Highlights
* Probabilistic learning is more adaptive in adolescents with higher IQ. * DLPFC and dACC are differentially sensitive to positive feedback. * Developmental differences in neural function differ depending on IQ level. * The dACC difference can partly be explained by school environment. * The findings have implications for educational neuroscience.
Keywords: IQ, Development, Learning, fMRI, Brain maturation
Abstract
Knowing how to adapt your behavior based on feedback lies at the core of successful learning. We investigated the relation between brain function, grey matter volume, educational level and IQ in a Dutch adolescent sample. In total 45 healthy volunteers between ages 13 and 16 were recruited from schools for pre-vocational and pre-university education. For each individual, IQ was estimated using two subtests from the WISC-III-R (similarities and block design). While in the magnetic resonance imaging (MRI) scanner, participants performed a probabilistic learning task. Behavioral comparisons showed that participants with higher IQ used a more adaptive learning strategy after receiving positive feedback. Analysis of neural activation revealed that higher IQ was associated with increased activation in DLPFC and dACC when receiving positive feedback, specifically for rules with low reward probability (i.e., unexpected positive feedback). Furthermore, VBM analyses revealed that IQ correlated positively with grey matter volume within these regions. These results provide support for IQ-related individual differences in the developmental time courses of neural circuitry supporting feedback-based learning. Current findings are interpreted in terms of a prolonged window of flexibility and opportunity for adolescents with higher IQ scores.
1. Introduction
1.1. The development of performance monitoring
Performance monitoring, which involves our ability to adjust our behavior following changing task demands, is one of the core components for successful learning. Performance monitoring involves the detection of errors or performance feedback in tasks where rules need to be inferred over trials, or when rules change unexpectedly. Across childhood and adolescence, there is a steady increase in the ability to monitor the outcomes of actions with the purpose of behavioral adjustment, which has been demonstrated using rule-switch tasks (Huizinga et al., 2006, Koolschijn et al., 2011) and probabilistic learning tasks (van den Bos et al., 2009, Eppinger et al., 2009). These findings have been interpreted in the context of slowly developing executive control functions, with steady advances in adolescence (Crone, 2009).
Neuroimaging studies have demonstrated that a network of regions in the lateral prefrontal (lat-PFC), superior parietal, and the dorsal anterior cingulate cortex (dACC) is engaged when individuals process performance feedback. These studies typically make use of simple learning paradigms, in which a stimulus requires a response, which is then followed by positive or negative feedback (Holroyd and Coles, 2002). The activation of these regions is typically related to negative feedback processing in adults (Holroyd et al., 2004, Zanolie et al., 2008). This has been associated with a monitoring system which signals that outcomes are worse than expected and that adjustment is necessary.
Given the developmental changes in performance monitoring until late adolescence, it would be expected that this error- or feedback monitoring system matures slowly. In support of this hypothesis, neuroimaging studies reported an age-related increase in activation following negative feedback in the lat-PFC, parietal cortex and dACC (Crone et al., 2008), showing that these regions are differentially engaged across development. These findings are also consistent with prior neuroimaging studies which have reported that the development of other executive control functions, such as working memory, task switching and response inhibition, is also associated with a protracted development of regions within the prefrontal cortex (for a review, see Bunge and Wright, 2007).
However, two prior studies reported not only less activation in lat-PFC and dACC following negative feedback, but also more activation in children in these areas following positive feedback (Van Duijvenvoorde et al., 2008, van den Bos et al., 2009). These neuroimaging findings were interpreted as pointing to a developmental shift from attention that is given to positive feedback (in childhood) towards attention that is given to negative feedback (in adulthood), with adolescence being a transition phase. Notably, these findings argue against a strict maturational viewpoint which predicts that the lateral PFC and dACC cannot be engaged due to structural immaturity (see the maturtional viewpoint by Johnson, 2011), instead, these regions are engaged under different task demands, consistent with skill learning or interactive specialization viewpoints (Johnson, 2011; see also Dumontheil et al., 2010).
1.2. Individual differences in education and IQ
Recently, much attention is given to the individual differences in learning performance, as children from different levels of education, and thus different levels of IQ, may benefit differently from learning cues. These differences may be particularly important for the field of educational neuroscience, in which one of the goals is to map changes in brain function to individual differences in learning trajectories (Goswami, 2006). Executive control functions, and associated brain activity, have been tightly linked to IQ differences in adults. For example, previous neuroimaging studies have reported that adults with higher fluid intelligence show stronger recruitment of the lat-PFC and dACC during executive control performance (Duncan, 2003, Gray et al., 2003).
Most developmental neuroimaging studies have not taken into account individual differences in IQ. Two sets of studies, focusing on functional and structural brain development, provide some insight into the developmental differences in relation to IQ. First, one functional neuroimaging studies compared math-gifted adolescents with a control group. The math-gifted group was tested for quantitative reasoning ability using the School and College Ability Test. These scores were converted to IQ scores, and their scores were in or above the 98th percentile. The control group had quantitative reasoning ability converted to IQ scores in the range 45–55th percentile. The math-gifted adolescents showed higher activation and higher effective connectivity in dorsolateral PFC, dACC and superior parietal cortex when performing mental rotation of complex 3D figures compared to a control group (O’Boyle et al., 2005, Prescott et al., 2010). Preusse et al. (2011), however, observed a different pattern. They showed that adolescents with high fluid intelligence, as measured with the Raven Advanced Progressive Matrices task, had stronger activation in parietal cortex when performing a geometric analogical reasoning task compared to adolescents with average fluid intelligence, whereas adolescents with average fluid intelligence had stronger activation in the dACC during this task compared to adolescents with high fluid intelligence. In sum, the findings of studies focusing on individual differences in IQ are still inconclusive, which could partly be due to the differences in measures of IQ (crystallized vs. fluid) that have been used in prior studies.
Second, structural neuroimaging studies have mapped cortical brain structures with respect to IQ in developing children and adolescents. These studies have found using longitudinal measures that there was a shift from predominantly negative relations between IQ and cortical thickness in children to positive correlations in adults, indicating that adolescence is an important transition phase. Specifically, IQ was positively associated with higher plasticity with a prolonged phase of cortical increase in adolescence. Together, these findings were interpreted in terms of a dynamic neuroanatomical expression of intelligence (Shaw et al., 2006, see also Karama et al., 2011, Pangelinan et al., 2011). Here, we test the hypothesis that the prolonged window of brain development for adolescents with higher IQ in comparison with adolescents with lower IQ is also observed in functional brain development.
1.3. The present study
The goal of this study was therefore to examine individual differences in brain function during performance monitoring in relation to IQ and level of education. In the Netherlands, adolescents participate in different school systems after the age of 12–13, based on scores on cognitive tasks that are completed at the end of elementary school (age 11 or 12). These school systems can be broadly divided in pre-vocational and pre-university education. Adolescents were recruited from these two school systems in order to sample a wide range of IQs and to further explore differences between levels of education. Given that the sampling from different school systems was a tool to recruit varying IQ levels, we did not have specific hypotheses concerning differences in education systems. Yet, we found it important to specifically test for this by comparing both differences related to IQ and differences related to school systems.
Participants from pre-university education and pre-vocational education completed a probabilistic learning task adopted from Frank et al. (2004; see also van den Bos et al., 2009). This task was chosen because it allows for a direct comparison with the literature where the developmental pattern has already been investigated (van den Bos et al., 2009). The task required the participants to learn a stimulus–response rules for two sets of stimuli, for which one stimulus–response set (containing stimuli A and B) was associated with an 80–20% and the second stimulus–response set (containing stimuli C and D) with a 70–30% reward mapping (see Fig. 1). During the task, functional neuroimaging data were acquired and the analyses focused on the processing of positive and negative feedback for high probability (80% and 70%, stimuli A and C) and low probability (20% or 30%, stimuli B and D) feedback.
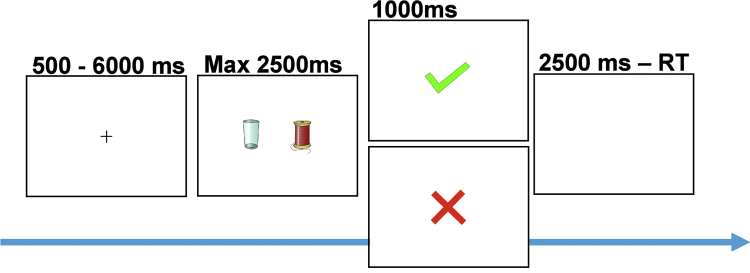
Fig. 1.
At the beginning of each trial a centrally located cue was presented with a jittered interval between 500 and 6000 ms, followed by a combined presentation of a stimulus pair and a response window of max. 2500 ms, after which feedback was presented for 1000 ms. After the feedback, a short filler was presented, in the form of a blank screen, in order to compensate for different reaction times between trials and between participants (filler duration = 2500 ms − reaction time). Participants chose one stimulus by pressing the left or right button and received positive or negative feedback according to probabilistic rules. Two pairs of stimuli were presented to the participants: (1) the AB pair with 80% positive feedback for A and 20% for B and (2) the CD pair with 70% positive feedback for C and 30% for D.
Based on prior studies, we expected adolescents from pre-vocational education to have lower estimated IQ scores and to have a slower learning curve in the probabilistic learning task, relative to adolescents from pre-university education. The prior imaging results of the developmental study using this task showed developmental differences in feedback processing in the lat PFC and the ACC, which will therefore be specific regions of interest in the current study. Using a probabilistic learning task, van den Bos et al. (2009) found that when participants explore a rule with low probability for reward, these rewards lead to more activation in lat-PFC and dACC in children and adolescents relative to adults. In contrast, this difference was not observed for reward trials when applying the rule with high probability for reward.
Under the assumption that adolescents with higher IQ (from pre-university education) relative to adolescents with lower IQ (from pre-vocational education) have a prolonged developmental trajectory of prefrontal cortex maturation (Preusse et al., 2011, Shaw et al., 2006, Karama et al., 2011), we predicted that high IQ adolescents would show an elevated response to positive feedback to the low probability reward rule.
In addition, we performed a voxel based morphometry (VBM) analyses in order to investigate the relation between IQ and cortical grey matter volumes. Based on previous studies we expected that IQ would be positively correlated with cortical grey matter volume (Shaw et al., 2006, Karama et al., 2011, Pangelinan et al., 2011), and we tested whether these differences could account for the differences in functional activation.
2. Methods
2.1. Participants
Forty-five healthy right-handed adolescents between ages 13 and 16 years (M age = 14.39; 22 female, 23 male) participated in the study. Participants were recruited from the community and with help of various local schools. Participants came from two categories based on the Dutch education system, where children are separated in different school systems based on test scores at the end of elementary school (approximately age 11–12). Pre-vocational education educates children for 4 years and prepares them for a working environment. Pre-university education educates children for 5–6 years and prepares them for further university education. In the current sample, 18 participants attended pre-vocational schools (M age = 14.5; 9 female, 9 male), and 27 participants attended pre-university schools (M age = 14.3; 13 female, 14 male). Part of the data was previously reported in a study on age comparisons (van den Bos et al., 2009).
Chi square analysis confirmed that the gender distribution was similar across the two groups (χ2(1) = .056, p = .82), and groups did not differ in mean age (t(44) = .541, p = .59). All participants reported normal or corrected-to-normal vision and participants or their caregivers reported the absence of specific learning disorder and neurological or psychiatric impairments. Parents filled out the Child Behavior Check List (CBCL, Achenbach, 1991) in order to screen for psychiatric conditions. All participants scored below clinical levels on all subscales of the CBCL, and had scores within 1 SD of the mean of a normative standardized sample. Participants and their caregivers gave informed consent for the study and all procedures were approved by the medical ethical committee of the Leiden University Medical Center. In accordance with Leiden University Medical Center policy, all anatomical scans were reviewed and cleared by the radiology department following each scan. No anomalous findings were reported.
2.2. Behavioral assessment of estimated IQ
Participants completed a verbal and non-verbal measure (similarities and block design subscales) of the Wechsler Intelligence Scale for Children (WISC) in order to obtain an estimate of their intelligence quotient (Wechsler, 1991, Wechsler, 1997). In the current sample, there was a high correlation between the estimated IQ on the two subscales (r = .87), therefore the estimated IQ score in subsequent analyses is the averaged IQ score of the two subscales.2 The minimum IQ score was 70 and the maximum IQ score was 130. As predicted, the pre-university group had higher IQ scores than the pre-vocational group, t(44) = 4.89, p < .001 (see Table 1).
Table 1.
Group statistics on IQ, reaction times (RTs), age and head motion. Groups are based on educational level, and subgroups are defined by educational level and IQ. Standard errors are presented in parentheses.
| IQ | Reaction times in ms | Head motion average in mm | Head motion max in mm | Age | |
|---|---|---|---|---|---|
| Pre-vocational education | 91.3 (2.4) | 805 (47) | .09 (.02) | 2.01 | 14.5 (.2) |
| Pre-university education | 107 (2.0) | 773 (39) | .08 (.01) | 2.21 | 14.3 (.2) |
| Subgroups | |||||
| I (IQ 70–90) | 80 (2.3) | 818 (74) | .11 (.02) | 2.01 | 15 (.4) |
| IIa (IQ 90–110) | 98.6 (1.3) | 799 (36) | .07 (.01) | 2.0 | 14.1 (.3) |
| IIb (IQ 90–110) | 99.3 (1.8) | 779 (41) | .08 (.01) | 2.21 | 14.6 (.4) |
| III (IQ 110–130) | 115.6 (2.6) | 764 (38) | .07 (.02) | .81 | 14.6 (.4) |
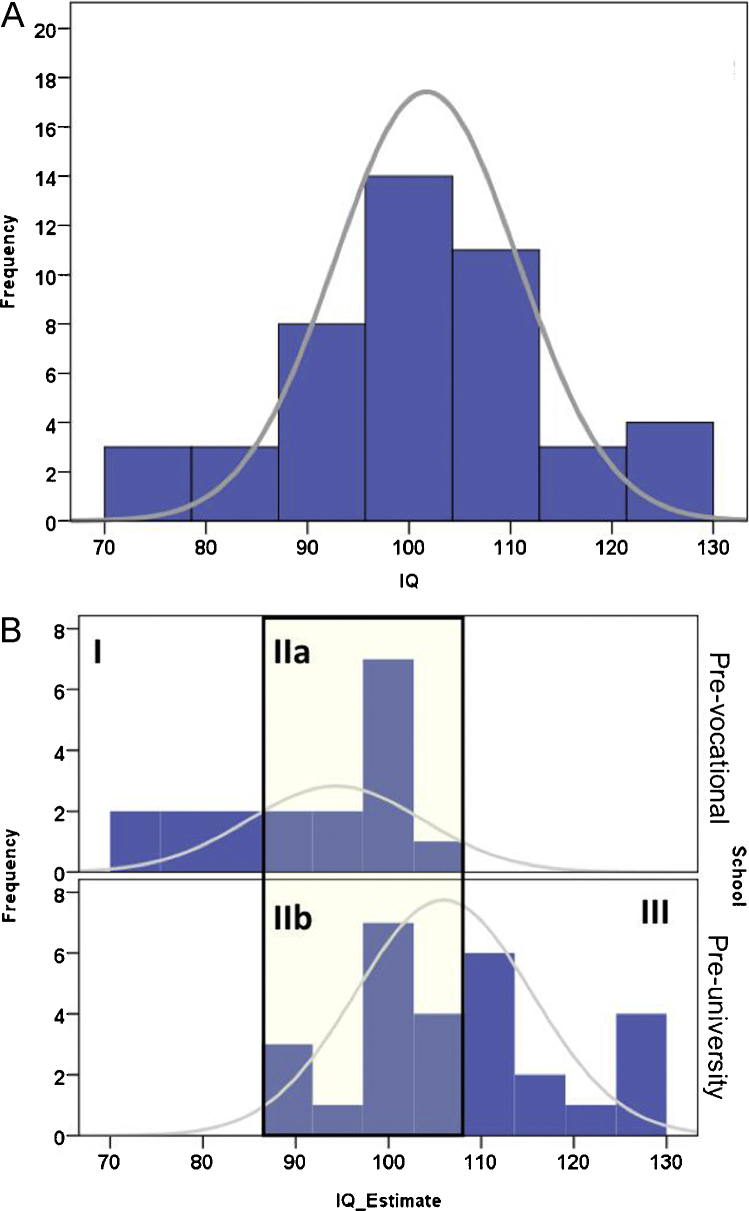
The scores were analyzed in more detail to examine how the estimated IQ scores related to school types. As can be seen in Fig. 2A, the estimated IQ scores are normally distributed. Fig. 2B shows that all adolescents who attend pre-vocational schools have IQ scores that fall in bins 70–110, and all adolescents from the pre-university schools have IQ scores that fall in bins 90–130. Thus, as expected, our IQ estimation is strongly related to educational level. Interestingly, the distribution of the IQ scores allows for a second type of categorization. That is, within the 90–110 bins there are participants who differ in school-level but have overlapping IQ estimations. This pattern allows us to disentangle the effects of school differences vs. IQ differences, by subdividing the participants in four groups; Low IQ/pre-vocational (n = 7; Group I; IQ[70–90]), Medium IQ/pre-vocational (n = 12; Group IIa; IQ[90–110]), Medium IQ/pre-university (n = 12; Group IIb; IQ[90–110]) and High IQ/pre-university (n = 14; Group III; IQ[110–130]) (see Fig. 2 and Table 1).
Fig. 2.
(A and B) IQ distribution for the pre-vocational and pre-university participants. These groups were further broken down to: a low IQ group (I; IQ 70–90), two average IQ groups (IIa and IIb; IQ 90–110) and a high IQ group (III; IQ 110–130).
2.3. Task procedure: probabilistic learning task
The procedure for the probabilistic learning task (Fig. 1; based on Frank et al., 2004, van den Bos et al., 2009) was as follows: The task consisted of two stimulus pairs (called AB and CD). The stimulus pairs consisted of pictures of everyday objects (e.g., a chair and a clock). Each trial started with the display of one of the two stimulus pairs and subsequently the participant had to choose one of the two stimuli (e.g., A or B), which were presented on the left or the right side of the screen. The stimulus pairs were presented in random order. Participants were instructed to choose either the left or the right stimulus by pressing a button with the index or middle finger of the right hand within a 2500 ms window, which was followed by a 1000ms feedback display. The feedback display consisted of a green ✓-signal for positive feedback and a red cross (×) for negative feedback. If no response was given within 2500 ms, the text “too slow” was presented on the screen. This occurred on less than 4% of the trials. Importantly, the school types did not differ in number of ‘too slow’ responses (t(43) = −.25, p = .79).
The feedback displayed was probabilistic. Choosing stimulus A led to positive feedback on 80% of AB trials, whereas choosing stimulus B led to positive feedback on 20% of these trials. The CD pair procedure was similar, but probability for reward was lower; choosing stimulus C led to positive feedback on 70% of CD trials, whereas choosing stimulus D led to positive feedback on 30% in these trials. Thus, the most optimal choice in order to obtain rewards was A or C (correct rule), whereas the least optimal choice was B or D (alternative rule).
Participants were instructed to earn as many points as possible (as indicated by receiving a positive feedback signal), but were also informed that it would not be possible to receive positive feedback on every trial. Further, participants were informed that although stimuli sometimes appeared on the right side and sometimes on the left side, this was an irrelevant dimension. After the instructions and right before the scanning session, the participants played 40 practice rounds on a computer in a quiet room to ensure that they understood the task.
In total, the task in the scanner consisted of two blocks of 100 trials each: 50 AB trials and 50 CD trials per block. The first and the second block consisted of different sets of pictures and therefore, participants had to learn a new mapping in both task blocks. The duration of each block was approximately 8.5 min. The stimuli were presented in pseudo-random order with a jittered interstimulus interval (min = 1000 ms, max = 6000 ms) optimized with OptSeq2 (surfer.nmr.mgh.harvard.edu/optseq/; Dale, 1999). In order to ensure that all blocks were completely independent, the stimuli were different in the practice block and the two experimental blocks. During inter-trial intervals, a central fixation cross was shown. Because previous studies revealed that behavioral strategies were dependent on rule type (correct or alternative rule), we differentiated between over-learned high probabilities (A and C trials collapsed, correct rule) and alternative low probabilities (B and D trials collapsed, alternative rule, cf. van den Bos et al., 2009) in the behavioral and imaging analyses.
2.4. Data acquisition
Participants were familiarized with the scanner environment on the day of the fMRI session through the use of a mock scanner, which simulated the sounds and environment of a real MRI scanner. Data were acquired using a 3.0T Philips Achieva scanner at the Leiden University Medical Center. Stimuli were projected onto a screen located at the head of the scanner bore and viewed by participants by means of a mirror mounted to the head coil assembly. First, a localizer scan was obtained for each participant. Subsequently, T2*-weighted Echo-Planar Images (EPI) (TR = 2.2 s, TE = 30 ms, 80 × 80 matrix, FOV = 220, 35 2.75 mm transverse slices with 0.28 mm gap) were obtained during 2 functional runs of 232 volumes each. The first two scans were discarded to allow for equilibration of T1 saturation effects. A high-resolution T1-weighted anatomical scan and a high resolution T2-weighted matched-bandwidth high-resolution anatomical scan, with the same slice prescription as the EPIs, were obtained from each participant after the functional runs. Stimulus presentation and the timing of all stimuli and response events were acquired using E-Prime software. Head motion was restricted by using a pillow and foam inserts that surrounded the head.
2.5. fMRI data analysis
Data were preprocessed using SPM5 (Wellcome Department of Cognitive Neurology, London). The functional time series were realigned to compensate for small head movements. Translational movement parameters never exceeded 1 voxel (<3 mm) in any direction for any subject or scan. There were no significant differences in movement parameters between the groups F(2, 45) = .89, p = .32 (see Table 1). Functional volumes were spatially smoothed using an 8 mm full-width half-maximum Gaussian kernel. Functional volumes were spatially normalized to EPI templates. The normalization algorithm used a 12-parameter affine transformation together with a nonlinear transformation involving cosine basis functions and resampled the volumes to 3 mm cubic voxels. The MNI305 template was used for visualization and all results are reported in the MNI305 stereotaxic space (Cosoco et al., 1997).
Statistical analyses were performed on individual participants’ data using the general linear model in SPM5. The fMRI time series data were modeled by a series of events convolved with a canonical haemodynamic response function (HRF). The presentation of the feedback screen was modeled as 0-duration events. The stimuli and responses were not modeled separately as these occurred in one prior or overlapping EPI images as feedback presentation.
In the model, feedback was further subdivided into correct (A and C) vs. alternative (B and D) rule and positive vs. negative feedback. These trial functions were used as covariates in a general linear model, along with a basic set of cosine functions that high-pass filtered the data, and a covariate for run effects. The least-squares parameter estimates of height of the best-fitting canonical HRF for each condition were used in pair-wise contrasts. The resulting contrast images, computed on a participant-by-participant basis, were submitted to group analyses. At the group level, contrasts between conditions were computed by performing one-tailed t-tests on these images, treating participants as a random effect.
We performed regression analyses with IQ as a covariate of interest to identify regions that showed IQ related differences in feedback processing. Subsequently we performed ROI analyses to further investigate the effects of school differences vs. IQ differences, using the four sub-groups described above.
2.6. Region-of-interest analyses
We used the Marsbar toolbox with SPM5 (http://marsbar.sourceforge.net; Brett et al., 2002) to perform Region of Interest (ROI) analyses to further characterize IQ and school type differences in patterns of activation and grey matter volume. We created ROIs of the regions that were identified in the whole brain analyses for illustration purposes or post hoc tests. The masks used to generate functional ROIs was based on the regression analyses with IQ as covariate across all participants (p < .001, >10 voxels).
For ROI analyses we focused on two a priori defined regions (right DLPFC and dACC), as a result, effects were considered significant at an α of .025, based on Bonferroni correction for multiple comparisons, p = .05/2, unless reported otherwise. These regions were chosen a priori based on our findings in our prior developmental studies (van den Bos et al., 2009).
2.7. Voxel based morphometry
To estimate the grey and white matter tissue volumes for each participant we used the SPM5 VBM5 toolbox (v1.15; http://dbm.neuro.uni-jena.de/vbm/). Two participants were excluded because of the poor quality of their structural T1 images. After each participant's structural T1 image was normalized to the MNI template, images were segmented into separate maps for cerebral spinal fluid, grey and white matter. Although the participants were not of adult age, segmentation was performed with use of prior probability maps because this increased the quality of the segmentation process. Additionally, the hand-selected anterior commissure of all brains was used as the origin for the registration and segmentation analyses. Furthermore, modulation for non-linear warping only was performed using the Jacobian determinants in order to adjust volume estimations for overall head size (O’Brien et al., 2006). Finally, images were resampled into 3 × 3 × 3 voxels and smoothed using an 8 mm full-width half-maximum Gaussian kernel, making the volume masks comparable to the functional activation maps.
3. Results
3.1. Behavior
3.1.1. Task performance and educational level
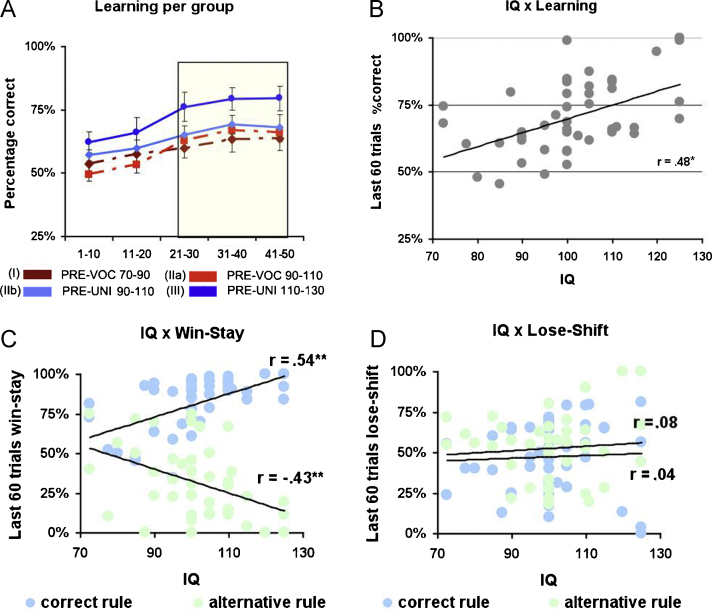
To investigate the differences in task performance for the different levels of education we calculated the percentage of correct choices (the high probability stimulus) per block of 20 trials for each participant, resulting in five blocks in total (Fig. 3A). Because the two runs in the scanner consisted of new stimulus pairs, the blocks of the two runs were collapsed.
Fig. 3.
(A) Learning curve showing average accuracy per 10 trial block for each of the four groups (I, IIa, IIb and III). (B) Correlation between IQ and accuracy on the last 60 trials of the task. (C) Correlation between IQ and percentage of win-stay choices per rule type. (D) Correlation between IQ and percentage of lose-shift choices per rule type.
To investigate whether the participants learned over time, we performed a repeated-measure ANOVA with task block as within-subjects variable and school level as between-subjects variable. The task block (5 blocks) × school type (2 types: pre-vocational vs. pre-university) ANOVA showed that participants learned to make more correct choices over time, as indicated by a main effect of task block (F(4,176) = 27.12, p < .001). However, there were no performance differences between school types (F(1, 44) = 3.34, p = .08), and there was no school type × task block interaction (F(4, 176) = .78, p = .98).
The analyses of performance further focused on the last 60 trails. In these last 60 trials the participants have acquired substantial knowledge of the probabilities associated with the different stimuli. Indeed, participants no longer showed differences in learning (main effect of task block, F(2, 88) = 2.75, p = .10). The task block (last 3 blocks) × school type (2 types: pre-vocational vs. pre-university) ANOVA showed that pre-university adolescents had higher accuracies than pre-vocational adolescents (main effect school type: F(1, 44) = 3.76, p < .05), but no interaction between task block and school type (p > .30).
To further investigate the differences between educational level and to dissociate effects of IQ and school type, we compared the two groups (IIa and IIb) from different educational levels but with the same level of IQ. The task block (3) × school type (IIa and IIb) revealed no significant effects (all p's > .2), suggesting that the performance differences between educational levels are mainly driven by IQ differences. As expected, correlation analyses with general performance revealed a positive relation between IQ and the number of correct choices in the last 60 trials (r = .48, p < .001, see Fig. 3B).
3.1.2. Behavioral strategy and IQ
To further examine IQ-related differences in task performance we explored the relation between IQ and behavioral strategies in the last 60 trials. For this analysis, we examined how often participants chose either the same stimulus after positive feedback (win-stay) or the shifted to the other stimulus after negative feedback (lose-shift).
There were IQ related differences in behavioral strategies following positive feedback; these analyses revealed a positive correlation between win-stay choices and IQ for the correct rule (r = .54, p < . 001), and a negative correlation between win-stay choices and IQ for the alternative rule (r = −.43, p < .001, see Fig. 3C). Thus, higher IQ was related to a more optimal shifting behavior after receiving positive feedback (it is optimal to stay with the correct rule and shift with the alternative rule). In contrast, there was no significant relation between IQ and lose-shift behavior (see Fig. 3D).
3.2. fMRI results
In the fMRI analyses we focused on patterns of activation in the last 60 trials of the task in order to be able to investigate the neural correlates of feedback processing for the different rule types and to make a direct comparison with the developmental literature (see also van den Bos et al., 2009).
3.2.1. Effects of feedback and rule type
First, we examined the brain regions involved in feedback processing across all participants. The comparison [positive feedback > negative feedback] resulted in activation in several regions, including the ventral striatum, VMPFC, bilateral DLPFC and the parietal cortex (see Table 2). The opposite contrast [negative feedback > positive feedback] resulted in activation in the bilateral insula and the dorsal ACC (see Table 2).
Table 2.
Brain regions revealed by whole brain contrasts. MNI coordinators for main effects, peak voxels reported at p < .001, uncorrected, at least 10 contiguous voxels.
| Anatomical region | L/R | Z | MNI coordinates |
||
|---|---|---|---|---|---|
| x | y | z | |||
| Positive > negative | |||||
| Striatum | L/R | 7.49 | 12 | 15 | −3 |
| Dorsolateral prefrontal cortex | R | 4.61 | 46 | 23 | 31 |
| Superior parietal cortex | R | 4.54 | 41 | −58 | 53 |
| Ventral medial PFC | L/R | 5.07 | 0 | 51 | −12 |
| Visual cortex | L/R | 4.60 | 31 | −93 | −7 |
| Negative > positive | |||||
| Dorsal anterior cingulate cortex | L/R | 4.43 | 6 | 21 | 31 |
| Anterior insula | L | 4.78 | 40 | 23 | 2 |
| R | 4.60 | −41 | 17 | −1 | |
| Positive alternative > positive correct | |||||
| Ventral striatum | L/R | 4.43 | −14 | 8 | −4 |
| Regression IQ [positive alternative > positive correct] | |||||
| Dorsal anterior cingulate cortex | L/R | 5.03 | −3 | 24 | 35 |
| Dorsolateral prefrontal cortex | R | 5.71 | 45 | 21 | 26 |
| Superior parietal cortex | R | 4.23 | 37 | −59 | 50 |
| Superior frontal gyrus | R | 4.11 | 19 | 58 | 21 |
| Middle occiptial gyrus | R | 4.16 | 24 | −78 | 15 |
Next, we investigated how feedback was modulated by rule type. Two rule types were distinguished: the correct rule (cor) and the alternative rule (alt). The comparison [positive feedback (alt) > positive feedback (cor)] resulted in activation in the ventral striatum (see Table 2), whereas the opposite contrast did not yield any significant effects. Analyses of the effect of rule type on negative feedback processing did not yield any significant results.
Thus, in the current study, the DLPFC was involved in processing positive feedback and the dACC in processing negative feedback. The striatum was particularly sensitive to positive feedback when it was least expected (i.e., a positive prediction error).
3.2.2. Effects of IQ on feedback processing
To examine how feedback processing was modulated by IQ differences, we first identified individual differences in neural activation of feedback processing by performing a regression analysis on the [positive feedback vs. negative feedback] contrast with estimated IQ as a predictor variable. This analysis did not yield any significant results.
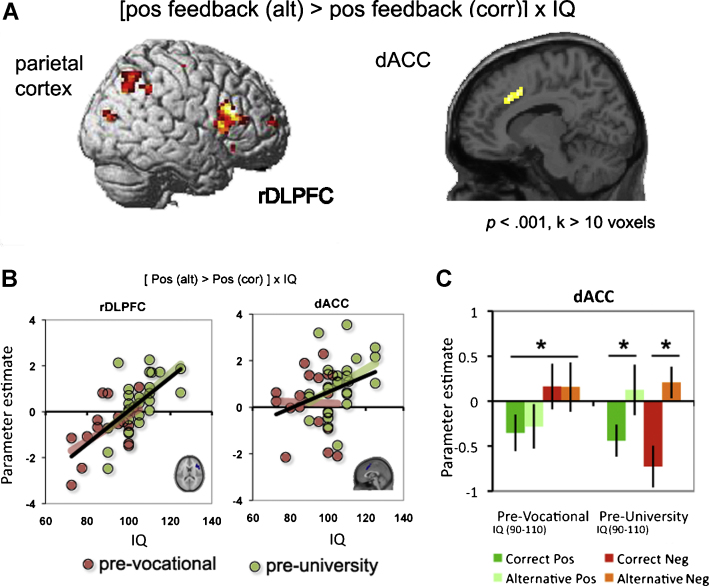
Second, we performed a regression analysis for each feedback type in the context of the correct and alternative rules. Examining feedback in the context of correct vs. alternative rules was previously found to be most powerful in dissociating effects for positive and negative feedback trials separately (van den Bos et al., 2009). The regression analysis on the [positive feedback (alt) > positive feedback (cor)] contrast revealed a positive correlation between estimated IQ and BOLD activity in regions previously implicated in performance monitoring, including the right DLPFC and the dACC (see Fig. 4A, Table 2). As can be seen in Fig. 4B, higher IQ was associated with more activity in right DLPFC and dACC following positive feedback after selecting the alternative rule compared to the correct rule. A regression analysis with negative feedback trials (alternative vs. correct rule) did not reveal regions that correlated with IQ and brain activation.
Fig. 4.
(A) Showing the parietal cortex, rDLPFC and dACC regions that were identified by the functional regression analyses with IQ, threshold at p < .001, k > 10 voxels. (B) Scatterplots showing the relation between IQ and parameter estimates for the [positive feedback (alternative) − positive feedback (correct)] contrast in the rDLPFC and dACC. Red colored dots and line represent the pre-vocational sub-group and the green dots and line represent the pre-university subgroup. (C) Graphs showing the differences in patterns of feedback related BOLD response in the dACC for the two different educational level (pre-vocational and pre-university) subgroups with similar IQ (IIa and IIb). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
To further explore the relation between IQ, level of education and activity in right DLPFC and dACC, we performed separate correlations between IQ and brain activity for each school group (see Fig. 4B; colored dots), based on ROIs which were derived from the whole-brain contrast [positive feedback (alt) > positive feedback (cor)] with IQ as regressor. These analyses showed that DPLFC activity was related to IQ in both the pre-vocational and the pre-university group (r = .54, p < .02 and r = .59, p < .01, respectively). However, for dACC, the correlation with IQ was only significant for the pre-university group (r = .44, p < .02), and not for the pre-vocational group (r = −.02, p = .93). These findings suggest that activity in dACC is possibly related to both IQ and educational level.
3.2.3. Educational level related differences in neural activity
Next, we explored whether neural activation patterns were specifically related to school type rather than individual differences in IQ. For these analyses we investigated the patterns of neural activity in the same ROIs (rDLPFC and dACC) but now for specific subgroups and for all feedback conditions. First, we ran 2 × 2 × 2 [school × valence × rule] ANOVAs for the two subgroups of selected adolescents with similar IQ but from different school types (groups IIa and IIb). The ANOVA resulted in a significant interaction between school type, valence and rule in the dACC (F(1,23) = 5.14, p < .01), but not in the rDLPFC (F(1,23) < 1.0, p = .32). Further analyses revealed that, when comparing the two school types of similar IQ, different feedback responses emerged. Specifically, in the pre-vocational group the dACC was valence sensitive such that activity was higher for negative feedback than for positive feedback (F(1,11) = 4.84, p < .02) but did not differentiate between rule types (F(1,11) < 1.0, p = .58). In contrast, in the pre-university group, the dACC was sensitive to rule type such that activity was higher following feedback from the alternative rule compared to the correct rule (F(1,11) = 7.23, p < .01) but not to valence (F(1,11) < 1.0, p = .41, see Fig. 4C).3 Taken together, activity following positive feedback in rDLPFC correlated with IQ, independent of the school from which the participants were recruited. Activity in the dACC also correlated with IQ, but seemed to have a slightly different activation pattern for the two school types.
3.3. Voxel based morphometry
VBM analyses yielded significant correlations between IQ and the estimated grey matter volume in several regions across the whole brain (see Supplementary Fig. S1). Most cortical regions showed a positive correlation between IQ and grey matter volume. This relation indicates that the children with a higher IQ have larger cortical grey matter volume than those with a lower IQ. A negative correlation between IQ and grey matter was found in bilateral hippocampus/amygdala, left ventral striatum, bilateral cerebellum, and left temporal pole. Thus, these structures have a higher grey matter volume for participants with a lower IQ compared to those with a higher IQ.
As can be seen in Fig. 5, the cortical brain areas for which the grey matter volume positively correlated with IQ overlap with the two functionally defined ROIs (dACC and rDLPFC). To further investigate the relation between IQ and grey matter volume in these ROIs, we extracted the regional estimated grey matter volume for each participant and correlated this with IQ level (see Fig. 5).
Fig. 5.
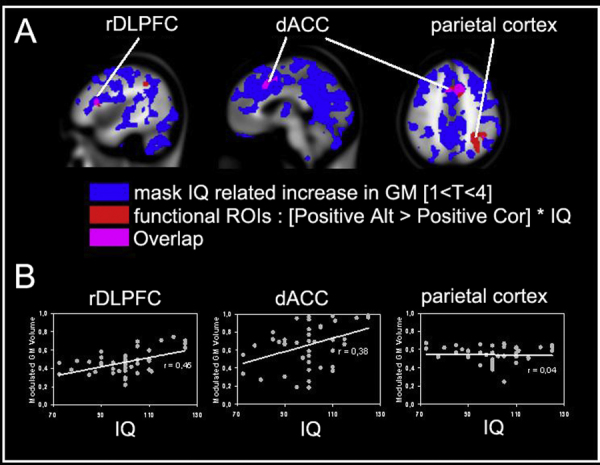
(A) Cortical map showing: (1) a blue colored mask that identifies all cortical areas that showed a significant correlation between grey matter volume and IQ, (2) a red colored mask showing rDPLFC, dACC and parietal cortex from the functional regression analyses, and (3) a purple mask showing the overlap between masks 1 and 2. (B) Representation of the correlation between IQ and average grey matter volumes for the three regions of interest. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
Given the IQ-related grey matter differences, we tested whether functional activation differences could be explained by local changes in grey matter volume using BPM. These analyses show that although there are IQ related differences in cortical gray matter, these do not explain the IQ related changes in functional activity (see Fig. S2).
4. Discussion
The goal of this study was to examine individual differences in performance monitoring in adolescents in relation to varying IQ scores and different educational backgrounds. The correlations between neural activity and IQ, and the comparisons of adolescents from pre-vocational and pre-university education resulted in a set of behavioral and neural differences. Behavioral analyses resulted in two important patterns: (1) IQ was positively correlated with overall accuracy on the last 60 trials of the task, and (2) these IQ related changes in learning were due to differences in shifting strategy in relation to positive, but not negative feedback. Specifically, the differences in shifting strategies showed that participants with a higher IQ were more likely to stay with the same rule after positive feedback when selecting the correct rule, but were more likely to switch away after positive feedback when selecting the alternative rule. Both are optimal shifting strategies and thus they contribute to the increased accuracy levels. The IQ difference in win-stay decisions in context of the correct rule are comparable to the age related increase in win-stay decisions that was reported in a prior developmental study with the same task (van den Bos et al., 2009).
4.1. Brain function in relation to IQ and education
Based on the results from previous functional and structural imaging studies we examined whether adolescents with higher IQ have a pattern of neural activation that allows for greater flexibility for learning (Shaw et al., 2006). Based on previous studies (van den Bos et al., 2009, Van Duijvenvoorde et al., 2008) differences were expected to occur in the key areas of the performance monitoring network: the right DLPFC and the dACC.
4.1.1. Right DLPFC
The results showed that for those participants with higher IQ there was more activity in the rDLPFC when receiving positive feedback after selecting the alternative vs. the correct rule. The pattern of activity for the higher IQ participants is most similar to that of 10–12-year-old children in a previous developmental study (see van den Bos et al., 2009). In addition, higher activation in DLPFC for positive feedback was also reported for younger (8–9 years) children relative to older children (11–13 years) and adults (18–25 years) in a different study on feedback-based rule learning (Van Duijvenvoorde et al., 2008). In that sense, the high IQ adolescents showed a pattern of activation that was tuned towards positive feedback, similar to younger children in prior studies. This comparison with prior studies suggests that the current results support a prolonged, rather than advanced maturation hypothesis of IQ related brain changes (see also Shaw et al., 2006). In adolescence, individuals with higher IQ may be more focused on exploring alternative actions.
Prior research suggests that developmental differences in positive and negative feedback adjustment are the result of differences in attention regulation (Somsen, 2007). Following this hypothesis, the current results suggest that the individual differences in IQ are related to a focus on different information value of positive feedback signals. Whereas adolescents with a higher IQ focus on positive feedback when exploring the alternative rule (thus the feedback containing informative value for exploration), the adolescents with a lower IQ seem to focus on positive feedback that confirms the already learned correct rule. Possibly, the adolescents with higher IQ were better at differentiating between positive feedback that is expected and positive feedback that is unexpected. An increased attention system for unexpected positive feedback can be advantageous when exploring alternative actions. The adolescents with higher IQ were also better able to switch following positive feedback when choosing the alternative rule. As a result, the adolescents with a lower IQ may be less able to update the relevant feedback information from alternative rules, and therefore they are possibly less flexible in selecting alternative actions.
4.1.2. dACC
Similar to the rDLPFC, the dACC also showed an IQ related increase in activity related to positive feedback after selecting the alternative rule. Again, this pattern of activation is similar to the developmental pattern reported previously (van den Bos et al., 2009, Van Duijvenvoorde et al., 2008), supporting the delayed, or explorative maturation hypothesis of IQ related brain changes. Interestingly, adolescents with higher IQ also showed an increase in activation following negative feedback when sampling the alternative rule. In previous studies, dACC activity has been related to a more general role in detecting conflict (Brown and Braver, 2005), and signaling the need for behavioral adjustment (Holroyd and Coles, 2008, Kerns et al., 2004, Rushworth, 2008). This suggests that the adolescents with a lower IQ are possibly less able to identify the relevant feedback information and they show an activation pattern in the dACC which is sensitive to negative feedback in general (also when it should be ignored), whereas the higher IQ adolescents only show dACC activity when there is need for behavioral adjustment (after selecting the alternative rule).
Exploratory analyses revealed that when comparing groups of similar IQ, but from different school systems, feedback related activity in the dACC was also differentially sensitive to the type/level of education. The exact reason for this difference is not yet understood but point in the direction of an influence of environmental factors, although it should be noted that the participants were all recruited from different schools. This suggests that the role of the school system on brain function and its development is an interesting avenue for future studies.
Finally, learning theories have suggested that two separate learning strategies contribute to feedback based learning (Daw et al., 2005, Maia, 2009): a model-based strategy that operates on explicit task representations, such as rules describing the reward contingencies given the current state (associated with DLPFC and dACC), and a model-free strategy that uses feedback directly to compute action values without any explicit model of the environment (associated with striatum and VMPFC). For the current study we decided to focus on the rule-based network given that the majority of the current literature on development of IQ is related to executive functioning (cf. van den Bos et al., 2009). However, a model-free reinforcement learning analysis of the same task suggests there are also important developmental changes in striatum-VMPFC connectivity that underlie differences in learning the reward contingencies (van den Bos et al., 2011). Although such analyses are beyond the scope of the current paper, the challenge for future studies will be to understand how differences in reinforcement learning mechanisms may contribute to IQ related changes in feedback learning.
4.2. Brain structure in relation to IQ and education
In the current study VBM analyses were performed, which revealed that grey matter volume was positively correlated with IQ in many parts of the cortex, including the functionally defined dACC and rDLPFC ROIs. Although several studies with adolescents have confirmed the positive relation between grey matter and intelligence (Shaw et al., 2006, Pangelinan et al., 2011), the exact relation between the quantity of grey matter and the quality of cognitive functions is still not well understood (Deary et al., 2010). Possibly, individuals with a higher IQ show a prolonged period of synaptic overproduction and pruning during adolescence, which is reflected by increased grey matter volumes (Shaw et al., 2006). In turn, this prolonged period of plasticity is thought to contribute to increased capacity for flexibility and learning (Johnston et al., 2009). The combined results of the functional and structural analyses in this study are in line with these hypotheses. First, both the rDLPFC and the dACC showed activation patterns that suggest that a high IQ is related to faster learning, increased flexibility and adaptive behavior. Second, the grey matter volume of these same areas correlated significantly with IQ. However, note that our analyses also revealed that grey matter volume by itself could not fully explain the changes in brain function. In future studies it will therefore be important to further investigate the relation between brain structure, function and IQ, for instance by incorporating structural connectivity analyses (Jung and Haier, 2007, Schmithorst et al., 2005).
4.3. Conclusions and implications for education
The current study has shown that whether higher intelligence is associated with advanced or less ‘mature’ patterns of brain activity depends on the specific component of the task being examined, as well as on the neural areas involved. We aimed to show that, rather than a static capacity difference, intelligence is related to the capacity of prefrontal areas to dynamically adapt to task demands and environmental contingencies. Therefore, our data support the hypothesis that the dynamic properties of these cortical areas are related to a prolonged developmental period of cortical plasticity associated with a higher IQ.
These results have potentially important implications for educational practice (Goswami, 2006, Blakemore, 2010). It has been shown that executive functions, even more than IQ (Blair and Razza, 2007), are strongly related to capacities deemed to be important in school learning such as numerical processing (Qin et al., 2004) and reading comprehension (Schlaggar and Church, 2009). Furthermore, a series of neuroimaging studies in adolescents and children have shown that these capacities rely on the same brain areas that are associated with executive functions (for a meta-analysis, see Houdé et al., 2010). A better understanding of the individual differences in functioning of the cognitive control network in relation to IQ and age period may contribute to a better understanding of how to develop specific training programs to improve school performance (Diamond et al., 2007).
Footnotes
The behavioral and fMRI analyses using each IQ measure separately yielded similar patterns of results to those using the combined score reported in this paper.
We have additionally performed the same set of analyses on the DLPFC and dACC ROIs that were extracted from the [POS-NEG] and [NEG-POS] contrasts (reported in Table 2). These analyses revealed the same results (all p's < .02) for both the effects of education and IQ.
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.dcn.2011.09.007.
Appendix A. Supplementary data
References
- Achenbach T.M. University of Vermont, Department of Psychiatry; Burlington, VT: 1991. Manual for the Child Behavior Checklist 4–18/and 1991 Profile. [Google Scholar]
- Blair C., Razza R.P. Relating effortful control, executive function, and false belief understanding to emerging math and literacy ability in kindergarten. Child Dev. 2007;78(2):647–663. doi: 10.1111/j.1467-8624.2007.01019.x. [DOI] [PubMed] [Google Scholar]
- Blakemore S.J. The developing social brain: implications for education. Neuron. 2010;65:744–747. doi: 10.1016/j.neuron.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett M.C., Anton J.L., Valabregue R., Poline J.B. Region of interest analysis using an spm toolbox. Neuroimage. 2002;16:497. [Google Scholar]
- Brown J.W., Braver T.S. Learned predictions of error likelihood in the anterior cingulate cortex. Science. 2005;307:1118–1121. doi: 10.1126/science.1105783. [DOI] [PubMed] [Google Scholar]
- Bunge S.A., Wright S.B. Neurodevelopmental changes in working memory and cognitive control. Curr. Opin. Neurobiol. 2007;17:243–250. doi: 10.1016/j.conb.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Cosoco C.A., Kollokian V., Kwan R.K.S., Evans A.C. Brainweb: online interface of a 3-D MRI simulated brain database. NeuroImage. 1997;5:425. [Google Scholar]
- Crone E.A. Executive functions in adolescence: inferences from brain and behavior. Dev. Sci. 2009;12:825–830. doi: 10.1111/j.1467-7687.2009.00918.x. [DOI] [PubMed] [Google Scholar]
- Crone E.A., Zanolie K., Van Leijenhorst L., Westenberg P.M., Rombouts S.A.R.B. Neural mechanisms supporting flexible performance adjustment during development. Cogn. Affect. Behav. Neurosci. 2008;2:165–177. doi: 10.3758/cabn.8.2.165. [DOI] [PubMed] [Google Scholar]
- Dale A.M. Optimal experimental design for event-related fMRI. Hum. Brain Mapp. 1999 doi: 10.1002/(SICI)1097-0193(1999)8:2/3<109::AID-HBM7>3.0.CO;2-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daw N.D., Niv Y., Dayan P. Uncertainty-based competition between prefrontal and dorsolateralstriatal systems for behavioral control. Nat. Neurosci. 2005;8:1704–1711. doi: 10.1038/nn1560. [DOI] [PubMed] [Google Scholar]
- Deary I.J., Penke L., Johnson W. The neuroscience of human intelligence differences. Nat. Rev. Neurosci. 2010;11:201–211. doi: 10.1038/nrn2793. [DOI] [PubMed] [Google Scholar]
- Diamond A., Barnett W.S. Preschool program improves cognitive control. Science. 2007;318:1387–1388. doi: 10.1126/science.1151148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumontheil I., Hassan B., Gilbert S., Blakemore S.J. Development of the selection and manipulation of self-generated thoughts in adolescence. J. Neurosci. 2010;30:7664–7671. doi: 10.1523/JNEUROSCI.1375-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J. Intelligence predicts brain responses to demanding tasks. Nat. Neurosci. 2003;6:207–208. doi: 10.1038/nn0303-207. [DOI] [PubMed] [Google Scholar]
- Eppinger B., Mock B., Kray J. Developmental differences in learning and error processing: evidence from ERPs. Psychophysiology. 2009;46:1043–1053. doi: 10.1111/j.1469-8986.2009.00838.x. [DOI] [PubMed] [Google Scholar]
- Frank M.J., Seeberger L.C., O’Reilly R.C. By carrot or by stick: cognitive reinforcement learning in parkinsonism. Science. 2004;306:1940–1943. doi: 10.1126/science.1102941. [DOI] [PubMed] [Google Scholar]
- Goswami U. Neuroscience and education: from research to practice? Nat. Neurosci. Rev. 2006;7:406–413. doi: 10.1038/nrn1907. [DOI] [PubMed] [Google Scholar]
- Gray J.R., Chabris C.F., Braver T.S. Neural mechanisms of general fluid intelligence. Nat. Neurosci. 2003;6:316–322. doi: 10.1038/nn1014. [DOI] [PubMed] [Google Scholar]
- Holroyd C.B., Coles M.G.H. The neural basis of human error processing: reinforcement learning, dopamine, and the error-related negativity. Psychol. Rev. 2002;109:679–709. doi: 10.1037/0033-295X.109.4.679. [DOI] [PubMed] [Google Scholar]
- Holroyd C.B., Coles M.G. Dorsal anterior cingulate cortex integrates reinforcement history to guide voluntary behavior. Cortex. 2008;44:548–559. doi: 10.1016/j.cortex.2007.08.013. [DOI] [PubMed] [Google Scholar]
- Holroyd C.B., Nieuwenhuis S., Yeung N., Nystrom L., Mars R.B., Coles M.G.H. Dorsal anterior cingulate cortex shows fMRI response to internal and external error signals. Nat. Neurosci. 2004;7:497–498. doi: 10.1038/nn1238. [DOI] [PubMed] [Google Scholar]
- Houdé O., Rossi S., Lubin A., Joliot M. Mapping numerical processing, reading, and executive functions in the developing brain: an fMRI meta-analysis of 52 studies including 842 children. Dev. Sci. 2010;13:876–885. doi: 10.1111/j.1467-7687.2009.00938.x. [DOI] [PubMed] [Google Scholar]
- Huizinga M., Dolan C.V., van der Molen M.W. Age-related change in executive function: developmental trends and a latent variable analysis. Neuropsychologia. 2006;44:2017–2036. doi: 10.1016/j.neuropsychologia.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Johnson M.H. Interactive specialization: a domain-general framework for human functional brain development. Dev. Cogn. Neurosci. 2011;1:7–21. doi: 10.1016/j.dcn.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston M.V., Ishida A., Ishida W.N. Plasticity and injury in the developing brain. Brain Dev. 2009;31:1–10. doi: 10.1016/j.braindev.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung R.E., Haier R.J. The parieto-frontal integration theory (P-FIT) of intelligence: converging neuroimaging evidence. Behav. Brain Sci. 2007;30:135–187. doi: 10.1017/S0140525X07001185. [DOI] [PubMed] [Google Scholar]
- Karama S., Colom R. Cortical thickness correlates of specific cognitive performance accounted for by the general factor of intelligence in healthy children aged 6 to 18. NeuroImage. 2011;55:1443–1453. doi: 10.1016/j.neuroimage.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerns J.G., Cohen J.D., MacDonald A.W., III, Cho R.Y., Stenger V.A., Carter C.S. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303:1023–1026. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- Koolschijn P.C., Schel M.A., De Rooij M., Rombouts S.A.R.B., Crone E.A. A 3-year longitudinal fMRI study on performance monitoring and test–retest reliability from childhood to early adulthood. J. Neurosci. 2011;31:4204–4212. doi: 10.1523/JNEUROSCI.6415-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maia T.V. Reinforcement learning, conditioning, and the brain: successes and challenges. Cogn. Affect. Behav. Neurosci. 2009;9:343–364. doi: 10.3758/CABN.9.4.343. [DOI] [PubMed] [Google Scholar]
- O’Boyle M.W., Cunningtond R., Silk T.J., Vaughan D., Jackson G., Syngeniotis A., Egan G.F. Mathematically gifted male adolescents activate a unique brain network during mental rotation. Cogn. Brain Res. 2005;25:583–587. doi: 10.1016/j.cogbrainres.2005.08.004. [DOI] [PubMed] [Google Scholar]
- O’Brien L.M., Ziegler D.A., Deutsch C.K., Kennedy D.N., Goldstein J.M., Seidman L.J., Hodge S., Makris N., Caviness V., Frazier J.A., Herbert M.R. Adjustment for whole brain and cranial size in volumetric brain studies: a review of common adjustment factors and statistical methods. Harv. Rev. Psych. 2006;14:141–151. doi: 10.1080/10673220600784119. [DOI] [PubMed] [Google Scholar]
- Pangelinan M.M., Zhang G., vanMeter J.W., Clark J.E., Hatfield B.D., Haufler A.J. Beyong age and gender: relationships between cortical and subcortical brain volume and cognitive-motor abilities in school-aged children. NeuroImage. 2011;54:3093–3100. doi: 10.1016/j.neuroimage.2010.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott J., Gavrilescu M., Cunningtond R., O’Boyle M.W., Egan G.F. Enhanced brain connectivity in math-gifted adolescents: an fMRI study using mental rotation. Cogn. Neurosci. 2010;iFirst:1–20. doi: 10.1080/17588928.2010.506951. [DOI] [PubMed] [Google Scholar]
- Preusse F., van der Meer E., Deshpande G., Krueger F., Wartenburger I. Fluid intelligence allows flexible recruitment of the parieto-frontal network in analogical reasoning. Front. Neurosci. 2011;5:1–14. doi: 10.3389/fnhum.2011.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Y., Carter C.S. The change of the brain activation patterns as children learn algebra equation solving. Proc. Natl. Acad. Sci. U.S.A. 2004;101:5686–5691. doi: 10.1073/pnas.0401227101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushworth M.F. Intention, choice and the medial frontal cortex. Ann. N. Y. Acad. Sci. 2008;1124:181–207. doi: 10.1196/annals.1440.014. [DOI] [PubMed] [Google Scholar]
- Schlaggar B.L., Church J.A. Functional neuroimaging insights into the development of skilled reading. Curr. Dir. Psychol. Sci. 2009;18:21–26. doi: 10.1111/j.1467-8721.2009.01599.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmithorst V.J., Wilke M., Dardzinski B.J., Holland S.K. Cognitive functions correlate with white matter architecture in a normal pediatric population: a diffusion tensor MRI study. Hum. Brain Mapp. 2005;26:139–147. doi: 10.1002/hbm.20149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P., Greenstein D., Lerch J., Clasen L., Lenroot R., Gogtay N., Evans A., Rapoport J., Giedd J. Intellectual ability and cortical development in children and adolescents. Nature. 2006;440:676–679. doi: 10.1038/nature04513. [DOI] [PubMed] [Google Scholar]
- Somsen R.J. The development of attention regulation in the wisconsin card sorting task. Dev. Sci. 2007;10:664–680. doi: 10.1111/j.1467-7687.2007.00613.x. [DOI] [PubMed] [Google Scholar]
- van den Bos W., Güroğlu B., van der Bulk B.G., Rombouts S.A.R.B., Crone E.A. Better than expected or as bad as you thought? The neurocognitive development of probabilistic feedback processing. Front. Neurosci. 2009;3:52. doi: 10.3389/neuro.09.052.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Bos W., Cohen M.X., Kahnt T., Crone E.A. Striatum-medial prefrontal cortex connectivity predicts developmental changes in reinforcement learning. Cerebral Cortex. 2011 doi: 10.1093/cercor/bhr198. (E-pub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Duijvenvoorde A., Zanolie K., Rombouts S.A.R.B., Raijmakers M., Crone E.A. Valuing the positive or adjusting the negative? A neurocognitive analysis of feedback-based learning. J. Neurosci. 2008;28:9495–9503. doi: 10.1523/JNEUROSCI.1485-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. The Psychological Corporation; San Antonio: 1991. Wechsler Intelligence Scale for Children—Third Edition. Manual. [Google Scholar]
- Wechsler D. The Psychological Corporation; San Antonio: 1997. Wechsler Adult Intelligence Scale—Third Edition. Administration and Scoring Manual. [Google Scholar]
- Zanolie K., Van Leijenhorst L., Rombouts S.A.R.B., Crone E.A. Separable neural mechanisms contribute to feedback processing in a rule-learning task. Neuropsychologia. 2008;46:117–126. doi: 10.1016/j.neuropsychologia.2007.08.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.