Highlights
* A systematic review of neuroscientific studies of mirror neuron systems in autism. * Data is mixed with no overall evidence of abnormal mirror responses. * It is time to move on from the broken mirror theory of autism. * Social top-down modulation of mirror systems is a critical factor in autism.
Keywords: Autism, Mirror neuron system, Imitation, Social cognition
Abstract
There is much interest in the claim that dysfunction of the mirror neuron system in individuals with autism spectrum condition causes difficulties in social interaction and communication. This paper systematically reviews all published studies using neuroscience methods (EEG/MEG/TMS/eyetracking/EMG/fMRI) to examine the integrity of the mirror system in autism. 25 suitable papers are reviewed. The review shows that current data are very mixed and that studies using weakly localised measures of the integrity of the mirror system are hard to interpret. The only well localised measure of mirror system function is fMRI. In fMRI studies, those using emotional stimuli have reported group differences, but studies using non-emotional hand action stimuli do not. Overall, there is little evidence for a global dysfunction of the mirror system in autism. Current data can be better understood under an alternative model in which social top-down response modulation is abnormal in autism. The implications of this model and future research directions are discussed.
1. Introduction
Individuals with autism spectrum condition (ASC) struggle with social interaction and communication, both in formal testing and in their everyday lives. Many different cognitive and brain-based theories have been proposed to account for these difficulties. Current proposals include difficulties in theory of mind (Frith, 2001), in social motivation (Chevallier et al., 2012) and in mirror neuron function (Dapretto and Iacoboni, 2006, Gallese et al., 2009, Rizzolatti et al., 2009). The latter is the focus of the present paper, where I examine the human mirror neuron system (MNS) and whether damage to this system might have relevance for autism.
The manuscript proceeds in three parts. First, I briefly describe the functions of the human mirror system and the different models of MNS dysfunction which have been proposed. Second, I systematically review the scientific literature on the MNS in ASC, focusing on studies which have used neuroscientific methods to assess the function of the MNS. Third, I provide an overall assessment of the field, and make suggestions for future directions in terms of both theory and experiment.
2. The mirror system and autism
The human mirror neuron system (MNS) can be defined as the set of brain regions which are active both when participants perform an action and when they observe another person performing the same action (Rizzolatti and Craighero, 2004). The core components of the human MNS are inferior frontal gyrus (IFG) and inferior parietal lobule (IPL). In the macaque monkey equivalents of these regions, single neurons have been recorded with mirror properties (Fogassi et al., 2005, Gallese et al., 1996). Though single units have not been recorded in the human MNS, detailed patterns of activation in fMRI studies leave little doubt that these areas contain mirror neuron populations (Kilner et al., 2009, Oosterhof et al., 2010). The human MNS is widely assumed to play a key role in action understanding and imitation, though this remains debated (Dinstein et al., 2008, Hickok, 2009). There are also a number of fMRI studies, some using emotional stimuli, which suggest the existence of a broader mirror neuron network (Caspers et al., 2010) that incorporates somatosensory and premotor cortex (Keysers et al., 2010), and possibly anterior insula (Wicker et al., 2003). The present paper will not consider this extended mirror network in detail.
The dominant theory of MNS function is based on a direct-matching model in which observed actions are directly mapped onto the observers own motor system (Rizzolatti and Sinigaglia, 2010). This framework stresses the idea that the MNS encodes the goal of an action, not just the basic motor features, and that this direct goal encoding provides a primary mechanism both for understanding other people and for imitating them. It is claimed that the MNS is critical for performing and understanding action sequences (Fogassi et al., 2005). The MNS might also make a contribution to theory of mind (Gallese, 2007) or to language (Gallese, 2008) but these claims are more speculative. Many other models of the MNS are possible and may even be more plausible (Michael, 2011), but the direct-matching model gives the basis for most theories linking the MNS to autism.
The claim that dysfunction of the MNS is a causal factor in poor social cognition in ASC is commonly called the broken mirror theory (BMT) of autism. Here I distinguish three variants of the BMT which make slightly different claims. First, extensive behavioural evidence of weak imitation skills in ASC (Williams et al., 2004) combined with the putative role of the MNS in imitation (Buccino et al., 2004) led to the idea that MNS dysfunction is the cause of poor imitation in ASC (Williams et al., 2001). The theory put forward by Williams explicitly links the MNS to the self-other mapping function described by Rogers and Pennington (1991) and suggests that failure of a basic self-other mapping in ASC could cause difficulties in both imitation and other aspects of social cognition such as theory of mind. A similar position is endorsed by Gallese et al. (2009), and I call this the imitation version of the BMT.
Second, the simulation version of the BMT is built on the idea that the MNS provides a basis for simulating other people. Such simulation could apply to actions, but also to emotions and to mental states. The simulation BMT claims that a failure of a basic simulation system in the MNS in autism would cause very widespread difficulties in theory of mind, language and empathy (Dapretto and Iacoboni, 2006, Oberman and Ramachandran, 2007). This theory predicts that comprehension of actions and emotions in the broader MNS should all be abnormal in ASC.
Third, the chaining version of the BMT is a more subtle theory which only on the claim that some mirror neurons represent action chains or sequences (Fogassi et al., 2005). These chaining mirror neurons are active only when the monkey sees or performs an action embedded in a particular sequence, for example, grasping-to-eat rather than grasping-to-place. Activation of chaining mirror neurons at the beginning of a sequence could potentially allow the monkey to predict how the sequence will unfold, and thus understand the intention of the actor (Fogassi et al., 2005). Rizzolatti and Fabbri-Destro (2010) suggest that only these action chaining mirror neurons are abnormal in ASC, and that dysfunction of these particular neurons leads to difficulties in other areas of social cognition. The chaining version of the broken mirror theory does not make direct claims about language or emotion comprehension impairment.
These three versions of the BMT share the claim that dysfunction of the MNS is a primary cause of poor social interaction in individuals with ASC. This claim is intuitively appealing and has received widespread attention, including in the popular press. Several studies suggest that brain-based explanations of behaviour are considered more convincing than psychological explanations (McCabe and Castel, 2008, Weisberg et al., 2008). This may explain why links between a seductively named brain system (the MNS) and a developmental disorder are appealing. Funding has been invested in this area and therapies based on improving MNS function have been proposed (Perkins et al., 2010, Wan et al., 2010). However, the evidence that the MNS is actually abnormal in individuals with ASC is not always clear.
Suitable evidence for abnormalities of the MNS in ASC could come from a variety of sources, both behavioural and brain-imaging studies. Behavioural studies are the most plentiful, and many have examined imitation and action comprehension in an effort to measure MNS function. Papers have reported abnormal imitation behaviour in children and adults with ASC (reviewed in Williams et al., 2004), with both reduced frequency of spontaneous imitation (Charman et al., 1997, Ingersoll, 2007) and reduced imitation accuracy (Rogers et al., 1996, Vivanti et al., 2008) observed. These have been reviewed in detail elsewhere (Hamilton, 2008, Kana et al., 2010, Williams et al., 2001) and overall provide mixed evidence for imitation difficulties. In particular, performance is worse on spontaneous imitation and imitation of non-object directed actions but is normal on goal-directed imitation and reaction-time tasks. A major difficulty in interpreting behavioural studies of imitation or action comprehension in ASC concerns the specificity of the result. Poor performance on an imitation could be caused by failure of the MNS, or by failure of other brain systems involved in visual processing, control of responding or motor performance. Similarly, good performance on a particular task could indicate an intact MNS or could reflect compensatory strategies. Thus, behavioural studies are limited in their ability to pinpoint the underlying cause of a behaviour in terms of particular brain systems.
This means we must turn to brain imaging studies to properly examine the BMT. As this theory is defined in terms of brain systems, it is appropriate to test it with reference to those same brain systems. Evidence in favour of the BMT should show reduced responses in the MNS regions of the brain in individuals with ASC compared to typical individuals. The present paper systematically reviews published studies on the function of the MNS in participants with ASC, with the aim of assessing the broken mirror theory of autism, and suggesting useful future directions for autism research.
3. Methodology of the review
Searches were conducted on Pubmed and GoogleScholar for the terms “autism”/“autism spectrum condition” and the terms “mirror neuron system”/“mirror system”, finding papers published up to March 2012. Additional searches located papers which cite some of the major papers on the topic (Dapretto et al., 2006, Williams et al., 2001). Papers were initially filtered by examination of the title/abstract to identify papers presenting original data (not reviews) which used brain-based rather than behavioural methods to compare typical and autistic participants. Further detailed reading identified 25 papers which directly contrast typical and autistic participants and measure brain responses in tasks relevant to the mirror neuron system. It is notable that this search also found 36 review papers on the topic of mirror neurons and autism have been published over the same period, which suggests that excitement outweighs data in this area.
The 25 empirical papers were grouped according to the method used for studying group differences in cognitive function, which include EEG, MEG, TMS and fMRI. No studies using near-infrared spectroscopy (NIRS) were identified. The review includes papers using eye tracking and muscle activation (EMG) measures because these feature prominently in this research area. However, studies using general behavioural measures (e.g. imitation tasks) were excluded; these have largely been reviewed elsewhere (Hamilton, 2008, Kana et al., 2010, Williams et al., 2001). Studies of reaction time during automatic imitation have also been reviewed elsewhere (Cook et al., 2012, Wang and Hamilton, 2012). Studies examining motor performance in ASC do not directly tap MNS function and have been examined elsewhere (Fournier et al., 2010, Gowen and Hamilton, 2012) so they were not included here. The final section of the review considers a number of other approaches which did not fit in the standard categorisation of tasks, and also the findings from structural MRI studies.
In recent years, there has been an increased focus on the importance of using appropriate and robust statistical methods in cognitive neuroscience (Kriegeskorte et al., 2009, Nieuwenhuis et al., 2011). Bearing these in mind, the present review will consider the robustness of the reported results in each paper, in addition to the headline claims. Methodological factors such as the number of participants, the stimulus design and possible file-drawer effects will also be considered. The 25 papers identified for detailed review are listed in Table 1, grouped by methodology. The results of each are summarised in terms of whether the paper provides evidence for an abnormal MNS in ASC, a normal MNS or evidence which is mixed. Mixed evidence can mean either that different components of the data suggest different things, or because the reported results are not entirely statistically robust. What follows is a narrative review of the studies listed in Table 1, describing first the methods used and how accurately they index MNS function, and then the patterns of data found in different studies.
Table 1.
Summary of studies of autism in this review.
| Modality | Paper | Number of ASC participants | Mean participant age (years) | Task | Results | Summary |
|---|---|---|---|---|---|---|
| EEG | Oberman et al. (2005) | 11 | 16.6 | Move hand; watch hand movement video; watch bouncing balls video; watch white noise | ASC do not show suppression for hand observation. Group by condition interaction not tested | Mixed |
| Oberman et al. (2008) | 13 | 10 | Watch videos of: stranger open/close hand; familiar person open/close hand; own hand open/close; bouncing balls | Main effect of familiarity; no familiarity by group interaction; simple effects in ASC for familiar only | Mixed | |
| Raymaekers et al. (2009) | 20 | 11.2 | Observe video of moving hand; bouncing balls; white noise; move own hand | Sig suppression to hand obs in both groups; no main effects of group or interactions; marginal age effects in ASC group | Normal | |
| Martineau et al. (2008) | 14 | 6 (approx.) | Observe 20s video of: white/lake/waterfall/person doing leg movements | Hemisphere by group by sequence interactions in theta1 only; left hemisphere differences | Abnormal | |
| Fan et al. (2010) | 20 | 17.7 | Observe+; observe hand manipulating object; observe moving white dot; perform hand manipulation on object | No main effect of group; no group by condition interactions; no correlations with age; correlation between mu and ADI-communication | Normal | |
| Bernier et al. (2007) | 14 | 23 | Rest/observe/execute/imitate of hand grasping block | No group by task interaction; but ASC-observe differs in simple effects; some correlations between mu and imitation performance | Mixed | |
| MEG | Avikainen et al. (1999) | 5 | 25 | Observe live object manipulation | No group differences | Normal |
| Honaga et al. (2010) | 7 | 26 | Observe live hand action for later execution, then execute actions. All object-directed familiar actions | No differences in sensorimotor cortex or visual cortex; group diffs in left sensorimotor; right premotor; ACC; right STS | Mixed | |
| Nishitani et al. (2004) | 8 | 29.9 | Imitate lip movements | Difference in timing of inferior frontal component | Abnormal | |
| TMS | Enticott et al. (2012) | 34 | 26 | Observe video of static hand; hand grasp mug; pantomime grasp | Main effect: smaller MEPs to transitive actions in ASC group | Abnormal |
| Théoret et al. (2005) | 10 | 39 | Observe index finger or thumb movements | Main effect of group. ASC show normal MEPS for allocentric actions but no MEP change for egocentric actions | Mixed | |
| EMG | Cattaneo et al. (2007) | 8 | 6.5 | Mouth muscle activity, doing/observing grasping | Group by time interaction for observation; delayed response in ASC in performance | Abnormal |
| Pascolo and Cattarinussi (2012) | 7 | 7.7 | Replicate cattaneo; record mouth muscle EMG when child grasps to eat | No anticipation in TD or ASC kids. No group differences; delay differs with food location and child arm length | Normal | |
| Eyetracking | Falck-Ytter (2009) | 18 | 5y10m | Observe person placing items in box | TD and ASC both show predictive gaze; no group differences | Normal |
| Falck-Ytter et al. (2012) | 40 | 5y10m | Observe actor who makes eye contact, then gazes or points at objects | TD look to indicated item more; ASC look less and slower; ASC performance correlates with Vineland communication | Normal | |
| Vivanti et al. (2011) | 18 | 13 | (1) Observe rational/irrational actions; (2) observe action sequences; (3) observe actress sorting objects, need to attend to emotions; (4) imitate actions after direct/averted gaze | (1) Both groups look to face in irrational; (2) ASC look less at face; ASC predict action based on sequence but not based on head turn; (3) no group differences; (4) both groups look more to direct gaze face; TD do better in direct gaze | Normal | |
| Vivanti et al. (2008) | 18 | 11y4m | Watch 6 meaningful/6 meaningless actions to imitate | ASC participants look less to the face and imitate less accurately. Both groups look more to meaningful than meaningless actions, with no group differences and no group by task interaction | Normal | |
| fMRI | Bastiaansen et al. (2011) | 21 | 30 | Observe disgust face/move face/taste disgust; ROI in RBA44 based on Dapretto, contrast obs face>rest | No main effects of group or emotion, activity in BA44 ROI interacts with age (low for young ASC) | Mixed |
| Dapretto et al. (2006) | 10 | 12 | Observe/imitate emotional facial expressions | Right pars opercularis shows less activity in ASC; left aIPS more activity in ASC | Abnormal | |
| Dinstein et al. (2010) | 13 | 27.4 | Observe/perform hand actions; RS analysis | Normal responses and normal adaptation in aIPS; ASC more variable | Normal | |
| Grèzes et al. (2009) | 12 | 26 | Observe static/dynamic people walking through door with neutral/fearful posture | Dynamic>static: both groups engage premotor, IPS and STS; fear>neutral: TD only engage rIFG, premotor and amygdala; DCM suggests differences driven by amygdala | Normal | |
| Marsh and Hamilton (2011) | 18 | 33 | Observation of rational and irrational actions | No differences in left or right aIPS; group differences in SMA/MCC; group by rationality interaction in mPFC | Normal | |
| Martineau et al. (2010) | 7 | 23 | Observe video of 1Hz hand flexion/extension v static hand; execute 1Hz hand flexion/extension v. static hand | (Observation>rest) by group interaction: bilateral IFG is MORE active in ASC | Abnormal | |
| Schulte-Rüther et al. (2011) | 14 | 27 | Observe happy/sad/neutral faces. Other task: “decide how he/she feels”. Self task: “decide how you feel” | No group differences in IFG, both groups robustly engage left IFG during the SELF task | Normal | |
| Williams et al. (2006) | 16 | 15;4 | Imitate finger lifting; symbolic cue; spatial cue | Groups similar for imitation; differences in fusiform/middle occipital/IPL; no BA44 effects in either group | Mixed | |
4. Studies which indirectly measure MNS function
4.1. EEG studies
A seminal paper by Muthukumaraswamy et al. (2004) defined a rhythm emanating from sensorimotor cortex which seems to change during performed and observed actions. This mu rhythm is defined as a signal between 8 and 13Hz recorded from electrodes C3, C4 and Cz. It is suppressed when participants perform an action, imagine performing an action or observe an action. Because the mu rhythm is suppressed for both performed and observed action, it has been taken as an index of mirror neuron function. Recent work combining EEG and fMRI shows that mu-suppression correlates with BOLD signal in inferior parietal lobule, dorsal premotor cortex and BA2 in the sensorimotor strip, but not in BA44 as previously assumed (Arnstein et al., 2011). BA2 is the strongest generator of the mu rhythm (Salmelin and Hari, 1994). The inferior parietal lobule is part of the MNS, while the dorsal premotor cortex and BA2 are part of the extended MNS (Caspers et al., 2010). Thus, the link between mu rhythm and the MNS is reasonable, though mu rhythm is unlikely to provide a pure assessment of the function of the frontal mirror systems in BA44.
Six studies have examined mu rhythm suppression in participants with ASC, testing a total of 92 participants (see Table 1). All but one of these studies focus on the classic mu rhythm recorded at 8–13Hz over C3, C4 and Cz, and all employ similar experimental designs based on Oberman et al. (2005). This means that comparisons across studies are relevantly easy. The basis of the design is that in different blocks of trials, participants perform hand movements; watch a video of hand movements; watch a video of a bouncing ball or watch a baseline video of white noise. Mu suppression is indexed by the difference in mu-rhythm power between observation of hand actions and baseline. Ideally, this should be revealed in a group by condition interaction, where participants with ASC show suppression in the performed action condition but not in the action observation condition. Of the five studies, none report this conclusive interaction. However, three studies testing 38 participants report a lack of suppression when participants with ASC observe actions, as indexed by simple effects (Bernier et al., 2007, Oberman et al., 2005, Oberman et al., 2008). The lack of a reported group by condition interaction in these papers unfortunately makes it very hard to properly interpret the data (Nieuwenhuis et al., 2011). A further two studies testing 40 participants found no differences in mu suppression in ASC and no group by condition interaction (Fan et al., 2010, Raymaekers et al., 2009). Finally, one study reported differences between typical and ASC participants in the 3–5.5Hz range when observing leg movements (Martineau et al., 2008). Overall, these studies do not present clear evidence for abnormal mu rhythm suppression in individuals with ASC when observing human actions.
4.2. MEG studies
Three studies have examined MEG signals during action observation or imitation in ASC. Two examined the rebound in the beta rhythm (15–25Hz) after median nerve stimulation during the observation of action. The relationship of this signal to the functioning of the mirror system is not known, but it is possible that it is similar to the mu rhythm (Salmelin and Hari, 1994). That is, the signal originates in the sensorimotor cortex and largely reflects the activation of these regions but is not a pure index of mirror system function. When beta rhythm rebound in typical and ASC adults was compared, group differences were found in one case (Honaga et al., 2010) but not another (Avikainen et al., 1999). Thus, there is no consistent evidence for differences here. The final study reported subtle differences in the timing of MEG components during a task requiring imitation of facial postures (Nishitani et al., 2004), with particular differences in IFG. This result suggests only small differences in MNS function. All these MEG studies have used very small sample sizes (5–8 participants) and none showed a conclusive task by group interaction, so the results must all be treated with caution.
4.3. TMS studies
Transcranial magnetic stimulation over human primary motor cortex results in a motor evoked potential (MEP) which can be recorded from muscles in the hand. Several studies have shown that the size of this MEP reflects the underlying excitability of primary motor cortex, and that cortical excitability can be modulated by a number of factors including prior TMS (Fitzgerald et al., 2006), medications (Ziemann, 2004), and motor or cognitive tasks (Rothwell et al., 1991). Of particular relevance here is the finding that MEP size is enhanced when typical participants observe an action compared to a control task (Fadiga et al., 1995, Strafella and Paus, 2001). This is often taken as an index of the function of mirror neuron system though like the mu rhythm it is a weakly localised measure.
Two studies have measured the excitability of primary motor cortex during the observation of action in adults with ASC (Enticott et al., 2012, Théoret et al., 2005). In both studies, motor evoked potentials (MEPs) were recorded from first dorsal interosseous (an index finger muscle) while participants observed hand actions. Theoret and colleagues reported that participants with ASC showed normal MEP enhancement when observing a hand oriented as if it belonged to someone else, but did not show MEP enhancement when the hand was oriented as if it belong to the participant. Enticott and colleagues reported that typical participants showed an MEP enhancement when watching a hand grasp a mug compared to watching a static hand, while participants with ASC did not show this enhancement. Thus, these studies both suggest abnormal excitability of primary motor cortex during action observation in ASC, which might indicate MNS abnormality.
4.4. Eyetracking studies
Recordings of eye movements while participants watch videos of human actions provide a rapid and implicit measure of a participant's interest in and understanding of the video. Typical adults show a distinctive pattern of predictive eye gaze when they perform hand actions, that is, the participant's eyes land on the target object around 200ms before the participant's hand reaches that object. This same pattern of predictive gaze is recorded when participants observe human hand actions, but not when they observe actions with no hand (Flanagan and Johansson, 2003). The matching of predictive eye movements between performed and observed hand actions has been taken as a measure of mirror system function. The close development of predictive gaze for performed and observed actions in infants (Rosander and von Hofsten, 2011) and the modulation of predictive gaze by the state of the motor system (Ambrosini et al., 2012) supports this claim.
Four studies have recorded gaze behaviour when individuals with ASC observe different hand actions. First, Falck-Ytter studied predictive gaze in a paradigm equivalent to Flanagan and Johansson, and found that both typical and autistic children showed the same pattern of predictive gaze (Falck-Ytter, 2009). In a second study, children watched videos where an actor makes eye contact with the camera and then looks or points to a particular object (Falck-Ytter et al., 2012). Participants with ASC looked to the indicated item less, showing that eye tracking measures are sensitive to differences in social gaze following in ASC. Third, Vivanti and colleagues recorded how children with ASC watched videos of meaningful and meaningless actions prior to imitating those actions (Vivanti et al., 2008). They found that participants with ASC spent slightly less time looking at the actor's face and showed worse imitation performance. Both typical and ASC participants showed the same pattern of looking more at the face during the meaningless action trials, and there were no group differences in the time spent looking at the hands. Finally, the same group studied how typical and autistic children respond when watching a variety of videos of action sequences. The pattern of results suggest that children with ASC sometimes looked less to the face of the actor, but showed the same degree of action prediction based on the sequence of hand actions or the rationality of the action (Vivanti et al., 2011).
Comparing across these studies is not straightforward, as all four involved different stimuli and methodologies. However, none of the studies provide evidence for differences in how participants with autism observe hand actions. Group differences only emerge when considering observation of the face or of social cues. While the contribution of the mirror system to eye gaze behaviour is not yet clear, it is much more likely to drive gaze towards the acting hand rather than to faces and other social cues. Thus, normal gaze towards hand actions in autism, and in particular normal predictive gaze, implies that the mirror systems supporting observation of other people's actions are also normal.
4.5. EMG studies
By studying the level and timing of muscle activation during motor tasks, it is possible to make inferences about the information processing underlying the action. Many studies of motor performance in participants with ASC have found evidence of difficulties in this domain (Fournier et al., 2010). The present review focuses only on studies which make claims about the ability of children with ASC to understand or respond to other people's actions, focusing on mirror systems. Most of these studies are based on the idea that some mirror neurons encode simple action sequences or chains (Fogassi et al., 2005), for example, picking up an object and then moving it to one's mouth. When typical individuals perform or observe such sequences, kinematic or EEG components which are required for the final action in the sequence are sometimes seen at an earlier stage, anticipating the final action and reflecting the idea that the different elements in a motor sequence are closely chained together (Rizzolatti and Fabbri-Destro, 2010). Two studies have attempted to measure this action chaining in individuals with ASC using EMG methods.
Cattaneo et al. (2007) recorded EMG from the mylohyoid muscle which opens the mouth while typical and autistic children picked up a small piece of food to eat or place upon their shoulder, or while they watched another person perform the same task. This action involves three components – reaching the food, grasping the food, and placing the food either in the mouth or on the shoulder. They report that typical, but not autistic, children activate the MH muscle during the grasping phase when performing the action, showing anticipation of the reaching phase. Furthermore, the typical children showed MH activity when watching someone else grasp whereas the autistic children did not. Similar results were reported with a task involving placing items in a pedal bin. Cattaneo and colleagues argue that the anticipatory activity of the MH muscle during performance and observation of action provides a measure of the functioning of ‘action chains’ in the parietal mirror system, and that the abnormal activity of this muscle in children with ASC must reflect abnormal mirror systems. However, a recent paper which attempted to replicate this result (Pascolo and Cattarinussi, 2012) found that neither typical nor ASC children showed anticipatory MH activity during performed actions. The authors suggested that differences in the lengths of children's arms and the distance they have to reach might impact on MH engagement in this task. Overall, these studies provide mixed evidence for difficulties in action chaining in children with ASC.
5. fMRI studies of the MNS in autism
The studies reviewed above all use weakly localised measures to examine the possible function of the mirror neuron system in individuals with ASC, which makes it hard to know if the results reflect only the function of the MNS or are influenced by other processes. The cleanest measure of MNS function is functional magnetic resonance imaging, because this method provides clearly localised activations within or outside the human MNS regions. The responses of inferior frontal gyrus (IFG) and inferior parietal lobule (IPL) are particularly important here. Eight studies have now examined the MNS in individuals with ASC using fMRI, and the results are reviewed here.
The first study to show MNS differences in ASC using fMRI examined children who were asked to observe or imitate emotional facial expressions during scanning (Dapretto et al., 2006). Right IFG showed stronger engagement in the typical children than in the autistic children during imitation of emotional facial expressions, while left anterior intraparietal sulcus showed the reverse pattern. Three studies since have examined mirroring responses to emotional stimuli in ASC. Grèzes et al. (2009) showed adults with ASC and typical adults movie of whole body actions displaying neutral or fearful behaviour. Both typical and autistic participants engaged mirror systems in response to the neutral stimuli, but the typical participants showed more activation of inferior frontal gyrus and amygdala in response to the fearful stimuli. The authors conclude that different responses to observation of emotional actions in autism are driven by differences in emotion processing in the amygdala, not by core differences in the mirror system.
A second study of emotion mirroring examined brain responses when participants observe a disgust expression or taste a disgusting taste (Bastiaansen et al., 2011); anterior insula responds in these conditions in typical adults (Wicker et al., 2003). In the participants with ASC, similar responses were seen at the whole group level with no systematic group differences. Using a region of interest analysis, Bastiaansen did find age-related changes in the engagement of right IFG across groups, with lower activation in the youngest participants with autism but no differences in older participants. They conclude that development of MNS function continues over the 18–55 years age range examined in their sample, and that improvement in MNS function in adulthood remains possible. Finally, a study by Schulte-Rüther et al. (2011) examined how typical and autistic participants respond to happy and sad facial expressions when asked to “decide how this person feels” or to “decide how you feel when you look at the face”. Group differences emerged in brain regions associated with theory of mind (mPFC and TPJ) but not in the inferior frontal cortex. In particular, both typical and autistic participants engaged left hemisphere IFG when instructed to attend to their own emotions, and the authors conclude that participants with ASC can engage their MNS when the task demands it. Together, these four studies of mirroring of emotional facial expressions in ASC provide a mixed picture, with only one study suggesting a clear deficit (Dapretto et al., 2006) while the other three show normal responses under neutral or instructed task conditions (Grèzes et al., 2009, Schulte-Rüther et al., 2011) or in some age groups (Bastiaansen et al., 2011). However, interpretation of all these studies is complicated by the use of emotional stimuli which almost certainly engage other brain systems beyond the MNS and cannot provide a pure index of MNS function.
Four studies have examined the integrity of the MNS in ASC using non-emotional tasks. Williams et al. (2006) used a simple finger movement imitation task in which participants observe, execute or imitate index finger movements to examine mirror systems in typical and autistic adults. This task was previously shown to engage IFG in typical adults (Iacoboni et al., 1999). The results from Williams et al. are hard to interpret because, unlike Iacoboni et al., they did not find engagement of IFG in either typical or autistic participants. Subtle differences were found in the responsiveness of occipital regions and part of parietal lobule, which the authors attribute to an abnormality of the parietal MNS. Martineau and colleagues examined observation of action in ASC (Martineau et al., 2010) and found a group by condition interaction, with more activation of the IFG in participants with ASC than in those without. Both groups of participants engaged parietal cortex though the precise regions activated differed slightly. Though these results are not exactly as predicted by the broken mirror hypothesis, the authors interpret them as evidence of atypical MNS engagement in ASC.
Dinstein et al. examined brain responses when participants with ASC observed still images of hand postures or executed those postures (Dinstein et al., 2010). Stimuli were presented in a systematic order to allow the measurement of neural selectivity by repetition suppression. This is based on the finding that when the same stimulus is presented twice in a row, BOLD responses are smaller on the second presentation, and this suppressed response indicates that populations of neurons are sensitive to the specific stimulus characteristics (Grill-Spector et al., 2006, Hamilton and Grafton, 2007). Dinstein and colleagues found that typical and autistic participants showed equivalent BOLD responses and equivalent patterns of repetition suppression across visual and motor brain regions include the aIPS component of the MNS. Marsh and Hamilton (2011) performed a similar study, recording brain responses while typical and autistic adults viewed videos of rational and irrational goal-directed hand actions. They found that both groups engaged aIPS when watching actions and showed repetition suppression in this region when the goal of the action was repeated. Differences between the groups emerged in middle cingulate cortex when observing actions and in medial prefrontal cortex when observing irrational actions. However, there was no evidence in either of these studies for abnormal MNS function in participants with ASC.
Together, these fMRI studies provide little evidence to support a specific and universal impairment of the MNS in ASC. Three studies report group differences in inferior frontal gyrus, and one found an increase in activation in ASC (Martineau et al., 2010) while the other found a decrease (Dapretto et al., 2006) and a third found age-related effects (Bastiaansen et al., 2011). Normal IFG responses were reported by two studies (Grèzes et al., 2009, Schulte-Rüther et al., 2011). Responses in the aIPS component of the MNS were entirely normal in the two studies which examined this region in detail (Dinstein et al., 2010, Marsh and Hamilton, 2011).
Finally, it is worth examining meta-analyses of engagement of the autistic brain in a broader range of tasks. If failure of the MNS is a cause of all social-cognitive difficulties in autism, this should be apparent in a variety of social contexts. A meta-analysis of autism fMRI studies (Di Martino et al., 2009) found consistent differences in medial prefrontal cortex in social tasks but not in any mirror neuron regions. A more recent and larger meta-analysis (Philip et al., 2012) similarly did not report any overall abnormality of MNS regions, in fact, right IFG was more active during complex social tasks in participants with ASC. Thus, fMRI meta-analyses also argue against the broken mirror model.
5.1. Structural MRI
If abnormality of the MNS is a core feature of ASC, one might expect to see structural abnormalities of the IFG and IPL, in particular when cortical thickness and structure is examined in detail. It is beyond the scope of the present review to examine all structural MRI studies of ASC in detail. Two papers report specific differences in mirror system regions in ASC. Yamasaki and colleagues manually traced the pars opercularis region in the structural MRI scans of participants with ASC (Yamasaki et al., 2010). They reported that the volume of right pars opercularis was reduced in ASC and that the size of this region correlated with the participants social and communication abilities. This suggests that MNS structure is related to social skills. Hadjikhani and colleagues examines cortical thickness across the whole brain (Hadjikhani et al., 2006) and found thinning in a number of areas in the ASC brain. These included the IFG, IPL and STS, as well as pre and postcentral gyrus, inferior occipital gyrus, orbitofrontal cortex, prefrontal cortex, middle and inferior temporal gyrus, and superior parietal lobule. These regions include important components of the MNS, but also many other brain regions which contribute to other forms of social cognition.
Contrasting with these two small studies, a voxel-based morphometry study of 65 adults with ASC revealed differences in medial temporal, fusiform and cerebellar regions but not IFG or IPL (Toal et al., 2010). Focusing on cortical thickness, Ecker and colleagues examined structural MRIs to determine what parameters and brain regions best distinguish autistic from typical brains (Ecker et al., 2010). They were able to develop a robust classification algorithm which drew on data from much of the cortical surface, with no particular contribution of MNS regions. A review of brain structure in ASC from 2003 identified differences in parieto-temporal cortex and cerebellum but not IPL or IFG (Brambilla et al., 2003). Similarly, a formal meta-analysis of grey and white matter differences reported in 22 studies found consistent group differences in medial prefrontal cortex, fusiform cortex, cingulate and insula but not in either IFG or IPL (Duerden et al., 2012). Together, these studies suggest that there are many subtle structural differences in the ASC brain, but changes in the MNS regions of IPL and IFG are certainly not the most prominent.
6. Discussion
The papers reviewed above reflect current knowledge of the integrity and function of the mirror neuron system in individuals with autism. Over the 25 studies reviewed, evidence in favour of a dysfunction of the MNS in autism is very limited. To explore the implications of these results, I first summarise the findings from weakly localised measures of MNS function (EEG/MEG/TMS/EMG/eyetracking) separately from more localised measures (fMRI/structural MRI). I then focus on what this means for the broken mirror theory (BMT) and consider possible alternative explanations for reduced imitation and abnormal social skills in ASC.
The weakly localised studies of MNS function in ASC yielded mixed results. Two TMS studies consistently suggested abnormal primary motor cortex excitability, while four eyetracking studies revealed normal behaviour. Of the eight EEG studies, only one reported a reliable group by task interaction and in the remainder evidence for abnormal MNS responses was weak or clearly absent. The three MEG and two EMG studies also showed mixed effects. Overall, this is a very variable dataset with no clear evidence favouring the BMT.
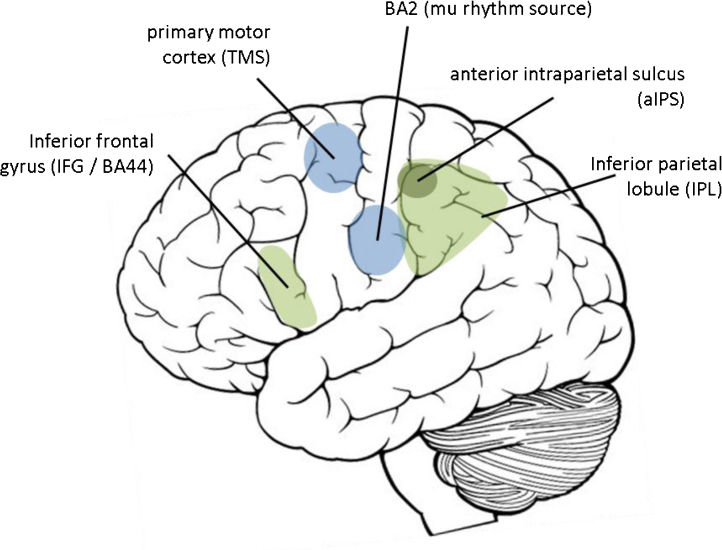
A major limitation of all these neurophysiological measures is that they do not provide precise localisation of the origin of the effects in the brain. This means that they do not provide a pure index of MNS function. The suppression of the mu rhythm in EEG correlates best with activation of the sensorimotor strip (Arnstein et al., 2011) not inferior frontal gyrus, and MEG measures are likely to be similar. TMS studies reveal the excitability of primary motor cortex but not the engagement of the MNS itself (see Fig. 1 for a summary of the relevant brain regions). Several recent studies have shown that activity in the sensorimotor cortex is modulated by social factors other than action observation. Cortical rhythms at 7–12Hz recorded with MEG (similar to the mu rhythm) are modulated by whether the actor looks at the participant or not, rather than by which arm he moves (Kilner et al., 2006). Similarly, MEP size recorded from primary motor cortex after TMS is larger when participants have just engaged in a social interaction than when they have not (Hogeveen and Obhi, 2012). These studies show that the broader social context has a substantial impact on the results obtained using weakly localised measures of ‘mirror neuron function’. It is very hard to equate factors such as level of social interaction and the amount of eye contact between typical and ASC participants, which means that differences between typical and ASC groups in studies using weakly localised measures could be due to extraneous factors, and might not reflect only the integrity of the MNS. Unless the impact of these top-down factors on the MNS can be ruled out (Southgate and Hamilton, 2008), weakly localised measures cannot provide clear evidence for an abnormal MNS in ASC.
Fig. 1.
Diagram of brain regions mentioned in text. The core human mirror neuron system comprises IFG and IPL, including the aIPS which is particularly sensitive to goal-directed actions. Measurements of mu rhythm are most sensitive to activation of BA2 (somatosensory cortex) while TMS measures are sensitive to the excitability of primary motor cortex.
fMRI studies directly measure the BOLD signal in MNS regions of the brain during cognitive tasks, and thus provide a much clearer test of the broken mirror theory. The eight studies reviewed provide a clear pattern. Four studies using emotional stimuli suggest at least some differences between typical and ASC participants, while three of the four studies using non-emotional stimuli did not find clear group differences (and the fourth found differences in the non-predicted direction). These results suggest possible differences in how individuals with ASC process emotional stimuli but not in how they process neutral action stimuli. Two studies which examined the responses of the autistic MNS to observation and performance of symbolic and goal-directed hand actions reported no differences (Dinstein et al.; Marsh and Hamilton). Similarly, structural MRI studies show that subtle differences between typical and autistic brains in structure and connectivity seem to be widely distributed and are not specific to the MNS. Thus, the present review does not provide any reason to believe that dysfunction of the MNS is a primary or universal problem in ASC.
6.1. Evaluating the broken mirror theory
These conclusions have important implications for the broken mirror theory. As described above, there are three slightly different versions of the BMT, so I consider each in turn. First, the imitation version of the BMT claimed that abnormal self-other mapping implemented in the mirror system is a core difficulty in ASC (Williams et al., 2001, Gallese et al., 2009). The data reviewed above provide no reason to support this theory. Behavioural evidence is also inconsistent, as the BMT predicts poor imitation and action understanding in ASC. Several studies report good imitation and understanding of action goals in young children with ASC (Aldridge et al., 2000, Carpenter et al., 2001, Hamilton et al., 2007) and in adults (Bird et al., 2007). This adds to the evidence that the classic, non-emotional MNS is not broken in autism, and suggests that it is time to move on from this theory.
The simulation version of the BMT is broader, encompassing both the classic MNS for hand actions and the broader MNS for emotional responses and claiming that dysfunction of a simulation mechanism in all these systems is present in autism (Dapretto and Iacoboni, 2006, Oberman et al., 2005). The studies reviewed above provide little or no evidence for abnormalities of the classic MNS in autism. There is some data suggesting abnormal responses to emotional stimuli, in particular in the fMRI studies (Dapretto et al., 2006, Grèzes et al., 2009) and this could be used to support the simulation BMT. However, a simulation-BMT which focuses only on emotional actions and does not account for good performance and normal brain activity in non-emotional tasks, loses much of its explanatory power. It cannot appeal to a general principle of simulation because that should apply to both emotional and non-emotional tasks. It then becomes hard to distinguish an emotion-simulation-BMT from other theories of autism that focus on emotion processing, for example the amygdala theory (Baron-Cohen et al., 2000). These factors all mean that a simulation-BMT theory seems unlikely to provide a fundamental explanation of social cognition in autism.
Finally, the chaining version of the BMT is based on the finding of mirror neurons which respond to action sequences (Fogassi et al., 2005), and suggests that the comprehension and production of action sequences is abnormal in ASC (Rizzolatti and Fabbri-Destro, 2010). This is the only version of the BMT which is unaffected by data showing that imitation of simple goal-directed actions (Hamilton et al., 2007) and brain responses to observation of goal-directed actions (Dinstein et al., 2010, Marsh and Hamilton, 2011) are entirely normal. The chaining BMT claims that these simple actions do not require chaining, whereas production and comprehension of more complex action sequences does require chaining and is abnormal in ASC. There are very few tests of this theory so far. Of all the studies reviewed above, only the two using EMG recordings directly address the chaining BMT, and those report contradictory results.
Evidence relevant to the chaining theory can also be found in studies of motor control and action understanding. Individuals with autism often have comorbid dyspraxia or other motor control difficulties (Fournier et al., 2010, Gowen and Hamilton, 2012), and these could be accounted for by difficulties in action chaining. Behavioural studies suggest that children with ASC find it hard to predict the next element in an action sequence. In these studies, the child sees a picture of an action and is asked ‘why is she doing it’ or ‘what is she doing?’. Autistic individuals made more errors on the ‘why’ questions than the ‘what’ questions (Boria et al., 2009) in contrast to individuals with Williams syndrome (Sparaci et al., 2012). It is claimed that understanding why an action is performed requires motor chaining abilities, and that failure to answer the ‘why’ question reflects a failure of action chaining in the MNS. However, these behavioural studies do not provide any way to localise the source of children's poor performance within the MNS (rather than another brain system). Studies of typical adults answering ‘what’ and ‘why’ questions during fMRI show that ‘why’ questions about actions engage mentalising brain regions, not the MNS (Spunt et al., 2010, Spunt et al., 2011). This means that children with ASC might give incorrect answers to the ‘why’ questions because of poor mentalising abilities, not abnormal action chaining abilities.
Overall, these studies provide equivocal evidence about the action chaining theory. The ideal test of the theory would be to have typical and autistic participants perform and observe action sequencing tasks during fMRI scanning. The chaining theory predicts reduced activation of parietal cortex in the participants with autism during this task. Such an experiment has yet to be performed. Even with the current data, there are reasons to be cautious about the action chaining theory. In particular, it is not clear how a specific difficulty in action sequencing could cause the problems with theory of mind which seems to best characterise ASC (Frith, 2012). Further work is necessary to test the action chaining theory and to understand the link between chaining and other types of social cognition if this theory is to be accepted.
6.2. Social response modulation: an alternative explanation
If researchers are to reject the broken mirror theory, it is essential to consider alternative explanations for present data. Many studies have reported reduced imitation frequency (Charman et al., 1997, Ingersoll, 2007) and reduced imitation accuracy (Rogers et al., 1996, Vivanti et al., 2008) in participants with ASC (reviewed in Williams et al., 2004). However, unusual imitation in the form of echolalia and echopraxia is also common in ASC (Grossi et al., 2012). A number of studies have also reported good imitation performance (Aldridge et al., 2000, Bird et al., 2007, Carpenter et al., 2001, Hamilton et al., 2007). Similarly, the neurophysiological studies reviewed above report mixed results, with abnormal brain responses when viewing emotional stimuli (Dapretto et al., 2006, Grèzes et al., 2009) but normal responses when viewing goal-directed actions (Dinstein et al., 2010, Grèzes et al., 2009, Marsh and Hamilton, 2011) (Fig. 2).
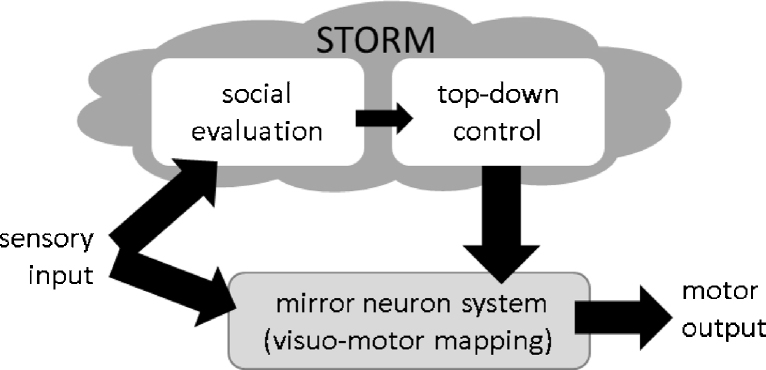
Fig. 2.
The social top-down response modulation (STORM) model. In this model, responses in the MNS to are subject to top-down control based on an evaluation of the current context and social situation. Failure of these top-down control signals could lead to abnormal imitation and abnormal MNS brain responses in autism.
This pattern of mixed results is not easy to reconcile with a standard broken mirror model. However, it is consistent with the social top-down response modulation (STORM) model (Southgate and Hamilton, 2008, Wang and Hamilton, 2012). This model has two core components, a basic visual-to-motor mapping and a top-down modulation system. The visuomotor mapping is implemented in a pathway of connections running from higher-order visual systems through inferior parietal cortex to premotor cortex and then on to motor cortex. In social psychology, this transformation has been termed the perception-behaviour expressway (Dijksterhuis and Bargh, 2001), while in motor control it is described in terms of action specification processes (Cisek and Kalaska, 2010). Within this visuomotor stream, the implementation of imitation responses (or other action responses) is highly dependent on past experience, that is, on learnt associations between visual representations of observed actions and motor representations of performed actions (Heyes, 2010). Such transformations can occur relatively fast and easily, in particular for familiar and practiced actions.
However, the implementation of imitation responses by this system is not inevitable, but is controlled by the demands of the social situation. There are numerous studies showing that imitation responses in typical adults are modulated by social cues. Imitation is enhanced by eye contact (Wang et al., 2010) and when interacting with individuals with high social status or those from an in-group (Lakin and Chartrand, 2003). In terms of neuroanatomy, the visuomotor stream is modulated by action selection processes originating elsewhere. Social signals from mPFC have been shown to modulate the MNS in social contexts (Wang et al., 2011), but it is possible that top-down modulation could also come from other parts of frontal cortex or from subcortical areas. In this way, STORM parallels the specification – selection model proposed for non-social control of movement (Cisek and Kalaska, 2010) and echoes older models of supervisory-attention systems (Norman and Shallice, 1986). The key difference is that STORM focuses particularly on modulation of the visuomotor stream by social cues and social signals.
There is initial evidence that modulation of imitation by social cues is abnormal in ASC. Participants with ASC can imitate actions that produce clear effects on objects (Ingersoll et al., 2003) but imitate less in contexts where imitation is spontaneous (rather than instructed) (Ingersoll, 2007). Whereas typical participants imitate more after priming with prosocial sentences, adults with ASC do not show any change in their mimicry behaviour (Cook and Bird, 2012). This demonstrates failure to modulate mimicry in the presence of normal mimicry responses in ASC. Children with autism similarly show normal automatic imitation but no enhancement of imitation following emotional cues (Grecucci et al., 2012). Finally, an interesting study from Spengler and colleagues shows that engagement of mPFC in a mentalising task in participants with ASC is correlated with these participants’ imitation performance (Spengler et al., 2010). This suggests that theory of mind abilities are linked to the control of imitation, which is important because difficulties in theory of mind are well established in autism (Baron-Cohen et al., 1985, Senju et al., 2009). Data from these initial studies are compatible with the STORM hypothesis.
The STORM model is also compatible with the results reviewed above. Abnormal engagement of the autistic MNS during emotional tasks (Dapretto et al., 2006, Grèzes et al., 2009) can be accounted for by the failure of social/emotional cues to modulate the MNS. Similar failure of top-down emotional modulation in autism has been reported for face processing (Bird et al., 2006). Differences in TMS and EEG responses in participants with autism (Enticott et al., 2012, Oberman et al., 2005) can be accounted for by between group differences in the sensitivity to social cues before and during the experimental session (Hogeveen and Obhi, 2012, Kilner et al., 2006). Reports of similar responses in typical and ASC participants are also coherent with the STORM model. In controlled situations where social cues are minimised, similar MNS responses are predicted in both typical and ASC participants as seen (Dinstein et al., 2010, Fan et al., 2010, Marsh and Hamilton, 2011). The findings that participants with ASC do show mu-suppression when observing familiar but not unfamiliar people (Oberman et al., 2008) can also be accounted for if we assume that people with ASC require stronger social engagement cues that typical individuals, but are not entirely immune to social signals. The only data which the STORM model does not account for are the results suggesting abnormal motor chaining in ASC (Cattaneo et al., 2007). These data may be better understood in terms of the comorbid dyspraxia which is common in ASC (Fournier et al., 2010) but which may be independent of difficulties in social cognition.
Thus, the STORM model is able to give an account of most current data on imitation behaviour and the functioning of the MNS in ASC. Further work is needed to test the theory in more detail. In particular, it is important to determine if individuals with autism fail to modulate imitation because they are not able to pick-up the relevant social cues such as eye contact, or if they fail because they do not implement the appropriate top-down control. It is also important to understand how top-down control of imitation relates to other cognitive domains such as theory of mind and executive function. STORM is congruent with the suggestion that top-down control in general is abnormal or weakened in ASC (Cook et al., 2012, Frith, 2003) and that weak top-down signals might lead to sensory differences in autism (Pellicano and Burr, 2012). Further study of these areas will yield important insights into imitation behaviour in ASC, allowing us to move beyond mirror neurons in our understanding of the social brain.
7. Conclusions and future directions
The data reviewed above demonstrates that despite over a decade of research, the broken mirror theory of autism cannot be supported in its standard form. Interventions based on this theory are unlikely to be helpful. Future studies of imitation and social responding in autism must take into account both the integrity of the MNS and the role of top-down control signals which modulate imitation responses. Understanding the relationship between action sequencing and other aspects of social cognition may also be valuable. Advances in these areas will lead to a better understanding of what is really different about action systems in autism, and will thus lead to more productive interventions to help individuals with autism imitate and interact with the social world.
Acknowledgments
The Hamilton lab receives funding from the Waterloo Foundation and the ESRC. I would like to thank Uta Frith for helpful comments on the manuscript. The author has no conflicts of interest.
References
- Aldridge M.A., Stone K.R., Sweeney M.H., Bower T.G.R. Preverbal children with autism understand the intentions of others. Developmental Science. 2000;3(3):294. [Google Scholar]
- Ambrosini E., Sinigaglia C., Costantini M. Tie my hands, tie my eyes. Journal of Experimental Psychology. Human Perception and Performance. 2012;38(2):263–266. doi: 10.1037/a0026570. [DOI] [PubMed] [Google Scholar]
- Arnstein D., Cui F., Keysers C., Maurits N.M., Gazzola V. {micro}-Suppression during action observation and execution correlates with BOLD in dorsal premotor, inferior parietal, and SI cortices. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2011;31(40):14243–14249. doi: 10.1523/JNEUROSCI.0963-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avikainen S., Kulomaki T., Hari R. Normal movement reading in Asperger subjects. Neuroreport. 1999;10(17):3467–3470. doi: 10.1097/00001756-199911260-00001. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S., Leslie A.M., Frith U. Does the autistic child have a theory of mind? Cognition. 1985;21(1):37–46. doi: 10.1016/0010-0277(85)90022-8. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S., Ring H.A., Bullmore E.T., Wheelwright S., Ashwin C., Williams S.C. The amygdala theory of autism. Neuroscience and Biobehavioral Reviews. 2000;24(3):355–364. doi: 10.1016/s0149-7634(00)00011-7. [DOI] [PubMed] [Google Scholar]
- Bastiaansen J.A., Thioux M., Nanetti L., van der Gaag C., Ketelaars C., Minderaa R., Keysers C. Age-related increase in inferior frontal gyrus activity and social functioning in autism spectrum disorder. Biological Psychiatry. 2011;69(9):832–838. doi: 10.1016/j.biopsych.2010.11.007. Society of Biological Psychiatry. [DOI] [PubMed] [Google Scholar]
- Bernier R., Dawson G., Webb S., Murias M. EEG mu rhythm and imitation impairments in individuals with autism spectrum disorder. Brain and Cognition. 2007;64(3):228–237. doi: 10.1016/j.bandc.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird G., Catmur C., Silani G., Frith C.D., Frith U. Attention does not modulate neural responses to social stimuli in autism spectrum disorders. NeuroImage. 2006;31(4):1614–1624. doi: 10.1016/j.neuroimage.2006.02.037. [DOI] [PubMed] [Google Scholar]
- Bird G., Leighton J., Press C., Heyes C. Intact automatic imitation of human and robot actions in autism spectrum disorders. Proceedings of the Royal Society B: Biological Sciences. 2007;274(1628):3027–3031. doi: 10.1098/rspb.2007.1019. doi: B717X703LR287633 [pii] 10.1098/rspb.2007.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boria S., Fabbri-Destro M., Cattaneo L., Sparaci L., Sinigaglia C., Santelli E., Rizzolatti G. Intention understanding in autism. PLoS One. 2009;4(5):e5596. doi: 10.1371/journal.pone.0005596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brambilla P., Hardan A., di Nemi S.U., Perez J., Soares J.C., Barale F. Brain anatomy and development in autism: review of structural MRI studies. Brain Research Bulletin. 2003;61(6):557–569. doi: 10.1016/j.brainresbull.2003.06.001. [DOI] [PubMed] [Google Scholar]
- Buccino G., Vogt S., Ritzl A., Fink G.R., Zilles K., Freund H., Rizzolatti G. Neural circuits underlying imitation learning of hand actions: an event-related fMRI study. Neuron. 2004;42(2):323–334. doi: 10.1016/s0896-6273(04)00181-3. [DOI] [PubMed] [Google Scholar]
- Carpenter M., Pennington B.F., Rogers S.J. Understanding of others’ intentions in children with autism. Journal of Autism and Developmental Disorders. 2001;31(6):589–599. doi: 10.1023/a:1013251112392. [DOI] [PubMed] [Google Scholar]
- Caspers S., Zilles K., Laird A.R., Eickhoff S.B. ALE meta-analysis of action observation and imitation in the human brain. NeuroImage. 2010;50(3):1148–1167. doi: 10.1016/j.neuroimage.2009.12.112. Elsevier B.V. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo L., Fabbri-Destro M., Boria S., Pieraccini C., Monti A., Rizzolatti G., Cossu G. Impairment of actions chains in autism and its possible role in intention understanding. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(45):17825–17830. doi: 10.1073/pnas.0706273104. doi:0706273104 [pii] 10.1073/pnas.0706273104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charman T., Swettenham J., Baron-Cohen S., Cox A., Baird G., Drew A. Infants with autism: an investigation of empathy, pretend play, joint attention, and imitation. Developmental Psychology. 1997;33(5):781–789. doi: 10.1037//0012-1649.33.5.781. [DOI] [PubMed] [Google Scholar]
- Chevallier C., Kohls G., Troiani V., Brodkin E.S., Schultz R.T. The social motivation theory of autism. Trends in Cognitive Sciences. 2012;16(4):231–239. doi: 10.1016/j.tics.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisek P., Kalaska J.F. Neural mechanisms for interacting with a world full of action choices. Annual Review of Neuroscience. 2010;33:269–298. doi: 10.1146/annurev.neuro.051508.135409. [DOI] [PubMed] [Google Scholar]
- Cook J., Barbalat G., Blakemore S.-J. Top-down modulation of the perception of other people in schizophrenia and autism. Frontiers in Human Neuroscience. 2012;6(June):175. doi: 10.3389/fnhum.2012.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook J., Bird G. Atypical social modulation of imitation in autism spectrum conditions. Journal of Autism and Developmental Disorders. 2012;42(6):1045–1051. doi: 10.1007/s10803-011-1341-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dapretto M., Davies M.S., Pfeifer J.H., Scott A.A., Sigman M., Bookheimer S.Y., Iacoboni M. Understanding emotions in others: mirror neuron dysfunction in children with autism spectrum disorders. Nature Neuroscience. 2006;9(1):28–30. doi: 10.1038/nn1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dapretto M., Iacoboni M. The mirror neuron system and the consequences of its dysfunction. Nature Reviews Neuroscience. 2006;7(12):942–951. doi: 10.1038/nrn2024. [DOI] [PubMed] [Google Scholar]
- Dijksterhuis A., Bargh J.A. The perception-behavior expressway: automatic effects of social perception on social behavior. Advances in Experimental Social Psychology. 2001;33:1–40. [Google Scholar]
- Di Martino A., Ross K., Uddin L.Q., Sklar A.B., Castellanos F.X., Milham M.P. Functional brain correlates of social and nonsocial processes in autism spectrum disorders: an activation likelihood estimation meta-analysis. Biological psychiatry. 2009;65(1):63–74. doi: 10.1016/j.biopsych.2008.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinstein I., Thomas C., Behrmann M., Heeger D. A mirror up to nature. Current Biology. 2008:3–8. doi: 10.1016/j.cub.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinstein I., Thomas C., Humphreys K., Minshew N.J., Behrmann M., Heeger D.J. Normal movement selectivity in autism. Neuron. 2010;66(3):461–469. doi: 10.1016/j.neuron.2010.03.034. Elsevier Ltd. doi: S0896-6273(10)00237-0 [pii] 10.1016/j.neuron.2010.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duerden E.G., Mak-Fan K.M., Taylor M.J., Roberts S.W. Regional differences in grey and white matter in children and adults with autism spectrum disorders: an activation likelihood estimate (ALE) meta-analysis. Autism Research: Official Journal of the International Society for Autism Research. 2012;5(1):49–66. doi: 10.1002/aur.235. [DOI] [PubMed] [Google Scholar]
- Ecker C., Marquand A., Mourao-Miranda J., Johnston P., Daly E.M., Brammer M.J., Maltezos S. Describing the brain in autism in five dimensions – magnetic resonance imaging-assisted diagnosis of autism spectrum disorder using a multiparameter classification approach. Journal of Neuroscience. 2010;30(32):10612–10623. doi: 10.1523/JNEUROSCI.5413-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enticott P.G., Kennedy H.A., Rinehart N., Tonge B.J., Bradshaw J.L., Taffe J.R., Daskalakis Z.J. Mirror neuron activity associated with social impairments but not age in autism spectrum disorder. Biological Psychiatry. Society of Biological Psychiatry. 2012;71(5):427–433. doi: 10.1016/j.biopsych.2011.09.001. [DOI] [PubMed] [Google Scholar]
- Fadiga L., Fogassi L., Pavesi G., Rizzolatti G. Motor facilitation during action observation: a magnetic stimulation study. Journal of neurophysiology. 1995;73(6):2608–2611. doi: 10.1152/jn.1995.73.6.2608. [DOI] [PubMed] [Google Scholar]
- Falck-Ytter T. Young children with autism spectrum disorder use predictive eye movements in action observation. Biology Letters. 2009;6(3):375–378. doi: 10.1098/rsbl.2009.0897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falck-Ytter T., Fernell E., Hedvall A.L., von Hofsten C., Gillberg C. Gaze performance in children with autism spectrum disorder when observing communicative actions. Journal of Autism and Developmental Disorders. 2012;42(10):2236–2245. doi: 10.1007/s10803-012-1471-6. [DOI] [PubMed] [Google Scholar]
- Fan Y.-T.T., Decety J., Yang C.-Y.Y., Liu J.-L.L., Cheng Y. Unbroken mirror neurons in autism spectrum disorders. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2010;51(9):981–988. doi: 10.1111/j.1469-7610.2010.02269.x. doi:JCPP2269 [pii] 10.1111/j.1469-7610.2010.02269.x. [DOI] [PubMed] [Google Scholar]
- Fitzgerald P.B., Fountain S., Daskalakis Z.J. A comprehensive review of the effects of rTMS on motor cortical excitability and inhibition. Clinical Neurophysiology: Official Journal of the International Federation of Clinical Neurophysiology. 2006;117(12):2584–2596. doi: 10.1016/j.clinph.2006.06.712. [DOI] [PubMed] [Google Scholar]
- Flanagan J.R., Johansson R.S. Action plans used in action observation. Nature. 2003;424(6950):769–771. doi: 10.1038/nature01861. [DOI] [PubMed] [Google Scholar]
- Fogassi L., Ferrari P.F., Gesierich B., Rozzi S., Chersi F., Rizzolatti G. Parietal lobe: from action organization to intention understanding. Science (New York, NY) 2005;308(5722):662–667. doi: 10.1126/science.1106138. [DOI] [PubMed] [Google Scholar]
- Fournier K.A., Hass C.J., Naik S.K., Lodha N., Cauraugh J.H. Motor coordination in autism spectrum disorders: a synthesis and meta-analysis. Journal of Autism and Developmental Disorders. 2010;40(10):1227–1240. doi: 10.1007/s10803-010-0981-3. [DOI] [PubMed] [Google Scholar]
- Frith C. Novartis Foundation Symposium, vol. 251. 2003. What do imaging studies tell us about the neural basis of autism? pp. 149–166. discussion 166–176, 281–297. [PubMed] [Google Scholar]
- Frith U. Mind blindness and the brain in autism. Neuron. 2001;32(6):969–979. doi: 10.1016/s0896-6273(01)00552-9. [DOI] [PubMed] [Google Scholar]
- Frith U. Why we need cognitive explanations of autism. Quarterly Journal of Experimental Psychology (2006) 2012;(September):37–41. doi: 10.1080/17470218.2012.697178. [DOI] [PubMed] [Google Scholar]
- Gallese V. Before and below theory of mind: embodied simulation and the neural correlates of social cognition. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences. 2007;362(1480):659–669. doi: 10.1098/rstb.2006.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallese V. Mirror neurons and the social nature of language: the neural exploitation hypothesis. Social Neuroscience. 2008;3(3–4):317–333. doi: 10.1080/17470910701563608. [DOI] [PubMed] [Google Scholar]
- Gallese V., Fadiga L., Fogassi L., Rizzolatti G. Action recognition in the premotor cortex. Brain. 1996;119(Pt 2):593–609. doi: 10.1093/brain/119.2.593. [DOI] [PubMed] [Google Scholar]
- Gallese V., Rochat M., Cossu G., Sinigaglia C. Motor cognition and its role in the phylogeny and ontogeny of action understanding. Developmental Psychology. 2009;45(1):103–113. doi: 10.1037/a0014436. [DOI] [PubMed] [Google Scholar]
- Gowen E., Hamilton A. Motor abilities in autism: a review using a computational context. Journal of Autism and Developmental Disorders. 2012 doi: 10.1007/s10803-012-1574-0. [DOI] [PubMed] [Google Scholar]
- Grecucci A., Brambilla P., Siugzdaite R., Londero D., Fabbro F., Rumiati R.I. Emotional resonance deficits in autistic children. Journal of Autism and Developmental Disorders. 2012 doi: 10.1007/s10803-012-1603-z. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K., Henson R., Martin A. Repetition and the brain: neural models of stimulus-specific effects. Trends in Cognitive Sciences. 2006;10(1):14–23. doi: 10.1016/j.tics.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Grossi D., Marcone R., Cinquegrana T., Gallucci M. On the differential nature of induced and incidental echolalia in autism. Journal of Intellectual Disability Research: JIDR. 2012:1–10. doi: 10.1111/j.1365-2788.2012.01579.x. [DOI] [PubMed] [Google Scholar]
- Grèzes J., Wicker B., Berthoz S., de Gelder B., Grezes J. A failure to grasp the affective meaning of actions in autism spectrum disorder subjects. Neuropsychologia. 2009;47(8–9):1816–1825. doi: 10.1016/j.neuropsychologia.2009.02.021. [DOI] [PubMed] [Google Scholar]
- Hadjikhani N., Joseph R.M., Snyder J., Tager-Flusberg H., Tager Flusberg H. Anatomical differences in the mirror neuron system and social cognition network in autism. Cerebral Cortex. 2006;16(9):1276–1282. doi: 10.1093/cercor/bhj069. [DOI] [PubMed] [Google Scholar]
- Hamilton A.F.d.C. Emulation and mimicry for social interaction: a theoretical approach to imitation in autism. Quarterly Journal of Experimental Psychology (2006) 2008;61(1):101–115. doi: 10.1080/17470210701508798. [DOI] [PubMed] [Google Scholar]
- Hamilton A.F.d.C., Brindley R.M., Frith U. Imitation and action understanding in autistic spectrum disorders: how valid is the hypothesis of a deficit in the mirror neuron system? Neuropsychologia. 2007;45(8):1859–1868. doi: 10.1016/j.neuropsychologia.2006.11.022. [DOI] [PubMed] [Google Scholar]
- Hamilton A.F.d.C., Grafton S.S. The motor hierarchy: from kinematics to goals and intentions. In: Haggard P., Rosetti Y., Kawato M., editors. Attention and Performance, vol. XXII. Oxford University Press; Oxford, UK: 2007. [Google Scholar]
- Heyes C. Where do mirror neurons come from? Neuroscience and Biobehavioral Reviews. 2010;34(4):575–583. doi: 10.1016/j.neubiorev.2009.11.007. Elsevier Ltd. [DOI] [PubMed] [Google Scholar]
- Hickok G. Eight problems for the mirror neuron theory of action understanding in monkeys and humans. Journal of Cognitive Neuroscience. 2009;21(7):1229–1243. doi: 10.1162/jocn.2009.21189. doi:10.1162/jocn.2009.21189 10.1162/jocn.2009.21189 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogeveen J., Obhi S.S. Social interaction enhances motor resonance for observed human actions. Journal of Neuroscience. 2012;32(17):5984–5989. doi: 10.1523/JNEUROSCI.5938-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honaga E., Ishii R., Kurimoto R., Canuet L., Ikezawa K., Takahashi H., Nakahachi T. Post-movement beta rebound abnormality as indicator of mirror neuron system dysfunction in autistic spectrum disorder: an MEG study. Neuroscience Letters. 2010;478(3):141–145. doi: 10.1016/j.neulet.2010.05.004. Elsevier Ireland Ltd. [DOI] [PubMed] [Google Scholar]
- Iacoboni M., Woods R.P., Brass M., Bekkering H., Mazziotta J.C., Rizzolatti G. Cortical mechanisms of human imitation. Science. 1999;286(5449):2526–2528. doi: 10.1126/science.286.5449.2526. [DOI] [PubMed] [Google Scholar]
- Ingersoll B. The effect of context on imitation skills in children with autism. Research in Autism Spectrum Disorders. 2007;2(2):332–340. [Google Scholar]
- Ingersoll B., Schreibman L., Tran Q.H. Effect of sensory feedback on immediate object imitation in children with autism. Journal of Autism and Developmental Disorders. 2003;33(6):673–683. doi: 10.1023/b:jadd.0000006003.26667.f8. [DOI] [PubMed] [Google Scholar]
- Kana R.K., Wadsworth H.M., Travers B.G. A systems level analysis of the mirror neuron hypothesis and imitation impairments in autism spectrum disorders. Neuroscience and Biobehavioral Reviews. 2010;35(3):1–9. doi: 10.1016/j.neubiorev.2010.10.007. Elsevier Ltd. [DOI] [PubMed] [Google Scholar]
- Keysers C., Kaas J.H., Gazzola V. Somatosensation in social perception. Nature Reviews. Neuroscience. 2010;11(6):417–428. doi: 10.1038/nrn2833. Nature Publishing Group. [DOI] [PubMed] [Google Scholar]
- Kilner J.M., Marchant J.L., Frith C.D. Modulation of the mirror system by social relevance. Social Cognitive and Affective Neuroscience. 2006;1(2):143–148. doi: 10.1093/scan/nsl017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilner J.M., Neal A., Weiskopf N., Friston K.J., Frith C.D. Evidence of mirror neurons in human inferior frontal gyrus. Journal of Neuroscience. 2009;29(32):10153–10159. doi: 10.1523/JNEUROSCI.2668-09.2009. doi:29/32/10153 [pii] 10.1523/JNEUROSCI.2668-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegeskorte N., Simmons W.K., Bellgowan P.S.F., Baker C.I. Circular analysis in systems neuroscience: the dangers of double dipping. Nature Neuroscience. 2009;12(5):535–540. doi: 10.1038/nn.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakin J.L., Chartrand T.L. Using nonconscious behavioral mimicry to create affiliation and rapport. Psychological Science. 2003;14(4):334–339. doi: 10.1111/1467-9280.14481. [DOI] [PubMed] [Google Scholar]
- Marsh L., Hamilton A.F.d.C. Dissociation of mirroring and mentalising systems in autism. NeuroImage. 2011;56(3):1511–1519. doi: 10.1016/j.neuroimage.2011.02.003. [DOI] [PubMed] [Google Scholar]
- Martineau J., Andersson F., Barthélémy C., Cottier J.-P.P., Destrieux C. Atypical activation of the mirror neuron system during perception of hand motion in autism. Brain Research. 2010;1320:168–175. doi: 10.1016/j.brainres.2010.01.035. Elsevier B.V. doi: S0006-8993(10)00105-8 [pii] 10.1016/j.brainres.2010.01.035. [DOI] [PubMed] [Google Scholar]
- Martineau J., Cochin S., Barthélémy C., Magne R. Impaired cortical activation in autistic children: is the mirror neuron system involved? International Journal of Psychophysiology: Official Journal of the International Organization of Psychophysiology. 2008;68(1):35–40. doi: 10.1016/j.ijpsycho.2008.01.002. [DOI] [PubMed] [Google Scholar]
- McCabe D.P., Castel A.D. Seeing is believing: the effect of brain images on judgments of scientific reasoning. Cognition. 2008;107(1):343–352. doi: 10.1016/j.cognition.2007.07.017. [DOI] [PubMed] [Google Scholar]
- Michael J. Four models of the functional contribution of mirror systems. Philosophical Explorations. 2011;14(2):185–194. [Google Scholar]
- Muthukumaraswamy S.D., Johnson B.W., McNair N.A. Mu rhythm modulation during observation of an object-directed grasp. Brain Research. Cognitive Brain Research. 2004;19(2):195–201. doi: 10.1016/j.cogbrainres.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S., Forstmann B.U., Wagenmakers E.-J. Erroneous analyses of interactions in neuroscience: a problem of significance. Nature Neuroscience. 2011;14(9):1105–1107. doi: 10.1038/nn.2886. Nature Publishing Group. [DOI] [PubMed] [Google Scholar]
- Nishitani N., Avikainen S., Hari R. Abnormal imitation-related cortical activation sequences in Asperger's syndrome. Annals of Neurology. 2004;55(4):558–562. doi: 10.1002/ana.20031. [DOI] [PubMed] [Google Scholar]
- Norman D.A., Shallice T. Attention to action: Willed and automatic control of behaviour. In: Davidson R.J., Schwartz G.E., Shapiro D., editors. Consciousness and Self-regulation: Vol. 4. Advances in Research and Theory. Plenum Press; New York: 1986. pp. 1–18. [Google Scholar]
- Oberman L.M., Hubbard E.M., McCleery J.P., Altschuler E.L., Ramachandran V.S., Pineda J.A. EEG evidence for mirror neuron dysfunction in autism spectrum disorders. Brain Research. Cognitive Brain Research. 2005;24(2):190–198. doi: 10.1016/j.cogbrainres.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Oberman L.M., Ramachandran V.S. The simulating social mind: the role of the mirror neuron system and simulation in the social and communicative deficits of autism spectrum disorders. Psychological bulletin. 2007;133(2):310–327. doi: 10.1037/0033-2909.133.2.310. [DOI] [PubMed] [Google Scholar]
- Oberman L.M., Ramachandran V.S., Pineda J.A. Modulation of mu suppression in children with autism spectrum disorders in response to familiar or unfamiliar stimuli: the mirror neuron hypothesis. Neuropsychologia. 2008;46(5):1558–1565. doi: 10.1016/j.neuropsychologia.2008.01.010. [DOI] [PubMed] [Google Scholar]
- Oosterhof N.N., Wiggett A.J., Diedrichsen J., Tipper S.P., Downing P.E. Surface-based information mapping reveals crossmodal vision-action representations in human parietal and occipitotemporal cortex. Journal of Neurophysiology. 2010;104(2):1077–1089. doi: 10.1152/jn.00326.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascolo P.B., Cattarinussi A. On the relationship between mouth opening and broken mirror neurons in autistic individuals. Journal of Electromyography and Kinesiology: Official Journal of the International Society of Electrophysiological Kinesiology. 2012;22(1):98–102. doi: 10.1016/j.jelekin.2011.07.009. [DOI] [PubMed] [Google Scholar]
- Pellicano E., Burr D. When the world becomes too real: a Bayesian explanation of autistic perception. Trends in Cognitive Sciences. 2012;16(10):504–510. doi: 10.1016/j.tics.2012.08.009. [DOI] [PubMed] [Google Scholar]
- Perkins T., Stokes M., McGillivray J., Bittar R. Mirror neuron dysfunction in autism spectrum disorders. Journal of Clinical Neuroscience: Official Journal of the Neurosurgical Society of Australasia. 2010;17(10):1239–1243. doi: 10.1016/j.jocn.2010.01.026. Elsevier Ltd. [DOI] [PubMed] [Google Scholar]
- Philip R.C.M., Dauvermann M.R., Whalley H.C., Baynham K., Lawrie S.M., Stanfield A.C. A systematic review and meta-analysis of the fMRI investigation of autism spectrum disorders. Neuroscience and Biobehavioral Reviews. 2012;36(2):901–942. doi: 10.1016/j.neubiorev.2011.10.008. [DOI] [PubMed] [Google Scholar]
- Raymaekers R., Wiersema J.R., Roeyers H. EEG study of the mirror neuron system in children with high functioning autism. Brain Research. 2009;1304:113–121. doi: 10.1016/j.brainres.2009.09.068. Elsevier B.V. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G., Craighero L. The mirror-neuron system. Annual Review of Neuroscience. 2004;27:169–192. doi: 10.1146/annurev.neuro.27.070203.144230. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G., Fabbri-Destro M. Mirror neurons: from discovery to autism. Experimental Brain Research. Experimentelle Hirnforschung. Expérimentation Cérébrale. 2010;200(3–4):223–237. doi: 10.1007/s00221-009-2002-3. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G., Fabbri-destro M., Cattaneo L. Mirror neurons and their clinical relevance. Nature Clinical Practice. Neurology. 2009;5(1):24–34. doi: 10.1038/ncpneuro0990. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G., Sinigaglia C. The functional role of the parieto-frontal mirror circuit: interpretations and misinterpretations. Nature Reviews Neuroscience. 2010;11(4):264–274. doi: 10.1038/nrn2805. Nature Publishing Group. doi:nrn2805 [pii] 10.1038/nrn2805. [DOI] [PubMed] [Google Scholar]
- Rogers S.J., Bennetto L., McEvoy R., Pennington B.F. Imitation and pantomime in high-functioning adolescents with autism spectrum disorders. Child Development. 1996;67(5):2060–2073. [PubMed] [Google Scholar]
- Rogers S.J., Pennington B.F. A theoretical approach to the deficits in infantile autism. Development and Psychopathology. 1991;3:137–162. [Google Scholar]
- Rosander K., von Hofsten C. Predictive gaze shifts elicited during observed and performed actions in 10-month-old infants and adults. Neuropsychologia. 2011;49(10):2911–2917. doi: 10.1016/j.neuropsychologia.2011.06.018. Elsevier Ltd. [DOI] [PubMed] [Google Scholar]
- Rothwell J.C., Thompson P.D., Day B.L., Boyd S., Marsden C.D. Stimulation of the human motor cortex through the scalp. Experimental physiology. 1991;76(2):159–200. doi: 10.1113/expphysiol.1991.sp003485. [DOI] [PubMed] [Google Scholar]
- Salmelin R., Hari R. Spatiotemporal characteristics of sensorimotor neuromagnetic rhythms related to thumb movement. Neuroscience. 1994;60(2):537–550. doi: 10.1016/0306-4522(94)90263-1. [DOI] [PubMed] [Google Scholar]
- Schulte-Rüther M., Greimel E., Markowitsch H.J., Kamp-Becker I., Remschmidt H., Fink G.R., Piefke M. Dysfunctions in brain networks supporting empathy: an fMRI study in adults with autism spectrum disorders. Social Neuroscience. 2011;6(1):1–21. doi: 10.1080/17470911003708032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senju A., Southgate V., White S.J., Frith U. Mindblind eyes: an absence of spontaneous theory of mind in Asperger syndrome. Science (New York, NY) 2009;325(5942):883–885. doi: 10.1126/science.1176170. [DOI] [PubMed] [Google Scholar]
- Southgate V., Hamilton A.F.d.C. Unbroken mirrors: challenging a theory of autism. Trends in Cognitive Sciences. 2008;12(6):225–229. doi: 10.1016/j.tics.2008.03.005. [DOI] [PubMed] [Google Scholar]
- Sparaci L., Stefanini S., Marotta L., Vicari S., Rizzolatti G. Understanding motor acts and motor intentions in Williams Syndrome. Neuropsychologia. 2012;50(7):1639–1649. doi: 10.1016/j.neuropsychologia.2012.03.019. Elsevier Ltd. [DOI] [PubMed] [Google Scholar]
- Spengler S., Bird G., Brass M. Hyperimitation of actions is related to reduced understanding of others’ minds in autism spectrum conditions. Biological Psychiatry. 2010;68(12):1148–1155. doi: 10.1016/j.biopsych.2010.09.017. Elsevier Inc. [DOI] [PubMed] [Google Scholar]
- Spunt R.P., Falk E.B., Lieberman M.D. Dissociable neural systems support retrieval of how and why action knowledge. Psychological Science. 2010;21(11):1593–1598. doi: 10.1177/0956797610386618. [DOI] [PubMed] [Google Scholar]
- Spunt R.P., Satpute A.B., Lieberman M.D. Identifying the what, why, and how of an observed action: an fMRI study of mentalizing and mechanizing during action observation. Journal of Cognitive Neuroscience. 2011;23(1):63–74. doi: 10.1162/jocn.2010.21446. [DOI] [PubMed] [Google Scholar]
- Strafella A.P., Paus T. Cerebral blood-flow changes induced by paired-pulse transcranial magnetic stimulation of the primary motor cortex. Journal of Neurophysiology. 2001;85(6):2624–2629. doi: 10.1152/jn.2001.85.6.2624. [DOI] [PubMed] [Google Scholar]
- Théoret H., Halligan E., Kobayashi M., Fregni F., Tager Flusberg H., Pascual-Leone A., Theoret H. Impaired motor facilitation during action observation in individuals with autism spectrum disorder. Current Biology. 2005;15(3):R84–R85. doi: 10.1016/j.cub.2005.01.022. [DOI] [PubMed] [Google Scholar]
- Toal F., Daly E.M., Page L., Deeley Q., Hallahan B., Bloemen O., Cutter W.J. Clinical and anatomical heterogeneity in autistic spectrum disorder: a structural MRI study. Psychological Medicine. 2010;40(7):1171–1181. doi: 10.1017/S0033291709991541. [DOI] [PubMed] [Google Scholar]
- Vivanti G., McCormick C., Young G.S., Abucayan F., Hatt N., Nadig A., Ozonoff S. Intact and impaired mechanisms of action understanding in autism. Developmental Psychology. 2011;47(3):841–856. doi: 10.1037/a0023105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivanti G., Nadig A., Rogers S.J., Ozonoff S. What do children with autism attend to during imitation tasks? Journal of Experimental Child Psychology. 2008;101(3):186–205. doi: 10.1016/j.jecp.2008.04.008. Elsevier Inc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan C.Y., Demaine K., Zipse L., Norton A., Schlaug G. From music making to speaking: engaging the mirror neuron system in autism. Brain Research Bulletin. 2010;82(3–4):161–168. doi: 10.1016/j.brainresbull.2010.04.010. Elsevier Inc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Hamilton A.F.D.C. Social top-down response modulation (STORM): a model of the control of mimicry in social interaction. Frontiers in Human Neuroscience. 2012;6(June):1–10. doi: 10.3389/fnhum.2012.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Newport R., Hamilton A.F.d.C. Eye contact enhances mimicry of intransitive hand movements. Biology Letters. 2010;7(1):7–10. doi: 10.1098/rsbl.2010.0279. doi:rsbl.2010.0279 [pii] 10.1098/rsbl.2010.0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Ramsey R., Hamilton A.F.d.C. The control of mimicry by eye contact is mediated by medial prefrontal cortex. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2011;31(33):12001–12010. doi: 10.1523/JNEUROSCI.0845-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisberg D.S., Keil F.C., Goodstein J., Rawson E., Gray J.R. The seductive allure of neuroscience explanations. Journal of Cognitive Neuroscience. 2008;20(3):470–477. doi: 10.1162/jocn.2008.20040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicker B., Keysers C., Plailly J., Royet J.P., Gallese V., Rizzolatti G. Both of us disgusted in My insula: the common neural basis of seeing and feeling disgust. Neuron. 2003;40(3):655–664. doi: 10.1016/s0896-6273(03)00679-2. [DOI] [PubMed] [Google Scholar]
- Williams J.H.G., Waiter G.D., Gilchrist A., Perrett D.I., Murray A.D., Whiten A. Neural mechanisms of imitation and mirror neuron functioning in autistic spectrum disorder. Neuropsychologia. 2006;44(4):610–621. doi: 10.1016/j.neuropsychologia.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Williams J.H.G., Whiten A., Singh T. A systematic review of action imitation in autistic spectrum disorder. Journal of Autism and Developmental Disorders. 2004;34(3):285–299. doi: 10.1023/b:jadd.0000029551.56735.3a. [DOI] [PubMed] [Google Scholar]
- Williams J.H.G., Whiten A., Suddendorf T., Perrett D.I. Imitation, mirror neurons and autism. Neuroscience and Biobehavioral Reviews. 2001;25(4):287–295. doi: 10.1016/s0149-7634(01)00014-8. [DOI] [PubMed] [Google Scholar]
- Yamasaki S., Yamasue H., Abe O., Suga M., Yamada H., Inoue H., Kuwabara H. Reduced gray matter volume of pars opercularis is associated with impaired social communication in high-functioning autism spectrum disorders. Biological Psychiatry. 2010;68(12):1141–1147. doi: 10.1016/j.biopsych.2010.07.012. Elsevier Inc. [DOI] [PubMed] [Google Scholar]
- Ziemann U. TMS and drugs. Clinical Neurophysiology: Official Journal of the International Federation of Clinical Neurophysiology. 2004;115(8):1717–1729. doi: 10.1016/j.clinph.2004.03.006. [DOI] [PubMed] [Google Scholar]