Highlights
* ERP correlates of individual differences in task switching were examined. * Children who switched flexibly had smaller N2 amplitudes than those who perseverated. * Reflection may allow children to resolve the conflict inherent in bivalent stimuli. * Research shows that children's executive function predicts academic outcomes. * Reflection may transform the way in which learning occurs.
Keywords: Executive function, Cognitive flexibility, Conflict monitoring, Electrophysiology
Abstract
To explore the neurocognitive mechanisms underlying individual differences in executive function during the preschool years, high-density electroencephalography (EEG) was used to record event-related potentials (ERPs) from 99 children (between 35 and 54 months of age) during performance on the Dimensional Change Card Sort (DCCS), a widely used measure of executive function in which participants are required to sort bivalent stimuli first by one dimension and then by another. ERP analyses comparing children who switched flexibly (passed) to those who perseverated on post-switch trials (failed) focused on the N2 component, which was maximal over fronto-central sites. N2 amplitude was smaller (less negative) for children who passed the DCCS than for children who failed, suggesting that the N2, often associated with conflict monitoring, may serve as a neural marker of individual differences in executive function. Implications for learning and education are discussed.
Executive function refers to the deliberate, top-down neurocognitive processes involved in the regulation of thought, action, and emotion – processes such as cognitive flexibility, inhibitory control, and working memory (Miyake et al., 2000). Individual differences in executive function in childhood have been found to predict important developmental outcomes, including math and reading skills in preschool and the early school grades (e.g., Blair and Razza, 2007), and SAT scores in adolescence (e.g., Shoda et al., 1990). Indeed, executive function is often a better predictor of achievement than is IQ, and teachers often report that the most important determinant of classroom success in kindergarten and early school grades is the extent to which children can sit still, pay attention, and follow rules (e.g., McClelland et al., 2007).
Children with poor executive function may be at a disadvantage in educational contexts for a number of reasons, including poor attention, poor emotional control, an increased likelihood of causing behavioral disruptions, and teachers’ diminished expectations of children's success. From a cognitive perspective, however, it has also been suggested that children with better executive function may approach learning opportunities in a more reflective, self-directed way that allows them to be goal-directed and proactive in seeking out new information instead of learning in a more passive, incremental fashion (Marcovitch et al., 2008). For example, children who are more likely to reflect upon and monitor their own knowledge may display greater cognitive flexibility and be better able to override the influence of habits or predispositions that interfere with learning.
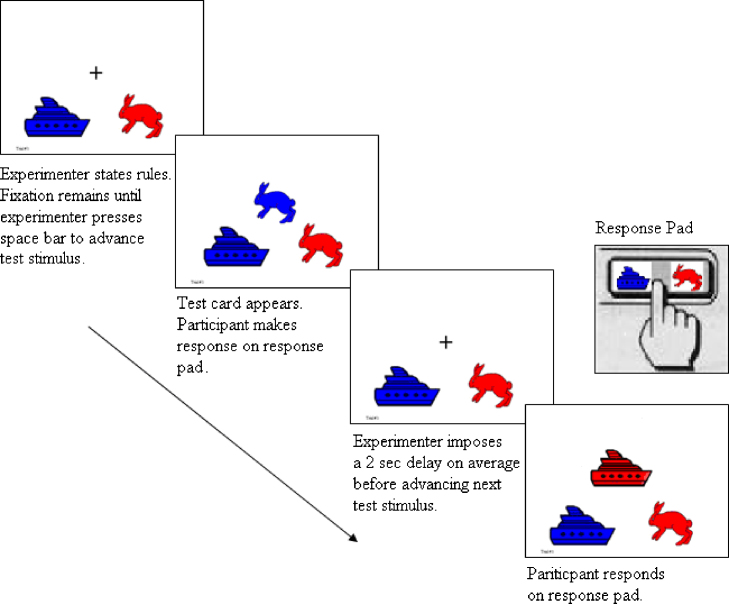
It is now well known that executive function undergoes particularly marked changes between the ages of 3 and 6 years (Zelazo et al., in press), just as children face sharp increases in the demands placed on their self-regulation (e.g., as they transition to school). A widely used measure of executive function during these years of rapid change is the Dimensional Change Card Sort (DCCS; Zelazo, 2006; see Fig. 1). In the standard version of this task, children are shown two target cards (e.g., a blue rabbit and a red boat) and asked to sort a series of bivalent test cards (e.g., red rabbits and blue boats) first according to one dimension (e.g., color) and then according to the other (e.g., shape). Regardless of which dimension is presented first, most 3-year-olds sort correctly on the pre-switch trials but then perseverate during the post-switch trials, continuing to sort test cards by the first dimension despite being told the new rules on every trial, and despite correctly answering questions about the post-switch rules. By 5 years of age, most children switch flexibly (e.g., Bialystok, 1999, Bohlmann and Fenson, 2005, Brace et al., 2006, Diamond et al., 2005, Dick et al., 2005, Kirkham et al., 2003, Kloo and Perner, 2005, Munakata and Yerys, 2001, Zelazo et al., 2003). The DCCS shows excellent test-retest reliability during this age range (ICCs = .90–.94; Beck et al., 2011).
Fig. 1.
Sequence of events for the computerized version of the Dimensional Change Card Sort.
How best to explain children's performance on the DCCS is currently a matter of debate. According to the Cognitive Complexity and Control theory-revised (CCC-r; Zelazo et al., 2003), for example, children who perseverate on the DCCS have difficulty reflecting on their rule representations and formulating a hierarchical rule system that resolves the conflict inherent in the bivalent stimuli. The rapid development of self-reflection during the preschool years allows children to understand that they know two different ways of approaching the task: “If I’m sorting by color, then the red rabbits go here; but if I’m sorting by shape, then they go there.” This approach suggests that performance on the DCCS should provide a measure of self-reflection, or monitoring one's rule knowledge, that will predict the efficiency of later learning. In contrast to the CCC-r theory, other approaches emphasize other cognitive processes, such as the need to maintain rules in working memory (e.g., Morton and Munakata, 2002) or to inhibit attention to pre-switch rules (e.g., Kirkham et al., 2003).
Regardless of how one characterizes the processes that make it possible for children to switch on the DCCS, however, there is general agreement that these processes depend importantly on neural networks involving lateral prefrontal cortex, as shown in several recent neuroimaging studies using the DCCS (Moriguchi and Hiraki, 2009, Morton et al., 2009, Waxer and Morton, 2011a). In the single study to date to examine preschoolers, Moriguchi and Hiraki (2009) used near-infrared spectroscopy (NIRS) to measure the concentration of oxygenated hemoglobin (oxy-Hb) in ventrolateral prefrontal cortex during performance on the task. Following presentation of the test cards, both 5-year-olds and those 3-year-olds who switched flexibly on the task showed an increase in oxy-Hb bilaterally, whereas 3-year-olds who failed the task did not.
Whereas lateral prefrontal cortex may mediate switching on the DCCS, performance on the task likely involves multiple cognitive processes (Waxer and Morton, 2011b). In a study with older children (9–11 years), adolescents, and adults that used a mixed block version of the DCCS (with switch trials followed by three repeat trials), Waxer and Morton (2011b) assessed the role of conflict processing by comparing performance on trials involving the usual bivalent test stimuli that could be sorted by either color or shape to trials involving univalent test stimuli that only matched one target stimulus. High-density (128-channel) electroencephalography (EEG) was used to record event-related potentials (ERPs), and the authors found that the N2 component of the stimulus-locked ERP was larger on bivalent than on univalent trials at all ages (although the authors referred to the second negative-going deflection as the N4 in children given its longer latency).
The N2 component of the ERP is usually observed at medial-frontal sites between 200 and 400 ms (with a longer latency for children than for adults) following stimulus presentation on various measures of executive function, including Go–Nogo tasks (e.g., Eimer, 1993, Falkenstein et al., 1999, Jodo and Kayama, 1992, Jonkman et al., 2003, Lamm et al., 2006) and versions of the Eriksen flanker task (e.g., Forster et al., 2011, Rueda et al., 2005, van Veen and Carter, 2002). The amplitude of the N2 has been found to vary as a function of conflict and the need for cognitive control. For example, amplitudes are larger (i.e., more negative) on Nogo trials than on Go trials in Go–Nogo tasks, they are larger when discriminability between Nogo and Go stimuli is difficult (see Folstein and van Petten, 2008, for review), and they increase as a function of target-distractor compatibility on a flanker task (Forster et al., 2011). For these and other reasons, the N2 is often taken as an index of executive function in general and conflict monitoring in particular (e.g., Botvinick et al., 2001, Botvinick, 2007, Lahat et al., 2010, Lamm et al., 2006, Nieuwenhuis et al., 2003, Rueda et al., 2004, Waxer and Morton, 2011a, Yeung and Nieuwenhuis, 2009). Conflict monitoring may initiate processes associated with lateral prefrontal cortex (e.g., Botvinick et al., 2001, Gehring and Knight, 2000, Ridderinkhof et al., 2004), such as higher order rule use (e.g., Bunge and Zelazo, 2006) or the representation of attention-guiding rules (e.g., Waxer and Morton, 2011a).
The current study used EEG to examine conflict-related processing during a standard version DCCS in preschool age children. In particular, the N2 component of the ERP was examined in relation to children's performance (passing vs. failing) on the post-switch phase of the task. In contrast to Go–Nogo tasks, in which greater conflict may be expected on Nogo versus Go trials (e.g., Crone et al., 2006), the standard version of the DCCS has the potential to elicit conflict on all trials (i.e., both pre- and post-switch trials) because all trials involve bivalent stimuli, which pull for competing response options. Source analysis of the N2 in adults and children has suggested cortical generators in both cingulate cortex and right orbitofrontal cortex (Bokura et al., 2001, Jonkman et al., 2007, Lewis et al., 2006, Lewis et al., 2008, Nieuwenhuis et al., 2003; for review, see van Veen and Carter, 2002), although in children the cingulate sources may be relatively posterior compared to older adolescents and adults (Lamm et al., 2006). Developmental research on the N2 has generally shown a decrease with age in both amplitude and latency (Davis et al., 2003, Johnstone et al., 2005, Johnstone et al., 2007, Jonkman, 2006, Jonkman et al., 2003, Lewis et al., 2006, Rueda et al., 2004), and individual differences in amplitude, in particular, appear to be related to executive function in school-age children and adolescents (Lamm et al., 2006).
A prediction derived from CCC-r theory is that children who understand the hierarchical structure of the task and effectively resolve the conflict inherent in the bivalent stimuli should not only switch flexibly but also show smaller N2 amplitudes on pre- and post-switch trials, consistent with the down-regulation of ACC-mediated conflict detection via top-down control (e.g., Forster et al., 2011). Children who fail to understand the hierarchical structure of the task should not only perseverate on the task but also display evidence of unresolved conflict between the competing affordances of the bivalent stimuli, manifested as larger N2 amplitudes. A similar prediction could be made on the basis of accounts emphasizing working memory (e.g., Morton and Munakata, 2002). According to inhibitory control accounts (e.g., Kirkham et al., 2003), however, children who perseverate on the DCCS understand the hierarchical structure of the task but simply cannot inhibit attention to the pre-switch rules on the post-switch phase (i.e., once the pre-switch rules have become prepotent through use). Therefore, inhibitory control accounts would presumably predict that compared to children who switch, children who perseverate should only experience higher levels of response conflict (and higher N2 amplitudes) on the post-switch phase, but not on the pre-switch phase. Do children who switch versus perseverate on the DCCS differ in N2 amplitude, and do these differences appear on the pre-switch phase, the post-switch phase, or both?
1. Method
1.1. Participants
The final sample included 99 children (36 males; aged 35–54 months: M age = 41.79; SD = 4.29). These children were predominantly (82%) Caucasian, from middle to upper-middle class backgrounds. Parents and their children were recruited through a database of parents who had expressed interest in participating in studies and through advertisements posted in the community. An additional 42 children (20 males; M age = 40.24, SD = 4.37) were tested but excluded from the final sample for the following reasons: (a) they failed to perform better than chance on the pre-switch phase of the DCCS and/or did not unambiguously pass or fail the post-switch phase (see pass/fail criteria below; n = 24); (b) they were inattentive and moved or vocalized excessively (n = 10); (c) their ERP data did not contain 15 or more usable post-switch trials (n = 8). Exclusion of all participants was carried out by an experimenter blind to children's pass/fail status.
1.2. Procedure
Children were tested individually (with their parent present) in a room decorated according to a space theme. They were shown an electrode sensor net, referred to as a “space hat”, and informed that they were going to drive a rocket ship once the experimenter put on the hat. When children were comfortable, the net was applied and children were seated 45 cm in front of a 17″ computer monitor, next to an experimenter. Overhead lights were turned off, and the room was illuminated by a 100-W lamp located 200 cm away from the monitor. Children were asked to sit as silently and as still as possible throughout the session. Children were administered a computerized-version of the DCCS.
The Dimensional Change Card Sort (DCCS). The DCCS was adapted from the standard version (Zelazo, 2006). Shape was always the pre-switch dimension and color was the post-switch dimension. On each trial, a test stimulus (blue rabbit or red boat) was presented at a central location above and between two target stimuli (blue boat on the left and red rabbit on the right). Test stimuli remained onscreen until children responded by pressing (using the index finger of their dominant hand) one of two laminated replicas of the target stimuli that were affixed to buttons on a response pad (see Fig. 1). After a response, the test stimulus disappeared while a fixation-cross appeared until the next trial. The experimenter initiated the next trial by pressing the space bar on a keyboard after a minimum of 1000 ms, with an average inter-trial interval of 2668.19 ms (SD = 418.86). Stimuli were presented in the same pseudo-random order for all children, such that a particular stimulus was never presented more than twice in a row and each of the two stimulus types (blue rabbit and red boat) were presented equally often on the post-switch phase.
To begin, children were given six practice trials. The pre-switch rules were stated before each of these trials. On the first practice trial, the experimenter demonstrated a correct response, emphasizing the importance of pressing gently and quietly. Children were then asked to respond without assistance on the five remaining practice trials and were rewarded with a sticker on each trial that they sat still, remained silent, and pressed the correct button without extraneous hand movements. If children hesitated or were unsure of the correct response, the experimenter pointed to the correct button. The practice trials were designed to ensure that children understood the task and practiced responding in an efficient fashion. Children then received 15 pre-switch and 30 post-switch trials that were administered in the same way as the last 5 training trials except that (a) children did not receive stickers or feedback of any kind, (b) children were only told the relevant rules on the initial trial of each phase and every 5th trial thereafter, and (c) if children hesitated, the experimenter provided only general encouragement to respond (and never pointed to the correct button). Between the pre- and post-switch phases, children were explicitly told to switch: “Now we're going to play a new game – the color game – and the color game is different. In this game if you see a blue one, press this one [the experimenter pointed to the correct button], but if you see a red one, press that one [the experimenter pointed to the correct button]. Okay?”
EEG Testing. EEG data were recorded using a 128-channel Geodesic Sensor Net (Tucker, 1993). Data were sampled at 250 Hz and recorded using NetStation 4.1.2 (EGI Software: EGI, Eugene, OR). Impedances were kept below 80 kΩ at the beginning of the session. All channels were referenced to Cz during recording and later rereferenced to an average reference. An FIR 1–30 Hz bandpass filter was applied. Data were stimulus-locked to the onset of the test stimulus. Segments were based on 400 ms pre-stimulus to 700 ms post-stimulus. For each segment, channels were marked as bad and replaced through interpolation of neighboring electrodes if the fast average amplitude exceeded 200 μV; the differential average amplitude exceeded 100 μV; and/or the channel had zero variance. Ocular artifacts were corrected using the ocular artifact removal tool in Net Station. The EOG sensitivity, which uses the slope of the eye blink to differentiate eye blinks from eye movements so that separate correction factors can be applied to every EEG channel (EGI; NetStation Waveforms Technical Manual, 2006), was set at 1.35 μV. This sensitivity level produced a good balance between rejection of unusable segments and preservation of segments relatively unaffected by artifacts. Data for each child were also individually inspected to ensure that the algorithms used were appropriate, with good trials included and bad trials excluded. Finally, segments were excluded if they contained more than 25 bad channels and/or eye blinks or eye movements (using a 100 μV threshold). As a result of the artifact detection process, 27% of pre-switch segments and 30% of post-switch segments were rejected (similar to rejection levels seen in previous research with young children, e.g., Rueda et al., 2004). Averaged ERPs from individual children were grand-averaged and adjusted using the 200 ms prior to stimulus as baseline.
As is common in research with young children (e.g., Lewis et al., 2006, Lewis et al., 2007, Rueda et al., 2004, Rueda et al., 2005, Todd et al., 2008), amplitude of the N2 was calculated based on the peak within the coded region (300–500 ms post-stimulus) that (a) occurred after the N1 and (b) had a fronto-central topography. Latency was calculated as the time in milliseconds from stimulus onset to peak amplitude. An experimenter blind to participants’ pass/fail status carried out the coding of the ERPs.
2. Results
2.1. Preliminary analyses
By design, all children included in the final sample passed the pre-switch phase (p ∼ .05, based on the binomial theorem), sorting correctly on 11 or more trials out of 15 (M = 13.92, SD = 1.17). Preschoolers’ post-switch performance on the DCCS is typically bimodal, with children either clearly passing or clearly failing, although the inclusion in the current version of many more trials than is usual, as well as the presence of the sensor net, was expected to reveal intermediate patterns of responding and/or increase the likelihood of occasional random responding due to distraction. Children were classified as passing the DCCS as a whole (i.e., passing the post-switch phase) if they were correct on 20 or more trials out of 30 on the post-switch phase (p < .05), and children were classified as failing the DCCS if they made 20 or more errors on the post-switch phase (p < .05). Altogether, 45 children passed, with an average of 26.38 (SD = 2.53) post-switch trials correct, whereas 54 children failed, making an average of 26.83 (SD = 2.55) post-switch errors.
Preliminary analyses did not reveal any significant differences between children who passed and children who failed the DCCS in terms of sex, ethnicity, mean number of pre-switch errors, median pre- or post-switch reaction times, or mean number of trials contributing to the ERP waveforms for the pre- or post-switch phases. Subsequent analyses collapsed across these variables. Children who passed were significantly older than children who failed, however, by approximately 3 months (passers: M = 43.40 months, SD = 4.11; failers: M = 40.44 months, SD = 3.99, F(1, 97) = 3.62, p < .01), so age was used as a covariate in the following analyses.
2.2. ERP analyses
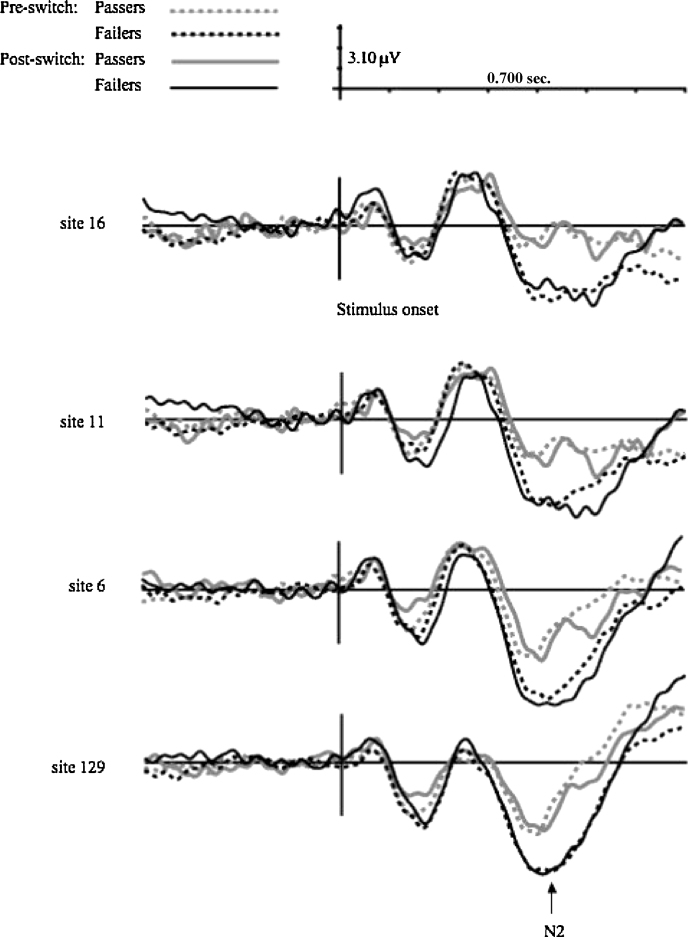
Analyses of ERPs during the post-switch trials were based on correct trials for children who switched (passers) and incorrect trials for children who perseverated (failers). The mean number of trials contributing to the post-switch N2s was 20.27 (SD = 4.22), comparable to trial counts used in previous neurodevelopmental research (e.g., Rueda et al., 2005, Todd et al., 2008). In order to increase the ratio of ERP signal to noise, however, we employed a much larger sample of participants than is typical. N2 amplitudes were examined at four frontal midline sites including sites 129, 6, 11, and 16, corresponding roughly to Cz, Fcz, Fz, and Afz respectively (Luu and Ferree, 2000), across which amplitudes were maximal. See Fig. 2 for grand-averaged waveforms at these sites, and Table 1 for mean N2 amplitudes for passers and failers.
Fig. 2.
Grand-averaged pre-switch and post-switch ERPs at sites: 129, 6, 11, and 16, for children who passed vs. failed the post-switch phase of the Dimensional Change Card Sort.
Table 1.
Mean N2 Amplitude (μV) and (SDs) at frontal-midline sites for children who passed vs. failed the post-switch phase of the DCCS.
| Phase | Site | Passers | Failers |
|---|---|---|---|
| Pre-switch | 129 | −5.51 (4.94) | −9.04 (6.10) |
| 6 | −6.46 (5.41) | −10.68 (7.55) | |
| 11 | −7.08 (6.16) | −10.35 (6.71) | |
| 16 | −6.52 (6.28) | −9.62 (6.35) | |
| Post-switch | 129 | −5.02 (4.25) | −7.73 (4.17) |
| 6 | −5.66 (4.84) | −8.13 (4.82) | |
| 11 | −5.75 (5.52) | −7.70 (6.07) | |
| 16 | −5.19 (5.74) | −7.56 (6.33) |
2.3. N2 amplitude
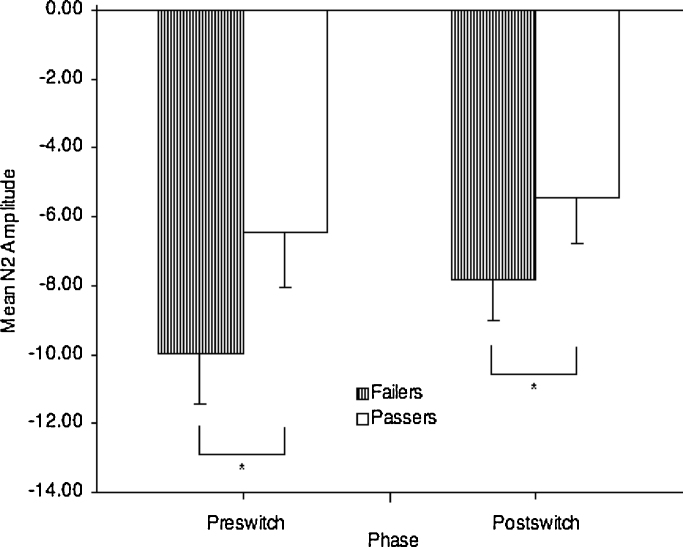
Post-switch N2 amplitudes (μV) at each of the four fronto-central sites were examined via a repeated measures analysis with performance status (passers vs. failers) as a between-subjects variable and age (in months) as a covariate. Results revealed a significant effect of performance status, F(1, 96) = 4.92, p = .03, , shown in Fig. 3. Univariate results were significant at three sites: site 129, F(1, 96) = 6.22, p = .01, , site 6, F(1, 96) = 3.82, p = .05, , and site 16, F(1, 96) = 3.93, p = .05, , but not at site 11, F(1, 96) = 2.38, p = .13, , with smaller N2 amplitudes for passers. Age was not a significant covariate, F(1, 96) < 1.00, and the same pattern of results was obtained when trial count or N2 latency was included as a covariate.
Fig. 3.
Mean pre-switch and post-switch N2 amplitudes across sites 129, 6, 11, and 16 for passers and failers (‘*’ indicates significant at p < .05; error bars represent 95% confidence intervals).
The mean number of pre-switch trials available for analysis was only 10.79 (SD = 2.48), so analysis of ERPs on pre-switch trials should be considered exploratory. Despite the low trial count, however, a repeated measures analysis (sites) with performance status (passers vs. failers) as a between-subjects variable and age (in months) as a covariate revealed a significant effect of performance status F(1, 96) = 10.71, p < .01. Univariate results were significant at all four sites: site 129, F(1, 96) = 7.95, p < .01, ; site 6, F(1, 96) = 8.67, p < .01, ; site 11, F(1, 96) = 8.64, p < .01, ; and site 16, F(1, 96) = 9.12, p < .01, , with smaller pre-switch amplitudes for passers. Age was not a significant covariate, F(1, 96) < 1.00, and the same pattern of results was obtained when trial count or N2 latency was included as a covariate.
2.4. N2 latencies
For all children, the peak of the N2 considered across both the pre-switch and post-switch phases and across all four sites occurred at 438.29 (SD = 48.40) ms post-stimulus. To determine whether N2 latencies differed between children who passed and children who failed the DCCS, a set of repeated measures analyses analogous to those used to examine amplitude were carried out on N2 latencies (i.e., repeated measures analyses with sites as a within-subjects variable, performance status as a between-subjects variable, and age as a covariate). Results revealed no significant effects of performance status at any site on either the pre- or the post-switch phase, all Fs(1, 96) < 2.55, p > .12 (see Table 2 for means and standard deviations). Age was not a significant covariate, F(1, 96) < 1.00, and the same pattern of results was obtained when trial count was included as a covariate.
Table 2.
Mean N2 latency (ms) and (SD) for children who passed vs. failed the post-switch phase of the DCCS.
| Phase | Site | Passers | Failers |
|---|---|---|---|
| Pre-switch | 129 | 427.11 (67.59) | 426.67 (68.42) |
| 6 | 452.27 (65.04) | 444.96 (71.16) | |
| 11 | 457.60 (70.86) | 447.78 (77.93) | |
| 16 | 444.53 (74.80) | 454.59 (79.85) | |
| Post-switch | 129 | 406.76 (59.04) | 427.11 (54.87) |
| 6 | 418.31 (61.91) | 435.56 (59.19) | |
| 11 | 442.84 (74.41) | 444.59 (66.31) | |
| 16 | 433.69 (75.53) | 444.37 (62.95) |
2.5. N2 topography and source analysis
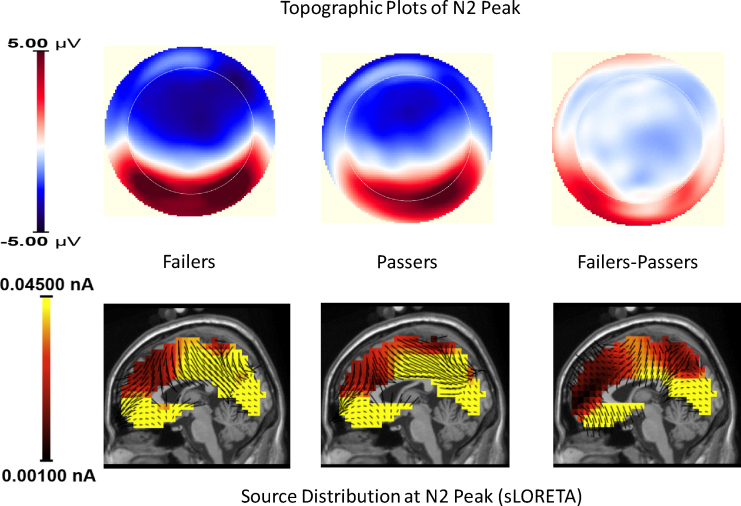
The topography of the N2 is shown separately for passers and failers in Fig. 4 (top row). Also shown is the topography of a difference wave created by subtracting the peak N2 amplitudes for passers from the peak N2 amplitudes for failers at each electrode site. This difference wave captures potential differences between failers and passers in N2 topography. As can be seen, there was a negativity across a broad fronto-central region, and differences between failers and passers were located within this region. EEG source imaging was performed using the standardized low resolution brain electromagnetic tomography (sLORETA) method (Pascual-Marqui, 2002), as implemented in the NetStation GeoSource 2.0 software package. This method relies on a Sun-Stok 4-shell Sphere head model with a Tikhonov regularization of 1 × 10−4. Results are shown in Fig. 4 (bottom), separately for failers and passers, and for the difference wave, which allowed us to focus on the sources of scalp electrical activity that differentiated children who passed versus failed the DCCS. As can be seen in the bottom right panel, there were several frontal and cingulate cortical source activations that likely contributed to the N2 differences. These sources included: ventral anterior cingulate cortex (BA 25, 24, and 32), central cingulate cortex (BA 23 and 31), posterior cingulate cortex (BA 29 and 30), medial orbitofrontal cortex (BA 11), and lateral orbitofrontal cortex (BA 47).
Fig. 4.
Topographic maps of the N2 at the latency of peak amplitude are presented for Failers, Passers, and the difference wave of Failers minus Passers. The scalp voltages were submitted to sLORETA, and the resulting source activations for each group are plotted below their respective topographic map.
The results of source analyses should always be interpreted with caution, and this is especially true when the analyses are based on EEG data from preschool age children because these data tend to be more variable than those of older participants and because there is no age-appropriate head model (i.e., model of how neural activation propagates from neural sources to the scalp). See Luck (2005) and Michel et al. (2004) for thoughtful reviews of EEG source analysis methodology.
3. Discussion
Compared to children who perseverated on the DCCS, children who switched flexibly on the DCCS showed smaller N2 amplitudes during the post-switch phase of the task. Exploratory analyses revealed that children who switched also showed smaller N2 amplitudes on pre-switch trials. Source analysis of the N2 suggested sources in cingulate and orbitofrontal regions. Consistent with previous research (e.g., Lamm et al., 2006), the cingulate sources were more posterior than is typically found with adults. The source analysis findings need to be replicated using pediatric head models when these models become available.
Previous research has found that the amplitude of the N2 generally decreases with age and is associated with EF (e.g., Lamm et al., 2006). In the current study, this more mature pattern of N2 amplitudes was observed in children who switched versus perseverated on the DCCS even when age, the number of trials contributing to the ERPs, and latency were controlled for statistically. No differences between children who switched and children who perseverated were observed in N2 latencies, although the N2 occurred later than is normally observed in adults, consistent with previous pediatric research on ERP latencies in general (Thierry, 2005).
Children who switched were older than children who perseverated by about 3 months, but age was used as a covariate in the analyses, so it is difficult to explain the differences in N2 amplitude in terms of age differences or incidental changes such as changes in skull thickness. In addition, the number of usable trials did not differ between children who switched and children who perseverated (i.e., artifacts were not more problematic for one group than the other), so it is unlikely that systematic differences in artifact contamination played a role. Instead, the smaller N2 amplitudes seen in children who switched versus perseverated support the suggestion that the N2 may be a reliable neural marker of early individual differences in performance on the DCCS, complementing previous work establishing a role for ventrolateral prefrontal cortex (Moriguchi and Hiraki, 2009). It should be noted, however, that recent research shows intra-subject variability in N2 amplitude depending on the specific processing context (Forster et al., 2011, Waxer and Morton, 2011a, Waxer and Morton, 2011b).
In adults, the N2 has been related to conflict monitoring (Carter et al., 1998) and detection of the need for top-down cognitive control mediated by lateral prefrontal cortex (e.g., Gehring and Knight, 2000, Ridderinkhof et al., 2004). Children who pass the DCCS may resolve the conflict inherent in the bivalent stimuli more efficiently than children who fail, resulting in smaller N2 amplitudes. This interpretation is consistent with the CCC-r theory (Zelazo et al., 2003), according to which children who switch flexibly are children who understand the hierarchical structure of the task. That is, according to this theory, children who pass the DCCS immediately resolve the conflict inherent in the bivalent stimuli (i.e., even during the pre-switch phase) by recognizing that they know two ways of sorting the stimuli.
Although the findings of this study are consistent with CCC-r theory, other interpretations are also possible. From a working memory perspective (e.g., Morton and Munakata, 2002), children who switch flexibly may resolve the conflict inherent in the stimuli by keeping the relevant rules firmly in mind. It is less clear, however, how the observed differences in N2 amplitude could be explained by accounts emphasizing inhibitory control (e.g., Kirkham et al., 2003). Although poor inhibitory control might lead to both perseveration and larger N2 amplitudes on the post-switch phase (i.e., in the presence of response conflict), there should be no differences in N2 amplitude during the pre-switch phase, in the absence of both response conflict and inhibitory demands.
The most obvious source of conflict in the DCCS is the conflict inherent in the bivalent stimuli, but for children who fail, there is additional conflict on the post-switch phase between the explicit post-switch instructions (i.e., sort by color) and children's perseverative behavior (i.e., sorting by shape). The finding that differences in N2 amplitudes were evident even on the pre-switch phase, while preliminary, suggests that the most relevant source of conflict may have been that inherent in the stimuli, however. Moriguchi and Hiraki (2009) also found that children who switch and children who perseverate differed on pre-switch as well as post-switch trials. In that study, children who perseverated on the task showed much less lateral prefrontal activation on both pre- and post-switch trials. In other words, it has now been found in two independent studies with preschoolers that what differentiates children who pass from children who fail the DCCS is not something that occurs only during the post-switch phase. Taken together with Moriguchi and Hiraki's finding, the current findings suggest the following account. Children who pass the DCCS detect the conflict inherent in the bivalent stimuli and then reflect upon their conflicting rule representations, recruiting lateral prefrontal cortex as they resolve the conflict by formulating (and keeping in mind) a representation of the hierarchical structure of the task (Bunge and Zelazo, 2006; cf. Badre and D’Esposito, 2007, Botvinick, 2008, Christoff and Gabrieli, 2000, Koechlin et al., 2003, Goldberg and Bilder, 1987) and/or by keeping the appropriate post-switch rules in mind (e.g., Waxer and Morton, 2011a). Children who perseverate on the DCCS also detect the conflict inherent in the stimuli but they fail to reflect upon their rule representations, fail to recruit lateral prefrontal cortex, and fail to understand the hierarchical structure of the task. For these children, unresolved conflict continues to be processed throughout the task, resulting in larger N2 amplitudes (current study) and reduced activation in lateral prefrontal cortex (Moriguchi and Hiraki, 2009). To confirm that N2 amplitudes as measured in the current study are indeed associated with conflict processing, it will be necessary to compare ERPs on trials involving bivalent stimuli to ERPs on trials involving univalent stimuli, as in the study Waxer and Morton (2011b). The current interpretation generates the strong prediction that both children who switch and children who perseverate would show greater N2 amplitudes on bivalent compared to univalent trials.
Future research should also address more directly the hypothesized link between indices of conflict monitoring, such as N2 amplitude, and indices of prefrontally mediated hierarchical rule use. It is possible that what develops during the preschool period is a functional network linking ACC to lateral prefrontal cortex. It is also possible that understanding the ways in which ACC interacts with lateral prefrontal cortex will shed light on the co-occurrence during early childhood of changes in executive function, error monitoring, metacognition, and uncertainty monitoring (reviewed in Lyons and Zelazo, 2011). Although these constructs are often studied independently of one another, similar patterns of development are observed across all four constructs, with substantial improvement observed in early childhood and more gradual improvements evident well into adolescence.
The current findings complement previous research pointing to the N2 as a neural marker of individual differences in executive function (e.g., Lamm et al., 2006), and extend previous work with adolescents and adults (e.g., Waxer and Morton, 2011a) by examining ERPs in relation to performance on a standard version of the DCCS in preschool-aged children. The findings support a characterization of performance on this task that highlights the role of conflict detection in a cascade of processes that eventuate in cognitive and behavioral flexibility.
Conflict detection and top-down executive function are such fundamental neurocognitive skills that it is perhaps not surprising that individual and developmental differences in these skills are associated with a wide range of developmental outcomes. Moffitt et al. (2011), for example, found that executive function in childhood predicts (as a gradient) physical health, substance dependence, socioeconomic status, and the likelihood of a criminal conviction at age 32 years, even after controlling for social class of origin and IQ. Although much remains to be learned about the nature of these far-reaching longitudinal correlations, the proposed account of individual differences in executive function as measured by the DCCS may help shed light at least on the relation between executive function and educational achievement (e.g., Blair, 2002, McClelland et al., 2006, McClelland et al., 2007). Reflecting on conflict, including gaps in one's understanding, may go hand in hand with the adoption of a more active, goal-directed, top-down approach to learning. Children with better executive function may spontaneously monitor their own understanding and seek actively to improve it. Further research might usefully investigate the conditions under which reflection is facilitated, in an effort not only to exercise children's executive function, but also potentially to transform the way in which children learn.
4. Conclusion
Children who switch flexibly on the DCCS, a key measure of executive function during the preschool years, show smaller N2 amplitudes, consistent with the down-regulation of ACC-mediated conflict detection in children who reflect upon their conflicting rule representations and apprehend the hierarchical structure of the task. These findings implicate conflict detection in flexible rule use during a period of rapid development in executive function, and they point to the possible emergence during this period of a functional neural network linking ACC and lateral prefrontal cortex.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgements
Funding for this research was provided by the Canadian Foundation for Innovation, the Canada Research Chairs Program, and the College of Education and Human Development, University of Minnesota. We thank Cheryl Leung, Shintula Wijeya, and Xenia Zheng for their assistance in data collection. We especially thank Katherine I. Murray and Jim Steiben, whose work on an earlier version of this study informed the research reported here.
References
- Badre D., D’Esposito M. Functional magnetic resonance imaging evidence for a hierarchical organization of the prefrontal cortex. Journal of Cognitive Neuroscience. 2007;19:2082–2099. doi: 10.1162/jocn.2007.19.12.2082. [DOI] [PubMed] [Google Scholar]
- Beck D.M., Schaefer C., Pang K., Carlson S.M. Executive function in preschool children: test–retest reliability. Journal of Cognition and Development. 2011;12:169–193. doi: 10.1080/15248372.2011.563485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bialystok E. Cognitive complexity and attentional control in the bilingual mind. Child Development. 1999;70:636–664. [Google Scholar]
- Blair C. School readiness: integrating cognition and emotion in a neurobiological conceptualization of child functioning at school entry. American Psychologist. 2002;57:111–127. doi: 10.1037//0003-066x.57.2.111. [DOI] [PubMed] [Google Scholar]
- Blair C., Razza R.A. Relating effortful control, executive function, and false belief understanding to emerging math and literacy ability in kindergarten. Child Development. 2007;78:647–663. doi: 10.1111/j.1467-8624.2007.01019.x. [DOI] [PubMed] [Google Scholar]
- Bohlmann N.L., Fenson L. The effects of feedback on perseverative errors in preschool aged children. Journal of Cognition and Development. 2005;6:119–131. [Google Scholar]
- Bokura H., Yamaguchi S., Kobayashi S. Electrophysiological correlates for response inhibition in a Go/NoGo task. Clinical Neurophysiology. 2001;112:2224–2232. doi: 10.1016/s1388-2457(01)00691-5. [DOI] [PubMed] [Google Scholar]
- Botvinick M.M. Conflict monitoring and decision making: reconciling two perspectives on anterior cingulate function. Cognitive, Affective, & Behavioral Neuroscience. 2007;7:356–366. doi: 10.3758/cabn.7.4.356. [DOI] [PubMed] [Google Scholar]
- Botvinick M.M. Hierarchical models of behavior and prefrontal function. Trends in Cognitive Sciences. 2008;12:201–208. doi: 10.1016/j.tics.2008.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick M.M., Braver T.S., Barch D.M., Carter C.S., Cohen J.D. Conflict monitoring and cognitive control. Psychological Review. 2001;108:624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Brace J.J., Morton J.B., Munakata Y. When actions speak louder than words: Improving children's flexibility in a card-sorting task. Psychological Science. 2006;17:665–669. doi: 10.1111/j.1467-9280.2006.01763.x. [DOI] [PubMed] [Google Scholar]
- Bunge S., Zelazo P.D. A brain-based account of the development of rule use in childhood. Current Directions in Psychological Science. 2006;15:118–121. [Google Scholar]
- Carter C.S., Braver T.S., Barch D.M., Botvinick M., Noll D., Cohen J.D. Anterior cingulate cortex, error detection, and the on-line monitoring of performance. Science. 1998;280:747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- Christoff K., Gabrieli J.D.E. The frontopolar cortex and human cognition: evidence for a rostrocaudal hierarchical organization within the human prefrontal cortex. Psychobiology. 2000;28:169–186. [Google Scholar]
- Crone E.A., Donohue S.E., Honomichl R., Wendelken C., Bunge S.A. Brain regions mediating flexible rule use during development. Journal of Neuroscience. 2006;26:11239–11247. doi: 10.1523/JNEUROSCI.2165-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis E.P., Bruce J., Snyder K., Nelson C. The X-trials: neural correlates of an inhibitory control task in children and adults. Journal of Cognitive Neuroscience. 2003;13:432–443. doi: 10.1162/089892903321593144. [DOI] [PubMed] [Google Scholar]
- Diamond A., Carlson S.M., Beck D.M. Preschool children's performance in task switching on the Dimensional Change Card Sort task: separating dimensions aids the ability to switch. Developmental Neuropsychology. 2005;28:689–729. doi: 10.1207/s15326942dn2802_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick A.S., Overton W.F., Kovacs S.L. The development of symbolic coordination: representation of imagined objects, executive function, and theory of mind. Journal of Cognition and Development. 2005;6:133–161. [Google Scholar]
- Eimer M. ERPs elicited by Go and Nogo stimuli: effects of attention and stimulus probability. Biological Psychology. 1993;35:123–138. doi: 10.1016/0301-0511(93)90009-w. [DOI] [PubMed] [Google Scholar]
- Electrical Geodesics, Inc. EGI; Eugene, OR: 2006. NetStation Waveform Tools Technical Manual (Tech. Rep. S-MAN-200-WTFR-001) [Google Scholar]
- Falkenstein M., Hoormann J., Hohnsbein J. ERP components in Go/Nogo tasks and their relation to inhibition. Acta Psychologica. 1999;101:267–291. doi: 10.1016/s0001-6918(99)00008-6. [DOI] [PubMed] [Google Scholar]
- Folstein J.R., van Petten C. Influence of cognitive control and mismatch on the N2 component of the ERP: a review. Psychophysiology. 2008;45:152–170. doi: 10.1111/j.1469-8986.2007.00602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster S.E., Carter C.S., Cohen J.D., Cho R.Y. Parametric manipulation of the conflict signal control-state adaptation. Journal of Cognitive Neuroscience. 2011;23:923–935. doi: 10.1162/jocn.2010.21458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring W.J., Knight R.T. Prefrontal-cingulate interactions in action monitoring. Nature Neuroscience. 2000;3:516–520. doi: 10.1038/74899. [DOI] [PubMed] [Google Scholar]
- Goldberg E., Bilder R.M. The frontal lobes and hierarchical organization of cognitive control. In: Perecman E., editor. The Frontal Lobes Revisited. The IRBN Press; New York: 1987. pp. 159–187. [Google Scholar]
- Jodo E., Kayama Y. Relation of a negative ERP component to response inhibition in a Go/No-go task. Electroencephalography and Clinical Neurophysiology. 1992;82:477–482. doi: 10.1016/0013-4694(92)90054-l. [DOI] [PubMed] [Google Scholar]
- Johnstone S.J., Dimoska A., Smith J.L., Barry R.J., Pleffer C.B., Chiswick D. The development of stop-signal and Go/Nogo response inhibition in children aged 7–12 years: performance and event-related potential indices. International Journal of Psychophysiology. 2007;63:25–38. doi: 10.1016/j.ijpsycho.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Johnstone S.J., Pleffer C.B., Barry R.J., Clarke A.R., Smith J.L. Development of inhibitory processing during the Go/NoGo task: a behavioral and event-related potential study of children and adults. Journal of Psychophysiology. 2005;19:11–23. [Google Scholar]
- Jonkman L.M. The development of preparation, conflict monitoring and inhibition from early childhood to young adulthood: a Go/Nogo ERP study. Brain Research. 2006;1097:181–193. doi: 10.1016/j.brainres.2006.04.064. [DOI] [PubMed] [Google Scholar]
- Jonkman L.M., Lansbergen M., Stauder J.E.A. Developmental differences in behavioral and event-related brain responses associated with response preparation and inhibition in a go/nogo task. Psychophysiology. 2003;40:752–761. doi: 10.1111/1469-8986.00075. [DOI] [PubMed] [Google Scholar]
- Jonkman L.M., Sniedt F.L.F., Kemner C. Source localization of the no-go-N2: a developmental study. Clinical Neurophysiology. 2007;118:1069–1077. doi: 10.1016/j.clinph.2007.01.017. [DOI] [PubMed] [Google Scholar]
- Kirkham N., Cruess L., Diamond A. Helping children apply their knowledge to their behavior on a dimension-switching task. Developmental Science. 2003;6:449–476. [Google Scholar]
- Kloo D., Perner J. Disentangling dimensions in the dimensional change card sorting task. Developmental Science. 2005;8:44–56. doi: 10.1111/j.1467-7687.2005.00392.x. [DOI] [PubMed] [Google Scholar]
- Koechlin E., Ody C., Frederique K. The architecture of cognitive control in the human prefrontal cortex. Science. 2003;302:1181–1185. doi: 10.1126/science.1088545. [DOI] [PubMed] [Google Scholar]
- Lahat A., Todd R.M., Mahy C.E.V., Zelazo P.D. Neurophysiological correlates of executive function: a comparison of European-Canadian and Chinese-Canadian 5-year-old children. Frontiers in Human Neuroscience. 2010;3(72):1–10. doi: 10.3389/neuro.09.072.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamm C., Zelazo P.D., Lewis M.D. Neural correlates of cognitive control in childhood and adolescence: disentangling the contributions of age and executive function. Neuropsychologia. 2006;44:2139–2148. doi: 10.1016/j.neuropsychologia.2005.10.013. [DOI] [PubMed] [Google Scholar]
- Lewis M.D., Granic I., Lamm C., Zelazo P.D., Stieben J., Todd R.M. Changes in the neural bases of emotion regulation associated with clinical improvement in children with behavior problems. Developmental Psychopathology. 2008;20:913–939. doi: 10.1017/S0954579408000448. [DOI] [PubMed] [Google Scholar]
- Lewis M.D., Lamm C., Segalowitz S., Stieben J., Zelazo P.D. Neurophysiological correlates of emotion regulation in children and adolescents. Journal of Cognitive Neuroscience. 2006;18:430–443. doi: 10.1162/089892906775990633. [DOI] [PubMed] [Google Scholar]
- Lewis M.D., Todd R.M., Honsberger M.J.M. Event-related potential measures of emotion regulation in early childhood. NeuroReport. 2007;18:61–65. doi: 10.1097/WNR.0b013e328010a216. [DOI] [PubMed] [Google Scholar]
- Luck S.J. MIT Press; Cambridge, MA: 2005. An Introduction to the Event-related Potential Technique. [Google Scholar]
- Luu, P., Ferree, T., 2000. Determination of the Geodesic Sensor Nets’ average electrode positions and their 10–10 international equivalents. (Technical Note, pp. 1–11). Eugene, OR: Electrical Geodesics Inc.
- Lyons K.E., Zelazo P.D. Monitoring, metacognition, and executive function: elucidating the role of self-reflection in the development of self-regulation. Advances in Child Development and Behavior. 2011;40:379–412. doi: 10.1016/b978-0-12-386491-8.00010-4. [DOI] [PubMed] [Google Scholar]
- Marcovitch S., Jacques S., Boseovski J.J., Zelazo P.D. Self-reflection and the cognitive control of behavior: implications for learning. Mind, Brain, and Education. 2008;2:136–141. [Google Scholar]
- McClelland M.M., Acock A.C., Morrison F.J. The impact of kindergarten learning-related skills on academic trajectories at the end of elementary school. Early Childhood Research Quarterly. 2006;18:225–237. [Google Scholar]
- McClelland M.M., Cameron C.E., Connor C.M., Farris C.L., Jewkes A.M., Morrison F.J. Links between behavioral regulation and preschoolers’ literacy, vocabulary, and math skills. Developmental Psychology. 2007;43:947–959. doi: 10.1037/0012-1649.43.4.947. [DOI] [PubMed] [Google Scholar]
- Michel C.M., Murray M.M., Lantz G., Gonzalez S., Spinelli L., Grave de Peralta R. EEG source imaging. Clinical Neurophysiology. 2004;115:2195–2222. doi: 10.1016/j.clinph.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Miyake A., Friedman N.P., Emerson M.J., Witzki A.H., Howerter A., Wager T.D. The unity and diversity of executive functions and their contributions to complex frontal lobe tasks: a latent variable analysis. Cognitive Psychology. 2000;41:49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- Moffitt T.E., Arseneault L., Belsky D., Dickson N., Hancox R., Harrington H.L., Houts R., Poulton R., Roberts B., Ross S., Sears M., Thomson W.M., Caspi A. A gradient of childhood self-control predicts health, wealth, and public safety. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:2693–2698. doi: 10.1073/pnas.1010076108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriguchi Y., Hiraki K. Neural origin of cognitive shifting in young children. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:6017–6021. doi: 10.1073/pnas.0809747106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton J.B., Bosma R., Ansari D. Age-related changes in brain activation associated with dimensional shifts of attention: an fMRI study. NeuroImage. 2009;46:336–358. doi: 10.1016/j.neuroimage.2009.01.037. [DOI] [PubMed] [Google Scholar]
- Morton J.B., Munakata Y. Active versus latent representations: a model of perseveration, knowledge-action dissociation, and décalage. Developmental Psychobiology. 2002;40:255–265. doi: 10.1002/dev.10033. [DOI] [PubMed] [Google Scholar]
- Munakata Y., Yerys B.E. All together now: when dissociations between knowledge and action disappear. Psychological Science. 2001;12:335–337. doi: 10.1111/1467-9280.00361. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S., Yeung N., Van den Wildenberg W., Ridderinkhof K.R. Electrophysiological correlates of anterior cingulate function in a Go/NoGo task: effects of response conflict and trial-type frequency. Cognitive, Affective & Behavioral Neuroscience. 2003;3:17–26. doi: 10.3758/cabn.3.1.17. [DOI] [PubMed] [Google Scholar]
- Pascual-Marqui R.D. Standardized low resolution brain electromagnetic tomography (sLORETA): technical details. Methods & Findings in Experimental & Clinical Pharmacology. 2002;24D:5–12. [PubMed] [Google Scholar]
- Ridderinkhof K.R., Ullsperger M., Crone E.A., Nieuwenhuis S. The role of medial frontal cortex in cognitive control. Science. 2004;306:443–447. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- Rueda M.R., Posner M.I., Rothbart M.K., Davis-Stober C.P. Development of the time course for processing conflict: an event-related potentials study with 4 year olds and adults. BMC Neuroscience. 2004;5:39. doi: 10.1186/1471-2202-5-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueda M.R., Rothbart M.K., McCandliss B.D., Saccomanno L., Posner M.I. Training, maturation, and genetic influences on the development of executive attention. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:14931–14936. doi: 10.1073/pnas.0506897102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoda Y., Mischel W., Peake P.K. Predicting adolescent cognitive and self-regulatory competencies from preschool delay of gratification: identifying diagnostic conditions. Developmental Psychology. 1990;26:978–986. [Google Scholar]
- Thierry G. The use of event-related potentials in the study of early cognitive development. Infant and Child Development. 2005;14:85–94. [Google Scholar]
- Todd R.M., Lewis M.D., Meusel L., Zelazo P.D. The time course of social-emotional processing in early childhood: ERP responses to facial affect and personal familiarity in a Go–Nogo task. Neuropsychologia. 2008;46:595–613. doi: 10.1016/j.neuropsychologia.2007.10.011. [DOI] [PubMed] [Google Scholar]
- Tucker D.M. Spatial sampling of head electrical fields: The Geodesic Sensor Net. Electroencephalography and Clinical Neurophysiology. 1993;87:154–163. doi: 10.1016/0013-4694(93)90121-b. [DOI] [PubMed] [Google Scholar]
- van Veen V., Carter C.S. The timing of action monitoring processes in the anterior cingulate cortex. Journal of Cognitive Neuroscience. 2002;14:593–602. doi: 10.1162/08989290260045837. [DOI] [PubMed] [Google Scholar]
- Waxer M., Morton B.J. The development of future-oriented control: an electrophysiological investigation. NeuroImage. 2011;3:1648–1654. doi: 10.1016/j.neuroimage.2011.02.001. [DOI] [PubMed] [Google Scholar]
- Waxer M., Morton B.J. Multiple processes underlying Dimensional Change Card Sort performance: a developmental electrophysiological investigation. Journal of Cognitive Neuroscience. 2011:3267–3279. doi: 10.1162/jocn_a_00038. [DOI] [PubMed] [Google Scholar]
- Yeung N., Nieuwenhuis S. Dissociating response conflict and error likelihood in anterior cingulate cortex. The Journal of Neuroscience. 2009;29:14506–14510. doi: 10.1523/JNEUROSCI.3615-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelazo P.D. The dimensional change card sort (DCCS): a method of assessing executive function in children. Nature Protocols. 2006;1:297–301. doi: 10.1038/nprot.2006.46. [DOI] [PubMed] [Google Scholar]
- Zelazo, P.D., Anderson, J.E., Richler, J., Wallner-Allen, K., Beaumont, J.L., Weintraub, S. NIH Toolbox Cognitive Function Battery (NIHTB-CFB): measuring executive function and attention. In: Zelazo, P.D., Bauer, P.J. (Eds.), National Institutes of Heath Toolbox Cognitive Function Battery (NIHTB-CHB): Validation for children between 3 and 15 years. Monographs of the Society for Research in Child Development, in press. [DOI] [PubMed]
- Zelazo, P.D., Muller, U., Frye, D., Marcovitch, S. (2003). The development of executive function in early childhood. Monographs of the Society for Research in Child Development, 68, Serial No. 274. [DOI] [PubMed]