Highlights
► A structural MRI study (N = 442): sex and age differences in typical development. ► Different age-related trajectories in subcortical brain volumes. ► Linear increase in white matter, non-linear decrease in gray matter volume with age. ► Sex-by-age interactions specifically in cortical surface measurements. ► Adolescence is characterized by large individual variance in brain maturation.
Keywords: Adolescence, Brain volumes, Cortical thickness, Surface area, Sex differences
Abstract
Recent advances in structural brain imaging have demonstrated that brain development continues through childhood and adolescence. In the present cross-sectional study, structural MRI data from 442 typically developing individuals (range 8–30) were analyzed to examine and replicate the relationship between age, sex, brain volumes, cortical thickness and surface area. Our findings show differential patterns for subcortical and cortical areas. Analysis of subcortical volumes showed that putamen volume decreased with age and thalamus volume increased with age. Independent of age, males demonstrated larger amygdala and thalamus volumes compared to females. Cerebral white matter increased linearly with age, at a faster pace for females than males. Gray matter showed nonlinear decreases with age. Sex-by-age interactions were primarily found in lobar surface area measurements, with males demonstrating a larger cortical surface up to age 15, while cortical surface in females remained relatively stable with increasing age. The current findings replicate some, but not all prior reports on structural brain development, which calls for more studies with large samples, replications, and specific tests for brain structural changes. In addition, the results point toward an important role for sex differences in brain development, specifically during the heterogeneous developmental phase of puberty.
1. Introduction
Brain development is an organized and highly dynamic multistep process, which is genetically determined, epigenetically directed and environmentally influenced (Tau and Peterson, 2010). This process continues both through childhood and adolescence, the developmental period during which the body and brain emerge from an immature state to adulthood (Spear, 2000, Steinberg and Morris, 2001). Although total brain size is approximately 90% of its adult size by age six, it is now well known that the gray and white matter subcomponents of the brain continue to undergo dynamic changes throughout adolescence (Giedd et al., 1999, Paus, 2005).
1.1. Age differences in brain structures
There is increasing consensus on the overall pattern of gray matter development over the course of childhood and adolescence: in childhood a global increase of cortical gray matter volume takes place, peaking around the onset of puberty, which is then followed by a gradual decrease in adolescence and early adulthood (Giedd and Rapoport, 2010, Gogtay and Thompson, 2010, Raznahan et al., 2011, Shaw et al., 2008, Taki et al., 2012). Cortical thinning occurs throughout adolescence and extends well into adulthood, but patterns (e.g. linear, quadratic, cubic) differ across brain regions and are also dependent on the studied age range (Østby et al., 2009, Raznahan et al., 2011, Shaw et al., 2008, Sowell et al., 2004, Sowell et al., 2007, Tamnes et al., 2009). In contrast, total white matter volume increases even until approximately the fifth decade of life and declines thereafter (Paus, 2010a, Paus et al., 2001, Walhovd et al., 2005). For subcortical regions, developmental patterns are less clear. For example, age-related volume increases for the hippocampus and amygdala (Østby et al., 2009, Taki et al., 2012), but see (Gogtay et al., 2004), and age-related volume decreases in the caudate, putamen, pallidum and accumbens have been reported (Østby et al., 2009, Sowell et al., 2002, Sowell et al., 2004).
1.2. Sex differences in brain structures
Sex differences account in part for the aforementioned different developmental growth trajectories. Cerebral and gray matter volume in the frontal and parietal lobes peak earlier in girls than in boys (though the exact ages vary depending on the subregion), a pattern which may relate to sex differences in timing of puberty (Lenroot et al., 2007). Moreover, sex differences have been demonstrated in the hippocampus (larger in females; but see (Bramen et al., 2011)), amygdala (larger in males) (Neufang et al., 2009) and thalamus (larger in males) (Bramen et al., 2011), but see (Sowell et al., 2002). Some of these findings have also been replicated in a VBM (voxel-based morphometry) study, showing pronounced sexual dimorphism (males larger than females) in amygdala, thalamus, putamen and insula (Peper et al., 2009).
Sex differences have also been reported in cortical thickness, indicating thicker cortices in parietal and temporal regions in females compared to males (age-range 8–87) (Sowell et al., 2007), but the opposite has also been reported (Raznahan et al., 2011). In a sample with a narrow age range (10–14 years), no sex differences were present in cortical thickness (Bramen et al., 2012). However, significant sex differences were present when gonadal hormones, in this case testosterone, were used as a predictor of cortical thickness (Bramen et al., 2012, Nguyen et al., 2012). Furthermore, maturational patterns for whole brain thickness show different trajectories between sexes (Raznahan et al., 2011).
These studies have provided important insights in the complex changes in brain development in late childhood and adolescence. However, there is still no general consensus on the developmental trajectories of all (sub)cortical brain structures in early and mid adolescence.
The aim of the current study was to perform a replication study focusing on cross-sectional age- and sex-related structural brain differences in a European sample (n = 442; 223 females, 219 males) in the age range of 8–30 years. Specifically, we used structural magnetic resonance imaging (MRI) to gain information on brain volumes, cortical thickness and surface area measurements. We had the following objectives: (1) examine age-related differences in (sub)cortical brain volumes, cortical thickness and surface area with possible sex-related differences. (2) The second goal was to examine the relationship between gray matter volume, cortical thickness and surface area with age and between sexes.
2. Materials and methods
2.1. Participants
We combined data sets from several different imaging studies performed at the Brain and Development Lab, Leiden University, between 2006 and 2010. The same scanner and scanner-protocols were utilized to create a large dataset of healthy participants. Four hundred forty-two (219 males; 223 females) unrelated typically developing children and young adults were included. The age range was between 8 and 30 with an about equal distribution across age cohorts (see Table 1 for subgroups).
Table 1.
Age and sex distribution of the sample.
| Age groups | Sex |
Total | |
|---|---|---|---|
| Females (N) | Males (N) | ||
| 8–9 | 16 | 20 | 36 |
| 10–11 | 23 | 31 | 54 |
| 12–13 | 30 | 31 | 61 |
| 14–15 | 31 | 28 | 59 |
| 16–17 | 27 | 31 | 58 |
| 18–20 | 46 | 33 | 79 |
| 21–23 | 34 | 31 | 65 |
| 24–29 | 16 | 14 | 30 |
| Total | 223 | 219 | 442 |
There were no differences in mean age between males (mean: 16.3 (SD = 4.74)) and females (mean: 17.0 (SD = 4.77; p = 0.08), and no differences in sex or age distribution and time of scan (all p's > 0.8). Participants had no self-reported history of neurological or psychiatric disorders, chronic illness, learning disabilities, or use of medicines known to affect nervous system functioning. They were required to be right handed and to have no MRI contraindications. Participants and primary caregivers (for minors) gave informed consent for the studies and received fixed payment for participation. All studies and procedures were approved by the Medical Ethics Committee of the Leiden University Medical Center.
2.2. Data acquisition
All participants were scanned with the same standard whole-head coil on the same 3-Tesla Philips Achieva MRI system (Best, The Netherlands). High-resolution T1-weighted anatomical scan were obtained: 3D-T1-weighted scan: TR = 9.717 ms; TE = 4.59 ms, flip angle = 8°, 140 slices, 0.875 mm × 0.875 mm × 1.2 mm, FOV = 224.000 × 168.000 × 177.333. All anatomical scans were reviewed and cleared by a radiologist.
2.3. (Sub)cortical volumes, thickness and area
Cortical reconstruction and volumetric segmentation was measured automatically using the software FreeSurfer version 5.0 (http://surfer.nmr.mgh.harvard.edu/) (Dale et al., 1999, Fischl and Dale, 2000, Fischl et al., 1999a). Until recently, manual tracing of brain regions by experts in neuroanatomy has been the accepted standard. However, as the size of the MRI datasets has increased, the time and cost required for the labor-intensive process of manual tracing has become prohibitive. It has been demonstrated that Freesurfer is sufficiently reliable and valid particularly in the context of larger sample sizes to detect associations with clinical or demographic variables (e.g. (Cherbuin et al., 2009, Dewey et al., 2010, Doring et al., 2011)).
Details of the surface-based cortical reconstruction and subcortical volumetric segmentation procedures have been documented in detail previously (Dale et al., 1999, Fischl and Dale, 2000, Fischl et al., 1999a, Fischl et al., 1999b, Fischl et al., 2001, Fischl et al., 2002, Han et al., 2006, Segonne et al., 2004). In short, for each subject the T1 MRI scan was used to construct a three-dimensional model of the cortical surface that included: (1) segmentation of the white matter; (2) tessellation of the gray/white matter boundary; (3) inflation of the folded, tessellated surface; and (4) correction of topological defects (Dale et al., 1999, Fischl et al., 1999a). Measures of cortical thickness are obtained from this surface reconstruction by estimating and then refining the gray/white boundary, deforming the surface outward to the pial surface, and measuring the distances from each point on the white matter surface to the pial surface (Fischl and Dale, 2000).
Volumetric subcortical segmentation and measurement was performed using automated procedures that have been validated as comparable in accuracy to much slower, labor-intensive manual tracing and labeling methods (Fischl et al., 2002, Fischl et al., 2004). This procedure automatically classifies brain tissue into multiple distinct structures such as cerebral and cerebellar gray and white matter, cerebrospinal fluid, basal ganglia, and other subcortical structures. Using probabilistic information derived from a manually labeled training data set, this approach automatically assigns a neuroanatomical label to each voxel in the MRI volume. First, data are rigid-body registered and morphed nonlinearly into a standard stereotactic space. Then, previously manually segmented images are used to calculate statistics about how likely a particular label is at any given location throughout the brain, and these data are used as Bayesian priors for estimating voxel identity in a given subject's brain. Three kinds of information are used by the segmentation to help disambiguate anatomical labels: (1) the prior probability of a given tissue class occurring at a specific location in the atlas space; (2) the image intensity likelihood given that tissue class; and (3) the probability of the local spatial configuration of the labels given the tissue class. Segmentations produced by this procedure can be visually inspected for accuracy and edited prior to inclusion in research analyses. For the purposes of the current study, automated image surfaces and segmentations were inspected and screened for quality control but were not manually edited, in order to maintain the objectivity of results. Intracranial volume was determined by a validated automated method known to be equivalent to manual intracranial volume estimation (Buckner et al., 2004).
Once the cortical models are complete the cerebral cortex was parcellated into units based on gyral and sulcal structure (Desikan et al., 2006). This parcellation method based on major sulci has been shown to be both valid and reliable, with high intraclass correlation coefficient between the manual and automated procedures for both cortical volume estimates and parcel boundaries. The parcellation produces 34 gyral regions subdivided into eleven frontal regions, nine temporal regions, five parietal regions, four occipital regions and four parts of the cingulate cortex (Desikan et al., 2006). In the present study, the subregions per lobe were combined to form one structure for comparison. The four parts of the anterior cingulate cortex were included in the frontal lobe segment (rostral and caudal part) and the temporal lobe segment (posterior and isthmus part), as suggested by the lobe mapping procedure of FreeSurfer (results were similar if these parts were excluded). Gray matter volume, cortical thickness and pial surface area (further referred to as surface area in this paper) were derived for each lobe. Procedures for the measurement of cortical thickness have been validated against histological analysis (Rosas et al., 2002) and manual measurements (Kuperberg et al., 2003, Salat et al., 2004). Freesurfer morphometric procedures have good test–retest reliability across scanner manufacturers and across field strengths (Han et al., 2006, Reuter et al., 2012).
2.4. Statistics
The volume of each subcortical structure, cerebral and cerebellar gray and white matter, was calculated by FreeSurfer as described in the previous section. The volumes of all structures described were averaged across hemisphere within subject. Variations with sex and (the interaction with) age or age-squared (i.e. non-linear trajectories) were estimated using a general linear model in those structures. Brain volumes, lobar gray matter and cortical thickness and surface area measures were corrected for intracranial volume (IC) as an estimate of head size, because the head size in males is in general about 10% larger than in females. Furthermore, in a review by Paus (2010b), it was demonstrated that without IC volume (overall brain size) correction sex differences were found in absolute volumes, but after correction only few differences remained. There is an ongoing discussion whether to correct for IC volume in cortical thickness and surface area measurements. However, to be stringent, the findings reported here include IC volume correction. In addition, information regarding Bonferroni correction for multiple comparisons is noted in Table 2, Table 3.
Table 2.
Volumes of cortical and subcortical brain structures.
| Structure | Raw volumes in ml (SD) |
Intracranial volume corrected |
||||||
|---|---|---|---|---|---|---|---|---|
| All | Females | Males | Sex | Age | Sex × age | Age2 | Sex × age2 | |
| N = 442 | N = 223 | N = 219 | p-Values | p-Values | p-Values | p-Values | p-Values | |
| Accumbens | 1.35 (0.21) | 1.28 (0.19) | 1.41 (0.21) | ns | ns | ns | ns | ns |
| Amygdala | 3.43 (0.38) | 3.25 (0.32) | 3.61 (0.35) | 0.023 | ns | ns | ns | ns |
| Caudate | 8.09 (1.06) | 7.71 (1.01) | 8.48 (0.97) | ns | ns | ns | ns | ns |
| Hippocampus | 9.2 (0.91) | 8.78 (0.78) | 9.62 (0.82) | ns | ns | ns | ns | ns |
| Pallidum | 3.65 (0.40) | 3.45 (0.33) | 3.85 (0.36) | ns | ns | ns | ns | ns |
| Putamen | 11.89 (1.34) | 11.26 (1.21) | 12.53 (1.14) | ns | <0.001a | ns | <0.001a | ns |
| Thalamus | 14.95 (1.44) | 14.25 (1.26) | 15.66 (1.25) | 0.045 | <0.001a | ns | <0.001a | ns |
| Lateral Ventricle | 11.95 (6.66) | 11.11 (6.26) | 12.81 (6.94) | ns | <0.001a | ns | <0.001a | ns |
| 3rd ventricle | 0.85 (0.21) | 0.8 (0.16) | 0.9 (0.23) | ns | <0.001a | 0.028 | <0.001a | 0.016 |
| 4th ventricle | 1.94 (0.58) | 1.82 (0.58) | 2.06 (0.56) | ns | ns | ns | ns | ns |
| Cerebellum GM | 89.55 (11.81) | 83.88 (10.24) | 95.33 (10.43) | 0.008 | <0.001a | ns | <0.001a | ns |
| Cerebellum WM | 31.29 (4.32) | 30.11 (4.06) | 32.5 (4.24) | ns | <0.001a | ns | <0.001a | ns |
| Cerebral GM | 741.93 (72.95) | 700.96 (59.26) | 783.64 (61.03) | <0.001a | <0.001a | <0.001a | <0.001a | <0.001a |
| Cerebral WM | 489.95 (55.61) | 461.16 (44.96) | 519.26 (49.86) | 0.002 | <0.001a | 0.013 | <0.001a | 0.022 |
| Total brain | 1231.88 (117.81) | 1162.12 (92.02) | 1302.9 (97.00) | <0.001a | 0.002 | ns | <0.001a | ns |
| Intracranium | 1573.02 (184.05) | 1464.54 (154.30) | 1683.48 (141.33) | <0.001a | ns | 0.001 | 0.045 | <0.001a |
| Frontal GM | 187.78 (21.01) | 177.27 (17.88) | 198.49 (18.43) | <0.001a | <0.001a | 0.001 | <0.001a | 0.001 |
| Parietal GM | 136.5 (16.75) | 128.96 (14.42) | 144.18 (15.44) | <0.001a | <0.001a | 0.001 | <0.001a | <0.001a |
| Temporal GM | 133.6 (15.31) | 125.88 (12.91) | 141.46 (13.47) | 0.002 | <0.001a | 0.018 | <0.001a | 0.016 |
| Occipital GM | 50.84 (6.72) | 47.98 (5.88) | 53.75 (6.27) | 0.002 | <0.001a | 0.014 | <0.001a | 0.014 |
| ACC GM | 24.56 (3.24) | 23.27 (2.85) | 25.88 (3.07) | 0.034 | <0.001a | ns | <0.001a | ns |
Abbreviations: GM, gray matter; WM, white matter; ACC, anterior cingulate cortex; ns, not significant.
Bonferroni p-Value = 0.002.
Survives Bonferroni correction.
Table 3.
Thickness and area measurements of the four lobes and cingulate cortex.
| Raw mean (SD) |
Intracranial volume corrected |
|||||||
|---|---|---|---|---|---|---|---|---|
| All | Females | Males | Sex | Age | Sex × age | Age2 | Sex × age2 | |
| N = 442 | N = 223 | N = 219 | p-Values | p-Values | p-Values | p-Values | p-Values | |
| Thickness (in mm) | ||||||||
| Frontal lobe | 2.85 (0.13) | 2.84 (0.13) | 2.85 (0.13) | ns | <0.001a | ns | <0.001a | ns |
| Parietal lobe | 2.58 (0.13) | 2.57 (0.13) | 2.59 (0.13) | ns | <0.001a | ns | <0.001a | ns |
| Temporal lobe | 2.94 (0.13) | 2.94 (0.13) | 2.95 (0.12) | ns | <0.001a | ns | <0.001a | ns |
| Occipital lobe | 2.13 (0.12) | 2.12 (0.12) | 2.14 (0.12) | ns | <0.001a | ns | <0.001a | ns |
| ACC | 2.97 (0.15) | 2.98 (0.15) | 2.95 (0.14) | 0.023 | <0.001a | ns | <0.001a | ns |
| Surface area (in mm2) | ||||||||
| Frontal lobe | 56.7 (6.01) | 53.6 (4.83) | 59.9 (5.36) | <0.001a | <0.001a | <0.001a | <0.001a | 0.001a |
| Parietal lobe | 46.5 (4.90) | 44.0 (3.96) | 49.1 (4.40) | <0.001a | <0.001a | <0.001a | <0.001a | <0.001a |
| Temporal lobe | 38.6 (4.13) | 36.4 (3.31) | 40.8 (3.66) | 0.001a | <0.001a | 0.001a | <0.001a | 0.001a |
| Occipital lobe | 22.4 (2.52) | 21.3 (2.20) | 23.5 (2.35) | 0.009 | 0.005 | ns | 0.015 | ns |
| ACC | 7.42 (0.95) | 7.0 (0.84) | 7.85 (0.88) | 0.01 | ns | 0.018 | <0.001a | 0.036 |
Abbreviations: ACC, anterior cingulate cortex; ns, not significant.
Bonferroni p-Value = 0.005.
Survives Bonferroni correction.
Model fits were calculated to examine developmental trajectories of brain volumes, cortical thickness and surface area measures. In case of significant sex or sex-by-age(-squared) interactions, model fits were calculated separately for both sexes as well as for the whole sample. F-tests were used to determine whether a linear or quadratic model significantly best fit the data. We used the extra sum-of-squares approach to obtain an F-ratio from the relative increase in the sum-of-squares and the relative increase in the degrees of freedom reflecting the number of parameters used for a linear or quadratic model (this information is available in the ANOVA table for each regression fit) (Motulsky and Christopoulos, 2004, Thomas et al., 2009).
3. Results
Raw brain volumes for the whole sample, and for males and females separately are reported in Table 2 along with the statistical results of comparisons between sex, age (/-squared) and their interactions. Table 3 lists gray matter volumes, thickness and area measurements for frontal, temporal, parietal and occipital lobes and the anterior cingulate cortex. All volume, thickness and area measurements were corrected for intracranial volume.
3.1. Age differences in brain volumes
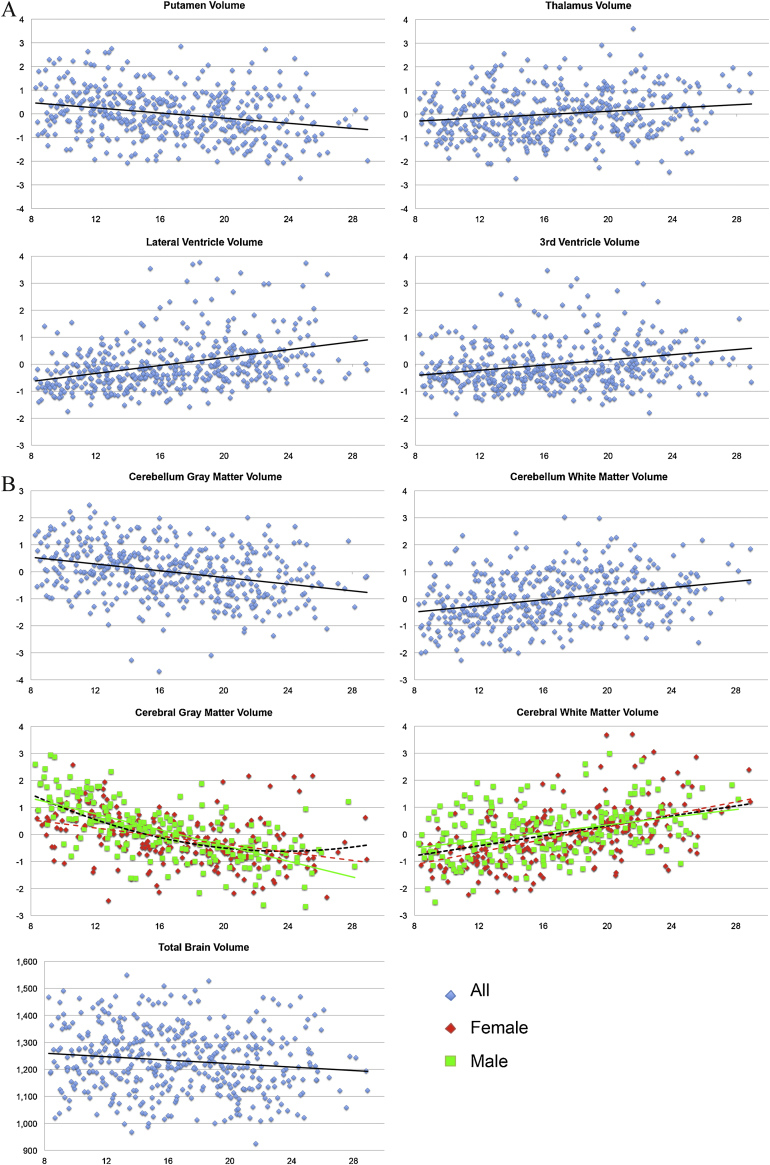
Analyses of subcortical volumes demonstrated that the only subcortical brain area showing a negative age association was the putamen and the only subcortical brain area showing a positive age association was the thalamus (Table 2). Positive associations with age were further found for the lateral and third ventricle volumes, and for cerebral and cerebellar white matter. Negative associations with age were found for gray matter volumes of the four lobes, ACC, cerebrum and cerebellum and total brain. Again, we calculated total gray matter-white matter (GM/WM) ratios. The total GM/WM-ratio decreased with age (p < 0.001) but there was no sex-by-age interaction.
3.2. Sex differences in brain volumes
As expected, significant sex differences were found in intracranial, total brain, cerebral gray and white matter, and cerebellar gray matter, with males showing larger volumes compared to females (Table 2). Analyses of subcortical volume differences showed that males had significantly larger amygdala and thalamus volumes than females. Second, gray matter volume within all four lobes as well as in ACC was larger in males than in females. Finally, we calculated total gray matter-white matter (GM/WM) ratios. This analysis revealed a larger total GM/WM-ratio in females than in males (p = 0.006).
3.3. Sex-by-age interaction and brain volumes
No sex-by-age interactions were found for any of the subcortical regions.
In contrast, significant sex-by-age interactions were found for lobar and cerebral gray matter volumes, with both groups showing gray matter volume loss with increasing age, but males demonstrated a general pattern of larger volume decreases with increasing age compared to females (see Fig. 1A and B). Males also showed a marginally larger third ventricle volume increases than females. Conversely, cerebral white matter volume increases were larger in females compared to males. Fig. 1A and B shows the significant relationships between age and brain volumes corrected for IC. Separate curves were fitted for males and females if the slopes were significantly different from each other (see Table 4). These slopes demonstrated that the quadratic fit was better than the linear fit for all structures. However, given that quadratic fits in cross-sectional studies may be affected by the age at which sampling started (or ended) (Fjell et al., 2010), we also displayed the linear fits to demonstrate the difference between both models.
Fig. 1.
(A and B) Regression plots showing the significant relationship between age (and sex in case of significant interaction effects (females in red, males in green)) and (sub)cortical brain volumes. With the exception of total brain volume (in ml), all indices were corrected for intracranial volume. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Table 4.
Model-fits of brain volumes, cortical thickness and surface area measures with age.
| Brain structure | Model fit |
|||||
|---|---|---|---|---|---|---|
| Linear |
Quadratica |
|||||
|
R-square |
R-square |
|||||
| All | Males | Females | All | Males | Females | |
| Amygdala | 0.0003 | |||||
| Caudate nucleus | 0.001 | |||||
| Nucleus accumbens | 0.007 | |||||
| Hippocampus | 0.001 | |||||
| Pallidum | 0.001 | |||||
| Putamen | 0.06*** | |||||
| Thalamus | 0.028*** | |||||
| Lateral ventricles | 0.123*** | |||||
| 3rd ventricle | 0.053*** | 0.066*** | 0.046** | |||
| 4th ventricle | 0.005 | |||||
| Cerebellum GM | 0.088*** | |||||
| Cerebellum WM | 0.073*** | |||||
| Total GMb | 0.283*** | 0.43*** | 0.15*** | 0.324*** | ||
| Total WMb | 0.193*** | 0.13*** | 0.278*** | |||
| Intracranium | 0.01* | 0.05 | 0.026** | |||
| Total brain | 0.029*** | |||||
| ACC surface area | 0.0002 | 0.015 | 0.011 | |||
| ACC GMb | 0.114*** | 0.123*** | ||||
| acc thickness | 0.215*** | |||||
| Frontal surface areab | 0.04*** | 0.144*** | 0.0001 | 0.055*** | 0.161*** | |
| Frontal GMb | 0.383*** | 0.49*** | 0.274*** | 0.405*** | 0.518*** | |
| Frontal thickness | 0.288*** | |||||
| Temporal surface areab | 0.027** | 0.103*** | 0.0001 | 0.06*** | 0.125*** | |
| Temporal GMb | 0.166*** | 0.255*** | 0.089*** | 0.198*** | 0.285*** | 0.114** |
| Temporal thickness | 0.169*** | |||||
| Parietal surface areab | 0.08*** | 0.211*** | 0.007 | 0.142*** | 0.273*** | |
| Parietal GMb | 0.401*** | 0.506*** | 0.296*** | 0.471*** | 0.574*** | 0.354*** |
| Parietal thickness | 0.404*** | 0.416*** | ||||
| Occipital surface areab | 0.011* | 0.055*** | ||||
| Occipital GMb | 0.192*** | 0.261*** | 0.123*** | 0.256*** | 0.318*** | 0.185*** |
| Occipital thickness | 0.389*** | 0.411*** | ||||
Abbreviations: GM, gray matter; WM, white matter. A non-significant R-square indicates that the regression line is not different from zero.
All quadratic fits were significantly better than linear fits based on F-ratio derived from “extra sum-of-squares method”. Obtained p-values of the F-ratio indicate if the simpler linear model is really correct, and the chance that randomly obtained data would show a better fit to a more complicated (quadratic) model. Low p-values indicate that the quadratic model is significantly better than the linear model Motulsky and Christopoulos (2004, p. 141).
Trajectories differed significantly between sexes.
p < 0.05.
p < 0.01.
p < 0.001.
3.4. Relationship between sex, age, cortical thickness and surface area
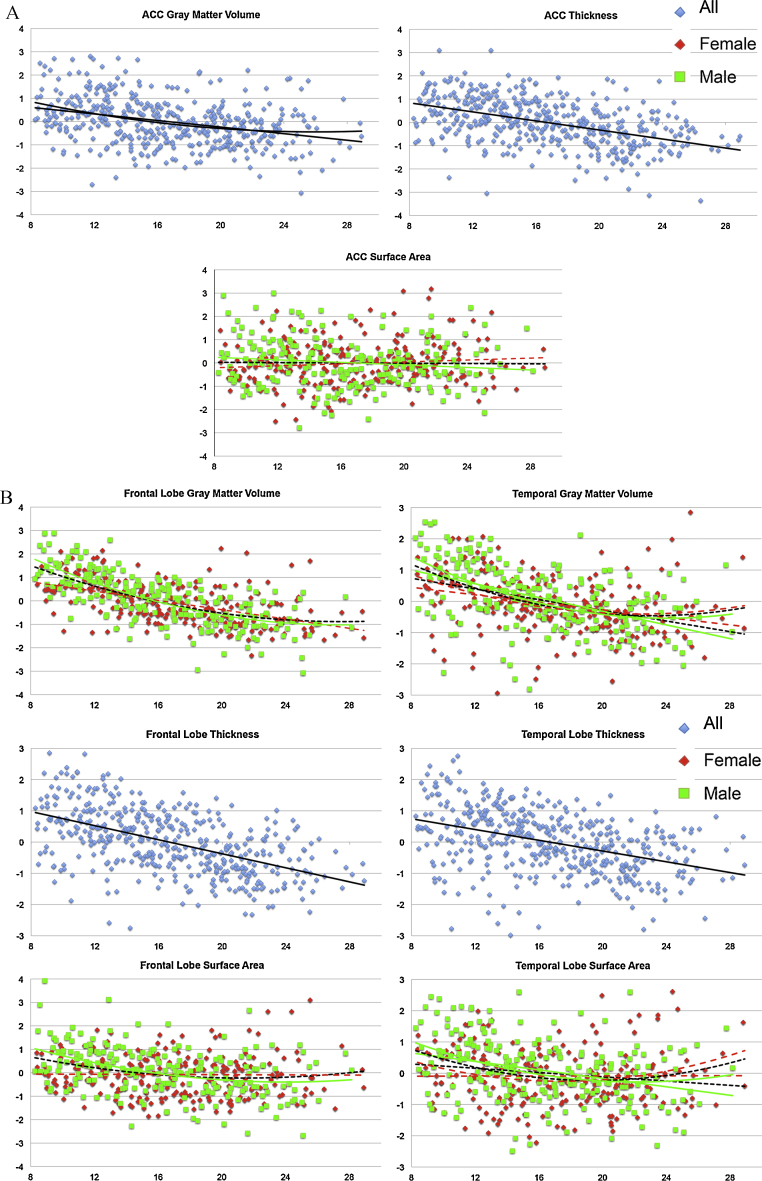
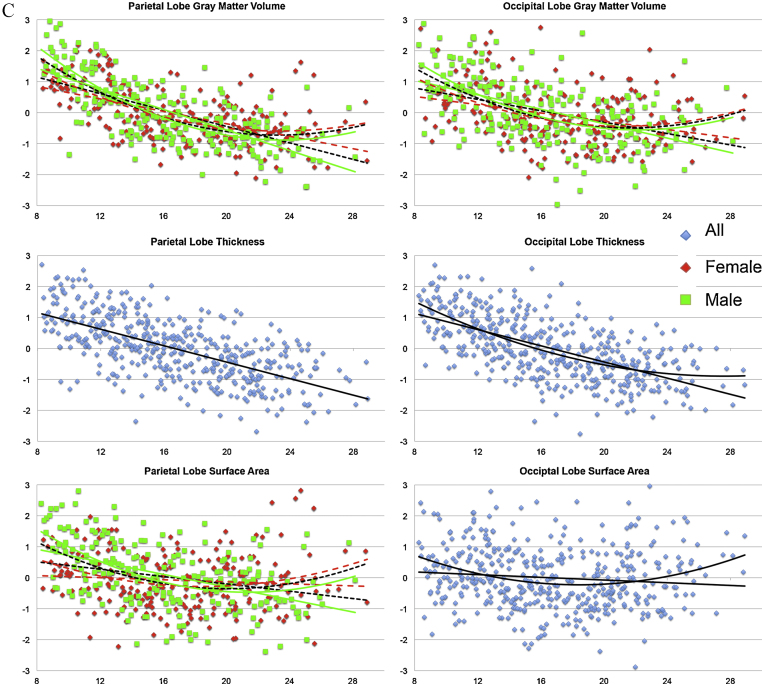
Besides volume differences, we also examined differences in cortical thickness and surface areas (Table 3 and Fig. 2A–C).
Fig. 2.
(A–C) Regression plots showing the relationship between age (and sex in case of significant interaction effects (females in red, males in green)) and of lobar gray matter volume, thickness and surface area. All indices were corrected for intracranial volume. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
As can be seen in Fig. 2A–C, gray matter volume and cortical thickness followed the same trajectory with increasing age, while there were only small age differences in surface area measurements. These associations are also expressed in terms of partial correlations between gray matter volume, cortical thickness and surface area in Table 5. Thus, age differences were most pronounced for gray matter volume and cortical thickness. Next, we tested whether these age differences were different for the sexes.
Table 5.
Partial correlations between lobar gray matter volumes, cortical thickness and surface area.
| Partial correlationsa |
|||
|---|---|---|---|
| GM volume–cortical thickness | GM volume–surface area | Cortical thickness–surface area | |
| Frontal cortex | 0.35 | 0.69 | −0.40 |
| Parietal cortex | 0.47 | 0.72 | −0.23 |
| Temporal cortex | 0.50 | 0.72 | −0.20 |
| Occipital cortex | 0.47 | 0.83 | ns |
| ACC | 0.20 | 0.86 | −0.29 |
Abbreviations: GM, gray matter; ACC, anterior cingulate cortex; IC, intracranial volume; ns, not significant.
Partial correlations were controlled for IC, sex and age.
All bold findings were significant: p < 0.0001.
Only surface area measurements showed significant sex-by-interactions on a lobar level, except for the occipital lobe and the ACC. Males demonstrated relatively large surface area contractions with increasing age, primarily in frontal, parietal and temporal cortex, while females showed only marginal or no surface contractions in these regions.
In contrast to the surface analyses, the lobar thickness measurement analyses were not sensitive to sex differences. That is, all lobar thickness measurements showed negative associations with increasing age, but no sex effects were found in cortical thickness of the four lobes (Table 3).
4. Discussion
This study aimed to replicate the effects of age, sex, and age by sex interactions on brain volumes, cortical thickness and surface area in a European sample of typically developing children, adolescents and young adults. Specific age and sex differences, and intriguing sex-by-age interactions were observed; these will be described in more detail below.
4.1. Age-effects and brain volumes
Total brain volume decreased with increasing age, and this was accompanied by strong cerebral and cerebellar gray matter volume decreases. In addition, cerebral and cerebellar white matter volume increased with age, replicating earlier findings on gray and white matter during adolescence (Brain Development Cooperative, 2012, Giedd et al., 1999). The four lobes and the ACC showed similar gray matter volume decreases with age. The steepness of the slopes differed between lobes. The larger temporal, parietal and frontal gray matter volume loss with increasing age may be associated with more protracted brain maturation; while the moderate occipital volume loss with age could indicate a more mature pattern of development (Casey et al., 2005).
The subcortical brain structures showed less marked age-related trajectories. In fact, with the exception of the thalamus and putamen, all subcortical volumes remained stable with age. This was unexpected, because a subset of studies has found small increases in amygdala and hippocampal volumes during adolescence (Neufang et al., 2009, Suzuki et al., 2005). Our findings are partly consistent with findings from Østby et al. (2009) who also reported an age-related decrease in the putamen, although they also reported small to moderate changes in several other subcortical regions. Notably, earlier reports from a longitudinal study including participants between ages 8–30 also showed that bilateral total hippocampal volume remained unchanged (Gogtay et al., 2006), but there were significant age differences in the development of hippocampal subregions (i.e. posterior vs. anterior portions). Despite differences in methodology between studies, these findings show that in this large sample only a subset of age effects could be replicated.
4.2. Sex differences and sex by age interaction effects
The second question concerned whether there were sex differences in brain volumes and whether these interacted with age differences in brain development. We replicated earlier findings on larger intracranial, total brain and total gray and white matter volume in males compared to females. On a subcortical level, we confirmed larger amygdala (Wilke et al., 2007) and thalamus (Sowell et al., 2002) volumes in males irrespective of age. Furthermore, males demonstrated larger gray matter volumes on all lobar levels, after covarying for intracranial volume to correct for differences in head size. The sex difference in hippocampal volumes and slopes could not be replicated (Bramen et al., 2011, Giedd et al., 1996, Neufang et al., 2009), but see other reports which also failed to replicate these findings (Giedd et al., 1997, Gogtay et al., 2006, Yurgelun-Todd et al., 2003). Thus, most of the predicted sex differences could be confirmed in the present study, although some inconsistencies remained.
Importantly, no sex-by-age interactions were observed for subcortical volumes. Yet, interesting sex-by-age interactions were found for cortical volumes, with males demonstrating larger total and lobar gray matter volume decreases with age than females. Concomitant white matter volume increase was larger in females. On these measures, all slopes were significantly different between sexes, indicating different developmental trajectories for gray and white matter development, although this should be confirmed in longitudinal research. The majority of these findings were predicted; however, white matter volume increase has been reported to be opposite to our current results, i.e. rapid white matter volume increases in males during adolescence (Giedd et al., 1999, Lenroot et al., 2007), or similar growth (Wilke et al., 2007). Prior studies have reported an important role of gonadal hormones on white matter development, a question which should be followed up in future research (Ladouceur et al., 2012, Perrin et al., 2008).
The sex-by-age interactions in cortical development were further supported by surface area analyses. Even though surface area measurements overall remained stable across development, there were sex-by-age interactions in the frontal, parietal and temporal lobe showing larger surface area in males between ages 8 and 15. This suggests that in boys there is still prolonged surface area expansion (but faster surface area loss with age), while in girls surface expansion seems to be completed (or slower). However, these findings should be interpreted with caution. Longitudinal research is necessary to determine how (change in) surface area expansion develops with increasing age.
Although volume is mathematically defined as area times height (in this case height can be seen as thickness), prior studies have suggested that cortical surface area and cortical thickness are (genetically) independent, both globally and regionally (Im et al., 2008, Pakkenberg and Gundersen, 1997, Panizzon et al., 2009, Winkler et al., 2009). This is also reflected by the low correlation between surface area and cortical thickness reported here and consistent with the idea that surface area reduction and cortical thinning are independent processes, yet not necessarily biologically independent (Panizzon et al., 2009, Winkler et al., 2009, Winkler et al., 2010). Moreover, the high correlations between volume and surface area measurements compared to volume and cortical thickness resemble those of a longitudinal twin-study in 9- and 12-year olds (van Soelen et al., 2012) and adolescence (Raznahan et al., 2011). Further evidence for differences in cortical thinning and surface area comes from patient studies. For example, in ADHD and dyslexia a decrease in surface area with intact cortical thickness has been reported (Frye et al., 2010, Wolosin et al., 2009). The exact relation between surface area and cortical thickness should be explored in more detail in future studies using longitudinal designs. The radial unit hypothesis of cortical development suggests that the cortical surface area is influenced by the number of columns, whereas cortical thickness is influenced by the number or the size of cells within a column (Rakic, 1988, Rakic, 2000). This would suggest cell loss (or shrinkage) within columns (reduction of cortical thickness) while the number of columns remains relatively stable (surface area) during the transition into adulthood.
It must be noted that the exact mechanism behind cortical thinning in adolescence is not well understood. Cortical thinning during puberty and adolescence has been associated with the loss of unneeded connections (synaptic pruning; (Huttenlocher and Dabholkar, 1997)), but pruning cannot fully account for the observed thinning (Paus, 2005). Interestingly, a postmortem study by Petanjek et al. (2011) reported decreases of dendritic spine density and elimination of synaptic spines starting during puberty, continuing well into the third decade of life. The cortical gray matter loss during adolescence is thought to be the result from the encroachment of continued white matter growth which normally extends into the 4th decade (Benes et al., 1994, Courchesne et al., 2000, Gogtay et al., 2008, Gogtay and Thompson, 2010, Raz et al., 2005, Westlye et al., 2010, Yakovlev and Lecours, 1967).
It is evident that longitudinal (replication) studies are needed to further disentangle the relationship and mechanisms of cortical thinning and surface area expansion during adolescence, possibly with additional measures of development besides age (e.g. puberty). In addition, the field may also benefit from the use of (ultra) high field MRI (7 T) to examine these ongoing changes in more detail (Duyn, 2011).
4.3. Limitations and future directions
There are several limitations and questions for future research that remain to be answered. First, we were not able to replicate all of the previously reported age differences in subcortical brain development. One reason for this lack of replication is that the current methodology is not sensitive enough to detect subtle age-related volume differences. Furthermore, it is possible that there are shape changes with different developmental patterns, which cancel out overall volumetric patterns. Shape analysis allows statistical assessment of the subregional anatomy of morphologically changed areas with 3D modeling of these structures, so that contracted or expanded subregions of, for example, the hippocampus can be identified (Patenaude et al., 2011). Second, heterogeneous effects found in cross-sectional studies provide a general estimation of age-related trajectories, but longitudinal studies represent a more stable measurement of individual change over time. However, our cross-sectional findings on cortical brain development mimic those from a recent longitudinal study (Raznahan et al., 2011). Third, pubertal influences may account in part for the heterogeneous effects. We aimed to create a large sample of unique individuals and examine brain morphology from childhood to adulthood. Unfortunately, in these prior neuroimaging studies we did not consistently collect measures of pubertal development. Therefore, we could not analyze effects of pubertal development in this study. In prior studies, sex differences have been reported in the hippocampus, amygdala, and cortical gray matter in more sexually mature adolescents based on physical pubertal maturity and circulating testosterone (for reviews see: Peper and Dahl, 2013, Peper et al., 2011). However, studies examining pubertal hormones are still underpowered in relation to sample size. The current study tried to overcome these issues by using a very large sample of children, (pre) adolescents and young adults. In future studies, it will be important to analyze effects of puberty in more detail, including longitudinal measurements of pubertal development.
Another limitation is that it is difficult to estimate the exact trajectories of change. The majority of brain regions showed linear relationships with age, but the large heterogeneity of this sample could mask curvilinear relationships. On the other hand, only large brain regions, i.e. lobar gray matter and total gray and white matter demonstrated significant curvilinear relationships with age. As mentioned earlier, caution must be taken when using quadratic model fits based on cross-sectional data, because the fit may be driven by sample characteristics at start (or end) of sampling age (Fjell et al., 2010). In addition, there may be biological reasons to reject a curvilinear fit. For example, in a normal population it is not expected that (lobar) gray matter volume increases after age 20.
5. Conclusion
In a large European sample, we described the role of developmental differences in a variety of structural brain indices from childhood to early adulthood. Consistent with prior research, we have shown that adolescence is characterized by large individual variance in brain volumes. Furthermore, sex differences were most prominent in cortical surface area measurements, and in gray and white matter, even after controlling for intracranial volume. The functional relevance of these structural findings in understanding typical brain development can be numerous. For example, thinning of frontal and parietal cortices has been linked to more mature brain activation patterns in children and adolescents (Lu et al., 2007, Lu et al., 2009). The goal for the future is to combine these studies effectively with behavioral, functional and hormonal measurements to disentangle the structure–function relationship and its development during the transition into adulthood.
Funding
This research was supported by a VIDI grant (no. 91786368) from the Netherlands Organization for Scientific Research (NWO) awarded to the author E.A. Crone.
Conflict of interest
None declared.
Acknowledgement
The authors are thankful to all Brain and Development members and alumni for their help with data acquisition.
Contributor Information
P. Cédric M.P. Koolschijn, Email: koolschijnpcmp@gmail.com.
Eveline A. Crone, Email: ecrone@fsw.leidenuniv.nl.
References
- Benes F.M., Turtle M., Khan Y., Farol P. Myelination of a key relay zone in the hippocampal formation occurs in the human brain during childhood, adolescence, and adulthood. Archives of General Psychiatry. 1994;51:477–484. doi: 10.1001/archpsyc.1994.03950060041004. [DOI] [PubMed] [Google Scholar]
- Brain Development Cooperative G. Total and regional brain volumes in a population-based normative sample from 4 to 18 years: the NIH MRI study of normal brain development. Cerebral Cortex. 2012;22:1–12. doi: 10.1093/cercor/bhr018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramen J.E., Hranilovich J.A., Dahl R.E., Chen J., Rosso C., Forbes E.E., Dinov I.D., Worthman C.M., Sowell E.R. Sex matters during adolescence: testosterone-related cortical thickness maturation differs between boys and girls. PLoS ONE. 2012;7:e33850. doi: 10.1371/journal.pone.0033850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramen J.E., Hranilovich J.A., Dahl R.E., Forbes E.E., Chen J., Toga A.W., Dinov I.D., Worthman C.M., Sowell E.R. Puberty influences medial temporal lobe and cortical gray matter maturation differently in boys than girls matched for sexual maturity. Cerebral Cortex. 2011;21:636–646. doi: 10.1093/cercor/bhq137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner R.L., Head D., Parker J., Fotenos A.F., Marcus D., Morris J.C., Snyder A.Z. A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: reliability and validation against manual measurement of total intracranial volume. Neuroimage. 2004;23:724–738. doi: 10.1016/j.neuroimage.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Casey B.J., Tottenham N., Liston C., Durston S. Imaging the developing brain: what have we learned about cognitive development? Trends in Cognitive Sciences. 2005;9:104–110. doi: 10.1016/j.tics.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Cherbuin N., Anstey K.J., Reglade-Meslin C., Sachdev P.S. In vivo hippocampal measurement and memory: a comparison of manual tracing and automated segmentation in a large community-based sample. PLoS ONE. 2009;4:e5265. doi: 10.1371/journal.pone.0005265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courchesne E., Chisum H.J., Townsend J., Cowles A., Covington J., Egaas B., Harwood M., Hinds S., Press G.A. Normal brain development and aging: quantitative analysis at in vivo MR imaging in healthy volunteers. Radiology. 2000;216:672–682. doi: 10.1148/radiology.216.3.r00au37672. [DOI] [PubMed] [Google Scholar]
- Dale A.M., Fischl B., Sereno M.I. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Desikan R.S., Segonne F., Fischl B., Quinn B.T., Dickerson B.C., Blacker D., Buckner R.L., Dale A.M., Maguire R.P., Hyman B.T., Albert M.S., Killiany R.J. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Dewey J., Hana G., Russell T., Price J., McCaffrey D., Harezlak J., Sem E., Anyanwu J.C., Guttmann C.R., Navia B., Cohen R., Tate D.F. Reliability and validity of MRI-based automated volumetry software relative to auto-assisted manual measurement of subcortical structures in HIV-infected patients from a multisite study. Neuroimage. 2010;51:1334–1344. doi: 10.1016/j.neuroimage.2010.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doring T.M., Kubo T.T., Cruz L.C., Jr., Juruena M.F., Fainberg J., Domingues R.C., Gasparetto E.L. Evaluation of hippocampal volume based on MR imaging in patients with bipolar affective disorder applying manual and automatic segmentation techniques. Journal of Magnetic Resonance Imaging. 2011;33:565–572. doi: 10.1002/jmri.22473. [DOI] [PubMed] [Google Scholar]
- Duyn J.H. The future of ultra-high field MRI and fMRI for study of the human brain. Neuroimage. 2011;62:1241–1248. doi: 10.1016/j.neuroimage.2011.10.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B., Dale A.M. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B., Liu A., Dale A.M. Automated manifold surgery: constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Transactions on Medical Imaging. 2001;20:70–80. doi: 10.1109/42.906426. [DOI] [PubMed] [Google Scholar]
- Fischl B., Salat D.H., Busa E., Albert M., Dieterich M., Haselgrove C., van der K.A., Killiany R., Kennedy D., Klaveness S., Montillo A., Makris N., Rosen B., Dale A.M. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fischl B., Sereno M.I., Dale A.M. Cortical surface-based analysis. II. Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Fischl B., Sereno M.I., Tootell R.B., Dale A.M. High-resolution intersubject averaging and a coordinate system for the cortical surface. Human Brain Mapping. 1999;8:272–284. doi: 10.1002/(SICI)1097-0193(1999)8:4<272::AID-HBM10>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B., van der Kouwe A., Destrieux C., Halgren E., Segonne F., Salat D.H., Busa E., Seidman L.J., Goldstein J., Kennedy D., Caviness V., Makris N., Rosen B., Dale A.M. Automatically parcellating the human cerebral cortex. Cerebral Cortex. 2004;14:11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- Fjell A.M., Walhovd K.B., Westlye L.T., Ostby Y., Tamnes C.K., Jernigan T.L., Gamst A., Dale A.M. When does brain aging accelerate? Dangers of quadratic fits in cross-sectional studies. Neuroimage. 2010;50:1376–1383. doi: 10.1016/j.neuroimage.2010.01.061. [DOI] [PubMed] [Google Scholar]
- Frye R.E., Liederman J., Malmberg B., McLean J., Strickland D., Beauchamp M.S. Surface area accounts for the relation of gray matter volume to reading-related skills and history of dyslexia. Cerebral Cortex. 2010;20:2625–2635. doi: 10.1093/cercor/bhq010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd J.N., Blumenthal J., Jeffries N.O., Castellanos F.X., Liu H., Zijdenbos A., Paus T., Evans A.C., Rapoport J.L. Brain development during childhood and adolescence: a longitudinal MRI study. Nature Neuroscience. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Giedd J.N., Castellanos F.X., Rajapakse J.C., Vaituzis A.C., Rapoport J.L. Sexual dimorphism of the developing human brain. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 1997;21:1185–1201. doi: 10.1016/s0278-5846(97)00158-9. [DOI] [PubMed] [Google Scholar]
- Giedd J.N., Rapoport J.L. Structural MRI of pediatric brain development: what have we learned and where are we going? Neuron. 2010;67:728–734. doi: 10.1016/j.neuron.2010.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd J.N., Snell J.W., Lange N., Rajapakse J.C., Casey B.J., Kozuch P.L., Vaituzis A.C., Vauss Y.C., Hamburger S.D., Kaysen D., Rapoport J.L. Quantitative magnetic resonance imaging of human brain development: ages 4–18. Cerebral Cortex. 1996;6:551–560. doi: 10.1093/cercor/6.4.551. [DOI] [PubMed] [Google Scholar]
- Gogtay N., Giedd J.N., Lusk L., Hayashi K.M., Greenstein D., Vaituzis A.C., Nugent T.F., III, Herman D.H., Clasen L.S., Toga A.W., Rapoport J.L., Thompson P.M. Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N., Lu A., Leow A.D., Klunder A.D., Lee A.D., Chavez A., Greenstein D., Giedd J.N., Toga A.W., Rapoport J.L., Thompson P.M. Three-dimensional brain growth abnormalities in childhood-onset schizophrenia visualized by using tensor-based morphometry. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:15979–15984. doi: 10.1073/pnas.0806485105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N., Nugent T.F., III, Herman D.H., Ordonez A., Greenstein D., Hayashi K.M., Clasen L., Toga A.W., Giedd J.N., Rapoport J.L., Thompson P.M. Dynamic mapping of normal human hippocampal development. Hippocampus. 2006;16:664–672. doi: 10.1002/hipo.20193. [DOI] [PubMed] [Google Scholar]
- Gogtay N., Thompson P.M. Mapping gray matter development: implications for typical development and vulnerability to psychopathology. Brain and Cognition. 2010;72:6–15. doi: 10.1016/j.bandc.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X., Jovicich J., Salat D., van der K.A., Quinn B., Czanner S., Busa E., Pacheco J., Albert M., Killiany R., Maguire P., Rosas D., Makris N., Dale A., Dickerson B., Fischl B. Reliability of MRI-derived measurements of human cerebral cortical thickness: the effects of field strength, scanner upgrade and manufacturer. Neuroimage. 2006;32:180–194. doi: 10.1016/j.neuroimage.2006.02.051. [DOI] [PubMed] [Google Scholar]
- Huttenlocher P.R., Dabholkar A.S. Regional differences in synaptogenesis in human cerebral cortex. Journal of Comparative Neurology. 1997;387:167–178. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Im K., Lee J.M., Lyttelton O., Kim S.H., Evans A.C., Kim S.I. Brain size and cortical structure in the adult human brain. Cerebral Cortex. 2008;18:2181–2191. doi: 10.1093/cercor/bhm244. [DOI] [PubMed] [Google Scholar]
- Kuperberg G.R., Broome M.R., McGuire P.K., David A.S., Eddy M., Ozawa F., Goff D., West W.C., Williams S.C., van der Kouwe A.J., Salat D.H., Dale A.M., Fischl B. Regionally localized thinning of the cerebral cortex in schizophrenia. Archives of General Psychiatry. 2003;60:878–888. doi: 10.1001/archpsyc.60.9.878. [DOI] [PubMed] [Google Scholar]
- Ladouceur C.D., Peper J.S., Crone E.A., Dahl R.E. White matter development in adolescence: the influence of puberty and implications for affective disorders. Developmental Cognitive Neuroscience. 2012;2:36–54. doi: 10.1016/j.dcn.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenroot R.K., Gogtay N., Greenstein D.K., Wells E.M., Wallace G.L., Clasen L.S., Blumenthal J.D., Lerch J., Zijdenbos A.P., Evans A.C., Thompson P.M., Giedd J.N. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. Neuroimage. 2007;36:1065–1073. doi: 10.1016/j.neuroimage.2007.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L., Leonard C., Thompson P., Kan E., Jolley J., Welcome S., Toga A., Sowell E. Normal developmental changes in inferior frontal gray matter are associated with improvement in phonological processing: a longitudinal MRI analysis. Cerebral Cortex. 2007;17:1092–1099. doi: 10.1093/cercor/bhl019. [DOI] [PubMed] [Google Scholar]
- Lu L.H., Dapretto M., O‘Hare E.D., Kan E., McCourt S.T., Thompson P.M., Toga A.W., Bookheimer S.Y., Sowell E.R. Relationships between brain activation and brain structure in normally developing children. Cerebral Cortex. 2009;19:2595–2604. doi: 10.1093/cercor/bhp011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motulsky H.J., Christopoulos A. Oxford University Press; NY, USA: 2004. Fitting Models to Biological Data Using Linear and Nonlinear Regression. [Google Scholar]
- Neufang S., Specht K., Hausmann M., Gunturkun O., Herpertz-Dahlmann B., Fink G.R., Konrad K. Sex differences and the impact of steroid hormones on the developing human brain. Cerebral Cortex. 2009;19:464–473. doi: 10.1093/cercor/bhn100. [DOI] [PubMed] [Google Scholar]
- Nguyen T.V., McCracken J., Ducharme S., Botteron K.N., Mahabir M., Johnson W., Israel M., Evans A.C., Karama S. Testosterone-related cortical maturation across childhood and adolescence. Cerebral Cortex. 2012 doi: 10.1093/cercor/bhs125. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Østby Y., Tamnes C.K., Fjell A.M., Westlye L.T., Due-Tonnessen P., Walhovd K.B. Heterogeneity in subcortical brain development: a structural magnetic resonance imaging study of brain maturation from 8 to 30 years. Journal of Neuroscience. 2009;29:11772–11782. doi: 10.1523/JNEUROSCI.1242-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakkenberg B., Gundersen H.J. Neocortical neuron number in humans: effect of sex and age. Journal of Comparative Neurology. 1997;384:312–320. [PubMed] [Google Scholar]
- Panizzon M.S., Fennema-Notestine C., Eyler L.T., Jernigan T.L., Prom-Wormley E., Neale M., Jacobson K., Lyons M.J., Grant M.D., Franz C.E., Xian H., Tsuang M., Fischl B., Seidman L., Dale A., Kremen W.S. Distinct genetic influences on cortical surface area and cortical thickness. Cerebral Cortex. 2009;19:2728–2735. doi: 10.1093/cercor/bhp026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patenaude B., Smith S.M., Kennedy D.N., Jenkinson M. A Bayesian model of shape and appearance for subcortical brain segmentation. Neuroimage. 2011;56:907–922. doi: 10.1016/j.neuroimage.2011.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T. Mapping brain maturation and cognitive development during adolescence. Trends in Cognitive Sciences. 2005;9:60–68. doi: 10.1016/j.tics.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Paus T. Growth of white matter in the adolescent brain: myelin or axon? Brain and Cognition. 2010;72:26–35. doi: 10.1016/j.bandc.2009.06.002. [DOI] [PubMed] [Google Scholar]
- Paus T. Sex differences in the human brain: a developmental perspective. Progress in Brain Research. 2010;186:13–28. doi: 10.1016/B978-0-444-53630-3.00002-6. [DOI] [PubMed] [Google Scholar]
- Paus T., Collins D.L., Evans A.C., Leonard G., Pike B., Zijdenbos A. Maturation of white matter in the human brain: a review of magnetic resonance studies. Brain Research Bulletin. 2001;54:255–266. doi: 10.1016/s0361-9230(00)00434-2. [DOI] [PubMed] [Google Scholar]
- Peper J.S., Brouwer R.M., Schnack H.G., van Baal G.C., van L.M., van den Berg S.M., Delemarre-Van de Waal H.A., Boomsma D.I., Kahn R.S., Hulshoff Pol H.E. Sex steroids and brain structure in pubertal boys and girls. Psychoneuroendocrinology. 2009;34:332–342. doi: 10.1016/j.psyneuen.2008.09.012. [DOI] [PubMed] [Google Scholar]
- Peper J.S., Dahl B.C. Surging hormones: brain–behavior interactions during puberty. Current Directions in Psychological Science. 2013 doi: 10.1177/0963721412473755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peper J.S., Pol H.E., Crone E.A., van H.J. Sex steroids and brain structure in pubertal boys and girls: a mini-review of neuroimaging studies. Neuroscience. 2011;191:28–37. doi: 10.1016/j.neuroscience.2011.02.014. [DOI] [PubMed] [Google Scholar]
- Perrin J.S., Herve P.Y., Leonard G., Perron M., Pike G.B., Pitiot A., Richer L., Veillette S., Pausova Z., Paus T. Growth of white matter in the adolescent brain: role of testosterone and androgen receptor. Journal of Neuroscience. 2008;28:9519–9524. doi: 10.1523/JNEUROSCI.1212-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petanjek Z., Judas M., Simic G., Rasin M.R., Uylings H.B., Rakic P., Kostovic I. Extraordinary neoteny of synaptic spines in the human prefrontal cortex. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:13281–13286. doi: 10.1073/pnas.1105108108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P. Specification of cerebral cortical areas. Science. 1988;241:170–176. doi: 10.1126/science.3291116. [DOI] [PubMed] [Google Scholar]
- Rakic P. Radial unit hypothesis of neocortical expansion. Novartis Found Symp. 2000;228:30–42. doi: 10.1002/0470846631.ch3. (Discussion 42–52) [DOI] [PubMed] [Google Scholar]
- Raz N., Lindenberger U., Rodrigue K.M., Kennedy K.M., Head D., Williamson A., Dahle C., Gerstorf D., Acker J.D. Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cerebral Cortex. 2005;15:1676–1689. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- Raznahan A., Shaw P., Lalonde F., Stockman M., Wallace G.L., Greenstein D., Clasen L., Gogtay N., Giedd J.N. How does your cortex grow? Journal of Neuroscience. 2011;31:7174–7177. doi: 10.1523/JNEUROSCI.0054-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter M., Schmansky N.J., Rosas H.D., Fischl B. Within-subject template estimation for unbiased longitudinal image analysis. Neuroimage. 2012;61:1402–1418. doi: 10.1016/j.neuroimage.2012.02.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosas H.D., Liu A.K., Hersch S., Glessner M., Ferrante R.J., Salat D.H., van der K.A., Jenkins B.G., Dale A.M., Fischl B. Regional and progressive thinning of the cortical ribbon in Huntington's disease. Neurology. 2002;58:695–701. doi: 10.1212/wnl.58.5.695. [DOI] [PubMed] [Google Scholar]
- Salat D.H., Buckner R.L., Snyder A.Z., Greve D.N., Desikan R.S., Busa E., Morris J.C., Dale A.M., Fischl B. Thinning of the cerebral cortex in aging. Cerebral Cortex. 2004;14:721–730. doi: 10.1093/cercor/bhh032. [DOI] [PubMed] [Google Scholar]
- Segonne F., Dale A.M., Busa E., Glessner M., Salat D., Hahn H.K., Fischl B. A hybrid approach to the skull stripping problem in MRI. Neuroimage. 2004;22:1060–1075. doi: 10.1016/j.neuroimage.2004.03.032. [DOI] [PubMed] [Google Scholar]
- Shaw P., Kabani N.J., Lerch J.P., Eckstrand K., Lenroot R., Gogtay N., Greenstein D., Clasen L., Evans A., Rapoport J.L., Giedd J.N., Wise S.P. Neurodevelopmental trajectories of the human cerebral cortex. Journal of Neuroscience. 2008;28:3586–3594. doi: 10.1523/JNEUROSCI.5309-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell E.R., Peterson B.S., Kan E., Woods R.P., Yoshii J., Bansal R., Xu D., Zhu H., Thompson P.M., Toga A.W. Sex differences in cortical thickness mapped in 176 healthy individuals between 7 and 87 years of age. Cerebral Cortex. 2007;17:1550–1560. doi: 10.1093/cercor/bhl066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell E.R., Thompson P.M., Leonard C.M., Welcome S.E., Kan E., Toga A.W. Longitudinal mapping of cortical thickness and brain growth in normal children. Journal of Neuroscience. 2004;24:8223–8231. doi: 10.1523/JNEUROSCI.1798-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell E.R., Trauner D.A., Gamst A., Jernigan T.L. Development of cortical and subcortical brain structures in childhood and adolescence: a structural MRI study. Developmental Medicine and Child Neurology. 2002;44:4–16. doi: 10.1017/s0012162201001591. [DOI] [PubMed] [Google Scholar]
- Spear L.P. The adolescent brain and age-related behavioral manifestations. Neuroscience and Biobehavioral Reviews. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Steinberg L., Morris A.S. Adolescent development. Annual Review of Psychology. 2001;52:83–110. doi: 10.1146/annurev.psych.52.1.83. [DOI] [PubMed] [Google Scholar]
- Suzuki M., Zhou S.Y., Hagino H., Niu L., Takahashi T., Kawasaki Y., Matsui M., Seto H., Ono T., Kurachi M. Morphological brain changes associated with Schneider's first-rank symptoms in schizophrenia: a MRI study. Psychological Medicine. 2005;35:549–560. doi: 10.1017/s0033291704003885. [DOI] [PubMed] [Google Scholar]
- Taki Y., Hashizume H., Thyreau B., Sassa Y., Takeuchi H., Wu K., Kotozaki Y., Nouchi R., Asano M., Asano K., Fukuda H., Kawashima R. Linear and curvilinear correlations of brain gray matter volume and density with age using voxel-based morphometry with the Akaike information criterion in 291 healthy children. Human Brain Mapping. 2012 doi: 10.1002/hbm.22033. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamnes C.K., Ostby Y., Fjell A.M., Westlye L.T., Due-Tonnessen P., Walhovd K.B. Brain maturation in adolescence and young adulthood: regional age-related changes in cortical thickness and white matter volume and microstructure. Cerebral Cortex. 2009;20:534–548. doi: 10.1093/cercor/bhp118. [DOI] [PubMed] [Google Scholar]
- Tau G.Z., Peterson B.S. Normal development of brain circuits. Neuropsychopharmacology. 2010;35:147–168. doi: 10.1038/npp.2009.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M.S., Annaz D., Ansari D., Scerif G., Jarrold C., Karmiloff-Smith A. Using developmental trajectories to understand developmental disorders. Journal of Speech, Language, and Hearing Research. 2009;52:336–358. doi: 10.1044/1092-4388(2009/07-0144). [DOI] [PubMed] [Google Scholar]
- van Soelen I., Brouwer R.M., van Baal G.C., Schnack H.G., Peper J.S., Collins D.L., Evans A.C., Kahn R.S., Boomsma D.I., Pol H.E. Genetic influences on thinning of the cerebral cortex during development. Neuroimage. 2012;59:3871–3880. doi: 10.1016/j.neuroimage.2011.11.044. [DOI] [PubMed] [Google Scholar]
- Walhovd K.B., Fjell A.M., Reinvang I., Lundervold A., Dale A.M., Eilertsen D.E., Quinn B.T., Salat D., Makris N., Fischl B. Effects of age on volumes of cortex, white matter and subcortical structures. Neurobiology of Aging. 2005;26:1261–1270. doi: 10.1016/j.neurobiolaging.2005.05.020. (Discussion 1268–1275) [DOI] [PubMed] [Google Scholar]
- Westlye L.T., Walhovd K.B., Dale A.M., Bjornerud A., Due-Tonnessen P., Engvig A., Grydeland H., Tamnes C.K., Ostby Y., Fjell A.M. Life-span changes of the human brain white matter: diffusion tensor imaging (DTI) and volumetry. Cerebral Cortex. 2010;20:2055–2068. doi: 10.1093/cercor/bhp280. [DOI] [PubMed] [Google Scholar]
- Wilke M., Krageloh-Mann I., Holland S.K. Global and local development of gray and white matter volume in normal children and adolescents. Experimental Brain Research. 2007;178:296–307. doi: 10.1007/s00221-006-0732-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler A.M., Kochunov P., Blangero J., Almasy L., Zilles K., Fox P.T., Duggirala R., Glahn D.C. Cortical thickness or grey matter volume? The importance of selecting the phenotype for imaging genetics studies. Neuroimage. 2010;53:1135–1146. doi: 10.1016/j.neuroimage.2009.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler A.M., Kochunov P., Fox P.T., Duggirala R., Almasy L., Blangero J., Glahn D.C. Heritability of volume, surface area and cortical thickness for anatomically defined cortical brain regions estimated in a large extended pedigree. Neuroimage. 2009:S162. http://www.glahngroup.org/Members/anderson/publications/HBM2009_h2_poster.pdf. [Google Scholar]
- Wolosin S.M., Richardson M.E., Hennessey J.G., Denckla M.B., Mostofsky S.H. Abnormal cerebral cortex structure in children with ADHD. Human Brain Mapping. 2009;30:175–184. doi: 10.1002/hbm.20496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakovlev P.I., Lecours A.R. The myelogenetic cycles of regional maturation of the brain. In: Minkowski A., editor. Regional Development of the Brain in Early Life. Blackwell; Boston: 1967. pp. 3–70. [Google Scholar]
- Yurgelun-Todd D.A., Killgore W.D., Cintron C.B. Cognitive correlates of medial temporal lobe development across adolescence: a magnetic resonance imaging study. Perceptual and Motor Skills. 2003;96:3–17. doi: 10.2466/pms.2003.96.1.3. [DOI] [PubMed] [Google Scholar]