Highlights
* fMRI data was collected from 29 children (4–9 years) and 15 adults. * Go/Nogo response cues were preceded by angry and happy faces. * Greater OFC activation found for happy faces in children when responses withheld. * This effect decreased parametrically with age group and was not observed in adults. * Age differences specific to interaction between emotional and control processes.
Keywords: Emotion, Cognition–emotion interactions, fMRI, Control, Development
Abstract
The modulation of control processes by stimulus salience, as well as associated neural activation, changes over development. We investigated age-related differences in the influence of facial emotion on brain activation when an action had to be withheld, focusing on a developmental period characterized by rapid social-emotional and cognitive change. Groups of kindergarten and young school-aged children and a group of young adults performed a modified Go/Nogo task. Response cues were preceded by happy or angry faces. After controlling for task performance, left orbitofrontal regions discriminated trials with happy vs. angry faces in children but not in adults when a response was withheld, and this effect decreased parametrically with age group. Age-related changes in prefrontal responsiveness to facial expression were not observed when an action was required, nor did this region show age-related activation changes with the demand to withhold a response in general. Such results reveal age-related differences in prefrontal activation that are specific to stimulus valence and depend on the action required.
1. Introduction
Questions about how emotional arousal and valence influence cognitive control are central to research on cognition and emotion: i.e., Do emotionally significant events help or hinder us when we have to perform an attentionally demanding task? Furthermore, how do interactions between the motivational significance of an event and demands for cognitive control change over development? In addressing the first question, the dual competition model (Pessoa, 2009) proposes that whether an emotional stimulus aids or hinders performance of a cognitive task depends in part on the arousal level of the stimulus, as well as whether the action tendency it evokes is consistent with the required response. For example, when one is performing an inhibition task, an angry face may signal the need to stop an action and thereby enhance performance, whereas a happy face may actually encourage a response, hindering performance (Blair et al., 1999, Hare et al., 2005, Roelofs et al., 2009). Applying the dual competition model to the question of developmental change, however, is complicated by findings that not only does the capacity for cognitive control continue to develop through adolescence (Bunge and Zelazo, 2006, Davidson et al., 2006), but preferential responses to positive vs. negative stimuli can also differ with developmental phase. For example, both older adults and young children have been found to show a “positivity bias” for positive relative to negative stimuli when compared to young adults (Boseovski and Lee, 2008, Mather et al., 2004, Mather and Carstensen, 2003). Our own research found that patterns of amygdala activation in response to facial expression differed across age groups. In a free-viewing condition, kindergarten (3.8–6.2 years) and early school-aged children (6.5–8.9 years) showed greater amygdala activation for happy vs. angry expressions (posed by both their mothers and matched strangers), whereas young adults did not (Todd et al., 2011). Further, amygdala sensitivity to angry expressions increased between the ages of 3 and 8 years. The goal of the present study was to follow up on this finding by examining whether a similar pattern would be found in prefrontal regions responsive to the interaction between social-emotional stimuli and the demand for cognitive control.
Convergent research indicates an important role for orbitofrontal regions of prefrontal cortex (PFC) in emotional modulation of cognitive control. Neural models of the influence of emotion on control processes emphasize the role of the orbitofrontal cortex (OFC) as an important node in such interactions (Pessoa, 2009). Recent models of OFC function stress its importance for flexible social behavior, which requires dynamic stimulus evaluation to guide ongoing action [for review see (Nelson and Guyer, 2011, Schoenbaum et al., 2009)]. This function includes guiding action in response to signals of approval or disapproval communicated by facial expression. Consistent with this model, in adults OFC has been found to be sensitive to the interaction between stimulus valence and response inhibition in a Go/Nogo task (Goldstein et al., 2007). More specifically, a region of left lateral OFC has consistently been found to be activated when instructions to approach/avoid an emotional face are incongruent with the facial expression (e.g., avoid a happy face) (Roelofs et al., 2009, Volman et al., 2011a, Volman et al., 2011b).
Such prefrontal sensitivity to facial expression during response inhibition may be present from a young age. Our previous ERP research found greatest frontocortical activation following emotional faces in a modified Go/Nogo task when young children (4–6 years) had to withhold a response (Todd et al., 2008). Thus, by the kindergarten years prefrontal activation, particularly in the OFC, may be responsive to the degree of control required to refrain from acting in the face of either affective responses (unpleasant vs. pleasant) or action tendencies (e.g., discouraging vs. encouraging of action) elicited by the facial expression (Blair et al., 1999).
Yet there is also evidence that prefrontal sensitivity to stimulus evaluation may differ with developmental phase. A recent fMRI study found age-related changes in OFC sensitivity to negative social feedback between late childhood (8–10 years), through adolescence (14–16 years, 16–17 years) and adulthood (19–25 years) (Gunther Moor et al., 2010). There may also be age-related differences in prefrontal sensitivity to the relation between stimulus evaluation and action. Increasing connectivity has been observed between regions of ventral prefrontal cortex and the amygdala over middle childhood (participants ranged in age from 5 to 11 years) in response to emotional challenge in a Go/Nogo task (Perlman and Pelphrey, 2010). This finding indicates that ventral prefrontal regions are increasingly co-activated with the amygdala when a negative affective state interacts with the demand for response inhibition. Thus, in the presence of a sustained negative mood, ventral PFC or OFC responses to task demand differ with age, possibly in tandem with the amygdala's role in motivational salience detection. However, whether age-related differences can be observed in PFC sensitivity to the valence of transient emotional stimuli in a manner that is modulated by task demand (act/withhold action) – and particularly in pre school-aged children – is still unknown.
The kindergarten and early school years are a time of ongoing changes in the capacity for social understanding and self-regulation [for review see Todd and Lewis (2008)]. Such behavioral changes are paralleled by significant structural brain development, much of it in prefrontal regions mediating social understanding and self-regulation processes. Between 3 and 6 years children show rapid growth in the anterior corpus collosum, a key white matter region for circuitry involved in sustained attention and planning and organizing action (Thompson et al., 2000). This growth spurt levels off after age 6, suggesting discontinuous development between pre-school and school-aged children. In contrast, prefrontal gray matter volume levels, which mature in a sequence running from medial to lateral and rostral to caudal (Gogtay et al., 2004), show a more continuous pattern of increasing volume from 4 years until puberty, followed by a decline in volume through adolescence, thought to reflect synaptic pruning. Specifically, orbitofrontal regions underlying social and motivational flexibility show a linear pattern of development between 4 and 9 years, with gray matter volume gradually increasing in early-mid childhood before beginning a process of decline to adult levels (Gogtay et al., 2004, O’Donnell et al., 2005). Thus, prefrontal structural development is characterized by both discontinuities and relative continuity over these years.
The present study investigated age-related differences in the influence of positive and negative facial expression on fMRI activation following response cues when an action must be withheld. Our focus was on age-related comparisons between kindergarten and early school aged children to capture a period characterized by significant social-emotional and cognitive change and structural brain development, and between these children and a group of young adults. Both children and adults performed a modified Go/Nogo task where response cues were preceded by emotional faces. To ensure that withholding a response would be sufficiently easy for all participants, the task was not speeded and had a 50/50 ratio of Nogo to Go trials. Based on convergent evidence, we hypothesized that activation in the OFC would show age-related differences in activation in response to facial expression when participants had to withhold a response. We further predicted that such activation patterns would reflect previously found age-related differences in amygdala responses to angry vs. happy faces in a free viewing condition (Todd et al., 2011).
2. Materials and methods
2.1. Subjects
37 children aged 3.8–9.0 years (26 female) and 15 adults aged 18–38 years (7 female), participated in the study after being screened for uncorrected visual impairments and psychiatric and neurological disorders (adult participant or parent report). Participants were recruited through flyers, advertisement, and word of mouth, and were from a variety of cultural and socio-economic backgrounds. Each family received $40 for participation and each child received a toy. Informed written consent was obtained from all adults and parents of the children, children gave verbal assent, and the study was approved by the Research Ethics Board at the Hospital for Sick Children. After removing the child participants with excessive motion (n = 3), those who did not complete two runs of the experimental task (n = 2), and those whose accuracy was less than 75% in any condition (n = 3), analyses were performed on 29 children (aged 4.4–9.0 years, 22 female).
All 15 adults’ data were included. To probe differences in brain activation related to age within the group of children, as in our previous study (Todd et al., 2011), children were divided into two groups based on entry into grade school: a younger kindergarten-aged group (4.4–6.5 years), and an older, but young school-aged group (6.5–9.0 years) (see Table 1 for demographic information). The cutpoint was initially selected for the Todd et al. (2011) study drawing from the same participant sample, based on a median split that corresponded with grade school entry. In Ontario children spend two years in kindergarten (starting at age 4) and can enter Grade 1 when they are 6. By 6.5 years most children have begun grade school, a transition accompanied by the numerous developmental and experiential changes between the preschool/kindergarten and early grade school years (Eccles et al., 1984). The group of children removed from analysis included the three youngest children we scanned (<4.4 years) and thus the lower tail of the age range scanned is not represented in the current study – as children below 4.4 years have difficulty staying still while performing a task in the scanner. Within the age windows that remained, 3 children were removed from the younger group 2 children from the older group (see Table 1 for demographic information). To examine age-related patterns of behavior and BOLD activation across all 3 age groups, data from all participants were entered into ANOVAs that included age-group as a between subject-factor. This analysis approach also allowed examination of planned comparisons between older children and younger children to probe age-related differences within this early-mid childhood period, and between each group of children and the group of adults.
Table 1.
Demographic information for participants in each age group and excluded participants.
| Group | N | Min age | Max age | Mean age | Sex |
|---|---|---|---|---|---|
| Young children | 10 | 4.4 | 6.4 | 5.4 | 7 female |
| Older children | 19 | 6.6 | 9 | 7.5 | 15 female |
| Adults | 15 | 18.6 | 38.5 | 25.8 | 7 female |
| Excluded: below 4.4 years | 3 | 3.8 | 4.2 | 4.1 | 2 female |
| Excluded: 4.4–6.4 years | 3 | 4.8 | 6.0 | 5.3 | 1 female |
| Excluded: 6.6–9 years | 2 | 6.8 | 7.3 | 7.1 | 2 female |
2.2. Stimuli
In order to maximize both ecological validity and stimulus salience for young children, facial stimuli included participants’ mothers and appearance-matched strangers posing happy and angry expressions. Emotionally expressive faces were photographed against a white background while looking straight at the camera. For each participant, five happy and five angry photographs were chosen of his/her mother, as well as five happy and five angry photographs of another mother, matched for age (mothers of children ranged between the ages of 20 and 45; mothers of adults ranged between 48 and 70), appearance, and emotional intensity. ANOVAs revealed no difference in raters’ perceived emotional intensity between mothers of children vs. adults, or between facial expressions (happy vs. angry), and no interaction between age group and expression (Fs < 1). Image contrast and luminance levels were also normalized.
2.3. Procedure
Mothers of participants either initially visited the laboratory to be photographed or emailed digital photos of themselves based on written instructions. The goal was to obtain faces expressing anger or disapproval that were typical of children's daily experience. Mothers were instructed to make faces that included the face that, “when they see it, the children know they're in trouble or had better stop what they are doing.” Mothers of adults were asked to make the face they had made when their children were young, and the instructions were phrased in the past tense. Instructions included a request to make both angry and happy faces with mouth open and mouth closed, to control for confounds between expression and amount of tooth showing. Photographs were rated by three adult raters for emotion type and intensity level. Raters were asked to indicate whether each photo was angry, happy, or other, and to assign an intensity rating of 1–5 for emotional faces (angry or happy), and intensity ratings were averaged across all 3 raters. Five photos with mean ratings for the most intensely angry or displeased expressions, including photos with mouth open and mouth closed, were chosen first and then happy faces were chosen that matched the angry faces in intensity. Photos that were not identified by all raters as angry or happy were rejected. As a measure of inter-rater reliability, intra-class correlation coefficients were calculated using a two-way random effects model on the reliability of the mean rating (Shrout and Fleiss, 1979), R = 0.77, F = 4.56, p < 0.001. Finally, photos of another mother were chosen that were matched for age, appearance and affective intensity. The same 5 happy and 5 angry photos that were used as mother's faces for one participant were used as stranger's faces for another participant.
Prior to entering the scanner, children were familiarized with pictures of the scanner and scanner sounds. Following instructions, read aloud by the experimenter, children completed a practice block of 18 trials outside of the scanner (repeated as necessary). Using a “practice scanner” made up of a child's play tube, children were then coached to remain still while lying on their backs and pressing a button. In the scanner, a response box was placed at each participant's dominant hand. Foam padding was used to constrain the participants’ heads. Participants watched cartoons through MR compatible goggles while structural images were obtained. The task was presented after acquisition of structural images.
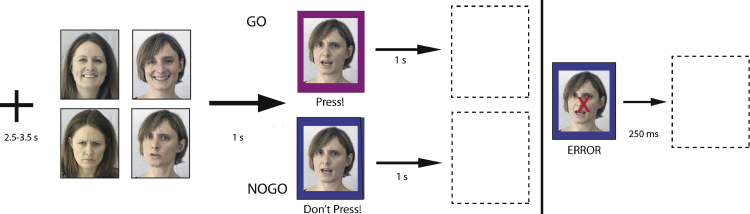
The experimental task was a rapid event-related design with random inter-trial intervals of between 2.5 and 3.5 s (3 s mean). Each trial could be Go, Nogo or null. In the Go and Nogo trials, a face would appear, and after 1000 ms, a colored frame appeared around the face (Fig. 1). The color of the frame (blue vs. purple, matched for luminance and counterbalanced across subjects) cued the participant to either press a button (Go) or withhold a button press (Nogo). In correct Go trials both face and frame disappeared immediately after the button was pressed. In correct Nogo trials, the frame remained around the face for 1000 ms, and then both face and frame disappeared. A green cross appeared on the screen for 250 ms following correct responses. A red X appeared and remained onscreen for 250 ms if the button was not pressed within 1000 ms of frame presentation (Go trials), or if the button was pressed incorrectly during a Nogo trial. Null trials consisted of a blank screen. 35 trials of each type (Go, Nogo, null) were randomly presented for each of 2 runs, for a total of 105 trials per run. For the Go and Nogo trials, there were 4 types of face stimulus: Mother-Happy, Mother-Angry, Stranger-Happy, and Stranger-Angry. Face presentation was pseudorandom: equal (as possible) numbers of each face type appeared over the course of the task but the order of presentation was unpredictable. The modification of the Go/Nogo task to have equal numbers of Go and Nogo trials was implemented so that the task would be easy enough for the youngest children to perform with high accuracy, and to maximize the number of correct Nogo trials for analysis. Altogether there were 70 Go and 70 Nogo trials, and both Go and Nogo cues could follow any of the 4 types of face. Key press responses were recorded with a handheld fiber optic keypad (Lumitouch, Burnaby, Canada). During the same session, participants viewed a single-run 7-min block design free viewing task in which the same set of faces was presented in single-condition blocks interleaved with scrambled faces. Results of this study are reported elsewhere (Todd et al., 2011). The order of task presentation (free viewing vs. Go/Nogo) was counterbalanced across participants.
Fig. 1.
Task Design: A single face was presented onscreen for 1 s, after which a frame appeared around the face. The color of the frame cued the participant to either press a button (Go condition, top), or withhold a press (NoGo condition, bottom). Four categories of face stimulus were used: Mother-Happy, Mother-Angry, Stranger-Happy, and Stranger-Angry. A red X appeared over the face on incorrect trials (incorrectly pressing, not pressing fast enough, or incorrectly withholding a press). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
2.4. fMRI data acquisition and analysis
Participants were scanned with a standard quadrature head coil on a 1.5 Tesla GE Excite HD scanner (GE Medical Systems, Milwaukee, WI). High resolution anatomical images were obtained using an axial 3D FSPGR sequence (TR/TE = 8.4/4.2 ms; FA = 15°; FOV = 240 mm; Voxel Size = 0.94 mm × 0.94 mm × 1.5 mm; 106 slices). Functional images were acquired using a T2*-weighted BOLD sensitive spiral in/out sequence (Glover and Thomason, 2004) (TR/TE = 2000/40 ms; FA = 90°; FOV = 240 mm; Voxel Size = 3.75 mm × 3.75 mm × 5 mm; 24 interleaved slices; 165 TRs per run).
2.4.1. First-level analysis
Data were analyzed using AFNI software (Cox, 1996). The first 3 volumes of each run were discarded and each run was analyzed separately. After slice timing and motion correction, functional data were aligned to an anatomical template using the standard coordinate space of Talairach and Tournoux (1988) and re-sampled into 3.75 mm cubic voxels. Functional data were also subjected to motion censoring (volumes with >3 mm of motion were dropped, and runs were dropped if >33% of volumes were censored). In the group of younger children, 4 out of 20 runs had spike motion which exceeded 3 mm (18, 13, 12, 5 scans). In the group of older children, 5 out of 38 total runs had >3 mm motion (6, 1, 1, 1, 1, scans). In the adult group, 2 out of 30 total runs had significant motion (3, 3 scans).
Data were then smoothed using a 6 mm FWHM Gaussian kernel, and converted to percent signal change. First-level general linear models (GLMs) included 3rd-order detrending and the 6 motion parameter estimates. Because we were interested in age and emotion-related differences in fMRI responses to cues indicating the demand to act or refrain from action, gamma functions were modeled for the response cue onsets (frames) that appeared around the face 1 s after face presentation and signaled whether the trial was Go or Nogo. Thus, fMRI activation linked to the cue onset was measured in correct Happy Go, Happy Nogo, Angry Go and Angry Nogo trials. Where appropriate, incorrect trials were modeled separately.
Our goal was to examine the influence of facial emotion on action withholding based on our two previous studies using variations of the same paradigm. First, in an ERP version of the same experiment we found that it was facial emotion rather than familiarity that interacted with task demand following the presentation of the Go/Nogo cue as examined here (Todd et al., 2008). Second, a free-viewing fMRI study looking at responses to familiarity and emotion of the faces themselves found that amygdala responsiveness to facial emotion, not familiarity, differed between age groups (Todd et al., 2011). Because we wanted to build on these findings to investigate the influence of facial expression on action demands in prefrontal regions densely linked to the amygdala, and to maximize the number of trials in each condition, we collapsed across mother and stranger faces.
2.4.2. Second-level analysis
At the group level, coefficients for each of the four conditions were entered into a 4-way mixed ANOVA using AFNI's GroupAna program implemented in MATLAB (Mathworks Inc., Natick, MA, USA), with Age Group (younger children, older children, adults), Emotion (happy vs. angry), Task (Go vs. Nogo) as fixed factors, and Subject as a random factor (2 repeats per subject). To correct for multiple comparisons, 3dClustSim was used to determine significant clusters at an individual voxel threshold of p < 0.02 and a minimum cluster extent of p < 0.05 (minimum of 31 voxels, volume of 1.634 cm3).
3. Results
3.1. Behavioral results
Response times for Go trials and accuracy for Go and Nogo trials were calculated for trials with angry vs. happy faces. RT was subjected to a repeated measures ANOVA with Emotion (Happy/Angry) as the within-subject variable and Age Group as the between-subject variable. Response time results revealed a main effect of Age Group, F = 28.36, p < 0.001. Planned contrasts revealed a linear effect, p < 0.001, showing typical decreasing response times with age (younger children, 646 ms; older children, 532 ms; adults, 409 ms). There was no effect of Emotion, or any significant Emotion × Age Group interaction, Fs < 0.1.
Accuracy data were subjected to a repeated measures ANOVA with Task Demand (Go/Nogo) and Emotion (Happy/Angry) as repeated measures and Age Group as the between-subject variable. Results showed an effect of Task Demand, F = 13.74, p < 0.001, with greater accuracy for Go than Nogo trials. There was also a main effect of Age Group, F = 8.49, p = 0.001. Planned contrasts revealed a linear effect, p = 0.001 showing increasing accuracy with age group. However, mean accuracy was near ceiling for each group (means: younger children, 96%; older children 97%, adults 99%). Thus, although significant, the differences in accuracy were very small. There was no effect of Emotion, or any significant Emotion × Age Group interaction, Fs < 0.1.
3.2. fMRI results
In order to examine activation differences related to the influence of emotional expression on prefrontal activation during action inhibition between early childhood and adulthood in, relation to the same baseline, fMRI data from all participants were subjected to a single voxelwise analysis with age-group as the between-subject variable. To investigate hypotheses about age-related differences between younger and older children, as well as between each group of children and adults, we examined pairwise group differences for the contrasts between cues following angry vs. happy faces in Nogo trials. To further probe significant effects identified by the between-group contrasts, we calculated mean signal change in 3 × 3 × 3 volumes centered on peak intensity in each significantly activated PFC region, and plotted activation for all subjects as a function of age group (Fig. 2B). Finally, to confirm that age effects showing valence differences in prefrontal activation for Nogo trial cues were not influenced by age-related differences in task difficulty or performance, follow-up ANCOVAs were performed in SPSS on mean percent signal change (angry–happy in Nogo trials) extracted from each 3 × 3 × 3 volume, with age group as the between subject factor and reaction time and accuracy as covariates.
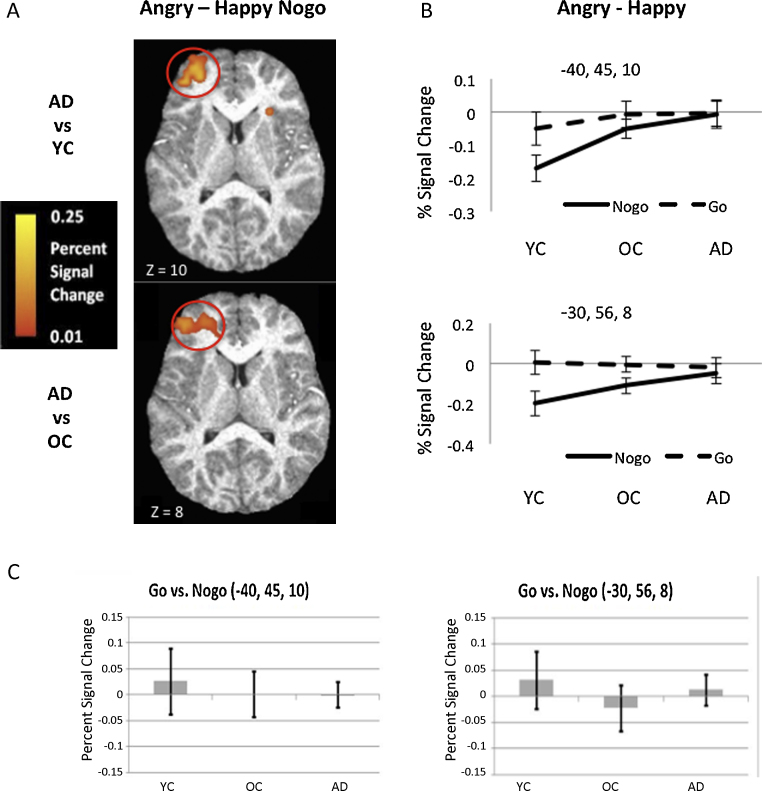
Fig. 2.
Left OFC brain activation peaking in left middle frontal gyrus (MFG) for response cue onset. (A) Activation maps from voxelwise analysis showing differences between adults and younger children (top) and between adults and older children (bottom) for the contrast Angry–Happy in Nogo trials. (B) Mean activation (% signal change) for left OFC (centered on activation for group contrasts illustrated in A) for the contrast Angry–Happy in Go and Nogo trials in each age group. Increasing activation was observed for cues following angry vs. happy faces with age group in Nogo trials. (C) Mean activation (% signal change) for left OFC regions for Go vs. Nogo trials in each age group. No age-related differences were found. YC, younger children; OC, older children; AD, adults.
3.2.1. Comparison 1: younger children vs. adults
The comparison between younger children and adults for the contrast [Angry > Happy] in Nogo trials revealed an orbitofrontal cluster in the left MFG (xyz: 40, 45, 10; Fig. 2A). Younger children showed greater activation for happy vs. angry faces in Nogo trials in this region, whereas adults did not. This finding was confirmed in follow-up analyses of the same contrast within each of the 3 age groups. When plotted across all 3 age groups, activation in this region showed a parametric effect of age group, with less contrast between happy and angry trials, due to greater relative activation for angry trials, with age (Fig. 2B). To examine the effects of behavioral performance on this parametric effect, one-way ANCOVAs were performed on percent signal change from this region with Age Group (3) as the between-subjects variable and accuracy and response time as covariates. Here the main effect of Age Group was trend-level, F (3,40) = 3.04, p = 0.06, yet there was a significant linear contrast, p = 0.02, showing increasing activation for angry relative to happy faces with age.
3.2.2. Comparison 2: older children vs. adults
The comparison between older children and adults for [Angry > Happy] in Nogo trials also revealed an orbitofrontal cluster in the left MFG near the peak for the contrast between younger children and adults (xyz: −30, 56, 8; Fig. 2A). Compared to adults, older children also showed greater activation for happy vs. angry faces in the left OFC in Nogo trials. This finding was confirmed in follow-up analyses of the same contrast in each group. Activation in this region also showed a linear effect of age group, with less contrast between happy and angry trials due to greater relative activation in angry Nogo trials with age. One-way ANCOVAs were again performed on percent signal change for this region, across all age groups, with accuracy and response time as covariates. Although the effect of Age Group was non-significant, F (3,01) = 2.35, p = 0.11, a significant linear contrast, p = 0.04, revealed increasing activation for angry relative to happy faces with age group for angry vs. happy faces in Nogo trials.
3.2.3. Comparison 3:younger vs. older children
The comparison between younger and older children for Angry > Happy in Nogo trials revealed an orbitofrontal cluster in the right MFG (xyz: 41, 45, 19; see Table 2 for all activations). In this region, younger children showed more activation for cues following happy relative to angry faces than older children, who did not discriminate between angry and happy faces in Nogo trials. This finding was confirmed in follow-up analyses of the same contrast within each group. Plotted across all 3 age groups, activation in this region showed a difference between younger children vs. older children and adults. Whereas contrasts between younger and older children remained significant after controlling for accuracy, p = 0.04, after controlling for response time the group difference was no longer significant, p = 0.09. When both measures were entered into the model, group differences were below trend level p > 0.1, suggesting that group differences in activation in right OFC may have been influenced by differences in task difficulty between younger and older children.
Table 2.
Regions activated in voxelwise analyses. Reported activations are corrected for multiple comparisons at an individual voxel threshold of p < 0.02 and a minimum cluster extent of p < 0.05 (minimum of 31 voxels, volume of 1.634 cm3).
| Contrast | Voxels | Intensity | Peak |
Region | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Nogo – Angry vs. Happy – AD vs. YC | 56 | 0.45 | −40 | 45 | 10 | L Middle Frontal Gyrus/BAs 46, 10 |
| Nogo – Angry vs. Happy – AD vs. OC | 35 | 0.67 | −30 | 56 | 8 | L Middle Frontal Gyrus/BA 10 |
| 34 | 0.24 | 30 | 19 | 1 | R Insula/BA 48 | |
| Nogo – Angry vs. Happy – OC vs. YC | 370 | 0.68 | 0 | 30 | 8 | Bilateral Anterior Cingulate/BAs 24, 25, 32 |
| 0.45 | 41 | 45 | 19 | R Middle Frontal Gyrus/BAs 10, 46 | ||
| 57 | 0.18 | −26 | −41 | 19 | L posterior Corpus Callosum | |
| 39 | 0.19 | 18 | −8 | 27 | L anterior Corpus Callosum | |
| Nogo – Angry vs. Happy – AD | 0 | |||||
| Nogo – Angry vs. Happy – OC | 605 | −0.70 | −38 | 60 | 12 | L Middle Frontal Gyrus/BA 10 |
| −0.4 | 30 | 60 | 12 | R Middle Frontal Gyrus/BA 10 | ||
| −0.3 | −2 | 56 | 12 | Middle Frontal Gyrus/BAs 9–10 | ||
| 130 | −0.27 | 64 | −34 | 12 | R Sup Temporal Gyrus/BA 22 | |
| −0.22 | 53 | −32 | 19 | R posterior Insula/BA 13 | ||
| 124 | −0.36 | −49 | 26 | 31 | L Middle Frontal Gyrus, BA 47 | |
| 107 | 0.37 | −34 | −26 | −18 | L Fusiform Gyrus/BA 20 | |
| 0.36 | −36 | −11 | −7 | L Insula/BA 21 | ||
| 72 | −0.23 | −41 | −15 | 8 | L Insula/Heschl Gyrus/BAs 13, 41 | |
| 65 | −0.22 | −56 | −64 | −3 | L Inferior Temporal Gyrus/BA 37 | |
| 55 | 0.14 | −22 | 8 | 34 | L Lingual Gyrus/BA 19 | |
| 51 | 0.37 | 0 | −34 | −14 | R Cerebellum | |
| 35 | 0.24 | 56 | −15 | −7 | R Middle Temporal Gyrus/BA 22 | |
| 33 | −0.29 | 23 | −60 | −11 | R fusiform gyrus/BA 19 | |
| 33 | −0.27 | −64 | −41 | −3 | L Middle Temporal Gyrus/BA 21 | |
| 31 | −0.70 | −4 | −79 | −22 | L Cerebellum | |
| Nogo – Angry vs. Happy – YC | 268 | −0.46 | 41 | 53 | 19 | R Middle – Sup Frontal Gyri/BA 46 |
| 184 | −0.93 | −30 | 64 | 19 | L Sup – Mid Frontal – Orbital Gyri/BAs 10, 46 | |
| 93 | −0.14 | 15 | −60 | 1 | R Precuneus/BA 23 | |
| 64 | −0.14 | 38 | 4 | 27 | R Precentral Gyrus – Operculum/BA 44 | |
| Go – Angry vs. Happy – AD vs. YC | 214 | −0.62 | 4 | −83 | 38 | L Cuneus – Precuneus/BAs 17, 18, 31 |
| 41 | −0.54 | 34 | 53 | 31 | R Middle Frontal Gyrus/BA 46 | |
| 41 | −1.34 | 4 | −49 | 64 | R Precuneus/BA 5 | |
| 40 | −0.31 | 0 | 41 | 23 | R Anterior Cingulate/BA 32 | |
| Go – Angry vs. Happy – AD vs. OC | 0 | |||||
| Go – Angry vs. Happy – OC vs. YC | 114 | −0.57 | 0 | −38 | 1 | R Lingual Gyrus – Cerebellum |
| 41 | −0.29 | −19 | −64 | −14 | L Cerebellum | |
| 34 | −0.13 | −15 | −23 | 8 | L Thalamus | |
| Go – Angry vs. Happy – AD | 0 | |||||
| Go – Angry vs. Happy – OC | 0 | |||||
| Go – Angry vs. Happy – YC | 152 | 0.47 | 19 | −83 | −22 | Cerebellum – Lingual Gyrus |
| 64 | 1.24 | 4 | −49 | 64 | R Precuneus/BA 5 | |
| 57 | 0.43 | 4 | −83 | 34 | R Cuneus/BA 18 | |
| 44 | 0.48 | 34 | 53 | 31 | R Middle Frontal Gyrus/BA 46 | |
| Go vs. Nogo – AD vs. OC | 33 | 0.26 | −45 | −49 | 53 | L Inferior Parietal Gyrus/BA 40 |
| Go vs. Nogo – AD vs. YC | 72 | 0.40 | −45 | −49 | 53 | L inferior Parietal Gyrus/BA 40 |
| Go vs. Nogo – OC vs. YC | 34 | 0.31 | −4 | 23 | −11 | Left Frontal Middle Orbital Gyrus/BA 11 |
| 32 | −0.24 | −23 | −30 | 57 | L Postcentral Gyrus/BA 3 | |
| Go vs. Nogo – Adults | 318 | 0.35 | −38 | −30 | 61 | L Postcentral – Precentral Gyrus/BAs 1–4 |
| 152 | 0.26 | −4 | −15 | 64 | Medial Frontal Gyrus/BA 6 | |
| 149 | 0.39 | 0 | −38 | 4 | Corpus Callosum | |
| 140 | −0.31 | 0 | −101 | −3 | Lingual Gyrus/BA 18 | |
| 76 | 0.23 | −4 | −79 | 38 | Precuneus/BA 19 | |
| 55 | 0.16 | 56 | −49 | 42 | R Inferior Parietal Gyrus/BA 21 | |
| 50 | 0.36 | 34 | −45 | −33 | R Cerebellum | |
| Go vs. Nogo – OC | 476 | 0.24 | −41 | −38 | 57 | L Postcentral – Precentral Gyrus/BAs 1–4 |
| 0.23 | 0 | −5 | 59 | Medial Frontal Gyrus/BA 6 | ||
| 182 | 0.19 | −30 | −60 | −26 | Bilateral Cerebellum | |
| 132 | −0.30 | 19 | −71 | −14 | Bilateral Lingual Gyrus | |
| 69 | 0.22 | 0 | −34 | 8 | Posterior Corpus Callosum | |
| 46 | 0.21 | −4 | 15 | 12 | L Caudate | |
| 33 | 0.13 | 38 | −8 | −11 | R Hippocampus | |
| Go vs. Nogo – YC | 477 | 1.18 | 0 | −11 | 68 | Medial Frontal Gyrus/BA 6 |
| 0.37 | −34 | −26 | 61 | L Precentral – Postcentral Gyrus/BAs 1- | ||
| 128 | 0.14 | −38 | 4 | 4 | L – R Insula/BA 48 | |
| 84 | 0.13 | 34 | 4 | 8 | R Insula | |
| 56 | −0.41 | 22 | −49 | 64 | R Superior Parietal Gyrus/BA 5 | |
| 49 | 0.24 | −49 | −23 | 16 | L Rolandic Operculum/BA 48 | |
Thus, both of the left OFC regions, which exhibited greater activation for angry vs. happy faces in Nogo trials with age, remained robust after controlling for differences in task performance. While suggestive, the finding of greater activation in right OFC for trials with happy vs. angry faces for younger vs. older children may have been confounded by age-related differences in task performance.
3.3. Task specificity of left MFG activation
To further examine whether age-related left OFC differences in activation for angry vs. happy faces were specific to the Nogo condition, activation for the contrast [Angry > Happy] in Go vs. Nogo trials was compared for both left MFG regions that showed robust effects of age group. Mean percent signal change from the 3 × 3 × 3 volumes centered on peak activation in these regions was subjected to repeated measures ANOVAs in SPSS with Task (Go vs. Nogo) as the within-subject factor and Age Group as the between-subject factor. For both regions, while there was no main effect of Age Group (ps > 1), there was an effect of Task showing greater differential activation between angry and happy faces for Nogo than Go trials F(1,41) = 4.38, p = 0.04 and F(1,41) = 9.79, p = 0.003, respectively. Although the interaction between Age Group and Task did not reach significance, planned contrasts again confirmed group differences for happy vs. angry faces in Nogo trials reported above (ps < 0.06). In contrast, none of the groups differed in activation for happy vs. angry faces in either left MFG region for Go trials (ps < 0.4) (Fig. 2B). Thus, follow-up analysis suggested that group differences in sensitivity to facial emotion were specific to Nogo trials.
3.4. Control analyses
To further ensure that our main results were not due to overall group differences in frontal activation related to task demand, voxelwise analyses were used to interrogate differences between Go and Nogo trials between each pair of groups as well as effects of task demand (Go vs. Nogo) within each group. As expected, the Go trials, which demanded a motor response, showed robust activation in left motor cortex that was greater for Go than Nogo trials in each separate group (see Table 2). There were no significant group activation differences in left OFC, and when investigated separately none of the groups showed significant activation for Go vs. Nogo in left OFC regions (Fig. 2C).
To determine whether our findings of age-related prefrontal valence differentiation were specific to trials in which responses were withheld, we further used voxelwise analyses to examine group differences between the angry vs. happy contrast within each age group separately, as well as between each pair of age groups for the contrast between angry and happy cues in Go trials. Again, no valence-related differences in OFC activation were found between groups, nor were there left OFC activations differentiating angry from happy faces within any group in Go trials. Valence-related differences were observed between older and younger children as well as between adults and younger children in visual cortex regions along the calcarine fissure, indicating greater activation for cues following angry than happy faces in Go trials for the youngest children only. However, our finding of age differences in frontal activation appears to be specific to the difference in fMRI response to angry vs. happy faces in a context in which a response must be withheld.
4. Discussion
Our results revealed novel developmental differences in prefrontal activation specific to stimulus valence, which in turn depended on the action demanded by the task. We found that, after we controlled for task performance, left lateral orbitofrontal regions discriminated Nogo cues in trials with happy vs. angry faces in children. This effect was larger in younger than in older children, and was not found in adults (Fig. 2). Such age-related changes in orbitofrontal responsiveness to facial expression were not observed in Go trials, nor did this region show age-related differences for Nogo trials in general.
The relative degree of left OFC activation for angry faces increased with age group in Nogo trials only – an activation pattern that mirrored our previously reported pattern of amygdala activation to stimulus valence alone, with greater amygdala activation for angry vs. happy faces in older participants (Todd et al., 2011). This convergence suggests that age-related differences in OFC responsiveness to stimulus valence, as modulated by task demand, may be partially driven by age-related changes in response to the facial expression itself. In addition to the left OFC activation that showed a linear change with age group, a region of right OFC was more sensitive to differences between happy and angry faces between kindergarten-aged and early school-aged children, suggesting this region may be more sensitive to changes that occur specifically within early-mid childhood. However, these results must be treated with caution as the effects were not significant after controlling for differences in behavioral performance.
In contrast to previous studies, which held salience relatively constant across age to look at differences in neural correlates of cognitive control (Lewis et al., 2006, Perlman and Pelphrey, 2010), the present study minimized age-related differences in cognitive control while tracking responses to the emotional valence of the preceding stimulus in relation to task demand. Between the ages of 4 and 9 the capacity for cognitive control is developing rapidly (Zelazo et al., 2003), and behavioral and neural correlates of cognitive control continue to develop through adolescence (Luna, 2009). However, by four years of age the capacity for using simple response rules in a non-speeded condition is well established (Jones et al., 2003). Thus, our task was well within the capacity of even our youngest children, as reflected by accuracy rates that were almost at ceiling. In contrast, our age distinction based on elementary school entry not only encapsulates a major shift in social development, marked by increased independence and higher social demands (Entwisle and Alexander, 1998), it also captures developmental changes in the salience of specific facial expressions – developmental changes that continue through adolescence (Durand et al., 2007, Gao and Maurer, 2009, Vida and Mondloch, 2009).
In addition to increasing salience or legibility of angry relative to happy faces with age, the degree of conflict between the action signaled by the face and the demands of the task may also be changing. There is evidence that smiling faces signal encouragement or approach and angry faces signal the need to stop or change a behavior or withdraw (Roelofs et al., 2009, Blair et al., 1999, Hare et al., 2005). The same region of left lateral OFC we report has been found to show greater activation in adults when participants had to override behavior evoked by facial emotion by pulling a lever to withdraw from happy faces and pushing it to approach angry faces (Roelofs et al., 2009). Recent studies have further found that de-activating this region increased error rates in the same task, suggesting left OFC is necessary for successful override of prepotent responses to facial emotion (Volman et al., 2011a, Volman et al., 2011b). Our data suggest that, in young children, the dissonance between the encouragement to act or approach signaled by a happy face and the demand to withhold an action elicits more activation in a region sensitive to such conflict than in adults. Our finding that adults did not show the same lateral OFC sensitivity to withholding a response for smiling faces may also reflect the fact that, with age, less conflict was generated between the action and expression because active withdrawal was not required in our task.
Reduced activation in left lateral OFC linked to the conflict between facial expression and task demand has also been associated with increased amygdala activation (Volman et al., 2011a, Volman et al., 2011b). Such findings in humans are consistent with non-human animal research suggesting the amygdala and OFC, which mediate associative learning, interact in linking stimulus evaluation and response selection (Saddoris et al., 2005, Schoenbaum et al., 2003). Taken with the parallel pattern of amygdala activation we found in a free viewing task (Todd et al., 2011), our own data are consistent with findings of a reciprocal relation between the amygdala and OFC in conditions of conflict between expression and required response. We can speculate that the pattern of amygdala activation we observed in young children is linked to a relatively automatic stimulus response association between smiling face and approach. The strength of this association may in turn be driven by the comparatively higher salience of smiling faces for younger children.
With development, the OFC is thought to respond to increasingly more complex combinations of stimulus evaluation and action requirements (Nelson and Guyer, 2011). Infants as young as 3–7 months show medial OFC sensitivity to vocal emotion (Blasi et al., 2011), and in one-year-olds OFC activation has been found to be sensitive to the reward of a mother's smile (Minagawa-Kawai et al., 2009). Whereas in infancy medial OFC activation may track the simple motivational salience of stimuli, our data provide novel evidence that, by 4–6 years, lateral OFC activation reflects a mid level of complexity–the interaction between stimulus valence and response demand. Such a pattern may reflect medial to lateral maturation of ventral prefrontal regions (Gogtay et al., 2004).
Additional results included an un-hypothesized finding of activation in visual cortex for Go cues, following angry faces, that was greater in younger than older children, but was not observed in adults. It is well established that enhanced visual cortex activation is linked to increased attention to a stimulus [for review see Corbetta and Shulman (2002)], suggesting the possibility that viewing angry faces increases attention to action cues more in younger children. This interpretation is consistent with findings that young children show more rapid ERP activation over visual cortex for threatening faces than for other facial expressions in oddball tasks requiring substantial action inhibition – an effect that is not observed in older age groups (Batty and Taylor, 2006). Future research will be required to test the effect of facial emotion on low-level features of a stimulus or cue in development.
4.1. Limitations and future directions
Several limitations to the study qualify our interpretation of the results and point to future research. First, with regard to the face stimuli used, the mothers of the adults were of course considerably older than those of the children, as were the matched stranger faces. There were no differences in rated facial expression intensity between mothers of adults and children and control analyses did not reveal age related differences in response to facial emotion that was not dependent on task demand, yet we cannot rule out that our results might be influenced by differences in maternal age. Moreover, a mothers’ expression of approval and disapproval likely has a different meaning for adults than for children, whose daily wellbeing is strongly influenced by maternal approval. Second, the generalizability of the results may be limited by demographic inequalities in the sample, especially given the relatively small sample size of the youngest group, and the preponderance of females. The exclusion of larger number of younger vs. older children due to poor performance and movement artifacts also raises the possibility that the remaining group of young children may not be representative. While we have no reason to believe that they differed, the groups were also not explicitly matched for SES and intelligence, and we cannot rule out that these factors influenced our findings. Third, despite the very high levels of accuracy across all participants, there were age-related differences in task performance that influenced results of interest in right OFC. In future studies it may be beneficial to employ an algorithm that adjusts accuracy to a specific level based on performance (Lamm et al., 2006, Leibenluft et al., 2007, Lewis et al., 2006), while still keeping accuracy rates near ceiling. This would allow us to better interpret fMRI activation as independent of task performance.
Although our findings showed a parametric effect across age groups, the specific nature of such a shift between mid childhood and adulthood is unknown. Future research should examine activation patterns that reflect differences in prefrontally mediated social-emotional processes between pre-adolescents and adolescents and adults, including OFC responsiveness to emotional faces (Monk et al., 2003), social evaluation (Blakemore, 2008), and reward-related behavior (Galvan et al., 2006). Finally, it is necessary to use caution in inferring developmental trends from a cross sectional study. Future studies should employ longitudinal designs to examine developmental changes in the interaction between stimulus salience and control processes within the same group of participants. Specifically, an important goal for future research will be to examine lateral OFC connectivity with the amygdala in the associative learning of stimulus evaluation and response demand, as well as the capacity to override such associations, as it develops over childhood and adolescence.
5. Conclusions
In conclusion, our findings of robust age-related modulation of OFC activation by emotional valence under the constraints of specific task requirements suggest that, with development, brain regions implicated in evaluation of the effects of social-emotional information on action are differently modulated by emotional salience as well as by changing capacity for cognitive control. Such findings point to the importance of integrating lifespan developmental changes into models of interactions between emotional salience and control processes.
Conflict of interest
None.
Acknowledgements
The authors would like to thank Evan Thompson for editorial guidance and Tammy Rayner for her expertise in scanning young participants. The research was funded by a National Science and Engineering Council postgraduate scholarship (RMT), the Sick Kids Foundation Scholarship Program (JWE), and a grant from the Canadian Institutes for Health Research (MJT).
References
- Batty M., Taylor M.J. The development of emotional face processing during childhood. Developmental Science. 2006;9(2):207–220. doi: 10.1111/j.1467-7687.2006.00480.x. [DOI] [PubMed] [Google Scholar]
- Blair R.J., Morris J.S., Frith C.D., Perrett D.I., Dolan R.J. Dissociable neural responses to facial expressions of sadness and anger. Brain. 1999;122(Pt 5):883–893. doi: 10.1093/brain/122.5.883. [DOI] [PubMed] [Google Scholar]
- Blakemore S.J. The social brain in adolescence. Nature Reviews Neuroscience. 2008;9(4):267–277. doi: 10.1038/nrn2353. [DOI] [PubMed] [Google Scholar]
- Blasi A., Mercure E., Lloyd-Fox S., Thomson A., Brammer M., Sauter D., Murphy D.G. Early specialization for voice and emotion processing in the infant brain. Current Biology. 2011;21(14):1220–1224. doi: 10.1016/j.cub.2011.06.009. [DOI] [PubMed] [Google Scholar]
- Boseovski J.J., Lee K. Seeing the world through rose-colored glasses? Neglect of consensus information in young children's personality judgments. Social Development. 2008;17(2):399–416. doi: 10.1111/j.1467-9507.2007.00431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunge S.A., Zelazo P.D. A brain-based account of the development of rule use in childhood. Current Directions in Psychological Science. 2006;15(3):118–121. [Google Scholar]
- Corbetta M., Shulman G.L. Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience. 2002;3(3):201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Cox R.W. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Davidson M.C., Amso D., Anderson L.C., Diamond A. Development of cognitive control and executive functions from 4 to 13 years: evidence from manipulations of memory, inhibition, and task switching. Neuropsychologia. 2006;44(11):2037–2078. doi: 10.1016/j.neuropsychologia.2006.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand K., Gallay M., Seigneuric A., Robichon F., Baudouin J.Y. The development of facial emotion recognition: the role of configural information. Journal of Experimental Child Psychology. 2007;97(1):14–27. doi: 10.1016/j.jecp.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Eccles J., Midgely C., Adler T.F. Grade-related changes in the school environment: effects on achievement motivation. In: Nichols J.G., editor. The Development of Achievement and Motivation. JAI Press; Greenwich, CT: 1984. pp. 283–331. [Google Scholar]
- Entwisle D.R., Alexander K.L. Facilitating the transition to first grade: the nature of transition and research on factors affecting it. Elementary School Journal. 1998;98(4):351–364. [Google Scholar]
- Galvan A., Hare T.A., Parra C.E., Penn J., Voss H., Glover G., Casey B.J. Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. Journal of Neuroscience. 2006;26(25):6885–6892. doi: 10.1523/JNEUROSCI.1062-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X., Maurer D. Influence of intensity on children's sensitivity to happy, sad, and fearful facial expressions. Journal of Experimental Child Psychology. 2009;102(4):503–521. doi: 10.1016/j.jecp.2008.11.002. [DOI] [PubMed] [Google Scholar]
- Glover G.H., Thomason M.E. Improved combination of spiral-in/out images for BOLD fMRI. Magnetic Resonance in Medicine. 2004;51(4):863–868. doi: 10.1002/mrm.20016. [DOI] [PubMed] [Google Scholar]
- Gogtay N., Giedd J.N., Lusk L., Hayashi K.M., Greenstein D., Vaituzis A.C., Thompson P.M. Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences United States of America. 2004;101(21):8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein M., Brendel G., Tuescher O., Pan H., Epstein J., Beutel M., Silbersweig D. Neural substrates of the interaction of emotional stimulus processing and motor inhibitory control: an emotional linguistic go/no-go fMRI study. Neuroimage. 2007;36(3):1026–1040. doi: 10.1016/j.neuroimage.2007.01.056. [DOI] [PubMed] [Google Scholar]
- Gunther Moor B., van Leijenhorst L., Rombouts S.A., Crone E.A., Van der Molen M.W. Do you like me? Neural correlates of social evaluation and developmental trajectories. Social Neuroscience. 2010:1–22. doi: 10.1080/17470910903526155. [DOI] [PubMed] [Google Scholar]
- Hare T.A., Tottenham N., Davidson M.C., Glover G.H., Casey B.J. Contributions of amygdala and striatal activity in emotion regulation. Biological Psychiatry. 2005;57(6):624–632. doi: 10.1016/j.biopsych.2004.12.038. [DOI] [PubMed] [Google Scholar]
- Jones L.B., Rothbart M.K., Posner M. Development of executive attention in preschool children. Developmental Science. 2003;6:498–504. [Google Scholar]
- Lamm C., Zelazo P.D., Lewis M.D. Neural correlates of cognitive control in childhood and adolescence: disentangling the contributions of age and executive function. Neuropsychologia. 2006;44(11):2139–2148. doi: 10.1016/j.neuropsychologia.2005.10.013. [DOI] [PubMed] [Google Scholar]
- Leibenluft E., Rich B.A., Vinton D.T., Nelson E.E., Fromm S.J., Berghorst L.H., Pine D.S. Neural circuitry engaged during unsuccessful motor inhibition in pediatric bipolar disorder. The American Journal of Psychiatry. 2007;164(1):52–60. doi: 10.1176/ajp.2007.164.1.A52. [DOI] [PubMed] [Google Scholar]
- Lewis M.D., Lamm C., Segalowitz S.J., Stieben J., Zelazo P.D. Neurophysiological correlates of emotion regulation in children and adolescents. Journal of Cognitive Neuroscience. 2006;18(3):430–443. doi: 10.1162/089892906775990633. [DOI] [PubMed] [Google Scholar]
- Luna B. Developmental changes in cognitive control through adolescence. [Review] Advances in Child Development and Behavior. 2009;37:233–278. doi: 10.1016/s0065-2407(09)03706-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather M., Canli T., English T., Whitfield S., Wais P., Ochsner K., Carstensen L.L. Amygdala responses to emotionally valenced stimuli in older and younger adults. Psychological Science. 2004;15(4):259–263. doi: 10.1111/j.0956-7976.2004.00662.x. [DOI] [PubMed] [Google Scholar]
- Mather M., Carstensen L.L. Aging and attentional biases for emotional faces. Psychological Science. 2003;14(5):409–415. doi: 10.1111/1467-9280.01455. [DOI] [PubMed] [Google Scholar]
- Minagawa-Kawai Y., Matsuoka S., Dan I., Naoi N., Nakamura K., Kojima S. Prefrontal activation associated with social attachment: facial-emotion recognition in mothers and infants. Cerebral Cortex. 2009;19(2):284–292. doi: 10.1093/cercor/bhn081. [DOI] [PubMed] [Google Scholar]
- Monk C.S., McClure E.B., Nelson E.E., Zarahn E., Bilder R.M., Leibenluft E., Pine D.S. Adolescent immaturity in attention-related brain engagement to emotional facial expressions. Neuroimage. 2003;20(1):420–428. doi: 10.1016/s1053-8119(03)00355-0. [DOI] [PubMed] [Google Scholar]
- Nelson E.E., Guyer A.E. The development of the ventral prefrontal cortex and social flexibilty. Developmental Cognitive Neuroscience. 2011;1(3):233–245. doi: 10.1016/j.dcn.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell S., Noseworthy M.D., Levine B., Dennis M. Cortical thickness of the frontopolar area in typically developing children and adolescents. Neuroimage. 2005;24(4):948–954. doi: 10.1016/j.neuroimage.2004.10.014. [DOI] [PubMed] [Google Scholar]
- Perlman S.B., Pelphrey K.A. Developing connections for affective regulation: age-related changes in emotional brain connectivity. Journal of Experimental Child Psychology. 2010 doi: 10.1016/j.jecp.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L. How do emotion and motivation direct executive control? Trends in Cognitive Sciences. 2009;13(4):160–166. doi: 10.1016/j.tics.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelofs K., Minelli A., Mars R.B., van Peer J., Toni I. On the neural control of social emotional behavior. Social Cognitive and Affective Neuroscience. 2009;4(1):50–58. doi: 10.1093/scan/nsn036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saddoris M.P., Gallagher M., Schoenbaum G. Rapid associative encoding in basolateral amygdala depends on connections with orbitofrontal cortex. [Research Support, U.S. Gov't, P.H.S.] Neuron. 2005;46(2):321–331. doi: 10.1016/j.neuron.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G., Roesch M.R., Stalnaker T.A., Takahashi Y.K. A new perspective on the role of the orbitofrontal cortex in adaptive behaviour. Nature Reviews Neuroscience. 2009;10(12):885–892. doi: 10.1038/nrn2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G., Setlow B., Saddoris M.P., Gallagher M. Encoding predicted outcome and acquired value in orbitofrontal cortex during cue sampling depends upon input from basolateral amygdala. Neuron. 2003;39(5):855–867. doi: 10.1016/s0896-6273(03)00474-4. [DOI] [PubMed] [Google Scholar]
- Shrout P.E., Fleiss J.L. Intraclass correlations: uses in assessing rater reliability. Psychological Bulletin. 1979;86(2):420–428. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- Talairach J., Tournoux P. Beorg Thieme Verlag; Stuttgart: 1988. Co-Planar Stereotactic Atlas of the Human Brain. [Google Scholar]
- Thompson P.M., Giedd J.N., Woods R.P., MacDonald D., Evans A.C., Toga A.W. Growth patterns in the developing brain detected by using continuum mechanical tensor maps. Nature. 2000;404(6774):190–193. doi: 10.1038/35004593. [DOI] [PubMed] [Google Scholar]
- Todd R.M., Evans J.W., Morris D., Lewis M.D., Taylor M.J. The changing face of emotion: age-related patterns of amygdala activation to salient faces. Social Cognitive and Affective Neuroscience. 2011;6(1):12–23. doi: 10.1093/scan/nsq007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd R.M., Lewis M.D. Self-regulation in the developing brain. In: Reed J., Warner-Rogers J., editors. Child Neuropsychology: Concepts, Theory, and Practice. Wiley-Blackwell; Chichester, UK: 2008. pp. 285–315. [Google Scholar]
- Todd R.M., Lewis M.D., Meusel L.A., Zelazo P.D. The time course of social-emotional processing in early childhood: ERP responses to facial affect and personal familiarity in a Go-Nogo task. Neuropsychologia. 2008;46(2):595–613. doi: 10.1016/j.neuropsychologia.2007.10.011. [DOI] [PubMed] [Google Scholar]
- Vida M.D., Mondloch C.J. Children's representations of facial expression and identity: identity-contingent expression aftereffects. Journal of Experimental Child Psychology. 2009;104(3):326–345. doi: 10.1016/j.jecp.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Volman I., Roelofs K., Koch S., Verhagen L., Toni I. Anterior prefrontal cortex inhibition impairs control over social emotional actions. Current Biology. 2011;21(20):1766–1770. doi: 10.1016/j.cub.2011.08.050. [DOI] [PubMed] [Google Scholar]
- Volman I., Toni I., Verhagen L., Roelofs K. Endogenous testosterone modulates prefrontal-amygdala connectivity during social emotional behavior. Cerebral Cortex. 2011;21(10):2282–2290. doi: 10.1093/cercor/bhr001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelazo P.D., Muller U., Frye D., Marcovitch S., Argitis G., Boseovski J., Sutherland A. The development of executive function in early childhood. Monographs of the Society for Research in Child Development. 2003;68(3):vii–vii137. doi: 10.1111/j.0037-976x.2003.00260.x. [DOI] [PubMed] [Google Scholar]