Highlights
* Face recognition examined in infants with and without family history of ASD. * Visual ERPs to mother/stranger faces and eye tracking to a stranger face recorded at age 9 months. * No group differences in eye gaze characteristics; both groups generate larger P400 and Nc responses to stranger face. * Only typical infants show delayed P400 to stranger faces. * Speed of familiarity detection correlates with interpersonal skills.
Keywords: ERP, Familiarity, Face processing, ASD, Infant
Abstract
The study investigated whether infant siblings of children with autism (sibs-ASD) process familiar and novel faces differently from typical infants and whether sensitivity to face familiarity is associated with infants’ social and communicative behaviors. Visual event-related potentials (ERPs) were recorded in 35 infants, age 9 months ± 15 days (20 typical infants, 15 sibs-ASD) using an oddball paradigm presenting photographs of infants’ mothers (70% of trials) and an unfamiliar female (30% of trials). Eye tracking responses to a different unfamiliar face were recorded to determine whether differences in gaze patterns might account for any ERP differences found. There were no group differences in the distribution, number or duration of fixations. Both infant groups differentiated between mothers and strangers, as reflected in amplitude modulations of posterior N290/P400 and frontal/central Nc responses. Group differences were present in the latency of the P400 response, where a delayed response to the stranger face was observed only in typical infants. Across both groups, shorter Nc latency to mother's face was associated with parental reports of stronger interpersonal skills. Individual differences in the speed of processing for novel vs. familiar faces may be an informative early marker of risk for atypical social development.
1. Introduction
Current prevalence estimates for autism spectrum disorders (ASD) are reported to be 1 in 110 children (CDC, 2009). Because early intervention programs can result in significant gains in cognitive, language, behavioral, and social functioning of children with ASD (CEICA, 2001, Harris and Handleman, 2000, Rogers, 1998, Zwaigenbaum et al., 2007), the early identification of children with autism has received a great deal of research attention. One strategy that has become increasingly popular is the prospective study of infant siblings of children with ASD (sibs-ASD), as their recurrence risk for receiving an autism spectrum diagnosis could be as high as 20% (Elsabbagh and Johnson, 2010, Landa and Garrett-Mayer, 2006, Ozonoff et al., 2011) and may thus yield information about early markers of the disorder (Ozonoff et al., 2010, Rogers, 2009). However, the outcomes of these high-risk infants are quite variable, and include not only typical development and ASD, but also a “broader phenotype,” observed in 10–37% of this group (Bolton et al., 1994, Constantino and Todd, 2000, Zwaigenbaum et al., 2005), who demonstrate behavioral features that resemble the symptoms of autism (e.g., social awkwardness, language symptoms) but do not reach clinically elevated levels (e.g., Zwaigenbaum et al., 2005, Landa and Garrett-Mayer, 2006). The heterogeneity seen in the sibs-ASD group is thought to hold important clues for understanding the genetic mechanisms underlying the etiology of the disorder.
The ability to remember and recognize faces is important for successful social functioning (Ellis and Young, 1998, Schults, 2005), and emerges very early in development. Newborns learn quickly to recognize their mother's face based on external and internal features (Pascalis et al., 1995), five-week-old infants can recognize their mother's face from internal features alone (Bartrip et al., 2001), and six-month-olds may engage different brain mechanisms to recognize their mother among strangers, depending on the difficulty of discrimination (de Haan and Nelson, 1997). Yet children and adults with autism are often reported to have difficulties with face processing (e.g., Blair et al., 2002, de Gelder et al., 1991, Gepner et al., 1996, Klin et al., 1999, Williams et al., 2005), including slower habituation to novel faces (Webb et al., 2010) and poor recognition of familiar faces (Boucher et al., 1998, Dawson et al., 2002, Klin et al., 1999). Moreover, deficits appear to be more pronounced in younger children with autism (Klin et al., 1999, Langdell, 1978). Face processing ability also has been correlated with social competence in persons with ASD (Dawson et al., 2005, Deruelle et al., 2004, Klin et al., 1999, Schultz et al., 2003, Teunisse and de Gelder, 2003, Volkmar et al., 1989). Therefore, examining face recognition processes in infants may be an informative approach to ascertaining risk for atypical social development.
Traditional behavioral assessments of face recognition may not be suitable for use with infants because they require participants to comprehend instructions and provide overt responses. Infant preferential looking paradigms may also be not sufficiently sensitive to individual differences (de Haan and Nelson, 1997). In contrast, recordings of electrical brain activity do not require behavioral responses and can be obtained at much younger ages. Several previous studies have successfully used event-related potentials (ERPs) to document recognition memory and/or face processing in typical infants (e.g., Courchesne et al., 1981, de Haan et al., 2003, Key et al., 2009, Nelson and Collins, 1992, Reynolds and Richards, 2005, Scott and Nelson, 2006) and more recently, in sibs-ASD (Elsabbagh and Johnson, 2010, McCleery et al., 2009, Luyster et al., 2011).
ERPs represent a portion of the ongoing brain activity time-locked to a stimulus presentation (e.g., picture of a face) and reflect the change in that activity associated with stimulus processing (Fabiani et al., 2000). Inferences about stimulus familiarity can be made by comparing brain responses to familiar (e.g., mother) or familiarized faces (e.g., frequently presented unknown faces) with responses to unfamiliar faces (e.g., strangers; infrequently presented novel faces). Existing ERP studies in children and adults with ASD reported slower than typical brain responses to faces (delayed N170 latencies; McPartland et al., 2004, Hileman et al., 2011) and no familiarity effects on amplitude of face-elicited ERP responses (P400 and Nc; Dawson et al., 2002). More recently, Webb et al. (2011) demonstrated increased P400 and Nc responses to familiar vs. novel faces in 18–30-month-old children with ASD that resembled ERPs of chronologically younger typical children matched on social ability, suggesting delayed rather than atypical face recognition processes.
In samples of infants under 12 months of age, ERP studies of face familiarity have most commonly examined the fronto-central Nc and the posterior N290/P400 responses. In typical 4–7-month-old infants, Courchesne et al. (1981) noted that the more familiar face (i.e., the one presented more frequently) was associated with a smaller anterior Nc response with shorter latency than the novel (infrequent) face. However, Nelson and Collins, 1991, Nelson and Collins, 1992 argued that the Nc response may reflect not just stimulus recognition due to familiarity but also a general orienting of attention to a rare novel event because the Nc modulation could be eliminated by manipulating the relative familiarity of the frequent and infrequent stimuli. More recently, Reynolds and Richards (2005) replicated and extended these findings in a sample of 5–8-month-olds by including an extended familiarization protocol and an infrequent familiar face in addition to an infrequent novel stimulus to demonstrate that the increased amplitude of the Nc response to the novel face was due specifically to stimulus novelty and not just its low probability.
Familiarity-related effects in infant ERPs were also reported for the posterior N290/P400 responses, thought to be a developmental precursor of the adult N170 response (de Haan et al., 2003). A longer N290 latency has been observed for habituated faces than for novel faces in typical 8-month-olds (Scott and Nelson, 2006), and a larger N290 as well as a smaller P400 amplitude were recorded for novel than familiar faces in 9-month-olds (Scott et al., 2006). Novelty-related effects were observed for the amplitude and latency measures of N290/P400 components in typical 9-month-olds even when familiar and novel faces differed only in a single feature (i.e., eyes or mouth; Key et al., 2009).
In light of the reported findings indicating that both novelty detection processes (indexed by Nc) and face-specific perceptual mechanisms (reflected by N290/P400 responses) may be sensitive to face familiarity, recording infants’ brain activity in responses to their mother's (i.e., an extensively familiar) face compared to a stranger's (i.e., novel) face would provide a good opportunity to examine face recognition processes in sibs-ASD. Although a recent study in 10-month-old typical infants and sibs-ASD reported no stimulus familiarity effects on ERP responses to faces or objects presented equally often in the same paradigm (McCleery et al., 2009), another study specifically examining face recognition in 12-month-old sibs-ASD and typical infants reported an increase in Nc mean amplitudes to strangers compared to mother faces but no group differences (Luyster et al., 2011).
It is possible that discrepant findings regarding group differences in ERP measures of face perception are attributable to differences in infant face scanning behaviors (e.g., fixating on more or less relevant features of the stimuli). A number of prior studies reported evidence of atypical distribution of fixations on face stimuli in children and adults with ASD compared to their typical peers (see Jemel et al., 2006 for review; but see also Bar-Haim et al., 2006, Rutherford and Towns, 2008). In sibs-ASD, reduced eye contact was present by 12 months in those later receiving the diagnosis (Zwaigenbaum et al., 2005). The only eye tracking study in sibs-ASD used mother's faces in a still-face paradigm to examine infants’ face scanning behaviors at 6 month of age (Merin et al., 2007). While there were no significant group differences between the typical infants and sibs-ASD, the authors did note greater frequency of fixations on the mouth in the high-risk group, yet this observation was not predictive of the ASD diagnosis (Young et al., 2009). No study to date has examined differences in face scanning in combination with ERP measures of face processing.
The purpose of the present study was to investigate whether: (1) 9-month-old sibs-ASD process familiar and novel faces differently from typical infants, as reflected in ERPs; and (2) individual differences in face recognition are associated with infants’ social and communicative behaviors. We hypothesized that discrimination of familiar vs. novel faces would be associated with differences in the amplitude and latency of the anterior Nc and posterior N290/P400 responses, with typical infants evidencing greater discrimination than sibs-ASD. Furthermore, such discrimination effects were expected to correlate with parental reports of communicative and interpersonal skills. In addition, to facilitate interpretation of the potential group differences in ERPs to familiar vs. novel faces, our study design included eye tracking to a stranger's face to examine whether sibs-ASD and typical infants looked at different parts of the face stimuli. The decision to focus on 9-month-olds was motivated by the fact that infants at this age have already acquired sufficient expertise with human faces (Schwarzer et al., 2007, Pascalis et al., 2002), while still developing social and communicative skills.
2. Method
2.1. Participants
A total of 35 infants, age 9 months, and their mothers participated in the study. The typical group included 20 infants (7 females; M age = 270.05 ± 10.92 days). All infants in this group were reported to have typical development and no family history of developmental disorders, including no first-degree relatives with an ASD diagnosis. The sibs-ASD group included 15 infants (5 females; M age = 277.93 ± 14.88 days) who had an older sibling diagnosed with ASD. Diagnoses of older siblings were made by licensed psychologists, and 11/15 (73%) of the sample received the ADOS either alone or in combination with the ADI-R to support the clinical diagnosis. Data were collected from an additional 5 typical infants and 2 sibs-ASD, but were excluded from analyses due to insufficient number of ERP trials retained after artifact detection. The two participant groups did not differ in proportion of males, age, receptive communication or interpersonal skills; however, the typical group had higher expressive communication skills as reported by parents using Vineland Adaptive Behavior Scales-II Parent/Caregiver Rating Form (VABS-II; Sparrow et al., 2005). Summary statistics for the study sample are presented in Table 1. Mothers of all infants provided written informed consent. The study was prospectively reviewed and approved by the Institutional Review Board.
Table 1.
Means (SDs) for sample characteristics.
| TD (n = 20) | Sibs-ASD (n = 15) | p-Value | |
|---|---|---|---|
| Age (days) | 270.05 (10.92) | 277.93 (14.88) | .10 |
| Receptive communicationa | 15.41 (2.15) | 14.47 (2.10) | .29 |
| Expressive communicationa | 14.60 (1.85) | 12.38 (1.96) | <.001 |
| Interpersonal relationshipsa | 14.33 (1.59) | 13.31 (2.02) | .11 |
v-scores from the VABS-II; mean = 15, SD = 3.
2.2. Study procedures
All data were collected in a single visit, with the eye tracking procedure preceding the ERP recording. The eye tracking paradigm was kept brief to avoid excessive familiarization with the stimuli that could have attenuated infants’ attention to the stimuli in one of the two ERP tasks (facial feature processing, not described here) included in the larger study. Infants completed all procedures in the same darkened sound-attenuated room while seated in the mother's lap.
2.2.1. Eye tracking
Stimuli: Eye-tracking was used to identify the presence of any group differences in face scanning behavior that might account for ERP differences. Stimuli comprised three color photographs of an unfamiliar smiling female face: one represented the original face, the other two depicted the same face with different eyes or a different mouth (Key et al., 2009). The stimuli were presented centrally on a 19 in. LCD monitor. The entire image size was 13.25 in. (w) × 10.5 in. (h), with the head measuring 6.50 in. (w) × 7.35 in. (h). Only eye tracking data for the original face (always presented first) were used in this study.
Procedure: Eye tracking data were collected using a table-top camera (Tobii x50 series) positioned 20 in. in front of the infant seated in the mother's lap (infant's eye to image distance was approximately 23 in.). Using ClearView software, each stimulus was presented twice for 5 s with a 3-s interstimulus black screen. Prior to the recording of eye gaze data, a 5-point calibration using infant-friendly moving images (colorful toys presented against black background) was performed to ensure accuracy of eye tracking data. Similar to the procedures described in Merin et al. (2007), calibration data were collected while a researcher in the room with the participant observed that the infant was looking at the screen and an eye-tracker operator in the control room verified that the eye tracker camera was detecting infant's eyes. After calibration, the plot of gaze data was examined and points with poor quality data were re-calibrated until usable calibration was obtained for each of the five regions of the screen.
2.2.2. ERP acquisition
Stimuli: Stimuli were color photographs of each infant's mother (a head shot with a smiling facial expression taken against a light-colored neutral background) and a stranger (photograph of another participant's mother, taken against the same background). Clothing details were masked using the same light-yellow drape for all pictures. Strangers were matched to mothers on race, eyewear and hair color. The photographs subtended a visual angle of 20.93° (w) × 16.75° (h), and therefore appeared close to life-size.
Procedure: A high-density array of 124 Ag/AgCl electrodes embedded in soft sponges (Geodesic Sensor Net, EGI, Inc., Eugene, OR) was used to record infant ERPs. Electrode impedance levels were adjusted to less than 40 kOhm. Data were sampled at 250 Hz with filters set to 0.1–30 Hz. During data collection, all electrodes were referred to Cz (re-referenced offline to an average reference). Each participant was tested while seated in the parent's lap in a darkened sound-attenuated room. ERPs were obtained using a passive (i.e., not requiring a behavioral response) oddball paradigm that included 100 trials. The mother's face served as the standard stimulus and was presented on 70% of the trials. The stranger's face served as the deviant and was presented on 30% of the trials. Each trial began with a 500 ms fixation point (black plus sign on a white background) followed by a 1000 ms presentation of the face stimuli. The stimuli were presented against a black background in the center of the computer screen positioned 90 cm in front of the participant. Interstimulus interval varied randomly between 1100 and 1600 ms to prevent habituation to stimulus onset.
Recording of the brainwaves was controlled by Net Station software (v. 4.1; EGI, Inc., Eugene, OR). Stimulus presentation was controlled by E-Prime (v. 1.1, PST, Inc., Pittsburgh, PA). During the entire test session, a researcher in the control room continuously monitored infants’ electroencephalogram (EEG) while another researcher present in the testing room observed infants’ behavior. Stimulus presentation occurred only when the EEG was free of motor artifact and the infant was quiet and looking at the monitor. During periods of inattention and/or motor activity, stimulus presentation was suspended and the researcher present in the testing room redirected infants to the computer screen using a battery-operated toy wand with flashing spinning lights. If that was not sufficient to attract infant's attention, the stimulus on the screen was temporarily replaced by a baby-friendly video (Baby Einstein series). Presentation of the face stimuli resumed as soon as the infant looked at the screen.
2.3. Measures of social and communicative functioning
During the visit, mothers of the infants completed three subscales of the Vineland Adaptive Behavior Scales-II Parent/Caregiver Rating Form (VABS-II; Sparrow et al., 2005): Receptive Communication, Expressive Communication, and Interpersonal Relationships. These subscales were selected a priori because they measure constructs that are most directly social and communicative behaviors, i.e., listening and understanding, using sounds and gestures to communicate, and relating to others. These subscales yield standardized v-scores with a mean of 15 and a standard deviation of 3. All infants in the study obtained v-scores within the average range (see Table 1). One out of 35 infants (2.8% of the sample) had missing data for two of the three subscales, and one infant (2.8% of the sample) had missing data for one of the three subscales. Missing data were single-imputed with the EM algorithm (Rubin, 1987). After imputation, the ratios of old to new means and standard deviations for each subscale were all between .988 and 1.02.
2.4. Data analysis
2.4.1. Eye tracking
Due to hardware failures or lack of infant cooperation (i.e., not looking at the screen), eye tracking data were available for 13 of the 20 typical infants (65%) and for 11 of the 15 infants in the sib-ASD group (73%). Means and standard deviations for number and duration of fixations combined across the two 5-s trials for infants who provided usable data are presented in Table 2. Using ClearView analysis tools, each infant's eye gaze data were quantified as the number and duration of fixations within the following regions of interest (ROIs): eyes, mouth, face (other than eyes and mouth), hair, neck, wall background (Fig. 1). A fixation was defined as having a radius of at least 50 pixels and the minimum duration of 100 ms. To control for individual differences in attention to the stimuli, data for the number and duration of fixations within regions of interest were expressed as proportion of the total fixations for the stimulus face. Also, to better reflect individual differences in looking to the eyes vs. the mouth region, an eyes–mouth index (EMI: a ratio of fixations to the eyes to the total fixations to the eyes and mouth combined) was computed using the procedures outlined by Merin et al. (2007). Group differences in the number and duration of fixations on the stranger's face were analyzed for each region of interest using Group (2) × ROI (6) repeated measures ANOVAs. Significant effects were followed up using paired t-tests. Group differences in EMIs were computed using two-tail independent group t-tests.
Table 2.
Means (SE) of fixation number and duration (expressed as % of the total fixations/looking time) on the stranger's face for sibs-ASD and TD infants.
| Region of interest | Fixation count |
Fixation duration |
||
|---|---|---|---|---|
| TD | Sibs-ASD | TD | Sibs-ASD | |
| Eyes | .258 (.079) | .249 (.069) | .258 (.087) | .274 (.076) |
| Mouth | .253 (.081) | .316 (.071) | .278 (.081) | .284 (.071) |
| Face (not eyes or mouth) | .385 (.076) | .363 (.066) | .389 (.080) | .383 (.070) |
| Hair | .039 (.022) | .005 (.019) | .032 (.018) | .006 (.016) |
| Neck | .053 (.037) | .049 (.033) | .036 (.029) | .038 (.026) |
| Wall | .011 (.012) | .018 (.011) | .007 (.009) | .014 (.008) |
| Eyes/mouth index | .415 (.126) | .466 (.110) | .411 (.128) | .415 (.112) |
Fig. 1.

Novel face used for eye tracking and corresponding regions of interest used for analyses.
2.4.2. ERPs
Individual ERPs were derived by segmenting the ongoing EEG on stimulus onset to include a 100-ms prestimulus baseline and a 700 ms post-stimulus interval. To avoid biasing the results due to a largely uneven number of standard and deviant trials (Thomas et al., 2004), only the standard trials preceding a deviant stimulus were selected for the analysis. Resulting segments were screened for artifacts using the default computer algorithms included in NetStation and then followed by a manual review. The automated screening criteria were set as follows: for the eye channels, voltage in excess of 140 μV was interpreted as an eye blink and voltage above 55 μV was considered to reflect eye movements. Any channel with voltage exceeding 200 μV was considered bad. Trials contaminated by eye or movement artifacts and trials with more than 15 bad channels were excluded from the analysis. The remaining ERPs were averaged, referenced to an average reference and baseline corrected. For a data set to be included in the statistical analyses, individual condition averages had to be based on at least 10 trials. Trial retention rates were generally similar across stimulus conditions and groups (Typical group: M standard = 18.00 ± 4.52, M stranger = 16.70 ± 3.39; sibs-ASD: M standard = 15.93 ± 5.01, M stranger = 13.73 ± 3.61), although compared to sibs-ASD, typical infants had more trials retained in the stranger condition, t(33) = 2.48, p = .02.
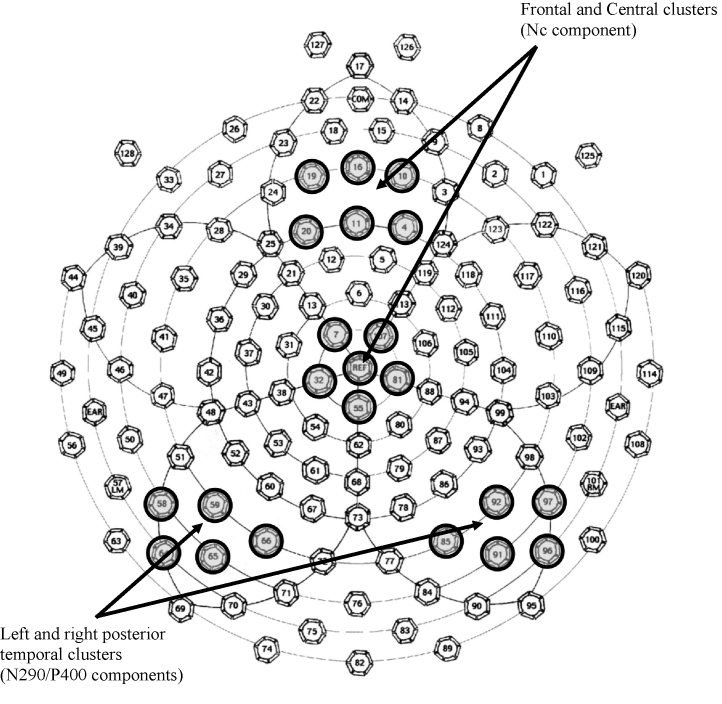
To reduce the number of variables in the statistical analyses, only electrodes corresponding to the locations commonly used to measure N290/P400 (left and right posterior temporal regions; Halit et al., 2003, Key et al., 2009, Scott and Nelson, 2006) and Nc responses (frontal/central midline; de Haan and Nelson, 1997, de Haan et al., 2003, Key et al., 2009) were selected a priori using the specific clusters identified by Reynolds and Richards (2005); see Fig. 2. Next, within each cluster, peak latency and mean amplitude measures were obtained for N290 (250–350 ms), P400 (350–450 ms), and Nc (400–600 ms) peaks using NetStation statistical extraction tool (see Table 3 for summary data). Latency windows were determined based on previously published studies and through the examination of the grand-averaged waveform. Data from individual electrodes within each cluster were averaged together. Resulting values were analyzed separately for amplitude and latency measures of each of the three ERP responses using repeated measures ANOVAs with Group (2: ASD, TD) × Condition (2: mother, stranger) × Electrode (2: posterior temporal left/right for N290/P400 or frontal/central midline for Nc) factors and Huynh–Feldt correction. Significant main effects and interactions were followed by planned comparisons targeting mother–stranger differences.
Fig. 2.
Layout of the 128-channel net and the electrode clusters used in the analyses.
Originally utilized by Reynolds and Richards (2005).
Table 3.
Mean and SD of average amplitude and latency measures.
| Mother |
Stranger |
|||||||
|---|---|---|---|---|---|---|---|---|
| TD |
Sibs-ASD |
TD |
Sibs-ASD |
|||||
| M | SD | M | SD | M | SD | M | SD | |
| N290 | ||||||||
| Mean amplitude | ||||||||
| Left temporal | 1.83 | 7.53 | −3.84 | 8.07 | 3.21 | 9.59 | .10 | 6.94 |
| Right temporal | −.79 | 8.14 | −2.42 | 6.57 | −1.51 | 9.44 | .99 | 7.55 |
| Latency | ||||||||
| Left temporal | 305.12 | 28.29 | 307.25 | 30.36 | 302.44 | 28.27 | 297.87 | 30.69 |
| Right temporal | 307.40 | 25.60 | 304.53 | 31.24 | 309.88 | 24.50 | 301.87 | 32.15 |
| P400 | ||||||||
| Mean amplitude | ||||||||
| Left temporal | 5.28 | 7.81 | −2.00 | 7.87 | 7.49 | 10.33 | 2.70 | 10.03 |
| Right temporal | 2.16 | 7.73 | .04 | 6.66 | 2.42 | 9.90 | 2.88 | 8.49 |
| Latency | ||||||||
| Left temporal | 405.44 | 24.94 | 414.24 | 33.34 | 420.64 | 19.23 | 404.80 | 35.89 |
| Right temporal | 407.96 | 26.74 | 397.07 | 32.69 | 423.24 | 20.92 | 399.20 | 29.70 |
| Nc | ||||||||
| Mean amplitude | ||||||||
| Frontal midline | −.21 | 5.73 | 1.38 | 5.82 | −.87 | 8.32 | −2.95 | 6.86 |
| Central midline | −2.68 | 7.26 | .21 | 5.58 | −3.12 | 6.03 | −1.13 | 4.04 |
| Latency | ||||||||
| Frontal midline | 482.37 | 54.79 | 503.69 | 60.408 | 477.73 | 43.66 | 486.22 | 61.78 |
| Central midline | 456.17 | 52.87 | 457.24 | 52.14 | 467.70 | 54.82 | 481.60 | 58.70 |
Additionally, we examined correlations between VABS-II V-scores on Receptive and Expressive Communication and Interpersonal Relationships subscales and ERP amplitude and latency measures.
3. Results
3.1. Eye tracking
There was a main effect of ROI for both number, F(5,105) = 13.436, p < .0001, partial η2 = .390, and duration F(5,105) = 13.879, p < .0001, partial η2 = .398, of fixations (see Table 2 for summary data). Follow-up post hoc analyses noted that infants fixated more on the stimulus face, its eyes and mouth than on hair, neck, and background wall (all ps < .003). There were no significant differences in fixations within these subgroups of the ROIs: for the eyes vs. mouth vs. face region, or the hair vs. neck vs. wall, all ps = .164–.780. There were no group differences in the EMI for the number or duration of fixations (ps = .760–.644).
3.2. ERPs
3.2.1. Face-specific N290/P400 responses
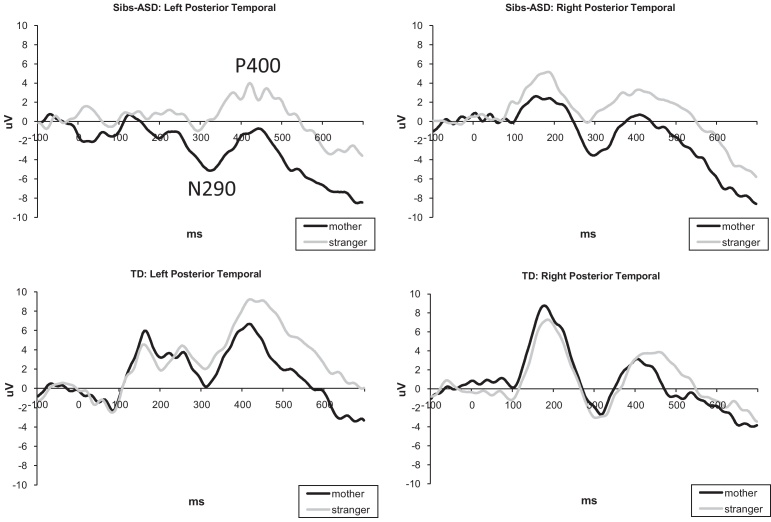
Amplitude: Overall, the main effect of Condition was observed for both peaks, N290: F(1,33) = 5.037, p = .032, partial η2. = .132, and P400: F(1,33) = 7.442, p = .010, partial η2. = .184. For all infants, regardless of risk group, responses to the stranger's face had smaller (less negative) N290 amplitudes, t(34) = 1.927, p = .06, d = .356, and larger (more positive) P400 amplitudes, t(34) = 2.523, p = .016, d = .427 (see Fig. 3). There were no group-related differences in the amplitude of N290/P400 responses.
Fig. 3.
ERPs in response to familiar and novel faces for left and right posterior temporal clusters in sibs-ASD (top row) and typical infants (bottom row).
Latency: There were no significant group or condition main effects for the N290 latency. However, a Condition × Group interaction was present for P400, F(1,33) = 4.892, p = .034, partial η2. = .129. Follow-up analyses indicated that the two infant groups differed in the latency of P400 to the stranger's face, F(1,33) = 6.748, p = .014 due to longer latency in the typical sample. Furthermore, only typical infants demonstrated a mother–stranger difference in P400 latency, such that the stranger's face was associated with a delayed response compared to the mother's face, t(19) = 3.462, p = .003, d = .774.
3.2.2. General familiarity/novelty Nc response
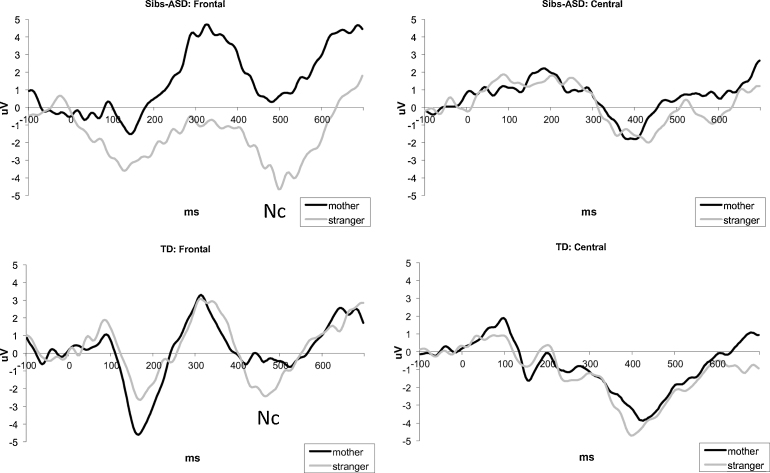
Amplitude: The main effect of Condition was observed for Nc, F(1,33) = 4.625, p = .039, partial η2. = .123, due to the stranger's face eliciting a larger (more negative) response, t(34) = 2.333, p = .06, d = .394 (Fig. 4). There were no significant main effects or interactions involving Group or Electrode factors.
Fig. 4.
ERPs in response to familiar and novel faces for frontal and central clusters in sibs-ASD (top row) and typical infants (bottom row).
Latency: There were no significant group or condition differences in the latency of Nc.
3.3. Brain–behavior relations
To examine the contribution of psychophysiological differences to the risk group assignment accuracy, a series of binary logistic regressions were conducted. Given significant group differences in parental reports of expressive communication skills (typical infants: M = 14.80 ± 1.51; sibs-ASD: M = 12.60 ± 1.80; p < .0001), the v-score for that subscale was entered into the regression first, followed by the latencies of P400 to mother's and stranger's face. The results are presented in Table 4. Overall, only the amplitude of the P400 response to the stranger's face was associated with an increase in the classification accuracy above and beyond what would be predicted based on group differences in expressive communication scores.
Table 4.
Summary statistics for the binary logistic regression analyses.
| Variable(s) entered | Step 1 |
Step 2 |
||||
|---|---|---|---|---|---|---|
| Model 1 | B | SE | χ2 | B | SE | χ2 |
| V expressive | 0.582 | 0.225 | 6.681* | 0.793 | 0.285 | 7.749* |
| P400 latency to stranger's face | 0.067 | 0.026 | 6.439* | |||
| P400 latency to mother's face | −.021 | .022 | .909 | |||
| R2 | 0.317 | 0.576 | ||||
| % correctly classified | ||||||
| Sibs-ASD | 66.7 | 73.3 | ||||
| Typical | 85 | 85 | ||||
Values significant at p < .015.
Examination of correlations between ERP measures in response to familiar and novel faces and parental report of social and communicative skills (VABS-II scores) indicated that better expressive communication and interpersonal skills were associated with smaller, less negative N290 (r = .410, p = .015 and r = .434, p = .009, respectively) and larger, more positive P400 (r = .434, p = .009 and r = .420, p = .012, respectively) responses to mother's face over the left hemisphere. Furthermore, shorter latency of the central Nc to mother's face was related to better interpersonal skills (r = −.385, p = .022). There were no significant correlations between VABS-II scores and ERP responses to stranger's face.
4. Discussion
This study examined psychophysiological responses to familiar and novel faces in sibs-ASD and in typical infants, and investigated whether sensitivity to face familiarity is associated with infants’ social and communicative behaviors. Our results indicate that the two infant groups are similar in their ability to detect differences between faces of their mothers vs. strangers, and that ERP responses to the mother's face are related to infants’ expressive communication and interpersonal relationship behaviors. The speed of P400 response to the stranger face was the only measure on which sibs-ASD and typical infants differed.
In line with previous reports (e.g., Merin et al., 2007), we observed no significant differences in the stranger face scanning behaviors of sibs-ASD and typical infants, as there were no group differences in the number or duration of fixations on the stimulus face in general or on its individual features (eyes or mouth). While it may be possible that more trials and/or a longer total viewing time (greater than 10 s) during the eye tracking would reveal more subtle group differences in face scanning strategies, the observed similarity in eye tracking data is consistent with a number of recently published papers identifying 12 months as the age after which behavioral symptoms of autism are more clearly and consistently present (Nadig et al., 2007, Zwaigenbaum et al., 2005). A recent eye tracking study in 2–4-year-old children with ASD also demonstrated that atypical face scanning becomes more pronounced over time (Chawarska and Shic, 2009). The lack of group differences in eye tracking data is also in line with findings in children and adults with ASD, indicating that alterations in face scanning (e.g., enhanced fixations on the mouth, Dalton et al., 2005, Joseph and Tanaka, 2003) are not universal and may be limited to certain cognitively demanding tasks (see Jemel et al., 2006 for review). Our eye tracking paradigm would not be considered demanding because only a single face was presented in its complete, natural form (i.e., no features missing or rearranged), there was no task related to stimulus viewing, and no behavioral response was required. Importantly, the lack of group differences in the distribution and duration of fixations observed in the present study also suggests that group differences present in ERP measures of face recognition are not due to differences in attention to facial elements across the two infant groups.
Analysis of the brain responses to the familiar and novel face stimuli revealed that, similar to previous infant studies of face processing (de Haan et al., 2003, Nelson, 2001, McCleery et al., 2009, Pascalis et al., 2002), posterior N290 and P400 peaks as well as fronto-central Nc responses were present in averaged ERPs of both infant groups. As a group, typical infants and sibs-ASD differentiated between their mothers and strangers, as reflected in amplitude modulations of face-specific posterior N290/P400 and the novelty-sensitive fronto-central Nc response.
The larger N290 to the familiar face observed in the present study is consistent with the interpretation of more extensive face processing given to familiar faces, as reported in prior studies in typical adults (Caharel et al., 2002, Caharel et al., 2005, Heisz and Shedden, 2008), infants (Scott et al., 2006), and in persons with ASD (Pierce et al., 2004). However, this finding appears to be at odds with a previous report of smaller N290 responses to familiarized than novel faces (Key et al., 2009). These differences in results may be due to the nature of the stimuli and the implicit task employed in the two studies. In the Key et al. (2009) study, all stimuli involved the same initially unfamiliar face, with one of its features occasionally replaced by the same feature from a different novel face. Prior studies in adults reported a reduction in the amplitude of N170 in response to repeated presentations of unfamiliar faces, while no reduction was observed for repetitions of the familiar, personally significant faces (Heisz et al., 2006, Heisz and Shedden, 2008). Furthermore, detection of the deviant stimulus in Key et al. (2009) required a featural level of processing. An increase in the N290 response was observed in response to the novel eyes, consistent with reports that the eyes are critical for the activation of face-specific perceptual mechanisms (Bentin et al., 2006). In the present study, we used faces of infants’ mothers (to ensure sufficiently extensive familiarity) and strangers; thus the difference between the stimuli was not limited to a single feature and could be detected at the holistic/configural level of processing because all facial elements and their arrangement varied across the familiar/novel conditions. A more familiar mother's face could also carry additional emotional significance, making it more easily recognizable as a face and activating face-specific perceptual mechanisms (Pizzagalli et al., 2002, Vuilleumier et al., 2004).
The finding of larger P400 amplitudes for the stranger than mother's face is also in line with previously reported results. In typical infants, larger amplitudes were observed for the less familiar faces (Scott et al., 2006) and for inverted mother's faces (Balas et al., 2010). Functionally, increase in the P400 amplitude can be attributed to greater reliance on featural processing (Balas et al., 2010) and greater visual attention or memory search and updating (Swingler et al., 2010). Similar amplitude modulations have been reported in typical adults and in adults with ASD for the P2 peak that immediately follows N170, with larger responses elicited by repeated novel than familiar faces (Caharel et al., 2002, Webb et al., 2010). However, Carver et al. (2003) reported that increased P400 to a stranger's compared to the mother's face is present only infants over 45 months of age, while infants under 24 months generate a larger P400 to their mother's faces. Our finding of a larger P400 to stranger's face in a younger infant sample could be attributed to the fact that all stimulus faces displayed a happy expression, while Carver et al. used neutral faces. Gross and Schwarzer (2010) demonstrated that in 7–9-month-old infants, facial recognition is enhanced when faces have emotional compared to the neutral expressions.
Mother–stranger fronto-central Nc amplitude differences observed in our sample are consistent with previous reports of larger amplitudes to novel faces (Courchesne et al., 1981, Reynolds and Richards, 2005). The lack of group differences on this measure may reflect the similarity in the overall perceptual and attentional strategies employed by 9-month-old sibs-ASD and typical infants for the purpose of familiarity/novelty stimulus classification. Similar findings have been reported for 12-month-old sibs-ASD and typical infants (Luyster et al., 2011).
Group differences were present only in the form of stimulus familiarity effects on the P400 latency. While infants in both groups demonstrated similar stimulus-related changes in the amplitude of ERP responses, there was a difference in the speed of P400 response to the stranger's face, as it peaked later in the typical group than in sibs-ASD. Furthermore, only the typical group demonstrated P400 latency differences between the mother's and stranger's face, whereas for the sibs-ASD group, both stimuli elicited P400 responses with nearly identical latencies. Including P400 latency data in a binary logistic regression improved risk group classification accuracy for sibs-ASD above and beyond what could be accomplished based on the parental report of expressive communication skills. Prolonged P400 to the stranger face observed in typical infants suggested that novel faces required a slightly longer perceptual analysis or more extensive memory search than the familiar face, perhaps due to underlying differences in the approach (e.g., holistic/configural approach for the familiar face vs. featural for the stranger face, e.g., Sterling et al., 2008). Indeed, prior studies in typical 12-month-olds reported longer P400 latency for monkey than human faces and for inverted vs. upright faces (Halit et al., 2003).
There are several possible explanations for the absence of this effect in sibs-ASD. First, it is possible that this group of infants is less likely to modulate their face perceptual processes based on familiarity and more likely to process stranger and mother faces in a similar holistic fashion by relying on only general perceptual properties (e.g., eyes above the mouth). Because one of the two stimuli used in the study was a highly familiar mother's face, a holistic approach could be sufficient for mother–stranger discrimination. In contrast, typical infants may engage in more flexible strategy selection by choosing potentially faster holistic processing for familiar faces and slower featural strategies for novel stimuli. Previously reported findings indicate that featural information is utilized for face discrimination even in the expert face processing of typical adults (same/different judgments; Rotshtein et al., 2007). From a social-emotional development standpoint, reduced ability of sibs-ASD to alter face processing strategy based on situational demands (e.g., differences in familiarity) may indicate reduced flexibility in their social information processing (and may potentially be related to the general preference for order and sameness and behavioral rigidity commonly noted in children and adults with ASD). Mother–stranger discrimination in the present study was a relatively simple task, and any one strategy (e.g., holistic or featural processing) could be successful. However, because everyday interactions are much more complex and dynamic in nature, sibs-ASD may be less able to adapt their behavior quickly based on available relevant information, and therefore more likely to be unsuccessful. Increased frequency of negative social outcomes could then lead to avoidance of social situations, lack of experience with faces and other social challenges.
It is also possible that P400 latency measures reflect more global differences in social development of the two infant groups. In a recent study of 6-month-old typical infants, prolonged P400 latency to stranger faces was associated with longer periods of searching for the mother during separation and interpreted to reflect individual differences in the developing mother–infant relationship (Swingler et al., 2010). A more established early social bond (e.g., a stronger preference for the mother) could affect how infants allocate neural resources for face processing by considering familiarity when selecting face processing strategies. An infant with less developed social ties may give less consideration to familiarity (despite the ability to detect physical differences in faces) and therefore not alter his/her face processing strategy. Future studies will need to include an objective measure of attachment to fully examine this possibility.
Alternatively, the lack of a mother–stranger latency difference in the sibs-ASD group could reflect faster habituation to and/or reduced interest in the novel stimulus. Prior studies utilizing an oddball paradigm report that infants as young as 4–7 months old are able to remember a frequently presented novel face (Courchesne et al., 1981). The infants in our study viewed the stranger's face 30 times over the course of 6–8 min, so the total looking time could be a high as 30 s, and in the Scott and Nelson (2006) study, 8-month-old infants were considered to become familiar with the face after 20 s of accumulated looking time. However, even if the stranger's face did lose some of its novelty, it still remained less familiar than the mother's face due to vast differences in the overall exposure time (seconds vs. months). The presence of mother–stranger differences in the amplitudes of the N290/P400 and in particular, the Nc response, which specifically reflects stimulus novelty (Reynolds and Richards, 2005), also argues against this explanation.
While risk group membership was best predicted using the speed of P400 response to the stranger's face, it was the response to the more familiar mother's face that correlated with parental reports of social-communicative behaviors. In particular, better interpersonal skills were associated with faster processing of the familiar face (reflected by the correlation with the Nc latency), reduced engagement of the face-specific perceptual mechanisms (smaller N290) and greater reliance on memory (larger P400) over left hemisphere. Prior infant studies of face processing associated left-hemisphere ERPs with featural processing (Deruelle and de Schonen, 1998, Scott and Nelson, 2006). Therefore, reduction in N290 and increase in P400 amplitudes over the left hemisphere observed in infants with more advanced social skills could be indicative of their shifting from featural face processing to more configural and memory-based processing of familiar faces.
Although many of our findings are consistent with the existing literature, the present study has several limitations. While not unusual in size for an ERP study, the sample of sibs-ASD was relatively small, and given the estimated prevalence rates for ASD among siblings of children with the diagnosis, unlikely to have included many children who will receive a later ASD diagnosis. Indeed, our group of sibs-ASD discriminated familiar from novel faces in a manner largely similar to that of typical infants, in contrast with previous findings that preschoolers with ASD failed to demonstrate P400 or Nc modulation in response to familiar vs. novel faces (Dawson et al., 2002). However, results from previously published studies of sibs-ASD suggest that early group differences may be due to the elevated risk status/genetic vulnerability of this group and are not necessarily driven by the minority who receive a later diagnosis of ASD (Stone et al., 2007). Thus, the observed differences in the ERP responses affected by stimulus condition manipulations (e.g., mother–stranger differences in P400 latency) suggest that even though most of sibs-ASD may not receive an ASD diagnosis, their brain mechanisms underlying face processing may be altered (e.g., see Dawson et al., 2005 for evidence of atypical face processing in unaffected parents of children with ASD).
Although the P400 latency to the stranger's face differed between the participant groups and increased risk group classification accuracy, it is possible that this ERP variable is not predictive of either an ASD diagnosis or other cognitive or behavioral differences at later ages (e.g., see Merin et al., 2007, Young et al., 2009). A follow-up diagnostic assessment as infants in our sample get older is the only way to determine the specific relation between individual differences in brain responses to stranger faces, parent-reported communicative skills and later developmental outcomes. A final limitation of the study is the reliance on parental report of social and communication skills. While the VABS-II is a valid measure, a more direct observations of early language and social skills (e.g., via the ESCS) would strengthen future work. Similarly, future studies should take advantage of recent technological developments allowing for co-registration of eye tracking and ERP data to allow for more precise characterization of behavioral and psychophysiological profiles of sibs-ASD and typical infants.
In conclusion, our results extend recent findings that infants at a higher than average risk for ASD discriminate familiar and novel faces by demonstrating that sibs-ASD differ from typical infants in their face processing strategies as reflected by the lack of P400 latency modulation across conditions. Furthermore, these strategy differences are not evident in face scanning behaviors (i.e., no detectable avoidance of the eye region or increased attention to the mouth area as frequently reported in older children with ASD), and are observed prior to 12 months of age. Additional longitudinal follow-up that includes a diagnostic assessment is needed to examine the predictive value of the observed individual differences in ERPs to familiar and novel face stimuli as a marker of risk for social impairments or autism spectrum disorders.
Conflict of interest statement
The authors have no conflict of interest to declare.
Acknowledgments
This work was supported in part by NICHD Grant P30 HD15052 to Vanderbilt Kennedy Center and by a Marino Autism Research Institute (MARI) Discovery Award to Dr. Alexandra Key. We would like to thank Ms. Stephanie Bradshaw and Ms. Katie Knoedelseder for their assistance in recruiting and testing the participants and Ms. Susan M. Williams for her help with data acquisition and processing.
Contributor Information
Alexandra P.F. Key, Email: sasha.key@vanderbilt.edu.
Wendy L. Stone, Email: stonew@u.washington.edu.
References
- Balas B., Nelson C., Westerlund A., Vodel-Farley V., Riggins T., Kuefner D. Personal familiarity influences the processing of upright and inverted faces in infants. Frontiers in Human Neuroscience. 2010;4:1–6. doi: 10.3389/neuro.09.001.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Haim Y., Shulman C., Lamy D., Reuveni A. Attention to eyes and mouth in high-functioning children with autism. Journal of Autism and Developmental Disorders. 2006;36(1):131–137. doi: 10.1007/s10803-005-0046-1. [DOI] [PubMed] [Google Scholar]
- Bartrip J., Morton J., de Schonen S. Responses to mother's face in 3-week to 5-month-old infants. British Journal of Developmental Psychology. 2001;19:219–232. [Google Scholar]
- Bentin S., Golland Y., Flevaris A., Robertson C., Moscovitch M. Processing the trees and the forest during initial stages of face perception: electrophysiological evidence. Journal of Cognitive Neuroscience. 2006;18(8):1406–1421. doi: 10.1162/jocn.2006.18.8.1406. [DOI] [PubMed] [Google Scholar]
- Blair R.J., Frith U., Smith N., Abell F., Cipolotti L. Fractionation of visual memory: agency detection and its impairment in autism. Neuropsychologia. 2002;40:108–118. doi: 10.1016/s0028-3932(01)00069-0. [DOI] [PubMed] [Google Scholar]
- Bolton P., Macdonald H., Pickles A., Rios P., Goode S., Crowson M. A case–control family history study of autism. Journal of Child Psychology and Psychiatry. 1994;35:877–900. doi: 10.1111/j.1469-7610.1994.tb02300.x. [DOI] [PubMed] [Google Scholar]
- Boucher J., Lewis V., Collis G. Familiar face and voice matching and recognition in children with autism. Journal of Child Psychology and Psychiatry. 1998;39:171–182. [PubMed] [Google Scholar]
- Caharel S., Courtay N., Bernard C., Lalonde R., Rebai M. Familiarity and emotional expression influence an early stage of face processing: an electrophysiological study. Brain and Cognition. 2005;59:96–100. doi: 10.1016/j.bandc.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Caharel S., Poiroux S., Bernard C., Thibaut F., Lalonde R., Rebai M. ERPs associated with familiarity and degree of familiarity during face recognition. International Journal of Neuroscience. 2002;112:1499–1512. doi: 10.1080/00207450290158368. [DOI] [PubMed] [Google Scholar]
- Carver L., Dawson G., Panagiotides H., Meltzoff A., McPartland J., Gray J., Munson J. Age-related differences in neural correlates of face recognition during the toddler and preschool years. Developmental Psychobiology. 2003;42:148–159. doi: 10.1002/dev.10078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) Prevalence of autism spectrum disorders—autism and developmental disabilities monitoring network, United States, 2006. Morbidity and Mortality Weekly Report. 2009;58(SS10):1–20. [PubMed] [Google Scholar]
- Chawarska K., Shic F. Looking but not seeing: atypical visual scanning and recognition of faces in 2 and 4-year-old children with autism spectrum disorder. Journal of Autism and Developmental Disorders. 2009;39:1663–1672. doi: 10.1007/s10803-009-0803-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Committee on Educational Interventions for Children with Autism . National Academy Press; Washington, DC: 2001. Educating Children with Autism. [Google Scholar]
- Constantino J., Todd R. Genetic structure of reciprocal social behavior. American Journal of Psychiatry. 2000;157(12):2043–2045. doi: 10.1176/appi.ajp.157.12.2043. [DOI] [PubMed] [Google Scholar]
- Courchesne E., Ganz L., Norcia A.M. Event-related brain potentials to human faces in infants. Child Development. 1981;52(3):804–811. [PubMed] [Google Scholar]
- Dalton K.M., Nacewicz B.M., Johnstone T., Schaefer H.S., Gernsbacher M.A., Goldsmith H.H., Alexander A.L., Davidson R.J. Gaze fixation and the neural circuitry of face processing in autism. Nature Neuroscience. 2005;8:519–526. doi: 10.1038/nn1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson G., Carver L., Meltzoff A.N., Panagiotides H., McPartland J., Webb S.J. Neural correlates of face and object recognition in young children with autism spectrum disorder, developmental delay, and typical development. Child Development. 2002;73(3):700–717. doi: 10.1111/1467-8624.00433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson G., Webb S., Wijsman E., Schellenberg G., Estes A., Munson J., Faja S. Neurocognitive and electrophysiological evidence of altered face processing in parents of children with autism: implications for a model of abnormal development of social brain circuitry in autism. Development and Psychopathology. 2005;17(3):679–697. doi: 10.1017/S0954579405050327. [DOI] [PubMed] [Google Scholar]
- de Gelder B., Vroomen J., Van der Heide L. Face recognition and lip-reading in autism. European Journal of Cognitive Psychology. 1991;3:69–86. [Google Scholar]
- de Haan M., Johnson M.H., Halit H. Development of face-sensitive event-related potentials during infancy: a review. International Journal of Psychophysiology. 2003;51(1):45–58. doi: 10.1016/s0167-8760(03)00152-1. [DOI] [PubMed] [Google Scholar]
- de Haan M., Nelson C.A. Recognition of the mother's face by six-month-old infants: a neurobehavioral study. Child Development. 1997;68(2):187–210. [PubMed] [Google Scholar]
- Deruelle C., de Schonen S. Do the right and left hemisphere attend to the same visuospatial information within a face in infancy? Developmental Neuropsychology. 1998;14:535–554. [Google Scholar]
- Deruelle C., Rondan C., Gepner B., Tardif C. Spatial frequency and face processing in children with autism and Asperger syndrome. Journal of Autism and Developmental Disorders. 2004;34(2):199–210. doi: 10.1023/b:jadd.0000022610.09668.4c. [DOI] [PubMed] [Google Scholar]
- Ellis H.D., Young A.W. Faces in their social and biological context. In: Young A.W., editor. Face and Mind. Oxford University Press; New York: 1998. pp. 67–95. [Google Scholar]
- Elsabbagh M., Johnson M.H. Getting answers from babies about autism. Trends in Cognitive Science. 2010;14(2):81–87. doi: 10.1016/j.tics.2009.12.005. [DOI] [PubMed] [Google Scholar]
- Fabiani M., Gratton G., Coles M.G.H. Event-related brain potentials: methods, theory, and applications. In: Cacioppo J.T., Tassinary L.G., Berntson G.G., editors. Handbook of Psychophysiology. second edition. Cambridge University Press; 2000. pp. 53–84. [Google Scholar]
- Gepner B., de Gelder B., de Schonen S. Face processing in autistics: evidence for a generalized deficit? Child Neuropsychology. 1996;2:123–139. [Google Scholar]
- Gross C., Schwarzer G. Face recognition across varying poses in 7- and 9-month-old infants: the role of facial expression. International Journal of Behavioral Development. 2010;34(5):417–426. [Google Scholar]
- Halit H., de Haan M., Johnson M.H. Cortical specialization for face processing: face-sensitive event-related potential components in 3- and 12-month-old infants. Neuroimage. 2003;19(3):1180–1193. doi: 10.1016/s1053-8119(03)00076-4. [DOI] [PubMed] [Google Scholar]
- Harris S.L., Handleman J.S. Age and IQ at intake as predictors of placement for young children with autism: a four-to-six-year follow-up. Journal of Autism and Developmental Disorders. 2000;30:137–142. doi: 10.1023/a:1005459606120. [DOI] [PubMed] [Google Scholar]
- Heisz J., Shedden J. Semantic learning modifies perceptual face processing. Journal of Cognitive Neuroscience. 2008;21(6):1127–1134. doi: 10.1162/jocn.2009.21104. [DOI] [PubMed] [Google Scholar]
- Heisz J., Watter S., Shedden J. Automatic face identity encoding at the N170. Vision Research. 2006;46:4604–4614. doi: 10.1016/j.visres.2006.09.026. [DOI] [PubMed] [Google Scholar]
- Hileman C., Henderson H., Mundy P., Newell L., Jaime M. Developmental and individual differences in the P1 and N170 ERP components in children with and without autism. Developmental Neuropsychology. 2011;36(2):214–236. doi: 10.1080/87565641.2010.549870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemel B., Mottron L., Dawson M. Impaired face processing in autism: fact or artifact? Journal of Autism and Developmental Disorders. 2006;36(1):91–106. doi: 10.1007/s10803-005-0050-5. [DOI] [PubMed] [Google Scholar]
- Joseph R.M., Tanaka J. Holistic and part-based face recognition in children with autism. Journal of Child Psychology and Psychiatry. 2003;44:529–542. doi: 10.1111/1469-7610.00142. [DOI] [PubMed] [Google Scholar]
- Key A., Stone W., Williams S. What do infants see in faces? ERP evidence of different roles of eyes and mouth for face perception in 9-month-old infants. Infant and Child Development. 2009;18:149–162. doi: 10.1002/icd.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klin A., Sparrow S.S., de Bildt A., Cicchetti D.V., Cohen D.J., Volkmar F.R. A normed study of face recognition in autism and related disorders. Journal of Autism and Developmental Disorders. 1999;29:499–508. doi: 10.1023/a:1022299920240. [DOI] [PubMed] [Google Scholar]
- Landa R., Garrett-Mayer E. Development in infants with autism spectrum disorders: a prospective study. Journal of Child Psychology and Psychiatry. 2006;47:629–638. doi: 10.1111/j.1469-7610.2006.01531.x. [DOI] [PubMed] [Google Scholar]
- Langdell T. Recognition of faces: an approach to the study of autism. Journal of Child Psychology and Psychiatry. 1978;19:255–268. doi: 10.1111/j.1469-7610.1978.tb00468.x. [DOI] [PubMed] [Google Scholar]
- Luyster R., Wagner J., Vogel-Farley V., Tager-Flusberg H., Nelson C. Neural correlates of familiar and unfamiliar face processing in infants at risk for autism spectrum disorders. Brain Topography. 2011 doi: 10.1007/s10548-011-0176-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCleery J., Akshoomoff N., Dobkins K., Carver L. Atypical face versus object processing and hemispheric asymmetries in 10-month-old infants at risk for autism. Biological Psychiatry. 2009;66:950–957. doi: 10.1016/j.biopsych.2009.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPartland J., Dawson G., Webb S.J., Panagiotides H., Carver L.J. Event-related brain potentials reveal anomalies in temporal processing of faces in autism spectrum disorder. Journal of Child Psychology and Psychiatry. 2004;45(7):1235–1245. doi: 10.1111/j.1469-7610.2004.00318.x. [DOI] [PubMed] [Google Scholar]
- Merin N., Young G., Ozonoff S., Rogers S. Visual fixation patterns during reciprocal social interaction distinguish a subgroup of 6-month-old infants at-risk for autism from comparison infants. Journal of Autism and Developmental Disorders. 2007;37(1):108–121. doi: 10.1007/s10803-006-0342-4. [DOI] [PubMed] [Google Scholar]
- Nadig A., Ozonoff S., Young G., Rozga A., Sigman M., Rogers S. A prospective study of response to name in infants at risk for autism. Archives of Pediatric & Adolescent Medicine. 2007;161(4):378–383. doi: 10.1001/archpedi.161.4.378. [DOI] [PubMed] [Google Scholar]
- Nelson C. The development and neural bases of face recognition. Infant and Child Development. 2001;10:3–18. [Google Scholar]
- Nelson C., Collins P. Neural and behavioral correlates of visual recognition memory in 4- and 8-month-old infants. Brain and Cognition. 1992;19(1):105–121. doi: 10.1016/0278-2626(92)90039-o. [DOI] [PubMed] [Google Scholar]
- Nelson C., Collins P. Event-related potential and looking-time analysis of infants’ responses to familiar and novel events: Implications for visual recognition memory. Developmental Psychology. 1991;27(1):50–58. [Google Scholar]
- Ozonoff S., Iosif A.M., Baguio F., Cook I.C., Hill M.M., Hutman T. A prospective study of the emergence of early behavioral signs of autism. Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49(3):256–266. [PMC free article] [PubMed] [Google Scholar]
- Ozonoff S., Young G., Carter A., Messinger D., YUrimiya N., Zwaigenbaum L., Bryson S., Carver L., Constantino J., Dobkins K., Hutman T., Iverson J., Landa R., Rogers S., Sigman M., Stone W. Recurrence risk for autism spectrum disorders: a baby siblings research consortium study. Pediatrics. 2011;128(3):e1–e7. doi: 10.1542/peds.2010-2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascalis O., de Schonen S., Morton J., Deruelle C., Fabre-Grenet M. Mother's face recognition by neonates: a replication and an extension. Infant Behavior and Development. 1995;18:79–85. [Google Scholar]
- Pascalis O., de Haan M., Nelson C.A. Is face processing species-specific during the first year of life? Science. 2002;296(5571):1321–1323. doi: 10.1126/science.1070223. [DOI] [PubMed] [Google Scholar]
- Pierce K., Haist F., Sedaghat F., Courchesne E. The brain response to personally familiar faces in autism: findings of fusiform activity and beyond. Brain. 2004;127:2703–2716. doi: 10.1093/brain/awh289. [DOI] [PubMed] [Google Scholar]
- Pizzagalli D.A., Lehman D., Hendrick A.M., Regard M., Pascual-Marqui R.D., Davidson R. Affective judgments of faces modulate early activity (∼160 ms) within the fusiform gyri. Neuroimage. 2002;16:663–677. doi: 10.1006/nimg.2002.1126. [DOI] [PubMed] [Google Scholar]
- Reynolds G., Richards J. Familiarization, attention, and recognition memory in infancy: an event-related potential and cortical source localization study. Developmental Psychology. 2005;41(4):598–615. doi: 10.1037/0012-1649.41.4.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers S.J. Neuropsychology of autism in young children and its implications for early intervention. Mental Retardation and Developmental Disabilities Research Reviews. 1998;4:104–112. [Google Scholar]
- Rogers S.J. What are infant siblings teaching us about autism in infancy? Autism Research. 2009;2:125–137. doi: 10.1002/aur.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotshtein P., Geng J., Driver J., Dolan R. Role of features and second-order spatial relations in face discrimination, face recognition, and individual face skills: behavioral and functional magnetic resonance imaging data. Journal of Cognitive Neuroscience. 2007;19:1435–1452. doi: 10.1162/jocn.2007.19.9.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin D. Wiley; New York: 1987. Multiple Imputation for Nonresponse in Sample Surveys. [Google Scholar]
- Rutherford M.D., Towns A. Scan path differences and similarities during emotion perception in those with and without autism. Developmental Disorders. 2008;38:1371–1381. doi: 10.1007/s10803-007-0525-7. [DOI] [PubMed] [Google Scholar]
- Schultz R.T., Grelotti D.J., Klin A., Kleinman J., Van der Gaag C., Marois R. The role of the fusiform face area in social cognition: implications for the pathobiology of autism. Philosophical Transactions of the Royal Society of London: Series B - Biological Science. 2003;358:415–427. doi: 10.1098/rstb.2002.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schults R. Developmental deficits in social perception in autism: the role of the amygdala and fusiform face area. International Journal of Developmental Neuroscience. 2005;23:125–141. doi: 10.1016/j.ijdevneu.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Schwarzer G., Zauner N., Jovanovic B. Evidence of a shift from featural to configural face processing in infancy. Developmental Science. 2007;10:452–463. doi: 10.1111/j.1467-7687.2007.00599.x. [DOI] [PubMed] [Google Scholar]
- Scott L., Nelson C. Featural and configural face processing in adults and infants: a behavioral and electrophysiological investigation. Perception. 2006;35:1107–1128. doi: 10.1068/p5493. [DOI] [PubMed] [Google Scholar]
- Scott L.S., Shannon R.W., Nelson C.A. Neural correlates of human and monkey face processing by 9-month-old infants. Infancy. 2006;10:171–186. doi: 10.1207/s15327078in1002_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparrow S.S., Cicchetti D.V., Balla D.A. second edition. AGS Publishing; Circle Pines, MN: 2005. Vineland Adaptive Behavior Scales. [Google Scholar]
- Sterling L., Dawson G., Webb S., Murias M., Munson J., Panagiotides H., Aylward E. The role of face familiarity in eye tracking of faces by individuals with autism spectrum disorders. Journal of Autism and Developmental Disorders. 2008;38:1666–1675. doi: 10.1007/s10803-008-0550-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone W.L., McMahon C.R., Yoder P.J., Walden T.A. Early social-communicative and cognitive development of younger siblings of children with autism spectrum disorders. Archives of Pediatrics and Adolescent Medicine. 2007;161:384–390. doi: 10.1001/archpedi.161.4.384. [DOI] [PubMed] [Google Scholar]
- Swingler M., Sweet M., Carver L. Brain–behavior correlations: relationships between mother–stranger face processing and infants’ behavioral responses to a separation from mother. Developmental Psychology. 2010;46(3):669–680. doi: 10.1037/a0018907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teunisse J.-P., de Gelder B. Face processing in adolescents with autistic disorder: the inversion and composite effects. Brain and Cognition. 2003;52:285–294. doi: 10.1016/s0278-2626(03)00042-3. [DOI] [PubMed] [Google Scholar]
- Thomas D., Grice J., Najm-Briscoe R., Miller J. The influence of unequal numbers of trials on comparisons of average event-related potentials. Developmental Neuropsychology. 2004;26:753–774. doi: 10.1207/s15326942dn2603_6. [DOI] [PubMed] [Google Scholar]
- Volkmar F., Sparrow S., Rende R., Cohen D. Facial perception in autism. Journal of Child Psychology and Psychiatry. 1989;30(4):591–598. doi: 10.1111/j.1469-7610.1989.tb00270.x. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P., Richardson M.P., Armony J.L., Driver J., Dolan R.J. Distant influences of amygdala lesion on visual cortical activation during emotional face processing. Nature Neuroscience. 2004;7:1271–1278. doi: 10.1038/nn1341. [DOI] [PubMed] [Google Scholar]
- Webb S.J., Jones E.J.H., Merkle K.M., Namkung J., Toth K., Greenson J. Increased attention to faces during habituation in toddlers with elevated autism symptoms. Child Neuropsychology. 2010;16:255–278. doi: 10.1080/09297041003601454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb S.J., Jones E.J.H., Merkle K.M., Venema K., Greenson J., Murias M. Developmental change in the ERP responses to familiar faces in toddlers with autism spectrum disorders versus typical development. Child Development. 2011 doi: 10.1111/j.1467-8624.2011.01656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D.L., Goldstein G., Minshew N.J. Impaired memory for faces and social scenes in autism: clinical implications of the memory disorder. Archives of Clinical Neuropsychology. 2005;20:1–15. doi: 10.1016/j.acn.2002.08.001. [DOI] [PubMed] [Google Scholar]
- Young G., Merin N., Rogers S., Ozonoff S. Gaze behavior and affect at 6 months: predicting clinical outcomes and language development in typically developing infants and infants at risk for autism. Developmental Science. 2009;12(5):798–814. doi: 10.1111/j.1467-7687.2009.00833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwaigenbaum L., Bryson S., Rogers T., Roberts W., Brian J., Szatmari P. Behavioral manifestations of autism in the first year of life. International Journal of Developmental Neuroscience. 2005;23:143–152. doi: 10.1016/j.ijdevneu.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Zwaigenbaum L., Thurm A., Stone W.L., Baranek G., Bryson S., Iverson J. Studying the emergence of autism spectrum disorders in high risk infants: methodological and practical issues. Journal of Autism and Developmental Disorders. 2007;37:466–480. doi: 10.1007/s10803-006-0179-x. [DOI] [PubMed] [Google Scholar]