Short abstract
This study aimed to investigate the effect of Angelica sinensis polysaccharides (ASP) on Alzheimer’s disease (AD) and its underlying mechanisms. In our study, we build the AD model by injecting Aβ25–35. Morris water maze (MWM) was applied to investigate learning and memory. Moreover, neurotransmitters, free radical, and inflammatory factors were also measured. Pathological change and neuronal death in hippocampus CA1, CA3, and DG region were detected by HE staining and Nissl staining. The neuronal apoptosis was detected by TUNEL. The expressions of caspase-3, Bcl-2 and Bax were measured by immunohistochemistry and Western blot. The expressions of BDNF, TrkB, p-Akt, Akt, p-CREB, and CREB were measured by Western blot. Our results showed that ASP could ameliorate spatial learning and memory deficiency in AD rats. ASP decreased AchE level and increased the levels of Ach and chAT in AD rats. ASP could increase the activity of SOD and CAT, decrease MDA activity, and inhibit the expression levels of inflammatory factors and neurons apoptosis in AD rats. Pathological change of hippocampus CA1, CA3, and DG region was ameliorated by ASP. In addition, the effects of ASP were reversed by K252a (TrkB inhibitor). Our study demonstrated that ASP could ameliorate memory impairment in AD rat through activating BDNF/TrkB/CREB pathway.
Impact statement
The present study demonstrated that ASP could ameliorate memory impairment through regulation of the balance of neurotransmitters, free radical metabolism, inflammation, and neurons apoptosis. Moreover, the mechanism of ASP on memory impairment may be related to BDNF/TrkB/CREB pathway in AD. Our research provides an innovatively regulatory mechanism about the ASP in AD rat and points a new way to the treatment of AD.
Keywords: Alzheimer’s disease, Angelica sinensis polysaccharides, Aβ25-35, BDNF/TrkB/CREB pathway, neurotransmitters, inflammatory factors
Introduction
Alzheimer’s disease (AD) is a chronic neurodegenerative disease that is characterized by progressive memory loss and cognitive disorder,1 which is one of the biggest health care challenges of the 21st century. AD is mainly characterized by formation of extracellular amyloid plaques and intracellular neurofibrillary tangles.2,3 β-amyloid (Aβ) is the main component of amyloid plaques, and the elevated Aβ is associated with the severity of cognitive deficits.4 Although numerous studies have focused on the investigation of diagnosis and pathogenesis of AD, the treatment protocol is limited.
Angelica sinensis, a commonly used traditional Chinese herbal, is widely used for nourishing the blood.5 Recent studies have indicated that extracts of Angelica sinensis have antioxidative and neuroprotective effects,6,7 and have been used in the treatment of gynecologic diseases, cardiovascular and cerebrovascular disease, and nervous system diseases.8 Angelica sinensis polysaccharides (ASPs) is one of the main extracted from the root of Angelica sinensis. However, the effect of ASP on AD has rarely been addressed.
Brain-derived neurotrophic factor (BDNF), an endogenous neurotrophin, is mainly synthesized by the brain and is expressed in the hippocampus and cerebral cortex.9 BDNF plays an important role in neuronal survival and learning and memory by binding its receptor tyrosine protein kinase B (TrkB).10,11 Subsequently, the activation of BDNF/TrkB signaling leads to the phosphorylation of the transcription factor cAMP response element binding protein (CREB).12 Previous researches have confirmed that Oleanolic acid could ameliorate memory deficit in AD rats by activating BDNF/TrkB/CREB pathway.13 However, the molecular mechanisms underlying ASP in AD and regulation of BDNF/TrkB/CREB pathway are rarely reported.
In this study, we investigated the effects of ASP on AD and its related molecular mechanisms, and found that ASP ameliorated memory impairment in AD rats through activating BDNF/TrkB/CREB pathway. Findings of our study may provide new theoretical foundation for deeply exploring the treatment of AD by using ASP.
Materials and methods
Animals
Male Sprague-Dawley rats (200 ± 20 g) were purchased from Hunan SJA Lab Animal Center of Changsha (Hunan, China). The rats were housed at a 12-h light/dark cycle under controlled temperature (22 ± 2°C) and humidity (50 ± 10%). All of the rats had free access to food and water. Moreover, the animals were allowed to adapt to the environment for seven days before the experiments. All animal experiments were conducted strictly in accordance with the guide for the care and use of laboratory animals and approved by the ethics committee of our hospital.
Modeling and administration
Sixty SD rats were randomly divided into six groups: Sham group, Model group, Donepezil HCL group, ASP group, Model + K252a group, and ASP + K252a group (n = 10 in each group). Rats were anaesthetized by intraperitoneal injection of pentobarbital sodium (50 mg/kg) and then fixed in the rat brain stereotaxic apparatus. Aβ25–35 (Sigma, USA) was dissolved in 0.9% sterile water at a concentration of 4 μg/μL and incubated for seven days at 37°C to cause aggregation. All rats (with the exception of the control group) were injected with 5 μL Aβ25–35 into bilateral CA1 subregion according to the stereotaxic coordinates of rat brain (3.3 mm posterior to bregma, 2.0 mm beside the sagittal suture and 3 mm beneath the surface of brain). The rats in the Sham group were administrated with sterile water using the same method. Three days after Aβ25–35 infusion, the rats of Donepezil HCL group, ASP group, and ASP + K252a group were separately given Donepezil HCL (0.251 mg/kg, bid., Eisai pharmaceutical Co., LTD, China) and ASP (50 mg/kg, bid., Jizhi biochemical Co., LTD, Shanghai, China) by intragastric administration for four weeks. The rats of Sham group, Model group, and Model + K252a group were given equal volume normal saline by intragastric administration. In addition, the animals of Model + K252a group and ASP + K252a group were intraperitoneally injected with K252a (100 μg/kg, Cayman, USA) 1 h before surgery.
Morris water maze test
After four weeks’ intragastric administration, spatial learning ability was investigated by using Morris water maze (MWM) test. Briefly, each rat was given four trials per day, with a maximum of 120 s allowed to find the submerged platform and stayed there for 10 s. If the rat could not find the platform within 120 s, the experimenter should guide it to the platform by hand and left it there for 30 s. This procedure was performed four times a day with 1-min interval for five consecutive days. The escape latency (time of reaching the hidden platform), the time spent in the target quadrant, and the frequency of rat crossing the virtual platform were recorded by the tracking system.
On the fifth day, the spatial probe trial was performed to assess the retention of spatial memory. The rat was put into the water maze and allowed to swim for 120 s. The escape latency, the time spent in the target quadrant, and the frequency of rat crossing the virtual platform were recorded by the tracking system.
Measuring SOD, CAT, and MDA in hippocampus tissue
The rats were sacrificed and the brain tissue was collected. For biochemical analysis, the brain tissue was homogenized and centrifuged at 4000 r/min for 10 min. Subsequently, the superoxide dismutase (SOD) and catalase (CAT) activity and malondialdehyde (MDA) levels were detected by using the corresponding kits (Beyotime, Shanghai, China) according to the manufacturer’s instructions.
Enzyme-linked immunosorbent assay
The rats were decapitated, and the brains were rapidly collected. Protein samples of brain tissue were then extracted. The levels of tumor necrosis factor α (TNF-α), interleukin-1β (IL-1β), and interleukin-6 (IL-6) were measured by using ELISA kits (Nanjing KeyGen Biotech Co., China) according to the methods provided by the manufacturer. In addition, the levels of AchE, Ach, and ChAT were measured according the ELISA kit instructions (AchE ELISA kit: Sigma, USA; Ach and ChAT ELISA kit: Merck, Germany).
Hematoxylin-eosin staining
The brains were collected and fixed in 4% formaldehyde solution overnight at 4°C. The paraffin-embedded tissues were sectioned to 5 μm thick sections after dehydration and vitrification. The tissue sections were deparaffined with xylene, rehydrated with gradient ethanol, and stained with HE dye in proper order. The histopathological changes of the hippocampus were visualized with an optical microscope (Olympus Optical Co Ltd; Tokyo, Japan) at 400 × magnification.
Nissl staining
The brain sections were dewaxed, rehydrated, and microwaved in 0.01 M sodium citrate buffer for 5 min. The sections were then cooled to room temperature and washed with PBS three times. After being stained with cresyl violet, the sections were dehydrated with 95% ethanol for 5 min, 100% ethanol for 10 min and xylene for 10 min, and fixed using mounting media. The histopathological changes of the hippocampus were visualized with an optical microscope (Olympus, Japan) at 400 × magnification.
Terminal dexynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) assay
Apoptosis cells were measured by using In Situ Cell Death Detection Kit (Roche, Mannheim, Germany). Briefly, the brains were collected and embedded in paraffin, and coronal sections (5 mm thick) were then stained according to the manufacturer’s instructions. Apoptotic cells were stained brown due to the binding of dUTP enzyme to their fragmented DNA. Apoptotic cells were counted for five fields per section under a magnification (400 ×) by a blinded manner.
Immunohistochemistry
Paraffin-embedded brain tissue was cut into 5 mm sections. The sections were incubated in peroxidase blocking reagent containing 3% H2O2 for 30 min to block endogenous peroxidase activity and nonspecific antigens. After washing, the sections were then blocked in normal goat serum for 15 min. Subsequently, the sections were incubated in the specific primary antibodies against Caspase 3 (1:100, Sigma, USA), Bcl-2 (1:100, Cell Signal, USA), and Bax (1:100, Cell Signal, USA) overnight. After washing, the sections were then incubated with the biotinylated secondary antibody at 37°C for 2 h. Diaminobenzidine (DAB) development and hematoxylin counter-staining were performed, followed by dehydration, clearing, and sectioning. Positive cells were stained brown and counted for five fields per section under a magnification (400 ×) by a blinded manner.
Western blot analysis
The rats were decapitated and total proteins of brain were extracted by using the protein extraction kits following the manufacturer’s instructions. Protein samples (50 μg) were subjected to 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred onto a nitrocellulose membrane. The membranes were blocked with 5% skimmed milk in TBST, followed by overnight incubations at 4°C with the corresponding primary antibody (BDNF, 1:1000, caspase 3, 1:1000, Sigma, USA; TrkB, 1:500, Abcam, USA; CREB, 1:1000, p-CREB, 1:1000, β-actin, 1:1000, Santa Cruz, USA; Akt, 1:1000, p-Akt, 1:1000, Bcl-2, 1:1000, Bax, 1:1000, Cell Signal, USA). Afterwards, the membranes were treated with the horseradish peroxidase-conjugated secondary antibodies: anti-rabbit antibody (1:5000, Cell Signal, USA). Immunoreactive proteins were visualized with an enhanced chemiluminescence (ECL) kit and the signal densitometry was quantified using a Western blotting detection system (Quantity One, Bio-Rad, USA).
Statistical analysis
All statistical analyses were performed using SPSS 22.0 statistical software (Chicago, IL, USA). The results were presented as mean ± standard deviation (SD). The two-tailed t test was used for comparison between two groups, while one-way ANOVA followed by Tukey's Multiple Comparison post-test was selected for comparison among multiple groups. All experiments were repeated three times in this study. P < 0.05 was considered to be statistically significant.
Results
ASP ameliorates spatial learning and memory deficiency in Aβ25–35-induced AD rats
The results of MWM showed that the escape latencies of Sham group, Model group, Donepezil HCL group, and ASP group were progressively decreased in successive trials. The escape latencies of Model group were longer than those of the Sham group during the last one session (P < 0.05) (Figure 1(a)). Besides, the escape latencies of Donepezil HCL group and ASP group were significantly shorter than those of Model group during the last one session (P < 0.05) (Figure 1(a)). The results above indicated that ASP could ameliorate spatial learning impairments.
Figure 1.
ASP ameliorated spatial learning and memory deficiency in AD rats. (a) Escape latency time of rats in each group. (b) Time spent in the target quadrant during the MWM probe test. (c) Number of times the rat crossed platform position during the MWM probe test. Data were presented as mean ± standard deviation with repeated for three times. *P < 0.05, vs. Sham group; #P < 0.05, vs. Model group.
Consistent with the above finding, both the time spent in the target quadrant and the frequency of rat crossing the virtual platform in Model group were decreased compared with Sham group (P < 0.05) (Figure 1(b) and (c)). When compared with Model group, both the time spent in the target quadrant and the frequency of rat crossing the virtual platform were significantly increased in Donepezil HCL group and ASP group (P < 0.05) (Figure 1(b) and (c)).
ASP regulates the balance of neurotransmitters in Aβ25–35-induced AD rats
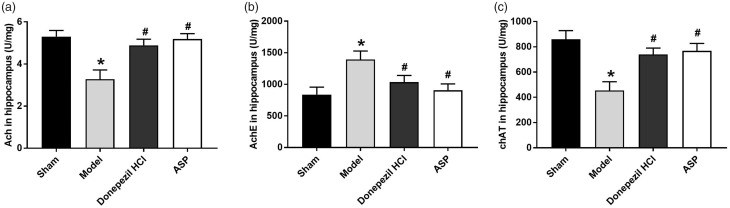
The results of ELISA showed that the levels of Ach and chAT in Model group were significantly decreased compared with Sham group (P < 0.05) (Figure 2(a) and (c)). On the contrary, the level of AchE in Model group was increased significantly than that in Sham group (P < 0.05) (Figure 2(b)). When compared with Model group, the levels of Ach and chAT were significantly increased in Donepezil HCL group and ASP group (P < 0.05) (Figure 2(a) and (c)), while AchE level was significantly decreased (P < 0.05) (Figure 2(b)). All the results suggested that ASP could increase the activities of Ach and chAT, and decrease the activities of AchE in Aβ25–35-induced AD rats.
Figure 2.
ASP regulated the balance of neurotransmitters in AD rats. (a) The activity of Ach in each group. (b) The activity of AchE in each group. (c) The activity of chAT in each group. Data were presented as mean ± standard deviation with repeated for three times. *P < 0.05, vs. Sham group; #P < 0.05, vs. Model group.
ASP decreases the free radical metabolism and inhibits the expressions of inflammatory factors in Aβ25–35-induced AD rats
As shown in Figure 3(a), the activity of SOD and CAT in Model group was significantly reduced than that in Sham group (P < 0.05). On the contrary, the activity of MDA in Model group was significantly increased than that in Sham group (P < 0.05). When compared with Model group, the activity of SOD and CAT was significantly increased in Donepezil HCL group and ASP group (P < 0.05), while MDA activity was significantly decreased (P < 0.05), suggesting that ASP could increase the activity of SOD and CAT and decrease MDA activity.
Figure 3.
ASP decreased the free radical metabolism and inhibited the expressions of inflammatory factors in AD rats. (a) The content of SOD, CAT, and MDA in each group. (b) The levels of IL-1β, IL-6, and TNF-α in each group. Data were presented as mean ± standard deviation with repeated for three times. *P < 0.05, vs. Sham group; #P < 0.05, vs. Model group.
The results of ELISA revealed that the levels of IL-1β, IL-6, and TNF-α in Model group were significantly increased compared with Sham group (P < 0.05). When ASP and Donepezil HCL were given, the levels of IL-1β, IL-6 and TNF-α were significantly decreased in comparison with Model group (P < 0.05) (Figure 3(b)). All the results above indicated that ASP could inhibit the expressions of inflammatory factors in Aβ25–35-induced AD rats.
ASP decreases hippocampus neurons damage in Aβ25–35-induced AD rats
The results of HE staining showed that the CA3 and DG region hippocampus neurons in Sham group were orderly arranged with normal structure, clear nuclei, obvious nucleoli, and abundant cytoplasm. A large number of swollen neurons with loosen structure, karyopyknosis, and forming some vacuolar structures could be observed in the Model group. When compared with Model group, the pathological changes of hippocampus neurons were ameliorated in both Donepezil HCL group and ASP group. In addition, CA1 region hippocampus neurons had no obvious pathological changes in different groups (Figure 4(a)). Next, we investigated the roles of ASP in hippocampus neurons survival by Nissl staining (Figure 4(b)). When compared with Sham group, the number of positive neurons cells in CA3 and DG region hippocampus was significantly decreased in Model group (P < 0.05). After administration of Donepezil HCL and ASP, the number of positive neurons cells in CA3 and DG region hippocampus was significantly increased compared with Model group (P < 0.05). All those results suggested that ASP could decrease hippocampus neurons damage in Aβ25–35-induced AD rats.
Figure 4.
ASP decreased hippocampus neurons damage in AD rats. (a) Pathological change of hippocampus CA1, CA3, and DG region were detected by HE staining (× 400). (b) Neuronal death in hippocampus CA1, CA3, and DG region was examined by Nissl staining (× 400). Data were presented as mean ± standard deviation with repeated for three times. *P < 0.05, vs. Sham group; #P < 0.05, vs. Model group. (A color version of this figure is available in the online journal.)
ASP inhibits neuronal apoptosis in Aβ25–35-induced AD rats
Neuronal apoptosis was detected by TUNEL assay and the positive cells were stained brown. When compared with Sham group, the number of positive cells was significantly increased in Model group (P < 0.05) (Figure 5(a)). After administration of Donepezil HCL and ASP, the number of positive cells was significantly decreased compared with Model group (P < 0.05). We further investigated the expressions of apoptosis-related proteins (caspase-3, Bcl-2 and Bax) by immunohistochemistry (Figure 5(b)) and Western blot (Figure 5(c) and (d)). Both immunohistochemistry and Western blot results showed that the expression levels of caspase-3 and Bax in Model group were significantly increased than those in Sham group (P < 0.05). On the contrary, Bcl-2 expression was reduced in Model group compared with Sham group (P < 0.05). The ratio of Bcl-2/Bax was significantly decreased in Model group compared with Sham group (P < 0.05). When ASP and Donepezil HCL were given, the expressions of caspase-3 and Bax were significantly decreased, while Bcl-2 expression and Bcl-2/Bax ratio were significantly increased in ASP and Donepezil HCL rats compared with Model group (P < 0.05). All the results above revealed that ASP could inhibit neuronal apoptosis in Aβ25–35-induced AD rats.
Figure 5.
ASP inhibited neuronal apoptosis in AD rats. (a) The neuronal apoptosis was detected by TUNEL. (b) The expressions of caspase-3, Bcl-2 and Bax were measured by immunohistochemistry. (c) The expressions of caspase-3, Bcl-2 and Bax were measured by Western blot. (d) Ratios of Western blot products relative to Bcl-2 and Bax. Data were presented as mean ± standard deviation with repeated for three times. Scale bar = 50 μm, arrows stand for positive cells. *P < 0.05, vs. Sham group; #P < 0.05, vs. Model group. (A color version of this figure is available in the online journal.)
ASP activates BDNF/TrkB/CREB pathway in the hippocampus of rats
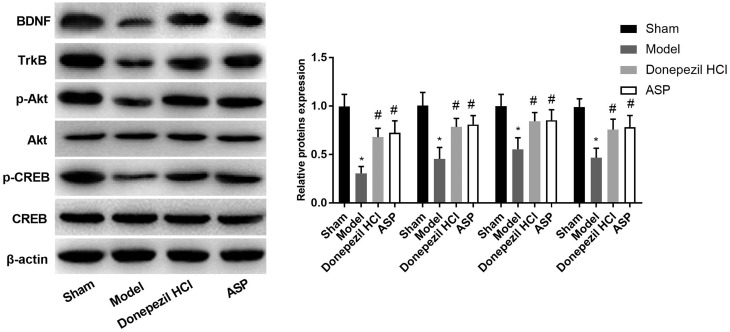
As shown in Figure 6, the expression levels of BDNF and TrkB in Model group were significantly decreased compared with Sham group (P < 0.05). When compared with Model group, BDNF and TrkB expression were significantly increased in Donepezil HCL group and ASP group (P < 0.05). In addition, the ratio of p-Art/Art and p-CREB/CREB in Model group was significantly decreased than that in Sham group (P < 0.05). When ASP and Donepezil HCL were given, the ratio of p-Art/Art and p-CREB/CREB was significantly increased, in comparison with Model group (P < 0.05). The results above suggested that ASP might protect Aβ25–35-induced AD by activating BDNF/TrkB/CREB pathway in rats.
Figure 6.
The expressions of BDNF, TrkB, p-Akt, Akt, p-CREB and CREB were measured by Western blot. Data were presented as mean ± standard deviation with repeated for three times. *P < 0.05, vs. Sham group; #P < 0.05, vs. Model group.
ASP protects Aβ25–35-induced AD by activating BDNF/TrkB/CREB pathway in rats
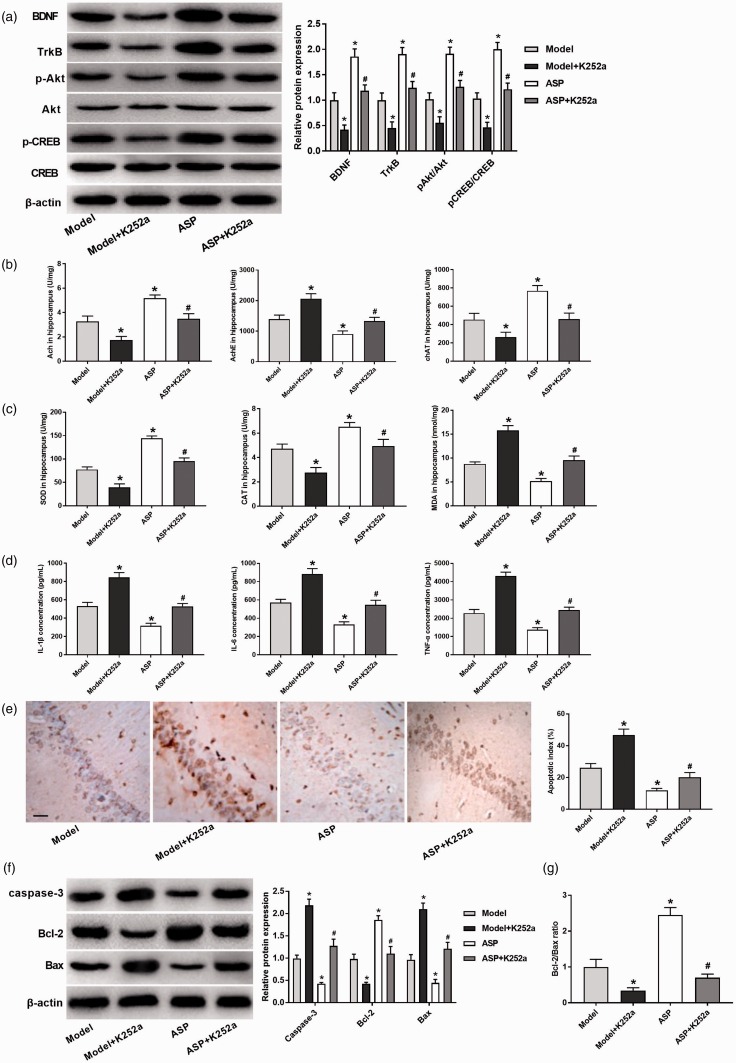
To further verify our hypothesis above, K252a (TrkB inhibitor) was injected to rats. As shown in Figure 7(a), the expression levels of BDNF, TrkB, p-Akt, and p-CREB in Model + K252a group were significantly decreased compared with Model group (P < 0.05). Meanwhile, the expression levels of BDNF, TrkB, p-Akt, and p-CREB in ASP + K252a group were significantly reduced than those in ASP group (P < 0.05). In addition, the levels of Ach and chAT were dramatically decreased and the levels of AchE were increased in Model + K252a group compared with Model group (P < 0.05). When compared with ASP group, the levels of Ach and chAT were significantly decreased in ASP + K252a group (P < 0.05), while AchE level was significantly increased (P < 0.05) (Figure 7(b)). As shown in Figure 7(c), the activity of SOD and CAT was significantly decreased and MDA was significantly increased in Model + K252a group than those in Model group (P < 0.05). When compared with ASP group, the activity of SOD and CAT was significantly decreased in ASP + K252a group (P < 0.05), while MDA activity was significantly increased (P < 0.05). The results of ELISA also revealed that the levels of IL-1β, IL-6, and TNF-α in Model + K252a group were significantly increased compared with Model group (P < 0.05). When compared with ASP group, the levels of IL-1β, IL-6 and TNF-α were markedly decreased in ASP + K252a group (P < 0.05) (Figure 7(d)). Figure 7(e) showed that cell apoptosis of Model + K252a group was markedly increased compared with Model group (P < 0.05), and the cell apoptosis of ASP + K252a group was markedly increased compared with ASP group (P < 0.05). These results were also confirmed in Western blot assay (Figure 7(f) and (g)). Caspase-3 and Bax expression in Model + K252a group were significantly increased than those in Model group (P < 0.05), and Bcl-2 expression and Bcl-2/Bax ratio were decreased in Model + K252a group compared with Model group (P < 0.05). Similar result was also found in ASP + K252a group in comparison with ASP group (P < 0.05). All the results above revealed that ASP could protect Aβ25–35-induced AD by activating BDNF/TrkB/CREB pathway in rats.
Figure 7.
ASP protected Aβ25–35-induced AD by activating BDNF/TrkB/CREB pathway in rats. (a) The expressions of BDNF, TrkB, p-Akt and p-CREB were measured by Western blot. (b) The activity of Ach, AchE, and chAT was detected in each group. (c) The content of SOD, CAT, and MDA was detected in each group. (d) The levels of IL-1β, IL-6, and TNF-α were measured in each group. (e) The neuronal apoptosis was detected by TUNEL. (f) The expressions of caspase-3, Bcl-2 and Bax were measured by Western blot. (g) Ratios of Western blot products relative to Bcl-2 and Bax. Data were presented as mean ± standard deviation with repeated for three times. Scale bar = 50 μm. *P < 0.05, vs. Model group; #P < 0.05, vs. ASP group. (A color version of this figure is available in the online journal.)
Discussion
AD is the most common form of dementia, which is characterized by progressive cognitive impairment, amnesia, and behavioral changes.14,15 ASP has been reported to play important role in preventing cell senescence of hematopoietic stem cell16 and protecting hypoxic-induced cytotoxicity neural stem cell.7 In the current study, after construction of Aβ25–35-induced AD rat model, we demonstrated that ASP could ameliorate memory impairment and reverse the upregulation of pro-inflammatory cytokine (TNF-α, IL-1β and TNFα) levels through activating BDNF/TrkB/CREB pathway.
As the initial extraction of Angelica sinensis, ASP has been reported to have beneficial effects in many diseases, including breast cancer,17 liver cancer,18 and acute myelogenous leukemia.19 Additionally, ASP has the ability to improve the memory impairment in rat, as well as inhibit the development of brain senility.20 In our study, MWM test confirmed that ASP could shorten the escape latencies, increase the activity time in platform quadrant and quadrant crossing times, suggesting its role in ameliorating spatial learning and memory deficiency in Aβ25–35-induced AD rats.
Acetylcholine (Ach) is a signal transmitter of cholinergic neurons. Researches have indicated that Ach in 90% of AD patients is deficient in the transmission of nerve impulse.21 The changes of chAT and AchE activity are considered to be important indicators to indirectly reflect the cholinergic biochemical changes in AD.22 In this study, ASP decreased AchE level and increased the levels of Ach and chAT, suggesting that ASP could regulate the balance of neurotransmitters in AD rats. Aβ-induced free radical and oxidative stress has been found to play a crucial part in the pathogenesis of AD.23 Sonnen et al.24 have reported that MDA is increased in hippocampus and plasma of AD patients, while SOD and CAT are decreased. Our results confirmed that ASP could increase the activity of SOD and CAT and decrease the MDA activity. Moreover, neuroinflammation also play important roles in the progression of AD.23 In AD, pro-inflammatory cytokines in cells including IL-1β and TNFα are released and are closely associated with neuronal function.25,26 Through decreasing the levels of IL-1β, IL-6, and TNF-a, and enhancing the antioxidant, APS improves neurogenesis and brain senescence in Chen et al.27 In this study, we found that ASP could significantly reduce the expressions of inflammatory factors in AD rats. Taken together, all of these studies suggested that ASP could ameliorate spatial learning and memory deficiency, and decrease free radical metabolism and neuroinflammation in AD rats.
BDNF is distributed in wide areas of the central nervous system.28 It is worth noting that BDNF inhibits neuronal apoptosis-induced Aβ.29 In AD, the expression of BDNF is significantly decreased in the hippocampus and partial cortical area.30 BDNF plays a substantial neuroprotective role via binding its specific receptor TrkB and then inducing Akt phosphorylation.31,32 Subsequently, the activation of BDNF/TrkB signaling leads to the phosphorylation of CREB.12 CREB is a major transcription factor involved in brain development, nerve survival, and neurogenesis, which regulates the transcription of BDNF and influences learning and memory processes through its phosphorylation caused by activating Akt signaling. Our results showed decreased BDNF, TrkB, p-Akt, and p-CREB expressions in Aβ25–35-induced AD rats, which were ameliorated by ASP treatment. Hence, we hypothesized that ASP could ameliorate memory impairment through activating BDNF/TrkB/CREB pathway in AD rat. To confirm the hypothesis above, K252a (an inhibitor of BDNF/TrkB pathway) was used, and we found that the effects of ASP on the balance of neurotransmitters, free radical metabolism, inflammation, and neurons apoptosis were reversed by K252a, suggesting that ASP could protect Aβ25–35-induced AD by activating BDNF/TrkB/CREB pathway in rats.
In conclusion, the present study demonstrated that ASP could ameliorate memory impairment through the regulation of the balance of neurotransmitters, free radical metabolism, and inflammation. Moreover, the mechanism of ASP on memory impairment may be related to the activation of BDNF/TrkB/CREB pathway in AD. Our research may provide an innovatively regulatory mechanism about the ASP in AD rat and a theoretical basis for the treatment of AD.
Authors’ contributions
All the authors participated in the design, interpretation of the studies and analysis of the data and review of the manuscript; Qian Du conducted the experiments, Xiaoyu Zhu statistical analysis of experimental results, Jieru Si wrote the manuscript.
DECLARATION OF CONFLICTING INTERESTS
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
FUNDING
The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD
References
- 1.Park SJ, Jung JM, Lee HE, Lee YW, Kim DH, Kim JM, Hong JG, Lee CH, Jung IH, Cho Y-B. The memory ameliorating effects of INM-176, an ethanolic extract of angelica gigas, against scopolamine-or Aβ1–42-induced cognitive dysfunction in mice. J Ethnopharmacol 2012; 143:611–20 [DOI] [PubMed] [Google Scholar]
- 2.Kumar S, Wirths O, Theil S, Gerth J. Early intraneuronal accumulation and increased aggregation of phosphorylated abeta in a mouse model of Alzheimer’s disease. Acta Neuropathol 125:699–709 [DOI] [PubMed]
- 3.Cotman CW, Tenner AJ, Cummings BJ. β-Amyloid converts an acute phase injury response to chronic injury responses. Neurobiol Aging 1996; 17:723–31 [DOI] [PubMed] [Google Scholar]
- 4.Ai LF, Cheng YZ, Xiang C. Thyroid hormone prevents cognitive deficit in a mouse model of Alzheimer’s disease. Neuropharmacology 2010; 58:722–9 [DOI] [PubMed] [Google Scholar]
- 5.Pei-Jou L. Hematopoietic effect of water-soluble polysaccharides from angelica sinensis on mice with acute blood loss. Exp Hematol 2010; 38:437–45 [DOI] [PubMed] [Google Scholar]
- 6.Xie X, Deng M, Yang Y, Zhang J, Xin J. Radix angelica sinensis that contains the component Z-Ligustilide promotes adult neurogenesis to mediate recovery from cognitive impairment. Curr Neurovasc Res 2013; 10:304–15 [DOI] [PubMed] [Google Scholar]
- 7.Lei T, Li H, Fang Z, Lin J, Wang S, Xiao L, Yang F, Liu X, Zhang J, Huang Z. Polysaccharides from angelica sinensis alleviate neuronal cell injury caused by oxidative stress. Neural Regen Res 2014; 9:260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xiao-Peng C. Phytochemical and pharmacological studies on radix angelica sinensis. Chin J Nat Med 2013; 6:577–87 [DOI] [PubMed] [Google Scholar]
- 9.Ivanova T, Beyer C. Pre- and postnatal expression of brain-derived neurotrophic factor mRNA/protein and tyrosine protein kinase receptor B mRNA in the mouse hippocampus. Neurosci Lett 2001; 307:21–4 [DOI] [PubMed] [Google Scholar]
- 10.Ai J, Sun L, Che H, Zhang R, Zhang T, Wu W, Su X, Chen X, Yang G, Li K, Wang N, Ban T, Bao Y, Guo F, Niu H, Zhu Y, Zhu X, Zhao S, Yang B. MicroRNA-195 protects against dementia induced by chronic brain hypoperfusion via its anti-amyloidogenic effect in rats. J Neurosci 2013; 33:3989–4001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu Q, Ji X, Chi T, Liu P, Jin G, Gu S, Zou L. Sigma 1 receptor activation regulates brain-derived neurotrophic factor through NR2A-CaMKIV-TORC1 pathway to rescue the impairment of learning and memory induced by brain ischaemia/reperfusion. Psychopharmacology 2015; 232:1779–91 [DOI] [PubMed] [Google Scholar]
- 12.Shang Y, Wang X, Li F, Yin T, Zhang J, Zhang T. rTMS ameliorates prenatal Stress-Induced cognitive deficits in Male-Offspring rats associated with BDNF/TrkB signaling pathway. Neurorehabil Neural Repair 2019; 33:271–83 [DOI] [PubMed] [Google Scholar]
- 13.Wang K, Sun W, Zhang L, Guo W, Xu J, Liu S, Zhou Z, Zhang Y. Oleanolic acid ameliorates Abeta25-35 injection-induced memory deficit in Alzheimer's disease model rats by maintaining synaptic plasticity. CNS Neurol Disord Drug Targets 2018; 17:389–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brookmeyer R, Kawas CH, Abdallah N, Paganini-Hill A, Kim RC, Corrada MM. Impact of interventions to reduce Alzheimer's disease pathology on the prevalence of dementia in the oldest-old. Alzheimers Dement 2016; 12:225–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fiest KM, Roberts JI, Maxwell CJ, Hogan DB, Smith EE, Frolkis A, Cohen A, Kirk A, Pearson D, Pringsheim T, Venegas-Torres A, Jetté N. The prevalence and incidence of dementia due to Alzheimer's disease: a systematic review and Meta-Analysis. Can J Neurol Sci 2016; 43:S51–82 [DOI] [PubMed] [Google Scholar]
- 16.Mu X, Zhang Y, Li J, Xia J, Chen X, Jing P, Song X, Wang L, Wang Y. Angelica sinensis polysaccharide prevents hematopoietic stem cells senescence in D-galactose-induced aging mouse model. Stem Cells Int 2017; 2017:3508907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou WJ, Wang S, Hu Z, Zhou ZY, Song CJ. Angelica sinensis polysaccharides promotes apoptosis in human breast cancer cells via CREB-regulated caspase-3 activation. Biochem Biophys Res Commun 2015; 467:562–9 [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y, Cui Z, Mei H, Xu J, Zhou T, Cheng F, Wang K. Angelica sinensis polysaccharide nanoparticles as a targeted drug delivery system for enhanced therapy of liver cancer. Carbohydr Polym 2019; 219:143–54 [DOI] [PubMed] [Google Scholar]
- 19.Liu J, Xu CY, Cai SZ, Zhou Y, Li J, Jiang R, Wang YP. Senescence effects of angelica sinensis polysaccharides on human acute myelogenous leukemia stem and progenitor cells. Asian Pac J Cancer Prev 2014; 14:6549–56 [DOI] [PubMed] [Google Scholar]
- 20.Chen XP, Li W, Xiao XF, Zhang LL, Liu CX. Phytochemical and pharmacological studies on radix angelica sinensis. Chin J Nat Med 2013; 11:577–87 [DOI] [PubMed] [Google Scholar]
- 21.Liu Y, Liu Z, Wei M, Hu M, Yue K, Bi R, Zhai S, Pi Z, Song F, Liu Z. Pharmacodynamic and urinary metabolomics studies on the mechanism of schisandra polysaccharide in the treatment of Alzheimer's disease. Food Funct 2019; 10:432–47 [DOI] [PubMed] [Google Scholar]
- 22.Mantzavinos V, Alexiou A. Biomarkers for Alzheimer's disease diagnosis. Curr Alzheimer Res 2017; 14:1149–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Azm SAN, Vafa M, Sharifzadeh M, Safa M, Mirshafiey A. Effects of M2000 (D-Mannuronic acid) on learning, memory retrieval, and associated determinants in a rat model of Alzheimer's disease. Am J Alzheimer Dis Other Dement 2016; 32:12–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sonnen JA, Breitner JC, Lovell MA, Markesbery WR, Quinn JF, Montine TJ. Free Radical-mediated damage to brain in Alzheimer's disease and its transgenic mouse models. Free Radic Biol Med 2008; 45:219–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rakic S, Hung YMA, Smith M, So D, Tayler HM, Varney W, Wild J, Harris S, Holmes C, Love S, Stewart W, Nicoll JAR, Boche D. Systemic infection modifies the neuroinflammatory response in late stage Alzheimer's disease. Acta Neuropathol Commun 2018; 6:018–0592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heneka MT, O'Banion MK, Terwel D, Kummer MP. Neuroinflammatory processes in Alzheimer's disease. J Neural Transm 2010; 117:919–47 [DOI] [PubMed] [Google Scholar]
- 27.Cheng X, Yao H, Xiang Y, Chen L, Xiao M, Wang Z, Xiao H, Wang L, Wang S, Wang Y. Effect of angelica polysaccharide on brain senescence of Nestin-GFP mice induced by D-galactose. Neurochem Int 2019; 122:149–56 [DOI] [PubMed] [Google Scholar]
- 28.Mariga A, Mitre M, Chao MV. Consequences of brain-derived neurotrophic factor withdrawal in CNS neurons and implications in disease. Neurobiol Dis 2017; 97:73–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arancibia S, Silhol M, Moulière F, Meffre J, Höllinger I, Maurice T, Tapia-Arancibia L. Protective effect of BDNF against beta-amyloid induced neurotoxicity in vitro and in vivo in rats. Neurobiol Dis 2008; 31:316–26 [DOI] [PubMed] [Google Scholar]
- 30.Zhang Z, Liu X, Schroeder JP, Chan C-B, Song M, Yu SP, Weinshenker D, Ye K. 7,8-dihydroxyflavone prevents synaptic loss and memory deficits in a mouse model of Alzheimer's disease. Neuropsychopharmacology 2014; 39:638–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yin C, Deng Y, Liu Y, Gao J, Yan L, Gong Q. Icariside II ameliorates cognitive impairments induced by chronic cerebral hypoperfusion by inhibiting the amyloidogenic pathway: involvement of BDNF/TrkB/CREB signaling and up-Regulation of PPARα and PPARγ in rats. Front Pharmacol 2018; 9:1211. [DOI] [PMC free article] [PubMed] [Google Scholar]