Highlights
* We study the effect of training executive attention in 5-year-old children. * Training improves fluid intelligence and aspects of affect regulation. * Training enhances efficiency of neural network involved in executive attention * Effect on neural function is still observed two months later without further training.
Keywords: Training, Executive attention, Conflict resolution, ERPs, Fluid intelligence, Affect regulation
Abstract
Executive attention is involved in the regulation of thoughts, emotions and responses. This function experiences major development during preschool years and is associated to a neural network involving the anterior cingulate cortex and prefrontal structures. Recently, there have been some attempts to improve attention and other executive functions through training. In the current study, a group of 5 years old children (n = 37) were assigned to either a training-group who performed ten sessions of computerized training of attention or a non-trained control group. Assessment of performance in a range of tasks, targeting attention, intelligence and regulation of affect was carried out in three occasions: (1) before, (2) after, and (3) two months after completion of training. Also, brain function was examined with a high-density electroencephalogram system. Results demonstrate that trained children activate the executive attention network faster and more efficiently than untrained children, an effect that was still observed two months after without further training. Also, evidence of transfer of attention training to fluid intelligence and, to a lesser degree, to regulation of affect was observed. Results show that efficiency of the brain system underlying self-regulation can be enhanced by experience during development, providing opportunities for curricular improvement.
1. Introduction
One of the major changes that take place over the course of human development occurs in the domain of executive control. With age, children go from external regulation of their behavior, often provided by caregivers and/or changes in stimulation, to an increased ability to self-regulate emotions and actions. Mechanisms of attention have been implicated in action-regulation from early theoretical models (James, 1890, Norman and Shallice, 1986). According to Posner's neurocognitive model, attention is related to the function of three brain networks involved in (1) reaching and/or maintaining the alerting state, (2) orienting attention and selecting the source of stimulation, and (3) regulating thoughts, emotions and action. The third function is carried out by the so-called executive attention network, which involves the anterior cingulate cortex (ACC) and prefrontal regions of the brain (Posner and Petersen, 1990, Posner et al., 2007). Functions associated with the executive attention network overlap to some extent with the more general domain of executive functions (EFs), which encompass a set of interrelated processes involved in planning and carrying out goal-directed actions, including working memory (WM), mental-set switching or attentional flexibility, inhibitory control, and conflict monitoring (Blair and Ursache, 2011, Welch, 2001, Welsh and Pennington, 1988). These cognitive abilities are required when it is necessary to hold information in mind, manage and integrate information and resolve conflict between sources of stimulation or response options.
Developmental studies have suggested that the three attention networks considered in Posner's model follow different maturational courses (Posner and Rueda, in press, Rueda et al., 2004a). The alerting and orienting networks appear to mature largely during infancy and early childhood, although both networks continue developing up to late childhood, showing improvements in the endogenous control of processes related to preparation and selectivity. The executive attention network appears to undergo a more progressive maturation, emerging at about the end of the first year of life and continuing during childhood into adolescence. However, despite the progressive improvement throughout childhood, executive attention shows a major period of development from about the end of the first year of life up to about 7 years of age (Rueda et al., 2004a, Rueda et al., 2005a). Maturation of this function is likely related to structural changes in brain areas that are part of the executive attention network and their connectivity patterns with other brain structures, in particular, the emergence of greater fronto-parietal functional connectivity over development (Power et al., 2010).
A widely used strategy to study executive attention in cognitive research is to utilize conflict tasks (Posner and DiGirolamo, 1998). These involve suppressing either processing or responding to information that elicits an incorrect or inappropriate response. One of these is the flanker task (Eriksen and Eriksen, 1974). In this task, a target stimulus is surrounded by stimulation that suggests either the same (congruent) or a different (incongruent) response than the one associated to the target. Suppressing the processing of the distracting information in the incongruent condition requires attentional control and activates the executive attention network to a greater extent than when there is no conflict between target and flankers (Fan et al., 2003).
The ability to regulate behavior in a flexible and controlled mode has proven to be central to many aspects of children development. Individual differences in efficiency of executive attention appear to play an important role in school competence and socialization (Checa et al., 2008, Eisenberg et al., 2010, Eisenberg et al., 2011, Rueda et al., 2010). Several studies have shown that children with greater executive attention efficiency (i.e. smaller conflict scores) show higher levels of competence at school, understanding competence as a combination of school achievement and adequate socio-emotional adjustment in the classroom (Blair and Razza, 2007, Bull and Scerif, 2001, Checa et al., 2008, Rueda et al., 2010). Recently, we have shown that the brain reaction to conflict, as measured with event-related potentials, also predict children's grades in math above and beyond general intelligence (Checa and Rueda, 2011). Moreover, higher level of attentional and effortful control helps on the prevention of developing psychopathologies, such as externalizing behavioral problems and ADHD (Eisenberg et al., 2005, Rothbart and Posner, 2006). All these data show the prospect for a potential benefit of promoting children's attention regulation skills through educational interventions.
In recent years, several studies have reported positive effects of training different aspects of attention in children. Most of the studies have used computer-based tasks or sets of exercises targeting particular abilities. Kerns et al. (1999) showed improvement in a number of attention measures in ADHD children after applying an intervention program combining vigilance, selective and executive attention requirements. Also, normally developing preschool children have been shown to benefit from a training program targeting executive attention (Rueda et al., 2005b). Other studies have also shown improvements in attentional flexibility after task-switching training in both children and adults (Karbach and Kray, 2009, Minear and Shah, 2008). Besides, an increasing number of studies have examined susceptibility of other EFs components to be enhanced by training. Training of WM has proven to have beneficial effects on WM abilities in normally developing children (Thorell et al., 2009), children with ADHD (Klingberg et al., 2005), and adults (Jaeggi et al., 2008).
Interestingly, most of the studies described above have also reported transfer effects of training to fluid intelligence. For instance, gains in reasoning skills have been found after training of WM (Jaeggi et al., 2008, Klingberg et al., 2005), task-switching (Karbach and Kray, 2009), and executive attention (Rueda et al., 2005a, Rueda et al., 2005b). This evidence speaks against the idea of fluid intelligence as a fixed trait (Horn and Cattell, 1966). Instead, it suggests that fluid intelligence skills can be changed, although it could be the case that reasoning skills are less subject to education than crystallized intelligence. Transfer to intelligence after training of executive functions is relatively unsurprising given the interrelated nature of EFs processes and the fact that many common regions of the frontal lobe are recruited by cognitive demands involved in general intelligence and the various processes under the umbrella of EFs (Duncan and Owen, 2000, Duncan et al., 2000).
Several studies have also examined the effect of training on brain function. Information on the neural effects of attention training mostly comes from research using rehabilitation programs with patients. Sustained attention training with neglect patients was found to produce increased activation in the superior parietal cortex, right and left frontal areas and ACC, areas known to be associated with all three attention networks (Sturm et al., 2006, Thimm et al., 2006). Evidence is also available for other EFs components. For instance, using fMRI, Olesen et al. (2004) reported increased activation in areas involved in WM processing (i.e. the superior and inferior parietal cortices as well as in the middle frontal gyrus) after WM training in adults. Moreover, changes in brain mechanisms affecting dopamine neurotransmission have also being reported after WM training in normal adults (McNab et al., 2009).
More limited evidence is available on the effect that EFs training produces in children's brain function. In a study carried by our group (Rueda et al., 2005b), the effect of training attention was characterized with event-related potentials. We found that 5 sessions of attention training in preschool children produced a pattern of brain activation that was more adult-like compared to the untrained group. Brain activation was registered while children performed a child-friendly flanker task, and changes in the brain reaction to conflict was characterized in two dimensions: (1) timing of the conflict effect, which showed a shorter latency after training, and (2) topography of the conflict effect, which moved to posterior leads in the frontal midline with training, the distribution that the flanker conflict effect shows in adults and that has been associated with a source of activation in the ACC (van Veen and Carter, 2002). Also, using ERPs with a selective attention paradigm, Stevens et al. (2008) reported an increased differentiation between attended and unattended processing in the brain signal (i.e. greater ability to filter-out irrelevant information) of children who underwent an intensive training program designed to improve language skills.
The aim of the present study was to further examine the effect of training attention on brain function in a group of preschool-aged children. Training focused on executive attention, although also included exercises targeting sustained and selective attention. To examine brain function, a high-density electroencephalogram system was used while children performed a flanker task before and after training. Moreover, in order to study durability of the training effect, a short-range follow-up session was carried out two months after completion of the training program. Finally, we were interested on examining possible transfer effects to untrained abilities that have been related to individual differences on executive attention efficiency. In particular, we assessed children's performance on tasks involving regulation of affect and intelligence in all sessions (PRE, POST1 and POST2).
Training was expected to result in increased executive attention efficiency both in performance of the conflict task and the underpinning brain activation. In terms of brain activation, increases in efficiency due to maturation appear to result in a reduction of the latency and duration of the conflict effect (Jonkman, 2006, Ridderinkhof and van der Molen, 1995). Also, prior imaging studies have shown that brain activations related to executive control become more focused and refined with maturation (Bunge et al., 2002, Casey et al., 1997). Therefore we expected that training would result in shortening the latency, amplitude and duration of the brain reaction to conflict. Besides, we predicted that the source of activations related to the ERP effects would also show more focused frontal activation after training.
Given previous evidence on the transfer of training EFs abilities to reasoning, we also expected our training to increase fluid intelligence. Finally, we hypothesized that increasing executive attention efficiency would generalize to tasks requiring regulation of responses involving rejection of immediate rewards in exchange for delayed but more favorable consequences. This hypothesis was based on evidence coming from both developmental and imaging studies. Developmental studies have pointed to the idea that children who show better attention control (i.e. smaller conflict interference in the flanker task) appear to be more able to regulate affect (Simonds et al., 2007). In addition, imaging studies have provided evidence for a role of the ACC, a major node of the executive attention network, in the regulation of affect (Bush et al., 2000, Ochsner et al., 2002, Ochsner and Gross, 2007).
2. Method
2.1. Participants and procedure
A total of 37 children (20 males; mean age: 64.7 months; SD: 3.2) recruited at an urban Primary School in Granada (Spain) participated in the study. Caregivers of all the children gave written consent to be involved in the study after being informed of its general purpose. All participants were Caucasian/European and had a similar social background. Prerequisites for participation were having normal or corrected-to-normal sensory capacities and no history of chronic illness and/or psychopathologies.
All participants carried out a first assessment session (PRE) at the Cognitive Neuroscience Lab of the Psychology Dept., University of Granada. During this session children were administered a set of pen and paper tasks including the Delay of Gratification and Children Gambling tasks and the Kaufman Brief Intelligence Test (K-BIT). After conclusion of these tasks, children were fitted with the 128-channels Geodesic Sensor Net (www.egi.com) and were asked to perform the child version of the Attention Network Task (ANT) while EEG was recorded. The duration of the session was 1 h approximately, including time for instructions and breaks between tasks. During this session, parents were also asked to fill out a temperament questionnaire.
After completion of the first session, children were pseudo-randomly assigned to either the experimental (to-be-trained) or the control (untrained) group. The assignment was made so children in each group would be matched by gender, average intelligence and flanker interference in the ANT. A total of 19 children (10 males; mean age: 65.1; SD: 3.73) were assigned to the experimental group, and 18 (10 males; mean age: 64.3; SD: 2.56) to the control group. Participants in the two groups did not differ in age (F > 1) or parental educational level, and they all attended the same school. Also, there were not significant differences (all t < 1; except t(35) = 1.7; p = .09 for orienting and t(35) = −1.12; p = .26 overall commission errors) between the groups in the scores of the assessment tasks obtained in the PRE session.
Children in the trained group went through intervention with a set of computerized exercises for a total of ten 45-min sessions that were carried out over a period of 5 weeks (2 sessions per week). Those sessions were conducted individually for each participant in a quiet room at the school. Participants assigned to the control group underwent the same number of sessions in similar conditions (individually with the experimenter and in a quiet room at school) but watched cartoon videos instead.
Once the intervention period was completed, children in both groups were invited to the lab in two more occasions. The first post-intervention (POST1) session was carried out within a period of one week after completion of the intervention. The second post-intervention (POST2) session was conducted two months after completion of the POST1 session. The tasks completed in these sessions and the procedure followed was identical to that of the PRE session.
3. Materials
3.1. Assessment tasks
3.1.1. Delay of gratification (DoG)
The DoG task administered in this study was a modified version of the task designed by Thompson et al. (1997). Children were instructed to choose between getting a prize immediately or waiting until the end of the task in order to (a) get two prizes instead of one (DoGself), or (b) have someone else (the experimenter) get a prize too (DoGother). Three different types of reward were used: stickers, 5-cents of euro coins, and candies. An example of a DoGself trial is “You can choose between having one sticker right now or getting two of them when we finish the game”. An example of the DoGother trial is “You can choose between having one coin for you right now or having one for you and one for me at the end of the game”. Two practice trials were used to explain the task. Experimental trials began only when it was clear that the child understood the instructions. Then, each participant completed 12 trials, 6 of each condition (DoGself/DoGother), four with each type of reward. The immediate-choice was presented in the first part of the statement in half of the trials and the delay-choice was presented first in the other half. The experimenter provided no feedback other than supplying the rewards immediately or putting them in an envelope that the child would get at completion of the task, depending on the child's choice. Percentage of delay choices in each condition was the dependent measure in this task.
3.1.2. Children gambling task
The gambling task administered in this study was a simplified version of the Iowa Gambling Task by Kerr and Zelazo (2004). Children had to pick one card at a time from one of two sets of cards in order to win candies. Each card could have smiling and sad faces printed on it. Children were told and shown that happy faces on the cards indicate the number of candies won, whereas sad faces indicate the number of candies lost. One of the sets provided a constant reward of one smiling face and either no sad faces or only one (Advantageous Set; ADV), consisting of a low-immediate-reward/low-win rate in the long run. The other set (Disadvantageous Set; DIS) provided a constant reward of two smiling faces and could have either zero, two, four or six sad faces, consisting of a high-immediate-reward/high-lost rate in the long run. The two sets of cards were placed in front of the child facing down so the child would have to figure the contingencies of each deck out progressively. In the beginning of the task, children were given a stake of 10 candies in order to start playing. There were 4 demonstration trials in which the experimenter sampled two cards from each deck. When a card was turned over, only the happy faces were visible while the sad faces were covered with a note. After the number of won candies was revealed to the child and the candies were supplied, the note was removed revealing the number of candies lost. Rewards were deposited into, and removed from (according to the number of happy and sad faces obtained in each trial), a transparent container situated in front of the child at an equal distance from each of the two decks. A total of 50 trials were administrated. The dependent variable was the number of picks from the ADV set minus the number of picks from the DIS set made in the last 40 trials. The location of the two decks and the design at the back of the decks were counterbalanced across participants.
3.1.3. K-BIT
The Kaufman Brief Intelligence Test (K-BIT; Kaufman and Kaufman, 1990) was used to measure general intelligence. The test provides a measure of crystallized (Verbal) intelligence and a measure of fluid reasoning (Matrices) as well as a composite intelligence (IQ) score. Administration of the test takes approximately 15 min with children aged 4–5 years.
3.1.4. Child ANT
We used the children version of the ANT task (Rueda et al., 2004a) to measure attention. In each trial of this task a row of five fish appearing either above or below a fixation point is presented. Children are told to press either the right or the left key on a panel depending on the direction in which the fish in the middle is pointing while ignoring the flanker fish, which point in either the same (congruent) or opposite (incongruent) direction as to the middle fish. Before the fish appear, visual signals are presented that inform either about the upcoming of the target (alerting cue) only or about the upcoming of the target as well as its location (orienting cue). Completion of the task allows calculation of three scores related to the efficiency of the attention networks by means of measuring how response times are influenced by alerting cues, orienting cues and congruency of the flankers. The alerting score is obtained by subtracting mean (or median) RT of trials with alerting signal from that of trials with no cue. The orienting score is obtained by subtracting RT from trials with orienting cue from trials in which the visual cue was presented at the location of the fixation point. Finally, the executive attention score is calculated by subtracting RT from trials with congruent flankers from that of trials with incongruent flankers. This is considered an index of the interference experienced by the participant when incongruous information is presented in the display along with the target. Larger interference scores indicate less efficiency of executive attention.
3.1.5. EEG recording and data processing
EEG was recorded using a high-density array of 128 Ag/AgCl electrodes arranged into a net (Geodesic Sensor Net, EGI Inc., Eugene, OR) while children performed the Child ANT during the pre- and post-training evaluation sessions. All 37 participants agreed to wear the sensor net. Impedances for each channel remained at or below 80 kΩ during testing. During acquisition, EEG recording was vertex-referenced and the signal was digitalized at 250 Hz. A time constant value of 0.01 Hz was used. Off-line data were filtered using a 0.3–12 Hz finite impulse response (FIR) band-pass filter and segmented into 200 ms pre-target and 1200 ms post-target epochs. Segmented files were scanned for eye and/or movement artifacts. One child (belonging to the control group) who had less than 12 clean segments per congruency condition was excluded from further processing. Segments were averaged across congruency conditions and re-referenced to the averaged (across channels) activation.
3.2. Training program
The training program was the same as the one used by Rueda et al. (2005b; also described in Rueda et al., 2007). The program consists of several computerized exercises divided in 4 categories: (1) Tracking/Anticipatory; (2) Attention Focusing/Discrimination; (3) Conflict Resolution; and (4) Inhibitory Control Exercises. In the current study the program was extended with three more exercises, one in the category of Attention Focusing/Discrimination, one in the Conflict Resolution category and another in a new category of Sustained Attention. Therefore, the new program consisted of a total of 11 exercises divided in 5 general categories. The exercises were programmed to be child-friendly and involved playing with a joystick or a mouse. All exercises required completion of a number of trials organized in increasing levels of difficulty. Most of the exercises had 7 levels of difficulty and in order to go from one level to the next the child must complete a minimum of correctly responded trials in a row (3, in most exercises).
Exercises in the Tracking/Anticipation category were designed to teach the children to track a cartoon cat on the computer screen by using the mouse, and monitor the position of other cartoons in the screen. In the Side exercise the child is asked to take the cat to the grass while avoiding going into the mud. As the child achieves higher levels, the mud area gets progressively bigger and the grass area gets smaller, increasing the difficulty to control the movement of the cat. In the Maze exercise, children help the cat to get food by navigating it through a maze to where the food is. Finally, in the Chase exercise children must anticipate the location where a duck that swims across a pond in a straight line will come across in order to chasing it. In a second version of the exercise (Chase Invisible), the duck becomes invisible when it goes into the pond, as if diving, so that its trajectory remains invisible.
The exercises in the Focusing/Discrimination category are of two types. The first type consists on matching-to-sample games, in which children have to click on the one of two pictures that looked exactly the same as a sample picture. Similarities between the two options increased progressively, requiring the child to pay closer attention. There are two versions of the exercise. In this first version (Portraits), the sample picture remains on the screen while the child selects the matching item. In the second version (Portraits Delay), the sample picture disappears forcing the child to keep in mind the attributes of the sample picture. The second type of exercise (Shapes) consists of the presentation of a number of overlapping figures and the child is to determine which are the ones presented by clicking on the appropriate buttons displayed on the sides of the screen. Difficulty is augmented in successive levels by increasing the number of overlapping shapes and the complexity of the patterns.
The Conflict exercises consisted on Stroop-like games with numbers. In the first one (Number of Numbers), children are presented with two sets of items. Their job is to click in the group composed by the larger amount of items. In the firsts levels of the exercise, sets consist of pictures of fruits and the number of items in each group differs by a large amount (e.g., two compared to eight). As the difficulty levels increase, the two sets are made of digits, and therefore trials can be congruent (when the larger set of digits is formed by digits of higher value, for example four numbers 8 vs. two numbers 1) or incongruent (when the larger set of digits is formed by digits of smaller value, for example six numbers 2 vs. four numbers 9). The second Stroop-like exercise (Value-Not-Size) also involves numbers, but in this case the conflicting dimensions are value and size. In successive trials, various numbers (either two, three or four), which differ in size, are presented and children are asked to click on the number of higher value disregarding the size. Again, there can be congruent (the larger number is the one with higher value) or incongruent (the larger number is not the one with higher value) trials. To go on from one difficulty level to the next children must correctly perform three incongruent trials in a row. Before performing these exercises, children completed another exercise in which their knowledge of Arabic digits was practiced.
The exercise (Farmer) included in the Inhibitory Control category consist in a Go/No-Go game in which the child's job is to help a farmer taking sheep inside a fence. The picture of a bale of hay is displayed in the middle of the computer screen. Children click on the bale of hay to find out whether the animal behind it is a sheep or a wolf. If the animal is a sheep the child is to click as quick as possible to make it go inside the fence, whereas the response must be hold to the wolf. In advanced levels, the wolf dresses-up as a sheep and only after a short interval it losses its mask and reveals its identity, making the response inhibition more challenging.
Finally, a category of Sustained Attention was included. This consists of one exercise (Frog) in which children are asked to help a frog catch flies that come out of a bottle at a particular time rate. The child must press a key as fast as possible in order to unroll the frog's tongue and catch the fly. In some trials, the fly makes a noise before coming out of the bottle. The requirement to sustain attention is increased across blocks of trials by enlarging the interval of time between flies.
4. Results
4.1. Effects on behavioral measures
To assess the effect of training on the various behavioral tasks included in the study we conducted a set of repeated measures ANOVAs with Session (PRE, POST1 and POST2) and Group (Untrained vs. Trained) as factors and each of the tasks scores as dependent measure. Then, changes in the PRE vs. POST sessions (PRE vs. POST1 and PRE vs. POST2) scores were examined with planned comparisons given that changes were predicted after intervention. For the measures that showed significant differences between groups at PRE test (i.e. orienting scores and % of commission errors in the ANT), pre scores were included as covariates in the ANOVAs. Results on the Group × Session interaction and planned contrast for each measure and group are summarized in Table 1.
Table 1.
Means and standard deviations (SD) of all the dependent variables (DV) included in the study for each group at session PRE, POST1, and POST2. The columns on the right show results of Group × Session interactions and planned contrasts performed between PRE vs. POST1 and PRE vs. POST2 scores for each group.
| Task | DV | Group | PRE | POST 1 | POST 2 | Group × Session interaction F value | Planned contrasts |
|
|---|---|---|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | PRE vs. POST1 | PRE vs. POST2 | ||||
| IQ | Matrices | Trained | 104 (9.6) | 110 (10.45) | 109 (11.6) | <1 | 4.9* | 2.8# |
| Control | 107 (10.6) | 112 (12.47) | 107 (10.8) | 2.5 ns | <1 | |||
| Vocabulary | Trained | 110 (10.8) | 110 (12.9) | 110 (10.7) | <1 | <1 | <1 | |
| Control | 107 (10.2) | 108.5 (11.1) | 106 (11.6) | <1 | <1 | |||
| Gambling | V-D last 40 trials | Trained | 6.9 (20.2) | 12.7 (18.2) | 16 (18.9) | <1 | <1 | 4.2* |
| Control | 5.11 (18.5) | 5.4 (22.8) | 3 (18.2) | <1 | <1 | |||
| DoG | Self | Trained | 77.2 (33.9) | 82.5 (32.1) | 84.2 (29.6) | 2.5# | 1.1 ns | 1.9 ns |
| Control | 70.6 (30.5) | 60.2 (39.2) | 68.5 (41.1) | 4.22* | <1 | |||
| Other | Trained | 63.1 (33.1) | 61.4 (42.3) | 61.4 (44.1) | <1 | <1 | <1 | |
| Control | 59.3 (33.4) | 43.5 (40.1) | 50.9 (44.4) | 4.43* | 1.15 ns | |||
| Child ANT | Overall RT | Trained | 1071.4 (241.6) | 934.5 (195.8) | 823.8 (129.6) | <1 | 9.9** | 33** |
| Control | 1064.1 (233) | 973.2 (303) | 809.47 (227) | 4.1* | 33** | |||
| Overall % commission errors | Trained | 5.9 (4.84) | 4.6 (5.8) | 3.6 (2.7) | <1 | 1.2 ns | 2.9# | |
| Control | 9 (10.8) | 6.4 (10.9) | 4.9 (4.9) | 4.2* | 8.3** | |||
| Overall % omission errors | Trained | 4.11 (4.87) | 3.54 (6.16) | 4.11 (4.8) | <1 | <1 | <1 | |
| Control | 4.36 (6.57) | 3.9 (5.74) | 4.28 (6.57) | <1 | 2.05 ns | |||
| Alerting | Trained | 12.8 (148.5) | 56.5 (111.8) | 63.3 (56.03) | <1 | 1.2 | 3.4# | |
| Control | 14.3 (95.6) | 17.9 (111.8) | 59.5 (54.9) | <1 | 2.5 ns | |||
| Orienting | Trained | 24.2 (125.5) | −21.6 (95.7) | 9.9 (105.7) | 2 ns | 1.3 ns | 1.1 ns | |
| Control | −61.4 (179) | −20.0 (130) | 6.5 (82.01) | <1 | 1.5 ns | |||
| Executive | Trained | 66.5 (168.6) | 53.5 (154.1) | 51.4 (63.3) | <1 | <1 | <1 | |
| Control | 84.5 (161.5) | 72.1 (183.6) | 80.1 (178.6) | <1 | <1 | |||
Significance level: ns: p < .10.
p < .05.
p < .01.
p < .10.
4.1.1. Child ANT
Attention network scores for Alerting, Orienting and Executive Attention were obtained. Also, general performance indexes such as overall RT and percentage of commission and omission errors were examined. Alerting scores were calculated by subtracting median RT to Double-Cue trials from median RT to No-Cue trials. The Orienting score was calculated by subtracting median RT in Spatial-Cue trials from median RT in Central-Cue trials. Finally, Executive Attention scores (flanker interference effect) were calculated by subtracting median RT for congruent trials from median RT for incongruent trials for each participant and session (see Table 1).
ANOVAs contrasting sessions PRE and POST1 revealed a significant main effect of Session for overall RT (F(1,35) = 13.34; p < .001) and for overall percentage of commission errors (F(1,35) = 4.97; p < .05). Planned contrasts showed a reduction in overall RT for both trained (F(1,35) = 9.9; p < .001) and control (F(1,35) = 4.1; p < .05) groups at POST1 compared to PRE. Also, we found a reduction between session PRE and POST1 in overall commission errors for the control group (F(1,35) = 4.17; p < .05) which was not present for the trained group (F(1,35) = 1.2; p = .27). No other contrast showed significant results.
The PRE vs. POST2 ANOVAs revealed a significant main effect of Session for overall RT (F(1,35) = 67; p < .001), overall percentage of commission errors (F(1,35) = 10.68; p < .01), and alerting scores (F(1,35) = 5.9; p < .05). No significant Group × Session interactions were obtained for any of the scores. However, contrasts showed significant reductions at POST2 compared to PRE for overall RT for both trained (F(1,35) = 33.5; p < .001) and untrained (F(1,35) = 33.5; p < .001) children. Also, the reduction in overall commission errors at session POST2 was significant for the control group (F(1,35) = 8.3; p < .01) and marginal for the trained group (F(1,35) = 2.94; p = .09). Finally, a marginal increase in alerting was observed for the trained group (F(1,35) = 3.4; p = .07).
4.1.2. K-BIT
When including Matrices scores as DV, only the main effect of Session for PRE vs. POST1 was significant (F(1,35) = 7.4; p < .01). No significant effects for PRE vs. POST2 were found. Despite the non-significant Group × Session interactions, planned contrasts showed a significant increase in the Matrices score following intervention only for the trained group (F(1,35) = 4.9; p < .05; F(1,35) = 2.6; p = .12 for the untrained group). The increase in Matrices was marginal for the trained group when comparing PRE to POST2 scores (F(1,35) = 2.8; p = .10) but did not approach significance (F < 1) for the control group. For the Vocabulary subscale, no significant effects were obtained in the ANOVAs or with planned comparisons.
4.1.3. Gambling task
None of the main effects or the Group × Session interaction was found significant when including the PRE and POST1 data in the ANOVA. However, the Group × Session interaction was marginally significant (F(1,35) = 3.07; p = .08) in the ANOVA including PRE vs. POST2 scores. Planned contrasts showed that children in the training group selected more advantageous choices than those in the control group (F(1,35) = 4.5; p < .05), and that trained children increased the percentage of total choices from the advantageous deck between sessions PRE and POST2 (F(1,35) = 4.17; p < .05).
4.1.4. Delay of gratification
ANOVAs were conducted separately for the scores (percentage of delay choices) obtained at the DoGself and the DoGother versions of the task. For the DoGself, main effects of Session or Group were not observed in any of the ANOVAs (PRE vs. POST1 or PRE vs. POST2). For the ANOVA with PRE vs. POST1 scores, the Group × Session interaction was significant in the DoGself task (F(1,35) = 4.91; p < .05). Planned contracts showed that untrained children decreased the percentage of delay choices between PRE and POST1 sessions (F(1,35) = 4.22; p < .05) and that the trained group had the tendency to delay more than the control group following intervention (F(1,35) = 3.58; p = .07). For the DoGother version of the task, the pattern of results was similar. The only significant difference observed occurred between percentage of delay choices from session PRE to POST1 for children in the control group (F(1,35) = 4.43; p < .05).
4.2. Effects on brain electrophysiology
4.2.1. ERPs
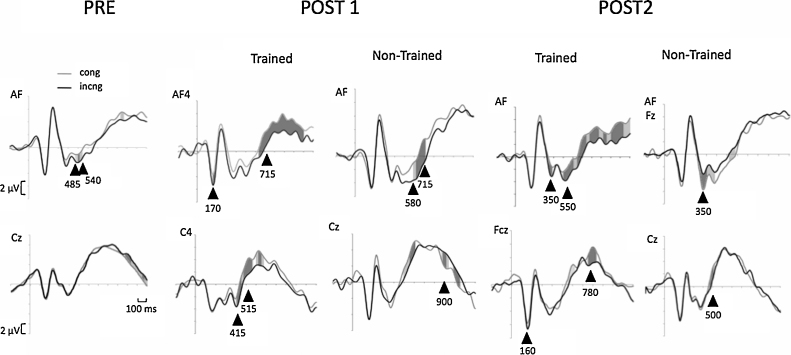
Target-locked ERPs per congruency condition are presented in Fig. 1 for the entire sample at session PRE and for children at the trained and non-trained groups at sessions POST1 and POST2. ERPs pertaining to the PRE intervention session showed amplitude differences between congruency conditions (i.e. larger negative amplitudes for incongruent trials) in leads situated in the frontal midline, as previously reported in the literature with the same (Rueda et al., 2004b) or similar tasks (Jonkman, 2006, van Veen and Carter, 2002). To asess possible differeces in this effect between groups at the PRE-intervention session, amplitude and latency effects of congruency at frontal midline channels in the time window ranging from 300 to 700 ms were tested with ANOVAs including Group (to be trained and control), Channel (AF, Fz, Fcz and Cz) and Condition (Congruent vs. Incongruent) for both amplitude (using both minimum amplitude within the time window and adaptive mean1 values) and latency measures. In any of these analyses the main effect of Group or any of its interactions with other factors approached significance (F < 1 in all cases except for the Channel × Cond × Group interaction for latency which reached an non-significant, p = .24, F value of 1.41). Given the absence of differences between groups the waveforms are presented for the entire sample at session PRE. The top row shows ERPs at prefrontal sites (AF/Fz) and the row at the bottom at fronto-parietal (Fcz/Cz) positions. Dependent-samples t tests of the differences in amplitude between congruent and incongruent conditions were carried out (using the t-test tool incorporated in the Net Station software, EGI, Eugene, OR) for each sample along the entire ERP segment. Areas of significant differences among conditions are shadowed in the waveforms presented in Fig. 1. Only differences in amplitude that were found significant in at least 10 consecutive samples (40 ms) were marked as significant in the waveforms. Also, topographic maps of the scalp distribution of significant incongruent vs. congruent differences at particular post-target times are shown in Fig. 2 (session POST1) and 3 (session POST2). At session POST1, trained children show the expected N2 effect (i.e. larger negative amplitude for incongruent compared to congruent trials) from around 400 ms after presentation of the target, whereas untrained children show the effect around 580 ms post-target. Also, the effect is observed in more posterior sites (around Fcz) for trained compared to non-trained children, who show the effect in the same sites (around Fz) as was observed in the PRE intervention session. This difference between trained and untrained children in the timing of the conflict effect is still present in data obtained at session POST2, where trained children show the expected N2 effect around 360 ms post-target, and children in the control group show the effect at about 500 ms after presentation of the target. We also found an early negative deflection around 170 ms after stimulus presentation that was significantly more negative for the incongruent condition at frontal sites in POST1 only in the trained group. This early effect was also evident at POST2 although its topography moved to more posterior channels (see waveforms in Fig. 1 and topo maps in Fig. 2, Fig. 3).
Fig. 1.
Plots of grand averaged ERPs waveforms to congruent and incongruent trials for all children at session PRE, and children assigned to the trained and non-trained groups at sessions POST1 and POST2. The top row shows ERPs at channels located in anterior frontal (AF) positions and the bottom row shows EPRs at channels in fronto-posterior (Fcz/Cz) leads. Shadowed areas between conditions show areas of significant amplitude differences between congruency conditions (dark grey: p < .01; light grey: p < .05). Arrow heads point to post-target times (in ms) for which topographic maps of t-tests differences were created (shown in Fig. 2, Fig. 3).
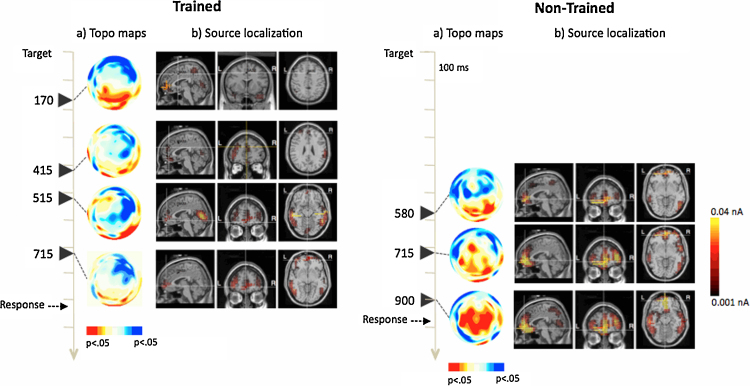
Fig. 2.
Topographic maps of significant amplitude differences between congruency conditions (a) and corresponding source solutions (b) for each group at session POST1. Arrowheads show the particular time points (in ms) between the presentation of the target and the averaged time of the response to which each topo map corresponds. Dark blue areas in the topographic maps indicate sites in which amplitude of the ERPs was significantly more negative for incongruent, compared to congruent, trials. The top part of the topo maps corresponds to the front of the head.
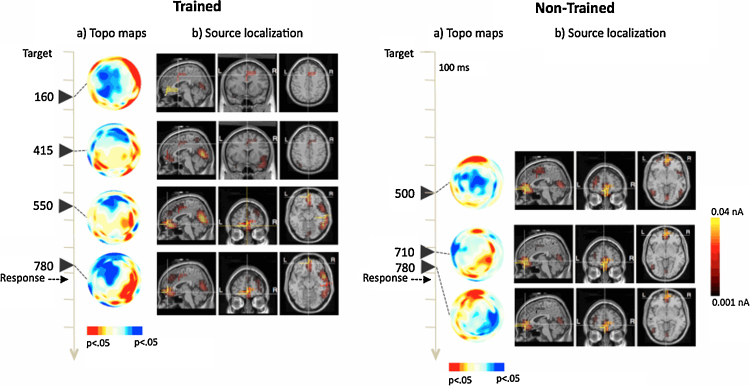
Fig. 3.
Topographic maps of significant amplitude differences between congruency conditions (a) and corresponding source solutions (b) for each group at session POST2. Arrowheads show the particular time points (in ms) between the presentation of the target and the averaged time of the response to which each topo map corresponds. Dark blue areas in the topographic maps indicate sites in which amplitude of the ERPs was significantly more negative for incongruent, compared to congruent, trials. The top part of the topo maps corresponds to the front of the head.
The topographic distribution of significant differences between flanker conditions changes differentially for trained and non-trained group in sessions POST1 and POST2. As shown in Fig. 2, the larger negativity associated with the incongruent condition moves from anterior sites in session PRE to a more posterior and right lateralized localization in sessions POST1 and POST2 for the trained group. However, this pattern of activation is not observed for children in the non-trained group who showed the conflict effect later in time and with a more anterior and left lateralized distribution in POST1, similar to the one observed in session PRE, and a more distributed effect in session POST2 (see Fig. 2, Fig. 3).
4.2.2. Source localization analyses
Grand averaged (across subjects) ERPs data for each group were used to compute the most likely cortical generators of the pattern of electrical fields registered on the scalp with a source localization software (GeoSource, EGI, Eugene, OR). A Finite Difference Model (FDM) was used for inverse modeling,2 in which the Minimum Norm Least Squares (MNLS) algorithm is used with a set of 2447 dipoles triples (comprising dipoles in the x, y, and z orientations) sampling the cerebral cortex. The Sun-Stok 4-Shell Sphere was used as a forward head model. Weighting was placed equally across locations with regularization carried out via TSVD (1 × 10−4) using LAURA (Local Auto-Regressive Average; Grave de Peralta Menendez et al., 2004) as a constraint. The Radius of influence was set to 12.2 mm with an exponent equal to 3.
Data corresponding to the entire sample at session PRE, and subsequently with trained and untrained groups at sessions POST1 and 2, were source analyzed at the post-target times in which significant differences between congruency conditions were found. With the PRE session data, the main sources were obtained at bilateral medial frontal gyrus (r and lMFG; BA10), medial inferior frontal gyrus (mIFG; BA11), bilateral medial temporal gyrus (lMTG and rMTG; BA21), and a weak source at dorsal ACC (BA32). A ROI montage was then created with these areas with a radius of 7 mm around the dipole of maximum activation, in order to trace changes in the intensity assigned to these dipoles in function of training at the POST sessions. Sources obtained at these ROIs (and additional areas) for trained and untrained groups are presented in Fig. 2 (session POST1) and Fig. 3 (session POST2).
5. Discussion
In this study, we extended the training program applied by Rueda et al. (2005b) with several new exercises, and increased from 5 to 10 the number of sessions used to train attention with a group of preschoolers. The study aimed to replicate and extend prior results on the effect of training attention on the efficiency of the executive attention network and related abilities. The specific goal was to investigate whether applying the extended training program has an effect on the efficiency of executive attention and the underlying brain mechanisms and to test durability of the effect in a short-term follow-up two months after completion of the training. We also aimed at examining whether training attention produces transfer effects to untrained abilities, although related to attention, such as intelligence and regulation of affect.
5.1. Effects of training on the performance of the child ANT
As revealed by the analyses, training had no significant effect on the behavioral performance of the ANT. Overall RT and percentage of errors data clearly show that children in both groups become more proficient performing the task in post sessions. Also, attention network scores, particularly the executive attention and alerting scores, show some evidence of increased efficiency. However, the fact that the pattern of change is very similar for trained and untrained groups indicates that repetition of the task itself is responsible for the improvement. Mean interference scores showed some evidence of a reduction of the conflict effect, indicating gains in efficiency of executive attention skills, for both the trained and untrained groups at session POST1. However, none of the observed reductions in conflict interference reached statistical significance. A similar pattern was observed for the Alerting score. The trained group exhibits an increase in alerting after training, which is marginally significant when comparing the PRE vs. POST2 scores. Mean scores of the untrained group also show an increase at session POST2, indicating that the mere repetition of the task is at least partially responsible for the effect. The increase in alerting scores would indicate that children become more able to prepare from the presence of warning cues, making faster responses when this happens. Nevertheless, despite showing changes in the network scores in the expected direction, those changes did not reach statistical significance. This is likely due to the high variability that children of the age included in the study show in reaction time when performing this task (large SDs, see Table 1). It is also probable that children reach a ceiling level of performance when carrying out the task in repeated occasions because the network scores obtained by the participants (particularly those in the trained group) in post-sessions are similar to the ones shown by adults (Conflict score: 61 ms; Alerting score: 30 ms) when performing the same task (Rueda et al., 2004a).
5.2. Transfer of training to fluid intelligence
Transfer of training to fluid intelligence was highly expected given prior results described in the literature. Both trained and untrained children showed and increase in the matrices subscale score at session POST1 as indicated by the significant main effect of Session. However, planned contrasts showed that only observed gains for trained children reached the significance level. This result replicates previous findings that training of executive attention improves non-trained reasoning abilities. The effect of training is specific for fluid intelligence and does not have an impact in the verbal scale related to learning-dependent or crystallized intelligence. Interestingly, we found some evidence (as indicated by a marginally significant PRE vs. POST2 contrast) suggesting that the generalization of training to fluid intelligence is still observed two months later without further training, whereas the matrices score returned to the initial level (F < 1 for the PRE vs. POST2 contrast) for the untrained group. Transfer to fluid intelligence skills was expected because it has been shown that there is an extended overlap between the brain structures implicated in general intelligence and those of the executive attention network (Duncan et al., 2000). Our data add on to evidence provided by other studies indicating that fluid intelligence can be improved with intervention (Jaeggi et al., 2008, Nutley et al., 2011, Rueda et al., 2005b). The modest effect observed in our study could be due to the fact that more extended interventions are required to change cognitive abilities such as reasoning that appear to be heritable to an important extent (Cattell, 1987, Gray and Thompson, 2004). However, data of this sort challenge the idea that fluid intelligence is not subject to the influence of education and socialization.
5.3. Transfer of training to regulation of affect
Another question that was addressed in this study was whether training of attention would generalize to tasks that are thought to rely on attention control skills. In our study, we included two tasks, Children's Gambling and Delay of Gratification (DoG), to examine transfer of training to regulation of affect and motivation (also known as “hot” executive function; Hongwanishkul et al., 2005). Regulation of motivational tendencies can be observed in situations in which inhibition of immediate rewards is required in the face of a higher price in the long term. We found that children trained with our program increased the number of advantageous choices in the Gambling Task when comparing performance at sessions PRE vs. POST2, whereas untrained children performed similarly in all three sessions (PRE, POST1 and POST2). In this task, it is necessary to reappraise the motivational significance of immediate rewards in order to learn to choose advantageously. Our results indicate that trained children became more able to control their choices according to the wins/looses contingencies of each deck, being more able to inhibit choosing from the dominant high-reward deck but subject to higher potential looses.
For the DoG task the pattern was somewhat different. In the two versions of the task used in our study (DoGself and DoGother), untrained children showed a significant decrease in the number of delay choices the second time they played the task (session POST1 compared to PRE). Trained children, however, did not show a decrease in the percentage of delay choices. A plausible explanation for the drop in the percentage of delay choices shown by children in the control group at the POST1 session is that the second time children perform the task they know how long the delay period is, because they experienced it at the PRE session, which may discourage them from choosing the delay option. This circumstance is also true for the trained group. However, in this case, training may have helped children to be more able to control dominant, yet unfavorable, responses, therefore preventing them from showing a decline in the percentage of delay choices in post-intervention sessions.
5.4. Effects of training on brain function
A major goal of our study was to advance understanding of the neural mechanisms of training and to examine whether the effect of the intervention on brain function shows signs of stability over time in a relatively short follow-up two months later. Brain electrical activity was recorded with a high-density electroencephalography system while children performed a child-friendly flanker task. As expected, the congruency of flankers modulated a negative component of the ERPs that was frontally distributed (often referred to as the N2). This component showed larger amplitude in trials involving conflict (i.e. with incongruent flankers). Prior studies had reported that preschool children show modulation of the frontal negativity sometime later than adults and at more anterior channels (Rueda et al., 2004b). In the current study, we observed modulation of the frontal negativity starting around 500 ms post-target at session PRE (see Fig. 1). Additionally, our results replicate previous findings that training affect both timing and topographic distribution of ERPs related to conflict monitoring (Rueda et al., 2005b). The effect of flankers on the amplitude of the ERPs at frontal leads is shown earlier and in more posterior channels for trained children compared to children in the control group (Fig. 1). Moreover, the pattern appears to be maintained two months later, as trained children show the expected larger negativity for incongruent trials earlier (from 360 ms post-target on) than non-trained children (see also topo maps of significant congruency effects in Fig. 2, Fig. 3). In adults, the conflict N2 effect has been associated with a source of activation in the ACC (van Veen and Carter, 2002). There is evidence suggesting that the ACC is involved in detecting the occurrence of conflict, which is then conveyed to prefrontal structures in charge of resolving the conflict (Botvinick et al., 2004, Walsh et al., 2011). Thus, a delayed frontal negativity may be indicative of poorer efficiency of the executive attention network related to a slower detection of conflict in children compared to adults. Data from our study suggest that one of the processes by which training may influence the executive attention network is by hastening the neural mechanisms supporting the detection and signaling of conflict from the ACC to prefrontal structures.
Data from source localization analyses are also consistent with the idea of a more efficient activation of the executive attention network in trained children. Modeled images of electrophysiology data identified the more likely neural generators of the effects observed in the ERPs. Fig. 2, Fig. 3 show the sources of activation modeled from the topographic maps of the incongruent minus congruent difference in electrical recordings at times in which significant differences in amplitude between those two conditions were observed. Overall, generators were localized at medial inferior frontal (BA11), medial–lateral PFC (mlPFC), medial frontal gyrus (BA10), and dorsal ACC (BA24/32). The pattern of activation is consistent with the one observed with similar tasks in somewhat older children using fMRI (Bunge et al., 2002, Konrad et al., 2005). Besides, source modeling data demonstrate that children in the trained group show earlier engagement of dorsal ACC than the untrained controls, who only show a clear ACC generator the third time they perform the task (session POST2). In addition, sources of activation in mlPFC become more lateralized to the right hemisphere as children gain experience with the task (POST2 compared to POST1). However, this lateralization to the right is only observed for the trained group. Imaging studies of interference suppression have reported activation in ventro-lateral PFC that is left lateralized in children but right lateralized in adults (Bunge et al., 2002, Konrad et al., 2005). Moreover, the right vlPFC activation in adults correlates positively with the ability to suppress interference (Bunge et al., 2002). These data suggest that children in the trained group may be showing a more adult-like pattern of brain activation while performing the task than children in the control group. However, comparison of source modeled ERPs data in children and fMRI data obtained with adults must be done with caution (see Note 2) and this interpretation should be subject to replication using more comparable brain imaging techniques.
A final remarkable result of the source localization data is related to the strength of activation and focalization of the modeled sources. Overall, children in the non-trained group show broader and stronger activations than children in the trained group. The idea that cortical function becomes less diffuse and more focal with maturation is well documented in recent neuroscience research (Casey et al., 2005, Durston and Casey, 2006). Moreover, tuning of activations appears to be dependent of the functional significance of the circuitry of brain structures, displaying attenuated activations in areas relatively less involved in a particular function and more focal activation of areas related to that function (Durston et al., 2006, Fair et al., 2007). Our source modeling results show more focused activation for trained children in nodes of the executive attention network, which suggests that activation of this network becomes more fine-tuned after training.
5.5. Limitations of the study
In our study, a video-watching non-active control group was used to examine the effect of training. The video-watching control may not be optimal for disentangling effects that are specific to the attention training aspects of the computerized program. However, the video-watching situation shares several aspects with the training experience, in that it involves interacting with a computer screen in the presence of the experimenter for a period of time. Also, prior studies reported no differences between active and passive control groups in training studies conducted with preschoolers (Thorell et al., 2009, Rueda et al., 2005b). In future studies, it may be more appropriate to include an active control group. The active control could either carry out a training program designed to improve a cognitive ability that is non-specific for attention or just perform the most basic levels of the exercises included in the training program.
To our knowledge, our study is the first to examine durability of training effects on cognitive and brain function in children. However, the amount of training in the current study was only moderate both in terms of number of sessions (a total of 10) and time (a maximum of 500 min in total over a period of 5 weeks). In spite of this, we found some evidence of lasting effects of training two months later on some of the measures used in the study, which were more clearly observed in brain function. We believe that producing a larger impact on cognition would require more extended interventions. As a matter of fact, there is evidence showing that curricular interventions based on Vygotsky's theory of development involving teacher–students exchanges or interactions between peers result in increased performance of conflict tasks and hence better executive attention efficiency (Diamond et al., 2007). Similarly, experiences that are likely to be more extended in time, such as being exposed to more than one language during development, appear to have a positive impact in attention control skills as measured by the ANT (Yang et al., 2011).
In our study, we did not find a clear effect of training on performance of the ANT. One question that emerges from the pattern of behavioral results in our study is why, despite not obtaining significant training effects on the task targeting the closest cognitive skills (the child ANT), significant effects were observed for intelligence and moderately for emotion regulation. Standard deviations of scores for each task presented in Table 1 suggest that differences in performance variability between the ANT task and the rest of behavioral measures included in the study could explain the fact that the effect of intervention is observed in the paper and pencil tasks and not in the ANT. Additionally, improvements observed for the control group in the ANT task at post sessions suggest that practicing may be another form of useful training. Also, the fact that scores obtained at post intervention sessions for both trained and untrained children reached adults levels of performance suggests the issue of a possible ceiling effect. For future studies it may be useful to include additional behavioral tasks designed to examine executive attention that are more challenging and sensitive to changes in efficiency for preschool-aged children.
6. Conclusions
Our results show that the brain circuitry involved in executive attention is activated faster and more efficiently after training. Training appears to accelerate mechanisms associated with monitoring of conflict supported by the dorsal division of the ACC, an effect that is still apparent two months after completion of training. More efficient engagement of the ACC is likely to be responsible of transfer of training to regulation of affect. The dorsal division of the ACC appears to be involved in reappraising the emotional value of events (Etkin et al., 2011). Affect regulation in our tasks require reappraising the positive value of immediate or high compensations or rewards taking into consideration that they lead to more negative consequences in the long term. Flexible allocation of attentional resources facilitates fluent reasoning and helps on connecting current decisions with future consequences. A major conclusion of our study is that efficiency of the executive attention network can be enhanced by means of an educational intervention and that other cognitive skills that rely on attention control or are associated with it may also benefit from this type of intervention.
6.1. Implications for education
Interventions of the type carried out in our study might be useful to help children get ready for school. They also have the potential to prevent children with poor attention skills from school failure, and even to help on the prevention of developing attention-related pathologies and conduct problems. It was discussed at the introduction that executive attention is important for a wide range of aspects in children's lives, including socialization and emotion regulation. In school, control of attention is important to adjust behavior in function of norms and goals, stay focused despite distractions, flexibly allocate attention on relevant information (either internal or external), and persist to complete difficult tasks even when rewards (i.e. learning, good grades, etc.) may take time to arrive. We think that showing evidence of the susceptibility of the executive attention network to be enhanced by training is only the start point, but that data of this sort will provide an opportunity for curricular improvement. Results of the current study point out the potential to produce larger and more durable impact in children's attention skills and related domains with more extended interventions.
Acknowledgements
This work was supported by a grant from the Spanish Ministry of Science and Innovation (ref. PSI2008.02955) awarded to the first author. Also supported by funds from the Consolider Ingenio 2010 Program of the MICINN on Cognition and Education. We are also grateful to children, families and staff of the Colegio Jesús-María Cristo de la Yedra of Granada who participated in the study.
Footnotes
The adaptive mean consists of the mean amplitude of a new time window created including 10 samples (40 ms) around the peak of minimum amplitude found within the larger time window of 300–700 ms.
The Finite Difference Model (FDM) that is used by GeoSource is based on the Montreal Neurological Institute (MNI) database for estimating its geometrical constraints. The MNI brain model is based on adults. By using GeoSource to isolate neural sources in preschool children we recognize that the source models may be subject to some error as brain size and level of maturation for cortical structures in children will differ from those of adults. Nevertheless, in our study the source modeling error derived from using an adult brain model remains constant for both the within group comparison as well as across groups, given the fact that children in the two groups were matched in age and gender.
References
- Blair C., Razza R.P. Relating effortful control, executive function, and false belief understanding to emerging math and literacy ability in kindergarten. Child Development. 2007;78(2 (March–April)):647–663. doi: 10.1111/j.1467-8624.2007.01019.x. [DOI] [PubMed] [Google Scholar]
- Blair C., Ursache A. A bidirectional model of executive functions and self-regulation. In: Vohs K.D., Baumeister R.F., editors. Handbook of Self-regulation: Research, Theory and Applications. 2nd ed. The Guilford Press; New York: 2011. pp. 300–320. [Google Scholar]
- Botvinick M.M., Cohen J.D., Carter C.S. Conflict monitoring and anterior cingulate cortex: an update. Trends in Cognitive Sciences. 2004;8(12):539–546. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Bull R., Scerif G. Executive functioning as a predictor of children's mathematics ability: inhibition, switching, and working memory. Developmental Neuropsychology. 2001;19(3):273–293. doi: 10.1207/S15326942DN1903_3. [DOI] [PubMed] [Google Scholar]
- Bunge S.A., Dudukovic N.M., Thomason M.E., Vaidya C.J., Gabrieli J.D.E. Immature frontal lobe contributions to cognitive control in children: evidence form fMRI. Neuron. 2002;33:1–20. doi: 10.1016/s0896-6273(01)00583-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush G., Luu P., Posner M.I. Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Sciences. 2000;4(6):215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Casey B., Tottenham N., Listen C., Durston S. Imaging the developing brain: what have we learned about cognitive development? Trends in Cognitive Sciences. 2005;9(3):104–110. doi: 10.1016/j.tics.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Casey B.J., Trainor R., Giedd J., Vauss Y. The role of the anterior cingulate in automatic and controlled processes: a developmental neuroanatomical study. Developmental Psychobiology. 1997;30(1):61–69. [PubMed] [Google Scholar]
- Cattell R.B. North-Holland; Amsterdam: 1987. Intelligence: Its Structure, Growth and Action. [Google Scholar]
- Checa P., Rodriguez-Bailon R., Rueda M.R. Neurocognitive and temperamental systems of self-regulation and early adolescents’ school competence. Mind, Brain and Education. 2008;2(4):177–187. [Google Scholar]
- Checa P., Rueda M.R. Behavioral and brain measures of executive attention and school competence in late childhood. Developmental Neuropsychology. 2011;36(8):1–15. doi: 10.1080/87565641.2011.591857. [DOI] [PubMed] [Google Scholar]
- Diamond A., Barnett W.S., Thomas J., Munro S. Preschool program improves cognitive control. Science. 2007;318(5855):1387–1388. doi: 10.1126/science.1151148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J., Owen A.M. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends in Neurosciences. 2000;23(10):475–483. doi: 10.1016/s0166-2236(00)01633-7. [DOI] [PubMed] [Google Scholar]
- Duncan J., Seitz R.J., Kolodny J., Bor D., Herzog H., Ahmed A. A neural basis for general intelligence. Science. 2000;289(5478):457–460. doi: 10.1126/science.289.5478.457. [DOI] [PubMed] [Google Scholar]
- Durston S., Casey B. What have we learned about cognitive development from neuroimaging? Neuropsychologia. 2006;44(11):2149–2157. doi: 10.1016/j.neuropsychologia.2005.10.010. [DOI] [PubMed] [Google Scholar]
- Durston S., Davidson M.C., Tottenham N., Galvan A., Spicer J., Fossella J.A. A shift from diffuse to focal cortical activity with development. Developmental Science. 2006;9(1):1–8. doi: 10.1111/j.1467-7687.2005.00454.x. [DOI] [PubMed] [Google Scholar]
- Eisenberg N., Sadovsky A., Spinrad T.L., Fabes R.A., Losoya S.H., Valiente C. The relations of problem behavior status to children's negative emotionality, effortful control, and impulsivity: concurrent relations and prediction of change. Developmental Psychology. 2005;41(1):193–211. doi: 10.1037/0012-1649.41.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg N., Smith C.L., Spinrad T.L. Effortful control: relations with emotion regulation, adjustment, and socialization in childhood. In: Vohs K.D., Baumeister R.F., editors. Handbook of Self-regulation. Research, Theory and Applications. 2nd ed. The Guilford Press; New York: 2011. pp. 263–283. [Google Scholar]
- Eisenberg N., Valiente C., Eggum N.D. Self-regulation and school readiness. Early Education and Development. 2010;21(5):681–698. doi: 10.1080/10409289.2010.497451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksen B.A., Eriksen C.W. Effects of noise letters upon the identification of a target letter in a nonsearch task. Perception & Psychophysics. 1974;16(1):143–149. [Google Scholar]
- Etkin A., Egner T., Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends in Cognitive Sciences. 2011;15(2):85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair D.A., Dosenbach N.U.F., Church J.A., Cohen A.L., Brahmbhatt S., Miezin F.M. Development of distinct control networks through segregation and integration. PNAS Proceedings of the National Academy of Sciences of the United States of America. 2007;104(33):13507–13512. [Google Scholar]
- Fan J., Flombaum J.I., McCandliss B.D., Thomas K.M., Posner M.I. Cognitive and brain consequences of conflict. Neuroimage. 2003;18(1):42–57. doi: 10.1006/nimg.2002.1319. [DOI] [PubMed] [Google Scholar]
- Grave de Peralta Menendez R., Murray M.M., Michel C.M., Martuzzi R., Gonzalez Andino S.L. Electrical neuroimaging based on biophysical constraints. Neuroimage. 2004;21:527–539. doi: 10.1016/j.neuroimage.2003.09.051. [DOI] [PubMed] [Google Scholar]
- Gray J.R., Thompson P.M. Neurobiology of intelligence: science and ethics. Nature Reviews Neuroscience. 2004;5(6):471–482. doi: 10.1038/nrn1405. [DOI] [PubMed] [Google Scholar]
- Hongwanishkul D., Happaney K.R., Lee W.S., Zelazo P.D. Assessment of hot and cool executive function in young children: age-related changes and individual differences. Developmental Neuropsychology. 2005;28(2):617–644. doi: 10.1207/s15326942dn2802_4. [DOI] [PubMed] [Google Scholar]
- Horn J.L., Cattell R.B. Refinement and test of the theory of fluid and crystallized general intelligences. Journal of Educational Psychology. 1966;57(5):253–270. doi: 10.1037/h0023816. [DOI] [PubMed] [Google Scholar]
- Jaeggi S.M., Buschkuehl M., Jonides J., Perring W.J. Improving fluid intelligence with training on working memory. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(19):6829–6833. doi: 10.1073/pnas.0801268105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James W. H. Holt and Company; New York: 1890. The Principles of Psychology. [Google Scholar]
- Jonkman L.M. The development of preparation, conflict monitoring and inhibition from early childhood to young adulthood; a Go/Nogo ERP study. Brain Research. 2006;1097(1):181–193. doi: 10.1016/j.brainres.2006.04.064. [DOI] [PubMed] [Google Scholar]
- Karbach J., Kray J. How useful is executive control training? Age differences in near and far transfer of task-switching training. Developmental Science. 2009;12(6):978–990. doi: 10.1111/j.1467-7687.2009.00846.x. [DOI] [PubMed] [Google Scholar]
- Kaufman A.S., Kaufman N.L. American Guidance Service; Circle Pines: 1990. Kaufman Brief Intelligence Test – Manual. [Google Scholar]
- Kerns K.A., Eso K., Thomson J. Investigation of a direct intervention for improving attention in young children with ADHD. Developmental Neuropsychology. 1999;16(2):273–295. [Google Scholar]
- Kerr A., Zelazo P.D. Development of “hot” executive function: the children's gambling task. Brain and Cognition. 2004;55:148–157. doi: 10.1016/S0278-2626(03)00275-6. [DOI] [PubMed] [Google Scholar]
- Klingberg T., Fernell E., Olesen P.J., Johnson M., Gustafsson P., Dahlstrom K. Computerized training of working memory in children with ADHD—a randomized, controlled trial. Journal of the American Academy of Child & Adolescent Psychiatry. 2005;44(2):177–186. doi: 10.1097/00004583-200502000-00010. [DOI] [PubMed] [Google Scholar]
- Konrad K., Neufang S., Thiel C.M., Specht K., Hanisch C., Fan J. Development of attentional networks: an fMRI study with children and adults. Neuroimage. 2005;28(2):429–439. doi: 10.1016/j.neuroimage.2005.06.065. [DOI] [PubMed] [Google Scholar]
- McNab F., Varrone A., Farde L., Jucaite A., Bystritsky P., Forssberg H. Changes in cortical dopamine D1 receptor binding associated with cognitive training. Science. 2009;323(5915):800–802. doi: 10.1126/science.1166102. [DOI] [PubMed] [Google Scholar]
- Minear M., Shah P. Training and transfer effects in task switching. Memory & Cognition. 2008;36(8):1470–1483. doi: 10.3758/MC.336.8.1470. [DOI] [PubMed] [Google Scholar]
- Norman D.A., Shallice T. Attention to action: willed and automatic control of behavior. In: Davison R.J., Schwartz G.E., Shapiro D., editors. Consciousness and Self-regulation. Plenum Press; New York: 1986. pp. 1–18. [Google Scholar]
- Nutley S.B., Soderqvist S., Bryde S., Thorell L.B., Humphreys K., Klingberg T. Gains in fluid intelligence after training non-verbal reasoning in 4-year-old children: a controlled, randomized study. Developmental Science. 2011;14(3):591–601. doi: 10.1111/j.1467-7687.2010.01022.x. [DOI] [PubMed] [Google Scholar]
- Ochsner K.N., Bunge S.A., Gross J.J., Gabrieli J.D. Rethinking feelings: an fMRI study of the cognitive regulation of emotion. Journal of Cognitive Neuroscience. 2002;14(8):1215–1229. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- Ochsner K.N., Gross J.J. Handbook of Emotion Regulation. Guilford Press; New York, NY: 2007. The neural architecture of emotion regulation. pp. 87–109. [Google Scholar]
- Olesen P.J., Westerberg H., Klingberg T. Increased prefrontal and parietal activity after training of working memory. Nature Neuroscience. 2004;7(1):75–79. doi: 10.1038/nn1165. [DOI] [PubMed] [Google Scholar]
- Posner M.I., DiGirolamo G.J. Executive attention: conflict, target detection, and cognitive control. In: Parasuraman R., editor. The Attentive Brain. MIT Press; Cambridge, MA: 1998. pp. 401–423. [Google Scholar]
- Posner M.I., Petersen S.E. The attention system of human brain. Annual Review of Neuroscience. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- Posner, M.I., Rueda, M.R. Development of attentional networks. In: Zelazo, P.D. (Ed.), Oxford Handbook of Developmental Psychology. Oxford University Press, Oxford, UK, in press.
- Posner M.I., Rueda M.R., Kanske P. Probing the mechanisms of attention. In: Cacioppo J.T., Tassinary J.G., Berntson G.G., editors. Handbook of Psychophysiology. 3rd ed. Cambridge University Press; Cambridge, UK: 2007. pp. 410–432. [Google Scholar]
- Power J.D., Fair D.A., Schlaggar B.L., Petersen S.E. The development of human functional brain networks. Neuron. 2010;67(5):735–748. doi: 10.1016/j.neuron.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridderinkhof K.R., van der Molen M.W. A psychophysiological analysis of developmental differences in the ability to resist interference. Child Development. 1995;66(4):1040–1056. [Google Scholar]
- Rothbart M.K., Posner M.I. 2nd ed. vol. 2. John Wiley & Sons Inc.; Hoboken, NJ: 2006. Temperament, attention, and developmental psychopathology. (Developmental Psychopathology: Developmental Neuroscience). pp. 465–501. [Google Scholar]
- Rueda M., Fan J., McCandliss B.D., Halparin J.D., Gruber D.B., Lercari L.P. Development of attentional networks in childhood. Neuropsychologia. 2004;42(8):1029–1040. doi: 10.1016/j.neuropsychologia.2003.12.012. [DOI] [PubMed] [Google Scholar]
- Rueda M., Posner M.I., Rothbart M.K. The development of executive attention: contributions to the emergence of self-regulation. Developmental Neuropsychology. 2005;28(2):573–594. doi: 10.1207/s15326942dn2802_2. [DOI] [PubMed] [Google Scholar]
- Rueda M.R., Checa P., Rothbart M.K. Contributions of attentional control to social emotional and academic development. Early Education and Development. 2010;21(5):744–764. [Google Scholar]
- Rueda M.R., Posner M.I., Rothbart M.K., Davis-Stober C.P. Development of the time course for processing conflict: an event-related potentials study with 4 year olds and adults. BMC Neuroscience. 2004;5(39):1–13. doi: 10.1186/1471-2202-5-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueda M.R., Rothbart M.K., McCandliss B.D., Saccomanno L., Posner M.I. Training, maturation, and genetic influences on the development of executive attention. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(41):14931–14936. doi: 10.1073/pnas.0506897102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueda M.R., Rothbart M.K., Saccomanno L., Posner M.I. Adolescent Psychopathology and the Developing Brain: Integrating Brain and Prevention Science. Oxford University Press; New York, NY: 2007. Modifying brain networks underlying self-regulation. pp. 401–419. [Google Scholar]
- Simonds J., Kieras J.E., Rueda M.R., Rothbart M.K. Effortful control, executive attention, and emotional regulation in 7–10-year-old children. Cognitive Development. 2007;22(4):474–488. [Google Scholar]
- Stevens C., Fanning J., Coch D., Sanders L., Neville H. Neural mechanisms of selective auditory attention are enhanced by computerized training: electrophysiological evidence from language-impaired and typically developing children. Brain Research. 2008;1205:55–69. doi: 10.1016/j.brainres.2007.10.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm W., Thimm M., Küst J., Fink G.R., Karbe H. Alertness-training in neglect: behavioral and imaging results. Restorative Neurology and Neuroscience. 2006;24(4–6):371–384. [PubMed] [Google Scholar]
- Thimm M., Fink G.R., Küst J., Sturm W., Karbe H. Impact of alertness training on spatial neglect: a behavioural and fMRI study. Neuropsychologia. 2006;44(7):1230–1246. doi: 10.1016/j.neuropsychologia.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Thompson C., Barries J., Moore C. The development of future-oriented prudence and altruims in preeschoolers. Cognitive Development. 1997;12:199–212. [Google Scholar]
- Thorell L.B., Lindqvist S., Nutley S.B., Bohlin G., Klingberg T. Training and transfer effects of executive functions in preschool children. Developmental Science. 2009;12(1):106–113. doi: 10.1111/j.1467-7687.2008.00745.x. [DOI] [PubMed] [Google Scholar]
- van Veen V., Carter C. The timing of action-monitoring processes in the anterior cingulate cortex. Journal of Cognitive Neuroscience. 2002;14:593–602. doi: 10.1162/08989290260045837. [DOI] [PubMed] [Google Scholar]
- Walsh B.J., Buonocore M.H., Carter C.S., Mangun G.R. Integrating conflict detection and attentional control mechanisms. Journal of Cognitive Neuroscience. 2011;23(9):2211–2221. doi: 10.1162/jocn.2010.21595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch M.C. The prefrontal cortex and the development of executive function in childhood. In: Kalverboer A.F., Gramsbergen A., editors. Handbook of Brain and Behavior in Human Development. Kluwer Academic; Dordrecht, The Netherlands: 2001. pp. 767–790. [Google Scholar]
- Welsh M.C., Pennington B.F. Assessing frontal lobe functioning in children: views from developmental psychology. Developmental Neuropsychology. 1988;4(3):199–230. [Google Scholar]
- Yang S.J., Yang H.J., Lust B. Early childhood bilingualism leads to advances in executive attention: dissociating culture and language. Bilingualism-Language and Cognition. 2011;14(3):412–422. [Google Scholar]