Highlights
-
•
Previous studies associate poor reading with unstable speech-evoked brainstem responses.
-
•
DCDC2 and KIAA0319 risk alleles form a strong genetic link with developmental dyslexia.
-
•
Genetic burden with KIAA0319 risk is related to unstable speech-evoked brainstem responses.
-
•
Genetic burden with DCDC2 risk is related to intact speech-evoked brainstem responses.
-
•
Revealed brain-gene relationships may inform the multifactorial pathophysiology of dyslexia.
Keywords: Developmental dyslexia, KIAA0319, DCDC2, Brainstem responses, Sound processing, Genetic risk
Abstract
Dyslexia is a reading disorder with strong associations with KIAA0319 and DCDC2. Both genes play a functional role in spike time precision of neurons. Strikingly, poor readers show an imprecise encoding of fast transients of speech in the auditory brainstem. Whether dyslexia risk genes are related to the quality of sound encoding in the auditory brainstem remains to be investigated. Here, we quantified the response consistency of speech-evoked brainstem responses to the acoustically presented syllable [da] in 159 genotyped, literate and preliterate children. When controlling for age, sex, familial risk and intelligence, partial correlation analyses associated a higher dyslexia risk loading with KIAA0319 with noisier responses. In contrast, a higher risk loading with DCDC2 was associated with a trend towards more stable responses. These results suggest that unstable representation of sound, and thus, reduced neural discrimination ability of stop consonants, occurred in genotypes carrying a higher amount of KIAA0319 risk alleles. Current data provide the first evidence that the dyslexia-associated gene KIAA0319 can alter brainstem responses and impair phoneme processing in the auditory brainstem. This brain-gene relationship provides insight into the complex relationships between phenotype and genotype thereby improving the understanding of the dyslexia-inherent complex multifactorial condition.
1. Introduction
Dyslexia is characterized by poor reading, writing, and spelling skills despite typical intelligence, no visual acuity problems, and appropriate education (ICD-10-CM, http://www.icd10data.com/ICD10CM/Codes/F01-F99/F80-F89/F81-/F81.0). Boys are 2–3 times more likely to be affected than girls, and cumulative incidence rates vary from 5–12% (Shaywitz et al., 1990). Dyslexia persists in 4–6% of adults (Schulte-Körne and Remschmidt, 2003) disadvantaging employment, and compromising participation in public life. Prevention requires early sensitive screenings and successful remediation, which are both still desirable.
Various cognitive domains support literacy acquisition. Thus, heterogeneous cognitive fingerprints of dyslexia phenotypes exist (Heim and Grande, 2012, Ramus and Ahissar, 2012) and multiple subtypes of dyslexia have been suggested (Bosse et al., 2007), but a bona fide theory of the underlying mechanisms has not been established yet. A widely accepted rationale bases dyslexia on an impairment of phonological representations (Snowling, 2001). Others advocate auditory processing deficits such as an impaired oscillatory phase locking for low frequency temporal coding in auditory cortex (Goswami, 2011), or a decreased sensitivity to rapidly changing phonological features (Benasich et al., 2002, Tallal, 1980). Auditory processing deficits might cause an impoverished distinction between speech sounds (Vandermosten et al., 2010), a deficient access to otherwise intact phonetic representations (Boets et al., 2013), or a deficient match between memory representations and auditory sensations (Díaz et al., 2012, Jaffe-Dax et al., 2015). Alternatively or additionally, visual attention, visual-magnocellular processing, or visual-auditory integration compose further cognitive problems (Heim et al., 2010, Stein and Walsh, 1997, Valdois et al., 2014, Widmann et al., 2012).
Dyslexia is moderately to highly heritable (Schumacher et al., 2007) with a multifactorial etiology (Fisher and DeFries, 2002) and a complex underlying genetic architecture. Evidence exists for multiple genes to contribute to the phenotype, with considerable genetic heterogeneity across individuals (Carrion-Castillo et al., 2013). Dyslexia is linked to several risk loci including nine so-called DYX-regions (DYX1-DYX9) (Carrion-Castillo et al., 2013, Giraud and Ramus, 2013, Peterson and Pennington, 2012, Poelmans et al., 2011), but a consistent genome-wide association is still missing. However, DYX2 on chromosome 6 is the best replicated susceptibility locus (Gabel et al., 2010), with DCDC2 (Lind et al., 2010, Ludwig et al., 2008, Meng et al., 2005, Newbury et al., 2011, Scerri et al., 2011, Schumacher et al., 2006, Wilcke et al., 2009) as well as KIAA0319 (Cope et al., 2005, Francks et al., 2004, Harold et al., 2006, Kaplan et al., 2002, Luciano et al., 2007, Meng et al., 2005, Paracchini et al., 2008, Scerri et al., 2011) as strongest candidate genes of this locus. Numerous studies evaluate the genetic origin of dyslexia, excellently compiled in recent reviews (Carrion-Castillo et al., 2013, Giraud and Ramus, 2013).
Despite considerable progress, complex gene-brain relations of KIAA0319 and DCDC2 are far from comprehensive, because studies elucidating the genes’ impact on cell anatomy and systems physiology are scarce. Animal experiments associate the functional role of both genes with neuronal migration (Burbridge et al., 2008, Meng et al., 2005, Paracchini et al., 2006, Peschansky et al., 2010) and, thus, a role in the formation of the neuronal cell assemblies during brain development. Furthermore, both genes are expressed in mature neurons after migration and contribute to protein binding.
More specifically, KIAA0319 encodes an integral transmembrane protein (Velayos-Baeza et al., 2010), and is a component in the early endosome, its membrane and the plasma membrane, possibly supporting a broader spectrum of signaling functions. In addition to neuronal migration, KIAA0319 is associated with a negative regulation of dendrite development. It regulates processes that stop, prevent or reduce the frequency, rate or extent of dendrite development (http://www.ncbi.nlm.nih.gov/gene/9856; Gene ID: 9856, updated on 6-Mar-2016). Animal studies indicated that in utero RNA interference (RNAi) targeting Kiaa0319 in male Wistar rats affected acoustic discrimination abilities of complex stimuli, which was associated with formation of heterotopias in white matter (Szalkowski et al., 2013). Electrophysiologically, a downregulation of Kiaa0319 expression was followed by a decreased response consistency to sound stimuli as measured from neurons in the primary auditory cortex, resulting in a reduced neuronal discrimination ability (Centanni et al., 2014a, Centanni et al., 2014b).
At the cellular level DCDC2 is involved in processes such as cellular defense response, dendrite morphogenesis, intracellular signal transduction, regulation of smoothened signaling pathway, regulation of Wnt signaling pathway, and regulation of cilium assembly. At the systems level, DCDC2 is correlated with visual learning and sensory perception of sound. DCDC2 is a component of axoneme, cytoplasm, cytoskeleton, kinocilium, nucleus and primary cilium. The doublecortin domain, to which DCDC2 belongs, has been shown to bind tubulin and enhance microtubule polymerization. Its function may affect the signaling of primary cilia (http://www.ncbi.nlm.nih.gov/gene/51473; Gene ID: 51473, updated on 6-Mar-2016). DCDC2 has been reported to be a deafness gene in a Tunisian family motivated by the considerations that hair cell kinocilia and cell primary cilia length regulation is likely influenced by DCDC2’s role in microtubule formation and stabilization (Szalkowski et al., 2012). Dcdc2 knockout mice showed a deficit in rapid auditory processing (Truong et al., 2014), which is consistent with the observation of degraded neural spike timing and, thus, difficulties in the encoding of rapid sequential sensory input as measured in the somatosensory cortex in the same mutants (Che et al., 2014).
Taken together, auditory processing deficits have been linked to gene homologues for both genes (Centanni et al., 2014a, Centanni et al., 2014b, Szalkowski et al., 2012, Truong et al., 2014). The physiological consequence of altered functions of KIAA0319 and DCDC2 is linked to imprecise neuronal temporal coding. It is plausible to assume that an imprecise encoding of acoustic input leads to processing deficits of ascending speech signals challenging the formation of robust phoneme representations in long-term memory. Thus, a temporal processing deficit might prevent the uncomplicated acquisition and consolidation of literacy skills as suggested by dominating theories (Goswami, 2011, Tallal, 2012).
A huge body of brain-behavior association studies report altered structural and functional correlates pointing to irregular auditory and phonological processes in dyslexia (Banai et al., 2005, Díaz et al., 2012, Hämäläinen et al., 2013, Hornickel and Kraus, 2013, Kujala et al., 2006, Paulesu et al., 2014, Schulte-Körne and Bruder, 2010). Several brain-gene studies considered KIAA0319 and DCDC2 in the context of literacy. Late electrophysiological responses to speech sounds (300–700 ms) are affected in rare variants in a region between KIAA0319 and DCDC2 (Czamara et al., 2010). A KIAA0319/TTRAP/THEM2 locus was associated with a reduced left-hemispheric functional asymmetry of posterior superior temporal sulcus during reading (Pinel et al., 2012). The KIAA0319 single nucleotide polymorphism rs2143340 was related to activation in the bilateral supramarginal gyri during a word rhyming task (Cope et al., 2012). In the same study, alleles of a DCDC2 complex tandem repeat were related to activation in the right lateral occipital temporal gyrus and the left supramarginal gyrus. These gene-related abnormal functional activations in the parietal lobes are consistent with the DCDC2-related reduced white matter volume, and a degraded cortical thickness in the same region (Darki et al., 2014).
The impact of dyslexia risk genes on early auditory processing is currently unknown. Interestingly, the sensation related processing of speech sounds has been found to be noisy at a very early stage in the auditory pathway of poor readers. Speech evoked brainstem responses (cABRs) were unstable and indistinctive in poor readers and in children with poor phonological skills (Banai et al., 2005, Chandrasekaran et al., 2009, Hornickel et al., 2009, Hornickel and Kraus, 2013, Strait et al., 2011, White-Schwoch and Kraus, 2013). Particularly striking is the sensitivity of cABRs in the phase of the formant transition of a given stimulus (Hornickel and Kraus, 2013). Formant transitions are fast changes of frequency bands that constitute important phonetic features because a correct encoding of formant transitions enables us to distinguish between stop consonants. Ultimately, we investigated how the two prominent dyslexia susceptible genes DCDC2 and KIAA0319 relate to the stability of speech-evoked brainstem responses in the phase of the formant transition of the syllable [da], which has been reported to be an electrophysiological marker of dyslexia in the early auditory pathway (Hornickel and Kraus, 2013). Here, we provide the first evidence that the dyslexia-associated gene KIAA0319 affects response consistency in the auditory brainstem and, thus, impairs phoneme encoding at a very early stage in the auditory pathway.
2. Materials and methods
2.1. Participants
One hundred fifty nine native German-speaking children were enrolled, consisting of 95 preliterate children, aged 4–7, participating in the Legascreen project (www.legascreen.de), along with 64 literate children, aged 11–13, who were former participants of the German Language Developmental Study (GLAD-Study) e.g. (Friedrich and Friederici, 2004). A summary of demographic, psychometric and electrophysiological data is given in Table 1. All participants had normal hearing, passing a hearing screening at a 25 dB hearing level (air conduction) for octaves from 250 to 4000 Hz. Click-evoked brainstem responses were normal. No neurological diseases were known or evident. All parents gave written informed consent, while children gave additional documented verbal assent to participate in the study. Experimental procedures were approved by the University of Leipzig Ethical Review Board.
Table 1.
Participant characteristics.
| Preliterate | Literate | |||
|---|---|---|---|---|
| N | 95 | M | 64 | M |
| Demographics | ||||
| Age (years) | 5.8 (0.9, 4.3–7.5) | – | 12.7 (0.6, 11.4–13.8) | – |
| Sex (male/female) | 48/47 | 39/25 | – | |
| Familial risk (no risk/risk) | 49/44 | 2 | 46/18 | – |
| Profession mother (median) | 3 | 8 | 2 | – |
| Profession father (median) | 3 | 8 | 2 | – |
| Brainstem measures | ||||
| Peak-V-latency | ||||
| Pre (ms) | 5.40 (0.23, 4.65–6.05) | 3 | 5.48 (0.20, 5.15–6.05) | 1 |
| Post (ms) | 5.42 (0.24, 4.55–6.05) | 3 | 5.51 (0.19, 5.15–5.95) | 1 |
| Response consistency (r) | 0.72 (0.13, 0.31–0.91) | – | 0.67 (0.22, −0.04–0.97) | – |
| Psychometrics | ||||
| Literacy | ||||
| DERET (mean PR) | 43.6 (29.5, 0–99) | 1 | ||
| LGVT speed (mean PR) | 37.1 (22.9, 5–98) | – | ||
| LGVT comp (mean PR) | 40.8 (24.7, 1–94) | – | ||
| WR (mean%correct) | 94.4 (11.5, 30–100) | – | ||
| WR speed (s) | 36.4 (27.3, 15–210) | – | ||
| NWR (mean%correct) | 79.2 (15.9, 23–100 | – | ||
| NWR reading speed (s) | 59.1 (36.9, 25–292) | – | ||
| Intelligence | ||||
| Intelligence (mean IQ) | 99 (12, 73–127) | – | 111 (10, 86–126) | 1 |
| Handedness | ||||
| (right/left/ambidexterity) | 83/9/- | 3 | 59/5/- | – |
M, missing data; DERET, standardized test writing skills; LGVT speed, standardized test reading speed; LGVT comp, standardized test reading comprehension; WR, word reading; NWR, nonword reading; professional education was operationalized with an ordinal scale with 1, without professional education; 2, Professional School, Vocational School; 3, Master Craftsman, Technical College, Bachelor, University of Cooperative Education; 4, Higher classes of civil service; 5, University of Applied Sciences; 6, University Degree; State Examination; values represent group averages (±SD, range) unless otherwise indicated.
2.2. Psychometrics
Literate children were tested for reading comprehension and reading speed (Lesegeschwindigkeits- und −verständnistest für die Klassen 6–12, LGVT, Schneider et al., 2007) as well as for performance in spelling and writing (Deutscher Rechtschreibtest, DERET, Stock and Schneider, 2008). To the best of our knowledge, none of the participants were formally diagnosed with a reading disorder. Non-verbal intelligence of literate children was determined by the Kaufman Assessment Battery for Children (K-ABC, Kaufman et al., 2009) and was missing in one participant. Preliterate children were tested with the Wechsler Preschool and Primary Scale of Intelligence (WPPSI-III, Wechsler et al., 2009). In the group of preliterate children 12 individuals yielded a non-verbal IQ <85. Because the brainstem measure varies with intelligence as reported in previous studies (Hornickel and Kraus, 2013, White-Schwoch and Kraus, 2013), we controlled for intelligence in all statistical tests across all participants.
2.3. Acoustic stimulus
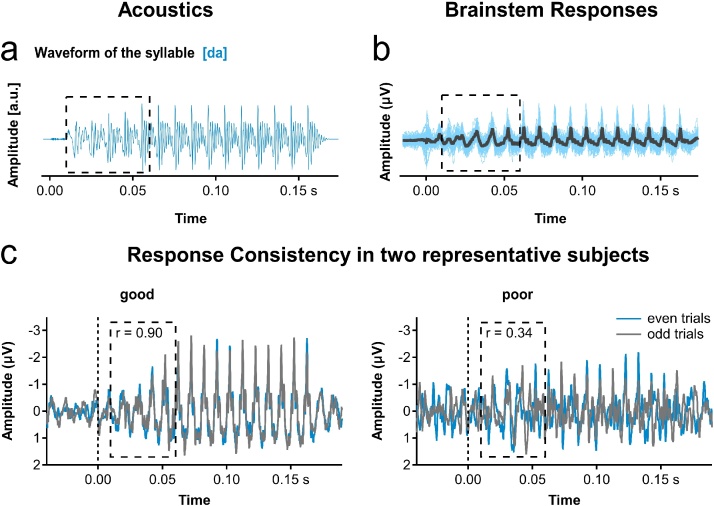
The Klatt-synthesized syllable [da] was provided by Nina Kraus (Hornickel et al., 2009, Hornickel and Kraus, 2013). The syllable was 170 ms long with a pitch onset (100 Hz) at 10 ms. The formant transition duration was 50 ms composed of a linear rising F1 (400–720 Hz), a linear falling F2 (1700–1240 Hz), F3 (2580–2500 Hz), and flat F4 (3300 Hz), F5 (3750 Hz), and F6 (4900 Hz). The steady-state vowel lasted 110 ms (Fig. 1a).
Fig. 1.
Acoustic stimulus and speech evoked brainstem responses. (a) Stimulus was a Klatt-synthesized [da] syllable of 170 ms duration with a 50 ms formant transition (dotted square) and a steady-state vowel of 110 ms duration. (b) The grand average of complex auditory brainstem responses (cABR, black lines) was calculated from 6000 stimulations per syllable and subject. Individual average waveforms are depicted in blue. (c) cABRs are shown for two representative subjects. High correlation coefficients between even and odd trials in the period of the formant transition (dotted squares) illustrate good response consistency in the subject depicted on the left. Small correlation coefficients illustrate a poor response consistency in the subject on the right.
2.4. Neurophysiological data recording and reduction
Children were seated comfortably in a reclining chair in an electrically shielded, soundproof booth and were allowed to watch a movie of their choice (SPL <45 dB). Throughout the recording session, the children were listening to the movie in the left ear and to the speech stimuli in the right ear, which is common practice (e.g. Hornickel and Kraus, 2013). Before and after stimulation with the target syllable a train of 2000 clicks was presented to test the integrity of the auditory pathway and to ensure stable recording conditions throughout the experiment. The syllable was presented to the right ear through Etymotic ER-3 insert earphones (Etymotic Research, Elk Grove Village, IL) at an intensity level of 80 dB SPL, at a rate of 4.35 Hz, and with both polarities (condensation and rarefaction). Brainstem responses were collected using BrainVision V-Amp in combination with an EP-PreAmp, an extremely low-level noise bipolar amplifier (BrainVision) at 20 kHz sampling rate. Three single multitrode Ag/AgCl electrodes were attached to the scalp from Cz to the ipsilateral earlobe, with the forehead as the ground. Impedances were down-regulated (<5 kΩ) and the inter-electrode impedance difference was not higher than 1.5 kΩ. The continuous signal was filtered off-line with the firfilt EEGLAB plugin (Windowed Sinc FIR-filter, bandpass 70–2000 Hz, Kaiser window, beta = 7.8572, filter order = 100300, fs = 20 kHz), epoched from −40 to 190 ms, and baseline corrected to a 40 ms interval preceding sound onset. Epochs with any activity exceeding the range of 35 μV were rejected and a total of 6000 epochs were considered for further analyses. A comparison of click-evoked wave V latencies across session and participants showed that recording parameters were comparable throughout the session and across participants (p > 0.4).
2.5. Neurophysiological data analysis
Response consistency was calculated for the portion of the response reflecting the formant transition, 7–60 ms post stimulus onset. Response consistency was the result of a correlation of the subaverages of an equal number of odd and even responses (Fig. 1c). Odd and even responses were determined after the summation of a single response to the original stimulus and a single response to the inverted stimulus resulting in the operationalization of the envelope FFR (Aiken and Picton, 2008, Hornickel and Kraus, 2013). Metrics were quantified with custom-written MATLAB scripts. For statistical analyses, a Fisher transformation was applied to the correlation coefficients by calculating the inverse of the hyperbolic tangent function to linearize the distribution of these coefficients (Fisher, 1921).
2.6. DNA extraction and genotyping
We selected single nucleotide polymorphisms (SNPs) of KIAA0319 (rs761100, rs2179515, rs2143340, rs3212236, rs9461045,and rs6935076) and DCDC2 (rs807724, rs1087266, rs807701, rs793842, rs1091047, rs6922023) because they were repeatedly associated with dyslexia in previous studies (Cope et al., 2005, Harold et al., 2006, Meng et al., 2005, Schumacher et al., 2006, Wilcke et al., 2009).
DNA was extracted from saliva samples using standard procedures (Quinque et al., 2006) or using Oragene DNA Genotek Kits (Kanata, Ontario, Canada). Genotyping was performed first with the matrix-assisted laser desorption/ionization time-of-flight mass spectrometry system iPLEX (Agena, Hamburg, Germany). Genotyping data had to fulfill the following quality measures: SNP-wise Hardy-Weinberg-Equilibrium (HWE; p > 0.05 Bonferroni corrected), SNP-wise call rate >97%, individual-wise: call-rate >90% and minor allele frequency (MAF) > 0.05. We excluded SNPs if any genotype comprised less than 5% of the cohort (see Table 2). Therefore, rs2143340, rs3212236, and rs9461045 were excluded from analyses.
Table 2.
Characteristics of the investigated set of single nucleotide polymorphisms.
| Nearest Gene | SNP | MAF |
N participants in Genotypic Groups |
Genotypic Groups |
Risk Allele | ||||
|---|---|---|---|---|---|---|---|---|---|
| Major | Hetero | Minor | Major | Hetero | Minor | ||||
| DCDC2 | rs807724 | 0.248 | 89 | 61 | 9 | AA | GA | GG | minor |
| DCDC2 | rs1087266 | 0.409 | 55 | 78 | 26 | CC | CT | TT | minor |
| DCDC2 | rs807701 | 0.399 | 59 | 73 | 27 | TT | TC | CC | minor |
| DCDC2 | rs793842 | 0.428 | 54 | 74 | 31 | GG | GA | AA | minor |
| DCDC2 | rs1091047 | 0.192 | 107 | 43 | 9 | GG | CG | CC | minor |
| DCDC2 | rs6922023 | 0.189 | 108 | 42 | 9 | AA | GA | GG | major |
| KIAA0319 | rs761100 | 0.428 | 50 | 82 | 27 | GG | GT | TT | minor |
| KIAA0319 | rs2179515 | 0.349 | 66 | 75 | 18 | AA | GA | GG | major |
| KIAA0319 | rs6935076 | 0.371 | 65 | 70 | 24 | CC | CT | TT | minor |
| KIAA0319 | rs2143340 | 0.151 | 117 | 36 | 6 | TT | TC | CC | minor |
| KIAA0319 | rs3212236 | 0.157 | 115 | 38 | 6 | AA | GA | GG | minor |
| KIAA0319 | rs9461045 | 0.157 | 115 | 38 | 6 | CC | TC | TT | minor |
SNP, Single Nucleotide Polymorphism; MAF, Minor Allele Frequency; Major, Homozygous Major Allele; Hetero, Heterozygous; Minor, Homozygous Minor Allele.
2.7. Statistical analyses
To test the reliability of the recordings throughout the session a repeated-measures mixed model ANCOVA was employed to the peak-V-latencies to clicks collected at the start and the end of the recording session. Across all participants, a principal component analysis (PCA) with a varimax rotation was employed considering the 9 selected DCDC2/KIAA0319 SNPs to account for the linkage disequilibrium between neighboring SNPs (Meng et al., 2005, Zondervan and Cardon, 2007). Any component with an eigenvalue greater than 1 was retained for further analyses (Kaiser, 1960). The extraction of the factor loadings of the main principal components allocated a weighting factor to every participant. This weighting factor compounds the burden of the genetic risk for each participant. This method has been adopted from Ge et al. (2012). To determine the association between the genetic risk and the electrophysiological measure a multiple regression was calculated with a repeated-measures ANCOVA considering PCA-components as within-subjects factor (DCDC2 and KIAA0319), and response consistency of the brainstem as well as age, gender, familial risk and intelligence as covariates. Additional partial correlations were calculated to determine the univariate amount of variance of response consistency explained by the risk genes; age, sex, familial risk, and intelligence were variables of no interest. We hypothesized that a higher genetic risk loading (meaning a larger sum of risk alleles across considered SNPs within an individual participant) would be associated with a worsened electrophysiological signature. Two-tailed p-values were reported. Statistical analyses were performed in SPSS (IBM).
Joint effects on literacy skills and dyslexia status for the SNPs of KIAA0319 and DCDC2 were calculated by comparing one model including the intercept and the co-variates (age, sex, familial risk and intelligence) against a second model including the intercept, the same co-variates and the SNPs of the gene. The comparison was carried out by a likelihood ratio test (R package ’lm test’, Zeileis and Hothorn, 2002) to investigate the additional information provided by the SNPs.
3. Results
We measured and analyzed cABRs to the [da] syllable in 64 literate children and in 95 preliterate children. Fig. 1b shows the averaged cABRs across participants for the [da] syllable. Fig. 1c exemplifies individual brainstem responses of two single participants. One participant showed a high response consistency with a Pearson correlation coefficient of r = 0.900 while the other participant showed a reduced response consistency with a Pearson correlation coefficient of r = 0.335. The supplementary material provides an alternative account of quantifying the physiological representation of sound.
For literate children we calculated the joint associations between the two genes KIAA0319 and DCDC2 and literacy skills. For KIAA0319, we observed significant association with writing and spelling performance (χ(3) = 13.37, p = 0.004) and no association with reading speed test (χ(3) = 6.88, p = 0.076) or reading comprehension test (χ(3) = 2.14, p = 0.544). For DCDC2, associations were observed for reading speed (χ(6) = 19.37, p = 0.004) as well as reading comprehension (χ(6) = 17.29, p = 0.008). No significant relations occurred between DCDC2 and performance in spelling and writing (χ(6) = 3.69, p = 0.718).
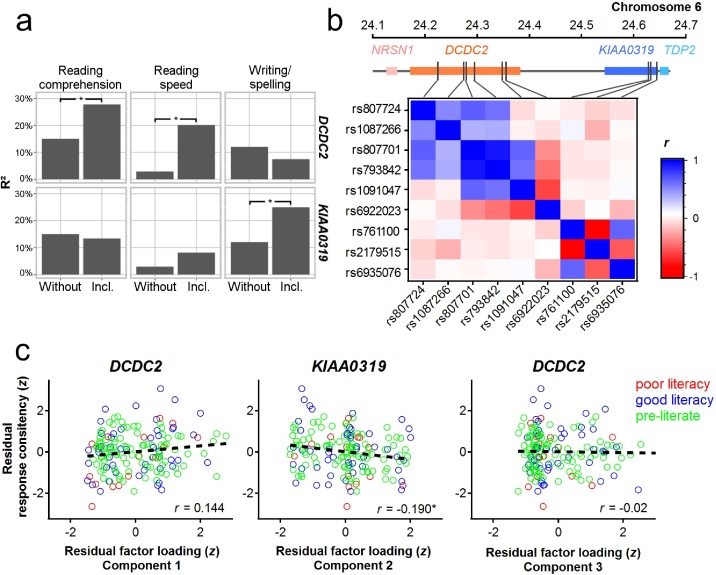
Fig. 2a shows how much of the variance of the three measures of literacy are explained by age, sex, familial risk, and intelligence alone or when additionally including genetic variance.
Fig. 2.
Genetic risk of dyslexia relates to speech evoked brainstem responses. (a) Shown is how much of the variance (adjusted R2) of three measures of literacy skills (reading comprehension, reading speed and writing/spelling) are explained by age, sex, familial risk and intelligence alone (left bars ‘without genetics’) or when additionally including variants of DCDC2 (right bars ‘incl. genetics’, upper panel) or variants of KIAA0319 (right bars ‘incl. genetics’, lower panel). Statistical significance of differences between the models without and including genetics were assessed using the likelihood ratio test. Significant differences between both models at the level of p ≤ 0.05 are marked with an asterisk. (b) Gene and marker locations on the top of the correlation matrix are proportional to physical distances on chromosome 6. The correlation matrix demonstrates the linkage disequilibrium of neighboring SNPs. (c) Response consistency showed a trend for a positive correlation with the individual factor load of the first principal component allocating the genetic risk loading of DCDC2. Response consistency was negatively correlated with the individual factor load of the second principal component allocating the genetic risk loading of KIAA0319. No correlation was evident between response consistency and the factor loadings of the third principal component. To illustrate the distribution of children with poor literacy skills compared to children with good literacy skills poor performers are depicted in red in case mean literacy across reading speed, reading comprehension and spelling performance was under the 25th percentile. Blue indicates good performers (≥25th percentile) and green indicates preliterate children. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
We performed a PCA to account for the linkage disequilibrium of SNPs located near each other (Fig. 2b). Three components displayed an eigenvalue greater than one. The first component had an eigenvalue of 3.2 and explained 26.8% of the allelic variance in the population with a factor loading allocating four DCDC2 SNPs (rs807724 = 0.925, rs1087266 = 0.747, rs80771 = 0.716, rs793842 = 0.678). The second component had an eigenvalue of 2.3 and explained 26.4 % of variance loading on all KIAA0319 SNPs (rs761100 = −0.943, rs2179515 = 0.898, rs6935076 = 0.793). The third component had an eigenvalue of 1.6 and explained 26.4 % of variance loading on the remaining two DCDC2 SNPs (rs1091047 = 0.923, rs6922023 = −0.807). The DCDC2 SNPs rs793842 and rs807701 did also load on component three (0.660, 0.631). Total variance explained by these three principal components was 79.6 %.
The repeated-measures ANCOVA yielded a Gene × Brainstem interaction (F(2153) = 4.35, p = 0.014). Tests of within-subjects contrasts indicated a quadratic relationship between genotypes and response consistency of the auditory brainstem (F(1153) = 6.7, p = 0.011). To further disentangle this gene-brain association we calculated post-hoc partial correlations between the response consistency at the level of the auditory brainstem and the factor loadings of the principal components extracted. Age, gender, familial risk, and intelligence were variables of control. Response consistency showed a trending positive correlation with the first principal component (r = 0.144, p = 0.075) allocating genetic risk of DCDC2, a significant negative correlation with the second principal component (r = −0.190, p = 0.018) allocating genetic risk of KIAA0319 (Fig. 2c), and no correlation with the third principal component (r = −0.02, p = 0.801).
4. Discussion
A PCA separated the genetic risk of dyslexia in our sample into three principal components. Two components explained the variance of risk loading associated with DCDC2 and one component explained the variance of risk loading of KIAA0319. Most interesting was the partial correlation showing that children with a higher amount of risk alleles of KIAA0319 have less stable speech evoked brainstem responses whereas children with lower KIAA0319 risk loading have more stable responses. Surprisingly, an opposite trend emerged for the partial correlation between DCDC2 and electrophysiology. A higher number of DCDC2 risk alleles was associated with more stable cABRs. This electrophysiology by gene interaction suggests that especially the KIAA0319 risk gene carriers are prone to noisy processing of speech stimuli at a very early stage of the auditory pathway. Conversely, the data suggest that DCDC2 risk variants might have a protective function for early auditory sensations. The different contribution of the risk factors to auditory sensations is unexpected, but most interesting. It supports reports of disparate cognitive profiles of dyslexia, when distinctively clustering the cognitive measures phonological, auditory, visual attention, and automatic skills, as first described by Heim and Grande (2012). It is highly plausible that this heterogeneity of underlying cognitive profiles could be associated with heterogeneous neurobiological mechanisms caused by specific genotypes (Cicchini et al., 2015).
Risk variants in KIAA0319 and DCDC2 have recently been shown to interact with each other and to influence dyslexia phenotypes in a non-additive manner (Powers et al., 2013). This trans-genetic interaction between DCDC2 risk haplotypes and KIAA0319 risk haplotypes is based on the strong linkage disequilibrium of two DCDC2 risk haplotypes to READ1 (regulatory element associated with dyslexia; GenBank accession No BV677278). READ1 regulates KIAA0319 expression through a KIAA0319 risk haplotype (Powers et al., 2016). Interestingly, Powers and colleagues suggest that READ1 alleles act in both ways, either deleterious or protective depending on length or structure of the allele. Long READ1 alleles with insertions in repeat unit 2 show significant associations with severe reading disability. Conversely, short READ1 alleles with a deletion of one copy of repeat 1 showed nominal associations not surviving a Bonferroni correction (Powers et al., 2016). Further combined analyses testing different combinations of READ1 and a KIAA0319 risk haplotype confirm the deleterious effect of READ1 allele 5 and READ1 allele 6 in synergy with the KIAA0319 risk haplotype. In contrast, READ1 allele 3 in combination with the same KIAA0319 risk haplotype was related to unaffected phenotypes. This gene–behaviour association demonstrates opposing effects of dyslexia risk genes on behaviour. In the light of these opposing effects it occurs likely that DCDC2 and KIAA0319 risk alleles could conversely act on brain-gene relationships. The present data suggest such a KIAA0319-DCDC2 interaction to have an impact on the response consistency of the auditory brainstem.
The neurobiological substrates dominantly contributing to the complex auditory brainstem potentials are the nuclei of the auditory midbrain including the inferior colliculus and the lateral lemniscus. However, it cannot be excluded that preceding central auditory processes e.g. in the auditory nerve, cochlear nucleus or the superior olive could contribute to irregular patterns in scalp-recorded responses, which aggregate phase-locked activity of the auditory midbrain (Bidelman, 2015, Glaser et al., 1976, Skoe and Kraus, 2010, Smith et al., 1975, Sohmer et al., 1977). Information about gene expression in the auditory pathway and especially in the inferior colliculus and the lateral lemniscus is fragmentary, and gene expression across mammals and life span is diverse. The Allen Brain Atlas (www.brain-map.org) provides the first neuroanatomical precise, genome-wide maps of transcript distributions. KIAA0319 and DCDC2 are highly expressed in the central nucleus of the inferior colliculus of the prenatal human brain relative to other tissues (Miller et al., 2014, Shen et al., 2012). In the Adult Human Brain Tissue Gene Expression Profiles such information is still missing (Hawrylycz et al., 2012).
How these genes eventually interact with neurophysiology especially in the region of the inferior colliculus and the lateral lemniscus and with behavior is largely unknown. Lately, animal studies started elucidating electrophysiological correlates of KIAA0319 and DCDC2 knockdown in cortical regions of rodents (Centanni et al., 2016, Centanni et al., 2014a, Centanni et al., 2014b, Che et al., 2014, Truong et al., 2014). In the context of auditory processing it is of particular interest that in utero RNAi of Kiaa0319 knockdown in rats introduced a decreased consistency of responses to speech combined with a reduced neural discrimination ability of speech sounds (Centanni et al., 2014a, Centanni et al., 2014b). The authors report that a reduced expression of Kiaa0319 increased neural excitability and input resistance when recording from affected neurons in the auditory cortex. These observations are in line with the current finding that a decreased response consistency was associated with an increased genetic burden of KIAA0319 risk alleles. A most recent electrophysiological study of the same group reports that suppression of the dyslexia risk variant of Dcdc2 in rats does not affect spike timing at the level of the auditory cortex, although animals lost the ability to identify speech sounds from a continuous stream (Centanni et al., 2016). This is reminiscent of the current findings. The present data show that DCDC2 risk alleles have a trending association with an increased response consistency. This indicates better phase-locking capabilities of neurons in the auditory midbrain in carriers of DCDC2 risk alleles. However, a different animal study showed that a Dcdc2 knockout impaired temporal encoding as evident in degraded neural spike timing during the encoding of rapid sequential sensory inputs to the somatosensory cortex (Che et al., 2014). This discrepancy might be disentangled on the background of above elaborated trans-gene interactions suggesting that certain DCDC2 haplotypes regulate KIAA0319 expression resulting in opposing behavioral effects.
Here, we present the first observation of an interaction between dyslexia risk genes and the encoding of sounds in the auditory brainstem in humans. The small number of participants makes it necessary to consider these observations as preliminary with a need for replication. To date, no studies exist elucidating the physiology of Kiaa0319 or Dcdc2 knockout or knockdown in auditory brainstem regions. Hence, future studies are necessary to further elucidate the here observed brain-gene interactions. The specification of such brain-gene interactions will ultimately lead to a better understanding of the origin of diverse dyslexia phenotypes.
Authors contributions
N.E.N. and A.F. conceived and designed the experiments, N.E.N., B.M., J.L., and G.S., performed the experiments; N.E.N, B.M., J.L. analyzed the data; N.E.N., M.G., I.K., N.K., H.K., A.W., M.A.S., J.B., F.E., and T.G., contributed unpublished analytic tools, N.E.N. wrote the paper. All authors commented on the paper.
Conflict of interest
None.
Acknowledgments
This work was supported by the Max Planck Society and the Fraunhofer Society (Legascreen (M.FE.A.NEPF0001) as a project within the framework of the “Pakt für Forschung und Innovation”). HK was also funded by the Leipzig Interdisciplinary Research Cluster of Genetic Factors, Clinical Phenotypes and Environment (LIFE Center, Universität Leipzig). We thank all members of the child laboratory for help with experiments and Clara Ekerdt for proofreading. Part of this work was previously presented at the Annual Meeting of the Society for Neuroscience, October 17–21, 2015, Chicago, USA; and at the Thirty-Fourth Workshop on Cognitive Neuropsychology, January 24–29, 2016, Bressanone, Italy.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.dcn.2017.01.008.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Aiken S.J., Picton T.W. Envelope and spectral frequency-following responses to vowel sounds. Hear. Res. 2008;245:35–47. doi: 10.1016/j.heares.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Banai K., Nicol T., Zecker S.G., Kraus N. Brainstem timing: implications for cortical processing and literacy. J. Neurosci. 2005;25:9850–9857. doi: 10.1523/JNEUROSCI.2373-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benasich A.A., Thomas J.J., Choudhury N., Leppänen P.H.T. The importance of rapid auditory processing abilities to early language development: evidence from converging methodologies. Dev. Psychobiol. 2002;40:278–292. doi: 10.1002/dev.10032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidelman G.M. Multichannel recordings of the human brainstem frequency-following response: scalp topography, source generators, and distinctions from the transient ABR. Hear. Res. 2015;323:68–80. doi: 10.1016/j.heares.2015.01.011. [DOI] [PubMed] [Google Scholar]
- Boets B., de Beeck H.P.O., Vandermosten M., Scott S.K., Gillebert C.R., Mantini D., Bulthé J., Sunaert S., Wouters J., Ghesquière P. Intact but less accessible phonetic representations in adults with dyslexia. Science. 2013;342:1251–1254. doi: 10.1126/science.1244333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosse M.-L., Tainturier M.J., Valdois S. Developmental dyslexia: the visual attention span deficit hypothesis. Cognition. 2007;104:198–230. doi: 10.1016/j.cognition.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Burbridge T.J., Wang Y., Volz A.J., Peschansky V.J., Lisann L., Galaburda A.M., Lo Turco J.J., Rosen G.D. Postnatal analysis of the effect of embryonic knockdown and overexpression of candidate dyslexia susceptibility gene homolog Dcdc2 in the rat. Neuroscience. 2008;152:723–733. doi: 10.1016/j.neuroscience.2008.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrion-Castillo A., Franke B., Fisher S.E. Molecular genetics of dyslexia: an overview. Dyslexia. 2013;19:214–240. doi: 10.1002/dys.1464. [DOI] [PubMed] [Google Scholar]
- Centanni T.C., Chen F., Booker A.M., Engineer C.T., Sloan A.M., Rennaker R.L., LoTurco J.J., Kilgard M.P. Speech sound processing deficits and training-induced neural plasticity in rats with dyslexia gene knockdown. PLoS One. 2014;9 doi: 10.1371/journal.pone.0098439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centanni T.M., Booker A.B., Sloan A.M., Chen F., Maher B.J., Carraway R.S., Khodaparast N., Rennaker R., LoTurco J.J., Kilgard M.P. Knockdown of the dyslexia-associated gene Kiaa0319 impairs temporal responses to speech stimuli in rat primary auditory cortex. Cereb. Cortex. 2014;24:1753–1766. doi: 10.1093/cercor/bht028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centanni T.C., Booker A.B., Chen F., Sloan A.M., Carraway R.S., Rennaker R.L., LoTurco J.J., Kilgard M.P. Knockdown of dyslexia-gene Dcdc2 interferes with speech sound discrimination in continuous streams. J. Neurosci. 2016;36:4895–4906. doi: 10.1523/JNEUROSCI.4202-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasekaran B., Hornickel J., Skoe E., Nicol T., Kraus N. Context-dependent encoding in the human auditory brainstem relates to hearing speech in noise: implications for developmental dyslexia. Neuron. 2009;64:311–319. doi: 10.1016/j.neuron.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Che A., Girgenti M.J., LoTurco J. The dyslexia-associated gene Dcdc2 is required for spike-timing precision in mouse neocortex. Biol. Psychiatry. 2014;76:387–396. doi: 10.1016/j.biopsych.2013.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchini G.M., Marino C., Mascheretti S., Perani D., Morrone M.C. Strong motion deficits in dyslexia associated with DCDC2 gene alteration. J. Neurosci. 2015;35:8059–8064. doi: 10.1523/JNEUROSCI.5077-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cope N., Harold D., Hill G., Moskvina V., Stevenson J., Holmans P., Owen M.J., O’Donovan M.C., Williams J. Strong evidence that KIAA0319 on chromosome 6p is a susceptibility gene for developmental dyslexia. Am. J. Hum. Genet. 2005;76:581–591. doi: 10.1086/429131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cope N., Eicher J.D., Meng H., Gibson C.J., Hager K., Lacadie C., Fulbright R.K., Constable R.T., Page G.P., Gruen J.R. Variants in the DYX2 locus are associated with altered brain activation in reading-related brain regions in subjects with reading disability. Neuroimage. 2012;63:148–156. doi: 10.1016/j.neuroimage.2012.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czamara D., Bruder J., Becker J., Bartling J., Hoffmann P., Ludwig K.U., Müller-Myhsok B., Schulte-Körne G. Association of a rare variant with mismatch negativity in a region between KIAA0319 and DCDC2 in dyslexia. Behav. Genet. 2010;41:110–119. doi: 10.1007/s10519-010-9413-6. [DOI] [PubMed] [Google Scholar]
- Díaz B., Hintz F., Kiebel S.J., von Kriegstein K. Dysfunction of the auditory thalamus in developmental dyslexia. Proc. Natl. Acad. Sci. U. S. A. 2012;109:13841–13846. doi: 10.1073/pnas.1119828109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darki F., Peyrard-Janvid M., Matsson H., Kere J., Klingberg T. DCDC2 polymorphism is associated with left temporoparietal gray and white matter structures during development. J. Neurosci. 2014;34:14455–14462. doi: 10.1523/JNEUROSCI.1216-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher S.E., DeFries J.C. Developmental dyslexia: genetic dissection of a complex cognitive trait. Nat. Rev. Neurosci. 2002;3:767–780. doi: 10.1038/nrn936. [DOI] [PubMed] [Google Scholar]
- Fisher R.A. On the probable error of a coefficient of correlation deduced from a small sample. Metron. 1921:3–32. [Google Scholar]
- Francks C., Paracchini S., Smith S.D., Richardson A.J., Scerri T.S., Cardon L.R., Marlow A.J., MacPhie I.L., Walter J., Pennington B.F., Fisher S.E., Olson R.K., DeFries J.C., Stein J.F., Monaco A.P. A 77-kilobase region of chromosome 6p22.2 is associated with dyslexia in families from the United Kingdom and from the United States. Am. J. Hum. Genet. 2004;75:1046–1058. doi: 10.1086/426404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich M., Friederici A.D. N400-like semantic incongruity effect in 19-month-olds: processing known words in picture contexts. J. Cogn. Neurosci. 2004;16:1465–1477. doi: 10.1162/0898929042304705. [DOI] [PubMed] [Google Scholar]
- Gabel L.A., Gibson C.J., Gruen J.R., LoTurco J.J. Progress towards a cellular neurobiology of reading disability. Neurobiol. Dis. 2010;38:173–180. doi: 10.1016/j.nbd.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge T., Feng J., Hibar D.P., Thompson P.M., Nichols T.E. Increasing power for voxel-wise genome-wide association studies: the random field theory, least square kernel machines and fast permutation procedures. NeuroImage. 2012;63:858–873. doi: 10.1016/j.neuroimage.2012.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraud A.-L., Ramus F. Neurogenetics and auditory processing in developmental dyslexia. Curr. Opin. Neurobiol. Neurogenet. 2013;23:37–42. doi: 10.1016/j.conb.2012.09.003. [DOI] [PubMed] [Google Scholar]
- Glaser E.M., Suter C.M., Dasheiff R., Goldberg A. The human frequency-following response: its behavior during continuous tone and tone burst stimulation. Electroencephalogr. Clin. Neurophysiol. 1976;40:25–32. doi: 10.1016/0013-4694(76)90176-0. [DOI] [PubMed] [Google Scholar]
- Goswami U. A temporal sampling framework for developmental dyslexia. Trends Cogn. Sci. 2011;15:3–10. doi: 10.1016/j.tics.2010.10.001. [DOI] [PubMed] [Google Scholar]
- Hämäläinen J.A., Salminen H.K., Leppänen P.H.T. Basic auditory processing deficits in dyslexia systematic review of the behavioral and event-related potential/field evidence. J. Learn. Disabil. 2013;46:413–427. doi: 10.1177/0022219411436213. [DOI] [PubMed] [Google Scholar]
- Harold D., Paracchini S., Scerri T., Dennis M., Cope N., Hill G., Moskvina V., Walter J., Richardson A.J., Owen M.J., Stein J.F., Green E.D., O’Donovan M.C., Williams J., Monaco A.P. Further evidence that the KIAA0319 gene confers susceptibility to developmental dyslexia. Mol. Psychiatry. 2006;11:1085–1091. doi: 10.1038/sj.mp.4001904. [DOI] [PubMed] [Google Scholar]
- Hawrylycz M.J., Lein E.S., Guillozet-Bongaarts A.L., Shen E.H., Ng L., Miller J.A., van de Lagemaat L.N., Smith K.A., Ebbert A., Riley Z.L., Abajian C., Beckmann C.F., Bernard A., Bertagnolli D., Boe A.F., Cartagena P.M., Chakravarty M.M., Chapin M., Chong J., Dalley R.A., Daly B.D., Dang C., Datta S., Dee N., Dolbeare T.A., Faber V., Feng D., Fowler D.R., Goldy J., Gregor B.W., Haradon Z., Haynor D.R., Hohmann J.G., Horvath S., Howard R.E., Jeromin A., Jochim J.M., Kinnunen M., Lau C., Lazarz E.T., Lee C., Lemon T.A., Li L., Li Y., Morris J.A., Overly C.C., Parker P.D., Parry S.E., Reding M., Royall J.J., Schulkin J., Sequeira P.A., Slaughterbeck C.R., Smith S.C., Sodt A.J., Sunkin S.M., Swanson B.E., Vawter M.P., Williams D., Wohnoutka P., Zielke H.R., Geschwind D.H., Hof P.R., Smith S.M., Koch C., Grant S.G.N., Jones A.R. An anatomically comprehensive atlas of the adult human brain transcriptome. Nature. 2012;489:391–399. doi: 10.1038/nature11405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim S., Grande M. Fingerprints of developmental dyslexia. Trends Neurosci. Educ. 2012;1:10–14. [Google Scholar]
- Heim S., Grande M., Pape-Neumann J., van Ermingen M., Meffert E., Grabowska A., Huber W., Amunts K. Interaction of phonological awareness and magnocellular processing during normal and dyslexic reading: behavioural and fMRI investigations. Dyslexia. 2010;16:258–282. doi: 10.1002/dys.409. [DOI] [PubMed] [Google Scholar]
- Hornickel J., Kraus N. Unstable representation of sound: a biological marker of dyslexia. J. Neurosci. 2013;33:3500–3504. doi: 10.1523/JNEUROSCI.4205-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornickel J., Skoe E., Nicol T., Zecker S., Kraus N. Subcortical differentiation of stop consonants relates to reading and speech-in-noise perception. Proc. Natl. Acad. Sci. U. S. A. 2009;106:13022–13027. doi: 10.1073/pnas.0901123106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe-Dax S., Raviv O., Jacoby N., Loewenstein Y., Ahissar M. A computational model of implicit memory captures dyslexics’ perceptual deficits. J. Neurosci. 2015;35:12116–12126. doi: 10.1523/JNEUROSCI.1302-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser H.F. The application of electronic computers to factor analysis. Educ. Psychol. Meas. 1960;20:141–151. [Google Scholar]
- Kaplan D.E., Gayán J., Ahn J., Won T.-W., Pauls D., Olson R.K., DeFries J.C., Wood F., Pennington B.F., Page G.P., Smith S.D., Gruen J.R. Evidence for linkage and association with reading disability, on 6p21. 3–22. Am. J. Hum. Genet. 2002;70:1287–1298. doi: 10.1086/340449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman A.S., Kaufman N.L., Melchers P., Preuß U. Swets & Zeitlinger; Frankfurt: 2009. Kaufman Assessment Battery for Children, Deutsche Version. [Google Scholar]
- Kujala T., Lovio R., Lepistö T., Laasonen M., Näätänen R. Evaluation of multi-attribute auditory discrimination in dyslexia with the mismatch negativity. Clin. Neurophysiol. 2006;117:885–893. doi: 10.1016/j.clinph.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Lind P.A., Luciano M., Wright M.J., Montgomery G.W., Martin N.G., Bates T.C. Dyslexia and DCDC2: normal variation in reading and spelling is associated with DCDC2 polymorphisms in an Australian population sample. Eur. J. Hum. Genet. 2010;18:668–673. doi: 10.1038/ejhg.2009.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciano M., Lind P.A., Duffy D.L., Castles A., Wright M.J., Montgomery G.W., Martin N.G., Bates T.C. A haplotype spanning KIAA0319 and TTRAP is associated with normal variation in reading and spelling ability. Biol. Psychiatry. 2007;62:811–817. doi: 10.1016/j.biopsych.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Ludwig K.U., Schumacher J., Schulte-Körne G., König I.R., Warnke A., Plume E., Anthoni H., Peyrard-Janvid M., Meng H., Ziegler A., Remschmidt H., Kere J., Gruen J.R., Müller-Myhsok B., Nöthen M.M., Hoffmann P. Investigation of the DCDC2 intron 2 deletion/compound short tandem repeat polymorphism in a large German dyslexia sample. Psychiatr. Genet. 2008;18:310–312. doi: 10.1097/YPG.0b013e3283063a78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng H., Smith S.D., Hager K., Held M., Liu J., Olson R.K., Pennington B.F., DeFries J.C., Gelernter J., O’Reilly-Pol T., Somlo S., Skudlarski P., Shaywitz S.E., Shaywitz B.A., Marchione K., Wang Y., Paramasivam M., LoTurco J.J., Page G.P., Gruen J.R. DCDC2 is associated with reading disability and modulates neuronal development in the brain. Proc. Natl. Acad. Sci. U. S. A. 2005;102:17053–17058. doi: 10.1073/pnas.0508591102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J.A., Ding S.-L., Sunkin S.M., Smith K.A., Ng L., Szafer A., Ebbert A., Riley Z.L., Royall J.J., Aiona K., Arnold J.M., Bennet C., Bertagnolli D., Brouner K., Butler S., Caldejon S., Carey A., Cuhaciyan C., Dalley R.A., Dee N., Dolbeare T.A., Facer B.A.C., Feng D., Fliss T.P., Gee G., Goldy J., Gourley L., Gregor B.W., Gu G., Howard R.E., Jochim J.M., Kuan C.L., Lau C., Lee C.-K., Lee F., Lemon T.A., Lesnar P., McMurray B., Mastan N., Mosqueda N., Naluai-Cecchini T., Ngo N.-K., Nyhus J., Oldre A., Olson E., Parente J., Parker P.D., Parry S.E., Stevens A., Pletikos M., Reding M., Roll K., Sandman D., Sarreal M., Shapouri S., Shapovalova N.V., Shen E.H., Sjoquist N., Slaughterbeck C.R., Smith M., Sodt A.J., Williams D., Zöllei L., Fischl B., Gerstein M.B., Geschwind D.H., Glass I.A., Hawrylycz M.J., Hevner R.F., Huang H., Jones A.R., Knowles J.A., Levitt P., Phillips J.W., Šestan N., Wohnoutka P., Dang C., Bernard A., Hohmann J.G., Lein E.S. Transcriptional landscape of the prenatal human brain. Nature. 2014;508:199–206. doi: 10.1038/nature13185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newbury D.F., Paracchini S., Scerri T.S., Winchester L., Addis L., Richardson A.J., Walter J., Stein J.F., Talcott J.B., Monaco A.P. Investigation of dyslexia and SLI risk variants in reading- and language-impaired subjects. Behav. Genet. 2011;41:90–104. doi: 10.1007/s10519-010-9424-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paracchini S., Thomas A., Castro S., Lai C., Paramasivam M., Wang Y., Keating B.J., Taylor J.M., Hacking D.F., Scerri T., Francks C., Richardson A.J., Wade-Martins R., Stein J.F., Knight J.C., Copp A.J., LoTurco J., Monaco A.P. The chromosome 6p22 haplotype associated with dyslexia reduces the expression of KIAA0319, a novel gene involved in neuronal migration. Hum. Mol. Genet. 2006;15:1659–1666. doi: 10.1093/hmg/ddl089. [DOI] [PubMed] [Google Scholar]
- Paracchini S., Steer C.D., Buckingham L.-L., Morris A.P., Ring S., Scerri T., Stein J., Pembrey M.E., Ragoussis J., Golding J., Monaco A.P. Association of the KIAA0319 dyslexia susceptibility gene with reading skills in the general population. Am. J. Psychiatry. 2008;165:1576–1584. doi: 10.1176/appi.ajp.2008.07121872. [DOI] [PubMed] [Google Scholar]
- Paulesu E., Danelli L., Berlingeri M. Reading the dyslexic brain: multiple dysfunctional routes revealed by a new meta-analysis of PET and fMRI activation studies. Front. Hum. Neurosci. 2014;8:830. doi: 10.3389/fnhum.2014.00830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peschansky V.J., Burbridge T.J., Volz A.J., Fiondella C., Wissner-Gross Z., Galaburda A.M., Turco J.J.L., Rosen G.D. The effect of variation in expression of the candidate dyslexia susceptibility gene homolog Kiaa0319 on neuronal migration and dendritic morphology in the rat. Cereb. Cortex. 2010;20:884–897. doi: 10.1093/cercor/bhp154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson R.L., Pennington B.F. Developmental dyslexia. Lancet. 2012;379:1997–2007. doi: 10.1016/S0140-6736(12)60198-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinel P., Fauchereau F., Moreno A., Barbot A., Lathrop M., Zelenika D., Bihan D.L., Poline J.-B., Bourgeron T., Dehaene S. Genetic variants of FOXP2 and KIAA0319/TTRAP/THEM2 locus are associated with altered brain activation in distinct language-related regions. J. Neurosci. 2012;32:817–825. doi: 10.1523/JNEUROSCI.5996-10.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poelmans G., Buitelaar J.K., Pauls D.L., Franke B. A theoretical molecular network for dyslexia: integrating available genetic findings. Mol. Psychiatry. 2011;16:365–382. doi: 10.1038/mp.2010.105. [DOI] [PubMed] [Google Scholar]
- Powers N.R., Eicher J.D., Butter F., Kong Y., Miller L.L., Ring S.M., Mann M., Gruen J.R. Alleles of a polymorphic ETV6 binding site in DCDC2 confer risk of reading and language impairment. Am. J. Hum. Genet. 2013;93:19–28. doi: 10.1016/j.ajhg.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers N.R., Eicher J.D., Miller L.L., Kong Y., Smith S.D., Pennington B.F., Willcutt E.G., Olson R.K., Ring S.M., Gruen J.R. The regulatory element READ1 epistatically influences reading and language, with both deleterious and protective alleles. J. Med. Genet. 2016;53:163–171. doi: 10.1136/jmedgenet-2015-103418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinque D., Kittler R., Kayser M., Stoneking M., Nasidze I. Evaluation of saliva as a source of human DNA for population and association studies. Anal. Biochem. 2006;353:272–277. doi: 10.1016/j.ab.2006.03.021. [DOI] [PubMed] [Google Scholar]
- Ramus F., Ahissar M. Developmental dyslexia: the difficulties of interpreting poor performance, and the importance of normal performance. Cogn. Neuropsychol. 2012;29:104–122. doi: 10.1080/02643294.2012.677420. [DOI] [PubMed] [Google Scholar]
- Scerri T.S., Morris A.P., Buckingham L.-L., Newbury D.F., Miller L.L., Monaco A.P., Bishop D.V.M., Paracchini S. DCDC2, KIAA0319 and CMIP are associated with reading-related traits. Biol. Psychiatry. 2011;70:237–245. doi: 10.1016/j.biopsych.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider W., Schlagmüller M., Ennemoser M. Hogrefe; 2007. LGVT 6–12 Lesegeschwindigkeits- und −verständnistest für die Klassen 6–12. [Google Scholar]
- Schulte-Körne G., Bruder J. Clinical neurophysiology of visual and auditory processing in dyslexia: a review. Clin. Neurophysiol. 2010;121:1794–1809. doi: 10.1016/j.clinph.2010.04.028. [DOI] [PubMed] [Google Scholar]
- Schulte-Körne G., Remschmidt H. Legasthenie-Symptomatik, Diagnostik, Ursachen, Verlauf und Behandlung. Dtsch. Ärzteblatt. 2003;7:A396–A406. [Google Scholar]
- Schumacher J., Anthoni H., Dahdouh F., König I.R., Hillmer A.M., Kluck N., Manthey M., Plume E., Warnke A., Remschmidt H., Hülsmann J., Cichon S., Lindgren C.M., Propping P., Zucchelli M., Ziegler A., Peyrard-Janvid M., Schulte-Körne G., Nöthen M.M., Kere J. Strong genetic evidence of DCDC2 as a susceptibility gene for dyslexia. Am. J. Hum. Genet. 2006;78:52–62. doi: 10.1086/498992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher J., Hoffmann P., Schmäl C., Schulte-Körne G., Nöthen M.M. Genetics of dyslexia: the evolving landscape. J. Med. Genet. 2007;44:289–297. doi: 10.1136/jmg.2006.046516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaywitz S.E., Shaywitz B.A., Fletcher J.M., Escobar M.D. Prevalence of reading disability in boys and girls: results of the connecticut longitudinal study. JAMA. 1990;264:998–1002. [PubMed] [Google Scholar]
- Shen E.H., Overly C.C., Jones A.R. The allen human brain atlas. Trends Neurosci. 2012;35:711–714. doi: 10.1016/j.tins.2012.09.005. [DOI] [PubMed] [Google Scholar]
- Skoe E., Kraus N. Auditory brainstem response to complex sounds: a tutorial. Ear Hear. 2010;31:302–324. doi: 10.1097/AUD.0b013e3181cdb272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J.C., Marsh J.T., Brown W.S. Far-field recorded frequency-following responses: evidence for the locus of brainstem sources. Electroencephalogr. Clin. Neurophysiol. 1975;39:465–472. doi: 10.1016/0013-4694(75)90047-4. [DOI] [PubMed] [Google Scholar]
- Snowling M.J. From language to reading and dyslexia1. Dyslexia. 2001;7:37–46. doi: 10.1002/dys.185. [DOI] [PubMed] [Google Scholar]
- Sohmer H., Pratt H., Kinarti R. Sources of frequency following responses (FFR) in man. Electroencephalogr. Clin. Neurophysiol. 1977;42:656–664. doi: 10.1016/0013-4694(77)90282-6. [DOI] [PubMed] [Google Scholar]
- Stein J., Walsh V. To see but not to read; the magnocellular theory of dyslexia. Trends Neurosci. 1997;20:147–152. doi: 10.1016/s0166-2236(96)01005-3. [DOI] [PubMed] [Google Scholar]
- Stock C., Schneider W. Hogrefe Verlag; Göttingen: 2008. Deutscher Rechtschreibtest für das dritte und vierte Schuljahr. [Google Scholar]
- Strait D.L., Hornickel J., Kraus N. Subcortical processing of speech regularities underlies reading and music aptitude in children. Behav. Brain Funct. 2011;7:1. doi: 10.1186/1744-9081-7-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szalkowski C.E., Fiondella C.G., Galaburda A.M., Rosen G.D., LoTurco J.J., Fitch R.H. Neocortical disruption and behavioral impairments in rats following in utero RNAi of candidate dyslexia risk gene Kiaa0319. Int. J. Dev. Neurosci. 2012;30:293–302. doi: 10.1016/j.ijdevneu.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szalkowski C.E., Fiondella C.F., Truong D.T., Rosen G.D., LoTurco J.J., Fitch R.H. The effects of Kiaa0319 knockdown on cortical and subcortical anatomy in male rats. Int. J. Dev. Neurosci. 2013;31:116–122. doi: 10.1016/j.ijdevneu.2012.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallal P. Auditory temporal perception, phonics, and reading disabilities in children. Brain Lang. 1980;9:182–198. doi: 10.1016/0093-934x(80)90139-x. [DOI] [PubMed] [Google Scholar]
- Tallal P. Improving neural response to sound improves reading. Proc. Natl. Acad. Sci. U. S. A. 2012;109:16406–16407. doi: 10.1073/pnas.1214122109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truong D.T., Che A., Rendall A.R., Szalkowski C.E., LoTurco J.J., Galaburda A.M., Holly Fitch R. Mutation of Dcdc2 in mice leads to impairments in auditory processing and memory ability. Genes Brain Behav. 2014;13:802–811. doi: 10.1111/gbb.12170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdois S., Peyrin C., Lassus-Sangosse D., Lallier M., Démonet J.-F., Kandel S. Dyslexia in a French–Spanish bilingual girl: behavioural and neural modulations following a visual attention span intervention. Cortex. 2014;53:120–145. doi: 10.1016/j.cortex.2013.11.006. [DOI] [PubMed] [Google Scholar]
- Vandermosten M., Boets B., Luts H., Poelmans H., Golestani N., Wouters J., Ghesquière P. Adults with dyslexia are impaired in categorizing speech and nonspeech sounds on the basis of temporal cues. Proc. Natl. Acad. Sci. U. S. A. 2010;107:10389–10394. doi: 10.1073/pnas.0912858107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velayos-Baeza A., Levecque C., Kobayashi K., Holloway Z.G., Monaco A.P. The dyslexia-associated KIAA0319 protein undergoes proteolytic processing with γ-secretase-independent intramembrane cleavage. J. Biol. Chem. 2010;285:40148–40162. doi: 10.1074/jbc.M110.145961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D., Petermann F., Lipsius M. third edition. Pearson; Frankfurt: 2009. Wechsler Preschool and Primary Scale of Intelligence. [Google Scholar]
- White-Schwoch T., Kraus N. Physiologic discrimination of stop consonants relates to phonological skills in pre-readers: a biomarker for subsequent reading ability? Front. Hum. Neurosci. 2013;7:899. doi: 10.3389/fnhum.2013.00899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widmann A., Schröger E., Tervaniemi M., Pakarinen S., Kujala T. Mapping symbols to sounds: electrophysiological correlates of the impaired reading process in dyslexia. Lang. Sci. 2012;3:60. doi: 10.3389/fpsyg.2012.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcke A., Weissfuss J., Kirsten H., Wolfram G., Boltze J., Ahnert P. The role of gene DCDC2 in German dyslexics. Ann. Dyslexia. 2009;59:1–11. doi: 10.1007/s11881-008-0020-7. [DOI] [PubMed] [Google Scholar]
- Zeileis A., Hothorn T. Diagnostic checking in regression relationships. R News. 2002;2:7–10. [Google Scholar]
- Zondervan K.T., Cardon L.R. Designing candidate gene and genome-wide case-control association studies. Nat. Protoc. 2007;2:2492–2501. doi: 10.1038/nprot.2007.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.