Short abstract
Staphylococcus aureus and Pseudomonas aeruginosa are primary pathogens in chronic rhinosinusitis (CRS), and the presence of S. aureus and P. aeruginosa biofilms has been associated with negative outcomes after surgery. This study investigated the inhibition effect of cetylpyridinium chloride (CPC)-quatsomes at low concentrations on both S. aureus and P. aeruginosa biofilms in vitro, as well as their toxicities towards cultured human airway epithelial (NuLi-1) cells. S. aureus ATCC 25923 and P. aeruginosa ATCC 15692 were used to establish biofilms. CPC-quatsome and CPC micelle solutions at concentrations of 0.01%, 0.025%, and 0.05% were prepared. AlamarBlue was used to test the viability of both planktonic S. aureus and P. aeruginosa and their biofilms after treatment for 5 min and 2 h, respectively. Confocal laser scanning microscopy (CLSM) was used to investigate the interactions between CPC-quatsomes and S. aureus and P. aeruginosa biofilms. A lactate dehydrogenase (LDH) assay was used to determine the toxicity of CPC-quatsomes on NuLi-1 cells. CPC-quatsome and CPC micelle solutions had significant inhibition effects at all tested concentrations on planktonic S. aureus and P. aeruginosa and their biofilms after 5-min exposure (P < 0.05). In the CLSM study, different interactions between CPC-quatsomes and S. aureus or P. aeruginosa biofilms were observed. After 2-h treatment, the size of S. aureus biofilms decreased, while the number of dead bacteria increased in P. aeruginosa biofilms. Neither CPC-quatsomes nor CPC micelle solutions showed significant toxicity on NuLi-1 cell at all tested CPC concentrations (P < 0.05). CPC-quatsomes at low concentrations inhibited S. aureus and P. aeruginosa in both planktonic form and biofilms. No adverse effects on NuLi-1 cells were observed, indicating their promising potential in the treatment of CRS.
Impact statement
Staphylococcus aureus and Pseudomonas aeruginosa biofilms are significant contributors to chronic rhinosinusitis (CRS), and are associated with poor prognosis. The killing effect of CPC-quatsomes on S. aureus biofilm at or above the CPC concentration of 0.5% (5 mg/mL) has been reported previously. This is the first study that showed the significant inhibition effect of CPC-quatsomes at low concentrations on both S. aureus and P. aeruginosa biofilms in vitro, and no adverse effects towards cultured human airway epithelial (NuLi-1) cells. In our study, CPC-quatsomes at concentrations of 0.01%, 0.025%, and 0.05% had significant inhibition effects on both planktonic and biofilms of S. aureus and P. aeruginosa. The result of this study indicates the promising potential of CPC-quatsome in the treatment of CRS.
Keywords: Chronic rhinosinusitis, Staphylococcus aureus, Pseudomonas aeruginosa, biofilm, cetylpyridinium chloride, CPC-quatsome
Introduction
Staphylococcus aureus and Pseudomonas aeruginosa are major pathogens in chronic rhinosinusitis (CRS) with 61% and 8% prevalence, respectively.1,2 Both S. aureus and P. aeruginosa biofilms have negative influence on postoperative outcomes and disease progression.3–6 The increasing burden of antimicrobial resistance and the reduced susceptibility of biofilms to antibiotics make mucosal biofilms particularly challenging to treat.7
Nanoparticulate drug delivery systems such as liposomes can improve the antimicrobial efficacy of encapsulated drugs against biofilms and recently have received increased attention.8–12 Besides the encapsulated drugs, the membranes themselves can harbor antimicrobial compounds and can demonstrate inherent anti-biofilm action making such delivery systems promising anti-biofilm strategies. Cetylpyridinium chloride (CPC) is widely used as a disinfectant and topical antiseptic for the skin and mucosa.13,14 In our previous study,15 the killing effect of quatsomes on S. aureus biofilm at or above the CPC concentration of 0.5% (5 mg/mL) was tested, killing >99% of S. aureus biofilms after 10-min exposure. A dose-dependent anti-biofilm effect was obtained at higher concentrations. In previous studies, even at very low concentrations, CPC displayed bactericidal activity against planktonic E. coli (0.025% CPC solution) and S. aureus (0.003% CPC solution).16,17 However, the anti-biofilm effect of CPC-quatsomes at low concentrations was not investigated.
Building on our initial report, the aim of this study was to systematically investigate the effect of CPC-quatsomes at concentrations lower than 0.5 mg/mL against S. aureus and P. aeruginosa biofilms in vitro, as well as their toxicities on cultured human airway epithelial cells. The 5-min exposure was set in our experiments in order to mimic nasal irrigation as standard delivery method of drugs into the sinonasal cavities. In addition, longer exposure times were also tested to confirm antimicrobial effects and to assess time dependency. Together, this study indicates the novel drug delivery systems have “built-in” anti-biofilm activity with the potential for translation into therapeutic products for the management of refractory CRS.
Materials and methods
CPC-quatsome and micelle solutions preparation
Quatsomes were prepared following the procedure published previously.15,18 Briefly, equimolar ratios of CPC and cholesterol were accurately weighed in glass vials. In order to avoid adverse effects in the solubility and critical micelle concentration (CMC) of CPC associated with the presence of electrolytes, 10 mL of Milli-Q water was added to the mixture and sonicated for 20 min in an ice bath (30/10 s on/off cycle, 40% amplitude). The quatsomes dispersions were stirred overnight on a magnetic stirring plate (400 r/min) at room temperature to equilibrate. A total of kthree quatsome dispersions were prepared with a final CPC concentration of 0.1, 0.25, and 0.5 mg/mL in Milli-Q water.19,20
Micelle solutions with corresponding CPC concentration (0.1, 0.25, and 0.5 mg/mL) were prepared by dissolving the appropriate amount of CPC in 10 mL of Milli-Q water followed by overnight stirring at room temperature.
Bacterial strains and biofilm formation
S. aureus American Type Culture Collection (ATCC) 25923 and P. aeruginosa (ATCC) 15692 (Manassas, VA, USA) were used in this study. The frozen strains were thawed and cultured at 37°C for 24 h on nutrient agar plates (Oxoid, SA, Australia). One single colony was picked and adjusted in sterile saline (0.9% NaCl) to obtain a bacterial suspension of 1McFarland unit. Then the suspension was diluted 1:15 in cerebrospinal fluid (CSF) broth (Thermo Fisher, Australia) to set up different models of biofilms.2
In addition, after revival, one single colony was transferred to a 50 mL centrifuge tube containing 5 mL of CSF broth and incubated at 200 r/min for 24 h in a 37°C incubator. The obtained bacterial suspension was diluted with CSF broth to have an absorbance of 0.05 at a wavelength of 600 nm (OD600). Then 10 mL of the bacterial suspension was transferred to a 50 mL centrifuge tube and incubated at 200 r/min in a 37°C incubator. The OD600 was measured hourly to prepare a standard growth curve of bacteria. Based on this, bacteria in the late logarithmic growth phase were selected for the anti-planktonic bacteria study.21,22
For the anti-biofilm experiments, flat clear-bottom 96 well microplates (Corning Incorporated, Corning NY, USA) were used to grow biofilms. The microplates containing 150 µL of the bacterial suspension in each well were incubated for 48 h at 37°C on a gyratory shaker (Ratek Instruments, Boronia, VIC, Australia) at 70 r/min to allow for biofilm formation.7,23
S. aureus and P. aeruginosa were grown in 8-well culture slides (BD Falcon, NSW, Australia) to obtain biofilm for observation under confocal laser scanning microscope, Leica TCS SP5 (Leica Microsystems, Wetzlar, Germany). For S. aureus biofilm formation, 300 µL of the bacterial suspension in each well was incubated at 37°C on a gyrorotary shaker (Ratek, Vic, Australia) at 70 r/min for 24 h. Then the same amount of fresh CSF broth was used to replace the media in order to maintain bacterial viability. The slides were then incubated at 37°C with 5% CO2 and 90% humidity for another 48 h. For P. aeruginosa, an air-liquid interface biofilm model was established. Slides containing 150 µL of the bacterial suspension in each well sitting at 45° angle from horizontal were cultured for 24 h at 37°C statically, and then 50 µL media was replaced by fresh CSF broth. Afterwards another 48-h incubation step was carried out using the same condition as S. aureus biofilm formation.
Antibacterial effects against planktonic bacteria
After incubation for 3.5 h under the condition mentioned above, both S. aureus and P. aeruginosa were at the late logarithmic growth phase; 1 mL of planktonic bacterial solution was centrifuged at 1020×g for 10 min at room temperature and the supernatant was discarded. Then the bacteria were resuspended in CPC micelle or quatsome solutions, and incubated for either 5 min or 2 h at 200 r/min in a 37°C incubator, respectively. Saline (0.9% NaCl) was selected as the non-treatment control for the maximum growth of the bacteria. After that, 0.5 mL of the treated bacterial suspension was centrifuged as above and resuspended in 1 mL saline. The washing step was repeated.
The obtained bacterial suspension was diluted 1:5 with saline and 100 µL was transferred to a black 96-well culture plate (Corning Incorporated, Corning NY, USA) in each well. Then 100 µL of CSF broth and 20 µL of alamarBlue (Invitrogen, CA, USA) were added per well. After 1-h incubation protected from light at 37°C, the fluorescence intensity of the sample was read using a FLUOstar OPTIMA plate reader (BMG Labtech, Vic, Australia), equipped with an excitation filter of 520–540 nm and an emission filter of 580–600 nm. All treatments were carried out in triplicate and the entire experimental procedure was repeated twice.
Anti-biofilm effects
Established S. aureus and P. aeruginosa biofilms in 96-well microplates were first washed twice with saline to remove planktonic bacteria11,24 and subsequently treated with CPC-quatsome and CPC micelle solutions, and saline (0.9% NaCl) as the non-treatment control for the maximum growth of the biofilms, from 5 min to 2 h at 37°C. Then 150 µL of CSF broth per well was added to allow biofilm recovery at 37°C for another 24 h. After each step, biofilms were rinsed twice with saline. Subsequently, the viability of the biofilms was determined by alamarBlue. The microplates were incubated with 20 µL of alamarBlue in 180 µL CSF broth in each well at 37°C for 1 h, and the fluorescence intensity of the samples was measured using a plate reader under the same conditions as described above. All treatments were carried out in quadruplicate and the experiments were repeated three times.
Confocal laser scanning microscopy
The interactions of CPC-quatsomes with S. aureus and P. aeruginosa biofilms were observed by CSLM. CPC-quatsomes were prepared containing 0.25% (w/w) of 1,1ʹ-dioctadecyl-3,3,3ʹ,3ʹ- tetramethy lindocarbo cyanine perchlorate (DiI, Molecular Probes, Invitrogen, Willow Creek, OR, USA). After removal of planktonic cells using two saline washes, biofilms formed on culture slides were treated with 300 µL of 0.05% DiI-labeled formulations for either 5 min or 2 h. Then the sample was fixed with 300 µL of 5% glutaraldehyde (Sigma Aldrich, St Louis, MO, USA) for 30 min at room temperature. After that, 300 µL per well of a 5 µm SYTO-9 and SYTOX (Invitrogen Molecular Probes, Vic, Australia) solution was applied to label live and dead cells in biofilms, respectively. The samples were incubated with each stain in the dark for 15 min at room temperature followed by CLSM examination. For the above-mentioned procedure, each well was rinsed twice with 300 µL saline. For S. aureus biofilms, the Leica TCS SP5 (Leica Microsystems, Wetzlar, Germany) was employed with a 63×/1.2 objective and 0.5 µm laser scanning step size. The fluorescence of DiI, SYTO-9 and SYTOX was detected at the excitation wavelengths of 561/476/504 nm, and emission of 570–600/500–520/520–540nm. The P. aeruginosa biofilm study was carried out using a Zeiss LSM700 confocal scanning laser microscope (Carl Zeiss Microscopy GmbH, Oberkochen, Germany) with a 63×/1.4 objective and 0.5 µm laser scanning step size, and the excitation/emission wavelength for DiI, SYTO-9 and SYTOX was 555 nm/∼570 nm, 488 nm/∼520nm, and 405 nm/∼480nm, respectively. The experiment was repeated twice and the software ZEN (ZEISS Microscope Software 2012) and ImageJ 1.43 (Wayne Rasband, National Institutes of Health, Bethesda, MD, USA) were applied for image processing and analysis.
Toxicity study
NuLi-1 cells (ATCC CRL-4011, Manassas, VA, USA) were grown in bronchial epithelial cell growth medium (BEGM) (Lonza, Mount Waverley, Australia) and used to determine the toxicities of CPC-quatsomes and CPC micelle solutions using the lactate dehydrogenase (LDH) assay.25 Briefly, 1 × 104 cells were inoculated in 96-well flat bottom plates (3 × 103 cells/mm2) and allowed to adhere overnight. Then the cells were exposed to different concentrations (0.01, 0.025, and 0.05%) of CPC-quatsomes and CPC micelle solutions diluted in 5% glucose (solvent used for solubilization of CPC-quatsomes and CPC micelle) in BEGM for 10 min or 30 min. NuLi-1 cells treated with 2% Triton x-100 were used as the positive control, and 5% glucose in BEGM as negative control; 100 µL supernatant from each well was transferred to a new plate, and incubated with 100 µL of LDH for 10 min protected from light at room temperature. The OD was measured by a FLUO star OPTIMA plate reader (BMG Labtech, Ortenberg, Germany) at 490 nm.
Statistical analysis
GraphPad Prism 5.0 (San Diego, CA, USA) was used for statistical analysis. Kruskal–Wallis test with Mann–Whitney test for post hoc comparisons were carried out for analyzing differences among selected pairwise treatment groups. A P value of <0.05 was considered as statistically significant.
Results
Antibacterial effects against planktonic bacteria
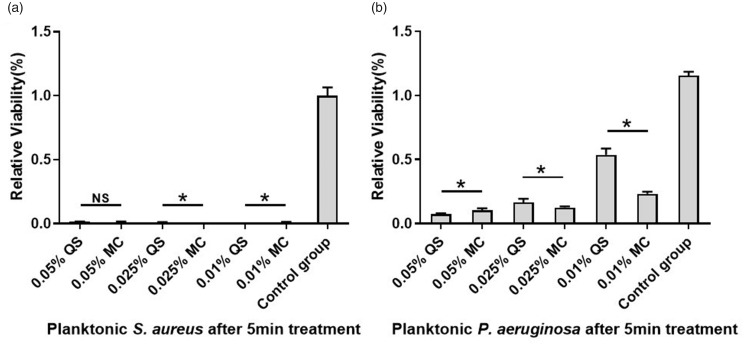
The data obtained from the alamarBlue assay demonstrated that 0.01%, 0.025%, and 0.05% CPC-quatsomes and CPC micelle solutions all had significant antimicrobial effects on planktonic forms of both S. aureus and P. aeruginosa (P < 0.05 compared with the control groups). CPC-quatsome and CPC micelle solutions at different concentrations (0.01%, 0.025%, and 0.05%) inhibited the growth of planktonic S. aureus over 98% after 5-min exposure; 0.01% and 0.025% CPC-quatsomes showed enhanced antimicrobial effect in comparison to CPC micelles (P < 0.05). With regard to planktonic P. aeruginosa, the inhibition rate for 0.01%, 0.025%, and 0.05% CPC-quatsome and CPC micelle solutions ranged from 54% to 94%, showing a tendency of CPC dose dependence; 0.05% CPC-quatsomes had better effects than the corresponding CPC micelles (P < 0.05) (Table 1 and Figure 1).
Table 1.
Relative viability of planktonic S. aureus and P. aeruginosa after 5-min treatments.
|
S. aureus |
P. aeruginosa |
|||||
|---|---|---|---|---|---|---|
| Relative viability | Inhibition ratio (%) | P | Relative viability | Inhibition ratio (%) | P | |
| 0.01% MC | 0.013 | 98.7 | 0.002 | 0.233 | 79.8 | 0.004 |
| 0.01% QS | 0.010 | 99.0 | 0.537 | 53.5 | ||
| 0.025% MC | 0.013 | 98.7 | 0.003 | 0.126 | 89.1 | 0.029 |
| 0.025% QS | 0.011 | 98.9 | 0.165 | 85.7 | ||
| 0.05% MC | 0.010 | 99.0 | 0.187 | 0.105 | 91.0 | 0.046 |
| 0.05% QS | 0.016 | 98.4 | 0.073 | 93.7 | ||
Note: All P values are determined with Mann–Whitney test.
MC: CPC micelle solution; QS: CPC-quatsome.
Figure 1.
Relative viabilities of planktonic S. aureus (a) and P. aeruginosa (b) after 5-min treatments with CPC-quatsomes and CPC micelle solutions. The cell viability was quantified by the alamarBlue assay. The results were from two independent measurements with n = 6. The histogram displayed medians with ranges, and the comparison was carried out by Kruskal–Wallis test with Mann–Whitney test for post hoc. *P < 0.05; NS: no statistical significance; MC: CPC micelle solution; QS: CPC-quatsome.
Anti-biofilm effects
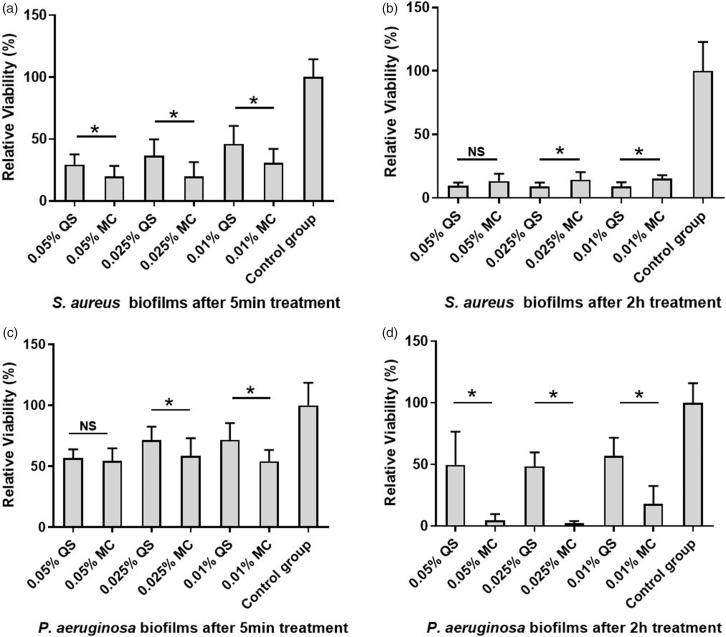
The data from the alamarBlue assay showed that all concentrations (0.01%, 0.025%, and 0.05%) of CPC-quatsomes and CPC micelle solutions had significant inhibition effects on S. aureus and P. aeruginosa biofilms after treatments for both 5 min and 2 h compared with the control group (P < 0.05), and there was a concentration and time-dependent tendency in their anti-biofilm effect. CPC micelle solutions at different concentrations (0.01%, 0.025%, and 0.05%) inhibited 69–80% growth of S. aureus biofilms, while CPC-quatsomes inhibited 54–71% S. aureus biofilms after 5-min exposure. After 2-h treatment, CPC-quatsomes inhibited up to 91% growth of S. aureus biofilm, and the inhibition rates of CPC micelle solutions were comparable at approximately 86% (Tables 2 and 3; Figure 2 and Supplementary Files 1 and 2).
Table 2.
Relative viability of S. aureus and P. aeruginosa biofilms after treatments for 5 min and 2 h.
|
S. aureus |
P. aeruginosa |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
5 min |
2 h |
5 min |
2 h |
|||||||||
| MC | QS | P | MC | QS | P | MC | QS | P | MC | QS | P | |
| 0.01% | 0.307 | 0.461 | 0.009 | 0.151 | 0.089 | 0.000 | 0.542 | 0.718 | 0.001 | 0.180 | 0.571 | 0.000 |
| 0.025% | 0.197 | 0.364 | 0.004 | 0.145 | 0.091 | 0.009 | 0.584 | 0.716 | 0.020 | 0.022 | 0.484 | 0.000 |
| 0.05% | 0.198 | 0.295 | 0.010 | 0.133 | 0.097 | 0.053 | 0.544 | 0.566 | 0.552 | 0.045 | 0.497 | 0.000 |
Note: All P values are determined with Mann–Whitney test.
MC: CPC micelle solution; QS: CPC-quatsome.
Figure 2.
Relative viabilities of S. aureus and P. aeruginosa biofilms after 5 min (a, c) and 2-h (b, d) treatments with CPC-quatsomes and CPC micelle solutions. The viabilities of biofilms were quantified by the alamarBlue assay. The results were from three independent measurements with n = 12. The histogram displayed medians with ranges, and the comparison was carried out by Kruskal–Wallis test with Mann–Whitney test for post hoc. *: P < 0.05; NS: no statistical significance; MC: CPC micelle solution, QS: CPC-quatsome.
After 5-min exposure, CPC micelle solutions inhibited 42–46% of P. aeruginosa biofilms, and CPC-quatsomes inhibited 28–43%. After 2-h treatment, over 82% of P. aeruginosa biofilms were killed by CPC micelle solutions, while CPC-quatsomes eliminated 43–52% of P. aeruginosa biofilms (Tables 2 and 3; Figure 2 and Supplementary Files 1 and 2).
Table 3.
Relative viability of S. aureus and P. aeruginosa biofilms after treatments for 5 min and 2 h.
|
S. aureus |
P. aeruginosa |
|||||
|---|---|---|---|---|---|---|
| 5 min | 2 h | P | 5 min | 2 h | P | |
| 0.01% MC | 0.307 | 0.151 | 0.000 | 0.542 | 0.180 | 0.000 |
| 0.01% QS | 0.461 | 0.089 | 0.000 | 0.718 | 0.571 | 0.019 |
| 0.025% MC | 0.197 | 0.145 | 0.176 | 0.584 | 0.022 | 0.000 |
| 0.025% QS | 0.364 | 0.091 | 0.000 | 0.716 | 0.484 | 0.016 |
| 0.05% MC | 0.198 | 0.133 | 0.041 | 0.544 | 0.045 | 0.000 |
| 0.05% QS | 0.294 | 0.097 | 0.000 | 0.566 | 0.497 | 0.047 |
Note: All P values are determined with Mann–Whitney test.
MC: CPC micelle solution; QS: CPC-quatsome.
Confocal laser scanning microscopy
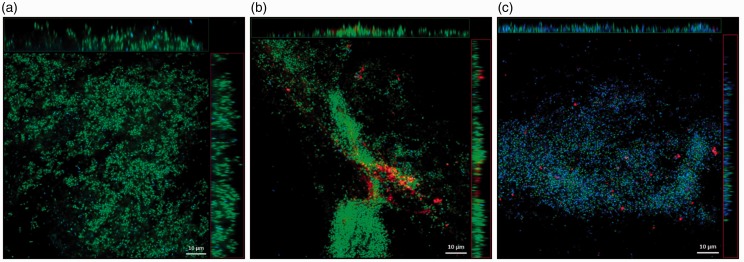
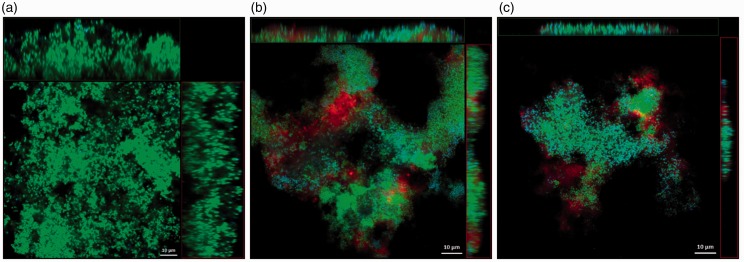
The CPC-quatsomes, live and dead bacteria were presented in red, green, and blue in confocal scanning laser micrographs, respectively. After 5-min treatment, CPC-quatsomes could be found within S. aureus and P. aeruginosa biofilms, evidenced from the x- and y-stacks, indicating deep penetration into biofilms. Compared to 5 min, the area and the thickness of S. aureus biofilms were reduced after exposure for 2 h to CPC-quatsomes, suggesting the decrease of biomass. For P. aeruginosa biofilms, the size reduction of biofilm was not as obvious as S. aureus biofilms. However, after the interaction with CPC-quatsomes for 2 h, the blue fluorescence (dead bacteria) was remarkably increased and the green fluorescence (live bacteria) had almost disappeared, indicating that more cells died in the biofilms (Figures 3 and 4).
Figure 4.
Confocal laser scanning microscopy images of P. aeruginosa biofilms before CPC-quatsome treatment (a), after 0.05% CPC-quatsome treatment for 5 min (b), and after 0.05% CPC-quatsome treatment for 2 h (c). Quatsome are stained red (DiI), live P. aeruginosa green (SYTO-9), dead P. aeruginosa blue (SYTOX). The large center images showed the X-Y view of the central layer from the z-stack; the top bars showed the X-Z view of the central layer from the y-stack, the vertical bars to the right showed the Y-Z view of the central layer from the x-stack for the same biofilm. (A color version of this figure is available in the online journal.)
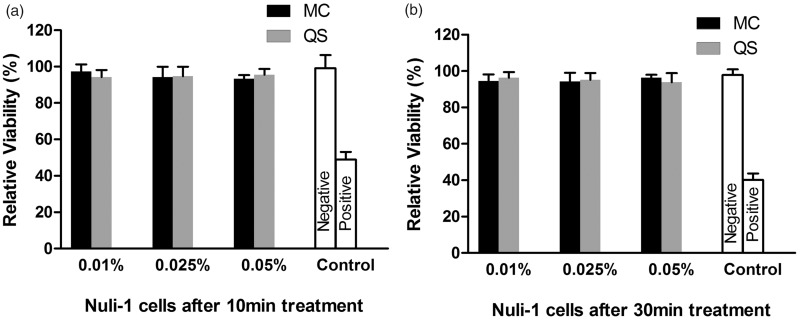
Figure 5.
Cell viability monitored by the LDH assay after 10-min (a) and 30-min (b) treatment of cultured human airway epithelial (Nuli-1) cells with CPC-quatsome and CPC micelle solutions. 2% Triton x-100 and 5% glucose in bronchial epithelial cell growth medium was used as the positive and negative control, respectively. The results were from two independent measurements with n = 6. The histogram displayed medians with ranges, and the comparison was carried out by Mann–Whitney test. MC: CPC micelle solution; QS: CPC-quatsome.
Toxicity to cultured human airway epithelial cells
Neither CPC-quatsome nor CPC micelle solutions showed a significant toxic effect on NuLi-1 cells after 10 min at all tested CPC concentrations (P > 0.05). On the other hand, exposure to a solution of 2% Triton x-100 demonstrated significant difference compared to negative controls (Figure 5). In addition, increased exposure time (up to 30 min) at the same concentrations of CPC-quatsomes and CPC micelle solutions also showed no significant influence on cell viability (P > 0.05) (Figure 5).
Figure 3.
Confocal laser scanning microscopy images of S. aureus biofilms before CPC-quatsome treatment (a), after 0.05% CPC-quatsome treatment for 5 min (b), and after 0.05% CPC-quatsome treatment for 2 h (c). Quatsomes were stained red (DiI), live S. aureus green (SYTO-9), and dead S. aureus blue (SYTOX). The large center images showed the X-Y view of the central layer from the z-stack; the top bars showed the X-Z view of the central layer from the y-stack, the vertical bars to the right showed the Y-Z view of the central layer from the x-stack for the same biofilm. (A color version of this figure is available in the online journal.)
Discussion
This study showed CPC-quatsomes at low concentrations were non-toxic and had significant antimicrobial effects on planktonic and biofilm forms of S. aureus and P. aeruginosa. Together with the results presented in our previous report,15 the systematic investigation of dose-dependent antimicrobial effects of CPC-quatsomes on S. aureus and P. aeruginosa planktonic cells and biofilms may provide a meaningful reference for future drug-loading studies.
In this study, the anti-biofilm effects were compared between different formulations and in different exposure times. Data from the antimicrobial efficacy experiments demonstrated that CPC micelles and quatsomes inhibited the growth of biofilms to a larger extent after treatment for 2 h than 5 min, confirming as expected that the anti-biofilm effect of the both CPC formulations was strongly dependent on the exposure time. For S. aureus biofilm, the CPC micelles showed a stronger “fast killing” effect than CPC-quatsomes within 5 min, but more biofilms were eradicated after 2-h exposure to CPC-quatsomes than CPC micelle solutions, which could be explained by the sustained-release feature of CPC-quatsome. It is more likely that biofilms in vivo could not be entirely cleared away just after the treatment for one time, so the CPC-quatsomes which could penetrate into the biofilm and release CPC gradually have more advantages over CPC micelles. In addition, the CLSM study confirmed that the adhesion and penetration of CPC-quatsomes to S. aureus biofilms occurred within as little as 5 min, which suggests the utility of CPC- quatsomes as carriers for other antimicrobial drugs in the context of a relatively short contact time with S. aureus biofilms associated with nasal irrigation.
Unlike S. aureus biofilm, the anti-biofilm effects of CPC-quatsomes against P. aeruginosa were less pronounced than those observed for CPC micelle solutions for both 5 min and 2-h treatments, suggesting that biofilms of different pathogens do not interact with CPC-quatsomes in the same way. In the CLSM study, the observation that after exposure to CPC-quatsomes after 2 h, the “dead bacteria” dominated in P. aeruginosa biofilms, but the size of biofilms did not decrease supported this point. It has been reported that the architecture and ultrastructure of biofilms, such as the surface and spatial arrangement of water-channels may account for different anti-biofilm efficacies.26,27 Whether the slime-like P. aeruginosa biofilms have more steric hindrance that impair the penetration of CPC-quatsomes and were not dispersed easily after treatment needs further studies. The different mechanisms of alamarBlue and SYTOX reacting with biofilms may partly explain the discrepancy between the anti-biofilm efficacy study and CLSM observation for P. aeruginosa. In the static observation under CLSM, SYTOX dyed the cells with impaired membrane, but the metabolism of the biofilm might not terminate immediately and could be detected by alamarBlue assay. Digital Holographic Microscope for live cells could be employed to test this assumption in future studies.
Our data established the foundation for further studies investigating the potential for synergistic effects of quatsomes loaded with drugs, but there were also some limitations. First, only the viability of the cultured airway cell for up to 30-min treatment was tested in the toxicity experiment. However, the mucociliary function is also important for sinonasal physiology,28 and a longer period of exposure would offer more information in understanding the potential adverse effects. Whether quatsomes affect the mucociliary function and architecture of epithelial cells in both the short and long term needs further study. Second, after the penetration of quatsomes in biofilms, the further interaction between quatsomes and the bacteria coated in the protective matrix of the biofilm was not able to be observed under CLSM in our study. The experiment to illuminate the in-depth anti-biofilm mechanism of the quatsome is necessary in future studies. In addition, it has been reported that biofilms in vivo possess structural and component characteristics that are different from the in vitro situation.26,27 Whether these differences alter their interactions with quatsomes remains unknown.29 Further anti-biofilm efficacy studies are needed to employ multi-bacterial biofilms growing on nasal mucosa rather than on glass or plastic surface.
Conclusion
CPC-quatsomes in low concentrations (0.01%, 0.025%, and 0.05%) inhibited S. aureus and P. aeruginosa in both planktonic form and biofilms, and had no adverse effects on cultured human airway epithelium, indicating their promising potential in the treatment of CRS related biofilms.
Supplemental Material
Supplemental material, EBM896779 Supplemental Material for Inhibition of Staphylococcus aureus and Pseudomonas aeruginosa biofilms by quatsomes in low concentrations by Dong Dong, Nicky Thomas, Mahnaz Ramezanpour, Alkis J Psaltis, Shuman Huang, Yulin Zhao, Benjamin Thierry, Peter-John Wormald, Clive A Prestidge and Sarah Vreugde in Experimental Biology and Medicine
Authors’ contributions
All authors participated in the design, interpretation of the studies and analysis of the data and review of the manuscript. Sarah Vreugde, Peter-John Wormald, Alkis James Psaltis, Zhao Yulin and Nicky Thomas designed research. Dong Dong, Mahnaz Ramezanpour, Benjamin Thierry and Clive A. Prestidge performed research. Shuman Huang, Dong Dong, Zhao Yulin and Sarah Vreugde wrote the paper.
DECLARATION OF CONFLICTING INTERESTS
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
FUNDING
Funding for this project was provided through a National Health and Medical Research Council of Australia (NHMRC) Project grant to SV, AJP, CP and NT (APP1164562), and the National Natural Science Foundation of China to YZ and DD (81570901).
ORCID iD
Dong Dong https://orcid.org/0000-0003-1691-3093
References
- 1.Boase S, Foreman A, Cleland E, Tan L, Melton-Kreft R, Pant H, Hu FZ, Ehrlich GD, Wormald PJ. The microbiome of chronic rhinosinusitis: culture, molecular diagnostics and biofilm detection. BMC Infect Dis 2013; 13:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dong D, Thomas N, Thierry B, Vreugde S, Prestidge CA, Wormald PJ. Distribution and inhibition of liposomes on Staphylococcus aureus and Pseudomonas aeruginosa biofilm. PLoS One 2015; 10:e0131806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singhal D, Foreman A, Jervis-Bardy J, Wormald PJ. Staphylococcus aureus biofilms: nemesis of endoscopic sinus surgery. Laryngoscope 2011; 121:1578–83 [DOI] [PubMed] [Google Scholar]
- 4.Zheng Z, Liu Q, Kim W, Tharmalingam N, Fuchs BB, Mylonakis E. Antimicrobial activity of 1,3,4-oxadiazole derivatives against planktonic cells and biofilm of Staphylococcus aureus. Future Med Chem 2018; 10:283–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bendouah Z, Barbeau J, Hamad WA, Desrosiers M. Biofilm formation by Staphylococcus aureus and Pseudomonas aeruginosa is associated with an unfavorable evolution after surgery for chronic sinusitis and nasal polyposis. Otolaryngol Head Neck Surg 2006; 134:991–6 [DOI] [PubMed] [Google Scholar]
- 6.Dong D, Yulin Z, Xiao W, Hongyan Z, Jia L, Yan X, Jia W. Correlation between bacterial biofilms and osteitis in patients with chronic rhinosinusitis. Laryngoscope 2014; 124:1071–7 [DOI] [PubMed] [Google Scholar]
- 7.Ha KR, Psaltis AJ, Butcher AR, Wormald PJ, Tan LW. In vitro activity of mupirocin on clinical isolates of Staphylococcus aureus and its potential implications in chronic rhinosinusitis. Laryngoscope 2008; 118:535–40 [DOI] [PubMed] [Google Scholar]
- 8.Meers P, Neville M, Malinin V, Scotto AW, Sardaryan G, Kurumunda R, Mackinson C, James G, Fisher S, Perkins WR. Biofilm penetration, triggered release and in vivo activity of inhaled liposomal amikacin in chronic Pseudomonas aeruginosa lung infections. J Antimicrob Chemother 2008; 61:859–68 [DOI] [PubMed] [Google Scholar]
- 9.Obonyo M, Zhang L, Thamphiwatana S, Pornpattananangkul D, Fu V. Antibacterial activities of liposomal linolenic acids against antibiotic-resistant Helicobacter pylori. Mol Pharm 2012; 9:2677–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Forier K, Raemdonck K, De Smedt SC, Demeester J, Coenye T, Braeckmans K. Lipid and polymer nanoparticles for drug delivery to bacterial biofilms. J Control Release 2014; 190:607–23 [DOI] [PubMed] [Google Scholar]
- 11.Ahmed K, Gribbon P, Jones MN. The application of confocal microscopy to the study of liposome adsorption onto bacterial biofilms. J Liposome Res 2002; 12:285–300 [DOI] [PubMed] [Google Scholar]
- 12.Forier K, Messiaen AS, Raemdonck K, Nelis H, Smedt SD, Demeester J, Coenye T, Braeckmans K. Probing the size limit for nanomedicine penetration into Burkholderia multivorans and Pseudomonas aeruginosa biofilms. J Control Release 2014; 195:21–8 [DOI] [PubMed] [Google Scholar]
- 13.McDonnell G, Russell AD. Antiseptics and disinfectants: activity, action, and resistance. Clin Microbiol Rev 1999; 12:147–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gilbert P, Moore LE. Cationic antiseptics: diversity of action under a common epithet. J Appl Microbiol 2005; 99:703–15 [DOI] [PubMed] [Google Scholar]
- 15.Thomas N, Dong D, Richter K, Ramezanpour M, Vreugde S, Thierry B, Wormald P-J, Prestidge CA. Quatsomes for the treatment of Staphylococcus aureus biofilm. J Mater Chem B 2015; 3:2770–7 [DOI] [PubMed] [Google Scholar]
- 16.Soumet C, Fourreau E, Legrandois P, Maris P. Resistance to phenicol compounds following adaptation to quaternary ammonium compounds in Escherichia coli. Vet Microbiol 2012; 158:147–52 [DOI] [PubMed] [Google Scholar]
- 17.Fromm-Dornieden C, Rembe J-D, Schäfer N, Böhm J, Stuermer EK. Cetylpyridinium chloride and miramistin as antiseptic substances in chronic wound management – prospects and limitations. J Med Microbiol 2015; 64:407–14 [DOI] [PubMed] [Google Scholar]
- 18.Cui Z-K, Bouisse A, Cottenye N, Lafleur M. Formation of pH-sensitive cationic liposomes from a binary mixture of monoalkylated primary amine and cholesterol. Langmuir 2012; 28:13668–74 [DOI] [PubMed] [Google Scholar]
- 19.Lidia FT, Evelyn MC, Mary CS, Marcel AA, Angelina A, Sylviane L, Susagna R, Jordi F, Nora V, Jaume V. Quatsomes: vesicles formed by self-assembly of sterols and quaternary ammonium surfactants. Langmuir 2013; 29:6519–28 [DOI] [PubMed] [Google Scholar]
- 20.Varade Joshi Aswal VK, Goyal PS, Hassan PA. Micellar behavior of mixtures of sodium dodecyl sulfate and dodecyldimethylamine oxide in aqueous solutions. Colloids Surf A Physicochem Eng Aspects 2005; 259:103–9 [Google Scholar]
- 21.de Almeida FA, Carneiro DG, de Oliveira Mendes TA, Barros E, Pinto UM, de Oliveira LL, Vanetti M. N-dodecanoyl-homoserine lactone influences the levels of thiol and proteins related to oxidation-reduction process in salmonella. PLoS One 2018; 13:e0204673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Balonova L, Hernychova L, Mann BF, Link M, Bilkova Z, Novotny MV, Stulik J. Multimethodological approach to identification of glycoproteins from the proteome of Francisella tularensis, an intracellular microorganism. J Proteome Res 2010; 9:1995–2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jardeleza C, Rao S, Thierry B, Gajjar P, Vreugde S, Prestidge CA, Wormald P-J. Liposome-encapsulated ISMN: a novel nitric oxide-based therapeutic agent against Staphylococcus aureus biofilms. PLoS One 2014; 9:e92117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peeters E, Nelis HJ, Coenye T. Comparison of multiple methods for quantification of microbial biofilms grown in microtiter plates. J Microbiol Methods 2008; 72:157–65 [DOI] [PubMed] [Google Scholar]
- 25.Richter K, Facal P, Thomas N, Vandecandelaere I, Ramezanpour M, Cooksley C, Prestidge CA, Coenye T, Wormald P-J, Vreugde S. Taking the silver bullet colloidal silver particles for the topical treatment of biofilm-related infections. ACS Appl Mater Interfaces 2017; 9:21631–8 [DOI] [PubMed] [Google Scholar]
- 26.Flemming H-C, Neu TR, Wozniak DJ. The EPS matrix: the “house of biofilm cells. J Bacteriol 2007; 189:7945–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bjarnsholt T, Alhede M, Alhede M, Eickhardt-Sørensen SR, Moser C, Kühl M, Jensen PØ, Høiby N. The in vivo biofilm. Trends Microbiol 2013; 21:466–74 [DOI] [PubMed] [Google Scholar]
- 28.Kucuksezer UC, Ozdemir C, Akdis M, Akdis CA. Chronic rhinosinusitis: pathogenesis, therapy options, and more. Expert Opin Pharmacother 2018; 19:1805–15 [DOI] [PubMed] [Google Scholar]
- 29.Thet NT, Wallace L, Wibaux A, Boote N, Jenkins A. Development of a mixed‐species biofilm model and its virulence implications in device related infections. J Biomed Mater Res Part B Appl Biomater 2019; 107:129–37 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, EBM896779 Supplemental Material for Inhibition of Staphylococcus aureus and Pseudomonas aeruginosa biofilms by quatsomes in low concentrations by Dong Dong, Nicky Thomas, Mahnaz Ramezanpour, Alkis J Psaltis, Shuman Huang, Yulin Zhao, Benjamin Thierry, Peter-John Wormald, Clive A Prestidge and Sarah Vreugde in Experimental Biology and Medicine