterised by a bulbous rootstock (Goldblatt, 1990). It is a native plant, widely distributed in the north of Brazil, popularly known as ‘batata roxa’, ‘alho-do-mato’ and ‘cebolinha-do-campo’. During the last years, we have extensively studied the pharmacological properties of the EE, fractions and isolated compounds from the bulbs of this plant. Our previous studies indicated a series of biological activities of C. paludosa including antinociceptive and anti-inflammatory (Lucena et al., 2007b), and antioxidant and antiglutamatergic (Lucena et al., 2007a) effects. More recently, we also demonstrated the in vivo effects of the major compounds (eleutherine and isoeleutherine) in different models of hypernociception and inflammation in mice (Tessele et al., 2011), justifying, at least in part, its popular therapeutic use for treating conditions related to inflammation and dolorous processes.
Of high interest, we observed recently that chronic treatment with the EE of C. paludosa attenuated the anxiety- and depression-like behaviours and the antioxidant deficits induced by prenatal MeHg exposure in rats (Lucena et al., 2010). Taking into account the absence of effective treatments for neurological deficits associated with MeHg exposure and the existence of previous findings demonstrating that C. paludosa may represent a valuable tool to attenuate long-lasting emotional defects induced by prenatal MeHg exposure, we investigated in the current study the effects of chronic administration of the EE of C. paludosa on the short- and long-term learning and memory impairments in adult rats born to dams treated with MeHg.
2. Materials and methods
2.1. Breeding and prenatal treatment
Three-month-old male and female Wistar rats, obtained from the Animal Facility of Centro Universitário de Brasília (UNICEUB, Brazil), were housed in groups of five animals/sex in polycarbonate cages under controlled conditions of temperature (23±1°C) and photoperiod (light:dark, 12:12h) with free access to food and water. For mating, individual females were placed overnight with a single male. Detection of a sperm plug the next morning denoted pregnancy, indicating gestation day one. Pregnant rats were housed individually in polycarbonate cages and assigned randomly to receive tap water or MeHg (8mg/kg) on gestational day 15. This day represents a critical developmental stage when the forebrain ventricular zone produces neurons in the embryonic cerebral cortex (DiCicco-Bloom and Sondell, 2005). Solutions were administered by means of intragastric intubation (gavage) at a volume of 1ml/kg of body weight dissolved in NaCl 0.9% (saline). The schedule of MeHg treatment was selected according to previous literature (Lucena et al., 2010, Maia et al., 2009, Zanoli et al., 1994) and it represents a model for the study of long-lasting behavioural impairments of acute poisoning during pregnancy.
2.2. Pregnancy outcome
The day of birth was designated as postnatal day one. After birth, the litters were culled to ten pups per litter and returned to their mothers until weaning on postnatal day 21, when they were then grouped (n=5 animals) by sex and regimen of treatment. During the lactation period, offspring were maintained with their respective dams to avoid interference related to maternal deprivation, which has been linked to behavioural alterations including anxiety, depression and cognitive dysfunction (Daniels et al., 2004, Huot et al., 2002).
All behavioural experiments were carried out with two-month-old male rats to avoid the well-described fluctuations of learning and memory performance of female rats across the oestrous cycle (Korol et al., 2004, Pompili et al., 2010). Moreover, previous epidemiological studies with children (Grandjean et al., 1998) and experimental studies with laboratory animals (Gimenez-Llort et al., 2001, Onishchenko et al., 2007, Rossi et al., 1997) on MeHg exposure have also reported more pronounced developmental effects in males than in females, although the mechanisms underlying such gender-related differences need to be further clarified. All procedures used in the present study were in compliance with the guidelines on animal care of the UnB Ethics Committee on the Use of Animals, which follows the “Principles of laboratory animal care” from NIH publication no. 85-23.
2.3. Preparation of the EE of C. paludosa and treatment
Plant material was collected at Porto Velho, State of Rondônia, Brazil. A voucher specimen was deposited at the Herbarium Dr. Ary Tupinambá Penna Pinheiro (Porto Velho, Brazil) under number 1782. Bulbs from C. paludosa were dried in a stove at 40°C and subsequently powdered using a mortar and pestle. The powder was incubated with ethanol (95%, v/v) at room temperature for approximately seven days. The supernatant was filtered on filter paper, and the bulb powder was submitted to a second extraction with ethanol. The supernatants from both ethanol extractions were combined, and the solvent was evaporated under reduced pressure at 40°C to obtain the crude EE.
EE of C. paludosa was dissolved in NaCl 0.9% (saline) plus Tween 80 (Merck, Darmstadt, Germany). The final concentration of Tween 80 did not exceed 2% and did not cause any per se effects. The control solution consisted of saline with 2% Tween 80 (vehicle). The doses of the EE of C. paludosa (1, 10 or 100mg/kg), selected according to previous literature (Lucena et al., 2007a, Lucena et al., 2010), were administered orally in a volume of 1ml/kg of body weight to adult male offspring rats (two months old) for 14 consecutive days. All behavioural experiments were carried out 1h following the last extract administration. The present treatment schedule was selected according to a previous study from our group (Lucena et al., 2010) and was designed to investigate the effects of chronic extract treatment on the long-lasting cognitive deficits induced by prenatal MeHg exposure.
2.4. Behavioural tests
Animals were acclimated to the experimental room for at least 2h before beginning the experimental procedures, which were carried out between 8a.m. and 12p.m. in order to avoid circadian influence and any kind of stress that could have interfered with the animals’ behaviour. The behavioural tests were conducted in independent groups of animals (n=10 animals in each group).
2.4.1. Social recognition task
Short-term social recognition memory was assessed with the social recognition task as previously described (Dantzer et al., 1987, Prediger et al., 2005). Adult rats were housed individually in plastic cages (42cm×34cm×17cm) and were used only after at least seven days of habituation to their new environment. Juvenile rats (25–30 days old, 100–150g) were kept in groups of 10 per cage and served as social stimuli for the adult rats. Animals were habituated for at least 1h before the beginning of the experiment. All juvenile rats were isolated in individual cages for 20min prior to the beginning of the experiment.
The social recognition task consisted of two successive presentations (5min each) separated by a delay period, during which a juvenile rat was placed in the home cage of the adult rat, and the time spent by the adult investigating the juvenile (nosing, sniffing, grooming or pawing) was recorded. At the end of the first presentation, the juvenile was removed and kept in an individual cage during the delay period and re-exposed to the adult rat after 30 or 120min. In this kind of test, if the delay period is less than 40min, adult male rats display recognition of this juvenile as indicated by a significant reduction of the social investigation time during the second presentation (Prediger and Takahashi, 2003, Prediger et al., 2005). However, when the same juvenile is re-exposed for a longer period of time (more than 60min) after the first presentation, the adult rat no longer recognises this juvenile; i.e. the social investigation time in the second presentation is similar to that observed during the first. Thus, 30-min and 120-min intervals were selected as temporal windows suitable for testing memory-disrupted and memory-enhancing treatments, respectively. An additional experiment was performed to discard non-memory related effects of the extract administration. In this experiment, an unfamiliar juvenile rat (i.e. different from that used in the first presentation) was exposed to the adult rat during the second encounter, with a similar expected duration of social investigation time (Prediger and Takahashi, 2003, Prediger et al., 2005).
2.4.2. Inhibitory avoidance task
The inhibitory avoidance apparatus was an acrylic box (50cm×25cm×25cm), the floor of which consisted of parallel stainless steel bars (1mm diameter) spaced 1cm apart. A platform (7-cm wide×2.5-cm high) was placed on the floor against the left wall. Animals were placed on the platform, and the time from latency to step-down on the grid with four paws was measured with an automatic device. Animals were submitted to the inhibitory avoidance task using a protocol similar to that described previously (Prediger et al., 2008). During training sessions, immediately after stepping down on the grid, the animals received a 0.4-mA, 1.0-s scrambled foot shock. During test sessions, no foot shock was administered, and the step-down latency (maximum 180s) was used as measure of retention. Animals were submitted to a single training session. In order to evaluate short- and long-term memory, test sessions were performed 1.5 and 24h after training, respectively. The last administration of EE or saline was performed by oral route 1h before training in the apparatus of inhibitory avoidance.
2.5. Statistical analysis
Data on the inhibitory avoidance task are shown as the medians (interquartile range) of step-down latencies. Comparisons of test session step-down latencies between groups were performed with a Kruskal–Wallis test followed by a Dunn's multiple comparison test. Data on the social recognition task were expressed as means±S.E.M, and statistical comparisons were carried out using two-way analysis of variance (ANOVA) with pre-treatment (MeHg or control) and treatment (EE of C. paludosa or vehicle) as independent variables. Following significant ANOVAs, multiple post hoc comparisons were performed using a Newman–Keuls test. The accepted level of significance for the tests was p≤0.05. All tests were performed using the Statistica® software package (StatSoft Inc., Tulsa, OK, USA).
3. Results
3.1. Social recognition task
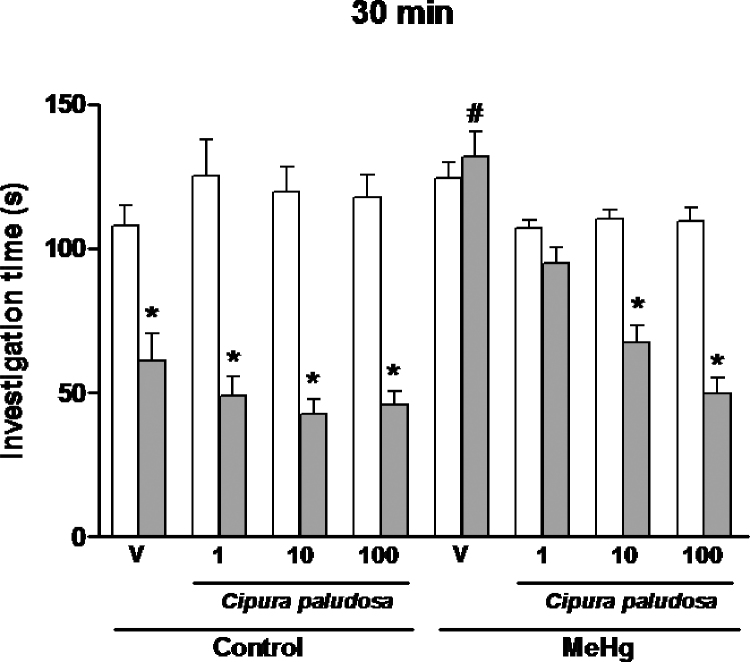
The results for the effects of chronic oral administration of the EE of C. paludosa (1, 10 or 100mg/kg) or vehicle on the social recognition memory of prenatal MeHg-exposed rats evaluated when the same juvenile rat was re-exposed after 30min are shown in Fig. 1. One-way ANOVA revealed a significant effect of the treatment factor with EE [F(15,159)=27.47, p<0.0001] on the investigation time during the second presentation of the familiar juvenile rat. Subsequent Newman–Keuls tests indicated that, at this time point, MeHg-treated rats were not able to recognise the juvenile rat because no significant reduction in the investigation time was observed during the second encounter. Importantly, repeated oral administration of the EE of C. paludosa (10 or 100mg/kg) reduced the investigation time of MeHg-treated rats during the second presentation of the familiar juvenile, indicating that treatment with the extract of C. paludosa was able to reverse the social recognition memory deficits associated with prenatal MeHg exposure.
Fig. 1.
Effects of repeated oral administration of the ethanolic extract of Cipura paludosa (1, 10 or 100mg/kg, once daily for 14 days) or vehicle (V) on social investigation times of prenatally methylmercury (MeHg)-exposed adult rats when the same juvenile was re-exposed at 30min after the first presentation (n=10 per group). Bars represent investigation times (mean±S.E.M., in seconds) in the first (white) and second presentation (grey). *p≤0.05 compared to the first presentation of the same group. #p≤0.05 compared to the second presentation of the vehicle-treated animals of the control group (Newman–Keuls test).
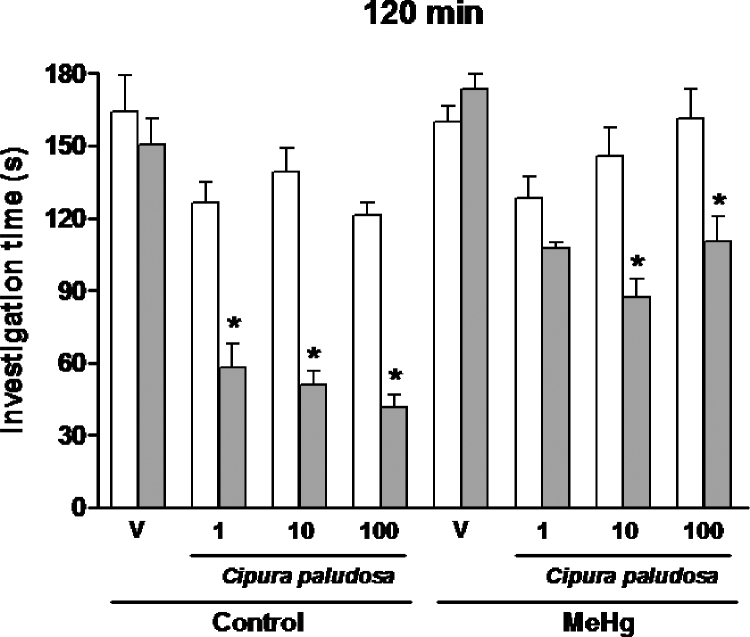
The effects of repeated oral administration of EE or vehicle on the social recognition memory of prenatal MeHg-exposed rats evaluated when the same juvenile rat was re-exposed after 120min are shown in Fig. 2. One-way ANOVA revealed a significant effect of the treatment factor with EE [F(15,159)=21.04; p<0.0001] on the investigation time during the second presentation of the familiar juvenile rat. Subsequent post hoc comparisons indicated that, at this time point, vehicle- or prenatal MeHg-exposed rats were not able to recognise the juvenile rat because no significant reduction in the investigation time was observed during the second encounter. Interestingly, all tested doses of the EE of C. paludosa (1, 10 or 100mg/kg) significantly decreased the investigation time displayed by prenatal vehicle-exposed rats in the forgetting procedure (exposure 120min later to a familiar juvenile), suggesting that EE facilitates short-term social memory. Moreover, repeated oral administration of the EE of C. paludosa (10 or 100mg/kg) reduced the investigation time of MeHg-treated rats during the second presentation of the familiar juvenile, suggesting that the social memory-enhancing properties of EE also extend to intrauterine MeHg-exposed rats.
Fig. 2.
Effects of repeated oral administration of the ethanolic extract of Cipura paludosa (1, 10 or 100mg/kg, once daily for 14 days) or vehicle (V) on social investigation times of prenatally methylmercury (MeHg)-exposed adult rats when the same juvenile was re-exposed at 120min after the first presentation (n=10 per group). Bars represent investigation times (mean±S.E.M., in seconds) in the first (white) and second presentation (grey). *p≤0.05 compared to the first presentation of the same group (Newman–Keuls test).
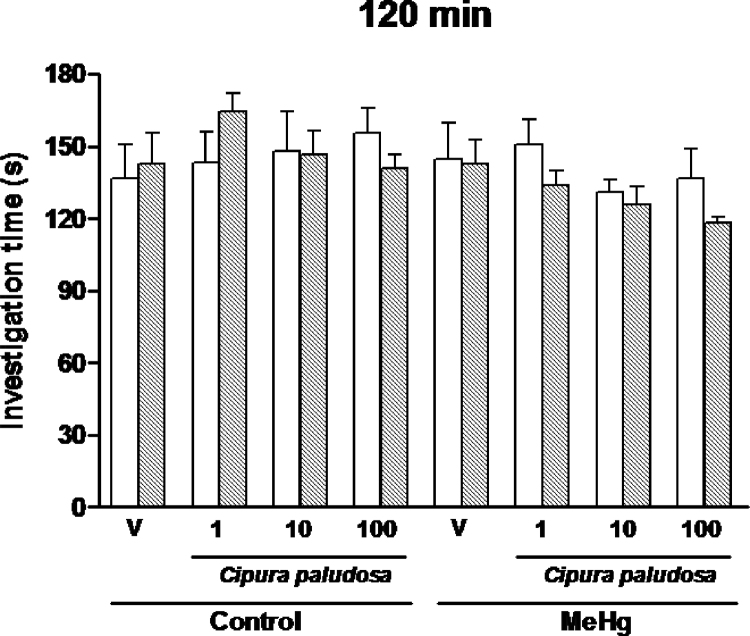
In addition, as shown in Fig. 3, when a different juvenile from the first encounter was used during the second presentation, no significant reduction in the investigation time was observed in the groups [F(15,159)=1.14; p=0.33]. These results suggest that the EE of C. paludosa given at the indicated doses specifically improves the long-lasting social recognition memory deficits of adult rats exposed to intrauterine MeHg.
Fig. 3.
Effects of repeated oral administration of the ethanolic extract of Cipura paludosa (1, 10 or 100mg/kg, once daily for 14 days) or vehicle (V) on social investigation times of adult rats exposed prenatally methylmercury (MeHg)-exposed adult rats when a different juvenile was re-exposed at 120min after the first presentation (n=10 per group). Bars represent investigation times (mean±S.E.M., in seconds) in the first (white) and second presentation (hatched).
3.2. Inhibitory avoidance task
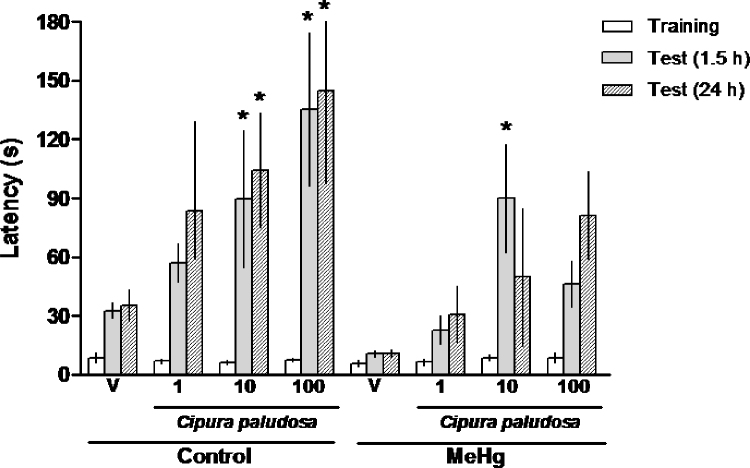
The effects of oral chronic treatment with the EE of C. paludosa (1, 10 or 100mg/kg) or vehicle on the short- and long-term memory of prenatal MeHg-exposed rats evaluated in the step-down inhibitory avoidance task are shown in Fig. 4. The Kruskal–Wallis non-parametric test did not reveal any significant effects of EE treatment on the step-down latencies during the training session (p>0.05). However, there was a significant effect of EE treatment on step-down latencies during the short-term [H(8, N=62.70); p<0.0001] and long-term [H(8, N=56.10); p<0.0001] test sessions.
Fig. 4.
Effects of repeated oral administration of the ethanolic extract of Cipura paludosa (1, 10 or 100mg/kg, once daily for 14 days) or vehicle (V) on the short-term (1.5h) and long-term (24h) memory evaluated in the step-down inhibitory avoidance task in adult rats exposed prenatally to methylmercury (MeHg). Data are shown as the medians (interquartile ranges) of latencies to step-down in the training (white) and test (1.5h: grey; 24h: hatched) sessions (n=10 animals per group). *p≤0.05 compared to the respective session of the control-treated group (Dunn's test).
The Dunn's test indicated that the administration of the EE of C. paludosa (10 or 100mg/kg), 60min before the training session, significantly increased the step-down latencies during the short- and long-term test session performed 1.5h and 24h after the training session, respectively. These results suggest that the extract of C. paludosa at the indicated doses specifically enhances the short- and long-term retention of a step-down inhibitory avoidance test in rats. Moreover, the EE of C. paludosa (10mg/kg) significantly increased the step-down latencies specifically during the short-term session in prenatal MeHg-exposed rats. These results reinforce previous findings obtained in the social recognition task that the extract of C. paludosa specifically improves the long-lasting short-term memory deficits of adult rats exposed to MeHg during intrauterine life.
4. Discussion
There is an increasing number of studies investigating the protective effects of plants or natural compounds on various neuropathological conditions. Remarkably, it has been demonstrated that plants/natural compounds are able to counteract metal-induced neurotoxicity under in vivo conditions (Farina et al., 2005, Franco et al., 2010, Gupta and Flora, 2006, Xu et al., 2005). For instance, Farina et al. (2005) showed the beneficial effects of the hydroalcoholic extract of plants of the genus Polygala against MeHg-induced neurotoxicity in mice. Interestingly, Black et al. (2011) have recently used a rat perinatal exposure model to investigate the modulation of developmental MeHg toxicity by an extract of Rhododendron tomentosum ssp. subarcticum, generally known as Labrador Tea, a plant rich in antioxidants traditionally consumed by Canadia Inuit. The authors observed that R. tomemtosum extract attenuated MeHg's effects on oxidative stress and brain glutamate N-methyl-d-aspartate (NMDA) NMDA receptor levels. However, despite the modulation of these molecular endpoints, R. tomemtosum extract did not lead to significant alterations of MeHg's effects on rat neurobehaviour (Black et al., 2011).
In addition, an in vitro study showed that quercetin, a well known flavonoid with antioxidant properties, prevented the mitotoxic effects of MeHg by inhibiting MeHg-induced reactive oxygen species formation (Franco et al., 2007). In the same study, other compounds such as coumarins and xanthones, did not display protective effects, suggesting that flavonoids may represent promising neuroprotective agents to counteract MeHg-induced neurotoxicity. Corroborating these findings, Franco et al. (2010) have demonstrated that the co-incubation of mouse brain mitochondria with two flavonoids (myricetin and myricitrin) derived from medicinal plants caused a concentration-dependent decrease of MeHg-induced mitochondrial dysfunction and oxidative stress. Therefore, consumption of these plants or natural compounds, which are already part of the cultural background of many communities, may therefore represent a convenient and practical method to increase phytochemical-based antioxidant intake in populations exposed to MeHg.
In this regard, previous findings from our group (Lucena et al., 2007a) indicated the protective effects of the EE of C. paludosa against MeHg-induced neurotoxicity in adult mice. Additionally, we observed recently that chronic treatment with the EE of C. paludosa attenuated the anxiety- and depression-like behaviours and the antioxidant deficits induced by prenatal MeHg exposure in rats (Lucena et al., 2010). Taking into account the absence of effective treatments for neurological deficits associated with MeHg exposure and the existence of previous findings demonstrating that C. paludosa may represent a valuable tool to attenuate long-lasting emotional changes induced by prenatal MeHg exposure, we investigated in the current study the effects of chronic administration of the EE of C. paludosa on the short- and long-term learning and memory impairments in adult rats born to dams treated with MeHg.
Behaviour is an important endpoint in studies on the effects of environmental agents on the nervous system of mammals and can be a useful tool for exploring whether toxic agents can interact to exacerbate behavioural aberrations such as learning and memory impairments (for review see Farina et al., 2011). The present findings show that a single oral dose of MeHg (8mg/kg) during intrauterine development interferes with long-lasting neurobehavioural responses in adult rat offspring, inducing marked learning and memory impairments, which reinforces previous findings from our group (Lucena et al., 2010, Maia et al., 2009, Maia et al., 2010) and others (Carratù et al., 2006, Ferraro et al., 2009, Onishchenko et al., 2007). Moreover, in the present study we extend the potential pharmacological properties of C. paludosa, demonstrating its cognitive enhancing activity on the social recognition memory and inhibitory avoidance short- and long-term memory of adult rats. Of high importance, the results of the current study demonstrate, for the first time, that the chronic oral treatment with the EE of C. paludosa reduced the long-lasting short-term learning and memory deficits induced by prenatal MeHg.
There is increasing concern about neurological defects in humans, and environmental contaminants have been proposed as possible causes of learning and emotional disturbances that manifest at a young age and neurodegenerative diseases later in life (Grandjean and Herz, 2011, Landrigan et al., 2005, National Research Council, 2000). MeHg is known to be an environmental neurotoxin that potentially causes neurological abnormalities, cognitive impairment, and behavioural disturbances in humans and laboratory animals (Bourdineaud et al., 2011, Yorifuji et al., 2011). More recently, developmental exposure to low doses of MeHg found in seafood was found to be a risk factor for cognitive and emotional disorders (e.g. attention, memory and emotional deficits) in children and adolescents in the fish-eating population of the Faroe Islands (Debes et al., 2006, Grandjean et al., 1997). In comparing the relevance of different animal models to human MeHg exposure, it should be noted that models of low-level exposure are more appropriate to simulate neurotoxic effects occurring in high fish-consuming populations. The most intriguing aspect is that, following MeHg exposure in utero, an infant who appears normal at birth may develop psychomotor deficits as the nervous system matures (Marsh et al., 1987). Indeed, the foetus appears to be more sensitive to toxic effects of MeHg than the mother, and adverse neurodevelopmental effects have been reported in the offspring of women showing little or no overt toxicity (Choi et al., 1978, Marsh et al., 1987).
The MeHg dose used in this study was selected on the basis of previous studies showing that, following maternal administration of 8mg/kg on gestational day 15, the brain concentration of MeHg in the offspring at birth is 60-fold higher than that in the brain of vehicle-treated rats, and it remains three-fold higher until postnatal day 60 (Cagiano et al., 1990, Maia et al., 2009, Maia et al., 2010). Moreover, using this same schedule of MeHg administration, we demonstrated recently that developmental exposure to low levels of MeHg induces long-lasting alterations in anxiety- and depression-like behaviour as well as inhibition of the antioxidant enzymes catalase (CAT) and glutathione peroxidase (GPx) in the cortex and cerebellum of rats (Lucena et al., 2010). These findings reinforce the notion of early exposure to environmental contaminants as a possible risk factor for neurodevelopmental disorders.
It has been well established that memories can be classified differently according to their duration in working memory (immediate memory lasting seconds or a few minutes), short-term memory (developing in a few seconds or minutes and lasting for several hours) and long-term memory (consolidating slowly and remaining relatively permanent) (for a review see Izquierdo et al., 1999). Short- and long-term memories are identified as separate entities (Izquierdo et al., 1999). These distinct types of memory can be evaluated in animal studies using different behavioural tasks. Thorpe et al. (2004) highlighted the importance of having more than one potential measure of memory since it is possible that null results are due to the test not being sensitive enough, rather than a failure in learning and/or memory. Therefore, a battery of behavioural assays, rather than a singe test, should be included in the investigation of the effects of substances and procedures on learning and memory.
In the present study we employed two widely used behavioural tasks (social recognition and inhibitory avoidance) for the investigation of the effects C. paludosa on learning and memory of rodents. As stated by Dantzer et al. (1987), a form of memory very similar to factual memory in humans that has received little attention in behavioural pharmacology is the so-called “social memory” or “recognition paradigm”, which is mainly generated from olfactory cues. This memory model is based on the fact that rodents spend more time investigating unfamiliar juvenile conspecifics more intensely than familiar ones. When the same juvenile is presented twice, the social investigation time decreases in the second presentation (Dantzer et al., 1987). Social memory is prolonged by repeated exposure to the stimulus of the juvenile rat and is impaired by retroactively interfering stimuli. It can be facilitated by memory-enhancing drugs and disrupted by pharmacological and pathophysiological models known to impair memory in rodents (Prediger et al., 2008, Prediger and Takahashi, 2005). In contrast to most other forms of learning and memory assessed in rodents, social recognition memory is longer than most tests of working memory but is shorter than various forms of declarative, emotional, or spatial memories, which can be detected 24h later. Additionally, the effects of the EE of C. paludosa on the short- and long-term spatial memories of rats were also investigated using the step-down inhibitory avoidance task, which is based on the animal's behaviour to refrain from executing a previous response. Several brain regions are involved in the learning of this task, including hippocampus, amygdala and prefrontal cortex (for review see Izquierdo et al., 2002).
The present results demonstrate that repeated oral treatment with the EE of C. paludosa (1, 10 or 100mg/kg) during 14 consecutive days decreases the investigation time of the same juvenile rat in the forgetting procedure (exposure 120min later to adult rats), indicating that the extract enhances short-term social memory in rats. This response cannot be attributed to non-memory related effects of the extract because no significant reduction in the investigation time was observed when a different juvenile was used for the second presentation. Furthermore, the positive effects of EE cannot be explained by a direct alteration in locomotor performance of the animals because no significant effects were observed in the total ambulation during the open field test in rats submitted to the same schedule of C. paludosa EE treatment (Lucena et al., 2010). It is important to note that the facilitation of the social recognition memory of adult rats induced by the extract cannot be solely explained by the improvement of the olfactory discrimination performance because EE administration did not alter the investigation/exploration time during the first presentation of the juvenile rat in the social recognition experiments. However, the fact that the specific neuroanatomical structures and neurochemical mechanisms underlying rodent social recognition remain unknown and the influence of motivational status on animals’ degree of interest in the investigation of both familiar and unfamiliar conspecifics represent important limitations of the social recognition task (Ferguson et al., 2002).
Consistent with the results obtained in the social recognition paradigm, a repeated pre-training administration of intermediate doses of the EE of C. paludosa (10 and 100mg/kg, gavage) significantly increased the step-down latencies during test sessions performed 1.5h and 24h after the training session, indicating a facilitation of the step-down inhibitory avoidance short- and long-term memory, respectively. As reviewed by Izquierdo and Medina (1997), the step-down inhibitory avoidance task constitutes an animal model of aversively motivated learning and memory. However, since the fear of foot shock is the main motivation of the step-down inhibitory avoidance task, other factors such as shock sensitivity, anxiety, and exploratory behaviour including sniffing, rearing and locomotor activity could confound partly the interpretation of the results of this task. Moreover, it must be acknowledged that it is not possible to determine the exact site of action or the molecular mechanisms underlying the ability of C. paludosa EE to improve short- and long-term memory in rats. Nevertheless, a hypothesis can be speculated. Ongoing studies from our group have pointed to the cognitive-enhancing properties of the EE of C. paludosa in adult rats and mice. These results have also shown a synergistic response following the co-administration of ‘sub-effective’ doses of the EE of C. paludosa with caffeine or the selective adenosine A2A receptor antagonist ZM241385 in the social recognition and step-down inhibitory avoidance tasks, suggesting that the blockage of adenosine receptors may be responsible for the present results. Although the role of adenosine receptor agonists and antagonists in learning and memory still needs to be elucidated, there is considerable evidence supporting our hypothesis (reviewed in Takahashi et al., 2008). Additional research is certainly necessary for determining the exact molecular mechanisms and anatomical sites involved in the mediation of the effects of the EE of C. paludosa on learning and memory, and this constitutes a very interesting field for future studies.
In the present study, we also demonstrate that a single oral dose of MeHg (8mg/kg) induces pronounced long-lasting impairments in the social recognition memory of adult male rats because they were not able to recognise a juvenile familiar rat after a short period of time (30min). Importantly, we observed that repeated administration of the EE of C. paludosa (10 and 100mg/kg, gavage) improves this cognitive impairment associated with prenatal MeHg exposure in rats, as indicated by a significant reduction in the investigation time during the second presentation of the juvenile rat. Consistent with the results obtained in the social recognition test, we also observed that repeated administration of the EE of C. paludosa (10 or 100mg/kg, gavage) was able to increase the step-down latencies during the short-term session in adult male rats exposed to MeHg. Reinforcing the current findings, previous studies have reported that other plants of the Iridaceae family may confer protection against cerebral ischaemia and learning and memory impairments in rodents (Papandreou et al., 2011, Pitsikas et al., 2007).
The neurochemical basis of MeHg-induced behavioural alterations may involve disturbances in a number of neurotransmitter systems, occurring initially during exposure and followed by long-lasting changes in brain function (Castoldi et al., 2001, Farina et al., 2011, Grandjean and Herz, 2011). Among the best characterised mechanisms of MeHg neurotoxicity are excitotoxicity and oxidative stress (Aschner et al., 2007, Farina et al., 2011, Yin et al., 2007, Yin et al., 2011). Perturbation of glutamate transport in astrocytes by MeHg can lead to over-stimulation and dysfunction of NMDA receptors (Yin et al., 2007). NMDA receptors are part of the glutamatergic system which is crucial for neuronal plasticity, learning and memory (Scheetz and Constantine-Paton, 1994). During foetal development, NMDA receptors are also involved in the establishment of neuronal circuitry (Haberny et al., 2002, Martel et al., 2009), which might constitute an additional target of MeHg toxicity. While NMDA receptors mediated excitotoxicity can lead to the generation of reactive oxygen species (Gasso et al., 2001), MeHg can also generate oxidative stress through direct perturbation of mitochondrial functions (Aschner et al., 2007, Franco et al., 2007). The resultant oxidative stress can lead to cell membrane damage, calcium deregulation, enzyme and cell signalling interference, microtubule disassembly, and ultimately, cell death (Farina et al., 2011).
Of particular importance, studies from our group have shown that C. paludosa EE abolishes MeHg-induced oxidative stress and the inhibition of catalase (CAT) and selenium-glutathione peroxidase (Se-GPx) activities in the cortical and cerebellar tissues in rodents (Lucena et al., 2007a, Lucena et al., 2010). These findings suggest that prenatal MeHg exposure, by causing oxidative stress, promotes the high specificity of MeHg to proteins in the central nervous system, which can alter behavioural and biochemical parameters in adult offspring rats. Therefore, the antioxidant properties of C. paludosa EE appear to represent a possible molecular mechanism involved in the observed protective effects of EE against long-lasting learning and memory deficits in prenatal MeHg-exposed rats. However, additional research is necessary to evaluate whether there exists any interaction between the adenosinergic and other neurotransmitter systems in the current effects of C. paludosa. Finally, a better evaluation of the potential cognitive-enhancing properties of C. paludosa extract and its isolated compounds using additional tasks of spatial memory (such as T-maze or water maze) is also indicated.
5. Conclusions
In conclusion, this study reinforces previous findings showing that acute low-level exposure to MeHg during gestation induces subtle cognitive dysfunctions and further emphasises that consumption of MeHg-contaminated food by pregnant women poses one of the most serious potential hazards for offspring. More importantly, the present results demonstrate for the first time that chronic treatment with the EE of C. paludosa reduced the long-lasting short-term learning and memory deficits induced by prenatal MeHg exposure. Therefore, in the present study we extend the potential pharmacological properties of this specie, demonstrating its cognitive enhancing activity. Experiments are currently in progress in our laboratory to identify the active constituents as well as the molecular mechanisms responsible for the observed protective effects of C. paludosa. Finally, we hope that this article may inspire other experimental research groups to further evaluate the cognitive enhancing properties of C. paludosa and its components on neuropathological conditions associated with exposure to environmental contaminants.
Conflict of interest statement
There are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Acknowledgements
This work was supported by grants from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), both from Brazil. The research was conducted in accordance with national and institutional guidelines for the protection of animal welfare. G.M.L. was supported by a scholarship from CAPES. R.D.S.P. is supported by a research fellowship from CNPq.
References
- Aschner M., Syversen T., Souza D.O., Rocha J.B., Farina M. Involvement of glutamate and reactive oxygen species in methylmercury neurotoxicity. Brazilian Journal of Medical and Biological Research. 2007;40:285–291. doi: 10.1590/s0100-879x2007000300001. [DOI] [PubMed] [Google Scholar]
- Aschner M., Yao C.P., Allen J.W., Tan K.H. Methylmercury alters glutamate transport in astrocytes. Neurochemistry International. 2000;37:199–206. doi: 10.1016/s0197-0186(00)00023-1. [DOI] [PubMed] [Google Scholar]
- Black P., Niu L., Sachdeva M., Lean D., Poon R., Bowers W.J., Chan H.M., Arnason J.T., Pelletier G. Modulation of the effects of methylmercury on rat neurodevelopment by co-exposure with Labrador Tea (Rhododendron tomentosum ssp. subarcticum) Food and Chemical Toxicology. 2011;49:2336–2342. doi: 10.1016/j.fct.2011.06.035. [DOI] [PubMed] [Google Scholar]
- Bourdineaud J.P., Fujimura M., Laclau M., Sawada M., Yasutake A. Deleterious effects in mice of fish-associated methylmercury contained in a diet mimicking the Western populations’ average fish consumption. Environment International. 2011;37:303–313. doi: 10.1016/j.envint.2010.09.003. [DOI] [PubMed] [Google Scholar]
- Cagiano R., de Salvia M.A., Renna G., Tortella E., Braghiroli D., Parenti C., Zanoli P., Baraldi M., Annau Z., Cuomo V. Evidence that exposure to methyl mercury during gestation induces behavioral and neurochemical changes in offspring of rats. Neurotoxicology and Teratology. 1990;12:23–28. doi: 10.1016/0892-0362(90)90108-o. [DOI] [PubMed] [Google Scholar]
- Campos-Esparza M.R., Sanchez-Gomez M.V., Matute C. Molecular mechanisms of neuroprotection by two natural antioxidant polyphenols. Cell Calcium. 2009;45:358–368. doi: 10.1016/j.ceca.2008.12.007. [DOI] [PubMed] [Google Scholar]
- Carratù M.R., Borracci P., Coluccia A., Giustino A., Renna G., Tomasini M.C., Raisi E., Antonelli T., Cuomo V., Mazzoni E., Ferraro L. Acute exposure to methylmercury at two developmental windows: focus on neurobehavioral and neurochemical effects in rat offspring. Neuroscience. 2006;141:1619–1629. doi: 10.1016/j.neuroscience.2006.05.017. [DOI] [PubMed] [Google Scholar]
- Castoldi A.F., Coccini T., Ceccatelli S., Manzo L. Neurotoxicity and molecular effects of methylmercury. Brain Research Bulletin. 2001;55:197–203. doi: 10.1016/s0361-9230(01)00458-0. [DOI] [PubMed] [Google Scholar]
- Choi B.H. The effects of methylmercury on the developing brain. Progress in Neurobiology. 1989;32:447–470. doi: 10.1016/0301-0082(89)90018-x. [DOI] [PubMed] [Google Scholar]
- Choi B.H., Lapham L.W., Amin-Zaki L., Saleem T. Abnormal neuronal migration, deranged cerebral cortical organization, and diffuse white matter astrocytosis of human fetal brain: a major effect of methylmercury poisoning in utero. Journal of Neuropathology and Experimental Neurology. 1978;37:719–733. doi: 10.1097/00005072-197811000-00001. [DOI] [PubMed] [Google Scholar]
- Daniels W.M., Pietersen C.Y., Carstens M.E., Stein D.J. Maternal separation in rats leads to anxiety-like behavior and a blunted ACTH response and altered neurotransmitter levels in response to a subsequent stressor. Metabolic Brain Disease. 2004;19:3–14. doi: 10.1023/b:mebr.0000027412.19664.b3. [DOI] [PubMed] [Google Scholar]
- Dantzer R., Bluthe R.M., Koob G.F., Le Moal M. Modulation of social memory in male rats by neurohypophyseal peptides. Psychopharmacology. 1987;91:363–368. doi: 10.1007/BF00518192. [DOI] [PubMed] [Google Scholar]
- Debes F., Budtz-Jørgensen E., Weihe P., White R.F., Grandjean P. Impact of prenatal methylmercury exposure on neurobehavioral function at age 14 years. Neurotoxicology and Teratology. 2006;28:363–375. doi: 10.1016/j.ntt.2006.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiCicco-Bloom E., Sondell M. vol. 8. Lippincott Williams & Wilkins; Philadelphia: 2005. (Neural Development and Neurogenesis. Kaplan & Sadock's Comprehensive Textbook of Psychiatry). [Google Scholar]
- Farina M., Dahm K.C., Schwalm F.D., Brusque A.M., Frizzo M.E., Zeni G., Souza D.O., Rocha J.B. Methylmercury increases glutamate release from brain synaptosomes and glutamate uptake by cortical slices from suckling rat pups: modulatory effect of ebselen. Toxicological Sciences. 2003;73:135–140. doi: 10.1093/toxsci/kfg058. [DOI] [PubMed] [Google Scholar]
- Farina M., Franco J.L., Ribas C.M., Meotti F.C., Missau F.C., Pizzolatti M.G., Dafre A.L., Santos A.R. Protective effects of Polygala paniculata extract against methylmercury-induced neurotoxicity in mice. Journal of Pharmacy and Pharmacology. 2005;57:1503–1508. doi: 10.1211/jpp.57.11.0017. [DOI] [PubMed] [Google Scholar]
- Farina M., Rocha J.B., Aschner M. Mechanisms of methylmercury-induced neurotoxicity: evidence from experimental studies. Life Sciences. 2011;89:555–563. doi: 10.1016/j.lfs.2011.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson J.N., Young L.J., Insel T.R. The neuroendocrine basis of social recognition. Frontiers in Neuroendocrinology. 2002;23:200–224. doi: 10.1006/frne.2002.0229. [DOI] [PubMed] [Google Scholar]
- Ferraro L., Tomasini M.C., Tanganelli S., Mazza R., Coluccia A., Carratù M.R., Gaetani S., Cuomo V., Antonelli T. Developmental exposure to methylmercury elicits early cell death in the cerebral cortex and long-term memory deficits in the rat. International Journal of Developmental Neuroscience. 2009;27:165–174. doi: 10.1016/j.ijdevneu.2008.11.004. [DOI] [PubMed] [Google Scholar]
- Franco J.L., Braga H.C., Stringari J., Missau F.C., Posser T., Mendes B.G., Leal R.B., Santos A.R., Dafre A.L., Pizzolatti M.G., Farina M. Mercurial-induced hydrogen peroxide generation in mouse brain mitochondria: protective effects of quercetin. Chemical Research in Toxicology. 2007;20:1919–1926. doi: 10.1021/tx7002323. [DOI] [PubMed] [Google Scholar]
- Franco J.L., Posser T., Missau F., Pizzolatti M.G., Dos Santos A.R., Souza D.O., Aschner M., Rocha J.B., Dafre A.L., Farina M. Structure–activity relationship of flavonoids derived from medicinal plants in preventing methylmercury-induced mitochondrial dysfunction. Environmental Toxicology and Pharmacology. 2010;30:272–278. doi: 10.1016/j.etap.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasso S., Cristofol R.M., Selema G., Rosa R., Rodriguez-Farre E., Sanfeliu C. Antioxidant compounds and Ca(2+) pathway blockers differentially protect against methylmercury and mercuric chloride neurotoxicity. Journal of Neuroscience Research. 2001;66:135–145. doi: 10.1002/jnr.1205. [DOI] [PubMed] [Google Scholar]
- Gimenez-Llort L., Ahlbom E., Daré E., Vahter M., Ögren S., Ceccatelli S. Prenatal exposure to methylmercury changes dopamine-modulated motor activity during early ontogeny: age and gender-dependent effects. Environmental Toxicology and Pharmacology. 2001;9:61–70. doi: 10.1016/s1382-6689(00)00060-0. [DOI] [PubMed] [Google Scholar]
- Goldblatt P. Phylogeny and classification of Iridaceae. Annals of the Missouri Botanical Garden. 1990;77:607–627. [Google Scholar]
- Grandjean P., Herz K.T. Methylmercury and brain development: imprecision and underestimation of developmental neurotoxicity in humans. Mount Sinai Journal of Medicine. 2011;78:107–187. doi: 10.1002/msj.20228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandjean P., Weihe P., White R.F., Debes F. Cognitive performance of children prenatally exposed to “safe” levels of methylmercury. Environmental Research. 1998;77:165–172. doi: 10.1006/enrs.1997.3804. [DOI] [PubMed] [Google Scholar]
- Grandjean P., Weihe P., White R.F., Debes F., Araki S., Yokoyama K., Murata K., Sørensen N., Dahl R., Jørgensen P.L. Cognitive deficit in 7-year-old children with prenatal exposure to methylmercury. Neurotoxicology and Teratology. 1997;19:417–428. doi: 10.1016/s0892-0362(97)00097-4. [DOI] [PubMed] [Google Scholar]
- Gupta R., Flora S.J. Effect of Centella asiatica on arsenic-induced oxidative stress and metal distribution in rats. Journal of Applied Toxicology. 2006;26:213–222. doi: 10.1002/jat.1131. [DOI] [PubMed] [Google Scholar]
- Haberny K.A., Paule M.G., Scallet A.C., Sistare F.D., Lester D.S., Hanig J.P., Slikker W., Jr. Ontogeny of the N-methyl-d-aspartate (NMDA) receptor system and susceptibility to neurotoxicity. Toxicological Sciences. 2002;68:9–17. doi: 10.1093/toxsci/68.1.9. [DOI] [PubMed] [Google Scholar]
- Huot R.L., Plotsky P.M., Lenox R.H., McNamara R.K. Neonatal maternal separation reduces hippocampal mossy fiber density in adult Long Evans rats. Brain Research. 2002;950:52–63. doi: 10.1016/s0006-8993(02)02985-2. [DOI] [PubMed] [Google Scholar]
- Izquierdo L.A., Barros D.M., Vianna M.R., Coitinho A., de David e Silva T., Choi H., Moletta B., Medina J.H., Izquierdo I. Molecular pharmacological dissection of short- and long-term memory. Cellular and Molecular Neurobiology. 2002;22:269–287. doi: 10.1023/A:1020715800956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo I., Medina J.H. Memory formation: the sequence of biochemical events in the hippocampus and its connection to activity in other brain structures. Neurobiology of Learning and Memory. 1997;68:285–316. doi: 10.1006/nlme.1997.3799. [DOI] [PubMed] [Google Scholar]
- Izquierdo I., Medina J.H., Vianna M.R., Izquierdo L.A., Barros D.M. Separate mechanisms for short- and long-term memory. Behavioural Brain Research. 1999;103:1–11. doi: 10.1016/s0166-4328(99)00036-4. [DOI] [PubMed] [Google Scholar]
- Korol D.L., Malin E.L., Borden K.A., Busby R.A., Couper-Leo J. Shifts in preferred learning strategy across the estrous cycle in female rats. Hormones and Behavior. 2004;45:330–338. doi: 10.1016/j.yhbeh.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Landrigan P.J., Sonawane B., Butler R.N., Trasande L., Callan R., Droller D. Early environmental origins of neurodegenerative disease in later life. Environmental Health Perspectives. 2005;113:1230–1233. doi: 10.1289/ehp.7571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang J., Inskip M., Newhook D., Messier C. Neurobehavioral effect of chronic and bolus doses of methylmercury following prenatal exposure in C57BL/6 weanling mice. Neurotoxicology and Teratology. 2009;31:372–381. doi: 10.1016/j.ntt.2009.08.007. [DOI] [PubMed] [Google Scholar]
- Lucena G.M., Franco J.L., Ribas C.M., Azevedo M.S., Meotti F.C., Gadotti V.M., Dafre A.L., Santos A.R.S., Farina M. Cipura paludosa extract prevent methyl mercury-induced neurotoxicity in mice. Basic and Clinical Pharmacology and Toxicology. 2007;101:127–131. doi: 10.1111/j.1742-7843.2007.00091.x. [DOI] [PubMed] [Google Scholar]
- Lucena G.M., Gadotti V.M., Maffi L.C., Silva G.S., Azevedo M.S., Santos A.R. Antinociceptive and anti-inflammatory properties from the bulbs of Cipura paludosa Aubl. Journal of Ethnopharmacology. 2007;112:19–25. doi: 10.1016/j.jep.2007.01.031. [DOI] [PubMed] [Google Scholar]
- Lucena G.M., Porto F.A., Campos E.G., Azevedo M.S., Cechinel-Filho V., Prediger R.D.S., Ferreira V.M. Cipura paludosa attenuates long-term behavioral deficits in rats exposed to methylmercury during early development. Ecotoxicology and Environment Safety. 2010;73:1150–1158. doi: 10.1016/j.ecoenv.2010.04.008. [DOI] [PubMed] [Google Scholar]
- Maia C.D., Ferreira V.M., Diniz J.S., Carneiro F.P., de Sousa J.B., da Costa E.T., Tomaz C. Inhibitory avoidance acquisition in adult rats exposed to a combination of ethanol and methylmercury during central nervous system development. Behavioural Brain Research. 2010;211:191–197. doi: 10.1016/j.bbr.2010.03.032. [DOI] [PubMed] [Google Scholar]
- Maia C.S., Lucena G.M., Corrêa P.B., Serra R.B., Matos R.W., Menezes F.C., Santos S.N., Sousa J.B., Costa E.T., Ferreira V.M. Interference of ethanol and methylmercury in the developing central nervous system. Neurotoxicology. 2009;30:23–30. doi: 10.1016/j.neuro.2008.11.008. [DOI] [PubMed] [Google Scholar]
- Manfroi C.B., Schwalm F.D., Cereser V., Abreu F., Oliveira A., Bizarro L., Rocha J.B., Frizzo M.E., Souza D.O., Farina M. Maternal milk as methylmercury source for suckling mice: neurotoxic effects involved with the cerebellar glutamatergic system. Toxicological Sciences. 2004;81:172–178. doi: 10.1093/toxsci/kfh201. [DOI] [PubMed] [Google Scholar]
- Marsh D.O., Clarkson T.W., Cox C., Myers G.J., Amin-Zaki L., Al-Tikriti S. Fetal methylmercury poisoning: relationship between concentration in single strands of maternal hair and child effects. Archives of Neurology. 1987;44:1017–1022. doi: 10.1001/archneur.1987.00520220023010. [DOI] [PubMed] [Google Scholar]
- Martel M.A., Wyllie D.J., Hardingham G.E. In developing hippocampal neurons, NR2B-containing N-methyl-d-aspartate receptors (NMDARs) can mediate signaling to neuronal survival and synaptic potentiation, as well as neuronal death. Neuroscience. 2009;158:334–343. doi: 10.1016/j.neuroscience.2008.01.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council . National Academy Press; Washington, DC: 2000. Toxicological Effects of Methylmercury. [Google Scholar]
- Onishchenko N., Tamm C., Vahter M., Hökfelt T., Johnson J.A., Johnson D.A., Ceccatelli S. Developmental exposure to methylmercury alters learning and induces depression-like behavior in male mice. Toxicological Sciences. 2007;97:428–437. doi: 10.1093/toxsci/kfl199. [DOI] [PubMed] [Google Scholar]
- Ou Y.C., White C.C., Krejsa C.M., Ponce R.A., Kavanagh T.J., Faustman E.M. The role of intracellular glutathione in methylmercury-induced toxicity in embryonic neuronal cells. Neurotoxicology. 1999;20:793–804. [PubMed] [Google Scholar]
- Papandreou M.A., Tsachaki M., Efthimiopoulos S., Cordopatis P., Lamari F.N., Margarity M. Memory enhancing effects of saffron in aged mice are correlated with antioxidant protection. Behavioural Brain Research. 2011;219:197–204. doi: 10.1016/j.bbr.2011.01.007. [DOI] [PubMed] [Google Scholar]
- Pitsikas N., Zisopoulou S., Tarantilis P.A., Kanakis C.D., Polissiou M.G., Sakellaridis N. Effects of the active constituents of Crocus sativus L. crocins on recognition and spatial rats’ memory. Behavioural Brain Research. 2007;183:141–146. doi: 10.1016/j.bbr.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Pompili A., Tomaz C., Arnone B., Tavares M.C., Gasbarri A. Working and reference memory across the estrous cycle of rat: a long-term study in gonadally intact females. Behavioural Brain Research. 2010;213:10–18. doi: 10.1016/j.bbr.2010.04.018. [DOI] [PubMed] [Google Scholar]
- Prediger R.D., Batista L.C., Takahashi R.N. Caffeine reverses age-related deficits in olfactory discrimination and social recognition memory in rats. Involvement of adenosine A1 and A2A receptors. Neurobiology of Aging. 2005;26:957–964. doi: 10.1016/j.neurobiolaging.2004.08.012. [DOI] [PubMed] [Google Scholar]
- Prediger R.D., Fernandes M.S., Rial D., Wopereis S., Pereira V.S., Bosse T.S., Da Silva C.D., Carradore R.S., Machado M.S., Cechinel-Filho V., Costa-Campos L. Effects of acute administration of the hydroalcoholic extract of mate tea leaves (Ilex paraguariensis) in animal models of learning and memory. Journal of Ethnopharmacology. 2008;120:465–473. doi: 10.1016/j.jep.2008.09.018. [DOI] [PubMed] [Google Scholar]
- Prediger R.D., Takahashi R.N. Ethanol improves short-term social memory in rats. Involvement of opioid and muscarinic receptors. European Journal of Pharmacology. 2003;462:115–123. doi: 10.1016/s0014-2999(03)01300-1. [DOI] [PubMed] [Google Scholar]
- Prediger R.D., Takahashi R.N. Modulation of short-term social memory in rats by adenosine A1 and A(2A) receptors. Neuroscience Letters. 2005;376:160–165. doi: 10.1016/j.neulet.2004.11.049. [DOI] [PubMed] [Google Scholar]
- Rossi A.D., Ahlbom E., Ögren S.O., Nicotera P., Ceccatelli S. Prenatal exposure to methylmercury alters locomotor activity of male but not female rats. Experimental Brain Research. 1997;117:428–436. doi: 10.1007/s002210050237. [DOI] [PubMed] [Google Scholar]
- Scheetz A.J., Constantine-Paton M. Modulation of NMDA receptor function: implications for vertebrate neural development. FASEB Journal. 1994;8:745–752. doi: 10.1096/fasebj.8.10.8050674. [DOI] [PubMed] [Google Scholar]
- Sirois J.E., Atchison W.D. Methylmercury affects multiple subtypes of calcium channels in rat cerebellar granule cells. Toxicology and Applied Pharmacology. 2000;167:1–11. doi: 10.1006/taap.2000.8967. [DOI] [PubMed] [Google Scholar]
- Takahashi R.N., Pamplona F.A., Prediger R.D. Adenosine receptor antagonists for cognitive dysfunction: a review of animal studies. Frontiers in Bioscience. 2008;13:2614–2632. doi: 10.2741/2870. [DOI] [PubMed] [Google Scholar]
- Tchounwou P.B., Ayensu W.K., Ninashvili N., Sutton D. Environmental exposure to mercury and its toxicopathologic implications for public health. Environmental Toxicology. 2003;18:149–175. doi: 10.1002/tox.10116. [DOI] [PubMed] [Google Scholar]
- Tessele P.B., Delle Monache F., Quintão N.L., da Silva G.F., Rocha L.W., Lucena G.M., Ferreira V.M., Prediger R.D., Cechinel Filho V. A new naphthoquinone isolated from the bulbs of Cipura paludosa and pharmacological activity of two main constituents. Planta Medica. 2011;77:1035–1043. doi: 10.1055/s-0030-1250745. [DOI] [PubMed] [Google Scholar]
- Thorpe C.M., Jacova C., Wilkie D.M. Some pitfalls in measuring memory in animals. Neuroscience and Biobehavioral Reviews. 2004;28:711–718. doi: 10.1016/j.neubiorev.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Yongjin L., Wei S., Yindong L., Yong Z., Xuefeng H., Chunmei S., Hongbo M., Changwen W., Yong L. Neuroprotective effects of chlorogenic acid against apoptosis of PC12 cells induced by methylmercury. Environmental Toxicology and Pharmacology. 2008;26:13–21. doi: 10.1016/j.etap.2007.12.008. [DOI] [PubMed] [Google Scholar]
- Yin Z., Lee E., Ni M., Jiang H., Milatovic D., Rongzhu L., Farina M., Rocha J.B., Aschner M. Methylmercury-induced alterations in astrocyte functions are attenuated by ebselen. Neurotoxicology. 2011;32:291–299. doi: 10.1016/j.neuro.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Z., Milatovic D., Aschner J.L., Syversen T., Rocha J.B., Souza D.O., Sidoryk M., Albrecht J., Aschner M. Methylmercury induces oxidative injury, alterations in permeability and glutamine transport in cultured astrocytes. Brain Research. 2007;1131:1–10. doi: 10.1016/j.brainres.2006.10.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yorifuji T., Tsuda T., Inoue S., Takao S., Harada M. Long-term exposure to methylmercury and psychiatric symptoms in residents of Minamata, Japan. Environment International. 2011;37:907–913. doi: 10.1016/j.envint.2011.03.008. [DOI] [PubMed] [Google Scholar]
- Xu Y., Li G., Han C., Sun L., Zhao R., Cui S. Protective effects of Hippophae rhamnoides L. juice on lead-induced neurotoxicity in mice. Biological and Pharmaceutical Bulletin. 2005;28:490–494. doi: 10.1248/bpb.28.490. [DOI] [PubMed] [Google Scholar]
- Zanoli P.C., Truzzi Veneri C., Braghiroli D., Baraldi M. Methylmercury during late gestation affects temporarily the development of cortical muscarinic receptors in rat offspring. Pharmacology and Toxicology. 1994;75:261–264. doi: 10.1111/j.1600-0773.1994.tb00358.x. [DOI] [PubMed] [Google Scholar]