Highlights
-
•
We investigated associations between 5-HTTLPR genotypes and selective attention.
-
•
Lower SES children aged 3–5 completed an ERP selective attention task.
-
•
Children with at least one short 5-HTTLPR allele had larger ERP attention effects.
-
•
The short allele is linked to enhanced neural mechanisms of attention in children.
-
•
This lays the groundwork for gene-by-environment studies involving cognitive skills.
Keywords: 5-HTTLPR, Selective attention, Preschoolers, ERP
Abstract
While a growing body of research has identified experiential factors associated with differences in selective attention, relatively little is known about the contribution of genetic factors to the skill of sustained selective attention, especially in early childhood. Here, we assessed the association between the serotonin transporter linked polymorphic region (5-HTTLPR) genotypes and the neural mechanisms of selective attention in young children from lower socioeconomic status (SES) backgrounds. Event-related potentials (ERPs) were recorded during a dichotic listening task from 121 children (76 females, aged 40–67 months), who were also genotyped for the short and long allele of 5-HTTLPR. The effect of selective attention was measured as the difference in ERP mean amplitudes elicited by identical probe stimuli embedded in stories when they were attended versus unattended. Compared to children homozygous for the long allele, children who carried at least one copy of the short allele showed larger effects of selective attention on neural processing. These findings link the short allele of the 5-HTTLPR to enhanced neural mechanisms of selective attention and lay the groundwork for future studies of gene-by-environment interactions in the context of key cognitive skills.
1. Introduction
Selective attention refers to the ability to prioritize relevant stimuli in the presence of irrelevant, competing distractors (Desimone and Duncan, 1995, Hillyard et al., 1973, Serences and Kastner, 2014). This ability is proposed to be fundamental for the foundations of language, memory, literacy, and mathematics (Astheimer and Sanders, 2012, Casco et al., 1998, Commodari and Di Blasi, 2014, Markant and Amso, 2014, Stevens and Bavelier, 2012). In addition, the neural mechanisms of selective attention have been associated with key cognitive skills, such as visual working memory and nonverbal intelligence, both in adults and children (Gazzaley, 2011, Giuliano et al., 2014, Isbell et al., 2016).
An extensive body of research has examined how early experiences relate to and modify the development of the neural mechanisms underlying selective attention. This research reveals the contributions of a wide range of experiential factors, including early sensory deprivation (Bavelier et al., 2000, Neville and Lawson, 1987), socioeconomic status (D'Angiulli et al., 2008; Stevens et al., 2014), music training (Strait et al., 2015), and targeted prevention and intervention programs (Neville et al., 2013, Stevens et al., 2008, Stevens et al., 2013). In comparison, relatively little is known about how genetic factors associate with the development of neural mechanisms of selective attention, especially in early childhood. Yet it has been argued that combining neuroscience methods with the study of alleles of specific candidate genes carries the potential to improve our understanding of how individual differences in cognitive abilities emerge and develop (Posner et al., 2007), as well as how both experiential and genetic factors contribute to the development of neural systems (Rueda et al., 2005). Further, it can allow for the investigation of gene-by-environment interactions, common in studies of social and emotional development and psychopathology (Belsky et al., 2009, Ellis et al., 2011, Manuck and McCaffery, 2014, Pluess and Belsky, 2013), and also emerging in the field of intervention research (Bakermans-Kranenburg and van IJzendoorn, 2015, Belsky and van Ijzendoorn, 2015).
The present study centered on the allelic variations of the serotonin transporter linked polymorphic region (5-HTTLPR) of the serotonin transporter gene, SLC6A4. Serotonin is the most widely distributed neurotransmitter in the brain, with the serotonin system originating from the raphe nuclei and projecting to a broad range of brain regions (for a review, see Lesch and Waider, 2012). One of these regions is prefrontal cortex (PFC), which receives direct projections of serotonergic neurons from raphe nuclei and hosts a dense distribution of serotonin receptor and transporter sites (Andrade, 2011, Puig and Gulledge, 2011). PFC is a fundamental component of neural mechanisms involved in selective attention (Degerman et al., 2006, Fritz et al., 2007, Pugh et al., 1996, Wu et al., 2007). PFC is generally considered a source of top-down attentional modulation, which can then influence sensory processing in site-specific regions (Petersen and Posner, 2012, Squire et al., 2013). Given the contributions of serotonin to the development, neuroplasticity and functioning of the frontal cortex in the mammalian brain (Andrade, 2011, Lesch and Waider, 2012, Puig and Gulledge, 2011) and the critical role of prefrontal cortex in selective attention (Bidet-Caulet et al., 2014, Knight et al., 1981), it is plausible that the serotonergic systems play a role in the development of selective attention.
A key controller of serotonin functioning in the brain is serotonin transporter (Murphy and Lesch, 2008). 5-HTTLPR is a polymorphic region in the gene that encodes serotonin transporter and is involved in serotonin reuptake from the synaptic cleft to presynaptic nerve terminals (Iurescia et al., 2015, Lesch et al., 1996). There are two predominant allelic variations of 5-HTTLPR: the short allele and the long allele (Heils et al., 1996). The short allele of 5-HTTLPR is generally associated with lower transcriptional activity of the serotonin transporter gene, but the precise neurobiological mechanisms through which these allelic variations contribute to brain functioning have yet to be determined (Iurescia et al., 2015). Despite this uncertainty in specific neurobiological mechanisms, 5-HTTLPR has been the most investigated genetic polymorphism in psychology (Caspi et al., 2010). Most previous research investigated the allelic variations of 5-HTTLPR in the context of susceptibility or resilience to psychopathology (Belsky and Pluess, 2009, Caspi et al., 2010, Karg et al., 2011, Pluess and Belsky, 2013). Accordingly, previous studies on 5-HTTLPR and attention focused predominantly on how this polymorphism relates to biased attention toward stimuli with positive or negative emotional valence, as a potential marker for susceptibility to psychopathology (Pergamin-Hight et al., 2012). Such studies commonly reported that the short allele, either in homozygous and/or heterozygous form, was associated with attentional bias toward emotionally valenced stimuli (Beevers et al., 2009, Fox et al., 2011, Lonsdorf et al., 2014, Osinsky et al., 2008, Thomason et al., 2010). However, little is known about the relationship between 5-HTTLPR and selective attention in young children, or in the absence of emotionally valenced stimuli.
In the present study, we assessed the relationship between 5-HTTLPR and the neural mechanisms of selective attention in a sample of 121 preschool-age children from lower socioeconomic status (SES) backgrounds. Event-related brain potentials (ERPs) were used to examine the neural mechanisms of selective auditory attention using a well-established, child-friendly dichotic listening task without emotional valence (Coch et al., 2005, Neville et al., 2013, Sanders et al., 2006, Stevens et al., 2009). We focused on children from lower SES backgrounds, whose selective attention abilities are at heightened risk for deficits (D'Angiulli et al., 2008, Stevens et al., 2009, Stevens et al., 2014), with notable individual differences in vulnerability (Isbell et al., 2016). Based on the structural and functional connections between serotonergic systems and PFC (Andrade, 2011, Lesch and Waider, 2012, Puig and Gulledge, 2011) and the role of PFC as a source of attentional modulation (Bidet-Caulet et al., 2014, Knight et al., 1981), we expected 5-HTTLPR to account for individual differences in the neural mechanisms of selective attention.
In the present study, no directional predictions were made regarding the association between 5-HTTLPR and selective attention, as the literature provides a basis for two very different predictions. On the one hand, across several studies, the short allele of 5-HTTLPR has been identified as a factor in vulnerability for unfavorable mental health outcomes, especially in the face of adversity (Caspi et al., 2010, Karg et al., 2011). While some reports contest the reliability of this link (Blakely and Veenstra-VanderWeele, 2011, Munafò et al., 2009, Risch et al., 2009), this finding is supported in a number of studies and meta-analyses (Conway et al., 2012, Jenness et al., 2011, Karg et al., 2011, Starr et al., 2014). As lower SES in childhood is commonly associated with adverse familial and environmental conditions (Baum et al., 1999, Evans, 2004), accordingly, it is possible that short allele carriers from lower SES backgrounds show greater sensitivity to such environmental adversity, and thus show attenuated neural indices of selective attention. On the other hand, it has also been argued that the short allele marks hypervigilance, defined as greater sensitivity to environmental stimuli that are motivationally relevant (Dobson and Brent, 2013, Homberg and Lesch, 2011). This framework draws from several studies which reported superior performance in short allele carriers compared to long homozygotes in various areas of cognition such as decision making, executive function, and reversal learning (e.g., Borg et al., 2009, Jedema et al., 2010, Roiser et al., 2006). It is argued that such results stem from hyperreactivity of the corticolimbic structures, including PFC, in short allele carriers (Homberg and Lesch, 2011). As claimed by this framework, such hyperreactivity results in increased vigilance in short allele carriers, which in turn is advantageous for cognitive tasks, especially in the absence of stimuli that can evoke emotional responses. As our selective attention task does not include any emotional or social valence, this framework predicts short allele carriers to show increased vigilance to the attended channel and correspondingly enhanced neural responses in our sustained selective attention task. Since our study exclusively focused on a lower SES sample and used a task without emotional or social valence, both of these frameworks (i.e. vulnerability to adversity versus hypervigilance) appear plausible. Therefore, no directional predictions were specified. Importantly, any main effects of the 5-HTTLPR observed in the lower SES sample provide the groundwork for future research examining gene-by-environment or gene-by-intervention interactions and provide information on the contribution of the 5-HTTLPR to the development of neural systems for attention in young children from lower SES backgrounds.
2. Method
2.1. Participants
The final sample included 121 children (76 females) between the ages of 40 and 67 months (Mean = 55 months, SD = 6.5 months). These were the same participants as reported in a previous study of neural mechanisms of selective attention in lower SES preschoolers (Isbell et al., 2016), excluding three participants from whom genetic data were not available. The participants were recruited in Oregon from 12 preschool sites of Head Start, a program for families living at or below the poverty line. Based on parent reports, children with diagnosed behavioral or neurological problems (e.g. attention-deficit and hyperactivity disorder, specific language impairment, epilepsy) and children taking psychoactive medications were excluded from recruitment for the present study. Children with an individualized family service plan (IFSP) or individualized education program (IEP), written documents outlining special education needs and services for a child with developmental delays or disorders, were also excluded from recruitment. All children from whom EEG data were collected were right-handed, monolingual, native English speakers and passed a hearing screening at 20 dB HL at 500, 1000, 2000 and 4000 Hz in both the right and left ears. From a total of 157 children who met these criteria, 23 were excluded due to low ERP data quality (excessive EEG artifacts and/or less than 75 trials per condition). In addition, 11 children were excluded for having less than 50% accuracy on the comprehension questions presented during the ERP task, described below. Three additional children for whom we did not have genetic information were also excluded. In the final sample of 121 children, parent reports indicated that 59% were White/Caucasian, 1% Black/African American, 4% American Indian or Alaskan, 15% more than one ethnicity, 1% unknown. In the sample, 20% of parents did not report the ethnicity of their children. Excluding the unknown/unreported children, our sample was predominantly (74%) White/Caucasian.1
Informed consent was obtained from parents or other caregivers. In addition, verbal assent was obtained from child participants. All families were paid for participation. Study procedures were approved by the University of Oregon Institutional Review Board.
2.2. Socioeconomic status (SES)
Although all children were considered lower SES by virtue of their eligibility for and participation in Head Start, parents/caregivers filled out a short questionnaire about the education level and profession of the primary caregivers as an additional metric of SES. The questionnaire was used to calculate a second index of child SES, which was coded by trained research assistants according to the Hollingshead Four Factor Index of Social Status (Hollingshead, 1975).
2.3. Electrophysiological assessment of selective auditory attention
2.3.1. ERP task
ERPs were recorded in a paradigm of spatial selective auditory attention, described in detail in previous studies (Isbell et al., 2016, Neville et al., 2013). Briefly, in this paradigm, children attended selectively to one of two simultaneously presented stories, differing in location (left/right audio speaker) and narration voice (female/male). Each story to be attended was accompanied by images relevant to the content of the story. Further, a small green arrow pointing to the left or right was superimposed at the bottom of the images to indicate the attended side.
ERPs were time-locked to probe stimuli superimposed on the attended and unattended stories. Half of the probe stimuli were linguistic and half were non-linguistic. The linguistic probe stimulus was a token of the syllable /ba/ spoken by a female voice (different from the female narrators) and edited to 100 ms. The nonlinguistic probe was created by scrambling the order of 4–6 ms segments of the linguistic probe to create a nonlinguistic probe with similar acoustic characteristics. Both probes were 100 ms in length and presented in a pseudorandom order at an interstimulus interval (ISI) of either 200, 500, or 1000 ms. Following our previous work with this paradigm in young children (Isbell et al., 2016, Neville et al., 2013, Stevens et al., 2009), all analyses were collapsed across the linguistic and non-linguistic probes. A total of 800 probe stimuli were presented during the experiment, with ∼400 stimuli in each of the attend and unattend conditions.
2.3.2. Procedure
Children arrived at the laboratory with their parent/caregiver(s) and were provided time to acclimate to the environment before placement of the electrode cap began. Once the EEG cap was in place, children were seated in a comfortable chair in an electrically shielded, sound-attenuating booth. Children were instructed not to move or lean from side to side. A trained research assistant sat in the booth with each child throughout the experiment to ensure the child remained centered in the chair. Two auditory loudspeakers were placed on either side of the participant (90° to the left and right of the chair). A computer monitor was positioned approximately 145 cm in front of the child. Before the data were recorded, children received a pre-recorded introduction to the paradigm and task, including instructions to attend to the story played from one speaker while ignoring the story presented on the other speaker. Children were also familiarized with the probe sounds (‘bas’ and ‘buzzes’) and told that these sounds could be ignored.
Children attended to a total of four narratives, attending twice to the right side and twice to the left side (order either RLLR or LRRL). All participants attended to two stories narrated by a female and two stories narrated by a male. For the duration of the experiment, participants were monitored by an intercom system and a video camera in addition to the trained research assistant in the booth. After each story, the experimenter asked the participant three basic comprehension questions about the attended story. The comprehension questions were always about the attended story and had two alternatives. A response of “I don’t know” was considered an incorrect response. Only children who performed with at least 50% accuracy on the comprehension questions were included in the EEG analyses.
2.3.3. EEG recording and analysis
EEG was recorded at a sampling rate of 512 Hz from 32 Ag-AgCl electrodes attached to an electrode cap and arranged according to the International 10/10 system. Recordings were made using the Active-Two system (Biosemi, Amsterdam, Netherlands), which does not require impedance measurements, an online reference, or gain adjustments. Additional electrodes were placed on the left and right mastoid, at the outer canthi of both eyes and below the right eye. Scalp signals were recorded relative to the Common Mode Sense (CMS) active electrode and then re-referenced off-line to the algebraic average of the left and right mastoid. Left and right horizontal eye channels were re-referenced to one another.
ERP analyses were carried out using EEGLAB (Delorme and Makeig, 2004) and ERPLAB (Lopez-Calderon and Luck, 2014). Data were down-sampled to 256 Hz to speed computation and band-pass filtered from 0.1 to 40 Hz. The EEG data were epoched offline between 100 ms prior to and 500 ms after stimulus onset, using the first 100 ms as the pre-stimulus-onset baseline. Artifact rejection was executed using a multi-step procedure. First, automatic artifact rejection was executed using a 200 ms window, moving at 50 ms increments with peak-to-peak rejection criteria of 100 μV for the eye channels and 200 μV for all other channels. On the basis of visual inspection of the epoched EEG data, if these automatic rejection parameters were insufficient for a participant (indicated by either clean trials being incorrectly rejected, or eyeblinks or eye movements failing to be correctly rejected), individual rejection parameters were selected and the rejection process was repeated with these individualized parameters. Then, trained research assistants performed a subsequent artifact rejection step to exclude additional epochs containing eye movements and muscle artifacts from further analyses. Out of ∼ 400 trials per attention condition, an average of 250 trials (SD = 63) per participant were accepted for the attend condition, and 250 trials (SD = 61) were accepted for the unattend condition.
For a total of three participants with otherwise clean EEG data, a single bad electrode was replaced with the average mean amplitude of the three neighboring electrodes. The neighboring electrodes were determined based on the rows described below, within the hemisphere of interest. The mean amplitudes of ERPs were measured between 100 and 200 ms post-stimulus onset, consistent with previous studies using this paradigm with young children from lower SES backgrounds (Isbell et al., 2016, Neville et al., 2013, Stevens et al., 2009). As shown in these previous studies, the 100–200 ms time window captures the attention effect across children. The appropriateness of this time window was confirmed by looking at individual subject averages prior to data analysis.
The ERP attention effect was operationalized as the mean amplitude difference between the ERPs to the probes in the stories when they were attended versus when they were unattended (attend − unattend). Three rows of 8 electrodes were created as follows: anterior: F7/8, F3/4, FT7/8, FC5/6; central: T7/8, C5/6, CP5/6, C3/4; posterior: P7/8, P3/4, PO3/4, O1/2. The configuration of the electrode cap is illustrated in Fig. 1. These electrode aggregates were identical to those used in previous studies that employed the same ERP paradigm, with the same EEG system/cap layout, and the same age group from lower SES backgrounds (Isbell et al., 2016, Neville et al., 2013).
Fig. 1.

Electrode configuration for event-related brain potential (ERP) recordings. The 24 electrodes included in analyses are bolded and specified in the text.
2.4. Genotyping
Buccal epithelial cells were collected with cotton collection swabs. For each child, two swabs were collected. Genotyping was conducted at the University of Oregon. Genomic DNA was isolated from the swabs using QuickExtract V1.0 (Epicentre Biotechnologies, Madison, WI) according to their protocol. Approximately 1% of this preparation was used for each amplification. The promoter region of SLC6A4 was amplified using the primers reported in Deckert et al. (Deckert et al., 1997).
Allele frequencies of 5-HTTLPR were 57% for the long (l) allele and 43% for the short (s) allele. According to the Hardy-Weinberg equilibrium, the expected distribution of 5-HTTLPR genotypes would be 33% for long-long, 49% for short-long, and 18% for short-short. In our sample, genotype frequencies were 29% for long–long (n = 35), 56% for short-long (n = 68), and 15% for short-short (n = 18). Chi-square tests revealed no significant differences between the observed frequencies and the expected frequencies according to the Hardy-Weinberg equilibrium (χ2(2) = 2.54, p = 0.28).
3. Results
Exploratory data analyses were conducted for the ERP data, for all children together, as well as independently for each 5-HTTLPR genotype group (long-long, short-long, and short-short). No outliers (+/− 3 SD) were detected. All children with acceptable ERP data, based on the criteria described above, were included in the analyses. Table 1 displays the descriptive statistics by the 5-HTTLPR genotype groups for age, SES, number of correct answers children gave for the comprehension questions asked during the ERP task, and number of clean ERP trials used in the analyses. SES information was missing for 11 children (3 long-long, 8 short-long children). Table 2 presents the mean amplitudes of the ERPs of the selective attention effect for each of the three genotype groups, in each of the three electrode aggregates.
Table 1.
Age, SES, story comprehension question accuracy, and number of ERP trials for the 5-HTTLPR genotypes.
| Age | SES | Comprehension Accuracy | Number of ERP trials | |
|---|---|---|---|---|
| 5-HTTLPR Genotypes | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) |
| Long-long (n = 35) | 4.48 | 30.79 | 8.00 | 504 |
| (0.51) | (10.74) | (1.41) | (123) | |
| Short-long (n = 68) | 4.51 | 28.98 | 8.62 | 507 |
| (0.56) | (11.90) | (1.54) | (121) | |
| Short-short (n = 18) | 4.81 | 31.11 | 8.83 | 468 |
| (0.52) | (11.96) | (1.38) | (130) |
Note. SES information was missing for 3 long-long and 8 short-long children (n = 11).
Table 2.
Mean amplitude differences (μV) for the ERPs elicited by identical probes embedded in stories when attended versus unattended for the long-long, short-long, and short-short 5-HTTLPR genotypes.
| Anterior Electrodes | Central Electrodes | Posterior Electrodes | All electrodes |
|
|---|---|---|---|---|
| 5-HTTLPR Genotypes | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) |
| Long-long (n = 35) | −0.17 | −0.17 | −0.55 | −0.30 |
| (1.37) | (1.42) | (1.70) | (1.23) | |
| Short-long (n = 68) | 0.48 | 0.44 | 0.02 | 0.31 |
| (1.48) | (1.36) | (1.43) | (1.11) | |
| Short-short (n = 18) | 0.56 | 0.76 | 0.56 | 0.63 |
| (1.49) | (1.33) | (1.81) | (1.26) |
Univariate ANOVAs were used to test whether age, SES, comprehension accuracy, or number of ERP trials varied as a function of 5-HTTLPR genotype. There were no main effects of 5-HTTLPR genotype on age, SES, comprehension accuracy, or number of ERP trials. The ANOVA statistics are reported in Table 3. Chi-square tests showed that there were also no significant differences in gender distribution between the 5-HTTLPR genotype groups, χ2 (2) = 0.03, p = 0.98. The parent reports of race/ethnicity were recoded as follows: white, not white, unknown/unreported, with subsequent chi-square tests revealing no significant differences in race/ethnicity between the 5-HTTLPR genotype groups, χ2 (4) = 0.78, p = 0.94. Similarly, when the children with unknown or unreported race/ethnicity information were excluded (n = 26), there were no significant differences in the distributions between the genotype groups, χ2 (2) = 0.12, p = 0.94.
Table 3.
Analyses of variance for age, SES, comprehension accuracy, and ERP mean amplitudes of the attention effect by 5-HTTLPR genotype (long-long, short-long, short-short).
| F | df | p | partial η2 | |
|---|---|---|---|---|
| Age | 2.60 | 2, 118 | 0.08 | 0.04 |
| SES | 0.38 | 2, 106 | 0.69 | <0.01 |
| Comprehension accuracy | 2.65 | 2, 118 | 0.08 | 0.04 |
| Number of ERP trials | 0.76 | 2, 118 | 0.47 | 0.01 |
| ERP mean amplitudes | ||||
| 5-HTTLPR | 3.05 | 2, 118 | 0.01* | 0.07 |
| Electrode location | 2.44 | 2, 236 | 0.09 | 0.02 |
| 5-HTTLPR x electrode location | 0.31 | 4, 236 | 0.78 | < 0.01 |
p < 0.05.
We used a mixed-model ANOVA to evaluate whether the ERPs of the selective attention effect varied as a function of 5-HTTLPR. The ANOVA included the between-group factor of three 5-HTTLPR genotypes (long-long, short-long, and short-short), and the within-group factor of three levels of electrode locations (anterior, central, posterior). Greenhouse-Geisser corrections were applied for all ANOVAs with greater than one degree of freedom. Uncorrected degrees of freedom but corrected p-values are reported. For the effect sizes in ANOVAs, partial η2 is reported.
In an initial ANOVA, age and gender were included as covariates. The effects of these covariates did not reach statistical significance in the ERP analyses. Consequently, these covariates were dropped from the final model for parsimony. The results of the final model are reported in Table 3.
Results indicated a significant main effect of 5-HTTLPR genotype on the ERP selective attention effect. There was neither a main effect of electrode location, nor an interaction between genotype groups and electrode location. Accordingly, Fig. 2 shows the ERP mean amplitudes of the selective attention effect for the 5-HTTLPR genotype groups, averaged across all electrode locations. The grand average ERP waveforms for all electrodes included in the analyses are illustrated separately for each genotype group as follows: long-long genotype in Fig. 3, short-long genotype in Fig. 4, and short-short genotype in Fig. 5.
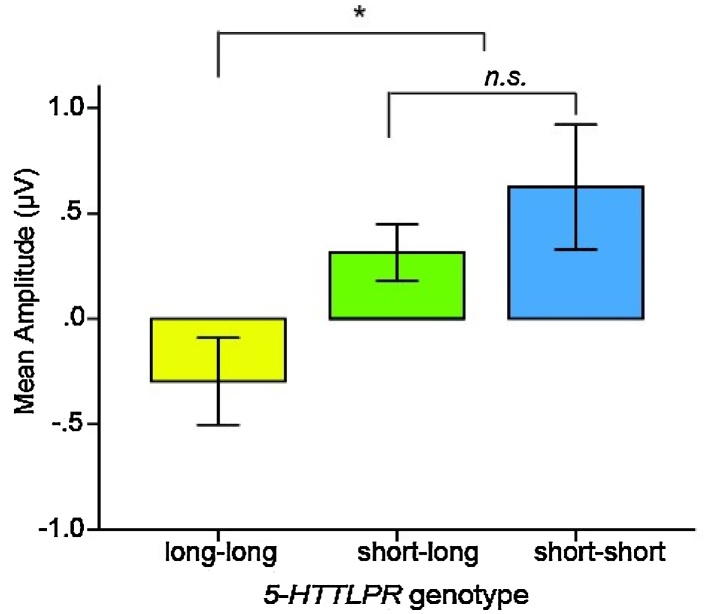
Fig. 2.
Mean amplitudes (μV) of ERP selection attention effect, averaged across all channels included in the analyses. Long-long children had smaller ERP mean amplitudes than children who carried at least one short allele. Error bars represent +/− 1 SE. * p < 0.05.
Fig. 3.
Grand-average ERP waveforms showing ERPs elicited by the attend and unattend conditions for children with the long-long genotype. For this, and all subsequent ERP figures, negative is plotted upward.
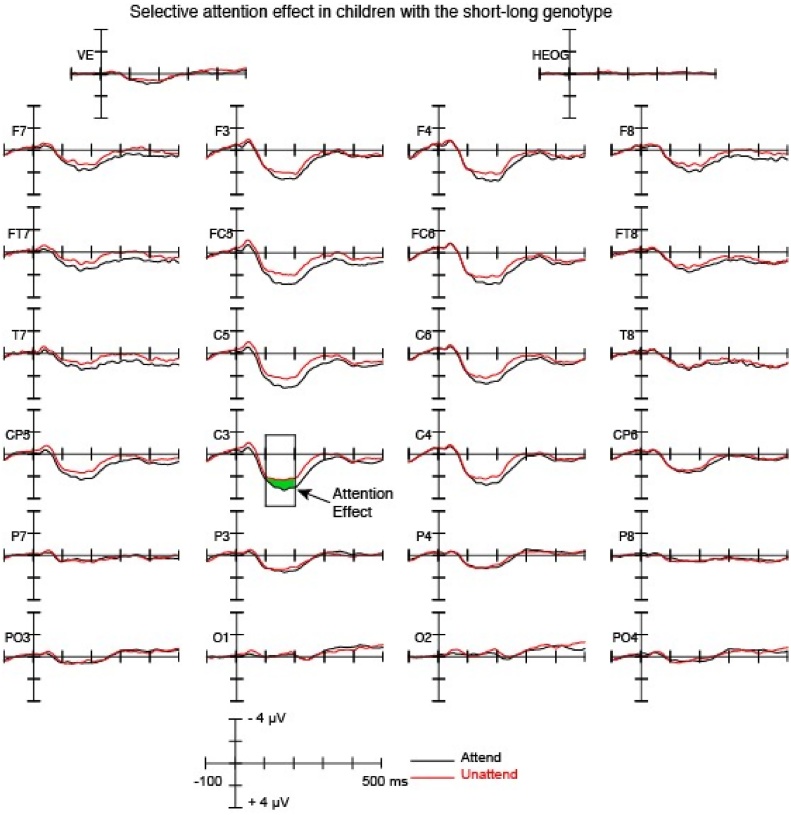
Fig. 4.
Grand-average ERP waveforms showing ERPs elicited by the attend and unattend conditions for children with the short-long genotype.
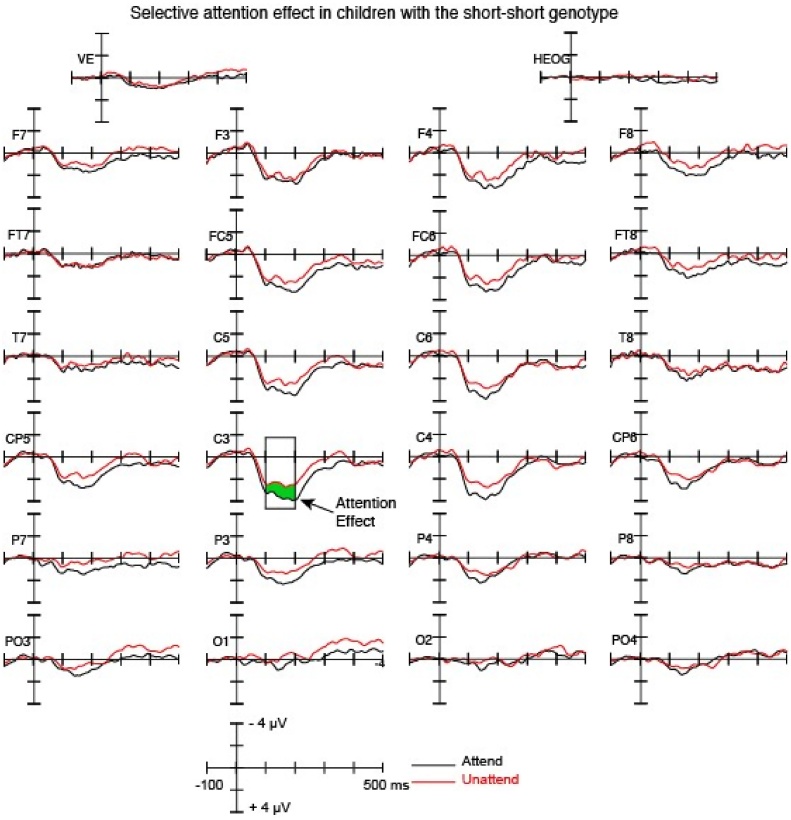
Fig. 5.
Grand-average ERP waveforms showing ERPs elicited by the attend and unattend conditions for children with the short-short genotype.
To unpack the main effect of 5-HTTLPR on ERPs of selective attention, we used Helmert contrasts. These planned orthogonal contrasts allowed us to directly test a) whether there were differences in the ERP selective attention effect between the long homozygotes and the short allele carriers, and b) between the children who carry one versus two copies of the short allele. The contrasts were constructed as follows: Comparison 1: longlong vs. short-long and short-short (contrast coefficients: −2, 1, 1); Comparison 2: short-long vs. short-short (contrast coefficients: 0, −1, 1). A Bonferroni correction was used to control for multiple comparisons, with the statistical significance level adjusted to p < 0.025. For effect sizes, Cohen’s d values were computed. Given the unequal sample sizes of the genotype groups, pooled standard deviation was used in Cohen’s d calculations.
The Helmert contrasts revealed a significant difference in the mean amplitudes of the selective attention effect between the children homozygous for the long allele (long-long) and the children who carry at least one copy of the short allele (short-long and short-short), t(118) = −3.05, p = 0.003, d = −0.58. The selective attention effect was attenuated (smaller, less positive in amplitude) in the long homozygotes relative to the short allele carriers. The second Helmert contrast indicated that the ERP selective attention effect did not differ significantly between the children who carry one versus two copies of the short allele (short-long vs. short-short), t(118) = −1.00, p = 0.32, d = −0.27.2
Following the primary analyses reported above, a supplemental analysis was conducted to rule out the possibility that the genotype effects for selective attention could be attributed to general heightened neural activity in short allele carriers. In this supplemental analysis, we ran an additional ANOVA in which attention (attend vs. unattend) was entered as a within-subjects factor. If the results stemmed from more general heightened neural activity, we would expect to find a main effect of genotype, regardless of condition (i.e. greater ERP mean amplitudes in short allele carriers in both the attend and unattend conditions). However, there was no main effect of 5-HTTLPR on overall amplitude (F(2, 118) = 0.96, p = 0.39). There was also no genotype x electrode location interaction (F(2, 118) = 0.79, p = 0.46), again indicating that there was no specificity in electrode location. These results suggest that the group differences in the attention effect favoring short allele carriers cannot be explained by more general heightened neural activity, across the scalp or over specific electrode locations.3
4. Discussion
The present study investigated the relationship between the 5-HTTLPR polymorphism and neural mechanisms of selective auditory attention in preschool children from lower SES backgrounds. We observed clear differences in a neural index of selective attention as a function of the 5-HTTLPR genotypes. Specifically, short allele carriers exhibited a larger effect of selective attention on ERPs compared to long homozygotes. In contrast, no differences were observed between children carrying one versus two copies of the short allele. As previous research indicates that larger effects of selective attention on ERPs are linked to better performance in nonverbal tasks of cognition in adults and children (Giuliano et al., 2014, Isbell et al., 2016), this suggests that the current findings can be interpreted as enhanced neural mechanisms of selective attention in short allele carriers.
The enhanced ERP attention effects in short carriers were broadly distributed across the scalp, with no evidence that the group effects were specific to more anterior versus posterior electrode sites. Indeed, although the source of attentional modulation is expected to be an anterior fronto-parietal network, including the PFC, our dependent measure captures the effects of that network on the underlying sensory processing. While previous research on ERP auditory selective attention effects suggests that anterior and central attention effects correlate most strongly with nonverbal IQ (Isbell et al., 2016), no such specificity was observed here. However, the overall distribution of the effect is consistent with previous research using the same dichotic listening paradigm, which showed that the ERP auditory selective attention effects in typically developing preschool aged children are broadly distributed across the scalp (Karns et al., 2015, Sanders et al., 2006). In addition, a supplemental analysis ruled out the possibility that short allele carriers simply showed generalized heightened neural activity, which would have been manifest as group differences regardless of attention condition (i.e., larger overall ERP amplitudes). Thus, our findings suggest an enhancement of the ERP attention effect in short allele carriers, which in preschool children is broadly distributed across the scalp.
Our results provide initial evidence for a relation between serotonergic systems, as indexed by the 5-HTTLPR polymorphism, and neural mechanisms of selective attention in young children. Given the critical role of PFC as a source of top-down attentional modulation (Petersen and Posner, 2012, Squire et al., 2013), it is plausible that this relation between serotonergic systems and selective attention is mediated by the structural and functional links between serotonergic systems and PFC (Andrade, 2011, Lesch and Waider, 2012, Puig and Gulledge, 2011). However, the ways in which the allelic variations of 5-HTTLPR contribute to brain functioning is still under investigation (Iurescia et al., 2015). Therefore, the precise neurobiological mechanisms that lead to enhanced selective attention in short allele carriers are to be determined.
Most previous research has examined 5-HTTLPR in relation to biased attention toward stimuli with emotional valence (for a review, see Pergamin-Hight et al., 2012). The most robust finding of these studies is that the short allele is associated with attentional bias toward positive valence, such as happy faces (Beevers et al., 2009, Fox et al., 2011), as well as negative emotional expressions, such as anger, fear, or sadness (Beevers et al., 2009, Fox et al., 2011, Lonsdorf et al., 2014, Thomason et al., 2010) or fear-relevant stimuli, such as spiders (Osinsky et al., 2008). The associations between the short allele and greater biased attention to negative valence are also observed in interaction with stressful life events and low social support (Jenness et al., 2015, Pearson et al., 2016). This greater attentional bias toward emotionally or socially salient stimuli in short allele carriers has been mainly discussed as a potential pathway of vulnerability toward affective disorders, such as depression and anxiety.
However, it has also been argued that the short allele, rather than being a marker for psychopathology, may be considered a marker of hypervigilance, displayed as elevated sensitivity to relevant environmental stimuli (Dobson and Brent, 2013, Homberg and Lesch, 2011). Accordingly, such hypervigilance can predict psychopathology or advantageous outcomes depending on the specific demands of tasks and relevant stimuli, as well as broader environmental context. To speculate, this hypervigilance framework may account for our findings of superior neural mechanisms of selective attention in short allele carriers, measured in a dichotic listening paradigm with no apparent positive or negative valence. The proposed hypervigilance of short allele carriers may manifest as pronounced attentional abilities in the absence of emotionally salient or threatening stimuli. For example, it has been posited that when the task relevant environmental cues are controllable and not emotionally or socially salient, the hypervigilance of short allele carriers can be observed as an overarching cognitive advantage. Indeed, there is a set of emerging studies suggesting that, under certain conditions, short allele carriers exhibit cognitive advantage in a diverse set of tasks mediated by the PFC, including working memory, executive function, and decision-making (Borg et al., 2009, Enge et al., 2011, Jedema et al., 2010, Strobel et al., 2007). However, a large-scale study failed to find such an association (Barnett et al., 2011). Therefore, any links between the short allele and a general cognitive advantage await further study that can identify the conditions under which the short allele is associated with improved performance.
Given the literature that linked the short allele to vulnerability for disadvantageous outcomes in the face of environmental adversity (Caspi et al., 2010, Karg et al., 2011) and that lower SES is generally associated with adverse familial and environmental conditions (Baum et al., 1999, Evans, 2004), it would be plausible to predict weaker selective attention effects in short allele carriers from lower SES families. Yet, our results did not support this prediction. Our findings may suggest that, at least early in development, short carrier status can act as a protective factor for abilities of selective attention in lower SES children. However, our findings may also stem from an underlying gene x environment interaction that we could not directly assess in our study, given our exclusive focus on a lower SES sample and the absence of any measure of environmental variability within our SES sample. It has also been asserted that short allele carriers are more sensitive not only to adversity, but also to supportive and enriching environments, and thus less prone to psychopathology under supportive environmental conditions, compared to long homozygotes (Belsky et al., 2009, Belsky and Pluess, 2009, Bogdan et al., 2014, Li et al., 2013). Although our sample was predominantly lower SES, we may have oversampled from a population of lower SES children with relatively supportive household environments. Indeed, as these children were also participating in part of a larger study (the present data were collected prior to any additional intervention or services), we may have selected those families with the willingness and capacity to sign up for participation in, and completion of, multiple research sessions. Another intriguing possibility is that the Head Start program, from which we recruited all of our participants, may act as a supportive environment for lower SES children and render short allele carriers, who are more sensitive to environmental conditions, more advantaged.
These possibilities call for several important directions for future investigation. First and foremost, it remains important to understand how the 5-HTTLPR genotypes interact with environmental factors in predicting selective attention. For instance, lower SES in childhood is associated with stressful familial experiences such as persistent economic hardship, crowding, family dissolution, and mobility, as well as neighborhood characteristics such as violence, crime, environmental hazards, and noise pollution (Bradley and Corwyn, 2002, Evans, 2004, Evans and Kim, 2010). Such chronic stress in childhood has been identified as a potential mechanism by which SES alters the development of the brain and, consequently, cognitive functioning (Blair, 2010, Blair et al., 2011). However, without an objective and validated measure of stress in young children, we cannot assess how 5-HTTLPR genotypes act under stress in predicting neural mechanisms of selective attention. Karg et al. (2011) reported that the genetic moderation by 5-HTTLPR in studies of depression was weaker if they included self-report questionnaires and stronger if an objective measure of stress or in-person interviews were included. Therefore, inclusion of a validated chronic stress measure for young children could uncover moderation of the link between 5-HTTLPR and selective attention. Furthermore, families largely differ in various protective factors that are predictive of cognitive outcomes, such as parental responsiveness and stimulating home environments (Bradley and Corwyn, 2002, Lengua et al., 2007, Tong et al., 2007). Again, inclusion of validated measures of supportive environments for young children could reveal genetic moderations we could not assess in our study. Incorporating indicators of protective factors, along with indices of risk, is an important future direction to assess the associations between 5-HTTLPR genotypes and neural mechanisms of selective attention. Such assessments would also appraise whether the stress reactivity or differential susceptibility frameworks can also be applied to neural mechanisms of selective attention.
While our results provide initial evidence for the association between 5-HTTLPR genotypes and individual differences in selective attention in typically developing children, certain limitations of our study require consideration. First, the participants in this study were recruited from lower SES families, as discussed above. While some studies reported stronger genetic influences in lower SES populations (Nobile et al., 2007, Nobile et al., 2010, Williams et al., 2008), others associated weaker genetic influences with lower SES (Schwartz, 2015, Tucker-Drob et al., 2011, Turkheimer et al., 2003). Further, a recent meta-analysis argued for large cross-national differences in gene-by-SES interactions (Tucker-Drob and Bates, 2015). Given a lower SES environment is itself a risk factor for poorer neural mechanisms of attention (D'Angiulli et al., 2008, Stevens et al., 2009), it remains to be investigated whether the effects of 5-HTTLPR and socioeconomic status are additive or interactive.
Second, based on parent reports, our participants were predominantly of Caucasian ancestry. This raises the question as to whether our findings would generalize beyond a sample of Caucasian ancestry. It has been demonstrated that the frequency and functional characteristics of 5-HTTLPR may differ across populations (Chiao and Blizinsky, 2010, Odgerel et al., 2013, van Ijzendoorn et al., 2012). For instance, being homozygous for the short allele was associated with lower serotonin function in the central nervous system in European-Americans, and higher serotonin function in African-Americans (Williams et al., 2003). As another example, differential susceptibility of the short allele to environmental factors was observed in samples composed of primarily White children (van Ijzendoorn et al., 2012), while in a sample of predominantly Black children, homozygous long allele carriers were found to show greater susceptibility to environmental effects (Davies and Cicchetti, 2014). Therefore, the extent to which our findings would generalize to more diverse populations of lower SES children remains to be investigated.
Third, although we did not find significant differences in the comprehension accuracy between the genotype groups, there was a trend favoring the short allele carriers. This suggests that there may be group differences, which we were either underpowered to detect or that our measure was not sensitive enough to capture. Our comprehension accuracy measure consists of forced-choice questions about the stories children were instructed to attend. We find this measure useful for our child-friendly ERP paradigm because it reinforces to children the goal of attending to a single story and is appropriate for children of diverse ages. The measure also provides a gross index of whether children were generally on task. At the same time, it is important to note that this measure is not designed to provide a sensitive assay of children’s behavioral performance. Thus, it is difficult to interpret the trend for group differences. Future work using more sensitive behavioral measures of attention will be important for determining how the 5-HTTLPR genotype is associated with behavioral performance of selective attention.
Another limitation of our study is the biallelic categorization of the 5-HTTLPR allelic variations. Here we focused on the two common allelic variants that occur either as a shorter sequence of 14 repeats (short allele) or a longer sequence of 16 repeats (long allele). However, other lengths have also been reported (Kraft et al., 2005, Nakamura et al., 2000). Furthermore, instead of a biallelic categorization, a triallelic classification has been proposed based on the single nucleotide variant (A to G) detected on the long allele (Hu et al., 2006, Kraft et al., 2005). The variant designated LA was associated with higher serotonin transporter binding, whereas the variant designated LG was associated with lower serotonin binding (Hu et al., 2006, Praschak-Rieder et al., 2007). According to this categorization, the LG variant is grouped together with the short allele, in comparison to the LA/LA genotype assigned to long homozygosity (Davies and Cicchetti, 2014, Enge et al., 2014, Mileva-Seitz et al., 2011). If we had used this triallelic categorization, children who carry the LG variant would be grouped together with short carriers, instead of long homozygotes. It will be important to test this triallelic variation approach in future studies of 5-HTTLPR genotype and brain functioning.
In addition, it bears repeating that as no single candidate gene can solely account for variability in any cognitive ability, thus it remains crucial to investigate how 5-HTTLPR polymorphism interacts with other polymorphisms linked to attentional abilities in children. In adults, single nucleotide polymorphisms (SNPs) of various genes have been linked to cognitive abilities (Green et al., 2008, Savitz et al., 2006). Among these, polymorphisms of several genes have been associated with attentional abilities (Stormer et al., 2012). These genes include, but are not limited to, catecholamine-O-methyltransferase (COMT) gene, cholinergic receptor, nicotinic alpha 4 (CHRNA4) gene, dopamine receptor D4 (DRD4) gene, and dopamine active transporter 1 gene (DAT1). In typically developing infants and children, variability in attentional abilities has also been linked to COMT and DAT1 polymorphisms (Holmboe et al., 2010, Markant et al., 2014, Rueda et al., 2005). A more comprehensive array of candidate genes, and assessment of their interactions with each other, would greatly advance our understanding of biological foundations of individual differences in neural mechanisms of selective attention.
5. Conclusion
The present study demonstrated a link between the 5-HTTLPR polymorphism and neural indices of selective attention in lower SES preschoolers. Compared to their long homozygote peers, children who carried at least one copy of the short allele displayed more pronounced attention effects, as measured by ERPs. These findings suggest that carrying at least one short allele of 5-HTTLPR may confer enhanced neural mechanisms of selective attention in preschool-age children from lower SES backgrounds. Further research is needed to understand the interactions between the 5-HTTLPR polymorphism and other candidate polymorphisms in the context of diverse environmental conditions. Future studies that address these issues can advance our understanding of the biological bases for neural mechanisms of selective attention, which are at risk in lower SES children. Additionally, the present study lays the groundwork for future research that can extend the gene-by-environment framework, common in research on vulnerability and resilience to psychopathology, to research on risk and resilience for key cognitive skills. Such efforts that combine neuroimaging with the study of genetic factors carry the potential to greatly improve our understanding of how individual differences in cognitive abilities emerge and develop (Posner et al., 2007) and how experiences shape the developing brain.
Acknowledgements
This research was made possible by Department of Education/Institute of Education Science Grant R305B070018 and National Institutes of Health/National Institute on Deafness and Other Communication Disorders Grant R01 DC000481 (to H.J.N.). We thank members of the Brain Development Lab for their support in data acquisition and processing, Pascale Voelker for her contributions to the genetic analyses, and Marie Braasch Chelberg for her helpful comments on an earlier version of the manuscript.
Footnotes
The main analyses reported below were also conducted using only the subset of children reported to be Caucasian, as a means of limiting the likelihood of possible population stratification explaining any observed associations. These analyses yielded results consistent with those conducted with the whole sample.
Per a reviewer comment, additional analyses were run, restricted to only those children answering 7 or more comprehension questions correctly (accuracy greater than 50%). The results of these analyses (n = 109) were consistent with those that included children with 50% accuracy. There was a main effect of 5-HTTLPR (F(2, 106) = 5.77, p = 0.004); short allele carriers showed a larger ERP attention effect than long-homozygotes, and no differences were found between children who carry one versus two copies of the short allele (p = 0.001 and p = 0.44 respectively).
Additional supplemental analyses, conducted separately for attend and unattend conditions, could not localize group differences specifically to distractor suppression (differences in the unattend condition) versus signal enhancement (differences in the attend condition).
References
- Andrade R. Serotonergic regulation of neuronal excitability in the prefrontal cortex. Neuropharmacology. 2011;61(3):382–386. doi: 10.1016/j.neuropharm.2011.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astheimer L.B., Sanders L.D. Temporally selective attention supports speech processing in 3-to 5-year-old children. Dev. Cognit. Neurosci. 2012;2(1):120–128. doi: 10.1016/j.dcn.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakermans-Kranenburg M.J., van IJzendoorn M.H. The hidden efficacy of interventions: gene x environment experiments from a differential susceptibility perspective. Annu. Rev. Psychol. 2015;6611:11–11.29. doi: 10.1146/annurev-psych-010814-015407. [DOI] [PubMed] [Google Scholar]
- Barnett J.H., Xu K., Heron J., Goldman D., Jones P.B. Cognitive effects of genetic variation in monoamine neurotransmitter systems: a population-based study of COMT, MAOA, and 5HTTLPR. Am. J. Med. Genet. Part B-Neuropsychiatr. Genet. 2011;2(156B):158–167. doi: 10.1002/ajmg.b.31150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum A., Garofalo J.P., Yali A.M. Socioeconomic status and chronic stress: does stress account for SES effects on health. Annu. N. Y. Acad. Sci. 1999;896:131–144. doi: 10.1111/j.1749-6632.1999.tb08111.x. [DOI] [PubMed] [Google Scholar]
- Bavelier D., Tomann A., Hutton C., Mitchell T., Corina D., Liu G., Neville H. Visual attention to the periphery is enhanced in congenitally deaf individuals. J. Neurosci. 2000;20(17):1–6. doi: 10.1523/JNEUROSCI.20-17-j0001.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beevers C.G., Wells T.T., Ellis A.J., McGeary J.E. Association of the serotonin transporter gene promoter region (5-HTTLPR) polymorphism with biased attention for emotional stimuli. J. Abnorm. Psychol. 2009;118(3):670–681. doi: 10.1037/a0016198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky J., Pluess M. Beyond diathesis stress: differential susceptibility to environmental influences. Psychol. Bull. 2009;135(6):885–908. doi: 10.1037/a0017376. [DOI] [PubMed] [Google Scholar]
- Belsky J., van Ijzendoorn M.H. What works for whom? Genetic moderation of intervention efficacy (Special Issue 01) Dev. Psychopathol. 2015;27:1–6. doi: 10.1017/S0954579414001254. [DOI] [PubMed] [Google Scholar]
- Belsky J., Jonassaint C., Pluess M., Stanton M., Brummett B., Williams R. Vulnerability genes or plasticity genes? Mol. Psychiatry. 2009;14(8):746–754. doi: 10.1038/mp.2009.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidet-Caulet A., Buchanan K.G., Viswanath H., Black J., Scabini D., Bonnet-Brilhault F., Knight R.T. Impaired facilitatory mechanisms of auditory attention after damage of the lateral prefrontal cortex. Cereb. Cortex. 2014 doi: 10.1093/cercor/bhu131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair C., Raver C.C., Granger D., Mills-Koonce R., Hibel L. Allostasis and allostatic load in the context of poverty in early childhood. Dev. Psychopathol. 2011;23(03):845–857. doi: 10.1017/S0954579411000344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair C. Stress and the development of self‐ regulation in context. Child Dev. Perspect. 2010;4(3):181–188. doi: 10.1111/j.1750-8606.2010.00145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakely R.D., Veenstra-VanderWeele J. Genetic indeterminism, the 5-HTTLPR, and the paths forward in neuropsychiatric genetics. Arch. Gen. Psychiatry. 2011;68(5):457–458. doi: 10.1001/archgenpsychiatry.2011.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdan R., Agrawal A., Gaffrey M.S., Tillman R., Luby J.L. Serotonin transporter-linked polymorphic region (5-HTTLPR) genotype and stressful life events interact to predict preschool-onset depression: a replication and developmental extension. J. Child Psychol. Psychiatry. 2014;55(5):448–457. doi: 10.1111/jcpp.12142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borg J., Henningsson S., Saijo T., Inoue M., Bah J., Westberg L., Farde Serotonin transporter genotype is associated with cognitive performance but not regional 5-HT1A receptor binding in humans. Int. J. Neuropsychopharmacol. 2009;12(6):783–792. doi: 10.1017/S1461145708009759. [DOI] [PubMed] [Google Scholar]
- Bradley R.H., Corwyn R.F. Socioeconomic status and child development. Annu. Rev. Psychol. 2002;53(1):371–399. doi: 10.1146/annurev.psych.53.100901.135233. [DOI] [PubMed] [Google Scholar]
- Casco C., Tressoldi P.E., Dellantonio A. Visual selective attention and reading efficiency are related in children. Cortex. 1998;34(4):531–546. doi: 10.1016/s0010-9452(08)70512-4. [DOI] [PubMed] [Google Scholar]
- Caspi A., Hariri A.R., Holmes A., Uher R., Moffitt T.E. Genetic sensitivity to the environment: the case of the serotonin transporter gene and its implications for studying complex diseases and traits. Am. J. Psychiatry. 2010;167(5):509–527. doi: 10.1176/appi.ajp.2010.09101452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiao J.Y., Blizinsky K.D. Culture-gene coevolution of individualism-collectivism and the serotonin transporter gene. Proc. R. Soc. B Biol. Sci. 2010;277(1681):529–537. doi: 10.1098/rspb.2009.1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coch D., Sanders L.D., Neville H.J. An event-related potential study of selective auditory attention in children and adults. J. Cogn. Neurosci. 2005;17(4):605–622. doi: 10.1162/0898929053467631. [DOI] [PubMed] [Google Scholar]
- Commodari E., Di Blasi M. The role of the different components of attention on calculation skill. Learn. Indiv. Diff. 2014;32(0):225–232. doi: 10.1016/j.lindif.2014.03.005. [DOI] [Google Scholar]
- Conway C.C., Keenan-Miller D., Hammen C., Lind P.A., Najman J.M., Brennan P.A. Coaction of stress and serotonin transporter genotype in predicting aggression at the transition to adulthood. J. Clin. Child Adolesc. Psychol. 2012;41(1):53–63. doi: 10.1080/15374416.2012.632351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Angiulli A., Herdman A., Stapells D., Hertzman C. Children's event-related potentials of auditory selective attention vary with their socioeconomic status. Neuropsychology. 2008;22(3):293–300. doi: 10.1037/0894-4105.22.3.293. [DOI] [PubMed] [Google Scholar]
- Davies P.T., Cicchetti D. How and why does the 5-HTTLPR gene moderate associations between maternal unresponsiveness and children's disruptive problems? Child Dev. 2014;85(2):484–500. doi: 10.1111/cdev.12148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deckert J., Catalano M., Heils A., DiBella D., Friess F., Politi E., Lesch Functional promoter polymorphism of the human serotonin transporter: lack of association with panic disorder. Psychiatr. Genet. 1997;7(1):45–48. doi: 10.1097/00041444-199700710-00008. [DOI] [PubMed] [Google Scholar]
- Degerman A., Rinne T., Salmi J., Salonen O., Alho K. Selective attention to sound location or pitch studied with fMRI. Brain Res. 2006;1077(1):123–134. doi: 10.1016/j.brainres.2006.01.025. [DOI] [PubMed] [Google Scholar]
- Delorme A., Makeig S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods. 2004;134(1):9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Desimone R., Duncan J. Neural mechanisms of selective visual attention? Annu. Rev. Neurosci. 1995;18(1):193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- Dobson S.D., Brent L.J.N. On the evolution of the serotonin transporter linked polymorphic region (5-HTTLPR) in primates. Front. Hum. Neurosci. 2013;7 doi: 10.3389/fnhum.2013.00588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis B.J., Boyce W.T., Belsky J., Bakermans-Kranenburg M.J., van Ijzendoorn M.H. Differential susceptibility to the environment: an evolutionaryneurodevelopmental theory. Dev. Psychopathol. 2011;23(1):7–28. doi: 10.1017/S0954579410000611. [DOI] [PubMed] [Google Scholar]
- Enge S., Fleischhauer M., Lesch K.P., Reif A., Strobel A. Serotonergic modulation in executive functioning: linking genetic variations to working memory performance. Neuropsychologia. 2011;49(13):3776–3785. doi: 10.1016/j.neuropsychologia.2011.09.038. [DOI] [PubMed] [Google Scholar]
- Enge S., Fleischhauer M., Lesch K.P., Reif A., Strobel A. Variation in key genes of serotonin and norepinephrine function predicts gamma-band activity during goal-directed attention. Cereb. Cortex. 2014;24(5):1195–1205. doi: 10.1093/cercor/bhs398. [DOI] [PubMed] [Google Scholar]
- Evans G.W., Kim P. Multiple risk exposure as a potential explanatory mechanism for the socioeconomic status–health gradient. Ann. N. Y. Acad. Sci. 2010;1186(1):174–189. doi: 10.1111/j.1749-6632.2009.05336.x. [DOI] [PubMed] [Google Scholar]
- Evans G.W. The environment of chilldhood poverty. Am. Psychol. 2004;59:77–92. doi: 10.1037/0003-066X.59.2.77. [DOI] [PubMed] [Google Scholar]
- Fox E., Zougkou K., Ridgewell A., Garner K. The serotonin transporter gene alters sensitivity to attention bias modification: evidence for a plasticity gene. Biol. Psychiatry. 2011;70(11):1049–1054. doi: 10.1016/j.biopsych.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz J.B., Elhilali M., David S.V., Shamma S.A. Auditory attention-focusing the searchlight on sound. Curr. Opin. Neurobiol. 2007;17(4):437–455. doi: 10.1016/j.conb.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Gazzaley A.1. Influence of early attentional modulation on working memory. Neuropsychologia. 2011;49(6):1410–1424. doi: 10.1016/j.neuropsychologia.2010.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuliano R.J., Karns C.M., Neville H.J., Hillyard S.A. Early auditory evoked potential is modulated by selective attention and related to individual differences in visual working memory capacity. J. Cogn. Neurosci. 2014;26(12):2682–2690. doi: 10.1162/jocn_a_00684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green A.E., Munafò M.R., DeYoung C.G., Fossella J.A., Fan J., Gray J.R. Using genetic data in cognitive neuroscience: from growing pains to genuine insights. Nat. Rev. Neurosci. 2008;9(9):710–720. doi: 10.1038/nrn2461. [DOI] [PubMed] [Google Scholar]
- Heils A., Teufel A., Petri S., Stober G., Riederer P., Bengel D., Lesch K.P. Allelic variation of human serotonin transporter gene expression. J. Neurochem. 1996;66(6):2621–2624. doi: 10.1046/j.1471-4159.1996.66062621.x. [DOI] [PubMed] [Google Scholar]
- Hillyard S.A., Hink R.F., Schwent V.L., Picton T.W. Electrical signs of selective attention in the human brain? Science. 1973;182(108):177–180. doi: 10.1126/science.182.4108.177. [DOI] [PubMed] [Google Scholar]
- Hollingshead A.B. Yale University; New Haven, CT: 1975. Four Factor Index of Social Status. Unpublished work. [Google Scholar]
- Holmboe K., Nemoda Z., Fearon R.M.P., Csibra G., Sasvari-Szekely M., Johnson M.H. Polymorphisms in dopamine system genes are associated with individual differences in attention in infancy. Dev. Psychol. 2010;46(2):404–416. doi: 10.1037/a0018180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homberg J.R., Lesch K.P. Looking on the bright side of serotonin transporter gene variation. Biol. Psychiatry. 2011;69(6):513–519. doi: 10.1016/j.biopsych.2010.09.024. [DOI] [PubMed] [Google Scholar]
- Hu X.Z., Lipsky R.H., Zhu G.S., Akhtar L.A., Taubman J., Greenberg B.D., Goldman Serotonin transporter promoter gain-of-function genotypes are linked to obsessive-compulsive disorder. Am. J. Hum. Genet. 2006;78(5):815–826. doi: 10.1086/503850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isbell E., Hampton Wray A., Neville H.J. Individual differences in neural mechanisms of selective auditory attention in preschoolers from lower socioeconomic status backgrounds: an event-related potentials study. Dev. Sci. 2016;19(6):865–880. doi: 10.1111/desc.12334. [DOI] [PubMed] [Google Scholar]
- Iurescia S., Seripa D., Rinaldi M. Role of the 5-HTTLPR and SNP promoter polymorphisms on serotonin transporter gene expression: a closer look at genetic architecture and in vitro functional studies of common and uncommon allelic variants. Mol. Neurobiol. 2015 doi: 10.1007/s12035-015-9409-6. [DOI] [PubMed] [Google Scholar]
- Jedema H.P., Gianaros P.J., Greer P.J., Kerr D.D., Liu S., Higley J.D., Bradberry C.W. Cognitive impact of genetic variation of the serotonin transporter in primates is associated with differences in brain morphology rather than serotonin neurotransmission. Mol. Psychiatry. 2010;15(5):512–522. doi: 10.1038/mp.2009.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenness J.L., Hankin B.L., Abela J.R.Z., Young J.F., Smolen A. Chronic family stress interacts with 5‐ HTTLPR to predict prospective depressive symptoms among youth. Depress. Anxiety. 2011;28(12):1074–1080. doi: 10.1002/da.20904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenness J.L., Hankin B.L., Young J.F., Smolen A. Stressful life events moderate the relationship between genes and biased attention to emotional faces in youth. Clin. Psychol. Sci. 2015:1–15. doi: 10.1177/2167702615601000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karg K., Burmeister M., Shedden K., Sen S. The serotonin transporter promoter variant (5-HTTLPR), stress, and depression meta-analysis revisited: evidence of genetic moderation. Arch. Gen. Psychiatry. 2011;68(5):444–454. doi: 10.1001/archgenpsychiatry.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karns C.M., Isbell E., Giuliano R.J., Neville H.J. Auditory attention in childhood and adolescence: an event-related potential study of spatial selective attention to one of two simultaneous stories. Dev. Cognit. Neurosci. 2015;13(0):53–67. doi: 10.1016/j.dcn.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight R.T., Hillyard S.A., Woods D.L., Neville H.J. The effects of frontal-cortex lesions on event-related potentials during auditory selective attention. Electroencephalogr. Clin. Neurophysiol. 1981;52(6):571–582. doi: 10.1016/0013-4694(81)91431-0. [DOI] [PubMed] [Google Scholar]
- Kraft J.B., Slager S.L., McGrath P.J., Hamilton S.P. Sequence analysis of the serotonin transporter and associations with antidepressant response. Biol. Psychiatry. 2005;58(5):374–381. doi: 10.1016/j.biopsych.2005.04.048. [DOI] [PubMed] [Google Scholar]
- Lengua L.J., Honorado E., Bush N.R. Contextual risk and parenting as predictors of effortful control and social competence in preschool children. J. Appl. Dev. Psychol. 2007;28(1):40–55. doi: 10.1016/j.appdev.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesch K.-P., Waider J. Serotonin in the modulation of neural plasticity and networks: implications for neurodevelopmental disorders? Neuron. 2012;76(1):175–191. doi: 10.1016/j.neuron.2012.09.013. [DOI] [PubMed] [Google Scholar]
- Lesch K.-P., Bengel D., Heils A., Sabol S.Z., Greenberg B.D., Petri S., Murphy D.L. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274(5292):1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- Li J.J., Berk M.S., Lee S.S. Differential susceptibility in longitudinal models of gene-environment interaction for adolescent depression. Dev. Psychopathol. 2013;25(4 Pt 1):991–1003. doi: 10.1017/S0954579413000321. [DOI] [PubMed] [Google Scholar]
- Lonsdorf T.B., Juth P., Rohde C., Schalling M., Ohman A. Attention biases and habituation of attention biases are associated with 5-HTTLPR and COMTval158met. Cognit. Affect. Behav. Neurosci. 2014;14(1):354–363. doi: 10.3758/s13415-013-0200-8. [DOI] [PubMed] [Google Scholar]
- Lopez-Calderon J., Luck S.J. ERPLAB: An open-source toolbox for the analysis of event-related potentials. Front. Hum. Neurosci. 2014;8(213) doi: 10.3389/fnhum.2014.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manuck S.B., McCaffery J.M. Gene-environment interaction. Annu. Rev. Psychol. 2014;65:41–470. doi: 10.1146/annurev-psych-010213-115100. [DOI] [PubMed] [Google Scholar]
- Markant J., Amso D. Leveling the playing field: attention mitigates the effects of intelligence on memory. Cognition. 2014;131(2):195–204. doi: 10.1016/j.cognition.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markant J., Cicchetti D., Hetzel S., Thomas K.M. Relating dopaminergic and cholinergic polymorphisms to spatial attention in infancy. Dev. Psychol. 2014;50(2):360–369. doi: 10.1037/a0033172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mileva-Seitz V., Kennedy J., Atkinson L., Steiner M., Levitan R., Matthews S.G., Fleming A.S. Serotonin transporter allelic variation in mothers predicts maternal sensitivity, behavior and attitudes toward 6-month-old infants. Genes Brain Behav. 2011;10(3):325–333. doi: 10.1111/j.1601-183X.2010.00671.x. [DOI] [PubMed] [Google Scholar]
- Munafò M.R., Durrant C., Lewis G., Flint J. Gene x environment interactions at the serotonin transporter locus. Biol. Psychiatry. 2009;65(3):211–219. doi: 10.1016/j.biopsych.2008.06.009. [DOI] [PubMed] [Google Scholar]
- Murphy D.L., Lesch K.-P. Targeting the murine serotonin transporter: insights into human neurobiology? Nat. Rev. Neurosci. 2008;9(2):85–96. doi: 10.1038/nrn2284. [DOI] [PubMed] [Google Scholar]
- Nakamura M., Ueno S., Sano A., Tanabe H. The human serotonin transporter gene linked polymorphism (5-HTTLPR) shows ten novel allelic variants. Mol. Psychiatry. 2000;5(1):32–38. doi: 10.1038/sj.mp.4000698. [DOI] [PubMed] [Google Scholar]
- Neville H.J., Lawson D. Attention to central and peripheral visual space in a movement detection task: an event-related potential and behavioral study. II. Congenitally deaf adults. Brain Res. 1987;405(2):268–283. doi: 10.1016/0006-8993(87)90296-4. [DOI] [PubMed] [Google Scholar]
- Neville H.J., Stevens C., Pakulak E., Bell T.A., Fanning J., Klein S., Isbell E. Family-based training program improves brain function, cognition, and behavior in lower socioeconomic status preschoolers. Proc. Natl. Acad. Sci. U. S. A. 2013;110(29):12138–12143. doi: 10.1073/pnas.1304437110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobile M., Giorda R., Marino C., Carlet O., Pastore V., Vanzin L., Battaglia M. Socioeconomic status mediates the genetic contribution of the dopamine receptor D4 and serotonin transporter linked promoter region polymorphisms to externalization in preadolescence. Dev. Psychopathol. 2007;19(4):1147–1160. doi: 10.1017/S0954579407000594. [DOI] [PubMed] [Google Scholar]
- Nobile M., Rusconi M., Bellina M., Marino C., Giorda R., Carlet O., Battaglia M. COMT Val158Met polymorphism and socioeconomic status interact to predict attention deficit/hyperactivity problems in children aged 10–14. Eur. Child Adolesc. Psychiatry. 2010;19(7):549–557. doi: 10.1007/s00787-009-0080-1. [DOI] [PubMed] [Google Scholar]
- Odgerel Z., Talati A., Hamilton S.P., Levinson D.F., Weissman M.M. Genotyping serotonin transporter polymorphisms 5-HTTLPR and rs25531 in european- and AfricanAmerican subjects from the national institute of mental health's collaborative center for genomic studies. Transl. Psychiatry. 2013;3:e307. doi: 10.1038/tp.2013.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osinsky R., Reuter M., Kupper Y., Schmitz A., Kozyra E., Alexander N., Hennig J. Variation in the serotonin transporter gene modulates selective attention to threat. Emotion. 2008;8(4):584–588. doi: 10.1037/a0012826. [DOI] [PubMed] [Google Scholar]
- Pearson R., McGeary J.E., Maddox W.T., Beevers C.G. Serotonin promoter polymorphism (5-HTTLPR) predicts biased attention for emotion stimuli: preliminary evidence of moderation by the social environment. Clin. Psychol. Sci. 2016;4(1):122–128. doi: 10.1177/2167702614562470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pergamin-Hight L., Bakermans-Kranenburg M.J., van Ijzendoorn M.H., Bar-Haim Y. Variations in the promoter region of the serotonin transporter gene and biased attention for emotional information: a meta-analysis. Biol. Psychiatry. 2012;71(4):373–379. doi: 10.1016/j.biopsych.2011.10.030. [DOI] [PubMed] [Google Scholar]
- Petersen S.E., Posner M.I. The attention system of the human brain: 20 years after. Annu. Rev. Neurosci. 2012;35:73–89. doi: 10.1146/annurev-neuro-062111-150525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluess M., Belsky J. Vantage sensitivity: individual differences in response to positive experiences. Psychol. Bull. 2013;139(4):901–916. doi: 10.1037/a0030196. [DOI] [PubMed] [Google Scholar]
- Posner M.I., Rothbart M.K., Sheese B.E. Attention genes. Dev. Sci. 2007;10(1):24–29. doi: 10.1111/j.1467-7687.2007.00559.x. [DOI] [PubMed] [Google Scholar]
- Praschak-Rieder N., Kennedy J., Wilson A.A., Hussey D., Boovariwala A., Willeit M., Meyer J.H. Novel 5-HTTLPR allele associates with higher serotonin transporter binding in putamen: a [(11)C] DASB positron emission tomography study. Biol. Psychiatry. 2007;62(4):327–331. doi: 10.1016/j.biopsych.2006.09.022. [DOI] [PubMed] [Google Scholar]
- Pugh K.R., offywitz B.A., Shaywitz S.E., Fulbright R.K., Byrd D., Skudlarski P., Gore J.C. Auditory selective attention: an fMRI investigation. Neuroimage. 1996;4(3 Pt 1):159–173. doi: 10.1006/nimg.1996.0067. [DOI] [PubMed] [Google Scholar]
- Puig M.V., Gulledge A.T. Serotonin and prefrontal cortex function: neurons, networks, and circuits. Mol. Neurobiol. 2011;44(3):449–464. doi: 10.1007/s12035-011-8214-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risch N., Herrell R., Lehner T., Liang K.Y., Eaves L., Hoh J., Merikangas Interaction between the serotonin transporter gene (5-HTTLPR), stressful life events, and risk of depression: a meta-analysis. JAMA. 2009;301(23):2462–2471. doi: 10.1001/jama.2009.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roiser J.P., Rogers R.D., Cook L.J., Sahakian B.J. The effect of polymorphism at the serotonin transporter gene on decision-making, memory and executive function in ecstasy users and controls. Psychopharmacology. 2006;188(2):213–227. doi: 10.1007/s00213-006-0495-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueda M.R., Rothbart M.K., McCandliss B.D., Saccomanno L., Posner M.I. Training, maturation, and genetic influences on the development of executive attention. Proc. Natl. Acad. Sci. 2005;102(41):14931–14936. doi: 10.1073/pnas.0506897102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders L.D., Stevens C., Coch D., Neville H.J. Selective auditory attention in 3to 5-year-old children: an event-related potential study. Neuropsychologia. 2006;44(11):2126–2138. doi: 10.1016/j.neuropsychologia.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Savitz J., Solms M., Ramesar R. The molecular genetics of cognition: dopamine, COMT and BDNF. Genes Brain Behav. 2006;5(4):311–328. doi: 10.1111/j.1601-183X.2005.00163.x. [DOI] [PubMed] [Google Scholar]
- Schwartz J.A. Socioeconomic status as a moderator of the genetic and shared environmental influence on verbal IQ: a multilevel behavioral genetic approach. Intelligence. 2015;52:80–89. [Google Scholar]
- Serences J.T., Kastner S. A multi-level account of selective attention. In: Nobre A.C., Kastner S., editors. The Oxford Handbook of Attention. Oxford University Press; New York, NY: 2014. pp. 76–104. [Google Scholar]
- Squire R.F., Noudoost B., Schafer R.J., Moore T. Prefrontal contributions to visual selective attention. Annu. Rev. Neurosci. 2013;36:451–466. doi: 10.1146/annurev-neuro-062111-150439. [DOI] [PubMed] [Google Scholar]
- Starr L.R., Hammen C., Conway C.C., Raposa E., Brennan P.A. Sensitizing effect of early adversity on depressive reactions to later proximal stress: moderation by polymorphisms in serotonin transporter and corticotropin releasing hormone receptor genes in a 20-year longitudinal study. Dev. Psychopathol. 2014;26(4pt2):1241–1254. doi: 10.1017/S0954579414000996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens C., Bavelier D. The role of selective attention on academic foundations: a cognitive neuroscience perspective. Dev. Cognit. Neurosci. 2012:S30–S48. doi: 10.1016/j.dcn.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens C., Fanning J., Coch D., Sanders L., Neville H. Neural mechanisms of selective auditory attention are enhanced by computerized training: electrophysiological evidence from language-impaired and typically developing children. Brain Res. 2008;1205:55–69. doi: 10.1016/j.brainres.2007.10.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens C., Lauinger B., Neville H. Differences in the neural mechanisms of selective attention in children from different socioeconomic backgrounds: an eventrelated brain potential study. Dev. Sci. 2009;12(4):634–646. doi: 10.1111/j.1467-7687.2009.00807.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens C., Harn B., Chard D.J., Currin J., Parisi D., Neville H. Examining the role of attention and instruction in at-risk kindergarteners: electrophysiological measures of selective auditory attention before and after an early literacy intervention. J. Learn. Disabil. 2013;46(1):73–86. doi: 10.1177/0022219411417877. [DOI] [PubMed] [Google Scholar]
- Stevens C., Paulsen D., Yasen A., Neville H.J. Atypical auditory refractory periods in children from lower socio-economic status backgrounds: ERP evidence for a role of selective attention. Int. J. Psychophysiol. 2014 doi: 10.1016/j.ijpsycho.2014.06.017. [DOI] [PubMed] [Google Scholar]
- Stormer V.S., Passow S., Biesenack J., Li S.C. Dopaminergic and cholinergic modulations of visual-spatial attention and working memory: insights from molecular genetic research and implications for adult cognitive development. Dev. Psychol. 2012;48(3):875–889. doi: 10.1037/a0026198. [DOI] [PubMed] [Google Scholar]
- Strait D.L., Slater J., O’Connell S., Kraus N. Music training relates to the development of neural mechanisms of selective auditory attention. Dev. Cognit. Neurosci. 2015;12(0):94–104. doi: 10.1016/j.dcn.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strobel A., Dreisbach G., Muller J., Goschke T., Brocke B., Lesch K.P. Genetic variation of serotonin function and cognitive control. J. Cogn. Neurosci. 2007;19(12):1923–1931. doi: 10.1162/jocn.2007.19.12.1923. [DOI] [PubMed] [Google Scholar]
- Thomason M.E., Henry M.L., Hamilton J.P., Joormann J., Pine D.S., Ernst M., Gotlib Neural and behavioral responses to threatening emotion faces in children as a function of the short allele of the serotonin transporter gene. Biol. Psychol. 2010;85(1):38–44. doi: 10.1016/j.biopsycho.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong S., Baghurst P., Vimpani G., McMichael A. Socioeconomic position, maternal IQ, home environment, and cognitive development. J. Pediatr. 2007;151(3):284–288. doi: 10.1016/j.jpeds.2007.03.020. (e281.) [DOI] [PubMed] [Google Scholar]
- Tucker-Drob E.M., Rhemtulla M., Harden K.P., Turkheimer E., Fask D. Emergence of a gene x socioeconomic status interaction on infant mental ability between 10 months and 2 years. Psychol. Sci. 2011;22(1):125–133. doi: 10.1177/0956797610392926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkheimer E., Haley A., Waldron M., D'Onofrio B., Gottesman I.I. Socioeconomic status modifies heritability of IQ in young children. Psychol. Sci. 2003;14(6):623–628. doi: 10.1046/j.0956-7976.2003.psci_1475.x. [DOI] [PubMed] [Google Scholar]
- Williams R.B., Marchuk D.A., Gadde K.M., Barefoot J.C., Grichnik K., Helms M.J., Siegler I.C. Serotonin-related gene polymorphisms and central nervous system serotonin function. Neuropsychopharmacology. 2003;28(3):533–541. doi: 10.1038/sj.npp.1300054. [DOI] [PubMed] [Google Scholar]
- Williams R.B., Marchuk D.A., Siegler I.C., Barefoot J.C., Helms M.J., Brummett B.H., Gadde K.M. Childhood socioeconomic status and serotonin transporter gene polymorphism enhance cardiovascular reactivity to mental stress. Psychosomatic Med. 2008;70(1):32–39. doi: 10.1097/PSY.0b013e31815f66c3. [DOI] [PubMed] [Google Scholar]
- Wu C.T., Weissman D.H., Roberts K.C., Woldorff M.G. The neural circuitry underlying the executive control of auditory spatial attention. Brain Res. 2007;1(1134):187198. doi: 10.1016/j.brainres.2006.11.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ijzendoorn M.H., Belsky J., Bakermans-Kranenburg M.J. Serotonin transporter genotype 5HTTLPR as a marker of differential susceptibility? A metaanalysis of child and adolescent gene-by-environment studies. Transl. Psychiatry. 2012:e147. doi: 10.1038/tp.2012.73. [DOI] [PMC free article] [PubMed] [Google Scholar]