Graphical abstract
Abbreviations: DTF, directed transfer function; TR, typical readers; MDD, moderately dysfluent dyslexic readers; SDD, severely dysfluent dyslexic readers
Keywords: Developmental dyslexia, Reading fluency, Visual word recognition, Directed functional connectivity, Directed transfer function
Highlights
-
•
Directed (EEG) connectivity shows altered visual word processing in dyslexic children.
-
•
Dyslexic children show reduced posterior-to-anterior connectivity.
-
•
Severely dysfluent dyslexic children show enhanced anterior-to-posterior connectivity.
-
•
Patterns of connectivity scale with the severity of reading (dys)fluency.
Abstract
Reading is a complex cognitive skill subserved by a distributed network of visual and language-related regions. Disruptions of connectivity within this network have been associated with developmental dyslexia but their relation to individual differences in the severity of reading problems remains unclear. Here we investigate whether dysfunctional connectivity scales with the level of reading dysfluency by examining EEG recordings during visual word and false font processing in 9-year-old typically reading children (TR) and two groups of dyslexic children: severely dysfluent (SDD) and moderately dysfluent (MDD) dyslexics. Results indicated weaker occipital to inferior-temporal connectivity for words in both dyslexic groups relative to TRs. Furthermore, SDDs exhibited stronger connectivity from left central to right inferior-temporal and occipital sites for words relative to TRs, and for false fonts relative to both MDDs and TRs. Importantly, reading fluency was positively related with forward and negatively with backward connectivity. Our results suggest disrupted visual processing of words in both dyslexic groups, together with a compensatory recruitment of right posterior brain regions especially in the SDDs during word and false font processing. Functional connectivity in the brain’s reading network may thus depend on the level of reading dysfluency beyond group differences between dyslexic and typical readers.
1. Introduction
Although reading is a complex cognitive function, 90–95% of all children master it without notable problems. However, children suffering from developmental dyslexia never attain fluent reading skills despite adequate intellectual abilities and educational opportunities (Blomert, 2005, Lyon et al., 2003). Among the reading-related deficits of developmental dyslexia, reading dysfluency poses the most pronounced and long lasting hurdle (Shaywitz et al., 2008), especially in relatively transparent orthographies, such as Dutch or German (Blomert, 2011, Wimmer and Schurz, 2010). At the same time, dyslexics show substantial inter-individual variability in their level of reading (dys)fluency (Katzir et al., 2008, Leinonen et al., 2001). One of the neurobiological signatures of dyslexia is an abnormal pattern of functional and/or anatomical connectivity within the brain’s reading network (Geschwind, 1965, Paulesu et al., 1996, Vandermosten et al., 2012, Wimmer and Schurz, 2010). Impaired connectivity could disrupt decoding or orthographic–phonological integration at word (Wimmer and Schurz, 2010), syllable and/or phoneme level (Blomert, 2011), causing slower and less accurate reading in dyslexics. In the present study we investigate whether atypical neural connectivity scales with differences in the level of reading (dys)fluency in dyslexic children.
Reading depends on efficient processing in large scale neural networks combining information of multiple and distinct processes in different brain regions. In particular, reading is subserved by brain networks for spoken language and visual processing that become closely linked over the first years of reading development (Hannagan et al., 2015, Schlaggar and McCandliss, 2007). Communication or connectivity between these regions may be mediated by the phase relation of cortical oscillations (Engel et al., 2001, Varela et al., 2001). In humans, these oscillations are typically measured at different scalp locations using electro-encephalography (EEG) or magneto-encephalography (MEG). There are several methods to determine EEG/MEG-based functional non-directed or directed connectivity. One of the first proposed measures of functional connectivity refers to EEG coherence, which provides the correlation between cortical oscillations across channels (Varela et al., 2001). More recently, directed connectivity measures have been developed, which provide additional information on the directionality of connectivity across channels. In the present study, we apply the directed transfer function (DTF) method. DTF is based on multivariate autoregressive models and Granger causality (Granger, 1969, Kamiński and Blinowska, 1991).
Directed connectivity measures have been successfully used to investigate the oscillatory dynamics of the reading network in typically reading adults (Bedo et al., 2014, Kujala et al., 2007, Woodhead et al., 2014). For example, the MEG study of Kujala et al. (2007) investigated directed connectivity by coherence-based detection of interconnected nodes in brain source space. This method enabled them to demonstrate that the processing of words in a rapid serial visual presentation (RSVP) paradigm involves long-range communication via alpha band (8–14 Hz), and in about half of the subjects also via beta band (15–30 Hz) activity. More specifically, their results showed activation propagation from posterior to anterior areas with the inferior occipito-temporal cortex and cerebellum as the main driving nodes, as well as more local frontal connectivity in areas related with visual recognition and working memory (Kujala et al., 2007). Furthermore, an EEG study applying phase synchrony and transfer entropy to analyze connectivity between independent components, reported ventral occipito-temporal cortex (vOT) as a central hub for word reading, with theta (3–7 Hz) and gamma (30–50 Hz) band connectivity between vOT and both early visual and language-processing areas (Bedo et al., 2014).
Several EEG/MEG studies investigated connectivity in the reading network of typically reading and dyslexic children or adults during rest and reading/attention related tasks. These studies have reported a mixed pattern of group differences, as reflected by connectivity, coherence, spectral power and network configuration measures (Arns et al., 2007, Dhar et al., 2010, Dimitriadis et al., 2013, Fraga González et al., 2016a, Frye et al., 2012, Frye et al., 2010, Leisman, 2002, Ligges et al., 2010, Milne et al., 2003, Stokić et al., 2011). In broad outline, however, these studies revealed altered EEG/MEG connectivity in dyslexic readers, including a general tendency for altered posterior-to-frontal connectivity (e.g. Arns et al., 2007, Milne et al., 2003) and a relatively stronger reliance on right hemisphere regions (e.g. Arns et al., 2007, Ligges et al., 2010).
The central aim of the present study is to investigate whether directed connectivity during reading scales with the level of dysfluency in dyslexic children. We investigate patterns of directed connectivity in the reading network in typically reading (n = 20) and two groups of dyslexic children: moderately dysfluent dyslexic (n = 18) and severely dysfluent dyslexic (n = 16) readers. The same groups of children were previously shown to exhibit different patterns of neural integration of letters and speech sounds as represented by mismatch negativity (MMN, 100–250 ms) and late negativity (LN, 600–750 ms) ERPs in a cross-modal oddball paradigm (Žarić et al., 2014). Specifically, both dyslexic groups exhibited reduced audiovisual integration of letters and speech sounds in the later time window (cross-modal LN), while severely dysfluent dyslexics additionally showed a reduction in the earlier perceptually driven audiovisual MMN response (Žarić et al., 2014). Here, we employ a visual word recognition paradigm (Fraga González et al., 2014, Fraga González et al., 2016b) to investigate whether directed connectivity during the processing of visual words and letter-like meaningless false font strings indicates a different functionality of the reading network in these groups. In particular, as the false fonts were designed to be letter-like, both word reading and false font ‘reading’ may be impaired in the severely dysfluent dyslexic group due to the weakest orthographic–phonological connectivity at word and/or subword level (Blomert, 2011, Wimmer and Schurz, 2010). Instead moderately dysfluent dyslexic children may more efficiently differentiate these stimulus types and exhibit impairments predominantly during the word reading condition. We quantify directed connectivity using the DTF which provides both the strength and direction of connectivity between selected electrodes (Kamiński and Blinowska, 1991). Focusing on the level of (dys)fluency in reading allowed us to examine whether children differing in fluency and letter-speech sound integration capability exhibit different patterns of directed connectivity that could potentially contribute to the mixed and inconclusive results obtained in previous functional connectivity studies.
2. Methods
2.1. Participants
Participants were 55 9-year-old children including 35 dyslexic and 20 typical readers, who also participated in a previous crossmodal oddball study (Žarić et al., 2014). Children were diagnosed as dyslexic at a specialized institute for dyslexia and reading problems using an extensive cognitive psycho-diagnostic test battery. Dyslexic children were included in our study based on their poor reading skills, i.e. below 10th percentile of the age appropriate group on 3DM (Dyslexia Differential Diagnosis) word and pseudoword reading fluency subtests (Blomert and Vaessen, 2009) and were divided into groups of severely dysfluent (SDD) and moderately dysfluent (MDD) dyslexics based on a composite score of reading fluency (including the 3DM word and pseudoword reading subtests (Blomert and Vaessen, 2009), the one-minute word reading test − EMT (Brus and Voeten, 1973), and the short story ‘De kat’ (‘The cat’) reading test (de Vos, 2007); for details see: Žarić et al., 2014). Children also completed other subtests of the 3DM battery, i.e. spelling, letter-speech sound identification, letter-speech sound discrimination, phoneme deletion, rapid automatized naming (RAN) and basic reaction time subtests (Blomert and Vaessen, 2009). Individual behavioral testing was performed on average (SD) 1.4 (1.2) months prior to the EEG experiment. In comparison to Žarić et al. (2014), data of two children in the severely dysfluent group could not be included, one due to an incomplete dataset, and the other because of the absence of correct responses in both experimental conditions. The present data thus includes 20 typical readers (TR), 18 moderately dysfluent dyslexics (MDD) and 16 severely dysfluent dyslexics (SDD). All children performed a battery of reading-related and cognitive behavioral tests (Table 1). As the exclusion of the 2 participants did not change the group differences on any of the behavioral tests, we refer to Žarić et al. (2014) for a more detailed description of these tests and behavioral scores. Note that the typical readers and a subset of the dyslexic children were also part of a previous investigation of early visual ERP responses using the same paradigm (Fraga González et al., 2014). In the present study, we included a larger number of dyslexic children which allowed for the division into moderately dysfluent and severely dysfluent groups (see also Žarić et al., 2014). Although reading fluency can be defined as accurate reading at a fast/conversational rate (e.g. Kim et al., 2014), in transparent orthographies such as Dutch, accuracy levels are high, even in dyslexic readers, after only one year of formal reading education (Blomert and Vaessen, 2009). Consequently, differences in fluency are mainly a reflection of differences in the speed of the reading process. Accordingly, on the 3DM reading task for which we obtained both accuracy and fluency measures, the MDD and SDD groups differed in reading fluency even after correction for accuracy differences, i.e. by counting the total number of correctly + incorrectly read words within 90 s (see Table 1).
Table 1.
Behavioral reading scores: Descriptive data and statistical group comparisons for typical readers, dysfluent and severely dysfluent dyslexic readers.
| Typical Readers | Moderately Dysfluent Readers | Severely Dysfluent Readers | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | 20 | 18 | 16 | |||||||||
| Age | 8.80 ± 0.38 | 9.02 ± 0.45 | 8.92 ± 0.41 | F(2,51) = 1.38 p = 0.260 |
||||||||
| Sex ratio (m:f) | 8:12 | 9:9 | 11:5 | |||||||||
| Handedness (L:R) | 2:18 |
3:15 |
3:13 |
Typical vs. Moderately Dysfluent |
Typical vs. Severely Dysfluent |
Dysfluent vs. Severely Dysfluent |
||||||
| M | SD | M | SD | M | SD | F(1,36)c | p | F(1,34) | P | F(1,32)c | P | |
| 3DM Total Reading Accuracy [%] | 95.22 | 3.64 | 90.99 | 4.76 | 78.66 | 9.60 | 10.97 | 0.002 | 52.69 | <0.001 | 23.39 | <0.001 |
| 3DM Total Reading Fluency [Nwords/90 sec] | 118.20 | 18.74 | 68.06 | 7.15 | 46.06 | 9.77 | 113.70 | <0.001 | 194.02 | <0.001 | 56.96 | <0.001 |
| 3DM Total correct + incorrect [Nwords/90 sec] | 123.41 | 16.40 | 74.83 | 7.44 | 58.69 | 11.89 | 133.06 | <0.001 | 175.11 | <0.001 | 23.07 | <0.001 |
| 3DM Total Reading Accuracy [T]a | 49.50 | 9.06 | 39.44 | 9.08 | 25.00 | 8.56 | 11.65 | 0.002 | 68.22 | <0.001 | 22.60 | <0.001 |
| 3DM Total Reading Fluency [T] | 53.95 | 9.34 | 33.83 | 2.41 | 26.25 | 3.87 | 78.62 | <0.001 | 123.22 | <0.001 | 48.19 | <0.001 |
| One minute test − EMT [C]b | 6.05 | 1.76 | 2.61 | 0.61 | 1.44 | 0.63 | 61.83 | <0.001 | 99.09 | <0.001 | 30.56 | <0.001 |
| Story reading fluency ‘ − De Kat’ [T] | 54.70 | 8.04 | 36.33 | 2.20 | 29.06 | 5.82 | 87.81 | <0.001 | 114.35 | <0.001 | 24.25 | <0.001 |
| Letter −speech sound coupling [T] | ||||||||||||
| 3DM Spelling − accuracy | 50.60 | 9.14 | 35.89 | 5.96 | 33.56 | 7.16 | 33.66 | <0.001 | 37.22 | <0.001 | 1.07 | 0.309 |
| 3DM Spelling − RT | 54.55 | 8.70 | 39.11 | 6.99 | 38.25 | 7.72 | 35.83 | <0.001 | 34.46 | <0.001 | 0.12 | 0.735 |
| L-SS identification − accuracy | 46.95 | 7.70 | 42.94 | 11.63 | 38.94 | 11.50 | 1.60 | 0.214 | 6.24 | 0.017 | 1.02 | 0.321 |
| L-SS discrimination − accuracy | 50.20 | 9.25 | 46.06 | 8.96 | 40.88 | 7.31 | 1.89 | 0.177 | 10.82 | 0.002 | 3.29 | 0.079 |
| L-SS identification − RT | 52.80 | 7.08 | 45.00 | 7.39 | 42.25 | 8.04 | 11.03 | 0.002 | 17.50 | <0.001 | 1.08 | 0.306 |
| L-SS discrimination − RT | 51.10 | 8.01 | 46.76 | 9.71 | 48.38 | 8.80 | 2.22 | 0.145 | 0.94 | 0.338 | 0.25 | 0.622 |
| Phoneme deletion − accuracy [T] | 52.70 | 7.63 | 41.94 | 7.18 | 36.44 | 8.39 | 19.28 | <0.001 | 36.97 | <0.001 | 4.12 | 0.051 |
| Rapid naming (RAN) [T] | 49.85 | 9.91 | 38.76 | 6.86 | 32.94 | 9.02 | 20.36 | <0.001 | 35.88 | <0.001 | 4.40 | 0.044 |
| Basic reaction time [T] | 60.70 | 9.61 | 62.29 | 7.70 | 66.63 | 11.22 | 0.302 | 0.586 | 2.91 | 0.097 | 1.69 | 0.203 |
| Raven matrices [C] | 7.04 | 1.49 | 6.81 | 1.68 | 7.28 | 1.32 | 0.21 | 0.651 | 0.24 | 0.624 | 0.81 | 0.375 |
T scores (M = 50, SD = 10).
C scores (M = 5, SD = 2).
For RAN, basic reaction time, phoneme deletion and L-SS discrimination degrees of freedom were: F(1,33) and F(1,31) as data for one subject was missing.
2.2. Stimuli
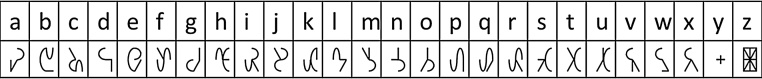
Visual stimuli consisted of 80 bisyllabic Dutch words and 80 corresponding false font strings. The words were selected in a two-step process. First a larger set of words was selected from two word lists (Ghyselinck et al., 2000, Schaerlakens et al., 1999) based on an age of acquisition (AOA) of 6-years or earlier. In the second step, three independent native Dutch adult speakers rated familiarity of the selected words, and only words that were rated as familiar by all 3 raters were further selected for the experiment. Half of the resulting 80 words had 4–5 letters and the other half had 6–7 letters. In the current study we averaged behavioral, ERP and connectivity values for short and long stimuli and investigated the effect of lexicality (i.e., words vs. false font strings) but not of stimulus length. We used bold lower-case “Arial” font, size 40, for the presentation of the words. The 80 words were then converted to 80 corresponding false font strings using a special “3elementSymbols-1600” false font (P.L. Cornelissen, personal communication October 2011). We used this false font (Fig. 1) as it consists of meaningless characters comparable to the Latin alphabet letters in spatial frequency, contrast characteristics and the number of line elements (Hansen et al., unpublished data). Words and false font strings were presented in white on a black background at the center of the screen, covering on average 6.4° (w) × 1.5° (h) of the visual angle.
Fig. 1.
False font used in the experiment.
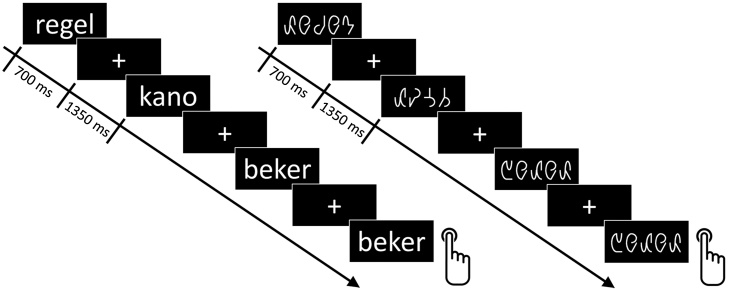
2.3. Experimental design
Stimuli were presented in a 2 × 2 blocked design with separate blocks per condition type (words, false font) and stimulus length (see also Fraga González et al., 2014). In total, 4 word and 4 false font blocks were presented in a pseudo-random order. Each block consisted of 44 stimulus trials. Stimuli were presented for 700 ms followed by a white fixation cross presented during the 1350 ms inter-stimulus interval (ISI). To keep participants attentive throughout the experimental session, they were asked to perform a one-back task on stimulus repetition (Fig. 2). Each block contained four target trials (i.e., immediate repetition of the preceding stimulus), presented pseudo-randomly, with 7–13 trials separating consecutive target trials. Participants’ task was to respond to the target trials by pressing any of the two buttons placed at the arms of the chair they were seated in. Target trials were excluded from the EEG analysis. The total recording time of the experiment was 12 min excluding breaks in between blocks given to the participants if necessary. The experiment was part of a longer experimental session that also included a crossmodal oddball paradigm (Žarić et al., 2014) and was performed during the second part of the experimental session after the first crossmodal oddball block.
Fig. 2.
Design of the experiment.
2.4. Behavioral data analysis
We calculated the percentages of correct target detections (i.e. button press up to 1550 ms after target trial onset), average reaction times and false alarms for words (short and long) and false font strings (short and long) to test whether the participants performed the task as intended and to evaluate possible behavioral differences between groups and conditions. To account for the fact that the distribution of accuracy data was left skewed, especially in the word condition (across groups: skewness − g1 and kurtosis −g2: g1w = −0.959 ± 0.325, g2w = 0.617 ± 0.639; g1ff = −0.240 ± 0.325, g2ff = −0.812 ± 0.639), condition differences were examined with the Wilcoxon signed-ranks test for one sample and group differences with the independent samples Mann–Whitney-U test. Finally, reaction times were analyzed with repeated measures ANOVA including Stimulus Type (words − false font) as the within subject factor and group pairs (TR vs MDD; TR vs SDD; MDD vs SDD) as the between subject factor (see also Fraga González et al., 2014).
2.5. EEG data recording and preprocessing
EEG data were recorded with the ‘Biosemi Active Two system’ (Biosemi, Amsterdam, Netherlands). Recordings were sampled at 1024 Hz with a bandwidth filter of DC–104 Hz (a highpass filter was not applied during recording, while the low pass filter had a 5th order sinc response with a −3 dB point at 1/5th of the selected sample rate). EEG was measured at 64 scalp electrodes using active-channels placed according to the 10–20 international system (Electro-cap International Inc.). Additionally, the cap provided locations for two more electrodes needed for recording of the DC signal in the vicinity of PO1 (Common Mode Sense, CMS) and PO2 (Driven Right Leg, DRL), and the CMS was used as a recording reference. Four ‘Flat-Type Active’ electrodes were used for the bipolar measurement of eye-movements, two of which were placed below and above left eye and two at the outer canthi of each eye. Two more electrodes on the left and right mastoids were used for offline re-referencing. We kept the offset band of the electrodes from −20 mV to 20 mV.
The data were preprocessed and analyzed using the v12.0.5b EEGLAB toolbox (Delorme and Makeig, 2004; http://www.sccn.ucsd.edu/eeglab), Fieldtrip (Oostenveld et al., 2011), BSMART toolbox (Cui et al., 2008) and custom Matlab scripts (MATLAB 2014a, The MathWorks, Natick, MA). Raw EEG signals were offline re-referenced to the average of the mastoids (Bastos and Schoffelen, 2016, Kaminski and Blinowska, 2014), band-pass-filtered from 0.5 to 100 Hz using a zero phase digital basic FIR filter (high-pass transition band width 0.08 Hz; low-pass transition band width 10 Hz), resampled to 256 Hz (to optimize the tradeoff between model complexity and the time-span covered by the multivariate autoregressive model, see e.g. Seth et al., 2015) and epoched between −500 to 900 ms with respect to stimulus events of the non-target trials of interest for the analysis. Epochs were baseline corrected with respect to the mean signal in the pre-stimulus period (500 ms). Epochs lasted until 900 ms post-stimulus because this time window includes the major responses to visual words/false fonts (Dambacher et al., 2012, Laszlo and Federmeier, 2014, Ligges et al., 2010, Rossell et al., 2003). Furthermore, visual inspection of our data revealed that a large percentage of eye-blinks started around 1000 ms post-stimulus. Epochs including signals with amplitudes higher than ±150 μV at the EOG and scalp electrodes were classified as artifactual and discarded from further analysis. As in some children power line noise was still present, we removed line noise at 50 Hz using CleanLine plug-in for EEGLAB that subtracts a time-domain sinusoid (the best estimate of the deterministic line noise) from the data (Mullen, 2012; http://www.nitrc.org/projects/cleanline). The final mean (SD) number of epochs per group included in the analysis corresponded to: TRs − 127 (20) for words and 134 (17) for false font strings; MDDs − 124 (20) for words and 121 (18) for false font; and SDDs − 124 (20) for words and 121 (23) for false fonts. Importantly, as we were interested in functional directed connectivity, we avoided preprocessing steps such as re-referencing to an average reference, artifact correction using independent component analysis, electrode interpolation or EEG source modeling that may affect the results of our analysis by artificially inducing shifts in the phase of the different spectral oscillations (Kaminski and Blinowska, 2014).
2.6. EEG data analysis
EEG functional connectivity analysis was performed using 9 electrodes over the left and right posterior to anterior sites (O1, O2, P7, P8, C3, Cz, C4, F5, F6). In rare cases of noisy signals at one of these electrodes the signal was replaced by an adjacent electrode. For two participants O1 was replaced by PO3, for one participant O2 was replaced by PO4 and for another participant we used F7 instead of F5. The 9 electrodes were selected based on the reported sensitivity of these sites to activity in different regions of the reading network, e.g. as reflected by reading-related ERPs (e.g. Coch and Meade, 2016, Dehaene, 1995, Hauk et al., 2006, Landi and Perfetti, 2007, Mahé et al., 2012, Savill and Thierry, 2011). Thus, we selected electrodes that are likely to be sensitive to activity in middle occipital areas (O1 and O2), occipito–temporal regions including inferior temporal areas (P7 and P8), auditory, somatosensory and motor areas (Cz, C3, C4) and inferior/middle frontal areas (F5 and F6) (Koessler et al., 2009, Mayhew et al., 2010). A complementary analysis of ERP activity at these electrodes is available as Supplementary material.
We investigated EEG connectivity during visual word and false font processing by calculating directed connectivity between channels using the directed transfer function (DTF, Kamiński and Blinowska, 1991). To calculate the directed connectivity between a given channel A and a given channel B, this method estimates the ratio between the input from channel A to channel B and all the inputs to channel B (Blinowska et al., 2004). DTF was applied across single trials using epoched data from 0 to 900 ms after stimulus presentation using signal oscillations between 1 and 70 Hz for each of the four conditions separately (short and long words, and short and long false font symbols). Coefficients of the multivariate autoregressive (MVAR) model were calculated using Brain − System for Multivariate AutoRegressive Time series (BSMART) toolbox as implemented in Fieldtrip (Cui et al., 2008, Oostenveld et al., 2011). The order of the model was estimated based on the Bayesian Information Criterion (Schwarz, 1978) representing the best trade-off between accuracy of fit and parsimony, as implemented in the ‘Granger causal connectivity analysis’ (GCCA) toolbox for Matlab (Seth, 2010). The mean (SD; range) model order over all trials, conditions and subjects was 4 (0.3; 3–7). The MVAR coefficients were then used to compute the spectral transfer matrix, which was used for the estimation of the DTF using Fieldtrip. DTF can be described as an expansion of Granger causality to an arbitrary number of signals/channels (Blinowska et al., 2004), and computes the ratio between the inflows from channel A to channel B and the inflows from all channels to the channel B. The resulting DTF value ranges from 0 to 1, where 1 indicates that all the inflows to channel B come exclusively from channel A. DTF is calculated as a function of frequency and is robust to constant phase disturbances and volume conduction because it ignores instantaneous (zero phase lag) correlations (Kamiński and Blinowska, 1991).
We used the time-invariant DTF (Blinowska, 2011) for epoched data as we employed a block design in which dynamic, time-variant properties could be attenuated by longer lasting cognitive states induced by the repetition of stimuli from the same category within blocks (Al-Aidroos et al., 2012). Examples of applications of DTF to EEG data include studies on attention and working memory (Blinowska et al., 2013, Blinowska et al., 2010, Brzezicka et al., 2011), motor tasks (Ewen et al., 2015, Ginter et al., 2001, Kuś et al., 2006), epilepsy (Franaszczuk et al., 1994, Papadopoulou et al., 2012), Parkinson’s disease (Androulidakis et al., 2008), rapid changes in connectivity in preterm children (Schumacher et al., 2015), and anesthesia (Papadopoulou et al., 2015).
DTF values were first analyzed using a repeated measures ANOVA with stimulus type (2 levels: words and false font) as within subjects factor and group (3 levels: TR, MDD, SDD) as between subjects factor per frequency (1–70 Hz) per electrode pair. Results were corrected for multiple comparisons per electrode pair using false discovery rate (FDR, Benjamini and Hochberg, 1995). Post-hoc comparisons were performed for frequencies and electrode pairs that yielded a significant main effect and/or group*stimulus interaction, using either independent samples t-tests (Group comparisons) or paired-samples t-tests (Stimulus effects within groups).
Finally, to investigate the relationship between EEG connectivity measures and individual differences in offline reading related behavioral scores, we applied Pearson’s correlations across the groups on the individual connectivity index from each channel pair and composite behavioral scores. As in our previous studies, behavioral composite scores were constructed for reading fluency (3DM word reading tests, the EMT, and “De Kat”, Žarić et al., 2014, Žarić et al., 2015) and letter-speech sound coupling accuracy and reaction time. To avoid a very large number of tests, correlations with behavioral scores were performed on averaged connectivity measures in the two frequency intervals that yielded largest group differences in connectivity, i.e. the 25–50 Hz and 60–70 Hz intervals. We performed FDR multiple comparison corrections.
3. Results
3.1. Behavioral performance
The percentages of correct responses (target trials) and false alarms (non-target trials), and mean reaction times for the correct responses across groups are given in Table 2. Behavioral measures of target detection (in total 16 targets per condition) suggest that for all three groups it was more difficult to recognize false font symbol string repetition than the repetition of words (Table 2).
Table 2.
Behavioral performance on the task M(SD). CR − correct responses, RT − reaction time for correct responses, FA − false alarms, MR − mean rank.
| Typical |
Moderately dysfluent |
Severely dysfluent |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| CR [%] | RT [s] | FA [%] | CR [%] | RT [s] | FA [%] | CR [%] | RT [s] | FA [%] | |
| Words | 90.00 (10.61) | 0.85 (0.12) | 0.25 (0.37) | 83.68 (15.77) | 0.96 (0.22) | 0.42 (0.91) | 72.27 (11.63) | 0.93 (0.13) | 2.38 (2.90) |
| False font | 64.69 (16.88) | 0.94 (0.11) | 6.97 (3.54) | 53.82 (14.72) | 0.95 (0.19) | 7.01 (3.96) | 58.59 (25.09) | 1.02 (0.19) | 10.59 (8.12) |
| Words − False Font Difference | Z = −3.85, p < 0.001, MRW>S = 10.95, MRW < S=2.00 | t(19) = −3.83, p=0.001 | Z = −3.93, p<0.001, MRW>S=0.00, MRW < S=10.50 | Z = −3.66, p<0.001, MRW>S = 9.00, MRW < S=0.00 | n.s. | Z = −3.624, p<0.001, MRW>S=0.500, MRW < S=9.00 | n.s | n.s. | Z = −3.520, p<0.001, MRW>S=0.00, MRW < S=8.50 |
A comparison of groups showed that MDDs were as accurate as TRs in detecting targets in the word repetition task but less accurate in the false font condition (U = 106.0, p = 0.030). Furthermore, comparison of reaction times between TRs and MDDs yielded a significant stimulus type*group interaction (F(1,36) = 5.33, p = 0.027), but post-hoc tests only showed a tendency for RT differences in the word condition (t(36) = −1.85, p = 0.073) and no differences in the false font condition. Compared to SDDs, both the typical readers and the MDDs were more accurate in the word conditions (TR versus SDD: CR: U = 34.5, p < 0.001; FA: U = 41.0, p < 0.001; MDD versus SDD: CR: U = 72.0, p = 0.012, FA: U = 46.5, p < 0.001), with TRs being faster than SDDs in both word and false font conditions (main effect of group: F(1,34) = 4.32, p = 0.045). Taken together, this pattern of results suggests that MDDs are successful in trading off speed for accuracy in the word conditions, while SDDs are both slower and less accurate.
3.2. EEG connectivity patterns for words and false font
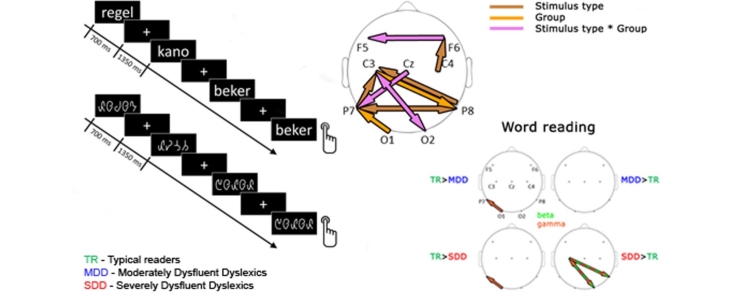
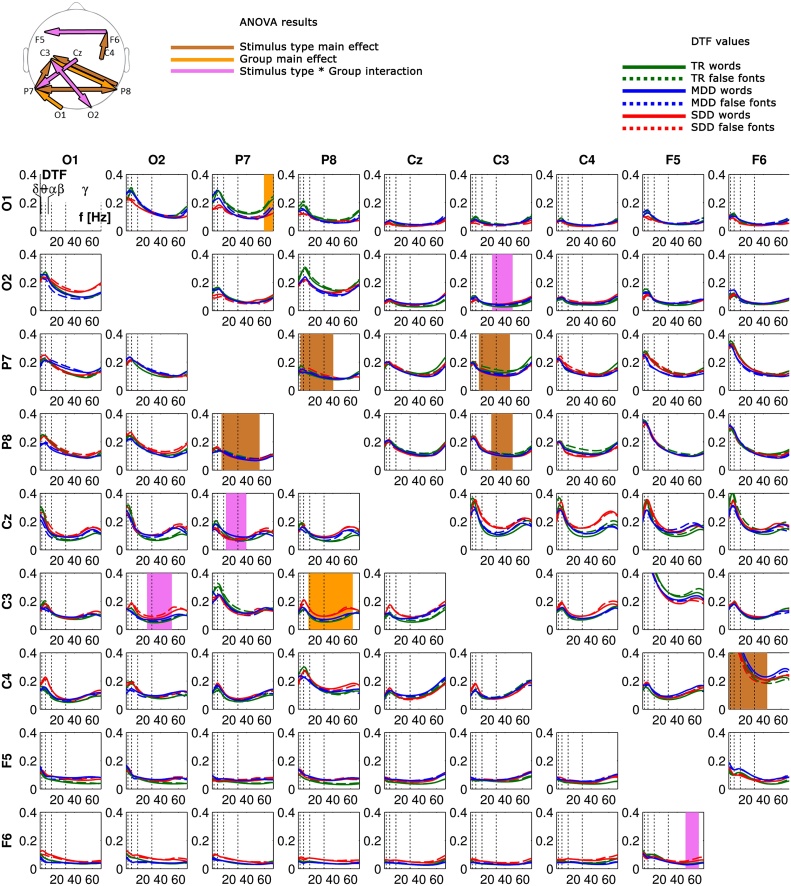
To examine oscillatory dynamics of brain activity during the processing of words and false font strings, we compared functional directed connectivity in the EEG signals (Fig. 3) elicited by the non-target stimuli in these two conditions across the three groups. To this end we used a repeated measures ANOVA with stimulus type (2 levels: words and false font) as within subjects factor and group (3 levels: TR, MDD, SDD) as between subjects factor and report effects that reach significance at qfdr < 0.05.
Fig. 3.
Overall pattern of directed connectivity results. Top − topographic representation of electrode pairs for which ANOVA yielded significant results. Brown − significant main effect of stimulus type; orange − significant main effect of group; violet − significant interaction between stimulus type and group. Bottom − connectivity index for DTF (y-axes) by frequency (x-axes). Vertical dotted lines − borders between frequency ranges (delta [δ] = 1–2 Hz, theta [θ] = 3–7 Hz, alpha [α] = 8–12 Hz, beta [β] = 13–30 Hz and gamma [γ] = 31–70 Hz). Rows − sources of connectivity; columns − sinks of connectivity. Typical readers (TR) − green, moderately dysfluent dyslexics (MDD) − blue, severely dysfluent dyslexics (SDD) − red. Words − solid lines, false font − dashed lines. Significant ANOVA results color coded as in the topoplot. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
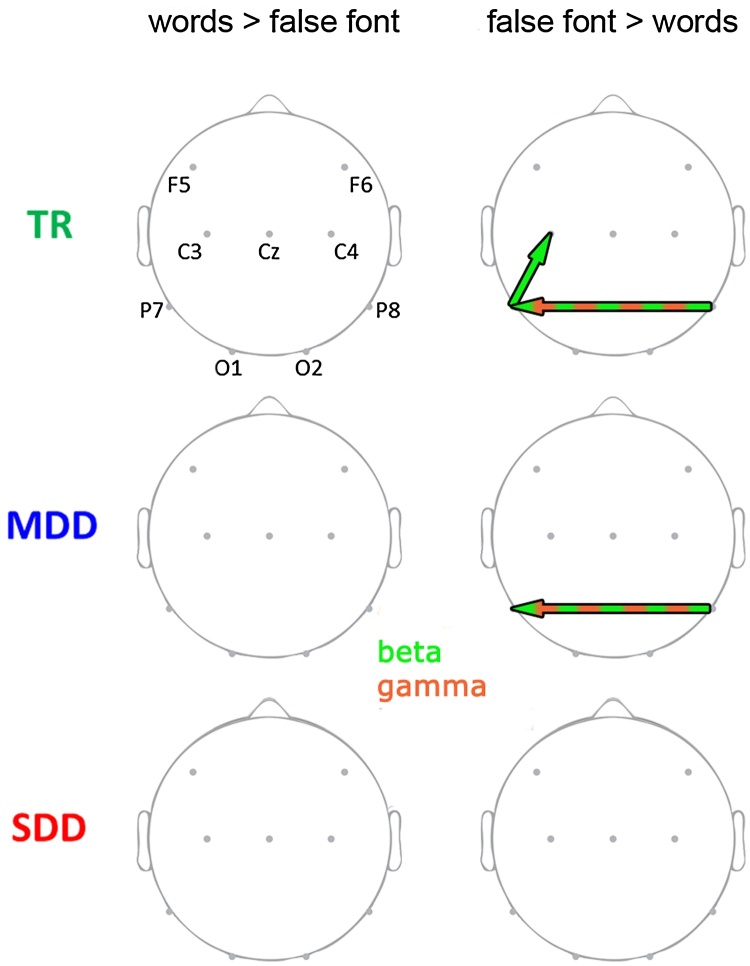
An increased functional connectivity for false fonts as compared to words led to a significant main effect of stimulus type in bidirectional broadband connectivity between the two inferior-temporal electrode sites (P7 to P8: 4–40 Hz and P8 to P7: 12–54 Hz). Further main effects of stimulus type showed stronger connectivity for false fonts from both inferior-temporal sites to the left central site (P7 to C3: 11–45 Hz and P8 to C3: 25–48 Hz), and for words from the right central to the right frontal site (C4 to F6: 2–44 Hz). Post-hoc t-tests (Fig. 4) for these frequencies and electrode pairs yielded stronger connectivity in the beta and gamma range for false fonts than for words from P8 to P7 in TRs and MDDs (TR: 16–40 Hz; MDD: 27–54 Hz) and stronger beta connectivity for false fonts from P7 to C3 in TRs (13–32 Hz). There were no differences between false fonts and words in the SDD group. Words did not elicit stronger connectivity than the false fonts in any of the groups.
Fig. 4.
Topographic visualization of differences in connectivity between words and false font strings per group. TR- typical readers, MDD − moderately dysfluent dyslexics, SDD − severely dysfluent dyslexics. Left column − Stronger connectivity for words than for false font strings, Right column − stronger connectivity for false font than for words. Arrows represent the direction of the connectivity. Frequency bands in which significant differences were found are color-coded: light green − beta (13–30 Hz), light red − gamma (31–70 Hz). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
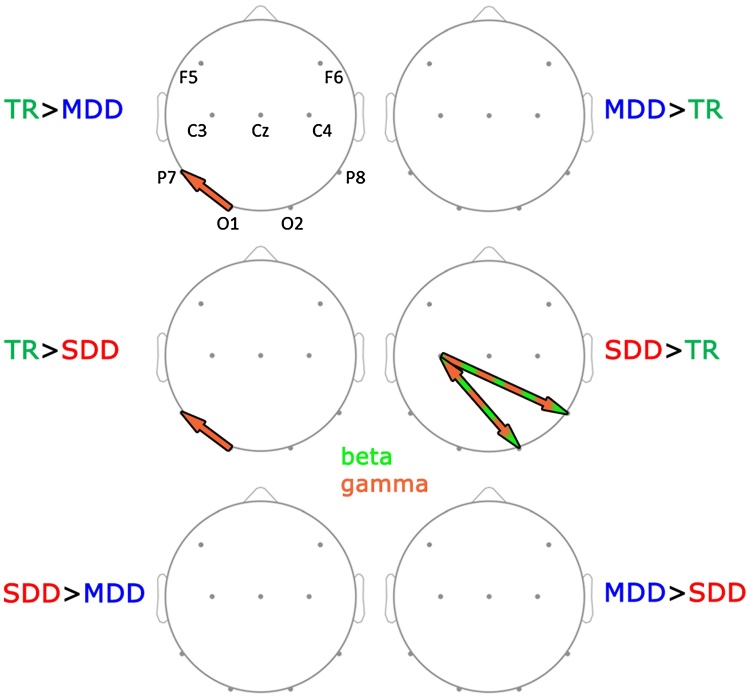
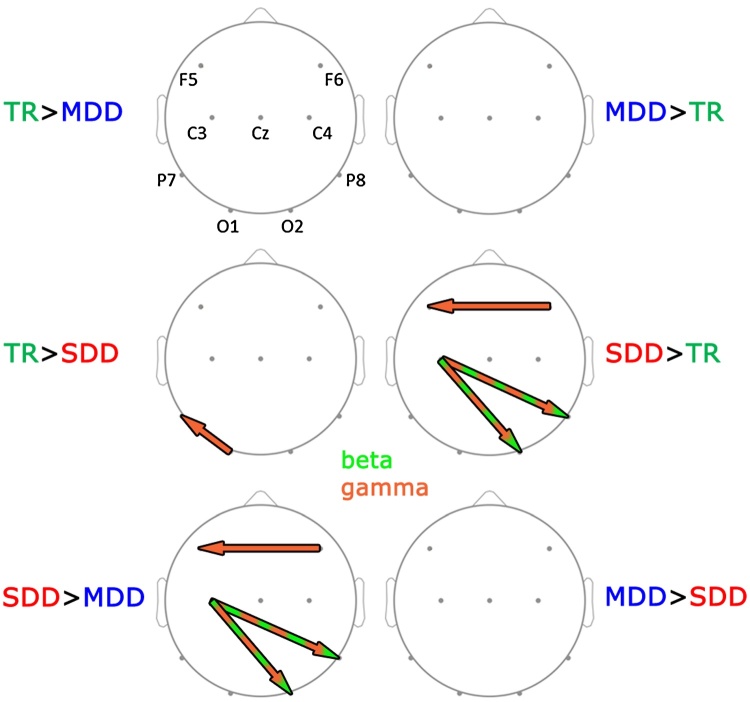
Next we investigated overall group differences. As can be seen in Fig. 3, there was a significant main effect of group in forward connectivity in the gamma range (60–70 Hz) from the left occipital site (O1) towards the left inferior-temporal site (P7) and in backward beta and gamma range (13–62 Hz) connectivity from the left central (C3) towards the right inferior-temporal site (P8). Post-hoc group comparisons for these frequencies and electrode pairs yielded stronger gamma range connectivity from O1 to P7 for words in TRs than in both dyslexic groups (TR/SDD: 60–70 Hz; TR/MDD: 60–65 Hz; Fig. 5), and the same connectivity was stronger in TRs than in SDDs for false fonts (60–70 Hz; Fig. 6). Conversely, SDDs showed stronger backward connectivity in the beta and gamma range from C3 to P8 than MDDs and TRs for false fonts (SDD/TR: 13–62 Hz; SDD/MDD: 28–61 Hz; Fig. 6), and than TRs for words (13–59 Hz; Fig. 5).
Fig. 5.
Topographic visualization of group differences in connectivity for words. TR- typical readers, MDD − moderately dysfluent dyslexics, SDD − severely dysfluent dyslexics. Stronger connectivity in one group relative to the other is represented with “>” (e.g. TR > MDD − stronger connectivity in TRs than MDDs). Arrows represent direction of the connectivity for which there is a group difference. Frequency bands in which significant group differences were found are color-coded: light green − beta (13–30 Hz), light red − gamma (31–70 Hz). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 6.
Topographic visualization of group differences in connectivity for false font strings. TR- typical readers, MDD − moderately dysfluent dyslexics, SDD − severely dysfluent dyslexics. Stronger connectivity in one group relative to the other is represented with “ > ” (e.g. TR > MDD − stronger connectivity in TRs than MDDs). Arrows represent direction of the connectivity for which there is a group difference. Frequency bands in which significant group differences were found are color-coded: light green − beta (13–30 Hz), light red − gamma (31–70 Hz). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Finally, our analysis yielded significant group*stimulus type interactions for connectivity in the beta and gamma range between the right occipital and the left central sites (bidirectional, O2 to C3: 26–48 Hz; C3 to O2: 25–52 Hz) and from the central to the left inferior-temporal site (Cz to P7: 17–39 Hz), as well as in the gamma range from the right to left frontal site (F6 to F5: 50–64 Hz). Post-hoc analysis for these frequencies and electrode-pairs indicated that for false font strings, SDDs, relative to both TRs and MDDs, had stronger backward connectivity in the beta and gamma range from C3 to O2 (SDD/TR: 25–52 Hz; SDD/MDD: 25–52 Hz; Fig. 6) and in the gamma range from F6 to F5 (SDD/TR: 50–64 Hz; SDD/MDD: 50–64 Hz). In the word condition, SDDs, relative to TRs, showed stronger bidirectional connectivity between O2 and C3 (O2 to C3: 26–48 Hz; C3 to O2: 25–52 Hz; Fig. 5).
In brief, our results showed overall stimulus effects in terms of stronger directed connectivity for false fonts than for words from bilateral inferior-temporal sites towards the left central site as well as between bilateral inferior-temporal sites. Words elicited stronger connectivity from the right central to the right frontal site, but only when all three groups were combined. Secondly, considering overall group differences, TRs showed stronger forward connectivity from the left occipital to the left inferior-temporal site than both dyslexic groups. SDDs instead showed stronger backward connectivity from the left central to the right inferior-temporal site than both TRs and MDDs. Thirdly, group*stimulus type interaction effects resulted from stronger connectivity during false font processing from the left central to the right occipital site, and from the right to the left frontal site for SDDs relative to the other two groups. Finally, in the word condition, SDDs, relative to TRs, exhibited stronger bidirectional connectivity between the left central and the right occipital site.
3.3. Correlations between directed connectivity and behavior
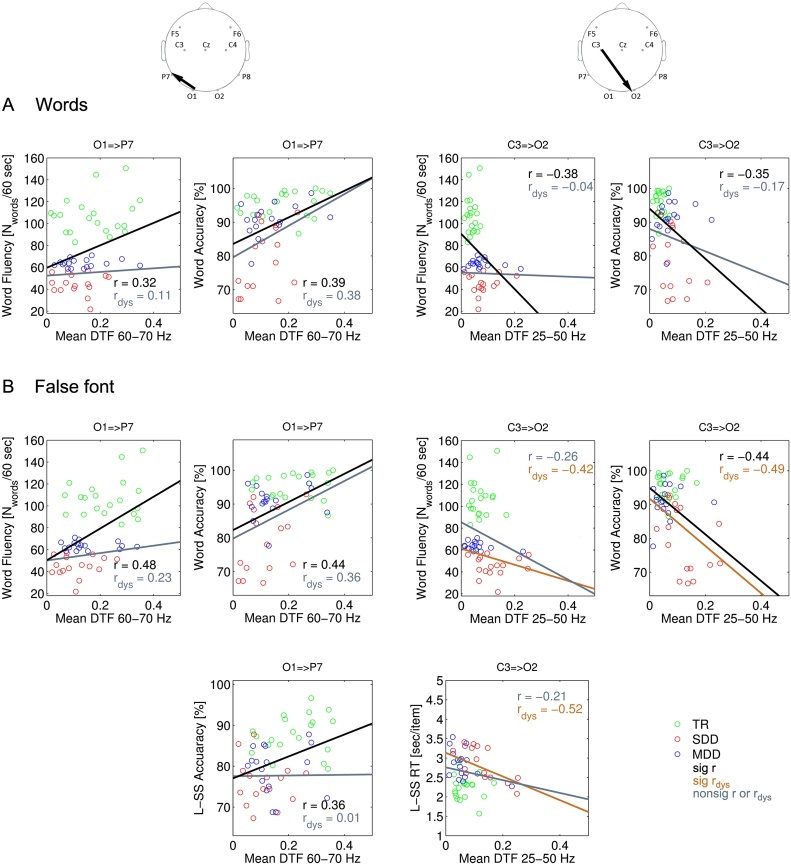
In a final analysis, we investigated whether individual differences in connectivity strength in the visual word and false font conditions relate to offline behavioral scores of reading fluency and accuracy and letter-speech sound coupling accuracy and speed. We restricted our analysis to the electrode pairs for which there was a main effect of group (O1 to P7 and C3 to O2). As most group differences in connectivity occurred in either the gamma range (forward connectivity) or in the beta and gamma range (backward connectivity), these analyses were restricted to two average connectivity indices, one in the higher gamma band (60–70 Hz) and the other across the beta and lower gamma bands (25–50 Hz). As illustrated in Fig. 7, both during word and false font processing, forward connectivity from the left occipital to the left inferior-temporal site (O1 to P7: 60–70 Hz), was positively correlated with reading fluency, reading accuracy and letter-speech sound coupling accuracy across the 3 groups, but not within the dyslexic groups alone. Furthermore, in the word condition, backward connectivity from the left central to the right occipital site (C3 to O2: 25–50 Hz) was negatively related to reading fluency and reading accuracy across the 3 groups. In the false font condition, this same backward connectivity was negatively related to reading accuracy across the 3 groups and to reading accuracy as well as fluency and letter speech sound reaction times across the two dyslexic groups.
Fig. 7.
Correlations between connectivity and behavior. Significant correlation (after FDR correction) of gamma range (60–70 Hz) forward and beta/gamma range (25–50 Hz) backward connectivity with reading related scores in A) word and B) false font condition. Green − typical readers; blue − moderately dysfluent dyslexics; red − severely dysfluent dyslexics; black lines − significant correlation across all three groups; brown lines − significant correlation across dyslexic groups; gray lines − nonsignificant correlations. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
We investigated EEG-based directed connectivity during visual word and false font processing in 9-year old typically reading (TR) and two groups of dyslexic children, moderately dysfluent dyslexics (MDD) and severely dysfluent dyslexics (SDD). In the visual word condition our results showed weaker forward connectivity from left occipital to left inferior-temporal sites in both dyslexic groups relative to TRs, suggesting reduced communication within the left posterior network for visual word processing. The strength of this occipital to inferior-temporal connectivity scaled positively with reading fluency across the three groups. In addition, SDDs, relative to TRs, exhibited stronger backward connectivity from left central to right inferior-temporal and occipital sites. In the false font condition, this connectivity, as well as connectivity from the right to left frontal site, differentiated SDDs from both TRs and MDDs. Furthermore, the strength of backward connectivity from the left central to the right occipital site scaled negatively with reading skills across the dyslexic groups. Finally, false fonts, relative to words, yielded stronger connectivity between inferior-temporal sites in TRs and MDDs, but not in SDDs. Together, these results suggest disrupted visual processing of words in both dyslexic groups, and a compensatory recruitment of right posterior brain regions especially in the SDDs during the processing of both visual words and false fonts.
4.1. Reduced connectivity from occipital to inferior-temporal sites during visual word and false font processing in both dyslexic groups
Dyslexics exhibited weaker left occipital to left inferior-temporal connectivity in the higher gamma range than TRs, and the strength of this connectivity was correlated with reading fluency across the three groups but not across the dyslexic groups alone. Post-hoc tests revealed a difference between TRs and MDDs only for word reading, while TRs differed from SDDs in both word and letter-like false font ‘reading’. The processing of visually presented words has been found to start in primary visual areas and proceed through higher visual cortex ventrally to the left occipito-temporal sulcus, where the putative visual word form area is located (Dehaene et al., 2005, McCandliss et al., 2003). Accordingly, an EEG connectivity study analyzing connectivity between brain sources during word reading in typically reading young adults, found feed-forward and feed-back visual signals between occipital and ventral occipito-temporal sites in theta and gamma ranges (Bedo et al., 2014). Similar to our results, a recent fMRI connectivity study in children found weaker connectivity along the visual pathway during a visual word- and non-word-rhyming task in 9 year old dyslexics relative to their typically reading peers (Finn et al., 2014). Another fMRI study investigated functional connectivity between the middle-occipital gyrus, the left occipito-temporal junction and left parietal cortex during word reading in healthy adults with variable reading performance (Levy et al., 2009). Interestingly, and resembling our current left occipital (O1) to left inferior-temporal (P7) connectivity findings, the strength of these bottom-up connections in the word and pseudoword conditions were significantly related to individual differences in reading fluency (Levy et al., 2009).
4.2. Increased connectivity from and towards central sites during visual word and false font processing in the severely dysfluent dyslexic group
Severely dysfluent dyslexics showed stronger EEG connectivity between posterior and central sites in the beta and lower gamma range than TRs in the word condition and relative to both other groups in the false font condition. This connectivity did not differ between MDDs and TRs. In the word condition, the group differences occurred from the right occipital to the left central site and backwards from the left central towards the right occipital and inferior-temporal sites. In the false font condition, the group differences were observed in backward connectivity, from the left central towards the right occipital and inferior-temporal sites. In typically reading young adults, word reading has been associated with bidirectional theta and gamma range connectivity between early and higher order visual regions in the left occipital and occipito-temporal cortex, and language related superior temporal and parietal regions (Bedo et al., 2014). In typical development, greater activation of temporo-parietal regions in children than in adults is taken as a sign of greater reliance on phonological processing during reading (Booth et al., 2001, Church et al., 2008). Accordingly, we hypothesize that increased connectivity from and towards central sites in dyslexic children may reflect a compensatory reliance on temporo-parietal regions related to, for example, phonological/semantic processing, sensorimotor and/or verbal working memory functions (Bedo et al., 2014, Blomert, 2011, Pugh et al., 2001, Pugh et al., 2000, Simos et al., 2002, van Atteveldt and Ansari, 2014). Furthermore, especially in the SDDs, additional recruitment of language and/or memory related temporo-parietal networks may compensate for diminished involvement of visually/orthographically driven left inferior-temporal regions due to the weaker orthographic-phonological connectivity (Wimmer and Schurz, 2010). Interestingly, an increase in coherence with and between the central sites has been observed in dyslexics after neurofeedback training, together with improvements in reading and phonological awareness (Nazari et al., 2012), while in another study a lack of reading improvement was coupled with a lack of changes in fronto-central coherence (Breteler et al., 2010). It would be interesting to investigate training effects in relation to fluency as we found stronger connectivity involving central sites in the most dysfluent dyslexics. Furthermore, and similar to our finding that SDDs exhibit stronger connectivity involving right hemisphere sites, hyperactivation and stronger connectivity of right hemisphere sites in dyslexics, relative to typical readers was observed in previous studies (Finn et al., 2014, Grünling et al., 2004, Shaywitz et al., 1998, Simos et al., 2007). Importantly, and further suggesting the inadequacy of compensatory mechanisms involving the right hemisphere regions in SDDs, a study showed that dyslexics who activated the right, instead of the left temporoparietal region in response to a reading intervention did not show adequate behavioral improvements (Simos et al., 2007). Finally, our pattern of results with forward connectivity in the higher gamma range and backward connectivity in the beta and lower gamma range is in general agreement with studies suggesting that feedforward and feedback information may be transferred by different frequencies, generally higher frequencies being related to feedforward and lower frequencies to feedback connectivity (Bastos et al., 2015, Bressler and Richter, 2015, van Kerkoerle et al., 2014).
4.3. Increased connectivity from right to left frontal sites during false font reading in severely dysfluent dyslexics
Our results further showed stronger anterior connectivity from the right frontal to the left frontal site in SDDs. Also this group difference occurred in the beta and lower gamma range. The stronger connectivity between frontal sites in SDDs is in line with previous research showing increased fMRI connectivity in dyslexic children and adults during resting state and reading related tasks between multiple frontal and parietal brain areas (Finn et al., 2014, Richards and Berninger, 2008, Schurz et al., 2014). In the present study children performed a one-back task involving attentional and working memory processes in addition to bottom-up visual processing of words and letter-like false fonts. Accordingly, the stronger connectivity between frontal sites in SDDs may suggest more effortful strategies, related to higher working memory load, involvement of phonological processing (Brunswick et al., 1999, Finn et al., 2014, Shaywitz et al., 1998) or increased attention (Gross et al., 2004). Alternatively, TRs and MDDs may have been more successful than SDDs in suppressing the communication with task irrelevant areas through desynchronization (Lachaux et al., 2008).
4.4. Increased connectivity from right to left inferior-temporal sites for false font versus word reading
Typical readers and moderately dysfluent dyslexics exhibited stronger connectivity between inferior-temporal sites during letter-like false font processing, relative to word processing. This difference was not present in SDD group. We investigated overall differences in functional connectivity within the reading network while children processed visual words or false fonts in a block design. This design did not yield stronger connectivity for visual words as compared to false fonts in any of the groups separately, although the overall ANOVA did indicate stronger connectivity for words from right central to right frontal site across the groups (main effect of Stimulus Type from C4 to F6). The false fonts did yield stronger connectivity as compared to the visual words from right inferior-temporal to left inferior temporal sites in TRs and MDDs and from left inferior-temporal to left central sites in TRs. In SDDs, functional connectivity patterns for words and false fonts did not significantly differ. Because at the same time SDDs did show a stronger backward connectivity relative to the TRs and MDDs, this lack of condition effects possibly indicates a sustained uncertainty and/or altered orthographic–phonological connectivity in SDDs (Wimmer and Schurz, 2010). In other words, the letter-likeness of the false fonts may have induced uncertainty as to whether the presented strings were orthographic or quasi-orthographic. In TRs and MDDs this uncertainty may have been resolved within posterior regions of the reading network, resulting in stronger connectivity between inferior-temporal sites.
Finally, we used a blocked presentation of visual words and false fonts that allowed the investigation of longer lasting cognitive states induced by the repetition of stimuli from the same category (Al-Aidroos et al., 2012), but could attenuate condition differences within narrower time windows (Woodhead et al., 2014). In future studies it would be relevant to investigate EEG connectivity during word reading using unblocked and unpredictable consecutive trials. Importantly, the application of time variant connectivity measures, such as the short-time directed transfer function (Blinowska, 2011), could be then employed to investigate the temporal signature of directed EEG connectivity, allowing the observation of dynamic network changes during reading.
5. Conclusions
According to our knowledge, this is the first study that investigates directed connectivity during word reading in 9-year old typically reading and dyslexic children with different levels of reading dysfluency. Our results point to different patterns of connectivity in the reading network of typical and dyslexic children and within dyslexic children as a function of the severity of their reading dysfluency. Specifically, moderately dysfluent dyslexics, who previously showed a less severe impairment in letter-speech sound coupling (Žarić et al., 2014), now showed an impairment in the left posterior network for visual word processing, but did not show disrupted connectivity during the processing of letter-like false fonts. Severely dysfluent dyslexics, who previously showed a lack of firm associations between letters and speech sounds (Žarić et al., 2014), now revealed a (1) disruption of connectivity in the left posterior network for visual word processing, (2) compensatory involvement of anterior and right hemisphere sites, and (3) a lack of electrophysiological differentiation between words and letter-like stimuli. Finally, severely dysfluent dyslexics differed in connectivity from moderately dysfluent dyslexics in the false font condition. The finding that the functional connectivity pattern in dyslexic children is related to their reading level, may in part explain the mixed results obtained in previous functional connectivity studies of dyslexia.
Acknowledgments
Unfortunately, our co-author, Professor Leo Blomert, passed away on November 25, 2012. His contributions to the project prior to this date were significant. This project, “Fluent reading acquisition neurocognitively decomposed: the case of dyslexia (HCMI 10–59)”, is funded by the National Initiative Brain and Cognition (NIHC), a part of the Netherlands Organization for Scientific Research (NWO) under grant number 056-14-015. We would like to thank Suzanne van Grieken, Marlena van Langevelde, Gert-Jan Munneke, Jitka Annen, Mandy Meijer, Helene Vos and Jorinde Wesseling for their help during data collection. Finally, we would like to express our gratitude to the children participating in the research and to their parents.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.dcn.2016.11.003.
Contributor Information
Gojko Žarić, Email: gojko.zaric@maastrichtuniversity.nl.
João M. Correia, Email: joao.correia@maastrichtuniversity.nl.
Gorka Fraga González, Email: G.FragaGonzalez@uva.nl.
Jurgen Tijms, Email: jurgentijms@iwal.nl.
Maurtis W. van der Molen, Email: M.W.vanderMolen@uva.nl.
Milene Bonte, Email: m.bonte@maastrichtuniversity.nl.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- Al-Aidroos N., Said C.P., Turk-Browne N.B. Top-down attention switches coupling between low-level and high-level areas of human visual cortex. Proc. Natl. Acad. Sci. 2012;109:14675–14680. doi: 10.1073/pnas.1202095109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Androulidakis A.G., Mazzone P., Litvak V., Penny W., Dileone M., Gaynor L.M.F.D., Tisch S., Di Lazzaro V., Brown P. Oscillatory activity in the pedunculopontine area of patients with Parkinson’s disease. Exp. Neurol. 2008;211:59–66. doi: 10.1016/j.expneurol.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Arns M., Peters S., Breteler R., Verhoeven L. Different brain activation patterns in dyslexic children: evidence from EEG power and coherence patterns for the double-deficit theory of dyslexia. J. Integr. Neurosci. 2007;6:175–190. doi: 10.1142/s0219635207001404. [DOI] [PubMed] [Google Scholar]
- Bastos A.M., Schoffelen J.-M. A tutorial review of functional connectivity analysis methods and their interpretational pitfalls. Front. Syst. Neurosci. 2016;9:1–23. doi: 10.3389/fnsys.2015.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastos A.M., Vezoli J., Bosman C.A., Schoffelen J.-M., Oostenveld R., Dowdall J.R., De Weerd P., Kennedy H., Fries P. Visual areas exert feedforward and feedback influences through distinct frequency channels. Neuron. 2015;85:390–401. doi: 10.1016/j.neuron.2014.12.018. [DOI] [PubMed] [Google Scholar]
- Bedo N., Ribary U., Ward L.M. Fast dynamics of cortical functional and effective connectivity during word reading. PLoS One. 2014;9 doi: 10.1371/journal.pone.0088940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y., Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B. 1995;57:289–300. [Google Scholar]
- Blinowska K.J., Kuś R., Kamiński M. Granger causality and information flow in multivariate processes. Phys. Rev. E. 2004;70:50902. doi: 10.1103/PhysRevE.70.050902. [DOI] [PubMed] [Google Scholar]
- Blinowska K.J., Kuś R., Kamiński M., Janiszewska J. Transmission of brain activity during cognitive task. Brain Topogr. 2010;23:205–213. doi: 10.1007/s10548-010-0137-y. [DOI] [PubMed] [Google Scholar]
- Blinowska K.J., Kamiński M., Brzezicka A., Kamiński J. Application of directed transfer function and network formalism for the assessment of functional connectivity in working memory task. Philos. Trans. A. Math. Phys. Eng. Sci. 2013;371:20110674. doi: 10.1098/rsta.2011.0614. [DOI] [PubMed] [Google Scholar]
- Blinowska K.J. Review of the methods of determination of directed connectivity from multichannel data. Med. Biol. Eng. Comput. 2011;49:521–529. doi: 10.1007/s11517-011-0739-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomert L., Vaessen A.A.A. Boom test uitgevers; Amsterdam: 2009. 3DM Differential Diagnostiek Van Dyslexie: Cognitieve Analyse Van Lezen En Spellen. [Google Scholar]
- Blomert L. Uitgeverij Nieuwezijds; Amsterdam: 2005. Dyslexie in Nederland. [Google Scholar]
- Blomert L. The neural signature of orthographic-phonological binding in successful and failing reading development. Neuroimage. 2011;57:695–703. doi: 10.1016/j.neuroimage.2010.11.003. [DOI] [PubMed] [Google Scholar]
- Booth J.R., Burman D.D., Van Santen F.W., Harasaki Y., Gitelman D.R., Parrish T.B., Marsel Mesulam M.M. The development of specialized brain systems in reading and oral-language. Child Neuropsychol. 2001;7:119–141. doi: 10.1076/chin.7.3.119.8740. [DOI] [PubMed] [Google Scholar]
- Bressler S.L., Richter C.G. Interareal oscillatory synchronization in top-down neocortical processing. Curr. Opin. Neurobiol. 2015;31:62–66. doi: 10.1016/j.conb.2014.08.010. [DOI] [PubMed] [Google Scholar]
- Breteler M.H.M., Arns M., Peters S., Giepmans I., Verhoeven L. Improvements in spelling after QEEG-based neurofeedback in dyslexia: a randomized controlled treatment study. Appl. Psychophysiol. Biofeedback. 2010;35:5–11. doi: 10.1007/s10484-009-9105-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunswick N., McCrory E., Price C.J., Frith C.D., Frith U. Explicit and implicit processing of words and pseudowords by adult developmental dyslexics: a search for Wernicke’s Wortschatz? Brain. 1999;122(Pt 1):1901–1917. doi: 10.1093/brain/122.10.1901. [DOI] [PubMed] [Google Scholar]
- Brus B.T., Voeten M.J.M. Berkenhout Testmateriaal; Nimegen: 1973. Eén-minuut Test, Vorm A En B; Verantwoording En Handleiding. [Google Scholar]
- Brzezicka A., Kamiński M., Kamiński J., Blinowska K.J. Information transfer during a transitive reasoning task. Brain Topogr. 2011;24:1–8. doi: 10.1007/s10548-010-0158-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church J. a., Coalson R.S., Lugar H.M., Petersen S.E., Schlaggar B.L. A developmental fMRI study of reading and repetition reveals changes in phonological and visual mechanisms over age. Cereb. Cortex. 2008;18:2054–2065. doi: 10.1093/cercor/bhm228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coch D., Meade G. N1 and P2 to words and wordlike stimuli in late elementary school children and adults. Psychophysiology. 2016;53:115–128. doi: 10.1111/psyp.12567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui J., Xu L., Bressler S.L., Ding M., Liang H. BSMART: A Matlab/C toolbox for analysis of multichannel neural time series. Neural Netw. 2008;21:1094–1104. doi: 10.1016/j.neunet.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dambacher M., Dimigen O., Braun M., Wille K., Jacobs A.M., Kliegl R. Stimulus onset asynchrony and the timeline of word recognition: event-related potentials during sentence reading. Neuropsychologia. 2012;50:1852–1870. doi: 10.1016/j.neuropsychologia.2012.04.011. [DOI] [PubMed] [Google Scholar]
- Dehaene S., Cohen L., Sigman M., Vinckier F. The neural code for written words: a proposal. Trends Cogn. Sci. 2005;9:335–341. doi: 10.1016/j.tics.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Dehaene S. Electrophysiological evidence for category-specific word processing in the normal human brain. Neuroreport. 1995;6:2153–2157. doi: 10.1097/00001756-199511000-00014. [DOI] [PubMed] [Google Scholar]
- Delorme A., Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- de Vos T. Boom test uitgevers; Amsterdam: 2007. Schoolvaardigheidstoets Technisch Lezen. [Google Scholar]
- Dhar M., Been P.H., Minderaa R.B., Althaus M. Reduced interhemispheric coherence in dyslexic adults. Cortex. 2010;46:794–798. doi: 10.1016/j.cortex.2009.09.006. [DOI] [PubMed] [Google Scholar]
- Dimitriadis S.I., Laskaris N. a., Simos P.G., Micheloyannis S., Fletcher J.M., Rezaie R., Papanicolaou a. C. Altered temporal correlations in resting-state connectivity fluctuations in children with reading difficulties detected via MEG. Neuroimage. 2013;83:307–317. doi: 10.1016/j.neuroimage.2013.06.036. [DOI] [PubMed] [Google Scholar]
- Engel K.a, Fries P., Singer W. Dynamic predictions: oscillations and synchrony in top-down processing. Nat. Rev. Neurosci. 2001;2:704–716. doi: 10.1038/35094565. [DOI] [PubMed] [Google Scholar]
- Ewen J.B., Lakshmanan B.M., Hallett M., Mostofsky S.H., Crone N.E., Korzeniewska A. Dynamics of functional and effective connectivity within human cortical motor control networks. Clin. Neurophysiol. 2015;126:987–996. doi: 10.1016/j.clinph.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn E.S., Shen X., Holahan J.M., Scheinost D., Lacadie C., Papademetris X., Shaywitz S.E., Shaywitz B.A., Constable R.T. Disruption of functional networks in dyslexia: a whole-brain, data-driven analysis of connectivity. Biol. Psychiatry. 2014;76:397–404. doi: 10.1016/j.biopsych.2013.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraga González G., Žarić G., Tijms J., Bonte M., Blomert L., van der Molen M.W. Brain-potential analysis of visual word recognition in dyslexics and typically reading children. Front. Hum. Neurosci. 2014;8:474. doi: 10.3389/fnhum.2014.00474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraga González G., Van der Molen M.J.W., Žarić G., Bonte M., Tijms J., Blomert L., Stam C.J., Van der Molen M.W. Graph analysis of EEG resting state functional networks in dyslexic readers. Clin. Neurophysiol. 2016;127:3165–3175. doi: 10.1016/j.clinph.2016.06.023. [DOI] [PubMed] [Google Scholar]
- Fraga González G., Žarić G., Tijms J., Bonte M.L., Blomert L., Leppänen P.H.T., van der Molen M.W. Responsivity to dyslexia training indexed by the N170 amplitude of the brain potential elicited by word reading. Brain Cogn. 2016;106:42–54. doi: 10.1016/j.bandc.2016.05.001. [DOI] [PubMed] [Google Scholar]
- Franaszczuk P.J., Bergey G.K., Kamiński M.J. Analysis of mesial temporal seizure onset and propagation using the directed transfer function method. Electroencephalogr. Clin. Neurophysiol. 1994;91:413–427. doi: 10.1016/0013-4694(94)90163-5. [DOI] [PubMed] [Google Scholar]
- Frye R.E., Wu M.-H., Liederman J., McGraw Fisher J. Greater pre-stimulus effective connectivity from the left inferior frontal area to other areas is associated with better phonological decoding in dyslexic readers. Front. Syst. Neurosci. 2010;4:156. doi: 10.3389/fnsys.2010.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye R.E., Liederman J., McGraw Fisher J., Wu M.H. Laterality of temporoparietal causal connectivity during the prestimulus period correlates with phonological decoding task performance in dyslexic and typical readers. Cereb. Cortex. 2012;22:1923–1934. doi: 10.1093/cercor/bhr265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geschwind N. Disconnexion syndromes in animals and man. I. Brain. 1965;88:237–294. doi: 10.1093/brain/88.2.237. [DOI] [PubMed] [Google Scholar]
- Ghyselinck M., De Moor W., Brysbaert M. Age-of-acquisition ratings for 2816 Dutch four- and five-letter nouns. Psychol. Belg. 2000;40:77–98. [Google Scholar]
- Ginter J., Blinowska K.J., Kamiński M., Durka P.J. Phase and amplitude analysis in time-frequency space-application to voluntary finger movement. J. Neurosci. Methods. 2001;110:113–124. doi: 10.1016/s0165-0270(01)00424-1. [DOI] [PubMed] [Google Scholar]
- Grünling C., Ligges M., Huonker R., Klingert M., Mentzel H.-J., Rzanny R., Kaiser W.a, Witte H., Blanz B. Dyslexia: the possible benefit of multimodal integration of fMRI- and EEG-data. J. Neural Transm. 2004;111:951–969. doi: 10.1007/s00702-004-0117-z. [DOI] [PubMed] [Google Scholar]
- Granger C.W.J. Investigating causal relations by econometric models and cross-spectral methods. Econometrica. 1969;37:424. [Google Scholar]
- Gross J., Schmitz F., Schnitzler I., Kessler K., Shapiro K., Hommel B., Schnitzler A. Modulation of long-range neural synchrony reflects temporal limitations of visual attention in humans. Proc. Natl. Acad. Sci. U. S. A. 2004;101:13050–13055. doi: 10.1073/pnas.0404944101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannagan T., Amedi A., Cohen L., Dehaene-Lambertz G., Dehaene S. Origins of the specialization for letters and numbers in ventral occipitotemporal cortex. Trends Cogn. Sci. 2015;19:374–382. doi: 10.1016/j.tics.2015.05.006. [DOI] [PubMed] [Google Scholar]
- Hansen, P.C., Andrews, P., Easby, R., Sullivan, F., Cornelissen, P.L., Pre-orthographic visual constraints on adult contextual reading speed, unpublished data.
- Hauk O., Davis M.H., Ford M., Pulvermüller F., Marslen-Wilson W.D. The time course of visual word recognition as revealed by linear regression analysis of ERP data. Neuroimage. 2006;30:1383–1400. doi: 10.1016/j.neuroimage.2005.11.048. [DOI] [PubMed] [Google Scholar]
- Kamiński M., Blinowska K.J. A new method of the description of the information flow in the brain structures. Biol. Cybern. 1991;65:203–210. doi: 10.1007/BF00198091. [DOI] [PubMed] [Google Scholar]
- Kaminski M., Blinowska K.J. Directed Transfer Function is not influenced by volume conduction-inexpedient pre-processing should be avoided. Front. Comput. Neurosci. 2014;8:61. doi: 10.3389/fncom.2014.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzir T., Kim Y.-S.Y.-S., Wolf M., Morris R., Lovett M.W. The varieties of pathways to dysfluent reading: comparing subtypes of children with dyslexia at letter, word, and connected text levels of reading. J. Learn. Disabil. 2008;41:47–66. doi: 10.1177/0022219407311325. [DOI] [PubMed] [Google Scholar]
- Kim Y.-S., Park C.H., Wagner R.K. Is oral/text reading fluency a bridge to reading comprehension? Read. Writ. 2014;27:79–99. doi: 10.1007/s11145-013-9434-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koessler L., Maillard L., Benhadid A., Vignal J.P., Felblinger J., Vespignani H., Braun M. Automated cortical projection of EEG sensors: anatomical correlation via the international 10-10 system. Neuroimage. 2009;46:64–72. doi: 10.1016/j.neuroimage.2009.02.006. [DOI] [PubMed] [Google Scholar]
- Kuś R., Ginter J.S., Blinowska K.J. Propagation of EEG activity during finger movement and its imagination. Acta Neurobiol. Exp. (Wars). 2006;66:195–206. doi: 10.55782/ane-2006-1607. [DOI] [PubMed] [Google Scholar]
- Kujala J., Pammer K., Cornelissen P., Roebroeck A., Formisano E., Salmelin R. Phase coupling in a cerebro-cerebellar network at 8–13 Hz during reading. Cereb. Cortex. 2007;17:1476–1485. doi: 10.1093/cercor/bhl059. [DOI] [PubMed] [Google Scholar]
- Lachaux J.P., Jung J., Mainy N., Dreher J.C., Bertrand O., Baciu M., Minotti L., Hoffmann D., Kahane P. Silence is golden: transient neural deactivation in the prefrontal cortex during attentive reading. Cereb. Cortex. 2008;18:443–450. doi: 10.1093/cercor/bhm085. [DOI] [PubMed] [Google Scholar]
- Landi N., Perfetti C.A. An electrophysiological investigation of semantic and phonological processing in skilled and less-skilled comprehenders. Brain Lang. 2007;102:30–45. doi: 10.1016/j.bandl.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Laszlo S., Federmeier K.D. Never seem to find the time: evaluating the physiological time course of visual word recognition with regression analysis of single item ERPs. Lang. Cogn. Process. 2014;29:642–661. doi: 10.1080/01690965.2013.866259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinonen S., Müller K., Leppänen P.H.T., Aro M., Ahonen T., Lyytinen H. Heterogeneity in adult dyslexic readers: relating processing skills to the speed and accuracy of oral text reading *. Read. Writ. 2001;14:265–296. [Google Scholar]
- Leisman G. Coherence of hemispheric function in developmental dyslexia. Brain Cogn. 2002;48:425–431. [PubMed] [Google Scholar]
- Levy J., Pernet C., Treserras S., Boulanouar K., Aubry F., Démonet J.-F., Celsis P. Testing for the dual-route cascade reading model in the brain: an fMRI effective connectivity account of an efficient reading style. PLoS One. 2009;4:e6675. doi: 10.1371/journal.pone.0006675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligges C., Ungureanu M., Ligges M., Blanz B., Witte H. Understanding the time variant connectivity of the language network in developmental dyslexia: new insights using Granger causality. J. Neural Transm. 2010;117:529–543. doi: 10.1007/s00702-010-0367-x. [DOI] [PubMed] [Google Scholar]
- Lyon G.R., Shaywitz S.E., Shaywitz B.A. A definition of dyslexia. Ann. Dyslexia. 2003;53:1–14. [Google Scholar]
- Mahé G., Bonnefond A., Gavens N., Dufour A., Doignon-Camus N. Impaired visual expertise for print in French adults with dyslexia as shown by N170 tuning. Neuropsychologia. 2012;50:3200–3206. doi: 10.1016/j.neuropsychologia.2012.10.013. [DOI] [PubMed] [Google Scholar]
- Mayhew S.D., Dirckx S.G., Niazy R.K., Iannetti G.D., Wise R.G. EEG signatures of auditory activity correlate with simultaneously recorded fMRI responses in humans. Neuroimage. 2010;49:849–864. doi: 10.1016/j.neuroimage.2009.06.080. [DOI] [PubMed] [Google Scholar]
- McCandliss B.D., Cohen L., Dehaene S. The visual word form area: expertise for reading in the fusiform gyrus. Trends Cogn. Sci. 2003;7:293–299. doi: 10.1016/s1364-6613(03)00134-7. [DOI] [PubMed] [Google Scholar]
- Milne R.D., Hamm J.P., Kirk I.J., Corballis M.C. Anterior-posterior beta asymmetries in dyslexia during lexical decisions. Brain Lang. 2003;84:309–317. doi: 10.1016/s0093-934x(02)00557-6. [DOI] [PubMed] [Google Scholar]
- Mullen T. 2012. NITRC: CleanLine: Tool/Resource Info. [Google Scholar]
- Nazari M.A., Mosanezhad E., Hashemi T., Jahan A. The effectiveness of neurofeedback training on EEG coherence and neuropsychological functions in children with reading disability. Clin. EEG Neurosci. 2012;43:315–322. doi: 10.1177/1550059412451880. [DOI] [PubMed] [Google Scholar]
- Oostenveld R., Fries P., Maris E., Schoffelen J.M. FieldTrip: open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput. Intell. Neurosci. 2011;2011 doi: 10.1155/2011/156869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulou M., Vonck K., Boon P., Marinazzo D. Mapping the epileptic brain with EEG dynamical connectivity: established methods and novel approaches. Eur. Phys. J. Plus. 2012;127:144. [Google Scholar]
- Papadopoulou M., Friston K., Marinazzo D. Estimating directed connectivity from cortical recordings and reconstructed sources. Brain Topogr. 2015 doi: 10.1007/s10548-015-0450-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulesu E., Frith U., Snowling M., Gallagher A., Morton J., Frackowiak R.S.J., Frith C.D. Is developmental dyslexia a disconnection syndrome? Brain. 1996;119:143–157. doi: 10.1093/brain/119.1.143. [DOI] [PubMed] [Google Scholar]
- Pugh K.R., Mencl W.E., Shaywitz B.A., Shaywitz S.E., Fulbright R.K., Constable R.T., Skudlarski P., Marchione K.E., Jenner R.a, Fletcher J.M., Liberman M.a, Shankweiler D.P., Katz L., Lacadie C., Gore J.C. The angular gyrus in developmental dyslexia: task-specific differences in functional connectivity within posterior cortex. Psychol. Sci. J. Am. Psychol. Soc./APS. 2000;11:51–56. doi: 10.1111/1467-9280.00214. [DOI] [PubMed] [Google Scholar]
- Pugh K.R., Mencl W.E., Jenner a R., Katz L., Frost S.J., Lee J.R., Shaywitz S.E., Shaywitz B.A. Neurobiological studies of reaing and reading disability. J. Commun. Disord. 2001;34:479–492. doi: 10.1016/s0021-9924(01)00060-0. [DOI] [PubMed] [Google Scholar]
- Richards T.L., Berninger V.W. Abnormal fMRI connectivity in children with dyslexia during a phoneme task: before but not after treatment. J. Neurolinguistics. 2008;21:294–304. doi: 10.1016/j.jneuroling.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossell S.L., Price C.J., Nobre A.C. The anatomy and time course of semantic priming investigated by fMRI and ERPs. Neuropsychologia. 2003;41:550–564. doi: 10.1016/s0028-3932(02)00181-1. [DOI] [PubMed] [Google Scholar]
- Savill N.J., Thierry G. Reading for sound with dyslexia: evidence for early orthographic and late phonological integration deficits. Brain Res. 2011;1385:192–205. doi: 10.1016/j.brainres.2011.02.012. [DOI] [PubMed] [Google Scholar]
- Schaerlakens A., Kohnstamm D., Lejaegere M. Gebaseerd op nieuw onderzoek in Nederland en België. Swets & Zeitlinger; Lisse: 1999. Streeflijst woordenschat voor zesjarigen. [Google Scholar]
- Schlaggar B.L., McCandliss B.D. Development of neural systems for reading. Annu. Rev. Neurosci. 2007;30:475–503. doi: 10.1146/annurev.neuro.28.061604.135645. [DOI] [PubMed] [Google Scholar]
- Schumacher E.M., Stiris T.A., Larsson P.G. Effective connectivity in long-term EEG monitoring in preterm infants. Clin. Neurophysiol. 2015 doi: 10.1016/j.clinph.2015.01.017. [DOI] [PubMed] [Google Scholar]
- Schurz M., Wimmer H., Richlan F., Ludersdorfer P., Klackl J., Kronbichler M. Resting-state and task-based functional brain connectivity in developmental dyslexia. Cereb. Cortex. 2014:1–13. doi: 10.1093/cercor/bhu184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz G. Estimating the dimension of a model. Ann. Stat. 1978;6:461–464. [Google Scholar]
- Seth A.K., Barrett A.B., Barnett L. Granger causality analysis in neuroscience and neuroimaging. J. Neurosci. 2015;35:3293–3297. doi: 10.1523/JNEUROSCI.4399-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth A.K. A MATLAB toolbox for Granger causal connectivity analysis. J. Neurosci. Methods. 2010;186:262–273. doi: 10.1016/j.jneumeth.2009.11.020. [DOI] [PubMed] [Google Scholar]
- Shaywitz S.E., Shaywitz B.A., Pugh K.R., Fulbright R.K., Constable R.T., Mencl W.E., Shankweiler D.P., Liberman M.a, Skudlarski P., Fletcher J.M., Katz L., Marchione K.E., Lacadie C., Gatenby C., Gore J.C. Functional disruption in the organization of the brain for reading in dyslexia. Proc. Natl. Acad. Sci. U. S. A. 1998;95:2636–2641. doi: 10.1073/pnas.95.5.2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaywitz S.E., Morris R., Shaywitz B.A. The education of dyslexic children from childhood to young adulthood. Annu. Rev. Psychol. 2008;59:451–475. doi: 10.1146/annurev.psych.59.103006.093633. [DOI] [PubMed] [Google Scholar]
- Simos P.G., Breier J.I., Fletcher J.M., Foorman B.R., Castillo E.M., Papanicolaou A.C. Brain mechanisms for reading words and pseudowords: an integrated approach. Cereb. Cortex. 2002;12:297–305. doi: 10.1093/cercor/12.3.297. [DOI] [PubMed] [Google Scholar]
- Simos P.G., Fletcher J.M., Sarkari S., Billingsley R.L., Denton C., Papanicolaou A.C. Altering the brain circuits for reading through intervention: a magnetic source imaging study. Neuropsychology. 2007;21:485–496. doi: 10.1037/0894-4105.21.4.485. [DOI] [PubMed] [Google Scholar]
- Stokić M., Milosavljević Z., Subotić M. Specific features of brain connectivity during silent reading in children with developmental dyslexia. Spec. Edukac. i Rehabil. 2011;10:479–492. [Google Scholar]
- Vandermosten M., Boets B., Wouters J., Ghesquière P. A qualitative and quantitative review of diffusion tensor imaging studies in reading and dyslexia. Neurosci. Biobehav. Rev. 2012;36:1532–1552. doi: 10.1016/j.neubiorev.2012.04.002. [DOI] [PubMed] [Google Scholar]
- Varela F., Lachaux J.P., Rodriguez E., Martinerie J. The brainweb: phase synchronization and large-scale integration. Nat. Rev. Neurosci. 2001;2:229–239. doi: 10.1038/35067550. [DOI] [PubMed] [Google Scholar]
- Wimmer H., Schurz M. Dyslexia in regular orthographies: manifestation and causation. Dyslexia. 2010;16:283–299. doi: 10.1002/dys.411. [DOI] [PubMed] [Google Scholar]
- Woodhead Z.V.J., Barnes G.R., Penny W., Moran R., Teki S., Price C.J., Leff P.a. Reading front to back: MEG evidence for early feedback effects during word recognition. Cereb. Cortex. 2014;24:817–825. doi: 10.1093/cercor/bhs365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Atteveldt N., Ansari D. How symbols transform brain function: a review in memory of Leo Blomert. Trends Neurosci. Educ. 2014:1–5. [Google Scholar]
- van Kerkoerle T., Self M.W., Dagnino B., Gariel-Mathis M.-A., Poort J., van der Togt C., Roelfsema P.R. Alpha and gamma oscillations characterize feedback and feedforward processing in monkey visual cortex. Proc. Natl. Acad. Sci. 2014;111:14332–14341. doi: 10.1073/pnas.1402773111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Žarić G., Fraga González G., Tijms J., van der Molen M.W., Blomert L., Bonte M. Reduced neural integration of letters and speech sounds in dyslexic children scales with individual differences in reading fluency. PLoS One. 2014;9:e110337. doi: 10.1371/journal.pone.0110337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Žarić G., Fraga González G., Tijms J., van der Molen M.W., Blomert L., Bonte M. Crossmodal deficit in dyslexic children: practice affects the neural timing of letter-speech sound integration. Front. Hum. Neurosci. 2015;9(369) doi: 10.3389/fnhum.2015.00369. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.