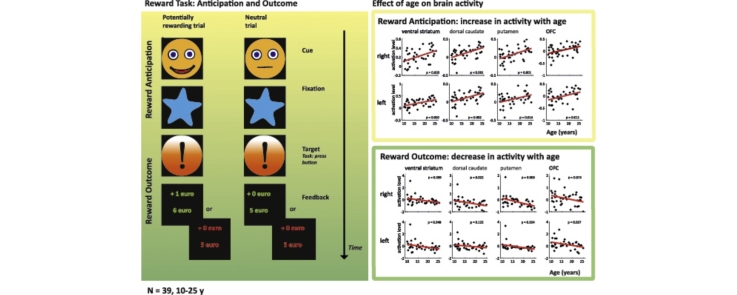
Graphical abstract
Keywords: Adolescence, Development, fMRI, Nucleus accumbens, Reward, Striatum
Highlights
-
•
We studied developmental changes in the brain reward system during adolescence.
-
•
Cross-sectional fMRI study in a sample of 39 participants aged 10–25.
-
•
Activity related to Reward Anticipation increased with age.
-
•
Activity related to Reward Outcome decreased with age.
-
•
We showed a shift in activation from Reward Outcome to Reward Anticipation with age.
Abstract
Typical adolescent behaviour such as increased risk-taking and novelty-seeking is probably related to developmental changes in the brain reward system. This functional MRI study investigated how brain activation related to two components of reward processing (Reward Anticipation and Reward Outcome) changes with age in a sample of 39 children, adolescents and young adults aged 10–25. Our data revealed age-related changes in brain activity during both components of reward processing. Activation related to Reward Anticipation increased with age, while activation related to Reward Outcome decreased in various regions of the reward network. This shift from outcome to anticipation was confirmed by subsequent analyses showing positive correlations between age and the difference in activation between Reward Anticipation and Reward Outcome. The shift was predominantly present in striatal regions and was accompanied by a significant effect of age on behaviour, with older participants showing more response speeding on potentially rewarding trials than younger participants. This study provides evidence for functional changes in the reward system which may underlie typical adolescent behaviour.
1. Introduction
Increased risk-taking and novelty-seeking are characteristics of adolescent behaviour (Casey et al., 2008a, Crone and Dahl, 2012, Ernst and Mueller, 2008, Spear, 2000, Steinberg, 2007). It has been suggested that these tendencies may be adaptive because they trigger adolescents to explore the world and become independent individuals (Crone and Dahl, 2012, Strang et al., 2013), but they could also lead to a substantial increase in morbidity related to dangerous behaviour (Casey et al., 2010a, Casey and Caudle, 2013) and an enhanced vulnerability for addiction (Gladwin et al., 2011, Schneider et al., 2012). The brain reward system is an important contributor to motivated behaviour (Somerville and Casey, 2010) and changes in the functioning of this circuit during adolescence are thought to underlie this typical adolescent behaviour. In fact, it has been suggested that the dopaminergic reward network, in particular the ventral striatum, is overactive in adolescents, making them hypersensitive to reward and leading to a greater motivational drive for novel, risky experiences (Chambers et al., 2003).
Indeed, increased ventral striatum activation is reported in adolescents in response to the actual receipt of reward (Ernst et al., 2005, Galvan et al., 2006, Van Leijenhorst et al., 2010a, Van Leijenhorst et al., 2010b), during an unexpected positive outcome (Cohen et al., 2010) and during rewarded trials in an incentive motivated antisaccade task (Padmanabhan et al., 2011). Other studies, however, have shown decreased ventral striatum activation during the anticipation of reward (Bjork et al., 2010, Bjork et al., 2004) and the assessment of a reward cue (Geier et al., 2010) in adolescents relative to adults.
These results indicate that the adolescent reward system is not simply hyper- or hypoactive compared to that of adults. Indeed, functional differences between the adult and adolescent reward system may depend on the component of reward processing that is considered (Bjork et al., 2010, Cohen et al., 2010, Geier and Luna, 2009, Geier et al., 2010, Van Leijenhorst et al., 2010b): the anticipation of a potential reward or the actual outcome, i.e. the receipt or omission of a reward (Knutson et al., 2001b).
Earlier studies in primates (Schultz et al., 2000) and humans (Knutson et al., 2001b) have shown that these different components of reward processing elicit dissociable brain responses in the reward system (see Haber and Knutson, 2010, for a review). In line with these results, previous work has suggested that the adolescent reward system may be characterized by different developmental trajectories for these two components of reward processing (Bjork et al., 2010, Geier and Luna, 2009, Geier et al., 2010). In fact, the hypothesis has been put forward that adolescents have an enhanced reactivity to the receipt of reward while displaying a decreased sensitivity to the anticipatorycues predicting reward (for reviews see Galván, 2010a, Spear, 2011).
However, direct evidence for this hypothesis is lacking: there are, to the best of our knowledge, no studies demonstrating age-related activation differences during both components of reward processing (i.e. during the anticipation and the actual outcome of reward). Moreover, combining the results of the available studies to substantiate the hypothesis is complicated because these studies used different tasks and included different age groups.
Here, we investigated age-related changes in reward-related brain activity in a sample of children, adolescents and adults aged 10–25 during anticipation and outcome of reward. We applied a modified version of the Monetary Incentive Delay task (Knutson et al., 2001a) which was optimized to analyse changes in brain activity related to the anticipation and outcome of reward separately (Figee et al., 2011, Van Hell et al., 2010). In addition, we used age as a continuous variable, in order to avoid confounds related to defining age groups (Luna et al., 2010) and enabling us to investigate both linear and non-linear effects of age (Casey, 2013). Activation changes were investigated in six predefined anatomical Regions of Interest (ROIs) which are all involved in the processing of reward (Knutson et al., 2001b): the bilateral ventral striatum, dorsal caudate, putamen, insula, cingulate cortex, and orbitofrontal cortex.
2. Materials and methods
2.1. Participants
Forty-two right-handed healthy volunteers aged 10–25 years (mean age 16.7 y, SD 4.8 y; 22 males) participated in the study. The study was approved by the Medical Ethics Committee of the University Medical Center Utrecht and all participants (and their parents in the case of minors) gave written informed consent. Data from three participants (1 male aged 14.9 y; 2 females aged 13.8 y and 23.8 y) were excluded from the analyses because they were outliers, with performance more than two standard deviations away from the group mean. This resulted in a sample of 39 participants.
Subjects received monetary compensation for participation: a fixed amount for participation and a flexible additional amount based on performance in the Reward Task.
Before scanning, participants who were scanner-naïve (except two adult participants, 1 male age 22.4 y, 1 female age 18.2 y) were familiarized with the scanning-procedure using a mock scanner in order to reduce scanner-related anxiety (Galván, 2010b).
2.2. Reward Task
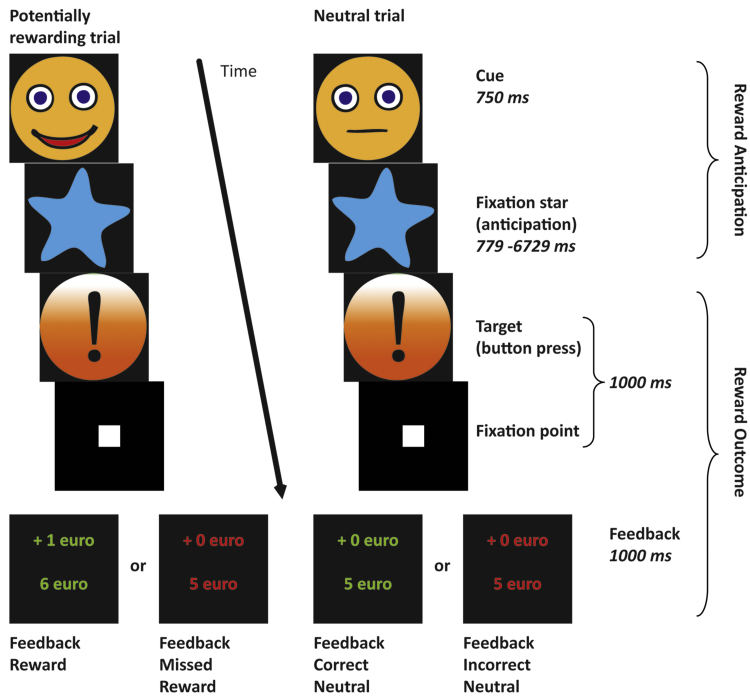
Participants performed a Reward Task (Fig. 1) based on the Monetary Incentive Delay task (Knutson et al., 2001a). Trials were potentially rewarding (30 trials) or neutral (30 trials), as indicated by a cue at the start of the trial. Following this cue and a fixation star, the target was presented. Participants were instructed to respond as fast as possible to this target by pressing a button, irrespective of cue type. Subsequent feedback notified participants of their performance, indicating if they had earned money, as well as their cumulative total at that moment. We told participants that they would receive the cumulative total amount of reward of the actual experiment in addition to the standard compensation for participation.
Fig. 1.
Schematic representation of the Reward Task, based on the Monetary Incentive Delay Task (Knutson et al., 2001a). Trials were potentially rewarding or neutral, as indicated by a cue at the start of the trial. Participants were instructed to respond as fast as possible to the target by pressing a button, irrespective of cue type. Subsequent feedback notified participants of their performance and indicated if they had earned money, as well as their cumulative total at that moment. Target duration was individually adjusted to ensure that each participant could succeed in 50% of the trials.
Target duration was individually adjusted to ensure that each participant could succeed in 50% of the trials. This adjustment was based on twenty practice trials, presented prior to the start of the experiment. From these practice data, the shortest reaction time to the target was used to determine the individual time limit for responses to the target. In 50% of the trials, the target was presented for the duration of the individual time limit plus 200 ms, enabling participants to be successful in these trials. In the other trials, the time limit was decreased with 150 ms, to make sure that participants could not respond in time.
The task was designed in such a way that maximum statistical power concerning the fMRI analyses could be reached in a relatively short time period: only one level of reward was used and no loss trials were included. Collinearity between the factors coding for anticipation (i.e. time between presentation of the cue and presentation of the target) and feedback was minimized by varying the duration of the anticipation time randomly (mean duration 3286 ms, range 779–6729 ms) and the inter-trial interval (mean duration 3535 ms, range 1029–6979 ms). This way, the blood-oxygen level-dependent (BOLD) signal in response to Reward Anticipation could be modelled independently from that to Reward Outcome (Figee et al., 2011, Van Hell et al., 2010). The actual task consisted of 60 trials with a mean duration of 9571 ms (range 4946–16107 ms), resulting in a total task duration of 9 min 35 s.
2.3. Image acquisition
The experiment was performed on a 3.0 T Philips Achieva MRI scanner (Philips Medical Systems, Best, the Netherlands) at the University Medical Center Utrecht. Images were acquired using an eight-channel sensitivity-encoding (SENSE) parallel-imaging head coil. Whole-brain T2*-weighted echo planar images (EPI) with BOLD contrast, oriented in a transverse plane tilted 20° over the left-right axis, were acquired in a single run (372 volumes; 30 slices per volume; interleaved acquisition; repetition time, 1600 ms; echo time, 23 ms; field of view: 208 mm × 120 mm × 256 mm; flip angle = 72.5°; 64 × 64 matrix; 4 mm × 4 mm in-plane resolution; 4 mm slice thickness; SENSE-factor, 2.4 (anterior–posterior)). A whole-brain three-dimensional fast field echo T1-weighted scan (185 slices; repetition time = 8.4 ms; echo time = 3.8 ms; flip angle = 8°; field of view, 252 mm × 288 mm × 185 mm; voxel size: 1 mm isotropic) was obtained for within-subject registration purposes.
2.4. Data analysis
2.4.1. Behavioural data
The relationship between age and behaviour (accuracy of potentially rewarding trials, mean reaction times and the difference in reaction time between the trial types) was investigated using Pearson correlation analyses.
2.4.2. Pre-processing and individual subject analysis
Image data were pre-processed and analyzed using SPM5 software (http://www.fil.ion.ucl.ac.uk/spm/software/spm5/). After realignment of the functional scans, the anatomical image was co-registered to the mean functional image. This image was segmented and normalization parameters were estimated. Using these parameters, the functional and anatomical images were matched to the Montreal Neurological Institute (MNI) T1-template brain. Functional images were spatially smoothed using an 8-mm full-width at half-maximum Gaussian kernel. Each participant's translation and rotation corrections were examined to ensure there was no excessive head motion (>2 mm in any direction between subsequent scans) (Van Dijk et al., 2012). The pre-processed time-series data for each individual were analyzed using a general linear model (GLM) regression analysis. The regression model consisted of six factors, representing haemodynamic changes which were event-related to (1) anticipation during and after the presentation of the reward cue (Anticipation Reward), (2) anticipation during and after the neutral cue (Anticipation Neutral), (3) feedback reflecting monetary reward (Feedback Reward), (4) feedback reflecting missed reward (Feedback Missed Reward), (5) feedback reflecting a correct response in a neutral trial (Feedback Correct Neutral) and (6) feedback reflecting an incorrect response in a neutral trial (Feedback Incorrect Neutral) (Fig. 1). The onset of the factors modelling anticipation (duration range 1529–7479 ms) was at the presentation of the cue, while the onset of the factors modelling feedback (duration: 2000 ms) was at the presentation of the target, including the button press to the target and the subsequent feedback (Fig. 1). To take residual head motion effects into account, motion parameters from the realignment procedure were included as regressors of no interest. Low frequency drifts were removed from the signal by applying a high-pass filter with a cut-off frequency of 1/204 Hz.
For each participant, statistical maps were generated for the contrasts (1) Anticipation Reward versus Anticipation Neutral (hereafter referred to as Reward Anticipation) and (2) Feedback Reward versus Feedback Correct Neutral (Reward Outcome). We used this Reward Outcome contrast instead of the more commonly applied Reward Hit contrast (defined as Feedback Reward versus Feedback Missed Reward; see for example Knutson et al., 2001b, Knutson et al., 2003) to investigate activity during the outcome phase of the Reward Task because we wanted to investigate age-related changes in brain activation related to the retrieval of reward independent from performance.
2.4.3. Whole-brain analyses
The individual statistical maps were used to perform whole-brain group-analyses investigating the relation between age and brain activation. These maps were tested for significance at a family-wise error (FWE) corrected cluster level of p = 0.05 (cluster-defining threshold of p = 0.001, cluster size of 40 voxels). These parameters were determined using SPM and a script (CorrClusTh.m, to be found on http://www2.warwick.ac.uk/fac/sci/statistics/staff/academic-research/nichols/scripts/spm), which uses estimated smoothness (estimated full width at half maximum (FWHM): 3.56 × 3.65 × 3.46 voxels) and random field theory to find these corrected thresholds.
2.4.4. Regions of interest analyses
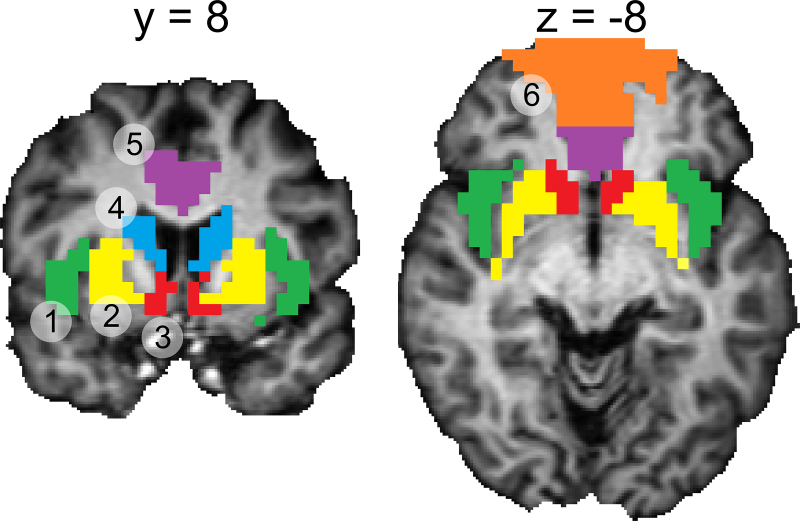
A Region of Interest (ROI) analysis was applied to investigate the relation between age and brain activation levels. Six bilateral anatomical ROIs were a priori selected, based on their known involvement in the anticipation and outcome of reward (Knutson et al., 2001b): the ventral striatum, dorsal caudate, putamen, insula, cingulate cortex and orbitofrontal cortex (Fig. 2). ROIs were based on definitions of the Anatomic Automatic Labeling (AAL) atlas (Tzourio-Mazoyer et al., 2002) and created using the WFU PickAtlas Toolbox implemented in SPM5. The dorsal caudate and ventral striatum were defined as those parts of the caudate nucleus above and below the z-coordinate of 0 mm, respectively. The orbitofrontal ROI consisted of the orbital part of both the middle and superior frontal gyrus; the cingulate cortex ROI was composed of the anterior and medial cingulate cortex. The other ROIs were identical to the anatomical regions of the AAL atlas.
Fig. 2.
Regions of interest: (1) insula; (2) putamen; (3) ventral striatum; (4) dorsal caudate; (5) cingulate cortex; (6) orbitofrontal cortex.
For each participant, the mean activation level (expressed as percent signal change) during the two contrasts of interest (Reward Anticipation and Reward Outcome) was calculated over all voxels in each ROI. Regression analyses were then performed for each ROI separately with activation level as dependent variable and age as predictor. Since the relation between age and activation is not necessarily a linear one, we looked for both linear and quadratic relations between activation and age. While linear relations indicate increases or decreases in activation during the transition from childhood to adulthood, quadratic relations represent increases or decreases in activation levels which are specific for adolescents (Casey, 2013, Cohen et al., 2010).
3. Results
3.1. Behavioural data
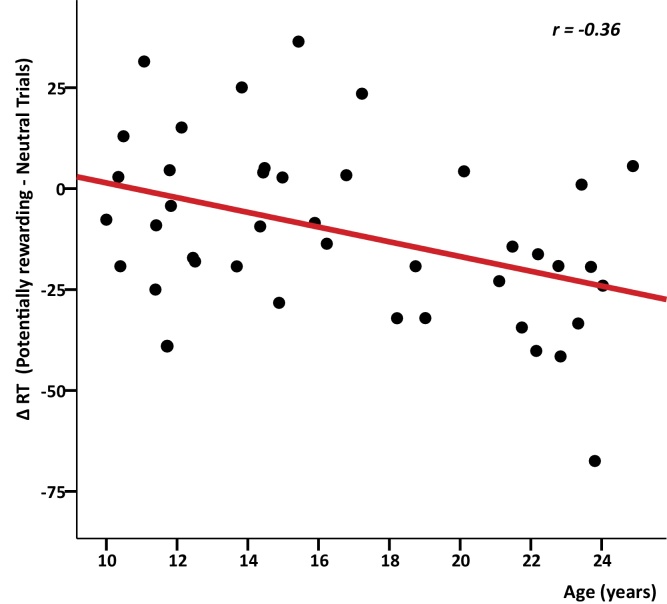
Means and standard deviations for accuracy and reaction times are provided in Table 1. Accuracy in potentially rewarding trials did not show a significant correlation with age, indicating that the manipulation to ensure equal performance across subjects was successful. Overall, subjects responded faster on potentially rewarding trials than on neutral trials (t(38) = −3.2, p = 0.003). This effect was correlated with age: older subjects showed more response speeding than younger subjects (Fig. 3; r = −0.36, p = 0.03).
Table 1.
Behavioural data.
| Mean ± SD | Correlation with age | |
|---|---|---|
| Accuracy (%) | ||
| Potentially rewarding trials | 48.5 ± 2.4# | r = 0.24, p = 0.15 |
| Reaction time (ms) | ||
| Potentially rewarding trials | 296.7 ± 30.7 | r = −0.26, p = 0.11 |
| Neutral trials | 306.6 ± 34.6 | r = −0.03, p = 0.85 |
| Potentially rewarding > neutral trials | −9.9 ± 19.2 | r = −0.36, p = 0.03* |
N = 39.
Significant at uncorrected threshold of p ≤ 0.05.
Note: Target accuracy was 50%.
Fig. 3.
Relation between age and the behavioural reward effect on reaction times (Potentially rewarding > neutral trials).
3.2. Whole-brain analyses
The main effects of task were in line with those reported in earlier work. Activation during Reward Anticipation (contrast Anticipation Reward versus Anticipation Neutral) was found in the ventral striatum, putamen, thalamus, anterior cingulate cortex, mid-cingulate cortex, insula and several frontal, temporal and parietal areas (Bjork et al., 2010, Bjork et al., 2004, Carter et al., 2009, Dillon et al., 2008, Ernst et al., 2004, Figee et al., 2011, Galvan et al., 2005, Hermans et al., 2010, Hommer et al., 2003, Knutson et al., 2001a, Knutson et al., 2001b, Knutson et al., 2003, Ossewaarde et al., 2011, Van Hell et al., 2010). Reward Outcome activity (contrast Feedback Reward versus Feedback Correct Neutral) was mainly found in the orbitofrontal cortex, caudate, posterior cingulate gyrus and bilateral parahippocampal gyri.
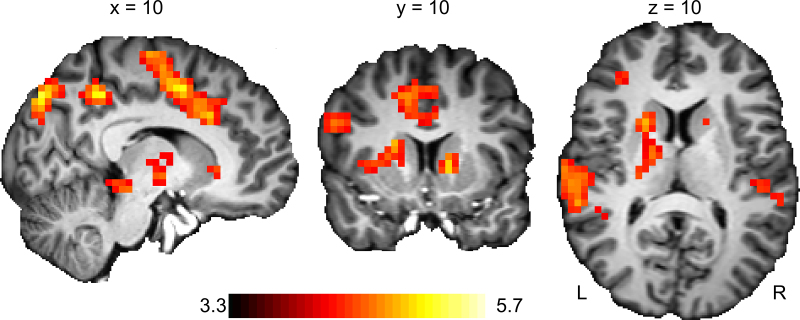
During Reward Anticipation, age showed a positive relation with activation in the dorsal caudate, supplementary motor area (SMA), bilateral primary motor cortex, parietal cortex and the precuneus (Table 2; Fig. 4). These regions are closely interconnected and, given their role in motor preparation and visuospatial attention (Cavanna and Trimble, 2006, Vink et al., 2005), parallel the behavioural observation that older subjects showed more response speeding than younger subjects during potentially rewarding versus neutral trials. During Reward Outcome, there was no activation significantly related with age.
Table 2.
Whole Brain effects of age.
| Region | BA | Left/right | # voxels | X | Y | Z | Max t-value |
|---|---|---|---|---|---|---|---|
| SMA | 6/24 | L/R | 556 | 16 | 16 | 40 | 5.45 |
| M1 | 4/6 | L | 128 | −36 | −12 | 56 | 5.11 |
| R | 161 | 60 | −24 | 32 | 5.13 | ||
| Thalamus | L/R | 240 | 4 | −12 | 0 | 4.69 | |
| Dorsal caudate | L/R | 57 | −12 | 12 | 4 | 4.65 | |
| Precuneus | 7 | R | 126 | 12 | −44 | 44 | 5.68 |
| Parietal lobe | 7 | R | 221 | 8 | −80 | 40 | 4.96 |
| Supramagrinal gyrus | 2 | L | 48 | −56 | −28 | 0 | 4.25 |
Whole-brain results of regions showing increased activation with age during Reward Anticipation. There were no regions showing significant decrease in activation with age.
BA, Brodmann area; SMA, supplementary motor area; M1, primary motor cortex.
Fig. 4.
Age-related activation changes in whole-brain activity during Reward Anticipation (colours represent t-values).
3.3. Regions of interest analyses
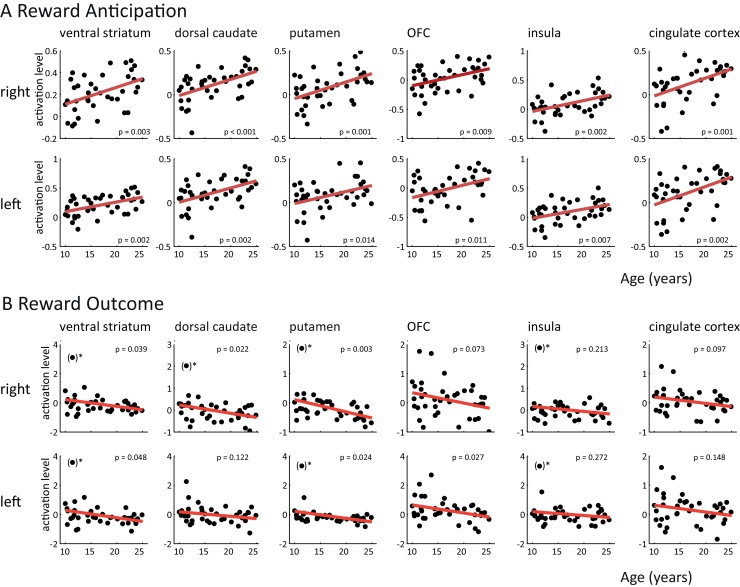
During Reward Anticipation, age showed a positive linear relation with activation in all regions of interest, indicating an increase in activation with increasing age (Table 3; Fig. 5A). In contrast, during Reward Outcome, activation levels decreased linearly with age in the right putamen, bilateral ventral striatum, right dorsal caudate, left putamen and left orbitofrontal cortex (Table 3; Fig. 5B). Thus, young subjects activated the reward regions mostly during Reward Outcome, while older subjects activated these regions predominantly during Reward Anticipation. A quadratic model did not provide a significantly better description of the relation between age and brain activation than a linear model (p > 0.3 in all ROIs).
Table 3.
Relation between age and reward activation.
| Left/right | Reward Anticipation |
Reward Outcome |
|||
|---|---|---|---|---|---|
| r | p | r | p | ||
| Ventral striatum | R | 0.46 | 0.003** | −0.33 | 0.039* |
| L | 0.47 | 0.002** | −0.32 | 0.048* | |
| Dorsal caudate | R | 0.52 | 0.001** | −0.37 | 0.022* |
| L | 0.48 | 0.002** | −0.25 | 0.122 | |
| Putamen | R | 0.50 | 0.001** | −0.46 | 0.003** |
| L | 0.39 | 0.014* | −0.36 | 0.024* | |
| Orbitofrontal | R | 0.41 | 0.009* | −0.29 | 0.073 |
| L | 0.41 | 0.011* | −0.36 | 0.027* | |
| Insula | R | 0.48 | 0.002** | −0.20 | 0.213 |
| L | 0.42 | 0.007* | −0.18 | 0.272 | |
| Cingulate cortex | R | 0.50 | 0.001** | −0.27 | 0.097 |
| L | 0.48 | 0.002** | −0.24 | 0.148 | |
N = 39.
Reward Anticipation: contrast Anticipation Reward versus Anticipation Neutral.
Reward Outcome: contrast Feedback Reward versus Feedback Correct Neutral.
Significant at uncorrected threshold of p ≤ 0.05.
Significant at adjusted threshold of p ≤ 0.004, Bonferroni corrected for multiple ROIs.
Fig. 5.
Age-related activation changes in Reward Anticipation (A) and Reward Outcome (B) in ROIs. Activation levels in percent signal change, p-values in figure for n = 39. Results for n = 38, without participant denoted by *: right ventral striatum: p = 0.060; left ventral striatum: p = 0.080; right putamen: p = 0.001; left putamen: p = 0.008; right insula: p = 0.551; left insula: p = 0.681.
Taken together, these data suggest that there is a shift in the reward network during development from childhood to adulthood: from activation being driven by Reward Outcome towards activation being driven by Reward Anticipation.
We formally tested this shift by calculating for each participant and in each ROI the difference-score between the level of activation during Reward Anticipation and Reward Outcome (ActivityReward Anticipation > ActivityReward Outcome). Subsequently, we investigated whether these difference-scores showed a relation with age. Results showed a positive correlation between the difference-score and age in the bilateral ventral striatum, bilateral dorsal caudate, bilateral putamen, bilateral orbitofrontal cortex, bilateral cingulate cortex and right insula (Table 4). These significant positive correlations indicate a relative shift in reward-related activation from Reward Outcome to Reward Anticipation with age in participants from 10 to 25 years.
Table 4.
Relation between age and Δ Activity Reward Anticipation > Activity Reward Outcome.
| Left/right | r | P | |
|---|---|---|---|
| Ventral striatum | R | 0.40 | 0.012* |
| L | 0.39 | 0.015* | |
| Dorsal caudate | R | 0.46 | 0.004** |
| L | 0.34 | 0.032* | |
| Putamen | R | 0.54 | <0.001** |
| L | 0.43 | 0.007* | |
| Orbitofrontal | R | 0.37 | 0.020* |
| L | 0.40 | 0.011* | |
| Insula | R | 0.34 | 0.035* |
| L | 0.27 | 0.092 | |
| Cingulate cortex | R | 0.42 | 0.007* |
| L | 0.36 | 0.024* |
N = 39.
Significant at uncorrected threshold of p ≤ 0.05.
Significant at adjusted threshold of p ≤ 0.004, Bonferroni corrected for multiple ROIs.
To exclude the possibility that this shift represents an inherent relation between the height of activation during Reward Anticipation and Reward Outcome, we investigated this relation in a subgroup of participants with a restricted age-range to exclude the effect of age (N = 10, mean age (SD) 23.1 (1.0) y, 5 males). This analysis did not yield any significant associations in any ROI (Supplementary Table 1).
Supplementary material related to this article can be found, in the online version, at http://dx.doi.org/10.1016/j.dcn.2013.08.004.
Correlation between Reward Anticipation and Reward Outcome.
4. Discussion
Here, we investigated how brain activation related to the anticipation and receipt of reward changes with age in a sample of 39 children, adolescents and young adults aged 10–25.Our results showed, for the first time, opposite-directed age-related changes in brain activity during both the anticipation and receipt of reward: brain activation during the anticipation of reward (Reward Anticipation) increased with age, while activation during the actual receipt of reward (Reward Outcome) decreased with age in several regions of interest (Table 3; Fig. 5). Thereby, our findings provide direct evidence for the hypothesis that the adolescent reward system is characterized by different developmental trajectories for these two components of reward processing. The increase in Reward Anticipation-related activity was accompanied by an age-related change in task performance: older subjects showed more response speeding than younger subjects in potentially rewarding trials relative to neutral trials (Table 1; Fig. 3). Whole-brain analyses showed that age correlated positively with activation related to Reward Anticipation in a broad network of areas involved in motor preparation and visuospatial attention (Table 2; Fig. 4), supporting this behavioural finding.
The opposite-directed age-related changes during the two components of reward processing suggest the presence of a shift in activity with age: from Reward Outcome to Reward Anticipation. Indeed, we found this shift from Reward Outcome to Reward Anticipation with age to be present in striatal regions (i.e. the bilateral ventral striatum, bilateral putamen and bilateral dorsal caudate), as well as in the bilateral orbitofrontal cortex, right insula, and bilateral cingulate cortex (Table 4). These results indicate that the relative height of activation shifts from Reward Outcome to Reward Anticipation during the development from childhood to adulthood.
Our finding that brain activation during Reward Anticipation (contrast Anticipation Reward versus Anticipation Neutral) increased with age, while activation in response to Reward Outcome (contrast Feedback Reward versus Feedback Correct Neutral) decreased with age is in line with previous literature. Several studies reported reduced recruitment of reward-related regions in adolescents during anticipating responding for a reward (Bjork et al., 2010, Bjork et al., 2004) and during the presentation of an incentive cue (Geier et al., 2010), with significant lower activation levels in the right ventral striatum (Bjork et al., 2010, Bjork et al., 2004, Geier et al., 2010), the right amygdala and right insula (Bjork et al., 2004). Other studies showed increased activity in the ventral striatum in adolescents compared to adults in response to the notification of reward (Ernst et al., 2005, Galvan et al., 2006, Padmanabhan et al., 2011, Van Leijenhorst et al., 2010b), during the notification of gain in a gambling task (Van Leijenhorst et al., 2010a) and during an unexpected positive outcome, also known as a positive prediction error (Cohen et al., 2010). Here, we replicate these findings and extend them by showing for the first time both opposite-directed age-related changes in reward-related brain activity in the same group of participants.
It should be noted that our finding of enhanced adolescent brain activation during Reward Outcome is inconsistent with the results of (Bjork et al., 2010), who also employed a modified Monetary Incentive Delay (MID)-task to investigate brain activation in adolescents and adults during the anticipation and outcome phase of reward processing. This study reported no group-differences in activation levels in the ventral striatum related to reward deliveries (Bjork et al., 2010). We speculate that differences in the design of the task have possibly contributed to these discrepant findings: the MID-task in (Bjork et al., 2010) had a parametric design involving multiple levels of reward and loss, while the Reward Task used here had only one level of reward in order to increase statistical power. In addition, the authors of (Bjork et al., 2010) compared pre-defined age groups of adolescents (age 12–17 y) and adults (age 22–42 y) whereas we used a more sensitive correlational approach to detect age-related effects in a sample of participants aged 10–25. These differences could have enabled us to detect a significant effect of age during the outcome component of reward processing.
Although we found a negative correlation between age and activation during Reward Outcome in the ROI analysis, there was no significant effect of age in the whole-brain analysis on this contrast. We hypothesize that this divergence relates to methodological differences between these analyses. That is, a strict statistical threshold was used in the whole-brain analysis: we applied a family-wise error (FWE) corrected cluster level of p = 0.05 (cluster-defining threshold of p = 0.001, with a cluster size of at least 40 voxels). This strict threshold could limit the ability to find very specific effects at the whole-brain level. In the ROI-analysis, on the other hand, we looked very specifically for effects of age in predefined brain areas. Because these areas were predefined, the use of a lower statistical threshold was allowed (Poldrack and Mumford, 2009, Poldrack, 2007). As a result, this ROI analysis was more sensitive to detect specific, localized effects of age.
Our results are in line with the ‘imbalance’ or dual processing model of adolescent brain development proposed by Casey and colleagues (Casey et al., 2008b, Casey et al., 2010b, Somerville et al., 2010). This model emphasizes that the speed of maturation of the adolescent brain is different across regions, with subcortical limbic areas (e.g. the ventral striatum) developing earlier than prefrontal cortical regions (see also Casey et al., 2005, Durston and Casey, 2006). This imbalance between the fast maturation of subcortical areas (involved in affective processing) and the relatively slow development of prefrontal areas (responsible for cognitive control over behaviour and planning) leads to an increase in impulsive, reward-seeking behaviour in adolescents.
Interestingly, we did not only observe subcortical hyperactivation, which would be predicted based on this model. Instead, we found both hyperactivation and hypoactivation of subcortical areas in adolescents related to the receipt of reward and the anticipation of reward, respectively. This is in line with earlier work showing component-specific activation differences between adolescents and adults (for example Bjork et al., 2010, Cohen et al., 2010, Geier et al., 2010).
Our findings may be understood in terms of a failure in younger participants to link the meaning of an abstract cue to anticipatory behaviour relevant to the outcome of this cue. Learning stimulus-response mappings is related to the ventral striatum (Vink et al., 2013). Moreover, establishing cue-outcome mappings requires the interplay of dopaminergic transmissions in both frontal and striatal regions (Shohamy, 2011). However, the frontal cortex as well as certain parts of the dopaminergic circuits are not completely developed in early adolescence (Garske et al., 2013). In addition, connections between the frontal cortex and the striatum are still maturing in younger participants, because the myelination of fibre tracts continues into the second decade of life (Klingberg et al., 1999). Interestingly, increased structural connectivity between frontal, parietal and striatal regions with age has been linked to improvements in cognitive functioning, like working memory capacity (Nagy et al., 2004, Olesen et al., 2003) and cognitive control (Liston et al., 2006); for a review see Geier and Luna (2009). In this light, we hypothesize that immaturities in frontal, parietal and striatal regions might possibly limit the ability of younger participants to couple the knowledge about the meaning of a cue to anticipatory brain activity and behaviour relevant to the outcome of this cue.
Indeed, we found that cues signalling a potential reward triggered the reward network and motor- and attention-related brain regions less in early adolescence than in adulthood (Table 2; Fig. 4). Moreover, younger subjects did not show as much response speeding on potentially rewarding trials as older subjects did, adding to the idea that they did not anticipate the rewarding outcome due to a reduced cue-outcome mapping (Table 1). Because of the potentially poor coupling between cue and outcome, and hence the relative absence of a prediction based on that cue, the response to actually obtaining a reward becomes elevated in adolescents (Fig. 5). This is in line with the data of (Cohen et al., 2010), showing increased striatal activity in adolescents related to a unexpected positive outcome during feedback of a probabilistic learning task. Interestingly, similar effects have been shown to occur during ageing, where elderly subjects show reduced activation during anticipation but heightened activation during receipt of reward (Schott et al., 2007). Taken together, these results stress the point that the functioning of cortical and subcortical regions involved in reward processing in adolescents is influenced by the part of reward processing that is examined (Luna et al., 2013).
It should be noted that the behavioural effect of age on behaviour reported here (more response speeding with age in rewarded versus neutral trials) differs from the results of Geier and colleagues, who showed that adolescents significant improved their performance in rewarded versus neutral trials while adults did not (Geier et al., 2010). We speculate that methodological dissimilarities between the studies could possibly account for these diverse results. In our task, participants were instructed to respond as soon as possible to a target by pressing a button, where participants in the study of Geier et al. (2010) were instructed to inhibit an eye saccade to a target. In addition, we used a correlational approach in participants aged 10–25, while Geier and colleagues compared predefined age groups (group of adolescents (13–17 y) versus a group of adults (18–30 y)). Finally, Geier et al. (2010) reported hit rate while we used reaction times to describe the behavioural effect. These dissimilarities in the tasks, the definition of the groups and the outcome measures could possibly explain the contrasting results.
The task used in this study was relatively basic and primarily designed to distinguish brain activity during Reward Anticipation from activity during Reward Outcome. Although the results of this study provide valuable insight in age-related changes in reward processing, there are some limitations. First, although the data presented here fit within the imbalance model as stated above, it should be noted that they do not provide a complete insight in the neural substrates of real-world adolescent behaviour. For example, adolescents show enhanced sensitivity to the influence of peers (for reviews see Albert et al., 2013, Casey et al., 2010a, Somerville et al., 2010, Spear, 2000, Van Duijvenvoorde and Crone, 2013) and their behaviour and even brain activity has shown to be affected by the presence of peers (Chein et al., 2011). Therefore, recent reviews have pointed to the importance of using an integrative approach when studying the neural correlates of adolescent behaviour, in which emotional as well as hormonal and social factors (like the influence of peers on risk-taking behaviour) are taken into account (Bjork et al., 2012, Crone and Dahl, 2012, Pfeifer and Allen, 2012). However, the design of the present study did not enable us to consider these influences.
Furthermore, we did not obtain individual ratings of pubertal development, which are potentially predictive for changes in brain activity during adolescence. Recently, Forbes et al. (2010) investigated the neural response of healthy adolescents to reward and reported that the stage of pubertal maturation influenced the level of striatal activation as measured with fMRI. However, the adolescents with a less advanced pubertal maturation were significantly younger than the adolescents with more advanced pubertal maturation (Forbes et al., 2010). This supports the idea that age and pubertal ratings are in general highly correlated, which is also described by Bjork et al. (2010); for a review see Blakemore et al. (2010). Consequently, we believe that using ratings of pubertal maturation instead of age would not have changed the results of this study.
Although we ensured that none of the participants in this experiment had any psychiatric illness, we did not acquire scores indicating the presence of behavioural and psychosocial symptoms. Recently, Bjork and colleagues showed that individual differences in a score of psychosocial and behavioural problems correlated positively with activation in the ventral striatum during reward anticipation in healthy adolescents (Bjork et al., 2011). This finding is interesting because it sheds more light on the relation between the functioning of the reward system during adolescence and the typical behaviour in this age-period. Including ratings of psychosocial and behavioural symptomatology could therefore be valuable for subsequent studies on this topic.
5. Conclusion
To conclude, this study is, to the best of our knowledge, the first to demonstrate opposite-directed age-related changes in brain activity during both the anticipation and receipt of reward. Activation levels during Reward Anticipation showed a positive relation with age, while activation levels during Reward Outcome decreased with age. Taken together, these findings indicated that activation shifts from being driven primarily by the receipt of reward to the anticipation of reward in participants aged 10–25. This developmental shift was accompanied by a significant effect of age on behaviour, with older subjects showing more response speeding than younger subjects when they anticipated a reward. These results provide insight in the functional changes in the brain reward circuitry during adolescence. This is important as developmental changes in this circuit may underlie typical adolescent behaviour. In addition, this reward network is implicated in various psychiatric illnesses which have their onset in early adolescence (Paus et al., 2008). Investigating the normal development may therefore be informative for identifying developmental abnormalities in an early stage.
Conflicts of interest
None of the authors have any conflicts of interest.
Acknowledgements
We would like to thank Eveline A. Crone and several anonymous reviewers for reading previous versions of this paper and giving valuable suggestions.
References
- Albert D., Chein J., Steinberg L. The teenage brain: peer influences on adolescent decision making. Current Directions in Psychological Science. 2013;22:114–120. doi: 10.1177/0963721412471347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork J.M., Knutson B., Fong G.W., Caggiano D.M., Bennett S.M., Hommer D.W. Incentive-elicited brain activation in adolescents: similarities and differences from young adults. The Journal of Neuroscience. 2004;24:1793–1802. doi: 10.1523/JNEUROSCI.4862-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork J.M., Lynne-Landsman S.D., Sirocco K., Boyce C.A. Brain maturation and risky behavior: the promise and the challenges of neuroimaging-based accounts. Child Development Perspectives. 2012;6:385–391. [Google Scholar]
- Bjork J.M., Smith A.R., Chen G., Hommer D.W. Adolescents, adults and rewards: comparing motivational neurocircuitry recruitment using fMRI. PLoS One. 2010;5:e11440. doi: 10.1371/journal.pone.0011440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork J.M., Smith A.R., Chen G., Hommer D.W. Psychosocial problems and recruitment of incentive neurocircuitry: exploring individual differences in healthy adolescents. Developmental Cognitive Neuroscience. 2011;1:570–577. doi: 10.1016/j.dcn.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore S.-J., Burnett S., Dahl R.E. The role of puberty in the developing adolescent brain. Human Brain Mapping. 2010;31:926–933. doi: 10.1002/hbm.21052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey B.J. The teenage brain: an overview. Current Directions in Psychological Science. 2013;22:80–81. doi: 10.1177/0963721413480170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey B.J., Caudle K. The teenage brain: self control. Current Directions in Psychological Science. 2013;22:82–87. doi: 10.1177/0963721413480170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey B.J., Tottenham N., Liston C., Durston S. Imaging the developing brain: what have we learned about cognitive development? Trends in Cognitive Sciences. 2005;9:104–110. doi: 10.1016/j.tics.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Casey B.J., Getz S., Galvan A. The adolescent brain. Developmental Review. 2008;28:62–77. doi: 10.1016/j.dr.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey B.J., Jones R.M., Hare T.A. The adolescent brain. Annals of the New York Academy of Sciences. 2008;1124:111–126. doi: 10.1196/annals.1440.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter R.M., Macinnes J.J., Huettel S.A., Adcock R.A. Activation in the VTA and nucleus accumbens increases in anticipation of both gains and losses. Frontiers in Behavioral Neuroscience. 2009;3:21. doi: 10.3389/neuro.08.021.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey B.J., Duhoux S., Malter Cohen M. Adolescence: what do transmission, transition, and translation have to do with it? Neuron. 2010;67:749–760. doi: 10.1016/j.neuron.2010.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey B.J., Jones R.M., Levita L., Libby V., Pattwell S.S., Ruberry E.J., Soliman F., Somerville L.H. The storm and stress of adolescence: insights from human imaging and mouse genetics. Developmental Psychobiology. 2010;52:225–235. doi: 10.1002/dev.20447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanna A.E., Trimble M.R. The precuneus: a review of its functional anatomy and behavioural correlates. Brain: A Journal of Neurology. 2006;129:564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Chambers R.A., Taylor J.R., Potenza M.N. Developmental neurocircuitry of motivation in adolescence: a critical period of addiction vulnerability. The American Journal of Psychiatry. 2003;160:1041–1052. doi: 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chein J., Albert D., O’Brien L., Uckert K., Steinberg L. Peers increase adolescent risk taking by enhancing activity in the brain's reward circuitry. Developmental Science. 2011;14:F1–F10. doi: 10.1111/j.1467-7687.2010.01035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J.R., Asarnow R.F., Sabb F.W., Bilder R.M., Bookheimer S.Y., Knowlton B.J., Poldrack R.A. A unique adolescent response to reward prediction errors. Nature Neuroscience. 2010;13:669–671. doi: 10.1038/nn.2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone E.A., Dahl R.E. Understanding adolescence as a period of social-affective engagement and goal flexibility. Nature Reviews Neuroscience. 2012;13:636–650. doi: 10.1038/nrn3313. [DOI] [PubMed] [Google Scholar]
- Dillon D.G., Holmes A.J., Jahn A.L., Bogdan R., Wald L.L., Pizzagalli D.A. Dissociation of neural regions associated with anticipatory versus consummatory phases of incentive processing. Psychophysiology. 2008;45:36–49. doi: 10.1111/j.1469-8986.2007.00594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durston S., Casey B.J. What have we learned about cognitive development from neuroimaging? Neuropsychologia. 2006;44:2149–2157. doi: 10.1016/j.neuropsychologia.2005.10.010. [DOI] [PubMed] [Google Scholar]
- Ernst M., Mueller S.C. The adolescent brain: insights from functional neuroimaging research. Developmental Neurobiology. 2008;68:729–743. doi: 10.1002/dneu.20615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M., Nelson E.E., McClure E.B., Monk C.S., Munson S., Eshel N., Zarahn E., Leibenluft E., Zametkin A., Towbin K., Blair J., Charney D., Pine D.S. Choice selection and reward anticipation: an fMRI study. Neuropsychologia. 2004;42:1585–1597. doi: 10.1016/j.neuropsychologia.2004.05.011. [DOI] [PubMed] [Google Scholar]
- Ernst M., Nelson E.E., Jazbec S., McClure E.B., Monk C.S., Leibenluft E., Blair J., Pine D.S. Amygdala and nucleus accumbens in responses to receipt and omission of gains in adults and adolescents. Neuroimage. 2005;25:1279–1291. doi: 10.1016/j.neuroimage.2004.12.038. [DOI] [PubMed] [Google Scholar]
- Figee M., Vink M., De Geus F., Vulink N., Veltman D.J., Westenberg H., Denys D. Dysfunctional reward circuitry in obsessive–compulsive disorder. Biological Psychiatry. 2011;69:867–874. doi: 10.1016/j.biopsych.2010.12.003. [DOI] [PubMed] [Google Scholar]
- Forbes E.E., Ryan N.D., Phillips M.L., Manuck S.B., Worthman C.M., Moyles D.L., Tarr J.A., Sciarrillo S.R., Dahl R.E. Healthy adolescents’ neural response to reward: associations with puberty, positive affect, and depressive symptoms. Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49 doi: 10.1097/00004583-201002000-00010. 162.e1–172.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galván A. Adolescent development of the reward system. Frontiers in Human Neuroscience. 2010;4:6. doi: 10.3389/neuro.09.006.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galván A. Neural plasticity of development and learning. Human Brain Mapping. 2010;31:879–890. doi: 10.1002/hbm.21029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan A., Hare T.A., Davidson M., Spicer J., Glover G., Casey B.J. The role of ventral frontostriatal circuitry in reward-based learning in humans. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2005;25:8650–8656. doi: 10.1523/JNEUROSCI.2431-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan A., Hare T.A., Parra C.E., Penn J., Voss H., Glover G., Casey B.J. Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. The Journal of Neuroscience. 2006;26:6885–6892. doi: 10.1523/JNEUROSCI.1062-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garske A.K., Lawyer C.R., Peterson B.M., Illig K.R. Adolescent changes in dopamine d1 receptor expression in orbitofrontal cortex and piriform cortex accompany an associative learning deficit. PLoS One. 2013;8:e56191. doi: 10.1371/journal.pone.0056191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geier C.F., Terwilliger R., Teslovich T., Velanova K., Luna B. Immaturities in reward processing and its influence on inhibitory control in adolescence. Cerebral Cortex. 2010;20:1613–1629. doi: 10.1093/cercor/bhp225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geier C., Luna B. The maturation of incentive processing and cognitive control. Pharmacology, Biochemistry, and Behavior. 2009;93:212–221. doi: 10.1016/j.pbb.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladwin T.E., Figner B., Crone Eveline A., Wiers R.W. Addiction, adolescence, and the integration of control and motivation. Developmental Cognitive Neuroscience. 2011;1:364–376. doi: 10.1016/j.dcn.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber S.N., Knutson Brian. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans E.J., Bos P.A., Ossewaarde L., Ramsey N.F., Fernández G., Van Honk J. Effects of exogenous testosterone on the ventral striatal BOLD response during reward anticipation in healthy women. Neuroimage. 2010;52:277–283. doi: 10.1016/j.neuroimage.2010.04.019. [DOI] [PubMed] [Google Scholar]
- Hommer D.W., Knutson B., Fong G.W., Bennett S., Adams C.M., Varnera J.L. Amygdalar recruitment during anticipation of monetary rewards: an event-related fMRI study. Annals of the New York Academy of Sciences. 2003;985:476–478. doi: 10.1111/j.1749-6632.2003.tb07103.x. [DOI] [PubMed] [Google Scholar]
- Klingberg T., Vaidya C.J., Gabrieli J.D., Moseley M.E., Hedehus M. Myelination and organization of the frontal white matter in children: a diffusion tensor MRI study. Neuroreport. 1999;10:2817–2821. doi: 10.1097/00001756-199909090-00022. [DOI] [PubMed] [Google Scholar]
- Knutson B., Adams C.M., Fong G.W., Hommer D. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. The Journal of Neuroscience. 2001;21:RC159. doi: 10.1523/JNEUROSCI.21-16-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B., Fong G.W., Adams C.M., Varner J.L., Hommer D. Dissociation of reward anticipation and outcome with event-related fMRI. Neuroreport. 2001;12:3683–3687. doi: 10.1097/00001756-200112040-00016. [DOI] [PubMed] [Google Scholar]
- Knutson B., Fong G.W., Bennett S.M., Adams C.M., Hommer D. A region of mesial prefrontal cortex tracks monetarily rewarding outcomes: characterization with rapid event-related fMRI. Neuroimage. 2003;18:263–272. doi: 10.1016/s1053-8119(02)00057-5. [DOI] [PubMed] [Google Scholar]
- Liston C., Watts R., Tottenham N., Davidson M.C., Niogi S., Ulug A.M., Casey B.J. Frontostriatal microstructure modulates efficient recruitment of cognitive control. Cerebral Cortex. 2006;16:553–560. doi: 10.1093/cercor/bhj003. (New York, NY: 1991) [DOI] [PubMed] [Google Scholar]
- Luna B., Velanova K., Geier C.F. Methodological approaches in developmental neuroimaging studies. Human Brain Mapping. 2010;31:863–871. doi: 10.1002/hbm.21073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna B., Paulsen D.J., Padmanabhan A., Geier C. The teenage brain: cognitive control and motivation. Current Directions in Psychological Science. 2013;22:94–100. doi: 10.1177/0963721413478416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy Z., Westerberg H., Klingberg T. Maturation of white matter is associated with the development of cognitive functions during childhood. Journal of Cognitive Neuroscience. 2004;16:1227–1233. doi: 10.1162/0898929041920441. [DOI] [PubMed] [Google Scholar]
- Olesen P.J., Nagy Z., Westerberg H., Klingberg T. Combined analysis of DTI and fMRI data reveals a joint maturation of white and grey matter in a fronto-parietal network. Brain Research. Cognitive Brain Research. 2003;18:48–57. doi: 10.1016/j.cogbrainres.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Ossewaarde L., Qin S., Van Marle H.J.F., Van Wingen G.A., Fernández G., Hermans E.J. Stress-induced reduction in reward-related prefrontal cortex function. Neuroimage. 2011;55:345–352. doi: 10.1016/j.neuroimage.2010.11.068. [DOI] [PubMed] [Google Scholar]
- Padmanabhan A., Geier C.F., Ordaz S.J., Teslovich T., Luna B. Developmental changes in brain function underlying the influence of reward processing on inhibitory control. Developmental Cognitive Neuroscience. 2011;1:517–529. doi: 10.1016/j.dcn.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T., Keshavan M., Giedd J.N. Why do many psychiatric disorders emerge during adolescence? Nature Reviews Neuroscience. 2008;9:947–957. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer J.H., Allen N.B. Arrested development? Reconsidering dual-systems models of brain function in adolescence and disorders. Trends in Cognitive Sciences. 2012;16:322–329. doi: 10.1016/j.tics.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack R.A. Region of interest analysis for fMRI. Social Cognitive and Affective Neuroscience. 2007;2:67–70. doi: 10.1093/scan/nsm006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack R.A., Mumford J.A. Independence in ROI analysis: where is the voodoo? Social Cognitive and Affective Neuroscience. 2009;4:208–213. doi: 10.1093/scan/nsp011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider S., Peters J., Bromberg U., Brassen S., Miedl S.F., Banaschewski T., Barker G.J., Conrod P., Flor H., Garavan H., Heinz A., Ittermann B., Lathrop M., Loth E., Mann K., Martinot J.-L., Nees F., Paus T., Rietschel M., Robbins T.W., Smolka M.N., Spanagel R., Ströhle A., Struve M., Schumann G., Büchel C. Risk taking and the adolescent reward system: a potential common link to substance abuse. The American Journal of Psychiatry. 2012;169:39–46. doi: 10.1176/appi.ajp.2011.11030489. [DOI] [PubMed] [Google Scholar]
- Schott B.H., Niehaus L., Wittmann B.C., Schütze H., Seidenbecher C.I., Heinze H.-J., Düzel E. Ageing and early-stage Parkinson's disease affect separable neural mechanisms of mesolimbic reward processing. Brain: A Journal of Neurology. 2007;130:2412–2424. doi: 10.1093/brain/awm147. [DOI] [PubMed] [Google Scholar]
- Schultz W., Tremblay L., Hollerman J.R. Reward processing in primate orbitofrontal cortex and basal ganglia. Cerebral Cortex. 2000;10:272–284. doi: 10.1093/cercor/10.3.272. [DOI] [PubMed] [Google Scholar]
- Shohamy D. Learning and motivation in the human striatum. Current Opinion in Neurobiology. 2011;21:408–414. doi: 10.1016/j.conb.2011.05.009. [DOI] [PubMed] [Google Scholar]
- Somerville L.H., Casey B.J. Developmental neurobiology of cognitive control and motivational systems. Current Opinion in Neurobiology. 2010;20:236–241. doi: 10.1016/j.conb.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville L.H., Jones R.M., Casey B.J. A time of change: behavioral and neural correlates of adolescent sensitivity to appetitive and aversive environmental cues. Brain and Cognition. 2010;72:124–133. doi: 10.1016/j.bandc.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear L.P. The adolescent brain and age-related behavioral manifestations. Neuroscience and Biobehavioral Reviews. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spear L.P. Rewards, aversions and affect in adolescence: emerging convergences across laboratory animal and human data. Developmental Cognitive Neuroscience. 2011;1:392–400. doi: 10.1016/j.dcn.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L. Risk taking in adolescence: new perspectives from brain and behavioral science. Current Directions in Psychological Science. 2007;16:55–59. [Google Scholar]
- Strang N.M., Chein J.M., Steinberg L. The value of the dual systems model of adolescent risk-taking. Frontiers in Human Neuroscience. 2013;7 doi: 10.3389/fnhum.2013.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N., Landeau B., Papathanassiou D., Crivello F., Etard O., Delcroix N., Mazoyer B., Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Van Dijk K.R.A., Sabuncu M.R., Buckner R.L. The influence of head motion on intrinsic functional connectivity MRI. Neuroimage. 2012;59:431–438. doi: 10.1016/j.neuroimage.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Duijvenvoorde A.C.K., Crone E.A. The teenage brain: a neuroeconomic approach to adolescent decision making. Current Directions in Psychological Science. 2013;22:108–113. [Google Scholar]
- Van Hell H.H., Vink M., Ossewaarde L., Jager G., Kahn R.S., Ramsey N.F. Chronic effects of cannabis use on the human reward system: an fMRI study. European Neuropsychopharmacology. 2010;20:153–163. doi: 10.1016/j.euroneuro.2009.11.010. [DOI] [PubMed] [Google Scholar]
- Van Leijenhorst L., Gunther Moor B., Op de Macks Z.A., Rombouts S.A.R.B., Westenberg P.M., Crone Eveline A. Adolescent risky decision-making: neurocognitive development of reward and control regions. Neuroimage. 2010;51:345–355. doi: 10.1016/j.neuroimage.2010.02.038. [DOI] [PubMed] [Google Scholar]
- Van Leijenhorst L., Zanolie K., Van Meel C.S., Westenberg P.M., Rombouts S.A.R.B., Crone Eveline A. What motivates the adolescent? Brain regions mediating reward sensitivity across adolescence. Cerebral Cortex. 2010;20:61–69. doi: 10.1093/cercor/bhp078. [DOI] [PubMed] [Google Scholar]
- Vink M., Kahn R.S., Raemaekers M., Ramsey N.F. Perceptual bias following visual target selection. Neuroimage. 2005;25:1168–1174. doi: 10.1016/j.neuroimage.2004.12.042. [DOI] [PubMed] [Google Scholar]
- Vink M., Pas P., Bijleveld E., Custers R., Gladwin T. Ventral striatum is related to within-subject learning performance. Neuroscience. 2013;250(October):408–416. doi: 10.1016/j.neuroscience.2013.07.034. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Correlation between Reward Anticipation and Reward Outcome.