Highlights
* We examine the relation between brain and behavior indices of empathy across age. * Self-report measures of empathy indicate greater responsivity by females, which increases with age. * Implicit hemodynamic and physiological measures do not demonstrate any gender-related patterns.
Keywords: Developmental neuroscience, Affective neuroscience, Empathy, Sex differences
Abstract
Behavioral research indicates that human females are more empathic than males, a disparity that widens from childhood to adulthood. Nevertheless, the extent to which such sex differences are an artifact of self-report indices is unclear. The present study compared age-related sex differences in both self-report and neurophysiological measures of empathic arousal, a primary building block of empathy. Participants included sixty-five 4–17-year-old children (mean 11.5±3.5 years) who completed the Bryant Empathy Scale, and were scanned while viewing animated clips depicting people being hurt. Female participants scored higher than males on self-reported dispositional empathy, a difference that increased with age. In contrast, no sex-related differential changes were detected in hemodynamic responses or in pupil dilation, with no interaction between sex and age. Results suggest a dissociation between explicit ratings and neurophysiological measures of empathic arousal. Past observed sex differences in empathy may reflect females’ greater willingness to report empathic experiences. Findings are also discussed in terms of discrepancies in the methods used to assess affective responding and how they relate to the multi-faceted construct of empathy.
1. Introduction
It is a commonly held belief that females are more interpersonally sensitive than males. Some scholars have suggested that empathy, defined as the ability to share and understand the emotions and feelings of others in relation to oneself, is a critical cognitive difference between females and males. In particular, past research demonstrates an augmentation of empathic ability in the former and suppression in the latter (Baron-Cohen and Wheelwright, 2004). Such a disparity in empathy levels between males and females is consistent with a biological disparity in parental investment, which would make it particularly advantageous for females to have a high level of empathy to protect and care for their young and to affiliate with kin (Decety and Sveltova, 2012, Decety et al., 2012b). Sex differences in empathy have also been hypothesized as resulting from sex-differentiated processes whereby males engage in more intrasexual competition, status striving, and attempts at resource accumulation than do females (Geary, 2002; but see Andersen et al., 2012), processes which are thought to require low levels of empathizing.
Given the empirical and theoretical evidence of sex differences in empathy, one might expect to observe divergences in empathy across a large range of measures. Indeed, empathy researchers have a wide variety of tools at their disposal including self and other- (e.g., parent or teacher) report questionnaires, facial or vocal indices, physiological measures and, more recently, neuroscience methods including functional magnetic resonance imaging (fMRI) and electroencephalography (EEG). Yet, existing research that compares across a wide range of measures reveals surprisingly inconsistent findings and mixed reports of sex differences. One challenge involves understanding and integrating across measures: it is possible that different measurement techniques may tap diverse aspects of empathy, and that sex differences may not be universally present across all empathic components. Thus, paradigms that investigate diverse measures of empathy, and seek to understand the nature of sex differences across indices are of interest. The current paper takes up this challenge by comparing measures of explicit, self-reported experiences of empathy with neurophysiological and autonomic responses to empathy-eliciting stimuli in the same paradigm to determine whether and how sex differences in empathic responses are observed across different analyses.
Across many tools of measurement, studies of empathy are inherently complex. Empathy is not a single ability but a complex socio-emotional competency that encompasses different components (Decety, 2011). Even though these components are intertwined and not independent of one another, past research suggests that they may also be dissociable. In particular, breaking down a larger empathy construct may be particularly fruitful in investigations of sex differences, as a more nuanced exploration of diverse empathic components would permit a detailed investigation of potential sex differences within each component. Empathic arousal, the first element of empathy to appear during ontogeny, refers to the contagious sharing of the affective state of another; empathic understanding entails the formation of an explicit representation of the feelings of another person as an intentional agent and emotion regulation enables the control of emotion, affect, drive and motivation (Decety, 2010, Singer and Lamm, 2009). Recent affective developmental neuroscience research with child and adult participants indicates that the affective, cognitive and regulatory aspects of empathy involve interacting, yet partially non-overlapping, neural circuits (Decety et al., 2012a). Furthermore, there is now evidence for age-related changes in these neural circuits which reveal how brain maturation influences reactions to the distress of others (Decety and Michalska, 2010).
The most robust evidence of sex differences in empathy that favor females are observed in studies that employ self-report measures of empathy. As illustration, in a well-known study with adults, women were found to score significantly higher than men on the empathizing quotient (EQ) self-report questionnaire (Baron-Cohen and Wheelwright, 2004), which measures attention to the needs and situations of others, as evidenced by perspective-taking and cooperativeness. Other studies relying on self-report data have similarly suggested that females might be more empathic than males (Cohen and Strayer, 1996, Davis and Franzoi, 1991, Rueckert and Naybar, 2008), and that these sex differences in self-reports emerge as early as 6–9 years of age (Chapman et al., 2006). The empathy disparity between the sexes has also been found to widen between early childhood and adulthood. In a series of experiments designed to evoke personal distress and empathic sadness either through watching videotapes or through mood induction in children and adults, both sex and age-related effects of self-reported emotions were observed. Females reported more distress and more empathy than did males, and the discrepancy between the sexes increased with age (Eisenberg et al., 1989).
Beyond self-reported empathy, some emotional judgment tasks provide supporting evidence for the claim of sex-related difference in empathy. For instance, research with the “Reading the Mind in the Eyes” test (an examination of recognition of mental states and emotions from the eye region of the face) finds that female children and adults score significantly higher than males (Chapman et al., 2006, Baron-Cohen et al., 2001; see McClure, 2000, for a meta-analysis of sex differences in facial cue detection). It has been suggested that exposure to prenatal androgens influences these post-natal social outcomes. As illustration, significant negative correlations have been reported between levels of fetal testosterone in mothers’ amniotic fluid and their school-aged children's ability to read emotional expressions (Chapman et al., 2006). In a laboratory setting, administration of testosterone in young women was found to induce a significant impairment in their cognitive empathy (Van Honk et al., 2011). Preschool-aged children's social relationships (Knickmeyer et al., 2005) and use of intentional language (Knickmeyer et al., 2006) has similarly been observed to correlate with fetal testosterone exposure.
Nonetheless, although the data reviewed above suggest that females display an advantage over males in their ability to correctly identify others’ emotional expressions, there are some qualifications to these observed tendencies. First, the female advantage is most evident when participants judge the face as opposed to other nonverbal channels such as body language and prosody (Hall and Mast, 2007). Second, while females may judge emotion cues more accurately than males, several studies report that females do not appear to display a corresponding advantage in their ability to infer the specific content of other people's thoughts and feelings (Hall, 1978, Hancock and Ickes, 1996, Ickes et al., 1990, Marangoni et al., 1995). Third, motivational factors strongly influence the perception of nonverbal cues. Past work has shown that women are relatively more empathically accurate than men when female gender roles are made salient before an empathic accuracy task (Klein and Hodges, 2001; see Ickes et al., 2000 for a comprehensive meta-analysis), suggesting that social roles and contexts may influence observed female advantages in empathy.
When nonverbal measures that rely on physiological or neural data are considered, reports of sex differences in empathy become even less clear. A review and meta-analysis of studies of empathy and related abilities using self-report and psychophysiological methods by Eisenberg and Lennon (1983) provides support for the role of gender-based response biases in self-reports of empathy (see also follow-up by Lennon and Eisenberg, 1987). This research demonstrated that changes in autonomic nervous system activity such as heart rate, electrodermal activity and blood pressure, as well as facial and gestural measures, showed no clear evidence for sex differences in either children or adults, while more explicit measures such as self-report questionnaires and (to a lesser extent) emotion-identification tasks did reveal sex differences. This research therefore suggests that sex differences may be differentially observed across implicit and explicit measures of empathic responding.
Considering additional implicit analyses, the past decade has seen an explosion of research that studies empathy through the use of functional neuroimaging techniques. Intriguingly, these initial investigations have revealed more similarities than disparities across the sexes (Lamm et al., 2011). Using meta-analytical techniques, Wager et al. (2003) examined 65 neuroimaging studies of responses to emotional stimuli and found that women did not show greater activation to viewing emotional material than men, contrary to prior behavioral findings. Although men showed greater lateralized activation in response to emotional stimuli (consistent with the generally greater hemispheric asymmetry of function that is sometimes observed for males; i.e., Killgore and Gangestad, 1999, Killgore, 2000), amygdala activity elicited by emotional stimuli was left-lateralized in both men and women. Only a handful of neuroimaging studies have demonstrated any evidence of sex effects on the neural mechanisms of empathic responding, particularly with regard to lateralization of function (Derntl et al., 2010, Killgore et al., 2001, Schulte-Rüther et al., 2008). In one fMRI study of empathy for pain in adults (Singer et al., 2006), both sexes showed activation of similar brain regions when inferring the feelings of others based on abstract visual cues indicating pain. In males, but not females, this response was attenuated when watching people in pain who had previously behaved unfairly, again suggesting that empathic reactions may be differentially mediated by motivational factors in men and women. In general, neuroimaging studies examining sex differences in empathic responses are in their early stages: many studies have examined brain activations in response to empathy-eliciting stimuli in either men or women, but they have seldom directly compared men and women within the same study, often because the sample sizes for each sex were insufficient.
In sum, there is an observed tension in the literature concerning the presence of sex differences in empathy. Data from dispositional and other self-report measures often provide evidence that females either intrinsically experience more empathy than do males, or use different strategies of cognitive and affective processing, which may contribute to observed sex differences. Yet other nonverbal and biological measures of empathy do not consistently show this difference. One possible interpretation for this discrepancy, proposed by Eisenberg and Lennon (1983), is that sex differences favoring females could be due to biases in self-reports, such that individuals may respond in ways to be consistent with sex-role stereotypes. Differential performance in empathy tasks may reflect sex differences in reluctance to report empathic experiences, stemming from societal expectations regarding the expression of emotion (which is often valued in women but not men), rather than intrinsic differences between males and females in levels of underlying empathy. Questions that frame dispositional surveys as tests of empathy may prompt responses influenced by participants’ identification with gender stereotypes. In support of this idea, effects of gender stereotype-congruent responding have been observed at both behavioral and neural levels in other domains (e.g., studies of male vs. female math and spatial abilities, Massa et al., 2005, Krendl et al., 2008, Mangels et al., 2012).
A second potential explanation for discordant findings across measures, which is not necessarily mutually exclusive with the first, is that the various measures assess different aspects of empathic experience, and variability in one measure is not synonymous with variability in another. As discussed above, empathy is composed of multiple facets, and it is conceivable that self-report measures and physiological responses to a distressing situation actually test different components of empathy (e.g., cognitive empathy or emotion understanding vs. affective arousal). It is thus possible that self-report biases are not the unique source of differences between boys’ and girls’ explicit avowals of empathy. Furthermore, it should be noted that different component processes of empathy may follow divergent developmental trajectories. Developmental changes have been observed in the functional organization of neural structures implicated in both cognitive and affective empathy from childhood to adulthood (Decety and Michalska, 2010, Killgore et al., 2001), so if sources of variability in empathic ability are to be better understood, age needs to be considered as a critical variable. Studies of age-related changes in both explicit and implicit measures of empathy within the same group of participants will be particularly useful in furthering our understanding of the multidimensional facets of empathic abilities within individuals and across sexes.
The current study examines how developmental changes in self reports of empathic responding compare with developmental changes in empathy-related brain function and pupil dilation. By including both multiple nonverbal and explicit reports, the study permits comparison across several different types of empathy measurements within the same individual and across males and females. Moreover, because the study uses the same set of measurements across a wide range of participant ages, it also can provide a clear view of developmental trends that emerge through this comparison.
The experimental paradigm that this study adopts is a paradigm developed in our laboratory consisting of dynamic visual empathy-eliciting stimuli that have been well validated and employed in previous work with children, adolescents and adults (Decety and Michalska, 2010, Decety et al., 2012a, Decety et al., 2009). This paradigm has also been employed in studies across different cultures, such as Japan (Moriguchi et al., 2007), Taiwan (Cheng et al., 2010) and Germany (Otti et al., 2010). The stimuli depict painful situations that have either been caused by accident or on purpose by another individual. Results from these studies indicate that attending to painful situations caused by accident is associated with activation of regions belonging to the so-called “pain matrix”, including the anterior midcingulate cortex (aMCC), supplementary motor area (SMA), anterior insular cortex (AIC), periaqueductal gray (PAG) and somatosensory cortex, whereas attending to similar situations caused intentionally activates these same regions, as well as additional areas that are commonly engaged in mental state understanding and affective evaluation (temporoparietal junction and medial and orbital frontal cortices). Neuroimaging studies with pediatric samples indicate that there are age-related changes in the hemodynamic response to both types of stimuli (Decety and Michalska, 2010, Decety et al., 2012a).
In the present study, we combined explicit measures of dispositional empathy and evaluations of empathy-eliciting stimuli with neuro-hemodynamic response and affective arousal, as measured by pupil dilation. If the developmental course of empathy differs universally for males and females, then both self-report and neuro-physiological measures should be correlated and demonstrate an increasing female advantage with age. If, however, implicit and explicit measures of empathy tap different constructs, or previously observed sex disparities in empathy primarily reflect response biases, then we would expect age-related trajectories to show a female advantage in the explicit measure only.
2. Materials and methods
2.1. Participants
Sixty-five children and adolescents from age 4 to 17 years (mean 11.5±3.5 years) participated in this experiment. Thirty participants (46.2%, mean age 11.5±3.8) were female, and thirty-five (53.8%, mean age 11.4±3.3 years) were male. The age distribution of participants was as follows: age 4–7 years, mean age 6.42±1.06 (N=13, 6 females); age 8–12, mean age 10.80±1.52 (N=30, 13 females); age 13–17, mean age 15.32±1.61 (N=22, 11 females). Participants in the present dataset were a subset of a larger fMRI study on the neural development of moral sensitivity (Decety et al., 2012a). Parents’ written informed consent was obtained in addition to children and adolescents’ verbal assent. All participants were paid for their participation. The study was approved by the University of Chicago Institutional Review Board and conducted in accordance with the Declaration of Helsinki.
2.2. Dispositional measures of empathy
First, participants were administered the Bryant Empathy Scale (Bryant, 1982), which the author defined and validated as measuring affective empathy, or the ability to respond affectively to the perceived emotional experiences of others, and is a widely used and reliable measure of children's empathic tendencies, which has moderate to strong internal consistency and demonstrates strong test–retest reliability (Bryant, 1982, De Wied et al., 2007). Analyses were focused on the empathic sadness (ES) subcomponent, comprised of five items, including items like “I get upset when I see a child being hurt,” and “Seeing another child who is crying makes me feel like crying”. Factor analytic studies have proposed that the ES scale is more relevant to affective empathy than the Attitude Scale subcomponent, which reflects negative attitudes toward emotional behavior (De Wied et al., 2007). The ES scale includes items that are common to girls and boys of all ages and is therefore well suited to the examination of sex differences and age trends in affective empathy across childhood, adolescence and young adulthood. This procedure was completed successfully by 56 children (28 females). The first 9 children tested did not receive the Bryant Scale, as it was not available at the time that they were scheduled to participate in the brain scan. All subsequent children completed this measure.1
2.3. Training in a mock scanner
Prior to MRI scanning, participants were acclimated to the experimental procedures in a mock scanner. They were asked to lie in the mock scanner while a documentary movie was played. When participants felt comfortable, they were presented with 24 stimuli (six per condition) depicting situations similar to, but not the same as, those they would watch in the actual scanning sessions. MRI noise was simulated through a recording played during the mock session.
2.4. FMRI paradigm
In the scanner, all participants were presented with a series of 60 dynamic visual scenarios belonging to one of two categories: (1) a person shown hurting another person intentionally and (2) a person shown hurting another unintentionally. A third category, which served as a control condition, depicted 30 additional scenarios where two people were shown in everyday social interactions without any infliction of pain (i.e., a child passing a notebook to another child).2
The clips showed situations of varying degrees of intensity, portrayed people of both sexes, and different races, as well as various ages. Importantly, the faces of the protagonists were not visible and thus there was no emotional reaction visible to participants. Stimuli were presented in an MRI scanner with E-prime software and a back-projection system. The duration of each clip was 2200ms and was presented in a block of six stimuli, with a jittered inter-stimulus interval (1.69–5.93ms), during which a black fixation cross was presented against a gray background. Active blocks were 19.8s in duration and baseline fixation blocks were 17.6s in duration. Intention (intentional/unintentional) of the person hurting the other was randomized within each block. Baseline blocks included six trials of No Pain control images. The order of movies that depicted this trial type was fixed.
Each dynamic stimulus consisted of 3 digital color pictures, which were edited to the same size (600×480 pixels) and presented in a successive manner to imply motion. The durations of the first, second, and third pictures in each animation were 1000, 200, and 1000ms, respectively. Participants were instructed to passively watch all the scenarios, which were shown in five short sessions to maintain their attention. All 65 participants completed this part of the study. These scenarios were previously validated with eye-tracking measures and behavioral evaluations of perceived agency and empathy and the intentional harm as compared to unintentional harm produced greater empathic sadness and distress (Hempel, 2009).
2.5. Imaging acquisition and fMRI data analysis
Magnetic resonance imaging was performed on a 3T Philips Achieva Quasar scanner at the University of Chicago Brain Research Imaging Center. The fMRI pulse sequence parameters include: TR/TE 2200/26, flip angle=80°, contiguous slices with 4mm thickness, no gap, 230mm×230mm FOV, 76×75 matrix.
Functional MRI data processing was carried out with SPM8 (Wellcome Department of Imaging Neuroscience, London, UK), implemented in MATLAB 7.0 (Mathworks Inc., Sherborn, MA). Volumes were coregistered to the EPI template, realigned and resliced to 2mm cubic voxels, then normalized to MNI space and smoothed with a 6mm full-width half-maximum isotropic Gaussian kernel. A mean T1 image was created from all participants who completed scanning. Structural T1 images were first coregistered to the mean EPI image for each participant. The coregistered T1 images were then spatially normalized and an average of these normalized T1 images of all the participants was created. All 65 subjects who completed scanning had less than 0.5 voxels of in-plane motion throughout the entire experiment. Individual subject data were analyzed using a fixed-effects model. For each participant, a general linear model incorporated regressors for each of the three event categories of person interaction (Intentional Pain, Unintentional Pain, No Pain control condition) as well as the fixation baseline condition, with movement parameters and session regressors included as covariates of no interest. Contrasts for Intentional Pain encoding, Unintentional Pain encoding, No Pain control condition encoding and the subtraction contrasts of Pain minus No Pain and Intentional minus Unintentional Pain (as well as the reverse contrasts) were entered into second-level random effects analyses for groupwise summary. Results for the group analysis were thresholded at p<0.001, and only clusters of 10 or more voxels were reported. The choice of this threshold was determined based on previous studies on empathy for pain and on power considerations for the current paradigm (Jackson et al., 2005, Lamm et al., 2007a). These included regions associated with empathy for pain (insula, ACC, mACC, PAG) and mentalizing (TPJ, medial prefrontal cortex, MPFC).
Complementary to the whole brain analyses, region of interest (ROI) analyses were performed using the rfxplot toolbox implemented in SPM8 (Gläscher, 2009). These analyses compared event-related hemodynamic responses in the following five a priori defined functional ROIs: amygdala, AIC, aMCC, vmPFC and inferior frontal gyrus (IFG), guided by previous work in our lab on empathy in children and adolescents, as well as two recent meta-analyses; one on 20 fMRI studies of insular and anterior cingulate cortex activation during the perception of pain (Jackson et al., 2006) and the other on 32 fMRI studies of empathy for pain (Lamm et al., 2011). Peak co-ordinates were determined on the basis of the whole brain analyses. Individual functional ROIs were delineated by determining the conjunction of the activation map (p=0.05, uncorrected) All Pain>No Pain, with All referring to activation being pooled across both intentionality conditions. Only co-ordinates that fell within the boundaries defined in the meta-analyses of pain perception were considered. Other regions were not included in our analyses. ROIs were defined as a 6-mm diameter spherical region centered on the following MNI coordinates: right amygdala: x=22, y=4, z=−16, left amygdala: x=−22, y=4, z=−16; right insula x=44, y=0, z=−6, left insula x=−44, y=0, z=−6; right aMCC: x=10, 20, 32, left aMCC: x=−10, 20, 32; right vmPFC x=10, y=50, z=−6, left vmPFC x=−2, y=58, z=−8; right IFG x=44, y=32, z=4, left IFG=−44, y=32, z=4. Statistical analyses of ROI data consisted of computing analyses of covariance (ANCOVA) for repeated measures on individual mean percent signal change (PSC) values to test for main effects of sex and sex-by-intent interactions at the group level, with age as a covariate. For statistical analyses of the ROI data SPSS was used (Statistical Package for the Social Sciences, Version 16 for Windows).
2.6. Pupillometry measurements and analysis
For the duration of the scanning, all participants’ eye gaze fixations and pupil dilation were recorded using ASL 6000 (Applied Science Laboratories, Beford, MA, USA). Eye gaze was tracked at a refresh rate of 120Hz. Fixations on the stimuli were measured as constant eye gaze that must be held within 1° of the visual angle for at least 100ms. Data were analyzed using ASL Results v. 1.17.09. Data were successfully collected from 36 participants (18 females), mean 12.4±3.6 years; age distribution: 4–7 years, mean age 6.49±0.88 (N=6, 4 females); age 8–12, mean age 11.01±1.45 (N=12, 5 females); age 13–17, mean age 15.32±1.58 (N=18, 9 females). Data on the remaining participants were not analyzable due to difficulties with calibration or eye movement. Pupil dilation analysis was constrained to the third frame for 2 reasons: first, subjects cannot differentiate between conditions in the first frame and second, using the third frame ensured that any change in pupillometry could be attributed to affective arousal instead of differing luminance between the animation and the previous fixation screen. To control for luminance of the photos between conditions, the average luminance of each of the third frames was measured in Adobe Photoshop. There was no significant difference in luminance between the two intentionality conditions t(29)=−1.58, p=0.12.
2.7. Empathy evaluations
Building on prior work investigating empathy in children, which differentiated between empathic sadness and personal distress (Eisenberg et al., 1989, Eisenberg et al., 1991), we administered two probes that assessed these components separately. After the scanning session, participants were presented with the same stimuli that they saw in the scanner on a computer desktop, and asked to respond to two questions probing empathic concern for the victim and personal distress using a computer based visual analog scale (VAS) ranging from 0 to 100: “How sad are you for the person who was hurt?” “How upset do you feel about what happened?” The child-friendly scale was a 100mm scale with descriptive anchors at each end. The left side was labeled as “not sad at all/not upset at all” and the opposite side was labeled as “extremely sad/extremely upset.” Participants were asked to move a cursor along the scale to express their empathic feelings. All children were trained on the scale with salient examples (i.e., “what is your favorite food? “How much do you like this favorite food?”) prior to answering these questions to ensure they had a clear grasp of the task. The training required that children accurately rate most favorite and least favorite food items, as well as items that children neither “liked nor disliked” to demonstrate an understanding of magnitude and ordinal position before beginning the empathy ratings. A research assistant sat next to the younger children to assist them in these evaluations.
3. Results
3.1. Dispositional measures
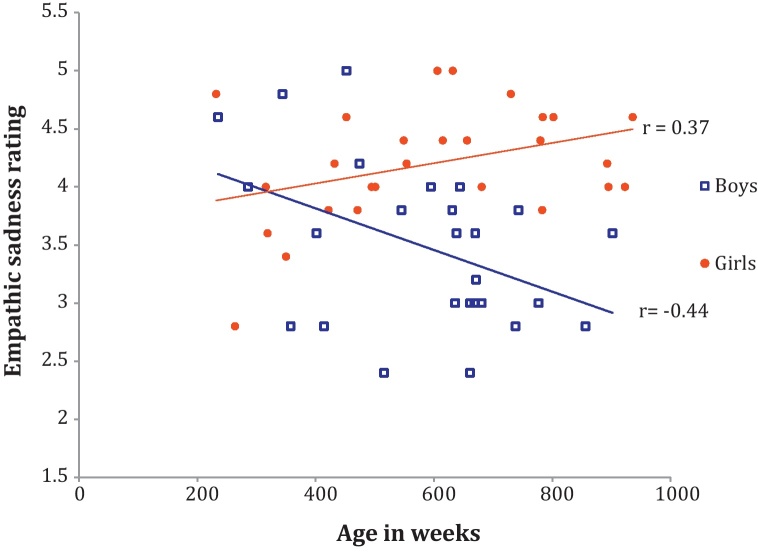
As illustrated in Fig. 1, males and females demonstrated noticeably different patterns of self-reported dispositional empathy (males: 3.48±0.13, females 4.2±0.1, t(54)=4.2, p<0.001). An ANCOVA was calculated with sex as a grouping factor, Bryant ES score as a dependent variable, and age as a covariate. Results revealed a significant age-by-sex interaction F(1,53)=10.0, p<0.005, but no main effect of age (F=2.0, p>0.05). Because of the inclusion of age as a continuous variable, follow-up analyses involved computing correlations rather than post hoc contrasts. Pearson correlation analyses revealed that male scores on the Bryant Empathy Index decreased significantly with age, such that males reported being less empathic as they got older, r(28)=−0.44, p<0.01. Females, in contrast, showed a significant pattern in the opposite direction; they reported being more empathic with age, as reflected by their significantly increasing Bryant scores (r(28)=0.37, p<0.05).
Fig. 1.
Male and female ratings on the Bryant Empathy Scale empathic sadness subscale across age.
3.2. Pupil dilation
A repeated-measures ANCOVA with intentionality (intentional, unintentional behaviors) as a within-subjects variable, sex as a between-subjects variable and age as a covariate, was conducted. A main effect of intention was found for pupil dilation, F(1,33)=12.459, p<0.005, indicating that participants had increased pupil dilation when watching people being hurt intentionally vs. by accident. No significant main effect of sex on pupil dilation was observed, F<1, p=0.5, nor any sex by intention interaction, p=15. With increasing age, the percent pupil dilation relative to subjects’ mean baseline pupil diameter decreased more for accidentally harmed people than intentionally harmed people, F(1,33)=17.307, p<0.01.
3.3. fMRI data
Across both sexes, when collapsing across both intentionality conditions, significant activation relative to the No Pain control condition, was detected in brain regions involved in action understanding such as the IFG (44, 32, 4), and those belonging to the pain matrix, including the AIC (44, 0, −6), the ACC (10, 44, 6 and −6, 46, 10) and aMCC (10, 20, 32), the left somatosensory cortex (−56, −14, 18) and PAG (6, 28, −19 and −6, −28, −19). See Table 1 in Supplementary material for further details. Age related decreases in this Pain vs. No Pain contrast were observed in the PAG, bilateral AIC, right amygdala, right aMCC and right IFG, whereas increases with age were seen in left medial frontal gyrus and left orbitofrontal cortex (OFC) (see Table 2 in Supplementary material). When participants viewed people being hurt intentionally vs. unintentionally, increased hemodynamic response was seen in the right amygdala (22, 4, −16), ventromedial prefrontal cortex (vmPFC, 10, 50, −6 and −2, 58, −8), right OFC (16, 66, −8), and left TPJ (−56, −38, 18) (see Table 3 in Supplementary material). For this Intentional vs. Accidental Pain contrast, age related decreases were observed in the amygdala and temporal pole, whereas greater activation with increasing age was seen in the vmPFC. These results are consistent with previous neuroimaging work examining the neural underpinnings of intentionally vs. accidentally caused pain in children and adolescents (Decety et al., 2008, Decety and Michalska, 2010). The inverse contrast (Accidental Pain–Intentional Pain) did not show significant differential activation.
A two-sample t-test at the whole-brain level revealed no significant differences between girls and boys in either contrast at both p<0.05 FWE corrected and p<0.001 uncorrected thresholds used for our other whole-brain contrasts. While this is consistent with one of our hypotheses, that sex disparities in empathic responding do not reflect any underlying differences in implicit responding to the observation of others in pain, it is nevertheless an argument from a null effect. On the one hand, it is unlikely that lack of power is the main issue given our large sample size. On the other hand, there may be significant regions in the current analysis evident on the slightly less significant side of the arbitrary threshold adopted here. To address this, we lowered the threshold to p<0.05, uncorrected, and assessed any group differences for the Pain>No Pain and Intentional Pain>Unintentional Pain contrasts. Even at this far more liberal threshold, no sex differences were detected in areas coding either the affective or cognitive components of the empathic response. The only differences that we observed were located in the right thalamus (10, −16, 4, t=2.67) and right cerebellum (6, −56, −48, t=3.08) for girls>boys, which are not regions that have been observed as contributing to affective empathy nor empathic understanding in past research. In sum, even with a very liberal statistical threshold, we find no evidence of sex differences in empathy-related brain regions between males and females.
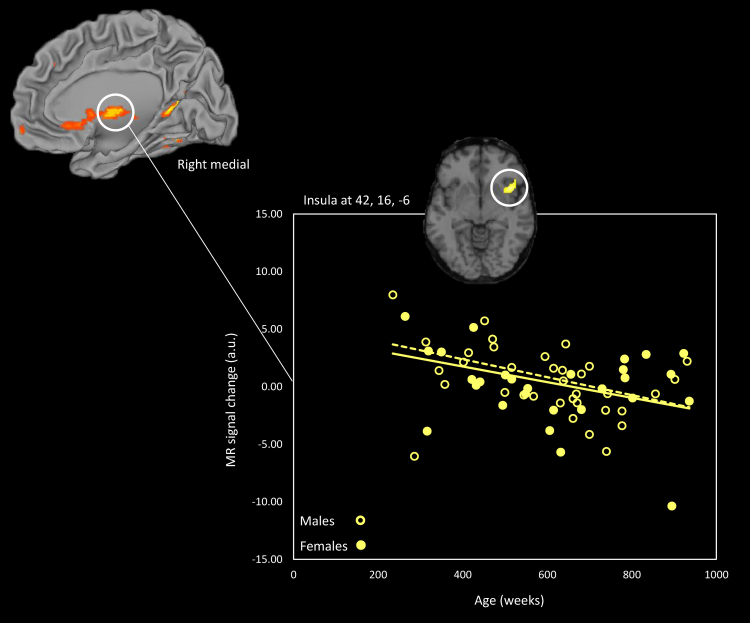
Post hoc ROI analyses comparing activation associated with each type of scenario to activation during the No Pain control condition provided detail regarding potential sex differences in patterns of performance. We specifically assessed activation in five (bilateral) ROIs hypothesized to reflect different kinds of affective information processing during empathy for pain. These analyses tested hypotheses about activation differences in a priori and functionally defined areas with higher sensitivity. For instance, activation of the anterior insula during affective processing in general as well as during the perception of pain in others is well-documented and appears to be related to interoceptive awareness and affective evaluation (Jackson et al., 2006). Analyses were restricted to changes in BOLD response within ten a priori defined 3mm radius ROIs including the AIC, aMCC, amygdala, IFG and vmPFC. Males and females did not differ in the pattern of activation within these regions during the perception of pain relative to the control condition, as reflected by a non-significant interaction between sex and activation in any of the ROIs. Even in the Insula ROI, where one might have the greatest reason to predict an interaction with sex, given its role in coding the affective-motivational aspects of pain perception, no effect of sex was found, as illustrated in Fig. 2. Moreover, no significant age×sex interaction was observed and no significant correlations with either Bryant dispositional ratings across both sexes or within each sex were found.
Fig. 2.
Male and female parameter estimates across age for the right insula when viewing painful scenarios vs. everyday actions. The surface-rendered image was created using CARET software and population-average, landmark-, surface-based atlases (Van Essen, 2005). Females: n=30; males: n=35.
3.4. Empathy evaluations
In contrast with the hemodynamic and pupil dilation measures, a main effect of sex for participants’ self-report of feeling upset while viewing the empathy-eliciting scenarios was observed. A repeated-measures ANCOVA with emotion (upset, sad) as one within-subjects variable, intentionality (intentional, unintentional behaviors) as another within-subjects variable, sex as a between-subjects variable and age as a covariate, was conducted. A main effect of intention was found (F=8.89, p<0.005), such that all participants, regardless of sex or age, reported being more upset and more sad when observing intentional vs. unintentional harm behaviors. Females reported being significantly more upset than males when viewing people being hurt (56.92 vs. 50.15 and 39.10 vs. 32.8 for intentional and unintentional harm, respectively), p<0.05. While girls reported feeling more sad than boys for the victim who was hurt both on purpose (59.25 vs. 52.75) and by accident (44.47 vs. 39.02), these differences only approached significance (p=0.1). Additional analyses revealed that there was a negative correlation with age for participants’ ratings of empathic sadness when observing both intentional and unintentional harm conditions (r=−0.2, p<0.05 and r=−0.25, p<0.01, respectively), although there were no sex by age interactions, and no significant three-way interactions, F<1, p=ns.
4. Discussion
The present study examined sex-related differences in both explicit (dispositional ratings and behavioral evaluations) and implicit (neuro-hemodynamic response and pupil dilation) measures of empathy across age in a large sample of children and adolescents. On a self-report dispositional scale of empathy (Bryant, 1982), female participants in the current study scored significantly higher with age, while male participant scores significantly decreased with age. Girls also reported feeling more upset than boys when viewing a person being hurt. Importantly, these behavioral and self-report differences were not accompanied by any significant differences in patterns of neural activity between the sexes. Indeed, in contrast to the dispositional and behavioral data, no sex-related differential activations in response to viewing people in pain were detected either in the hemodynamic response in a priori defined regions of interest, or in affective arousal, as measured by pupillometry, and no interaction between sex and age in either of these measures was observed.
With respect to the mechanisms that give rise to the discrepant findings between the types of measures, our findings suggest several interpretations. One possibility is that differences in self-reported empathy may reflect females’ greater willingness with age to report empathic behavior (and males decreasing willingness to report empathic feelings), rather than real sex differences in underlying experiences of empathy. It is probable that self-report measures such as the Bryant Empathy Index, similar to the EQ (Baron-Cohen and Wheelwright, 2004) and IRI in adults (Davis, 1983), assess an individual's beliefs about their experience of empathy, or social signaling, which likely reflects gender-relevant societal norms and expectations about emotion expression. This assumption is in line with behavioral evidence demonstrating that sex differences in empathy seem to correlate better with gender roles than with biological sex (Karniol et al., 1998) and that self-report measures of empathy are associated with social desirability (Cialdini et al., 1987). The idea that women's beliefs about their own empathic abilities may motivate them to be more empathic is also consistent with the findings of Klein and Hodges (2001), who demonstrate that sex differences in empathic accuracy disappear under testing conditions that hold constant the motivational context. The assessment of sex differences in the present study would have benefitted from the inclusion of a measure of gender-role beliefs in order to have a clearer pattern as observed in the study by Klein and Hodges (2001).
Sex differences in dispositional self-report ratings may also reflect the fact that measures used to evaluate children's empathic and prosocial tendencies tend to include a disproportionate number of sex biased items favoring girls (i.e., items pertaining to stereotypically female activities) and that including items that reflect male activities would diminish this divergence (Zarbatany et al., 1985). While the self-report questionnaire that we used (Bryant) was designed to include items relevant to both girls and boys, it is possible that some of these items, such as “seeing another child who is crying makes me feel like crying” may not be gender neutral, due to gender-related expectations about crying.
An additional possibility that may explain discrepant observations of sex differences across measures is that each of the dependent measures index separate aspects of an individual's response to and experience of an empathy-eliciting event, and may each be differentially influenced by sex. Of the components that collectively contribute to the construct of empathy, neuro-hemodynamic and physiological measures in response to perceiving individuals in distress and/or pain may largely be indexing empathic arousal, while behavioral and dispositional measures may reflect other components, such as empathic understanding and empathic concern. In previous fMRI studies of empathy for pain, activation in the affective-motivational component of the pain matrix (specifically, anterior insula and anterior cingulate cortex) correlated most strongly with emotional contagion scores, whereas the correlation between the IRI empathic concern subscale yielded no significant clusters in these regions (Lamm et al., 2007b). The present data similarly failed to find correlations between hemodynamic responses and dispositional ratings of empathic sadness, despite the large sample size combined with focused region of interest analyses. The finding that the affective response at both the physiological and neural levels did not vary by sex may suggest that males and females do not differ in their initial responsiveness to the perception of pain. This observation is consistent with meta-analytic assessments of sex differences in empathy for pain paradigms in adults, which similarly fail to find differences across the sexes (Lamm et al., 2011, but see Derntl et al., 2010).
Open questions remain concerning potential differences and similarities in the processing of pain across sexes. Although we observe no evidence of sex differences in neural responses to witnessing painful events experienced by others, some past research does suggest that men and women's first-hand experience of pain can differ (Coll et al., 2012, Mogil and Bailey, 2010). Evidence from positron emission tomography studies indicates that while there is substantial overlap between sexes in the patterns of cerebral activation in response to nociceptive stimuli, females perceive painful stimuli as more intense than do males and exhibit greater activation in the thalamus and anterior insula (Paulson et al., 1998). Furthermore, there is robust evidence across multiple fMRI studies that overlap of the neural network involved in the direct experience of pain with the empathic network constitutes a core network for pain empathy (Lamm et al., 2011). In the current study, we observed that even though female participants had similar hemodynamic and pupillary responses as males when viewing individuals in painful situations, they reported being significantly more upset by them. The lack of congruence between the neural and physiological measures on the one hand, and the dispositional measures and self-reported post scan ratings on the other, suggests that males and females may appraise their initial (similar) response to painful situations differently, thus reflecting potentially real differences in phenomenological experiences.
More generally, future research can consider the extent to which the kinds of stimuli frequently used in neuroimaging studies of empathy for pain may be an imperfect probe of interpersonal sensitivity or of empathic concern. Instead, these stimuli may trigger an aversive response in the observer associated with more general survival mechanisms such as aversion and withdrawal when exposed to danger and threat (Yamada and Decety, 2009). In fact, based on a systematic review of electroencephalographic and functional MRI studies that examined neural response triggered by nociceptive stimuli, it has been proposed that activity of the “pain matrix” reflects a system involved in detecting, processing, and reacting to the occurrence of salient sensory events, regardless of the sensory channel through which these events are conveyed (Legrain et al., 2011). Supporting this argument, a recent study which directly contrasted young children's behavioral expressions of empathy for situations of sadness and pain showed that they were more upset by another's sadness than another's pain and engaged in more behaviors representative of concern and prosocial responses to sadness than to pain (Bandstra et al., 2011). This suggests that young children respond differently to others’ pain than others’ sadness, and they may in fact be less responsive to others’ pain than to others’ emotions. Neuroimaging research on empathy so far has favored employing the perception, anticipation or imagination of acute physical pain because pain is an ecologically valid stimulus that warns of physical threat. Most of these studies typically employ stimuli depicting body parts being injured and facial expressions of pain both with children (e.g., Decety and Michalska, 2010) and adults (Akitsuki and Decety, 2009, Cheng et al., 2010, Decety and Porges, 2011, Gu et al., 2010, Lamm et al., 2010). This research focus inadvertently puts a lot of emphasis on the noxious stimulus, the sensory aspects of pain and the reflexive aspects of pain behavior. Creating evocative scenarios of potentially greater relevance to affective empathy in addition to these stimuli will enable researchers to assess empathy more specifically as a reaction to others’ emotions.
Despite the prevailing view that females are more empathic and prosocial than males, the developmental empirical evidence is equivocal. Studies with very young infants report that there are no sex differences in emotion contagion, like contagious crying (Geangu et al., 2010) and expressions of distress and concerned affect (Roth-Hanania et al., 2011, but see Knafo et al., 2008). Based on the current findings, it appears that while there are no observed differences in physiological indices of empathy, overt dispositional expressions of empathy are increasingly suppressed with age in males and increasingly enhanced in females from age 4 throughout adolescence. Although many previous studies with children have used a single criterion measure as their operational definition of empathy, the present study investigates four different potential measures of affective empathy (pupil dilation, neuro-hemodynamic, dispositional measures and evaluations of scenarios). Probing areas of overlap and divergence between males and females on these various assessments of empathic responding, particularly across age, can teach us something about sex differences in empathy and how they develop, but it also has constructive implications for our understanding of how we measure these differences. It is difficult to tell which measure (self-reports, physiology, behavior, or specific brain activations) is the best at assessing emotion in different situations (Eisenberg and Lennon, 1983, Wager and Ochsner, 2005). The current study suggests important methodological considerations regarding the assessment of sex differences in empathy in particular, and assessments of empathy more broadly: (i) the development of a questionnaire that specifically queries the willingness to respond to other's emotions (rather than a more general questionnaire probing social desirability) would be of benefit to future studies of sex differences in empathy; (ii) attempts should be made to translate self-report measures of dispositional empathy to more standardized observations with less gender-biased protocols; (iii) in addition to presenting images of people in painful situations, future fMRI studies need to include stimuli depicting emotions such as sadness, fear or happiness and examine correlations between such responses and convergent measures of affective empathy, cognitive empathy and empathic concern.
Finally, to express doubt about the validity of self-reports questionnaire measures of dispositional empathy is not to express doubt that individual differences affect the experience of empathy, especially empathic concern, and that there may be genuine sex differences that moderate empathy (Batson, 2011). Other dispositional moderators of empathic responding include general emotionality, the regulation of emotion, as well as attachment styles, all of which would be worth including in future research. While differences in dispositional and post-scan ratings in our study do not predict neurophysiological correlates (both pupil dilation and hemodynamic response), the observation that males and females differ in their endorsement of empathic beliefs is potentially useful in informing our understanding of sex differences in communication and empathic expression.
Conflict of interest statement
The authors do not have any interests that might be interpreted as influencing the research.
Ethical approval
APA ethical standards were followed in the conduct of the research.
Acknowledgments
The study was supported by an NSF award (BCS-0718480) to Jean Decety. We are grateful to Alexa Tompary for assistance in scanning and data analysis. We thank three anonymous reviewers for their constructive comments.
Footnotes
As part of the larger study, participants also completed the Nachamie's Child Mach, which was not central to the research question addressed here and results are therefore not reported.
All participants also saw two other types of scenarios involving objects that were not relevant to the research reported here. These will not be discussed further.
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.dcn.2012.08.001.
Appendix A. Supplementary data
The following is the supplementary data to this article:
References
- Akitsuki Y., Decety J. Social context and perceived agency modulate brain activity in the neural circuits underpinning empathy for pain: an event-related fMRI study. NeuroImage. 2009;47:722–734. doi: 10.1016/j.neuroimage.2009.04.091. [DOI] [PubMed] [Google Scholar]
- Andersen, S., Ertac, S., Gneezy, U., Liszt, J.A., Maximiano, S., 2012. Gender, competitiveness and socialization at a young age: evidence from a matrilineal and a patriarchal society. Review of Economics and Statistics, Epub ahead of print.
- Bandstra N.F., Chambers C.T., McGrath P.J., Moore C. The behavioural expression of empathy to others’ pain versus others’ sadness in young children. Pain. 2011;152:1074–1182. doi: 10.1016/j.pain.2011.01.024. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S., Wheelright S., Hill J., Raste Y., Plumb I. The Reading the Mind in the Eyes Test revised version: a study with normal adults and adults with Asperger syndrome or high-functioning autism. Journal of Child Psychology and Psychiatry. 2001;42:241–251. [PubMed] [Google Scholar]
- Baron-Cohen S., Wheelwright S. The empathy quotient: an investigation of adults with Asperger syndrome or high functioning autism, and normal sex differences. Journal of Autism and Developmental Disorders. 2004;34:163–175. doi: 10.1023/b:jadd.0000022607.19833.00. [DOI] [PubMed] [Google Scholar]
- Batson C.D. Oxford University Press; New York: 2011. Altruism in Humans. [Google Scholar]
- Bryant B. An index of empathy for children and adolescents. Child Development. 1982;53:413–425. [Google Scholar]
- Chapman E., Baron-Cohen S., Auyeung B., Taylor K., Hackett G. Fetal testosterone and empathy: evidence from the Empathy Quotient (EQ) and the Reading the Mind in the Eyes Test. Social Neuroscience. 2006:135–148. doi: 10.1080/17470910600992239. [DOI] [PubMed] [Google Scholar]
- Cheng Y., Chen C.Y., Lin C.P., Chou K.H., Decety J. Love hurts: an fMRI study. NeuroImage. 2010;51:923–929. doi: 10.1016/j.neuroimage.2010.02.047. [DOI] [PubMed] [Google Scholar]
- Cialdini R.B., Schaller M., Houlihan D., Arps K., Fultz J., Beaman A.L. Empathy-based helping: is it selflessly or selfishly motivated? Journal of Personality and Social Psychology. 1987;52:749–758. doi: 10.1037//0022-3514.52.4.749. [DOI] [PubMed] [Google Scholar]
- Cohen D., Strayer J. Empathy in conduct disordered and comparison youth. Developmental Psychology. 1996;32:988–998. [Google Scholar]
- Coll M.P., Budell L., Rainville P., Decety J., Jackson P.L. The role of gender in the interaction between self-pain and the perception of pain in others. Journal of Pain. 2012;13:695–703. doi: 10.1016/j.jpain.2012.04.009. [DOI] [PubMed] [Google Scholar]
- Davis M.H. Measuring individual differences in empathy: evidence for a multidimensional approach. Journal of Personality and Social Psychology. 1983;44:113–126. [Google Scholar]
- Davis M.H., Franzoi S.L. Stability and change in adolescent self-consciousness and empathy. Journal of Research in Personality. 1991;25:70–87. [Google Scholar]
- De Wied M., Maas C., van Goozen S., Vermande M., Engels R., Meeus W., Matthys W., Goudena P. Bryant's Empathy Index: a closer examination of its internal structure. European Journal of Psychological Assessment. 2007;23:99–104. [Google Scholar]
- Decety J. The neurodevelopment of empathy in humans. Developmental Neuroscience. 2010;32:257–267. doi: 10.1159/000317771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decety J. Dissecting the neural mechanisms mediating empathy. Emotion Review. 2011;3:92–108. [Google Scholar]
- Decety J., Michalska K.J., Kinzler K.D. The contribution of emotion and cognition to moral sensitivity: a neurodevelopmental study. Cerebral Cortex. 2012;22:209–220. doi: 10.1093/cercor/bhr111. [DOI] [PubMed] [Google Scholar]
- Decety J., Michalska K.J., Akitsuki Y. Who caused the pain? A functional MRI investigation of empathy and intentionality in children. Neuropsychologia. 2008;46:2607–2614. doi: 10.1016/j.neuropsychologia.2008.05.026. [DOI] [PubMed] [Google Scholar]
- Decety J., Michalska K.J. Neurodevelopmental changes in the circuits underlying empathy and sympathy from childhood to adulthood. Developmental Science. 2010;13:886–899. doi: 10.1111/j.1467-7687.2009.00940.x. [DOI] [PubMed] [Google Scholar]
- Decety J., Michalska K.J., Akitsuki Y., Lahey B. Atypical empathic responses in adolescents with aggressive conduct disorder: a functional MRI investigation. Biological Psychology. 2009;80:203–211. doi: 10.1016/j.biopsycho.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decety J., Norman G.J., Berntson G.G., Cacioppo J.T. A neurobehavioral evolutionary perspective on the mechanisms underlying empathy. Progress in Neurobiology. 2012;98:38–48. doi: 10.1016/j.pneurobio.2012.05.001. [DOI] [PubMed] [Google Scholar]
- Decety J., Porges E.C. Imagining being the agent of actions that carry different moral consequences: an fMRI study. Neuropsychologia. 2011;49:2994–3001. doi: 10.1016/j.neuropsychologia.2011.06.024. [DOI] [PubMed] [Google Scholar]
- Decety J., Sveltova M. Putting together phylogenetic and ontogenetic perspectives on empathy. Developmental Cognitive Neuroscience. 2012;2:1–24. doi: 10.1016/j.dcn.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derntl B., Finkelmeyer A., Eickhoff S., Kellerman T., Flakenberg D.I., Schneider F., Habel U. Multidimensional assessment of empathic abilities: neural correlates and gender differences. Psychoneuroendocrinology. 2010;35:67–82. doi: 10.1016/j.psyneuen.2009.10.006. [DOI] [PubMed] [Google Scholar]
- Eisenberg N., Fabes R.A., Schaller M., Miller P.A. Sympathy and personal distress: development, gender differences, and interrelations of indexes. New Directions for Child Development. 1989;44:107–126. doi: 10.1002/cd.23219894408. [DOI] [PubMed] [Google Scholar]
- Eisenberg N., Lennon R. Sex differences in empathy and related capacities. Psychological Bulletin. 1983;94:100–131. [Google Scholar]
- Eisenberg N., Miller P.A., Shell R., McNalley S., Shea C. Prosocial development in adolescence: a longitudinal study. Developmental Psychology. 1991;27:849–857. [Google Scholar]
- Geangu E., Benga O., Stahl D., Striano T. Contagious crying beyond the first days of life. Infant Behavior and Development. 2010;33:279–288. doi: 10.1016/j.infbeh.2010.03.004. [DOI] [PubMed] [Google Scholar]
- Geary D.C. Sexual selection and sex differences in social cognition. In: McGillicuddy-De Lisi A.V., De Lisi, editors. Biology, Society and Behavior: The Development of Sex Differences in Cognition. Ablex/Greenwood; Greenwich, CT: 2002. pp. 23–53. [Google Scholar]
- Gläscher J. Visualization of group inference data in functional neuroimaging. Neuroinformatics. 2009;7:73–82. doi: 10.1007/s12021-008-9042-x. [DOI] [PubMed] [Google Scholar]
- Gu X., Liu X., Guise K.G., Naidich T.P., Hof P.R., Fan J. Functional dissociation of the frontoinsular and anterior cingulate cortices in empathy for pain. Journal of Neuroscience. 2010;30:3739–3744. doi: 10.1523/JNEUROSCI.4844-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J.A. Gender effects in decoding nonverbal cues. Psychological Bulletin. 1978;85:845–857. [Google Scholar]
- Hall J.A., Mast M.S. Sources of accuracy in the empathic accuracy paradigm. Emotion. 2007:438–446. doi: 10.1037/1528-3542.7.2.438. [DOI] [PubMed] [Google Scholar]
- Hancock M., Ickes W. Empathic accuracy: when does the perceiver–target relationship make a difference? Journal of Social and Personal Relationships. 1996;13:179–199. [Google Scholar]
- Hempel, J., 2009. Eye-tracking as a method to investigate empathy and sympathy. Honors Thesis under the direction of Dr. J. Decety, The University of Chicago.
- Ickes W., Stinson L., Bissonnette V., Garcia S. Naturalistic social cognition: empathic accuracy in mixed-sex dyads. Journal of Personality and Social Psychology. 1990;59:730–742. [Google Scholar]
- Ickes W., Gesn P.R., Graham T. Gender differences in empathic accuracy: differential ability or differential motivation? Personal Relationships. 2000;7:95–109. [Google Scholar]
- Jackson P.L., Meltzoff A.N., Decety J. How do we perceive the pain of others: a window into the neural processes involved in empathy. NeuroImage. 2005;24:771–779. doi: 10.1016/j.neuroimage.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Jackson P.L., Rainville P., Decety J. To what extent do we share the pain of others? Insight from the neural bases of pain empathy. Pain. 2006;125:5–9. doi: 10.1016/j.pain.2006.09.013. [DOI] [PubMed] [Google Scholar]
- Karniol R., Gabay R., Ochion Y., Harari Y. Is gender or gender-role orientation a better predictor of empathy in adolescence? Sex Roles. 1998;39:45–59. [Google Scholar]
- Killgore W.D.S., Oki M., Yurgelun-Todd D.A. Sex-specific developmental changes in amygdala responses to affective faces. Neuroreport. 2001;12:427–433. doi: 10.1097/00001756-200102120-00047. [DOI] [PubMed] [Google Scholar]
- Killgore W.D.S., Gangestad S.W. Sex differences in asymmetrically perceiving the intensity of facial expressions. Perceptual and Motor Skills. 1999;89:311–314. doi: 10.2466/pms.1999.89.1.311. [DOI] [PubMed] [Google Scholar]
- Killgore W.D.S. Sex differences in identifying the facial affect of normal and mirror-reversed faces. Perceptual and Motor Skills. 2000;91:525–530. doi: 10.2466/pms.2000.91.2.525. [DOI] [PubMed] [Google Scholar]
- Klein K.J.K., Hodges S.D. Gender differences, motivation and empathic accuracy: when it pays to understand. Personality and Social Psychology Bulletin. 2001;27:720–730. [Google Scholar]
- Knafo A., Zahn Waxler C., VanHulle C., Robinson J.L., Rhee S.H. The developmental origins of a disposition toward empathy: genetic and environmental contributions. Emotion. 2008;8:737–752. doi: 10.1037/a0014179. [DOI] [PubMed] [Google Scholar]
- Knickmeyer R., Baron-Cohen S., Raggatt P., Taylor K. Foetal testosterone, social relationships, and restricted interests in children. Journal of Child Psychology and Psychiatry. 2005;46:198–210. doi: 10.1111/j.1469-7610.2004.00349.x. [DOI] [PubMed] [Google Scholar]
- Knickmeyer R., Baron-Cohen S., Raggat P., Taylor K., Hackett G. Fetal testosterone and empathy. Hormones and Behavior. 2006;49:282–292. doi: 10.1016/j.yhbeh.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Krendl A.C., Richeson J.A., Kelley W.M., Heatherton T.F. The negative consequences of threat: a functional magnetic resonance imaging investigation of the neural mechanisms underlying women's underperformance in math. Psychological Science. 2008;19:168–175. doi: 10.1111/j.1467-9280.2008.02063.x. [DOI] [PubMed] [Google Scholar]
- Lamm C., Batson C.D., Decety J. The neural substrate of human empathy: effects of perspective-taking and cognitive appraisal. Journal of Cognitive Neuroscience. 2007;19:42–58. doi: 10.1162/jocn.2007.19.1.42. [DOI] [PubMed] [Google Scholar]
- Lamm C., Decety J., Singer T. Common and distinct neural networks associated with directly experienced pain and empathy for pain. NeuroImage. 2011;54:2492–2502. doi: 10.1016/j.neuroimage.2010.10.014. [DOI] [PubMed] [Google Scholar]
- Lamm C., Meltzoff A.N., Decety J. How do we empathize with someone who is not like us? Journal of Cognitive Neuroscience. 2010;2:362–376. doi: 10.1162/jocn.2009.21186. [DOI] [PubMed] [Google Scholar]
- Lamm C., Nusbaum H.C., Meltzoff A.N., Decety J. What are you feeling? Using functional magnetic resonance imaging to assess the modulation of sensory and affective responses during empathy for pain. PLoS ONE. 2007;12:e1292. doi: 10.1371/journal.pone.0001292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legrain V., Iannetti G.D., Plaghki L., Moureaux A. The pain matrix reloaded: a salience detection system for the body. Progress in Neurobiology. 2011;93:111–124. doi: 10.1016/j.pneurobio.2010.10.005. [DOI] [PubMed] [Google Scholar]
- Lennon R., Eisenberg N. Gender and age differences in empathy and sympathy. In: Eisenberg N., Strayer J., editors. Empathy and its Development. Cambridge University Press; Cambridge, UK: 1987. pp. 195–217. [Google Scholar]
- Mangels J.A., Good C., Whiteman R.C., Maniscalo B., Dweck C.S. Emotion blocks the path to learning under stereotype threat. Social Cognitive and Affective Neuroscience. 2012;7:230–241. doi: 10.1093/scan/nsq100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marangoni C., Garcia S., Ickes W., Teng G. Empathic accuracy in a clinically relevant setting. Journal of Personality and Social Psychology. 1995;68:854–869. doi: 10.1037//0022-3514.68.5.854. [DOI] [PubMed] [Google Scholar]
- Massa L.J., Mayer R.E., Bohon L.M. Individual differences in gender role beliefs influence spatial ability test performance. Learning and Individual Differences. 2005;15:99–111. [Google Scholar]
- McClure E.B. A meta-analytic review of sex differences in facial expression processing and their development in infants, children, and adolescents. Psychological Bulletin. 2000;126:424–453. doi: 10.1037/0033-2909.126.3.424. [DOI] [PubMed] [Google Scholar]
- Mogil J.S., Bailey A.L. Sex and gender differences in pain and analgesia. Progress in Brain Research. 2010;186:141–175. doi: 10.1016/B978-0-444-53630-3.00009-9. [DOI] [PubMed] [Google Scholar]
- Moriguchi Y., Decety J., Ohnishi T., Maeda M., Matsuda H., Komaki G. Empathy and judging other's pain: an fMRI study of alexithymia. Cerebral Cortex. 2007;17:2223–2234. doi: 10.1093/cercor/bhl130. [DOI] [PubMed] [Google Scholar]
- Otti A., Guendel H., Laeerb L., Wohlschlaegerb A., Lane R., Decety J., Zimmer C., Henningsen P., Noll-Hussong M. I know the pain you feel—how the human brain's default mode predicts our resonance to another's suffering. Neuroscience. 2010;169:143–148. doi: 10.1016/j.neuroscience.2010.04.072. [DOI] [PubMed] [Google Scholar]
- Paulson P.E., Minoshima S., Morrow T.J., Casey K.L. Gender differences in pain perception and patterns of cerebral activation during noxious heat stimulation in humans. Pain. 1998;76:223–229. doi: 10.1016/s0304-3959(98)00048-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth-Hanania R., Davidov M., Zahn-Waxler C. Empathy development from 8 to 16 months: early signs of concerned for others. Infant Behavior and Development. 2011;34:447–458. doi: 10.1016/j.infbeh.2011.04.007. [DOI] [PubMed] [Google Scholar]
- Rueckert L., Naybar N. Gender differences in empathy: the role of the right hemisphere. Brain and Cognition. 2008;67:162–167. doi: 10.1016/j.bandc.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Schulte-Rüther H.J., Markowitsch N.J., Shah G.R., Piefke M. Gender differences in brain networks supporting empathy. NeuroImage. 2008;42:393–403. doi: 10.1016/j.neuroimage.2008.04.180. [DOI] [PubMed] [Google Scholar]
- Singer T., Lamm C. The social neuroscience of empathy. Annals of the New York Academy of Sciences. 2009;1156:81–96. doi: 10.1111/j.1749-6632.2009.04418.x. [DOI] [PubMed] [Google Scholar]
- Singer T., Seymour B., O’Doherty J.P., Stephan K.E., Dolan R.J., Frith C.D. Empathic neural responses are modulated by the perceived fairness of others. Nature. 2006;439:466–469. doi: 10.1038/nature04271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Honk J., Schutter D.J., Bos P.A., Kruijt A-W., Lentjes E.G., Baron-Cohen S. Testosterone administration impairs cognitive empathy in women depending on second-to-fourth digit ratio. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:3448–3452. doi: 10.1073/pnas.1011891108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen D.C. A population-average, landmark- and surface-based (PALS) atlas of human cerebral cortex. Neuroimage. 2005;28:635–662. doi: 10.1016/j.neuroimage.2005.06.058. [DOI] [PubMed] [Google Scholar]
- Wager T.D., Phan K.L., Liberzon I., Taylor S.F. Valence, gender and lateralization of functional brain anatomy in emotion: a meta-analysis of findings from neuroimaging. NeuroImage. 2003;19:513–531. doi: 10.1016/s1053-8119(03)00078-8. [DOI] [PubMed] [Google Scholar]
- Wager T.D., Ochsner K.N. Sex differences in the emotional brain. Neuroreport. 2005;16:85–87. doi: 10.1097/00001756-200502080-00001. [DOI] [PubMed] [Google Scholar]
- Yamada M., Decety J. Unconscious affective processing and empathy: an investigation of subliminal priming on the detection of painful facial expressions. Pain. 2009;143:71–75. doi: 10.1016/j.pain.2009.01.028. [DOI] [PubMed] [Google Scholar]
- Zarbatany L., Hartmann D.P., Gelfand D.M., Vinciguerra P. Gender differences in altruistic reputation: are they artifactual? Developmental Psychology. 1985;21:97–101. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.