Highlights
► Evoked responses to biological motion (BM) were measured in children aged 6–15 years. ► These were measured at one-year intervals for two years. ► Three components were specified. ► Enhanced responses to BM were observed in all age groups. ► Evoked responses change from 8-year-olds to 12-year-olds.
Keywords: Point-light walker, Biological motion, Event-related potential (ERP), Longitudinal study, Development, Children
Abstract
Event-related potentials were measured in twenty-four children aged 6–15 years, at one-year intervals for two years, to investigate developmental changes in each subject's neural response to a point-light walker (PLW) and a scrambled PLW (sPLW) stimulus. One positive peak (P1) and two negative peaks (N1 and N2) were observed in both occipitotemporal regions at approximately 130, 200, and 300–400 ms. The amplitude and latency of the P1 component measured by the occipital electrode decreased during development over the first one-year period. Negative amplitudes of both N1 and N2, induced by the PLW stimulus, were significantly larger than those induced by the sPLW stimulus. Moreover, for the P1–N1 amplitude, the values for the eight-year-old children were significantly larger than those for the twelve-year-old children. N1 and N2 latency at certain electrodes decreased with age, but no consistent changes were observed. These results suggest that enhanced electrophysiological responses to PLW can be observed in all age groups, and that the early components were changed even over the course of a single year at the age of twelve.
1. Introduction
Biological motion (BM) is a phenomenon whereby one can perceive vivid actions with only a dozen points of light representing the joints (Johansson, 1973). Interestingly, much information can be extracted from point-light motion, such as individual identification (Cutting and Kozlowski, 1977, Troje et al., 2005), gender (Kozlowski and Cutting, 1977, Troje, 2002), direction (Beintema and Lappe, 2002, Bertenthal and Pinto, 1994, Mather et al., 1992, Troje and Westhoff, 2006) or emotion (Dittrich, 1993, Pollick et al., 2001).
Previous neuroimaging studies have revealed that the posterior part of the superior temporal sulcus (pSTS) plays an important role in the perception of BM (Bonda et al., 1996, Grossman and Blake, 2001, Grossman et al., 2000, Michels et al., 2009, Pelphrey et al., 2003, Peuskens et al., 2005, Vaina et al., 2001). Further, along with pSTS regions, the middle temporal/V5 complex (hMT/V5+) (Grezes et al., 2001, Howard et al., 1996), fusiform gyrus (Grossman and Blake, 2002, Grossman et al., 2004, Santi et al., 2003), amygdala (Bonda et al., 1996), frontal region (Saygin, 2007, Saygin et al., 2004), kinetic-occipital (KO) (Servos et al., 2002, Vaina et al., 2001), and cerebellum (Grossman et al., 2000, Jokisch et al., 2005b) are also involved in BM processing. A recent fMRI study demonstrated that the fusiform body area (FBA) and the extrastriate body area (EBA) are the two main regions involved in the processing of the point-light walker (PLW) stimulus (Jastorff and Orban, 2009).
Several event-related potential (ERP) (Hirai et al., 2003, Hirai et al., 2005, Jokisch et al., 2005a, Krakowski et al., 2011) and magnetoencephalography (MEG) studies (Hirai et al., 2008, Pavlova et al., 2006, Virji-Babul et al., 2007) have also shown the neural dynamics of BM processing. ERP studies (Hirai et al., 2003, Hirai et al., 2005, Jokisch et al., 2005a, Krakowski et al., 2011) have demonstrated that two negative components are specified at around 200 ms and 240–330 ms after stimulus onset in the bilateral occipitotemporal region. These results suggest that the first component, which was estimated in the vicinity of the KO/hMT region (Krakowski et al., 2011), reflects the processing of motion (Hirai and Kakigi, 2008) or a pattern of moving dots that represent a familiar human form, such as body-sensitive neural responses that are observed at 190 ms (Peelen and Downing, 2007, Stekelenburg and de Gelder, 2004, Thierry et al., 2006). The second component (∼500 ms), which was estimated in the vicinity of the pSTS region (Hirai et al., 2008, Krakowski et al., 2011) or the superior temporal gyrus and fusiform gyrus regions (Jokisch et al., 2005a) might be sensitive to coherent human forms, rather than object forms as mediated by global motion information (Safford et al., 2010, Virji-Babul et al., 2007). This concept of a two-stage processing model in BM seems to be compatible with the framework regarding a hierarchical model of BM processing (Troje, 2008).
The processing of BM presents at the early stages of development and changes from birth to later childhood. Previous behavioral evidence also indicates that infants aged from 3 to 9 months show a differential preference toward a BM stimulus as well as other kinds of motion pattern stimuli (Bertenthal et al., 1984, Bertenthal et al., 1987, Fox and McDaniel, 1982). Moreover, a recent study demonstrated that even newborn infants can distinguish a BM stimulus from other kinds of motion stimuli (Simion et al., 2008), implying that the preference for BM may be equipped innately and the sensitivity to the BM changes by age 9 (Blake et al., 2003, Freire et al., 2006, Jordan et al., 2002, Pavlova et al., 2001). Moreover, a recent study showed that the performance on the emotional detection from point-lights motion improves drastically until 8.5 years of age, followed by a much slower improvement rate through late childhood and adolescence (Ross et al., 2012).
From the view of atypical development, a behavioral study revealed that children with autism aged 8–10 years did not perform as well as their normal counterparts; however, performance of the static version of the form-from-motion task was equal between the groups (Blake et al., 2003). The atypical processing of BM in children and adolescents with autism has been consistently reported in recent studies (Annaz et al., 2012, Kaiser et al., 2010b, Klin et al., 2009, Koldewyn et al., 2010). However, for adults with autism, it is still controversial whether or not the processing of BM is typical (Murphy et al., 2009, Saygin et al., 2010) or atypical (Cook et al., 2009, Kaiser et al., 2010a). These studies imply that the atypical processing of BM is consistently observed in children with autism, however whether this persists in adulthood is controversial.
Results from these behavioral studies have demonstrated that BM-detecting mechanisms emerge during an early stage of development and that they change remarkably during childhood. Supporting these findings, several ERP, MEG, or fMRI studies on BM perception in infants or children indicate that the differential neural activities were observed between BM and other kinds of motion stimuli in 5-month-old infants (Marshall and Shipley, 2009) and 8-month-old infants (Hirai and Hiraki, 2005, Reid et al., 2006) and the neural activity changes by the age of 10 (Carter and Pelphrey, 2006, Hirai et al., 2009).
Despite the fact that the neural response to the BM stimuli in children aged from 6 to 10 years old changed dramatically during their development, previous ERP studies did not follow how the neural responses to BM change during this period. Moreover, children with autism (average age, 8.4 years) were significantly impaired during the BM detection task, when compared against typically developed children (average age, 7.9 years) (Blake et al., 2003). Thus, to aid in further studies, an electrophysiological index of BM processing during childhood needs to be established, to determine how the component(s) would be modulated by autistic or atypically developed children aged between 7 and 10 years.
To address these issues, ERPs were measured in twenty-four children, aged 6–14 years, to compare the developmental changes in the neural responses to BM over a two-year period, at one-year intervals. Specifically, the developmental changes of P1, N1, and N2 ERP components related to BM processing were evaluated in each child for two years.
2. Methods
2.1. Participants
Twenty-four children participated in the present study at one-year intervals for two years. The period of examination during the two years was approximately one year for each child (12.0 ± 0.8 months, Mean ± SD). Japanese children were recruited from elementary and junior high schools in Okazaki, Japan. Children in this study had no history of neurological disorders. All children and their parents provided informed consent to participate, and the experimental protocol was approved by the Ethics Committee of the National Institute for Physiological Sciences. Children were divided into three groups (8-year-olds, 10-year-olds, and 12-year-olds), as indicated in Table 1.
Table 1.
Study participants in the first year.
| Group | Number | Age range, mean age (SD) | Female | Male |
|---|---|---|---|---|
| 8-year-olds | 8 | 6–9 years 8 years 4 months (±12 months) |
1 | 7 |
| 10-year-olds | 8 | 9–11 years 10 years 1 months (±5 months) |
5 | 3 |
| 12-year-olds | 8 | 11–14 years 12 years 1 months (±11 months) |
1 | 7 |
2.2. Experimental stimuli and tasks
Two forms of visual stimuli (PLW and sPLW) were used, as had been in previous studies (Grossman et al., 2000, Hirai et al., 2003). The point-light walker (PLW; basic stimulus) was generated from computer algorithms developed by Cutting (1978). The animation was comprised of 11 moving point-lights attached to the head and main body joints; the animation appeared as if a person was walking on a treadmill. Two different walker animations were created (one facing to the left and one facing to the right). For a control, a scrambled PLW (sPLW) stimulus was used. Each sPLW point had the same velocity vector (i.e., the same speed and direction for each point) as the PLW points, and there were the same number of point-lights, although the initial spatial position was randomized. Thus, the only difference between the PLW and sPLW stimuli was the spatial configuration of point-lights; that is, random versus ordered. In each stimulus, ten different stimulus patterns were created by shifting the starting frame from the original stimulus. The two kinds of walker (namely, left and right facing) were presented randomly. In this experiment, speed of gait was 2.0 steps per second and frame duration was 33 ms, producing a smooth animation. Each stimulus was presented for 500 ms and the inter-stimulus interval varied randomly between 960 and 1440 ms. The animation was displayed subtending a visual angle of 3° × 3° on a 21-in. CRT monitor (SONY, GDM-F520), at a viewing distance of 150 cm. All points were white against a black background. A red fixation point was presented at the center of the monitor during the stimulus presentation and the inter-stimulus interval, and participants were required to fixate on this point. The fixation point was placed in the center of both the PLW and sPLW stimuli. The experimental equipment, environment, and procedures were identical at all points in time.
The experiment consisted of seven blocks. In a single block, each experimental stimulus (PLW and sPLW) was randomly presented 12 times. Two kinds of tasks were required; one during and one after the experiment. To maintain their attention on the center of the monitor during the experiment, participants were instructed to press a button with their right thumb when a target (static point-lights) was presented instead of the animation. One frame was extracted from the PLW or sPLW animation to create the target. Following experimentation, children were presented with each visual stimulus and were required to verbally describe what the presented stimuli (both PLW and sPLW) looked like. In the verbal task, children were allowed to describe freely whatever came to mind. During a single block, a static point-light stimulus was randomly presented twice. The experimental task and procedures were also identical at all points in time.
2.3. EEG recording and data analysis
In both the first and second year, electroencephalograms (EEGs) were recorded using Ag/AgCl disk electrodes placed on the scalp at 21 locations: Nose, A1, A2, O1, O2, P3, P4, Pz, T3, T4, C3, C4, Cz, F3, F4, Fz, FCz, T5, T6, T5′, and T6′, according to the International 10–20 System. T5′ and T6′ were located 2 cm below T5 and T6, as they had been placed in our previous studies (Watanabe et al., 2003). Two electrodes, HEOG (right temple) and VEOG (above the right eye), were used to record electro-oculograms (EOGs) for the identification of horizontal and vertical eye movements. Impedance was maintained at less than 5 kΩ. All EEG signals were collected on a signal processor (EEG-1100, Nihon-Kohden, Tokyo, Japan). The bandpass filter was set at 0.1–100 Hz. All recordings were initially referenced to C3 and C4 (based on system settings), but later to the tip of the nose to match previous ERP studies (Hirai and Kakigi, 2008, Hirai et al., 2009, Jokisch et al., 2005a). Electrical potential was digitized at a 1000-Hz sampling rate, and data was stored on a computer disk for offline analysis.
2.4. Data analysis (ERP waveform analysis)
In the off-line analysis of EEG recordings, a 0.1–30 Hz bandpass filter (24 dB/octave) was applied to the data. Trials in which the EEG or EOG signal variation exceeded a value of ±75 μV were discarded. The analysis window was extended for 500 ms following the onset of each stimulus. The mean amplitude during the 100 ms prior to stimulus presentation was used as the baseline and applied to individual data. Only ERP data from participants that exhibited signal variation of less than ±75 μV for each stimulus in over 30 trials were analyzed; a grand-averaged waveform for each group was calculated. All participants passed the above-mentioned criteria for both years. During the first year, the average number of accepted trials was as follows: 8-year-olds (PLW: 45.5 ± 8.1, sPLW: 46.8 ± 7.3 trials; mean ± SD), 10-year-olds (PLW: 50.5 ± 9.3, sPLW: 51.2 ± 9.1 trials), and 12-year-olds (PLW: 54.9 ± 15.4, sPLW: 56.8 ± 17.9 trials). During the second year, the number of accepted trials was as follows: 8-year-olds (PLW: 46.9 ± 13.5, sPLW: 48.4 ± 14.8 trials; mean ± SD), 10-year-olds (PLW: 62.3 ± 11.2, sPLW: 61.3 ± 12.5 trials), and 12-year-olds (PLW: 60.7 ± 12.8, sPLW: 64.2 ± 9.3 trials).
The data analysis focused on three ERP components (P1, N1, and N2), which were observed at approximately 130, 200, and ∼400 ms, respectively for the following reasons. First, in our previous study (Hirai et al., 2009), we focused on these three ERP components (P1, N1 and N2) and found for these components, both conditional differences (i.e. PLW vs. sPLW motion) and developmental changes in latencies. Moreover, a recent ERP study with adults by Krakowski et al. (2011) also found that these three components (P1, N1 and N2) were modulated by the stimuli. Therefore, we think it is reasonable to focus on these three ERP components in the present study to elucidate the developmental changes in the neural activities underlying the perception of BM. Secondly, we already know some things with regard to their generator(s): it is known that the N1 component is generated from the vicinity area of the hMT/V5 (Jokisch et al., 2005a, Krakowski et al., 2011) and the generator of the N2 component is estimated in the STG/STS region (Hirai et al., 2008, Jokisch et al., 2005a, Krakowski et al., 2011). Thus, we would be able to discuss the developmental changes of BM processing regarding not only the changes in the waveform shape but also the strength or timing of the source of each component.
The peak of each component was determined using the following time windows: P1 component (80–160 ms), N1 component (160–240 ms), and N2 component (240–440 ms). As in previous studies (Hirai et al., 2003, Hirai et al., 2005, Jokisch et al., 2005a), one positive component (P1) and two negative components (N1 and N2) were observed at the T5′/T6′ electrodes, and P1 and N1 were also observed at the O1/O2 electrodes. Thus, a statistical analysis was performed on the amplitude and latency of P1, N1, and N2 at the T5′/T6′ electrodes, as well as P1 and N1 at the O1/O2 electrodes. In addition to the analysis of each component, we analyzed the peak-to-peak (P1–N1) amplitude to address the large differences in P1 amplitude across age groups.
Both the behavioral and ERP data were analyzed using a mixed-design repeated measures analysis of variance (ANOVA), with the Greenhouse–Geisser epsilon correction for nonsphericity; Tukey's HSD was applied for multiple comparisons. For the behavioral data, a two-way ANOVA was applied to the percentages of correct performances. Group was used as an inter-subject factor (8-year-olds, 10-year-olds, and 12-year-olds) and Year was used as an intra-subject factor (first and second year).
Both the P1 and N1 components and the peak-to-peak (P1–N1) amplitude were analyzed using mixed-design repeated measures ANOVAs, with the Greenhouse–Geisser epsilon correction for nonsphericity; Tukey's HSD was applied for multiple comparisons. A five-way ANOVA was applied to the amplitude and latency of each component (P1 and N1) and P1–N1 amplitude. Group was used as an intersubject factor (8-year-olds, 10-year-olds, and 12-year-olds) and Hemisphere (Left Hemisphere and Right Hemisphere), Stimulus (PLW and sPLW), Electrode (Occipital and Occipitotemporal) and Year (first and second) were used as intrasubject factors. For the N2 component, a three-way ANOVA was applied to the amplitude and latency of N2. Group was used as an intersubject factor (8-year-olds, 10-year-olds, and 12-year-olds) and Hemisphere (Left Hemisphere and Right Hemisphere), Stimulus (PLW and sPLW) and Year (first and second) were used as intrasubject factors.
In the analysis, if the sphericity assumption was violated in Mauchly's sphericity test, the Greenhouse–Geisser epsilon coefficient was used to correct the degrees of freedom, then F and p values were recalculated. We considered statistical significance to be p < 0.05.
Because the number of averaged trials was different across groups, one might think that it would affect the results. In our preliminary analysis, we applied analysis of covariance (ANCOVA) to both the amplitude and the latency of each component, while the number of trials as covariate. However, we did not confirm a parallelism except for the N2 amplitude, thus it would not be appropriate to applying ANCOVA in the current results.
3. Results
3.1. Behavioral performance
In the first and second year, the percentages of correct performances for the target-detection task (detecting the static version of the stimulus) were as follows: (8-year-olds: 100.0 ± 0, 10-year-olds: 90.0 ± 5.3, 12-year-olds: 100.0 ± 0%; mean ± SE) in the first year and (8-year-olds: 97.5 ± 2.5, 10-year-olds: 97.9 ± 2.1, 12-year-olds: 100.0 ± 0%) in the second year. Taken together, no significant effect was observed when groups and years were applied in a two-way ANOVA analysis [Fs < 2.7, ps > 0.09], suggesting that all the groups successfully performed the task over two years.
Over a single year of development, nearly all participants reported that the PLW stimulus appeared to be a ‘walking human figure’. In contrast, it was difficult for all participants to perceive a human figure from the sPLW stimulus. The present results are in accordance with previous behavioral studies in children (Blake et al., 2003, Freire et al., 2006, Pavlova et al., 2001). These studies suggest that children as young as 5 years of age perform as well as adults in detecting unmasked BM. In the present study, all children in the 8-year-old group (mean age: 8 years and 4 months) reported that the PLW stimulus looked like a ‘walking human figure’.
3.2. ERP data
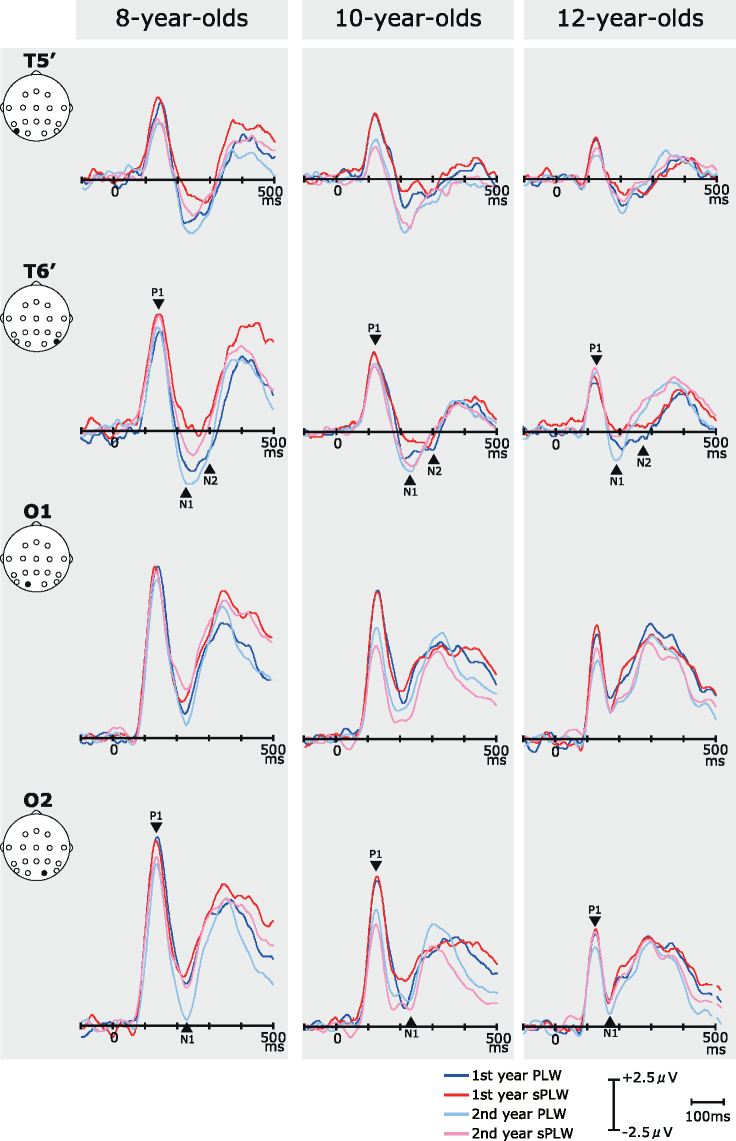
Fig. 1 shows the grand-averaged ERPs across the age groups for PLW and sPLW between the first and second year. Two prominent components (P1 and N1) at the O1/O2 electrodes, and three components (P1, N1 and N2) at the T5′/T6′ electrodes, were observed in response to both PLW and sPLW stimuli, which are similar to results from previous studies (Hirai et al., 2003, Hirai et al., 2005, Hirai et al., 2009, Jokisch et al., 2005a, Krakowski et al., 2011). A statistical analysis was performed on the latency and amplitude of each component.
Fig. 1.
Grand-averaged ERPs at four electrodes (O1, O2, T5′, T6′) in response to (A) PLW and (B) sPLW stimuli displayed to subjects in three age groups. Each color indicates a stimulus condition in the first or second year. (PLW condition in the first year (blue) and the second year (aqua), and the sPLW condition in the first year (red) and the second year (pink).) Three prominent components, P1, N1 and N2, were observed at around 130, 200 and 300–400 ms after the stimulus onset, respectively. The P1 component was observed at the O1/O2 electrodes, and its amplitude decreased significantly with development. The latency of the P1 component at the O1/O2 electrodes was not modulated by development. The N1 and N2 components were mainly observed at the T5′/T6′ electrodes. Both negative amplitudes induced by the PLW stimulus were significantly larger than those induced by the sPLW stimulus. The N1 latency decreased significantly with development, but the N2 latency did not.
3.2.1. P1 component
As shown in Fig. 2, Fig. 3, for the P1 component, both amplitude and latency decreased during development over the first one-year period. In addition, we also found that the stimulus type modulated the P1 latency, but not the P1 amplitude.
Fig. 2.
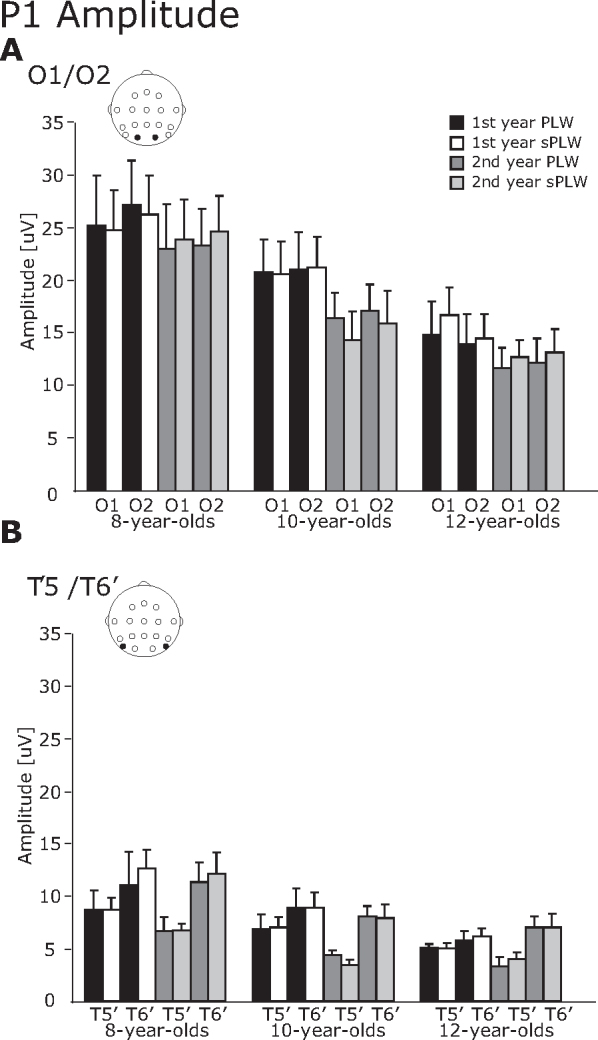
The averaged amplitude of the P1 component. (A) O1/O2 electrodes and (B) T5′/T6′ electrodes. The error bars indicate the standard errors (SEs) of the mean.
Fig. 3.
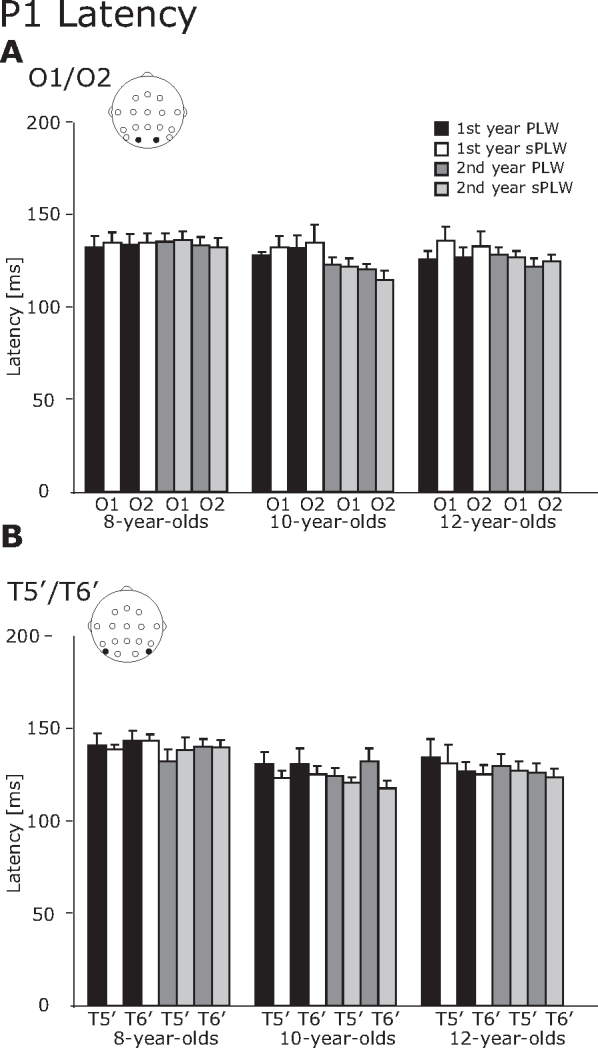
The averaged latency of the P1 component. (A) O1/O2 electrodes and (B) T5′/T6′ electrodes. The error bars indicate the standard errors (SEs) of the mean.
For the P1 amplitude, the interaction of Year × Hemisphere [F(1, 21) = 7.6, p < 0.05] and Year × Electrode [F(1, 21) = 5.6, p < 0.05], and Hemisphere × Electrode [F(1, 21) = 11.8, p < 0.05] were significant. Also a main effect of Group was significant [F(2, 21) = 4.1, p < 0.05]. Subsequent analysis revealed that the amplitude in the right hemisphere was significantly larger than in the left hemisphere for the first year (13.7 vs. 14.8 μV) [F(1, 46) = 5.47, p < 0.05] and for the second year (10.9 vs. 13.3 μV) [F(1, 46) = 26.0, p < 0.01]. For the left hemisphere, the amplitude at the second year was significantly smaller than at the first year (13.7 vs. 10.9 μV) [F(1, 46) = 11.3, p < 0.01]. Regarding the electrodes, the amplitude at the occipital electrode was significantly larger than at the occipitotemporal electrodes for the first year [F(1, 46) = 85.2, p < 0.01] and the second year [F(1, 46) = 58.7, p < 0.01]. At the occipital electrodes, the amplitude in the first year was significantly larger than in the second year (20.5 vs. 17.3 μV) [F(1, 46) = 12.4, p < 0.01]. For the interaction between hemisphere and electrode, the amplitudes at the occipital electrodes were significantly larger than at the occipitotemporal electrodes in the left hemisphere (18.7 vs. 5.8 μV) [F(1, 46) = 91.4, p < 0.01] and the right hemisphere (19.2 vs. 8.9 μV) [F(1, 46) = 57.9, p < 0.01]. These interhemispheric differences were also seen at the occipitotemporal electrodes; the amplitudes in the right hemisphere were significantly larger than in the left hemisphere (8.9 vs. 5.8 μV) [F(1, 46) = 31.4, p < 0.01]. Regarding the developmental changes, the amplitudes in the 8-year-olds were significantly larger than in the 12-year-olds (17.2 vs. 9.6 μV).
For the P1 latency, a four-way interaction of Year × Hemisphere × Electrode × Stimulus was significant [F(1, 21) = 5.0, p < 0.05] and a two-way interaction between Group × Stimulus was significant [F(1, 21) = 4.0, p < 0.05]. Regarding the four-way interaction, a follow-up analysis revealed that the P1 latency at the occipitotemporal electrode was significantly longer than at the occipital electrode for the PLW condition (132.8 vs. 128.4 ms) [F(1, 46) = 4.84, p < 0.05]. Moreover, the P1 latency induced by the PLW stimulus was significantly greater than that was induced by the sPLW stimulus at the occipitotemporal electrode (132.8 vs. 129.7 ms) [F(1, 46) = 4.3, p < 0.05]. Regarding the two-way interaction between Group × Stimulus, the P1 latency induced by the PLW stimulus was significantly greater than by the sPLW stimulus for the 10-year-olds (127.7 vs. 124.0 ms) [F(1, 42) = 7.23, p < 0.05]. For the sPLW stimulus, the effect of group was significant [F(1, 42) = 3.5, p < 0.05]. The following Tukey's HSD analysis revealed that the P1 latency in the 8-year-olds was significantly longer than in the 10-year-olds (137.6 vs. 128.7 ms, p < 0.05).
3.2.2. N1 component
For the N1 component, the N1 amplitude was modulated by the stimulus type while the N1 latency changed across all groups (Fig. 4, Fig. 5).
Fig. 4.
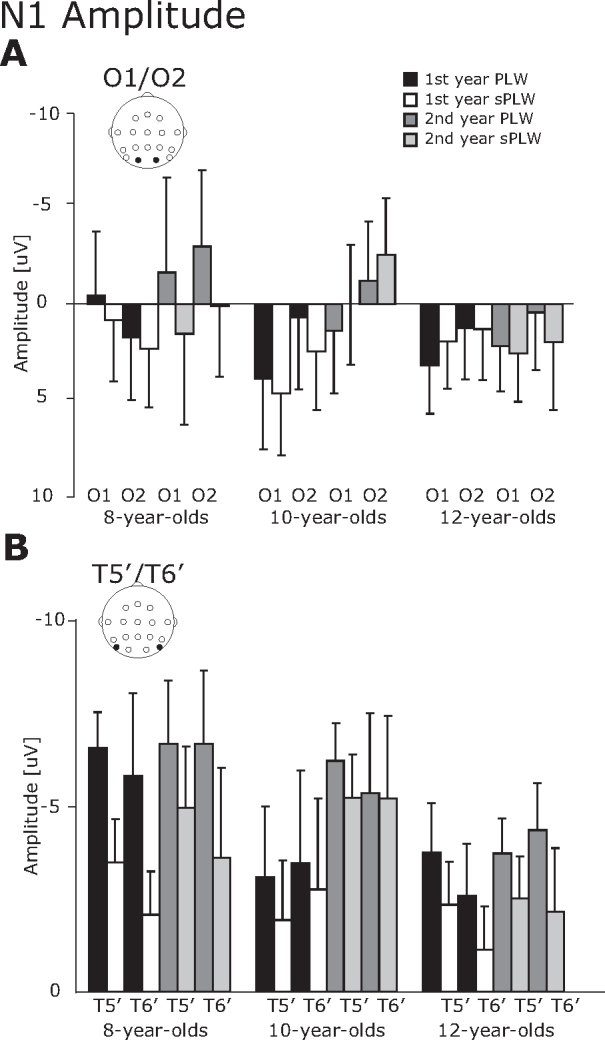
The averaged amplitude of the N1 component. (A) O1/O2 electrodes and (B) T5′/T6′ electrodes. The error bars indicate the standard errors (SEs) of the mean.
Fig. 5.
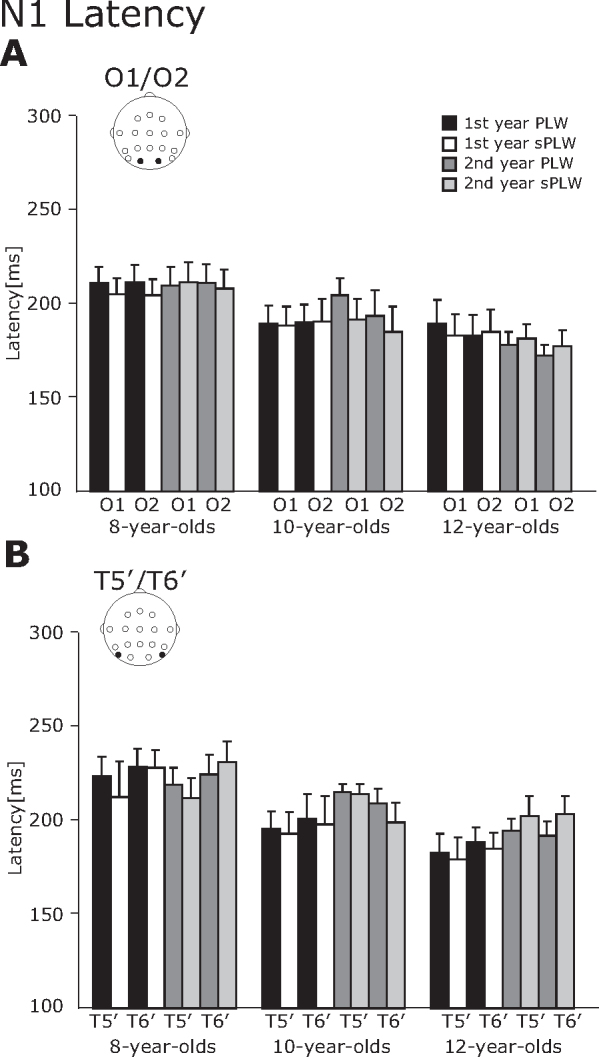
The averaged latency of the N1 component. (A) O1/O2 electrodes and (B) T5′/T6′ electrodes. The error bars indicate the standard errors (SEs) of the mean.
For the N1 amplitude, a four-way interaction of Year × Electrode × Stimulus × Group was significant [F(2, 21) = 4.4, p < 0.05]. A follow-up analysis revealed significant main effects of Electrode [F(1, 21) = 13.4, p < 0.01] and Stimulus [F(1, 21) = 12.3, p < 0.01], but did not reach the statistical significance for other interactions [Fs < 3.7, ps > 0.06]. It suggests that the N1 amplitude for the PLW stimulus was significantly larger than that for the sPLW stimulus (−2.1 vs. −0.8 μV), and the N1 amplitude at the occipitotemporal electrode was significantly larger than that at the occipital electrode (−4.0 vs. 1.1 μV).
For the N1 latency, a three-way interaction of Year × Stimulus × Group was significant [F(2, 21) = 4.6, p < 0.05] and a two-way interaction of Hemisphere × Electrode was significant [F(2, 21) = 4.8, p < 0.05]. A follow-up analysis of the three-way interaction revealed that the N1 latency at the occipital electrode was significantly shorter than that at the occipitotemporal electrode in both hemispheres (Left Hemisphere: 156.4 vs. 163.1 ms, p < 0.05; Right Hemisphere: 154.3 vs. 166.1 ms, p < 0.01). Furthermore, a main effect of group was significant [F(2, 21) = 3.7, p < 0.05], suggesting that the latency in the 8-year-olds was significantly longer than in the 12-year-olds (215.9 vs. 186.4 ms).
3.2.3. P1–N1 component
As shown in Fig. 6, the P1–N1 amplitude was modulated by the type of stimulus, the year and the group. We also found an inter-hemispheric difference as well. The four-way interactions between Year × Hemisphere × Electrode × Stimulus [F(2, 21) = 5.47, p < 0.05] and Year × Electrode × Stimulus × Group [F(2, 21) = 5.23, p < 0.05] were significant. Subsequent analysis of the four-way interaction between Year × Hemisphere × Electrode × Stimulus revealed a two-way interaction between Year × Hemisphere [F(1, 23) = 17.7, p < 0.01]. For the interaction of Year × Hemisphere, follow-up analysis revealed that the amplitude in the right hemisphere was significantly larger than that in the left hemisphere in the first year (14.3 vs. 15.5 μV) [F(1, 46) = 4.22, p < 0.05] and the second year (12.8 vs. 16.0 μV) [F(1, 46) = 29.2, p < 0.01]. Moreover, significant main effects of electrode [F(1, 23) = 26.1, p < 0.01] and stimulus [F(1, 23) = 6.1, p < 0.05]. This suggests that the amplitude at the occipital electrode was significantly larger than at the occipitotemporal electrode (17.8 vs. 11.4 μV) and the amplitude induced by the PLW was significantly larger than by the sPLW (15.1 vs. 14.1 μV).
Fig. 6.
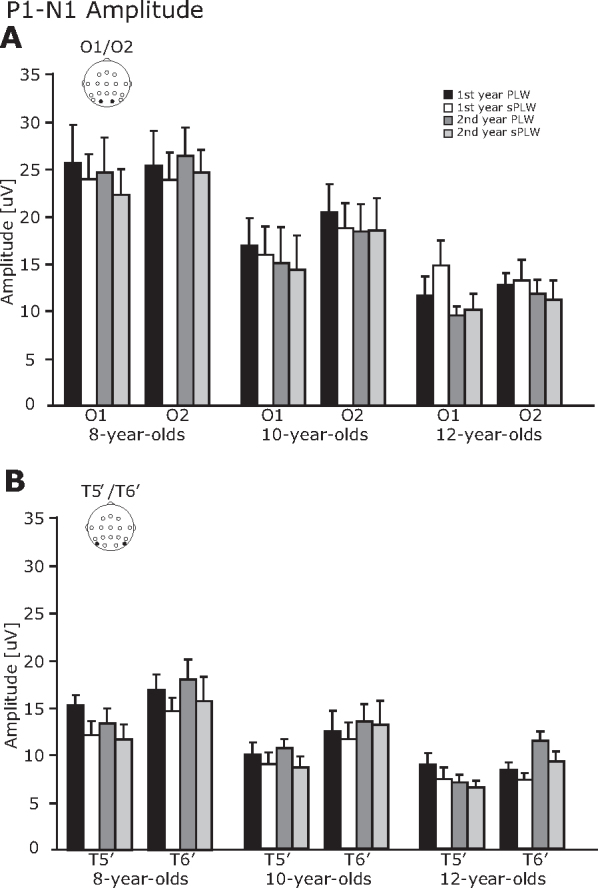
The averaged P1–N1 amplitude. (A) O1/O2 electrodes and (B) T5′/T6′ electrodes. The error bars indicate the standard error (SE) of the mean.
Subsequent analysis of a four-way interaction of Year × Electrode × Stimulus × Group revealed a significant two-way interaction of Electrode × Stimulus [F(1, 21) = 4.4, p < 0.05]. For the interaction of Electrode × Stimulus, subsequent analysis revealed that the amplitude at the occipital electrode was significantly larger than that at the occipitotemporal electrode for PLW stimulus (18.1 vs. 12.2 μV) [F(1, 46) = 21.5, p < 0.01] and for sPLW stimulus (17.6 vs. 10.6 μV) [F(1, 46) = 29.1, p < 0.01]. Moreover, the amplitude induced at the occipitotemporal electrode by the PLW stimulus was significantly larger than that by the sPLW stimulus (12.2 vs. 10.6 μV) [F(1, 46) = 9.90, p < 0.01]. Regarding the group difference, the amplitudes in the 8-year-olds were significantly larger than that in the 12-year-olds (19.6 vs. 10.1 μV, p < 0.01).
3.2.4. N2 component
For the N2 component (Fig. 7), both amplitude and latency were modulated by the stimulus type, but only the N2 latecy was modulated by the year and group.
Fig. 7.
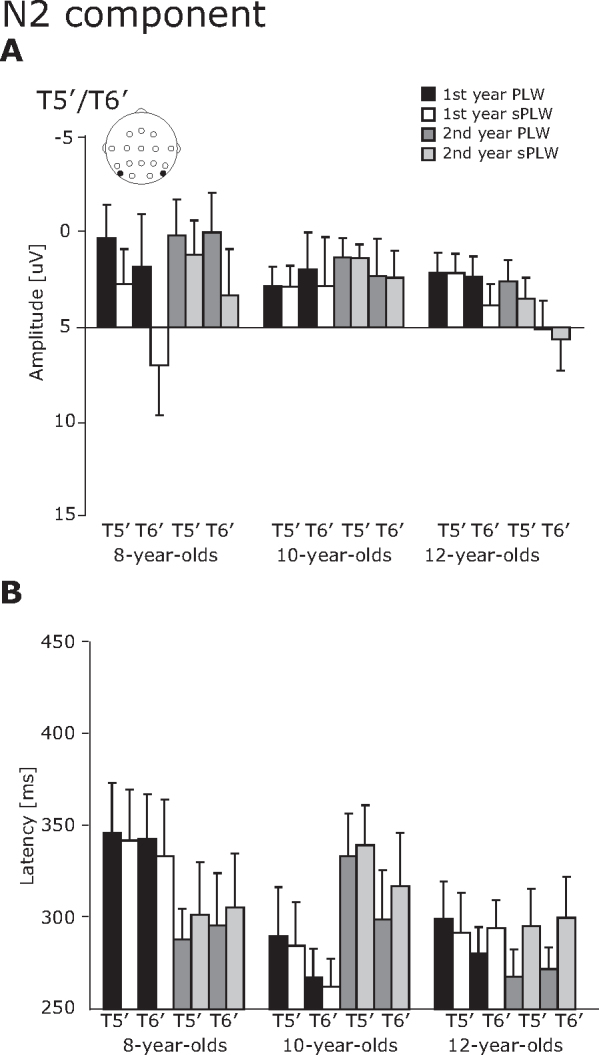
The averaged amplitude and latency of the N2 component at the T5′/T6′ electrodes. The error bars indicate the standard errors (SEs) of the mean.
For the N2 amplitude, a main effect of stimulus was significant [F(1, 23) = 15.9, p < 0.01]. This indicates that the N2 amplitudes induced by the PLW stimulus were significantly larger than those induced by the sPLW stimulus (−3.1 vs. −1.7 μV). For the N2 latency, the interaction of Year × Group [F(2, 21) = 4.91, p < 0.05] and Year × Stimulus [F(1, 21) = 7.16, p < 0.05] was significant. Subsequent analysis revealed that the second-year latencies for the sPLW stimulus were significantly longer than those for the PLW stimulus (309.8 vs. 292.7 ms) [F(1, 46) = 9.94, p < 0.01]. Moreover, the latencies in the first year was significantly longer than in the second year for the 8-year-olds (341.0 vs. 298 ms) [F(1, 21) = 4.6, p < 0.05], but opposite for the 10-year-olds (276.1 vs. 322 ms) [F(1, 21) = 5.1, p < 0.05].
4. Discussion
We measured the ERP's of twenty four children each year over a two-year period in order to track individual developmental changes in the temporal profile of the neural responses to PLW and sPLW stimuli during childhood. One positive component (P1) and two negative components (N1 and N2) were observed, which are related to the processing of PLW stimuli, as previously reported in adult ERP studies (Hirai et al., 2003, Hirai et al., 2005, Hirai et al., 2009, Jokisch et al., 2005a, Krakowski et al., 2011). Our findings can be summarized as follows: Regarding the amplitudes, (1) the P1 amplitudes at the occipital and occipitotemporal electrodes were altered by age group and year but not by stimulus, while P1 latency was altered by stimulus, (2) the P1–N1 amplitudes were different between the 8-year-old and the 12-year-old groups and were modulated by the stimulus as well, (3) the N1 and N2 amplitudes were not changed by age group or year, but differed at the occipitotemporal electrodes according to the stimulus. As for the latencies, the P1 and N1 latency at the occipitotemporal electrodes decreased by age group but not by year. For N2, the latency was affected by year, group and stimulus at the occipitotemporal electrodes.
These findings suggest that enhanced electrophysiological responses to PLW can be observed in all age groups, and that the early component was changed even over the course of a single year at the age of twelve.
4.1. P1 component
In a group analysis of the present study, age group and year had an influence on the P1 amplitude but stimulus did not. Regarding developmental changes in P1 amplitude, our current finding appears consistent with previous ERP findings showing that the P1 amplitude during development decreases from visual stimulus, such as color, motion, and face (Coch et al., 2005, Itier and Taylor, 2004, Mitchell and Neville, 2004, Taylor et al., 2001, Taylor et al., 2004). This decrement of the P1 amplitude would reflect developmental changes in the encoding of low-level visual properties (McCarthy et al., 1999) and changes in the cortical structure (Courchesne, 1990, Gogtay et al., 2004). Here, the P1 amplitude was significantly smaller in the second year than it was in the first in all groups. This result implies that the primary visual cortex is developing rapidly during this period; therefore, differences in amplitude should be detectable between the first and second year.
Regarding the stimulus effect on the P1 amplitude, our current finding was not consistent with previous ERP studies showing the stimulus effect was found in 7-year-olds (Hirai et al., 2009) and adults (Krakowski et al., 2011). The differences between the current study and previous studies were the age of the group (7-year-olds in the previous study) (Hirai et al., 2009) or the use of different stimulus sets (Krakowski et al., 2011). In future studies, we need to address which information within a PLW can affect the P1 component and how this changes throughout development.
For the P1 latencies at the T5′/T6′ electrodes, those of 8-year-olds were significantly longer than those of 10-year-olds and a conditional difference of P1 latency was only observed in the 10-year-old group. The group difference is consistent with previous studies such as color, motion and face stimuli (Coch et al., 2005, Mitchell and Neville, 2004). These results imply that the changes in P1 latency are non-monotonic.
4.2. N1 component
The N1 amplitude at T5′/T6′ was modulated by the stimulus condition while N1 latency was not. Consistent with previous ERP studies (Hirai et al., 2003, Jokisch et al., 2005a), the N1 amplitude induced by the PLW stimulus was significantly larger in all age groups than that induced by the sPLW stimulus, and there were no conditional differences in latency.
Several cross-sectional ERP studies have investigated developmental changes of the N1 component related to the processing of motion, color (Coch et al., 2005, Mitchell and Neville, 2004), face (Taylor et al., 2001, Taylor et al., 2004), and body (Gliga and Dehaene-Lambertz, 2005) stimuli. Contrary to these studies, the present study does not show a consistent change in N1 amplitude through development. Several ERP studies demonstrate that the motion-processing N1 component (Coch et al., 2005), as well as the face-specific N170 component (Taylor et al., 1999, Taylor et al., 2004), remains unchanged through development. In the current experiment, we did not see group differences in the N1 amplitude, but we found a group effect on the P1–N1 amplitudes. It is possible that the N1 component is affected by development, however, the preceding P1 component varied extensively across age groups, thus we did not observe a significant developmental effect in the N1 analysis, but observed it in the peak-to-peak (P1–N1) analysis. As a result, the P1–N1 amplitude across the occipitotemporal electrodes is significantly larger in the 8-year-olds than in the 12-year-olds. We have previously shown that the P1–N1 amplitude for both stimuli in the right hemisphere is significantly smaller in adults than in 9, 10 and 11-year-olds, but is not significantly smaller than that of the 13-year-olds (Hirai et al., 2009). Consistent with this, the P1–N1 amplitudes of the 8-year-olds are significantly larger than those of the 12-year-olds, but not in the 10-year-olds.
We also found that N1 latency decreases significantly with age at the T5′/T6′ electrodes, which appears consistent with findings from a previous study (Mitchell and Neville, 2004). When using a unidirectional linear motion stimuli (Langrova et al., 2006) or face stimuli, the developmental changes in latency are also reported (Itier and Taylor, 2004, Taylor et al., 2001).
Two ERP studies for the perception of BM demonstrate that the first negative component is estimated in the vicinity of the dorsal visual stream, in close proximity to regions associated with general motion processing such as hMT (Krakowski et al., 2011) or lingual gyrus (Jokisch et al., 2005a). This implies that the timing of the neural activity generated from the hMT or the lingual gyrus can change until age 12.
4.3. N2 component
Consistent with other ERP studies, the N2 amplitude induced by the PLW stimulus is significantly larger than that induced by the sPLW stimulus (Hirai et al., 2003, Jokisch et al., 2005a, Krakowski et al., 2011), but the amplitude at the T5′/T6′ electrodes was not changed by development. Several ERP and MEG studies show that the sources of the N2 component are estimated to be in the fusiform gyrus (FG), anterior cingulate gyrus, medial frontal gyrus, and superior temporal gyrus (STG) (Jokisch et al., 2005a) and in the vicinity of the pSTS (Hirai et al., 2008, Krakowski et al., 2011, Safford et al., 2010). The importance of the pSTS and FG regions in the perception of BM are observed in many fMRI and neuropsychological studies (Bonda et al., 1996, Grossman and Blake, 2002, Grossman et al., 2000, Saygin, 2007).
Our study does not observe age-related changes in N2 amplitude, but does find non-monotonic developmental changes in corresponding N2 latency. The developmental changes in activity in the fusiform face area (FFA) differ in response between a 5–8-year old group and a 11–14-year old group, when given a face stimulus (Passarotti et al., 2003, Scherf et al., 2007). Another fMRI study investigates the developmental changes in the neural response to a BM stimulus in school-aged children, 7–10 years old (Carter and Pelphrey, 2006), although the point-light motion technique is not employed, instead using a biological figure (a walking human), as well as the following: BM by a non-biological figure (a walking robot); disorganized, non-BM by a disjointed mechanical figure; and organized, non-BM by a grandfather clock. They found that the STS region responds greater to the biological stimulus than to the non-BM stimulus, and increases in specificity for BM with development. However, contrary to their findings, we found non-monotonic developmental changes for the N2 latency and did not observe an age-related change in the N2 amplitude and latency as well. It is likely that the preceding ERP components vary extensively across age groups, thus we find significant developmental changes, as reported in the P1–N1 analysis, but not in the N1 analysis. Moreover, the sample size of participants is small in our current study, and this might affect the non-monotonical developmental changes for the N2 latency.
To reveal the developmental changes in this later component, we should apply the adaptation paradigm to extract a single component which is related to the later ERP component, used in our previous study (Hirai and Kakigi, 2008).
4.4. Developmental changes and possible other effects
Our current findings of developmental changes for the neurophysiological responses to BM stimuli during childhood seem to be consistent with a recent behavioral finding of recognition of basic emotions from body movement (Ross et al., 2012) (and see Slaughter and Brownell, 2011). They found that the performance on emotional recognition from point-light motion improved steeply until 8.5 year of age followed by a much slower improvement rate through late childhood and adolescence. This finding suggests that the processing of BM can change from childhood to adolescence not only the processing of action perception from point-lights motion, but also retrieving basic emotions from point-light motion.
Moreover, we found a significant effect of Year in all components. In our current study, we presented the same visual stimuli in both first and second year, thus one might think that the modulation of each component by the Year might be due to the familiarity effect of the visual stimulus. A series of ERP studies have shown that intensive training can in fact modulate the N170 component (e.g. Rossion et al., 2002), however in our current experiment, we presented 84 trials per year Moreover, the second experiment was performed at one-year interval. Thus, we presume that the effect of the Year reflects changes of cortical development, not reflect the familiarity to the stimulus itself.
Because more female children participated in the 10-year-old group than other groups, we therefore also considered gender effects in this group. We reanalyzed the data including the gender as an inter-subject factor (male and female). The gender effect was manifested in both N2 amplitude and latency. For the N2 amplitude, we found a two-way interaction between Year × Gender [F(1, 6) = 16.03, p < 0.01]. This indicates that the N2 amplitudes in male group were significantly larger than those in female group (−5.5 vs. −0.5 μV) at the first year, but not significant at the second year (−3.0 vs. −3.2 μV). For N2 latency, a three-way interaction between Year × Hemisphere × Gender was significant [F(1, 6) = 8.5, p < 0.05]. This indicates that the N2 latency in male group was significantly longer than that female group in the left hemisphere at the first year (346.5 ms vs. 251.6 ms). Regarding the gender effect on the perception of BM, a recent behavioral study showed that males surpass in recognition accuracy of happy actions, whereas the performance in females on recognition of hostile angry knocking was better than that in males. Advantage of women in recognition accuracy of neutral actions suggests that females are better tuned to the lack of emotional content in body actions (Sokolov et al., 2011). The gender differences on the perception of BM is an interesting topic, however, the number of participants are rather small in our current experiment, further studies should be addressed this effect.
5. Conclusion
The processing of BM is a fundamental component of social cognition, emerging very early in life. Here, we have focused on the longitudinal developmental changes in the electrophysiological responses to BM stimuli during childhood. We identified three ERP components related to BM perception and found differential developmental trajectories in as little as a single year. Recent studies show that children with autism have difficulty in the detection of BM, however these findings are controversial. We hope that our current findings will help shed some light on the atypical processing of BM in children with ASD by giving a comparative baseline.
Role of the funding source
M.H. was supported by a Grant-in-Aid for JSPS Fellows No. 18-11826 from the Ministry of Education, Science, Sports, and Culture, Japan.
Conflict of interest statement
The authors declare no conflict of interest.
Acknowledgements
We thank all the children and parents for their participation. We also thank Mr. Y. Takeshima, and Ms. M. Teruya for technical support.
References
- Annaz D., Campbell R., Coleman M., Milne E., Swettenham J. Young children with autism spectrum disorder do not preferentially attend to biological motion. Journal of Autism and Developmental Disorders. 2012;42:401–408. doi: 10.1007/s10803-011-1256-3. [DOI] [PubMed] [Google Scholar]
- Beintema J.A., Lappe M. Perception of biological motion without local image motion. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:5661–5663. doi: 10.1073/pnas.082483699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertenthal B.I., Pinto J. Global processing of biological motions. Psychological Science. 1994;5:221–225. [Google Scholar]
- Bertenthal B.I., Proffitt D.R., Cutting J.E. Infant sensitivity to figural coherence in biomechanical motions. Journal of Experimental Child Psychology. 1984;37:213–230. doi: 10.1016/0022-0965(84)90001-8. [DOI] [PubMed] [Google Scholar]
- Bertenthal B.I., Proffitt D.R., Kramer S.J., Spetner N.B. Infants’ encoding of kinetic displays varying in relative coherence. Developmental Psychology. 1987;23:171–178. [Google Scholar]
- Blake R., Turner L.M., Smoski M.J., Pozdol S.L., Stone W.L. Visual recognition of biological motion is impaired in children with autism. Psychological Science. 2003;14:151–157. doi: 10.1111/1467-9280.01434. [DOI] [PubMed] [Google Scholar]
- Bonda E., Petrides M., Ostry D., Evans A. Specific involvement of human parietal systems and the amygdala in the perception of biological motion. Journal of Neuroscience. 1996;16:3737–3744. doi: 10.1523/JNEUROSCI.16-11-03737.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter E.J., Pelphrey K.A. School-aged children exhibit domain-specific responses to biological motion. Social Neuroscience. 2006;1:396–411. doi: 10.1080/17470910601041382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coch D., Skendzel W., Grossi G., Neville H. Motion and color processing in school-age children and adults: an ERP study. Developmental Science. 2005;8:372–386. doi: 10.1111/j.1467-7687.2005.00425.x. [DOI] [PubMed] [Google Scholar]
- Cook J., Saygin A.P., Swain R., Blakemore S.J. Reduced sensitivity to minimum-jerk biological motion in autism spectrum conditions. Neuropsychologia. 2009;47:3275–3278. doi: 10.1016/j.neuropsychologia.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courchesne E. Oxford University Press; New York: 1990. Chronology of Postnatal Human Brain Development: Event-Related Potential, Positron Emission Tomography, Myelogenesis, and Synaptogenesis Studies. [Google Scholar]
- Cutting J.E. A program to generate synthetic walkers as dynamic pointlight displays. Behavior Research Methods, Instruments, & Computers. 1978;10:91–94. [Google Scholar]
- Cutting J.E., Kozlowski L.T. Recognizing friends by their walk: gait perception without familiarity cues. Bulletin of the Psychonomic Society. 1977;9:353–356. [Google Scholar]
- Dittrich W.H. Action categories and the perception of biological motion. Perception. 1993;22:15–22. doi: 10.1068/p220015. [DOI] [PubMed] [Google Scholar]
- Fox R., McDaniel C. The perception of biological motion by human infants. Science. 1982;218:486–487. doi: 10.1126/science.7123249. [DOI] [PubMed] [Google Scholar]
- Freire A., Lewis T.L., Maurer D., Blake R. The development of sensitivity to biological motion in noise. Perception. 2006;35:647–657. doi: 10.1068/p5403. [DOI] [PubMed] [Google Scholar]
- Gliga T., Dehaene-Lambertz G. Structural encoding of body and face in human infants and adults. Journal of Cognitive Neuroscience. 2005;17:1328–1340. doi: 10.1162/0898929055002481. [DOI] [PubMed] [Google Scholar]
- Gogtay N., Giedd J.N., Lusk L., Hayashi K.M., Greenstein D., Vaituzis A.C., Nugent T.F., 3rd, Herman D.H., Clasen L.S., Toga A.W., Rapoport J.L., Thompson P.M. Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grezes J., Fonlupt P., Bertenthal B., Delon-Martin C., Segebarth C., Decety J. Does perception of biological motion rely on specific brain regions? Neuroimage. 2001;13:775–785. doi: 10.1006/nimg.2000.0740. [DOI] [PubMed] [Google Scholar]
- Grossman E.D., Blake R. Brain activity evoked by inverted and imagined biological motion. Vision Research. 2001;41:1475–1482. doi: 10.1016/s0042-6989(00)00317-5. [DOI] [PubMed] [Google Scholar]
- Grossman E.D., Blake R. Brain areas active during visual perception of biological motion. Neuron. 2002;35:1167–1175. doi: 10.1016/s0896-6273(02)00897-8. [DOI] [PubMed] [Google Scholar]
- Grossman E.D., Blake R., Kim C.Y. Learning to see biological motion: brain activity parallels behavior. Journal of Cognitive Neuroscience. 2004;16:1669–1679. doi: 10.1162/0898929042568569. [DOI] [PubMed] [Google Scholar]
- Grossman E.D., Donnelly M., Price R., Pickens D., Morgan V., Neighbor G., Blake R. Brain areas involved in perception of biological motion. Journal of Cognitive Neuroscience. 2000;12:711–720. doi: 10.1162/089892900562417. [DOI] [PubMed] [Google Scholar]
- Hirai M., Fukushima H., Hiraki K. An event-related potentials study of biological motion perception in humans. Neuroscience Letters. 2003;344:41–44. doi: 10.1016/s0304-3940(03)00413-0. [DOI] [PubMed] [Google Scholar]
- Hirai M., Hiraki K. An event-related potentials study of biological motion perception in human infants. Brain Research – Cognitive Brain Research. 2005;22:301–304. doi: 10.1016/j.cogbrainres.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Hirai M., Kakigi R. Differential cortical processing of local and global motion information in biological motion: an event-related potential study. Journal of Vision. 2008;8(2):1–17. doi: 10.1167/8.16.2. [DOI] [PubMed] [Google Scholar]
- Hirai M., Kaneoke Y., Nakata H., Ryusuke K. Neural responses related to point-light walker perception: a magnetoencephalographic study. Clinical Neurophysiology. 2008;119:2775–2784. doi: 10.1016/j.clinph.2008.09.008. [DOI] [PubMed] [Google Scholar]
- Hirai M., Senju A., Fukushima H., Hiraki K. Active processing of biological motion perception: an ERP study. Brain Research – Cognitive Brain Research. 2005;23:387–396. doi: 10.1016/j.cogbrainres.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Hirai M., Watanabe S., Honda Y., Kakigi R. Developmental changes in point-light walker processing during childhood and adolescence: an event-related potential study. Neuroscience. 2009;161:311–325. doi: 10.1016/j.neuroscience.2009.03.026. [DOI] [PubMed] [Google Scholar]
- Howard R.J., Brammer M., Wright I., Woodruff P.W., Bullmore E.T., Zeki S. A direct demonstration of functional specialization within motion-related visual and auditory cortex of the human brain. Current Biology. 1996;6:1015–1019. doi: 10.1016/s0960-9822(02)00646-2. [DOI] [PubMed] [Google Scholar]
- Itier R.J., Taylor M.J. Face recognition memory and configural processing: a developmental ERP study using upright, inverted, and contrast-reversed faces. Journal of Cognitive Neuroscience. 2004;16:487–502. doi: 10.1162/089892904322926818. [DOI] [PubMed] [Google Scholar]
- Jastorff J., Orban G.A. Human functional magnetic resonance imaging reveals separation and integration of shape and motion cues in biological motion processing. Journal of Neuroscience. 2009;29:7315–7329. doi: 10.1523/JNEUROSCI.4870-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson G. Visual perception of biological motion and a model for its analysis. Perception and Psychophysics. 1973;14:201–211. [Google Scholar]
- Jokisch D., Daum I., Suchan B., Troje N.F. Structural encoding and recognition of biological motion: evidence from event-related potentials and source analysis. Behavioural Brain Research. 2005;157:195–204. doi: 10.1016/j.bbr.2004.06.025. [DOI] [PubMed] [Google Scholar]
- Jokisch D., Troje N.F., Koch B., Schwarz M., Daum I. Differential involvement of the cerebellum in biological and coherent motion perception. European Journal of Neuroscience. 2005;21:3439–3446. doi: 10.1111/j.1460-9568.2005.04145.x. [DOI] [PubMed] [Google Scholar]
- Jordan H., Reiss J.E., Hoffman J.E., Landau B. Intact perception of biological motion in the face of profound spatial deficits: Williams syndrome. Psychological Science. 2002;13:162–167. doi: 10.1111/1467-9280.00429. [DOI] [PubMed] [Google Scholar]
- Kaiser M.D., Delmolino L., Tanaka J.W., Shiffrar M. Comparison of visual sensitivity to human and object motion in autism spectrum disorder. Autism Research. 2010;3:191–195. doi: 10.1002/aur.137. [DOI] [PubMed] [Google Scholar]
- Kaiser M.D., Hudac C.M., Shultz S., Lee S.M., Cheung C., Berken A.M., Deen B., Pitskel N.B., Sugrue D.R., Voos A.C., Saulnier C.A., Ventola P., Wolf J.M., Klin A., Vander Wyk B.C., Pelphrey K.A. Neural signatures of autism. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:21223–21228. doi: 10.1073/pnas.1010412107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klin A., Lin D.J., Gorrindo P., Ramsay G., Jones W. Two-year-olds with autism orient to non-social contingencies rather than biological motion. Nature. 2009;459:257–261. doi: 10.1038/nature07868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koldewyn K., Whitney D., Rivera S.M. The psychophysics of visual motion and global form processing in autism. Brain. 2010;133:599–610. doi: 10.1093/brain/awp272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlowski L.T., Cutting J.E. Recognizing the sex of a walker from a dynamic point-light display. Perception and Psychophysics. 1977;21:575–580. [Google Scholar]
- Krakowski A.I., Ross L.A., Snyder A.C., Sehatpour P., Kelly S.P., Foxe J.J. The neurophysiology of human biological motion processing: a high-density electrical mapping study. Neuroimage. 2011;56:373–383. doi: 10.1016/j.neuroimage.2011.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langrova J., Kuba M., Kremlacek J., Kubova Z., Vit F. Motion-onset VEPs reflect long maturation and early aging of visual motion-processing system. Vision Research. 2006;46:536–544. doi: 10.1016/j.visres.2005.06.024. [DOI] [PubMed] [Google Scholar]
- Marshall J.C., Shipley T.F. Event-related potentials to point-light displays of human actions in 5-month-old infants. Developmental Neuropsychology. 2009;34:368–377. doi: 10.1080/87565640902801866. [DOI] [PubMed] [Google Scholar]
- Mather G., Radford K., West S. Low-level visual processing of biological motion. Proceedings: Biological Sciences. 1992;249:149–155. doi: 10.1098/rspb.1992.0097. [DOI] [PubMed] [Google Scholar]
- McCarthy G., Puce A., Belger A., Allison T. Electrophysiological studies of human face perception. II: response properties of face-specific potentials generated in occipitotemporal cortex. Cerebral Cortex. 1999;9:431–444. doi: 10.1093/cercor/9.5.431. [DOI] [PubMed] [Google Scholar]
- Michels L., Kleiser R., de Lussanet M.H., Seitz R.J., Lappe M. Brain activity for peripheral biological motion in the posterior superior temporal gyrus and the fusiform gyrus: dependence on visual hemifield and view orientation. Neuroimage. 2009;45:151–159. doi: 10.1016/j.neuroimage.2008.10.063. [DOI] [PubMed] [Google Scholar]
- Mitchell T.V., Neville H.J. Asynchronies in the development of electrophysiological responses to motion and color. Journal of Cognitive Neuroscience. 2004;16:1363–1374. doi: 10.1162/0898929042304750. [DOI] [PubMed] [Google Scholar]
- Murphy P., Brady N., Fitzgerald M., Troje N.F. No evidence for impaired perception of biological motion in adults with autistic spectrum disorders. Neuropsychologia. 2009;47:3225–3235. doi: 10.1016/j.neuropsychologia.2009.07.026. [DOI] [PubMed] [Google Scholar]
- Passarotti A.M., Paul B.M., Bussiere J.R., Buxton R.B., Wong E.C., Stiles J. The development of face and location processing: an fMRI study. Developmental Science. 2003;6:100–117. [Google Scholar]
- Pavlova M., Krageloh-Mann I., Sokolov A., Birbaumer N. Recognition of point-light biological motion displays by young children. Perception. 2001;30:925–933. doi: 10.1068/p3157. [DOI] [PubMed] [Google Scholar]
- Pavlova M., Marconato F., Sokolov A., Braun C., Birbaumer N., Krageloh-Mann I. Periventricular leukomalacia specifically affects cortical MEG response to biological motion. Annals of Neurology. 2006;59:415–419. doi: 10.1002/ana.20762. [DOI] [PubMed] [Google Scholar]
- Peelen M.V., Downing P.E. The neural basis of visual body perception. Nature Reviews Neuroscience. 2007;8:636–648. doi: 10.1038/nrn2195. [DOI] [PubMed] [Google Scholar]
- Pelphrey K.A., Mitchell T.V., McKeown M.J., Goldstein J., Allison T., McCarthy G. Brain activity evoked by the perception of human walking: controlling for meaningful coherent motion. Journal of Neuroscience. 2003;23:6819–6825. doi: 10.1523/JNEUROSCI.23-17-06819.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peuskens H., Vanrie J., Verfaillie K., Orban G.A. Specificity of regions processing biological motion. European Journal of Neuroscience. 2005;21:2864–2875. doi: 10.1111/j.1460-9568.2005.04106.x. [DOI] [PubMed] [Google Scholar]
- Pollick F.E., Paterson H.M., Bruderlin A., Sanford A.J. Perceiving affect from arm movement. Cognition. 2001;82:B51–B61. doi: 10.1016/s0010-0277(01)00147-0. [DOI] [PubMed] [Google Scholar]
- Reid V.M., Hoehl S., Striano T. The perception of biological motion by infants: an event-related potential study. Neuroscience Letters. 2006;395:211–214. doi: 10.1016/j.neulet.2005.10.080. [DOI] [PubMed] [Google Scholar]
- Ross P.D., Polson L., Grosbras M.H. Developmental changes in emotion recognition from full-light and point-light displays of body movement. PLoS ONE. 2012;7:e44815. doi: 10.1371/journal.pone.0044815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossion B., Gauthier I., Goffaux V., Tarr M.-J., Crommelinck M. Expertise training with novel objects leads to face-like electrophysiological responses. Psychological Science. 2002;13:250–257. doi: 10.1111/1467-9280.00446. [DOI] [PubMed] [Google Scholar]
- Safford A.S., Hussey E.A., Parasuraman R., Thompson J.C. Object-based attentional modulation of biological motion processing: spatiotemporal dynamics using functional magnetic resonance imaging and electroencephalography. Journal of Neuroscience. 2010;30:9064–9073. doi: 10.1523/JNEUROSCI.1779-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santi A., Servos P., Vatikiotis-Bateson E., Kuratate T., Munhall K. Perceiving biological motion: dissociating visible speech from walking. Journal of Cognitive Neuroscience. 2003;15:800–809. doi: 10.1162/089892903322370726. [DOI] [PubMed] [Google Scholar]
- Saygin A.P. Superior temporal and premotor brain areas necessary for biological motion perception. Brain. 2007;130:2452–2461. doi: 10.1093/brain/awm162. [DOI] [PubMed] [Google Scholar]
- Saygin A.P., Cook J., Blakemore S.J. Unaffected perceptual thresholds for biological and non-biological form-from-motion perception in autism spectrum conditions. PLoS ONE. 2010;5:e13491. doi: 10.1371/journal.pone.0013491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saygin A.P., Wilson S.M., Hagler D.J., Jr., Bates E., Sereno M.I. Point-light biological motion perception activates human premotor cortex. Journal of Neuroscience. 2004;24:6181–6188. doi: 10.1523/JNEUROSCI.0504-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherf K.S., Behrmann M., Humphreys K., Luna B. Visual category-selectivity for faces, places and objects emerges along different developmental trajectories. Developmental Science. 2007;10:F15–F30. doi: 10.1111/j.1467-7687.2007.00595.x. [DOI] [PubMed] [Google Scholar]
- Servos P., Osu R., Santi A., Kawato M. The neural substrates of biological motion perception: an fMRI study. Cerebral Cortex. 2002;12:772–782. doi: 10.1093/cercor/12.7.772. [DOI] [PubMed] [Google Scholar]
- Simion F., Regolin L., Bulf H. A predisposition for biological motion in the newborn baby. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:809–813. doi: 10.1073/pnas.0707021105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slaughter V., Brownell C. Cambridge University Press; Cambridge, UK: 2011. Early Development of Body Representations. [Google Scholar]
- Sokolov A.A., Kruger S., Enck P., Krageloh-Mann I., Pavlova M.A. Gender affects body language reading. Frontiers in Psychology. 2011;2:16. doi: 10.3389/fpsyg.2011.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stekelenburg J.J., de Gelder B. The neural correlates of perceiving human bodies: an ERP study on the body-inversion effect. Neuroreport. 2004;15:777–780. doi: 10.1097/00001756-200404090-00007. [DOI] [PubMed] [Google Scholar]
- Taylor M.J., Batty M., Itier R.J. The faces of development: a review of early face processing over childhood. Journal of Cognitive Neuroscience. 2004;16:1426–1442. doi: 10.1162/0898929042304732. [DOI] [PubMed] [Google Scholar]
- Taylor M.J., Edmonds G.E., McCarthy G., Allison T. Eyes first! Eye processing develops before face processing in children. Neuroreport. 2001;12:1671–1676. doi: 10.1097/00001756-200106130-00031. [DOI] [PubMed] [Google Scholar]
- Taylor M.J., McCarthy G., Saliba E., Degiovanni E. ERP evidence of developmental changes in processing of faces. Clinical Neurophysiology. 1999;110:910–915. doi: 10.1016/s1388-2457(99)00006-1. [DOI] [PubMed] [Google Scholar]
- Thierry G., Pegna A.J., Dodds C., Roberts M., Basan S., Downing P. An event-related potential component sensitive to images of the human body. Neuroimage. 2006;32:871–879. doi: 10.1016/j.neuroimage.2006.03.060. [DOI] [PubMed] [Google Scholar]
- Troje N.F. Decomposing biological motion: a framework for analysis and synthesis of human gait patterns. Journal of Vision. 2002;2:371–387. doi: 10.1167/2.5.2. [DOI] [PubMed] [Google Scholar]
- Troje N.F. Biological motion perception. In: al A.B.e., editor. The Senses: A Comprehensive Reference. Elsevier; Amsterdam, Netherlands: 2008. pp. 231–238. [Google Scholar]
- Troje N.F., Westhoff C. The inversion effect in biological motion perception: evidence for a life detector? Current Biology. 2006;16:821–824. doi: 10.1016/j.cub.2006.03.022. [DOI] [PubMed] [Google Scholar]
- Troje N.F., Westhoff C., Lavrov M. Person identification from biological motion: effects of structural and kinematic cues. Perception and Psychophysics. 2005;67:667–675. doi: 10.3758/bf03193523. [DOI] [PubMed] [Google Scholar]
- Vaina L.M., Solomon J., Chowdhury S., Sinha P., Belliveau J.W. Functional neuroanatomy of biological motion perception in humans. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:11656–11661. doi: 10.1073/pnas.191374198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virji-Babul N., Cheung T., Weeks D., Kerns K., Shiffrar M. Neural activity involved in the perception of human and meaningful object motion. Neuroreport. 2007;18:1125–1128. doi: 10.1097/WNR.0b013e32821c5470. [DOI] [PubMed] [Google Scholar]
- Watanabe S., Kakigi R., Puce A. The spatiotemporal dynamics of the face inversion effect: a magneto- and electro-encephalographic study. Neuroscience. 2003;116:879–895. doi: 10.1016/s0306-4522(02)00752-2. [DOI] [PubMed] [Google Scholar]