Highlights
* We investigated neural substrates supporting rule use in children and adults. * Functional MRI showed recruitment of frontal and parietal cognitive control regions. * These regions responded to both rule switching and representing difficult rules. * Children and adults engaged similar regions, but children updated rules more slowly. * Development may shift temporal dynamics in common networks for executive functions.
Keywords: Cognitive control, Executive function, Task-switching, Development, fMRI
Abstract
Flexible rule-guided behavior develops gradually, and requires the ability to remember the rules, switch between them as needed, and implement them in the face of competing information. Our goals for this study were twofold: first, to assess whether these components of rule-guided behavior are separable at the neural level, and second, to identify age-related differences in one or more component that could support the emergence of increasingly accurate and flexible rule use over development. We collected event-related fMRI data while 36 children aged 8–13 and adults aged 20–27 performed a task that manipulated rule representation, rule switching, and stimulus incongruency. Several regions – left dorsolateral prefrontal cortex (DLPFC), left posterior parietal cortex, and pre-supplementary motor area – were engaged by both the rule representation and the rule-switching manipulations. These regions were engaged similarly across age groups, though contrasting timecourses of activation in left DLPFC suggest that children updated task rules more slowly than did adults. These findings support the idea that common networks can contribute to a variety of executive functions, and that some developmental changes take the form of changes in temporal dynamics rather than qualitative changes in the network of brain regions engaged.
1. Introduction
From infancy through adolescence, children show remarkable developments in the ability to control their thoughts and actions. Their behaviors become less tied to sensory input and habitual responses, and increasingly driven by higher-order goals that can help to override more automatic responses (Diamond, 1985, Luna et al., 2004, Munakata et al., in press, Zelazo, 2004). Such cognitive control relies upon the integrity of prefrontal cortical regions and associated networks (Bunge and Wright, 2007, Crone, 2009, Casey et al., 2005, Diamond, 1990, Luna et al., 2010). Cognitive control comprises a number of functions, including the ability to represent appropriate rules for behavior in a variety of contexts, to flexibly switch between rules, and to deal with conflict when competing rules indicate different courses of action.
One factor in children's improvements in cognitive control is the ability to use increasingly higher-order task rules to select the appropriate action (Bunge and Zelazo, 2006, Jacques and Zelazo, 2005, Kharitonova and Munakata, 2011, Rougier et al., 2005, Snyder and Munakata, 2010). The prolonged developmental progression of rule-guided behavior has been hypothesized to reflect different rates of development among distinct prefrontal cortical regions (Bunge and Zelazo, 2006). According to this framework, infants and toddlers make use of stimulus-outcome rules (e.g., strawberries bring pleasure), which depend on orbitofrontal cortex, while older children can also use conditional rules (e.g., stop when a light is red, go when the light is green), which depend on ventrolateral and dorsolateral prefrontal regions (Bunge et al., 2005).
There is some evidence for a partial dissociation between the brain regions involved in representing complex conditional rules and in switching flexibly between these rules (Crone et al., 2006a, Crone et al., 2006b). In one previous study, rule representation was examined by contrasting bivalent rules, in which stimulus-response mappings depended on a current context, with univalent rules, in which stimulus-response mappings were fixed (Crone et al., 2006b). Rule representation and rule-switching both engaged a core set of brain regions, including lateral prefrontal cortex (lPFC), pre-supplementary motor area (pre-SMA), and posterior parietal cortex (PPC) (Crone et al., 2006b). However, there was also evidence for a partial dissociation between the regions involved in representing rules and those involved in switching flexibly between them. Specifically, pre-SMA demonstrated a greater effect of rule switching whereas lPFC demonstrated a greater effect of rule complexity. These two regions demonstrated different developmental trajectories, with delayed maturation of lPFC-mediated rule representation relative to pre-SMA mediated rule switching (Crone et al., 2006a).
An open question is whether this neural dissociation observed between rule representation and switching, and the prolonged developmental progression for rule representation, hold for other aspects of rule representation in addition to the complexity of the rule structure (Bunge and Zelazo, 2006). For example, task rules can also vary in terms of whether the stimulus-response mappings are arbitrary; pushing a left button for a green light and a right button for a red light (an arbitrary mapping) is more difficult than pushing a left button for a left-pointing arrow and pushing a right button for a right-pointing arrow (a non-arbitrary mapping). Rather than requiring the representation of higher-order rules, the more difficult rule in this case may require greater maintenance in working memory, in order to guide the less automatic response (Miller and Cohen, 2001).
Another open question concerns the temporal dynamics of brain regions that support rule-guided behavior, and how these dynamics may change with development. For example, developmental transitions have been observed in the temporal dynamics of cognitive control, with children transitioning from a reactive to a proactive form of cognitive control across development (Andrews-Hanna et al., 2011, Chatham et al., 2009, Finn et al., 2010). That is, children appear more likely to retrieve rule information reactively from long-term memory as needed, while adults are more likely to employ a proactive form of cognitive control, maintaining rules in working memory until they are needed (Munakata et al., in press).
To examine these issues regarding the neural underpinnings of rule-guided behavior and its development, we conducted an event-related fMRI study in school-aged children and young adults. We used the Nemo task (Baym et al., 2008), which requires participants to switch flexibly from one task rule to another, with one of these task rules involving an arbitrary response mapping while the other does not. The task involves three distinct manipulations: (1) Rule Type: a manipulation of rule representation, comparing arbitrary with non-arbitrary stimulus-response mappings, (2) Switching: whether the rule switches or repeats, and (3) Incongruency: whether a stimulus would elicit the same response or a different response depending on whether participants are required to make a judgment based on the color or the orientation of the stimulus.
This fMRI task design allowed us to address three key questions. First, do distinct networks support the ability to represent arbitrary rules, switch flexibly between rules, and implement rules in the face of distracting information? Second, are there differences between children and adults in the networks engaged for these various aspects of rule use? Third, are there differences between children and adults in the temporal dynamics of activation of brain regions involved in flexible rule use?
2. Materials and methods
2.1. Participants
Thirty-six healthy, native English-speaking, right-handed volunteers aged 8–13 (M = 10.56; 11 males, 9 females) and 20–27 (M = 22.44; 5 males, 11 females) were recruited using local advertisements and through the University of California, Davis. Informed consent was obtained from each participant (and the primary caregiver for children) in accordance with the Declaration of Helsinki, and the study was approved by the Internal Review Board at the University of California, Davis. The majority of the children in this sample served as controls for an fMRI study of Tourette syndrome (Baym et al., 2008).
2.2. Task design
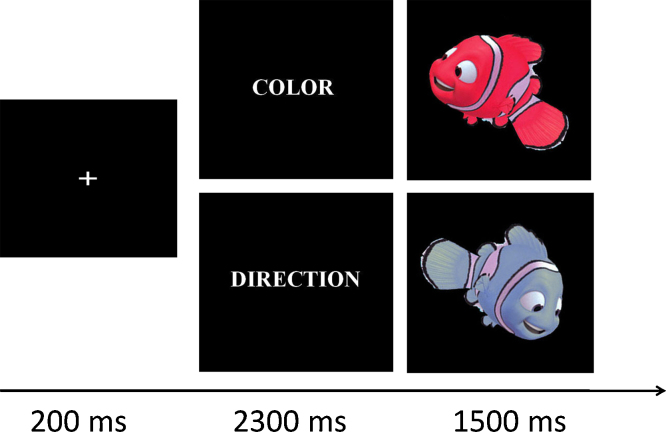
Participants were instructed on a trial-by-trial basis to use one of two visually presented rule cues to determine the appropriate response for a given target stimulus (Fig. 1). The words “COLOR” and “DIRECTION”, presented onscreen, served as the rule cues. The target stimuli were characters from the Disney movie “Finding Nemo”, manipulated in Adobe Photoshop CS2 to produce 16 stimuli in various shades of red and blue, facing to the left or right of the screen at various angles.
Fig. 1.
On each trial of the Nemo task, participants were instructed to respond with a left or right button press to each stimulus, based on the relevant rule for that trial (Color or Direction). 200 ms before the start of a trial, a crosshair alerted participants to fixate to the center of the screen. The instructional cue, “Color” or “Direction”, appeared for 2300 ms, followed immediately by a cartoon stimulus. Participants had 1500 ms to respond to this target stimulus. Each trial was followed by a variable-duration inter-trial interval (ITI) of 2–8 s.
On Direction trials, participants were to press a left button in response to a leftward facing stimulus, and to press a right button for a rightward facing stimulus. On Color trials, participants were to press one button for red stimuli and the other button for blue stimuli. Half of the participants were taught that a red stimulus indicated a left-button press and a blue stimulus indicated a right-button press, and half were taught the reverse. Participants used the index and middle fingers of their right hand to respond.
On each trial, a fixation cross appeared for 200 ms, followed by the instructional cue for 2300 ms, and then a target stimulus for 1500 ms. Color and Direction trials were pseudo-randomly ordered throughout a scan, with variable inter-trial intervals interspersed between them. The order of trial type presentation and jittered fixation was determined using Optseq2, an optimizing program designed to allow for maximal efficiency in deconvolving trials from each condition and baseline activation (Dale, 1999). Inter-trials intervals ranged from 2 to 8 s, as determined by the Optseq algorithm.
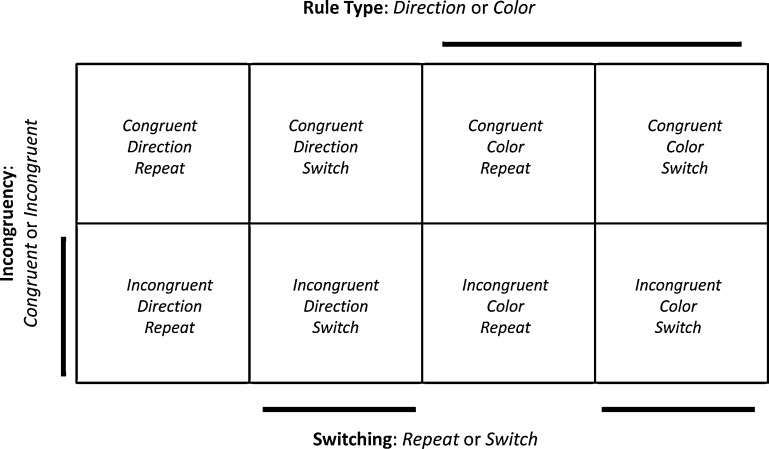
The task involved a 2 × 2 × 2 factorial design with independent manipulations of Rule Type, Switching, and Incongruency (Fig. 2). The Rule Type factor (Direction or Color) was determined by whether a trial involved the Color rule, for which the associated response mapping was arbitrary, or the Direction rule, for which it was not. The Switching factor (Repeat or Switch) was determined by whether the rule type of the current trial was the same of different compared to the rule type of the previous trial. The Incongruency factor (Congruent or Incongruent) was determined by whether or not the response associated with both the Color and the Direction rules was the same or different for the given stimulus.
Fig. 2.
A depiction of the factorial design, involving three factors: Rule Type (Color vs. Direction), Switching (Switch vs. Repeat), and Incongruency (Incongruent vs. Congruent). The cells in the factorial design comprise the eight experimental conditions.
2.3. Training procedure
Children were introduced to the scanner environment with a mock scanner at the UC Davis Imaging Research Center, where they were trained to lie still while listening to MRI pulse sequences. During the training session, participants learned the stimulus-response mappings for the Color and Direction rules, and practiced applying them both on paper and in a 3-min computerized test. Adults did not participate in a mock scan, but were taught the Color and Direction rules in the same manner.
2.4. Data acquisition
Participants completed 192 trials of the Nemo task over the course of four 4.5-min fMRI scans, with an equal number of trials for each bin within the 2 × 2 × 2 factorial design. Scan lists were counterbalanced across participants.
Imaging was performed using an 8-channel phased-array coil on a 3-T Siemens Trio MRI scanner (Siemens Medical Solutions, Erlangen, Germany) at the UC Davis Imaging Research Center. Participants viewed stimuli back-projected onto a projection screen with a mirror mounted on the head coil and responded using a button box held in their right hand. The scan session included a T2 localizer scan, four 4.5-min fMRI runs, and a high-resolution three-dimensional T1 MPRAGE anatomical scan.
Blood Oxygen Level-Dependent (BOLD) data were collected using a gradient-echo echo-planar pulse sequence (TR = 2000 ms, TE = 25 ms, 34 axial slices, no inter-slice gap, 3.4 mm × 3.4 mm × 4 mm voxels, flip angle = 90°, field of view = 220 mm, 135 volumes per run). The first four volumes from each functional scan were removed from our analysis to account for magnetic field equilibration. To minimize the effects of head movement on the fMRI data, we implemented the gradient-echo echo-planar pulse Prospective Acquisition Correction (3D-PACE) sequence which prospectively adjusts scan parameters throughout a run on the basis of real-time assessment of head motion (Siemens Medical Solutions).
2.5. Data analysis
We conducted a 4-way ANOVA on the behavioral results, with Rule Type (Color vs. Direction), Switching (Switch vs. Repeat), and Incongruency (Incongruent vs. Congruent) as within-subject factors, and age group (Children vs. Adults) as a between-subject factor.
fMRI data were preprocessed and analyzed using SPM5 (Wellcome Department of Cognitive Neurology, London, UK). Functional volumes from each participant were first corrected for interleaved slice acquisition, and submitted to a rigid-body motion correction. These motion-corrected functional images were then spatially normalized to an EPI template and resampled to 3 mm × 3 mm × 4 mm voxels. Both children and adult participants were normalized to this template, as the SPM EPI template has been validated for use in normalization of brain volumes for children aged 6 and up (Burgund et al., 2002, Kang et al., 2003). Finally, functional images were smoothed using an 8-mm full-width at half maximum isotropic Gaussian kernel.
Statistical analyses were performed using the general linear model in SPM5. Functional MRI time-series data were modeled as a series of events, time-locked to the onset of the instruction cue at the beginning of each trial, and were convolved with SPM's canonical hemodynamic response function (HRF). Each of the eight cells in the factorial design was modeled as a separate condition, and incorrect trials were modeled separately from correct trials. The resulting functions were used as covariates in a general linear model, along with covariates of no interest including their first and second temporal derivatives, six motion covariates (head translation and rotation along three dimensions), and a covariate for session effects. The least-squares parameter estimates, for each condition, of height of the best-fitting response function were used in whole-brain contrasts, and the resulting contrast images (each of the 8 conditions vs. fixation baseline), computed on a subject-by-subject basis, were submitted to group analyses.
Group analyses were conducted as whole-brain ANOVAs in SPM5, which can include up to three factors. We consider results from two whole-brain mixed ANOVAs: the first included Rule Type and Switching as within-subjects factors, and age group as a between-subjects factor; the second ANOVA was similar, but included Incongruency instead of Switching as a within-subjects factor. Including age group as a factor in each ANOVA served to balance the contribution of each group to the overall parameter estimates, given that we had data for 20 children but only 16 adults. Results that involved Incongruency were obtained from the second ANOVA; all other results were obtained from the first ANOVA. Within-subjects statistics, in the context of SPM5's ANOVA routine, were obtained by normalizing the contrast data to the group means. For each examined contrast, we report all activation clusters that survived thresholding at p < .001 (uncorrected), with an extent threshold of 20 voxels. Conjunction analysis was performed using the minimum statistic, i.e. by taking the minimum T-value across multiple contrasts (Nichols et al., 2004).
ROI analyses were conducted using Marsbar. For cortical regions that demonstrated significant engagement for one or more of the three manipulations (Rule Type, Switching, or Incongruency) in the mixed ANOVAs (as described above), we identified functional ROIs. In each ROI, we extracted condition-specific parameter estimates and subjected these to a four-way mixed ANOVA (Rule Type × Switch × Incongruency × Group) using R. We tested for main effects of the experimental manipulations (apart from those used to define the ROI), effects of age group, and potential interactions. In addition, event-related timecourses were obtained for selected ROIs, using the finite impulse response (FIR) method. This method involved fitting a separate impulse regressor to each 2-s interval of data within the 12-s window of data following each trial onset.
3. Results
3.1. Behavioral results
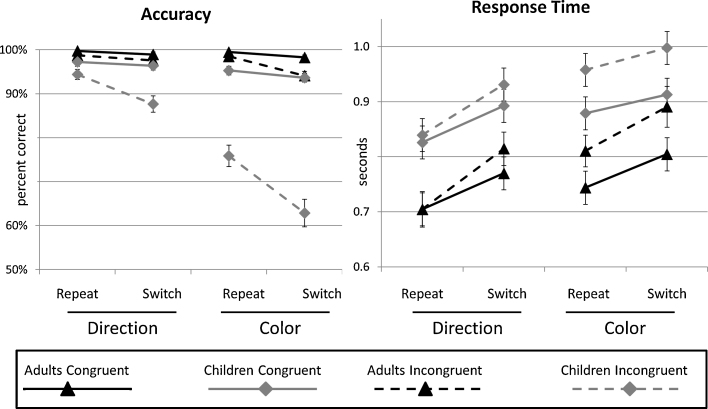
Children performed fairly well overall on the Nemo task, although they were less accurate than adults (85% vs. 97% correct; p = .008), and exhibited slower response times (RTs) (891 vs. 755 ms; p < .0001). We had predicted that participants, and in particular children, would experience relatively greater difficulty when (1) the relevant stimulus-response mapping was arbitrary rather than non-arbitrary (Rule Type manipulation), (2) the target stimulus was incongruent (Incongruency manipulation), and/or (3) the rule switched from the previous trial (Switching manipulation). All of these predictions were borne out (Fig. 3). All three manipulations affected accuracy and response times in both age groups (all p's < 0.05). Moreover, the overall accuracy difference between children and adults was driven by significant interactions between age group and each of the three experimental factors, such that children's accuracy was impacted more than adults’ by each of the manipulations (Age × Incongruency: p < .001; Age × Switching: p = .005; Age × Rule Type: p < .001).
Fig. 3.
Mean accuracy (left) and response time (right) for each experimental condition, for children and adults.
In addition to these main effects and interactions with age group, there were interactions between each of the experimental factors (Rule Type × Switching, Rule Type × Incongruency, and Switching × Incongruency; all p's < 0.01). For each of these interactions, the effect of one factor was greater for the more difficult condition of the second manipulation; for example, there was a greater effect of Incongruency for Color trials than for Direction trials. Notably, both Rule Type and Switching had a greater impact on the Incongruency effect in children than in adults (p's < 0.001). Thus, we observed strong interactions between the manipulations.
3.2. fMRI results: whole-brain analyses
3.2.1. Question 1: Are there common or distinct brain networks for representing arbitrary rules, switching flexibly between rules, and implementing rules in the face of distracting stimulus information?
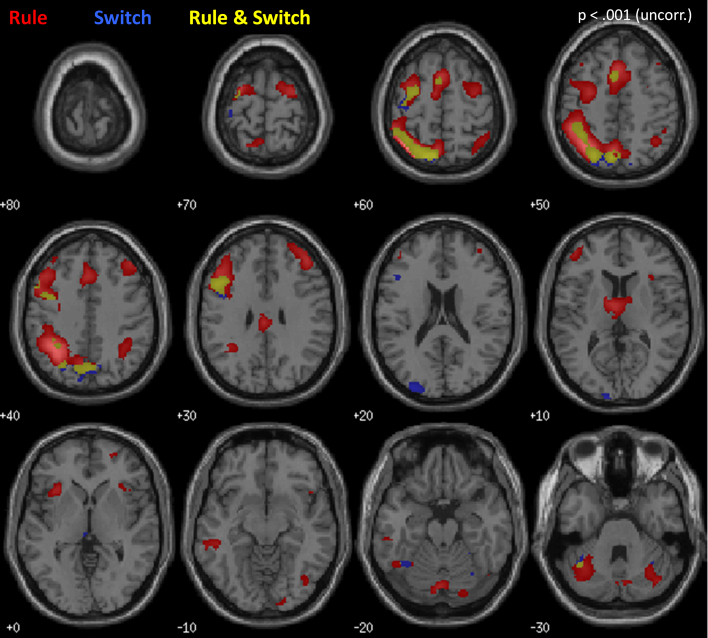
To identify regions associated with each experimental factor, we examined the main effect contrasts within the whole-brain ANOVAs (Fig. 4 and Table 1). The main effect of Rule Type (Color > Direction; red in Fig. 4) yielded activation across a bilateral fronto-parietal network, and was particularly strong in left-hemisphere PPC, DLPFC, and premotor cortex (PMC), and bilateral pre-SMA (Table 1A). The main effect of Switching (Switch > Repeat; blue in Fig. 4) produced a highly similar pattern of activation, with maximal engagement in left PPC, left DLPFC, and bilateral pre-SMA (Table 1B). The conjunction of Rule Type and Switching (yellow in Fig. 4) yielded activation in left DLPFC, left PPC, and PMC. The main effect of Incongruency (Incongruent > Congruent) yielded no areas of activation.
Fig. 4.
Whole brain activation, across all participants, associated with the Rule (red) and Switch (blue) manipulations. Yellow indicates areas of overlap. Results are displayed at p < .001 (uncorrected). MNI z-coordinates are provided next to each brain image.
Table 1.
Activation clusters for select contrasts, each thresholded at p < .001 (uncorrected) with a minimum cluster-size of 20 voxels. The following contrasts failed to produce activation clusters at this threshold, and so are excluded from the table: Incongruency (main effect), Group × Rule Type, Group × Incongruency. DLPFC = dorsolateral prefrontal cortex, PMC = premotor cortex, pre-SMA = pre-supplementary motor area, MOG = middle occipital gyrus, STG = superior temporal gyrus, MTG = middle temporal gyrus, SPL = superior parietal lobe, IPL = inferior parietal lobe.
| Region | B.A. | x, y, z (peak) | T-value (peak) | # Voxels |
|---|---|---|---|---|
| Main effects | ||||
| A. Rule Type (Color > Direction) | ||||
| L. DLPFC | 9 | −45, 24, 32 | 4.8 | 164 |
| R. DLPFC | 9 | 45, 36, 36 | 5.0 | 62 |
| L. PMC | 6 | −27, −3, 68 | 5.0 | 98 |
| R. PMC | 6 | 24, −3, 68 | 3.9 | 38 |
| Pre-SMA | 8, 6 | 0, 12, 56 | 5.4 | 143 |
| L. SPL, IPL, precuneus | 7, 40 | −27, −69, 60 | 7.8 | 705 |
| Thalamus | −3, −9, 12 | 4.8 | 35 | |
| B. Switching (Switch > Repeat) | ||||
| L. DLPFC | 9 | −48, 15, 32 | 4.1 | 95 |
| L. PMC | 6 | −30, −6, 64 | 4.0 | 41 |
| Pre-SMA | 8, 6 | −3, 6, 56 | 3.9 | 31 |
| L. SPL, IPL, precuneus | 7, 40 | −9, −72, 56 | 5.2 | 365 |
| L. MOG | 19 | −30, −90, 20 | 4.1 | 23 |
| L. Cerebellum | 37 | −39, −54, −24 | 3.7 | 32 |
| Differential effects (Rule Type vs. Switching) | ||||
| C. Rule Type > Switching ((Color − Direction) > (Switch − Repeat)) | ||||
| R. DLPFC | 9 | 39, 52, 24 | 4.1 | 71 |
| D. Switching > Rule Type ((Switch − Repeat) > (Color − Direction)) | ||||
| L. MOG, cuneus | 18, 17, 19 | −12, −93, 4 | 7.5 | 198 |
| Age group: main effects and interactions | ||||
| E. Group (Adults > Children) | ||||
| Pre-SMA | 6, 8 | 9, 12, 52 | 4.9 | 99 |
| L. PMC | 6 | −36, −12, 60 | 5.1 | 136 |
| R. PMC | 6 | 27, 0, 60 | 5.2 | 136 |
| L. IPL | 40 | −48, −36, 52 | 4.5 | 144 |
| L. MOG | 18 | −21, −93, −8 | 4.4 | 40 |
| R. MOG | 18 | 9, −90, 24 | 3.8 | 41 |
| F. Group (Children > Adults) | ||||
| R. IPL | 40 | 54, −66, 40 | 4.5 | 45 |
| Cingulate gyrus | 31 | 3, −27, 44 | 3.9 | 24 |
| G. Group × Switching | ||||
| L. STG | 22 | −57, −27, 4 | 4.3 | 64 |
| R. MTG | 21 | 60, −18, −4 | 4.9 | 72 |
Given the apparent similarity between the Rule Type and Switching manipulations, we conducted a follow-up analysis designed to identify any significant differences between them. Specifically, we computed for each participant the contrast between Rule Type and Switching effects, i.e. (Color > Direction) > (Switch > Repeat),3 and submitted the resulting contrast images to a one-sample t-test. This contrast yielded activation in right DLPFC that was engaged more strongly by the Rule Type manipulation (Table 1C), and a cluster in left middle occipital gyrus (MOG) that was engaged more strongly by the Switching manipulation (Table 1D).
3.2.2. Question 2: Are there differences between children and adults in the neural substrates of rule-guided behavior?
We sought next to compare and contrast the activation patterns for the two age groups. Overall, children and adults showed highly similar patterns of task-related activation; however, probing for a main effect of Group (in the Group × Rule Type × Switching ANOVA) did reveal several regions with greater overall activation in adults than in children, including bilateral pre-SMA and PMC and left PPC (Table 1E), as well as regions demonstrating greater activation in children than in adults, including right inferior parietal lobe and a cluster within the cingulate gyrus (Table 1F). At the whole-brain level, no regions were significantly activated by the interaction between Group and Rule or by the interaction between Group and Incongruency. There was activation associated with the interaction between Group and Switching, in left superior temporal gyrus as well as in right middle temporal gyrus (Table 1G). Children, but not adults, engaged these regions more for Switch trials than for Repeat trials.
3.3. fMRI results: ROI analyses
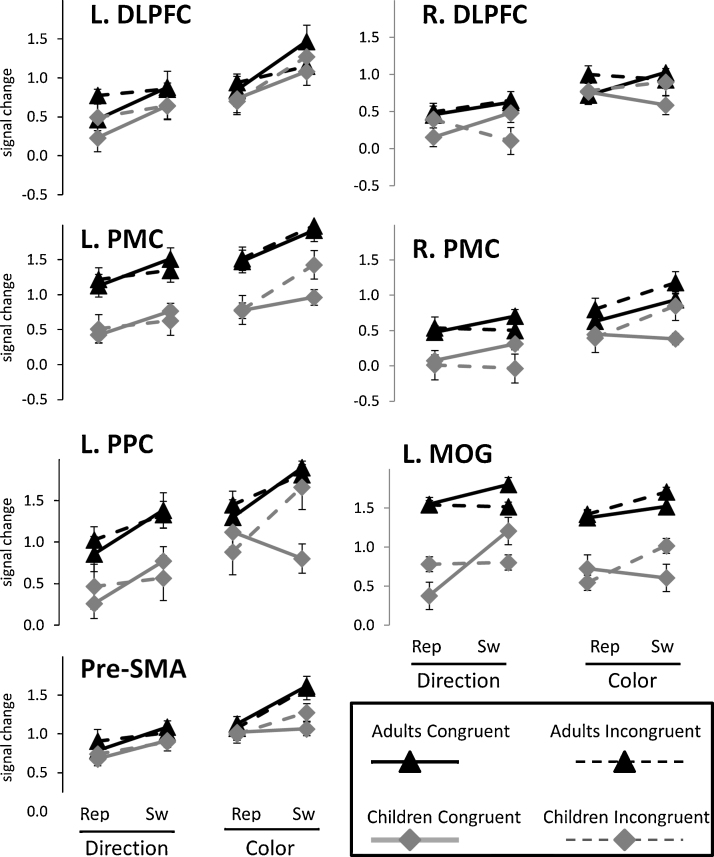
From the whole-brain ANOVAs, we selected for further examination each cortical cluster that demonstrated significant activation for Rule Type and/or Switching. No clusters were selected for Incongruency, since none were significantly engaged by this manipulation, and subcortical clusters were not included in this analysis. Functional ROIs based on the overlap between effects of Rule Type and Switching (i.e. based on the minimum statistic conjunction analysis) were located in left DLPFC (79 voxels), left PMC (41 voxels), left PPC (300 voxels), and pre-SMA (31 voxels). Functional ROIs based on the main effect of Rule Type were located in right DLPFC (62 voxels) and right PMC (38 voxels), and an ROI based on the main effect of Switching was located in left MOG (23 voxels). Parameter estimates from each of these functional ROIs are shown in Fig. 5.
Fig. 5.
Parameter estimates for each experimental condition, for each group, from seven selected ROIs: left DLPFC, left PMC, left PPC, and pre-SMA (obtained from the overlap between Rule Type and Switching contrasts); right DLPFC and right PMC (obtained from the Rule Type contrast), and left MOG (obtained from the Switching contrast).
By definition, four of the ROIs that we examined were activated for both Rule Type and Switching, and together constitute a common network for representing arbitrary rules and switching between them. Right PMC, which was identified from the Rule Type manipulation, also demonstrated a marginal effect of Switching (F = 3.7, p = .06). On the other hand, right DLPFC, identified from the same contrast, demonstrated no effect of Switching. Left MOG, identified from the Switching manipulation, demonstrated no effect of Rule Type. None of the ROIs that we examined demonstrated a significant effect of Incongruency. However, right PMC did demonstrate a Rule Type × Incongruency interaction (F = 9.5, p = .004), such that Incongruency had a greater effect for Color trials than for Direction trials. A similar trend was observed in left PMC (F = 3.1, p = .09).
Overall, both age groups showed highly similar patterns of task-related activation in these ROIs. A significant or marginal main effect of group was observed in left PMC (F = 9.8, p = .003), right PMC (F = 4.2, p = .04), left PPC (F = 6.4, p = .01), and left MOG (F = 3.7, p = .06). In each of these regions, parameter estimates were higher for adults than for children across all conditions.4 However, there were no interactions between age group and any of the three manipulations for any of the ROIs, indicating that children and adults engaged these regions in a similar fashion during performance of the Nemo task.
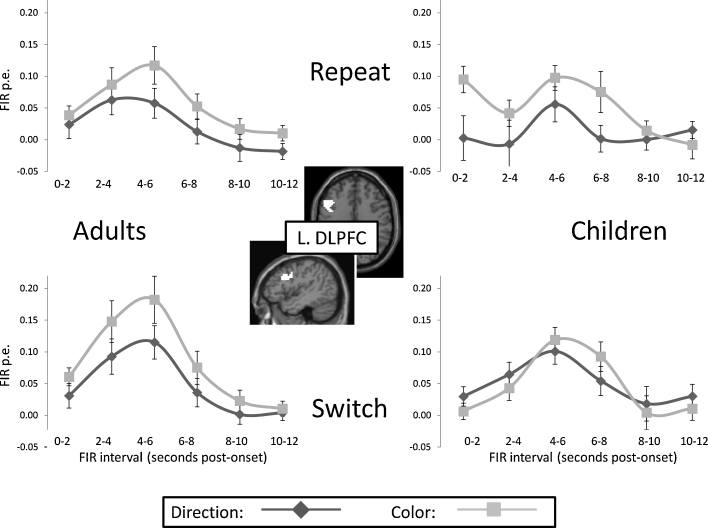
In addition to calculating average parameter estimates for each of the functional ROIs, we extracted event-related timecourses to test for possible differences between children and adults in temporal dynamics. Across all four of the regions that were engaged for both Rule Type and Switching (left DLPFC, left PMC, left PPC, and pre-SMA), we observed an intriguing pattern: in children, but not in adults, activation at the beginning of each trial appears to have been driven by the previous trial's rule, such that it was higher when switching from or repeating the Color rule and lower when switching from or repeating the Direction rule.
We tested whether this effect was significant in one or more of these ROIs by submitting activation values from the first 2-s time bin (from 0 to 2 s, time-locked to the presentation of the rule cue) to a three-way ANOVA (Rule Type × Switching × Group). We observed a significant effect in left DLPFC (Rule Type × Switching × Group: p = .04; Rule Type × Switching (children): p = .03; see Fig. 6), and trend-level effects in the other three regions (left PMC, SMA, and left PPC); the effect in left DLPFC does not survive the p < .007 threshold that the Bonferroni correction for seven ROI analyses would impose, but the consistency of the pattern of age-related differences in temporal dynamics for regions engaged by Rule Type and Switching warrants further investigation.
Fig. 6.
BOLD activation FIR timecourses from the left DLPFC ROI, for correct congruent trials, separately for children and adults. At the onset of each trial, activation in children but not in adults reflected the rule of the previous trial.
4. Discussion
As participants performed the Nemo task, several cognitive control regions – left PPC, left DLPFC, left PMC, and pre-SMA – responded to the manipulations of both Rule Type and Switching. These cognitive control regions were engaged similarly across children and adults, even though children performed the task less accurately and more slowly than adults. The primary differences between these age groups may take the form of distinct temporal dynamics in the activation of these cognitive control regions, rather than differences in the set of regions engaged. We discuss each of these points in turn, together with their implications and relations to the existing literature.
4.1. Shared neural substrates for rule-guided behavior and task-switching
The high degree of overlap in regions responding to the Rule Type and Switch manipulations – left PPC, left DLPFC, left PMC, and pre-SMA – is consistent with the idea that the process of switching to a new rule engages the same regions that support the representation of arbitrary task rules in the absence of a switch. The fact that these regions are engaged by both manipulations may reflect the general role of these regions in the sustained activation of task-relevant information in working memory, which can provide top-down support when task performance is not automatic, e.g., during task-switching (Cohen and Servan-Schreiber, 1992, Morton and Munakata, 2002) and during the retrieval of an arbitrary task rule that is not yet well-learned (Donohue et al., 2005, Rougier et al., 2005, Souza et al., 2009).
More broadly, our finding of shared neural substrates for rule-guided behavior and task-switching is consistent with theoretical frameworks that emphasize the role of task-relevant activation in supporting a variety of executive functions, including shifting, inhibition, updating, and monitoring (e.g., Miller and Cohen, 2001, Munakata et al., 2011, Velanova et al., 2009). For example, a factor analysis of executive function reveals a common factor that is tapped across a wide range of executive function tasks (including Stroop, Stop Signal, Antisaccade, Keep-Track, N-Back, and task-switching), in addition to more specific factors; a parsimonious interpretation is that the common factor reflects active goal maintenance in prefrontal cortical regions, which supports a variety of executive functions (Friedman et al., 2007).
Although the behavioral data indicate that participants were sensitive to the Incongruency manipulation, the whole-brain contrast testing for a main effect of Incongruent > Congruent trials revealed no clusters at p < .001 uncorrected. This lack of an effect of Incongruency could reflect the fact that this manipulation was relevant to the response period only, which took place a couple of seconds after the appearance of the rule cues relevant to the manipulations of Rule Type and Switching. However, PMC did demonstrate an interaction between Rule Type and Incongruency, such that the effect of Incongruency was greater for Color trials than for Direction trials (significant for right PMC, and trend-level for left PMC). This finding reflects the behavioral results, and supports the hypothesis that selection of the appropriate motor response (mediated in part by PMC) is more difficult on Color than Direction trials because the Direction rule comes to mind more automatically than the Color Rule, and therefore the direction of the stimulus is likely to interfere with performance on Color trials. Similarly, on the Stroop task, the less-automatic response (naming the color of the word) is more vulnerable to distracting information from the more-automatic response (reading the word) than the other way around.
4.2. Similar cognitive control regions across children and adults
Cognitive control regions were engaged similarly across children and adults, even though adults performed the task more accurately and more rapidly. In contrast, other studies have demonstrated shifts in the functional networks recruited for cognitive control across development (Brown et al., 2005, Dosenbach et al., 2010, Scherf et al., 2006), or changes in the activation profiles within the same functional networks across development (Bunge et al., 2002, Rubia et al., 2006). Our finding of similar engagement of cognitive control regions across children and adults may make sense in the context of our finding of overlapping regions responding to the Rule and Switch manipulations. That is, the high degree of overlap in regions responding to the Rule and Switch manipulations suggests their role in maintenance of information in working memory. However, the primary developmental differences in activation of DLPFC are observed during manipulation of information in working memory rather than in maintenance (Crone et al., 2006c, Jolles et al., 2011). Thus, children and adults may show similar recruitment of cognitive control regions to the extent that those regions support the maintenance of information in working memory.
4.3. Distinct temporal dynamics in activation of cognitive control regions across children and adults
The primary differences between children and adults in our task may take the form of distinct temporal dynamics in the activation of cognitive control regions. Specifically, in children but not adults, initial DLPFC activation appeared to be driven by the previous trial's rule, starting higher for a preceding Color trial than for a preceding Direction trial, regardless of whether the current trial was Color or Direction. Further testing will be necessary to confirm this pattern in DLPFC, and to test whether it extends to other cognitive control regions, as the data suggest.
These timecourse findings are consistent with other findings highlighting the “sluggishness” of prefrontal rule representations early in development. For example, such sluggishness during development has been measured behaviorally, using factor analyses of executive function that reveal poor shifting-specific abilities, which have been interpreted in terms of mental “stickiness”. Such stickiness during development is associated with better behavioral outcomes, such as fewer attention problems (Friedman et al., 2007) and better self-restraint (Friedman et al., 2011). The sluggishness of children's rule representations could also play a role in improvements in cognitive control that are observed when children are asked to wait before responding (Diamond et al., 2002), and in children showing larger carry-over effects from previous trials than adults in task-switching paradigms (Cepeda et al., 2001, Crone et al., 2006d).
In addition, such stickiness during development has been motivated computationally. In a neural network model of the development of task-switching (Morton and Munakata, 2002), a slow updating of prefrontal representations needed to be incorporated to simulate children's behavior in response to negative feedback (Chatham et al., in press). Specifically, if children receive negative feedback when they fail to switch to new task rules, some children respond by adopting an “opposites” game, sticking with their old rules instead of switching to the new rules, but doing the opposite of what they were doing before. Simulating such effects required the models to be slow to update to the current rules, so that they could apply their learning to the prior rules, suggesting that such sluggishness might support the behaviors observed in children.
Our timecourse findings are also consistent with other evidence of developmental transitions in the temporal dynamics of cognitive control, specifically, a transition from reactive to proactive control across development (Andrews-Hanna et al., 2011, Chatham et al., 2009, Finn et al., 2010). Together, these results suggest that children are more likely than adults to maintain prior rule information when it is no longer relevant, and to retrieve current rule information reactively rather than maintaining it proactively.
5. Summary
More generally, our findings highlight informative similarities and differences across brain regions and across development. Several cognitive control regions responded similarly to the manipulations of rule difficulty and rule-switching. These regions responded similarly across children and adults, with the exception of DLPFC being slower to update to the current rule in children. These patterns of similarities and differences observed in our study add to a growing literature suggesting that common mechanisms can contribute to a variety of executive functions, and that certain developmental changes may be evident not in changes in brain regions involved, but rather in changes in the timing of engagement of these regions.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgments
This research was supported by a John Merck Scholarship in the Biology of Developmental Disabilities in Children (SB), and a University of Colorado Faculty Fellowship (YM). We thank Sarah Donahue, Robin Libove, and Samantha Wright for assistance with data collection and analysis, Hannah Snyder and Melanie Stollstorff for comments on the manuscript, and Eden Davis for assistance with manuscript preparation.
Footnotes
This contrast reduces to Color Repeat > Direction Switch, thus targeting the regions that distinguish between the Rule Type and Switching manipulations.
While our ANOVA-based whole-brain analysis aimed to balance the contribution of each group to the overall parameter estimates, the functional ROIs obtained from this would be completely unbiased with respect to group only in the unlikely case that both groups had equivalent levels of noise. In fact, the voxels identified in this way will be biased toward showing greater signal change in the group with higher noise levels (Kriegeskorte et al., 2009). The slightly smaller size of the adult group could contribute to higher noise levels in adults; on the other hand, the child group had higher average variance (looking across each ROI and condition). Group effects reported for the ROIs should be considered with this caveat in mind.
Contributor Information
Carter Wendelken, Email: cwendelken@berkeley.edu.
Yuko Munakata, Email: munakata@colorado.edu.
References
- Andrews-Hanna J.R., Seghete K.L.M., Claus E.D., Burgess G.C., Ruzic L., Banich M.T. Cognitive control in adolescence: neural underpinnings and relation to self-report behaviors. PLoS One. 2011;6:e21598. doi: 10.1371/journal.pone.0021598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baym C.L., Corbett B.A., Wright S.B., Bunge S.A. Neural correlates of tic severity and cognitive control in children with Tourette syndrome. Brain. 2008;131:165–179. doi: 10.1093/brain/awm278. [DOI] [PubMed] [Google Scholar]
- Brown T.T., Lugar H.M., Coalson R.S., Miezin F.M., Petersen S.E., Schlaggar B.L. Developmental changes in human cerebral functional organization for word generation. Cereb. Cortex. 2005;15:275–290. doi: 10.1093/cercor/bhh129. [DOI] [PubMed] [Google Scholar]
- Bunge S.A., Dudukovic N.M., Thomason M.E., Vaidya C.J., Gabrieli J.D.E. Immature frontal lobe contributions to cognitive control in children: evidence from fMRI. Neuron. 2002;33:301–311. doi: 10.1016/s0896-6273(01)00583-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunge S.A., Wallis J.D., Parker A., Brass M., Crone E.A., Hoshi E., Sakai K. Neural circuitry underlying rule use in humans and nonhuman primates. J. Neurosci. 2005;25:10347–10350. doi: 10.1523/JNEUROSCI.2937-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunge S.A., Wright S.B. Neurodevelopmental changes in working memory and cognitive control. Curr. Opin. Neurobiol. 2007;17:243–250. doi: 10.1016/j.conb.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Bunge S.A., Zelazo P.D. A brain-based account of the development of rule use in childhood. Curr. Dir. Psychol. Sci. 2006;15:118–121. [Google Scholar]
- Burgund E.D., Kang H.C., Kelly J.E., Buckner R.L., Snyder A.Z., Petersen S.E., Schlaggar B.L. The feasibility of a common stereotactic space for children and adults in fMRI studies of development. Neuroimage. 2002;17:184–200. doi: 10.1006/nimg.2002.1174. [DOI] [PubMed] [Google Scholar]
- Casey B.J., Tottenham N., Liston C., Durston S. Imaging the developing brain: what have we learned about cognitive development? Trends Cogn. Sci. 2005;9:104–110. doi: 10.1016/j.tics.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Cepeda N.J., Kramer A.F., Gonzalez de Sather J.C. Changes in executive control across the life span: examination of task-switching performance. Dev. Psychol. 2001;37:715–730. [PubMed] [Google Scholar]
- Chatham C.H., Frank M.J., Munakata Y. Pupillometric and behavioral markers of a developmental shift in the temporal dynamics of cognitive control. Proc. Natl. Acad. Sci. U.S.A. 2009;106:5529–5533. doi: 10.1073/pnas.0810002106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatham, C.H., Yerys, B.E., Munakata, Y. Why don't you do what I want? The informative failures of children and models. Cognitive Dev., in press. [DOI] [PMC free article] [PubMed]
- Cohen J.D., Servan-Schreiber D. Context, cortex, and dopamine: a connectionist approach to behavior and biology in schizophrenia. Psychol. Rev. 1992;99:45–77. doi: 10.1037/0033-295x.99.1.45. [DOI] [PubMed] [Google Scholar]
- Crone E.A. Executive functions in adolescence: inferences from brain and behavior. Dev. Sci. 2009;12:825–830. doi: 10.1111/j.1467-7687.2009.00918.x. [DOI] [PubMed] [Google Scholar]
- Crone E.A., Donohue S.E., Honomichl R., Wendelken C., Bunge S.A. Brain regions mediating flexible rule use during development. J. Neurosci. 2006;26:11239–11247. doi: 10.1523/JNEUROSCI.2165-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone E.A., Wendelken C., Donohue S.E., Bunge S.A. Neural evidence for dissociable components of task-switching. Cereb. Cortex. 2006;16:475–486. doi: 10.1093/cercor/bhi127. [DOI] [PubMed] [Google Scholar]
- Crone E.A., Wendelken C., Donohue S., van Leijenhorst L., Bunge S.A. Neurocognitive development of the ability to manipulate information in working memory. Proc. Natl. Acad. Sci. U.S.A. 2006;103:9315–9320. doi: 10.1073/pnas.0510088103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone E.A., Bunge S.A., van der Molen M.W., Ridderinkhof K.R. Switching between tasks and responses: a developmental study. Dev. Sci. 2006;9:278–287. doi: 10.1111/j.1467-7687.2006.00490.x. [DOI] [PubMed] [Google Scholar]
- Dale A. Optimal experimental design for event-related fMRI. Hum. Brain Mapp. 1999;8:109–114. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<109::AID-HBM7>3.0.CO;2-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond A. Development of the ability to use recall to guide action, as indicated by infants’ performance on AB. Child Dev. 1985;56:868–883. [PubMed] [Google Scholar]
- Diamond A. Rate of maturation of the hippocampus and the developmental progression of children's performance on the delayed non-matching to sample and visual paired comparison tasks. Ann. N.Y. Acad. Sci. 1990;608:394–433. doi: 10.1111/j.1749-6632.1990.tb48904.x. [DOI] [PubMed] [Google Scholar]
- Diamond A., Kirkham N., Amso D. Conditions under which young children can hold two rules in mind and inhibit a prepotent response. Dev. Psychol. 2002;38:352–362. [PubMed] [Google Scholar]
- Donohue S., Wendelken C., Crone E.A., Bunge S.A. Retrieving rules for behavior from long-term memory. Neuroimage. 2005;26(4):1140–1149. doi: 10.1016/j.neuroimage.2005.03.019. [DOI] [PubMed] [Google Scholar]
- Dosenbach N.U.F., Nardos B., Cohen A.L., Fair D.A., Power J.D., Church J.A., Nelson S.M., Wig G.S., Vogel A.C., Lessov-Schlaggar C.N., Barnes K.A., Dubis J.W., Feczko E., Coalson R.S., Pruett J.R., Barch D.M., Petersen S.E., Schlaggar B.L. Prediction of individual brain maturity using fMRI. Science. 2010;329:1358–1361. doi: 10.1126/science.1194144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn A.S., Sheridan M.A., Kam C., Hinshaw S., D’Esposito M. Longitudinal evidence for functional specialization of the neural circuit supporting working memory in the human brain. J. Neurosci. 2010;30:11062–11067. doi: 10.1523/JNEUROSCI.6266-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman N.P., Haberstick B.C., Willcutt E.G., Miyake A., Young S.E., Corley R.P., Hewitt J.K. Greater attention problems during childhood predict poorer executive functioning in late adolescence. Psychol. Sci. 2007;18:893–900. doi: 10.1111/j.1467-9280.2007.01997.x. [DOI] [PubMed] [Google Scholar]
- Friedman N.P., Miyake A., Robinson J.L., Hewitt J.K. Developmental trajectories in toddlers’ self-restraint predict individual differences in executive function 14 years later: a behavioral genetic analysis. Dev. Psychol. 2011;47:1410–1430. doi: 10.1037/a0023750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacques S., Zelazo P.D. On the possible socio-communicative roots of cognitive flexibility. In: Homer B., Tamis-Lemonda C., editors. The Development of Social Understanding and Communication. Lawrence Erlbaum Associates; Mahwah, NJ: 2005. pp. 53–81. [Google Scholar]
- Jolles D.D., Kleibeuker S.W., Rombouts S.A.R.B., Crone E.A. Developmental differences in prefrontal activation during working memory maintenance and manipulation for different memory loads. Develop. Sci. 2011;14(4):713–724. doi: 10.1111/j.1467-7687.2010.01016.x. [DOI] [PubMed] [Google Scholar]
- Kang H.C., Burgund E.D., Lugar H.M., Petersen S.E., Schlaggar B.L. Comparison of functional activation foci in children and adults using a common stereotactic space. Neuroimage. 2003;19:16–28. doi: 10.1016/s1053-8119(03)00038-7. [DOI] [PubMed] [Google Scholar]
- Kharitonova M., Munakata Y. The role of representations in executive function: investigating a developmental link between flexibility and abstraction. Front. Psychol. 2011;2:347. doi: 10.3389/fpsyg.2011.00347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegeskorte N., Simmons W.K., Bellgowan P.S., Baker C.I. Circular analysis in systems neuroscience: the dangers of double dipping. Nat. Neurosci. 2009;12(5.):535–540. doi: 10.1038/nn.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna B., Garver K.E., Urban T.A., Lazar N.A., Sweeney J.A. Maturation of cognitive processes from late childhood to adulthood. Child Dev. 2004;75:1357–1372. doi: 10.1111/j.1467-8624.2004.00745.x. [DOI] [PubMed] [Google Scholar]
- Luna B., Padmanabhan A., O’Hearn K. What has fMRI told us about the development of cognitive control through adolescence? Brain Cogn. 2010;72:101–113. doi: 10.1016/j.bandc.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller E.K., Cohen J.D. An integrative theory of prefrontal cortex function. Annu. Rev. Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Morton J., Munakata Y. Active versus latent representations: a neural network model of perseveration, dissociation, and decalage. Dev. Psychobiol. 2002;40:255–265. doi: 10.1002/dev.10033. [DOI] [PubMed] [Google Scholar]
- Munakata Y., Herd S.A., Chatham C.H., Depue B.E., Banich M.T., O’Reilly R.C. A unified framework for inhibitory control. Trends Cogn. Sci. 2011:453–459. doi: 10.1016/j.tics.2011.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munakata, Y., Snyder, H.R., Chatham, C.H. Developing cognitive control: three key transitions. Curr. Dir. Psychol. Sci., in press. [DOI] [PMC free article] [PubMed]
- Nichols T., Brett M., Andersson J., Wager T. Valid conjunction inference with the minimum statistic. Neuroimage. 2004;25:653–660. doi: 10.1016/j.neuroimage.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Rougier N.P., Noelle D.C., Braver T.S., Cohen J.D., O’Reilly R.C. Prefrontal cortex and flexible cognitive control: rules without symbols. Proc. Natl. Acad. Sci. U.S.A. 2005;102:7338–7343. doi: 10.1073/pnas.0502455102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K., Smith A.B., Woolley J., Nosarti C., Heyman I., Taylor E., Brammer M. Progressive increase of frontostriatal brain activation from childhood to adulthood during event-related tasks of cognitive control. Hum. Brain Mapp. 2006;27:973–993. doi: 10.1002/hbm.20237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherf K.S., Sweeney J.A., Luna B. Brain basis of developmental change in visuospatial working memory. J. Cogn. Neurosci. 2006;18:1045–1058. doi: 10.1162/jocn.2006.18.7.1045. [DOI] [PubMed] [Google Scholar]
- Souza M.J., Donohue S.E., Bunge S.A. Controlled retrieval and selection of action-relevant knowledge mediated by partially overlapping regions in left ventrolateral prefrontal cortex. Neuroimage. 2009;46(1):299–307. doi: 10.1016/j.neuroimage.2009.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder H.R., Munakata Y. Becoming self-directed: abstract representations support endogenous flexibility in children. Cognition. 2010;116:155–167. doi: 10.1016/j.cognition.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velanova K., Wheeler M.E., Luna B. The maturation of task set-related activation supports late developmental improvements in inhibitory control. J. Neurosci. 2009;29:12558–12567. doi: 10.1523/JNEUROSCI.1579-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelazo P.D. The development of conscious control in childhood. Trends Cogn. Sci. 2004;8:12–17. doi: 10.1016/j.tics.2003.11.001. [DOI] [PubMed] [Google Scholar]