Abstract
Substantial hormonal and neurobiological changes occur during puberty, and are widely argued to render this period of life a sensitive period in terms of risk for mental health problems. However, there is a paucity of research focusing on adrenarche, the earlier phase of pubertal development. Furthermore, there is a limited understanding of the association between adrenarche and neural development during this phase of life. We systematically reviewed research examining human adrenarcheal development as operationalized by hormonal levels of DHEA and DHEA-S, in relation to indices of mental health (Systematic Review 1). We then reviewed the limited amount of literature that has examined the association between adrenarcheal development and brain structure or function (Systematic Review 2). In general, studies showed that earlier timing of adrenarche was associated with greater mental health symptoms, and there is emerging support that brain development plays a role in this relationship. However, several methodological inconsistencies were noted. We propose that future research in this area test a theoretical model of adrenarche as a sensitive period of neurobiological development, whereby timing of exposure to hormones interacts with brain development, biological sex, and psychosocial stress to influence environmental sensitivity and risk for mental health problems through adolescence.
Keywords: Puberty, Adrenarche, Sensitive periods, Mental health, Brain development
1. Introduction
Substantial changes occur during puberty, including social and physical development, increases in hormones and hormonal reactivity, and related neurobiological development. These changes are widely argued to render this period of life a sensitive period in terms of risk for mental health problems (Ladouceur et al., 2012, Paus et al., 2008). However, there are two separate phases in pubertal development: adrenarche, which is triggered by the maturation of the zona reticularis of the adrenal gland, and gonadarche, associated with the maturation of the hypothalamic-pituitary-gonadal axis. A link between puberty and mental health has been mainly demonstrated with respect to the second phase of pubertal development, gonadarche, which begins with the secretion of gonadotropin-releasing hormone (GnRH) from the hypothalamus at approximately 10–11 years of age, and triggers a rise in testosterone and estradiol, the maturation of primary and secondary sexual characteristics, and menarche in girls (Dorn, 2006). Individual differences in puberty can be measured in three ways. First, pubertal status, the developmental stage at which an individual is at a given point of time, can be measured by physical characteristics such as Tanner stage. Tanner stage can be assessed via self-report, parent-report, or a physical examination by a physician. Second, pubertal timing, which is pubertal status relative to same-age and −sex peers, can be measured by comparing stage/status via physical characteristics to peers, or by comparing levels of pubertal hormones to peers. Third, pubertal tempo, which is how quickly an individual passes through pubertal stages, can be measured over time (i.e., longitudinally) to examine the rate of maturation via physical characteristics or levels of hormones. We note that there is a paucity of studies that examine pubertal tempo in relation to mental health. Pubertal stage (gonadarche), on the other hand, has shown to be associated with mental health (Angold et al., 1998, Oldehinkel et al., 2011), and, in particular, it is pubertal timing that appears to be especially salient in predicting the onset of mental health problems (e.g., Angold and Costello, 2006, Kaltiala-Heino et al., 2003b, Mendle et al., 2010), and may be associated with different symptoms compared to pubertal stage alone (Oldehinkel et al., 2011). Early timing of gonadarche has been associated with depression (Copeland et al., 2010, Graber et al., 2004), anxiety (Hayward et al., 1992, Patton et al., 1996, Zehr et al., 2007), and eating (Zehr et al., 2007) and behavioral disorders (Copeland et al., 2010, Lynne et al., 2007, Stattin and Magnusson, 1990), especially for girls (Ge et al., 2001b), while the evidence for boys is more mixed (Ge et al., 2001a, Graber et al., 1997, Kaltiala-Heino et al., 2003a).
1.1. Adrenarche in human development
Considerably less work has focused on how adrenarche, the earlier phase of pubertal development associated with a dramatic increase in the level of androgens secreted by the adrenal cortex, affects psychological and neural functioning. This is surprising given that 1) early timing of adrenarche is a known risk factor for poor physical health later in life (Ibáñez et al., 2006), and 2) adrenarche does not occur in species other than human beings and some higher primates (Conley et al., 2012), and therefore may have specific evolutionary significance that might be associated with patterns of neural development particular to these species. Adrenarche typically begins around five to seven years of age when levels of the androgens dehydroepiandrosterone (DHEA) and its sulfate (DHEA-S), secreted from the adrenal glands, begin to increase, before the hypothalamic-pituitary-gonadal axis is re-activated (Parker et al., 1978, Rainey et al., 2002, Remer et al., 2005). DHEA is converted to DHEA-S through a sulfation process and is also more stable as DHEA-S (Maninger et al., 2009). Adrenarche and gonadarche are separate periods that are activated and controlled by independent mechanisms (Counts et al., 1987). Importantly, the external physical changes associated with adrenarche (increased skin oil production, body odor, and skeletal maturation; Dorn and Chrousos, 1997) may not be obvious until well after the initial rise in adrenarcheal hormones has begun (Wan, 2012) and as of yet there are no references values for DHEA/DHEA-S that match the physical manifestations of adrenarche (Uçar, 2015). This means that measurement of these hormones as objective indications of this phase is vital for research examining adrenarche.
1.2. Possible links between adrenarcheal and brain development
Moreover, there is limited understanding of the association between adrenarche and neural development during this phase of life. However, it has been proposed that adrenarche serves an evolutionary purpose for humans to extend brain development and promote synaptogenesis for social learning that is necessary starting from this age (Campbell, 2006). Indeed, there is evidence suggesting that adrenarcheal hormones may be key factors in brain development during the transition from childhood to early adulthood. For example, animal research has demonstrated that DHEA and DHEA-S have pleiotropic roles in the brain including stimulating neurite growth and neurogenesis, modifying neural activity via direct and indirect effects (via conversion to testosterone, di-hydrotestosterone and estrogen) on pre- and post-synaptic receptor and binding sites, and neuroprotective effects (via anti-glucocorticoid, anti-oxidant and inhibition of apoptosis; Maninger et al., 2009). Rodent work has demonstrated that DHEA administration decreases cognitive impairments and putatively depressive behaviors by enhancing neurogenesis in the hippocampus (Moriguchi et al., 2013, Moriguchi et al., 2011). In adults, administration of DHEA appears to reduce activity in the amygdala and hippocampus, enhance connectivity between the amygdala and hippocampus, and enhance activity in the rostral anterior cingulate cortex (rACC) during emotional processing and regulation (Sripada et al., 2013).
Furthermore, the timing of adrenarcheal hormones may follow patterns in brain development, suggesting that these hormones may have organizational and activation roles in the brain, although more research is needed. For example, DHEA-S levels are high at birth, decrease rapidly after birth, begin to increase again at the beginning of adrenarche around five to six years of age, and peak in the mid-20s (Rainey et al., 2002, Sulcová et al., 1997) – although it should be noted that other research has shown that DHEA begins to increase closer to seven years of age (Remer et al., 2005), or later depending on sex (Sulcová et al., 1997). Timing of adrenarche is variable, and in particular, consensus regarding definitions of premature adrenarche (PA) vary (Idkowiak et al., 2011, Utriainen et al., 2015), although the consensus as of yet is that PA is defined by increasing levels of adrenarcheal hormones before age 8 in girls and 9 in boys in conjunction with physical signs such as pubarche (Idkowiak et al., 2011). Little is known about underlying causes of variation in the timing of adrenarche, although it has been associated with sex, ethnicity and body mass (for review, see Utriainen et al., 2015), and prenatal stress (Belsky et al., 2015). Therefore, further longitudinal research following children from ages five through to gonadarche is needed to determine the average age, range, and individual differences associated with the onset of adrenarche.
Nevertheless, this timing of the onset of adrenarche, as measured by DHEA-S, appears to be similar to that observed for cortical glucose utilization rates, which peak from four to eight years of age (with one study showing a single peak at 7.8 years; Muzik et al., 1999), and is also similar to when the brain reaches its adult volume (for review see Campbell, 2011). Furthermore, maturation of grey matter in the cerebral cortex continues into the early 20s (Gogtay et al., 2004), and as stated earlier, levels of DHEA-S peak after age 20 (Rainey et al., 2002, Sulcová et al., 1997). In sum, similar patterns in the increase in DHEA and DHEA-S and indices of brain maturation, which are nicely visualized in a review by Campbell (2011), are suggestive of the hypothesis that adrenarche may have salient effects on brain development beginning from childhood and throughout adolescence and early adulthood.
1.3. The case for adrenarche as a sensitive period in mental health development
Although studies of child and adolescent brain development have mainly focused on changes associated with chronological age (Mutlu et al., 2013, Tamnes et al., 2010, Vijayakumar et al., 2016, Wierenga et al., 2014), it has been suggested that patterns of brain development might be better explained by pubertal (particularly gonadal) development (Crone and Dahl, 2012, Giedd et al., 1999). Further, it has been suggested that periods of marked brain development during the transition from childhood to adolescence might render the brain particularly sensitive to factors that contribute to the development of mental health problems (Blakemore et al., 2010). We posit that in addition to gonadarche, the adrenarcheal period may be a particularly sensitive period of development when hormones, neurobiological maturation, and environmental risk factors interact to influence psychosocial development. This sensitive period may begin as early as age five, when levels of adrenarcheal hormones rise, but more research measuring adrenarcheal hormones over time is needed to confirm this. However, during the transition from childhood to adolescence, the brain continues to experience rapid growth and reorganization, second only to infancy in terms of its significance (Spear, 2000). It becomes more plastic and hence sensitive to internal and external factors that may influence neurodevelopmental trajectories (Andersen and Teicher, 2008). The period of adrenarche, starting in childhood, is characterized by significant changes across the cortex, including development of prefrontal and limbic regions that support emotional and behavioral regulation (Kesek et al., 2009), as well as the connections between these regions (Cunningham et al., 2002).
Therefore, neurodevelopmental mechanisms are likely to be important mediators of the association between adrenarche and mental health outcomes. A review of the extant research on adrenarche and both mental health and brain development is timely to establish whether there are consistent patterns and associations. This review will also highlight research directions and methodological issues that require further investigation.
1.4. The aim of this review
In this review paper, we will use PRISMA guidelines to systematically review research that has examined human adrenarcheal development, as measured by levels of the adrenarcheal hormones DHEA and DHEA-S, in relation to indices of mental health (Systematic Review 1). We will then review the more limited amount of literature (including a series of studies by our group) that has examined the association between adrenarcheal development and brain structure or function (Systematic Review 2). We will comment on potentially important factors that influence associations between adrenarcheal development, brain structure/function, and mental health, such as timing of exposure to adrenal hormones, and biological sex. Finally, we propose a theoretical model of adrenarche as a sensitive period of neurobiological development, whereby brain development can either interact with, or mediate the association between timing of exposure to hormones, environmental sensitivity, and risk for mental health problems through adolescence. In addition to exploring the prospective effects of adrenarche on subsequent neurobiological and psychosocial development, we will explore factors associated with earlier timing of adrenarche, such as psychosocial stress (e.g., Ellis and Essex, 2007), to build a theoretical framework that can guide future investigation.
2. Material and methods
2.1. Methods of systematic review (SR) 1: adrenarcheal development and mental health
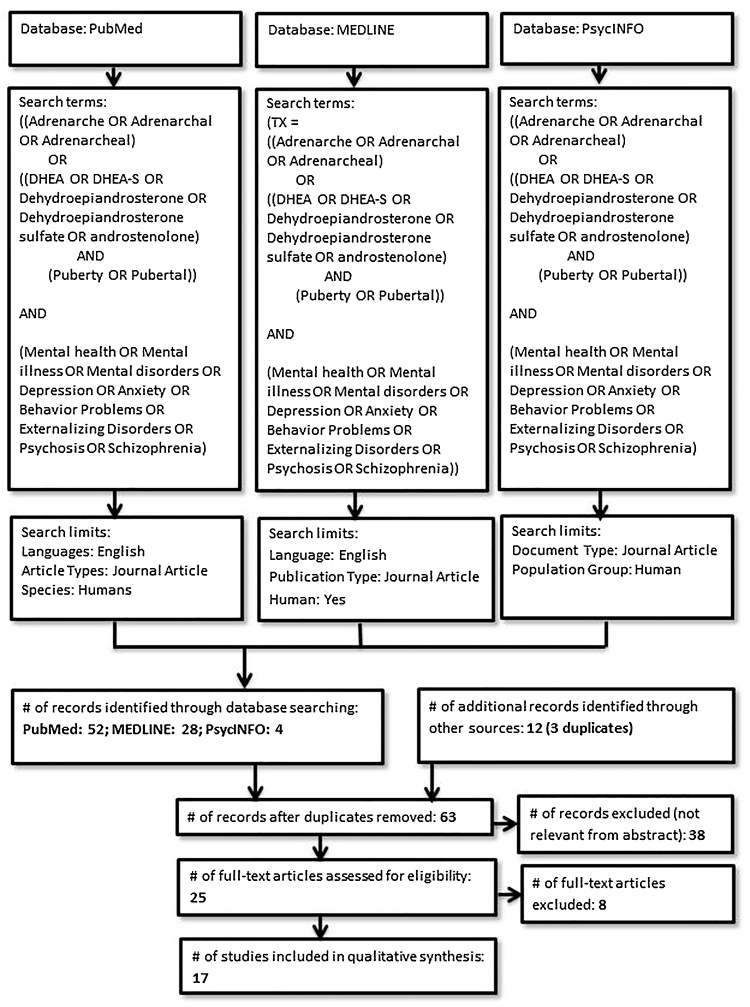
The systematic reviews were conducted according to the PRISMA guidelines (Moher et al., 2009). Online searches of the PubMed, MEDLINE (EBSCOhost), and PsycINFO (APA PsycNET) databases were performed in August 2016. Fig. 1 presents a flowchart and details of the procedure for SR1.
Fig. 1.
Procedure used for study selection in Systematic Review 1 (Adrenarche and mental health).
2.1.1. Literature search of SR1
Abstracts were reviewed for references to measurement of DHEA/DHEA-S during puberty and mental health. If the study was relevant, the full text was retrieved. Additional studies were retrieved through other methods, including reviewing bibliographies of articles identified through the search terms.
2.1.2. Inclusion and exclusion criteria of SR1
Journal articles were included in SR1 if they reported on studies that 1) measured adrenarche from five years (Rainey et al., 2002) onwards1 through basal levels of adrenarcheal hormones DHEA or DHEA-S, and 2) measured any Axis-I mental health symptoms or diagnosis of a mental illness either through self-report questionnaires, parent or teacher questionnaires, or researcher observed scales. We included studies that either 1) conducted analyses between continuous levels of adrenarcheal hormones and mental health problems, or 2) placed participants into adrenarcheal groups based on levels of adrenarcheal hormones and examined group differences in mental health. The age range for mental health measurement was not limited to allow for the inclusion of longitudinal studies that investigated long term mental health outcomes associated with adrenarche. Studies that measured overall pubertal development (status, timing, or tempo) but did not differentiate between adrenarche and gonadarche (i.e., studies that only measured total score on the Pubertal Development Scale or Tanner staging and did not measure levels of DHEA or DHEA-S) were not included. Further inclusion and exclusion criteria for SR1 are presented in Fig. 1.
2.2. Methods of SR2: adrenarcheal development and brain structure or function
Fig. 2 presents a flowchart and details of the procedure for SR2.
Fig. 2.
Procedure used for study selection in Systematic Review 2 (Adrenarche and Brain Structure/Function).
2.2.1. Literature search of SR2
Abstracts were reviewed for references to measurement of DHEA/DHEA-S during puberty and brain structure or function. If the study was relevant, the full text was retrieved. Additional studies were retrieved through other methods, including reviewing bibliographies of articles identified through the search terms.
2.2.2. Inclusion and exclusion criteria of SR2
Journal articles were included in SR2 if they reported on studies that 1) measured adrenarche from five years onwards through basal levels of adrenarcheal hormones DHEA or DHEA-S, and 2) measured brain structure using MRI, or brain function during emotional or social processing using fMRI or PET. We did not include EEG studies in our review as they would not be as informative as to the specific circuits and regions involved during adrenarche. Again, studies that measured overall pubertal development (stage, timing, or tempo) but did not differentiate between adrenarche and gonadarche (i.e., studies that only measured total score on the Pubertal Development Scale or Tanner staging and did not measure levels of DHEA or DHEA-S) were not included. Further inclusion and exclusion criteria for SR2 are presented in Fig. 2.
3. Results
3.1. Results of SR1: adrenarcheal development and mental health
3.1.1. Methods of measuring adrenarche in SR1
A summary of each study is presented in Table 1. Studies measured adrenarche in different ways. First, five studies measured levels of adrenarcheal hormones (DHEA and/or DHEA-S) alone (Belsky et al., 2015, Constantino et al., 1993, Goodyer et al., 2000, Goodyer et al., 1996, Van Goozen et al., 1998), and twelve studies measured both hormones and physical characteristics associated with adrenarche, such as Tanner stage of pubic hair development (Dorn et al., 2008, Dorn et al., 1999, Klauser et al., 2015, Mundy et al., 2015, Murray et al., 2016, Nottelmann et al., 1987, Shirtcliff et al., 2007, Sontag-Padilla et al., 2012, Susman et al., 1996, Susman et al., 1987, van Goozen et al., 2000, Whittle et al., 2015). Amongst the latter, three compared groups of children with “premature adrenarche” (PA) with on-time children based on Tanner pubic hair stage, but then confirmed PA status by showing that levels of adrenarcheal hormones were significantly higher in the PA compared to the on-time group (Dorn et al., 2008, Dorn et al., 1999, Sontag-Padilla et al., 2012), consistent with the definition of PA (Idkowiak et al., 2011). We considered these studies to be measuring differences in adrenarcheal hormones in a similar way that Klauser et al. (2015) placed participants into early vs. late adrenarcheal timing based on levels of DHEA and testosterone, or Belsky et al. (2015) defined a pre-adrenarcheal group as DHEA below a detection threshold.
Table 1.
Summary of studies from Systematic Review 1 (SR1).
| Authors (year); PubMed ID | Sample | Measure(s) of adrenarche | Measure(s) of mental health | Summary of findings |
|---|---|---|---|---|
|
Murray et al. (2016) PMID: 26600008 |
100 children who completed an MRI scan, out of 128 9-year-old children, selected based on high vs. low DHEA and testosterone levels in saliva 6 months prior | Continuous levels of DHEA, DHEA-S, and testosterone were measured again in saliva (averaged across 2 mornings) | Self-reported SCAS (anxiety symptoms) | SCAS scores were not significantly associated with hormone measures Note: Associations between adrenarche and brain structure were found, reported in SR2. |
|
Whittle et al. (2015) PMID: 25678548 |
83 children who completed an fMRI task, out of 128 9-year-old children, selected based on high vs. low DHEA and testosterone levels in saliva 6 months prior | Continuous levels of DHEA and testosterone were measured again in saliva (averaged across 2 mornings) | Parent-report CBCL (externalizing symptoms only), self-reported CDI (depressive symptoms), and self-reported SCAS (anxiety symptoms) | In females only, CBCL externalizing score was positively associated with DHEA (r = 0.364, p < 0.05). There were no significant associations between DHEA and mental health in males. Note: Associations between adrenarche and brain function were found, reported in SR2. |
|
Klauser et al. (2015) PMID: 25459897 |
41 early developing (mean age 9.64 ± 0.35) and 44 late developing (9.48 ± 0.30) children based on DHEA and testosterone levels | Early vs. late adrenarche based on levels of DHEA and Testosterone approximately 6 months earlier; Tanner stage based on parent-report Sexual Maturity Status line drawings | Self-reported CDI (depressive symptoms) and self-reported SCAS (anxiety symptoms) | Controlled for Tanner stage to exclude effect of early gonadarche, and for age. No significant differences between early and late children were found for CDI or SCAS scores, for boys and girls together, or for boys or girls separately. Note: Associations between adrenarche and brain structure were also found, reported in SR2. |
|
Belsky et al. (2015) PMID: 25915592 |
73 females in Grade 1 (aged 6.8–7.8 years, mean age = 7.25 years) | DHEA measured in saliva 4 times at home visit following Grade 1. Preadrenarcheal (41% of sample) = 6/8 assays below detection threshold of 10 pg/ml and all <16 pg/ml | At age 18 (mean age 17.84 yrs), self-report MacArthur Health and Behavior Questionnaire (internalizing and externalizing) | Longitudinal study found path for prenatal stress → maternal depression & negative parenting in infancy → increased cortisol at 4.5 yrs → accelerated adrenarcheal development → more physical and mental health problems age 18. Adrenarche & mental health correlated at 0.32 (p < = 0.01) |
|
Mundy et al. (2015) PMID: 26592329 |
Population based study of 1124 children aged 8–9. | DHEA, DHEA-S, and testosterone measured in saliva. Hormone levels were standardized by age (separately by sex), then categorized into minimal, intermediate, and advanced development. | Parent-report Strengths and Difficulties Questionnaire (Difficulties scales: Emotional symptoms, conduct problems, hyperactivity/inattention, peer problems; Other scale: prosocial behavior) | In females, only higher levels of DHEA-S were positively associated with peer problems only. In males: higher levels of DHEA were associated with more peer problems and emotional symptoms; higher levels of DHEA-S were associated with more total difficulties, conduct problems, hyperactivity/inattention, and peer problems; higher levels of testosterone were associated with more total difficulties, peer problems, and emotional symptoms. |
|
Sontag-Padilla et al. (2012) PMID: 22293005 |
76 girls mean age 7.50 years, SD = 0.85. Two groups: 40 with PA (recruited from pediatric endocrine clinics), 36 on time (recruited via community) | Premature adrenarche (PA) vs. on-time adrenarche. PA documented by a pediatric endocrinologist, Tanner 1 breast, Tanner 2 or greater pubic hair (On-time were Tanner 1 breast and Tanner 1 pubic hair). Adrenal hormones DHEA-S and androstendione were measured in serum to confirm PA status. |
Parent report of the CBCL (internalizing and externalizing); Child self-report CDI (depressive symptoms), child self-report STAI-C (anxiety symptoms) |
Main effects – PA girls had higher levels of CBCL internalizing scores [50.70 (10.45) vs. 46.06 (8.99), t = −2.01, d = 0.48, p < 0.05], and higher CBCL externalizing scores [50.28 (9.02) vs. 45.00 (8.67), t = −2.53, d = 0.60, <0.01] Interactions – PA interacted with: lower levels of executive functioning to predict higher externalizing and anxiety symptoms; increased cortisol to predict externalizing symptoms; and decreased cortisol to predict depressive symptoms |
|
Dorn et al. (2008) PMID: 18655525 |
Same sample as Sontag-Padilla et al. (2012) | See Sontag-Padilla et al. (2012). This study also examined testosterone in blood | DISC (diagnostic interview); teacher and parent report CBCL (internalizing and externalizing); child self-report CDI (depressive symptoms), child self-report STAI-C (anxiety symptoms), child self-report relational aggression | Compared to on-time girls, PA girls had: − higher rate of diagnosis of ODD in the past year (20% vs 3.1%; p = 0.04), past month (17.5% vs 0%; p = 0.02), and lifetime (20% vs 3.1%; p = 0.04); − higher symptoms scores for separation anxiety (3.13 ± 2.43 vs. 1.77 ± 1.93), specific phobia (1.40 ± 1.13 vs. 0.59 ± 0.S4), GAD (3.08 ± 2.38 vs. 1.59 ± 1.74), panic disorder, OCD (0.48 ± 0.91 vs. 0.03 ± 0.18), MDD (4.93 ± 3.71 vs. 2.09 ± 1.91), ADHD (5.10 ± 4.87 vs. 2.59 ± 3.23), and ODD (6.45 ± 3.07 vs. 4.59 ± 3.10); − higher scores of parent-report Social Problems (55.2 ± 7.3 vs. 51.8 ± 4.6), Anxious/Depressed (54.2 ± 5.3 vs. 51.8 ± 3.4), Aggressive Behavior (54.0 ± 5.4 vs. 51.5 ± 3.2), total Externalizing Behavior (50.3 ± 9.0* 45.0 ± 8.7), total Internalizing Behavior (50.7 ± 10.5* 46.1 ± 9.0) and total Behavior Problems (50.0 ± 11.2** 4l.S ± 14.3) on the CBCL; − and higher scores of teacher-report aggressive behavior on the CBCL (p = 0.03) |
|
Shirtcliff et al. (2007) PMID: 17537074 |
106 boys and 107 girls, mean age 13.7 years (SD = 1.7) | Continuous baseline levels (prior to a stress task) of DHEA in saliva (also measured pubertal development via Tanner stage) | Internalizing and externalizing symptoms with the parent- and child-report CBCL and DISC interview |
Girls with more internalizing problems had lower levels of baseline DHEA. No significant associations between baseline DHEA and symptoms for boys. |
|
Goodyer et al. (2000) PMID: 11102323 |
73 boys, 107 girls, mean age 13.5 years (range 12.2–16.5) at high risk for psychopathology (due to recent negative life events, high emotionality, or parental history) | DHEA in saliva at 08:00 and 20:00, averaged across 4 days, i.e., mean morning DHEA and mean evening DHEA. | Self-report depressive symptoms (Mood and Feelings Questionnaire); DSM-IV criteria for MDD with the K-SADS interview at a 12 month follow-up. | Mean DHEA (either morning or evening) did not predict if participants developed MDD at follow-up; however, associations between mean DHEA and self-report symptoms at baseline were not measured. |
|
van Goozen et al. (2000) PMID: 11068901 |
3 groups of children, 24 with ODD, 42 psychiatric controls (PC), and 30 normal controls (NC; 16 boys), aged between 6 and 12 (ODD mean = 10.1, PC mean = 9.3, NC mean = 10.1). | DHEA-S measured in plamsa. Tanner stage measured for pubic hair and breast or male gential development | DSM-IV criteria via semistructured diagnostic interview for ODD and other psychiatric disorders | DHEA-S: Controlling for age and Tanner stage, there was a main effect of group [F (2,76) = 5.65, p < 0.01], with the ODD group having higher levels of DHEA-S (ODD = 3.01 μmol/L ± 1.7, PC = 1.63 ± 1.4, NC = 2.03 ± 1.1). |
|
Dorn et al. (1999) PMID: 9988243 |
Pilot study of children 6–9 years old. 9 PA (8 girls, enrolled from pediatric endocrine clinics), 20 on-time (8 girls, recruited from the community). | Premature adrenarche (PA) vs. on-time adrenarche. PA documented by a pediatric endocrinologist, Tanner 2 or 3 pubic hair (On-time were Tanner 1 breast or genital and Tanner 1 pubic hair). Adrenal hormones DHEA, DHEA-S and delta-4-androstenedione were measured in serum and were higher in PA group. |
Parent-report DISC interview for DSM-III disorders, parent-report CBCL, self-report CDI (depressive symptoms), and self-report STAI-C (anxiety symptoms) | PA children had higher CDI scores at the trend level only [10.2 ± 5.1 vs. 5.4 ± 5.1, t(24) = 2.03, p = 0.05]. PA children had higher scores on the parent-report CBCL for Somatic complaints (56.8 ± 8.5 vs. 51.4 ± 3.4, t = 2.42, p = 0.02), Withdrawal (56.9 ± 10.4 vs. 51.3 ± 3.9, t = 2.13, p = 0.04), Social Problems (54.9 ± 6.2 vs. 50.9 ± 2.6, t = 2.50, p = 0.02), total Internalizing (52.6 ± 13.5 vs. 42.5 ± 9.9, t = 2.20, p = 0.04), total Externalizing (54.1 ± 9.7 vs. 42.8 ± 9.6, t = 2.83, p = 0.01), and total Behavior Problems (53.5 ± 11.9 vs. 40.3 ± 10.7, t = 2.86, p = 0.01). 44% of children in the PA group had 1 or more diagnoses, while only 1 child in the on-time group had a diagnosis. |
|
Van Goozen et al. (1998) PMID: 9474448 |
2 groups of boys, 15 with conduct or oppositional defiant disorder and 25 controls, aged 8–12 years (mean age CD: 10.2, controls: 9.6) | Androstenedione, testosterone, and DHEA-S measured in plasma | DSM-IV criteria for CD or ODD; Parent- and teacher-report CBCL (Aggression and Delinquency scales) | CD participants had a significantly higher level of DHEA-S [2.85 nmol/l ± 1.1 vs. 1.46 ± 0.8, F (1,38) = 22.68, p < 0.0001] and a higher level of androstenedione at trend level [1.03 ± 0.4 vs. 0.81 ± 0.3, F (1,38) = 3.82, p < 0.06], but no difference in testosterone [0.90 ± 1.1 vs. 0.86 ± 1.5, F (1,38) = 0.01, NS]. DHEA-S was significantly associated with parent-report Delinquency (rho = 0.33) and Aggression (rho = 0.46) and teacher-report Delinquency (rho = 0.39) and Aggression (rho = 0.48). |
| Goodyer et al. (1996) | 3 groups of adolescents aged 8–16, 82 with MDD, 25 non-MDD psychiatric controls, and 40 healthy controls | DHEA in saliva, averaged over 2 days at 08:00, 12:00, and 20:00. | DSM-II criteria using the K-SADS diagnostic interview: 1) for MDD, 2) other psychiatric disorder, or 3) no disorder. | MDD group had lower morning DHEA than the other groups. No group differences in DHEA-S. |
|
Susman et al. (1996) PMID: 8853589 |
108 healthy adolescents, 56 ten- to 15-year-old boys (mean age 12.7), 52 nine- to 15-year old girls (mean age 11.9) | Plasma concentrations of DHEA, DHEA-S, and other gonadal hormones | Externalizing: CBCL, Anxiety: total number of anxiety symptoms from the DISC | Externalizing symptoms and DHEA-S were significantly negatively associated in girls. Anxiety symptoms and DHEA were significantly positively associated in boys. |
|
Constantino et al. (1993) PMID: 8282667 |
18 boys, aged 4–10 years, that were hospitalized for aggressive behavior and had Conduct Disorder, and 18 age- and race-matched controls | Serum concentrations of DHEA and DHEA-S, and other hormones. | Boys in the aggressive group met DSM-III criteria for Conduct Disorder and scored >98th percentile on the aggression subscale of the CBCL | There were no significant group differences in concentrations of any hormones. |
|
Susman et al. (1987) PMID: 3608660 |
56 boys and 52 girls, aged 9–14 years (all 5 stages of gonadal development) | Serum levels of gonadotropins, gonadal steroids, adrenal androgens (including DHEA and DHEA-S), and testosterone-estradiol binding globulin | Emotional dispositions: self-reported anger, nervousness, sadness, and impulse control. Aggressive attributes: mother-reported acting out, aggressive behavior problems, and rebellious and nasty characteristics |
For DHEA/DHEA-S only: Girls: DHEA-S positively associated with self-reported calmness, and negatively associated with parent-reported aggressiveness and nasty behavior. Boys: DHEA positively associated with self-reported sadness and parent-reported rebelliousness, and DHEA-S negatively associated with self-reported impulse control and parent-reported delinquency |
|
Nottelmann et al. (1987) PMID: 3819952 |
Same sample as Susman et al. (1987). | See Susman et al. (1987). | “Self-image problems”/adjustment from the self-report Offer Self-Image Questionnaire for Adolescents. Mother-report of internalizing and externalizing symptoms on the CBCL. |
For DHEA/DHEA-S only (analyses only reported separately by sex): Girls: Negative association between DHEA-S and social self-image problems (controlling for age and pubertal status), and internalizing and externalizing symptoms (not controlling for age and pubertal status). Boys: No significant associations between DHEA/DHEA-S and self-image problems, but a negative association between DHEA-S and hyperactive symptoms (only when not controlling for chronological age and Tanner pubertal status). |
Second, studies handled the issue of adrenarcheal timing in different ways. For example, some measured levels of hormones across different ages and did not control for exact age, which could be seen as measuring adrenarcheal stage instead of timing (Constantino et al., 1993, Dorn et al., 2008, Dorn et al., 1999, Goodyer et al., 2000, Goodyer et al., 1996, Sontag-Padilla et al., 2012, Susman et al., 1996, Susman et al., 1987, Van Goozen et al., 1998). Although two of these studies limited the age range of the participants to approximately two to three years (Dorn et al., 2008, Sontag-Padilla et al., 2012), most had wider age ranges (Constantino et al., 1993, Goodyer et al., 2000, Goodyer et al., 1996, Susman et al., 1996, Susman et al., 1987, Van Goozen et al., 1998). Furthermore, one of these studies compared a group of children aged six to nine years with PA to those with on-time adrenarche, and although the authors did not statistically control for age, they found no significant age difference between groups (Dorn et al., 1999). Another two similar studies age-matched on-time children with PA children within six months (Dorn et al., 2008, Sontag-Padilla et al., 2012), and these could be argued to have measured adrenarcheal timing instead of stage. Three studies (Mundy et al., 2015, Nottelmann et al., 1987, van Goozen et al., 2000) measured levels of hormones but controlled for chronological age in their statistical models, which could therefore be considered to be adrenarcheal timing (i.e., developmental differences standardized by age;), although one of these studies controlled for both age and Tanner stage pubertal development (Nottelmann et al., 1987). Another study measured Tanner stage in addition to DHEA levels but, rather than controlling for Tanner stage, this variable was examined as a separate dependent variable in the association with DHEA levels (Shirtcliff et al., 2007). Yet, other studies measured adrenarcheal timing explicitly by design by restricting the age range and classifying early vs. late timing based on comparative levels of adrenarcheal hormones. For example, Belsky et al. (2015) measured DHEA in salvia in Grade 1 girls aged 6.8–7.8 years and classified girls with six out of eight undetectable assays as pre-adrenarcheal. A further three studies (Klauser et al., 2015, Murray et al., 2016, Whittle et al., 2015) included data from the “Imaging brain development in the Childhood to Adolescence Transition Study” (iCATS). This study recruited children from a larger cohort (CATS; Mundy et al., 2015, Mundy et al., 2013) based on earlier or later timing of adrenarche by measuring levels of salivary DHEA and testosterone in children approximately nine years of age, with the crossover area of the upper tertiles of DHEA/testosterone characterized as relatively earlier development, and the lower tertiles as relatively later development (Simmons et al., 2014).
3.1.2. Summary of overall patterns of results in SR1
In many studies, children with higher levels of DHEA or DHEA-S (or earlier timing of adrenarche based on higher levels of these hormones compared to peers) had higher levels of mental health symptoms or a greater likelihood of having a mental disorder (Belsky et al., 2015, Dorn et al., 2008, Dorn et al., 1999, Mundy et al., 2015, Sontag-Padilla et al., 2012, Susman et al., 1996, Susman et al., 1987, van Goozen et al., 2000, Van Goozen et al., 1998, Whittle et al., 2015). However, there were a significant number of studies that showed opposite associations, that lower levels of DHEA/DHEA-S were associated with mental health problems (Nottelmann et al., 1987, Shirtcliff et al., 2007, Goodyer et al., 1996). Further breakdown of these findings based on mental health problems are presented in section 3.1.4. Interestingly, of the studies that found a negative association, Goodyer et al. (1996) found that it was specifically morning DHEA (measured at 08:00 h) that was lower in a group of adolescents with MDD, suggesting that the diurnal variation in DHEA is a methodological concern for future research. Furthermore, Nottelmann et al. (1987) found that when controlling for Tanner pubertal status and chronological age, only the negative association between DHEA-S and social self-image problems in girls survived. A few studies found no significant associations between DHEA/DHEA-S and mental health (Constantino et al., 1993, Goodyer et al., 2000, Murray et al., 2016). Of these, Goodyer et al. (2000) only measured DHEA to predict if participants developed MDD over a 12 month follow-up, but did not conduct cross-sectional analyses of DHEA and self-report symptoms at baseline. Another study found no associations between anxiety symptoms and levels of DHEA or DHEA-S, but found that earlier Tanner stage within a cohort (which could be interpreted as later pubertal timing) was associated with higher obsessive compulsive disorder symptoms (Murray et al., 2016).
3.1.3. Summary of patterns by sex in SR1
Studies that differentiated by sex mainly showed that higher levels of DHEA and DHEA-S (or earlier timing of adrenarche) was associated with higher levels of mental health symptoms (including externalizing) amongst girls (Dorn et al., 2008, Mundy et al., 2015, Sontag-Padilla et al., 2012, Whittle et al., 2015), although one study found that higher levels of internalizing symptoms were associated with lower levels of DHEA in girls only (Shirtcliff et al., 2007), two studies found similar negative associations between DHEA-S and externalizing symptoms (Susman et al., 1996, Susman et al., 1987), and one found negative associations between DHEA-S and both internalizing and externalizing symptoms (only when not controlling for age and Tanner pubertal status; Nottelmann et al., 1987). For boys, results were similar; one study found that higher levels of DHEA were associated with higher levels of anxiety symptoms (Susman et al., 1996), and another found the same for self-reported sadness (Susman et al., 1987), in boys only and not girls. Another found that boys with conduct disorder or ODD had higher levels of DHEA-S than controls, and DHEA-S was positively associated with symptoms of delinquency and aggression (Van Goozen et al., 1998). However, one study found that levels of DHEA-S were negatively associated with self-reported impulse control and parent-reported delinquency in boys (Susman et al., 1987). Finally, two studies found no significant associations for boys: one study found no significant differences in depressive or anxiety symptoms between early and late developers (based on hormone levels), for girls and boys together, or for boys and girls separately (Klauser et al., 2015); another study of boys with conduct disorder and controls showed no group differences in levels of DHEA(S) (Constantino et al., 1993). It should be noted that the latter study had a wide range of ages (4–10 years) and was not designed to measure DHEA as a marker of pubertal development specifically.
3.1.4. Summary of patterns by measurement of mental health in SR1
The measurement of mental health also varied. Some studies measured symptoms: six studies measured anxiety symptoms (Dorn et al., 2008, Dorn et al., 1999, Murray et al., 2016, Sontag-Padilla et al., 2012, Susman et al., 1996, Whittle et al., 2015); four measured depressive symptoms (Dorn et al., 2008, Dorn et al., 1999, Sontag-Padilla et al., 2012, Whittle et al., 2015); eight measured internalizing symptoms more broadly (Belsky et al., 2015, Dorn et al., 2008, Dorn et al., 1999, Mundy et al., 2015, Nottelmann et al., 1987, Shirtcliff et al., 2007, Sontag-Padilla et al., 2012, Susman et al., 1987); and 11 measured externalizing symptoms (Belsky et al., 2015, Dorn et al., 2008, Dorn et al., 1999, Mundy et al., 2015, Nottelmann et al., 1987, Shirtcliff et al., 2007, Sontag-Padilla et al., 2012, Susman et al., 1996, Susman et al., 1987, Van Goozen et al., 1998, Whittle et al., 2015). Of the studies that measured internalizing symptoms, two found that they were not associated with hormones (Klauser et al., 2015, Murray et al., 2016), one found a negative association (Shirtcliff et al., 2007), and seven found a positive association (Belsky et al., 2015, Dorn et al., 2008, Dorn et al., 1999, Mundy et al., 2015, Sontag-Padilla et al., 2012, Susman et al., 1996, Susman et al., 1987). Of the studies measuring externalizing symptoms, seven found that they were positively associated with hormones (Belsky et al., 2015, Dorn et al., 2008, Dorn et al., 1999, Mundy et al., 2015, Sontag-Padilla et al., 2012, Van Goozen et al., 1998, Whittle et al., 2015), and two found a negative association (Susman et al., 1996, Susman et al., 1987). Some studies measured diagnosis of a mental disorder from DSM criteria: three measured a large range of diagnoses (Dorn et al., 2008, Dorn et al., 1999, van Goozen et al., 2000), two measured Conduct or Oppositional Defiant Disorder (Constantino et al., 1993, Van Goozen et al., 1998), and two measured MDD (Goodyer et al., 2000, Goodyer et al., 1996). Several studies overlapped across measurement of diagnoses and/or types of symptoms.
We totaled studies that measured symptoms (internalizing vs. externalizing) by biological sex. We did not include studies that only measured diagnoses since there were not enough of those to ascertain patterns. If studies did not differentiate results by sex, but included both sexes, we counted the study as a result for males and females. The totals and the citations are listed in Table 2. For both males and females, a slight majority of studies found a positive association between hormones and both internalizing and externalizing symptoms. However, there were a significant number of conflicting studies that may be due to differences in age ranges and the method of measuring adrenarcheal status or timing compared to peers.
Table 2.
Cross tab description of studies measuring internalizing and externalizing symptoms by biological sex – direction of association with levels of adrenarcheal hormones.
3.2. Results of SR 2: adrenarcheal development and brain structure or function
Seven studies measured adrenarche or DHEA/DHEA-S during puberty and brain structure or function, summarized in Table 3 One study measured cortical thickness (CTh; Nguyen et al., 2013), one measured white and gray matter volume (Klauser et al., 2015), one measured pituitary volume (Murray et al., 2016), one measured white matter mean diffusivity using DTI (Menzies et al., 2015), and three measured brain activity during emotional tasks using fMRI (Goddings et al., 2012, Klapwijk et al., 2013, Whittle et al., 2015). Of note, Klapwijk et al. (2013) specifically measured functional connectivity during an emotional task. In these studies, levels of adrenarcheal hormones were positively associated with CTh in prefrontal areas (Nguyen et al., 2013) and volume of the pituitary (Murray et al., 2016), but negatively correlated with white matter volume in prefrontal areas (Klauser et al., 2015). Hormones were also positively associated with activity during an emotional face-viewing task in several prefrontal areas, the insula, and the striatum, especially for females (Whittle et al., 2015), and during social emotional processing compared to basic emotion processing in the left anterior temporal cortex (Goddings et al., 2012). However, two studies found no association between levels of DHEA/DHEA-S and either white matter diffusivity or functional connectivity (Klapwijk et al., 2013, Menzies et al., 2015). It should be noted that three studies (Klauser et al., 2015, Murray et al., 2016, Whittle et al., 2015) are all separate analyses from the same study of adrenarcheal timing discussed in the Results of SR 1 above (iCATS; Simmons et al., 2014). These three studies measured adrenarcheal timing by design, whereas the study by Nguyen et al. (2013) measured basal levels of hormones over the course of four years in participants aged four to 22 years of age, although the authors controlled for the effects of age in the regression models and also tested group differences at each age. Three studies (Goddings et al., 2012, Klapwijk et al., 2013, Menzies et al., 2015) did not specifically measure adrenarche (participants were aged 11–16 years and differences in puberty were likely measuring differences in gonadarche), however, results from Goddings et al. (2012) showed positive associations between levels of DHEA and brain activity during social emotional processing.
Table 3.
Summary of studies from Systematic Review 2 (SR2).
| Authors (year); PubMed ID | Sample | Measure(s) of adrenarche | Measure(s) of brain structure or function | Summary of findings |
|---|---|---|---|---|
|
Murray et al. (2016) PMID: 26600008 |
100 children who completed an MRI scan, out of 128 9-year-old children, selected based on high vs. low DHEA and testosterone levels in saliva 6 months prior | Continuous levels of DHEA, DHEA-S, and testosterone were measured again in saliva (averaged across 2 mornings); Tanner stage based on parent-report Sexual Maturity Status line drawings | Structural MRI to measure pituitary gland. | Pituitary volume was positively associated with levels of DHEA (r = 0.28, p < 0.01), DHEA-S (r = 0.32, p < 0.01), and testosterone (r = 0.24, p < 0.05). Note: Associations between adrenarche and anxiety symptoms were also found, reported in SR1. |
|
Whittle et al. (2015) PMID: 25678548 |
83 children who completed an fMRI task, out of 128 9-year-old children, selected based on high vs. low DHEA and testosterone levels in saliva 6 months prior | Continuous levels of DHEA were measured again in saliva (averaged across 2 mornings); Tanner stage (nuisance factor) based on parent-report Sexual Maturity Status line drawings | fMRI during an emotional face-viewing task. ROIs: amygdala, hippocampus, cingulate cortex, insula, dlPFC, and striatum | Levels of DHEA were negatively associated with activity in the right insula and mid-cingulate cortex during fear compared to calm faces, in the right mid-cingulate during angry compared to calm faces, and in the left dorsal cingulate during happy compared to calm faces. For females, these associations were present in the insula, cingulate, and dlPFC for fear faces, right mid-cingulate and putamen for angry faces, and insula and right cingulate for happy faces. Females also had a positive association between DHEA and activity in the left subgenual and right vmPFC for happy faces. No associations for males. Note: Associations between adrenarche and mental health symptoms were also found, reported in SR1. |
|
Klauser et al. (2015) PMID: 25459897 |
41 early developing (mean age 9.64 ± 0.35) and 44 late developing (9.48 ± 0.30) children based on DHEA and testosterone levels | Early vs. late adrenarche based on levels of DHEA and Testosterone approximately 6 months earlier; Continuous levels of DHEA and testosterone were measured again in saliva (averaged across 2 mornings); Tanner stage based on parent-report Sexual Maturity Status line drawings | Structural MRI to measure gray and white matter volume | Controlled for age, and for Tanner stage to exclude effect of early gonadarche. No group differences in total brain volume, total gray matter volume, total white matter volume, or regional gray matter volume in the whole sample, or for boys and girls separately. The early group had decreased white matter volume on left anterior corona radiata (frontal lobe) for the whole sample (peak t = 4.21, corrected p = 0.046), and in a subsample of males (peak t = 4.43, corrected p = 0.019), but not females. Current DHEA levels were also negatively correlated with white matter volume in left corona radiata in the whole sample (peak t = 3.98, corrected p = 0.022). |
|
Menzies et al. (2015) PMID: 25454416 |
61 boys aged 12.7–16.0 years | Levels of DHEA (as well as testosterone and estradiol) in saliva (for post hoc analyses). Authors also grouped boys into early-mid puberty (gonadarche) and late-post puberty via Tanner stage (for a priori analyses). | White matter microstructure (DTI): white matter mean diffusivity (MD) and fractional anisotropy (FA) | There were no associations between FA and gonadarche status based on Tanner stage, so no post hoc analyses were conducted for FA. For MD, there were no significant associations with DHEA (but there was a significant negative association with testosterone) |
|
Nguyen et al. (2013) PMID: 23804104 |
Longitudinal study of 255 healthy children from 4 to 22 years old (143 females, 112 males) | Levels DHEA (and testosterone for 234 participants) measured in saliva over the course of 4 years at each MRI visit; Self-report PDS used to differentiate between pre- and post-gonadarche participants |
Repeated MRI scans every 2 years – measured cortical thickness (CTh). | DHEA associated with increased CTh in left frontal, right temporal, and right parietal lobes in the pre-gonadarche group (r = 0.15). No significant DHEA-CTh associations in the post-gonadarche group. Age analysis showed DHEA associated with increased CTh between 4–13 years (all rs = 0.2): in the DLPFC from 4 to 8, right premotor cortex from 5 to 11, right temporoparietal junction from 7 to 12, and right entorhineal/perirhinal cortex from 4 to 13. After age 13, no significant associations. In the complete sample, significant interaction between DHEA and testosterone for the right ACC (r = 0.2). In the pre-gonadarche group, significant interaction between DHEA and testosterone in the right posterior cingulate gyrus and occipital pole (r = 0.3 for both). No significant interaction between DHEA and testosterone in the post-gonadarche group. No interactions with sex. |
|
Klapwijk et al. (2013) PMID: 23998674 |
35 female adolescents aged 11.1–13.7 years (mean 12.6; SD 0.7), 33 of which also had DHEA measured in saliva | Levels of DHEA in saliva (as well as testosterone and estradiol). Other separate indicators of puberty were Tanner stages and self-reported menarcheal status, and the latter two were combined to create early and late groups. | fMRI during an emotion evocation task (two social and two basic emotions); functional connectivity between the dmPFC and other social brain regions (pSTS, TPJ and ATC) | No associations between DHEA and functional connectivity. |
|
Goddings et al. (2012) PMID: 23106734 |
42 female adolescents mean age 12.5 years, range 11.1–13.7 years. | This study did not measure adrenarche specifically (early/late pubertal groups were based on Tanner stages and menarche – likely measures of pre/post gonadarche, especially in the age range of the sample), but they did measure salivary levels of testosterone, estradiol, and DHEA. Results are shown for DHEA associations only. | fMRI during emotional evocation (social vs. basic emotions). ROIs: MPFC, precuneus, right pSTS/TPJ, left ATC, left DMPFC. | Levels of DHEA positively associated with activity in the left anterior temporal cortex (ATC) during the social emotion tasks compared to the basic emotion tasks. |
As mentioned above, two out these five studies (Murray et al., 2016, Whittle et al., 2015) also measured mental health symptoms in relation to adrenarche and brain development (discussed in SR1). In particular, these studies assessed whether brain structure or function mediated the association between adrenarche and mental health symptoms. One of these studies (Whittle et al., 2015) found that, for females, higher levels of externalizing symptoms were associated with a medium to large effect on decreased activation in the anterior insula and a medium effect on increased activation in the ventromedial prefrontal cortex while viewing happy faces. Also, decreased activity in the insula while viewing happy faces partially mediated the association between higher levels of DHEA and higher levels of externalizing symptoms in females. The other study (Murray et al., 2016) found that larger pituitary volumes were associated with a medium effect on higher levels of social anxiety symptoms. Mediation analyses showed that when controlling for age, sex, and BMI, larger pituitary volumes partially mediated the association between higher levels of DHEA/DHEA-S and higher levels of social anxiety symptoms.
4. Discussion
Overall, this review suggests that measures of adrenarche are associated with mental health symptoms or diagnoses. Brain development may play a role in this association, but studies are limited. However, future research may be justified in testing a model by which changes in brain development might mediate this association between adrenarche and mental health. In general, studies showed that earlier timing of adrenarche and higher levels of adrenarcheal hormone levels were associated with more mental health problems, but there were a number of conflicting studies that may be due to differences in methodology. For studies that examined results by sex, a pattern of results is beginning to emerge for females whereby earlier timing of adrenarche (or higher levels of adrenarcheal hormones) is associated with greater mental health problems. There is less research on males, but findings thus far are similar. Some studies found that higher levels of adrenarcheal hormones were associated with higher levels of internalizing symptoms, either anxiety symptoms (Susman et al., 1996) or emotional symptoms more broadly (Mundy et al., 2015). Other studies found that higher levels of hormones were associated with externalizing disorders and symptoms (Mundy et al., 2015, Van Goozen et al., 1998), although not always in the expected direction (Susman et al., 1987). More studies that include both boys and girls and examine sex differences will help to elucidate if patterns remain similar across sexes.
Only seven studies measured adrenarche and brain structure or function, and each study measured a different aspect of the brain (function (ROI or connectivity), volume, diffusion, or CTh). Therefore, strong amalgamation of findings is not possible; however, patterns are beginning to emerge. The limited data from these studies of adrenarcheal hormones, brain structure and function, and mental health symptoms shows that associations between these variables are likely to be present and suggest that further work in this area is justified. In particular, both studies that examined mediational paths (albeit cross-sectional) found that measures of brain structure and function mediated the association between adrenarche and mental health symptoms (Murray et al., 2016, Whittle et al., 2015).
There are several methodological issues that need to be addressed in future research on adrenarche, brain development, and mental health. First, as discussed above, studies should have enough power to analyze results by sex. There is some suggestion that the prevalence of PA (Utriainen et al., 2015) and the general timing of adrenarche may be different for girls and boys (e.g., the increase in DHEA appears to continue longer into adulthood for males compared to females; Worthman, 1999), and previous research has also suggested that risk for mental health problems is sex differentiated, especially for depression (for review, see Piccinelli and Wilkinson, 2000), and that this differentiation emerges at gonadarche (Angold et al., 1998, Patton et al., 1996). Therefore, given these possible differences in developmental timing, examining effects uniquely by sex is critical to understanding unique risk trajectories for mental health problems.
Second, as outlined in the results, some studies measured stage and others measured timing (stage compared to same age- and sex-peers). Within those that measured timing, studies defined timing in different ways – either by comparing levels of hormones in a restricted age range, or by controlling for age a covariate in statistical models. Relatedly, the issue of clinical vs. normal variation in adrenarcheal development needs to be considered. Some studies measured normal variations in adrenarche while others included participants that had been clinically diagnosed with PA and compared them to normally developing controls. None measured tempo of adrenarche, which is the rate of maturation. Studies of pubertal (gonadarche) tempo have shown that faster tempo are associated with more mental health symptoms in both boys (Mendle et al., 2010) and girls (Marceau et al., 2011). Therefore, tempo may also be an especially salient factor in the association between adrenarche and mental health.
Third, it is not yet clear if it is better to measure adrenarche with physical characteristics (e.g., physical signs such as presence of pubic hair), with biological hormones such as DHEA/DHEA-S, or a combination of both. For example, of those studies that measured hormones and Tanner stage, most found conflicting results for associations with mental health. Murray et al. (2016) found that anxiety was associated with earlier Tanner stage (i.e., less developed) but not with levels of hormones. Whittle et al. (2015) found that for females, both later Tanner stage and higher levels of hormones (i.e., more developed) were associated with more externalizing symptoms, but for males, only earlier Tanner stage (i.e., less developed) was associated with more internalizing symptoms. Klauser et al. (2015) controlled for Tanner stage and found no associations between mental health symptoms and levels of hormones. van Goozen et al. (2000) found earlier Tanner stage (i.e., less developed) to be associated with externalizing disorder, but when controlling for age and Tanner stage, higher levels of hormones were associated with externalizing disorder. On the other hand, several studies that selected PA vs. on-time children based on Tanner pubic hair stage confirmed that PA children had higher levels of adrenarcheal hormones (Dorn et al., 2008, Dorn et al., 1999, Sontag-Padilla et al., 2012). It is likely that adrenarcheal hormones do not produce some physical changes until gonadarche (Wan, 2012), and therefore it is not possible to separate adrenarche and gonadarche by measuring physical changes alone. On the other hand, although cutoff values for DHEA-S in serum have recently been proposed to match age of onset of biochemical adrenarche with the physical manifestation (pubarche; Guran et al., 2015), others have suggested that these DHEA-S reference values cannot be validated without longitudinal research (see commentary by Uçar, 2015). Future studies should measure both adrenarcheal hormones and physical characteristics (especially pubic hair stage) in order to further understand how these two variables are related to development.
Fourth, concurrent DHEA/DHEA-S and mental health assessment may show DHEA/DHEA-S reactivity to stress and mental health disorder, rather than the measurement of adrenarcheal levels being indicative of a pubertal process alone. For example, animal research has shown that both acute and chronic stress are associated with increases in DHEA-S (Maninger et al., 2010). Much of the earlier work in this area was not necessarily designed to measure DHEA/DHEA-S as a marker of adrenarcheal development, and the vast majority of studies reviewed here measured adrenarcheal hormones and mental health symptoms or disorder at the same time (Constantino et al., 1993, Dorn et al., 2008, Dorn et al., 1999, Goodyer et al., 1996, Klauser et al., 2015, Mundy et al., 2015, Murray et al., 2016, Nottelmann et al., 1987, Shirtcliff et al., 2007, Sontag-Padilla et al., 2012, Susman et al., 1996, Susman et al., 1987, van Goozen et al., 2000, Van Goozen et al., 1998, Whittle et al., 2015). Indeed, only two studies measured mental health outcomes longitudinally. One found that in girls, earlier adrenarcheal timing at ages six to seven was associated with more internalizing and externalizing symptoms at age 18 (Belsky et al., 2015). Interestingly, this study also found that prenatal stress predicted earlier timing in these girls at age six to seven. However, another study found that baseline DHEA levels did not predict the emergence of MDD over a year-long follow-up (Goodyer et al., 2000). Further longitudinal research is needed to elucidate the associations between stress, mental health, and adrenarcheal hormones.
Fifth, there is currently no consensus on an “end point” of adrenarche. This is due to the gradual increase of adrenarcheal hormones, ending in early adulthood. Therefore, it is difficult to assess when a sensitive period for brain development may end. It is possible that adrenarche “ends” in early adulthood; however, a sensitive period for brain development and the onset of mental illness may end earlier than that. For example, the most sensitive period for brain development may be closer to the onset of adrenarche, especially if adrenarche is seen as an event (i.e., when the sharp increase in adrenarcheal hormones begins) rather than a period of time. In other words, although DHEA and DHEA-S continue to increase steadily across adolescence and into early adulthood, it is possible that the initial increase of these hormones is more salient for brain and psychological development. However, this has not been empirically tested. Again, longitudinal research could measure levels of hormones, brain development, and mental health across childhood (from age five) and adolescence (up to the early 20s) to answer some of these questions.
Sixth, these studies measured different types of adrenarcheal hormone(s). Although adrenarche is most commonly defined as increases in secretion of DHEA and DHEA-S, there are other metabolites of this process that are related to adrenarcheal development, such as testosterone, that may also be associated with brain development and mental health. Furthermore, although DHEA and DHEA-S are associated, there is a diurnal variation in DHEA (Hucklebridge et al., 2005) that does not appear to be synchronous with variation in DHEA-S (Carlström et al., 2002). Therefore, studies that measure one of these hormones without the other may not be directly comparable, and the reasons for measuring one or the other are not clear from many of the research studies reviewed here. Future research should either measure both DHEA and DHEA-S or have clear, justified hypotheses about one of the hormones. It is not yet clear from the synthesized research if one or the other is more salient in brain or mental health development. Furthermore, as with cortisol, there is evidence that diurnal DHEA hyper-/hypo-secretion may be important: one study reviewed here found that low morning levels of DHEA were associated with MDD in children eight to 16 years of age (Goodyer et al., 1996). Additionally, interactions between hormones may be important. For example, research has shown that DHEA/DHEA-S and cortisol interact to predict mental health symptoms in adults (Goodyer et al., 1998, Michael et al., 2000). Interactions between hypothalamic–pituitary–adrenal (HPA) and hypothalamic–pituitary–gonadal (HPG) axes seem to be especially salient for adolescent mental health (for review, see Marceau et al., 2015). In the current review, Nguyen et al. (2013) found that levels of DHEA interacted with levels of testosterone to predict cortical thickness of the right cingulate cortex and occipital pole, especially in participants that were pre-pubertal on measures of physical development. Therefore, future studies should consider measuring how adrenarcheal hormones interact with other hormones such as cortisol or testosterone.
Seventh, the term “mental health problems” is broad. Some of the results differed depending on the type of psychopathology, especially regarding internalizing compared to externalizing symptoms (e.g., Susman et al., 1996, Susman et al., 1987). Furthermore, it is unclear whether adrenarche is associated with threshold, clinical-level diagnoses of a mental illness, or if it is associated with subclinical variations in mental health symptoms. Subclinical symptoms for many mental illnesses in adolescence, such as depressive symptoms, often predict the development of clinical episodes later in life (Pine et al., 1999). However, future research should examine both levels of symptoms, which can be measured via self- or parent-report questionnaires, and clinical diagnoses, which can be measured via established clinical cut off scores on questionnaires, or by semi-structured diagnostic interviews administered by trained researchers (usually considered the gold standard).
Finally, longitudinal studies in this area are especially critical. Only studies that measure changes in adrenarche over time are able to assess the importance of adrenarcheal tempo compared to stage or timing. Also, in order to formally test brain development as a mediator of the association between adrenarche and mental health, longitudinal studies that measure adrenarcheal development, brain structure and function, and mental health across time are needed. Although we reviewed two longitudinal studies (Belsky et al., 2015, Nguyen et al., 2013), neither examined all three variables.
Nevertheless, based on an integration of the literature reviewed here, we propose a hypothetical model of adrenarche as a potentially sensitive period of neurobiological development (refer to Fig. 3). In this model, timing of exposure to adrenarcheal hormones interacts with biological sex to influence changes in brain development (a), which, in turn, are associated with risk for mental health problems (b), the type of which can also interact with sex. We note that brain development might only be one of many indirect pathways from adrenarcheal timing to mental health outcomes, but we argue that the existing evidence justifies this as the next step for research to test empirically. Importantly, this model also indicates that these processes could potentially be both influenced by stress, especially early stressful family environments (c), as well as influence environmental sensitivity to psychosocial stress and increase risk for mental health problems (d). For example, for path c in the model, life history theory suggests that humans are sensitive especially to early childhood experiences and environments such that increased stress early in life may alter biological processes to produce different reproductive strategies (Charnov, 1993, Roff, 1992, Stearns, 1992). This environmental stress early in life may act as a cue to biological systems to favor earlier reproduction. Indeed, one longitudinal study showed that more stressful early childhood environments were associated with earlier timing of adrenarche as measured by adrenal hormones (Ellis and Essex, 2007). Furthermore, for path d in the model, early timing of adrenarche could render an individual sensitive to stress via effects on brain development, especially psychosocial stress, as one study showed that levels of adrenarcheal hormones were associated with brain activity specifically during social emotional processing (Goddings et al., 2012). Earlier timing could be related to potentially maladaptive patterns of brain development, and therefore result in greater sensitivity to stress. However, this hypothetical pathway remains to be empirically tested.
Fig. 3.
Proposed model of adrenarche as a potential sensitive period of neurobiological and psychosocial development.
To comprehensively test this model, future research should be longitudinal and prospective in nature, and should objectively measure early family environments and ongoing psychosocial stress. Multiple measurements of adrenarcheal hormones and physical characteristics across time are needed to assess both timing and tempo of development. Measures of brain structure and function, and of mental health problems, should also be assessed multiple times to allow examination of change in these variables and to formally test brain development as a mediator of the association between adrenarche and mental health (i.e., an indirect association of adrenarche and mental health via brain development). Furthermore, this research should test conditional indirect effects whereby biological sex may moderate this indirect association of adrenarche and mental health, either in the association between adrenarche and brain development, or between brain development and mental health. Also, psychosocial stress should be tested as a moderator in the association between brain development and mental health.
5. Conclusions
This review highlights the small but growing number of research studies that show an emerging pattern whereby timing of adrenarche is associated with increased risk for mental health problems. It also highlights the few studies that have begun to examine brain development as a potential mechanism of this association. Future longitudinal research that formally tests mediation models, considers biological sex and psychosocial stress as moderators, and assesses early life stress may provide evidence to support adrenarche as a sensitive period for neurobiological development. This research will have important clinical implications and may identify novel risk and protective factors for mental health problems across adolescence.
Source of funding
Sarah Whittle was supported by a National Health and Medial Council (NHMRC) Career Development Fellowship (ID: 1007716).
Conflict of interest
None.
Footnotes
We did not include a specific upper age limit to define the end of adrenarche because there is currently no consensus on the end status of adrenarche.
References
- Andersen S.L., Teicher M.H. Stress, sensitive periods and maturational events in adolescent depression. Trends Neurosci. 2008;31:183–191. doi: 10.1016/j.tins.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Angold A., Costello E.J. Puberty and depression. Child Adolesc. Psychiatr. Clin. N. Am. 2006;15:919–937. doi: 10.1016/j.chc.2006.05.013. ix. [DOI] [PubMed] [Google Scholar]
- Angold A., Costello E.J., Worthman C.M. Puberty and depression: the roles of age, pubertal status and pubertal timing. Psychol. Med. 1998;28:51–61. doi: 10.1017/s003329179700593x. [DOI] [PubMed] [Google Scholar]
- Belsky J., Ruttle P.L., Boyce W.T., Armstrong J.M., Essex M.J. Early adversity, elevated stress physiology, accelerated sexual maturation, and poor health in females. Dev. Psychol. 2015;51:816–822. doi: 10.1037/dev0000017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore S.-J., Burnett S., Dahl R.E. The role of puberty in the developing adolescent brain. Hum. Brain Mapp. 2010;31:926–933. doi: 10.1002/hbm.21052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell B. Adrenarche and the evolution of human life history. Am. J. Hum. Biol. 2006;18:569–589. doi: 10.1002/ajhb.20528. [DOI] [PubMed] [Google Scholar]
- Campbell B. Adrenarche in comparative perspective. Am. J. Hum. Biol. 2011;23:44–52. doi: 10.1002/ajhb.21111. [DOI] [PubMed] [Google Scholar]
- Carlström K., Karlsson R., Von Schoultz B. Diurnal rhythm and effects of oral contraceptives on serum dehydroepiandrosterone sulfate (DHEAS) are related to alterations in serum albumin rather than to changes in adrenocortical steroid secretion. Scand. J. Clin. Lab. Invest. 2002;62:361–368. doi: 10.1080/00365510260296519. [DOI] [PubMed] [Google Scholar]
- Charnov E.L. Oxford University Press; Oxford, England: 1993. Life History Invariants. [Google Scholar]
- Conley A.J., Bernstein R.M., Nguyen A.D. Adrenarche in nonhuman primates: the evidence for it and the need to redefine it. J. Endocrinol. 2012;214:121–131. doi: 10.1530/JOE-11-0467. [DOI] [PubMed] [Google Scholar]
- Constantino J.N., Grosz D., Saenger P., Chandler D.W., Nandi R., Earls F.J. Testosterone and aggression in children. J. Am. Acad. Child Adolesc. Psychiatry. 1993;32:1217–1222. doi: 10.1097/00004583-199311000-00015. [DOI] [PubMed] [Google Scholar]
- Copeland W., Shanahan L., Miller S., Costello E.J., Angold A., Maughan B. Outcomes of early pubertal timing in young women: a prospective population-based study. Am. J. Psychiatry. 2010;167:1218–1225. doi: 10.1176/appi.ajp.2010.09081190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Counts D.R., Pescovitz O.H., Barnes K.M., Hench K.D., Chrousos G.P., Sherins R.J., Comite F., Loriaux D.L., Cutler G.B. Dissociation of adrenarche and gonadarche in precocious puberty and in isolated hypogonadotropic hypogonadism. J. Clin. Endocrinol. Metab. 1987;64:1174–1178. doi: 10.1210/jcem-64-6-1174. [DOI] [PubMed] [Google Scholar]
- Crone E.A., Dahl R.E. Understanding adolescence as a period of social–affective engagement and goal flexibility. Nat. Rev. Neurosci. 2012;13:636–650. doi: 10.1038/nrn3313. [DOI] [PubMed] [Google Scholar]
- Cunningham M.G., Bhattacharyya S., Benes F.M. Amygdalo-cortical sprouting continues into early adulthood: implications for the development of normal and abnormal function during adolescence. J. Comp. Neurol. 2002;453:116–130. doi: 10.1002/cne.10376. [DOI] [PubMed] [Google Scholar]
- Dorn L.D., Chrousos G.P. The neurobiology of stress: understanding regulation of affect during female biological transitions. In: Seminars in Reproductive Endocrinology. 1997 doi: 10.1055/s-2008-1067965. [DOI] [PubMed] [Google Scholar]
- Dorn L.D., Hitt S.F., Rotenstein D., Maloney M.J. Biopsychological and cognitive differences in children with premature vs on-time adrenarche. J. Am. Acad. Child Adolesc. Psychiatry. 1999;38:1062. doi: 10.1001/archpedi.153.2.137. [DOI] [PubMed] [Google Scholar]
- Dorn L.D., Rose S.R., Rotenstein D., Susman E.J., Huang B., Loucks T.L., Berga S.L. Differences in endocrine parameters and psychopathology in girls with premature adrenarche versus on-time adrenarche. J. Pediatr. Endocrinol. Metab. 2008;21:439–448. doi: 10.1515/jpem.2008.21.5.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorn L.D. Measuring puberty. J. Adolesc. Health. 2006;39:625–626. doi: 10.1016/j.jadohealth.2006.05.014. [DOI] [PubMed] [Google Scholar]
- Ellis B.J., Essex M.J. Family environments, adrenarche, and sexual maturation: a longitudinal test of a life history model. Child Dev. 2007;78:1799–1817. doi: 10.1111/j.1467-8624.2007.01092.x. [DOI] [PubMed] [Google Scholar]
- Ge X., Conger R.D., Elder G.H., Jr. The relation between puberty and psychological distress in adolescent boys. J. Res. Adolesc. 2001;11:49–70. [Google Scholar]
- Ge X., Conger R.D., Elder G.H. Pubertal transition, stressful life events, and the emergence of gender differences in adolescent depressive symptoms. Dev. Psychol. 2001;37:404–417. doi: 10.1037//0012-1649.37.3.404. [DOI] [PubMed] [Google Scholar]
- Giedd J.N., Blumenthal J., Jeffries N.O., Castellanos F.X., Liu H., Zijdenbos A., Paus T., Evans A.C., Rapoport J.L. Brain development during childhood and adolescence: a longitudinal MRI study. Nat. Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Goddings A.L., Burnett Heyes S., Bird G., Viner R.M., Blakemore S.J. The relationship between puberty and social emotion processing. Dev. Sci. 2012;15:801–811. doi: 10.1111/j.1467-7687.2012.01174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N., Giedd J.N., Lusk L., Hayashi K.M., Greenstein D., Vaituzis A.C., Nugent T.F., Herman D.H., Clasen L.S., Toga A.W., Rapoport J.L., Thompson P.M. Dynamic mapping of human cortical development during childhood through early adulthood. Proc. Natl. Acad. Sci. U. S. A. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodyer I.M., Herbert J., Altham P.M.E., Pearson J., Secher S.M., Shiers H.M. Adrenal secretion during major depression in 8- to 16-year-olds, I. Altered diurnal rhythms in salivary cortisol and dehydroepiandrosterone (DHEA) at presentation. Psychol. Med. 1996;26:245. doi: 10.1017/s0033291700034644. [DOI] [PubMed] [Google Scholar]
- Goodyer I.M., Herbert J., Altham P.M. Adrenal steroid secretion and major depression in 8- to 16-year-olds, III. Influence of cortisol/DHEA ratio at presentation on subsequent rates of disappointing life events and persistent major depression. Psychol. Med. 1998;28:265–273. doi: 10.1017/s0033291797006314. [DOI] [PubMed] [Google Scholar]
- Goodyer I.M., Herbert J., Tamplin A., Altham P.M.E. Recent life events, cortisol, dehydroepiandrosterone and the onset of major depression in high-risk adolescents. Br. J. Psychiatry. 2000;177:499–504. doi: 10.1192/bjp.177.6.499. [DOI] [PubMed] [Google Scholar]
- Graber J.A., Lewinsohn P.M., Seeley J.R., Brooks-Gunn J. Is psychopathology associated with the timing of pubertal development? J. Am. Acad. Child Adolesc. Psychiatry. 1997;36:1768–1776. doi: 10.1097/00004583-199712000-00026. [DOI] [PubMed] [Google Scholar]
- Graber J.A., Seeley J.R., B.-G J., Lewinsohn P.M. Is pubertal timing associated with psychopathology in young adulthood? J. Am. Acad. Child Adolesc. Psychiatry. 2004;43:718–726. doi: 10.1097/01.chi.0000120022.14101.11. [DOI] [PubMed] [Google Scholar]
- Guran T., Firat I., Yildiz F., Kaplan Bulut I., Dogru M., Bereket A. Reference values for serum dehydroepiandrosterone-sulphate in healthy children and adolescents with emphasis on the age of adrenarche and pubarche. Clin. Endocrinol. (Oxf.) 2015;82:712–718. doi: 10.1111/cen.12612. [DOI] [PubMed] [Google Scholar]
- Hayward C., Killen J.D., Hammer L.D., Litt I.F., Wilson D.M., Simmonds B., Taylor C.B. Pubertal stage and panic attack history in sixth- and seventh-grade girls. Am. J. Psychiatry. 1992;149:1239–1243. doi: 10.1176/ajp.149.9.1239. [DOI] [PubMed] [Google Scholar]
- Hucklebridge F., Hussain T., Evans P., Clow A. The diurnal patterns of the adrenal steroids cortisol and dehydroepiandrosterone (DHEA) in relation to awakening. Psychoneuroendocrinology. 2005;30:51–57. doi: 10.1016/j.psyneuen.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Ibáñez L., Ong K., Dunger D.B., de Zegher F. Early development of adiposity and insulin resistance after catch-up weight gain in small-for-gestational-age children. J. Clin. Endocrinol. Metab. 2006;91:2153–2158. doi: 10.1210/jc.2005-2778. [DOI] [PubMed] [Google Scholar]
- Idkowiak J., Lavery G.G., Dhir V., Barrett T.G., Stewart P.M., Krone N., Arlt W. Premature adrenarche: novel lessons from early onset androgen excess. Eur. J. Endocrinol. 2011;165:189–207. doi: 10.1530/EJE-11-0223. [DOI] [PubMed] [Google Scholar]
- Kaltiala-Heino R., Kosunen E., Rimpelä M. Pubertal timing, sexual behaviour and self-reported depression in middle adolescence. J. Adolesc. 2003;26:531–545. doi: 10.1016/s0140-1971(03)00053-8. [DOI] [PubMed] [Google Scholar]
- Kaltiala-Heino R., Marttunen M., Rantanen P., Rimpelä M. Early puberty is associated with mental health problems in middle adolescence. Soc. Sci. Med. 2003;57:1055–1064. doi: 10.1016/s0277-9536(02)00480-x. [DOI] [PubMed] [Google Scholar]
- Kesek, A., Zelazo, P.D., Lewis, M.D., 2009. The development of executive cognitive function and emotion regulation in adolescence. In: Allen, N.B., Sheeber, L. (Eds.), Adolescent Emotional Development and the Emergence of Depressive Disorders, pp. 135–155.
- Klapwijk E.T., Goddings A.L., Burnett Heyes S., Bird G., Viner R.M., Blakemore S.J. Increased functional connectivity with puberty in the mentalising network involved in social emotion processing. Horm. Behav. 2013;64:3140322. doi: 10.1016/j.yhbeh.2013.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klauser P., Whittle S., Simmons J.G., Byrne M.L., Mundy L.K., Patton G.C., Fornito A., Allen N.B. Reduced frontal white matter volume in children with early onset of adrenarche. Psychoneuroendocrinology. 2015;52:111–118. doi: 10.1016/j.psyneuen.2014.10.020. [DOI] [PubMed] [Google Scholar]
- Ladouceur C.D., Peper J.S., Crone E.A., Dahl R.E. White matter development in adolescence: the influence of puberty and implications for affective disorders. Dev. Cogn. Neurosci. 2012;2:36–54. doi: 10.1016/j.dcn.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynne S.D., Graber J.A., Nichols T.R., Brooks-Gunn J., Botvin G.J. Links between pubertal timing, peer influences, and externalizing behaviors among urban students followed through middle school. J. Adolesc. Health. 2007;40 doi: 10.1016/j.jadohealth.2006.09.008. 181.e7-181.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maninger N., Wolkowitz O.M., Reus V.I., Epel E.S., Mellon S.H. Neurobiological and neuropsychiatric effects of dehydroepiandrosterone (DHEA) and DHEA sulfate (DHEAS) Front. Neuroendocrinol. 2009;30:65–91. doi: 10.1016/j.yfrne.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maninger N., Capitanio J.P., Mason W.A., Ruys J.D., Mendoza S.P. Acute and chronic stress increase DHEAS concentrations in rhesus monkeys. Psychoneuroendocrinology. 2010;35:1055–1062. doi: 10.1016/j.psyneuen.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marceau K., Ram N., Houts R.M., Grimm K.J., Susman E.J. Individual differences in boys’ and girls’ timing and tempo of puberty: modeling development with nonlinear growth models. Dev. Psychol. 2011;47:1389–1409. doi: 10.1037/a0023838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marceau K., Ruttle P.L., Shirtcliff E.A., Essex M.J., Susman E.J. Developmental and contextual considerations for adrenal and gonadal hormone functioning during adolescence: implications for adolescent mental health. Dev. Psychobiol. 2015;57:742–768. doi: 10.1002/dev.21214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendle J., Harden K.P., Brooks-Gunn J., Graber J.A. Development’s tortoise and hare: pubertal timing, pubertal tempo, and depressive symptoms in boys and girls. Dev. Psychol. 2010;46:1341–1353. doi: 10.1037/a0020205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzies L., Goddings A.L., Whitaker K.J., Blakemore S.J., Viner R.M. The effects of puberty on white matter development in boys. Dev. Cogn. Neurosci. 2015;11:116–128. doi: 10.1016/j.dcn.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael A., Jenaway A., Paykel E.S., Herbert J. Altered salivary dehydroepiandrosterone levels in major depression in adults. Biol. Psychiatry. 2000;48:989–995. doi: 10.1016/s0006-3223(00)00955-0. [DOI] [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J., Altman D.G., The PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriguchi S., Yamamoto Y., Ikuno T., Fukunaga K. Sigma-1 receptor stimulation by dehydroepiandrosterone ameliorates cognitive impairment through activation of CaM kinase II, protein kinase C and extracellular signal-regulated kinase in olfactory bulbectomized mice. J. Neurochem. 2011;117:879–891. doi: 10.1111/j.1471-4159.2011.07256.x. [DOI] [PubMed] [Google Scholar]
- Moriguchi S., Shinoda Y., Yamamoto Y., Sasaki Y., Miyajima K., Tagashira H., Fukunaga K. Stimulation of the sigma-1 receptor by DHEA enhances synaptic efficacy and neurogenesis in the hippocampal dentate gyrus of olfactory bulbectomized mice. PLoS One. 2013;8:1–10. doi: 10.1371/journal.pone.0060863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundy L.K., Simmons J.G., Allen N.B., Viner R.M., Bayer J.K., Olds T., Williams J., Olsson C., Romaniuk H., Mensah F., Sawyer S.M., Degenhardt L., Alati R., Wake M., Jacka F., Patton G.C. Study protocol: the Childhood to Adolescence Transition Study (CATS) BMC Pediatr. 2013;13:160. doi: 10.1186/1471-2431-13-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundy L.K., Romaniuk H., Canterford L., Hearps S., Viner R.M., Bayer J.K., Simmons J.G., Carlin J.B., Allen N.B., Patton G.C. Adrenarche and the emotional and behavioral problems of late childhood. J. Adolesc. Health. 2015;57:608–616. doi: 10.1016/j.jadohealth.2015.09.001. [DOI] [PubMed] [Google Scholar]
- Murray C.R., Simmons J.G., Allen N.B., Byrne M.L., Mundy L.K., Seal M.L., Patton G.C., Olsson C.A., Whittle S. Associations between dehydroepiandrosterone (DHEA) levels, pituitary volume, and social anxiety in children. Psychoneuroendocrinology. 2016;64:31–39. doi: 10.1016/j.psyneuen.2015.11.004. [DOI] [PubMed] [Google Scholar]
- Mutlu A.K., Schneider M., Debbané M., Badoud D., Eliez S., Schaer M. Sex differences in thickness, and folding developments throughout the cortex. Neuroimage. 2013;82:200–207. doi: 10.1016/j.neuroimage.2013.05.076. [DOI] [PubMed] [Google Scholar]
- Muzik O., Janisse J., Ager J., Shen C., Chugani D.C., Chugani H.T. A mathematical model for the analysis of cross-sectional brain glucose metabolism data in children. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 1999;23:589–600. doi: 10.1016/s0278-5846(99)00018-4. [DOI] [PubMed] [Google Scholar]
- Nguyen T.-V., McCracken J.T., Ducharme S., Cropp B.F., Botteron K.N., Evans A.C., Karama S. Interactive effects of dehydroepiandrosterone and testosterone on cortical thickness during early brain development. J. Neurosci. 2013;33:10840–10848. doi: 10.1523/JNEUROSCI.5747-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nottelmann E.D., Susman E.J., Inoff-Germain G., Cutler G.B., Loriaux D.L., Chrousos G.P. Developmental processes in early adolescence: relationships between adolescent adjustment problems and chronologic age, pubertal stage, and puberty-related serum hormone levels. J. Pediatr. 1987;110:473–480. doi: 10.1016/s0022-3476(87)80521-8. [DOI] [PubMed] [Google Scholar]
- Oldehinkel A.J., Verhulst F.C., Ormel J. Mental health problems during puberty: Tanner stage-related differences in specific symptoms. The TRAILS study. J. Adolesc. 2011;34:73–85. doi: 10.1016/j.adolescence.2010.01.010. [DOI] [PubMed] [Google Scholar]
- Parker L.N., Sack J., Fisher D.A., Odell W.D. The adrenarche: prolactin, gonadotropins, adrenal androgens, and cortisol. J. Clin. Endocrinol. Metab. 1978;46:396–401. doi: 10.1210/jcem-46-3-396. [DOI] [PubMed] [Google Scholar]
- Patton G.C., Hibbert M.E., Carlin J., Shao Q., Rosier M., Caust J., Bowes G. Menarche and the onset of depression and anxiety in Victoria, Australia. J. Epidemiol. Community Health. 1996;50:661–666. doi: 10.1136/jech.50.6.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T., Keshavan M., Giedd J.N. Why do many psychiatric disorders emerge during adolescence? Nat. Rev. Neurosci. 2008;9:947–957. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccinelli M., Wilkinson G. Gender differences in depression: critical review. Br. J. Psychiatry. 2000;177:486–492. doi: 10.1192/bjp.177.6.486. [DOI] [PubMed] [Google Scholar]
- Pine D.S., Cohen E., Cohen P., Brook J. Adolescent depressive symptoms as predictors of adult depression: moodiness or mood disorder? Am. J. Psychiatry. 1999;156:133–135. doi: 10.1176/ajp.156.1.133. [DOI] [PubMed] [Google Scholar]
- Rainey W.E., Carr B.R., Sasano H., Suzuki T., Mason J.I. Dissecting human adrenal androgen production. Trends Endocrinol. Metab. 2002;13:234–239. doi: 10.1016/s1043-2760(02)00609-4. [DOI] [PubMed] [Google Scholar]
- Remer T., Boye K.R., Hartmann M.F., Wudy S.A. Urinary markers of adrenarche: reference values in healthy subjects, aged 3–18 years. J. Clin. Endocrinol. Metab. 2005;90:2015–2021. doi: 10.1210/jc.2004-1571. [DOI] [PubMed] [Google Scholar]
- Roff D. Chapman and Hall; New York: 1992. The Evolution of Life Histories: Theory and Analysis. [Google Scholar]
- Shirtcliff E.A., Zahn-Waxler C., Klimes-Dougan B., Slattery M. Salivary dehydroepiandrosterone responsiveness to social challenge in adolescents with internalizing problems. J. Child Psychol. Psychiatry. 2007;48:580–591. doi: 10.1111/j.1469-7610.2006.01723.x. [DOI] [PubMed] [Google Scholar]
- Simmons J.G., Whittle S.L., Patton G.C., Dudgeon P., Olsson C., Byrne M.L., Mundy L.K., Seal M.L., Allen N.B. Study protocol: imaging brain development in the Childhood to Adolescence Transition Study (iCATS) BMC Pediatr. 2014;14:115. doi: 10.1186/1471-2431-14-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sontag-Padilla L.M., Dorn L.D., Tissot A., Susman E.J., Beers S.R., Rose S.R. Executive functioning, cortisol reactivity, and symptoms of psychopathology in girls with premature adrenarche. Dev. Psychopathol. 2012;24:211–223. doi: 10.1017/S0954579411000782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear L.P. The adolescent brain and age-related behavioral manifestations. Neurosci. Biobehav. Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Sripada R.K., Marx C.E., King A.P., Rajaram N., Garfinkel S.N., Abelson J.L., Liberzon I. DHEA enhances emotion regulation neurocircuits and modulates memory for emotional stimuli. Neuropsychopharmacology. 2013;38:1798–1807. doi: 10.1038/npp.2013.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stattin H., Magnusson D. Lawrence Erlbaum Associates, Inc; Hillsdale, NJ: 1990. Pubertal Maturation in Female Development. [Google Scholar]
- Stearns S. Oxford University Press; Oxford, England: 1992. The Evolution of Life Histories. [Google Scholar]
- Sulcová J., Hill M., Hampl R., Stárka L. Age and sex related differences in serum levels of unconjugated dehydroepiandrosterone and its sulphate in normal subjects. J. Endocrinol. 1997;154:57–62. doi: 10.1677/joe.0.1540057. [DOI] [PubMed] [Google Scholar]
- Susman E.J., Inoff-Germain G., Nottelmann E.D., Loriaux D.L., Cutler G.B., Chrousos G.P. Hormones, emotional dispositions, and aggressive attributes in young adolescents. Child Dev. 1987;58:1114–1134. [PubMed] [Google Scholar]
- Susman E.J., Granger D.A., Murowchick E., Ponirakis A., Worrall B.K. Gonadal and adrenal hormones. Developmental transitions and aggressive behavior. Ann. N. Y. Acad. Sci. 1996;794:18–30. doi: 10.1111/j.1749-6632.1996.tb32506.x. [DOI] [PubMed] [Google Scholar]
- Tamnes C.K., Østby Y., Fjell A.M., Westlye L.T., Due-Tønnessen P., Walhovd K.B. Brain maturation in adolescence and young adulthood: regional age-related changes in cortical thickness and white matter volume and microstructure. Cereb. Cortex. 2010;20:534–548. doi: 10.1093/cercor/bhp118. [DOI] [PubMed] [Google Scholar]
- Uçar A. Age-related serum dehydroepiandrosterone sulphate (DHEAS) levels per se should not be considered a reliable surrogate parameter for clinical presentation of adrenarche. Clin. Endocrinol. (Oxf.) 2015;82:912–913. doi: 10.1111/cen.12689. [DOI] [PubMed] [Google Scholar]
- Utriainen P., Laakso S., Liimatta J., Jääskeläinen J., Voutilainen R. Premature adrenarche – a common condition with variable presentation. Horm. Res. Paediatr. 2015;83:221–231. doi: 10.1159/000369458. [DOI] [PubMed] [Google Scholar]
- van Goozen S.H., van den Ban E., Matthys W., Cohen-Kettenis P.T., Thijssen J.H., van Engeland H. Increased adrenal androgen functioning in children with oppositional defiant disorder: a comparison with psychiatric and normal controls. J. Am. Acad. Child Adolesc. Psychiatry. 2000;39:1446–1451. doi: 10.1097/00004583-200011000-00020. [DOI] [PubMed] [Google Scholar]
- Van Goozen S.H.M., Matthys W., Cohen-Kettenis P.T., Thijssen J.H.H., Van Engeland H. Adrenal androgens and aggression in conduct disorder prepubertal boys and normal controls. Biol. Psychiatry. 1998;43:156–158. doi: 10.1016/S0006-3223(98)00360-6. [DOI] [PubMed] [Google Scholar]
- Vijayakumar N., Allen N.B., Youssef G., Dennison M., Yücel M., Simmons J.G., Whittle S. Brain development during adolescence: a mixed-longitudinal investigation of cortical thickness, surface area, and volume. Hum. Brain Mapp. 2016;37:2027–2038. doi: 10.1002/hbm.23154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan W. Pubertal pathways and the relationship to anthropometric changes in childhood: the Fels longitudinal study. Open J. Pediatr. 2012;2:118–126. doi: 10.4236/ojped.2012.22020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittle S., Simmons J.G., Byrne M.L., Strikwerda-Brown C., Kerestes R., Seal M.L., Olsson C.A., Dudgeon P., Mundy L.K., Patton G.C., Allen N.B. Associations between early adrenarche, affective brain function and mental health in children. Soc. Cogn. Affect. Neurosci. 2015;10:1282–1290. doi: 10.1093/scan/nsv014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierenga L.M., Langen M., Oranje B., Durston S. Unique developmental trajectories of cortical thickness and surface area. Neuroimage. 2014;87:120–126. doi: 10.1016/j.neuroimage.2013.11.010. [DOI] [PubMed] [Google Scholar]
- Worthman C.M. The epidemiology of human development. In: Worthman C.M., Panter-Brick C., editors. Hormones, Health and Behavior. Cambridge University Press; Cambridge: 1999. pp. 47–104. [Google Scholar]
- Zehr J.L., Culbert K.M., Sisk C.L., Klump K.L. An association of early puberty with disordered eating and anxiety in a population of undergraduate women and men. Horm. Behav. 2007;52:427–435. doi: 10.1016/j.yhbeh.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]