Highlights
* 3- to 5-year-old children direct attention to times that contain word onsets during speech perception. * Attention probes presented in conjunction with word onsets elicit a more negative early ERP response than control probes. * Results resemble temporally selective effects previously observed in adults. * Despite polarity differences, results resemble spatially selective attention effects in children.
Keywords: Speech perception, Development, Selective attention, Temporal orienting, Event-related potential
Abstract
Recent event-related potential (ERP) evidence demonstrates that adults employ temporally selective attention to preferentially process the initial portions of words in continuous speech. Doing so is an effective listening strategy since word-initial segments are highly informative. Although the development of this process remains unexplored, directing attention to word onsets may be important for speech processing in young children who would otherwise be overwhelmed by the rapidly changing acoustic signals that constitute speech. We examined the use of temporally selective attention in 3- to 5-year-old children listening to stories by comparing ERPs elicited by attention probes presented at four acoustically matched times relative to word onsets: concurrently with a word onset, 100 ms before, 100 ms after, and at random control times. By 80 ms, probes presented at and after word onsets elicited a larger negativity than probes presented before word onsets or at control times. The latency and distribution of this effect is similar to temporally and spatially selective attention effects measured in adults and, despite differences in polarity, spatially selective attention effects measured in children. These results indicate that, like adults, preschool aged children modulate temporally selective attention to preferentially process the initial portions of words in continuous speech.
1. Introduction
Selective attention allows listeners to preferentially process the most relevant information in complex environments that would otherwise overwhelm perceptual systems. Through the use of auditory spatially selective attention, sometimes labeled the “cocktail party effect” (Cherry, 1953), listeners can process sounds from one location in detail while ignoring competing sounds from other locations. Auditory attention can be deployed as a finely tuned gradient around the location of the selected source (Teder-Salejarvi et al., 1999), and listeners can further hone the selection process by using other features such as frequency and pitch (Woods and Alain, 1993, Woods et al., 1991).
Event-related brain potential (ERP) studies have shown that selective attention affects auditory processing at an early, perceptual stage. In a now classic study of auditory attention, ERPs elicited by tone pips presented at attended and unattended locations were compared; sounds presented at the attended location elicited a larger negativity beginning around 100 ms after onset (N1) (Hillyard et al., 1973). In addition to a larger N1, attended stimuli elicit a sustained negativity termed the processing negativity or negative difference (Nd) (Näätänen, 1982, Näätänen and Michie, 1979, Woldorff et al., 1987) that may reflect additional processing of attended sounds.
Typical N1 and Nd attention effects have been observed when listeners attend to a variety of stimuli, including syllables and environmental sounds (Hansen et al., 1983, Hink et al., 1978, Hink et al., 1977). The Hillyard paradigm has also been adapted to examine spatial attention during speech perception by presenting probes at the same location as two competing narratives during a dichotic listening task. Probes presented to the same ear as the attended narrative elicit a larger N1 than probes presented along with the unattended narrative (Hink and Hillyard, 1976). These results indicate that spatially selective attention modulates the processing of simple and complex auditory stimuli, including speech, in a similar manner.
Research has also characterized at least some aspects of the development of auditory selective attention. There is considerable evidence that the control of selective attention continues to develop throughout adolescence (Berman and Friedman, 1995, Kannass et al., 2006). However, several recent studies have demonstrated that children as young as 3 years of age can selectively attend to sounds at a particular location when provided with sufficient motivation, an engaging task, and multiple cues. For example, when children between the ages of 3 and 8 years attend to one of two simultaneously presented stories narrated by different speakers and presented at distinct locations, probes presented at the same location as the attended story elicit a larger positivity beginning around 100 ms after onset compared to probes from the same location as the unattended story (Coch et al., 2005, Sanders et al., 2006). This larger positivity is more prolonged in 3- to 5-year-old children, while 6- to 8-year-old children also show a later negativity in response to attended probes. Although the polarity of this early positive attention effect is different than the negative attention effects observed in adults, the latency and scalp distribution are remarkably similar. This indicates that spatially selective attention modulates auditory processing at a perceptual stage in both children and adults, even for complex sounds such as continuous speech.
Although spatially selective attention is clearly important for speech processing when there are multiple talkers at different locations, a single continuous stream of speech also presents significant challenges to even fully developed perceptual systems. The rapidly changing nature of speech means it may not be possible to fully process all of the segments in a continuous stream in detail. As such, listeners may benefit from attending to critical times when listening to a single speaker rather than particular locations. Recent evidence indicates that adults do direct attention to selected times, which improves processing of stimuli presented at those compared to unattended times (Coull et al., 2000, Coull and Nobre, 1998, Griffin et al., 2002). ERP evidence demonstrates that sounds presented at attended times elicit a larger N1 than sounds at unattended times (Lange et al., 2003, Sanders and Astheimer, 2008). Temporally selective attention modulates auditory processing in a manner similar to spatially selective attention.
For temporally selective attention to benefit speech comprehension, listeners would need to direct attention to the times at which the most critical portions of the speech signal are presented. Although it is not yet clear how to best define the ‘most critical’ segments in speech, it has been shown that listeners rely more heavily on word onsets than other segments during auditory word recognition (Connine et al., 1993, Marslen-Wilson and Zwitserlood, 1989, Salasoo and Pisoni, 1985). Further, ERP evidence indicates that word-initial segments elicit a larger N1 than word-medial segments in continuous speech (Sanders and Neville, 2003a, Sanders et al., 2002), a difference that closely resembles auditory temporally selective attention effects. A recent study adapted the classic Hillyard spatial attention paradigm to directly examine the use of temporal attention during speech perception by inserting attention probes at various acoustically matched times relative to word onsets. Speech-like probes presented within the first 150 ms of a word elicited a larger N1 than probes presented before a word onset or at random control times (Astheimer and Sanders, 2009). Critically, the same ERP effect was not observed when pure tones were used as attention probes. Evidently, listeners ignore the entire stream of nonlinguistic probes when the probes and speech are easily discriminated. The lack of effects for the pure-tone probes also indicates that the attempt to present attention probes in acoustically matched portions of the speech stream was successful since any physical differences in the compared conditions were present with both linguistic and nonlinguistic probes. The results of this study demonstrate that adults direct attention to the initial portions of words in continuous speech. Since the initial portions of words are particularly important for speech comprehension, attending to the times at which these segments are presented may allow listeners to extract as much information as possible from the overwhelming number of acoustic changes in speech.
The observation that adults direct selective attention to word onsets during continuous speech processing raises questions about the relationship between the ability to allocate attention and language skills in children. There is considerable evidence that language experience impacts the manner in which temporally selective attention is allocated during speech processing. Native Japanese speakers who learn English relatively late (after 12 years of age) do not evidence the difference in N1 amplitude in response to word and syllable onsets in English sentences (Sanders and Neville, 2003b). Further, only listeners who demonstrate high performance on behavioral tests of word recognition in artificial language learning paradigms evidence early processing differences of word and syllable onsets after training (Sanders et al., 2002). Perhaps, then, children with less language experience than adults may not be able to direct attention to the initial portions of words in continuous speech. Conversely, since selective attention is most important for comprehension when listeners are confronted with more information than can be processed in detail, children may have to be even more selective than adults when listening to continuous speech to compensate for other limits on processing rapidly changing information.
The current study examined the use of temporally selective attention during continuous speech processing in 3- to 5-year-old children by adapting the attention probe paradigm previously described by Astheimer and Sanders (2009). Children in this age range were included since they can employ spatially selective attention in a manner that affects auditory perceptual processing (Sanders et al., 2006), but their ability to use temporally selective attention to support language processing is unknown. Children listened to a narrative containing linguistic attention probes presented during acoustically matched portions of the speech stream at various times relative to word onsets: concurrently with, 100 ms before, and 100 ms after word onsets, and at random control times with no systematic relationship to word boundaries. If children, like adults, direct selective attention to the times when the initial segments of words are presented, linguistic probes presented at those times will elicit larger auditory evoked potentials than the same probes presented at other times.
2. Methods
2.1. Participants
Eighteen children (7 females) between the ages of 3 and 5 years (M = 4 years, 4 months) provided data included in analysis. Fourteen of the participants were right-handed, and all were native English speakers with no history of auditory, language, or other neurological disorders. An additional three participants completed the experiment but their data were excluded due to poor EEG quality caused by excessive eye blinks (n = 2) and motion artifacts (n = 1). A parent or guardian of each child provided written informed consent, and all children were compensated with a small toy and a $10 gift card.
2.2. Stimuli
The attention probe paradigm described by Astheimer and Sanders (2009) was adapted for child participants. Six stories from Margaret and H.A. Rey's The Complete Adventures of Curious George were recorded by a female speaker in a child-directed manner. The stories ranged in duration from 4 to 8 min. The entire 44-min recording was divided at natural pauses between sentences or phrase boundaries into 168 10–20 s segments and saved in the left channel of stereo WAV files. Linguistic attention probes were created by extracting a 50 ms excerpt of the narrator pronouncing the syllable “ba.” Three-hundred attention probes were added to the right channel of the narrative in each of four conditions: coincident with a word onset, 100 ms before a word onset, 100 ms after a word onset, and at random control times not systematically associated with a word onset (Fig. 1), for a total of 1200 attention probes. Probe position was also constrained by not presenting more than one probe within 1.5 s. Word onsets were defined as the earliest indication of a new phoneme based on visual inspection of sound waveforms and listening to sentences using a gating procedure. Only onsets that three independent raters identified as occurring at the same time (within 8 ms) were used as anchors for attention probes. The specific word onsets that attention probes were associated with were selected such that (1) all were open-class words important for understanding the content of the story, (2) the words were not in the first 1.5 s or the last .5 s of a sound file, (3) there were not pauses immediately preceding the words, and (4) the acoustic properties of the narrative were similar across conditions. Specifically, there were no significant differences (p's > .20) in average intensity, peak intensity, average pitch, amount of pitch change, or direction of pitch change in the segments of the narrative 100–50 ms before probe onset, 50 ms before probe onset, during the 50 ms probe presentation, or 50–100 ms after probe onset for the four conditions.
Fig. 1.
Experimental paradigm. Children listened to stories with linguistic attention probes (a 50 ms excerpt of the syllable “ba”) presented during acoustically matched portions of the narrative at four times: concurrently with word onsets, 100 ms before word onsets, 100 ms after word onsets, and at random control times. Images corresponding to the story were presented on a computer monitor.
The narrative with attention probes was presented over two Dell speakers placed directly in front of participants and connected to a Dell computer using E-Prime software. The narrative and probes were presented with a peak intensity of 65 dB SPL (A-weighted) measured at the location of participants. Small illustrations from the Curious George stories were presented at the center of a black background on a computer monitor 152 cm in front of the participant, with a new image presented at the beginning of each sound file. Since attention probes were not presented at the very beginning or end of a sound file, they were never presented close in time to the transitions between images. Images were presented at a visual angle of 3.0° such that participants could view an entire photograph without making eye movements.
2.3. Procedure
All procedures were approved by university and department review boards at the University of Massachusetts, Amherst. Participants were fitted with a 128-channel HydroCel Geodesic Sensor Net (Electrical Geodesics, Inc., Eugene OR) containing electrodes imbedded in small sponges soaked in a potassium chloride saline solution. Additional saline was used to maintain impedances at each electrode site below 100 kΩ throughout the experiment. Continuous EEG was recorded at a bandwidth of .01–80 Hz while participants listened to the narrative and viewed illustrations on the monitor. Following each of the six stories, children answered two short-answer comprehension questions on topics ranging from simple recall (i.e. What gift did the man give to George?) to causal explanations (i.e. Why did George have to go to jail?). Although the illustrations displayed during the stories may have improved performance, the questions specifically assessed narrative comprehension and could not be answered based on the images alone. All participants with data included in analysis provided correct answers to at least 80% of the comprehension questions.
EEG was digitized (250 Hz) and segmented into epochs 100 ms before to 800 ms after attention probe onset, with the 100 ms pre-stimulus interval serving as a baseline. Trials containing eye blinks, eye movements, and head movements, as determined by maximum amplitude criteria and visual inspection were excluded from individual subject averages. Only data from participants with at least 50 artifact-free trials in each condition were included.
Mean amplitude was measured in time windows 80–140 ms and 200–350 ms after stimulus onset, based on visual inspection of the waveforms. Measurements were made at 60 electrode sites broadly distributed across the entire scalp and combined into 30 pairs of electrodes in a 5 (Left–Right position, or LR) × 6 (Anterior–Posterior position, or AP) grid (Fig. 2) and entered into 4 (Probe Time) × 5 (LR) × 6 (AP) repeated-measures ANOVAs (Greenhouse-Geisser adjusted). Post-hoc analyses were conducted for all significant (p < .05) main effects and interactions. To further characterize the latency of the observed effects, mean amplitude across electrodes that showed the largest effect of probe time was measured in 50 ms bins staggered by 10 ms. The first of three consecutive bins to show a statistically significant difference between probes at word onset versus control times was considered the onset of the attention effect, and the last bin to show a significant effect was considered the offset. The same latency analysis was applied to data from adults who participated in a similar study (Astheimer and Sanders, 2009) to allow for a direct comparison of the latency of attention effects in adults and children.
Fig. 2.
Electrode array. Approximate scalp location of the 128 recording electrodes. Measurements were taken from the 60 electrodes shown in black and combined into 30 pairs arranged in a 5 (Left/Right position) × 6 (Anterior/Posterior position) grid.
3. Results
3.1. Behavior
Children listened to between 3 and 6 out of the six possible stories (M = 3.68) for a total of 14–32 min. Although the number of comprehension questions completed depended on the number of stories children listened to, proportion correct ranged from .83 to 1.0 (M = .93).
3.2. Event-related potentials
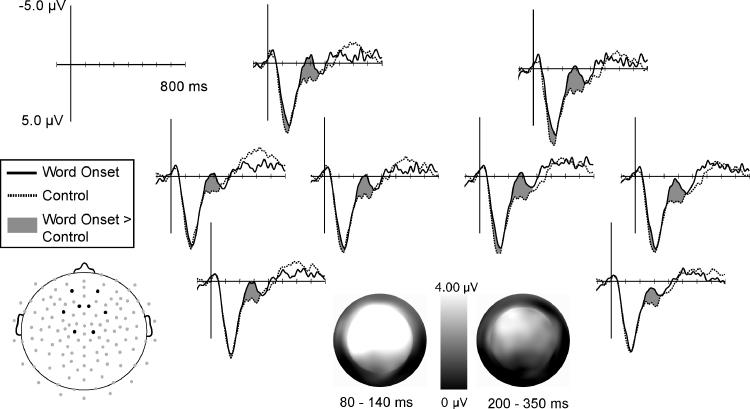
Attention probes elicited a broad positivity that peaked around 115 ms and was largest over left medial, anterior electrodes (LR × AP: F(20,340) = 3.14, p < .01). The observed attention effects in this early time window had a similar, although less lateralized, distribution as the auditory evoked potentials themselves. Probe time modulated mean amplitude 80–140 ms after onset at anterior electrode sites (Time × AP: F(15,255) = 3.27, p < .04). At these anterior electrodes (AP levels 1–4), there was a main effect of probe time (F(3,51) = 3.60, p < .03) such that probes presented at word onset elicited a smaller positivity than probes presented at random times (F(1,17) = 4.54, p ≤ .05; Fig. 3). As shown in Fig. 4, probes presented at word onset also elicited a smaller positivity than probes presented 100 ms before word onset (F(1,17) = 6.792, p < .02). However, there was no difference between probes presented at word onset and 100 ms after word onset in this early time window (p > .25).
Fig. 3.
Word onset versus control times. ERPs elicited by probes presented at word onsets (solid line) and at random control times (dotted line). Data are shown from eight medial, anterior electrode sites indicated on the electrode map. Probes played at word onset elicited a more negative response from 80 to 140 ms and from 200 to 350 ms. Differences in mean amplitude (control minus word onset) in both time windows are shown in the topographic plots.
Fig. 4.
Attention before and after word onsets. ERPs elicited by probes presented at word onsets (solid line), 100 ms before word onsets (dotted line), and 100 ms after word onsets (dashed line). Data are shown from eight medial, anterior electrode sites indicated on the electrode map. During the early positivity, probes presented at and after word onset elicited a more negative waveform than probes played before word onset. During the later time window, probes presented at word onset elicited a more negative response than all other conditions.
A different pattern of results was observed in the 200–350 ms window surrounding the later negativity, which peaked around 250 ms and was largest over all anterior electrodes (AP: F(5,85) = 24.07, p < .001). As seen in the topographic plot in Fig. 3, in this later window the effect of probe time had a more focal, left anterior distribution (Time × LR × AP: F(60,1020) = 2.28, p < .02). Among these electrode sites (LR levels 1–3, AP levels 1–3) there was a main effect of probe time (F(3,51) = 3.52, p < .03) such that probes presented at word onset elicited a larger negativity than probes presented at all other times (Fig. 3, Fig. 4).
Based on the left medial and anterior distribution of the observed attention effects, amplitude was averaged across all electrodes in that region for the latency bin analysis. Among children, the earliest of three consecutive bins with a significant difference between probes at word onset versus control began in the 50–100 ms time window; this difference ended in the 80–130 ms time window (Fig. 5). The difference observed during the late negativity began in the 210–260 ms time window and ended in the 260–310 ms time window. An identical analysis was conducted on adult data from Astheimer and Sanders (2009). The earliest indication of an attention effect began in the 50–100 ms window encapsulating the N1, and ended in the 170–220 ms window following the P2 (Fig. 5).
Fig. 5.
Latency bin analysis. Mean amplitude difference between probes presented at word onset versus control times in staggered 50 ms time windows beginning 0–200 ms after probe onset. Data shown are from left medial and anterior electrode sites (indicated by shaded region on the electrode map) recorded from 3- to 5-year-old children in the current study (gray bars) and adults (black bars) in a similar paradigm (Astheimer and Sanders, 2009). Brackets indicate time windows with significant differences (p < .05) between word onset and control times (upper gray bracket = children in the current study, lower black bracket = adults in previous experiment).
4. Discussion
The current study examined the use of temporally selective attention during narrative comprehension to test whether, like adults, 3- to 5-year-old children direct attention to times that contain word onsets during continuous speech processing. Linguistic attention probes presented within the first 150 ms of a word onset elicited a larger negativity beginning around 50 ms after probe onset compared to probes presented at random control times. These results indicate that children differentially process information presented at times that do and do not include word-initial segments during continuous speech comprehension.
Differences in the pattern of auditory evoked potentials measured in the early and later time windows provide some insight into the manner in which young children direct temporally selective attention during speech processing. As shown in Fig. 4, during the early time window probes presented at and 100 ms after word onset elicited a more negative waveform that was broadly distributed over anterior electrodes. This response matches the pattern of N1 results observed in Astheimer and Sanders (2009) and suggests that, like adults, 3- to 5-year-old children attend not only to word onsets, but to at least the first 100 ms of words. This may be an effective listening strategy given previous evidence that word recognition relies more heavily on word-initial segments (Connine et al., 1993, Marslen-Wilson and Zwitserlood, 1989, Salasoo and Pisoni, 1985). In the later time window, only probes presented at word onset evidenced a larger negativity, which was more focally distributed over left frontal regions (Fig. 3). The distinct pattern of results for the probe-time conditions may reflect a two-step process in which selective attention, as indexed by the early differences in ERP amplitude, is directed to times that include word-initial segments until enough information is gathered to differentiate the word from other possibilities. However, the additional linguistic processing indexed by ERP amplitude in the later time-window (e.g. lexical access) is consistently time-locked to the beginnings of words. Alternatively, the later negativity could reflect a disruption or phonological mismatch when an attention probe is presented concurrently with word onset, similar to the phonological mapping negativity (PMN) observed in adults (Connolly et al., 1992, Connolly and Phillips, 1994). Either interpretation points to the relative importance of the initial portions of words for auditory language comprehension.
The effects of temporally selective attention on auditory evoked potentials in the current study share many similarities with temporally selective attention effects observed in adult studies. Although children show a broad positivity around 100 ms after sound onset rather than the typical adult P1–N1–P2 peaks, probes presented at word onset elicited a larger negativity (or a smaller positivity) between 80 and 140 ms, followed by a larger negativity between 200 and 350 ms. Despite the fact that the attention effects are evident on qualitatively different auditory onset components in children and adults, the latency, polarity, and scalp distribution of the differences between conditions are quite similar for temporally selective attention in the two groups (Astheimer and Sanders, 2009, Lange et al., 2003, Sanders and Astheimer, 2008) and for spatially selective attention in adults (Hansen et al., 1983, Hillyard et al., 1973, Näätänen, 1982, Woldorff et al., 1987). The similarities in the ERP indices indicate that children and adults modulate temporally selective attention during continuous speech processing in a manner that affects auditory perceptual processing in both groups.
Results of the latency analysis provide further support that temporally selective attention affects early perceptual processing during speech comprehension in children, with the earliest differences between conditions observed at the same time (50 ms after onset, as seen in Fig. 5) as previously observed in adults (Astheimer and Sanders, 2009). Although the early latency of this effect in children seems somewhat surprising given their nascent language skills, the difficulty of speech processing in children actually provides a potential explanation for such an early effect. Previous studies of visual temporally selective attention demonstrate earlier-latency attention effects during difficult tasks that include rapidly presented stimuli (Bush and Sanders, 2008) or challenging perceptual discrimination tasks (Correa et al., 2006). Similarly, rapidly speech streams like those used in the current study may necessitate the use of temporally selective attention in a manner that modulates auditory processing at a very early perceptual stage.
The negative attention effect observed in the current study differs in polarity from that reported in previous studies of spatially selective attention in children of the same ages (Sanders et al., 2006, Stevens et al., 2008, Stevens et al., 2006). This unexpected difference in polarity could be interpreted in at least three ways. First, spatially and temporally selective attention may affect auditory evoked potentials in an identical manner regardless of whether one or two stories are presented. If so, the smaller positivity for probes presented concurrently with word onsets observed in the current study would have to be interpreted as children allocating less attention to times that include word initial segments in continuous speech. This interpretation seems highly unlikely since children as young as 18 months of age demonstrate incremental speech processing, such that they are able to recognize words based only on initial segments (Fernald et al., 2001). Second, children could be directing attention to times that contain word onsets in speech, but the electrophysiological indices of spatially and temporally selective attention are distinct. This possibility also seems unlikely since the ERP effects of spatially and temporally selective attention observed in adults are virtually identical (Hillyard et al., 1973, Lange et al., 2003, Näätänen, 1982, Sanders and Astheimer, 2008). More likely, the differences in auditory evoked potentials and the polarity of the attention effects are due to complex interactions between maturity of auditory cortical processing, selective attention, and both the information and sensory density of the acoustic environments.
A growing body of evidence supports the idea of a relationship between the shape of auditory evoked potentials, auditory density, and the polarity of attention effects across development. The latency and amplitude of the typical P1–N1 auditory evoked potentials are not fully developed until the age of 17–18 years. Across development, a broad positivity that dominates the auditory evoked potentials in 5- to 6-year-old children narrows in time and decreases in amplitude as the N1 broadens and becomes more negative (Ponton et al., 2000). Although some indication of an N1 is observable in 6- to 8-year-old children listening to sounds preceded by silence, this waveform is not evident for sounds presented in dense auditory environments that include two simultaneously presented stories and two types of attention probes (Coch et al., 2005, Sanders et al., 2006) suggesting that the N1 peak is highly refractory in children. In these same studies, auditory spatially selective attention resulted in a larger broad positivity for sounds at attended locations. In the current study, children listened to a single story with one type of attention probe presented from a single location, a far sparser auditory environment that may have resulted in a more adult-like negative attention effect in 3- to 5-year-old children.
Regardless of the differences in polarity of the selective attention effects in children, the current study demonstrates that 3- to 5-year-old children modulate temporally selective attention during continuous speech processing. These results contribute to a growing body of evidence demonstrating a link between selective attention and language processing skills in children. Several behavioral studies have reported deficits in various aspects of selective attention in children with dyslexia or specific language impairment (SLI) (Asbjørnsen and Bryden, 1998, Cherry, 1981, Sperling et al., 2005, Ziegler et al., 2005). In the Hillyard spatial attention paradigm, children with SLI show no ERP differences in response to probes presented at the same location as attended and unattended stories (Stevens et al., 2006). However, following a computerized training program designed to improve rapid auditory processing and language skills (Fast ForWord) both language impaired and typically developing children show larger effects of spatially selective attention on auditory evoked potentials (Stevens et al., 2008). The potential causal relationship between training, receptive language skill, and allocation of spatially selective attention needs further exploration. However, our results suggest that another form of selective attention is directly involved in speech processing in children. Temporally selective attention may allow children to process the most relevant acoustic changes in rapidly changing speech signals, thus providing a mechanistic link between selective attention and receptive language processing in children.
5. Conclusions
The results of the present study indicate that, like adults, 3- to 5-year-old children direct attention to times at which word-initial segments are presented in continuous speech, enhancing perceptual processing of these critical sounds. Although the use of temporally selective attention across development and across individuals with varying language abilities needs to be explored, the current results raise the possibility that temporally selective attention is necessary for efficient speech processing. It may therefore be beneficial to design treatments for receptive language impairments that explicitly train selective attention. Understanding the cues that children and adults employ to direct attention to word onsets in continuous speech will be essential for making interventions designed to improve language processing across the lifespan as efficient as possible.
Acknowledgments
This study was funded by a John Merck Scholars Fellowship to L.D.S. to study the Biology of Developmental Disabilities in Children. We thank Mara Breen, Lindsay Demers, and Benjamin Zobel for assistance with data collection, and two anonymous reviewers for their comments on an earlier version of this manuscript.
References
- Asbjørnsen A.E., Bryden M.P. Auditory attentional shifts in reading-disabled students: quantification of attentional effectiveness by the Attentional Shift Index. Neuropsychologia. 1998;36(2):143–148. doi: 10.1016/s0028-3932(97)00090-0. [DOI] [PubMed] [Google Scholar]
- Astheimer L., Sanders L. Listeners modulate temporally selective attention during natural speech processing. Biol. Psychol. 2009;80(1):23–34. doi: 10.1016/j.biopsycho.2008.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman S., Friedman D. The development of selective attention as reflected by event-related brain potentials. J. Exp. Child Psychol. 1995;59(1):1–31. doi: 10.1006/jecp.1995.1001. [DOI] [PubMed] [Google Scholar]
- Bush W., Sanders L. Temporally selective attention modulates early perceptual processing of images presented in rapidly changing streams. Poster Presented at the 15th Annual Meeting of the Cognitive Neuroscience Society, April; San Francisco, CA ; 2008. [Google Scholar]
- Cherry E. Some experiments on the recognition of speech, with one and two ears. J. Acoust. Soc. Am. 1953;25:975–979. [Google Scholar]
- Cherry R.S. Development of selective auditory attention skills in children. Percept. Mot. Skills. 1981;52(2):379–385. doi: 10.2466/pms.1981.52.2.379. [DOI] [PubMed] [Google Scholar]
- Coch D., Sanders L., Neville H. An event-related potential study of selective auditory attention in children and adults. J. Cogn. Neurosci. 2005;17(4):605–622. doi: 10.1162/0898929053467631. [DOI] [PubMed] [Google Scholar]
- Connine C., Blasko D., Titone D. Do the beginnings of spoken words have a special status in auditory word recognition? J. Mem. Lang. 1993;32(2):193–210. [Google Scholar]
- Connolly J.F., Phillips N.A., Stewart S.H., Brake W.G. Event-related potential sensitivity to acoustic and semantic properties of terminal words in sentences. Brain Lang. 1992;43(1):1–18. doi: 10.1016/0093-934x(92)90018-a. [DOI] [PubMed] [Google Scholar]
- Connolly J.F., Phillips N.A. Event-related potential components reflect phonological and semantic processing of the terminal word of spoken sentences. J. Cogn. Neurosci. 1994;6(3):256–266. doi: 10.1162/jocn.1994.6.3.256. [DOI] [PubMed] [Google Scholar]
- Correa A., Lupiáñez J., Madrid E., Tudela P. Temporal attention enhances early visual processing: a review and new evidence from event-related potentials. Brain Res. 2006;1076(1):116–128. doi: 10.1016/j.brainres.2005.11.074. [DOI] [PubMed] [Google Scholar]
- Coull J., Frith C., Buchel C., Nobre A. Orienting attention in time: behavioural and neuroanatomical distinction between exogenous and endogenous shifts. Neuropsychologia. 2000;38(6):808–819. doi: 10.1016/s0028-3932(99)00132-3. [DOI] [PubMed] [Google Scholar]
- Coull J., Nobre A. Where and when to pay attention: the neural systems for directing attention to spatial locations and to time intervals as revealed by both PET and fMRI. J. Neurosci. 1998;18(18):7426–7435. doi: 10.1523/JNEUROSCI.18-18-07426.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernald A., Swingley D., Pinto J.P. When half a word is enough: infants can recognize spoken words using partial phonetic information. Child Dev. 2001;72(4):1003–1015. doi: 10.1111/1467-8624.00331. [DOI] [PubMed] [Google Scholar]
- Griffin I., Miniussi C., Nobre A. Multiple mechanisms of selective attention: differential modulation of stimulus processing by attention to space or time. Neuropsychologia. 2002;40(13):2325–2340. doi: 10.1016/s0028-3932(02)00087-8. [DOI] [PubMed] [Google Scholar]
- Hansen J., Dickstein P., Berka C., Hillyard S. Event-related potentials during selective attention to speech sounds. Biol. Psychol. 1983;16(3–4):211–224. doi: 10.1016/0301-0511(83)90025-x. [DOI] [PubMed] [Google Scholar]
- Hillyard S., Hink R., Schwent V., Picton T. Electrical signs of selective attention in the human brain. Science. 1973;182(108):177–180. doi: 10.1126/science.182.4108.177. [DOI] [PubMed] [Google Scholar]
- Hink R., Hillyard S. Auditory evoked potentials during listening to dichotic speech messages. Percept. Psychophys. 1976;20:236–242. [Google Scholar]
- Hink R., Hillyard S., Benson P. Event-related brain potentials and selective attention to acoustic and phonetic cues. Biol. Psychol. 1978;6(1):1–16. doi: 10.1016/0301-0511(78)90002-9. [DOI] [PubMed] [Google Scholar]
- Hink R., Van Voorhis S., Hillyard S., Smith T. The division of attention and the human auditory evoked potential. Neuropsychologia. 1977;15(4–5):597–605. doi: 10.1016/0028-3932(77)90065-3. [DOI] [PubMed] [Google Scholar]
- Kannass K.N., Oakes L.M., Shaddy D.J. A longitudinal investigation of the development of attention and distractibility. J. Cogn. Dev. 2006;7(3):381–409. [Google Scholar]
- Lange K., Rösler F., Röder B. Early processing stages are modulated when auditory stimuli are presented at an attended moment in time: an event-related potential study. Psychophysiology. 2003;40(5):806–817. doi: 10.1111/1469-8986.00081. [DOI] [PubMed] [Google Scholar]
- Marslen-Wilson W., Zwitserlood P. Accessing spoken words: the importance of word onsets. J. Exp. Psychol. Hum. Percept. Perform. 1989;15(3):576–585. [Google Scholar]
- Näätänen R. Processing negativity: an evoked-potential reflection of selective attention. Psychol. Bull. 1982;92(3):605–640. doi: 10.1037/0033-2909.92.3.605. [DOI] [PubMed] [Google Scholar]
- Näätänen R., Michie P. Early selective-attention effects on the evoked potential: a critical review and reinterpretation. Biol. Psychol. 1979;8(2):81–136. doi: 10.1016/0301-0511(79)90053-x. [DOI] [PubMed] [Google Scholar]
- Ponton C.W., Eggermont J.J., Kwong B., Don M. Maturation of human central auditory system activity: evidence from multi-channel evoked potentials. Clin. Neurophysiol. 2000;111(2):220–236. doi: 10.1016/s1388-2457(99)00236-9. [DOI] [PubMed] [Google Scholar]
- Salasoo A., Pisoni D. Interaction of knowledge sources in spoken word identification. J. Mem. Lang. 1985;24(2):210–231. doi: 10.1016/0749-596X(85)90025-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders L., Astheimer L. Temporally selective attention modulates early perceptual processing: event-related potential evidence. Percept. Psychophys. 2008;70(4):732–742. doi: 10.3758/pp.70.4.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders L., Neville H. An ERP study of continuous speech processing I. Segmentation, semantics, and syntax in native speakers. Brain Res. Cogn. Brain Res. 2003;15(3):228–240. doi: 10.1016/s0926-6410(02)00195-7. [DOI] [PubMed] [Google Scholar]
- Sanders L., Neville H. An ERP study of continuous speech processing II. Segmentation, semantics, and syntax in non-native speakers. Brain Res. Cogn. Brain Res. 2003;15(3):214–227. doi: 10.1016/s0926-6410(02)00194-5. [DOI] [PubMed] [Google Scholar]
- Sanders L., Newport E., Neville H. Segmenting nonsense: an event-related potential index of perceived onsets in continuous speech. Nat. Neurosci. 2002;5(7):700–703. doi: 10.1038/nn873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders L., Stevens C., Coch D., Neville H. Selective auditory attention in 3- to 5-year-old children: an event-related potential study. Neuropsychologia. 2006;44(11):2126–2138. doi: 10.1016/j.neuropsychologia.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Sperling A.J., Lu Z., Manis F.R., Seidenberg M.S. Deficits in perceptual noise exclusion in developmental dyslexia. Nat. Neurosci. 2005;8(7):862–863. doi: 10.1038/nn1474. [DOI] [PubMed] [Google Scholar]
- Stevens C., Fanning J., Coch D., Sanders L., Neville H. Neural mechanisms of selective auditory attention are enhanced by computerized training: electrophysiological evidence from language-impaired and typically developing children. Brain Res. 2008;1205:55–69. doi: 10.1016/j.brainres.2007.10.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens C., Sanders L., Neville H. Neurophysiological evidence for selective auditory attention deficits in children with specific language impairment. Brain Res. 2006;1111(1):143–152. doi: 10.1016/j.brainres.2006.06.114. [DOI] [PubMed] [Google Scholar]
- Teder-Salejarvi W., Hillyard S., Röder B., Neville H. Spatial attention to central and peripheral auditory stimuli as indexed by event-related potentials. Brain Res. Cogn. Brain Res. 1999;8(3):213–227. doi: 10.1016/s0926-6410(99)00023-3. [DOI] [PubMed] [Google Scholar]
- Woldorff M., Hansen J., Hillyard S. Evidence for effects of selective attention in the mid-latency range of the human auditory event-related potential. Electroencephalogr. Clin. Neurophysiol. Suppl. 1987;40:146–154. [PubMed] [Google Scholar]
- Woods D.L., Alain C. Feature processing during high-rate auditory selective attention. Percept. Psychophys. 1993;53(4):391–402. doi: 10.3758/bf03206782. [DOI] [PubMed] [Google Scholar]
- Woods D.L., Alho K., Algazi A. Brain potential signs of feature processing during auditory selective attention. Neuroreport. 1991;2(4):189–192. doi: 10.1097/00001756-199104000-00007. [DOI] [PubMed] [Google Scholar]
- Ziegler J.C., Pech-Georgel C., George F., Alario F., Lorenzi C. Deficits in speech perception predict language learning impairment. Proc. Natl. Acad. Sci. U.S.A. 2005;102(39):14110–14115. doi: 10.1073/pnas.0504446102. [DOI] [PMC free article] [PubMed] [Google Scholar]