Highlights
* We examined the impact of potential losses and gains on decisions under risk. * Losses influence choice more strongly than gains for both adolescents and adults. * Adolescents show more frontostriatal activation than adults when rejecting gambles. * Real-world risk taking correlates with mPFC activity differently between age groups. * Differences in neural activation underlie similar behaviors during development.
Keywords: Loss aversion, Risk, Decision-making, Adolescence, Development, fMRI
Abstract
Individuals are frequently faced with risky decisions involving the potential for both gain and loss. Exploring the role of both potential gains and potential losses in predicting risk taking is critical to understanding how adolescents and adults make the choice to engage in or avoid a real-life risk. This study aimed to examine the impact of potential losses as well as gains on adolescent decisions during risky choice in a laboratory task. Adolescent (n=18) and adult (n=16) participants underwent functional magnetic resonance imaging (fMRI) during a mixed gambles task, and completed questionnaires measuring real-world risk-taking behaviors. While potential loss had a significantly greater effect on choice than potential gain in both adolescents and adults and there were no behavioral group differences on the task, adolescents recruited significantly more frontostriatal circuitry than adults when choosing to reject a gamble. During risk-seeking behavior, adolescent activation in medial prefrontal cortex (mPFC) was negatively correlated with self-reported likelihood of risk taking. During risk-avoidant behavior, mPFC activation of in adults was negatively correlated with self-reported benefits of risk-taking. Taken together, these findings reflect different neural patterns during risk-taking and risk-avoidant behaviors in adolescents and adults.
1. Introduction
Adolescence is often described as a period of increased risk-taking behavior (e.g. reckless driving, substance use, risky sexual practices) (Arnett, 1992, Arnett, 1999, Dahl, 2004, Steinberg, 2008). Many psychological theories of adolescence pose that a sense of invulnerability is normative in this developmental phase (e.g. Lapsley and Hill, 2010), and suggest that this causes adolescents to underweight possible negative consequences when they make risky decisions. However, economic models of risk-taking, such as prospect theory (Kahneman and Tversky, 1979), have suggested that losses “loom larger” than gains for most individuals – the aversiveness of a potential loss is greater than the desirability of an equal potential gain, a behavioral phenomenon known as loss aversion. The relationships between theories of risk originating in behavioral economics and those originating in developmental psychology have not been extensively studied, and integrating these literatures is necessary to expand our understanding of the effects of loss on adolescent decision-making. Exploring the role of both potential gains and potential losses in predicting risk-taking is critical to understanding how adolescents and adults make the choice to engage in or avoid a real-life risk, and why these choices may differ across development.
Few behavioral studies of risk-taking behavior have focused specifically on adolescent responses to potential loss. Both children and adults have been shown to be more risk-seeking when choosing between a guaranteed small loss and the chance of a larger loss than when choosing between a guaranteed small gain and the chance of a larger gain (e.g. Levin and Hart, 2003, Levin et al., 2007); however, in other studies this pattern has been observed only in adults (Weller et al., 2011) and in younger children (age 5–8) and older children (age 9–13) but not in adolescents (age 14–20) or adults (age 21–64) (Harbaugh et al., 2002). On a similar task where participants selected between two gambles, adolescents have been shown to prefer a lower probability of a large loss to a higher probability of a small loss, but reverse this preference in the domain of gains (Rao et al., 2011). This response pattern is consistent with the same economic theories that predict loss aversion, but loss aversion itself has not been measured in adolescents. One study (Harbaugh et al., 2001) found that both children and adults display similar levels of the endowment effect (a behavioral phenomenon where participants demand more money to sell a good in their possession than to buy the same good, which is typically believed to be driven by loss aversion); However, there remains the possibility that loss aversion in a risky context would differ from the riskless context in which the endowment effect is measured, and that loss aversion would show nonlinear developmental trends. Therefore, the measurement of loss aversion and sensitivity to potential loss in adolescents remain an important and open area of study.
Evidence from developmental neuroscience has mostly focused on rewards, and consistently demonstrates increased neural sensitivity to gains during adolescence (but see Bjork et al., 2010, Bjork et al., 2004). An early study of children and adolescents responding to monetary gains and losses found increased activation in ventral striatum (VS) and lateral and medial orbitofrontal cortex (OFC) for gains relative to losses (May et al., 2004), a finding consistent with similar studies conducted in adults (e.g. Delgado et al., 2000, Rolls, 2000). In a direct comparison of children, adolescents and adults responding to positive reward outcomes of varying magnitudes, adolescents showed significantly greater activation in VS relative to children and adults (Galván et al., 2006); this activation was associated with self-reported risk taking (Galván et al., 2007). Increased VS activation in response to reward for adolescents relative to children and adults has been replicated in other studies (Geier et al., 2010, Van Leijenhorst et al., 2010a, Van Leijenhorst et al., 2010b), supporting an inverted U-shaped function of striatal sensitivity to reward that peaks in mid adolescence. Dual systems models of adolescent brain development (Casey et al., 2008, Steinberg et al., 2008) suggest that adolescents show heightened reward sensitivity relative to other age groups due to the late developmental trajectory of the PFC and its interaction with maturational changes in the striatum across adolescence and into early adulthood.
These reward studies have led to important advancements in understanding the role that potential gains play in risk-taking in adolescence. Surprisingly, however, the findings are less clear about the role of potential losses in influencing adolescent risk-taking. Most fMRI studies of monetary loss have focused on how the adolescent brain responds to a loss outcome (Helfinstein et al., 2011, Van Leijenhorst et al., 2010b) or to a cue predicting a loss (Guyer et al., 2006), but it is unclear how a potential loss may sway risky choice in adolescents. Exploring the role of both potential gains and potential losses in predicting risk taking is critical to understanding how adolescents and adults make the choice to engage in or avoid a real-life risk, why these choices may differ across development, and how they may be influenced.
Tom et al. (2007) examined the neural representation of potential gains and potential losses during risky decision-making using a mixed gambles task (gambles with a 50/50 chance of a gain or loss of varying amounts) commonly implemented in the behavioral economics literature (e.g. Rabin and Thaler, 2001, Tversky and Kahneman, 1992). They did not find separate brain systems for gains and losses, but found areas in the brain, including the VS, ventromedial prefrontal cortex (VMPFC), ventral anterior cingulate cortex (ACC), and medial OFC, that were sensitive to the potential for both gains and losses, in which activation increased parametrically with increasing potential gains and decreased parametrically with increasing potential losses. Furthermore, the negative slope of the decrease in activation in VS and VMPFC for increasing losses was greater than the corresponding positive slope of the increase in activation in the same regions for increasing gains; this finding was consistent with the pattern of loss aversion, the tendency of individuals to prefer avoiding losses over seeking gains, which has been demonstrated in behavioral research (Kahneman and Tversky, 1979, Kahneman and Tversky, 1984).
In the current study, our goal was to investigate the poorly understood impact of potential losses and loss aversion on adolescent decision making and neural response using functional magnetic resonance imaging (fMRI) and the mixed gambles task described previously (Tom et al., 2007). We aimed to examine the impact of potential losses as well as gains on adolescent behavior during risky choice, and to observe how behavioral and neural responses to potential gains and potential losses differ between adolescents and adults. We also investigated whether neural responses to potential losses would be predictive of actual risk-taking in these participants. We hypothesized that adolescents would display less loss aversion than adults, and that their choices on the mixed gambles task would be more strongly influenced by potential gains. We also predicted that adolescents would show more activation than adults in VS and VMPFC when accepting gambles, and that this risk-based neural activation would be associated with higher self-reported risk taking. We predicted that adolescents would reject fewer trials overall than adults, and that when rejecting gambles they would show more activation in prefrontal cortex than adults, consistent with requiring greater behavioral inhibition to avoid risk-taking.
2. Methods
2.1. Participants
Sixteen healthy right-handed adult participants (ages 25–30, mean age 28.1 years, SD=1.8 years, and 9 females) and 19 healthy right-handed adolescent participants (ages 13–17, mean age 15.5 years, SD=1.3 years, and 10 females) were recruited through poster and internet advertisements approved through the UCLA Institutional Review Board (IRB) and through the Galván Lab participant database. All participants provided informed consent, and participants under the age of 18 provided assent while their parent or guardian completed the informed consent procedure. Participants were excluded from participation if they had a previous diagnosis of psychiatric or neurologic illness or developmental delay, were taking psychoactive medication at the time of the study, or had metal in their bodies.
2.2. Materials
2.2.1. Risk-taking measures
Participants completed three self-report questionnaires during an initial behavioral testing session. Both adolescent and adult participants completed the Adolescent Risk Taking scale (Alexander et al., 1990), a 6-item scale in which they reported the number of times in their life they had engaged in risky activities, such as shoplifting and riding in a car with a dangerous driver, by selecting from one of three options: “never,” “once or twice,” or “several times”. Participants also completed the Domain-Specific Risk Taking Scale (DOSPERT; Weber et al., 2002, Fiigner and Weber, 2011), a well-validated 40-item measure of one's perceived risk of, benefit of, and likelihood of engaging in risky events. Versions of the DOSPERT for adults, adolescents (ages 14–17) and children (ages 9–13) were administered based on participant age (Figner and Weber, 2011). For example, the child version of the DOSPERT investigates ethical risk-taking by asking participants to consider the scenario, “stealing someone else's best friend,” while adolescents are asked to consider “dating someone else's boyfriend or girlfriend” and adults are asked to consider “having an affair with a married man or woman.” The DOSPERT uses a 7-point Likert scale for each of the assessment dimensions (“not at all risky” to “extremely risky,” “no benefits at all” to “great benefits,” and “extremely unlikely” to “extremely likely”) and includes scenarios in the domains of financial, ethical, recreational, social, and health risk.
2.2.2. Monetary experience questionnaire
For this study, we created a questionnaire to investigate the valence and arousal of participants’ feelings toward receiving $20 and the possibility of gaining or losing that sum. The purpose of this questionnaire was to encourage participants to feel connected to the money with which they were endowed during the behavioral testing session, in order to prevent the “house money effect” (increased risk-taking behavior that is observed when the money at stake is not the participant's own; Thaler and Johnson, 1990). In addition, the results of this questionnaire were used to verify that participants of different ages have a similar understanding of and appreciation for money. Participants responded to each question using a 5-point Likert scale, with each point represented by a face icon depicting the corresponding emotion (from a very unhappy face to a very happy face) or degree of arousal (from a very calm face to a very excited face). In addition to reporting these feelings, participants wrote a brief statement about what they would do with the money if they won it, and answered questions about how much money they receive from employment, allowance, and other sources.
2.2.3. Mixed gambles fMRI task
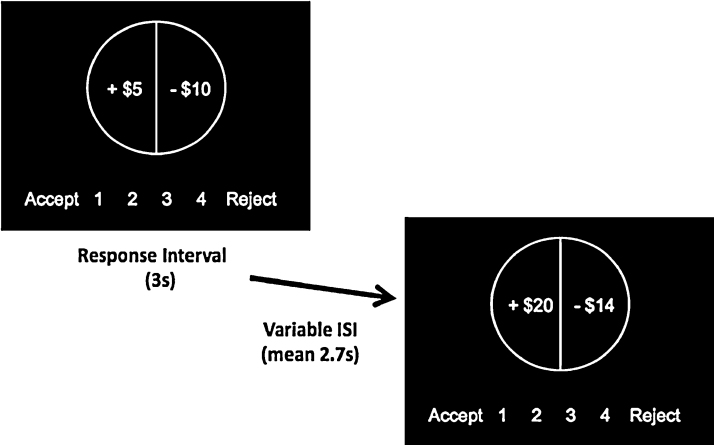
During the fMRI scan, participants completed a novel version of the mixed gambles task originally designed by Tom et al. (2007). The version implemented in the current study was modified to be developmentally appropriate, through the addition of a scale showing the response options at the bottom of each trial presentation and the use of white text on a black screen to avoid attentional biases (see Fig. 1).
Fig. 1.
Example of a trial from the mixed gambles task. Participants had 3000ms in which to respond to the gamble by pressing one of four keys. A jittered inter-stimulus interval followed, after which participants viewed and responded to a new gamble. Participants did not experience the outcomes of the gambles during the scan.
In the task, participants were presented with a series of gambles with a 50% probability of gaining the amount shown on one side of a “spinner” and a 50% probability of losing the amount shown on the other side. During the response interval of 3000ms, participants responded whether they accepted that gamble for real money, by pressing one of four buttons corresponding to a 4-point Likert scale (strongly accept, weakly accept, weakly reject, and strongly reject). Rather than a binary response, four responses were used to make it more difficult for participants to default to a simple choice rule; this response design was previously used in the task from Tom et al. (2007). However, for data analysis purposes the responses were binarized such that both strong and weak accept responses were coded as 1 and both weak and strong reject responses were coded as 0. The gain and loss amounts were independently manipulated, with gain amounts ranging from +$5 to +$20 in $1 increments and loss amounts ranging from −$5 to −$20 in $1 increments, for a total of 144 trials. Randomly interspersed within these trials were 24 gain-only trials and 24 loss-only trials, with values drawn from the same range, for a total of 192 trials across four runs. These gain-only and loss-only trials provided confirmation that participants were engaged with the task, as they should reject all loss-only trials and accept all gain-only trials. The side of the “spinner” in which the gain and loss appeared and the order of the stimuli was counterbalanced across participants. A variable “jittered” inter-stimulus interval then followed, averaging 2700ms, before the next gamble was presented in the same fashion.
The participants were informed that they would never see the outcomes of the gambles during the experiment, and that at the end of the experiment one gamble would be selected at random to be played for real money. If the participant had rejected the selected gamble during the experiment it would have no effect on their payment, and if they had accepted the gamble during the experiment its outcome would be resolved through a random coin-flip program, with the participant winning or losing the amount in the gamble depending on the outcome of the coin flip. Participants were told that they had the opportunity to lose or gain up to $20 (based on the theoretical possibility that the gamble with the highest gain or highest loss could be selected) and that their payment depended on their responses to the gambles in the task. This served to encourage participant engagement in the task and convince them of the veracity of the experimental protocol. Participants were instructed to bring $20 (which they were paid during the behavioral testing session) to the scan, which was matched by $20 of the experimenter's money.
2.3. Procedure
2.3.1. Behavioral testing session
A behavioral testing session was held approximately 1 week prior to the fMRI scan. All participants began by completing the appropriate informed consent/assent form for their age group. Adult participants and the parents/guardians of the adolescent participants completed an fMRI screening form and study intake form to ensure participant eligibility. All participants then completed a 1-h behavioral testing session consisting of the Adolescent Risk Taking scale, the DOSPERT, and a brief index of IQ (i.e. the Wechsler Abbreviated Scale of Intelligence, vocabulary and matrix reasoning subscales, adolescent M=104, SD=14.3, adult M=110, SD=15.2). Following completion of the tasks, participants were paid $20. Participants were informed in advance of the risk of gaining or losing money during the fMRI portion of the experiment, as described above. Thus, the $20 constituted a portion of the participants’ payment for the entire experiment, while endowing them with the payment in advance was intended to prevent the “house money effect” from influencing their task performance. Participants completed the monetary feelings questionnaire after receiving their payment. Adolescent participants were acclimated to the scanning environment with a mock MRI scanner and to hear the sounds of various functional and structural sequences.
2.3.2. fMRI session
Approximately 1 week after the behavioral testing session, participants returned for the fMRI portion of the study, which lasted ∼60min. Prior to entering the scanner, participants were instructed in the rules of the task and completed a block of 10 practice trials, ensuring that all participants understood the task fully. Participants had the opportunity to clarify any questions and to complete the practice block again if further practice was needed. In the scanner, participants completed four 4-min runs of the mixed gambles task (48 trials per run, for a total of 192 trials). Participants viewed a movie while structural MRI scans were collected. Following completion of the scan, participants were paid for their completion of the task; payment was designed so that no participant actually lost money, ensuring that all participants received at least $25 for their completion of the fMRI session (in accordance with the UCLA institutional review board payment scale). However, to elicit naturalistic risk-taking behavior, participants were unaware of this during completion of the loss aversion task.
2.3.3. Imaging procedure
Scanning was performed on a 3-Tesla Siemens Trio MRI machine in the Ahmanson-Lovelace Brain Mapping Center at UCLA. For the functional runs, 140 T2*-weighted echoplanar images (EPIs) were collected (33 slices; slice thickness, 4mm; TR, 2000ms; TE, 30ms; flip angle, 90°; matrix, 64×64; and field of view, 200). Two structural MRI images were collected as well: a T2-weighted matched-bandwidth high-resolution scan (following the same slice prescription as the EPIs) and a T1-weighted magnetization-prepared rapid- acquisition gradient echo image (MPRAGE; 160 sagittal slices; slice thickness, 1mm; TR, 2000ms; TE, 2100ms; matrix, 192×192; and field of view, 256).
2.3.4. Imaging data preprocessing and analysis
Data preprocessing and analysis were conducted using FSL version 4.1 (www.fmrib.ox.ac.uk/fsl). Images were motion-corrected using MCFLIRT and denoised using MELODIC independent components analysis. Data were smoothed using a 5mm full-width-half-maximum Gaussian kernel and filtered with a nonlinear high-pass filter (66s cutoff). A three-step registration process was used to align individual participant data into standard Montreal Neurological Institute (MNI) space. EPI images were first registered to the matched-bandwidth image, then to the MPRAGE image, and finally to MNI space. Data from participants whose head movements exceed 3mm in translational or rotational movement was not included in the analyses. One adolescent participant was excluded on the basis of motion, and behavioral and neural analyses were completed using the remaining eighteen adolescent participants (10 females, age M=15.4 years, and SD=1.4 years) and all sixteen adult participants. For the participants included, there were no significant differences between adolescents and adults in translational motion (adolescent M=.17mm, SD=.15mm, adult M=.13mm, SD=.10mm, t(32)=.980, p=.335) or rotational motion (adolescent M=.003mm, SD=.003mm, adult M=.002mm, SD=.001mm, t(32)=1.468, p=.152).
Data analysis was conducted using FEAT, first at an individual subject-level and then using a mixed-effects model at the group analysis level. Z-statistic images were thresholded at a cluster-level of z>2.3 and a corrected significance threshold of p≤0.05.
Statistical analyses were performed on each participant's data using a general linear model. For each participant, we separately modeled the onsets of the trials they accepted and the trials they rejected, using a 1-s duration. Six motion parameters were also included as covariates in the model for each run for each of the participants. At the group level, the main effects of trials that participants accepted and trials that they rejected were each modeled relative to an implicit baseline (all remaining activation that is not explicitly included in the model), and contrasts between accepted and rejected trials were computed for all participants and independently for adolescents and adults. In addition, whole-brain contrasts between adolescents and adults were computed for all accepted trials and for all rejected trials separately using two-tailed t-tests.
To ensure that there were no baseline differences between groups, we performed an analysis of resting activation when the participant was viewing a blank screen (i.e. not performing the task). Participants viewed a blank screen at the end of each run after the last trial was completed. Because of the jittered design, the amount of time from the last trial until the end of the run ranged from 10 to 24s on each run (M=16s). No significant differences in baseline activation were observed between adolescent and adult participants. This analysis convinces us that the observed neural differences between groups is not driven by baseline differences and instead are due to differences in response to the task.
2.3.5. Loss aversion
We computed a behavioral measure of loss aversion using logistic regression. This regression technique allows for the prediction of a binary response variable (i.e. the choice to accept or reject each gamble, coded as 1 or 0) from the independent variables of gain amount and loss amount. The logistic regression yielded regression coefficients (β) that represent the size of the contribution of the gain amount and loss amount to the participant's decision. The coefficient of loss aversion, lambda (λ) was then calculated from the regression coefficients using the following formula: λ=−βloss/βgain.
Larger values of λ reflect greater sensitivity to losses relative to gains, and values of λ>1 reflect loss aversion. Correlational analyses were conducted to determine whether loss aversion varied as a function of age. In addition, we created a hierarchical linear model, with gain amount and loss amount as level 1 predictors, age group as a level 2 predictor, and binary choice as the outcome variable, to test whether the extent to which gain and loss amounts influenced choice differed between age groups.
3. Results
3.1. Behavioral results
3.1.1. Monetary experience questionnaire
Upon receiving the $20 endowment, adolescent and adult participants reported similar levels of happiness (adolescent M=4.33, SD=.77, adult M=4.12, SD=.89, t(32)=.735, p=.467) and arousal (adolescent M=2.89, SD=1.08, adult M=2.88, SD=1.20, t(32)=.035, p=.972). The amount of monthly spending money participants reported was not significantly correlated with happiness (adolescent r=−.003, p=.990; adult r=.028, p=.919) or arousal (adolescent r=−.024, p=.927, adult r=−.088, p=.746) upon endowment. Adolescent and adult participants also did not differ from one another in their happiness (adolescent M=4.22, SD=.65, adult M=4.25, SD=.68, t(32)=−.122, p=.904) or arousal (adolescent M=3.56, SD=.92, adult M=3.38, SD=1.10, t(32)=.524, p=.604) after receiving their payment for the task. Neither adolescents nor adults showed a significant difference between their happiness upon receiving the initial endowment and upon receiving their final payment (adolescent t(17)=−.622, p=.542, adult t(15)=.620, p=.544). Both groups reported greater excitement following receipt of their final payment than their initial endowment (adolescent t(17)=2.61, p=.018, adult t(15)=3.16, p=.006); this may be due to the fact that the final payment was guaranteed, while the endowment was at risk during the task, as well as to the fact that all participants received more than $20 as their final payment (adolescent M=$26.89, SD=$1.08, adult M=$27.81, SD=$1.42). Neither age nor amount of money received had an effect on how happy participants were after receiving payment for the task, bage=.003, t(31)=.016, p=.987, bamount=.189, t(31)=.995, p=.328.
3.1.2. Risk taking questionnaires
Adolescent and adult participants did not differ from one another in their total real-world risk-taking behavior on the Adolescent Risk Taking scale (adolescent M=4.82, SD=2.86, adult M=5.81, SD=2.74, t(31)=−1.01, p=.318). On the DOSPERT scale, adolescent and adult participants showed no differences in their reported likelihood of risk-taking (adolescent M=3.40, SD=.69, adult M=3.56, SD=1.18, t(32)=−.487, p=.630), perceived riskiness (adolescent M=4.32, SD=.77, adult M=4.29, SD=.83, t(32)=.097, p=.92), and perceived benefits (adolescent M=2.84, SD=.77, adult M=3.19, SD=.96, t(32)=−1.17, p=.251).
For adolescent participants, scores on the Adolescent Risk Taking scale were positively correlated with perceived riskiness (r=484, p=.049), while for adult participants they were positively correlated with likelihood of risk-taking (r=.595, p=.015). When both age groups were combined, Adolescent Risk Taking scale scores correlated positively with both likelihood of risk-taking (r=.469, p=.006) and perceived benefits (r=.389, p=.025).
Across both age groups, male and female participants did not differ from one another in Adolescent Risk Taking scale scores (male M=5.93, SD=3.35, female M=4.78, SD=2.21, t(31)=−1.19, p=.244), or DOSPERT ratings of likelihood (male M=3.64, SD=.82, female M=3.34, SD=1.04, t(32)=−.909, p=.370), riskiness (male M=4.35, SD=.82, female M=4.27, SD=.78, t(32)=−.284, p=.779), or benefits (male M=3.08, SD=.91, female M=2.95, SD=.85, t(32)=−.436, p=.667).
3.1.3. Mixed gambles task
Adolescent and adult participants performed similarly on the mixed gambles task. Independent samples t-tests revealed that adolescents and adults showed no differences in reaction time to accept a gamble (adolescent M=1460ms, SD=330ms, adult M=1410ms, SD=310ms, t(32)=.469, p=.642) or to reject a gamble (adolescent M=1460ms, SD=310ms, adult M=1330ms, SD=270ms, t(32)=1.362, p=.183). Adolescents and adults also did not differ in the percentage of overall trials they accepted (adolescent M=35.9%, SD=18.3%, adult M=35.1%, SD=14.0%, t(32)=.149, p=.882) or in the mean expected value of the trials they accepted (adolescent M=$1.96, SD=$0.97, adult M=$1.88, SD=$1.14, t(32)=.208, p=.836) and the trials they rejected (adolescent M=−$1.12, SD=$0.69, adult M=−$1.06, SD=$0.83, t(32)=−.24, p=.81). In addition, adolescents and adults did not differ in the percentage of gain-only trials they accepted (adolescent M=69.3%, SD=18.6%, adult M=57.0%, SD=28.0%, t(32)=1.52, p=.138) or the percentage of loss-only trials they rejected (adolescent M=87.2%, SD=15.5%, adult M=81.2%, SD=16.3%, t(32)=1.11, p=.275). Taken together, these findings demonstrate that adolescents and adults had a similar understanding of the expectations of the task and completed it in a similar way.
Performance on the mixed gambles task did not show any sex differences. Female and male participants did not differ in their reaction times to accept (female M=1423ms, SD=308ms, male M=1447ms, SD=337ms, t(32)=−.224, p=.824) or to reject a gamble (female M=1370ms, SD=262ms, male M=1432ms, SD=340ms, t(32)=−.598, p=.554). They also did not differ in the percentage of overall trials they accepted (female M=38.3%, SD=14.7%, male M=32.4%, SD=17.6%, t(32)=.249, p=.291) or in the mean expected value of the trials they accepted (female M=$1.85, SD=$0.90, male M=$2.00, SD=$1.20, t(32)=−.402, p=.690) and the trials they rejected (female M=−$1.13, SD=$0.61, male M=−$1.05, SD=$0.89, t(32)=−.314, p=.755). Female and male participants did not differ in the percentage of gain-only trials they accepted (female M=64.3%, SD=23.2%, male M=62.7%, SD=25.5%, t(32)=.183, p=.856) or the percentage of loss-only trials they rejected (female M=83.5%, SD=16.5%, male M=85.3%, SD=15.8%, t(32)=−.330, p=.743).
3.1.4. Loss aversion
A behavioral coefficient of loss aversion (λ) was computed for each participant using the logistic regression procedure described above. After the exclusion of one statistical outlier from the adolescent population (who accepted too few gambles to generate an accurate λ term using logistic regression), no significant differences in loss aversion were observed between adolescents (M=.99, SD=1.98) and adults (M=1.11, SD=1.47), t(31)=−.205, p=.84. Both adolescents and adults demonstrated a range of behavioral patterns from loss seeking (willing to accept gambles where the loss amount was greater than the gain amount) to loss averse (only willing to accept gambles where the loss amount was less than the gain amount), with coefficients of loss aversion for adolescents between −4.9 and 5.7, and those for adults between −3.0 and 3.3. No significant differences in loss aversion were observed between male participants (M=1.05, SD=.70) and female participants (M=1.04, SD=2.28), t(31)=−.016, p=.987.
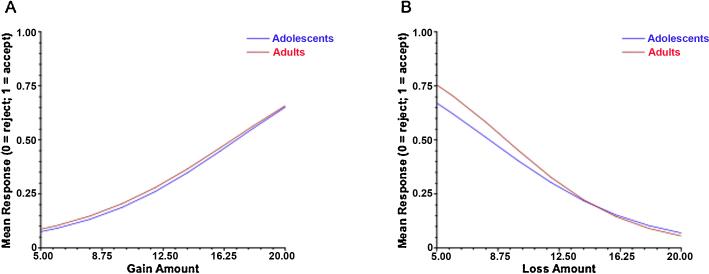
Across all participants, hierarchical linear modeling revealed a significant effect of the slope of gains on outcome (β=.20, t(33)=5.69, and p<.001) and the slope of losses on outcome (β=−.24, t(33)=−7.35, and p<.001), where increasing potential gains increased the likelihood of an accept response while increasing potential losses decreased it (Fig. 2). Furthermore, post hoc analyses revealed that the coefficient for losses is significantly different from the coefficient for gains, χ2(1)=3.86, p=.047, such that increasing loss amounts have a significantly greater effect on choice than increasing gain amounts do. However, age group had no effect on the slope for gains (β=−.01, t(32)=−.11, and p=.91) or for losses (β=−.04, t(32)=−.63, and p=.53).
Fig. 2.
The effects of increasing gain amounts and loss amounts on response choice for adolescents and adults. For both age groups, increasing gain amounts increased the likelihood of accepting a gamble (A) while increasing loss amounts decreased the likelihood of accepting a gamble (B). The magnitude of the slope for losses was significantly greater than that for gains.
3.1.5. Relationship between self-report questionnaires and mixed gambles behavior
Across all participants, the percentage of mixed gamble trials that were accepted showed no significant correlation with scores on the Adolescent Risk Taking scale (r=.124, p=.491) or with the DOSPERT likelihood (r=.176, p=.326), perceived riskiness (r=−.031, p=.863), or perceived benefits (r=.084, p=.636) scales. Similarly, across all participants the coefficient of loss aversion did not correlate with Adolescent Risk Taking (r=.106, p=.563), likelihood (r=.090, p=.620), perceived riskiness (r=.009, p=.959), or perceived benefits (r=.182, p=.310) scores. When the data for adolescent and adult participants are analyzed separately, these correlations remain not significant.
3.2. fMRI results
3.2.1. Accept trials
On trials in which participants accepted the presented gambles, significant activation was observed relative to an implicit baseline. Whole-brain omnibus analyses of the contrast of Accepted Trials>Baseline revealed activation in anterior cingulate cortex (ACC), frontal pole, VS, insula, precentral gyrus, and occipital cortex (see coordinates in Table 1). Direct comparisons to investigate sex differences revealed significantly greater activation for male participants than female participants in ACC, precuneous corex and cerebellum (see coordinates in Table 1). Direct group comparisons between adolescents and adults for the contrasts Accepted Trials>Baseline revealed no significant differences in activation between adolescents and adults on accept trials.
Table 1.
Significant regions identified in whole-brain analyses for accepted and rejected trials and contrasts.
| Region | X | Y | Z | Max Z | |
|---|---|---|---|---|---|
| Accepted trials | |||||
| Occipital cortex | R/L | 26 | −90 | −12 | 9.50 |
| −18 | −98 | 0 | 8.40 | ||
| Frontal pole | R/L | 46 | 36 | 20 | 6.75 |
| −46 | 36 | 20 | 5.14 | ||
| Precentral gyrus | R/L | 46 | 6 | 26 | 7.41 |
| −58 | 6 | 30 | 6.18 | ||
| Anterior cingulate cortex | R/L | 10 | 30 | 20 | 5.66 |
| −8 | 26 | 28 | 4.98 | ||
| Ventral striatum | R/L | 18 | 14 | −2 | 7.08 |
| −20 | 6 | 4 | 7.12 | ||
| Insula | R/L | 42 | −2 | 8 | 3.65 |
| −42 | −4 | 8 | 5.85 | ||
| Accepted trials – men>women | |||||
| Anterior cingulate cortex | R | 12 | 34 | 16 | 3.74 |
| Precuneous cortex | R | 6 | −60 | 38 | 3.63 |
| Cerebellum | L | −28 | −56 | −44 | 3.48 |
| Rejected trials | |||||
| Occipital cortex | R/L | 26 | −90 | −10 | 8.98 |
| −18 | −98 | 0 | 8.57 | ||
| Frontal pole | R/L | 52 | 40 | 18 | 6.14 |
| −40 | 40 | 14 | 4.94 | ||
| Precentral gyrus | R/L | 48 | 8 | 28 | 7.25 |
| −44 | 4 | 28 | 6.87 | ||
| Anterior cingulate cortex | R/L | 6 | 24 | 32 | 6.48 |
| −4 | 22 | 34 | 6.92 | ||
| Ventral striatum | R/L | 20 | 10 | 2 | 6.45 |
| −22 | 8 | −4 | 6.70 | ||
| Insula | R/L | 42 | 0 | 4 | 4.06 |
| −42 | 4 | 0 | 4.51 | ||
| Rejected trials – men>women | |||||
| Frontal pole | R | 32 | 40 | 32 | 3.72 |
| Cerebellum | L | −50 | −50 | −44 | 3.66 |
| Accepted>rejected | |||||
| Angular gyrus | R/L | 42 | −56 | 44 | 5.09 |
| −42 | −58 | 50 | 5.37 | ||
| Middle frontal gyrus | R | 40 | 26 | 46 | 4.32 |
| Superior frontal gyrus | R/L | 22 | 30 | 50 | 3.89 |
| −18 | 28 | 50 | 4.30 | ||
| Anterior cingulate cortex | R/L | 12 | 34 | 18 | 4.11 |
| −6 | 40 | 16 | 4.22 | ||
| Ventral striatum | R | 12 | 16 | 0 | 4.60 |
| Accepted>rejected – men>women | |||||
| Angular gyrus | R/L | 46 | −50 | 40 | 2.61 |
| −32 | −72 | 46 | 2.25 | ||
| Rejected>accepted | |||||
| Temporal pole | L | −44 | 10 | −40 | 4.10 |
| Postcentral gyrus | L | −12 | −38 | 56 | 4.16 |
| Superior temporal gyrus | R | 62 | −14 | 0 | 3.42 |
| L hippocampus | L | −26 | −14 | −24 | 3.48 |
3.2.2. Reject trials
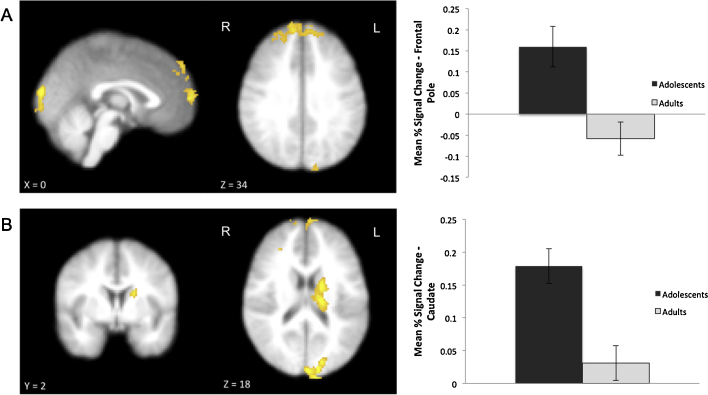
On trials in which participants rejected the presented gambles, significant activation was observed relative to an implicit baseline. Whole-brain omnibus analyses for the Rejected Trials>Baseline contrast revealed activation in regions similar to those observed for accepted trials (ACC, frontal pole, VS, insula, precentral gyrus, occipital cortex; see coordinates in Table 1). Direct comparisons to investigate sex differences revealed significantly greater activation for male participants than female participants in frontal pole and cerebellum (see coordinates in Table 1). Direct group comparison between adolescents and adults for the contrast Rejected Trials>Baseline revealed significantly greater activation for adolescents than for adults in the left caudate (peak activation at x, y, z MNI coordinates in mm: −16, 18, 18), bilateral frontal pole (0, 64, 8), and right occipital pole (−12, −94, 18) (Fig. 3). Significantly greater activation was observed for adults than for adolescents in the postcentral gyrus (−54, −20, 28).
Fig. 3.
The contrast Rejected Trials>Baseline for Adolescents>Adults. (A) Greater activation is observed in adolescents than adults in the frontal pole, p<.001, cluster size=1080 voxels. (B) The difference in percent signal change between the two age groups is shown for 6-mm spherical ROI centered on the local maximum peak voxel in the frontal pole (B; x=30, y=50, and z=38). (C) Greater activation is observed in adolescents than adults in the caudate, p<.02, cluster size=486 voxels. (D) The difference in percent signal change between the two age groups is shown for 6-mm spherical ROI centered on the local maximum peak voxel in the caudate (D; x=−16, y=18, and z=18). All activation is cluster corrected for multiple comparisons.
3.2.3. Contrasts between accepted and rejected trials
To examine the specific activation to accepted trials compared to rejected trials, a contrasts of Accepted Trials>Rejected Trials and Rejected Trials>Accepted Trials were examined. Significantly greater activation was observed for accepted trials than for rejected trials in bilateral ACC, right VS, bilateral angular gyrus, bilateral superior frontal gyrus, and right middle frontal gyrus, while significantly greater activation was observed for rejected trials than for accepted trials in left temporal pole, left postcentral gyrus, right superior frontal gyrus, and left hippocampus (Table 1). Direct comparisons to investigate sex differences revealed significantly greater activation for male participants than female participants in angular gyrus for the contrast Accepted Trials>Rejected Trials (Table 1). No significant differences between male and female participants were observed for the contrast Rejected Trials>Accepted Trials. Direct group comparisons between adolescents and adults for the contrasts Accepted Trials>Baseline, Accepted Trials>Rejected Trials, and Rejected Trials>Accepted Trials revealed no significant differences in activation between adolescents and adults.
3.2.4. Neural activation and risk-taking
To initially investigate the relationship between neural activation on the mixed gambles task and the DOSPERT as a measure of real-life risk-taking, whole brain analyses were conducted for the entire sample. Whole-brain omnibus analyses revealed a negative correlation between scores on the DOSPERT likelihood scale and activation in the superior frontal gyrus for both accepted trials and rejected trials (see coordinates in Table 2). Whole-brain omnibus analyses also revealed a negative correlation between scores on the DOSPERT benefits scale and activation in paracingulate gyrus, superior frontal gyrus, lateral occipital cortex, and postcentral gyrus for rejected trials, and between DOSPERT benefits scores and activation in paracingulate gyrus and postcentral gyrus for accepted trials (see coordinates in Table 2). No significant correlations were observed between neural activation and scores on the DOSPERT risks scale for either trial type.
Table 2.
Regions identified in whole-brain analyses that correlate negatively with scores on DOSPERT scales (likelihood, benefits and risks) for accepted and rejected trials.
| Measure | Region | X | Y | Z | Max Z | |
|---|---|---|---|---|---|---|
| Accepted trials | ||||||
| DOSPERT likelihood | Sup. frontal gyrus | R/L | 2 | 46 | 36 | 4.11 |
| −6 | 38 | 34 | 4.21 | |||
| DOSPERT benefits | Paracingulate gyrus | R/L | 8 | 36 | 38 | 4.57 |
| −2 | 46 | 34 | 4.21 | |||
| Postcentral gyrus | R | 52 | −12 | 58 | 3.79 | |
| Rejected trials | ||||||
| DOSPERT likelihood | Sup. frontal gyrus | R/L | 6 | 50 | 26 | 4.40 |
| −2 | 42 | 34 | 4.19 | |||
| DOSPERT benefits | Paracingulate gyrus | R/L | 8 | 40 | 34 | 4.54 |
| −4 | 38 | 32 | 4.48 | |||
| Sup. frontal gyrus | R | −20 | 28 | 46 | 3.99 | |
| Lat. occipital cortex | L | −60 | −68 | 26 | 4.50 | |
| Postcentral gyrus | R | 52 | −12 | 58 | 4.34 | |
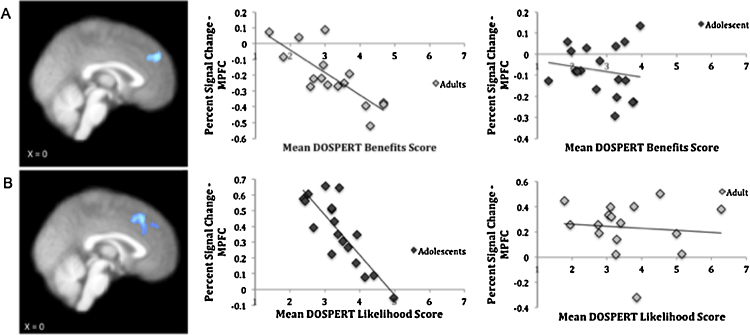
In order to investigate age-related differences in the relationship between neural activation on the task and real-world risk-taking (as measured by the DOSPERT), separate whole-brain regression analyses were conducted for adolescents and adults (Fig. 4). For adults, a significant negative correlation was observed between activation from the Rejected Trials>Baseline contrast and the benefits of risk-taking DOSPERT scale in medial prefrontal cortex (mPFC; peak voxel x=−2, y=48, and z=34) and precentral gyrus (x=34, y=−34, and z=70). This relationship was not significant for adolescent participants. A Fisher's z transformation revealed that the peak voxel correlation between mPFC and the DOSPERT benefits scale was significantly greater for adults (r=−.791, p<.001) than adolescents (r=−.164, p=.517), z=−2.398, p=.016. In adolescents, there was a significant negative correlation between activation from the Accepted Trials>Baseline contrast and the likelihood of risk-taking DOSPERT scale in mPFC (x=4, y=26, and z=42). This relationship was not significant for adult participants. A Fisher's z transformation revealed that the peak voxel correlation between mPFC and the DOSPERT likelihood scale was significantly greater for adolescents (r=−.831, p<.001) than adults (r=−.095, p=.727), z=−2.893, p=.004. There were no other significant correlations for adults between neural activation and the DOSPERT risks scales when rejecting trials, nor were there significant correlations between neural activation and any of the DOSPERT scales when accepting trials. For adolescents, there were no significant correlations between neural activation and any of the DOSPERT scales when rejecting trials, nor were there significant correlations with the DOSPERT likelihood or risk scales when accepting trials.
Fig. 4.
(A) Peak voxel neural activation in MPFC (cluster size=436 voxels, p<.03) and precentral gyrus (cluster size=704 voxels, p<.01) in the Rejected Trials>Baseline contrast correlated negatively with self-reported benefits of risk-taking (measured on a Likert scale from 1 to 7) in adults (left) but not in adolescents (right). (B) Peak voxel neural activation in MPFC (cluster size=559 voxels, p<.001) in the Accepted Trials>Baseline contrast correlated negatively with self-reported likelihood of risk-taking (measured on a Likert scale from 1 to 7) in adolescents (left) but not in adults (right).
4. Discussion
The behavioral findings from this study are the first to directly compare quantifiable measures of adolescent and adult loss aversion under risk. We found that adolescents and adults are similarly loss-averse when considering mixed gambles. Across age groups, loss amounts were shown to have a greater impact on choice than gain amounts. While prospect theory has established that losses loom larger than gains during adult decision-making (Kahneman and Tversky, 1979), these findings suggest that the same dictum can hold true for adolescents as well. Although initially surprising, this finding is consistent with the idea that adolescents and adults do not differ in risk perception or appraisal (Steinberg, 2004). Because risk aversion is generally considered to be caused by loss aversion (Kobberling and Wakker, 2005), behavioral similarities in aversion to loss may contribute to adolescents displaying the same cognitive understanding of risk as adults.
Adolescents and adults performed similarly on other behavioral measures of the mixed gambles task as well; they accepted and rejected similar proportions of mixed gambles, and did not differ significantly in the expected value of the trials they accepted and rejected. Although these findings deviate from our initial hypotheses, they are consistent with other gambling tasks that have not observed behavioral differences between adolescents and adults (e.g. Bjork et al., 2004, Eshel et al., 2007). The lack of behavioral differences observed on the mixed gambles task may be explained by the theory that performance on these types of tasks reflects maturity in risk perception among adolescents; because they perceive risk similarly, adolescents and adults are willing to accept similar amounts of risk on this risk-taking task. It is also interesting to note that regardless of age, the behavior of participants on the non-mixed gambles (gain-only and loss-only) deviated from what would be considered normatively optimal by accepting a small percentage of loss-only trials and rejecting a small percentage of gain-only trials. These deviations may have been due to the difficulty of overriding a prepotent response of evaluating mixed gambles, since gain-only and loss-only trials made up only 25% of all trials in the task (i.e. participants may have responded to the trials as though they were mixed gambles, and only realized their error after responding).
While adolescents and adults responded similarly to mixed gambles on a behavioral level and used a similar neural network while accepting gambles during the task, they demonstrated different underlying neural responses to the process of rejecting gambles. Though they rejected the same proportion of trials as adults, adolescents displayed greater corticostriatal recruitment (i.e. greater activation in the caudate and frontal pole) than adults to achieve this behavioral performance. These findings suggest a difference in neural development during the avoidance of risk; although neuroimaging studies have examined the choice between risky and certain options in gambling tasks (e.g. Levin and Hart, 2003), this study directly explored the choice between accepting and avoiding risk in adolescents and adults. It is possible that adding affectively arousing components to a choice (e.g. peer influences, dynamic task designs that increase tension and exhilaration) overwhelm the reward-sensitive regions of the adolescent decision-making system and lead to increased risk-seeking behavior, similar to the elevated risk-taking observed in other arousing tasks (Gardner and Steinberg, 2005, Fiigner et al., 2009). Similarly, although men and women did not differ on their behavioral task performance, men showed greater neural activation than women in a variety of regions during multiple aspects of the task. Interpreting these differences is challenging due to their domain-generality and limited power, but such findings are consistent with the observation of greater neural activation for men than women across regions during multiple cognitive tasks (Bell et al., 2006) and reward-seeking tasks (Lighthall et al., 2012; but cf. Lee et al., 2009).
In addition, the relationship between measured real-world risk-taking and reported perceptions of risk-taking differed between adolescents and adults. For adults, the likelihood of risk-taking measure of the DOSPERT predicted reported real-world risk-taking on the Adolescent Risk Taking scale. For adolescents, likelihood was not associated with real-world risk-taking; instead, scores on the Adolescent Risk Taking scale were positively correlated with perceived riskiness. Because most of the risky behaviors measured on the Adolescent Risk Taking scale typically only occur during adolescence (e.g. sneaking out of the house, acting on a dare), these findings may capture separate aspects of the experience of risk-taking across development. The adult data suggest that having had a propensity for risk-taking in adolescence is related to having a propensity for risk-taking as an adult. For adolescent participants, who are still in the process of establishing their risk-taking tendencies, a different relationship is seen. Adolescents who identify the most risk in situations are also those who are most likely to have engaged in typically adolescent risk behaviors, suggesting that they may in fact actively seek out risky activities while having accurate risk perceptions, consistent with other studies of adolescent risk behavior (Reyna and Farley, 2006, Steinberg, 2004).
The relationship between behavioral measures of risk-taking and neural activation while accepting and rejecting gambles also differed for adolescents and adults. For adolescents, higher reported likelihoods of risk-taking were associated with decreased MPFC activation when accepting gambles. For adults, no neural activation correlated with likelihood of risk taking. In adults, higher reported benefits of risk-taking were associated with decreased MPFC activation when rejecting gambles, but no relationship was seen between neural activation and benefits of risk-taking in adolescents. These findings suggest that developmental changes in both brain and behavior may lead to shifts in what information is most important to individuals when assessing risk. Because the MPFC has been implicated in the representation of value during risky decision-making (e.g. Hare et al., 2008, Levy et al., 2010), this finding may suggest that adolescents who are more inclined toward real-world risk-taking rely less on value assessments when evaluating choices than less risk-prone adolescents do. Risk-taking adolescents may rely instead on “hot” cues such as affective arousal that are not captured by the mixed gambles task. Future studies are necessary to test this possibility.
The experimental paradigm employed here has several strengths. It provides the opportunity to observe both risk-seeking and risk-averse behaviors, and because each gamble is treated as an independent event and the outcomes of the gambles are not displayed, the results are not confounded by prediction error or learning. However, the procedure also has some limitations. Although adolescent and adult participants reported similar emotional responses to receiving their monetary endowment for the task, it is still possible that monetary risk is less meaningful for adolescents than adults because they are responsible for fewer expenses in their daily lives. In addition, the relatively small sample size in this study precluded examination of age-related differences within the adolescent population. Other studies have observed peaks in risk-taking behavior and neural reward sensitivity during middle adolescence (e.g. Van Leijenhorst et al., 2010a), which a larger adolescent sample would provide the opportunity to explore.
This study provides valuable insight into the differing patterns of neural activation underlying behaviorally similar levels of loss aversion in adolescents and adults. The increased neural activation required by adolescents to perform in an adult manner on a non-emotionally arousing task may help to resolve some of the mixed findings within the adolescent risk-taking literature: adolescents may have the ability to refrain from elevated levels of risk-taking, but require additional cognitive and neural resources to do so. Contrary to the popular perception of adolescents as disregarding the potential negative consequences of risk-taking, these behavioral and neural findings suggest that adolescents can be averse to loss and adept at risk avoidance. For adolescents, the choice to take a risk may be weighted by the addition of social or affective factors under certain experimental tasks or real-world circumstances. Recognizing the interplay of these systems, and the conditions that may bias adolescents toward successful avoidance or maladaptive seeking of risk, is a critical step toward understanding when and how to intervene in adolescent behavior to encourage healthy outcomes.
Conflicts of interest statement
The authors report no conflicts of interest.
Acknowledgements
EBL was supported in part by the UCLA Pre-Doctoral Training Program in the Translational Neuroscience of Drug Abuse, and LL was supported by a Rubicon grant from the Netherlands Organization for Scientific Research (NWO). The authors would like to thank Jennifer Krull and Craig Fox for their invaluable advice on data analysis, Kristine McGlennen for her assistance with data collection, Bernd Figner and Elke Weber for adolescent versions of the DOSPERT, and all of the participating families for their time and effort.
Contributor Information
Emily E. Barkley-Levenson, Email: ebarkley@ucla.edu.
Linda Van Leijenhorst, Email: lleijenhorst@fsw.leidenuniv.nl.
Adriana Galván, Email: agalvan@ucla.edu.
References
- Alexander C.S., Kim Y.J., Ensminger M., Johnson K.E., Smith B.J., Dolan L.J. A measure of risk taking for young adolescents: reliability and validity assessments. Journal of Youth and Adolescence. 1990;19(6):559–569. doi: 10.1007/BF01537176. [DOI] [PubMed] [Google Scholar]
- Arnett J.J. Reckless behavior in adolescence: a developmental perspective. Developmental Review. 1992;12(4):339–373. [Google Scholar]
- Arnett J.J. Adolescent storm and stress, reconsidered. American Psychologist. 1999;54(5):317–326. doi: 10.1037//0003-066x.54.5.317. [DOI] [PubMed] [Google Scholar]
- Bell E.C., Willson M.C., Wilman A.H., Dave S., Silverstone P.H. Males and females differ in brain activation during cognitive tasks. Neuroimage. 2006;30(2):529–538. doi: 10.1016/j.neuroimage.2005.09.049. [DOI] [PubMed] [Google Scholar]
- Bjork J.M., Knutson B., Fong G.W., Caggiano D.M., Bennett S.M., Hommer D.W. Incentive-elicited brain activation in adolescents: similarities and differences from young adults. Journal of Neuroscience. 2004;24(8):1793–1802. doi: 10.1523/JNEUROSCI.4862-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork J.M., Smith A.R., Chen G., Hommer D.W. Adolescents, adults and rewards: comparing motivational neurocircuitry recruitment using fMRI. PLoS ONE. 2010;5(7):e11440. doi: 10.1371/journal.pone.0011440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey B.J., Jones R.M., Hare T.A. The adolescent brain. Annals of the New York Academy of Sciences. 2008;1124:111–126. doi: 10.1196/annals.1440.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl R.E. Adolescent brain development: a period of vulnerabilities and opportunities. Annals of the New York Academy of Sciences. 2004;1021:1–22. doi: 10.1196/annals.1308.001. [DOI] [PubMed] [Google Scholar]
- Delgado M.R., Nystrom L.E., Fissell C., Noll D.C., Fiez J.A. Tracking the hemodynamic responses to reward and punishment in the striatum. Journal of Neurophysiology. 2000;84(6):3072–3077. doi: 10.1152/jn.2000.84.6.3072. [DOI] [PubMed] [Google Scholar]
- Eshel N., Nelson E.E., Blair J., Pine D.S., Ernst M. Neural substrates of choice selection in adults and adolescents: development of the ventrolateral prefrontal and anterior cingulate cortices. Neuropsychologia. 2007;45:1270–1279. doi: 10.1016/j.neuropsychologia.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figner B., Mackinlay R.J., Wilkening F., Weber E.U. Affective and deliberative processes in risky choice: age differences in risk taking in the Columbia Card Task. Journal of Experimental Psychology: Learning Memory and Cognition. 2009;35(3):709–730. doi: 10.1037/a0014983. [DOI] [PubMed] [Google Scholar]
- Figner B., Weber E.U. Who takes risks and why? Determinants of risk-taking. Current Directions in Psychological Science. 2011;20:211–216. [Google Scholar]
- Galván A., Hare T., Parra C., Penn J., Voss H., Glover G., Casey B.J. Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. Journal of Neuroscience. 2006;26(25):6885. doi: 10.1523/JNEUROSCI.1062-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galván A., Hare T., Voss H., Glover G., Casey B.J. Risk-taking and the adolescent brain: who is at risk? Developmental Science. 2007;10(2):F8–F14. doi: 10.1111/j.1467-7687.2006.00579.x. [DOI] [PubMed] [Google Scholar]
- Gardner M., Steinberg L. Peer influence on risk taking, risk preference, and risky decision making in adolescence and adulthood: An experimental study. Developmental Psychology. 2005;41(4):625–635. doi: 10.1037/0012-1649.41.4.625. [DOI] [PubMed] [Google Scholar]
- Geier C.F., Terwilliger R., Teslovich T., Velanova K., Luna B. Immaturities in reward processing and its influence on inhibitory control in adolescence. Cerebral Cortex. 2010;20(7):1613–1629. doi: 10.1093/cercor/bhp225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer A.E., Nelson E.E., Perez-Edgar K., Hardin M.G., Roberson-Nay R., Monk C.S., Fox N.A., Ernst M. Striatal function alteration in adolescents characterized by early childhood behavioral inhibition. Journal of Neuroscience. 2006;26(24):6399–6405. doi: 10.1523/JNEUROSCI.0666-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbaugh W.T., Krause K., Vesterlund L. Are adults better behaved than children? Age, experience and the endowment effect. Economics Letters. 2001;70:175–181. [Google Scholar]
- Harbaugh W.T., Krause K., Vesterlund L. Risk attitudes of children and adults: choices over small and large probability gains and losses. Experimental Economics. 2002;5:53–84. [Google Scholar]
- Hare T.A., O’Doherty J., Camerer C.F., Schultz W., Rangel A. Dissociating the role of the orbitofrontal cortex and the striatum in the computation of goal values and prediction errors. Journal of Neuroscience. 2008;28(22):5623–5630. doi: 10.1523/JNEUROSCI.1309-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfinstein S.M., Benson B., Perez-Edgar K., Bar-Haim Y., Detloff A., Pine D.S., Fox N.A., Ernst M. Striatal responses to negative monetary outcomes differ between temperamentally inhibited and non-inhibited adolescents. Neuropsychologia. 2011;49(3):479–485. doi: 10.1016/j.neuropsychologia.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahneman D., Tversky A. Prospect theory: an analysis of decision under risk. Econometrica. 1979;47(2):263–291. [Google Scholar]
- Kahneman D., Tversky A. Choices values and frames. American Psychologist. 1984;39:341–350. [Google Scholar]
- Kobberling V., Wakker P.P. An index of loss aversion. Journal of Economic Theory. 2005;122:119–131. [Google Scholar]
- Lapsley D.K., Hill P.L. Subjective invulnerability optimism bias and adjustment in emerging adulthood. Journal of Youth and Adolescence. 2010;39:847–857. doi: 10.1007/s10964-009-9409-9. [DOI] [PubMed] [Google Scholar]
- Lee T.M.C., Chan C.C.H., Leung A.W.S., Fox P.T., Gao J.H. Seks-related differences in neural activity during risk taking: an fMRI study. Cerebral Cortex. 2009;19(6):1303–1312. doi: 10.1093/cercor/bhn172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin I.P., Hart S.S. Risk preferences in young children: early evidence of individual differences in reaction to potential gains and losses. Journal of Behavioral Decision Making. 2003;16(5):397–413. [Google Scholar]
- Levin I.P., Hart S.S., Weller J.A., Harshman L.A. Stability of choices in a risky decision-making task: a 3-year longitudinal study with children and adults. Journal of Behavioral Decision Making. 2007;20:241–252. [Google Scholar]
- Levy I., Snell J., Nelson A.J., Rustichini A., Glimcher P.W. Neural representation of subjective value under risk and ambiguity. Journal of Neurophysiology. 2010;103:1036–1047. doi: 10.1152/jn.00853.2009. [DOI] [PubMed] [Google Scholar]
- Lighthall N.R., Sakaki M., Vasunilashorn S., Nga L., Somayajula S., Chen E.Y., Samii N., Mather M. Gender differences in reward-related decision processing under stress. Social Cognitive and Affective Neuroscience. 2012;7(4):476–484. doi: 10.1093/scan/nsr026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May J.C., Delgado M.R., Dahl R.E., Stenger V.A., Ryan N.D., Fiez J.A., Carter C.S. Event-related functional magnetic resonance imaging of reward-related brain circuitry in children and adolescents. Biological Psychiatry. 2004;55(4):359–366. doi: 10.1016/j.biopsych.2003.11.008. [DOI] [PubMed] [Google Scholar]
- Rabin M., Thaler R.H. Anomalies: risk aversion. Journal of Economic Perspectives. 2001;15(1):219–232. [Google Scholar]
- Rao U., Sidhartha T., Harker K.R., Bidesi A.S., Chen L.A., Ernst M. Relationship between adolescent risk preferences on a laboratory task and behavioral measures of risk-taking. Journal of Adolescent Health. 2011;48:151–158. doi: 10.1016/j.jadohealth.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyna V.F., Farley F. Risk and rationality in adolescent decision making: Implications for theory, practice and public policy. Psychological Science in the Public Interest. 2006;71(1):1–44. doi: 10.1111/j.1529-1006.2006.00026.x. [DOI] [PubMed] [Google Scholar]
- Rolls E.T. The orbitofrontal cortex and reward. Cerebral Cortex. 2000;10(3):284–294. doi: 10.1093/cercor/10.3.284. [DOI] [PubMed] [Google Scholar]
- Steinberg L. Risk taking in adolescence: what changes, and why? Annals of the New York Academy of Sciences. 2004;1021:51–58. doi: 10.1196/annals.1308.005. [DOI] [PubMed] [Google Scholar]
- Steinberg L. A social neuroscience perspective on adolescent risk-taking. Developmental Review. 2008;28(1):78–106. doi: 10.1016/j.dr.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L., Albert D., Cauffman E., Banich M., Graham S., Woolard J. Age differences in sensation seeking and impulsivity as indexed by behavior and self-report: evidence for a dual systems model. Developmental Psychology. 2008;44:1764–1778. doi: 10.1037/a0012955. [DOI] [PubMed] [Google Scholar]
- Thaler R.H., Johnson E.J. Gambling with the house money and trying to break even: the effects of prior outcomes on risky choice. Management Science. 1990;36(6) [Google Scholar]
- Tom S., Fox C.R., Trepel C., Poldrack R.A. The neural basis of loss aversion in decision-making under risk. Science. 2007;315(5811):515–518. doi: 10.1126/science.1134239. [DOI] [PubMed] [Google Scholar]
- Tversky A., Kahneman D. Advances in prospect theory: cumulative representation of risk and uncertainty. Journal of Risk and Uncertainty. 1992;5:297–323. [Google Scholar]
- Van Leijenhorst L., Zanolie K., Van Meel C.S., Westenberg P.M., Rombouts S.A.R.B., Crone E.A. What motivates the adolescent? Brain regions mediating reward sensitivity across adolescence. Cerebral Cortex. 2010;20(1):61–69. doi: 10.1093/cercor/bhp078. [DOI] [PubMed] [Google Scholar]
- Van Leijenhorst L., Gunther Moor B., Op de Macks Z.A., Rombouts S.A.R.B., Westenberg P.M., Crone E.A. Adolescent risky decision making: neurocognitive development of reward and control regions. Neuroimage. 2010;51:345–355. doi: 10.1016/j.neuroimage.2010.02.038. [DOI] [PubMed] [Google Scholar]
- Weber E.U., Blais A.R., Betz N.E. A domain-specific risk-attitude scale: measuring risk perceptions and risk behaviors. Journal of Behavioral Decision Making. 2002;15(4):263–290. [Google Scholar]
- Weller J.A., Levin I.P., Denburg N.L. Trajectory of risky decision making for potential gains and losses from ages 5 to 85. Journal of Behavioral Decision Making. 2011;24(4):331–344. [Google Scholar]