Abstract
Zebrafish (Danio rerio) are highly social animals that engage in a diverse variety of nonreproductive social behaviors that emerge as early as 14 days postfertilization (dpf). However, we observe considerable behavioral variability at this stage, and comparisons across studies are potentially complicated both by chronological gaps in measurements and inconsistencies in developmental staging. To address these issues, we adapted our assay for social orienting and cueing in the adult zebrafish and used it to probe behavior in a critical window of larval development. In addition, we performed measurements of body length and tested a cohort of larvae with impaired growth to understand if this morphological feature is predictive of individual sociality. We report that zebrafish exhibit increasingly complex social behaviors between 10 and 16 dpf, including place preference, orienting, and social cueing. Furthermore, social behavior is related to standard length on an individual basis beginning at 14 dpf, such that developmentally stunted 14 dpf zebrafish raised on dry feed do not exhibit social behaviors, suggesting some morphological features are more predictive than chronological age. This highly variable and early stage in development provides an opportunity to further understand how genetic and environmental factors affect the assembly of neural circuits underlying complex behaviors.
Keywords: zebrafish, collective behavior, development, ontogeny, feeding
Introduction
Zebrafish (Danio rerio) are highly social animals that engage in a diverse variety of nonreproductive social behaviors. An increasingly extensive literature describes many of these behaviors in depth.1–3 The experimental tractability and the genetic similarity between zebrafish and humans renders zebrafish an attractive model for neurodevelopmental disorders that affect social interactions.4 Social behaviors in the zebrafish share many similarities with other vertebrates, including mammals, and some of the brain regions driving these behaviors may be evolutionarily conserved.5–9 Attraction toward conspecifics is proposed to begin as early as 7 days postfertilization (dpf) and interactions rapidly increase in complexity. However, how they eventually develop into orienting routines, reciprocal interactions driven by social cues, and experience-dependent preferences for shoalmates with specific visual characteristics had not yet been investigated.10–15
A full description of social development in zebrafish will be useful for understanding how early genetic and environmental perturbations affect behavioral outcomes.16 Several groups have studied social ontogeny, or the developmental stages at which zebrafish begin to reliably engage in these behaviors. By 14 dpf, zebrafish begin to exhibit features of adult social interactions and these are robust by ∼21 dpf. These findings are consistent across different experimental paradigms, including open field contexts where fish are able to interact freely, physically separated animals where the social stimulus is purely visual, and virtual stimuli that mimic biological motion.11,13,14,17
Considerable behavioral variability is observed at these early stages. Furthermore, comparisons across these studies are potentially complicated by inconsistencies in developmental staging criteria, as chronological age may not be a reliable reflection of true developmental state—are morphological features more predictive of individual behavior than age? Similarly, there are often considerable chronological gaps between when these measurements are made—is social ontogeny a continuous process, or one that occurs very rapidly over developmental time?
To address these questions, we adapted our assay for social orienting and cueing in the zebrafish and used it to probe a narrow chronological window that we expected to be relevant given the findings of other groups. In addition, we performed measurements of body length to understand if this morphological feature is predictive of individual sociality.18 Finally, we investigated the effect of early nutrition by comparing social ontogeny in zebrafish reared with different feeding practices.19
Materials and Methods
Fish husbandry
ABxTU strain zebrafish were maintained in standard conditions as described in the Zebrafish Book, on a 14 h light cycle.20 Unless otherwise noted, zebrafish were introduced to food at 4 dpf and fed rotifers three times daily. All procedures carried out in this study were approved by the University of Oregon Institutional Animal Care and Use Committee.
Social behavior
Socially motivated place preference and orienting behavior of larval zebrafish was measured using a modified version of our dyad assay for juveniles and adults.15 Zebrafish are placed in isolated tanks (50 mm length × 20 mm width × 20 mm depth) separated by an opaque divider and allowed to habituate for 5 min, then the divider is removed and the animals are allowed to interact for an additional 5 min. Both the pre- and social stimulus periods were recorded in a subset of animals to determine the baseline exploratory behavior in the tanks. Recordings were obtained from below at 10 fps using a Mightex SME-B050-U camera and illuminated by an overhead white LED panel (Environmental Lights). Fish that spent <10% of the experiment in motion (as defined by moving at least one-third of their total body length per frame) were not included in subsequent analyses. Frames where the animal was not effectively segregated from the background were also discarded, and experiments with >10% detection errors were excluded from analysis (33 discarded/407 total experiments).
Social interaction is parameterized as the average relative distance from the divider and the percentage of time spent at 45°–90° using our previously described software written in Python (Fig. 1; available from https://github.com/stednitzs/daniopen). Refinement of orienting behavior was measured using vector strength in a subset of animals, where no frames were discarded due to detection errors. Polar histogram plots were collapsed about the 180° axis to generate “calzone” plots, and vector strength calculated relative to 45° for each animal using Python (Fig. 2A–C). Social cueing was quantified using time-lag cross-correlation in Python for a subset of zebrafish dyads where no frames were discarded due to detection errors in either fish. Latency was calculated for each dyad by measuring the time from time 0 to the peak correlation (Fig. 2D–F).
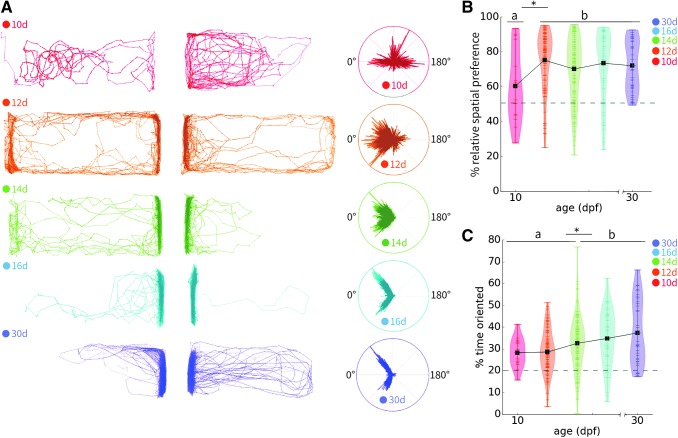
FIG. 1.
Progression of spatial preference and orienting behavior by age. (A) Representative dyad traces and polar histograms of body orientation at each age assayed. (B) Relative spatial preference arises and plateaus at 12 dpf. Preference is calculated as the inverse of the average relative distance from the divider throughout the recording period. The dotted line represents chance as determined by the grand mean in the prestimulus period (49.83%). (C) Percentage of time oriented between 45° and 90° increases gradually over time. The dotted line represents chance as determined by the grand mean in the prestimulus period (19.88%). Lowercase letters indicate homogenous subsets as determined by Tukey's b post hoc tests such that these groups differ significantly at p < 0.05 (*). Violin plots show individual data points as horizontal tick marks, and the width of each plot represents the density of data points along the distribution. The group mean is indicated by a black square. Dotted lines represent chance values, as determined by the grand mean in the prestimulus period. Sample sizes for each age are the following: 10d: 16, 12d: 86, 14d: 126, 16d: 50, and 30d: 36. dpf, days postfertilization.
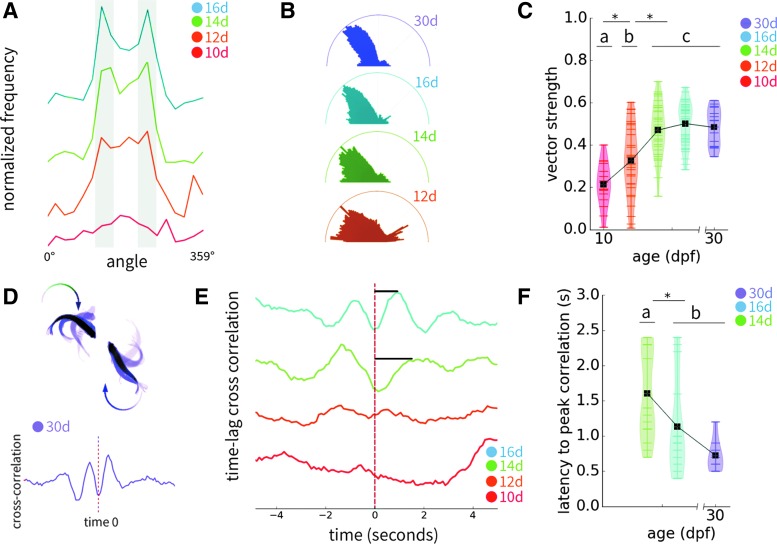
FIG. 2.
Refinement of orienting behavior and social cueing. (A) Normalized frequency of orienting behavior at 10–16 dpf. Portions highlighted in gray indicate the 45°–90° region. (B) Average polar plots across each age group sampled collapsed about the 180° axis to generate “calzone” plots. Vector strength was calculated from these data based on 45° relative to the divider. (C) Vector strength increases across time and plateaus at 14 dpf. (D) Example of time lag cross-correlation structure for 30 dpf zebrafish, showing that angles are highly correlated between dyads with a slight latency, n = 8 pairs. Black bar indicates latency from time 0 to peak. (E) Developmental timeline of time-lag cross-correlation, indicating that correlated structures begin to occur at 14 dpf and decrease in latency by 16 dpf. Black bars indicate latency from time 0 to peak. (F) Quantification of latency to peak correlation in 14, 16, and 30 dpf zebrafish dyads. Lowercase letters indicate homogenous subsets as determined by Tukey's b post hoc tests such that these groups differ significantly at p < 0.05 (*). Violin plots show individual data points as horizontal tick marks, and the width of each plot represents the density of data points along the distribution. The group mean is indicated by a black square. Sample sizes for each age are the following unless otherwise noted: 10d: 16, 12d: 31, 14d: 38, 16d: 34, and 30d: 16. Only dyads where no frames were dropped due to detection errors are included.
Morphological features
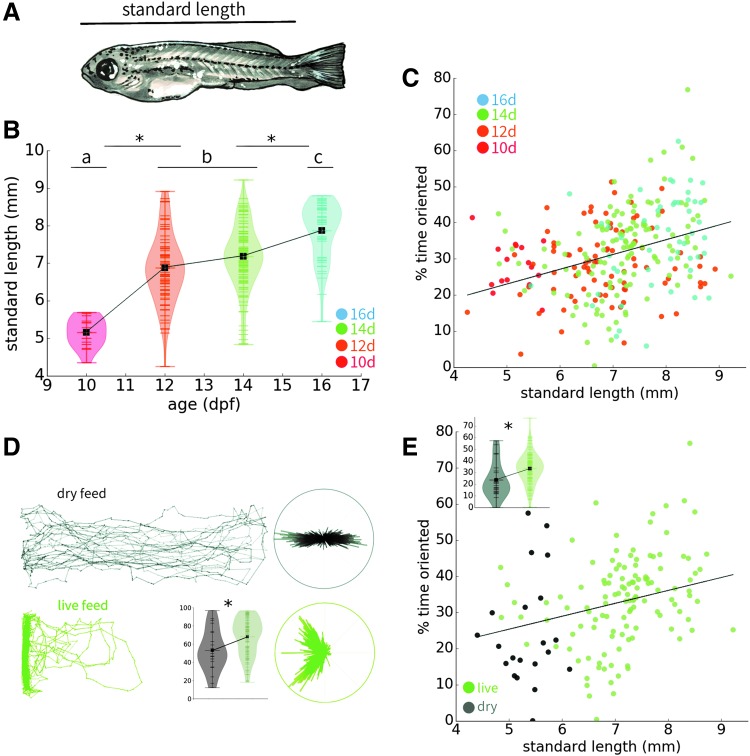
After behavioral experiments, individual zebrafish were anesthetized in MS-222 and imaged on a stereomicroscope (Leica M205 FA). Animals were imaged alive, as the fixation process was expected to alter the length of the animal. We measured the standard length as described by Parichy et al., from the tip of the nose to the most posterior end of the body, excluding the fins (Fig. 3A).18
FIG. 3.
Length is predictive of individual social behavior. Sample sizes for each age are the following unless otherwise noted: 10d: 16, 12d: 85, 14d: 126, and 16d: 50. (A) Diagram of standard length measurements, from the tip of the nose to the end of the tail, excluding the tail fins. (B) Standard length plotted by age. (C) Orienting behavior plotted by standard length for individual animals, showing an increase in orienting behavior with increasing standard length. (D) Representative traces of 14 dpf zebrafish reared on dry versus live food (n = 22 and 126, respectively). Inset shows violin plot by condition for spatial preference, not considering standard length. (E) Orienting behavior plotted by standard length for 14 dpf zebrafish reared on dry versus live food. Inset shows violin plot by condition of the same data set, not considering standard length. Lowercase letters indicate homogenous subsets as determined by Tukey's b post hoc tests such that these groups differ significantly at p < 0.05 (*). Violin plots show individual data points as horizontal tick marks, and the width of each plot represents the density of data points along the distribution. The group mean is indicated by a black square.
Nutrition
We investigated the role of nutrition, a major factor known to influence larval development. We measured social behavior and standard length as previously described, but instead reared zebrafish on GemmaMicro dry feed, feeding three times daily as performed in Carvalho et al.19 All other conditions (feeding schedule, light cycle, water quality, and temperature) remained constant.
Statistics
Statistical analyses were performed in Python or SPSS version 24. A one-way analysis of variance was used to compare different ages with Tukey's B post hoc tests to correct for multiple comparisons. Correlation analyses were performed using linear regression. Sample sizes for each age are the following unless otherwise noted in the figure legends: 10d: 16, 12d: 86, 14d: 126, 16d: 50, and 30d: 36.
Results
Social behavior
We measured a number of distinct parameters of social behavior in zebrafish between 10 and 30 dpf, which encompasses stages before the flexion (or dorsal bending) of the notochord through to the juvenile stage (Fig. 4). Spatial preference refers to an animal's tendency to prefer the side of the tank where conspecifics are visible. Orienting behavior was measured by calculating the head angle for every frame. Finally, we measured social cueing by measuring the extent to which orienting turns in one fish influenced orienting turns in the partner fish using cross-correlation analysis.
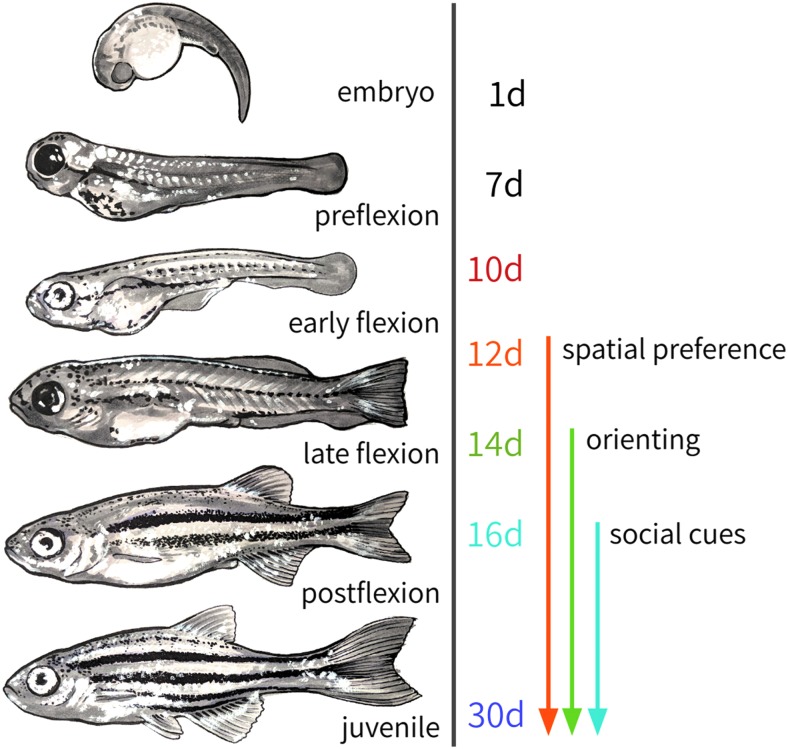
FIG. 4.
Behavioral-developmental timeline. Approximate developmental stages of zebrafish, and the ages at which specific social behaviors are first detectable as described in this article.
We observed no differences in orienting behavior by age in the prestimulus period, before the divider was removed and conspecifics were not visible (Supplementary Fig. S1C). In contrast, spatial preferences differed among ages such that younger larvae are somewhat more likely to be located adjacent to the opaque divider, suggesting that orienting and spatial preference are distinct (Supplementary Fig. S1A, B).
At 10 dpf, we found that preflexion larvae are largely asocial and do not have increased spatial preference or orienting behavior in the social stimulus phase of the experiment relative to the prestimulus period (p = 0.581, Fig. 1A). Spatial preference for the side of the tank adjacent to conspecifics increased at 12 dpf relative to 10 dpf, such that 12 dpf zebrafish begin to exhibit interest in conspecifics and are more likely to be found adjacent to the divider when a social stimulus is visible (p = 0.021; Fig. 1A). Spatial preference plateaued by 12 dpf and remained constant through the juvenile stage (Fig. 1B).
Orienting behavior is weakly, although significantly, correlated to chronological age (R2 = 0.047, p < 0.001), and at 14 dpf larvae begin to more reliably exhibit orienting compared with 12 dpf (Fig. 1C). By 16 dpf the percentage of time spent at 45°–90° is statistically indistinguishable from that of 30 dpf postflexion larvae (p = 0.709, Fig. 1C). The progression of orienting behavior is gradual, such that two homogeneous subsets can be identified through post hoc tests: pre- and early flexion (10, 12, and 14 dpf) and late flexion/juvenile stages (14, 16, and 30 dpf; Fig. 1C). These results indicate that social behavior as measured by our dyad assay parameters develop rapidly.
Interestingly, we observed a refinement of the stereotyped 45°–90° orienting behavior we previously described in adults over this timescale.15 Although the preferred angle is consistently within this window, variability decreases over time (Fig. 2A). We quantified the refinement of social orienting by calculating the vector strength at this characteristic angle across ages (Fig. 2B). We found that vector strength increased significantly at each time point measured until 14 dpf, and remained constant thereafter (Fig. 2C). These results suggest that orienting behavior observed in juveniles and breeding adults can be fully established as early as 14 dpf.
Next, we probed social cueing across chronological age by measuring time lag cross-correlation of orientation between social partners. Juvenile and adult zebrafish mirror one another's orienting behavior such that the turn of one animal elicits a corresponding turn in the other in <1 s (average 0.73 s; Fig. 2D). We applied the same analysis across developmental time and found that the latency to peak correlation decreased and the time lag between turns decreased (Fig. 2E). Notably, these turning events have a greater latency in 14 dpf larvae relative to 16 dpf (p = 0.067) and 30 dpf juveniles (p = 0.005), but by 16 dpf zebrafish exhibit a similar average latency to juveniles (p = 0.284; Fig. 2F). Although the speed of animals increases such that average distance traveled per frame increases by age (p < 0.001), speed is not significantly correlated to latency and does not account for the decreased response time (R2 = 0.055, p = 0.123). These results suggest that zebrafish larvae actively attend to the behavior of conspecifics by 16 dpf.
Morphological features
Chronological age influences overall standard body length (as measured from the front of the face to the end of the tail, excluding fins; Fig. 3A), and we report an increase in standard body length from 10 to 16 dpf (R2 = 0.229, p < 0.001; Fig. 3B).
Standard length was weakly predictive of orienting behavior (R2 = 0.067, p < 0.001; Fig. 3C) and spatial preference (R2 = 0.049, p < 0.001). However, when each age group is considered separately, length is only predictive of spatial preference at 12 dpf (R2 = 0.058, p = 0.01). Similarly, length is only predictive of orienting at 14 and 16 dpf (R2 = 0.050 and 0.134, p < 0.012 and 0.009, respectively). The effect of length on orienting is greatest at 16 dpf, indicating that size is most related to sociality at the late flexion stage. When these data are analyzed using multiple regression with both age and standard length as predictors of orienting behavior, length is still significantly related, but age is not (p = 0.002 and p = 0.113, respectively), suggesting that size, and therefore actual developmental stage, is at least partially responsible for driving the effect of chronological age.
Considerable variability in developmental features and behavior between laboratories could be due to differences in rearing practices. To further explore the relationship between standard length, age, and social behavior, we raised a cohort of larvae to 14 dpf using a dry food regimen that impairs the rate of growth relative to live food (n = 22). We observed social deficits in the dry food cohort relative to our larvae reared on live food (p = 0.002; Fig. 3D), and reduced standard lengths (p < 0.001; Fig. 3E). Considering the entire 14 dpf data set, length remained significantly correlated to orienting behavior (R2 = 0.131, p < 0.001). These findings suggest that standard length has superior predictive power than chronological age, especially when considered across multiple nutrition regimens.
Discussion
Zebrafish rapidly acquire complex social behaviors between 10 and 16 dpf. Furthermore, this is related to standard length on an individual basis beginning at 14 dpf. This highly variable and early stage in development represents an opportunity to further understand how genetic and environmental factors affect the assembly of the neural circuits underlying complex behaviors.
Spatial preference for conspecifics is the first social behavior observed in our assay, occurring at 12 dpf. Orienting behavior is exhibited at 14 dpf, which gradually increases in precision. Zebrafish begin attending to cues from conspecifics by 14 dpf, and they respond more quickly to these cues by 16 dpf. Altogether, these findings suggest a sequential acquisition of progressively more complex social behaviors over a rapid timescale (Fig. 4).
Standard length of larval zebrafish is predictive of individual variability in social orienting behaviors, concurring with previous study showing other developmental features such as fin morphology and pigment formation is predicted by length.18 Interestingly, this effect of standard length only occurs at 14 dpf and beyond, suggesting a critical period before which orienting is unlikely to occur. Similarly, when zebrafish were developmentally delayed by a nutritionally restricted diet, both their size and social behavior were impaired. In light of these results, we propose that standard length should be reported in conjunction with chronological age in behavioral studies of early and late flexion larvae.
The neuronal mechanisms behind these rapid behavioral changes remain an outstanding question. Although zebrafish can detect and pursue small prey by 4 dpf,21 preflexion larvae do not respond to biological motion, a complex and salient visual feature of social behavior.14 The receptive field size in the optic tectum is reported to decrease between 14 and 20 dpf, providing a potential neuronal mechanism for refined visual behaviors during this time period.22 Specific visual social cues influence social preferences in the adult, such as local features of the head, direction of motion, and pigmentation patterns,23,24 but the developmental progression of these effects are as yet unexplored.
Previous study shows that developmental perturbations through chemical insults, social isolation, or genetic mutations can have profound effects on the social behavior of animals later in life, including zebrafish.16,25–29 The refinement of sensorimotor processing during critical periods may be governing the rapid changes in social behavior. Given our increasing knowledge about the neuroanatomical correlates of social behavior in zebrafish, 12–16 dpf is a promising time period to investigate how these circuits may be affected by developmental disturbances known to influence neurodevelopmental disorders in humans.
Supplementary Material
Acknowledgments
We thank Kip Keller and Avinash Singh Bala for advice on data analysis. We also thank Adam Christensen, John Dowd, and the University of Oregon Aquatic Animal Care Services for fish husbandry, and Judith Eisen for comments on the article.
Authors' Contributions
Conceptualization and writing was done by S.J.S. and P.W.; methodology, investigation, and data analysis were the responsibilities of S.J.S.; and supervision was by P.W.
Disclosure Statement
No competing financial interests exist.
Funding Information
This work was supported by National Institutes of Health grant R33MH104188.
Supplementary Material
References
- 1. Engeszer RE, Patterson LB, Rao AA, Parichy DM. Zebrafish in the wild: a review of natural history and new notes from the field. Zebrafish 2007;4:21–40 [DOI] [PubMed] [Google Scholar]
- 2. Orger MB, de Polavieja GG. Zebrafish Behavior: opportunities and challenges. Annu Rev Neurosci 2017;40:125–147 [DOI] [PubMed] [Google Scholar]
- 3. Suriyampola PS, Shelton DS, Shukla R, Roy T, Bhat A, Martins EP. Zebrafish social behavior in the wild. Zebrafish 2016;13:1–8 [DOI] [PubMed] [Google Scholar]
- 4. Howe K, Clark MD, Torroja CF, Torrance J, Berthelot C, Muffato M, et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature 2013;496:498–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mueller T, Dong Z, Berberoglu MA, Guo S. The dorsal pallium in zebrafish, Danio rerio (Cyprinidae, Teleostei). Brain Res 2011;1381:95–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nieuwenhuys R. The development and general morphology of the telencephalon of actinopterygian fishes: synopsis, documentation and commentary. Brain Struct Funct 2011;215:141–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. O'Connell LA, Hofmann HA. Evolution of a vertebrate social decision-making network. Science 2012;336:1154–1157 [DOI] [PubMed] [Google Scholar]
- 8. Shinozuka K, Watanabe S. Effects of telencephalic ablation on shoaling behavior in goldfish. Physiol Behav 2004;81:141–148 [DOI] [PubMed] [Google Scholar]
- 9. Teles MC, Cardoso SD, Oliveira RF. Social plasticity relies on different neuroplasticity mechanisms across the brain social decision-making network in zebrafish. Front Behav Neurosci 2016;10:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Abril-de-Abreu R, Cruz J, Oliveira RF. Social eavesdropping in zebrafish: tuning of attention to social interactions. Sci Rep 2015;5:12678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dreosti E, Lopes G, Kampff AR, Wilson SW. Development of social behavior in young zebrafish. Front Neural Circuits 2015;9:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Engeszer RE, Da Barbiano LA, Ryan MJ, Parichy DM. Timing and plasticity of shoaling behaviour in the zebrafish, Danio rerio. Anim Behav 2007;74:1269–1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hinz RC, de Polavieja GG. Ontogeny of collective behavior reveals a simple attraction rule. Proc Natl Acad Sci U S A 2017;114:2295–2300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Larsch J, Baier H. Biological motion as an innate perceptual mechanism driving social affiliation. Curr Biol 2018;28:3523–3532.e4. [DOI] [PubMed] [Google Scholar]
- 15. Stednitz SJ, McDermott EM, Ncube D, Tallafuss A, Eisen JS, Washbourne P. Forebrain control of behaviorally driven social orienting in zebrafish. Curr Biol 2018;28:2445–2451.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Buske C, Gerlai R. Early embryonic ethanol exposure impairs shoaling and the dopaminergic and serotoninergic systems in adult zebrafish. Neurotoxicol Teratol 2011;33:698–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Buske C, Gerlai R. Shoaling develops with age in Zebrafish (Danio rerio). Prog Neuropsychopharmacol Biol Psychiatry 2011;35:1409–1415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Parichy DM, Elizondo MR, Mills MG, Gordon TN, Engeszer RE. Normal table of postembryonic zebrafish development: staging by externally visible anatomy of the living fish. Dev Dyn 2009;238:2975–3015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Carvalho AP, Araujo L, Santos MM. Rearing zebrafish (Danio rerio) larvae without live food: evaluation of a commercial, a practical and a purified starter diet on larval performance. Aquacult Res 2006;37:1107–1111 [Google Scholar]
- 20. Westerfield M. The Zebrafish Book, 4th Edition; A Guide for the Laboratory Use of Zebrafish (Danio rerio). University of Oregon Press, Eugene OR, 2000 [Google Scholar]
- 21. Patterson BW, Abraham AO, MacIver MA, McLean DL. Visually guided gradation of prey capture movements in larval zebrafish. J Exp Biol 2013;216(Pt 16):3071–3083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bergmann K, Meza Santoscoy P, Lygdas K, Nikolaeva Y, MacDonald RB, Cunliffe VT, et al. Imaging neuronal activity in the optic tectum of late stage larval zebrafish. J Dev Biol 2018;6:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Engeszer RE, Ryan MJ, Parichy DM. Learned social preference in zebrafish. Curr Biol CB 2004;14:881–884 [DOI] [PubMed] [Google Scholar]
- 24. Neri P. Feature binding in zebrafish. I-Perception 2012;3:391 [Google Scholar]
- 25. Baronio D, Puttonen HAJ, Sundvik M, Semenova S, Lehtonen E, Panula P. Embryonic exposure to valproic acid affects the histaminergic system and the social behaviour of adult zebrafish (Danio rerio). Br J Pharmacol 2018;175:797–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liu C-X, Li C-Y, Hu C-C, Wang Y, Lin J, Jiang Y-H, et al. CRISPR/Cas9-induced mutant zebrafish display autism-like behaviors. Mol Autism 2018;9:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shams S, Amlani S, Buske C, Chatterjee D, Gerlai R. Developmental social isolation affects adult behavior, social interaction, and dopamine metabolite levels in zebrafish. Dev Psychobiol 2018;60:43–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Weber DN, Ghorai JK. Experimental design affects social behavior outcomes in adult zebrafish developmentally exposed to lead. Zebrafish 2013;10:294–302 [DOI] [PubMed] [Google Scholar]
- 29. Zimmermann FF, Gaspary KV, Leite CE, De Paula Cognato G, Bonan CD. Embryological exposure to valproic acid induces social interaction deficits in zebrafish (Danio rerio): a developmental behavior analysis. Neurotoxicol Teratol 2015;52(Pt A):36–41 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.