Abstract
Aims: It is known that mitochondrial reactive oxygen species generation ([ROS]m) causes the release of Ca2+ via ryanodine receptor-2 (RyR2) on the sarcoplasmic reticulum (SR) in pulmonary artery smooth muscle cells (PASMCs), playing an essential role in hypoxic pulmonary vasoconstriction (HPV). In this study, we sought to determine whether hypoxia-induced RyR2-mediated Ca2+ release may in turn promote [ROS]m in PASMCs and the underlying signaling mechanism.
Results: Our data reveal that application of caffeine or norepinephrine to induce Ca2+ release increased [ROS]m in PASMCs. Likewise, exogenous Ca2+ augmented ROS generation in isolated mitochondria and at complex III from PASMCs. Inhibition of mitochondrial Ca2+ uniporter (MCU) with Ru360 attenuated agonist-induced [ROS]m. Ru360 produced a similar inhibitory effect on hypoxia-induced [ROS]m. Rieske iron–sulfur protein (RISP) gene knockdown inhibited Ca2+- and caffeine-induced [ROS]m. Inhibition of RyR2 by tetracaine or RyR2 gene knockout suppressed hypoxia-induced [ROS]m as well.
Innovation: In this article, we present convincing evidence that Ca2+ release following hypoxia or RyR simulation causes a significant increase in MCU, and the increased MCU subsequently RISP-dependent [ROS]m, which provides a positive feedback mechanism to enhance hypoxia-initiated [ROS]m in PASMCs.
Conclusion: Our findings demonstrate that hypoxia-induced mitochondrial ROS-dependent SR RyR2-mediated Ca2+ release increases MCU and then RISP-dependent [ROS]m in PASMCs, which may make significant contributions to HPV and associated pulmonary hypertension.
Keywords: intracellular calcium, reactive oxygen species, mitochondria, ryanodine receptor, hypoxia, pulmonary artery smooth muscle cells
Introduction
Hypoxia causes strong vasoconstriction in pulmonary arteries (PAs), termed hypoxic pulmonary vasoconstriction (HPV). This unique response is an important adaptive mechanism for pulmonary ventilation/perfusion matching in the lungs but can also become a crucial pathological factor for pulmonary hypertension. HPV may result from an increase in intracellular Ca2+ concentration ([Ca2+]i), which is mediated by multiple ion channels in pulmonary artery smooth muscle cells (PASMCs). A series of our studies and others have revealed that ryanodine receptors (RyRs), the Ca2+ release channels localized on the sarcoplasmic reticulum (SR), are important for the hypoxia-induced increase in [Ca2+]i and contraction in PASMCs (13, 33, 40, 41, 52). We have further shown that all three subtypes of RyRs (RyR1, RyR2, and RyR3) are involved in hypoxic Ca2+ and contractile responses in PASMCs; however, RyR2 is the most valuable player (17, 18, 51).
Innovation
We and others have disclosed that hypoxia induces Rieske iron–sulfur protein (RISP)-dependent mitochondrial reactive oxygen species generation ([ROS]m) and then Ca2+ release from the sarcoplasmic reticulum (SR) via ryanodine receptor-2 (RyR2) in pulmonary artery smooth muscle cells (PASMCs), thereby leading to hypoxic pulmonary vasoconstriction (HPV) and pulmonary hypertension. However, it is unknown whether and how RyR2-mediated Ca2+ release may in turn promote [ROS]m in PASMCs. In this article, we present novel evidence that Ca2+ release from the SR following the opening of RyR by its agonist caffeine or the opening of inositol-1,4,5-trisphosphate receptor (IP3R) by the vascular neurotransmitter norepinephrine significantly increases mitochondrial Ca2+ uptake and RISP-dependent [ROS]m in PASMCs. Hypoxic stimulation produces a similar response. Agonist- and hypoxia-induced Ca2+ release-mediated RISP-dependent [ROS]m are RyR2 determined and uniquely occur in PASMCs, but not in systemic arterial smooth muscle cells. Collectively, the interaction of RyR2-mediated Ca2+ release with RISP-dependent mitochondrial ROS serves as an important signaling mechanism for HPV and associated pulmonary hypertension.
The hypoxic increase in [Ca2+]i in PASMCs has been thought to be attributed to the enhanced intracellular reactive oxygen species generation ([ROS]i) due to the specific effects of ROS on ion channels (31, 33, 40, 41, 52). The hypoxic increase in [ROS]i mainly occurs in mitochondria and at NADPH oxidase. We have revealed that mitochondria are a primary source of the hypoxic increase in [ROS]i, which enhances the activity of protein kinase C-ɛ and then NADPH oxidase, leading to further [ROS]i, termed ROS-induced ROS generation (RIRG) in PASMCs (28, 29, 37). The hypoxia-induced mitochondrial ROS generation ([ROS]m) is predominantly produced at complex III, in which Rieske iron–sulfur protein (RISP) acts as an indispensable molecule (16, 45).
Our studies have further shown that hypoxia causes activation of RyRs and Ca2+ release in PASMCs, which are blocked by pharmacological and genetic inhibition of [ROS]m, and mimicked by exogenous ROS (18). Thus, the specific mitochondrial ROS-induced RyR2-mediated Ca2+ release, that is, ROS-induced Ca2+ release (RICR), serves as an essential mechanism for hypoxic cellular responses in PASMCs.
RyR2-mediated Ca2+ release can be taken up by mitochondria, which is likely to be central to mitochondrial functions including ROS generation in cardiac myocytes (7, 11). These important findings in cardiac cells, with our revolutionary discoveries on the essential role mitochondria-SR interaction-mediated RICR in PASMCs as described above, have led us to propose a novel hypothesis that the hypoxia-induced ROS-initiated RyR2-dependnet Ca2+ release may in turn promotes [ROS]m, termed Ca2+-induced ROS generation (CIRG), which provides a positive feedback mechanism to further enhance the hypoxic ROS generation, thereby contributing to attendant Ca2+ and contractile responses in PASMCs. Indeed, Ca2+ uptake is correlated with ROS generation in mitochondria isolated from cardiac ventricular myocytes (35), and Ca2+ addition results in a significant increase in ROS generation at mitochondrial complex III isolated from the heart (3).
To test our hypothesis, we first sought to determine whether direct Ca2+ addition could increase ROS generation in isolated mitochondria and complex III from PASMCs. RyR-mediated Ca2+ release plays an important role in cellular responses in PASMCs (36, 52); thus, we next examined whether Ca2+ release from the SR following application of caffeine, a classic and widely used RyR agonist (12, 21, 30), augmented [ROS]m in PASMCs. In complement to the effect of caffeine-evoked RyR activation, subsequent experiments were performed to test the effect of blockage of RyR2-mediated Ca2+ release with its antagonist tetracaine and gene knockout (KO) on hypoxia-induced [ROS]m in PASMCs.
With the intention of defining a potential mechanism for the enhanced [ROS]m by caffeine-induced RyR-dependent Ca2+ release, we investigated whether Ca2+ release-dependent [ROS]m might be a result of the increased mitochondrial Ca2+ uniporter (MCU) by assessing the effect of its inhibitor Ru360 on caffeine-induced ROS generation. RISP in mitochondrial complex III is an essential molecule for [ROS]m in PASMCs as stated above. To further determine the molecular mechanism for RyR Ca2+ release-mediated [ROS]m, we accordingly conducted a set of experiments to assess whether genetic suppression of RISP using specific lentiviral short hairpin RNA (shRNA)-mediated gene knockdown (KD) could block caffeine-caused ROS generation in PASMCs and mitochondria.
Finally, we sought to determine whether hypoxia-induced mitochondrial ROS-initiated RyR2-dependent Ca2+ release might further enhance the hypoxic [ROS]m in PASMCs and also whether this hypoxic response is attributable to the MCU-associated RISP signaling.
Results
Application of caffeine, norepinephrine, and hypoxia to elevate [Ca2+]i significantly increased [ROS]i and [ROS]m in PASMCs
Here, we first examined whether RyR-mediated Ca2+ release might induce [ROS]i, that is, CIRG in PASMCs. In these experiments, cells were loaded with chloromethyldihydrodichlorofluorescein diacetate (CM-H2DCF/DA) to measure [ROS]i (mainly H2O2) (37, 41) and then treated with the classic RyR agonist caffeine (20 mM) for 5 min to induce Ca2+ release from the SR. As shown in Figure 1A, DCF-derived fluorescence intensity was increased remarkably in caffeine-treated cells compared with untreated cells, indicating that caffeine-induced RyR-mediated Ca2+ release causes a significant increase in [ROS]i in PASMCs.
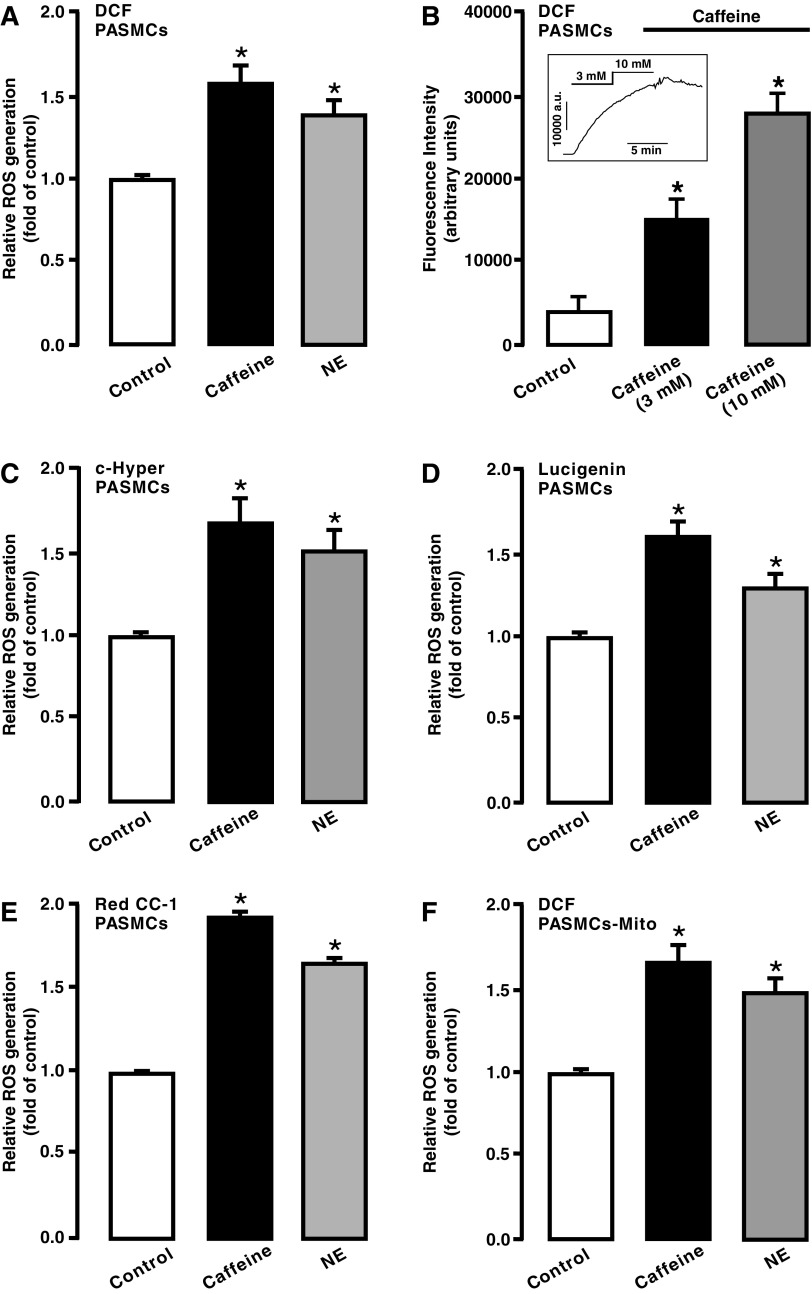
FIG. 1.
Elevation of [Ca2+]i by caffeine or norepinephrine increased ROS generation in PASMCs and mitochondria. PASMCs were treated with CM-H2DCF/DA (DCF) (A, B), pHyPer-cyto vector (C), lucigenin (D), and Red CC-1 (E) to determine ROS generation and then exposed to caffeine (20 mM) or norepinephrine (100 μM) for 5 min to examine either of their effect except in (B) as indicated. In (F), PASMCs were exposed to caffeine (20 mM) or norepinephrine (100 μM) for 5 min, mitochondria were isolated, and ROS generation was measured using H2DCF/DA (DCF). The mean data were obtained from four different experiments. *p < 0.05 compared with control. [Ca2+]i, intracellular Ca2+ concentration; CM-H2DCF/DA, chloromethyldihydrodichlorofluorescein diacetate; PASMCs, pulmonary artery smooth muscle cells; ROS, reactive oxygen species.
Norepinephrine, a major vascular neurotransmitter, can increase [Ca2+]i by largely inducing Ca2+ release from the SR via inositol-1,4,5-trisphosphate receptors (IP3Rs) in vascular smooth muscle cells (SMCs) (21, 24, 50, 51). Accordingly, we sought to test whether treatment with norepinephrine also increased ROS generation in PASMCs. Like caffeine, the application of norepinephrine (100 μM) for 5 min resulted in a large increase in [ROS]i in PASMCs (Fig. 1A).
We also found that caffeine at 3 and 10 mM caused qualitative increases in DCF-derived fluorescence in PASMCs (Fig. 1B). Taken together, our findings provide evidence that caffeine can, in a concentration-dependent manner, increase [ROS]i in PASMCs.
Caffeine- and norepinephrine-induced [ROS]i were also detected by using pHyPer-cyto, a mammalian expression vector encoding a fluorescent ROS sensor HyPer (specifically assessing H2O2) (2, 16). As shown in Figure 1C, both caffeine and norepinephrine increased [ROS]i in PASMCs.
As depicted in Figure 1D and E, the similar effect of caffeine and norepinephrine on [ROS]i in PASMCs was confirmed by the chemiluminescent reagent lucigenin (mainly for measuring O2−) and the fluorescent dye RedoxSensor Red CC-1 (for measuring both O2− and H2O2) (37, 41). Taken together, caffeine and norepinephrine elicited ROS generation by releasing Ca2+ from the SR in PASMCs.
It is known that mitochondria are the primary sources for [ROS]i in PASMCs (18, 28, 29, 37, 42, 43, 46–48). As such, we examined ROS generation in isolated mitochondria from PASMCs following caffeine or norepinephrine exposure. The results indicated that [ROS]m was increased as well (Fig. 1F).
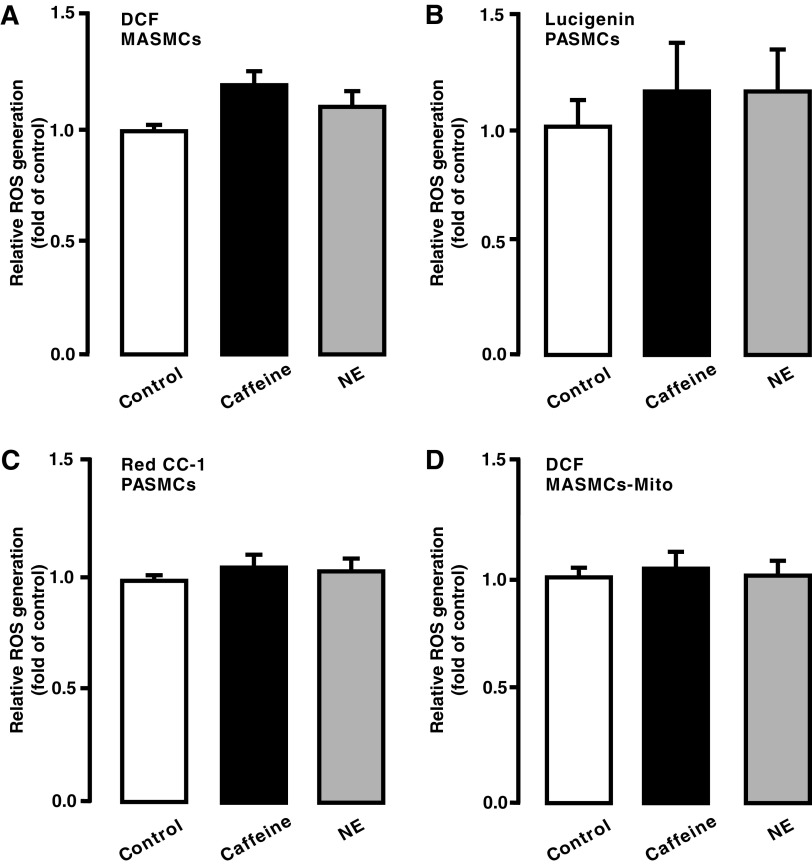
Hypoxia causes differential ROS, Ca2+, and contractile responses in PASMCs and systemic artery SMCs, thereby leading to significant vasoconstriction in pulmonary arteries, but not in systemic arteries (40). As such, we thought that CIRG might only occur in the former cells, rather than in the latter. In support, as shown in Figure 2A–D, neither caffeine nor norepinephrine induced ROS generation in mesentery (systemic) artery smooth muscle cells (MASMCs) and isolated mitochondria from MASMCs.
FIG. 2.
Caffeine- or norepinephrine-induced increase in [Ca2+]i did not affect ROS generation in MASMCs. MASMCs were loaded with CM-H2DCF/DA (DCF) (A), lucigenin (B), and Red CC-1 (C) and then treated with caffeine (20 mM) or norepinephrine (100 μM) for 5 min. In (D), cells were exposed to caffeine (20 mM) or norepinephrine (100 μM) for 5 min, isolated mitochondria were obtained, and ROS generation was assessed. The mean data were obtained from three different experiments. Note that no statistically significant difference between control and treatment was found. MASMCs, mesentery (systemic) artery smooth muscle cells.
Caffeine, norepinephrine, or hypoxia increased intramitochondrial Ca2+ concentration in PASMCs
To further illustrate the role of Ca2+ in [ROS]m, we investigated the effect of caffeine, norepinephrine, and hypoxia on intramitochondrial Ca2+ concentration [Ca2+]m. The mitochondria-targeted double-mutated aequorin (mit-2mutAEQ) Ca2+ sensor was used to assess [Ca2+]m, as described in a previous publication (6). The correlation of the mit-2mutAEQ-derived relative luminescence (luminescence/total residual luminescence, L/Lmax) with different free Ca2+ concentrations was constructed, as shown in Figure 3A. This correlation curve was then used to calibrate the data.
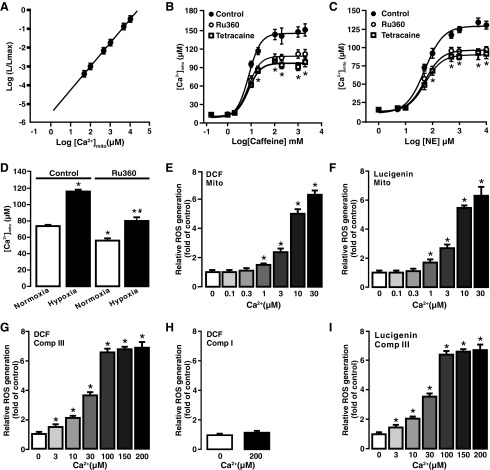
FIG. 3.
Caffeine, norepinephrine, and hypoxia elevated [Ca2+]m that causes [ROS]m in PASMCs. (A) The correlation curve between mit-2mutAEQ-derived relative luminescence and different free Ca2+ concentration was constructed and then used to quantify the effect of caffeine, norepinephrine, and hypoxia on [Ca2+]m in PASMCs. The mit-2mutAEQ-derived relative luminescence was determined using pcDNA3.1+/mit-2mutAEQ vector as described in the Materials and Methods section. (B) Application of caffeine at various concentrations for 5 min induced a large elevation of [Ca2+]m in PASMCs. [Ca2+]m was by quantified by fitting the data into the correlation curve for mit-2mutAEQ-derived luminescence at various free Ca2+ concentrations. Caffeine-induced increase in [Ca2+]m was concentration-dependent and suppressed by pretreatment with the mitochondria Ca2+ uniporter inhibitor Ru360 (10 μM) for 5 min. (C) Norepinephrine, similar to caffeine, also induced a concentration-dependent and Ru360-inhibited elevation of [Ca2+]m in PASMCs. (D) Hypoxia exposure for 5 min caused a significant elevation of [Ca2+]m in PASMCs compared with normoxic exposure. The hypoxic response was inhibited by treatment with Ru360 (10 μM) for 5 min as well. (E) Exogenous free Ca2+ at concentrations ranged from 1 to 30 μM resulted in a concentration-dependent increase in ROS generation in freshly isolated mitochondria from PASMCs. ROS generation was determined by measuring DCF-derived fluorescence by the mean of H2DCF/DA (DCF). (F) Ca2+ concentration-dependent ROS generation in freshly isolated mitochondria was observed by using the chemiluminescent dye lucigenin. (G) Application of Ca2+ concentration-dependent increased ROS generation in isolated complex III from PASMCs. ROS generation was determined by DCF-derived fluorescence. (H) Ca2+ (200 μM) failed to increase ROS generation (DCF-derived fluorescence) in isolated complex I from PASMCs. (I) Ca2+-dependent ROS generation in isolated complex III from PASMCs was further observed using lucigenin. All data were obtained from three separate experiments. In (B, C), *p < 0.05 compared with control; in (D), *p < 0.05 compared with control in normoxia condition. #p < 0.05 compared with control in hypoxia condition; in (E–H), *p < 0.05 compared with no Ca2+-treated group. [Ca2+]m, intramitochondrial Ca2+ concentration; mit-2mutAEQ, mitochondria-targeted double mutated aequorin; [ROS]m, mitochondrial reactive oxygen species generation.
To test the effect of norepinephrine and caffeine, cells were first transfected with pcDNA3.1+/mit-2mutAEQ and then exposed to different concentrations of norepinephrine (1 μM–10 mM) and caffeine (0.2 mM–2 M). As presented in Figure 3B and C, both caffeine and norepinephrine increased [Ca2+]m in concentration-dependent manner. Interestingly, caffeine- or norepinephrine-induced increase in [Ca2+]m was significantly inhibited by pretreatment with the MCU inhibitor Ru360 (10 μM) for 5 min. Similarly, hypoxia also induced a large increase in [Ca2+]m compared with normoxia, and the hypoxia-induced effect was suppressed by treatment with Ru360 (10 μM) for 5 min (Fig. 3D).
Exogenous Ca2+ application significantly increased ROS generation in mitochondria and complex III isolated from PASMCs
A previous publication (22) has reported that cell stimulation largely increases local [Ca2+]i (20–40 μM) in chromaffin cells, whereas the rest of the cell has a lower [Ca2+]i (1–2 μM). To further understand the Ca2+-dependent [ROS]m in PASMCs, we sought to test the effect of different free Ca2+ concentrations on ROS generation in isolated mitochondria. Various free Ca2+ concentrations were obtained using different EGTA and Ca2+ concentrations with a widely used online program (10). Application of exogenous Ca2+ caused a significant increase in [ROS]m, determined by DCF-derived florescence (Fig. 3E) and lucigenin-derived chemiluminescence (Fig. 3F), in a concentration-dependent manner (1–30 μM) in isolated mitochondria from PASMCs.
[ROS]m in PASMCs primarily occurs at complex III (4, 14, 16, 37, 41, 45); thus, we next examined Ca2+-dependent ROS generation in isolated complex III using H2DCF/DA. Application of exogenous free Ca2+ induced a concentration-dependent increase in ROS generation (DCF-derived fluorescence) in isolated complex III (Fig. 3G). However, isolated complex I from PASMCs did not show the same Ca2+-induced ROS response as complex III did (Fig. 3H). This suggests that complex I may not play a significant role in Ca2+-induced [ROS]m in PASMCs. Ca2+-dependent ROS generation in isolated complex III was also detected by lucigenin, similar to H2DCF/DA (Fig. 3I).
RISP KD inhibited caffeine- and hypoxia-induced ROS generation in PASMCs and Ca2+-evoked ROS generation in mitochondria from PASMCs
We and other investigators have shown that RISP is indispensable for the hypoxic ROS generation in PASMCs (16, 45); thus, we sought to determine the role of RISP in CIRG. As described in our previous reports (16, 48), infection with lentiviral shRNAs specific for RISP largely knocked down its protein expression in PASMCs (Fig. 4A). Similar results were observed in three different experiments. In contrast, infection of lentiviral nonsilencing (NS) shRNAs did not significantly suppress RISP expression.
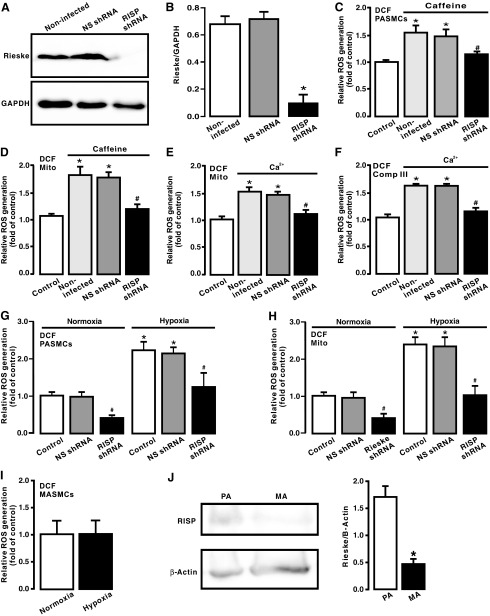
FIG. 4.
RISP KD blocked caffeine- and hypoxia-induced ROS generation in PASMCs and Ca2+-evoked ROS generation in mitochondria. (A) Western blot image of RISP expression in PASMCs uninfected, infected with lentiviral RISP shRNAs, and infected with lentiviral NS shRNAs. (B) Summary of Western blot analysis of RISP expression levels in PASMCs uninfected, infected with lentiviral RISP shRNAs, and infected with lentiviral NS shRNAs. (C) Caffeine (20 mM)-evoked ROS generation, determined by assessing DCF-derived fluorescence, in PASMCs uninfected, infected with lentiviral RISP shRNAs, and infected with lentiviral NS shRNAs. (D) ROS generation (DCF-derived fluorescence) in isolated mitochondria from PASMCs uninfected and untreated with caffeine as well as uninfected, infected with lentiviral RISP shRNAs, and infected with lentiviral NS shRNAs followed by treatment with caffeine (20 mM) for 5 min. (E) ROS generation in isolated mitochondria from PASMCs uninfected and untreated with exogenous Ca2+, as well as uninfected, infected with lentiviral RISP shRNAs, and infected with lentiviral NS shRNAs followed by treatment with Ca2+ (3 μM) for 5 min. (F) ROS generation in complex III from PASMCs uninfected and untreated with Ca2+, as well as uninfected, infected with lentiviral RISP shRNAs, and infected with lentiviral NS shRNAs followed by treatment with Ca2+ (3 μM) for 5 min. (G) ROS generation in PASMCs uninfected, infected with lentiviral RISP shRNAs, and infected with lentiviral NS shRNAs followed by normoxic or hypoxic exposure for 5 min. (H) ROS generation in isolated mitochondria from PASMCs uninfected, infected with lentiviral RISP shRNAs, and infected with lentiviral NS shRNAs followed by normoxic or hypoxic exposure for 5 min. (I) ROS generation in MASMC following normoxic or hypoxic exposure for 5 min. (J) Western blot analysis of RISP expression in PASMCs and MASMCs. All data were obtained from at least three separate experiments. *p < 0.05 compared with control; #p < 0.05 compared with caffeine- or Ca2+-treated samples. KD, knockdown; NS, nonsilencing; RISP, Rieske iron–sulfur protein; shRNA, short hairpin RNA.
Corresponding to the suppressed RISP expression, caffeine-evoked ROS generation was abolished in PASMCs infected with lentiviral RISP shRNAs (Fig. 4B). Similarly, ROS generation was completely blocked in isolated mitochondria from cells infected with lentiviral RISP shRNAs followed by treatment with caffeine (Fig. 4C).
Moreover, we observed that exogenous CIRG was eliminated in isolated mitochondria and complex III from PASMCs infected with lentiviral RISP shRNAs, whereas ROS generation was not affected in isolated mitochondria and complex III from cells infected with lentiviral NS shRNAs (Fig. 4D, E).
We also demonstrated that hypoxia-induced ROS generation was significantly inhibited in RISP KD PASMCs, isolated mitochondria, and complex III. RISP KD also slightly suppressed ROS generation under normoxic conditions (Fig. 4F, G). Additionally, we showed that hypoxia-induced ROS generation only occurred in PASMCs, but not in MASMCs (Fig. 4H), which are consistent with our previous reports (28, 29, 34, 37, 39, 48, 49). Interestingly, RISP expression level was significantly higher in PASMCs than in MASMCs (Fig. 4I). All these findings support our new concept that CIRG only functions in PASMCs, but not in systemic arterial smooth muscle cells; thus, RISP plays a primary role in mediating hypoxia-induced CIRG in PASMCs.
Inhibition of mitochondrial Ca2+ uptake blocked Ca2+-evoked [ROS]m in PASMCs
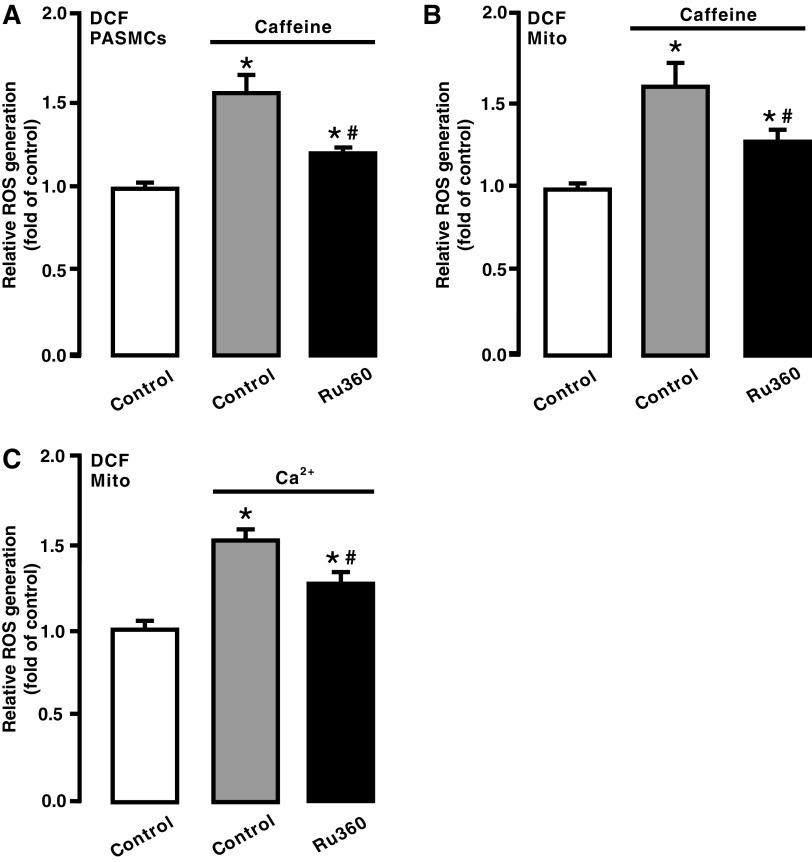
We assumed that caffeine-induced Ca2+ release from the SR via RyRs might cause mitochondrial Ca2+ uptake and accordingly mediate mitochondrial CIRG in PASMCs. To test this assumption, we assessed the effect of the MCU inhibitor Ru360. Following treatment with Ru360 (10 μM) for 5 min, cells were exposed to caffeine (20 mM) for 5 min in the continued presence of Ru360. As shown in Figure 5A, treatment with Ru360 attenuated caffeine-elicited ROS generation in PASMCs. ROS generation was also prevented in isolated mitochondria from cells exposed to caffeine after pretreatment with Ru360 (Fig. 5B).
FIG. 5.
Treatment with Ru360 prevented caffeine-induced ROS generation in PASMCs and mitochondria. (A) ROS generation in PASMCs untreated, treated with caffeine (20 mM) for 5 min, and treated with Ru360 (10 μM) for 5 min followed by caffeine (20 mM) for 5 min in the continued presence of Ru360. (B) ROS generation in isolated mitochondria from PASMCs untreated, treated with caffeine, or treated with Ru360 followed by caffeine. (C) ROS generation in isolated mitochondria from untreated, treated with Ca2+ (3 μM) for 5 min, or treated with Ru360 followed by exogenous Ca2+. Data were obtained from three separate experiments. *p < 0.05 compared with control, and #p < 0.05 compared with caffeine- or Ca2+-treated group.
In agreement with its effect on caffeine-induced responses, treatment with Ru360 blocked Ca2+-evoked ROS generation in isolated mitochondria (Fig. 5C).
Inhibition of mitochondrial Ca2+ uptake attenuated hypoxia-induced ROS generation in PASMCs and mitochondria
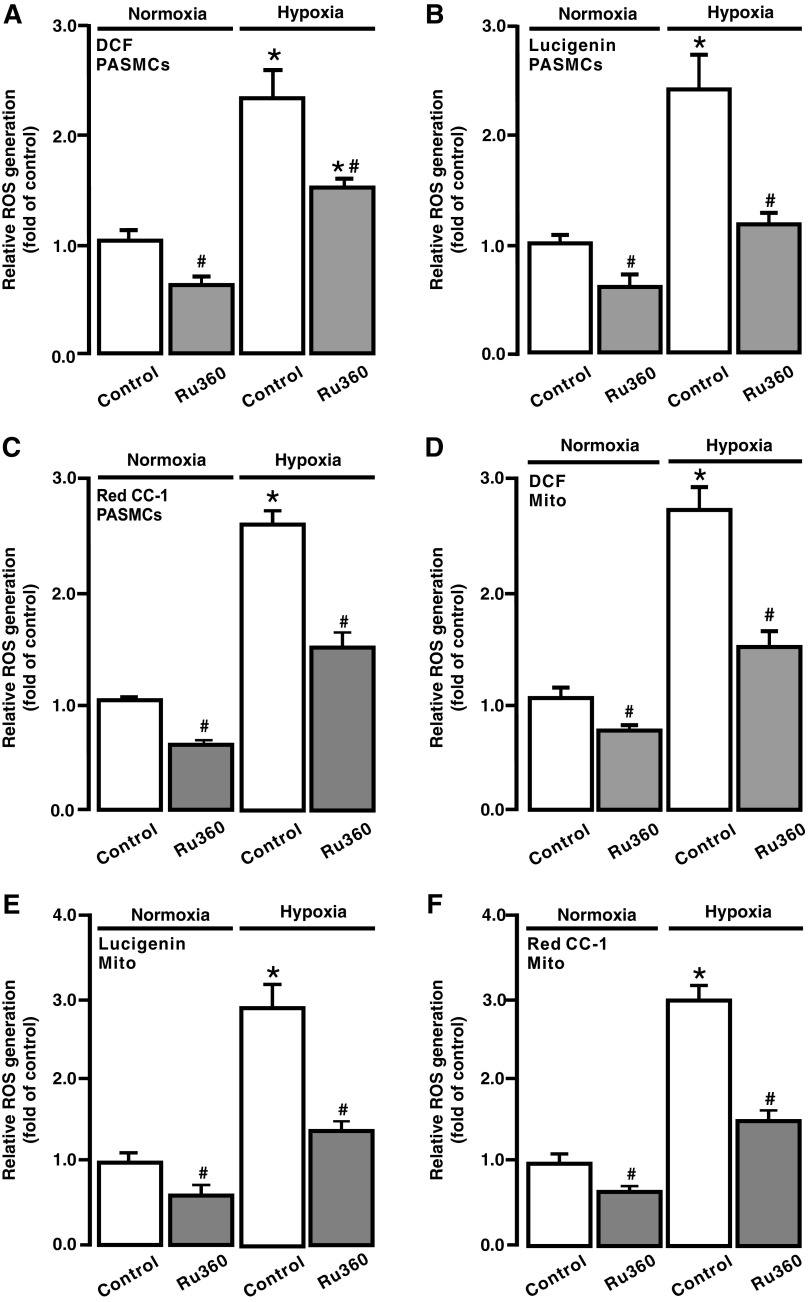
Consistent with our previous reports (18, 28, 29, 37, 48), exposure to acute hypoxia for 5 min caused a large increase in ROS generation in isolated PASMCs, detected by DCF (Fig. 6A), Red CC-1 (Fig. 6B), and lucigenin (Fig. 6C). The hypoxic ROS generation was inhibited in cells following treatment with Ru360 (10 μM) for 5 min. In addition, Ru360 had an inhibitory effect on the resting ROS generation in PASMCs under normoxic conditions.
FIG. 6.
Ru360 attenuated hypoxia-induced ROS generation in PASMCs and mitochondria. ROS generation, determined using H2DCF/DA (DCF) (A), lucigenin (B), and Red CC-1 (C), in PASMCs untreated and treated with Ru360 (10 μM) for 5 min followed by normoxia or hypoxia for 5 min. ROS generation, assessed using DCF (D), lucigenin (E), and Red CC-1 (F), in isolated mitochondria from PASMCs untreated and treated with Ru360 (10 μM) for 5 min followed by normoxia or hypoxia for 5 min. Data were obtained from three separate experiments. *p < 0.05 compared with normoxic control, and #p < 0.05 compared with hypoxic control.
To further evaluate [ROS]m, isolated mitochondria were treated with Ru360 (10 μM) for 5 min followed by hypoxic exposure for 5 min. As shown in Figure 6D–F, hypoxia induced a much smaller ROS generation in isolated mitochondria following treatment with Ru360. The resting [ROS]m was also slightly inhibited after treatment with Ru360.
Inhibition of RyRs attenuated the hypoxic ROS generation in PASMCs
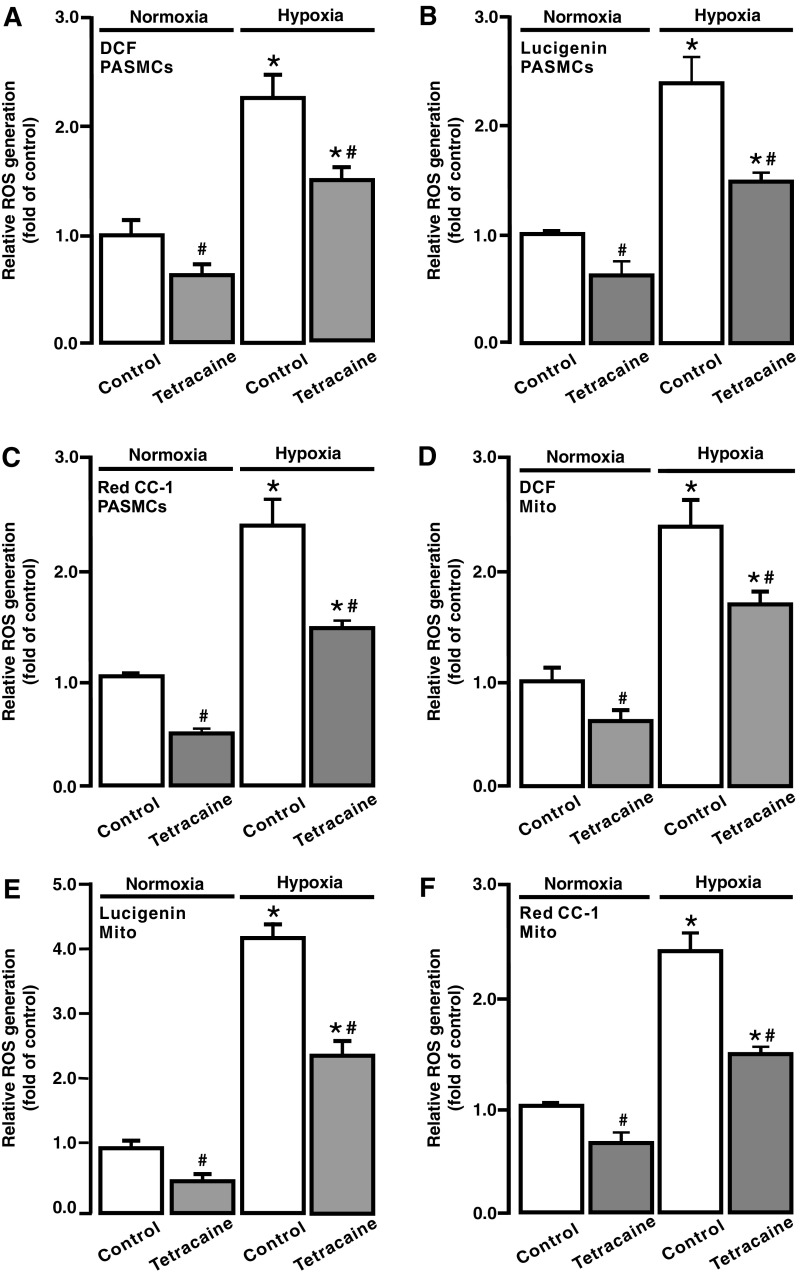
ROS-dependent RyR-mediated Ca2+ release is essential for the hypoxic increase in [Ca2+]i in PASMCs (41, 52); thus, we studied whether RyRs may play a significant role in the hypoxic ROS generation in PASMCs. Cells were treated with the RyR antagonist tetracaine (10 μM) for 5 min and then exposed to hypoxia with tetracaine for 5 min. ROS generation was largely suppressed in PASMCs after treatment with tetracaine; the resting ROS generation was also somewhat inhibited in cells under normoxic conditions (Fig. 7A–C).
FIG. 7.
Inhibition of RyRs with tetracaine blocked hypoxic ROS generation in PASMCs and mitochondria. ROS generation, measured using H2DCF/DA (DCF) (A), lucigenin (B), and Red CC-1 (C), in PASMCs treated without and with tetracaine (10 μM) for 5 min following normoxia or hypoxia for 5 min. ROS generation, evaluated using DCF (D), lucigenin (E), and Red CC-1 (F), in isolated mitochondria from PASMCs untreated and treated with tetracaine (10 μM) for 5 min followed by normoxia or hypoxia for 5 min. Data were obtained from three separate experiments. *p < 0.05 compared with normoxic control, and #p < 0.05 compared with hypoxic control. RyR, ryanodine receptor.
We next assessed the effect of tetracaine on [ROS]m. To achieve this, cells were treated with tetracaine, mitochondria were isolated, and ROS generation was measured. Hypoxia-induced and resting [ROS]m were attenuated in cells pretreated with tetracaine (Fig. 7D–F).
RyR2 gene KO blocked the hypoxia-induced [ROS]m in PASMCs
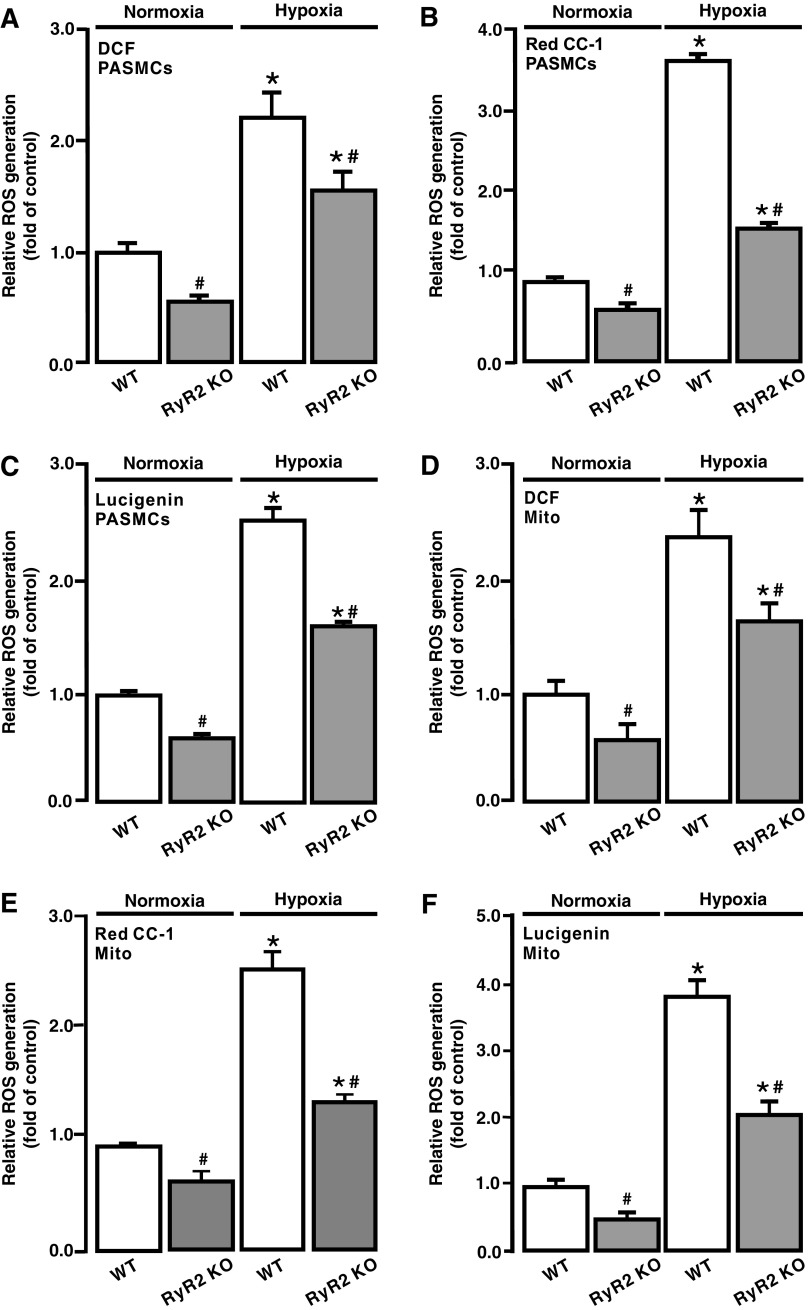
Our previous studies have shown that RyR1, RyR2, and RyR3 all are expressed and mediate acute hypoxic Ca2+ and contractile responses in PASMCs; however, the role of RyR2 dominates over that of RyR1 and RyR3 (17, 18, 51). In complement to the effect of pharmacological inhibition of RyRs, we examined the effect of RyR2 gene KO on the hypoxic ROS generation in PASMCs. As shown in Figure 8A–C, acute hypoxic exposure for 5 min caused a much smaller ROS generation in PASMCs isolated from RyR2 KO mice than control (wild type [WT]) animals. The resting ROS generation was also reduced in RyR2 KO PASMCs.
FIG. 8.
RyR2 gene knockout blocked hypoxia-induced ROS generation in PASMCs and mitochondria. ROS generation, assessed using DCF (A), lucigenin (B), and Red CC-1 (C), in PASMCs from WT and RyR2 KO mice. ROS generation, examined using H2DCF/DA (DCF) (A), lucigenin (B), and Red CC-1 (C), in PASMCs from WT and RyR2 KO mice. ROS generation, assessed using DCF (D), lucigenin (E), and Red CC-1 (F), in isolated mitochondria from WT and RyR2 KO mouse PASMCs. Data were obtained from three separate experiments. *p < 0.05 compared with normoxic WT group, and #p < 0.05 compared with hypoxic WT group. KO, knockout; WT, wild type.
The hypoxic increase in ROS generation was significantly suppressed in isolated mitochondria from RyR2 KO PASMCs, and the resting [ROS]m was decreased as well (Fig. 8D–F).
Discussion
One of the major novel findings resulting from this study is that the application of the classic RyR agonist caffeine to activate RyRs on the SR can cause a significant [ROS]m (i.e., CIRG) in isolated PASMCs. This discovery complements our previous reports, in which increasing [ROS]i (e.g., application of H2O2) opens RyRs and induces SR Ca2+ release (i.e., RICR), leading to an increase in [Ca2+]i in PASMCs (18, 28, 29, 37). ROS-mediated increase in [Ca2+]i plays an important role in hypoxia-induced pulmonary vasoconstriction, vasoremodeling, and hypertension (1, 41, 53). Presumably, the crosstalk between Ca2+ and ROS signaling may enhance the hypoxia-induced ROS generation in PASMCs, which provides a positive feedback mechanism to mediate the hypoxic ROS generation and associated cellular responses.
In this study, we have also found that the application of norepinephrine, a major neurotransmitter in vascular SMCs to activate α-adrenergic receptors and increase [Ca2+]i (21, 24, 50, 51), can result in a large increase in ROS generation as well in PASMCs. It is well known that the activation of α-adrenergic receptors induces SR Ca2+ release by IP3Rs (21, 24, 50, 51). Ca2+ release via IP3Rs may activate adjacent RyRs to induce further SR Ca2+ release, that is, a local Ca2+-induced Ca2+ release (CICR) process (51), in PASMCs. Thus, the role of norepinephrine to induce ROS generation is likely to be, at least in part, implemented by RyR-dependent Ca2+ release as a result of local IP3R/RyR interaction-mediated CICR (19).
We and other investigators have reported that mitochondria are a major source for ROS generation in PASMCs, and mitochondrial ROS are primarily generated at complex III (18, 28, 29, 37, 42, 43, 46, 47). Consistent with these previous reports, the current study has shown that ROS generation is largely increased in isolated mitochondria from PASMCs pretreated with caffeine to induce Ca2+ release due to activation of RyRs. Likewise, ROS generation is also augmented in isolated mitochondria from PASMCs after treatment with norepinephrine to induce Ca2+ release from the SR as a result of the opening of IP3Rs (19, 51).
Moreover, by using mitochondria-targeted Ca2+ sensor, we successfully measured and quantified the changes in [Ca2+]m in cells following exposure of different concentrations of caffeine or norepinephrine. In complement to caffeine- and norepinephrine-induced responses, different concentrations of exogenous Ca2+ also significantly increase ROS generation in isolated mitochondria and complex III from PASMCs. These data not only reveal that Ca2+ release from the SR may cause [ROS]i (CIRG) primarily at mitochondrial complex III in PASMCs but also further support our novel concept that the SR can locally communicate with closely neighboring mitochondria in the format of CIRG in PASMCs.
Our recent work has unveiled that RISP KD at complex III blocks the hypoxic ROS generation in isolated PASMCs, mitochondria, and complex III, whereas its gene overexpression produces the opposite effects; thus, RISP is a crucial molecule for the hypoxic ROS generation in PASMCs (16, 48). In agreement, we have also shown that RISP plays an important role in hypoxia-induced increase in [Ca2+]i in PASMCs and vasoconstriction in PAs (16, 48). Supporting our findings, Waypa et al. have also found that RISP KO abolishes the hypoxia-induced increase in [Ca2+]i in PAs and right ventricular systolic pressure (45). In line with the important role of RISP, in the current study, we have discovered that RISP KD inhibits Ca2+- and caffeine-induced ROS generation in isolated mitochondria and/or complex III from PASMCs. Further experiments are needed to determine whether Ca2+ may cause [ROS]m by producing a direct or an indirect effect on RISP in PASMCs.
We have surmised that the increased [Ca2+]i may give rise to an increase in mitochondrial Ca2+ uptake, leading to ROS generation in mitochondria and at complex III. Consistent with our conjecture, treatment with Ru360, a specific mitochondrial Ca2+ uptake inhibitor (1), prevents caffeine from inducing ROS generation in PASMCs. Likewise, caffeine-induced ROS generation is also blocked in mitochondria from PASMCs after treatment with Ru360. More interestingly, Ru360 inhibits hypoxia-induced [ROS]m. These results reveal that mitochondrial Ca2+ uptake serves as a critical step for Ca2+-dependent [ROS]m, thereby playing an important role in hypoxic cellular responses in PASMCs.
Previous investigations from our laboratory and others have demonstrated that RyRs play an important role in hypoxia-induced increase in [Ca2+]i in PASMCs and pulmonary vasoconstriction in PAs (17, 18, 25–27, 36, 51). Hypoxia may inhibit voltage-dependent K+ (Kv) channels and activate store-operated Ca2+ (SOC) channels, leading to Ca2+ and contractile responses in PASMCs; however, the hypoxic inhibition of Kv channels and the activation of SOC channels may be secondary to SR Ca2+ release, presumably via RyRs (25–27). These data reinforce the importance of RyRs in hypoxic cellular responses in PASMCs.
Our laboratory and others have demonstrated that both pharmacological and genetic inhibition of [ROS]m almost completely inhibit hypoxia-induced Ca2+ and contractile responses in PASMCs (8, 16, 18, 28, 29, 37, 42–46, 48). As such, we have assumed that hypoxia-induced, ROS-initiated RICR may cause further ROS generation (CIRG), providing a positive feedback mechanism to contribute to hypoxia-evoked physiological and pathological functions in PASMCs. Indeed, in this study, we have observed that treatment with tetracaine to block RyRs remarkably inhibits the hypoxic ROS generation in PASMCs. The hypoxic response is also reduced in isolated mitochondria from PASMCs after pretreatment with tetracaine.
Our previous investigations have demonstrated that RyR1 or RyR3 KO can partially inhibit, whereas RyR2 KO completely inhibits, the hypoxia-induced increase in [Ca2+]i in PASMCs and pulmonary vasoconstriction in PAs (17, 18, 51). Thus, all three known RyR subtypes are involved in hypoxic Ca2+ and contractile responses in PASMCs; however, RyR2 is the most valuable player. In agreement with this notion, we have unveiled that hypoxia causes a much smaller increase in ROS generation in PASMCs from RyR2 KO mice than control (WT) mice. Furthermore, the hypoxic ROS generation is significantly reduced in isolated mitochondria from RyR2 KO mice. These pharmacological and genetic findings for the first time demonstrate that RyR2 functions as a key molecule to implement the focal communication from the SR to mitochondria, leading to CIRG in PASMCs during hypoxic stimulation.
In summary, our current study proves a concept that Ca2+ release from the SR via RyR2 can increase [Ca2+]i, mitochondrial Ca2+ uptake, and RISP-dependent mitochondrial CIRG. Previously, we have discovered that hypoxia first causes ROS generation and then the opening of RyRs to induce Ca2+ release from the SR in PASMCs, leading to HPV and associated pulmonary hypertension (16, 18, 28, 29, 37, 48, 49). Similar findings have been reported by Waypa et al. (43, 45, 46). Here, we present evidence that hypoxia-induced SR Ca2+ release is uptaken by MCUs, which in turn increases [Ca2+]m and subsequently leads to RISP-dependent [ROS]m, providing a positive feedback mechanism to contribute to the hypoxia-induced increase in [ROS]i in PASMCs. This novel communication from the SR to mitochondria in the form of CIRG may play an important role in the overall hypoxia-induced Ca2+ and contractile responses in PASMCs.
Materials and Methods
Preparation of PASMCs
PASMCs were prepared from mouse resistance PAs as we have described previously (16–18, 28, 29, 37, 48, 51). All experiments using mice were conducted in compliance with the National Institutes of Health guidelines and approved by the Animal Care and Use Committee of Albany Medical College. Resistance (third and smaller intralobar) PAs were dissected, with removal of the endothelium and connective tissues, from male Swiss Webster mice at 2 months old. PASMCs were isolated from the dissected PAs using the two-step enzymatic digestion method, in which the arteries were digested with papain and then collagenase in physiological saline solution (PSS). The harvested PASMCs were cultured in modified Dulbecco's minimal essential medium for three passages and used in experiments.
PASMCs from smooth muscle-conditional RyR2 KO mice (C57/B6 background) were obtained using the same method described above. RyR2 KO mice were generated by crossbreeding RyR2flox/flox mice with smooth muscle-specific myosin heavy-chain Cre recombinase mice. The KO mice were genotyped by polymerase chain reaction analysis of tail tip DNAs. Western blot analysis revealed that these mice showed the absence of RyR2 expression in PAs.
Detection of ROS generation
ROS generation in PASMCs was first measured using CM-H2DCF/DA (Molecular Probes), as this fluorescent dye has been most widely used to determine [ROS]i (mainly H2O2) in living cells (5, 32, 37, 41). The measurement was conducted, as described in our previous publications (16, 28, 29, 37). A suspension of PASMCs was incubated with CM-H2DCF/DA (5 μM) at 37°C for 30 min, followed by washing for 10 min. The dye was excited at 485 nm, and the emitted fluorescence at 532 nm was collected using a FlexStation-III spectrophotometer (Molecular Devices). Fluorescent intensity was normalized to control.
There are the criticisms of the use of H2DCF due to its oxidation and cytotoxicity; however, this probe at low concentrations (1–10 μM) in serum-free media is suitable for the ROS measurement in almost all cell types (9, 15, 23, 41). Despite this fact, we utilized the chemiluminescent probe lucigenin (mainly for measuring O2−) as a supplementary approach to examine [ROS]i in PASMCs (37). Lucigenin (20 μM; Molecular Probes) was added to one well of a 96-well plate containing 1.5 × 105 PASMCs. The emitted chemiluminescence derived by lucigenin was detected. The initial (maximal) values of lucigenin-derived chemiluminescence were normalized to the control group. The fluorescent Redox Sensor Red CC-1 was also used to measure both O2− and H2O2 generation (37). Cells were loaded with Red CC-1 (1 μM; Molecular Probes) at 37°C for 30 min. Fluorescence derived from Red CC-1 was measured using an excitation wavelength of 544 nm and emission wavelength of 612 nm. [ROS]i was determined by the difference in fluorescence between the wells containing the assay buffer with and without cells.
To detect ROS generation in isolated mitochondria, we utilized the same method reported in our previous publication (16). Isolated mitochondrial samples (50 μg protein) were added in microplate wells containing 5 mM pyruvate, 2.5 mM malate, 10 μM H2DCF/DA, and 5 μM horseradish peroxidase (HRP). After 10 min of incubation, different concentrations of caffeine, norepinephrine, and free Ca2+ were added to medium. Fluorescence was measured at 37°C using the FlexStation-III spectrophotometer with an excitation wavelength of 485 nm and emission wavelength of 532 nm. ROS generation was determined by subtracting the fluorescence intensity measured in control wells that contained assay buffer without mitochondria, H2DCF/DA, and HRP. Lucigenin was also used in detecting ROS generation in mitochondria. Fifty micrograms of isolated mitochondrial sample was incubated with lucigenin (20 μM), 5 mM pyruvate, and 2.5 mM malate for 10 min. Lucigenin-derived chemiluminescence was detected in an absorbance of 550 nm after the addition of different concentrations of free Ca2+ to the medium.
To detect ROS generation in complex III, immunocapture kit specific for complex III (Abcam) was used to isolate according to the manufacturer's instruction, as we have described previously (16). Freshly isolated complex III (3 μg) was incubated in the respiration buffer containing 40 μM reduced decylubiquinone, 2 mM potassium cyanide, 50 μM cytochrome c, 10 μM H2DCF/DA, and 5 μM HRP for 10 min. Different concentrations of free Ca2+ or the hypoxic gas mixture were applied to respiration buffer, and ROS were detected at 37°C using the FlexStation-III spectrophotometer. Lucigenin was also used in detecting ROS generation in complex III. Three micrograms of isolated complex III was incubated with buffer containing 40 μM reduced decylubiquinone, 2 mM potassium cyanide, 50 μM cytochrome c, and 20 μM lucigenin for 10 min. Different concentrations of free Ca2+ were added to medium, and lucigenin-derived chemiluminescence was detected by luminometer in an absorbance of 550 nm.
ROS generation in complex I was assessed using a similar procedure to that used in the aforementioned complex III experiments. Complex I was isolated using its specific immunocapture kit (Abcam), and ROS were measured in buffer containing 8 mM MgCl2, 2.5 mg/mL bovine serum albumin, 0.15 mM NADH, and 1 mM potassium cyanide (16, 20).
Intracellular ROS were also assessed using the specific ROS biosensor pHyPer-cyto (2, 16). Primary PASMCs were transfected with pHyPer-cyto plasmid vector. After transfection for 48 h, caffeine or norepinephrine was added to induce ROS generation. HyPer was alternatively excited at 420 and 500 nm. Emitted fluorescence at 510 nm was measured at 37°C using the FlexStation-III spectrophotometer.
[Ca2+]m detection with mutant aequorin
Mitochondria-targeted Ca2+ sensor pcDNA3.1+/mit-2mutAEQ (Asp119 to Ala, Asn28 to Leu points mutations on aequorin) was a gift from Javier Alvarez-Martin (plasmid No. 45539; Addgene, Watertown, MA). Following the procedure provided by Addgene (6), pcDNA3.1+/mit-2mutAEQ was purified and transfected to primary cultured mouse PASMCs for 48 h. After transfection, PASMCs were incubated for 2 h at room temperature in standard medium (145 mM NaCl, 5 mM KCl, 1 mM MgCl2, 1 mM CaCl2, 10 mM glucose, and 10 mM HEPES [pH 7.4]) with 2 μM coelenterazine w (Biotium).
After incubation, PASMCs were treated with different concentrations of caffeine and norepinephrine for 5 min. Emitted luminescence was recorded by a luminometer and then calibrated for the Ca2+ concentration by following a previous publication (6).
Rieske iron–sulfur protein KD
Lentiviral shRNAs specific for RISP were used to KD its expression in PASMCs, as described in our previous publications (16, 48). Lentiviruses containing RISP shRNAs and nonsilence shRNAs were purchased from Thermo Scientific OpenBiosystems and then packaged using pCMV-dR8.2 dvpr and pCMV-VSV-G packing vectors. The efficiency of RISP KD was determined using Western blot analysis.
Hypoxia
To induce hypoxic responses, cell, mitochondrion, or complex preparations were treated with a normoxic PSS (110 mM NaCl, 3.4 mM KCl, 2.4 mM CaCl2, 0.8 mM MgSO4, 25.8 mM NaHCO3, 1.2 mM KH2PO4, and 5.6 mM glucose [pH 7.4]) that was aerated with a 21% O2, 5% CO2, and 74% N2 mixture for 10 min and then hypoxic PSS gassed with a 1% O2, 5% CO2, and 94% N2 mixture for 5 min, as we have reported previously (16–18, 38, 48, 51). As a control, preparations were treated with normoxic PSS alone.
Statistical analysis
Levels of statistical significance was evaluated with data from at less three independent experiments using one- or two-way analysis of variance with an appropriate post hoc test. Data are shown as means ± standard error of the mean. The differences between the means of data at p < 0.05 were considered statistically significant.
Abbreviations Used
- [Ca2+]i
intracellular Ca2+ concentration
- [Ca2+]m
intramitochondrial Ca2+ concentration
- CICR
Ca2+-induced Ca2+ release
- CIRG
Ca2+-induced reactive oxygen species generation
- CM-H2DCF/DA
chloromethyldihydrodichlorofluorescein diacetate
- HPV
hypoxic pulmonary vasoconstriction
- HRP
horseradish peroxidase
- IP3R
inositol-1,4,5-trsiphosphate receptor
- KD
knockdown
- KO
knockout
- Kv
voltage-dependent K+
- MASMCs
mesentery (systemic) artery smooth muscle cells
- MCU
mitochondrial Ca2+ uniporter
- mit-2mutAEQ
mitochondria-targeted double-mutated aequorin
- NS
nonsilencing
- PA
pulmonary artery
- PASMC
pulmonary artery smooth muscle cell
- PSS
physiological saline solution
- RICR
reactive oxygen species-induced Ca2+ release
- RISP
Rieske iron–sulfur protein
- ROS
reactive oxygen species
- [ROS]i
intracellular ROS generation
- [ROS]m
mitochondrial reactive oxygen species generation
- RyR
ryanodine receptor
- shRNA
short hairpin RNA
- SOC
store-operated Ca2+
- SR
sarcoplasmic reticulum
- WT
wild type
Author Disclosure Statement
No competing financial interests exist.
Funding Information
This work was supported by the American Heart Association (AHA) Scientist Development Grant 0630236N (Y.-M.Z.) and Established Investigator Award 0340160N (Y.-X.W.), National Natural Science Foundation of China 81160011 (Z.Y.), and National Institutes of Health R01HL108232 and R01HL122865 (Y.-X.W.).
References
- 1. Aggarwal S, Gross CM, Sharma S, Fineman JR, and Black SM. Reactive oxygen species in pulmonary vascular remodeling. Compr Physiol 3: 1011–1034, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Belousov VV, Fradkov AF, Lukyanov KA, Staroverov DB, Shakhbazov KS, Terskikh AV, and Lukyanov S. Genetically encoded fluorescent indicator for intracellular hydrogen peroxide. Nat Methods 3: 281–286, 2006 [DOI] [PubMed] [Google Scholar]
- 3. Cadenas E and Boveris A. Enhancement of hydrogen peroxide formation by protophores and ionophores in antimycin-supplemented mitochondria. Biochem J 188: 31–37, 1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chandel NS, McClintock DS, Feliciano CE, Wood TM, Melendez JA, Rodriguez AM, and Schumacker PT. Reactive oxygen species generated at mitochondrial complex III stabilize hypoxia-inducible factor-1alpha during hypoxia: a mechanism of O2 sensing. J Biol Chem 275: 25130–25138, 2000 [DOI] [PubMed] [Google Scholar]
- 5. Chiou YS, Huang Q, Ho CT, Wang YJ, and Pan MH. Directly interact with Keap1 and LPS is involved in the anti-inflammatory mechanisms of (-)-epicatechin-3-gallate in LPS-induced macrophages and endotoxemia. Free Radic Biol Med 11: 1–16, 2016 [DOI] [PubMed] [Google Scholar]
- 6. de la Fuente S, Fonteriz RI, de la Cruz PJ, Montero M, and Alvarez J. Mitochondrial free [Ca2+] dynamics measured with a novel low-Ca2+ affinity aequorin probe. Biochem J 445: 371–376, 2012 [DOI] [PubMed] [Google Scholar]
- 7. De la Fuente S and Sheu SS. SR-mitochondria communication in adult cardiomyocytes: a close relationship where the Ca2+ has a lot to say. Arch Biochem Biophys 663: 259–268, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Desireddi JR, Farrow KN, Marks JD, Waypa GB, and Schumacker PT. Hypoxia increases ROS signaling and cytosolic Ca2+ in pulmonary artery smooth muscle cells of mouse lungs slices. Antioxid Redox Signal 12: 595–602, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dikalov S, Griendling KK, and Harrison DG. Measurement of reactive oxygen species in cardiovascular studies. Hypertension 49: 717–727, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dong L, Zheng YM, Van RD, Rathore R, Liu QH, Singer HA, and Wang YX. Functional and molecular evidence for impairment of calcium-activated potassium channels in type-1 diabetic cerebral artery smooth muscle cells. J Cereb Blood Flow Metab 28: 377–386, 2008 [DOI] [PubMed] [Google Scholar]
- 11. Feissner RF, Skalska J, Gaum WE, and Sheu SS. Crosstalk signaling between mitochondrial Ca2+ and ROS. Front Biosci (Landmark Ed) 14: 1197–1218, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fill M and Copello JA. Ryanodine receptor calcium release channels. Physiol Rev 82: 893–922, 2002 [DOI] [PubMed] [Google Scholar]
- 13. Guibert C, Ducret T, and Savineau J-P. Ionic channels as therapeutic targets in pulmonary hypertension. In: Recent Advances in Pulmonary Vascular Biology, edited by Wang Y-X. Trivandrum: Research Signpost, 2011, pp. 57–80 [Google Scholar]
- 14. Guzy RD, Hoyos B, Robin E, Chen H, Liu L, Mansfield KD, Simon MC, Hammerling U, and Schumacker PT. Mitochondrial complex III is required for hypoxia-induced ROS production and cellular oxygen sensing. Cell Metab 1: 401–408, 2005 [DOI] [PubMed] [Google Scholar]
- 15. Halliwell B and Whiteman M. Measuring reactive species and oxidative damage in vivo and in cell culture: how should you do it and what do the results mean? Br J Pharmacol 142: 231–255, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Korde AS, Yadav VR, Zheng YM, and Wang YX. Primary role of mitochondrial Rieske iron-sulfur protein in hypoxic ROS production in pulmonary artery myocytes. Free Radic Biol Med 50: 945–952, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Li XQ, Zheng YM, Rathore R, Ma J, Takeshima H, and Wang YX. Genetic evidence for functional role of ryanodine receptor 1 in pulmonary artery smooth muscle cells. Pflugers Arch 457: 771–783, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liao B, Zheng YM, Yadav VR, Korde AS, and Wang YX. Hypoxia induces intracellular Ca2+ release by causing reactive oxygen species-mediated dissociation of FK506-binding protein 12.6 from ryanodine receptor 2 in pulmonary artery myocytes. Antioxid Redox Signal 14: 37–47, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liu QH, Zheng YM, Korde AS, Yadav VR, Rathore R, Wess J, and Wang YX. Membrane depolarization causes a direct activation of G protein-coupled receptors leading to local Ca2+ release in smooth muscle. Proc Natl Acad Sci U S A 106: 11418–11423, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lopez-Fabuel I, Le Douce J, Logan A, James AM, Bonvento G, Murphy MP, Almeida A, and Bolanos JP. Complex I assembly into supercomplexes determines differential mitochondrial ROS production in neurons and astrocytes. Proc Natl Acad Sci U S A 113: 13063–13068, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mei L, Zheng YM, and Wang YX. Ryanodine and inositol trisphosphate receptors/Ca2+ release channels in airway smooth muscle cells. In: Calcium Signaling in Airway Smooth Muscle Cells, edited by Wang YX. Cham: Springer International Publishing, 2014, pp. 1–20 [Google Scholar]
- 22. Montero M, Alonso MT, Carnicero E, Cuchillo-Ibanez I, Albillos A, Garcia AG, Garcia-Sancho J, and Alvarez J. Chromaffin-cell stimulation triggers fast millimolar mitochondrial Ca2+ transients that modulate secretion. Nat Cell Biol 2: 57–61, 2000 [DOI] [PubMed] [Google Scholar]
- 23. Munzel T, Afanas'ev IB, Kleschyov AL, and Harrison DG. Detection of superoxide in vascular tissue. Arterioscler Thromb Vasc Biol 22: 1761–1768, 2002 [DOI] [PubMed] [Google Scholar]
- 24. Narayanan D, Adebiyi A, and Jaggar JH. Inositol trisphosphate receptors in smooth muscle cells. Am J Physiol Heart Circ Physiol 302: H2190–H2210, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ng LC, Wilson SM, and Hume JR. Mobilization of sarcoplasmic reticulum stores by hypoxia leads to consequent activation of capacitative Ca2+ entry in isolated canine pulmonary arterial smooth muscle cells. J Physiol 563: 409–419, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ng LC, Wilson SM, McAllister CE, and Hume JR. Role of InsP3 and ryanodine receptors in the activation of capacitative Ca2+ entry by store depletion or hypoxia in canine pulmonary arterial smooth muscle cells. Br J Pharmacol 152: 101–111, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Post JM, Gelband CH, and Hume JR. [Ca2+]i inhibition of K+ channels in canine pulmonary artery. Novel mechanism for hypoxia-induced membrane depolarization. Circ Res 77: 131–139, 1995 [DOI] [PubMed] [Google Scholar]
- 28. Rathore R, Zheng YM, Li XQ, Wang QS, Liu QH, Ginnan R, Singer HA, Ho YS, and Wang YX. Mitochondrial ROS-PKCɛ signaling axis is uniquely involved in hypoxic increase in [Ca2+]i in pulmonary artery smooth muscle cells. Biochem Biophys Res Commun 351: 784–790, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rathore R, Zheng YM, Niu CF, Liu QH, Korde A, Ho YS, and Wang YX. Hypoxia activates NADPH oxidase to increase [ROS]i and [Ca2+]i through the mitochondrial ROS-PKCɛ signaling axis in pulmonary artery smooth muscle cells. Free Radic Biol Med 45: 1223–1231, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Santulli G, Lewis D, des Georges A, Marks AR, and Frank J. Ryanodine receptor structure and function in health and disease. Subcell Biochem 87: 329–352, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shimoda LA and Undem C. Interactions between calcium and reactive oxygen species in pulmonary arterial smooth muscle responses to hypoxia. Respir Physiol Neurobiol 174: 221–229, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shin HJ, Kwon HK, Lee JH, Anwar MA, and Choi S. Etoposide induced cytotoxicity mediated by ROS and ERK in human kidney proximal tubule cells. Sci Rep 6: 34064, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sylvester JT, Shimoda LA, Aaronson PI, and Ward JP. Hypoxic pulmonary vasoconstriction. Physiol Rev 92: 367–520, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Truong L, Zheng YM, and Wang YX. Mitochondrial Rieske iron-sulfur protein in pulmonary artery smooth muscle: a key primary signaling molecule in pulmonary hypertension. Arch Biochem Biophys 664: 68–75, 2019 [DOI] [PubMed] [Google Scholar]
- 35. Viola HM, Arthur PG, and Hool LC. Transient exposure to hydrogen peroxide causes an increase in mitochondria-derived superoxide as a result of sustained alteration in L-type Ca2+ channel function in the absence of apoptosis in ventricular myocytes. Circ Res 100: 1036–1044, 2007 [DOI] [PubMed] [Google Scholar]
- 36. Wang J, Shimoda LA, and Sylvester JT. Ca2+ responses of pulmonary arterial myocytes to acute hypoxia require release from ryanodine and inositol trisphosphate receptors in sarcoplasmic reticulum. Am J Physiol Lung Cell Mol Physiol 303: L161–L168, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wang QS, Zheng YM, Dong L, Ho YS, Guo Z, and Wang YX. Role of mitochondrial reactive oxygen species in hypoxia-dependent increase in intracellular calcium in pulmonary artery myocytes. Free Radic Biol Med 42: 642–653, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wang YX, Dhulipala PK, and Kotlikoff MI. Hypoxia inhibits the Na+/Ca2+ exchanger in pulmonary artery smooth muscle cells. FASEB J 14: 1731–1740, 2000 [DOI] [PubMed] [Google Scholar]
- 39. Wang YX and Korth M. Effects of doxorubicin on excitation-contraction coupling in guinea pig ventricular myocardium. Circ Res 76: 645–653, 1995 [DOI] [PubMed] [Google Scholar]
- 40. Wang YX and Zheng YM. Role of ROS signaling in differential hypoxic Ca2+ and contractile responses in pulmonary and systemic vascular smooth muscle cells. Respir Physiol Neurobiol 174: 192–200, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wang YX and Zheng YM. ROS-dependent signaling mechanisms for hypoxic Ca2+ responses in pulmonary artery myocytes. Antioxid Redox Signal 12: 611–623, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Waypa GB, Chandel NS, and Schumacker PT. Model for hypoxic pulmonary vasoconstriction involving mitochondrial oxygen sensing. Circ Res 88: 1259–1266, 2001 [DOI] [PubMed] [Google Scholar]
- 43. Waypa GB, Guzy R, Mungai PT, Mack MM, Marks JD, Roe MW, and Schumacker PT. Increases in mitochondrial reactive oxygen species trigger hypoxia-induced calcium responses in pulmonary artery smooth muscle cells. Circ Res 99: 970–978, 2006 [DOI] [PubMed] [Google Scholar]
- 44. Waypa GB, Marks JD, Guzy R, Mungai PT, Schriewer J, Dokic D, and Schumacker PT. Hypoxia triggers subcellular compartmental redox signaling in vascular smooth muscle cells. Circ Res 106: 526–535, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Waypa GB, Marks JD, Guzy RD, Mungai PT, Schriewer JM, Dokic D, Ball MK, and Schumacker PT. Superoxide generated at mitochondrial complex III triggers acute responses to hypoxia in the pulmonary circulation. Am J Respir Crit Care Med 187: 424–432, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Waypa GB, Marks JD, Mack MM, Boriboun C, Mungai PT, and Schumacker PT. Mitochondrial reactive oxygen species trigger calcium increases during hypoxia in pulmonary arterial myocytes. Circ Res 91: 719–726, 2002 [DOI] [PubMed] [Google Scholar]
- 47. Waypa GB and Schumacker PT. Oxygen sensing in hypoxic pulmonary vasoconstriction: using new tools to answer an age-old question. Exp Physiol 93: 133–138, 2008 [DOI] [PubMed] [Google Scholar]
- 48. Yadav VR, Song T, Joseph L, Mei L, Zheng YM, and Wang YX. Important role of PLC-gamma1 in hypoxic increase in intracellular calcium in pulmonary arterial smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 304: L143–L151, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yadav VR, Song T, Mei L, Joseph L, Zheng YM, and Wang YX. PLCgamma1-PKCepsilon-IP3R1 plays an important role in hypoxia-induced calcium response in pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 314: L724–L735, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zheng YM, Mei QB, Wang QS, Abdullaev I, Lai FA, Xin HB, Kotlikoff MI, and Wang YX. Role of FKBP12.6 in hypoxia- and norepinephrine-induced Ca2+ release and contraction in pulmonary artery myocytes. Cell Calcium 35: 345–355, 2004 [DOI] [PubMed] [Google Scholar]
- 51. Zheng YM, Wang QS, Rathore R, Zhang WH, Mazurkiewicz JE, Sorrentino V, Singer HA, Kotlikoff MI, and Wang YX. Type-3 ryanodine receptors mediate hypoxia-, but not neurotransmitter-induced calcium release and contraction in pulmonary artery smooth muscle cells. J Gen Physiol 125: 427–440, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zheng YM and Wang YX. Ryanodine receptors/Ca2+ release channels in pulmonary artery smooth muscle cells. In: Recent Advances in Pulmonary Vascular Biology, edited by Wang YX. Trivandrum: Research Signpost, 2011, pp. 91–114 [Google Scholar]
- 53. Zuo L, Rose BA, Roberts WJ, He F, and Banes-Berceli AK. Molecular characterization of reactive oxygen species in systemic and pulmonary hypertension. Am J Hypertens 27: 643–650, 2014 [DOI] [PubMed] [Google Scholar]