Highlights
* Spatially selective attention affects early auditory processing in 4- and 5-year-old children. * Young children have mechanisms by which attention can modulate perception. * Attended sounds elicit a larger negativity by 80 ms in children and adults. * Nonverbal paradigm can be used to define the relationship between attention and language skill.
Keywords: Auditory, Selective attention, Nonlinguistic, Development, Event-related potentials
Abstract
Under some conditions 4- and 5-year-old children can differentially process sounds from attended and unattended locations. In fact, the latency of spatially selective attention effects on auditory processing as measured with event-related potentials (ERPs) is quite similar in young children and adults. However, it is not clear if developmental differences in the polarity, distribution, and duration of attention effects are best attributed to acoustic characteristics, availability of non-spatial attention cues, task demands, or domain. In the current study adults and children were instructed to attend to one of two simultaneously presented soundscapes (e.g., city sounds or night sounds) to detect targets (e.g., car horn or owl hoot) in the attended channel only. Probes presented from the same location as the attended soundscape elicited a larger negativity by 80 ms after onset in both adults and children. This initial negative difference (Nd) was followed by a larger positivity for attended probes in adults and another negativity for attended probes in children. The results indicate that the neural systems by which attention modulates early auditory processing are available for young children even when presented with nonverbal sounds. They also suggest important interactions between attention, acoustic characteristics, and maturity on auditory evoked potentials.
1. Introduction
Selective attention allows for the preferential processing of information that is most relevant to current goals. Since the amount of information available at one time almost always exceeds the processing capacity of human neural systems, selective attention is critical to our ability to learn in complex environments. The relationship between selective attention and learning is clearly important for young children who are still acquiring many perceptual and cognitive skills. However, at least some aspects of selective attention are not fully developed until the second decade of life (Berman and Friedman, 1995, Couperus et al., 2011, Elliott, 1979, Gomes et al., 2007, Lane and Pearson, 1982, Sexton and Geffen, 1979, Sussman and Steinschneider, 2009). As such, it is important to understand the conditions under which young children can differentially process attended and unattended information.
Much of the research on the development of selective attention has employed dichotic listening paradigms to determine the extent to which auditory spatially selective attention affects the ability of children and adults to report information presented to one ear while avoiding intrusions of information presented to the other ear (Doyle, 1973, Hiscock and Kinsbourne, 1980, Määttä et al., 2005, Pearson and Lane, 1991). Both the abilities to correctly respond to attended sounds and to ignore unattended sounds have been shown to have a prolonged developmental time course. Further, the age at which children are first able to allocate selective attention in an adult-like manner is affected by the paradigm (Bahrick et al., 2004, D’Angiulli et al., 2008, Dixon et al., 2010, Hanania and Smith, 2010, van der Molen, 2000), stimulus modality (Bartgis et al., 2003, Coch et al., 2005, Couperus et al., 2011, Huang-Pollock et al., 2002, Ruff and Capozzoli, 2003), selection criterion (Amso and Johnson, 2005, Geffen and Sexton, 1978, Gomes et al., 2007, McKay et al., 1994, Taylor and Khan, 2000), and domain of the to-be-attended information (Berman and Friedman, 1995, Drake et al., 2000, Maccoby and Konrad, 1966, Pérez-Edgar and Fox, 2005, Sanders et al., 2006).
Behavioral studies provide important information about the extent to which children and adults can extract meaning from attended stimuli in complex environments. Event-related brain potentials (ERPs) provide additional information about the levels of processing that are modulated by attention. Evidence that selective attention affects early perceptual processing, as indexed by ERPs in the first 250 ms after stimulus onset, suggests a greater impact of attention on subsequent processing of attended and ignored information. In adults asked to sustain attention at one location in a classic Hillyard auditory attention paradigm, sounds from both attended and unattended locations elicit a positive-negative-positive series of peaks; the auditory evoked potentials elicited by sounds from attended locations are larger in amplitude (Hansen et al., 1983, Hillyard, 1981, Woldorff et al., 1987). Specifically, attended sounds elicit a larger first negative peak (N1) by 100 ms after onset. Under some conditions, this negative difference (Nd) in response to attended and unattended sounds is sustained across the following positive peak (P2) (Näätänen, 1990, Schröger and Eimer, 1997). However, in other studies both the negative (N1) and positive (P2) evoked potentials are larger in response to attended sounds; the later attention effect has a positive polarity (Coch et al., 2005, Hink and Hillyard, 1976, Picton et al., 1971).
Auditory evoked potentials are not fully mature until at least 16 years of age (Bruneau et al., 1997, Ponton et al., 2000, Ponton et al., 2002, Tonnquist-Uhlén et al., 1995). Among other differences, in response to sounds presented in isolation the first negative peak evident in children has a longer latency, smaller amplitude, and more central distribution than what is observed in adults. Further, this first negative peak may be particularly sensitive to the amount of auditory information that is presented. When sounds are presented in acoustically dense environments, such as two simultaneously presented narratives, the N1 observed in adults is smaller in amplitude than the preceding P1 such that this first negative deflection does not reach the baseline amplitude measured during a pre-stimulus interval. Further, under these conditions there is no evidence of a negative peak between 50 and 250 ms after onset in 3- to 8-year-old children (Coch et al., 2005, Sanders et al., 2006, Stevens et al., 2009). To the extent that early ERP attention effects reflect top-down modulation of auditory perceptual processing, conditions that affect auditory evoked potentials are also likely to impact the attention effects observed on those waveforms.
Indeed, the polarity, distribution, and duration of spatially selective attention effects on auditory evoked potentials may be affected by interactions between maturity of the auditory system and acoustic characteristics in addition to the availability of other attention cues, task demands, and domain. That is, in the classic ERP studies of spatially selective attention, adults were asked to monitor one of two streams of simple, nonverbal sounds (e.g., tones) that differed only in location to detect deviants in the attended stream (Hillyard, 1981, Näätänen, 1990, Woldorff et al., 1987). As described above, standard sounds elicited a larger negativity by 100 ms after onset. When adolescents were presented with similar listening conditions, sounds from attended locations elicited a larger negativity that sometimes differed in amplitude or latency from what was reported in adults (Berman and Friedman, 1995, D’Angiulli et al., 2008, Loiselle et al., 1980, Lovrich and Stamm, 1983, Zambelli et al., 1977). More striking developmental differences were evident when 3- to 8-year-old children and adults were asked to attend to one of two simultaneously presented narratives for comprehension (Coch et al., 2005, Sanders et al., 2006). Although these experiments differed in several important ways from the classic studies described above, adults showed a larger negativity in response to behaviorally irrelevant probe stimuli presented from the same location as the attended narrative by 100 ms after onset. In these and similar studies with other populations (Stevens et al., 2006, Stevens et al., 2008, Stevens et al., 2009), children showed a larger positivity in response to probes from the same location as the attended narrative by 100 ms after onset that was sometimes extended in duration and with a broader distribution than what was observed for adults. One interpretation offered by the authors was that both the larger negativity observed in adults and the larger positivity observed in children by 100 ms after probe onset indexed a larger perceptual response to attended sounds. However, they also noted differences in this and the classic Hillyard paradigm in addition to acoustic characteristics: the attended and unattended narratives could be differentiated by features other than location (i.e., content and narrator), listeners were not specifically instructed to attend to the probe stimuli, participants were asked to remember the content of the attended story to answer subsequent questions, and the sounds people were asked to attend were verbal.
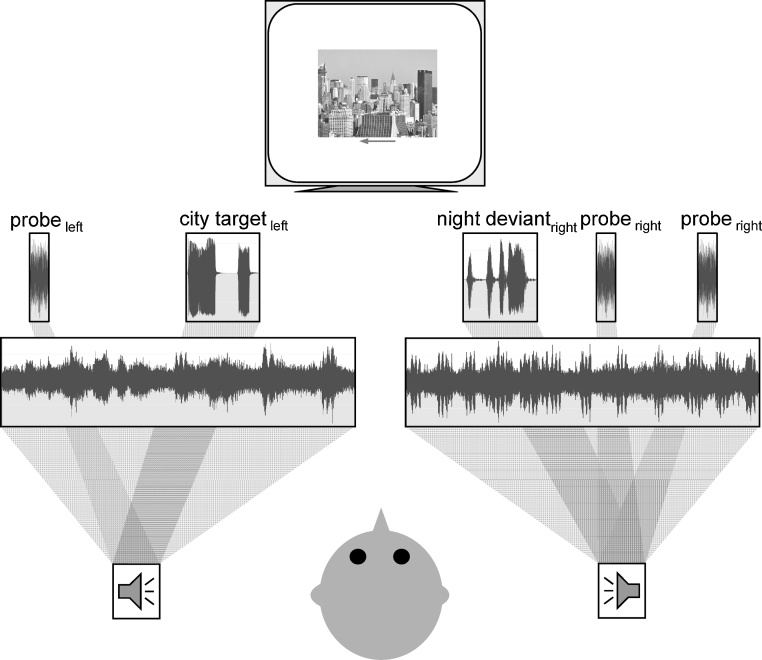
It was not possible to engage young children in precisely the same auditory spatially selective attention ERP experiment originally designed for adults that requires listening to meaningless tones for an extended period of time. As such, the current study was designed to begin building a bridge between the classic Hillyard paradigm and previous experiments that have been successful in demonstrating the effects of spatially selective attention on early auditory processing in young children. Specifically, adults and typically developing 4- and 5-year-old children were asked to attend to one of two simultaneously presented nonverbal soundscapes to detect target sounds in the attended stream (Fig. 1). The soundscapes were created from multiple sounds that related to a single theme (city, night, jungle, or ocean) to provide meaningful information that encouraged both adults and children to employ sustained, selective attention. These nonverbal environments were presented in pairs along with images that related to the theme of the to-be-attended sounds. Occasionally, deviant sounds that were also related to the themes of the soundscapes (city: truck horn; night: owl hoot; jungle: bird call; ocean: ship horn) were presented. These deviants were designed to provide a behavioral task that both adults and children could accomplish. Listeners were asked to press a button in response to a deviant sound only when it was presented as part of the attended environment; deviants presented along with the unattended environment could be ignored. The effects of selective attention on perceptual processing were measured by comparing ERPs elicited by unrelated attention probes that never required a behavioral response and that were presented from the same locations as the attended and ignored soundscapes. Since the only difference between probes in the attended and unattended channel was location, differences in ERPs can be interpreted as the effects of spatially selective attention on auditory processing. This design is more similar to the classic Hillyard paradigm than the studies that used two simultaneous narratives in that: (1) it includes a sparser acoustic environment, (2) participants are engaged in a continuous monitoring task, and (3) only nonverbal sounds are presented. However, the design shares features with the child-directed studies that are hypothesized to be important for engaging young children: (1) it employs meaningful sounds, and (2) there are multiple, continuously present cues indicating which stream should be attended.
Fig. 1.
Paradigm. A nonverbal soundscape, related deviant sounds, and unrelated probe sounds were presented from both a loudspeaker to the left and to the right of adults and children. Participants were instructed to attend to the sounds from one location to detect the deviant sounds in that environment only (i.e., targets). Images that matched the theme of the attended soundscape and an arrow pointing in the direction of attended sounds were presented on a computer monitor.
2. Methods
2.1. Participants
Sixteen adults (5 women) between the ages of 21 and 31 years (M = 25.5 years, SD = 2.8) participated in the study. All participants reported normal hearing, normal or corrected to normal vision, being right-handed, having no neurological disorders, and having avoided psychoactive medication within the past six months.
Twenty 4- and 5-year-old children participated in the study. Data from two children were excluded from analysis because there were not a sufficient number of artifact-free trials (> 45) to include in individual subject averages. The final sample of 18 children (9 girls; M age = 5.0 years, SD = 0.6) provided ERP data from 47 to 182 trials per condition (M = 100). Parents or guardians reported that all of these children had normal hearing, normal or corrected to normal vision, had no neurological disorders, and had not taken any psychoactive medication. Most of the children were described as being right-handed, but one was left-handed and one was ambidextrous.
2.2. Stimuli
Soundscapes were 5.5 min audiotracks of nonverbal sounds, all of which were consistent with a city, night, jungle, or ocean environment. The city soundscape was primarily traffic noise. The night soundscape featured crickets, frogs, and nocturnal birds. The jungle soundscape included a dense array of insect, bird, and mammal calls. The ocean soundscape was constructed from the sounds of waves, gulls, and rocking boats. Each soundscape faded in at the beginning and out at the end over 2 s spans. City and night soundscapes were paired, as were the jungle and ocean soundscapes. The sounds of paired environments were pasted into different channels of 16-bit, stereo WAV files with a 44.1 kHz sampling rate. The purpose of the soundscapes was to provide meaningful information that encouraged both adults and children to employ sustained, selective attention.
It was important to design a behavioral task that could provide a measure of compliance with the instruction to attend to one of the two simultaneously presented soundscapes and that could be accomplished by both adults and young children. Deviant sounds were created for each environment and defined as the target that required a behavioral response when presented in an attended soundscape. These sounds fit with the theme of the soundscapes (city: truck horn; night: owl hoot; jungle: bird call; ocean: ship horn), but were distinct from the other included sounds. Deviants were 2 s sequences with large spectro-temporal variability (examples shown in Fig. 1). To ensure that 4- and 5-year-old children could detect at least some of the targets while making the task difficult enough to engage attention in adults, each deviant was presented at three different volumes (soft, medium, and loud) relative to the matching soundscape. Ability to detect the targets was more closely related to the abruptness of amplitude changes within a deviant sound and the spectro-temporal relationship between each deviant and the environment in which it was presented than with peak or mean amplitude measures taken across the 2 s durations of the deviants. As such, the deviant volumes were selected using perceptual rather than physical measurements. Several adults were initially asked to adjust the volume of each deviant to clearly stand out from and then blend in with its associated environment. The consistently selected volumes were defined as the loud and soft deviants; medium deviants were set at the midpoint between the two.
To measure the effects of selective attention on perceptual processing, it was important to present sounds from the same locations as the attended and ignored soundscapes that never required a behavioral response. Attention probes to be presented from the same location as attended and unattended soundscapes were constructed separately for each nonverbal environment. A 50 ms sample of pink noise with 4-sample onset and offset ramps was first selected. The spectrum of each soundscape was measured by calculating the average power in narrow frequency bands across the entire 5.5 min stream. These functions were then used to filter the pink noise such that the probes presented from the same location as a specific environment matched the spectral characteristics of that soundscape.
In addition to the auditory stimuli, videos matching the content of each soundscape were created to keep participants engaged and to provide a continuous reminder of the soundscape to be attended throughout the experiment. Videos included short live-action movies and still images with smooth transitions created by dissolving one image into another. All videos were presented within a 7 cm × 12 cm area on the computer monitor such that they did not extend beyond 4 degrees of visual angle. An arrow shown under the video pointed towards the loudspeaker where the related, attended soundscape was presented. Since participants were asked to attend to the same environment from different locations in different blocks of the experiment, two distinct videos were created to accompany each soundscape.
2.3. Procedure
The soundscape to be attended in the first block of trials was initially introduced in isolation and given a one-word label (i.e., city, night, jungle, or ocean). The soundscape was presented from the same location during practice and the first test block such that listeners were familiar both with the sounds and with the location to be attended. The participant was asked to think about sounds likely to occur in that environment and to identify any familiar sounds while listening to the soundscape. The experimenter then introduced the target sound for the attended environment by playing that sound in isolation from the same location and helping the participant to find an appropriate label (i.e., truck, owl, bird, or boat). Participants were told that some of the targets would seem closer (sound played at louder volume) and others far away. Regardless of loudness, participants were instructed to press a button on a response box any time the target sound was played. Children were given practice pressing the button in response to the softer and louder targets played in isolation. Next, participants were given practice detecting the target sound in the environment that was to be attended. The competing soundscape with its deviant sound was then introduced from the other location. Participants were reminded to listen carefully to one of the soundscapes from a particular location to detect the targets in that environment only while ignoring the other sounds. Finally, the probes from both the attended and unattended location were added with the instruction that these “extra” sounds would be presented but were not important for the task.
For all test blocks, participants were instructed to listen to one of the soundscapes and to press a button as quickly as possible if they heard the target sound from the attended location only. Button presses within 3000 ms after the presentation of a target were considered hits. All other responses were considered to be false alarms. Adults were asked to complete 8 test blocks (attending to each of the four environments once from the left and once from the right location); children were only asked to complete 4 blocks (attending to the two environments within a pair from both locations). All participants attended to the same soundscape for two subsequent blocks (e.g., attend jungle left then attend jungle right), but the initial location and environment to be attended as well as the pair of soundscapes that were presented were balanced across participants. Brief instructions with a short practice were given between blocks to indicate a shift of location for the same environment (e.g., “now all of the jungle sounds you have been listening to and the bird sounds you have responded to will be coming from over here.”). The full set of instructions and practice were repeated between blocks that required attention to a new environment and responses to new targets.
Each video was started manually as the two simultaneous soundscapes faded in. As such, the images were not changing at the same times relative to the soundscapes across participants. The environments were presented at 65 dBA and began at least 3 s before the first targets and probes. Attention probes were played 10 dB louder than the average for the environments. Targets in the attended environment and deviants presented in the unattended environment were presented at three levels: soft, medium, and loud. Each target, deviant, and probe in a block was preceded by a different interval between 250 and 2219 ms (180 different interstimulus intervals in 11 ms steps). Within each block, 72 probes were presented from the same location as the attended soundscape and 72 from the location of the unattended soundscape; eighteen targets were presented in the attended environment and 18 deviants were presented in the unattended environment (six at each loudness level). Adults heard a total of 576 probes from both the attended and unattended locations and had the opportunity to respond to 144 targets (12 at each loudness level in each environment). Children heard half those total numbers of probes and targets, but still had the opportunity to respond to 12 targets at each loudness level in the two environments they heard.
EEG was recorded continuously from 128 electrodes (EGI, Eugene OR) and digitized at 250 Hz with a bandwidth of 0.01–100 Hz. A 60 Hz notch filter was applied offline before data were segmented into 600 ms epochs beginning 100 ms before the presentation of a probe, target, or deviant. Averaged epochs were limited to this short span such that an additional probe, target, or deviant was never presented during the pre-stimulus interval and was included in the post-stimulus interval for less than 18% of the trials. Trials containing artifacts from eye blinks, eye movements, and head movements established by visual inspection and by maximum amplitude criteria set separately for adults (75 μV s) and each child (100–200 μV s) were excluded from analysis. Trials on which artifacts were evident at any one of the central or mastoid electrodes were excluded from analyses to avoid interpolation at these critical cites. Data from remaining trials were averaged separately for each participant, attention condition, and electrode. The averaged recording from the two mastoid electrodes was used as a reference to facilitate comparisons with previously published studies. The 100 ms before probe, target, or deviant onset was used as a baseline.
There are very few previous ERP studies of auditory selective attention that include children as young as 4 and 5 years of age (but see Sanders et al., 2006, Stevens et al., 2009). As such, it was not possible to define specific regions-of-interest and measurement time windows in which the effects of selective attention on perceptual processing as indexed by ERPs were likely to be observed in this group. Further, assumptions about which peaks in ERP waveforms from children and adults correspond to the same underlying components are not warranted without longitudinal data from a large group of participants across a broad range of ages. Instead, ERP data were analyzed using a data-driven approach to specifically address the questions of whether spatially selective attention directed to nonverbal sounds affects perceptual processing in young children, and if so, the timing of the effects of attention on auditory processing.
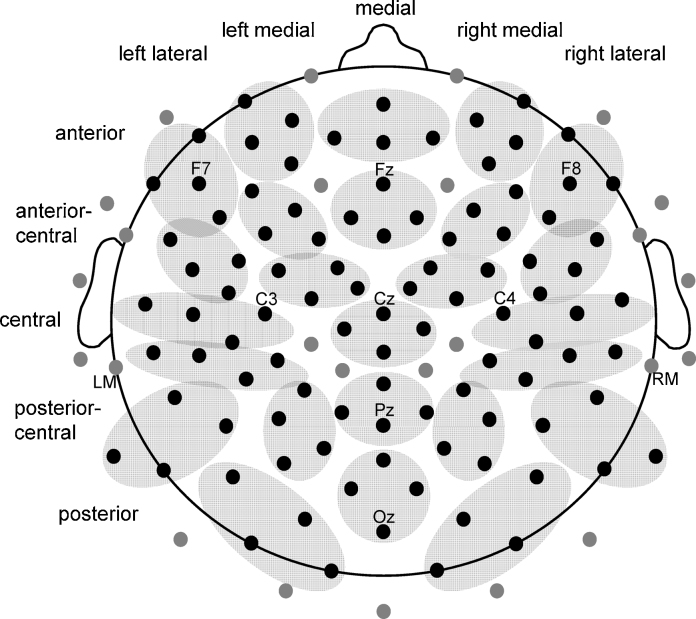
ERP data were averaged across groups of four electrodes arranged in a 5 × 5 grid across the scalp (Fig. 2). Averaging data across contiguous electrodes in small regions provided the increased signal-to-noise ratio necessary for analyzing ERPs elicited by the relatively small number of targets and deviants. Further, this approach decreases the chance of finding spurious attention by electrode position interactions that are not supported by main effects of attention at any subset of electrodes. For both children and adults, mean amplitude measures were taken in four broad time windows 25–55, 85–135, 150–250, and 350–500 ms after onset. To better capture the auditory evoked potentials evident in children, an additional mean amplitude measurement 50–120 ms after onset was taken in this group. Mean amplitude was selected rather than peak amplitude to avoid measures that are necessarily influenced by noise to a greater extent in conditions and individuals in which fewer trials contribute to individual subject averages and which equate differences in amplitude that are actually observed at different times in each participant. To determine the timing of the onset and offset of any attention effects, mean amplitude measures were also taken starting every 10 ms between 0 and 220 ms after onset in 30 ms epochs (i.e., 0–30, 10–40, 20–50 ms, etc.). This approach was deemed more conservative than measuring peak latencies, which always reflects the largest summation of signal and noise.
Fig. 2.
Electrode locations. Black circles indicate the approximate positions of the 100 electrodes that provided data included in analysis. Data were averaged across the four electrodes shown within a gray oval. Electrode positions were treated as two five-level factors in ANOVAs. The labels indicate the specific electrodes where main effects of attention were significant as described in the text.
Data were initially analyzed using repeated-measures ANOVAS (Greenhouse–Geisser adjusted): Attention (attended, unattended) × Left/Medial/Right electrode position (LMR: left lateral, left medial, medial, right medial, right lateral) × Anterior/Central/Posterior electrode position (ACP: anterior, anterior central, central, posterior central, posterior). Significant interactions (p < .05) between Attention and electrode position factors motivated follow-up analyses to determine if there were main effects of Attention at the subset of electrodes where the difference in mean amplitude was the largest. This approach avoids conducting a large number of statistical tests yet ensures that significant effects of Attention with different distributions than previously reported will not be missed.
3. Results
3.1. Behavior
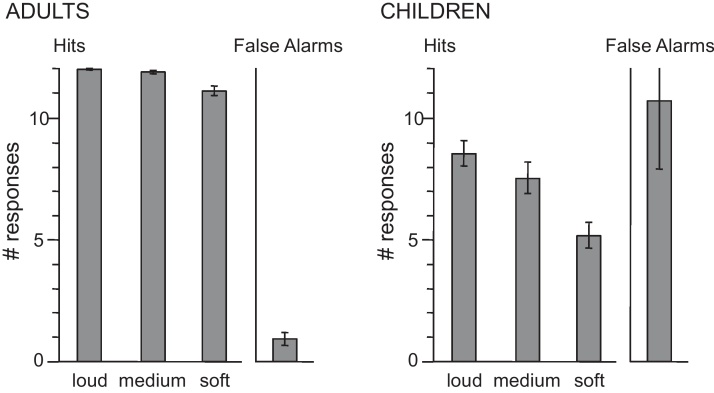
As shown in Fig. 3, adults’ detection of targets that were presented as part of the attended soundscape depended on loudness (F(2,30) = 19.54, p < .001). There were more hits for targets presented at the loud and medium levels (M = 11.97 and 11.84 of 12) than at the soft level (M = 11.09). Performance for adults, who attended to all four soundscapes in separate blocks, also depended on the specific environment presented. There were fewer hits (M = 11.37) for the bird call presented as part of the jungle environment (F(3,45) = 3.74, p < .05) and also more false alarms (M = 1.75) in this condition (F(3,45) = 2.83, p < .05).
Fig. 3.
Behavioral Data. Bars show the average number of button presses in the 3000 ms following the presentation of a target sound (hits) and at other times (false alarms) within a single block of trials. Each soundscape included 12 targets presented at the loud, at the medium, and at the soft level. Standard Error bars are shown.
Each child only listened to two of the four environments (city and night or jungle and ocean). However, the pair of sounds did not affect performance (p's > .50). As was true of adults, loudness of the targets affected the number of hits (F(2,36) = 41.92, p < .001). There were more responses to loud and medium targets (M = 8.48 and 7.48 of 12) than for soft targets (M = 5.12). Children made more false alarms (M = 10.62) than they did hits (M = 7.02). However, the probability of a response in the 3000 ms after a rarely presented target (0.58) was much higher than the probability of a response during the segments of equal length that did not follow a target (0.13).
3.2. Event-related potentials
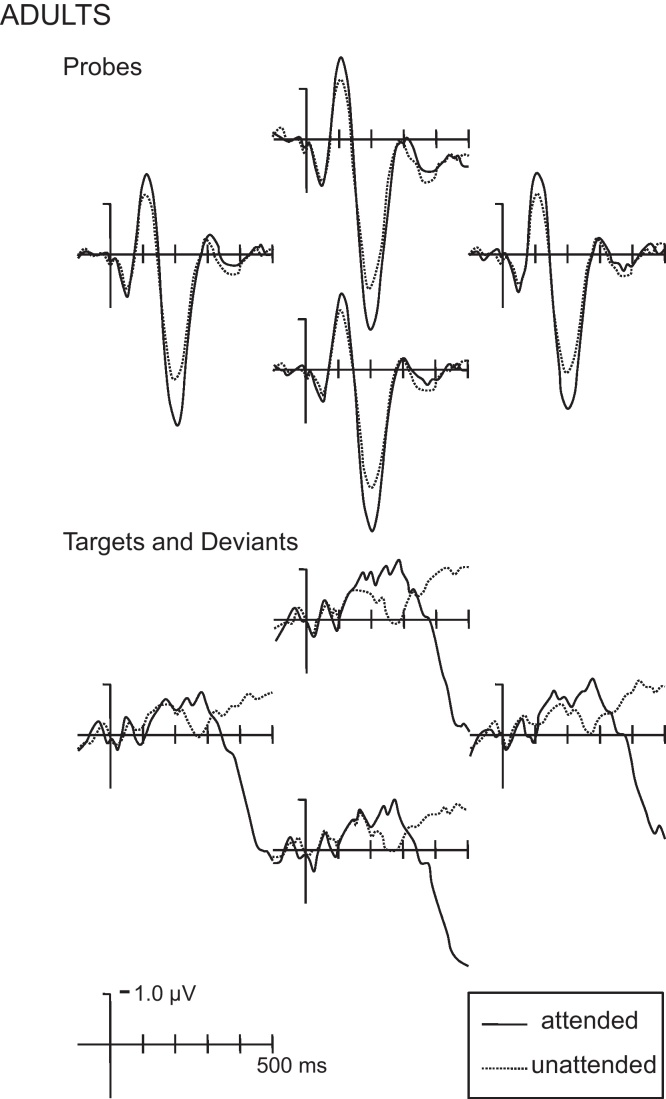
In adults, attention probes presented from the same locations as both attended and unattended soundscapes elicited the typical positive-negative-positive series of auditory evoked potentials (Fig. 4). There was no effect of attention on the amplitude of the first positive peak (P1) 25–55 ms after onset. However, during the first negative peak (N1), there were interactions between Attention and electrode position factors on mean amplitude measures 85–135 ms taken across the entire scalp (Attention × ACP: F(4,60) = 3.09, p < .05; Attention × LMR: F(4,60) = 2.85, p < .05). At the subset of four medial and central electrodes (positions shown in Fig. 2, waveforms in Fig. 4), probes presented from the same location as attended environments elicited a larger negativity (Attention: F(1,15) = 12.23, p < .001). The onset/offset analysis on mean amplitude in 30 ms long time windows at these medial and central sites indicated the attention effect began 80–110 ms and extended through 100–130 ms after probe onset.
Fig. 4.
ERPs from adults. Top panel: ERPs recorded at the medial and central electrodes elicited by behaviorally irrelevant probe stimuli presented at the same location as the attended (solid lines) and unattended (dotted lines) soundscape are shown. Probes from attended locations elicited a larger N1 and P2. Bottom panel: ERPs recorded at the medial and posterior-central electrodes elicited by deviants presented as part of the attended soundscape (targets, solid lines) and as part of the unattended soundscape (dotted lines) are shown. Targets elicited a larger P3 across all posterior-central sites regardless of laterality.
Similarly, there were interactions between Attention and electrode position factors on the second positivity (P2) 150–250 ms after onset (Attention × ACP: F(4,60) = 3.82, p < .05; Attention × LMR: F(4,60) = 5.39, p < .05; Attention × ACP × LMR: F(16,240) = 4.69, p < .001) in adults. Probes from attended locations elicited a larger positivity (P2) over medial and central regions (Attention: F(1,15) = 40.54, p < .001). This positive attention effect began by 140–170 ms and extended through 220–250 ms after onset.
No differences in ERPs for probes in the latest time window were suggested by the measurements taken across the entire scalp in adults. However, there were interactions of Attention and an electrode position factor for mean amplitude measured 350–500 ms after the onset of deviant sounds (Attention × ACP: F(4,60) = 15.08, p < .001). Across all posterior-central electrodes regardless of laterality (positions shown in Fig. 2) targets elicited a larger positivity compared to deviant sounds in unattended environments (Attention: F(1,15) = 9.24, p < .01). The small number of deviant sounds presented for each participant precluded conducting an analysis with either loudness (soft, medium, and loud) or correctness included as a factor. Further, the differences in ERPs observed for targets and deviants presented in the unattended environment must be interpreted with caution since these conditions differ both in selective attention and requiring a behavioral response.
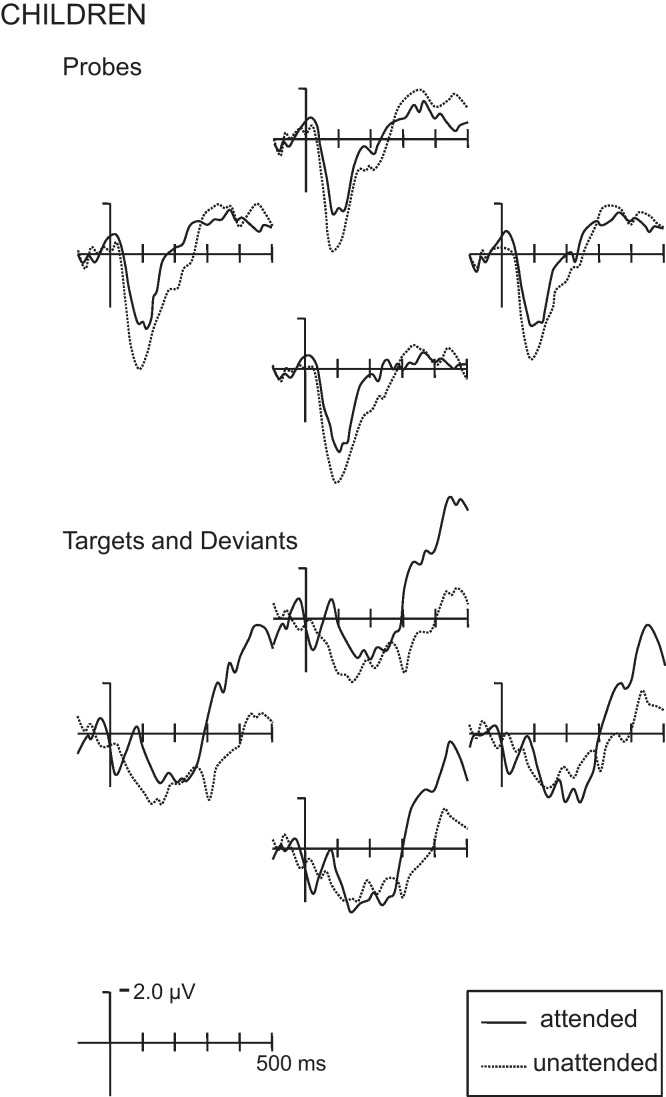
In children, probes elicited a broadly distributed positivity that peaked around 100 ms after onset rather than the more mature positive-negative-positive oscillation (Fig. 5). However, there was some indication of a first negative peak in children by 150 ms. Despite the differences in the auditory evoked potentials for children and adults, the attention effects were quite similar. Specifically, there were no differences in ERPs elicited by probes presented from the same locations as attended and unattended soundscapes 25–55 ms after onset. However, there was an interaction between Attention and electrode position factors on mean amplitude measured in the time window 50–120 ms after onset selected specifically to best capture early attention effects in children (Attention × ACP × LMR: F(16,272) = 2.70, p < .05). Over medial and central regions (positions show in Fig. 2, waveforms in Fig. 5), sounds from attended locations elicited a larger negativity (i.e., a smaller positivity) (Attention: F(1,17) = 4.84, p < .01). The onset/offset analysis on measurements made at these sites suggested this effect began 50–80 ms and extended through 70–100 ms after probe onset.
Fig. 5.
ERPs from 4- and 5-year-old children. Large amplitude ERPs recorded in children are shown at half the scale used for adult data. Top panel: ERPs recorded at the medial and central electrodes elicited by behaviorally irrelevant probe stimuli presented at the same location as the attended (solid lines) and unattended (dotted lines) soundscape are shown. Probes from attended locations elicited a larger negativity 50–120 and 150–250 ms after onset. Bottom panel: ERPs recorded at the medial and anterior-central electrodes elicited by deviants presented as part of the attended soundscape (targets, solid lines) and as part of the unattended soundscape (dotted lines) are shown. Targets elicited a larger anterior negativity 350–500 ms after onset.
In children, measurements taken across the entire scalp did not reveal significant Attention by electrode position interactions on mean amplitude 150–250 ms after onset. However, to facilitate comparisons between children and adults an analysis was conducted on measurements taken at the subset of medial and central electrodes in this time window. As was true in the earlier time window for children, probes from attended locations elicited a larger negativity (Attention: F(1,17) = 3.80, p < .05). Analysis of mean amplitude in 30 ms windows at the medial and central sites suggested the later negative attention effect began by 150–180 ms and continued through 220–250 ms after onset.
There was no evidence for a difference in ERPs elicited by probes after 250 ms. However, as was true for adults, there were Attention by electrode position interactions on measurements taken 350–500 ms after the onset of deviants (Attention × ACP: F(4,68) = 2.62, p < .05 and Attention × LMR: F(4,68) = 2.96, p < .05). At anterior-central electrodes regardless of laterality (positions show in Fig. 2) targets elicited a larger negativity than deviants presented in unattended environments (Attention: F(1,17) = 4.67, p < .05; Attention × LMR at anterior-central electrodes: p > .75). Again, the small number of deviant sounds presented for each participant precluded conducting an analysis with either loudness or correctness as factors.
4. Discussion
Adults were asked to listen to one of two simultaneously presented soundscapes that differed in content (e.g., jungle and ocean) and location of presentation to detect target sounds (e.g., a bird call) in the attended environment only. Behaviorally irrelevant probes that shared a location with the attended sounds elicited larger auditory evoked potentials by 80 ms after onset. Specifically, probes from attended locations elicited a larger first negative (N1) and second positive (P2) peak. Although listeners could have used any number of selection criteria to differentiate between the two soundscapes, differences in ERPs elicited by probes presented at the same locations as the attended and unattended environments indicates they were using location as one of those selection criteria. Thus, the data add to a growing body of literature indicating that ERP probe paradigms can be used to index the allocation of attention without explicitly instructing listeners to attend to the probes themselves (Astheimer and Sanders, 2009, Astheimer and Sanders, 2012, Coch et al., 2005, Hink and Hillyard, 1976, Stevens et al., 2009). In order for responses elicited by probes to be used to index attention based on one selection criterion (e.g., location), it is important that the probes are not easily differentiated from attended sounds on the basis of another simple feature (e.g., pitch). If probes differ enough to be processed as a separate auditory stream they can simply be ignored, resulting in no differences in ERPs elicited by probes that do and do not share a simple feature with attended sounds.
Children evidenced an attention effect that began at least as early as that observed for adults. There was some evidence that the larger negativity in response to probes from the same location as attended soundscapes began earlier in children (i.e., 50 ms after onset). At least some of the previous studies of auditory spatially selective attention in adults reported effects by 50 ms after onset (White and Yee, 2006, Woldorff et al., 1987, Woldorff and Hillyard, 1991). In these studies, attended sounds elicited a larger first positive peak (P1). For adults in the current study, mean amplitude in the P1 window was numerically larger for probes presented from attended locations. The positive and negative attention effects in the first 250 ms after sound onset may partially overlap resulting in a lack of statistical significance for the earliest attention effects. The negative attention effect observed in 4- and 5-year-old children 50–120 ms after sound onset and observed again between 150 and 250 ms may also overlap spatially and temporally, but in this case, additively.
Previous studies employing two simultaneously presented narratives, rather than nonverbal soundscapes, also reported spatially selective attention effects with similar onset latencies (Coch et al., 2005, Sanders et al., 2006, Stevens et al., 2006, Stevens et al., 2008, Stevens et al., 2009). However, in these studies the earliest effects of attention on ERPs were opposite in polarity for children and adults. For children, the larger auditory evoked potentials elicited by sounds from the same location as an attended narrative was observed as a positivity by 100 ms after onset. One possible factor determining the polarity of the attention effects in children is the domain of the sounds to be attended (i.e., verbal or nonverbal). However, it seems unlikely that spatially selective attention directed to verbal and nonverbal sounds would impact early auditory processing in fundamentally different ways, and only in children. Further, there is also evidence of negative temporally selective attention effects by 100 ms after onset in 3- to 5-year-old children listening to a single narrative (Astheimer and Sanders, 2012).
A second possible explanation for differences in the polarity of auditory spatially selective attention effects in young children listening to narratives and nonverbal environments are task demands. During the narrative task in which a positive attention effect was observed, children were asked to listen to the story from the attended location in order to answer questions presented later in the session (Coch et al., 2005, Sanders et al., 2006, Stevens et al., 2006, Stevens et al., 2008, Stevens et al., 2009). In the current study in which a negative attention effect was observed, children were asked to continuously monitor the attended stream for a target sound–a task more similar to that employed in the Hillyard paradigm. However, it is important to recognize that in both paradigms employed with young children the task demands are identical during presentation of the probes from attended and unattended locations. It is not clear why the task would affect the amplitude of auditory evoked potentials only for sounds from the attended or unattended location in such a way that the polarity of the difference between the two would switch.
A more likely explanation involves interactions between maturity of the auditory system, the amount of sound presented, and the effects of attention on auditory evoked potentials. Sounds presented in isolation elicit, in adults, an N1 that is 4 to 5 times larger than the P1 (Alho et al., 1986, Hillyard, 1981, Näätänen, 1982, Woldorff et al., 1987) and, in children, a negative deflection around 150 ms after onset that is larger in amplitude than the first positive peak (Albrecht et al., 2000, Bruneau et al., 1997, Martin et al., 1988, Ponton et al., 2000, Ponton et al., 2002). However, probes presented from two locations along with two simultaneously presented narratives create an acoustically dense and dynamic environment. These probes elicit, in adults, an N1 that is sometimes smaller in amplitude than the P1 and, in children, only a positive deflection during the first 250 ms after onset (Coch et al., 2005, Sanders et al., 2006, Stevens et al., 2006, Stevens et al., 2008, Stevens et al., 2009). The pattern of auditory evoked potentials elicited by probes in the current study fell between that observed for sounds presented in isolation and probes presented along with two narratives; in adults, N1 amplitude was about twice that of P1 amplitude and, in children, the negative deflection between 150 and 250 ms after onset was smaller than the first positive peak. In previous studies, the conditions that resulted in a larger positivity for attended sounds–children listening to an acoustically dense environment–were also the conditions that resulted in a complete lack of a negative deflection in the first 250 ms after sound onset. Observing differences on early auditory evoked potentials suggests that physical characteristics of sound may be the key factor in determining the polarity of attention effects in young children. However, it will also be important to explore the ways in which higher-level task demands (e.g., target detection compared to listening for comprehension) interact with ERP indices of selective attention.
Neither adults nor children evidenced differences in ERPs elicited by probes from attended and unattended locations more than 250 ms after onset. In contrast, both groups showed late differences in ERPs elicited by targets presented in the attended channel and deviants presented as part of the unattended soundscape. Late ERP effects were evident in both groups, even though children were clearly worse at the target detection task. In adults, targets elicited a late posterior positivity. This effect is likely related to the P300 reported across a wide range of target detection tasks, especially those with relatively rare targets and accurate performance (Donald and Little, 1981, Roth et al., 1976, Squires et al., 1975). The fact that probes did not elicit a P300 suggests these sounds were easy to distinguish from the targets. In children, targets elicited an anterior negativity between 350 and 500 ms after onset. The polarity and distribution of this effect suggest children may have engaged in distinct cognitive processes to perform the behavioral task (Courchesne, 1978).
Previous and current results suggest that mechanisms by which selective attention can modulate early auditory processing are in place by 3-years of age in typically developing children, even though the executive control that allows listeners to flexibly and optimally allocate attention are not. However, this conclusion does not hold for all children. Three- to eight-year-old children who were categorized as specifically language impaired (SLI) based on standardized scores of receptive language processing did not evidence any effect of spatially selective attention on early auditory evoked potentials in a narrative paradigm identical to the one described above (Stevens et al., 2006). After computerized training on several speech-related protocols, similar SLI children did show a larger positivity in response to sounds from attended locations (Stevens et al., 2008).
There are many potential explanations for the relationship between selective attention and language skill. For example, most of the settings in which language acquisition takes place are not ideal environments in which the only sound is from one talker. The ability to selectively attend to one person's speech while ignoring other sounds might be important for optimal language acquisition. However, it is equally plausible that children who are struggling with language processing might be less inclined or less able to selectively attend to verbal material. Instead of problems with allocating attention in a manner that affects early perceptual processing, perhaps language-impaired children have a more specific deficit in differentially processing complex verbal material. To determine if the general ability to use auditory spatially selective attention impacts language processing, or if problems with language processing affect both standardized tests and ERP measures that rely on verbal material, it will be important to measure the effects of selective attention directed to nonverbal sounds, such as those used in the current study, in children with language impairment.
4.1. Conclusions
Children as young as 4- and 5-years of age evidence effects of spatially selective attention on auditory evoked potentials when listening to one of two simultaneously presented nonverbal soundscapes. The results indicate that the mechanisms by which attention modulates perceptual processing are in place by this age. In contrast, the behavioral performance and effects of attention on later processing of target sounds suggest the control of attentional resources and the many other cognitive processes indexed by these behavioral measures and later ERPs are far from mature. Given the importance of selective attention for the acquisition of other perceptual and cognitive skills across verbal and nonverbal domains, a complete understanding of children's ability to allocate attention in a manner that modulates early perceptual processing will be crucial for developing more ideal learning environments.
Conflict of interest
There were no conflicts of interests.
Acknowledgments
This study was funded by a John Merck Scholars Fellowship to L.D.S. to study the Biology of Developmental Disabilities in Children. We thank Lori Astheimer, Mara Breen, and Lindsay Demers for assistance with data collection.
References
- Albrecht R., Suchodoletz W.V., Uwer R. The development of auditory evoked dipole source activity from childhood to adulthood. Clinical Neurophysiology. 2000;111:2268–2276. doi: 10.1016/s1388-2457(00)00464-8. [DOI] [PubMed] [Google Scholar]
- Alho K., Paavilainen P., Reinikainen K., Sams M., Näätänen R. Separability of different negative components of the event-related potential associated with auditory stimulus processing. Psychophysiology. 1986;23:613–623. doi: 10.1111/j.1469-8986.1986.tb00680.x. [DOI] [PubMed] [Google Scholar]
- Amso D., Johnson S. Selection and inhibition in infancy: evidence from the spatial negative priming paradigm. Cognition. 2005;95:B27–B36. doi: 10.1016/j.cognition.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Astheimer L., Sanders L. Listeners modulate temporally selective attention during natural speech processing. Biological Psychology. 2009;80:23–34. doi: 10.1016/j.biopsycho.2008.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astheimer L., Sanders L. Temporally selective attention supports speech processing in 3- to 5-year-old children. Developmental Cognitive Neuroscience. 2012;2:120–128. doi: 10.1016/j.dcn.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahrick L., Lickliter R., Flom R. Intersensory redundancy guides the development of selective attention, perception, and cognition in infants. Current Directions in Psychological Science. 2004;13:99–102. [Google Scholar]
- Bartgis J., Lilly A., Thomas D. Event-related potential and behavioral measures of attention in 5-, 7-, and 9-year-olds. Journal of General Psychology. 2003;130:311–335. doi: 10.1080/00221300309601162. [DOI] [PubMed] [Google Scholar]
- Berman S., Friedman D. The development of selective attention as reflected by event-related brain potentials. Journal of Experimental Child Psychology. 1995;59:1–31. doi: 10.1006/jecp.1995.1001. [DOI] [PubMed] [Google Scholar]
- Bruneau N., Roux S., Guérin P., Barthélémy C., Lelord G. Temporal prominence of auditory evoked potentials (N1 wave) in 4–8-year-old children. Psychophysiology. 1997;34:32–38. doi: 10.1111/j.1469-8986.1997.tb02413.x. [DOI] [PubMed] [Google Scholar]
- Coch D., Sanders L., Neville H. An event-related potential study of selective auditory attention in children and adults. Journal of Cognitive Neuroscience. 2005;17:605–622. doi: 10.1162/0898929053467631. [DOI] [PubMed] [Google Scholar]
- Couperus J., Hunt R., Nelson C., Thomas K. Visual search and contextual cueing: differential effects in 10-year-old children and adults. Attention, Perception, & Psychophysics. 2011;73:334–348. doi: 10.3758/s13414-010-0021-6. [DOI] [PubMed] [Google Scholar]
- Courchesne E. Neurophysiological correlates of cognitive development: changes in long-latency event-related potentials from childhood to adulthood. Electroencephalography and Clinical Neurophysiology. 1978;45:468–482. doi: 10.1016/0013-4694(78)90291-2. [DOI] [PubMed] [Google Scholar]
- D’Angiulli A., Herdman A., Stapells D., Hertzman C. Children's event-related potentials of auditory selective attention vary with their socioeconomic status. Neuropsychology. 2008;22:293–300. doi: 10.1037/0894-4105.22.3.293. [DOI] [PubMed] [Google Scholar]
- Dixon M., Zelazo P., De Rosa E. Evidence for intact memory-guided attention in school-aged children. Development Sciences. 2010;13:161–169. doi: 10.1111/j.1467-7687.2009.00875.x. [DOI] [PubMed] [Google Scholar]
- Donald M., Little R. The analysis of stimulus probability inside and outside the focus of attention as reflected by the auditory N1 and P3 components. Canadian Journal of Psychology. 1981;35:175–187. doi: 10.1037/h0081153. [DOI] [PubMed] [Google Scholar]
- Doyle A.-B. Listening to distraction: a developmental study of selective attention. Journal of Experimental Child Psychology. 1973;15:100–115. doi: 10.1016/0022-0965(73)90134-3. [DOI] [PubMed] [Google Scholar]
- Drake C., Jones M.R., Baruch C. The development of rhythmic attending in auditory sequences: attunement, referent period, focal attending. Cognition. 2000;77:251–288. doi: 10.1016/s0010-0277(00)00106-2. [DOI] [PubMed] [Google Scholar]
- Elliott L. Performance of children aged 9 to 17 years on a test of speech intelligibility in noise using sentence material with controlled word predictability. Journal of the Acoustical Society of America. 1979;66:651–653. doi: 10.1121/1.383691. [DOI] [PubMed] [Google Scholar]
- Geffen G., Sexton M. The development of auditory strategies of attention. Developmental Psychology. 1978;14:11–17. [Google Scholar]
- Gomes H., Duff M., Barnhardt J., Barrett S., Ritter W. Development of auditory selective attention: event-related potential measures of channel selection and target detection. Psychophysiology. 2007;44:711–727. doi: 10.1111/j.1469-8986.2007.00555.x. [DOI] [PubMed] [Google Scholar]
- Hanania R., Smith L. Selective attention and attention switching: towards a unified developmental approach. Development Sciences. 2010;13:622–635. doi: 10.1111/j.1467-7687.2009.00921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen J., Dickstein P., Berka C., Hillyard S. Event-related potentials during selective attention to speech sounds. Biological Psychology. 1983;16:211–224. doi: 10.1016/0301-0511(83)90025-x. [DOI] [PubMed] [Google Scholar]
- Hillyard S. Selective auditory attention and early event-related potentials: a rejoinder. Canadian Journal of Psychology. 1981;35:159–174. doi: 10.1037/h0081155. [DOI] [PubMed] [Google Scholar]
- Hink R., Hillyard S. Auditory evoked potentials during selective listening to dichotic speech messages. Perception and Psychophysics. 1976;20:236–242. [Google Scholar]
- Hiscock M., Kinsbourne M. Asymmetries of selective listening and attention switching in children. Developmental Psychology. 1980;16:70–82. [Google Scholar]
- Huang-Pollock C., Carr T., Nigg J. Development of selective attention: perceptual load influences early versus late attentional selection in children and adults. Developmental Psychology. 2002;38:363–375. [PubMed] [Google Scholar]
- Lane D., Pearson D. The development of selective attention. Merrill-Palmer Quarterly. 1982;28:317–337. [Google Scholar]
- Loiselle D., Stamm J., Maitinsky S., Whipple S. Evoked potential and behavioral signs of attentive dysfunctions in hypteractive boys. Psychophysiology. 1980;17:193–201. doi: 10.1111/j.1469-8986.1980.tb00134.x. [DOI] [PubMed] [Google Scholar]
- Lovrich D., Stamm J. Event-related potential and behavioral correlates of attention in reading retardation. Journal of Clinical Neurophysiology. 1983;5:13–37. doi: 10.1080/01688638308401148. [DOI] [PubMed] [Google Scholar]
- Määttä S., Pääkkönen A., Saavalainen P., Partanen J. Selective attention event-related potential effects from auditory novel stimuli in children and adults. Clinical Neurophysiology. 2005;116:129–141. doi: 10.1016/j.clinph.2004.07.023. [DOI] [PubMed] [Google Scholar]
- Maccoby E., Konrad K. Age trends in selective listening. Journal of Experimental Child Psychology. 1966;3:113–122. doi: 10.1016/0022-0965(66)90086-5. [DOI] [PubMed] [Google Scholar]
- Martin L., Barajas J., Fernandez R., Torres E. Auditory event-related potentials in well-characterized groups of children. Electroencephalography and Clinical Neurophysiology. 1988;71:375–381. doi: 10.1016/0168-5597(88)90040-8. [DOI] [PubMed] [Google Scholar]
- McKay K., Halperin J., Schwartz S., Sharma V. Developmental analysis of three aspects of information processing: sustained attention, selective attention, and response organization. Developmental Neuropsychology. 1994;10:121–132. [Google Scholar]
- Näätänen R. Processing negativity: an evoked-potential reflection of selective attention. Psychological Bulletin. 1982;92:605–640. doi: 10.1037/0033-2909.92.3.605. [DOI] [PubMed] [Google Scholar]
- Näätänen R. The role of attention in auditory information processing as revealed by event-related potentials and other brain measures of cognitive function. Behavioral and Brain Sciences. 1990;13:201–288. [Google Scholar]
- Pearson D., Lane D. Auditory attention switching: a developmental study. Journal of Experimental Child Psychology. 1991;51:320–334. doi: 10.1016/0022-0965(91)90039-u. [DOI] [PubMed] [Google Scholar]
- Pérez-Edgar K., Fox N. A behavioral and electrophysiological study of children's selective attention under neutral and affective conditions. Journal of Cognition and Development. 2005;6:89–118. [Google Scholar]
- Picton T., Hillyard S., Galambos R., Schift M. Human auditory attention: a central or peripheral process? Science. 1971;173:351–353. doi: 10.1126/science.173.3994.351. [DOI] [PubMed] [Google Scholar]
- Ponton C., Eggermont J., Khosla D., Kwong B., Don M. Maturation of human central auditory system activity: separating auditory evoked potentials by dipole source modeling. Clinical Neurophysiology. 2002;113:407–420. doi: 10.1016/s1388-2457(01)00733-7. [DOI] [PubMed] [Google Scholar]
- Ponton C., Eggermont J., Kwong B., Don M. Maturation of human central auditory system activity: evidence from multi-channel evoked potentials. Clinical Neurophysiology. 2000;111:220–236. doi: 10.1016/s1388-2457(99)00236-9. [DOI] [PubMed] [Google Scholar]
- Roth W., Ford J., Lewis S., Kopell B. Effects of stimulus probability and task-relevance on event-related potentials. Psychophysiology. 1976;13:311–317. doi: 10.1111/j.1469-8986.1976.tb03082.x. [DOI] [PubMed] [Google Scholar]
- Ruff H., Capozzoli M. Development of attention and distractibility in the first 4 years of life. Developmental Psychology. 2003;39:877–890. doi: 10.1037/0012-1649.39.5.877. [DOI] [PubMed] [Google Scholar]
- Sanders L., Stevens C., Coch D., Neville H. Selective auditory attention in 3- to 5-year-old children: an event-related potential study. Neuropsychologia. 2006;44:2126–2138. doi: 10.1016/j.neuropsychologia.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Schröger E., Eimer M. Endogenous covert spatial orienting in audition: cost-benefit analyses of reaction times and event-related potentials. Quarterly Journal of Experimental Psychology. A, Human Experimental Psychology. 1997;50:457–474. [Google Scholar]
- Sexton M., Geffen G. Development of three strategies of attention in dichotic monitoring. Developmental Psychology. 1979;15:299–310. [Google Scholar]
- Squires K., Squires N., Hillyard S. Decision-related cortical potentials during and auditory signal detection task with cued observation intervals. Journal of Experimental Psychology: Human Perception and Performance. 1975;1:268–279. doi: 10.1037//0096-1523.1.3.268. [DOI] [PubMed] [Google Scholar]
- Stevens C., Fanning J., Coch D., Sanders L., Neville H. Neural mechanisms of selective auditory attention are enhanced by computerized training: electrophysiological evidence from language-impaired and typically developing children. Brain Research. 2008;1205:55–69. doi: 10.1016/j.brainres.2007.10.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens C., Lauinger B., Neville H. Differences in the neural mechanisms of selective attention in children from different socioeconomic backgrounds: an event-related brain potential study. Development Sciences. 2009;12:634–646. doi: 10.1111/j.1467-7687.2009.00807.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens C., Sanders L., Neville H. Neurophysiological evidence for selective auditory attention deficits in children with Specific Language Impairment. Brain Research. 2006;1111:143–152. doi: 10.1016/j.brainres.2006.06.114. [DOI] [PubMed] [Google Scholar]
- Sussman E., Steinschneider M. Attention effects on auditory scene analysis in children. Neuropsychologia. 2009;47:771–785. doi: 10.1016/j.neuropsychologia.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor M., Khan S. Top-down modulation of early selective attention processes in children. International Journal of Psychophysiology. 2000;37:135–147. doi: 10.1016/s0167-8760(00)00084-2. [DOI] [PubMed] [Google Scholar]
- Tonnquist-Uhlén I., Borg E., Spens K. Topography of auditory evoked long-latency potentials in normal children, with particular reference to the N1 component. Electroencephalography and Clinical Neurophysiology. 1995;95:34–41. doi: 10.1016/0013-4694(95)00044-y. [DOI] [PubMed] [Google Scholar]
- van der Molen M. Developmental changes in inhibitory processing: evidence from psychophysiological measures. Biological Psychology. 2000;54:207–239. doi: 10.1016/s0301-0511(00)00057-0. [DOI] [PubMed] [Google Scholar]
- White P., Yee C. P50 sensitivity to physical and psychological state influences. Psychophysiology. 2006;43:320–328. doi: 10.1111/j.1469-8986.2006.00408.x. [DOI] [PubMed] [Google Scholar]
- Woldorff M., Hansen J., Hillyard S. Evidence for effects of selective attention in the mid-latency range of the human auditory event-related potential. Current Trends in Event-related Potential Research Electroencephalography and Clinical Neurophysiology Supplement. 1987;40:146–154. [PubMed] [Google Scholar]
- Woldorff M., Hillyard S. Modulation of early auditory processing during selective listening to rapidly presented tones. Electroencephalography and Clinical Neurophysiology. 1991;79:170–191. doi: 10.1016/0013-4694(91)90136-r. [DOI] [PubMed] [Google Scholar]
- Zambelli A., Stamm J., Maitinsky S., Loiselle D. Auditory evoked potentials and selective auditory attention in formerly hypteractive adolescent boys. American Journal of Psychiatry. 1977;134:742–747. doi: 10.1176/ajp.134.7.742. [DOI] [PubMed] [Google Scholar]