Short abstract
Hemodynamic forces have an important role in venous intimal hyperplasia, which is the main cause of arteriovenous fistula dysfunction. Endothelial cells (ECs) constantly exposed to the shear stress of blood flow, converted the mechanical stimuli into intracellular signals, and interacted with the underlying vascular smooth muscle cells (VSMCs). Caveolin-1 is one of the important mechanoreceptors on cytomembrane, which is related to vascular abnormalities. Extracellular signal-regulated kinase1/2 (ERK1/2) pathway is involved in the process of VSMCs proliferation and migration. In the present study, we explore the effects of Caveolin-1-ERK1/2 pathway and uremia toxins on the endothelial cells and VSMCs following shear stress application. Different shear stress was simulated with a ECs/VSMCs cocultured parallel-plate flow chamber system. Low shear stress and oscillating shear stress up-regulated the expression of fibroblast growth factor-4, platelet-derived growth factor-BB, vascular endothelial growth factor-A, ERK1/2 phosphorylation in endothelial cells, and proliferation and migration of VSMCs but down-regulated the Caveolin-1 expression in endothelial cells. Uremia toxin induces the proliferation and migration of VSMCs but not in a Caveolin-1-dependent manner in the static environment. Low shear stress-induced proliferation and migration of VSMCs is inhibited by Caveolin-1 overexpression and ERK1/2 suppression. Shear stress-regulated VSMC proliferation and migration is an endothelial cells-dependent process. Low shear stress and oscillating shear stress exert atherosclerotic influences on endothelial cells and VSMCs. Low shear stress modulated proliferation and migration of VSMCs through Caveolin-1-ERK1/2 pathway, which suggested that Caveolin-1 and ERK1/2 can be used as a new therapeutic target for the treatment of arteriovenous fistula dysfunction.
Impact statement
Venous intimal hyperplasia is the leading cause of arteriovenous fistula (AVF) dysfunction. This article reports that shear stress-regulated vascular smooth muscle cells (VSMCs) proliferation and migration is an endothelial cell (EC)-dependent process. Low shear stress (LSS) and oscillating shear stress (OSS) exert atherosclerotic influences on the ECs and VSMCs. LSS-induced proliferation and migration of VSMCs is inhibited by Caveolin-1 overexpression and extracellular signal-regulated kinase1/2 (ERK1/2) suppression, which suggested that Caveolin-1 and ERK1/2 can be used as a new therapeutic target for the treatment of AVF dysfunction.
Keywords: Arteriovenous fistula, shear stress, endothelial cells, vascular smooth muscle cells, coculture, caveolin-1, extracellular signal-regulated kinase1/2, platelet-derived growth factor, vascular endothelial growth factor, fibroblast growth factor, uremia toxins
Introduction
Chronic kidney disease (CKD) is an important public health problem that threatened human health and received worldwide attention.1 Maintenance hemodialysis (MHD) is one of the most important way of renal replacement therapy in patients with end stage renal disease (ESRD). Arteriovenous fistula (AVF) is the preferred vascular access for MHD treatment.2,3 However, as patients age, and with the increasing rate of hypertension, diabetes, and hyperlipidemia, the incidence of AVF dysfunction increased, which imposes a heavy burden on both families and society.4,5
Many studies revealed that the main cause of AVF dysfunction is venous intimal hyperplasia (VIH).6,7 The important feature of VIH in AVF is the non-uniform distribution.8 The VIH occurs in the sites matching with special hemodynamics patterns due to the alterative vessel geometry following anastomosis creation.9 This suggests that local hemodynamic changes play an important role in the formation and development of VIH. The wall shear stress (WSS) is the friction along blood flow and directly regulates the function of endothelial cells (ECs). Both low and disturbed shear stress are attributed to proatherogenic influences which is related to ECs dysfunction. Conversely, the laminar shear stress has the effect of maintaining endothelial function and anti-atherosclerosis.10,11 In our previous study,9 we also found that the neointimal hyperplasia in the low and disturbed WSS region is more evident compared to high and laminar WSS region in the AVF. However, the mechanism how hemodynamic forces cause VIH is not fully understood.
ECs injury is an important prerequisite for VIH. As the first barrier between blood and vessel walls, ECs constantly exposed to the shear stress of blood flow and converted the mechanical stimuli into intracellular signals.12,13 The known mechanoreceptors included receptor of tyrosine kinase, integrins, G protein, caveolin-1, and some ion channels.14,15 It has been shown that Caveolin-1 is an important mechanoreceptor related to vascular abnormalities (such as atherosclerosis, cardiac hypertrophy, etc.).16,17
Shear stress acting on ECs also changes the phenotype of underlying vascular smooth muscle cells (VSMCs) and induces VSMCs proliferation and migration with the subsequent alterations in the secretions of different cytokines from ECs, including fibroblast growth factor (FGF), platelet-derived growth factor (PDGF), vascular endothelial growth factor (VEGF), etc.18–21 Extracellular signal-regulated kinase1/2 (ERK1/2) pathway not only is one of the most important signal transduction pathways involved in the process of VSMCs proliferation and migration,22,23 but also plays an important role in the mechanical transduction of ECs for shear stress.24 Therefore, we hypothesized that Caveolin-1-ERK1/2 pathway will be involved in the ECs regulating proliferation and migration of VSMCs induced by shear stress.
Furthermore, the uremic toxins also played a significant role in AVF dysfunction of MHD patients.25 In the following study, we also simulate the uremia environment in vitro and explore the role of uremic toxins in VIH.
In the present study, we investigate how shear stress influences ECs and the role they play in cocultured VSMCs using an EC/VSMCs cocultured parallel-plate flow chamber system. Further exploration of the effects of Caveolin-1-ERK1/2 pathway and uremia toxins on the ECs and VSMCs following the application of shear stress provides new insights for the understanding of the molecular mechanisms of VIH.
Materials and methods
Materials
Polyclonal antibodies against Caveolin-1, phospho-ERK1/2, and total-ERK1/2 were purchased from Cell Signaling Technologies. Polyclonal antibodies against alpha smooth muscle actin (α-SMA), PDGF-BB, VEGF-A, and FGF-4 were purchased from Abcam. All other antibodies and chemicals of reagent grade were obtained from Santa Cruz Biotechnology or Sigma.
Cell culture
Both ECs and VSMCs were isolated from fresh human umbilical veins by the explanted technique.26 ECs were characterized by Von Willebrand factor (Abcam) and VSMCs were characterized by α-SMA (Abcam).
Lentiviral caveolin-1 vector construction and transfection
Recombinant lentivirus overexpressing Caveolin-1 (LV/Cav-1, Vigene Biosciences) was constructed by inserting wild-type Caveolin-1 cDNA into a shuttle plasmid. A control vector (LV/Ctrl, Vigene Biosciences) carrying cDNA modifying enhanced green fluorescence protein (EGFP) was also prepared. We transfected ECs with a lentiviral vector that expressed both Caveolin-1 and EGFP (LV/Cav-1) or a lentiviral vector that expressed EGFP alone as a control (LV/Ctrl).
Cocultured parallel plate flow chamber system and hemodynamic analysis
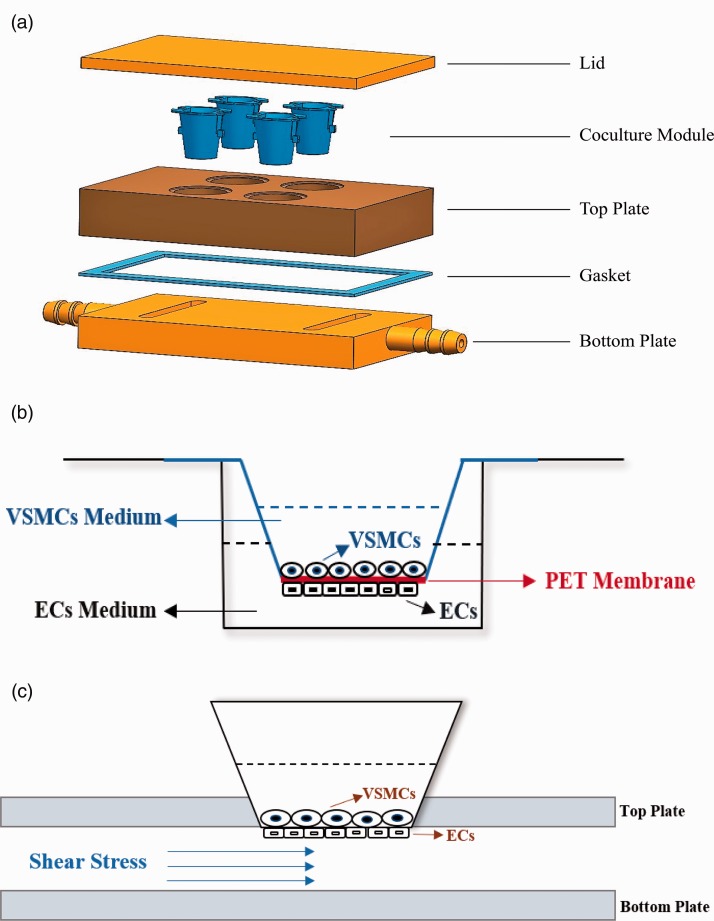
To simulate the shear stress, we built a parallel plate coculture flow chamber like the system designed by Nackman et al.27 The design and construction of the chamber are illustrated in Figure 1(a). The coculture system was established by seeding cells on the two sides of a 10-μm-thick porous polyethylene terephthalate (PET) membrane (Figure 1(b)). ECs were first plated to the outside of the PET membrane and grown over 6 h. Then, VSMCs were seeded on the opposite side of the PET membrane. The EC side of the coculture was subsequently applied to the designated shear stress, but the VSMCs side was maintained under static conditions (Figure 1(c)). We performed hemodynamic analysis with ANSYS Workbench and Fluent software.
Figure 1.
(a) The components of the three-dimensional models of ECs/VSMCs coculture parallel plate flow chamber system. (b) The model of ECs/VSMCs coculture system. ECs and VSMCs are on the two sides of a porous polyethylene terephthalate membrane. (c). The model of parallel plate flow chamber system. The EC side of the coculture was applied to the designated shear stress, whereas the opposite VSMCs side was maintained under static conditions. (A color version of this figure is available in the online journal.)
Experimental protocol
Different experimental conditions were designed for the purpose of this study.
In order to determine the effect of different shear stress on ECs and VSMCs
(1) VSMC/EC, VSMCs cocultured with ECs under static conditions; (2) VSMC/EC + LSS, VSMCs cocultured with ECs and low shear stress (LSS, 4 dyn/cm2) was applied to the EC side for 12 h; (3) VSMC/EC+NSS, VSMCs cocultured with ECs and normal shear stress (NSS, 12 dyn/cm2) was applied to the EC side for 12 h; (4) VSMC/EC + HSS, VSMCs cocultured with ECs and high shear stress (HSS, 20 dyn/cm2) was applied to the EC side for 12 h; (5) VSMC/EC+OSS, VSMCs cocultured with ECs and oscillating shear stress (OSS, 0 ± 4 dyn/cm2) was applied to the EC side for 12 h. Additionally, in order to determine the effect of different treating time, the LSS group was subdivided as follows: VSMCs were cocultured with ECs that were treated with LSS for 0 h, 6 h, 12 h or 24 h.
In order to determine the effect of uremic toxins on ECs and VSMCs
(1) VSMC/EC, VSMCs cocultured with ECs using normal growth culture medium under static conditions; (2) VSMC/EC (uremia), VSMCs cocultured with ECs using uremic serum under static conditions; (3) VSMC/EC+LSS (uremia), VSMCs cocultured with ECs using uremic serum and LSS (4 dyn/cm2) was applied to the EC side for 12 h; (4) VSMC/EC+NSS (uremia), VSMCs cocultured with ECs using uremic serum and NSS (12 dyn/cm2) was applied to the EC side for 12 h; (5) VSMC/EC + HSS (uremia), VSMCs cocultured with ECs using uremic serum and HSS (20 dyn/cm2) was applied to the EC side for 12 h; (6) VSMC/EC+OSS (uremia), VSMCs cocultured with ECs using uremic serum and OSS (0 ± 4 dyn/cm2) was applied to the EC side for 12 h. Normal growth culture medium was Dulbecco’s modified Eagle medium (DMEM) (Hyclone) with 10% fetal bovine serum (FBS) (Gibco) and uremia conditions was simulated using DMEM with 10% the serum from ESRD patients. Collecting serum of ESRD patient was in accordance with the ethical standards of the Institutional and National Research Committee on Human Experimentation and with the Helsinki Declaration of 1975 (revised in 2013), which was obtained from residual blood samples used for clinical routine test and information of the patient was anonymized.
In order to determine the effect of Caveolin-1-ERK1/2 pathway on ECs and VSMCs under LSS treatment
Before cocultured with VSMCs, ECs were performed with different pretreatment: (1) control group, ECs with LV/Ctrl transfection; (2) LV/Cav-1 group, ECs with LV/Cav-1 transfection; (3) PD98059 group, ECs incubated with PD98059 (Selleck Chemicals) at the concentration of 10 uM for 1 h; and (4) LV/Cav-1 and PD98059 group, ECs were pretreated with both LV/Cav-1 transfection and PD98059 incubation at the concentration of 10 uM for 1 h.
Histology and immunohistochemistry
We performed histological and immunohistochemistry analysis with fistulae specimen from previous canine model, which complied with the Guide for the Care and Use of Laboratory Animals. Immunohistochemistry analysis was performed with anti-Caveolin-1(1:800) and anti-α-SMA (1:200) antibodies.
Western blotting analysis
Tissues or cells lysates were separated with 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The phosphorylation of ERK1/2 was measured with the ratio of phospho-ERK1/2 to total-ERK1/2 and the density of other protein was measured relative to GAPDH.
Cell migration and proliferation
VSMCs proliferation was analyzed with the BrdU kit (Roche Diagnostics). VSMCs migration assay was performed with the Transwell system (Costar).
Statistical analysis
Each experiment was performed at least in quadruplicate and values were shown as mean ± SD. *P < 0.05 and **P < 0.001 indicate statistically significant.
Results
Caveolin-1 and ERK1/2 were involved in neointimal hyperplasia of AVF in the canine model
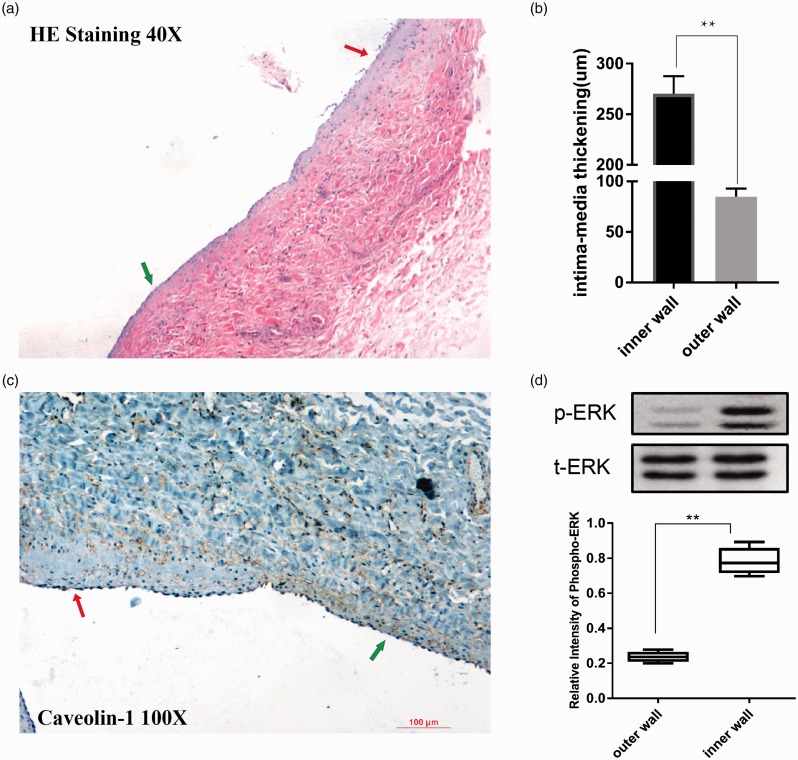
As is shown in Figure 2(a), HE staining shows VIH predisposed to the inner wall of AVF. The intima-media thickening in the inner wall of AVF is more compared to the outer wall, which is shown in Figure 2(b). However, positive immunostaining for Caveolin-1 was obviously adjacent to the endothelium of AVF. It is thought that Caveolin-1 is produced by endothelial cells. The expression of Caveolin-1 was decreased in the areas of intimal hyperplasia of AVF (Figure 2(c)), which has a reverse relationship with VIH. Therefore, we found that the positive immunostaining for Caveolin-1 was inapparent adjacent to the inner wall of anastomosis, compared to the outer wall.
Figure 2.
(a) The image shows 40× magnified hematoxylin and eosin-stained venous segments near the distal end of anastomosis (red arrow points to the inner wall and the green arrow points to the outer wall). The inner wall has evident neointimal hyperplasia. (b) The graph shows the intima-media thickening of the venous segments at the inner wall and outer wall near the distal end of anastomosis. **P < 0.001 indicates statistically significant. (c) The 100×magnified Caveolin-1 stained pictures of venous segments near the distal end of anastomosis (red arrow points to the inner wall and the green arrow points to the outer wall). The positive immunostaining for Caveolin-1 was more apparent in the outer wall. (d) Western blotting to detect phosphorylation of ERK1/2 between the inner wall and outer wall near the distal end of anastomosis. ERK1/2 phosphorylation was up-regulated in the inner wall. **P < 0.001 indicates statistically significant. (A color version of this figure is available in the online journal.)
Western blotting was used to detect the expression of ERK1/2. Compared to the outer wall, ERK1/2 phosphorylation was significantly up-regulated in the inner wall of AVF, which had opposite changes to Caveolin-1 (Figure 2(d)).
Hemodynamic analysis of ECs/VSMCs cocultured parallel plate flow chamber system
We used the computational fluid dynamics applied to three-dimensional models of ECs/VSMCs cocultured flow chamber system to estimate the velocity and WSS.
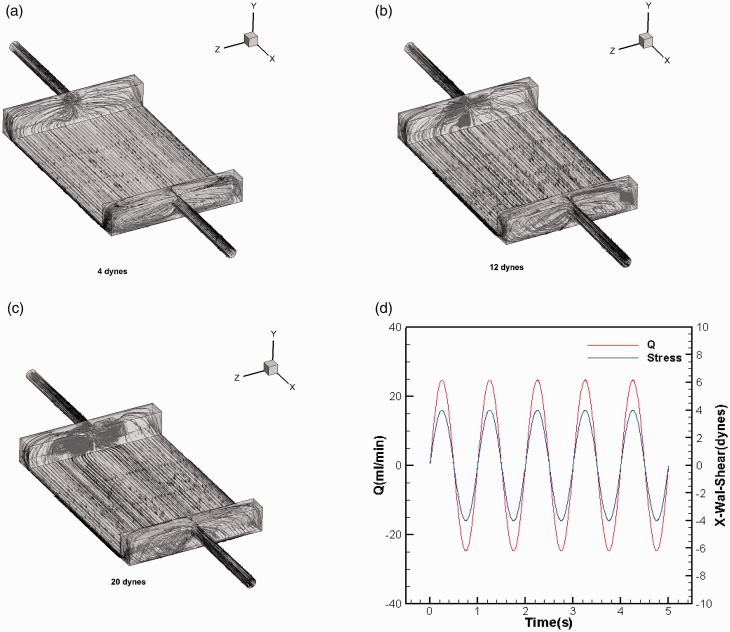
In the mode of steady flow, we analyze LSS (4 dyn/cm2), NSS (12 dyn/cm2), and HSS (20 dyn/cm2). The flow rates of the fluid have a positive relationship with WSS. Correspondingly, the flow rates were 25 mL/min, 75 mL/min, and 125 mL/min. The streamline diagram for the above different flow rates is shown in Figure 3(a) to (c). The flow trajectory of the fluid particle demonstrated that the flow moved along the X axis and showed parabolic distribution, which was symmetrical along the Z = 0 plane. Although the gradient is large at the entrance and exit, it is quickly stabilized. The central region has an essentially steady flow and a uniform distribution, which shows Poiseuille flow and laminar WSS in the parallel plate flow chamber.
Figure 3.
(a) The image shows a streamline diagram of fluid at 25 mL/min flow rates (4 dyn/cm2). (b) The image shows a streamline diagram of fluid at 75 mL/min flow rates (12 dyn/cm2). (c) The image shows a streamline diagram of fluid at 125 mL/min flow rates (20 dyn/cm2). (d) The changing curves of velocity and wall shear stress with time under unsteady flow conditions. The sine functions with a frequency of 1 Hz and amplitudes of 4 dyn/cm2. (A color version of this figure is available in the online journal.)
Under unsteady flow conditions, sine functions with a frequency of 1 Hz and amplitudes of 25 mL/min were used as speed entry conditions to make their flow rate changing with time. The curves of velocity and WSS changed sinusoidally with time, as shown in Figure 3(d). When the time is 0.25 s, the WSS peaks are 4 dyn/cm2, respectively. We definite it as oscillating shear stress (OSS, 0 ± 4 dyn/cm2).
LSS and OSS modulated expression of caveolin-1, ERK1/2 phosphorylation, PDGF-BB, VEGF-A, FGF-4 in ECs and induced migration and proliferation of cocultured VSMCs
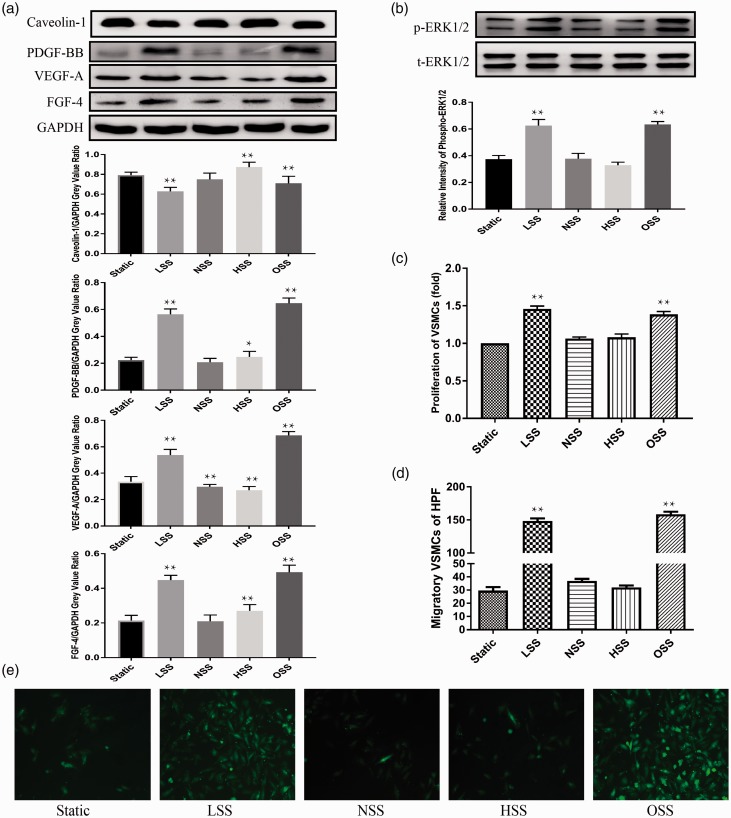
To demonstrate the interactions between ECs and VSMCs exposed to different shear stress, a ECs/VSMCs cocultured parallel-plate flow chamber system was used. In ECs, we found that Caveolin-1 expression in ECs reduced exposed to LSS and OSS, compared with the static group (Figure 4(a)). However, both the expressions of PDGF-BB, VEGF-A, FGF-4, and the ERK1/2 phosphorylation were significantly up-regulated in ECs following LSS and OSS, showing opposite changes to Caveolin-1 (Figure 4(a) and (b)). Furthermore, the expression of VEGF-A, PDGF-BB, FGF-4, and phosphorylated ERK1/2 increased and Caveolin-1 also decreased in a time-dependent fashion following the application of LSS (Figure 5(a) and (b)). Compared to the static group, NSS treatment decreased the expression of VEGF-A (Figure 4(a)) in ECs. HSS treatment increased the expression of Caveolin-1, PDGF-BB, FGF-4 and decreased the expression of VEGF-A in ECs (Figure 4(a)).
Figure 4.
Western blot shows expression level of protein in ECs treated with different shear stress for 12 h. In ECs, application of LSS and OSS suppressed expression of Caveolin-1 (a), up-regulated the expressions of PDGF-BB, VEGF-A, FGF-4 (a) and increased the phosphorylation of ERK1/2 (b), compared with the static group. The proliferation (c) and migration (d and e) of cocultured VSMCs were significantly up-regulated following ECs treated with LSS and OSS, compared to the static group. NSS treatment decreased expression of VEGF-A in ECs (a). HSS treatment increased expression of Caveolin-1, PDGF-BB, FGF-4 and decreased expression of VEGF-A in ECs (a), but had no significant effects on cocultured VSMCs (c, d and e). Values were expressed as mean ± SD for each condition from five independent experiments (*P < 0.05 vs. static; **P < 0.001 vs. static). (A color version of this figure is available in the online journal.)
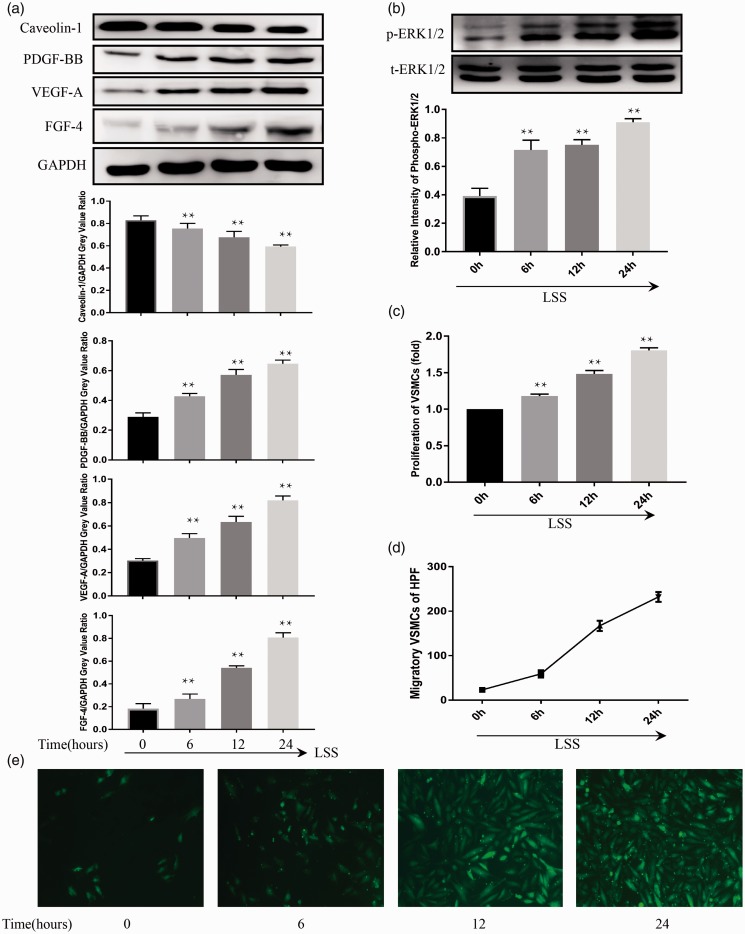
Figure 5.
In ECs, LSS treatment suppressed the expression of Caveolin-1 and increased the expression of VEGF-A, PDGF-BB, FGF-4, and phospho-ERK1/2 in a time-dependent fashion (a, b). The migration and proliferation of cocultured VSMCs increased gradually as time increased following the application of LSS to the ECs (c, d, e), reaching a peak level at 24 h. Compared to 0 h group, statistically significant differences were found at 6 h, 12 h, and 24 h. Values were expressed as mean ± SD for each condition from four independent experiments. (**P < 0.001 vs. 0 h). (A color version of this figure is available in the online journal.)
Compared to the static group, the proliferation (Figure 4(c)) and migration (Figure 4(d) and (e)) of cocultured VSMCs were significantly up-regulated following the ECs treated with LSS and OSS. The proliferation and migration of VSMCs were also increased in a time-dependent manner following the application of LSS (Figure 5(c) to (e)). However, NSS and HSS treatment had no significant effects on cocultured VSMCs (Figure 4(c) to (e)).
Uremia toxin induces the proliferation and migration of VSMCs in the static environment but not in a caveolin-1-dependent fashion
The uremia toxin increased the expression of VEGF-A, PDGF-BB, FGF-4, and ERK1/2 phosphorylation in ECs (Figure 6(a) and (b)) and up-regulated the proliferation (Figure 6(c)) and migration (Figure 6(d) and (e)) of VSMCs, compared to normal serum static group. However, uremia toxin had no specific effects on the expression of Caveolin-1 in ECs (Figure 6(a)). Uremia toxin induces the migration and proliferation of VSMCs without depending on Caveolin-1 manner in the static environment.
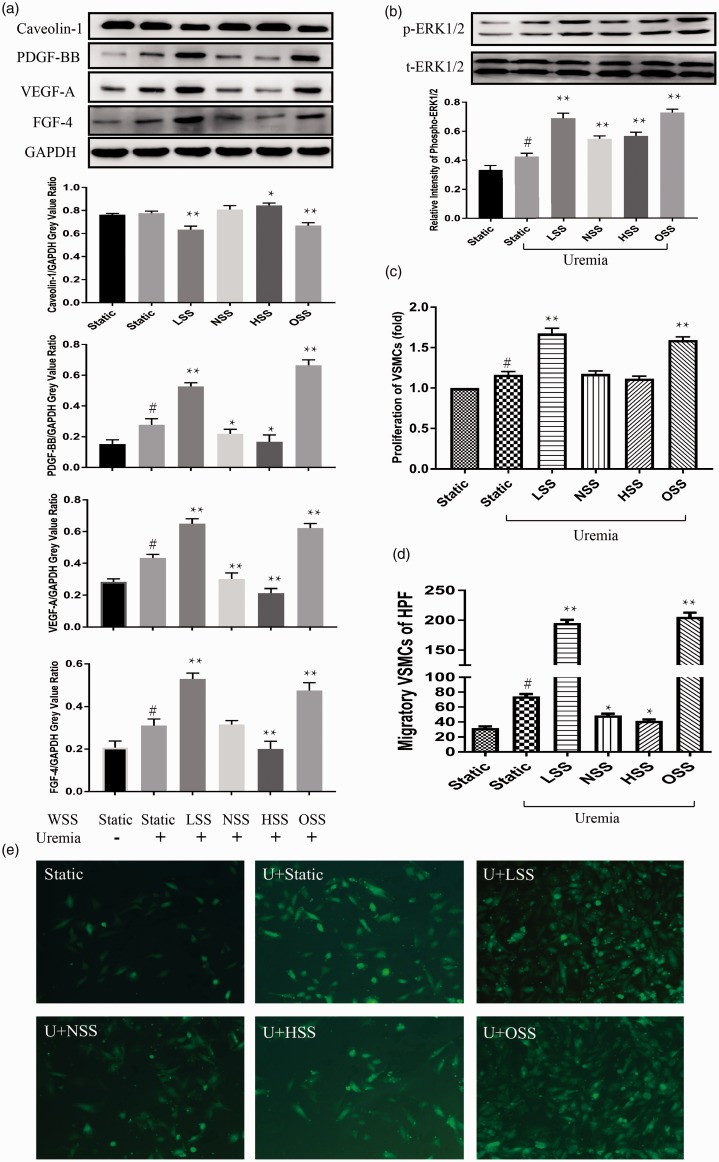
Figure 6.
The uremia toxin increased the expression of VEGF-A, PDGF-BB, FGF-4 and phospho-ERK1/2 (a, b) in ECs and up-regulated proliferation (c) and migration (d, e) of VSMCs, compared to normal serum static group. However, uremia toxin had no notable effects on expression of Caveolin-1 in ECs (a), compared to normal serum static group. Under uremic environment, LSS and OSS showed similar effects on ECs and cocultured VSMCs as in normal growth culture medium (a–e). NSS up-regulated the phosphorylation of ERK1/2, decreased VEGF-A, PDGF-BB in ECs and increased migration of VSMCs (a, b d, e). HSS increased the expression of Caveolin-1, phospho-ERK1/2, decreased the expression of VEGF-A, PDGF-BB, FGF-4 in ECs and down-regulated the migration of cocultured VSMCs (a, b d, e). However, both NSS and HSS had no effect on proliferation (c) in VSMCs. Values were expressed as mean ± SD for each condition from four independent experiments (#P < 0.05 compared to the normal serum static group; *P < 0.05 compared to the uremic static group; **P < 0.001 compared to the uremic static group). (A color version of this figure is available in the online journal.)
Like the normal serum group, LSS and OSS have similar effect on ECs and cocultured VSMCs in the uremia environment. The expression of VEGF-A, PDGF-BB, and FGF-4 increased, ERK1/2 pathway was activated, Caveolin-1 decreased in ECs, and the migration and proliferation of VSMCs were also up-regulated following the application of LSS and OSS in the uremia environment, compared to the uremic static group (Figure 6(a) to (e)). However, both NSS and HSS had no consistent effect on ECs and VSMCs (Figure 6(a) to (e)).
LSS-induced proliferation and migration of VSMCs is inhibited by the pretreatment of cocultured ECs with LV-Cav-1 or PD98059
We hypothesized that Caveolin-1-ERK1/2 pathway might play a crucial role in the interactions between ECs and VSMCs during the process of shear stress-induced vascular pathologies. We overexpressed Caveolin-1 with LV-Cav-1 and used PD98059 to inhibit the bioactivity of ERK1/2 to determine whether the Caveolin-1-ERK1/2 pathway was involved in the process of EC-regulated migration and proliferation of cocultured VSMCs following the application of LSS. The expressions of PDGF-BB, VEGF-A, FGF-4, and phosphor-ERK1/2 in ECs were down-regulated (Figure 7(a) and (b)), and migration and proliferation of cocultured VSMCs (Figure 7(c) to (e)) were suppressed by using both LV-Cav-1 and PD98059.
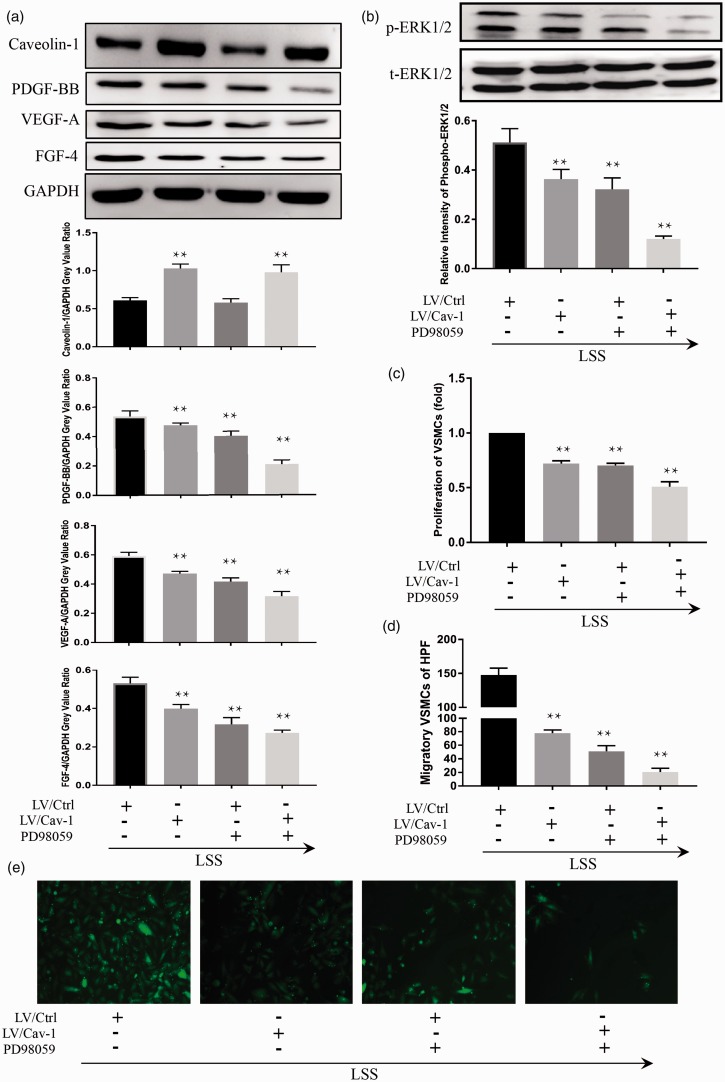
Figure 7.
The effect of Caveolin-1-ERK pathway on the LSS-induced migration and proliferation of cocultured VSMCs could be reduced by LV-Cav-1 and PD98059. In ECs, the expressions of PDGF-BB, VEGF-A, FGF-4, and phospho-ERK1/2 were down-regulated by pretreatment with LV-Cav-1 and PD98059 (a and b). Overexpressed Caveolin-1 suppressed the bioactivity of ERK1/2 in ECs also markedly repressed proliferation (c) and migration (d and e) of cocultured VSMCs. Incubation of both LV-Cav-1 and PD98059 in ECs resulted in the most significant difference. Compared to LV/Ctrl ECs, the phosphorylation of ERK1/2 was repressed in LV/Cav-1-infected ECs following the application of LSS (b). Incubation of PD98059 in ECs decreased the production of PDGF-BB, VEGF-A, FGF-4 in ECs (a), and suppressed the migration and proliferation of VSMCs (c, d and e) by LSS stimulation, but the expression of Caveolin-1 had no specific change (a). Values were expressed as mean ± SD for each condition from four independent experiments (**P < 0.001 vs. LV/Ctrl). (A color version of this figure is available in the online journal.)
Compared to mock transfection cells, the Caveolin-1-overexpressing cells showed hyporesponsiveness on ERK1/2 activation to LSS. LV/Ctrl cells up-regulated bioactivity of ERK1/2 following the application of LSS, whereas the phosphorylation of ERK1/2 was repressed in LV/Cav-1-infected ECs following LSS treatment (Figure 7(b)). Interestingly, accompanying the suppressed bioactivity of ERK1/2 by PD98059 in ECs, the production of PDGF-BB, VEGF-A, FGF-4 in ECs was decreased (Figure 7(a) and (b)), and the migration and proliferation of VSMCs were repressed (Figure 7(c) to (e)) by the LSS stimulation, but the expression of Caveolin-1 had no specific change (Figure 7(a)).
Discussion
In this study, we investigated the role of the shear stress-Caveolin-1-ERK1/2 pathway and uremia toxins in neointimal hyperplasia of AVF. First, we revealed a reduction in Caveolin-1 expression and an increase in ERK1/2 phosphorylation in neointimal hyperplasia region matched with LSS and OSS in the AVF of canine model. Second, LSS and OSS regulated the expression of PDGF-BB, VEGF-A, FGF-4, Caveolin-1, and phospho-ERK1/2 in EC and induced the migration and proliferation of cocultured VSMC in parallel-plate flow chamber system. Third, we found that both Caveolin-1 overexpression and inhibition of ERK1/2 pathway in ECs reduced the proliferation and migration of cocultured VSMCs by LSS. Furthermore, ERK1/2 phosphorylation is a downstream signaling event that was modulated by Caveolin-1. Finally, our results also indicate that uremic toxin induces the migration and proliferation of VSMC but not in a Caveolin-1-dependent manner.
The relationship between shear stress, caveolin-1, ERK1/2, and neointimal hyperplasia was disclosed based on animal model
In our previous study,9 we found that the WSS levels have an inverse relationship with neointimal hyperplasia in the AVF. Compared to the outer wall of AVF (high and laminar WSS region), VIH predisposed to occur in the inner wall (low and disturbed WSS region) of the anastomosis sites.9 There is enough evidence to suggest a link between the location of VIH and regional changes in hemodynamic patterns in AVF.28,29 Shear stress acting on the EC results in altered gene expression and production of several growth factors that interact with the underlying VSMCs.28,30,31 ECs dysfunction and VSMCs proliferation and migration are the main factors leading to the formation of VIH.32,33
In the present study, by using animal models, Caveolin-1 and ERK1/2 were differentially expressed between different sites matched to different shear stresses. We found that Caveolin-1 and ERK1/2 were involved in the development of AVF dysfunction. The down-regulation of Caveolin-1 and the up-regulation of ERK1/2 are involved in the neointimal hyperplasia of AVF in the canine model. Many studies have reported that the growth factors like PDGF-BB,34,35 VEGF-A,36–38 and FGF-421,39 participated in the migration and proliferation of VSMC and had a relationship with ERK1/2 pathway. The three secretory molecules PDGF-BB, VEGF-A, and FGF-4 were selected to further investigate the interaction of ECs and VSMC at different shear stress levels.
LSS and OSS are pathologically inducing factors for proliferation and migration of VSMC in cocultured systems
A parallel plate flow chamber system was used to study how the shear stress affects EC and their role in VSMC. ECs and VSMCs were grown on opposite sides of the PET membrane. Shear stress was applied to the EC side, while VSMC on the opposite side remains static, which is closer to the physical environment of vascular wall in the human body. Although the direct contact of EC and VSMC in co-culture is prevented, they act in a paracrine manner through producing transferable humoral factor.
In our cocultured system, LSS and OSS are pathologically inducing factors for vascular pathology by upregulating the VSMC proliferation and migration. Activation of ERK 1/2 pathway and down-regulation of Caveolin-1 may be involved in the effects of shear stress on migration and proliferation of VSMCs. The application of LSS and OSS to EC also resulted in increased expression of PDGF-BB, VEGF-A, FGF-4 in EC, compared to control group.
The possible effect of close communication between the cocultured cells is via humoral transmission. Therefore, we may postulate that the enhanced release of humoral substance derived from ECs responded to shear stress acts as modulators of VSMC proliferation and migration. In our co-culture system, where VSMC and EC are separated by a very thin membrane film, growth factors released from the EC can reach VSMC at very high concentrations for a limited time and distance, thus producing significant results. In view of this, it can be speculated that ECs induced by LSS and OSS produce PDGF-BB, and VEGF-A, FGF-4 may be involved in the regulation of proliferation and migration of adjacent VSMC by paracrine or autocrine action from EC.
Caveolin-1-ERK1/2 pathway is involved in EC-modulated VSMC proliferation and migration in response to LSS
The caveolae is the intrinsic cell surface plasma membrane invagination found in ECs. It is well known that caveolae play a significant role in cell biology and has been implicated in the development of neointimal hyperplasia and atherosclerosis.40 As a key component of the caveolae structure protein, Caveolin-1 has been extensively studied. Several studies have investigated the role of Caveolin-1 in cell migration.17,41 Our animal model demonstrates that LSS and OSS-induced VSMC proliferation and migration may be Caveolin-1 dependent. However, the underlying molecular mechanism remains elusive and further research is needed. There is currently some evidence that Caveolin-1 plays a role in promoting cell function through ERK 1/2 pathway. Engelman et al.42 reported that Caveolin-1 directly interacts with ERK 1/2 through its residues 32 to 95. Peptides from this region inhibit the activity of ERK1/2. Li et al.43 reported that interaction between Caveolin-1/Polymerase I and transcript release factors of PDGF receptor leads to inhibition of ERK1/2 phosphorylation. We hypothesized that the Caveolin-1-ERK1/2 pathway may be involved in neointimal hyperplasia and then explored the potential mechanisms in vitro.
Using the ECs/VSMCs co-cultured parallel plate flow chamber system, we further explored whether changes in Caveolin-1 and ERK1/2 expression affect the proliferation and migration of cocultured VSMC following shear stress. In fact, we found that LSS-induced proliferation and migration of VSMC was inhibited by the pre-treatment of cocultured EC with LV-Cav-1 or PD98059. Both the Caveolin-1 overexpression and ERK1/2 pathway suppression in ECs markedly decreased the expressions of PDGF-BB, VEGF-A, FGF-4 in ECs and reduced the migration and proliferation of cocultured VSMCs following the application of LSS, suggesting that Caveolin-1 and ERK1/2 play an important role in ECs-regulated migration and proliferation of VSMCs following the application of shear stress. We also found that Caveolin-1 overexpression in ECs downregulated the expression of phospho-ERK1/2 following LSS, but the ERK1/2 phosphorylation knockdown showed no notable effects on expression of Caveolin-1. Therefore, ERK1/2 phosphorylation is a downstream signaling event that was modulated by Caveolin-1. We found that Caveolin-1 regulates VSMC proliferation or migration through the ERK1/2 signaling pathway in response to LSS. These results, which are fully consistent with the results from the animal model study, indicate that Caveolin-1-ERK1/2 pathway is involved in EC-modulated VSMC migration and proliferation under LSS condition.
Uremia toxin induces the migration and proliferation of VSMCs without depending on caveolin-1 manner
MHD patients who are different from the general population, usually have microinflammation, oxidative stress, coagulation abnormalities, calcium and phosphorus metabolism disorders, which are unfavorable factors for AVF maturation and dysfunction.25 With the continuous deterioration of CKD, the glomerular filtration rate (GFR) decreases, the excretion of uremic toxins decreases and the accumulation of toxins increases in the body, resulting in dysfunction of ECs. Studies have found that low levels of GFR are associated with inflammation and potential ECs dysfunction.44
Our study demonstrates that uremia toxin upregulated the proliferation and migration of VSMCs and increased the expression of VEGF-A, PDGF-BB, FGF-4, and ERK1/2 phosphorylation in EC, compared to the normal serum static group. However, uremia toxin had no specific effects on expression of Caveolin-1. Therefore, uremia toxin induces the migration and proliferation of VSMCs but not in a Caveolin-1-dependent manner.
In the present study, our results suggest that shear stress-regulated VSMC proliferation and migration is a ECs-dependent process. LSS and OSS exert atherosclerotic influences on the ECs and VSMCs, which was consistent with our previous reports that LSS and OSS are the vascular wall virulence factor that induces neointimal hyperplasia in the animal model. We further explored the mechanism of LSS induced proliferation and migration of VSMCs by Caveolin-1-ERK1/2 pathway in vitro and suggested that Caveolin-1 and ERK1/2 can be used as a new therapeutic target for the treatment of AVF dysfunction.
Disadvantages
There are some shortcomings in this study. First, this study simulates the effects of hemodynamic changes in vivo on ECs and VSMCs with a parallel plate flow chamber. For the settings of our hemodynamic model, blood is usually assumed to be a single-phase Newtonian fluid and the vessel wall is assumed to be a uniformly deformable elastomer or a non-deformable rigid body. These two assumptions are different from the actual situation of blood flow in human body. Second, this study was to explore how ECs transmit the mechanical stimuli to VSMCs following the application of different shear stress (4 dyn/cm2, 12 dyn/cm2, 20 dyn/cm2 and 0 ± 4 dyn/cm2). However, the blood flow pattern may change with time during the process of AVF maturation and the actual hemodynamic situation in the human body is more complicated than the experiment. Third, uremia conditions were simulated by the culture medium with 10% serum from ESRD patients. Although the result may change with different concentration of uremic serum, the effect of uremic toxins on ECs and VSMCs has a consistent trend.
Authors' contributions
All authors participated in the design, interpretation of the studies and analysis of the data and review of the manuscript; Lan Jia, Zhe Wang, Haiyan Chen, Haibo Yu and Bo Wang conducted the experiments, Lan Jia, Lihua Wang, Fang Wei and Aili Jiang wrote the manuscript, and Chen Li contributed to perform hemodynamic analysis.
DECLARATION OF CONFLICTING INTERESTS
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
FUNDING
This study was supported by the National Natural Science Foundation of China (NSFC) (Grant number: 81600591); Natural Science Foundation of Tianjin (Grant number: 16JCQNJC11500); General Program of Tianjin Medical University (No:2016KYZM11); Project of Chinese Society Of Blood Purification Administration (No:CHABP201605); Informatization Special Funds of Tianjin City Commission for Industry and Commerce (No: 201708229).
ORCID iD
References
- 1.Saran R, Robinson B, Abbott KC, Agodoa LYC, Bragg-Gresham J, Balkrishnan R, Bhave N, Dietrich X, Ding Z, Eggers PW, Gaipov A, Gillen D, Gipson D, Gu H, Guro P, Haggerty D, Han Y, He K, Herman W, Heung M, Hirth RA, Hsiung JT, Hutton D, Inoue A, Jacobsen SJ, Jin Y, Kalantar-Zadeh K, Kapke A, Kleine CE, Kovesdy CP, Krueter W, Kurtz V, Li Y, Liu S, Marroquin MV, McCullough K, Molnar MZ, Modi Z, Montez-Rath M, Moradi H, Morgenstern H, Mukhopadhyay P, Nallamothu B, Nguyen DV, Norris KC, O'Hare AM, Obi Y, Park C, Pearson J, Pisoni R, Potukuchi PK, Repeck K, Rhee CM, Schaubel DE, Schrager J, Selewski DT, Shamraj R, Shaw SF, Shi JM, Shieu M, Sim JJ, Soohoo M, Steffick D, Streja E, Sumida K, Kurella Tamura M, Tilea A, Turf M, Wang D, Weng W, Woodside KJ, Wyncott A, Xiang J, Xin X, Yin M, You AS, Zhang X, Zhou H, Shahinian V. US renal data system 2018 annual data report: epidemiology of kidney disease in the United States. Am J Kidney Dis 2019; 73:A7–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jemcov TK, Van Biesen W. Optimal timing for vascular access creation. J Vasc Access 2017; 18:29–33 [DOI] [PubMed] [Google Scholar]
- 3.Berger JR, Hedayati SS. Renal replacement therapy in the elderly population. Clin J Am Soc Nephrol 2012; 7:1039–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ismail H, Abdul Manaf MR, Abdul Gafor AH, Mohamad Zaher ZM, Ibrahim A. Economic burden of ESRD to the Malaysian health care system. Kidney Int Rep 2019; 4:1261–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mandel EI, Bernacki RE, Block SD. Serious illness conversations in ESRD. Clin J Am Soc Nephrol 2017; 12:854–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roy-Chaudhury P, Arend L, Zhang J, Krishnamoorthy M, Wang Y, Banerjee R, Samaha A, Munda R. Neointimal hyperplasia in early arteriovenous fistula failure. Am J Kidney Dis 2007; 50:782–90 [DOI] [PubMed] [Google Scholar]
- 7.Alpers CE, Imrey PB, Hudkins KL, Wietecha TA, Radeva M, Allon M, Cheung AK, Dember LM, Roy-Chaudhury P, Shiu YT, Terry CM, Farber A, Beck GJ, Feldman HI, Kusek JW, Himmelfarb J. Histopathology of veins obtained at hemodialysis arteriovenous fistula creation surgery. J Am Soc Nephrol 2017; 28:3076–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sivanesan S, How TV, Bakran A. Sites of stenosis in AV fistulae for haemodialysis access. Nephrol Dial Transplant 1999; 14:118–20 [DOI] [PubMed] [Google Scholar]
- 9.Jia L, Wang L, Wei F, Yu H, Dong H, Wang B, Lu Z, Sun G, Chen H, Meng J, Li B, Zhang R, Bi X, Wang Z, Pang H, Jiang A. Effects of wall shear stress in venous neointimal hyperplasia of arteriovenous fistulae. Nephrology 2015; 20:335–42 [DOI] [PubMed] [Google Scholar]
- 10.Morbiducci U, Kok AM, Kwak BR, Stone PH, Steinman DA, Wentzel JJ. Atherosclerosis at arterial bifurcations: evidence for the role of haemodynamics and geometry. Thromb Haemost 2016; 115:484–92 [DOI] [PubMed] [Google Scholar]
- 11.Franzoni M, Cattaneo I, Longaretti L, Figliuzzi M, Ene-Iordache B, Remuzzi A. Endothelial cell activation by hemodynamic shear stress derived from arteriovenous fistula for hemodialysis access. Am J Physiol Heart Circ Physiol 2016; 310:H49–59 [DOI] [PubMed] [Google Scholar]
- 12.Roy-Chaudhury P, Wang Y, Krishnamoorthy M, Zhang J, Banerjee R, Munda R, Heffelfinger S, Arend L. Cellular phenotypes in human stenotic lesions from haemodialysis vascular access. Nephrol Dial Transplant 2009; 24:2786–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davies PF, Barbee KA, Volin MV, Robotewskyj A, Chen J, Joseph L, Griem ML, Wernick MN, Jacobs E, Polacek DC, dePaola N, Barakat AI. Spatial relationships in early signaling events of flow-mediated endothelial mechanotransduction. Annu Rev Physiol 1997; 59:527–49 [DOI] [PubMed] [Google Scholar]
- 14.Chen LJ, Wei SY, Chiu JJ. Mechanical regulation of epigenetics in vascular biology and pathobiology. J Cell Mol Med 2013; 17:437–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zaragoza C, Marquez S, Saura M. Endothelial mechanosensors of shear stress as regulators of atherogenesis. Curr Opin Lipidol 2012; 23:446–52 [DOI] [PubMed] [Google Scholar]
- 16.Frank PG, Lisanti MP. Role of caveolin-1 in the regulation of the vascular shear stress response. J Clin Invest 2006; 116:1222–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shihata WA, Michell DL, Andrews KL, Chin-Dusting JP. Caveolae: a role in endothelial inflammation and mechanotransduction? Front Physiol 2016; 7:628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee T, Misra S. New insights into dialysis vascular access: molecular targets in arteriovenous fistula and arteriovenous graft failure and their potential to improve vascular access outcomes. Clin J Am Soc Nephrol 2016; 11:1504–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yan G, Wang Q, Hu S, Wang D, Qiao Y, Ma G, Tang C, Gu Y. Digoxin inhibits PDGF-BB-induced VSMC proliferation and migration through an increase in ILK signaling and attenuates neointima formation following carotid injury. Int J Mol Med 2015; 36:1001–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cardus A, Parisi E, Gallego C, Aldea M, Fernandez E, Valdivielso JM. 1,25-Dihydroxyvitamin D3 stimulates vascular smooth muscle cell proliferation through a VEGF-mediated pathway. Kidney Int 2006; 69:1377–84 [DOI] [PubMed] [Google Scholar]
- 21.Yang X, Liaw L, Prudovsky I, Brooks PC, Vary C, Oxburgh L, Friesel R. Fibroblast growth factor signaling in the vasculature. Curr Atheroscler Rep 2015; 17:509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu S, Chen Y, Chen S, Ye N, Li Y, Sun Y. Klotho inhibits proliferation and migration of angiotensin II-induced vascular smooth muscle cells (VSMCs) by modulating NF-kappaB p65, Akt, and extracellular signal regulated kinase (ERK) signaling activities. Med Sci Monit 2018; 24:4851–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Srivastava T, Dai H, Heruth DP, Alon US, Garola RE, Zhou J, Duncan RS, El-Meanawy A, McCarthy ET, Sharma R, Johnson ML, Savin VJ, Sharma M. Mechanotransduction signaling in podocytes from fluid flow shear stress. Am J Physiol Renal Physiol 2018; 314:F22–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ruze A, Zhao Y, Li H, Gulireba X, Li J, Lei D, Dai H, Wu J, Zhao X, Nie Y. Low shear stress upregulates the expression of fractalkine through the activation of mitogen-activated protein kinases in endothelial cells. Blood Coagul Fibrinol 2018; 29:361–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kokubo T, Ishikawa N, Uchida H, Chasnoff SE, Xie X, Mathew S, Hruska KA, Choi ET. CKD accelerates development of neointimal hyperplasia in arteriovenous fistulas. J Am Soc Nephrol 2009; 20:1236–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grobmyer SR, Kuo A, Orishimo M, Okada SS, Cines DB, Barnathan ES. Determinants of binding and internalization of tissue-type plasminogen activator by human vascular smooth muscle and endothelial cells. J Biol Chem 1993; 268:13291–300 [PubMed] [Google Scholar]
- 27.Nackman GB, Fillinger MF, Shafritz R, Wei T, Graham AM. Flow modulates endothelial regulation of smooth muscle cell proliferation: a new model. Surgery 1998; 124:353–60 discussion 60–1 [PubMed] [Google Scholar]
- 28.Brahmbhatt A, Remuzzi A, Franzoni M, Misra S. The molecular mechanisms of hemodialysis vascular access failure. Kidney Int 2016; 89:303–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krishnamoorthy MK, Banerjee RK, Wang Y, Zhang J, Sinha Roy A, Khoury SF, Arend LJ, Rudich S, Roy-Chaudhury P. Hemodynamic wall shear stress profiles influence the magnitude and pattern of stenosis in a pig AV fistula. Kidney Int 2008; 74:1410–9 [DOI] [PubMed] [Google Scholar]
- 30.Li L, Terry CM, Blumenthal DK, Kuji T, Masaki T, Kwan BC, Zhuplatov I, Leypoldt JK, Cheung AK. Cellular and morphological changes during neointimal hyperplasia development in a porcine arteriovenous graft model. Nephrol Dial Transplant 2007; 22:3139–46 [DOI] [PubMed] [Google Scholar]
- 31.Redmond EM, Cullen JP, Cahill PA, Sitzmann JV, Stefansson S, Lawrence DA, Okada SS. Endothelial cells inhibit flow-induced smooth muscle cell migration: role of plasminogen activator inhibitor-1. Circulation 2001; 103:597–603 [DOI] [PubMed] [Google Scholar]
- 32.Kelly BS, Heffelfinger SC, Whiting JF, Miller MA, Reaves A, Armstrong J, Narayana A, Roy-Chaudhury P. Aggressive venous neointimal hyperplasia in a pig model of arteriovenous graft stenosis. Kidney Int 2002; 62:2272–80 [DOI] [PubMed] [Google Scholar]
- 33.Wang Y, Krishnamoorthy M, Banerjee R, Zhang J, Rudich S, Holland C, Arend L, Roy-Chaudhury P. Venous stenosis in a pig arteriovenous fistula model–anatomy, mechanisms and cellular phenotypes. Nephrol Dial Transplant 2008; 23:525–33 [DOI] [PubMed] [Google Scholar]
- 34.Kingsley K, Huff JL, Rust WL, Carroll K, Martinez AM, Fitchmun M, Plopper GE. ERK1/2 mediates PDGF-BB stimulated vascular smooth muscle cell proliferation and migration on laminin-5. Biochem Biophys Res Commun 2002; 293:1000–6 [DOI] [PubMed] [Google Scholar]
- 35.Qi YX, Jiang J, Jiang XH, Wang XD, Ji SY, Han Y, Long DK, Shen BR, Yan ZQ, Chien S, Jiang ZL. PDGF-BB and TGF-{beta}1 on cross-talk between endothelial and smooth muscle cells in vascular remodeling induced by low shear stress. Proc Natl Acad Sci U S A 2011; 108:1908–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parenti A, Brogelli L, Filippi S, Donnini S, Ledda F. Effect of hypoxia and endothelial loss on vascular smooth muscle cell responsiveness to VEGF-A: role of flt-1/VEGF-receptor-1. Cardiovasc Res 2002; 55:201–12 [DOI] [PubMed] [Google Scholar]
- 37.Parenti A, Bellik L, Brogelli L, Filippi S, Ledda F. Endogenous VEGF-A is responsible for mitogenic effects of MCP-1 on vascular smooth muscle cells. Am J Physiol Heart Circ Physiol 2004; 286:H1978–84 [DOI] [PubMed] [Google Scholar]
- 38.Yang B, Janardhanan R, Vohra P, Greene EL, Bhattacharya S, Withers S, Roy B, Nieves Torres EC, Mandrekar J, Leof EB, Mukhopadhyay D, Misra S. Adventitial transduction of lentivirus-shRNA-VEGF-A in arteriovenous fistula reduces venous stenosis formation. Kidney Int 2014; 85:289–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vazquez-Padron RI, Lasko D, Li S, Louis L, Pestana IA, Pang M, Liotta C, Fornoni A, Aitouche A, Pham SM. Aging exacerbates neointimal formation, and increases proliferation and reduces susceptibility to apoptosis of vascular smooth muscle cells in mice. J Vasc Surg 2004; 40:1199–207 [DOI] [PubMed] [Google Scholar]
- 40.Lamaze C, Tardif N, Dewulf M, Vassilopoulos S, Blouin CM. The caveolae dress code: structure and signaling. Curr Opin Cell Biol 2017; 47:117–25 [DOI] [PubMed] [Google Scholar]
- 41.Nunez-Wehinger S, Ortiz RJ, Diaz N, Diaz J, Lobos-Gonzalez L, Quest AF. Caveolin-1 in cell migration and metastasis. Curr Mol Med 2014; 14:255–74 [DOI] [PubMed] [Google Scholar]
- 42.Engelman JA, Chu C, Lin A, Jo H, Ikezu T, Okamoto T, Kohtz DS, Lisanti MP. Caveolin-mediated regulation of signaling along the p42/44 MAP kinase Cascade in vivo. A role for the caveolin-scaffolding domain. FEBS Lett 1998; 428:205–11 [DOI] [PubMed] [Google Scholar]
- 43.Li Q, Bai L, Liu N, Wang M, Liu JP, Liu P, Cong YS. Increased polymerase I and transcript release factor (cavin-1) expression attenuates platelet-derived growth factor receptor signalling in senescent human fibroblasts. Clin Exp Pharmacol Physiol 2014; 41:169–73 [DOI] [PubMed] [Google Scholar]
- 44.Bro S, Bentzon JF, Falk E, Andersen CB, Olgaard K, Nielsen LB. Chronic renal failure accelerates atherogenesis in apolipoprotein E-deficient mice. J Am Soc Nephrol 2003; 14:2466–74 [DOI] [PubMed] [Google Scholar]