Abstract
The present study is the first to investigate whether cerebral blood flow in the hippocampus relates to aerobic fitness in children. In particular, we used arterial spin labeling (ASL) perfusion MRI to provide a quantitative measure of blood flow in the hippocampus in 73 7- to 9-year-old preadolescent children. Indeed, aerobic fitness was found to relate to greater perfusion in the hippocampus, independent of age, sex, and hippocampal volume. Such results suggest improved microcirculation and cerebral vasculature in preadolescent children with higher levels of aerobic fitness. Further, aerobic fitness may influence how the brain regulates its metabolic demands via blood flow in a region of the brain important for learning and memory. To add specificity to the relationship of fitness to the hippocampus, we demonstrate no significant association between aerobic fitness and cerebral blood flow in the brainstem. Our results reinforce the importance of aerobic fitness during a critical period of child development.
Keywords: Arterial spin labeling, Childhood, Development, Hippocampus, Perfusion, Physical activity
1. Introduction
Participation in physical activity and higher levels of aerobic fitness are associated with superior scholastic achievement, cognitive control, and memory in children (Buck et al., 2008, Castelli et al., 2007, Chaddock et al., 2010a, Chaddock et al., 2010b, Chaddock et al., 2011, Chomitz et al., 2009, Hillman et al., 2009, Pontifex et al., 2011, Voss et al., 2011). Still, little is known about the neural mechanisms by which aerobic fitness influences the developing brain during childhood. Volumetric and functional magnetic resonance imaging (MRI) techniques provide some clues, such that higher fit children show larger brain volumes in the hippocampus and basal ganglia (Chaddock et al., 2010a, Chaddock et al., 2010b), as well as differences in blood oxygenation level dependent (BOLD) fMRI brain activation in areas of frontal and parietal cortex (Chaddock et al., 2012, Voss et al., 2011), relative to their lower fit peers. Non-human animal work raises the possibility that, mechanistically, children with higher levels of aerobic fitness may have increased growth and expansion of neural tissue and/or increased vasculature (see Voss et al., 2013 for a review).
The present study is the first to investigate whether increased cerebral blood flow (CBF) in the hippocampus is associated with aerobic fitness during childhood. This hypothesis cannot be directly tested with traditional BOLD techniques, given that BOLD can change depending on a number of factors related to local metabolism and neural function, including blood volume, perfusion, blood velocity, and cerebral metabolic rate of oxygen (Hoge et al., 1999). Hence, here we use arterial spin labeling (ASL) perfusion MRI to provide a quantitative measure of blood flow and a more direct link to local neuronal activity (Alsop and Detre, 1996). Specifically, an ASL signal arises from the delivery of magnetically tagged arterial water into an imaging slice of interest, where the blood water exchanges in the tissue. The output measure of CBF, or the blood supply to a brain area in a given time (mL/100 g/min), is known to provide information regarding how the brain meets and regulates its metabolic demands via the delivery of metabolites, oxygen and nutrients to activated neurons (Hales et al., 2014, Sokoloff et al., 1977) (see ASL Scan Acquisition section in the Method for more information about ASL).
We specifically focused on CBF in the hippocampus, in view of converging evidence that demonstrates positive physical activity-related brain changes in the hippocampus in rodents and humans across the lifespan (Bugg and Head, 2011, Burdette et al., 2010, Chaddock et al., 2010a, Erickson et al., 2009, Erickson et al., 2011, Honea et al., 2009, Pereira et al., 2007, van Praag et al., 1999b). For example, voluntary wheel running in rodents has been found to enhance learning and memory (van Praag et al., 2005) as well as induce angiogenesis and increased vascular density (Black et al., 1990, Clark et al., 2009, Kleim et al., 2002, Rhyu et al., 2010), and the growth of new neurons in the hippocampus (van Praag et al., 1999a). In humans, physical activity and aerobic fitness are associated with a greater number of small-caliber vessels (Bullitt et al., 2009), increased cerebral blood volume in the hippocampus in middle-aged adults (Pereira et al., 2007; age 21–45) and increased hippocampal blood flow in older adults (Burdette et al., 2010). Cognitively, increased hippocampal CBF has been linked to higher task performance on a spatial memory task in middle-aged and older adults (Heo et al., 2010, Pereira et al., 2007). It is possible that these findings extend to children, such that aerobic fitness relates to greater perfusion in the hippocampus, which may suggest improved microcirculation, cerebral vasculature, and function.
We hypothesized that aerobic fitness in 7- to 9-year-old preadolescent children would be associated with increased resting CBF in the hippocampus. We explored anterior and posterior subsections of the hippocampus to examine whether aerobic fitness had selective effects on hippocampal blood flow, and to investigate distinct contributions of different anatomical regions within the hippocampus given that functional distinctions have been described along the anterior/posterior axis of the hippocampus (i.e., spatial versus relational processing) (Giovanello et al., 2009, Sperling et al., 2003). To provide additional specificity to a fitness-CBF relationship, we also measured CBF in the brainstem as a control region. Like the hippocampus, the brainstem is a subcortical structure in the midbrain included in our ASL slice acquisition, yet this region has not been found to relate to aerobic fitness. Thus, we did not predict an association between aerobic fitness and brainstem CBF. Given these hypotheses, the present study will provide insight into a potential cerebrovascular mechanism by which aerobic fitness enhances brain health in children.
2. Method
2.1. Participants
Children were recruited from schools in East-Central Illinois. Eligible children were required to (1) report an absence of school-related learning disabilities (i.e., individual education plan related to learning), adverse health conditions, physical incapacities, or neurological disorders, (2) qualify as prepubescent (Tanner pubertal timing score ≤2; Taylor et al., 2001), (3) report no use of medications that influence central nervous system function, (4) demonstrate right handedness (as measured by the Edinburgh Handedness Questionnaire; Oldfield, 1971), (5) complete a mock MRI session successfully to screen for claustrophobia in an MRI machine, and (6) sign an informed assent approved by the University of Illinois at Urbana-Champaign. A legal guardian also provided written informed consent in accordance with the Institutional Review Board of the University of Illinois at Urbana-Champaign. The guardian was asked to provide information regarding participants’ socioeconomic status, as determined by: (1) participation in free or reduced-price lunch program at school, (2) the highest level of education obtained by the mother and father, and (3) number of parents who worked full-time (Birnbaum et al., 2002).
Ninety-one children were eligible for the study. Eighteen children were excluded from the analysis due to excessive head motion during the ASL scan. Seventy-three children (41 girls, 32 boys), ages 7–9 years (M = 8.63 years, SD = 0.54) were included in the ASL analysis.
2.2. Aerobic fitness testing
Children completed a VO2max test to assess aerobic fitness. The aerobic fitness of each child was measured as maximal oxygen consumption (VO2max) during a graded exercise test (GXT). The GXT employed a modified Balke Protocol and was administered on a LifeFitness 92T motor-driven treadmill (LifeFitness, Schiller Park, IL) with expired gases analyzed using a TrueOne2400 Metabolic Measurement System (ParMedics, Sandy, Utah). Children walked and/or ran on a treadmill at a constant speed with increasing grade increments of 2.5% every 2 min until volitional exhaustion occurred.
Oxygen consumption was measured using a computerized indirect calorimetry system (ParvoMedics True Max 2400) with averages for VO2 and respiratory exchange ratio (RER) assessed every 20 s. A polar heart rate (HR) monitor (Polar WearLink+ 31; Polar Electro, Finland) was used to measure HR throughout the test, and ratings of perceived exertion (RPE) were assessed every 2 min using the children’s OMNI scale (Utter et al., 2002). Maximal oxygen consumption was expressed in mL/100 g/min and VO2max was based upon maximal effort as evidenced by (1) a plateau in oxygen consumption corresponding to an increase of less than 2 mL/kg/min despite an increase in workload; (2) a peak HR ≥ 185 beats per minute (American College of Sports Medicine, 2014) and an HR plateau (Freedson and Goodman, 1993); (3) RER ≥ 1.0 (Bar-Or, 1983); and/or (4) a score on the children’s OMNI ratings of perceived exertion (RPE) scale ≥ 8 (Utter et al., 2002). Our sample consisted of relatively lower fit children (average VO2max percentile = 33.5%).
2.3. Arterial spin labeling (ASL) scan acquisition
Quantitative resting CBF in the child sample was measured using multi-slice pseudo-continuous arterial spin labeling (pCASL) (Wu et al., 2007). A number of studies have reported successful use of the ASL technique in children (Helton et al., 2009, Thomason et al., 2009, van den Tweel et al., 2009, Wang et al., 2003). In general, during an ASL scan, one or more radiofrequency (RF) pulses excite water molecules in arterial blood water in upstream blood (i.e., below the slice or region of interest), thus “labeling” or “tagging” the blood. Following a period of time, the labeled blood enters the imaging plane and alters the signal in the image, which is referred to as the “tag image.” Then, this acquisition is repeated, without the RF labeling, and a “control image” is created without the added signal contribution from tagged arterial blood. The difference between the tag image and the control image is the perfusion image, which reflects only blood flow, or CBF. Therefore, the perfusion image quantifies the amount of arterial blood delivered to each voxel in the slice within the post-label delay. This is affected by the arterial transit time (ATT), which is the time it takes for blood to travel from labeling plane to image voxel.
During the pCASL scan, the slices were oriented axially, perpendicular to the vertebral arteries. The top slice was positioned superior to the corpus callosum so that the slices covered the temporal lobes of the brain, including the hippocampus. Prior to acquisition, shimming was performed over a region that extended from the imaging slices to the tagging plane. The acquisition parameters included: multi-slice, gradient-echo echo-planar imaging (EPI) sequence, repetition time (TR) = 4000 ms, echo time (TE) = 19 ms, field-of-view (FOV) = 220 mm × 220 mm, matrix = 64 × 64, in-plane resolution = 3.4 mm, 16 slices, slice thickness = 4 mm, slice gap = 1 mm, bandwidth = 3004 Hz/Pixel, flip angle = 90°, no background suppression, ascending slice acquisition. The acquisition also included a fat saturation pulse to remove signal contamination from subcutaneous fat. Spins were tagged with a series of 1640 RF pulses for a total tagging duration of 1500 ms, with a tagging efficiency of ∼85% (Wu et al., 2007). The offset between the center of the imaging slices and the labeling plane was 70 mm and a post-label delay of 700 ms was used, although this delay has since been considered short for perfusion studies with pCASL (Alsop et al., 2015; see limitations). A series of 60 images (30 tag and 30 control) was acquired for a total scan duration of 4 min.
To assist with registration and anatomical identification for the ASL scan, a high-resolution T1-weighted image was acquired over the entire brain using a 3D MPRAGE (Magnetization Prepared Rapid Gradient Echo) protocol with 0.9 mm isotropic resolution (1900/2.32/900 ms repetition/echo/inversion times). All images were collected on a Siemens Magnetom Trio 3T whole-body MRI scanner with 12-channel receiver head coil (Siemens Medical Solutions; Erlangen, Germany).
2.4. ASL analysis
To analyze the ASL data, we used BASIL, a collection of tools in FMRIB’s Software Library (FSL) version 5.0.1 that creates quantitative CBF images from ASL data (www.fmrib.ox.ac.uk/fsl/basil) (Jenkinson et al., 2012). First, the ASL data were reconstructed and motion corrected via a rigid body algorithm in MCFLIRT (Jenkinson et al., 2002) and participants with excessive head motion (relative motion >4.0 mm) were excluded as it was expected data quality would be compromised. Next, the Oxford ASL (oxford_asl) tool within BASIL was applied in order to quantify CBF (Chappell et al., 2009). The tool performed a tag-control subtraction to remove the static tissue contribution, and the resulting time series was used to calculate relative CBF by inversion of the standard model for delivery of the ASL label (Buxton et al., 1998) using a Bayesian model inversion technique (Chappell et al., 2009) and an ATT of 700 ms, T1 relaxation time of arterial blood and tissue of 1.6 and 1.3 s, respectively, and a whole brain blood-tissue partition coefficient of 0.9 (Herscovitch and Raichle, 1985). Resulting CBF maps were visually inspected for data quality and clearly corrupted images were excluded.
Relative CBF (in scanner native units) was then converted to absolute physiological units (mL/100 g/min) by estimation of the equilibrium magnetization of arterial blood. This was estimated in a separate calibration process using the unlabeled (control) images from the data by measuring the equilibrium magnetization in the CSF in the ventricles and converting to the equivalent blood value accounting for differences in T1 relaxation time and proton densities (MacIntosh et al., 2008, Wong et al., 2006). The ventricles were automatically masked in the ASL image space by first segmenting the MPRAGE image using FMRIB’s Automated Segmentation Tool (FAST) (Zhang et al., 2001). The resulting tissue-type partial volume estimate was masked using a lateral ventricle template in the MNI152 standard brain image that was linearly transformed into the same space as each participant’s MPRAGE image. The resulting ventricle CSF mask was then transformed into the low-resolution ASL image space and finally thresholded at 90% to identify pure CSF voxels in the ventricles (the resulting mask was inspected for correctness in each subject). The resulting mask for each participant was used to calculate equilibrium magnetization in the ventricles. We also corrected for spatial intensity variations (or bias field or RF inhomogeneities) by calculating the bias field via FAST and scaling the CBF images accordingly. To account for inversion efficiency, we scaled the final CBF values by 1/0.85.
Segmentation and volumetric analysis of the hippocampus and brainstem were performed using a semi-automated, model-based subcortical tool (FIRST; FMRIB’s Integrated Registration and Segmentation Tool) (Patenaude et al., 2011). A two-stage affine registration to a standard space template (MNI space) with 1 mm resolution using 12° of freedom and a subcortical mask to exclude voxels outside the subcortical regions was first performed on each participant’s MPRAGE scan. Next, the hippocampus and brainstem were segmented with 30 and 40 modes of variation, respectively. To achieve accurate segmentation, the FIRST methodology models 317 manually segmented and labeled T1-weighted brain images from normal children, adults, and pathological populations (obtained from the Center for Morphometric Analysis, Massachusetts General Hospital, Boston) as a point distribution model with the geometry and variation of the shape of each structure submitted as priors. Volumetric labels are parameterized by a 3D deformation of a surface model based on multivariate Gaussian assumptions. FIRST searches through linear combinations of shape modes of variation for the most probable shape (i.e., brain structure) given the intensity distribution in the T1-weighted image, and specific brain regions are extracted (see Patenaude et al., 2011 for further description of the method). Modes of variation are optimized based on leave-one-out cross-validation on the training set, and they increase the robustness and reliability of the results (Patenaude et al., 2011).
Anterior and posterior sections of the hippocampus were calculated by determining the center of gravity for both the left and right hippocampus for each participant. The y coordinate from the center-of-gravity calculation was used to divide the region into anterior and posterior sections (Erickson et al., 2011). All segmentations were visually checked for errors. The volume of each participant’s left and right hippocampus (average, anterior, posterior) was measured in cubic millimeters.
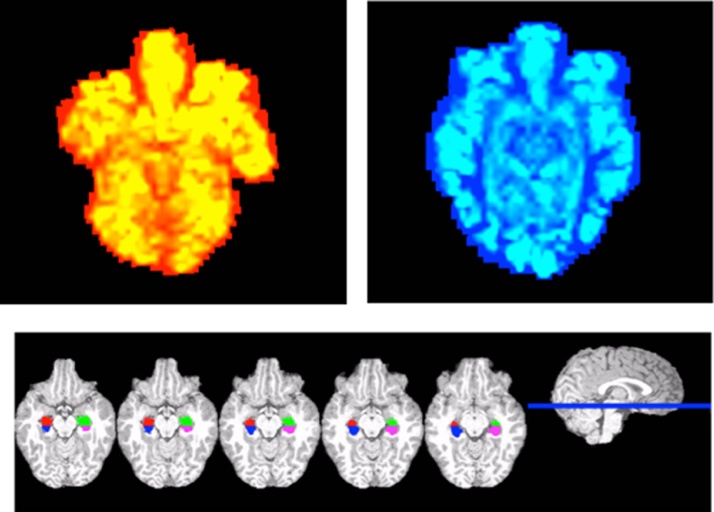
The hippocampus and brainstem segmentations were converted into ASL space via FMRIB’s Linear Image Registration Tool (FLIRT) affine linear registration with trilinear interpolation (Jenkinson and Smith, 2001, Jenkinson et al., 2002). The transformation was used in the final calculation of CBF in absolute units. See Fig. 1 for sample CBF maps and anterior/posterior hippocampal segmentations.
Fig. 1.
Sample CBF perfusion maps for a relatively lower fit (red) and higher fit (blue) participant, and sample anterior/posterior hippocampal segmentations in native structural space for one participant. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
2.5. Statistical analysis
Because there were significant and robust correlations between hemispheres (all r > 0.55, p < 0.0001), and no differences between left and right hippocampal CBF or left and right hippocampal volume (t < 1, p > 0.3), we averaged hippocampal CBF values across left and right hemispheres and averaged hippocampal volumes across left and right hemispheres. Average hippocampal CBF and average hippocampal volume were used in subsequent analyses.
Bivariate correlation analyses were conducted using Pearson product-moment correlation coefficients between the descriptive variables (age, sex, SES), aerobic fitness (VO2max), hippocampal CBF, hippocampal volume, brainstem CBF, and brainstem volume to determine appropriate covariates. Linear regressions were employed to test associations between aerobic fitness and hippocampal CBF (average, anterior, posterior) and brainstem CBF, when controlling for age, sex, and volume. T-scores and standardized betas (β) are presented. The alpha level for all tests was set at p < 0.05.
3. Results
Younger age was associated with greater CBF in the hippocampus (r = −0.393; p = 0.01) and brainstem (r = −0.289; p = 0.013), and aerobic fitness was associated with sex such that females had lower fitness scores than males (r = 0.311, p = 0.007). Thus, we included age and sex as covariates in the regression model. Despite no significant correlations between hippocampal CBF and hippocampal volume (r = 0.004, p > 0.05), or brainstem CBF and brainstem volume (r = −0.015, p > 0.05), we also included volume as a covariate to confirm that blood flow effects were independent of the size of the brain region.
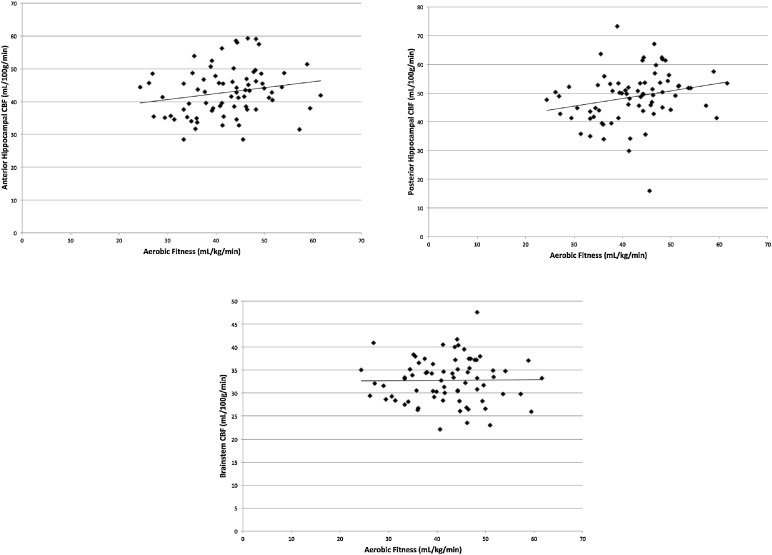
Higher aerobic fitness predicted greater CBF in the hippocampus (β = 0.235, t = 2.085, p = 0.041) when controlling for age, sex, and average hippocampal volume (Fig. 2). In particular, higher aerobic fitness predicted greater CBF in the posterior hippocampus (β = 0.241, t = 2.163, p = 0.034), when controlling for age, sex, and posterior hippocampal volume (Fig. 2). Higher aerobic fitness was marginally associated with CBF in the anterior hippocampus (β = 0.201, t = 1.723, p = 0.090), when controlling for age, sex, and anterior hippocampal volume (Fig. 2). As hypothesized, aerobic fitness did not predict CBF in the brainstem (β = 0.017, t = 0.136, p = 0.892), when controlling for age, sex, and brainstem volume (Fig. 2).
Fig. 2.
Scatterplots of the associations among aerobic fitness, anterior and posterior hippocampal CBF, and brainstem CBF.
4. Discussion
Consistent with our predictions, aerobic fitness was associated with greater cerebral blood flow in children. Specifically, using ASL perfusion techniques, we demonstrate that higher levels of aerobic fitness are associated with increased cerebral blood flow in the microvasculature of the hippocampus in 7- to 9-year-old children, independent of age, sex, and hippocampal volume. We also explored specificity of the relationship of fitness to the hippocampus and demonstrated, as predicted, no significant association between aerobic fitness and brainstem CBF.
Our results raise the possibility that aerobic fitness plays a role in vascularization of the hippocampus during childhood. Studies suggest a positive benefit of aerobic exercise on brain vasculature in animals (Black et al., 1990, Clark et al., 2009, Kleim et al., 2002, Rhyu et al., 2010) as well as cerebral blood flow measures in middle-aged and older humans (Bullitt et al., 2009, Burdette et al., 2010, Pereira et al., 2007). Here we suggest that these associations may extend to a child population during a critical period of maturation. In fact, angiogenesis has been directly coupled with cerebral blood volume (Dunn et al., 2004, Jiang et al., 2005, Lin et al., 2002, Maia et al., 2005, Sugahara et al., 1998). Although we measured cerebral blood flow rather than cerebral blood volume, we expect perfusion and blood volume to be closely related via the Central Volume Theorem (Newman et al., 2006, Stewart, 1893). A tissue with higher perfusion likely has higher blood volume to sustain the perfusion; however, flow also depends on how quickly the blood passes through the tissue, usually quantified in terms of mean transit time. We postulate that increased blood water delivery and availability in the hippocampus, as a function of higher aerobic fitness, may be due to more blood vessels in this region.
Yet as a variety of molecular and cellular cascades accompany hippocampal changes with aerobic exercise, we can only speculate about the biological mechanisms underlying increased perfusion. For example, in addition to changes in vasculature, aerobic exercise is known to increase cell proliferation and cell survival (Cotman and Berchtold, 2002, Ding et al., 2006), dendritic structure (Redila and Christie, 2006), growth factors (Neeper et al., 1996), and gliogenesis (Uda et al., 2006) in the hippocampus. In fact, angiogenesis and neurogenesis are tightly linked (Louissaint et al., 2002, Palmer et al., 2000). For instance, blocking the secretion of vascular endothelial growth factor (VEGF), a neurotrophic molecule involved in blood vessel growth (Lopez-Lopez et al., 2004), has been found to abolish exercise-induced neurogenesis (Fabel et al., 2003). Further, measures of cerebral blood volume have been said to provide an in vivo correlate of neurogenesis (Pereira et al., 2007). It is possible that some fitness-related differences in cerebral blood flow may be mediated, in part, by neurogenesis.
We are the first to explore the plasticity of perfusion in the anterior and posterior hippocampus in children. Our results do not suggest compelling specificity of fitness on anterior or posterior perfusion (independent of volume), with a significant positive association between fitness and posterior hippocampal CBF and a marginal positive relationship between fitness and anterior hippocampus. Given functional distinctions along the anterior/posterior axis of the hippocampus (Giovanello et al., 2009, Sperling et al., 2003), future studies should integrate a relational memory task to explore the links among aerobic fitness, cerebrovascular function in sections of the hippocampus, and cognitive function specific to the hippocampus.
It is interesting that the relationship between aerobic fitness and hippocampal CBF was independent of hippocampal volume, and resting CBF in the hippocampus was not related to the volume of the hippocampus. This lack of CBF-volume association is also supported by a study (Mozolic et al., 2010) that reported significant increases in resting cerebral blood flow with participation in a cognitive training program, but no associations among cognitive training, CBF, and changes in brain structure (via VBM). Together, the data raise the possibility that mechanisms underlying plasticity of blood flow and brain volume in humans are partially independent, and increased blood flow is not solely driven by a larger size of the brain region.
Additionally, our data raise the possibility that extreme group differences in aerobic fitness (e.g., 70th percentile versus 30th percentile VO2max) may be needed to demonstrate hippocampal volume effects (Chaddock et al., 2010a), whereas CBF differences may be a more sensitive marker to understand how small relative differences in aerobic fitness influence brain health, particularly in terms of microcirculation, during development. We did not observe a significant relationship between aerobic fitness and hippocampal volume in the present study, which included a relatively lower fit sample of children (average VO2max percentile = 33.5%). However, our previous work demonstrates that higher fit children (>70th percentile VO2max) show larger hippocampal volumes compared to their lower fit peers (<30th percentile VO2max) (Shvartz and Reibold, 1990). Thus, different child fitness spectrums in each study may lead to different results and outcomes. Whereas the present study provides a first step in understanding the predictive power of aerobic fitness in hippocampal perfusion during child development, questions still remain regarding the associations among individual differences in aerobic fitness, hippocampal structure and function, and performance on specific memory tasks (e.g., relational, spatial) which require additional research.
Despite our result that aerobic fitness is related to increased hippocampal blood flow in children, we acknowledge limitations of the study, including the choice of an ATT of 700 ms used in post-processing CBF quantification. This value is used in the FSL software based on experience with adult ASL studies (Chappell et al., 2009), although it may not be appropriate for the study of a pediatric population. However, choice of ATT simply provides a scaling factor in CBF calculations and would not affect the relationships presented here. Future work may look to incorporate a multi-delay labeling scheme to simultaneously estimate ATT and CBF, thus providing a more quantitatively accurate measure. We also note that the parameters of the ASL sequence in this study used a short post-label delay for the tagging scheme employed. Specifically, we used a pCASL acquisition as it is recognized to offer the highest signal to noise ratio of all ASL labeling schemes, but with a post-label delay of 700 ms. Subsequent to the present study, the consensus recommendation in the field of ASL acquisition was a longer post-labeling delay for routine pCASL perfusion studies of 1500 ms for children (Alsop et al., 2015). A likely consequence of a shorter post-label delay is that some labeled blood may remain in the arterial vasculature, thereby not being delivered to the tissue at the time of imaging. At the voxel level, the image intensity may not be a pure measure of perfusion, but also include a contribution from arterial blood volume (Chappell et al., 2010). Thus, it is possible that the region of interest measurements are a mixture of CBF and arterial blood volume, with the amount of arterial blood in the signal dependent on the ATT and post-label delay. In addition, as we did not account for partial volume effects in the hippocampus, this may have contributed to the fitness associations we report. A goal for future research will be to use partial volume correction methods on ASL data derived from the hippocampus to determine whether the effects change after accounting for this factor.
This research has important implications, as physical activity is decreasing in and out of the school environment (Troiano et al., 2008), and children are becoming increasingly unfit (Centers for Disease Control and Prevention, 2009). We provide additional evidence to suggest that the developing brain may be plastic and sensitive to individual differences in aerobic fitness levels. Specifically, aerobic fitness may influence how the brain regulates its metabolic demands via blood flow in a region of the brain important for learning and memory.
Acknowledgements
The study was supported by the National Institute of Child Health and Human Development (NICHD) RO1 grant to Dr. Kramer and Dr. Hillman. We would like to thank the Biomedical Imaging Center for their help with data collection and the design of the ASL imaging protocol, in particular Ryan Larsen, Brad Sutton, Holly Tracy, and Nancy Dodge.
References
- Alsop D.C., Detre J.A. Reduced transit-time sensitivity in noninvasive magnetic resonance imaging of human cerebral blood flow. J. Cereb. Blood Flow Metab. 1996;16:1236–1249. doi: 10.1097/00004647-199611000-00019. [DOI] [PubMed] [Google Scholar]
- Alsop D.C., Detre J.A., Golay X., Gunther M., Hendrikse J., Hernandez-Garcia L., Lu L., MacIntosh H., Parkes B.J., Smits L.M., van Osch M., Wang M.J.P., Wong E.C., Zaharchuk G. Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: a consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magn. Reson. Med. 2015;73:102–116. doi: 10.1002/mrm.25197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American College of Sports Medicine . 9th ed. Wolters Kluwer/Lippincott Williams & Wilkins.; Philadelphia, PA: 2014. ACSM’s Guidelines for Exercise Testing and Prescription. [Google Scholar]
- Bar-Or O. Springer-Verlag; New York, NY: 1983. Pediatric Sports Medicine for the Practitioner: From Physiologic Principles to Clinical Applications. [Google Scholar]
- Birnbaum A.S., Lytle L.A., Murray D.M., Story M., Perry C.L., Boutelle K.N. Survey development for assessing correlates of young adolescents’ eating. Am. Acad. Health Behav. 2002;26:284–295. doi: 10.5993/ajhb.26.4.5. [DOI] [PubMed] [Google Scholar]
- Black J.E., Isaacs K.R., Anderson B.J., Alcantara A.A., Greenough W.T. Learning causes synaptogenesis, whereas motor activity causes angiogenesis, in cerebellar cortex of adult rats. Proc. Natl. Acad. Sci. U. S. A. 1990;87:5568–5572. doi: 10.1073/pnas.87.14.5568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck S.M., Hillman C.H., Castelli D.M. The relation of aerobic fitness to stroop task performance in preadolescent children. Med. Sci. Sports Exerc. 2008;40:166–172. doi: 10.1249/mss.0b013e318159b035. [DOI] [PubMed] [Google Scholar]
- Bugg J.M., Head D. Exercise moderates age-related atrophy of the medial temporal lobe. Neurobiol. Aging. 2011;32:506–514. doi: 10.1016/j.neurobiolaging.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullitt E., Rahman F.N., Smith J.K., Kim E., Zeng D., Katz L.M., Marks B.L. The effect of exercise on the cerebral vasculature of healthy aged subjects as visualized by MR angiography. Am. J. Neuroradiol. 2009;30:1857–1863. doi: 10.3174/ajnr.A1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdette J.H., Laurienti P.J., Espeland M.A., Morgan A., Telesford Q., Vechlekar C.D., Hayasaka S., Jennings J.M., Katula J.A., Kraft R.A., Rejeski W.J. Using network science to evaluate exercise-associated brain changes in older adults. Front. Aging Neurosci. 2010;2:1–10. doi: 10.3389/fnagi.2010.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxton R., Frank L., Wong E., Siewert B., Warach S., Edelman R. A general kinetic model for quantitative perfusion imaging with arterial spin labeling. Magn. Reson. Med. 1998;40:383–396. doi: 10.1002/mrm.1910400308. [DOI] [PubMed] [Google Scholar]
- Castelli D.M., Hillman C.H., Buck S.M., Erwin H.E. Physical fitness and academic achievement in third- and fifth-grade students. J. Sport Exerc. Psychol. 2007;29:239–252. doi: 10.1123/jsep.29.2.239. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention . 2009. Chronic Diseases and Health Promotion.http://www.cdc.gov/chronicdisease/overview/ (Retrieved from) [Google Scholar]
- Chaddock L., Erickson K.I., Prakash R.S., Kim J.S., Voss M.W., VanPatter M., Pontifex M.B., Raine L.B., Konkel A., Hillman C.H., Cohen N.J., Kramer A.F. A neuroimaging investigation of the association between aerobic fitness, hippocampal volume and memory performance in preadolescent children. Brain Res. 2010;1358:172–183. doi: 10.1016/j.brainres.2010.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaddock L., Erickson K.I., Prakash R.S., VanPatter M., Voss M.V., Pontifex M.B., Raine L.B., Hillman C.H., Kramer A.F. Basal ganglia volume is associated with aerobic fitness in preadolescent children. Dev. Neurosci. 2010;32:249–256. doi: 10.1159/000316648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaddock L., Hillman C.H., Buck S.M., Cohen N.J. Aerobic fitness and executive control of relational memory in preadolescent children. Med. Sci. Sports Exerc. 2011;43:344–349. doi: 10.1249/MSS.0b013e3181e9af48. [DOI] [PubMed] [Google Scholar]
- Chaddock L., Erickson K.I., Prakash R.S., Voss M.V., VanPatter M., Pontifex M.B., Hillman C.H., Kramer A.F. A functional MRI investigation of the association between childhood aerobic fitness and neurocognitive control. Biol. Psychol. 2012;89:260–268. doi: 10.1016/j.biopsycho.2011.10.017. [DOI] [PubMed] [Google Scholar]
- Chappell M.A., Groves A.R., Whitcher B., Woolrich M.W. Variational Bayesian inference for a non-linear forward model. IEEE Trans. Signal Process. 2009;57:223–236. [Google Scholar]
- Chappell M.A., MacIntosh B.J., Donahue M.J., Guenther M., Jezzard P., Woolrich M.W. Separation of macrovascular signal in multi-inversion time arterial spin labelling MRI. Magn. Reson. Med. 2010;63:1357–1365. doi: 10.1002/mrm.22320. [DOI] [PubMed] [Google Scholar]
- Chomitz V.R., Slining M.M., McGowan R.J., Mitchell S.E., Dawson G.F., Hacker K.A. Is there a relationship between physical fitness and academic achievement?: : Positive results from public school children in the northeastern United States. J. School Health. 2009;79:30–37. doi: 10.1111/j.1746-1561.2008.00371.x. [DOI] [PubMed] [Google Scholar]
- Clark P.J., Brzezinska W.J., Puchalski E.K., Krone D.A., Rhodes J.S. Functional analysis of neuromuscular adaptations to exercise in the dentate gyrus of young adult mice associated with cognitive gain. Hippocampus. 2009;19:937–950. doi: 10.1002/hipo.20543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotman C.W., Berchtold N.C. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends Neurosci. 2002;25:295–301. doi: 10.1016/s0166-2236(02)02143-4. [DOI] [PubMed] [Google Scholar]
- Ding Q., Vaynman S., Akhavan M., Ying Z., Gomez-Pinilla F. Insulin-like growth factor I interfaces with brain-derived neurotrophic factor-mediated synaptic plasticity to modulate aspects of exercise-induced cognitive function. Neuroscience. 2006;140:823–833. doi: 10.1016/j.neuroscience.2006.02.084. [DOI] [PubMed] [Google Scholar]
- Dunn J.F., Roche M.A., Springett R., Abajian M., Merlis J., Daghlian C.P., Lu S.Y., Makki M. Monitoring angiogenesis in brain using steady-state quantification of DeltaR2 with MION infusion. Magn. Reson. Med. 2004;51:55–61. doi: 10.1002/mrm.10660. [DOI] [PubMed] [Google Scholar]
- Erickson K.I., Prakash R.S., Voss M.W., Chaddock L., Hu L., Morris K.S., White S.M., Wójcicki T.R., McAuley E., Kramer A.F. Aerobic fitness is associated with hippocampal volume in elderly humans. Hippocampus. 2009;19:1030–1039. doi: 10.1002/hipo.20547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson K.I., Voss M.V., Prakash R.S., Basak C., Szabo A., Chaddock L., Kim J.S., Heo S., Alves H., White S.M., Wójcicki T.R., Mailey E., Vieira V.J., Martin S.A., Pence B.D., Woods J.A., McAuley E., Kramer A.F. Exercise training increases size of hippocampus and improves memory. Proc. Natl. Acad. Sci. U. S. A. 2011;108:3017–3022. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabel K., Fabel K., Tam B., Kaufer D., Baiker A., Simmons N., Kuo C.J., Palmer T.D. VEGF is necessary for exercise-induced adult hippocampal neurogenesis. Eur. J. Neurosci. 2003;18:2803–2812. doi: 10.1111/j.1460-9568.2003.03041.x. [DOI] [PubMed] [Google Scholar]
- Freedson P.S., Goodman T.L. Measurement of oxygen consumption. In: Rowland T.W., editor. Pediatric Laboratory Exercise Testing: Clinical Guidelines. Human Kinetics; Champaign, IL: 1993. pp. 91–113. [Google Scholar]
- Giovanello K.S., Schnyer D., Verfaellie M. Distinct hippocampal regions make unique contributions to relational memory. Hippocampus. 2009;19:111–117. doi: 10.1002/hipo.20491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hales P.W., Kawadler J.M., Aylett S.E., Kirkham F.J., Clark C.A. Arterial spin labeling characterization of cerebral perfusion during normal maturation from late childhood into adulthood: normal ‘reference range’ values and their use in clinical studies. J. Cereb. Blood Flow Metab. 2014;34:776–784. doi: 10.1038/jcbfm.2014.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helton K.J., Paydar A., Glass J., Weirich E.M., Hankins J., Li C.S., Smeltzer M.P., Wang W.C., Ware R.E., Ogg R.J. Arterial spin-labeled perfusion combined with segmentation techniques to evaluate cerebral blood flow in white and gray matter of children with sickle cell anemia. Pediatr. Blood Cancer. 2009;52:85–91. doi: 10.1002/pbc.21745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heo S., Prakash R.S., Voss M.W., Erickson K.I., Ouyang C., Sutton B.P., Kramer A.F. Resting hippocampal blood flow: spatial memory and aging. Brain Res. 2010;1315:119–127. doi: 10.1016/j.brainres.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herscovitch P., Raichle M. What is the correct value for the brain-blood partition coefficient for water? J. Cereb. Blood Flow Metab. 1985;5:65–69. doi: 10.1038/jcbfm.1985.9. [DOI] [PubMed] [Google Scholar]
- Hillman C.H., Buck S.M., Themanson J.R., Pontifex M.B., Castelli D.M. Aerobic fitness and cognitive development: event-related brain potential and task performance of executive control in preadolescent children. Dev. Psychol. 2009;45:114–129. doi: 10.1037/a0014437. [DOI] [PubMed] [Google Scholar]
- Hoge R., Atkinson J., Gill B., Crelier G., Marrett S., Pike G. Investigation of BOLD signal dependence on CBF and CMRO2: the deoxyhemoglobin dilution model. Magn. Reson. Med. 1999;42:849–863. doi: 10.1002/(sici)1522-2594(199911)42:5<849::aid-mrm4>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Honea R.A., Thomas G.P., Harsha A., Anderson H.S., Donnelly J.E., Brooks W.M., Burns J.M. Cardiorespiratory fitness and preserved medial temporal lobe volume in Alzheimer’s disease. Alzheimer Dis. Assoc. Disord. 2009;23:188–197. doi: 10.1097/WAD.0b013e31819cb8a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M., Smith S.M. A global optimisation method for robust affine registration of brain images. Med. Image Anal. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Jenkinson M., Bannister P.R., Brady J.M., Smith S.M. Improved optimisation for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Jenkinson M., Beckmann C.F., Behrens T.E., Woolrich M.W., Smith S.M. FSL. Neuroimage. 2012;62:782–790. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Jiang Q., Zhang Z.G., Ding G.L., Zhang L., Ewing J.R., Wang L., Zhang R., Li L., Lu M., Meng H., Arbab A.S., Hu J., Li Q.J., PourabdollahNejad D.S., Athiraman H., Chopp M. Investigation of neural progenitor cell induced angiogenesis after embolic stroke in rat using MRI. Neuroimage. 2005;28:698–707. doi: 10.1016/j.neuroimage.2005.06.063. [DOI] [PubMed] [Google Scholar]
- Kleim J.A., Cooper N.R., VandenBerg P.M. Exercise induces angiogenesis but does not alter movement representations within rat motor cortex. Brain Res. 2002;934:1–6. doi: 10.1016/s0006-8993(02)02239-4. [DOI] [PubMed] [Google Scholar]
- Lin T.N., Sun S.W., Cheung W.M., Li F., Chang C. Dynamic changes in cerebral blood flow and angiogenesis after transient focal cerebral ischemia in rats. Evaluation with serial magnetic resonance imaging. Stroke. 2002;33:2985–2991. doi: 10.1161/01.str.0000037675.97888.9d. [DOI] [PubMed] [Google Scholar]
- Lopez-Lopez C., LeRoith D., Torres-Aleman I. Insulin-like growth factor I is required for vessel remodeling in the adult brain. Proc. Natl. Acad. Sci. U. S. A. 2004;101:9833–9838. doi: 10.1073/pnas.0400337101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louissaint A., Rao S., Leventhal C., Goldman S.A. Coordinated interaction of neurogenesis and angiogenesis in the adult songbird brain. Neuron. 2002;34:945–960. doi: 10.1016/s0896-6273(02)00722-5. [DOI] [PubMed] [Google Scholar]
- MacIntosh B.J., Pattinson K.T., Gallichan D., Ahmad I., Miller K.L., Feinberg D.A., Wise R.G., Jezzard P. Measuring the effects of remifentanil on cerebral blood flow and arterial arrival time using 3D GRASE MRI with pulsed arterial spin labelling. J. Cereb. Blood Flow Metab. 2008;28:1514–1522. doi: 10.1038/jcbfm.2008.46. [DOI] [PubMed] [Google Scholar]
- Maia A.C., Malheiros S.M., da Rocha A.J., da Silva C.J., Gabbai A.A., Ferraz F.A., Stávale J.N. MR cerebral blood volume maps correlated with vascular endothelial growth factor expression and tumor grade in nonenhancing gliomas. Am. J. Neuroradiol. 2005;26:777–783. [PMC free article] [PubMed] [Google Scholar]
- Mozolic J.L., Hayasaka S., Laurienti P.J. A cognitive training intervention increases resting cerebral blood flow in healthy older adults. Front. Hum. Neurosci. 2010;12:1–10. doi: 10.3389/neuro.09.016.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neeper S.A., Gomez-Pinilla F., Choi J., Cotman C.W. Physical activity increases mRNA for brain-derived neurotrophic factor and nerve growth factor in rat brain. Brain Res. 1996;726:49–56. [PubMed] [Google Scholar]
- Newman G.C., Hospod F.E., Fain S.B., Cook T.D. Optimizing dynamic T2* MR imaging for measurement of cerebral blood flow using infusions for cerebral blood volume. Am. J. Neuroradiol. 2006;27:1239–1240. [PMC free article] [PubMed] [Google Scholar]
- Oldfield R.C. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Palmer T.D., Willhoite A.R., Gage F.H. Vascular niche for adult hippocampal neurogenesis. J. Comp. Neurol. 2000;425:479–494. doi: 10.1002/1096-9861(20001002)425:4<479::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Patenaude B., Smith S.M., Kennedy D., Jenkinson M. A Bayesian model of shape and appearance for subcortical brain. Neuroimage. 2011;56:907–922. doi: 10.1016/j.neuroimage.2011.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira A.C., Huddleston D.E., Brickman A.M., Sosunov A.A., Hen R., McKhann G.M., Sloan R., Gage F.H., Brown T.R., Small S.A. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proc. Natl. Acad. Sci. U. S. A. 2007;104:5638–5643. doi: 10.1073/pnas.0611721104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontifex M.B., Raine L.B., Johnson C.R., Chaddock L., Voss M.W., Cohen N.J., Kramer A.F., Hillman C.H. Cardiorespiratory fitness and the flexible modulation of cognitive control in preadolescent children. J. Cogn. Neurosci. 2011;23:1332–1345. doi: 10.1162/jocn.2010.21528. [DOI] [PubMed] [Google Scholar]
- Redila V.A., Christie B.R. Exercise-induced changes in dendritic structure and complexity in the adult hippocampal dentate gyrus. Neuroscience. 2006;137:1299–1307. doi: 10.1016/j.neuroscience.2005.10.050. [DOI] [PubMed] [Google Scholar]
- Rhyu I.J., Bytheway J.A., Kohler S.J., Lange H., Lee K.J., Boklewski J., McCormick K., Williams N.I., Stanton G.B., Greenough W.T., Cameron J.L. Effects of aerobic exercise training on cognitive function and cortical vascularity in monkeys. Neuroscience. 2010;167:1239–1248. doi: 10.1016/j.neuroscience.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shvartz E., Reibold R.C. Aerobic fitness norms for males and females aged 6–75 years: a review. Aviat. Space Environ. Med. 1990;61:3–11. [PubMed] [Google Scholar]
- Sokoloff L., Reivich M., Kennedy C., Rosiers M.H.D., Patlak C.S., Pettigrew K.D., Sakurada O., Shinohara M. The [14c] deoxyglucose method for the measurement of local cerebral glucose utilization: theory, procedure, and normal values in the conscious and anesthetized albino rat. J. Neurochem. 1977;28:897–916. doi: 10.1111/j.1471-4159.1977.tb10649.x. [DOI] [PubMed] [Google Scholar]
- Sperling R., Chua E., Cocchiarella A., Rand-Giovannetti E., Poldrack R., Schacter D.L., Albert M. Putting names to faces: successful encoding of associative memories activates the anterior hippocampal formation. Neuroimage. 2003;20:1400–1410. doi: 10.1016/S1053-8119(03)00391-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart G.N. Researches on the circulation time in organs and on the influences which affect it: parts I–III. J. Physiol. (Lond.) 1893;15:1–89. doi: 10.1113/jphysiol.1893.sp000462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugahara T., Korogi Y., Kochi M., Ikushima I., Hirai T., Okuda T., Shigematsu Y., Liang L., Ge Y., Ushio Y., Takahashi M. Correlation of MR imaging-determined cerebral blood volume maps with histologic and angiographic determination of vascularity of gliomas. Am. J. Roentgenol. 1998;171:1479–1486. doi: 10.2214/ajr.171.6.9843274. [DOI] [PubMed] [Google Scholar]
- Taylor S.J.C., Whincup P.H., Hindmarsh P.C., Lampe F., Odoki K., Cook D.G. Performance of a new pubertal self-assessment questionnaire: a preliminary study. Paediatr. Perinat. Epidemiol. 2001;15:88–94. doi: 10.1046/j.1365-3016.2001.00317.x. [DOI] [PubMed] [Google Scholar]
- Thomason M.E., Waugh C.E., Glover G.H., Gotlib I.H. COMT genotype and resting brain perfusion in children. Neuroimage. 2009;48:217–222. doi: 10.1016/j.neuroimage.2009.05.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troiano R.P., Berrigan D., Dodd K.W., Mâsse L.C., Tilert T., McDowell M. Physical activity in the United States measured by accelerometer. Med. Sci. Sports Exerc. 2008;40:181–188. doi: 10.1249/mss.0b013e31815a51b3. [DOI] [PubMed] [Google Scholar]
- Uda M., Ishido M., Kami K., Masuhara M. Effects of chronic treadmill running on neurogenesis in the dentate gyrus of the hippocampus of adult rat. Brain Res. 2006;1104:64–72. doi: 10.1016/j.brainres.2006.05.066. [DOI] [PubMed] [Google Scholar]
- Utter A.C., Robertson R.J., Nieman D.C., Kang J. Children’s OMNI scale of perceived exertion: walking/running evaluation. Med. Sci. Sports Exerc. 2002;34:139–144. doi: 10.1097/00005768-200201000-00021. [DOI] [PubMed] [Google Scholar]
- van Praag H., Christie B.R., Sejnowski T.J., Gage F.H. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc. Natl. Acad. Sci. U. S. A. 1999;96:13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H., Kempermann G., Gage F.H. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat. Neurosci. 1999;2:266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- van Praag H., Shubert T., Zhao C., Gage F.H. Exercise enhances learning and hippocampal neurogenesis in aged mice. J. Neurosci. 2005;25:8680–8685. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Tweel X.W., Nederveen A.J., Majoie C.B., van der Lee J.H., Wagener-Schimmel L., van Walderveen M.A., The B.T.P., Nederkoorn P.J., Heijboer H., Fijnvandraat K. Cerebral blood flow measurement in children with sickle cell disease using continuous arterial spin labeling at 3.0-Tesla MRI. Stroke. 2009;40:795–800. doi: 10.1161/STROKEAHA.108.523308. [DOI] [PubMed] [Google Scholar]
- Voss M.W., Chaddock L., Kim J.S., VanPatter M., Pontifex M.B., Raine L.B., Kramer A.F. Aerobic fitness is associated with greater efficiency of the network underlying cognitive control in preadolescent children. Neuroscience. 2011;199:166–176. doi: 10.1016/j.neuroscience.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss M.W., Vivar C., Kramer A.F., van Praag H. Bridging animal and human models of exercise-induced brain plasticity. Trends Cogn. Sci. 2013;17:525–544. doi: 10.1016/j.tics.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J.J., Licht D.J., Jahng G., Liu C., Rubin J.T., Haselgrove J., Zimmerman R.A.…Detre J.A. Pediatric perfusion imaging using pulsed arterial spin labeling. J. Magn. Reson. Imaging. 2003;18:404–413. doi: 10.1002/jmri.10372. [DOI] [PubMed] [Google Scholar]
- Wong E.C., Cronin M., Wu W.C., Inglis B., Frank L.R., Liu T.T. Velocity-selective arterial spin labeling. Magn. Reson. Med. 2006;55:1334–1341. doi: 10.1002/mrm.20906. [DOI] [PubMed] [Google Scholar]
- Wu W.C., Fernández-Seara M., Detre J.A., Wehrli F.W., Wang J. A theoretical and experimental investigation of the tagging efficiency of pseudocontinuous arterial spin labeling. Magn. Reson. Med. 2007;58:1020–1027. doi: 10.1002/mrm.21403. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Brady M., Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans. Med. Imaging. 2001;20:45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]