Abstract
The two-component framework of episodic memory (EM) development posits that the contributions of medial temporal lobe (MTL) and prefrontal cortex (PFC) to successful encoding differ across the lifespan. To test the framework’s hypotheses, we compared subsequent memory effects (SME) of 10–12 year-old children, younger adults, and older adults using functional magnetic resonance imaging (fMRI). Memory was probed by cued recall, and SME were defined as regional activation differences during encoding between subsequently correctly recalled versus omitted items. In MTL areas, children’s SME did not differ in magnitude from those of younger and older adults. In contrast, children’s SME in PFC were weaker than the corresponding SME in younger and older adults, in line with the hypothesis that PFC contributes less to successful encoding in childhood. Differences in SME between younger and older adults were negligible. The present results suggest that, among individuals with high memory functioning, the neural circuitry contributing to successful episodic encoding is reorganized from middle childhood to adulthood. Successful episodic encoding in later adulthood, however, is characterized by the ability to maintain the activation patterns that emerged in young adulthood.
Keywords: Aging, Development, Episodic memory, fMRI, Lifespan, Subsequent memory
1. Introduction
Episodic memory (EM) emerges and increases during childhood (e.g., Schneider and Pressley, 1997) and deteriorates in aging (e.g., Rönnlund et al., 2005). On the surface, children in middle childhood and older adults show comparable memory levels, with both groups performing worse than younger adults (Li et al., 2004). However, direct lifespan comparisons of neural correlates of EM are entirely lacking, leaving an untested assumption that neural mechanisms underlying memory in children and older adults are the same, given their similarities in performance (but see behavioral comparisons by Brehmer et al., 2007, Fandakova et al., 2013, Shing et al., 2008). Therefore, lifespan studies are strongly needed in the field of developmental cognitive neuroscience, which has tended to focus either on comparisons on the lower end (children vs. young adults) or the higher end (younger vs. older adults) of the developmental spectrum. Here, we examined the neural correlates of subsequent-memory effects (SME), defined as differences in fMRI activation between subsequently remembered and omitted trials during encoding, in 10–12 years old children, younger adults between 21 and 26 years, and older adults above 60 years of age. Our focus was to compare SME within the memory systems of the various age groups when they operate successfully to form durable memory representation.
According to the two-component framework of EM development, the ontogeny of EM reflects interactions between associative and strategic components (Shing et al., 2010, Shing et al., 2008, Werkle-Bergner et al., 2006). The associative component refers to binding mechanisms that integrate features of episodes into coherent representations (Treisman, 1996, Zimmer et al., 2006), and the strategic component refers to cognitive-control processes that aid and regulate memory functions (Simons and Spiers, 2003). In line with established conceptions of EM (Eichenbaum, 2002, Moscovitch, 1992, Simons and Spiers, 2003) and meta-analyses of fMRI studies on SME (Kim, 2011, Spaniol et al., 2009), we assume that the lateral PFC and MTL (particularly the hippocampus, HC) support strategic and associative components of EM, respectively. The HC and associated structures contribute to the formation of memory representations, particularly establishing associations between features (e.g., Davachi, 2006). Lateral PFC, on the other hand, supports cognitive control processes in service of memory (Fletcher and Henson, 2001), such as implementation of attention selection processes in the VLPFC and organization of information in working memory in the DLPFC (e.g., Blumenfeld and Ranganath, 2007).
Both PFC and MTL undergo profound reorganization in childhood (Johnson, 2001, Nelson, 2001) and aging (Buckner, 2004, Cabeza et al., 2004). The structural integrity of PFC (particularly the dorsolateral regions) undergoes maturational changes well into adolescence. On the other hand, MTL regions mature at faster rates particularly in the first few years of life (Gogtay et al., 2006, Sowell et al., 2003). Therefore, we assume that successful memory formation in children should rely more on the associative component of MTL and less on the strategic component of PFC that is still developing (Ofen, 2012, Shing et al., 2010; but see Ghetti and Bunge, 2012). Thus far, results are mixed regarding MTL differences between childhood and young adulthood in SME, with some studies finding age differences (e.g., Ghetti et al., 2010) and others not (e.g., Ofen et al., 2007). This stands in contrast to the more consistent age differences found in PFC across development. Therefore, our empirical investigation will shed light on this topic.
On the other hand, lower EM performance in aging is assumed to reflect senescent changes in the associative as well as strategic components (Shing et al., 2010). Gray matter differences are especially pronounced in both HC and PFC regions (Fjell et al., 2009, Raz et al., 2005; but see Raz et al., 2010 for lack of longitudinal change in PFC). However, findings regarding functional alterations in these regions during episodic encoding in old age have been mixed. In part, the inconsistencies across studies may reflect the heterogeneity of memory functioning in old age (Persson et al., 2011, Raz et al., 2010, Rönnlund et al., 2005). This partly gave rise to a maintenance view on EM proposed by Nyberg et al. (2012), underscoring the notion that preserved brain functioning is the primary characteristic of successful memory aging. While this view focuses on between-person differences, a within-person aspect of maintenance would suggest that, among healthy older adults, successful episodic encoding should engage both the associative and strategic components optimally, reflecting a state that is youth-like. Therefore, our study aimed to shed lights on the mixed findings by comparing age groups across the lifespan to identify their commonalities and differences in neural mechanisms contributing to successful memory formation.
Importantly, in contrast to most SME studies that tested memory with a recognition procedure, we opted for cued recall in which participants studied word pairs (e.g. dog-crown) and subsequently had to recall the target (crown) when shown the cue (dog). This required individuals’ memory system to operate at its best during encoding in order to form strong, distinctive memory representations that can be recalled later on. In younger adults, compared to recognition, cued-recall imposes stronger demands on both PFC and MTL (Habib and Nyberg, 2007, Staresina and Davachi, 2006). Based on our framework, we expected that SME in children, relative to younger and older adults, would rely more on MTL and less on PFC regions. In contrast, SME-related activation of healthy older adults should be largely similar to those of younger adults. This is because the neural circuitry contributing to SME, particularly for forming strong memory representations, should not alter fundamentally in healthy aging (cf. Maillet and Rajah, 2014). As a secondary goal, we also explored potential age differences in functional connectivity in EM networks. Thus far little is known about memory-related connectivity in children, while older adults seem to show increased connectivity in fronto-temporal networks for successful memory encoding (Dennis et al., 2008, Oh and Jagust, 2013). Therefore, in addition to functional activation, our analyses will extend the two-component framework of EM lifespan development to examine MTL-PFC interactions.
2. Methods
2.1. Participants
This fMRI study was part of a larger scale EM training study. The initial sample consisted of 95 children aged 10–12 years (fifth-graders in the German Gymnasium education track), 49 younger adults aged 21–26 years (university or college students), and 165 older adults aged 63–74 years (retired community dwellers living in Berlin). These participants took part in a screening session and were invited to participate in the fMRI study if they fulfilled all of the following criteria: (a) a minimum raw score of 34 correctly solved symbols on the digit symbol test (maximum score 94; Wechsler, 1955); (b) recalling at least 3 word pairs from a list of 10 pairs; and additionally for adults only (c) more than 27 points on the Mini-Mental State Exam (maximum score 30; Folstein et al., 1975); (d) 30 points or higher on the CES-D scale on depression (Radloff, 1977). The first two criteria were established after extensive piloting and were adopted to increase the likelihood that our scanned participants would produce enough remembered trials for the fMRI analyses.
56 children, 35 younger adults, and 55 older adults fulfilled the screening criteria and participated in the fMRI assessment. These participants were right-handed, native German speakers, and reported not having neurological or psychiatric disorders (e.g. Alzheimer, multiple sclerosis, Parkinson’s disease, dyslexia, mood disorders etc.). For the current analysis of SME, three children had to be excluded due to excessive motion artifacts, and one younger adult due to technical error in scanning. Furthermore, we included only those participants who provided sufficient numbers of remembered as well as omitted responses for the fMRI analysis (i.e. a minimum of two runs with at least six trials per run; see Murphy and Garavan, 2005 on number of events for fMRI designs). Therefore, for the analyses below, we used data from 31 children (Mage = 11.09, SDage = 0.39), 33 younger adults (Mage = 24.0, SDage = 1.33), and 25 older adults (Mage = 66.8, SDage = 2.15). As shown in Table 1, compared to the excluded sample, the final sample was positively selected on memory performance, particularly in the case of children and older adults. Within each age group, the excluded and final samples were comparable in terms of gender radio, performance on a marker test of crystallized intelligence (verbal knowledge; Lehrl, 1977), a marker test of fluid intelligence (digit symbol; Wechsler, 1955), and years of education. We found expected lifespan patterns with respect to crystallized and fluid intelligence, namely (a) a continuous increase in verbal knowledge across the lifespan, and (b) an inverted U-shaped lifespan function for the Digit Symbol scores, with children and older adults showing lowered performance in comparison to younger adults.
Table 1.
Basic demographic and cognitive performance of participants excluded from and included in final analyses.
| Age Group | Excluded |
Final |
||
|---|---|---|---|---|
| Mean | SE | Mean | SE | |
| Female Ratio | ||||
| CH | 0.31 | – | 0.40 | – |
| YA | 0.33 | – | 0.49 | – |
| OA | 0.58 | – | 0.40 | – |
| Years of Formal Education | ||||
| CH | 5 | – | 5 | – |
| YA | 16.25 | 0.75 | 16.27 | 0.49 |
| OA | 15.68 | 0.72 | 17.32 | 0.73 |
| Digit Symbol | ||||
| CH | 41.15 | 1.47 | 40.24 | 1.48 |
| YA | 68.67 | 9.68 | 70.24 | 1.80 |
| OA | 50.90 | 1.63 | 49.68 | 1.54 |
| Vocabulary Knowledge | ||||
| CH | 9.30 | 0.95 | 9.91 | 0.76 |
| YA | 24.17 | 1.10 | 22.23 | 0.63 |
| OA | 28.06 | 0.53 | 28.70 | 0.56 |
| Memory Performance | ||||
| CH | 0.06 | 0.01 | 0.24 | 0.02 |
| YA | 0.29 | 0.15 | 0.41 | 0.03 |
| OA | 0.12 | 0.03 | 0.32 | 0.02 |
Notes: CH = children, YA = younger adults, OA = older adults. Scale of digit symbol ranged between 0 and 94, and the score refers to number of symbols correctly solved. Scale of vocabulary knowledge ranged between 0 and 35, and the score refers to number of items correctly solved. Memory performance refers to percentage correct. Excluded and final sample only differed significantly on memory performance, particularly in children, t(51) = −6.43, p < 0.001 and older adults, t(49) = −4.93, p < 0.001. Note that the number of younger adults in the excluded sample was very small (n = 2).
2.2. Materials and procedure
Stimuli were highly imaginable concrete German nouns paired together to form unrelated word pairs. Words were drawn from German norm databases and previous studies had established their comprehensibility for children of similar ages as those included here (see Brehmer et al., 2004, Brehmer et al., 2007, Hasselhorn et al., 1990, Shing et al., 2008). Screened by several raters, word pairs were checked against semantic or associative relatedness, having the same starting letter, or rhyming, as such pairings may be easier to remember. Length of study list was adjusted to render the task comparably difficult for each age group (Luna et al., 2010, Nagel et al., 2009, Poldrack, 2015) while facilitate having sufficient number of trials for fMRI analysis. As the present study was embedded in a training project (Brehmer et al., 2016), we aimed for a baseline performance level of between 25 and 45 percent to provide room for training-induced improvement. Based on extensive piloting with variations in list length, we settled on 72 pairs for children, 96 pairs for younger adults, and 28 pairs for older adults. In order to boost the number of trials for fMRI analysis, we repeated the encoding run twice for children and younger adults and four times for older adults (each run contained unique word pairs; i.e. a total of 144 pairs for children, and 192 pairs for younger adults, 112 pairs for older adults). Two filler word pairs were added at the beginning and end of each encoding run to minimize primacy and recency effects.
Before scanning, participants were trained on getting comfortable with the MRI environment and practiced on the encoding task inside a scanner simulator (NordicNeuroLab). Motion was detected with a camera and feedback was given when they moved too much. In the next session, participants performed the actual word-pair encoding task inside the scanner. Each word pair was presented for six seconds, with one word appearing at the top and the other at the bottom of the screen. This was followed by a question for two seconds (‘how sure are you that you will later remember the word pair?’) to ensure that participants remained attentive to the task. Participants responded by pressing ‘very sure’, ‘sure’, or ‘unsure’. An explicit baseline condition was included (data not used in the current analyses), in which participants saw pairs of letter strings (e.g., xxxxx–kkkkk). Six trials of such letter string pairs (lasting for one second each) were presented sequentially, forming one block. In 1/3 of these blocks, there were trials where the pairs of letter strings consisted of the same letters (e.g., xxxxx–xxxxx). Participants were asked to monitor the occurrence of these trials and to press a button when they saw one. In 2/3 of the blocks, all trials consisted of pairs of letter strings with different letters (e.g. xxxxx–kkkkk), hence no button presses were required and these blocks constituted the explicit baseline condition. Scanning time for the baseline task was roughly 1/3 of scanning time of the actual memory task. Trials were separated by a jittered fixation period, ranging from 500 to 1500 msec (in 500 ms steps).
After each encoding list, participants were tested in a cued-recall procedure, which differed slightly for children compared to younger and older adults. For younger and older adults, retrieval was done inside the scanner without scanning. For children, retrieval was done on a computer in a quiet room close to the scanner. Based on piloting, lying in the scanner for both the encoding and retrieval phases was too strenuous for children, lowering scanner quality due to movement artifacts. Therefore, children entered the scanner again after completing retrieval of the first list. Participants saw one word at the top of the screen and had to recall the other word. They indicated whether they could remember the word by pressing either ‘yes’ or ‘no’. When ‘yes’ was pressed, they were asked to verbally report the answer. Participants were also asked to indicate their confidence in getting the answer correct by pressing ‘very sure’, ‘sure’, or ‘unsure’. When ‘no’ was pressed, the next trial appeared. After finishing the memory task, participants were asked to describe the strategies they had used for encoding the word pairs in an open-ended format. These strategies were later on coded into three broad categories based on Dunlosky and Hertzog (1998), namely visual, semantic, and shallow strategies (such as rote repetition or focusing on graphemic aspects of the words). Participants could report using these strategies in any combination (e.g. using one of them exclusively, or combining some of them). Each participant received a score for each of the strategy category, denoting the proportion of using strategies in the respective category relative to all strategies reported by the individual.
2.3. fMRI data acquisition and preprocessing
Whole-brain MRI data were collected with a Siemens 3 T Trio Magnetom. Functional data were acquired using an echo-planar imaging sequence (TR = 2000 ms; TE = 30 ms; flip angle = 80°; FOV = 216 mm; matrix = 72 × 72; voxel size = 3 × 3 x 3 mm3; 36 slices). Before acquisition of each functional sequence, a new localizer was acquired to adjust the slice orientation (alignment based on genu-splenium of the corpus callosum). For registration of functional images, 2 structural sequences were collected; one T2-weighted turbo-spin echo sequence (TR = 8170 ms; TE = 93 ms; matrix = 256 × 256; in-plane resolution = 1 × 1 mm, slice thickness = 3 mm) in the same orientation as the functional sequences; and one high resolution T1-weighted MPRAGE sequence (TR = 1550 ms; TE = 2.34 ms; matrix = 256 × 256, in-plane resolution = 1 mm, slice thickness = 1 mm). Gradient echo images were also measured for correction of magnetic field inhomogeneities.
Quality of the functional data was first checked by inspecting spikes using dataQuality, a Matlab-based tool (http://cbi.nyu.edu/software/dataQuality.php). In short, each slice of each volume was divided into brain, ghosting, or background regions. A small region of interest in the background was defined to detect spikes, that is when the signal was larger than 10 standard deviations above the mean (across time). Detected spikes were corrected by substituting the value with an average of the intensity of the two time points before and after a problematic time point, but only if they themselves were free from spikes. Repaired data were then checked again to ensure that no more spikes were found. Within the runs detected with spikes, the number of spikes occurred between 1 and 3 times. Therefore, the occurrence of spikes was likely random.
2.4. fMRI data analysis
Data of each run from each participant were then preprocessed and analyzed using FEAT in FSL (Version 5.98, FMRIB’s Software Library, http://www.fmrib.ox.ac.uk/fsl, Smith et al., 2004). Preprocessing included nonbrain tissue removal, slice time and motion correction, correction of field inhomogeneity, and spatial smoothing using an 8-mm full-width half-maximum Gaussian filter. Runs with motion more than 3 mm (absolute displacement) in any direction were handled by either removing that particular run entirely if motion was detected throughout, or leaving out (without replacement) the volumes affected by motion (and the subsequent ones) if it had occurred in the second half of the measurement. This affected 2 children (1 had one run in which the last 47 vol of the 497 vols were removed, 1 had one run removed entirely and in another run the last 246 vol were removed) and 3 younger adult (1 had one run in which the last 244 vol of the 648 vol were removed, 1 had one run in which the last 224 vol were removed, 1 had one run removed entirely). Mean framewise displacement showed a significant age effect, F(2, 84) = 5.97, p = 0.004. Despite the age difference, all three age groups showed relatively low framewise displacement (children: mean = 0.07, range = 0.03–0.27; younger adults: mean = 0.05, range = 0.03–0.11; older adults: mean = 0.09, range = 0.04–0.24). Post-hoc comparison with Tukey HSD indicated that the age effect was driven by older adults showing higher motion than younger adults (p < 0.05). Children did not differ from younger or older adults (ps > 0.05). To control for differences due to motion, each individual’s framewise displacement was entered as a regressor of no interest in group-level analyses. A pre-whitening technique was used to account for the intrinsic temporal autocorrelation in functional imaging. Low-frequency artifacts were removed by applying a high-pass temporal filter (Gaussian-weighted straight-line fitting, sigma = 50 s). Age-specific brain templates were created from participants’ T1 images using the non-liner registration ANTS program (Avants et al., 2011) following the iterative procedures of Sanchez et al. (2012). Functional scans were registered to their own T1 scans, then to the age-specific brain templates, and finally to the MNI standard space.
First-level statistical analyses were performed on each individual’s data from each run using the general linear model. Time series were modeled with separate regressors for the encoding phase of each trial, separately for subsequently remembered, omissions, and wrongly answered trials (post-hoc sorting based on the response participants gave during cued-recall). Other regressors were included to model the question phase, the explicit baseline phase (with and without button press, separately, see Supplementary Material), and filler pairs at the beginning and end of the run. The regressors were generated by convolving the impulse function related to the onsets and lengths of events of interest with a Gamma hemodynamic response function. The critical contrast for analyzing SME was correctly remembered > omissions (R > O). Incorrectly answered trials were not included in the contrast due to ambiguity in the reasons for getting a trial wrong (e.g., intrusion vs. interference error; see behavioral error analysis). Additionally six motion parameters were included as regressors of no interest. Contrast images were computed for each run per subject, spatially normalized, transformed into MNI space (2 mm isotropic voxel) and submitted to a within-subject fixed-effects analysis (i.e. averaging) across runs. Higher-level analysis across subjects was carried out using a mixed-effect model in FSL (FLAME). First, to characterize overall SME, we computed a whole-brain contrast for R > O across all participants, regardless of age. Participants’ mean-centered exact age and memory performance (number of correctly remembered trials over total number of trials) were entered as additional regressors. Functional activation was determined by thresholding the Gaussianized T-statistic image of the group analysis, using clusters determined by Z > 2.3 (p < 0.01) and a corrected cluster significance threshold of p <0.05. All coordinates are reported in MNI space.
Next, region of interest (ROI) analyses were performed to examine age differences in SME within regions that were most relevant to our hypotheses. These regions included left lateral PFC (superior, middle, and inferior frontal gyri), bilateral parahippocampal gyrus (PHG), and bilateral hippocampi. First, we prepared anatomical ROIs using the Harvard-Oxford Cortical and Subcortical Structural Atlas (http://www.cma.mgh.harvard.edu/fsl_atlas.html), with voxels thresholded at a minimum 25% probability of being within a specific region. A conjunction was then made between the anatomical ROIs and the group-level SME functional map across all runs and participants (as described above). This ensured that percent signal change (PSC) for each ROI is extracted from active voxels within that region. PSC of each participant from each ROI was subjected to subsequent ANOVAs (with age group as a factor). Effects were considered significant at an alpha level of 0.01 (after adjusting from 0.05 for multiple comparisons for five PFC and MTL ROIs). Outliers were identified at p < 0.001 (i.e., 3 standard deviations beyond the mean, 2-tailed test). One child was identified as an outlier across all ROIs with unusually high values above the mean of whole sample, hence was excluded from the fMRI analysis. Next, to complement the ROI analysis, age effects in SME were explored in all task-positive regions, using the general subsequent-memory activation map from all participants (as described above) as a pre-threshold mask. An F-test with age group as a factor was conducted.
Finally, to explore possible age differences in functional connectivity of MTL as a function of memory formation, we conducted a generalized psycho-physiological interaction (PPI) analysis (McLaren et al., 2012). We identified left and right hippocampal seed ROIs for the PPI analyses based on a sphere with a radius of 4 mm around the peak hippocampal voxel (separately for left and right hemispheres) that were active at the group-level R > O map [coordinates of left hippocampal peak voxel: −26, −26, −12; coordinates of right hippocampal peak voxel: 32, −16, −18]. The generalized PPI analysis compared the temporal correlation between the hippocampal seeds and other brain regions for remembered versus omissions trials. At the first level, the PPI design matrix contained three types of regressors: (a) ‘psychological’ regressors that represent the full design including all event types (i.e. remembered, omission errors, wrongly answered, and question); (b) a time-series ‘physiological’ variable that represents the time course of the hippocampal seeds; and (c) interaction variables that represent the interaction of the psychological and physiological variables. A direct contrast to capture difference in connectivity was formed by contrasting remember trials from omissions. Additionally, six motion parameters were included as regressors of no interest. Individual beta images of the contrast term were then entered into a group-level whole-brain analysis, treating participants as random. Participants’ mean-centered percentage correct recall, sex, and framewise motion displacement were entered as additional regressors. To constrain the search space to task-positive regions, the PPI analyses were constrained using a binary mask from the group-level subsequent-memory network. Connectivity maps were thresholded at 2.3 (p < 0.01) at the voxel level and p < 0.05 at the cluster level.
3. Results
3.1. Behavioral results
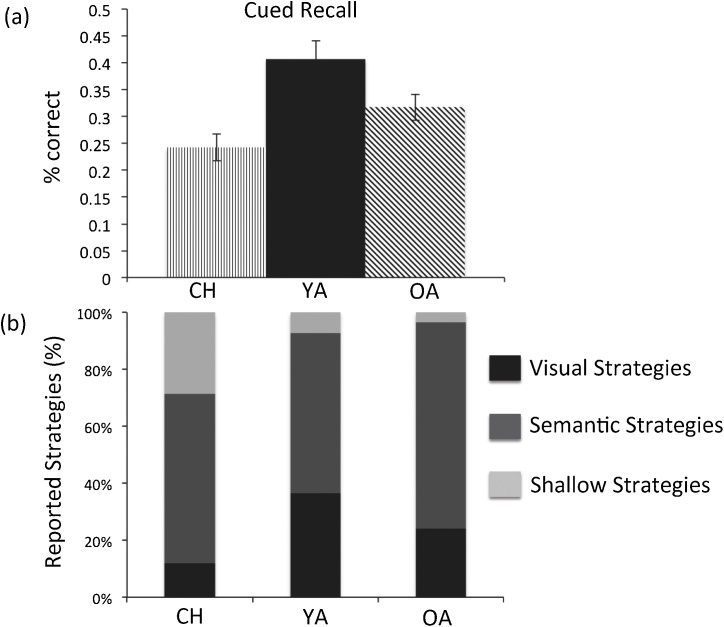
Percentage cued recall for each age group is presented in Fig. 1a. Note that performance comparisons across age groups should be taken cautiously, as we adjusted list length for each age group with the aim of obtaining accuracy levels between 25 and 45 percent. Nevertheless, an ANOVA revealed a significant effect of age, F(2, 84) = 7.93, p < 0.05. Post-hoc comparisons using Tukey HSD indicated that children (M = 0.25, SE = 0.03) showed lower performance than younger adults (M = 0.41, SE = 0.03), p < 0.05. Older adults (M = 0.32, SE = 0.03) showed a trend of lower performance than younger adults, p = 0.09, whereas children and older adults did not differ reliably, p > 0.05. For any incorrect trial, participants could either produce an incorrect answer (intrusions), an answer that was paired with another word from the current or preceding encoding list (interference), or no response (omission). A mixed ANOVA (within person: error type; between person: age group) revealed main effects of error type, F(2, 83) = 551.93, p < 0.05, and of age group, F(2, 84) = 7.93, p < 0.05. The most frequent error type was omissions (M = 0.60, SE = 0.02), followed by intrusions (M = 0.05, SE = 0.01), and interference errors (M = 0.03, SE = 0.003). Children (M = 0.25, SE = 0.01) and older adults (M = 0.23, SE = 0.01) produced more errors than younger adults (M = 0.20, SE = 0.01). We also found a significant interaction between age group and error type, F(4, 166) = 2.88, p < 0.05. To follow up on this interaction, we examined the effect of age group in each error type separately. Specifically, the three age groups differed significantly in omission [F(2, 84) = 4.69, p < 0.05], but not in intrusion [F(2, 84) = 1.02, p > .05] or interference error [F(2, 84) = 1.08, p < 0.05]. Post-hoc comparisons on omission error using Tukey HSD indicated that children (M = 0.67, SE = 0.03) had more omissions than younger adults (M = 0.53, SE = 0.03; p < 0.05), whereas younger and older adults (M = 0.61, SE = 0.03) did not differ reliably (p < 0.05).
Fig. 1.
(a) Percentage correct cued recall across age (error bars represent standard errors); (b) Percentage reported strategies (over all reported strategies) across age. CH = Children, YA = Younger Adults, OA = Older Adults.
Next, we examined strategy use to characterize how the three age groups might differ in how they encoded the pairs (see Fig. 1b). In a mixed ANOVA (within person: strategy type; between person: age group), a significant main effect of strategy type [F(2, 81) = 44.9, p < 0.05] and an interaction between strategy type and age group [F(4, 162) = 4.83, p < 0.01] were found. The most commonly reported strategy was semantic (M = 0.67, SE = 0.04), followed by visual (M = 0.22, SE = 0.04), and shallow strategies (M = 0.11, SE = 0.03). To trace the source of the interaction, we examined age differences for each strategy type separately. Significant age effects were found for visual [F(2, 82) = 4.27, p < 0.05] and shallow strategies [F(2, 82) = 6.87, p < 0.05]. For visual strategies, post-hoc comparisons indicated that younger adults (M = 0.36, SE = 0.08) reported significantly more use of this strategy than children (M = 0.09, SE = 0.05; p < 0.05); older adults (M = 0.21, SE = 0.07) did not differ from either of the other age groups (p > 0.10). For shallow strategies, post-hoc comparisons indicated that children (M = 0.25, SE = 0.06) reported use of shallow strategies more often than both younger (M = 0.06, SE = 0.04) and older adults (M = 0.02, SE = 0.02), ps < 0.05, with the two adult groups not differing from each other.
Next, we examined whether participants reporting using more or less effective strategies indeed differed in their performance. Each participant was classified as either reporting using only shallow strategies (N = 4), using both effective and shallow strategies (N = 12), or using only effective strategies (visual and semantic strategies combined; N = 70). There was a significant performance difference among the three groups of participants, F (2, 82) = 3.42, p < 0.05, after controlling for chronological age. Post-hoc comparisons indicated that participants reporting using only effective strategies (Madjusted = 0.35, SE = 0.02) showed better performance than those reporting using only shallow strategies (Madjusted = 0.16, SE = 0.09), p < 0.05. Those using both effective and shallow strategies (Madjusted = 0.26, SE = 0.05) did not differ from the other two groups (p > 0.05).
Finally, we also examined JOL during encoding according to whether the trial was subsequently remembered or forgotten. In a mixed ANOVA (within person: trial type; between person: age group), significant main effects of trial type [F(1, 84) = 129.04, p < 0.01] and age group [F(2, 84) = 16.91, p < 0.01] were found. Subsequently remembered trials (M = 1.66, SE = 0.04) were rated higher in JOL than subsequently omitted trials (M = 1.35, SE = 0.03). Children (M = 1.74, SE = 0.05) showed higher average JOL than younger (M = 1.5, SE = 0.05) and older adults (M = 1.28, SE = 0.06), p < 0.05, with the two adult groups also being significantly different from each other, p < 0.05. The lack of interaction between trial type and age group [F(2, 84) = 0.29, p = 0.75] suggests that relative JOL is age invariant from middle childhood to old age.
3.2. Subsequent memory effects (R > O) across all participants: whole-brain contrasts
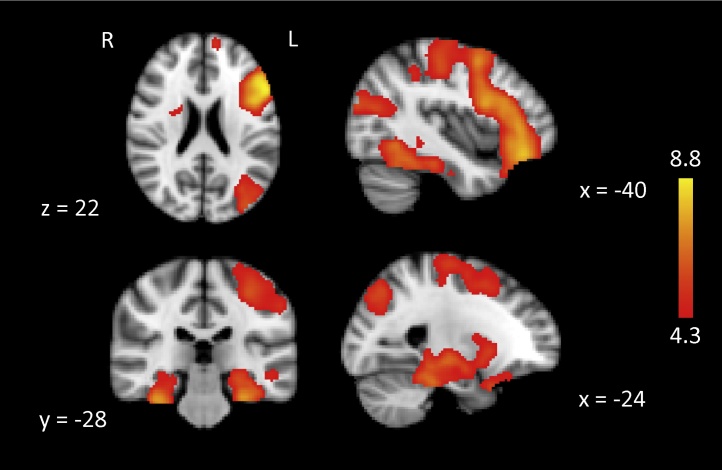
First, to characterize overall activation differences between remembered items and omission errors, we computed whole-brain fMRI contrasts for SME (R > O) across all participants, including mean-centered exact age and performance (percentage correct) as covariates. In line with the literature (Spaniol et al., 2009), SME were associated with significant activation in left lateral PFC (spanning across superior, middle, and inferior frontal gyri), left lateral parietal cortex (postcentral and supramarginal gyri), and bilateral temporal regions (both lateral and medial walls), as well as in subcortical regions, including both hippocampi (Fig. 2, see coordinates of clusters in Table 2). To ensure that the overall SME map encompasses the SME of each age group, we calculated the spatial overlap between the overall activation map (A) and each age group’s activation map (B), which is A∩B. We then calculated, for each group, the proportion of activation overlapping with the overall activation map relative to their own entire activation (i.e. A∩B/B). This ratio measure was 0.86, 0.93, and 0.86 for children, younger adults, and older adults, respectively. Therefore, most of the activation of each age group was spatially represented within the overall activation map. In particular, all three age groups showed robust SME in left lateral PFC, the hippocampi, and PHG, regions that we examined next as ROIs.
Fig. 2.
Subsequent-memory activation (whole brain contrast of R > O) across all participants, after controlling for exact age and percentage correct. Activation maps are rendered on MNI standard brain, with coordinates presented at the bottom of each section. Sagittal views are shown from the left side of brain. A z-value scale is presented on the right. Activations were thresholded at a voxel-level threshold of Z > 2.3, corrected at the cluster-level at p < 0.05. To visualize clusters, we depict activations that survived a voxel-level threshold of Z > 4.3, corrected at the cluster-level p < 0.05.
Table 2.
Peak activations for subsequent-memory effects (correctly remembered > omissions, R > O) across all participants, with exact age and memory performance as covariates. Activation maps were threshold at a voxel-level threshold of z > 2.3, corrected at the cluster-level with FWE, p < 0.05. To better characterize the peak clusters, we list clusters that survived a voxel-level threshold of z > 4.3, corrected at the cluster-level with FWE, p < 0.05.
| Cluster | Region | BA | Z max | MNI coordinates (mm) |
Number of voxels |
||
|---|---|---|---|---|---|---|---|
| X | Y | Z | |||||
| 1 | Left inferior frontal gyrus | 44/45/46 | 8.7 | −54 | 20 | 20 | 12981 |
| Left middle frontal gyrus | 6/9 | 7 | −42 | 4 | 52 | ||
| Left postcentral gyrus | 40 | 6.27 | −32 | −26 | 52 | ||
| 2 | Left inferior temporal gyrus | 20/37 | 8.09 | −48 | −54 | −18 | 7067 |
| Left temporal fusiform cortex | 35/36 | 7.84 | −30 | −34 | −24 | ||
| Bilateral putamen | n.a. | 7.39 | −16 | 10 | −2 | ||
| Left parahippocampal gyrus/hippocampus | n.a. | 6.17 | −26 | −32 | −14 | ||
| 3 | Right parahippocampal gyrus/hippocampus | n.a. | 5.46 | 36 | −18 | −22 | 2392 |
| Right temporal fusiform cortex | 35/36 | 7.40 | 36 | −32 | −26 | ||
| 4 | Left lateral superior occipital cortex | 19 | 6.11 | −28 | −70 | 44 | 1843 |
| 5 | Right lingual gyrus | 18 | 5.15 | 14 | −84 | 0 | 290 |
| 6 | Right frontal orbital cortex | 47 | 5.51 | 28 | 32 | −14 | 145 |
| 7 | Bilateral caudate | n.a. | 4.86 | 20 | 2 | 16 | 72 |
| 8 | Left frontal medial cortex | 11 | 4.93 | −4 | 42 | −24 | 61 |
3.3. Age comparisons of lateral PFC and MTL: ROI analyses
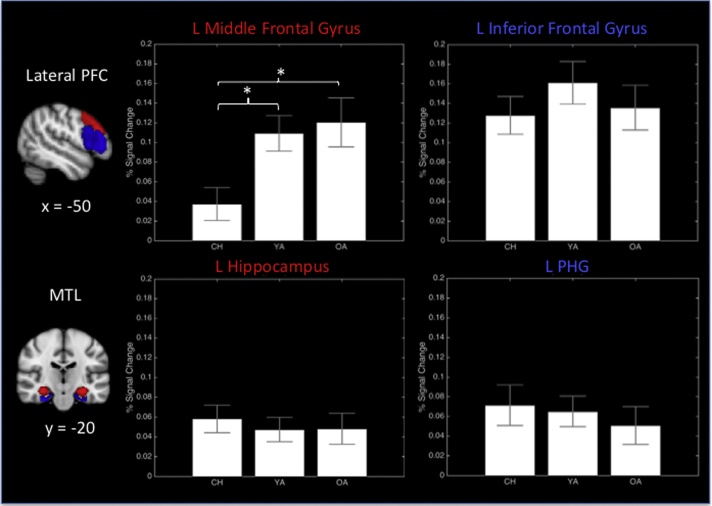
Based on the SME map defined across all participants, we conducted age comparisons in the ROIs, namely left lateral PFC (superior, middle, and inferior frontal gyri) and bilateral MTL (HC and PHG), in which PSC was extracted for each participant. We examined age differences in PSC in these regions using one-way ANOVAs (with age group as a factor). Across all ROIs, the only region that showed a significant age effect was left middle frontal gyrus, F (2, 84) = 4.96, p < 0.01 (Fig. 3). Particularly, post-hoc comparisons indicated that children showed lower SME in this region (M = 0.04, SE = 0.02) than both younger (M = 0.11, SE = 0.02, p < 0.01) and older (M = 0.12, SE = 0.02, p < 0.01) adults, with no difference between the two adult groups (p > 0.10). The age difference remained after controlling for mean centered memory performance (percent correct), gender, framewise motion, and relative JOL (i.e., the difference in JOL for remembered versus omitted trials) [F(2, 80) = 5.72, p < 0.01]. The age difference also remained including absolute (i.e., mean level across remembered and omitted trials) instead of relative JOL as a covariate [F(2, 80) = 6.26, p < 0.01]. For superior and inferior frontal gyrus as well as MTL, there were no age differences in SME (ps > 0.20). To further examine the MTL, we also tested for age differences using an anatomical MTL mask for small-volume correction, which did not yield significant age difference in SME within the MTL.
Fig. 3.
Extracted percent signal change for subsequent-memory effects (R > O) for regions in the ROI analysis. Significant age differences were found in left middle frontal gyrus, with children showing weaker SME than younger and older adults. CH = Children, YA = Young Adults, OA = Older Adults.
We also explored age differences with an F-test in all task-positive (SME) regions. There was no cluster that survived the threshold (Z > 2.3 and a p < 0.05 cluster threshold). Finally, we had examined the relationship between memory performance and subsequent memory effect within each group as well as across groups controlling for age. There was no consistent pattern of relationship that emerged.
3.4. Hippocampal connectivity as a function of subsequent memory
To characterize overall connectivity, a PPI analysis was run across all participants (with left and right HC as seed regions), entering participants’ sex, recall accuracy, and framewise motion (mean centered as previous models) as additional regressors. At a voxel threshold of 2.3 (p < 0.01) and a cluster threshold of p < 0.05, no reliable memory-dependent (i.e. R > O) variation in functional connectivity was observed. This was also the case when the model was run for each age group separately. There were also no reliable group differences in connectivity, with or without recall accuracy as covariate.
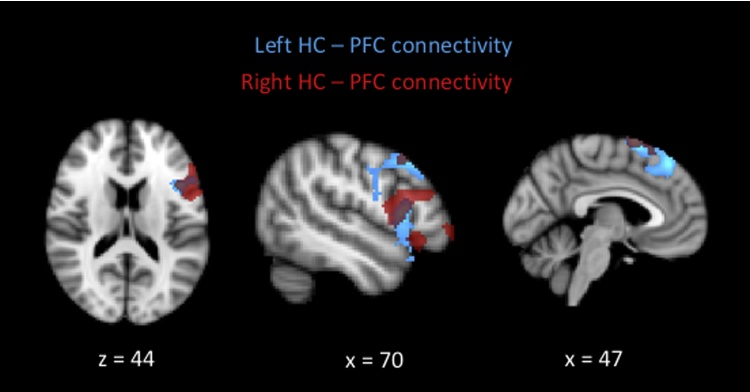
The difficulty in identifying consistent connectivity pattern may be related to individual differences in the encoding processes that participants engaged in (both within and across age groups), leading to differential patterns of connectivity across participants (Schott et al., 2013). As differential task processing is related to memory performance, we then explored the extent to which functional connectivity was related to performance across participants, controlling for gender and motion (mean-centered). As shown in Fig. 4, for left HC, we found a performance-related increase in connectivity to left lateral PFC (middle and inferior frontal gyri, BA 44) and left medial PFC (superior frontal gyrus, BA 6). Similarly, for right HC, we found a performance-related increase in connectivity to left lateral PFC (middle and inferior frontal gyri, BA 9/46) and left medial PFC (superior frontal gyrus, BA 6). These regions overlapped with those found for left HC. Therefore, individuals with better performance showed stronger connectivity between HC and lateral as well as medial PFC for remembered compared to omitted trials.
Fig. 4.
Performance-related increase in connectivity between bilateral hippocampi and left lateral PFC (middle and inferior frontal gyrus) as well as left medial PFC (superior frontal gyrus). Blue color denotes clusters found with left hippocampus as seed, red color denotes clusters found with right hippocampus as seed (overlaid on top at 60% transparency). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
This study investigated age-related differences in activation patterns during encoding that predict successful memory formation for word pairs. We directly compared the extreme ends of lifespan and probed memory with cued recall, a less commonly used procedure in SME studies. This is an important extension to past research, because cued recall requires stronger and more accessible traces than recognition. In line with our predictions, activation patterns related to SME differed between 10 and 12 year-old children and adults, but not between younger and older adults. In children, PFC activations during study were less predictive of successful recall than in adults. Specifically, children, compared to the adult groups, showed smaller SME in left middle frontal gyrus. In contrast, MTL activations were equally predictive of successful recall in all three age groups.
The finding that children showed weaker SME in middle frontal gyrus is consistent with the two-component framework of EM (Shing and Lindenberger, 2011, Shing et al., 2010), which states that memory functioning in children entails less strategic elaboration than in adults. This reflects the maturational lag between frontal and MTL regions in neural development. In this line, Ofen et al. (2007) showed that activation associated with successful memory formation increased with age in PFC, but not MTL (Chiu et al., 2006, Guler and Thomas, 2013). Supporting the notion that increase in strategic elaboration parallels PFC involvement across development, we observed that children who reported using only effective strategies (n = 18), relative to their age peers who reported using shallow strategies (exclusively or in combination with effective strategies, n = 12), showed stronger SME in a cluster within the middle frontal gyrus (37 voxels, z = 2.3, uncorrected), the region in which age differences in SME were found. This effect was not found in any other ROIs. Thus, children who showed some extent of SME in PFC appeared to have started earlier than their age peers in using effective strategies. It is important to treat this effect as preliminary due to the small effect and limited sample size. However, it is conceivable that children are less capable of forming mediators for encoding the word pairs because they possess fewer mental schemata to support semantic elaboration, while at the same time there is large individual difference in this aspect among children even of the same age (Brod et al., 2013, Craik and Bialystok, 2006). We assume that, based on the extensive memory development literature, children undergo progression in strategy use across time, starting from using shallow, rote repetition to deep, elaborative strategies. Age-related difference in strategy use has been shown to be an important factor that drives memory development (e.g., Schneider and Pressley, 1997). Such progress is likely supported by development in the structural and functional integrities of the PFC, explaining our observation of children showed less SME in the middle frontal gyrus. To strengthen this observation, future studies with a longitudinal design and larger sample sizes are needed to address individual differences in the progression of children’s strategy use and its relations to memory performance at the neural level.
Findings on the involvement of MTL in memory development in childhood are not unequivocal. Evidence suggests that there may be protracted maturation for some, but not all, MTL functions (Ghetti et al., 2010, Maril et al., 2010). For example, memory processes involving detailed memories with contextual information are suggested to be associated with age differences in MTL activation (Ghetti and Bunge, 2012). We acknowledge that our paradigm differs in important ways from the one of Ghetti et al. (2010), which included pictorial stimuli presented with perceptual details. Nevertheless, as cued recall relies upon memories that likely contain contextual details, our data seems to be at odds with this view. Therefore, the organizing principles of when MTL activations do or do not increase during development remain to be clarified. Future studies should manipulate strength and details in memory representations experimentally to dissociate the effects of these two factors and the age differences therein.
On the aging side, we found little differences between older and younger adults’ SME, suggesting that high-functioning older adults, such as those in our study, engage much of the same neural circuitry as younger adults when forming representations that can be subsequently recalled. Such finding may seem at odd with the two-component framework that posits a dual deficit in associative and strategic components in aging (following evidence of structural decline in the PFC and MTL). However, while there may be deterioration in the two components, the framework does not preclude a within-person maintenance pattern of successful encoding in aging, such that the neural substrate that contributes to memory formation, when successful, remains largely similar. Such age-invariant SME in left frontal and MTL regions was previously reported (de Chastelaine et al., 2011, Duverne et al., 2009, Morcom et al., 2003). It would be interesting to examine the potential dissociation between age-related differences in the two memory components in general (such as attempting to remember when one is in encoding mode) versus age differences in the two components in relation to encoding success (when memory formation is successful). However, our data is not optimally suitable for this purpose, as the encoding success trials are a large subset of the encoding mode trials. In future studies, one would ideally include an independent memory task that taps into encoding mode with a block design, or a mixed block and event-related design to tease apart sustained (i.e. mode) versus transient (i.e. success) processes within the same task (Petersen and Dubis, 2012).
Our results also stand in contrast to findings of increased or more bilateral PFC SME in older adults, starting from middle age (Park et al., 2013). These divergences may reflect differences in the nature of the tasks. Successful memory formation, assessed with the present cued-recall procedure, may impose higher demands on the quality of memory representations needed to reach the threshold for producing a correct response. Our results suggest that, in both younger and older adults, such high-quality engrams are formed by involving both PFC and MTL during encoding. A recognition procedure, on the other hand, may more readily probe partially accessible memory representations. Interestingly, a meta-analysis by Maillet and Rajah (2014) showed that regions consistently over-recruited by older adults are either overlapping or close to regions involved in unsuccessful encoding in younger adults (e.g. medial frontal gyrus, precuneus). This may reflect an age-related shift away from perceptual details towards evaluative and personal thoughts and feelings during encoding. However, these operations are less efficient means of encoding information (e.g., Hashtroudi et al., 1995, Kensinger, 2009). Memory representations bound in such ways may be sufficiently accessible in a recognition but not in a cued-recall procedure, explaining why older adults in our sample did not show engagement of additional regions reported in the aging literature on SME (Maillet and Rajah, 2014).
Our procedure of adapting list length minimized task performance difference across groups, which carries important advantages for interpreting group differences in activation (see Nagel et al., 2009, Luna et al., 2010, Poldrack, 2015). However, there are limitations to consider. First, we could not fully equalize the total number of to-be-remembered word pairs across age groups, as additional runs would have been too strenuous for the children and the older adults. This might have lowered the power of detecting SME in older adults due to having the least number of trials. However, given that older adults showed as robust an SME as younger adults, we think that the validity of the present results is not compromised. We also ran validation analyses by leaving out trials in children and younger adults so that all age groups were represented by the same number of trials for the first level R > O contrast (see Supplementary Material). Results remained largely unchanged, with similar SME regions found as well as same patterns of age differences in ROI analysis, supporting the validity of the analyses. Nevertheless, future lifespan comparisons will benefit from other routes to adapt task difficulty without confounding number of trials.
The second limitation refers to the fact that our samples, particularly the older adults, were highly selective. In total, 97 out of 165 older adults were not invited to participate in the fMRI study due to failure in meeting the screening criteria. Similarly, children retained were possibly less representative of their cohort than the younger adults (although the latter were university students and hence also positively selected). It is likely that the selection procedures used in this study, while contributing to its internal validity, reduced the generalizability of the present findings to the general population. For older adults, Salami et al., (2012) showed that more activity in cognitive-control networks (including frontoparietal cortices) correlated negatively with memory performance. That is, among older adults, better performing persons showed less additional engagement of the control network relative to younger adults. In light of this finding, it is conceivable that the high-functioning nature of the older adults has contributed to the similarity of their SME patterns to the patterns observed in younger adults. This is in line with the between-person aspect of brain maintenance proposed by Nyberg et al. (2012). Given that maintenance likely does not exist in absolute terms, future studies are needed to delineate the boundary conditions within which healthy older adults may exhibit youth-like neural patterns. Likewise, the observed fMRI differences between children and younger adults may not be restricted to differences in successful memory formation. In particular, the high-performing children participating in the present study may not have under-recruited the middle frontal gyrus during SME relative to younger adults, but rather may have been quicker to disengage from encoding after the memory was formed.
Finally, to reduce movement artifacts that are particularly prominent in children, their retrieval was conducted outside the scanner, leading to a change in retrieval context and slightly longer delay between encoding and retrieval that might have lowered children’s performance. Although we think that this cannot fully account the age difference found uniquely in middle frontal gyrus, but not other frontal or MTL regions, such difference in procedure is best avoided in future studies. Taken together, it is a challenging task to investigate age-related differences in memory mechanisms across the lifespan. Participants of different ages differ in a wealth of factors in addition to the phenomenon of interest (see Shing and Lindenberger, 2011). Our study is the first attempt at creating experimental setups that allow meaningful cross-sectional age comparisons in successful memory formation across the human lifespan. We believe that the utility and feasibility of such an approach are demonstrated, while the challenges and limitations that we encountered, particularly the selection bias across age groups and procedural differences as confounds, serve as important lessons for future studies, and indicate that there is room for further improvements in research design.
Despite finding robust SME in all groups, we did not detect a consistent pattern of functional connectivity at the group level, both for the entire sample or within each age group. The difficulty in detecting functional connectivity likely reflects large individual differences in how participants processed the task. Such differences also posit a challenge for mean-activation detection, but are magnified for connectivity analysis due to the need for more precise temporal covariation. Varying level of processing, Schott et al. (2013) showed that successful encoding during deep versus shallow processing was accompanied by greater connectivity of left HC to ventrolateral PFC and temporoparietal cortex. Therefore, specific processes that one engages in during encoding are associated with differential patterns of connectivity, supporting our reasoning for not finding a consistent connectivity patterns at the group level. Interestingly, better performance was related to greater connectivity between HC and medial and lateral frontal gyrus (in particular, BA9/44/46). Based on previous literature, we assume that this has to do with these regions’ roles in the organization of multiple pieces of information in working memory such as building semantic relations between pairs of words, which in turn enhances memory for associations among items in the HC. Such deep processing is shown to benefit memory performance, potentially accounting for our observation that individual difference in performance is associated with the strength of connectivity between HC and PFC regions.
In conclusion, our findings demonstrated that, in high-functioning children, the neural circuitry contributing to successful episodic encoding is reorganized during the transition from middle childhood to adulthood, progressing from reliance on the MTL to increasing engagement of the PFC in forming durable memory representations. On the other hand, older adults with high memory functioning show preserved ability to engage the PFC and MTL when memory formation is successful, supporting the proposition that maintenance of youth-like activation patterns contributes positively to EM functioning in old age (Fandakova et al., 2015, Lindenberger, 2014, Nyberg et al., 2012).
Conflict of interest statement
We declare no potential financial and personal conflict of interest.
Acknowledgements
This work was supported by the German Research Foundation (grant number DFG SH550/2-1) to YLS and YB, as well as by a grant from the Swedish Research, an Alexander von Humboldt Research Award, and a donation from the af Jochnick Foundation to LB, and by grants from the Max Planck Society, the Federal Ministry of Education and Research, and a Gottfried Wilhelm Leibniz Award 2010 of the German Research Foundation (DFG) to UL. YB also received postdoctoral funding from ERA-AGE, Future Leaders of Ageing Research in Europe (FLARE)/Swedish Council of Working Life and Social Research. The study was conducted within the “Cognitive and Neuronal Dynamics of Memory across the Lifespan” (CONMEM) project at the Center for Lifespan Psychology, Max Planck Institute for Human Development. We thank Annette Rentz and Lene-Marie Gassner for their invaluable help for the study.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.dcn.2016.06.003.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Avants B.B., Tustison N.J., Song G., Cook P.A., Klein A., Gee J.C. A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage. 2011;54(3):2033–2044. doi: 10.1016/j.neuroimage.2010.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenfeld R.S., Ranganath C. Prefrontal cortex and long-term memory encoding: an integrative review of findings from neuropsychology and neuroimaging. Neuroscientist. 2007;13(3):280–291. doi: 10.1177/1073858407299290. [DOI] [PubMed] [Google Scholar]
- Brehmer Y., Stoll G., Bergner S., Benoit R., von Oertzen T., Lindenberger U. 2004. Selection of Unambiguous Visual Words Appropriate for Children in Age-comparable Memory Experiments: Results of a Pilot Study.http://psydok.sulb.uni-saarland.de/volltexte/2004/189/ [Google Scholar]
- Brehmer Y., Li S.-C., Müller V., von Oertzen T., Lindenberger U. Memory plasticity across the lifespan: uncovering children’s latent potential. Dev. Psychol. 2007;43(2):465–478. doi: 10.1037/0012-1649.43.2.465. [DOI] [PubMed] [Google Scholar]
- Brehmer Y., Shing Y.L., Heekeren H.R., Lindenberger U., Bäckman L. Training-induced changes in subsequent memory effects: No major differences among children, younger adults, and older adults. Neuroimage. 2016;131:214–225. doi: 10.1016/j.neuroimage.2015.11.074. [DOI] [PubMed] [Google Scholar]
- Brod G., Werkle-Bergner M., Shing Y.L. The influence of prior knowledge on memory: a developmental cognitive neuroscience perspective. Front. Behav. Neurosci. 2013;7:1–13. doi: 10.3389/fnbeh.2013.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner R.L. Memory and executive function in aging and AD: Multiple factors that cause decline and reserve factors that compensate. Neuron. 2004;44(1):195–208. doi: 10.1016/j.neuron.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Cabeza R., Nyberg L., Park D.C. University Press; New York: Oxford: 2004. Cognitive Neuroscience of Aging: Linking Cognitive and Cerebral Aging. [Google Scholar]
- Chiu C.Y., Schmithorst V.J., Brown R.D., Holland S.K., Dunn S. Making memories: a cross-sectional investigation of episodic memory encoding in childhood using FMRI. Dev. Neuropsychol. 2006;29(2):321–340. doi: 10.1207/s15326942dn2902_3. [DOI] [PubMed] [Google Scholar]
- Craik F.I.M., Bialystok E. Cognition through the lifespan: mechanisms of change. Trends Cognit. Sci. 2006;10(3):131–139. doi: 10.1016/j.tics.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Davachi L. Item: context and relational episodic encoding in humans. Curr. Opin. Neurobiol. 2006;16:693–700. doi: 10.1016/j.conb.2006.10.012. [DOI] [PubMed] [Google Scholar]
- de Chastelaine M., Wang T.H., Minton B., Muftuler L.T., Rugg M.D. The effects of age, memory performance: and callosal integrity on the neural correlates of successful associative encoding. Cereb. Cortex. 2011;21:2166–2176. doi: 10.1093/cercor/bhq294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis N.A., Hayes S.M., Prince S.E., Madden D.J., Huettel S.A., Cabeza R. Effects of aging on the neural correlates of successful item and source memory encoding. J. Exp. Psychol. 2008;34(4):791–808. doi: 10.1037/0278-7393.34.4.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlosky J., Hertzog C. Aging and deficits in associative memory: what is the role of strategy production? Psychol. Aging. 1998;13(4):597–607. doi: 10.1037//0882-7974.13.4.597. [DOI] [PubMed] [Google Scholar]
- Duverne S., Motamedinia S., Rugg M.D. The relationship between aging, performance, and the neural correlates of successful memory encoding. Cereb. Cortex. 2009;19:733–744. doi: 10.1093/cercor/bhn122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H. University Press; New York: Oxford: 2002. The Cognitive Neuroscience of Memory: An Introduction. [Google Scholar]
- Fandakova Y., Shing Y.L., Lindenberger U. Differences in binding and monitoring mechanisms contribute to lifespan age differences in false memory. Dev. Psychol. 2013;49:1822–1832. doi: 10.1037/a0031361. [DOI] [PubMed] [Google Scholar]
- Fandakova Y., Lindenberger U., Shing Y.L. Maintenance of youth-like processing protects against false memory in later adulthood. Neurobiol. Aging. 2015;36:933–941. doi: 10.1016/j.neurobiolaging.2014.10.022. [DOI] [PubMed] [Google Scholar]
- Fjell A.M., Westlye L.T., Amlien I., Espeseth T., Reinvang I., Raz N., Walhovd K.B. High consistency of regional cortical thinning in aging across multiple samples. Cereb. Cortex. 2009;19:2001–2012. doi: 10.1093/cercor/bhn232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher P.C., Henson R.N.A. Frontal lobes and human memory: insights from functional neuroimaging. Brain. 2001;124:849–881. doi: 10.1093/brain/124.5.849. [DOI] [PubMed] [Google Scholar]
- Folstein M.F., Folstein S.E., McHugh P.R. Mini-mental state: a practical method for grading the cognitive state of patients for the clinician. J. Psychiatry Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Ghetti S., Bunge S.A. Neural changes underlying the development of episodic memory during middle childhood. Dev. Cognit. Neurosci. 2012;2(4):381–395. doi: 10.1016/j.dcn.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghetti S., DeMaster D.M., Yonelinas A.P., Bunge S.A. Developmental differences in medial temporal lobe function during memory encoding. J. Neurosci. 2010;30(28):9548–9556. doi: 10.1523/JNEUROSCI.3500-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N., Nugent T.F.I., Herman D.H., Ordonez A., Greenstein D., Hayashi K.M., Thompson P.M. Dynamic mapping of normal human hippocampal development. Hippocampus. 2006;16:664–672. doi: 10.1002/hipo.20193. [DOI] [PubMed] [Google Scholar]
- Guler O.E., Thomas K.M. Developmental differences in the neural correlates of relational encoding and recall in children: an event-related fMRI study. Dev. Cognit. Neurosci. 2013;3:106–116. doi: 10.1016/j.dcn.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habib R., Nyberg L. Neural correlates of availability and accessibility in memory. Cereb. Cortex. 2007;18:1720–1726. doi: 10.1093/cercor/bhm201. [DOI] [PubMed] [Google Scholar]
- Hashtroudi S., Johnson M.K., Vnek N., Ferguson S.A. Aging and the effects of affective and factual focus on source monitoring and recall. Psychol. Aging. 1995;9:160–170. doi: 10.1037//0882-7974.9.1.160. [DOI] [PubMed] [Google Scholar]
- Hasselhorn M., Jaspers A., Hernando M.-D. Typizitätsnormen zu zehn Kategorien für Kinder von der Vorschule bis zur vierten Grundschulklasse [Typicality norms concerning 10 semantic categories provided for 6- to 10-year-old children] Sprache Kognition. 1990;9:92–108. [Google Scholar]
- Johnson M.H. Functional brain development in humans. Nat. Rev. Neurosci. 2001;2:475–483. doi: 10.1038/35081509. [DOI] [PubMed] [Google Scholar]
- Kensinger E.A. How emotion affects older adults’ memories for event details. Memory. 2009;17:208–219. doi: 10.1080/09658210802221425. [DOI] [PubMed] [Google Scholar]
- Kim H. Neural activity that predicts subsequent memory and forgetting: a meta-analysis of 74 fMRI studies. Neuroimage. 2011;54:2446–2461. doi: 10.1016/j.neuroimage.2010.09.045. [DOI] [PubMed] [Google Scholar]
- Lehrl S. Straube; Erlangen, Germany: 1977. Mehrfachwahl-Wortschat-Test b (MTY-b) [Multiple-choice vocabulary test] [Google Scholar]
- Li S.-C., Lindenberger U., Hommel B., Aschersleben G., Prinz W., Baltes P.B. Transformations in the couplings among intellectual abilities and constituent cognitive processes across the life span. Psychol. Sci. 2004;15:155–163. doi: 10.1111/j.0956-7976.2004.01503003.x. [DOI] [PubMed] [Google Scholar]
- Lindenberger U. Human cognitive aging: corriger la fortune? Science. 2014;346:572–578. doi: 10.1126/science.1254403. [DOI] [PubMed] [Google Scholar]
- Luna B., Velanova K., Geier C.F. Methodological approaches in developmental neuroimaging studies. Hum. Brain Mapp. 2010;31:863–871. doi: 10.1002/hbm.21073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maillet D., Rajah M.N. Age-related differences in brain activity in the subsequent memory paradigm: a meta-analysis. Neurosci. Biobehav. Rev. 2014;45:246–257. doi: 10.1016/j.neubiorev.2014.06.006. [DOI] [PubMed] [Google Scholar]
- Maril A., Davis P.E., Koo J.J., Reggev N., Zuckerman M., Ehrenfeld L., Rivkin M.J. Developmental fMRI study of episodic verbal memory encoding in children. Neurology. 2010;75:2110–2116. doi: 10.1212/WNL.0b013e318201526e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaren D.G., Ries M.L., Xu G., Johnson S.C. A generalized form of context-dependent psychophysiological interactions (gPPI): a comparison to standard approaches. Neuroimage. 2012;61(4):1277–1286. doi: 10.1016/j.neuroimage.2012.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morcom A.M., Good C.D., Frackowiak R.S.J., Rugg M.D. Age effects on the neural correlates of successful memory encoding. Brain. 2003;126:213–229. doi: 10.1093/brain/awg020. [DOI] [PubMed] [Google Scholar]
- Moscovitch M. Memory and working-with-memory: a component process model based on modules and central systems. J. Cogn. Neurosci. 1992;4:257–267. doi: 10.1162/jocn.1992.4.3.257. [DOI] [PubMed] [Google Scholar]
- Murphy K., Garavan H. Deriving the optimal number of events for an event-related fMRI study based on the spatial extent of activation. Neuroimage. 2005;27:771–777. doi: 10.1016/j.neuroimage.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Nagel I.E., Preuschhof C., Li S.-C., Nyberg L., Bäckman L., Lindenberger U., Heekeren H.R. Performance level modulates adult age differences in brain activation during spatial working memory. Proc. Natl. Acad. Sci. U. S. A. 2009;106:22552–22557. doi: 10.1073/pnas.0908238106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson C.A. The ontogeny of human memory: a cognitive neuroscience perspective. In: Johnson M.H., Munakata Y., Gilmore R.O., editors. Brain Development and Cognition: A Reader. Blackwell Publishing; Oxford, UK: 2001. pp. 151–178. [Google Scholar]
- Nyberg L., Lovden M., Riklund K., Lindenberger U., Backman L. Memory aging and brain maintenance. Trends Cogn. Sci. 2012;16:292–305. doi: 10.1016/j.tics.2012.04.005. [DOI] [PubMed] [Google Scholar]
- Ofen N., Kao Y.-C., Sokol-Hessner P., Kim H., Whitfield-Gabrieli S., Gabrieli J.D.E. Development of the declarative memory system in the human brain. Nat. Neurosci. 2007;10:1198–1205. doi: 10.1038/nn1950. [DOI] [PubMed] [Google Scholar]
- Ofen N. The development of neural correlates for memory formation. Neurosci. Biobehav. Rev. 2012;36:1708–1717. doi: 10.1016/j.neubiorev.2012.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh H., Jagust W.J. Frontotemporal network connectivity during memory encoding is increased with aging and disrupted by beta-amyloid. J. Neurosci. 2013;33:18425–18437. doi: 10.1523/JNEUROSCI.2775-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H., Kennedy K.M., Rodrigue K.M., Hebrank A., Park D.C. An fMRI study of episodic encoding across the lifespan: changes in subsequent memory effects are evident by middle-age. Neuropsychologia. 2013;51:448–456. doi: 10.1016/j.neuropsychologia.2012.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson J., Pudas S., Lind J., Kauppi K., Nilsson L.-G., Nyberg L. Longitudinal structure-function correlates in elderly reveal MTL dysfunction with cognitive decline. Cereb. Cortex. 2011;22:2297–2304. doi: 10.1093/cercor/bhr306. [DOI] [PubMed] [Google Scholar]
- Petersen S.E., Dubis J.W. The mixed block/event-related design. Neuroimage. 2012;62:1177–1184. doi: 10.1016/j.neuroimage.2011.09.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack R.A. Is efficiency a useful concept in cognitive neuroscience? Dev. Cogn. Neurosci. 2015;11:12–17. doi: 10.1016/j.dcn.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rönnlund M., Nyberg L., Bäckman L., Nilsson L.G. Stability, growth, and decline in adult life span development of declarative memory: cross-sectional and longitudinal data from a population-based study. Psychol. Aging. 2005;20:3–18. doi: 10.1037/0882-7974.20.1.3. [DOI] [PubMed] [Google Scholar]
- Radloff L.S. The CES-D scale: a self-report depression scale for research in the general population. J. Appl. Psychol. Meas. 1977;1:385–401. [Google Scholar]
- Raz N., Lindenberger U., Rodrigue K.M., Kennedy K.M., Head D., Williamson A., Acker J.D. Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cereb. Cortex. 2005;15:1676–1689. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- Raz N., Ghisletta P., Rodrigue K.M., Kennedy K.M., Lindenberger U. Trajectories of brain aging in middle-aged and older adults: regional and individual differences. Neuroimage. 2010;51:501–511. doi: 10.1016/j.neuroimage.2010.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salami A., Eriksson J., Nyberg L. Opposing effects of aging on large-scale brain systems for memory encoding and cognitive control. J. Neurosci. 2012;32:10749–10757. doi: 10.1523/JNEUROSCI.0278-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez C.R., Richards J.E., Almli C.R. Age-specific MRI templates for pediatric neuroimaging. Dev. Neuropsychol. 2012;37:379–399. doi: 10.1080/87565641.2012.688900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider W., Pressley M. 2nd ed. Erlbaum; Mahwah, NJ: 1997. Memory Development Between Two and Twenty. [Google Scholar]
- Schott B.H., Wüstenberg T., Wimber M., Fenker D.B., Zierhut K.C., Seidenbecher C.I., Richardson-Klavehn A. The relationship between level of processing and hippocampal-cortical functional connectivity during episodic memory formation in humans. Hum. Brain Mapp. 2013;34:407–424. doi: 10.1002/hbm.21435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shing Y.L., Lindenberger U. The development of episodic memory: lifespan lessons. Child Dev. Perspect. 2011;5:141–155. [Google Scholar]
- Shing Y.L., Werkle-Bergner M., Li S.-C., Lindenberger U. Associative and strategic components of episodic memory: a lifespan dissociation. J. Exp. Psychol. Gen. 2008;137(3):495–513. doi: 10.1037/0096-3445.137.3.495. [DOI] [PubMed] [Google Scholar]
- Shing Y.L., Werkle-Bergner M., Brehmer Y., Müller V., Li S.-C., Lindenberger U. Episodic memory across the lifespan: the contributions of associative and strategic components. Neurosci. Biobehav. Rev. 2010;34:1080–1091. doi: 10.1016/j.neubiorev.2009.11.002. [DOI] [PubMed] [Google Scholar]
- Simons J.S., Spiers H.J. Prefrontal and medial temporal lobe interactions in long-term memory. Nat. Rev. Neurosci. 2003;4:637–648. doi: 10.1038/nrn1178. [DOI] [PubMed] [Google Scholar]
- Smith S.M., Jenkinson M., Woolrich M.W., Beckmann C.F., Behrens T.E.J., Johansen-Berg H., Matthews P.M. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23:208–219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Sowell E.R., Peterson B.S., Thompson P.M., Welcome S.E., Henkenius A.L., Toga A.W. Mapping cortical change across the human life span. Nat. Neurosci. 2003;6(3):309–314. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- Spaniol J., Davidson P.S.R., Kim A.S.N., Han H., Moscovitch M., Grady C.L. Event-related fMRI studies of episodic encoding and retrieval: meta-analyses using activation likelihood estimation. Neuropsychologia. 2009;47:1765–1779. doi: 10.1016/j.neuropsychologia.2009.02.028. [DOI] [PubMed] [Google Scholar]
- Staresina B.P., Davachi L. Differential encoding mechanisms for subsequent associative recognition and free recall. J. Neurosci. 2006;26:9162–9172. doi: 10.1523/JNEUROSCI.2877-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treisman A. The binding problem. Curr. Opin. Neurobiol. 1996;6:171–178. doi: 10.1016/s0959-4388(96)80070-5. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Psychological Corporation; New York: 1955. Wechsler Adult Intelligence Scale Manual. [Google Scholar]
- Werkle-Bergner M., Müller V., Li S.-C., Lindenberger U. Cortical EEG correlates of successful memory encoding: implications for lifespan comparisons. Neurosci. Biobehav. Rev. 2006;30:839–854. doi: 10.1016/j.neubiorev.2006.06.009. [DOI] [PubMed] [Google Scholar]
- Zimmer, H.D., Mecklinger, A., Lindenberger, U. (Eds.), 2006. Handbook of binding and Handbook of binding and memory: Perspectives from cognitive neuroscience, Oxford University Press, Oxford, UK.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.