Highlights
* Extensive practice improved working memory performance in children and adults. * Performance improvements lasted over a 6-month period. * Before practice, children showed immature frontoparietal activation during working memory manipulation relative to maintenance. * After practice, performance and activation differences between children and adults were considerably reduced. * These findings suggest that the developing frontoparietal network is highly flexible.
Keywords: Working memory, Cognitive control, Development, Training, Children, Functional magnetic resonance imaging
Abstract
Functions that rely on dorsolateral prefrontal and parietal cortex, including working memory manipulation, are among the latest functions to mature. Yet, several behavioral studies have shown that children may improve on these functions after extensive practice. In this pilot study, we examined whether children would be able to demonstrate increased frontoparietal brain activation after practice. Twelve-year-old children and young adults practiced for 6 weeks with a working memory manipulation task. Before and after practice, functional magnetic resonance imaging data were acquired. Both children and adults demonstrated better performance, lasting at least up to 6 months after the practice period. Before practice, children showed immature frontoparietal activation for manipulation of information in working memory relative to pure maintenance, specifically during the delay period of the task. After practice, the activation differences between children and adults were considerably reduced, suggesting that children may show increased frontoparietal activation if given extensive practice. These preliminary findings argue against the hypothesis that certain brain structures cannot be engaged because of immaturity. Yet, future studies with larger samples should further examine flexibility in the developing brain, and establish what can and cannot be expected of children across school-aged development.
1. Introduction
Several studies have demonstrated that complex cognitive functions mediated by the dorsolateral prefrontal cortex (DLPFC) and superior parietal cortex show a protracted development (Diamond, 2002). For example, task switching, inhibition, and working memory manipulation (i.e., the ability to hold information in mind and work with it) improve up to late adolescence (Huizinga et al., 2006). In addition, functional magnetic resonance imaging (fMRI) studies have shown that children have immature activation patterns in DLPFC and parietal cortex during cognitive control tasks (Bunge and Wright, 2007, Crone et al., 2006, Klingberg, 2006). A fundamental question in current research on cognitive development concerns the extent to which these findings can be directly attributed to the protracted structural maturation of these regions or whether they can be reduced as a result of practice (Bunge and Crone, 2009, Casey et al., 2005, Durston and Casey, 2006). In the present study, we examined the flexibility of frontoparietal activation in children (relative to adults) by investigating the effects of extensive practice with a working memory manipulation task.
Behavioral studies have already demonstrated that children can improve their performance on complex cognitive tasks after extensive practice (Holmes et al., 2009, Karbach and Kray, 2009, Klingberg, 2010, Mackey et al., 2011). However, it is still unclear whether children will also demonstrate increased brain activation in the same regions that are task-relevant in adults (i.e., the frontoparietal network), or whether they will use compensatory brain regions. It is expected that the effects of practice on brain function depend on the maturation of the underlying brain structure. Longitudinal research examining changes in brain structure over development has shown that changes in cortical grey and white matter are still taking place until late adolescence (e.g., Giedd et al., 2009, Gogtay et al., 2004). Specifically higher order association areas in the DLPFC and parietal cortex are among latest regions to mature (Giedd et al., 2009). It is therefore possible that the immature neural circuitry prevents children from learning a specific task or, if children do learn the task, they might rely on compensatory brain regions (Luna, 2004, Scherf et al., 2006). On the other hand, an immature brain might allow plasticity, suggesting even stronger effects of practice in children (Luna, 2004, Qin et al., 2004).
There is already some evidence from neuroimaging research that experience or practice may influence brain activation in children (Aylward et al., 2003, Haier et al., 2009, Qin et al., 2004, Shaywitz et al., 2004, Simos et al., 2002, Temple et al., 2003). For example, it has been demonstrated that brain activation in children with developmental disorders, such as dyslexia, may normalize as a result of training (Aylward et al., 2003, Shaywitz et al., 2004, Simos et al., 2002, Temple et al., 2003). In the present pilot study, we used the same approach in typically developing children to examine whether they engage the same neural circuitry as adults when given extensive training (Bunge and Crone, 2009, Casey et al., 2005).
The pilot study involved 10 children (age 11–13; 6 female) and 15 young adults (age 19–25; 8 female), who practiced for 6 weeks, two to three times a week, with a working memory manipulation task. Before and after practice, participants were scanned with fMRI. The working memory manipulation task was selected because of the consistent age differences that were found in prior research (Crone et al., 2006, Jolles et al., 2011), and the effects of practice in young adults (Jolles et al., 2010). That is, unlike adults, 8- to 12-year-olds failed to recruit frontoparietal regions (right DLPFC in particular) for manipulation of information in working memory relative to pure maintenance (Crone et al., 2006, Jolles et al., 2011). Yet, it has been demonstrated that adults showed increased activation for manipulation relative to maintenance in these regions after extensive practice, specifically when the task load was high (Jolles et al., 2010). The present pilot study aimed to examine whether it is possible to train frontoparietal brain regions in 12-year-old children or whether children will recruit a different set of regions after practice. Future studies with larger samples should validate and extend the current findings by examining flexibility of brain function across a wide range of tasks and age groups.
2. Materials and methods
2.1. Participants
Eleven children and 15 adults participated in the current study. One child was excluded from the analyses because he got engaged in an accident in between practice sessions, resulting in a group of 10 children (children: Mage = 12.35 years (SD = .67), 6 female; adults: Mage = 22.04 years (SD = 1.85), 8 female). A chi-square analysis confirmed that the sex distribution did not differ between age groups (χ2(1, n = 25) = .11, p = .74). All participants gave written informed consent for participation in the study. Parents of children that participated in the study gave written informed consent as well. Prior to enrollment, participants were screened for psychiatric or neurological conditions, history of head trauma, and history of attention or learning disorders. No deviances were reported. Parents of the children filled out the Child Behavior Checklist (CBCL) (Achenbach, 1991) to screen for psychiatric symptoms. All children scored below clinical levels on all subscales of the CBCL. Participants completed two subscales (similarities and block design) of either the Wechsler Adult Intelligence Scale (WAIS) (Wechsler, 1997) or the Wechsler Intelligence Scale for Children (WISC) (Wechsler, 1991) to obtain an estimate of their IQ. The estimated IQ scores did not differ between age groups (children: 108.5 (SD = 11.0); adults: 113.0 (SD = 9.0); F(1,23) = 1.26; p = .27, η2 = .05).
In addition, we recruited 8 control group children, who participated in two test sessions that were separated by 6 weeks, but did not receive any instructions between these sessions (n = 8; Mage = 12.66 years (SD = .10); 3 female). There were no differences between children of the practice group and children of the control group groups in terms of age (F(1,16) = 1.61; p = .22, η2 = .09), sex (χ2(1, n = 18) = .90; p = .34) and estimated IQ scores (practice group: 108.5 (SD = 11.0); control group: 110.0 (SD = 14.8); F(1,16) = .06; p = .81, η2 = .004). Due to technical difficulties and head motion, fMRI data of two control participants were lost. The fMRI data of the other participants are presented in Supplement D. Finally, there was an adult control group, but these data are reported elsewhere (Jolles et al., 2010).
Adults received financial compensation for participation. Children received a gift and their parents received a monetary compensation for travel costs. The experiment was approved by the Central Committee on Research involving Human Subjects in the Netherlands.
2.2. Practice procedure and tasks
All participants practiced with the working memory task for 6 weeks, two to three times a week, and they were scanned before and after practice using fMRI (see also Jolles et al., 2010). Six months after the experiment, there was a (behavioral) follow-up session to assess the durability of performance improvements. One adult participant did not take part in the follow-up session (no response).
2.2.1. Working memory task
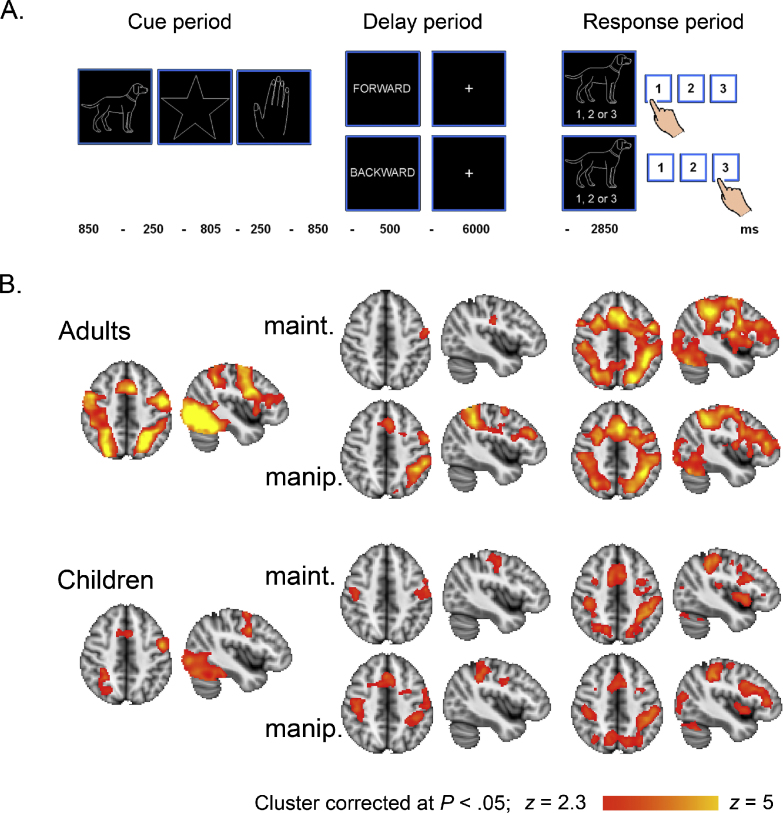
In the working memory task, each trial started with a 250 ms fixation cross, followed by three, four, or five sequentially presented objects in the centre of the screen (Fig. 2A). Each object was shown for 850 ms with a period of 250 ms in between. After the last object, the instruction “forward” or “backward” was presented for 500 ms. The forward instruction indicated that participants had to rehearse the objects in the presented order during a 6000 ms delay; the backward instruction indicated that participants had to rehearse the objects in the reversed order. After the delay, one of the target objects was presented for 2850 ms and participants had to indicate the location of the target object in the forward or backward sequence. During scanning, there were jittered periods of fixation between the trials based on an optimal sequencing program designed to maximize the efficiency of recovery of the blood oxygenation level dependent (BOLD) response (Dale, 1999).
Fig. 2.
(A) Working memory task (B) Working memory related activation before practice for Cue > fixation, Delay > fixation (maintenance and manipulation conditions separately), and Response > fixation (maintenance and manipulation conditions separately). Images are overlaid on axial (z = 46) and sagittal (x = −42) slices of a standard anatomical image. The left of the image is the right of the brain.
Each session consisted of three blocks of 30 trials each, in which 15 forward and 15 backward items were intermixed; one block with sequences of three objects (i.e., load 3), one block with sequences of four objects (i.e., load 4) and one block with sequences of five objects (i.e., load 5). During scanning, the order of runs was counterbalanced across participants, but it was the same for each participant before and after practice. The duration of the load 3 task block was 8.14 min; the duration of the load 4 task block was 8.84 min; and the duration of the load 5 task block was 9.53 min (i.e., the blocks had a different duration because the trials were longer depending on the number of objects that were presented during the stimulus phase). The total scan time was on average 45 min per session. In the present study, we only analyzed the blocks in which participants had to remember sequences of three or four objects. Data were collapsed across these blocks to increase power. When we entered load as a separate variable in the region of interest (ROI) analyses, we did not find a main effect of load or an interaction between load and condition before or after practice (all ps ≥ .11), nor did we find an interaction with time (all ps ≥ .58). The third block, in which participants had to remember sequences of five objects, was not included in the analyses because there were indications that participants were using a different strategy during this block (Jolles et al., 2010, Jolles et al., 2011), which makes it difficult to average activation across different blocks. More specifically, several participants indicated that they used grouping or chunking to memorize high-load forward sequences, suggesting that the high-load forward trials could not be regarded as pure maintenance trials.
We used four sets of stimuli, each consisting of 150 pictures of simple objects, to reduce familiarization effects. Every object could appear only once during each task block and the combination of objects within a sequence was randomly determined. Throughout the practice sessions two sets of colored pictures were used, which alternated every week. One set consisted of hand drawn pictures (Rossion and Pourtois, 2004) and the other set comprised photographs of simple objects. During scanning, two sets of black and white pictures were used, taken from the Max Planck Institute's picture database (www.mpi.nl). The selection of stimuli used before and after practice was randomized across subjects. Before scanning, participants were shown all pictures and they were asked to name each object out loud. They were instructed that there was no right or wrong answer, but they should name the objects with one or two-syllable words. Children and adults could name the objects without difficulties.
Before the first scan, the participants performed a number of trials to make sure that they understood the task instructions. There were five pre-scan blocks, which were presented in the following order: one block with four maintenance trials, one block with four manipulation trials and three blocks with eight trials in which maintenance and manipulation trials were mixed. In these blocks, sequences consisted of three, four, or five objects.
2.2.2. Practice procedure
Once a week, the participants performed the task under the supervision of a trained experimenter. The supervised practice session took place at the school of the participants (children) or at Leiden University (adults). The other practice sessions could be completed at home via the Internet. The participants could flexibly choose when to practice the task, under the restriction that they had to perform the task on three separate days during a week. They were explicitly instructed to perform the practice sessions by themselves (without help from their parents). On average, the children practiced 15 times (SD = 2.69) during the 6-week period and the adults practiced 16 times (SD = 1.73). The number of practice sessions did not differ significantly between groups (F(1,23) = 2.42; p = .13, η2 = .10). Practice sessions lasted approximately 25 min each.
Performance during the unsupervised sessions was recorded and monitored. If participants did not practice for two or more days, they received an e-mail to encourage them to start a new practice session. Combined across load 3 and load 4, children performed with an accuracy of 76.9% (SD = 18.0) during the unsupervised practice sessions, compared to 78.4% (SD = 14.7) during the supervised practice sessions; adults performed with an accuracy of 90.3% (SD = 6.6) during the unsupervised practice sessions, compared to 92.3% (SD = 5.1) during the supervised practice sessions. These findings indicate that the participants were seriously involved in the practice sessions. However, adults performed better during the supervised practice sessions than during the unsupervised practice sessions (F(1,14) = 9.68; p < .01, η2 = .41). In children there was no significant difference between practice sessions (F(1,9) = .58; p = .47, η2 = .06).
2.2.3. Transfer tasks
In addition, to learn more about the specific skills that were being trained, we investigated whether performance improvements generalized to unpracticed executive function tasks (e.g., Klingberg, 2010). Seven transfer tasks were administered to assess generalization effects. Previously, we described that transfer effects were absent in the adults (Jolles et al., 2010). In the present study, we examined whether transfer effects were present in the children, by comparing their performance to performance of a control group who participated in the two test sessions before and after practice, but did not receive any instructions during the 6 weeks between these sessions. In addition to the practiced working memory task, all children performed Raven Standard Progressive Matrices (RSPM; Raven et al., 1998), odd numbered items before practice and even numbered items after practice or the other way around (Jaeggi et al., 2008) and the Digit Span task of the WISC (Wechsler, 1991) both before and after the practice period. In addition, five other tasks were administered after the practice period only. These tasks included a spatial variant of the working memory task that was practiced and four tasks of an executive function test battery (Huizinga et al., 2006) (i.e., 1. the Mental Counters task to assess updating in working memory, 2. the Local–Global task to assess cognitive flexibility and inhibition, 3. the Wisconsin Card Sorting Task (WCST) and 4. the Tower of London (TOL) as complex executive function indices) The details about these transfer tasks are described in Jolles et al. (2010).
2.3. fMRI data acquisition
Scanning was performed with a standard whole-head coil on a 3-T Philips Achieva MRI system. A total of 222 and 241 T2*-weighted whole-brain EPIs were acquired (for the task blocks with sequences of three or four objects respectively), including two dummy scans preceding each scan to allow for equilibration of T1 saturation effects (TR = 2.2 s; TE = 30 ms, flip angle = 80°, 38 transverse slices, 2.75 × 2.75 × 2.75 mm (+10% inter-slice gap)). Visual stimuli were projected onto a screen that was viewed through a mirror at the head end of the magnet. After the functional scans, a high-resolution EPI scan and a T1-weighted anatomical scan were obtained for registration purposes (EPI scan: TR = 2.2 ms; TE = 30 ms, flip angle = 80°, 84 transverse slices, 1.964 × 1.964 × 2 mm; 3D T1-weighted scan: TR = 9.717 ms; TE = 4.59 ms, flip angle = 8°, 140 slices, .875 × .875 × 1.2 mm, FOV = 224.000 × 168.000 × 177.333). All anatomical scans were reviewed and cleared by a radiologist. No anomalous findings were reported.
We used a mock scanner to acclimate the participants to the scanner environment and we used cushions to reduce head movement in the scanner. Before practice adults showed a mean absolute displacement of .276 mm (SE .04), and children of .276 mm (SE .05); after practice adults showed a mean absolute displacement of .351 mm (SE .12), and children of .380 mm (SE .15). There were no differences between children and adults in mean absolute displacement (before practice: F(1,23) < .001, p = .996, η2 < .001; after practice: F(1,23) = .02, p = .88, η2 = .001). However, relative displacement was higher in children on both occasions (before practice: F(1,23) = 23.60, p < .001, η2 = .51; after practice: F(1,23) = 4.68, p < .05, η2 = .17). Children showed a mean relative displacement of .115 mm (SE .007) before practice and .115 mm (SE .01) after practice, compared with .069 mm (SE .006) before practice and .083 mm (SE .09) after practice in adults. The children with the largest values still showed a mean relative displacement of <.2 mm. To control for the influence of head movement on the fMRI data, motion parameters were added to the general linear model (GLM).
2.4. fMRI data analysis
Data analysis was carried out using FEAT (fMRI Expert Analysis Tool) Version 5.98, part of FSL (FMRIB's Software Library, www.FMRIb.ox.ac.uk/fsl (Smith et al., 2004). The following prestatistics processing was applied: motion correction (Jenkinson et al., 2002); non-brain removal (Smith, 2002); spatial smoothing using a Gaussian kernel of FWHM 8.0 mm; grand-mean intensity normalization of the entire 4D dataset by a single multiplicative factor; high-pass temporal filtering (Gaussian-weighted least-squares straight line fitting, with sigma = 50.0 s). Functional scans were registered to high-resolution EPI images, which were registered to T1 images, which were registered to standard MNI space (Jenkinson et al., 2002, Jenkinson and Smith, 2001). Independent Component Analysis (with MELODIC implemented in FSL) was used in two participants (one before practice and one after practice) to remove scanner artifacts (i.e., signal inhomogeneities) from the data. However, practice effects did not change depending on whether or not Independent Component Analysis was run on the two participants’ data.
In native space, the fMRI time-series were analyzed using an event-related approach in the context of the general linear model with local autocorrelation correction (Woolrich et al., 2001). Within each run, cue period, delay period, and response period were modeled separately. Each effect was modeled on a trial-by-trial basis as a concatenation of square-wave functions: the cue period started with the presentation of the first memory item and lasted until the last memory item disappeared (3050 ms or 4150 ms for sequences of three or four objects respectively); the delay period started with the instruction and lasted until the target item appeared (6500 ms); and the response period started with the presentation of the target item and lasted until the participant made a response (≤2850 ms). Delay- and response periods of maintenance and manipulation trials were modeled separately. If present, erroneous trials were included in the model (delay- and response periods separately), but excluded from the contrasts of interest. Hence, there were either five or seven square-wave functions (i.e., cue, delay maintenance, response maintenance, delay manipulation, response manipulation, delay error, response error). Each of these square-wave functions was convolved with a canonical hemodynamic response function and its temporal derivative. In addition, we included the six motion parameters as confound regressors in our model. The model was high-pass filtered (Gaussian-weighted least-squares straight line fitting, with sigma = 50.0 s).
Based on prior reports, we mainly focused on delay period activation for manipulation relative to maintenance trials (Crone et al., 2006, Jolles et al., 2010), but cue- and response periods were analyzed as well. For each run within each session, the following contrast images were created: 1. Cue > fixation; 2. Delay > fixation (maintenance condition); 3. Delay > fixation (manipulation condition); 4. Delay manipulation > delay maintenance; 5. Response > fixation (maintenance condition); 6. Response > fixation (manipulation condition); 7. Response manipulation > response maintenance. Next, the contrast images of the two runs (load 3 and load 4) within a scanning session were combined using fixed-effects analyses on a subject-by-subject and session-by-session basis (Beckmann et al., 2003, Woolrich et al., 2004). Finally, the resulting second-level contrast images were used in third-level whole brain analyses (all contrasts) and region of interest analyses (contrasts 2 and 3 only).
2.4.1. Whole brain mixed-effects group analyses
To examine activation differences between children and adults at the whole brain level, second-level contrast images were submitted to third-level mixed-effects group analyses, which were performed separately for the sessions before and after practice. In addition, a comparison was made between both sessions using a time × group GLM. The statistical parametric images were thresholded using clusters determined by z > 2.3 and a cluster corrected significance threshold of p < .05 (Worsley, 2001).
2.4.2. Region of interest analyses
Next, we performed region of interest analyses to examine the effects of practice in children in more detail. For these analyses, we concentrated on the delay period activation in (bilateral) DLPFC because prior studies have reported that immature activation was most pronounced in this region (Crone et al., 2006, Jolles et al., 2011). The location of the ROIs was functionally defined using an unbiased whole brain delay > fixation contrast (which is combined across maintenance and manipulation conditions) in a group of seven adults and seven children who did not take part in the working memory training. First, the obtained statistical map was thresholded using clusters determined by z > 2.3 and a cluster corrected significance threshold of p < .05. Then, it was masked by an anatomical ROI of the middle frontal gyrus from the Harvard–Oxford cortical atlas (FMRIb.ox.ac.uk/fsl/data/atlasdescriptions.html#ho). The ROIs that we found were slightly more superior than the ROIs in a previous study (Crone et al., 2006) but they were similar to the ROIs in two other studies (Jolles et al., 2010, Jolles et al., 2011). Mean z-values were calculated for second-level contrasts 2 and 3 (i.e., the delay > fixation contrasts for the maintenance condition and manipulation condition) for each session (i.e., before and after practice) in each participant, using Featquery (FMRIb.ox.ac.uk/fsl/feat5/featquery.html). Finally, the mean z-values were entered in a repeated-measures ANOVA with time (before and after practice) and condition (maintenance and manipulation) as within-subjects variables.
3. Results
3.1. Performance
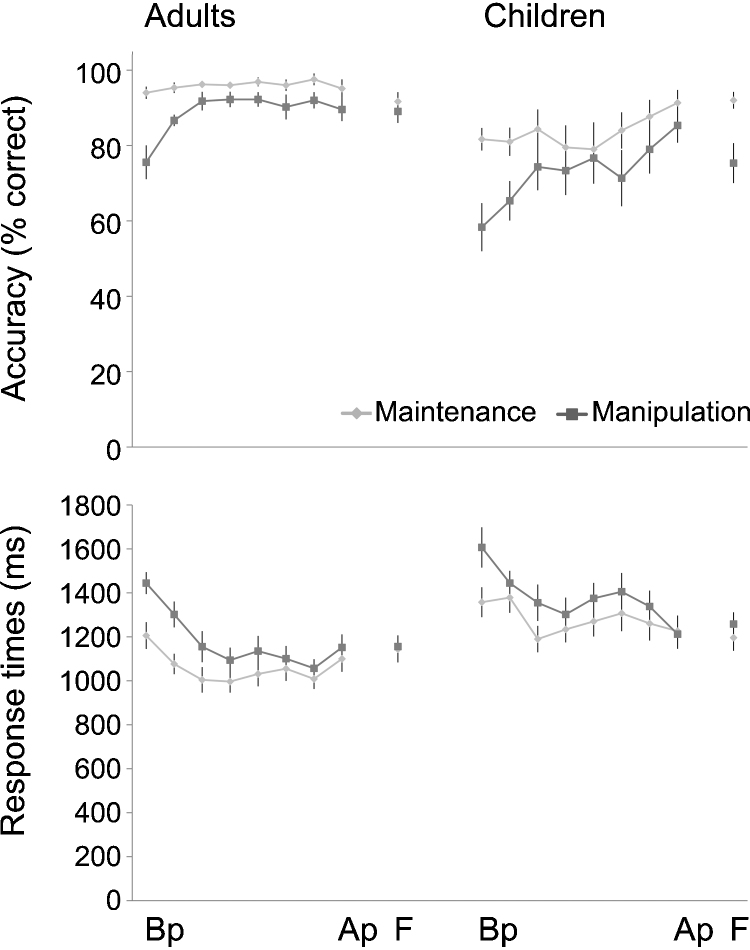
Performance on the practiced working memory task was analyzed for both accuracy (defined as the percentage of correct responses) and response times. Response times were calculated for correct trials only. To examine the effects of practice, we used a repeated-measures ANOVA with time (before and after practice) and condition (maintenance and manipulation) as within-subjects variables and age group (children and adults) as the between-groups factor. Participants responded faster and more accurately after practice (accuracy: F(1,23) = 25.62, p < .001, η2 = .53; RT: F(1,23) = 40.02, p < .001, η2 = .64; Fig. 1), specifically for manipulation trials (accuracy: F(1,23) = 18.08, p < .001, η2 = .44; RT: F(1,23) = 38.43, p < .001, η2 = .63). For accuracy, there was a time × group interaction (F(1,23) = 4.44, p < .05, η2 = .16), indicating that before practice adults performed better than children (F(1,23) = 9.26, p < .01, η2 = .29), but after practice the group difference was no longer significant (F(1,23) = .78, p = .39, η2 = .03). As can be seen in Fig. 1, the adults performed at ceiling already early in the training, but children showed a more linear improvement. During a follow-up session 6 months after the experiment, accuracy and response times were still better than before practice in both groups (all ps < .001; Fig. 1), demonstrating the durability of practice effects. A time × condition × age interaction was found when performance at the follow-up session was compared with the session after practice (F(1,22) = 5.34, p < .05, η2 = .20), indicating that there was an accuracy decrease for manipulation relative to maintenance in children, but not in adults.
Fig. 1.
Accuracy and response times for adults and children before practice (Bp), after practice (Ap), and during the follow-up session (F), as well as during the six supervised weekly practice sessions. Performance differences between children and adults were no longer significant after practice. Performance was still better during the follow-up session 6 months after the experiment compared to the session before practice. Error bars show SEM.
In addition, we examined whether performance improvements generalized to unpracticed executive function tasks. In a prior report, we already described that transfer effects were absent in the adults (Jolles et al., 2010). To test whether transfer effects were present in the children (n = 10), we compared their performance to performance of an age-matched control group (n = 8). These analyses can be considered preliminary because of the small sample sizes, but they may provide important directions for future research. First, we examined whether children's performance improvements on the practiced working memory task were larger than improvements of the control group. Before practice there were no differences between groups (all ps ≥ .25). After practice, children in the practice group were faster than children of the control group (F(1,16) = 6.25, p < .05, η2 = .28), which was confirmed by a time × group interaction (F(1,16) = 5.88, p < .05, η2 = .27). There were no significant differences between groups in terms of accuracy (main effect of group after practice: F(1,16) = .34, p = .57, η2 = .02; time × group interaction: F(1,16) = 4.30, p = .06, η2 = .21). Second, we investigated whether performance improvements generalized to unpracticed executive function tasks by comparing performance of the practice group and the control group on seven transfer tasks. It was demonstrated that after practice, children performed better on the digit span task of the WISC (F(1,16) = 6.25, p < .05, η2 = .28), but there was no significant difference between the practice group and the control group (F(1,16) = .05, p = .82, η2 = .003). Only one of the tasks showed a slight advantage for the children of the practice group: the Mental Counters working memory task (F(1,15) = 6.55, p < .05, η2 = .30; see also Supplement A). However, this effect did not survive Bonferroni correction for the number of tests performed.
3.2. Whole brain analyses
Using whole brain contrasts, we investigated age differences in working-memory related brain activation, both before and after the practice period. We examined activation during cue, delay, and response periods relative to fixation, as well as activation differences between manipulation and maintenance trials (for delay and response periods separately). We were specifically interested in manipulation > maintenance during the delay period, where we expected developmental differences to be most evident (Crone et al., 2006). The statistical parametric images were thresholded using clusters determined by z > 2.3 and a cluster corrected significance threshold of p < .05 (Worsley, 2001).
3.2.1. Before practice
In general, the task activated a bilateral frontoparietal network, including lateral PFC, anterior insula, anterior cingulate cortex, supplementary motor area, superior parietal cortex, supramarginal gyrus and lateral occipital cortex. Most of these regions were found during all phases of the task, in adults as well as children (Fig. 2B). In addition to the frontoparietal network, we also found activation in lower occipital regions, mainly during the cue and response periods of the task. Group comparisons showed increased activation for adults compared to children in left lateral PFC during cue and response periods (maintenance condition). During the cue period, we also found increased activation for adults compared to children in occipital regions.
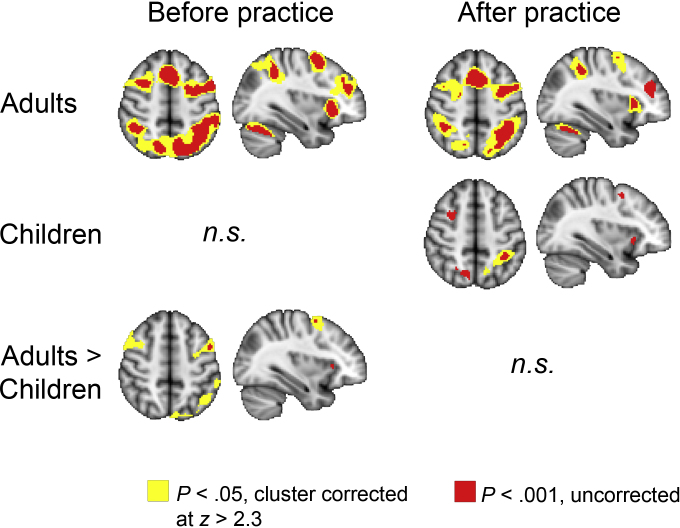
As expected, adults showed increased frontoparietal activation for manipulation > maintenance during the delay period (Fig. 3; Supplement B), but not during the response period of the task. Consistent with prior results (Crone et al., 2006), children did not show more activation for manipulation relative to maintenance, even when the threshold was lowered to p < .001, uncorrected for multiple comparisons. This developmental difference was confirmed by a condition × group effect at the whole brain level (Fig. 3; Supplement B).
Fig. 3.
Delay period activation for manipulation > maintenance. Images are overlaid on axial (z = 50) and sagittal (x = 34) slices of a standard anatomical image. The left of the image is the right of the brain.
3.2.2. After practice
After practice, the task activated very similar frontal and parietal regions, as well as occipital regions (mainly during cue and response periods). There were some differences between the groups when comparing task activation to fixation. That is, during the cue period, adults showed increased activation in the occipital cortex; during the delay period (manipulation condition), adults showed increased activation in the superior parietal cortex/lateral occipital cortex; and during the response period (maintenance condition), adults showed increased activation in the supplementary motor area.
However, with respect to the delay period manipulation > maintenance contrast there were no longer statistically significant differences between the age groups at the cluster corrected threshold (z > 2.3, p < .05) or at the uncorrected threshold of p < .001. Children showed increased activation for manipulation relative to maintenance in superior parietal cortex and lingual gyrus (cluster corrected at z > 2.3, p < .05) and when the threshold was lowered to p < .001 uncorrected, right DLPFC and bilateral anterior insula were found as well (Fig. 3; Supplement B). Children did not recruit additional regions compared with adults, arguing against the possibility of recruitment of compensatory brain regions. Hence, after practice activation in children was increased in the same regions as those that are used by adults. However, it should be noted that the effects of time did not reach significance at the whole brain level.
3.2.3. Performance-matched analyses
An additional analysis was carried out to examine whether the observed frontoparietal activation in children after practice was caused by an increased number of data points, associated with more correct trials. For each child, we selected a random subset of correct trials after practice to match the number of correct trials before practice. The remaining trials were modeled as a covariate of no interest (which also included the incorrect trials). For this analysis, children showed increased activation for manipulation relative to maintenance in lingual gyrus (cluster corrected at z > 2.3, p < .05) and when the threshold was lowered to p < .001 uncorrected, superior parietal cortex and dorsolateral prefrontal cortex were found as well (Supplement B). Moreover, there were no significant differences between the age groups. These findings indicate that children's increased activation for manipulation > maintenance after practice could not fully be explained by an increased number of data points.
3.2.4. Correction for grey matter volume
Because higher order association areas such as the dorsolateral PFC and superior parietal cortex show a particularly long grey matter development, we were interested whether the age differences in neural activation were influenced by underlying differences in grey matter density (or possible misregistrations). To this end, we repeated the third-level whole-brain analyses using grey matter volume information as a voxelwise covariate (Oakes et al., 2007). These analyses gave very similar results as the analyses without grey matter correction (see Supplement C), suggesting that it is unlikely that the results can be explained by differences in grey matter volume alone.
3.3. Region of interest analyses
Finally, we performed an ROI analysis to investigate the effects of practice in children in more detail, focusing on delay activation in left and right DLPFC. For both ROIs we performed a repeated-measures ANOVA with time (before and after practice) and condition (maintenance and manipulation) as within-subject variables.
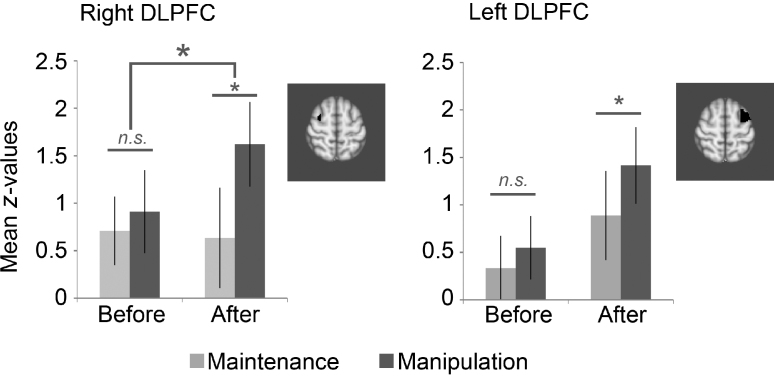
For right DLPFC, we found a time × condition interaction, indicating that activation increased after practice for manipulation trials relative to maintenance trials (Fig. 4; F(1,9) = 6.08, p < .05, η2 = .40). Post hoc tests illustrated that before practice there was no significant difference between conditions (F(1,9) = .66, p = .44, η2 = .07), but after practice, activation was increased for manipulation relative to maintenance (F(1,9) = 12.25, p < .01, η2 = .58). A second set of post hoc tests was carried out to examine whether the time × condition effect was primarily caused by increased activation during manipulation trials or by decreased activation during maintenance trials, but neither effect was significant (maintenance: F(1,9) = .03, p = .87, η2 = .003; manipulation F(1,9) = 2.60, p = .14, η2 = .22). For left DLPFC, there was a main effect of condition (Fig. 4; F(1,9) = 5.17, p < .05, η2 = .37), but the effects of time were not significant (time: F(1,9) = 2.50, p = .15, η2 = .22; time × condition: F(1,9) = 1.59, p = .24, η2 = .15). Yet, when the sessions before and after practice were examined separately, the effect of condition was only significant after practice (before practice: F(1,9) = 1.41, p = .27, η2 = .14; after practice: F(1,9) = 5.39, p < .05, η2 = .38). For the control group, we did not observe any effects of time and/or condition in bilateral DLPFC (see Supplement D).
Fig. 4.
Delay period activation for children in right DLPFC and left DLPFC ROIs (error bars show SEM). Both regions showed increased activation for manipulation relative to maintenance after practice, but not before practice. In right DLPFC there was also a time × condition interaction.
4. Discussion
In the present study, we examined the malleability of brain function as a result of working memory practice in a pilot sample of 12-year-old children, compared with young adults. In agreement with prior studies, practice resulted in better performance (Holmes et al., 2009, Karbach and Kray, 2009, Klingberg, 2010, Mackey et al., 2011), which lasted at least up to 6 months after the practice period. Moreover, performance differences between children and adults were no longer significant after practice, indicating that children in this age group are able to perform this particular task at an adult level.
4.1. Frontoparietal activation before and after practice
Before practice, working memory-related brain activation was present in a frontoparietal network, including dorsolateral PFC, superior parietal cortex, and anterior cingulate cortex/supplementary motor area. Frontoparietal regions were found during all phases of the task, both in adults and in children. Although there were some age differences during the cue- and response periods, age differences were most pronounced during the delay period. During this period, adults showed increased frontoparietal activation for the manipulation condition relative to the maintenance condition, which is thought to be associated with the reordering of memory items in response to the backward instruction (D’Esposito et al., 1999, Owen, 2000, Smith and Jonides, 1999, Wagner et al., 2001, Wendelken et al., 2008). As predicted, children failed to show significant delay period activation for manipulation above and beyond the regions they used for pure maintenance (e.g., see also Crone et al., 2006). It is important to note that frontoparietal activation was found for other contrasts, which indicates that the under-recruitment during delay period manipulation relative to maintenance trials was not purely a power issue. Moreover, these findings argue against the Maturational Viewpoint of functional brain development (as described in Johnson, 2011), which suggests that these regions are not yet “functional” due to immature neural circuitry.
After practice, the observed age differences in delay period activation were reduced. That is, after training children showed increased activation in similar regions as were seen in adults, including DLPFC, anterior insula and superior parietal cortex. Children did not rely on any additional, compensatory brain regions. It is important to stress that based on null-results (i.e., a lack of group differences after training), we cannot conclude that there is indeed no difference between children and adults. Nevertheless, the present findings may serve as a proof of principle, illustrating flexibility in children's brain activation as a result of training.
Although time effects did not reach significance at the whole brain level, ROI analyses showed an increase for manipulation trials relative to maintenance trials in the right DLPFC. Post hoc analyses demonstrated that before practice there was no difference between conditions, but after practice activation was increased for manipulation relative to maintenance. A similar pattern was found for left DLPFC, although the effect of time was not significant for this region. From the present data, it is not clear whether the increase for manipulation trials relative to maintenance trials was mainly caused by increased activation during manipulation trials or by decreased activation during maintenance trials, and it might have been a combination of both. It should be noted that there was a large variability between participants, and it is possible that some participants predominantly showed activation increases for manipulation trials, while others mainly showed activation decreases during maintenance trials. Future studies with larger samples should further examine the effects of training on working memory manipulation and maintenance processes, and explore the relation between individual differences in training-related activation changes and individual differences in performance gain.
For adults, there were no changes in brain activation within the frontoparietal network for load 3 and load 4. Even though performance was already at ceiling in week 3, after practice activation for manipulation trials was still increased relative to maintenance trials, suggesting that controlled processing was still required (cf. Jolles et al., 2010). However, as described in our prior study, the adults showed practice-related activation changes at load 5. That is, adults showed increased frontoparietal activation for manipulation relative to maintenance trials after practice, comparable to the effect that was observed in children during load 3 and load 4. This finding suggests that training effects depend on the specific task requirements and the difficulty of the task.
4.2. Flexibility or plasticity
There are two possible explanations for the observed activation increases for manipulation relative to maintenance trials. On the one hand, the activation changes could reflect flexibility of brain function that takes place within the limits of the current structural constraints of the brain (Lövdén et al., 2010, Posner and Rothbart, 2005). For example, changes in delay period activation might have occurred if children adopted a different strategy. That is, activation changes could reflect an increased use of control processes during manipulation trials and/or the choice for a more reactive strategy during maintenance trials (Jolles et al., 2010). On the other hand, the practice effects may indicate plastic changes in the underlying brain structure (Lövdén et al., 2010, Posner and Rothbart, 2005). It has been demonstrated that experience can induce neural changes like myelination, synaptic pruning, or synaptic strengthening (Changeux and Danchin, 1976, Fields, 2008, Huttenlocher, 2002). These processes may improve processing efficiency (e.g., increased working memory capacity, speed of processing, etc.), and lead to changes in neural activation, suggesting that the observed activation changes in the present study might also have a structural basis. It is, however, unlikely that the present results are directly caused by changes in grey matter volume, because the results were almost unaffected when we included grey matter volume as a voxelwise covariate in the analysis. In line with these findings, a prior study showed that structural and functional brain changes after practice did not occur in the same regions, indicating that functional changes are not necessarily a direct reflection of grey matter changes as measured with MRI (Haier et al., 2009).
4.3. Familiarity, expectancy, and motivation
It was demonstrated that performance improvements were larger in the trained participants than in participants of a passive control group, who only participated in the scanning sessions before and after practice. These findings are important because they suggest that training effects could not simply be attributed to familiarity with the task. However, it should be noted that the inclusion of a passive control group does not take into account expectancy effects or effects of motivation. For example, it is possible that participants in the training group improved more than participants in the control group simply because the training had increased their confidence in task performance or because they put in more effort after training. To rule out the effects of expectancy and motivation, future studies should consider including an active control group, which receives a placebo treatment that is similar to the training program but not effective (e.g., Klingberg, 2010, Morrison and Chein, 2010). Alternatively, future studies could compare the effects of two training programs that focus on different cognitive functions (e.g., Mackey et al., 2011). This approach would make it possible to examine the specificity of training effects in the frontoparietal network.
4.4. Transfer effects
To learn more about the specific skills that were being trained, we examined whether performance improvements transferred to untrained executive function tasks. We found improved performance on the digit span task of the WISC, but there were no significant differences between the practice group and the control group in terms of training gain. Yet, the practice group did show a slight advantage on the Mental Counters working memory task (that was only administered after practice). None of the other transfer tasks showed a significant group effect, suggesting that transfer effects were specific to working memory tasks. Yet, these results should be interpreted with caution because the effect did not survive Bonferroni correction for the number of tests performed. Moreover, the results are based on a small number of participants and therefore they must be replicated in future studies using larger samples.
There are some prior studies that have found transfer effects (Dahlin et al., 2008, Holmes et al., 2009, Karbach and Kray, 2009, Klingberg et al., 2002, Klingberg et al., 2005, Li et al., 2008, Mackey et al., 2011; but see also Owen et al., 2010), but it still remains to be determined what are the optimal task conditions leading to transfer. In general, transfer effects are expected to be specific to tasks that engage overlapping cognitive processes and brain networks as the task that is practiced. For example, Dahlin et al. (2008) demonstrated transfer to an n-back working memory task after 5 weeks of training in updating, but they did not find transfer to a task that did not involve updating processes or engage the same brain regions. Factors that might also be important include the complexity of the learning paradigm, the variability of tasks that are trained, and the adaptation of difficulty to a level that is appropriate for the individual (Green and Bavelier, 2008, Klingberg, 2010, Lövdén et al., 2010). Finally, it is important to determine the effectiveness of training and transfer in relation to the environmental input that an individual receives. For example, if children receive optimal training and support from their environment, they may perform close to the maximal possible level given their age and biological potential, suggesting that additional training will not have large effects (Denney, 1984).
4.5. Conclusion
Taken together, the present study provides preliminary evidence that children are able to show a more “mature” pattern of brain activation if given extensive training. It remains to be determined whether these changes reflect flexibility (i.e., changes within the limits of the current functional capacity) or structural plasticity (i.e., changes of those limits, associated with structural brain changes) (Lövdén et al., 2010). However, the absence of (far) transfer to untrained executive function tasks suggests that the practice effects reflect flexible changes specific to the task/function that was trained. One limitation of the current pilot study was the small number of children that participated in the training, which may have resulted in low power. With respect to the fMRI analyses, we performed several additional analyses to demonstrate the robustness of the results, such as matching of the number of correct trials before and after practice, and including cue- and response periods as a quality check of the data. These analyses indicated that it was unlikely that the absence of activation for manipulation relative to maintenance during the first session was purely related to low power. However, it is important to validate and extent the present results in a larger group of children, examining the effects of age, intervention, and other factors that may influence training results.
To conclude, although based on a small sample, these findings indicate that age differences in neural activation can reduce as a result of practice. These results provide the building blocks for further investigation of flexibility and plasticity in the developing brain, and the existence of sensitive periods for learning (Galvan, 2010, Huttenlocher, 2002, Luna, 2004, Qin et al., 2004). By understanding the potential of children's brain systems in the context of the developing brain, eventually we might be able to help optimizing education programs (Diamond et al., 2007, Gathercole et al., 2006, Posner and Rothbart, 2005).
Acknowledgements
S.A.R.B.R. and E.A.C are supported by grants from the Netherlands Organization for Scientific Research (NWO, VIDI grant nr 91786368 and 45207011). This work was also supported by the Gratama stichting and Leids Universiteits Fonds (granted to E.A.C.). The funding sources had no involvement in the study design, data collection, and preparation of the article. The authors thank J. van Driel, C. Willemse and D. Douwes for their help with the data collection.
Footnotes
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.dcn.2011.09.001.
Contributor Information
Dietsje D. Jolles, Email: d.d.jolles@lumc.nl.
Mark A. van Buchem, Email: m.a.van_buchem@lumc.nl.
Serge A.R.B. Rombouts, Email: s.a.r.b.rombouts@lumc.nl.
Eveline A. Crone, Email: ecrone@fsw.leidenuniv.nl.
Appendix A. Supplementary data
References
- Achenbach T.M. University of Vermont, Department of Psychiatry; Burlington: 1991. Manual for the Child Behavior Checklist/4-18 and 1991 Profile. [Google Scholar]
- Aylward E.H., Richards T.L., Berninger V.W., Nagy W.E., Field K.M., Grimme A.C., Richards A.L., Thomson J.B., Cramer S.C. Instructional treatment associated with changes in brain activation in children with dyslexia. Neurology. 2003;61:212–219. doi: 10.1212/01.wnl.0000068363.05974.64. [DOI] [PubMed] [Google Scholar]
- Beckmann C.F., Jenkinson M., Smith S.M. General multilevel linear modeling for group analysis in fMRI. Neuroimage. 2003;20:1052–1063. doi: 10.1016/S1053-8119(03)00435-X. [DOI] [PubMed] [Google Scholar]
- Bunge S.A., Crone E.A. Neural correlates of the development of cognitive control. In: Rumsey J.M., Ernst M., editors. Neuroimaging in Developmental Clinical Neuroscience. Cambridge University Press; Cambridge: 2009. pp. 22–37. [Google Scholar]
- Bunge S.A., Wright S.B. Neurodevelopmental changes in working memory and cognitive control. Curr. Opin. Neurobiol. 2007;17:243–250. doi: 10.1016/j.conb.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Casey B.J., Tottenham N., Liston C., Durston S. Imaging the developing brain: what have we learned about cognitive development? Trends Cogn. Sci. 2005;9:104–110. doi: 10.1016/j.tics.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Changeux J.P., Danchin A. Selective stabilisation of developing synapses as a mechanism for the specification of neuronal networks. Nature. 1976;264:705–712. doi: 10.1038/264705a0. [DOI] [PubMed] [Google Scholar]
- Crone E.A., Wendelken C., Donohue S., van Leijenhorst L., Bunge S.A. Neurocognitive development of the ability to manipulate information in working memory. Proc. Natl. Acad. Sci. U.S.A. 2006;103:9315–9320. doi: 10.1073/pnas.0510088103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlin E., Neely A.S., Larsson A., Backman L., Nyberg L. Transfer of learning after updating training mediated by the striatum. Science. 2008;320:1510–1512. doi: 10.1126/science.1155466. [DOI] [PubMed] [Google Scholar]
- Dale A.M. Optimal experimental design for event-related fMRI. Hum. Brain Mapp. 1999;8:109–114. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<109::AID-HBM7>3.0.CO;2-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denney N.W. A model of cognitive development across the life span. Dev. Rev. 1984;4:171–191. [Google Scholar]
- D’Esposito M., Postle B.R., Ballard D., Lease J. Maintenance versus manipulation of information held in working memory: an event-related fMRI study. Brain Cogn. 1999;41:66–86. doi: 10.1006/brcg.1999.1096. [DOI] [PubMed] [Google Scholar]
- Diamond A. Normal development of prefrontal cortex from birth to young adulthood: cognitive functions, anatomy, and biochemistry. In: Stuss D.T., Knight R.T., editors. Principles of Frontal Lobe Function. Oxford University Press; New York: 2002. pp. 466–503. [Google Scholar]
- Diamond A., Barnett W.S., Thomas J., Munro S. Preschool program improves cognitive control. Science. 2007;318:1387–1388. doi: 10.1126/science.1151148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durston S., Casey B.J. A shift from diffuse to focal cortical activity with development: the authors’ reply. Dev. Sci. 2006;9:18–20. doi: 10.1111/j.1467-7687.2005.00458.x. [DOI] [PubMed] [Google Scholar]
- Fields R.D. White matter in learning, cognition and psychiatric disorders. Trends Neurosci. 2008;31:361–370. doi: 10.1016/j.tins.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan A. Neural plasticity of development and learning. Hum. Brain Mapp. 2010;31:879–890. doi: 10.1002/hbm.21029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gathercole S.E., Lamont E., Alloway T.P. Working memory in the classroom. In: Pickering S.J., editor. Working Memory and Education. Elsevier Press; Oxford: 2006. pp. 219–240. [Google Scholar]
- Giedd J.N., Lalonde F.M., Celano M.J., White S.L., Wallace G.L., Lee N.R., Lenroot R.K. Anatomical brain magnetic resonance imaging of typically developing children and adolescents. J. Am. Acad. Child Adolesc. Psychiatry. 2009;48:465–470. doi: 10.1097/CHI.0b013e31819f2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N., Giedd J.N., Lusk L., Hayashi K.M., Greenstein D., Vaituzis A.C., Nugent T.F., Herman D.H., Clasen L.S., Toga A.W., Rapoport J.L., Thompson P.M. Dynamic mapping of human cortical development during childhood through early adulthood. Proc. Natl. Acad. Sci. U.S.A. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green C.S., Bavelier D. Exercising your brain: a review of human brain plasticity and training-induced learning. Psychol. Aging. 2008;23:692–701. doi: 10.1037/a0014345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haier R.J., Karama S., Leyba L., Jung R.E. MRI assessment of cortical thickness and functional activity changes in adolescent girls following three months of practice on a visual-spatial task. BMC Res. Notes. 2009;2:174. doi: 10.1186/1756-0500-2-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes J., Gathercole S.E., Dunning D.L. Adaptive training leads to sustained enhancement of poor working memory in children. Dev. Sci. 2009;12:F9–F15. doi: 10.1111/j.1467-7687.2009.00848.x. [DOI] [PubMed] [Google Scholar]
- Huizinga M., Dolan C.V., van der Molen M.W. Age-related change in executive function: developmental trends and a latent variable analysis. Neuropsychologia. 2006;44:2017–2036. doi: 10.1016/j.neuropsychologia.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Huttenlocher P.R. Harvard University Press; Cambridge: 2002. Neural Plasticity: The Effects of Environment on the Development of the Cerebral Cortex (Perspectives in Cognitive Neuroscience) [Google Scholar]
- Jaeggi S.M., Buschkuehl M., Jonides J., Perrig W.J. Improving fluid intelligence with training on working memory. Proc. Natl. Acad. Sci. U.S.A. 2008;105:6829–6833. doi: 10.1073/pnas.0801268105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M., Smith S. A global optimisation method for robust affine registration of brain images. Med. Image Anal. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Jenkinson M., Bannister P., Brady M., Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Johnson M.H. Interactive Specialization: a domain-general framework for human functional brain development? Dev. Cogn. Neurosci. 2011;1:7–21. doi: 10.1016/j.dcn.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolles D.D., Grol M.J., van Buchem M.A., Rombouts S.A., Crone E.A. Practice effects in the brain: changes in cerebral activation after working memory practice depend on task demands. Neuroimage. 2010;52:658–668. doi: 10.1016/j.neuroimage.2010.04.028. [DOI] [PubMed] [Google Scholar]
- Jolles D.D., Kleibeuker S.W., Rombouts S.A.R.B., Crone E.A. Developmental differences in prefrontal activation during working memory maintenance and manipulation for different memory loads. Dev. Sci. 2011;14:713–724. doi: 10.1111/j.1467-7687.2010.01016.x. [DOI] [PubMed] [Google Scholar]
- Karbach J., Kray J. How useful is executive control training? Age differences in near and far transfer of task-switching training. Dev. Sci. 2009;12:978–990. doi: 10.1111/j.1467-7687.2009.00846.x. [DOI] [PubMed] [Google Scholar]
- Klingberg T. Development of a superior frontal-intraparietal network for visuo-spatial working memory. Neuropsychologia. 2006;44:2171–2177. doi: 10.1016/j.neuropsychologia.2005.11.019. [DOI] [PubMed] [Google Scholar]
- Klingberg T. Training and plasticity of working memory. Trends Cogn. Sci. 2010;14:317–324. doi: 10.1016/j.tics.2010.05.002. [DOI] [PubMed] [Google Scholar]
- Klingberg T., Forssberg H., Westerberg H. Training of working memory in children with ADHD. J. Clin. Exp. Neuropsychol. 2002;24:781–791. doi: 10.1076/jcen.24.6.781.8395. [DOI] [PubMed] [Google Scholar]
- Klingberg T., Fernell E., Olesen P.J., Johnson M., Gustafsson P., Dahlstrom K., Gillberg C.G., Forssberg H., Westerberg H. Computerized training of working memory in children with ADHD – a randomized, controlled trial. J. Am. Acad. Child Adolesc. Psychiatry. 2005;44:177–186. doi: 10.1097/00004583-200502000-00010. [DOI] [PubMed] [Google Scholar]
- Li S.C., Schmiedek F., Huxhold O., Rocke C., Smith J., Lindenberger U. Working memory plasticity in old age: practice gain, transfer, and maintenance. Psychol. Aging. 2008;23:731–742. doi: 10.1037/a0014343. [DOI] [PubMed] [Google Scholar]
- Lövdén M., Bäckman L., Lindenberger U., Schaefer S., Schmiedek F. A theoretical framework for the study of adult cognitive plasticity. Psychol. Bull. 2010;136:659–676. doi: 10.1037/a0020080. [DOI] [PubMed] [Google Scholar]
- Luna B. Algebra and the adolescent brain. Trends Cogn. Sci. 2004;8:437–439. doi: 10.1016/j.tics.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Mackey A.P., Hill S.S., Stone S.I., Bunge S.A. Dissociable effects of reasoning and speed training in children. Dev. Sci. 2011;14:582–590. doi: 10.1111/j.1467-7687.2010.01005.x. [DOI] [PubMed] [Google Scholar]
- Morrison A.B., Chein J.M. Does working memory training work? The promise and challenges of enhancing cognition by training working memory. Psychon. Bull. Rev. 2010;18:46–60. doi: 10.3758/s13423-010-0034-0. [DOI] [PubMed] [Google Scholar]
- Oakes T.R., Fox A.S., Johnstone T., Chung M.K., Kalin N., Davidson R.J. Integrating VBM into the general linear model with voxelwise anatomical covariates. Neuroimage. 2007;34:500–508. doi: 10.1016/j.neuroimage.2006.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen A.M. The role of the lateral frontal cortex in mnemonic processing: the contribution of functional neuroimaging. Exp. Brain Res. 2000;133:33–43. doi: 10.1007/s002210000398. [DOI] [PubMed] [Google Scholar]
- Owen A.M., Hampshire A., Grahn J.A., Stenton R., Dajani S., Burns A.S., Howard R.J., Ballard C.G. Putting brain training to the test. Nature. 2010;465:775–778. doi: 10.1038/nature09042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner M.I., Rothbart M.K. Influencing brain networks: implications for education. Trends Cogn. Sci. 2005;9:99–103. doi: 10.1016/j.tics.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Qin Y., Carter C.S., Silk E.M., Stenger V.A., Fissell K., Goode A., Anderson J.R. The change of the brain activation patterns as children learn algebra equation solving. Proc. Natl. Acad. Sci. U.S.A. 2004;101:5686–5691. doi: 10.1073/pnas.0401227101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raven J., Raven J.C., Court J.H. Harcourt Assessment; San Antonio: 1998. Manual for Raven's Progressive Matrices and Vocabulary Scales. Section 1: General Overview. [Google Scholar]
- Rossion B., Pourtois G. Revisiting Snodgrass and Vanderwart's object pictorial set: the role of surface detail in basic-level object recognition. Perception. 2004;33:217–236. doi: 10.1068/p5117. [DOI] [PubMed] [Google Scholar]
- Scherf K.S., Sweeney J.A., Luna B. Brain basis of developmental change in visuospatial working memory. J. Cogn. Neurosci. 2006;18:1045–1058. doi: 10.1162/jocn.2006.18.7.1045. [DOI] [PubMed] [Google Scholar]
- Shaywitz B.A., Shaywitz S.E., Blachman B.A., Pugh K.R., Fulbright R.K., Skudlarski P., Mencl W.E., Constable R.T., Holahan J.M., Marchione K.E., Fletcher J.M., Lyon G.R., Gore J.C. Development of left occipitotemporal systems for skilled reading in children after a phonologically-based intervention. Biol. Psychiatry. 2004;55:926–933. doi: 10.1016/j.biopsych.2003.12.019. [DOI] [PubMed] [Google Scholar]
- Simos P.G., Fletcher J.M., Bergman E., Breier J.I., Foorman B.R., Castillo E.M., Davis R.N., Fitzgerald M., Papanicolaou A.C. Dyslexia-specific brain activation profile becomes normal following successful remedial training. Neurology. 2002;58:1203–1213. doi: 10.1212/wnl.58.8.1203. [DOI] [PubMed] [Google Scholar]
- Smith S.M. Fast robust automated brain extraction. Hum. Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith E.E., Jonides J. Neuroscience – storage and executive processes in the frontal lobes. Science. 1999;283:1657–1661. doi: 10.1126/science.283.5408.1657. [DOI] [PubMed] [Google Scholar]
- Smith S.M., Jenkinson M., Woolrich M.W., Beckmann C.F., Behrens T.E., Johansen-Berg H., Bannister P.R., De L.M., Drobnjak I., Flitney D.E., Niazy R.K., Saunders J., Vickers J., Zhang Y., De S.N., Brady J.M., Matthews P.M. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Temple E., Deutsch G.K., Poldrack R.A., Miller S.L., Tallal P., Merzenich M.M., Gabrieli J.D. Neural deficits in children with dyslexia ameliorated by behavioral remediation: evidence from functional MRI. Proc. Natl. Acad. Sci. U.S.A. 2003;100:2860–2865. doi: 10.1073/pnas.0030098100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner A.D., Maril A., Bjork R.A., Schacter D.L. Prefrontal contributions to executive control: fMRI evidence for functional distinctions within lateral prefrontal cortex. Neuroimage. 2001;14:1337–1347. doi: 10.1006/nimg.2001.0936. [DOI] [PubMed] [Google Scholar]
- Wechsler D. The Psychological Corporation; San Antonio: 1991. Wechsler Intelligence Scale for Children-third edition. Manual. [Google Scholar]
- Wechsler D. The Psychological Corporation; San Antonio: 1997. Wechsler Adult Intelligence Scale-third edition. Administration and Scoring Manual. [Google Scholar]
- Wendelken C., Bunge S.A., Carter C.S. Maintaining structured information: an investigation into functions of parietal and lateral prefrontal cortices. Neuropsychologia. 2008;46:665–678. doi: 10.1016/j.neuropsychologia.2007.09.015. [DOI] [PubMed] [Google Scholar]
- Woolrich M.W., Ripley B.D., Brady M., Smith S.M. Temporal autocorrelation in univariate linear modeling of FMRI data. Neuroimage. 2001;14:1370–1386. doi: 10.1006/nimg.2001.0931. [DOI] [PubMed] [Google Scholar]
- Woolrich M.W., Behrens T.E., Beckmann C.F., Jenkinson M., Smith S.M. Multilevel linear modelling for FMRI group analysis using Bayesian inference. Neuroimage. 2004;21:1732–1747. doi: 10.1016/j.neuroimage.2003.12.023. [DOI] [PubMed] [Google Scholar]
- Worsley K.J. Statistical analysis of activation images. In: Jezzard P.M., Matthews P.M., Smith S.M., editors. Functional MRI: An Introduction to Methods. Oxford University Press; Oxford: 2001. pp. 251–270. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.