Highlights
-
•
The Nc is seen as an indicator of the orienting response and is also regarded as an indicator of attentional processing.
-
•
Infants at 9 months are able to discriminate between animate and inanimate motion based on motion cues alone.
-
•
Infants most likely allocate more attentional resources to the inanimate than the animate motion.
Keywords: Infant, EEG/ERP, Nc, Attention, Animacy
Abstract
Simple geometric shapes moving in a self-propelled manner, and violating Newtonian laws of motion by acting against gravitational forces tend to induce a judgement that an object is animate. Objects that change their motion only due to external causes are more likely judged as inanimate. How the developing brain is employed in the perception of animacy in early ontogeny is currently unknown. The aim of this study was to use ERP techniques to determine if the negative central component (Nc), a waveform related to attention allocation, was differentially affected when an infant observed animate or inanimate motion. Short animated movies comprising a marble moving along a marble run either in an animate or an inanimate manner were presented to 15 infants who were 9 months of age. The ERPs were time-locked to a still frame representing animate or inanimate motion that was displayed following each movie. We found that 9-month-olds are able to discriminate between animate and inanimate motion based on motion cues alone and most likely allocate more attentional resources to the inanimate motion. The present data contribute to our understanding of the animate–inanimate distinction and the Nc as a correlate of infant cognitive processing.
1. Introduction
The detection and discrimination of agents is of primary importance for our understanding of the social world. The development of the concept of animacy is probably one of the most important foundational elements of social cognition formed in the first year of life. Adults, preschoolers and young infants readily perceive animacy in displays that consist of simple moving geometric shapes that can even lead to mental state attributions (e.g., Castelli et al., 2000, Heider and Simmel, 1944, Springer et al., 1996). Objects moving in a self-propelled manner (e.g., Mandler, 1992, Premack, 1990) and violating Newtonian laws of motion such as acting against gravitational forces (Steward, 1982, Tremoulet and Feldman, 2000) tend to evoke the perception of animacy. An object's motion which is consistent with Newtonian laws, such as changing motion vectors due to external cause, leads to a judgement of inanimacy. Contextual cues can further assist observers to determine whether the object was set in motion due to internal or external cause (Gelman et al., 1995, Tremoulet and Feldman, 2006).
To date there is a sizable lack of literature in the infancy domain that investigates animacy. Existing behavioural work suggests that the ability to distinguish unfamiliar entities based on motion cues is a skill that appears relatively early during development (Luo and Baillargeon, 2005). From the earliest months of life, infants are sensitive to biological components of motion from point-light displays of a moving person in comparison with unfamiliar biological motion such as a moving spider (Bertenthal, 1993), scrambled motion (Hirai and Hiraki, 2005, Marshall and Shipley, 2009) or inverted human movement (Reid et al., 2006). In a habituation paradigm infants, aged 8–10 months seem to endow animate entities with an internal energy which may help to discriminate agentive from non-agentive actions such as chasing from non-chasing in displays that contain simple moving geometric shapes (Rochat et al., 2004). Elsner and Gerber (2010) showed in another habituation paradigm discrimination abilities in 7-and 9-month-olds on the basis of a single moving object as represented by longer looking times to animate motion patterns. When combined, the behavioural literature suggests that infants from the earliest months of life are capable of discriminating animate from inanimate motion, although evidence derives from a disparate literature.
Additional work on how infants process visual stimuli may help to clarify these behavioural findings. Physiological measures, such as the use of an electroencephalogram (EEG) to investigate infant brain function, can be used as an indicator of attention. The event-related brain potential technique (ERP) that derives from EEG is particularly suitable for use with infants, as it allows the opportunity to directly investigate the neural systems involved in animacy processing even in the absence of overt behaviour (Reid, 2012). To date, no research has been conducted on the pattern of neural activity associated with the discrimination of animate and inanimate motion during early ontogeny. This is due to the limited behavioural paradigms available when investigating this population, which typically assess looking time duration or active measures of behaviour. ERPs can yield responses in the absence of overt behaviour, with the implication that in some paradigms, ERPs may well be more sensitive than other measures (Hoehl and Wahl, 2012).
In order to investigate electrophysiological correlates of attentional processes related to animacy detection as found in previous behavioural studies (e.g., Elsner and Gerber, 2010), we focussed on one of the most studied components in infant ERP research. The mid-latency negative central component (Nc) is a negative deflection occurring around 300–700 ms after stimulus onset, and is most prominent over fronto-central sites. Reynolds and Richards (2005) used source analysis techniques on infant Nc data and found that the likely generator of this component is the anterior cingulate.
In studies using an oddball paradigm, the Nc has been found to be greater in its negativity to the infrequently presented oddball stimulus. This has been interpreted as an attentional orientation towards the novel or more unexpected event (Courchesne et al., 1981). Nelson and Dukette (1998) propose that the Nc is part of a general orienting system that is not necessarily related to stimulus novelty. Evidence for familiarity effects and recognition processes related to the Nc derive from de Haan and Nelson, 1997, de Haan and Nelson, 1999. In their studies the Nc was found to be greater in amplitude at 6 months to the baby's own mother's than a stranger's face (de Haan and Nelson, 1997), and was found to be greater to familiar compared to novel toys (de Haan and Nelson, 1999), when presenting faces and objects with equal frequency. In a longitudinal study by Webb et al. (2005) with 4- to 12-month-olds, the Nc has also been used to assess familiarity. Infants were tested every two months, and a significant developmental change in the ERP response to visual stimuli during the first postnatal year was found, with the Nc amplitude becoming more negative with increasing age. However this effect failed to show a consistent pattern at all time points. According to the authors, the variability found might be due to stimulus probability, stimulus repetition, stimulus type, or participants’ experience (Webb et al., 2005). Currently available evidence suggests that the Nc reflects attentional processes that are affected by novelty, familiarity and recognition of the stimulus and may undergo a non-linear development during the first postnatal years. It can, however, be stated that our current understanding of attentional processes related to the Nc component are accompanied by a certain degree of difficulty in interpreting differences between various types of stimuli.
The latency of a component is considered to reflect the timing of neural activation towards a particular cognitive process (Webb et al., 2005). The latency to peak for the Nc in an oddball paradigm is often longer for the oddball when contrasted to the standard stimulus (Courchesne, 1978). Stimuli presented with an equal probability have also revealed significant latency differences in the Nc peak latency. For example, with 4-month-old infants, the peak of the Nc occurred later for the averted eye gaze condition when contrasted with directed eye gaze (Hoehl et al., 2008). These authors suggested that a shorter latency reflected the relative ease of processing of a specific condition.
Behavioural work suggests that infants are able to discriminate animate and inanimate moving stimuli objects with a clear preference for animacy (e.g., Elsner and Gerber, 2010). These studies can be very helpful to direct infant electrophysiological studies, for example in terms of choosing an appropriate age group or stimulus material. Furthermore, behavioural results can be related to ERP results to provide a behavioural correlate for an electrophysiological response. Thus, they can provide a link for interpreting the functional significance of ERP components and EEG activity (de Haan, 2007). In the present study we chose to present a small disc moving through a “marble run” course. On parallel paths the disc moved either consistent with Newtonian principles “inanimate motion” or not “animate motion”.
To date, no research has been conducted on the pattern of neural activity associated with the perception of animate and inanimate motion during early ontogeny. Understanding how infants process animate and inanimate moving stimuli may help to further clarify behavioural findings. Importantly, electrophysiological measures can be used as an indicator of attention and are therefore informative regarding the deployment of these mechanisms during the observation of animate and inanimate motion. In the present study we expected differences in attentional mechanisms as reflected in the Nc amplitude at fronto-central leads between the animate and inanimate condition. We further predicted latency to Nc peak differences between conditions reflecting processing speed. No predictions regarding the direction of differences in the Nc amplitude or latency were made due to the utilization of a novel paradigm employing priming techniques while using stimuli of equal frequency. Specifically, we first showed a video of animate or inanimate motion, and then showed a still frame image that depicted a salient element of the presented condition to which the ERPs were time-locked.
2. Materials and methods
2.1. Participants
The final analysis comprised of the data of fifteen 9-month-old infants (10 male, 5 female), with an average age of 275 days (range: 269–280 days). All infants were born full term (37–42 weeks gestation), were of normal birth weight (>2500 g), and parents did not report any neurological or neonatal problems with any of the children. All subjects were recruited from the area in and around Stockton-on-Tees, North East England.
An additional 10 infants were tested but excluded, most commonly due to inattentiveness resulting in strong body movement, therefore providing fewer than the required minimum number of artefact-free trials per condition for data analysis (n = 8) or due to overall noisiness of the channels (n = 2). A meta-analysis on 149 published infant EEG studies revealed an average attrition rate of 49.2% in those studies (Stets et al., 2012). Therefore an attrition rate of 40% as present in this study is within relative norms for this field. Two participants who had to be excluded from the final sample were excluded due to technical issues which lead to overall noisy channels. Consequently this would lead to an attrition rate of 32% when attention only is put into consideration. The experiment was conducted with the understanding and the written consent of each participant's parent.
2.2. Stimuli and apparatus
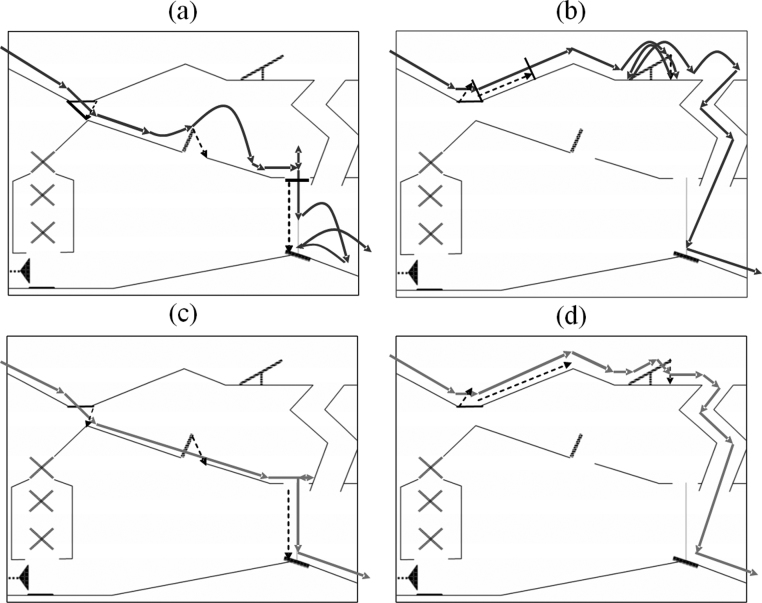
The experimental stimuli comprised four short silent movies each of which showed the same two-dimensional “marble run” course. A four-coloured disc “marble” followed two possible routes “top path” and “middle path” of the course in either an animate or an inanimate way (see Fig. 1).
Fig. 1.
2-D marble run, depicting both routes and movement types: (a) animate middle path, (b) animate top path, (c) inanimate middle path, and (d) inanimate top path. Top line displays the position and amount of jumps and direction changes of the animate condition. Bottom line illustrates the two possible paths of the inanimate condition.
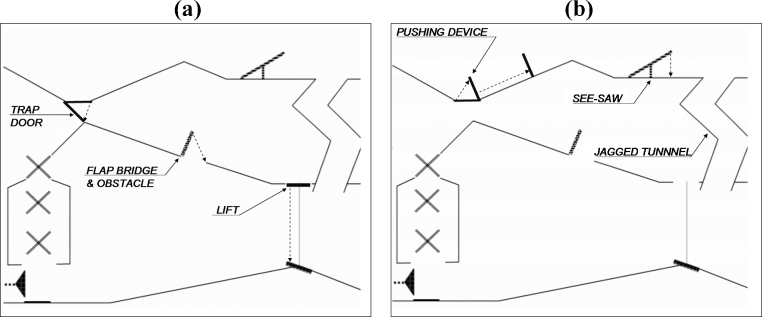
Both paths featured three different devices that moved in both conditions. The middle path included a trap door, a flap bridge and a lift. The top path contained a pushing device, a see-saw and a jagged tunnel (see Fig. 2). All six devices moved in the same manner across conditions.
Fig. 2.
Embedded devices: (a) depicts the middle path and (b) the top path.
To produce the perception of animacy, the marble was required to violate Newton's laws of motion. The marble in the animate condition performed sudden stops, starts from rest, jumps and changes in direction without any visible external cause. Thus, the marble in the animate condition interacted with the environmental properties of the marble run. The marble therefore leaped over obstacles, stopped suddenly in order to avoid collision and rolled up a hill independently. The impression of inanimacy was induced each time the marble appeared to be influenced by Newtonian laws, the principle of gravity and the set-up of the marble run. Hence, obstacles forced the marble to stop, a gap in the ground caused the marble to fall and a hilltop was only reached via the help of an external pushing device.
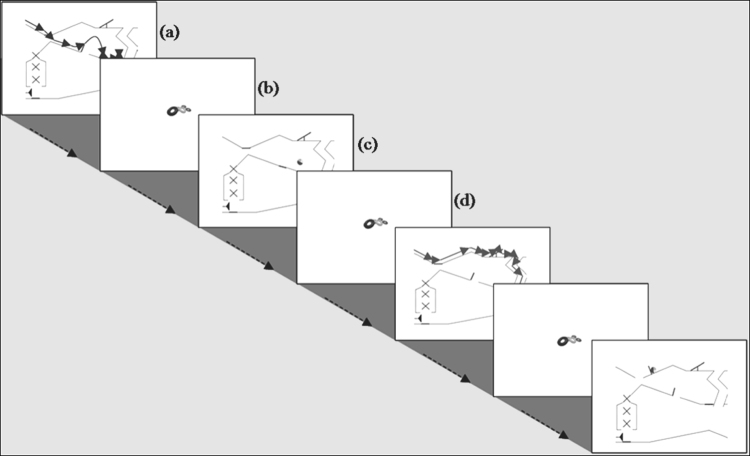
Infants always saw the marble in motion when continuously appearing on the top left side of the screen and disappearing on the bottom right side of the screen. This was in order to decrease the possibility of an unintentional perception of animacy induced by self-propulsion in the inanimate condition. Each movie (4 s) was always followed by a fixation stimulus of a “coloured-rings toy” (1 s) and then by a still image (1 s) obtained from the previously seen movie displaying a frame that depicted either an animate or an inanimate movement by the marble (see Fig. 3 for an example of the stimuli sequence). For instance a still frame displaying the marble in the air was a feature of animacy, while the still frame of the marble forced to move by gravity by rolling downwards was a feature of rigid principles of physics and therefore inanimate. It was to these images that we time-locked the resultant ERPs for the data analysis. Subsequently the same “toy” fixation stimulus appeared on screen (1 s) before the next movie was presented.
Fig. 3.
An example of the stimuli sequence presented to the participants: from top left to bottom right: (a) animate middle path movie (4 s), followed by (b) the fixation stimulus (1 s), (c) still frame to which the ERP was time-locked (1 s) and (d) another fixation stimulus (1 s). The duration of one sequence (a–d) is 7 s.
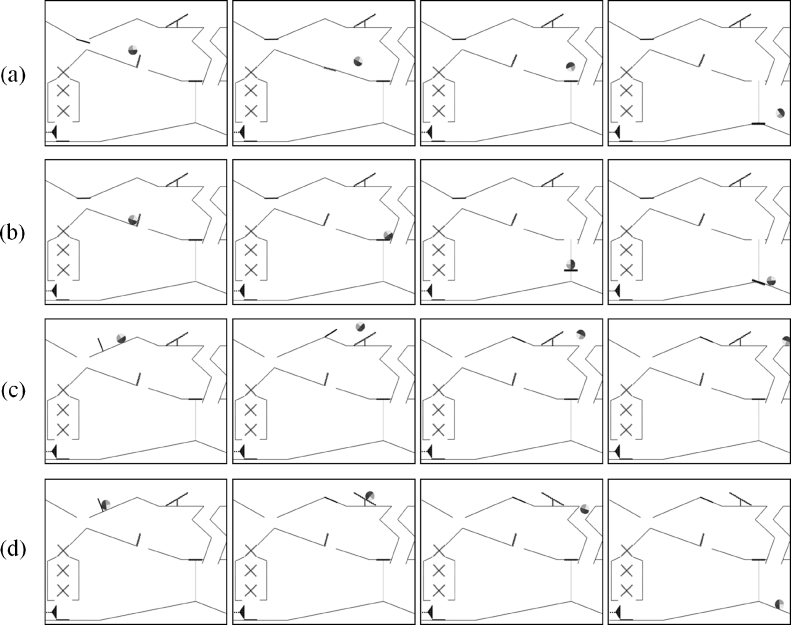
In total, four different still frames were shown for each condition (animate and inanimate) and path (top and middle) resulting in 16 different still frames (see Fig. 4). The movies and still frames were pseudo-randomized and repeated 8 times. This resulted in a maximum presentation of 128 videos and still frames. Infants saw a mean of 71.9 viewed presentations and their subsequent still frames. We constrained randomization by not presenting one condition three times consecutively. Different still frames were chosen in order to extend each infant's attention to the stimuli in an attempt to keep the task interesting throughout the testing session. The study was programmed using the Stim2-Gentask computer software package by Neuroscan Compumedics (Charlotte, USA).
Fig. 4.
Presented still frames to which the ERPs were time-locked: (a) animate middle path, (b) inanimate middle path, (c) animate top path, and (d) inanimate top path.
2.3. Design and procedure
Infants sat on the parent's lap in a dimly lit 2 × 2 m testing area which was separated from the rest of the laboratory by black coloured room dividers. The infant was seated directly in front of a 60 Hz 17-inch stimulus monitor at a viewing distance of 90 cm. Videos and their subsequent still frames were presented at 22 cm × 19 cm. This produced a visual angle subtending 13.9 degrees (horizontal) and 12 degrees (vertical). A towel between the parent and infant served to keep a distance between them in order to reduce artefacts caused by skin contact or parental heartbeat. Parents were instructed to remain still, quiet and if necessary to restrain their child from touching or pulling the cap. If the infant became fussy or uninterested in the stimuli, but prior to the acquisition of a minimum number of trials for ERP averaging, the experimenter gave the infant a short break before attempting to continue the study. Otherwise the experimenter remained silent and out of the participant's sight outside the testing area. The testing session ended when the infant's attention could no longer be attracted to the screen. EEG was recorded continuously, and the behaviour of the infants was video-recorded throughout the session in order to edit data on the basis of infant attention to the screen.
2.4. EEG recording and editing
EEG activity was recorded continuously from 32 scalp locations according to the 10–20 system, referenced online to AFz using Ag-AgCl ring electrodes with a sampling rate of 1 kHz. Horizontal and vertical electro-oculogram (HEOG± and VEOG±) were recorded bipolarly, and EEG data were amplified via a Neuroscan 32-channel amplifier. For additional data editing the raw data were first filtered with a 0.3–30 Hz bandpass filter and re-referenced offline to the linked mastoids. All trials in which the infants did not see the movie and the subsequent still frame were rejected from further analysis. All remaining trials were visually and manually edited for artefacts caused by eye or body movements and were segmented into epochs of waveform that comprised 200 ms prior to 1000 ms after onset of the still frame. Finally, following norms in the field (Hoehl and Wahl, 2012) those trials which were retained for analysis for each participant were baseline corrected and averaged in order to create individual averages for each participant.
For the looking behaviour a second coder who was naive to the aims of the study re-analyzed six randomly selected participants. The inter-rater agreement was high as evaluated by Spearman's rs = .902. The correlation was significant at the 0.01 level (two-tailed).
2.5. ERP analysis
The minimum criterion for inclusion was 3 trials for each of the 4 different stimulus sets (every stimulus set was presented for a maximum of 32 times). This was based on the results of Stets and Reid (2011) who examined the development of the Nc and showed that reliable and interpretable Nc data could be obtained from as few as three trials per condition for a standard visual ERP paradigm with infant participants. However each infant contributed to their average with 3–7 (M = 4.53; SD = 1.36) trials for the animate top path, 3–9 (M = 5.6; SD = 1.88) trials for the animate middle path, 4–9 (M = 6; SD = 1.51) trials for the inanimate top path and 3–9 (M = 4.73; SD = 2.05) trials for the inanimate middle path. As one trial comprised of the video (4 s), inter-stimulus interval (1 s) and the subsequent still frame (1 s), data were included for analysis when the infant gazed towards the screen for the priming video for at least 90% of the total presentation and observed the still frame to which we time-locked the ERP's (see Fig. 3 for stimulus sequence). Due to habituation effects and a generally short infant attention span, a criterion of 3 trials per condition was chosen.
Subsequent analyses were based on visual inspection of the waveforms and were directed by the few papers in the developmental cognitive neuroscience literature that have identified ERP components associated with social information processing in infants (e.g., Hoehl et al., 2008, Stets and Reid, 2011, Kobiella et al., 2008, Striano et al., 2006). This analysis revealed one time window and two scalp regions of interest. In addition, after Quinn et al. (2006), targeted analyses were used in lieu of more general ANOVAs because the comparison of conditions is dictated by the nature of the present paradigm.
Stimuli that contain an element of social processing appear to manifest an Nc that is lateralized to left electrodes in early development (see e.g., Carver and Vaccaro, 2007). Laterality could potentially relate to the detection of social information, or it could be a manifestation of developmental processes related to attention, where left lateralization is simply how attention mechanisms are manifested in early development (i.e., in the first 12 months) with change occurring later in development that displays a bilateral Nc effect. Left frontal and central (F3, C3) and right frontal and central (F4, C4) channels were computed, and a time window of 350 ms–600 ms was chosen after inspecting individual averages around the negative peaks for the two conditions. Fz and Cz could not be considered in the analysis due to high frequency noise. Paired sample t-tests (two-tailed) were conducted for further analysis.
3. Results
Paired sample t-tests investigating the top path revealed that the peak Nc amplitudes between animate and inanimate differed significantly for left hemisphere electrodes, t(14) = 2.42, p = .03, with the inanimate condition inducing a more negative peak amplitude for the Nc (M = −12.62 μV, SE = 3.09) when contrasted with the animate condition (M = −2.44 μV, SE = 3.19). This result therefore indicated a greater allocation of attention for inanimate motion for the top path.
Visual inspection of the top path ERPs further suggested a latency effect between conditions in the right hemisphere. We consequently performed a t-test which resulted in significant differences for the right hemisphere, t(14) = 2.18, p = .047, with the inanimate condition peaking earlier in time (M = 428 ms, SE = 15.63) than the animate condition (M = 474 ms, SE = 16.82).
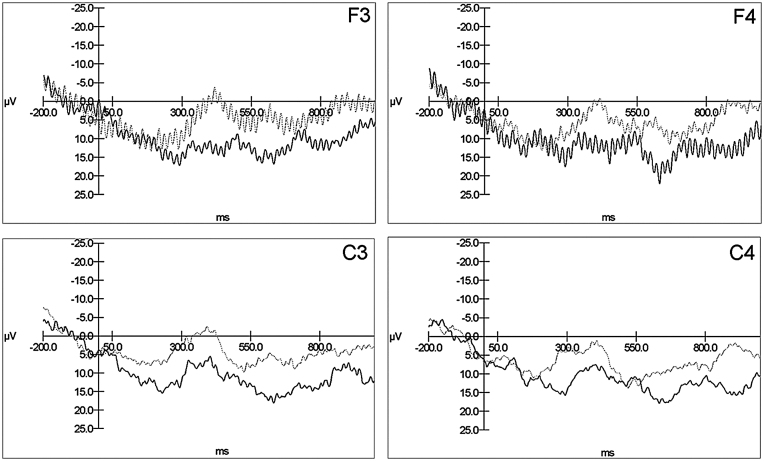
The grand averages and individual averages did not suggest that any other effects were evident at any scalp location, with statistical analyses indicating no significant effects (all ps > .05). Fig. 5 displays the grand averages comprised of the individual averages of the 15 infants separate for the left-frontal (F3), right-frontal (F4), left-central (C3), and right-central (C4) channels for the top path. Channels F3 and F4 contain some high frequency noise due to the laboratory not containing electrical shielding. The same analyses performed on the middle path stimuli suggested no significant differences between conditions.
Fig. 5.
Grand averages: comprised of the single averages of 15 infants separate for left-frontal (F3), right-frontal (F4), left-central (C3), and right-central (C4) channels for the top path.
4. Discussion
In the present study, the differences in the Nc component when perceiving animate and inanimate motion by fifteen 9-month-old infants was investigated. It could be shown for the first time on an electrophysiological level that 9-month-olds are capable of discriminating between animate and inanimate objects based on motion cues alone in an environmental context. The amount of attentional resources allocated to a stimulus is thought to be reflected in the size of the mid-latency negative central (Nc) component (Richards, 2003). In the present study the Nc amplitude elicited by the inanimate motion was larger than the Nc elicited by the animate motion for one of the two sets of stimuli. For the Nc amplitude the effect reached significant values on left fronto-central electrode sites which have been indicated in social information processing in other paradigms (e.g., Parise et al., 2008). Furthermore, the latency to the Nc peak was found to be shorter for the inanimate condition in the right hemisphere when contrasted to the animate, suggesting that inanimate motion was processed more rapidly by the infants. It should be noted that Fz and Cz could not be analyzed due to high frequency noise, thereby constraining interpretation of topographical effects with the present data.
This data therefore provides some evidence for the notion that infants seem to supply more attentional resources to inanimate rather than animate motion. The inanimate top path, where the marble was first pushed up a hill by a moving device, then rolled over a see-saw, fell down the jagged tunnel and then disappeared from the screen, induced the allocation of significantly more attention by the infant than the animate top path. Here the marble moved up the same hill without external help and performed several autonomous jumps and direction changes near the see-saw and the gap in the ground. The observed difference between condition did not reach a significant level for a second stimuli set where the marble travelled the middle path. Further for the top path, latency effects within the infant ERPs of the Nc component were observed in the right hemisphere indicating that the inanimate condition was processed more rapidly than the animate condition (Hoehl et al., 2008).
Based on previous behavioural studies with infants, it is possible that infants require more attentional resources to assimilate the information from the inanimate stimuli as animate motion may be more salient (e.g., Elsner and Gerber, 2010). For reasons associated with evolutionary pressures, fewer cues may be required to identify the nature of agentive motion (Scholl and Tremoulet, 2000), thereby requiring fewer attentional resources once identification has taken place. This would manifest itself as a reduced Nc amplitude for the animate condition when contrasted with the inanimate condition. This processing scenario only relates to the detection of animacy via movement cues, but not to mechanisms related to engagement with cognitive features of the agent, such as intentional mechanisms. The processing of such social-cognitive information may require an increased orienting response (Nelson and Monk, 2001). The effect reported in the present study therefore suggests that in these infants, detection of animate–inanimate distinctions occurred at a relatively perceptual rather than cognitive level.
Differences between behavioural studies and this present study may be attributed to the nature of habituation in ERP studies. Stets and Reid (2011) in a trial-by-trial analysis of an eye gaze paradigm showed that differences in the Nc between conditions were fundamentally different for the initial processing of conditions, with the trials of the second half of the experimental session producing the opposite effect. The implication of Stets and Reid (2011) was that averaging all trials from across the session effectively hid the on-line changes in processing that were occurring. The effects reported in the present study focus on the initial trials. Following Stets and Reid (2011), it is plausible that the later trials attenuate earlier effects or, alternatively, produce the opposite effect – with increased amplitude effects for animate stimuli. This would mirror behavioural findings that utilize habituation (e.g., Elsner and Gerber, 2010) although it is not possible to assess this in the present study given the mean number of trials contributed to their average by each infant.
The nature of the paradigm, specifically, the construction of the marble run, may also have played a role in the outcome of this study. Although research shows that the context of a movement may favour the perception of animacy (e.g., Gelman et al., 1995, Tremoulet and Feldman, 2006), it is possible that the additional moving elements in the presented movies suppressed the perception of animate movement. For example, when two things move together, which one shall be seen as the source of emitted energy and which is observed to be in action as a consequence of the movement? The slow moving pushing device of the top path may introduce ambiguity into the movie and cause insufficient information to be available for the infant in order for a full determination of the origin of movement to be made. Potentially it was this insufficiency that induced the greater attention towards the inanimate motion in this stimuli set, as infants needed to initially detect the cause of motion.
Furthermore, the animated movies were designed two-dimensionally. This means that only the height and width were visible to the infants. In the real world moving objects have more dimensions, including depth. The inclusion of an additional dimension may increase the complexity of the display. This could make it more difficult for young infants to process all information. An additional dimension could also raise infants’ interest and may extend the infant's attention span, which may allow the collection of more artefact-free trials (Stets et al., 2012). However, if infants did not see the marble run as being presented in a vertical plane (i.e., front view), but, rather, in a horizontal plane (i.e., view from above), (e.g., Heider and Simmel, 1944), then infants would not be able to detect the laws of gravitation in the present displays. This could in part explain the differences observed between the top and middle path, as the middle path featured relatively more falling characteristics overall.
5. General conclusion
The findings of the present study represent a first contribution to our understanding of the neural mechanisms associated with initial encoding of animate–inanimate distinctions. This shows that infants discriminate these forms of information on the basis of attention. Moreover, this is the first study to indicate differences in condition with relatively few trials included in the ERP average, as proposed by Stets and Reid (2011). Consequently this may drive differences between this study and prior behavioural work.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Acknowledgements
We would like to thank the participants and their parents for taking part in our study.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.dcn.2013.05.003.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- Bertenthal B.I. Infants’ perception of biomechanical motions: intrinsic image and knowledge-based constraints. In: Granrud C., editor. Visual Perception and Cognition in Infancy. Erlbaum; Hillsdale, NJ: 1993. pp. 175–214. [Google Scholar]
- Carver L.J., Vaccaro B.G. 12-month-old infants allocate increased neural resources to stimuli associated with negative adult emotion. Developmental Psychology. 2007;43(1):54–69. doi: 10.1037/0012-1649.43.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelli F., Happé F., Frith U., Frith C.D. Movement and mind: a functional imaging study of perception and interpretation of complex intentional movement pattern. Neuroimage. 2000;12:314–325. doi: 10.1006/nimg.2000.0612. [DOI] [PubMed] [Google Scholar]
- Courchesne E. Neurophysiological correlates of cognitive development: changes in long-latency event-related potentials from childhood to adulthood. EEG and Clinical Neurophysiology. 1978;45:468–482. doi: 10.1016/0013-4694(78)90291-2. [DOI] [PubMed] [Google Scholar]
- Courchesne E., Ganz L., Norcia M.E. Event-related brain potentials to human faces in infants. Child Development. 1981;52:804–811. [PubMed] [Google Scholar]
- de Haan M., Nelson C.A. Recognition of the mother's face by six-month old infants: a neurobehavioral study. Child Development. 1997;68:187–210. [PubMed] [Google Scholar]
- de Haan M., Nelson C.A. Brain activity differentiates face and object processing by 6-month-old infants. Developmental Psychology. 1999;34:1114–1121. doi: 10.1037//0012-1649.35.4.1113. [DOI] [PubMed] [Google Scholar]
- de Haan M. Current and future directions in infant electrophysiology. In: de Haan M., editor. Infant EEG and Event-Related Potentials. Psychology Press; Hove and New York: 2007. pp. 312–316. [Google Scholar]
- Elsner B., Gerber K. Baltimore; MD: 2010 March. Dead or Alive? Processing of Animate Motion Cues in the First Year of Life. Poster presented at the XVII Biennial International Conference on Infant Studies. [Google Scholar]
- Gelman R., Durgin F., Kaufman L. Distinguishing between animates and inanimates: not by motion alone. In: Sperber D., Premack D., Premack A.J., editors. Causal Cognition: A Multidisciplinary Debate. Oxford University Press; Oxford, NY: 1995. pp. 150–184. [Google Scholar]
- Heider F., Simmel M. An experimental study of apparent behaviour. American Journal of Psychology. 1944;57:243–259. [Google Scholar]
- Hirai M., Hiraki K. An event-related potentials study of biological motion perception in human infants. Brain Research: Cognitive Brain Research. 2005;22:301–304. doi: 10.1016/j.cogbrainres.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Hoehl S., Reid M.V., Mooney J., Striano T. What are you looking at? Infants’ neural processing of an adult's object-directed eye gaze. Developmental Science. 2008;11:10–16. doi: 10.1111/j.1467-7687.2007.00643.x. [DOI] [PubMed] [Google Scholar]
- Hoehl S., Wahl S. Recording infant ERP data for cognitive research. Developmental Neuropsychology. 2012;37(3):187–209. doi: 10.1080/87565641.2011.627958. [DOI] [PubMed] [Google Scholar]
- Kobiella A., Grossman T., Reid V.M., Striano T. The discrimination of angry and fearful facial expressions in 7-month-old infants: an event-related potential study. Cognition and Emotion. 2008;22:134–146. [Google Scholar]
- Luo Y., Baillargeon R. Can a self-propelled box have a goal? Psychological reasoning in 5-month-old infants. Psychological Science. 2005;16:601–608. doi: 10.1111/j.1467-9280.2005.01582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandler J.M. How to build a baby: II, Conceptual primitives. Psychological Review. 1992;99(4):587–604. doi: 10.1037/0033-295x.99.4.587. [DOI] [PubMed] [Google Scholar]
- Marshall P.J., Shipley T.F. Event-related potentials to point-light displays of human actions in 5-month-old infants. Developmental Neuropsychology. 2009;34(3):368–377. doi: 10.1080/87565640902801866. [DOI] [PubMed] [Google Scholar]
- Nelson C.A., Dukette D. In: Cognitive Neuroscience of Attention: A Developmental Perspective. Richards J.E., editor. Erlbaum; Hillsdale, NJ: 1998. pp. 327–362. [Google Scholar]
- Nelson C.A., Monk C.S. The use of event-related potentials in the study of cognitive development. In: Nelson C.A., Luciana M., editors. Developmental Cognitive Neuroscience. MIT Press; Cambridge, MA: 2001. pp. 125–135. [Google Scholar]
- Parise E., Reid V.M., Stets M., Striano T. Direct eye contact influences the neural processing of objects in 5-month-old infants. Social Neuroscience. 2008;3:141–150. doi: 10.1080/17470910701865458. [DOI] [PubMed] [Google Scholar]
- Premack D. The infant's theory of self-propelled objects. Cognition. 1990;36:1–16. doi: 10.1016/0010-0277(90)90051-k. [DOI] [PubMed] [Google Scholar]
- Reid V.M., Hoehl S., Striano T. The perception of biological motion by infants: an event-related potential study. Neuroscience Letters. 2006;395:211–214. doi: 10.1016/j.neulet.2005.10.080. [DOI] [PubMed] [Google Scholar]
- Reid V.M. How infants detect information in biological motion. In: Slaughter V., Brownell C.A., editors. Early Development of Body Representation. Cambridge University Press; Cambridge: 2012. pp. 146–162. [Google Scholar]
- Reynolds G.D., Richards J.E. Familiarization, attention, and recognition memory in infancy: an event-related potential and cortical source location study. Developmental Psychology. 2005;41(4):598–615. doi: 10.1037/0012-1649.41.4.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards J.E. Attention affects the recognition of briefly presented visual stimuli in infants: an ERP study. Developmental Science. 2003;6(3):312–328. doi: 10.1111/1467-7687.00287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochat P., Striano T., Morgan R. Who is doing what to whom? Young infants’ developing sense of social causality in animated displays. Perception. 2004;33:355–369. doi: 10.1068/p3389. [DOI] [PubMed] [Google Scholar]
- Scholl B.J., Tremoulet P.D. Perceptual causality and animacy. Trends in Cognitive Science. 2000;4(8):299–309. doi: 10.1016/s1364-6613(00)01506-0. [DOI] [PubMed] [Google Scholar]
- Springer K., Meier J.A., Berry D. Nonverbal bases of social perception: developmental change in sensitivity to patterns of motion that reveal interpersonal events. Journal of Nonverbal Behavior. 1996;20(4):199–211. [Google Scholar]
- Stets M., Reid V.M. Infant ERP amplitudes change over the course of an experimental session: implications for cognitive processes and methodology. Brain and Development. 2011;33(7):558–568. doi: 10.1016/j.braindev.2010.10.008. [DOI] [PubMed] [Google Scholar]
- Stets M., Stahl D., Reid V.M. A meta-analysis investigating factors underlying attrition rates in infant ERP studies. Developmental Neuropsychology. 2012;37(3):226–252. doi: 10.1080/87565641.2012.654867. [DOI] [PubMed] [Google Scholar]
- Steward J.A. University of Pennsylvania; 1982. Perception of Animacy. (Unpublished Dissertation) [Google Scholar]
- Striano T., Reid V.M., Hoehl S. Short communication neural mechanisms of joint attention in infancy. European Journal of Neuroscience. 2006;23:2819–2823. doi: 10.1111/j.1460-9568.2006.04822.x. [DOI] [PubMed] [Google Scholar]
- Tremoulet P.D., Feldman J. Perception of animacy from the motion of a single object. Perception. 2000;29:943–951. doi: 10.1068/p3101. [DOI] [PubMed] [Google Scholar]
- Tremoulet P.D., Feldman J. The influence of spatial context and the role of intentionality in the interpretation of animacy from motion. Perception and Psychophysics. 2006;68(6):1047–1058. doi: 10.3758/bf03193364. [DOI] [PubMed] [Google Scholar]
- Quinn P.C., Westerlund A., Nelson C.A. Neural markers of categorization in 6-month-old infants. Psychological Science. 2006;17:59–66. doi: 10.1111/j.1467-9280.2005.01665.x. [DOI] [PubMed] [Google Scholar]
- Webb S.J., Long J.D., Nelson C.A. A longitudinal investigation of visual event-related potentials in the first year of life. Developmental Science. 2005;8(6):605–616. doi: 10.1111/j.1467-7687.2005.00452.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.