Highlights
-
•
Chinese dyslexia showed a smaller morpheme-word incongruency effect in left IFG.
-
•
The smaller effect suggests less sensitivity to morphemes in the dyslexics.
-
•
Higher skill in dyslexics was related with smaller incongruency effect in left IFG.
-
•
Higher skill dyslexics may rely on a compensatory whole-word strategy.
-
•
Morpheme effect was specific as it was not found for a phonological task.
Keywords: Functional magnetic resonance imaging (fMRI), Chinese dyslexia, Morphological processing
Abstract
Previous behavioral studies have suggested that morphological awareness is impaired in Chinese children with reading disability (RD), but how this is reflected in brain alterations is not known. Using functional magnetic resonance imaging (fMRI), the current study compared morphological processing in a RD group (11–13 years old) to an age-matched typically developing (TD) group. Participants made semantic relatedness judgments to incongruent word pairs that were either semantically related but did not share a morpheme or semantically unrelated but did share a morpheme. This was compared to conditions where semantic relatedness and morphemic information was congruent. A smaller incongruency effect was found in left dorsal posterior (BA9) and ventral anterior (BA47) inferior frontal gyrus (IFG) in the RD compared to the TD, suggesting that the RD is less sensitive to morphological information. This was a specific deficit as a phonological control task that manipulated congruency between orthography and phonology did not show group differences in the IFG. Moreover, brain activation in the IFG for the incongruency effect in the semantic task was negatively correlated with reading skill for the RD group only, suggesting that higher skill children with RD may rely on a compensatory whole-word strategy by ignoring the morphemic information.
1. Introduction
Dyslexia is defined as a reading difficulty which cannot be accounted by poor intelligence or lacking sufficient education. Estimates of the dyslexia prevalence in children in Mainland China range from 4.5% to 8.0% (Zhang et al., 1996). Chinese children with dyslexia consistently demonstrate difficulties in reading and writing Chinese characters and words (Ho et al., 2004, Shu et al., 2003).
In modern Chinese, 95% of the commonly used words are compounds composed of two or more morphemes (Zhang, 2011). For example, different kinds of alcohol in Chinese are written as  (beer),
(beer),  (liquor),
(liquor),  (wine), etc. These words do not share any orthographic or phonological similarity in English, however in Chinese those words share an identical morpheme
(wine), etc. These words do not share any orthographic or phonological similarity in English, however in Chinese those words share an identical morpheme  , which is pronounced /jiu3/ (number indicates tone) and means ‘alcohol’. At the character level, the majority of Chinese characters are composed of a semantic radical and a phonological radical. For example,
, which is pronounced /jiu3/ (number indicates tone) and means ‘alcohol’. At the character level, the majority of Chinese characters are composed of a semantic radical and a phonological radical. For example,  (river),
(river),  (lake),
(lake),  (ocean),
(ocean),  (wine) and
(wine) and  (thirsty) all share an identical morpheme (the semantic radical ‘
(thirsty) all share an identical morpheme (the semantic radical ‘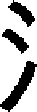 ’, which means water). Due to this, the Chinese language has been defined as a language of compounds (Su, 1994). Behavioral studies have suggested that Chinese compound words have both morphemic and whole-word levels of representation in the mental lexicon (Zhou and Marslen-Wilson, 1994, Zhou and Marslen-Wilson, 1995). In contrast, in alphabetic languages, like English, morphology involves not only compounding, but also inflectional and derivational affixation.
’, which means water). Due to this, the Chinese language has been defined as a language of compounds (Su, 1994). Behavioral studies have suggested that Chinese compound words have both morphemic and whole-word levels of representation in the mental lexicon (Zhou and Marslen-Wilson, 1994, Zhou and Marslen-Wilson, 1995). In contrast, in alphabetic languages, like English, morphology involves not only compounding, but also inflectional and derivational affixation.
Due to the morphological nature of Chinese language, many studies on reading development and impairment have suggested an important role of morphological awareness in Chinese reading. Morphological awareness refers to the ability to reflect on and manipulate morphemes and word formation rules in a language (Kuo and Anderson, 2006). Some studies reported that morphological awareness uniquely predicted Chinese children's reading performance for both typically developing children and dyslexics even after controlling for several predictors including phonological awareness (Chung et al., 2011, Shu et al., 2006). Further, these studies reported that dyslexic readers were less competent than controls in morphological awareness (Chung et al., 2011, Shu et al., 2006, Wong et al., 2013) and that dyslexic readers were best distinguished from age-matched controls with tasks of morphological awareness (Shu et al., 2006). Similarly, studies on children with a familial risk for dyslexia reported that compared to the controls, those with risk performed significantly worse on morphological awareness (McBride-Chang et al., 2008) and that measures of morphological awareness in 5-year-olds with risk can distinguish those with dyslexia from those without dyslexia at 7 years old (McBride-Chang et al., 2011).
A few neuroimaging studies have investigated the neural basis of Chinese dyslexia. Most of them have focused on phonological processing and have revealed structural and functional differences in left middle frontal gyrus (Siok et al., 2008, Siok et al., 2004). Others have investigated visuo-spatial (Siok et al., 2009) and semantic processing (Hu et al., 2010), showing brain differences in left intra-parietal sulcus (Siok et al., 2009) and in posterior middle temporal and angular gyri (Hu et al., 2010). However, none of these studies investigated morphological processing in Chinese dyslexia.
Though there have been behavioral studies suggesting a deficit in morphological awareness in Chinese dyslexia, how this is reflected in alterations in the brain is not known. The goal of the current study was to investigate the brain basis underlying the morphological awareness deficit in Chinese dyslexia and how this was related to reading skill. A semantic relatedness judgment task was used that tapped into morphological processing by manipulating the congruency between semantic relatedness and morphemic overlap in pairs of words. We expected that typically developing readers would show sensitivity to congruency in that they would show greater activation for incongruent compared to congruent pairs of words. Incongruent pairs consisted of words that were either semantically related but did not share a morpheme or were not semantically related but did share a morpheme. Congruent pairs were either words that were semantically related and shared a morpheme or words that were not semantically related and did not share a morpheme. If dyslexic children have a morphological awareness deficit, they should be less sensitive to morphemic information, thereby showing a smaller incongruency effect as compared to typically developing children. A control phonological judgment task manipulating the congruency between orthography and phonology was included to ensure that any group differences were specific to morphological processing.
2. Methods
2.1. Participants
Fourteen typically developing (TD) children (M age = 11.7 years, SD = 0.31, range from 11 to 13; 9 males) and 14 reading disabled (RD) children (M age = 11.9 years, SD = 0.53, range from 11 to 13; 10 males) participated in the study. The participants were all in fifth or sixth-grade and were recruited from three primary schools in Beijing. Informed consent was obtained from all participants and informed consent procedures were approved by the Institutional Review Board at Beijing Normal University and Northwestern University.
Inclusionary criteria for both TD and RD groups were: (1) native Chinese speakers; (2) right-handed; (3) normal hearing and normal/corrected-to-normal vision; (4) no neurological disease or psychiatric disorders; (5) not taking medication affecting the central nervous system, and (6) no Attention Deficit Hyperactivity Disorder (ADHD).
Standardized and informal tests were administered to examine mental and reading ability. Mental ability was measured with the Chinese version of the Wechsler Scale of Intelligence for Children (WISC-R) (Wechsler, 1974). Scoring procedures were based by local norms established by (Lin and Zhang, 1986). Reading ability was measured by the Character Recognition Measure and Assessment Scale for Primary School Children (CRM) (Wang and Tao, 1993). CRM is a widely used standardized test for screening Mandarin-speaking Chinese children with reading disability. In this test, children were asked to make words using given Chinese characters. The task of making words rather than naming characters were used because of the many homophones in Chinese, hence correct pronunciation itself cannot assure the understanding character meaning. The task of making words in the CRM ensures an assessment of the understanding of character meaning. The score on this test reflects how many characters a child can understand among the 3500 commonly used Chinese characters. Because there is not a standardized test for reading fluency in Mainland China, we used an informal character reading fluency (CRF) test as in several previous studies (Siok and Fletcher, 2001, Siok et al., 2004). The CRF consists of 135 high frequency Chinese characters. The characters are all from primary school Chinese language textbooks and were arranged into 5 columns from easy to hard. Children were asked to read aloud the characters as fast and as accurately as possible. Reading fluency was computed as the number of correctly pronounced characters divided by completion time in seconds. The CRF was not used for screening children with dyslexia because it has not been standardized.
RD children met the following inclusionary criteria: (1) Performance IQ above 90 and (2) 1.5 years below their corresponding grade in the CRM test. The age-matched control children met the following criteria: (1) Performance IQ above 90 and (2) no more than 1.5 years below than their corresponding grade in the CRM.
Table 1 presents the means and standard deviations of the mental and reading ability tests in the TD and RD groups for the semantic and phonological tasks. The same children participated in both tasks. The TD and RD groups were matched on age (t(26) = −1.145, p = 0.160), gender (X2(1) = 0.164, p = 0.686) and Performance IQ (t(26) = 1.140, p = 0.265). There were significant differences between groups on both Verbal IQ (t(26) = 2.915, p = 0.007) and reading ability measures: CRM (t(26) = 8.510, p < 0.001) and CRF (t(26) = 6.093, p < 0.001). To rule out the influence of VIQ difference, VIQ were co-varied out in the following group difference analysis for both behavioral and neuroimaging data for both tasks.
Table 1.
Demographic information, mental and reading ability for the typically developing (TD) and reading disability (RD) groups.
| TD | RD | p | |
|---|---|---|---|
| Age (months) | 140.4(3.7) | 143.2(6.4) | >0.05 |
| Gender | 9M 5F | 10M 4F | >0.05 |
| PIQ | 107.7(12.8) | 103.1(8.2) | >0.05 |
| VIQ | 113.8(14.2) | 98.3 (14.2) | <0.01 |
| CRM (out of 3500) | 2989(102) | 2453(212) | <0.001 |
| CRF (character/s) | 1.07(0.17) | 0.63(0.21) | <0.001 |
Note: PIQ = Performance Intelligence Quotient; VIQ = Verbal Intelligence Quotient; CRM = Character Recognition Measure; CRF = Character Reading Fluency. Numbers in parentheses are standard deviations.
2.2. Experimental procedure
The semantic task: for the lexical conditions, two two-character Chinese words were visually presented sequentially with each word presented for 800 ms followed by a 200 ms blank interval. A red fixation cross (+) appeared on the screen after the second stimulus was removed, indicating the need to make a response during the subsequent interval jittered equally between 2200, 2600 and 3000 ms. The participants were asked to judge whether the two words were related in meaning. They were given examples of pairs to aid in their understanding of the instructions, e.g.  (blue sky) &
(blue sky) &  (white clouds) are related, however,
(white clouds) are related, however,  (blue sky) &
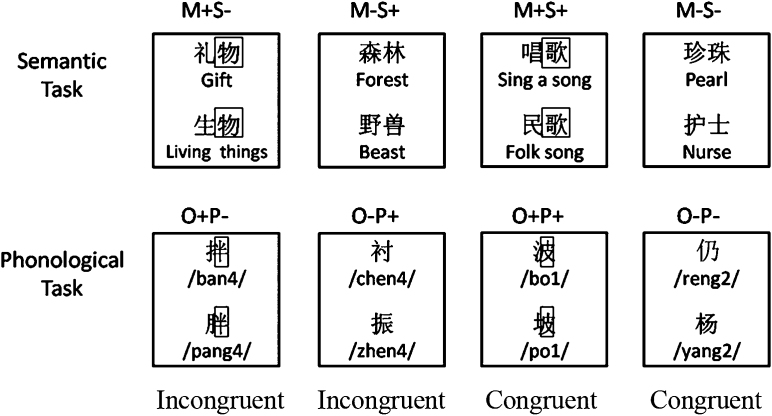
(blue sky) &  (desk) are unrelated. Half of the word pairs were semantically related and half were not. If a pair of words was semantically related, the participants were asked to press a “yes” button with the right index finger; and if the pair of characters was semantically unrelated, the participants were asked to press a “no” button with the right middle finger. Four lexical conditions that independently manipulated the morphemic overlap and semantic relatedness information were included, resulting in two congruent conditions and two incongruent conditions (see Fig. 1). In the two incongruent conditions, the two words either shared an identical morpheme but were not semantically related ((M+S−, e.g.,
(desk) are unrelated. Half of the word pairs were semantically related and half were not. If a pair of words was semantically related, the participants were asked to press a “yes” button with the right index finger; and if the pair of characters was semantically unrelated, the participants were asked to press a “no” button with the right middle finger. Four lexical conditions that independently manipulated the morphemic overlap and semantic relatedness information were included, resulting in two congruent conditions and two incongruent conditions (see Fig. 1). In the two incongruent conditions, the two words either shared an identical morpheme but were not semantically related ((M+S−, e.g.,  [gift] and
[gift] and  [living things], sharing the same morpheme
[living things], sharing the same morpheme  [goods]), or did not share a morpheme but were semantically related (M-S+, e.g.,
[goods]), or did not share a morpheme but were semantically related (M-S+, e.g.,  [forest] and
[forest] and  [beast]). In the two congruent conditions, the two words either shared an identical morpheme and were semantically related (M+S+, e.g.,
[beast]). In the two congruent conditions, the two words either shared an identical morpheme and were semantically related (M+S+, e.g.,  [sing a song] and
[sing a song] and  [folk song], sharing the same morpheme
[folk song], sharing the same morpheme  [song]), or did not share a morpheme and were not semantically related (M−S−, e.g.,
[song]), or did not share a morpheme and were not semantically related (M−S−, e.g.,  [pearl] and
[pearl] and  ± [nurse]).
± [nurse]).
Fig. 1.
Examples of stimuli for the lexical conditions in the semantic task and the phonological task. The small rectangle highlights the shared morpheme or phonetic radical.
There were two control conditions for the lexical conditions. The two kinds of control trials were designed to account for activation due to visual analysis, decision making, and motor control in the lexical conditions. For one control condition (‘perceptual’), two symbols were visually presented sequentially and the participants were asked to judge whether the pair of symbols was identical or not. The symbols were unfamiliar to all the participants. If a pair of symbols (e.g., 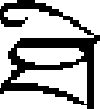 and
and  ) was identical, the participants were asked to press a “yes” button with the right index finger, and if not (e.g.,
) was identical, the participants were asked to press a “yes” button with the right index finger, and if not (e.g.,  and
and  ), the participants were asked to press a “no” button with the right middle finger. Half of the symbol pairs were identical and half were not. The timing for a perceptual trial was the same as for a character trial. For the null trials, two black fixation-crosses were visually presented sequentially with each black fixation-cross presented for 800 ms followed by a 200 ms blank interval. A blue fixation cross appeared on the screen after the second black fixation-cross was removed. Participants were asked to press a yes button with the right index finger when the second black fixation-cross turned blue during the subsequent interval jittered equally between 2200, 2600 and 3000 ms.
), the participants were asked to press a “no” button with the right middle finger. Half of the symbol pairs were identical and half were not. The timing for a perceptual trial was the same as for a character trial. For the null trials, two black fixation-crosses were visually presented sequentially with each black fixation-cross presented for 800 ms followed by a 200 ms blank interval. A blue fixation cross appeared on the screen after the second black fixation-cross was removed. Participants were asked to press a yes button with the right index finger when the second black fixation-cross turned blue during the subsequent interval jittered equally between 2200, 2600 and 3000 ms.
The phonological task: The phonological task used the same design as the semantic relatedness task. However, the participants were asked to judge whether the two characters rhymed or not. Half of the character pairs rhymed and half did not. If a pair of characters rhymed, participants were asked to press a yes button with the right index finger; if the pair of characters did not rhyme, the participants were asked to press a no button with the right middle finger. Four conditions independently manipulating the orthography and phonology were included, resulting in two congruent conditions and two incongruent conditions (see Fig. 1). In the two incongruent conditions, two characters in a pair either shared an identical phonetic radical but did not rhyme (O+P−, e.g.,  and
and  , sharing the phonetic radical
, sharing the phonetic radical  , pronounced as /ban4/ and /pang4/), or did not share a phonetic radical but rhymed (O−P+, e.g.,
, pronounced as /ban4/ and /pang4/), or did not share a phonetic radical but rhymed (O−P+, e.g.,  /chen4/ and
/chen4/ and  /zhen4/). In the two congruent conditions, two characters in a pair either shared an identical phonetic radical and rhymed (O+P+, e.g.,
/zhen4/). In the two congruent conditions, two characters in a pair either shared an identical phonetic radical and rhymed (O+P+, e.g.,  and
and  , sharing the phonetic radical
, sharing the phonetic radical  , pronounced as /bo1/ and /po1/), or did not share a phonetic radical and did not rhyme (O−P−, e.g.,
, pronounced as /bo1/ and /po1/), or did not share a phonetic radical and did not rhyme (O−P−, e.g., 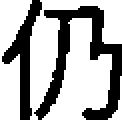 /reng2/ and
/reng2/ and  /yang2/). The two control conditions (‘perceptual’ and ‘null’) were the same as those in the semantic task, except that the symbols used in the perceptual condition were single symbols (
/yang2/). The two control conditions (‘perceptual’ and ‘null’) were the same as those in the semantic task, except that the symbols used in the perceptual condition were single symbols (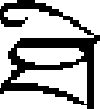 and
and  ) because the phonological task involved single characters.
) because the phonological task involved single characters.
We used an event-related design with four 6 min 44 s runs including two runs of each task. In each run, there were 48 pairs of lexical stimuli, 12 pairs of perceptual stimuli, and 24 pairs of null stimuli. The order of lexical and control trials was optimized for event-related design using OptSeq (http://www.surfer.nmr.mgh.harvard.edu/optseq). Before each run, a short instruction (‘semantic task’ or ‘rhyming task’) was presented to indicate the coming task. The administration of the two tasks was counterbalanced across participants. In each run, there was a 12 s equilibration period at the beginning, and a 22 s period at the end in order to be able to deconvolve the whole hemodynamic response function (HRF) for the last trial. Before the fMRI scanning, a short practice was given to the subjects to get them familiar with the experimental procedure and task requirements.
2.3. Stimulus characteristics
The semantic task: semantic association strength between the first and the second word was assessed using a 7-point scale. Thirty subjects in Beijing were asked to judge to what extent pairs of characters were related. An average score across subjects below 3 was considered unrelated (M = 2.05), whereas an average score over 4.5 was considered related (M = 5.75). Semantic association strengths between the word and their two morphemes were also assessed using a 7-point scale in the same thirty subjects. Only words with strong associations (M > 4) to their two morphemes were included. A strong association between the morpheme and whole word meanings allowed us to effectively examine the interaction between morpheme and word processing.
Several lexical variables were controlled. First, all words were from Chinese language textbooks for primary school. 89.1% of the stimuli were from textbooks for Grade 1–Grade 4, and 10.9% from Grade 5. Second, the words were matched for frequency, acquisition term, and strokes across the four lexical conditions (see Table 2). One way ANOVAs separately on the first words and second words showed no main effect of condition for frequency, acquisition term, or strokes. Third, conditions were matched for word association strength separately for the yes responses and no responses. Two sample t-tests for word association strength between the members of the pair revealed no significant differences between the two yes response conditions or between the two no response conditions. Fourth, conditions were matched for morpheme-word association strength for each word within the pair separately for the yes responses and no responses. 2 morpheme order (first, second) by 2 condition (congruent, incongruent) ANOVAs for morpheme-word association strength revealed no main effects or interactions for the yes or no responses calculated separately for the first and second words.
Table 2.
Stimuli characteristics for the semantic task.
| Frequency |
Acquisition term |
Strokes |
Morpheme-word association |
Word association | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Word 1 | Word 2 | Word 1 | Word 2 | Word 1 | Word 2 | M1W1 | M2W1 | M1W2 | M2W2 | - | |
| M+S+ | 11.2(13.9) | 11.3(12.4) | 4.8(2.6) | 4.8(2.3) | 15.9(4.3) | 15.5(4.2) | 5.3(0.7) | 5.7(0.5) | 5.2(0.6) | 5.6(0.4) | 5.7(0.5) |
| M+S− | 8.2(6.9) | 8.0(7.5) | 5.6(2.6) | 5.4(2.5) | 15.0(5.1) | 15.3(5.9) | 5.3(0.6) | 5.1(0.6) | 5.2(0.7) | 5.1(0.6) | 2.2(0.3) |
| M−S+ | 11.2(9.3) | 10.6(10.4) | 4.9(2.3) | 4.8(2.4) | 15.7(4.4) | 17.1(4.6) | 5.2(0.7) | 5.8(0.4) | 5.5(0.8) | 5.6(0.7) | 5.8(0.4) |
| M−S− | 10.3(7.5) | 10.3(10.8) | 5.3(2.8) | 4.8(2.4) | 16.5(4.3) | 15.9(3.5) | 5.3(0.7) | 5.2(0.9) | 5.2(0.8) | 5.3(0.7) | 1.9(0.4) |
Note: Frequency is the number of times a word shown in Chinese language textbooks from Grade 1 to Grade 6 in primary school. Acquisition term is the term when a word is first shown in Chinese language textbooks. Stroke is the sum of the strokes of the two characters in a word. Morpheme-Word Association and Word association are on a 7-point scale. M1W1 = first morpheme of the first word; M2W1 = second morpheme of the first word; M1W2 = first morpheme of the second word; M2W2 = second morpheme of the second word.
The phonological task: several lexical variables were controlled. First, all characters were from Chinese language textbooks for primary school. 91.1% of the stimuli were from textbooks for Grade 1–Grade 4, and 8.9% from Grade 5. Second, the characters were matched for frequency, acquisition term, and strokes across the four lexical conditions and across presentation order (see Table 3). One way ANOVAs separately on the first characters and second characters showed no main effect of condition for frequency, acquisition term, or strokes. Third, both phonological and orthographic consistency (Bolger et al., 2008a) was matched across congruent and incongruent conditions, and across the presentation order. 2 conditions (congruent, incongruent) by 2 presentation order (first, second) ANOVAs revealed no main effects or interactions for the phonological and orthographic consistency.
Table 3.
Stimuli characteristics for the phonological task.
| Frequency |
Acquisition term |
Strokes |
Phonological consistency |
Orthographic consistency |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cha 1 | Cha 2 | Cha 1 | Cha 2 | Cha 1 | Cha 2 | Cha 1 | Cha 2 | Cha 1 | Cha 2 | |
| O+P+ | 29.8(27.6) | 28.5(29.1) | 5.6(2.2) | 5.3(2.5) | 9.7(2.2) | 9.5(2.5) | 0.85(0.24) | 0.85(0.24) | 0.27(0.21) | 0.30(0.27) |
| O+P− | 29.7(20.1) | 30.1(57.2) | 4.5(2.2) | 5.3(2.1) | 10.5(3.0) | 10.3(3.3) | 0.42(0.25) | 0.46(0.27) | 0.28(0.28) | 0.27(0.20) |
| O−P+ | 32.9(29.7) | 33.3(33.1) | 4.8(1.9) | 5.7(2.5) | 10.0(3.2) | 10.6(2.5) | 0.85(0.21) | 0.84(0.27) | 0.30(0.18) | 0.30(0.17) |
| O−P− | 33.9(34.0) | 30.4(36.9) | 5.1(2.4) | 5.1(2.8) | 10.1(2.3) | 9.6(2.0) | 0.42(0.24) | 0.44(0.22) | 0.25(0.24) | 0.26(0.19) |
Note: Cha = Character. Frequency is the number of times of a character shown in Chinese language textbooks from Grade 1 to Grade 6 in primary school. Acquisition term is the term when a character is first shown in Chinese language textbooks. Stroke is the sum of the strokes in a character. Phonological (orthographic) consistency was computed as the ratio of phonological (orthographic) friends to the sum of phonological (orthographic) friends and enemies (i.e. friends/(friends + enemies)) based on the characters in Chinese language textbooks from Grade 1 to Grade 6. Phonological enemies were defined as the number of characters with the same phonological radical but different pronunciation to the stimulus and orthographic enemies were defined as the number of characters with the same pronunciation but different phonological radicals to the stimulus. Friends were defined as the number of characters with the same phonological radical and pronunciation as the stimulus.
2.4. Data collection
All images were acquired using a 3 T Siemens scanner at Beijing Normal University. For the functional imaging, a susceptibility weighted single-shot EPI (echo planar imaging) method with BOLD (blood oxygenation level-dependent) was adopted. The following scan parameters were used: TR = 2000 ms; TE = 20 ms; flip angle = 80; slice thickness = 3 mm, gap = 0.48 mm; number of slices = 32; FOV = 220 × 206.25; matrix = 128 × 120 × 32; voxel size = 1.71875 × 1.71875 × 3.48 mm. In addition, a high resolution, T1 weighted 3D image was acquired (MPRAGE, axial slices = 160; slice thickness = 1 mm; FOV = 256 × 256; matrix = 256 × 256 × 160; voxel size = 1 × 1 × 1 mm; TR = 2300 ms; TE = 3.36 ms).
2.5. Data analysis
Data analysis was performed using SPM5 (Statistical Parametric Mapping) (http://www.fil.ion.ucl.ac.uk/spm). Image pre-processing included: slice timing, realignment, co-registration, tissue segmentation, normalization, and smoothing. Specifically, the functional images were corrected for differences in slice-acquisition time to the middle slice and were realigned to the first volume in the scanning session. All the subjects included had head movement no larger than the voxel size. Subjects’ functional images were then co-registered with their corresponding high-resolution structural MRI images. The co-registered high-resolution structural MRI images were segmented and normalized to a standardized tissue probability template (based on Montreal Neurologic Institute (MNI) stereotactic space; http://www.bic.mni.mcgill.ca). Using the parameters from the structural MRI image segmentation and normalization, the functional images were normalized with a resample voxel size of 2 × 2 × 2 mm and then smoothed with Gaussian filter of FWHM (full width half max) = 4 × 4 × 8 mm.
The general linear model was used to estimate condition effects in the semantic task for each subject using an event-related analysis procedure. Four lexical conditions ‘M+S+’, ‘M−S+’, ‘M+S-’, ‘M-S-’, and two control conditions ‘perceptual’, and ‘null’ were modeled using a canonical HRF (hemodynamic response function). For each subject, four contrasts of interest were computed: incongruent > congruent ([M−S+, M+S−] vs. [M+S+, M−S−]), congruent > incongruent ([M+S+, M−S−] vs. [M−S+, M+S−]), incongruent > null ([M−S+, M+S−] vs. null), and congruent > null ([M+S+, M−S−] vs. null). Although the null condition does not as effectively control for complex visual analysis compared to perceptual condition, we chose the null rather than the perceptual conditions as the baseline because the null condition was exactly the same for the semantic and phonological tasks. In addition, the null condition had higher accuracy and less variability than perceptual condition (See Section 3.1).
Parameter estimates from contrasts in single subject models were entered into random-effects analysis. Parameter estimates from the incongruent > congruent contrast and from the congruent > incongruent contrast were entered separately into a one-sample design to test for differences within groups and a two-sample design to test differences between groups. Because the focus of the current paper is the role of morphological information in word processing, we mainly report incongruency (incongruent > congruent) and congruency effects (congruent > incongruent). First, the incongruency and congruency effects within TD and RD groups and between groups are reported. Second, brain activation values are extracted separately for TD and RD from those regions showing the group differences in the incongruency or congruency effects. Regions of interest were defined as the cluster showing the group differences in the incongruency or congruency effect. Brain-behavior analyses for those regions of interest were used to test for a correlation between brain activation in the incongruency or congruency effect and reading performance separately for each group. The correlation analyses were conducted separately for each group because previous studies (Pernet et al., 2009, Phinney et al., 2007) have shown differential brain-behavior correlations between the two groups, suggesting that dyslexia reflects a qualitative difference rather than the low end of the reading ability distribution.
A similar procedure as for the semantic task was used in the analysis of the phonological task. Four lexical conditions ‘O+P+’, ‘O−P+’, ‘O+P−’, ‘O−P−’, and two control conditions ‘perceptual’, and ‘null’ were modeled using a canonical HRF. For each subject, four contrasts of interest were computed: incongruent > congruent ([O−P+, O+P−] vs. [O+P+, O−P−]), congruent > incongruent ([O+P+, O−P−] vs. [O−P+, O+P-]), incongruent > null ([O−P+, O+P−] vs. null), and congruent > null ([O+P+, O−P−] vs. null). Parameter estimates from contrasts in single subject models were entered into random-effects analysis. Parameter estimates from the incongruent > congruent contrast and from congruent > incongruent were entered separately into a one-sample design to test for differences within groups and a two-sample design to test differences between groups.
Because the role of the phonological task is to rule out a phonological component from the morphological processing, the anatomical regions showing the group differences in the morphological incongruency effect were used as mask for the phonological incongruency effect analysis. In addition, region of interest analyses were used to examine the role of phonological processing in the areas showing the group differences in the morphological incongruency effect. Regions of interest were defined as the clusters showing group activation differences for the incongruency or congruency effect in the semantic task.
3. Results
3.1. Behavioral results
Table 4 presents behavioral data for the semantic task. A 2 group (TD, RD) by 2 condition (congruent, incongruent) ANOVA with VIQ as covariates was calculated separately on accuracy and reaction times. Analyses on accuracy revealed significant main effect of group (F(1,25) = 10.701, p = 0.003), indicating that the RD group was less accurate. This analysis revealed neither significant main effect of condition (F(1,25) = 1.024, p = 0.321) nor significant group × condition interaction (F(1,25) = 0.072, p = 0.790). In reaction time analyses, values larger or smaller than 3 standard deviations from mean for each subject were treated as outliers and replaced with the cut-off value. Analyses on reaction times revealed significant main effect of condition (F(1,25) = 5.884, p = 0.023), indicating incongruent condition was slower than congruent condition. This analysis revealed neither significant effect of group (F(1,25) = 2.366, p = 0.137), nor a group × condition interaction (F(1,25) = 0.237, p = 0.631). Univariate analysis of variance with VIQ as covariates was used to test whether there were group differences for perceptual and null conditions. No significant group differences were found on accuracy or reaction times for either condition.
Table 4.
Mean accuracy and reaction time (and standard deviations) for the lexical and control trials in the semantic task for the typically developing (TD) and reading disability (RD) groups.
| Group | Accuracy (%) |
Reaction time (ms) |
||||||
|---|---|---|---|---|---|---|---|---|
| Lexical |
Control |
Lexical |
Control |
|||||
| Incongruent | Congruent | Perceptual | Null | Incongruent | Congruent | Perceptual | Null | |
| TD | 78.6 | 81.5 | 93.9 | 97.2 | 1261 | 1177 | 886 | 1320 |
| (16.0) | (11.3) | (8.3) | (4.2) | (469) | (448) | (378) | (329) | |
| RD | 65.2 | 73.8 | 92.6 | 93.4 | 1280 | 1138 | 848 | 1438 |
| (12.3) | (11.5) | (8.0) | (6.9) | (406) | (324) | (313) | (185) | |
Table 5 presents behavioral data for the phonological task. A 2 group (TD, RD) by 2 condition (congruent, incongruent) ANOVA with VIQ as covariates was calculated separately on accuracy and reaction times. Analyses on accuracy revealed significant main effect of group (F(1,25) = 6.005, p = 0.022), indicating that the RD group was less accurate. This analysis revealed neither significant main effect of condition (F(1,25) = 0.254, p = 0.619) nor a significant group × condition interaction (F(1,25) = 0.618, p = 0.439). In the reaction time analyses, values larger or smaller than 3 standard deviations from the mean for each subject were treated as outliers and replaced with the cut-off value. Analyses on reaction times revealed significant main effect of group (F(1,25) = 5.510, p = 0.023) and a trend toward significant main effect of condition (F(1,25) = 3.984, p = 0.057), indicating that the TD group and the incongruent condition were the slowest. This analysis revealed no significant group × condition interaction (F(1,25) = 0.875, p = 0.359). Univariate analysis of variance with VIQ as covariates was used to test whether there were group differences for perceptual and null conditions. No significant group differences were found on accuracy or reaction times for either condition.
Table 5.
Mean accuracy and reaction time (and standard deviations) for the lexical and control trials in the phonological task for the typically developing (TD) and reading disability (RD) groups.
| Group | Accuracy (%) |
Reaction Time (ms) |
||||||
|---|---|---|---|---|---|---|---|---|
| Lexical |
Control |
Lexical |
Control |
|||||
| Incongruent | Congruent | Perceptual | Null | Incongruent | Congruent | Perceptual | Null | |
| TD | 66.4 | 77.2 | 96.7 | 97.2 | 1480 | 1402 | 847 | 1364 |
| (13.5) | (8.5) | (5.5) | (6.6) | (579) | (581) | (314) | (283) | |
| RD | 53.2 | 66.8 | 89.90 | 95 | 1426 | 1331 | 868 | 1418 |
| (7.2) | (9.2) | (10.5) | (5.7) | (530) | (457) | (316) | (196) | |
3.2. fMRI activation results
We did not find group differences for the contrast lexical-null in the semantic task, however we found group differences in left inferior temporal gyrus (x = −50, y = −56, z = −18, T = 4.52, voxels = 23) and in right middle occipital gyrus (x = 42, y = −68, z = −16, T = 4.00, voxels = 10) in this contrast for the rhyme task at p < 0.001 uncorrected.
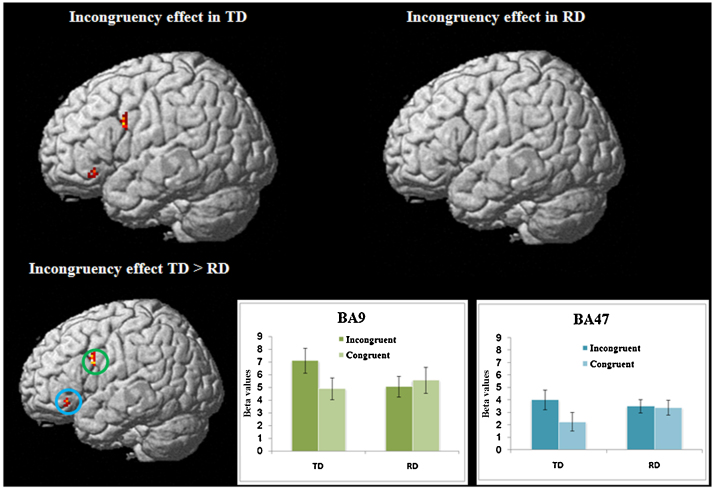
Although we did not find group differences in brain activation for lexical > null contrast, we found significant group difference for incongruent > congruent contrast. Table 6 presents incongruency effects within each group and the differences between the typically developing (TD) group and reading disabled (RD) group in the semantic task. The incongruent condition showed greater activation than congruent condition for the TD group in left inferior frontal gyrus (BA9 and BA47). No brain regions showed incongruency effects for the RD group. Greater incongruency effects were revealed in TD than RD in left inferior frontal gyrus (BA9 and BA47). To better demonstrate the incongruency effect, beta values were extracted from those two regions (BA9 and BA47) for the congruent > null and incongruent > null contrasts for TD and RD separately (see Fig. 2). The group differences remain similar when VIQ was partialed out. No group differences were revealed in the congruency (congruent > incongruent) effect.
Table 6.
Brain regions showing incongruency effects (incongruent > congruent) within the typically developing (TD) and reading disability (RD) groups, and differences in the congruency effect between groups in the semantic task.
| Regions | H | BA | T | Voxels | x | y | z |
|---|---|---|---|---|---|---|---|
| TD | |||||||
| Inferior frontal gyrus | L | 9 | 4.44 | 61 | −38 | 8 | 28 |
| Inferior frontal gyrus | L | 47 | 4.41 | 17 | −34 | 30 | −10 |
| RD | |||||||
| – | – | – | – | – | – | – | |
| TD–RD | |||||||
| Inferior frontal gyrus | L | 9 | 4.01 | 61 | −36 | 8 | 26 |
| Inferior frontal gyrus | L | 47 | 3.97 | 14 | −36 | 32 | −8 |
Note: H = hemisphere; L = left; BA = Brodmann's Areas; x, y, z: Montreal Neurological Institute (MNI) coordinates. Clusters presented are more than 10 contiguous voxels surviving a threshold of 0.001 (uncorrected) or 0.05 (fdr corrected).
Fig. 2.
Brain regions showing incongruency effect for the typically developing (TD) and reading disability (RD), and greater incongruency effects for the TD compared to the RD groups in the semantic task. Bar graph demonstrated the beta values for the incongruent and congruent condition separately in TD and RD in the two brain regions (BA9 and BA47) showing group difference in the incongruency effect.
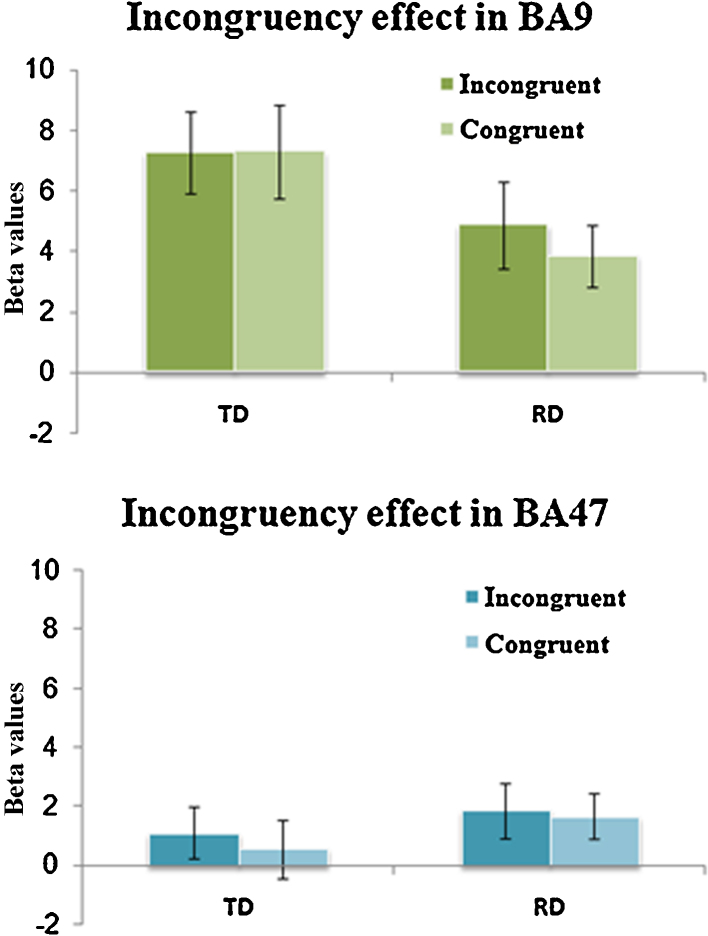
To investigate whether the incongruency effect in the semantic task in the left inferior frontal gyrus (IFG) was specific to incongruence between semantic relatedness and morphemic overlap, an IFG anatomical mask based on aal template was used to investigate the incongruency effect between orthography and phonology in the phonological task. No incongruency effect was revealed in IFG in this task. To further investigate this issue, regions of interest analysis on those two regions showing the morphological incongruency effect in the semantic task was conducted to determine whether there was an incongruency effect between orthography and phonology in this region for the phonological task. Beta values were extracted from those two regions (BA9 and BA47) for the congruent > null and incongruent > null contrasts in the phonological task. As demonstrated in Fig. 3, neither TD nor RD showed an incongruency effect between orthography and phonology in BA9 and BA47.
Fig. 3.
Incongruency effects in the phonological task. Neither TD nor RD showed incongruency effect in BA9 or BA47.
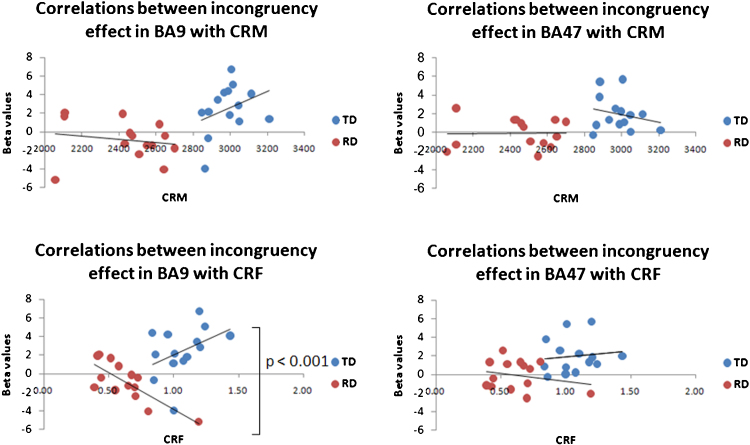
Correlations between brain activation for the incongruency effects in the semantic task and reading performance were also examined in these two regions of inferior frontal gyrus (BA9 and BA47). As demonstrated in Fig. 4, a significant group difference (Z = 3.42, p < 0.001) was only revealed in the correlations between CRF and the incongruency effect in the BA9. Specifically, RD showed a significant negative brain-CRF correlation in BA9 (r = −0.770, p = 0.001) whereas TD showed a trend toward significant positive correlation (r = 0.411, p = 0.144).
Fig. 4.
Correlation of the incongruency effect (incongruent > congruent) in the semantic task with reading performance in TD and RD. Correlations were significantly different between TD and RD in BA9 for Character Reading Fluency (CRF). CRM: Character Recognition Measure.
4. Discussion
Using fMRI techniques, this study revealed that Chinese children with dyslexia showed a smaller incongruency effect between semantic relatedness and morphemic overlap in left inferior frontal gyrus (IFG) as compared to typically developing (TD) children, suggesting children with reading disability (RD) are less sensitive to the morphological information. Further, the group differences in left IFG were specific to morphological processing because there was no group difference in the incongruency effect for a rhyming task that manipulated the overlap between orthography and phonology. Our study also showed that the magnitude of the incongruency effect was modulated by reading skill only in the children with dyslexia, with higher skill being associated with a smaller incongruency effect, suggesting less reliance on morphological information because of the use of a whole-word strategy.
The altered morphological effects for Chinese dyslexic children in the current study are consistent with previous behavioral studies suggesting a morphological awareness deficit in Chinese dyslexic (Chung et al., 2011, Shu et al., 2006, Wong et al., 2013) and at risk children (McBride-Chang et al., 2008, McBride-Chang et al., 2011). However, our study provides the first neural evidence for a deficit in morphological processing in Chinese dyslexic children and shows that the neural abnormality in Chinese children is not limited to the visuo-orthographic and phonological processing (Meng et al., 2007, Siok et al., 2008, Siok et al., 2009). This study is also consistent with several previous studies on dyslexia in alphabetic languages that have examined morphological processing (Aylward et al., 2003, Casalis et al., 2004, Egan and Tainturier, 2011, Leikin and Hagit, 2006, Schiff and Raveh, 2007, Schiff et al., 2011). For example, a fMRI study (Aylward et al., 2003) reported reduced activation in left middle frontal gyrus and several brain regions in the right hemisphere in English dyslexic children during a morpheme mapping task. In contrast to the brain activation data, behavioral measures in the current study failed to show a group difference in the morphological incongruency effect, which might be due to large individual variability and/or the relatively small number of subjects. This suggests that even in the absence of a significant behavioral effect, the neural measures sometimes are more sensitive in detecting group differences. Indeed, previous research has suggested that neuroimaging data can provide additional information beyond behavioral indices (Hoeft et al., 2011, Hoeft et al., 2007b).
The role of left IFG in morphological processing is supported by both neuropsychological and neuroimaging studies in alphabetic languages. Neuropsychological studies on left hemisphere non-fluent patients, typically with damage to inferior frontal regions, showed impaired ability in processing inflectional morphemes (e.g. -ed in ‘joined’) (Tyler et al., 2002a, Tyler et al., 2002b). Recent neuroimaging studies have also supported the role of left IFG in inflectional (Pulvermuller et al., 2006, Tyler et al., 2005a, Tyler et al., 2005b) and derivational morpheme (e.g. -ness in ‘happiness’) processing (Bozic et al., 2007, Marangolo et al., 2006). Our study extends these findings suggesting that IFG may be involved in the processing of ‘free’ morphemes rather than only bound morphemes like -ed in ‘joined’ or -ly in ‘bravely’. Although we propose a morphological deficit hypothesis, several different ideas have been proposed for the alteration of left IFG in dyslexics in alphabetic languages. Some (Hoeft et al., 2006, Hoeft et al., 2007a, Shaywitz et al., 1998, Shaywitz et al., 2003) have reported over-activation in dyslexics in this region and argued that this may reflect compensatory reliance on effortful pronunciations in word recognition. Others (Booth et al., 2007, Cao et al., 2006, Georgiewa et al., 1999) have reported under-activation in dyslexics in this region and suggested that this may reflect a dysfunction in access to lexical and sublexical phonological representations. In fact, recent meta-analyses suggest that over activation appears to be more dorsal, whereas underactivation seems to be more ventral in the IFG (Richlan et al., 2009, Richlan et al., 2011). Although our study reported alterations of the incongruency effect in both dorsal and ventral IFG, it is interesting to note that we found group differences in the correlation of character reading fluency with the incongruency effect only for the more dorsal region, suggesting a potential role of output phonology in resolving this conflict.
Our previous study on Chinese dyslexia (Liu et al., 2012) reported overall underactivation in left IFG (BA44, peak at x = −58, y = 14, z = 20) in the rhyming task, possibly reflecting a deficit in efficient access to lexical phonological representations. The current study, using about one half of the same participants, also showed numerically reduced activation in left IFG in the rhyming task (BA44, peak at x = −60, y = 16, z = 18). The current study, however, found no phonological incongruency effect in the rhyming task in IFG regions that showed a semantic incongruency effect (i.e. BA9 or BA47). It could be that different parts of left IFG have different roles. Studies have suggested that the ventral part of left IFG is more involved in the semantic processing whereas the dorsal part of left IFG is more involved in the phonological processing (Devlin et al., 2003, Fiez, 1997, Poldrack et al., 1999) and that the anterior part of the left IFG is more involved in controlled retrieval of lexical representations in posterior cortex based on top-down information, whereas posterior IFG is more involved in the selection between active lexical representations (Badre et al., 2005, Badre and Wagner, 2007, Cao et al., 2010).
The specificity of the morphological processing deficit in Chinese dyslexia shown in the current study is also supported by previous behavioral studies (Chung et al., 2011, Shu et al., 2006). These studies show that morphological awareness uniquely predicts Chinese dyslexic children's reading performance after controlling for several predictors including phonological awareness. This finding is also consistent with studies in alphabetic languages (Carlisle, 2000, Singson et al., 2000), which have reported a unique contribution of morphological awareness to reading after controlling the effect of phonological abilities.
Morphological deficits in children with dyslexia may alter their reading strategies. Our study showed that higher skill in children with dyslexia is correlated with a smaller incongruency effect. We assumed that the smaller incongruency effect in RD may be due to that RD may tend to use more whole-word mapping strategy than morpheme decomposing strategy due to their morphological deficit. Our findings suggest that Chinese children with dyslexia may use a whole-word strategy to compensate for their poor morphological awareness and those with greater compensation are able to achieve a better level of reading performance. Another possible explanation for the negative correlation between the incongruency effect and reading skill in RD could be that those RD with better skill more effectively engage the reading network when processing congruent compared to incongruent words because the formers tends to be easier. This could be similar to an effect found in a study of English speaking children with RD who showed stronger responses to consistent compared to inconsistent words (Bolger et al., 2008b). Further studies are needed to address this issue.
Previous studies have suggested that skilled readers can automatically decompose words into their constituent morphemes (Feldman et al., 1995, Zhang et al., 2011), whereas readers with lower skill may have difficulty in decomposition (Chung et al., 2011, Egan and Tainturier, 2011, McBride-Chang et al., 2003, Sangster and Deacon, 2011, Shu et al., 2006, Tsesmeli and Seymour, 2006). Studies in alphabetic languages have suggested that dyslexics, compared to their age- or reading-level controls, have difficulty in parsing a stem morpheme from a word spelling, e.g. kiss-kissed, final-finally (Egan and Tainturier, 2011, Tsesmeli and Seymour, 2006). Previous studies on Chinese children have shown that older children performed better than younger children (McBride-Chang et al., 2003) and typically developing children performed better than dyslexic children (Chung et al., 2011, Shu et al., 2006) in morpheme parsing using a variety of tasks including identification and discrimination. In summary, studies show that higher skill is associated with better morphological awareness in typical children and that dyslexia is associated with deficits in morphological processing. The novel finding in our study is that higher reading skill in Chinese children with dyslexia may be associated with a whole word reading strategy because of their deficit in morphological processing.
The present study is, of course, limited in the questions it can answer about Chinese dyslexia. One limitation is the lack of a reading-level matched group. Due to the lack of this group, the data cannot address whether the reduced activation in the left inferior frontal gyrus in RD is due to the lower reading level or dyslexia per se. Some previous studies (Hoeft et al., 2006, Hoeft et al., 2007a) proposed that a dysfunction of frontal regions may reflect compensatory process to overcome reading difficulty, however in these studies frontal regions showed a pattern of hyper-activation in RD, which is different from the hypo-activation pattern in RD demonstrated in the current study. Another limitation of the current study is that we only investigated one type of morphological processing in Chinese (i.e. word-level). Whether the neural mechanism for character-level morphological processing (i.e. the semantic radical effect) is similar to that for word-level morphological processing also needs to be investigated. One recent behavioral study suggested that word-level and character-level morphological processing may be different in Chinese children (Liu et al., 2010).
To conclude, the current study suggests that Chinese children with dyslexia have a neural abnormality in morphological processing. They showed a reduced incongruency effect in left inferior frontal gyrus during a semantic relatedness judgment task as compared to typically developing children. The deficit in morphological processing seems to be independent of orthographic and phonological processing, as we did not show differences in frontal regions for a rhyming task. Finally, our results suggest that higher skill in Chinese children with dyslexia may be associated with the use of a compensatory whole-word strategy by ignoring morphemic information. The investigation of the brain basis underlying the morphological awareness deficit in Chinese dyslexia is helpful in understanding the cause of this disorder, revealing potential biomarkers to diagnose this disorder, and providing possible brain measures to examine in various intervention methods (Shaywitz and Shaywitz, 2008). Studies have shown the value of neuroimaging data for predicting reading gains (Hoeft et al., 2007b, Hoeft et al., 2011).
Conflict of interest
None.
Acknowledgements
This study was supported by grants to Dr. Li Liu from National Natural Science Foundation of China (31000500, 31070899) and the Fundamental Research Funds for the Central Universities, and by grants to Dr. James R. Booth from the National Institute of Child Health and Human Development (HD042049).
Contributor Information
Danling Peng, Email: pdl3507@bnu.edu.cn.
James R. Booth, Email: j-booth@northwestern.edu.
References
- Aylward E.H., Richards T.L., Berninger V.W., Nagy W.E., Field K.M., Grimme A.C., Richards A.L., Thomson J.B., Cramer S.C. Instructional treatment associated with changes in brain activation in children with dyslexia. Neurology. 2003;61:212–219. doi: 10.1212/01.wnl.0000068363.05974.64. [DOI] [PubMed] [Google Scholar]
- Badre D., Poldrack R.A., Pare-Blagoev E.J., Insler R.Z., Wagner A.D. Dissociable controlled retrieval and generalized selection mechanisms in ventrolateral prefrontal cortex. Neuron. 2005;47:907–918. doi: 10.1016/j.neuron.2005.07.023. [DOI] [PubMed] [Google Scholar]
- Badre D., Wagner A.D. Left ventrolateral prefrontal cortex and the cognitive control of memory. Neuropsychologia. 2007;45:2883–2901. doi: 10.1016/j.neuropsychologia.2007.06.015. [DOI] [PubMed] [Google Scholar]
- Bolger D.J., Hornickel J., Cone N.E., Burman D.D., Booth J.R. Neural correlates of orthographic and phonological consistency effects in children. Hum. Brain Mapp. 2008;29:1416–1429. doi: 10.1002/hbm.20476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolger D.J., Minas J., Burman D.D., Booth J.R. Differential effects of orthographic and phonological consistency in cortex for children with and without reading impairment. Neuropsychologia. 2008;46:3210–3224. doi: 10.1016/j.neuropsychologia.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth J.R., Bebko G., Burman D.D., Bitan T. Children with reading disorder show modality independent brain abnormalities during semantic tasks. Neuropsychologia. 2007;45:775–783. doi: 10.1016/j.neuropsychologia.2006.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozic M., Marslen-Wilson W.D., Stamatakis E.A., Davis M.H., Tyler L.K. Differentiating morphology, form, and meaning: neural correlates of morphological complexity. J. Cogn. Neurosci. 2007;19:1464–1475. doi: 10.1162/jocn.2007.19.9.1464. [DOI] [PubMed] [Google Scholar]
- Cao F., Bitan T., Chou T.L., Burman D.D., Booth J.R. Deficient orthographic and phonological representations in children with dyslexia revealed by brain activation patterns. J. Child Psychol. Psychiatry. 2006;47:1041–1050. doi: 10.1111/j.1469-7610.2006.01684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao F., Lee R., Shu H., Yang Y., Xu G., Li K., Booth J.R. Cultural constraints on brain development: evidence from a developmental study of visual word processing in mandarin Chinese. Cereb. Cortex. 2010;20:1223–1233. doi: 10.1093/cercor/bhp186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlisle J.F. Awareness of the structure and meaning of morphologically complex words: impact on reading. Read. Writ. 2000;12:169–190. [Google Scholar]
- Casalis S., Cole P., Sopo D. Morphological awareness in developmental dyslexia. Ann. Dyslexia. 2004;54:114–138. doi: 10.1007/s11881-004-0006-z. [DOI] [PubMed] [Google Scholar]
- Chung K.K.H., Ho C.S.H., Chan D.W., Tsang S.M., Lee S.H. Cognitive skills and literacy performance of Chinese adolescents with and without dyslexia. Read. Writ. 2011;24:835–859. doi: 10.1007/s11145-010-9227-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlin J.T., Matthews P.M., Rushworth M.F. Semantic processing in the left inferior prefrontal cortex: a combined functional magnetic resonance imaging and transcranial magnetic stimulation study. J. Cogn. Neurosci. 2003;15:71–84. doi: 10.1162/089892903321107837. [DOI] [PubMed] [Google Scholar]
- Egan J., Tainturier M.J. Inflectional spelling deficits in developmental dyslexia. Cortex. 2011;47:1179–1196. doi: 10.1016/j.cortex.2011.05.013. [DOI] [PubMed] [Google Scholar]
- Feldman L.B., Pnini T., Frost R. Decomposing words into their constituent morphemes—evidence from English and Hebrew. J. Exp. Psychol. Learn. Mem. Cogn. 1995;21:947–960. doi: 10.1037//0278-7393.21.4.947. [DOI] [PubMed] [Google Scholar]
- Fiez J.A. Phonology, semantics, and the role of the left inferior prefrontal cortex. Hum. Brain Mapp. 1997;5:79–83. [PubMed] [Google Scholar]
- Georgiewa P., Rzanny R., Hopf J.M., Knab R., Glauche V., Kaiser W.A., Blanz B. fMRI during word processing in dyslexic and normal reading children. Neuroreport. 1999;10:3459–3465. doi: 10.1097/00001756-199911080-00036. [DOI] [PubMed] [Google Scholar]
- Ho C.S.-H., Chan D.W.-O., Lee S.-H., Tsang S.-M., Luan V.H. Cognitive profiling and preliminary subtyping in Chinese developmental dyslexia. Cognition. 2004;91:43–75. doi: 10.1016/s0010-0277(03)00163-x. [DOI] [PubMed] [Google Scholar]
- Hoeft F., Hernandez A., McMillon G., Taylor-Hill H., Martindale J.L., Meyler A., Keller T.A., Siok W.T., Deutsch G.K., Just M.A., Whitfield-Gabrieli S., Gabrieli J.D. Neural basis of dyslexia: a comparison between dyslexic and nondyslexic children equated for reading ability. J. Neurosci. 2006;26:10700–10708. doi: 10.1523/JNEUROSCI.4931-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeft F., McCandliss B.D., Black J.M., Gantman A., Zakerani N., Hulme C., Lyytinen H., Whitfield-Gabrieli S., Glover G.H., Reiss A.L., Gabrieli J.D. Neural systems predicting long-term outcome in dyslexia. Proc. Natl. Acad. Sci. U. S. A. 2011;108:361–366. doi: 10.1073/pnas.1008950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeft F., Meyler A., Hernandez A., Juel C., Taylor-Hill H., Martindale J.L., McMillon G., Kolchugina G., Black J.M., Faizi A., Deutsch G.K., Siok W.T., Reiss A.L., Whitfield-Gabrieli S., Gabrieli J.D. Functional and morphometric brain dissociation between dyslexia and reading ability. Proc. Natl. Acad. Sci. U. S. A. 2007;104:4234–4239. doi: 10.1073/pnas.0609399104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeft F., Ueno T., Reiss A.L., Meyler A., Whitfield-Gabrieli S., Glover G.H., Keller T.A., Kobayashi N., Mazaika P., Jo B., Just M.A., Gabrieli J.D. Prediction of children's reading skills using behavioral, functional, and structural neuroimaging measures. Behav. Neurosci. 2007;121:602–613. doi: 10.1037/0735-7044.121.3.602. [DOI] [PubMed] [Google Scholar]
- Hu W., Lee H.L., Zhang Q., Liu T., Geng L.B., Seghier M.L., Shakeshaft C., Twomey T., Green D.W., Yang Y.M., Price C.J. Developmental dyslexia in Chinese and English populations: dissociating the effect of dyslexia from language differences. Brain. 2010;133:1694–1706. doi: 10.1093/brain/awq106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo L.J., Anderson R.C. Morphological awareness and learning to read: a cross-language perspective. Educ. Psychol. 2006;41:161–180. [Google Scholar]
- Leikin M., Hagit E.Z. Morphological processing in adult dyslexia. J. Psycholinguist. Res. 2006;35:471–490. doi: 10.1007/s10936-006-9025-8. [DOI] [PubMed] [Google Scholar]
- Lin C.D., Zhang H.C. Beijing Teachers College Press; Beijing China: 1986. The Chinese Version of WSIC-R. [Google Scholar]
- Liu L., Wang W., You W., Li Y., Awati N., Zhao X., Booth J., Peng D. Similar alterations in brain function for phonological and semantic processing to visual characters in Chinese dyslexia. Neuropsychologia. 2012;50:2224–2232. doi: 10.1016/j.neuropsychologia.2012.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P.D., Chung K.K., McBride-Chang C., Tong X. Holistic versus analytic processing: evidence for a different approach to processing of Chinese at the word and character levels in Chinese children. J. Exp. Child. Psychol. 2010;107:466–478. doi: 10.1016/j.jecp.2010.06.006. [DOI] [PubMed] [Google Scholar]
- Marangolo P., Piras F., Galati G., Burani C. Functional anatomy of derivational morphology. Cortex. 2006;42:1093–1106. doi: 10.1016/s0010-9452(08)70221-1. [DOI] [PubMed] [Google Scholar]
- McBride-Chang C., Lam F., Lam C., Chan B., Fong C.Y.C., Wong T.T.Y., Wong S.W.L. Early predictors of dyslexia in Chinese children: familial history of dyslexia, language delay, and cognitive profiles. J. Child Psychol. Psychiat. 2011;52:204–211. doi: 10.1111/j.1469-7610.2010.02299.x. [DOI] [PubMed] [Google Scholar]
- McBride-Chang C., Lam F., Lam C., Doo S., Wong S.W.L., Chow Y.Y.Y. Word recognition and cognitive profiles of Chinese pre-school children at risk for dyslexia through language delay or familial history of dyslexia. J. Child Psychol. Psychiat. 2008;49:211–218. doi: 10.1111/j.1469-7610.2007.01837.x. [DOI] [PubMed] [Google Scholar]
- McBride-Chang C., Shu H., Zhou A. Morphological awareness uniquely predicts young children's Chinese character recognition. J. Educ. Psychol. 2003;95:743–751. [Google Scholar]
- Meng X., Tian X., Jian J., Zhou X. Orthographic and phonological processing in Chinese dyslexic children: an ERP study on sentence reading. Brain Res. 2007;1179:119–130. doi: 10.1016/j.brainres.2007.08.046. [DOI] [PubMed] [Google Scholar]
- Pernet C., Andersson J., Paulesu E., Demonet J.F. When all hypotheses are right: a multifocal account of dyslexia. Hum. Brain Mapp. 2009;30:2278–2292. doi: 10.1002/hbm.20670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phinney E., Pennington B.F., Olson R., Filley C.M., Filipek P.A. Brain structure correlates of component reading processes: implications for reading disability. Cortex. 2007;43:777–791. doi: 10.1016/s0010-9452(08)70506-9. [DOI] [PubMed] [Google Scholar]
- Poldrack R.A., Wagner A.D., Prull M.W., Desmond J.E., Glover G.H., Gabrieli J.D. Functional specialization for semantic and phonological processing in the left inferior prefrontal cortex. Neuroimage. 1999;10:15–35. doi: 10.1006/nimg.1999.0441. [DOI] [PubMed] [Google Scholar]
- Pulvermuller F., Shtyrov Y., Ilmoniemi R.J., Marslen-Wilson W.D. Tracking speech comprehension in space and time. Neuroimage. 2006;31:1297–1305. doi: 10.1016/j.neuroimage.2006.01.030. [DOI] [PubMed] [Google Scholar]
- Richlan F., Kronbichler M., Wimmer H. Functional abnormalities in the dyslexic brain: a quantitative meta-analysis of neuroimaging studies. Hum. Brain Mapp. 2009;30:3299–3308. doi: 10.1002/hbm.20752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richlan F., Kronbichler M., Wimmer H. Meta-analyzing brain dysfunctions in dyslexic children and adults. Neuroimage. 2011;56:1735–1742. doi: 10.1016/j.neuroimage.2011.02.040. [DOI] [PubMed] [Google Scholar]
- Sangster L., Deacon S.H. Development in children's sensitivity to the role of derivations in spelling. Can. J. Exp. Psychol. 2011;65:133–139. doi: 10.1037/a0018569. [DOI] [PubMed] [Google Scholar]
- Schiff R., Raveh M. Deficient morphological processing in adults with developmental dyslexia: another barrier to efficient word recognition? Dyslexia. 2007;13:110–129. doi: 10.1002/dys.322. [DOI] [PubMed] [Google Scholar]
- Schiff R., Schwartz-Nahshon S., Nagar R. Effect of phonological and morphological awareness on reading comprehension in Hebrew-speaking adolescents with reading disabilities. Ann. Dyslexia. 2011;61:44–63. doi: 10.1007/s11881-010-0046-5. [DOI] [PubMed] [Google Scholar]
- Shaywitz S.E., Shaywitz B.A. Paying attention to reading: the neurobiology of reading and dyslexia. Dev. Psychopathol. 2008;20:1329–1349. doi: 10.1017/S0954579408000631. [DOI] [PubMed] [Google Scholar]
- Shaywitz S.E., Shaywitz B.A., Fulbright R.K., Skudlarski P., Mencl W.E., Constable R.T., Pugh K.R., Holahan J.M., Marchione K.E., Fletcher J.M., Lyon G.R., Gore J.C. Neural systems for compensation and persistence: young adult outcome of childhood reading disability. Biol. Psychiatry. 2003;54:25–33. doi: 10.1016/s0006-3223(02)01836-x. [DOI] [PubMed] [Google Scholar]
- Shaywitz S.E., Shaywitz B.A., Pugh K.R., Fulbright R.K., Constable R.T., Mencl W.E., Shankweiler D.P., Liberman A.M., Skudlarski P., Fletcher J.M., Katz L., Marchione K.E., Lacadie C., Gatenby C., Gore J.C. Functional disruption in the organization of the brain for reading in dyslexia. Proc. Natl. Acad. Sci. U. S. A. 1998;95:2636–2641. doi: 10.1073/pnas.95.5.2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu H., McBride-Chang C., Wu S., Liu H.Y. Understanding Chinese developmental dyslexia: morphological awareness as a core cognitive construct. J. Educ. Psychol. 2006;98:122–133. [Google Scholar]
- Shu H., Meng X., Lai A. The lexical representation and processing in Chinese-speaking poor readers. In: McBride-Chang C., Chen H.-C., editors. Reading Development in Chinese Children. Praeger Press; Westport CT: 2003. pp. 199–214. [Google Scholar]
- Singson M., Mahony D., Mann V. The relation between reading ability and morphological skills: evidence from derivational suffixes. Read. Writ. 2000;12:219–252. [Google Scholar]
- Siok W.T., Fletcher P. The role of phonological awareness and visual-orthographic skills in Chinese reading acquisition. Dev. Psychol. 2001;37:886–899. [PubMed] [Google Scholar]
- Siok W.T., Niu Z., Jin Z., Perfetti C.A., Tan L.H. A structural-functional basis for dyslexia in the cortex of Chinese readers. Proc. Natl. Acad. Sci. U. S. A. 2008;105:5561–5566. doi: 10.1073/pnas.0801750105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siok W.T., Perfetti C.A., Jin Z., Tan L.H. Biological abnormality of impaired reading is constrained by culture. Nature. 2004;431:71–76. doi: 10.1038/nature02865. [DOI] [PubMed] [Google Scholar]
- Siok W.T., Spinks J.A., Jin Z., Tan L.H. Developmental dyslexia is characterized by the co-existence of visuospatial and phonological disorders in Chinese children. Curr. Biol. 2009;19:R890–R892. doi: 10.1016/j.cub.2009.08.014. [DOI] [PubMed] [Google Scholar]
- Su P. Peking University Press; Beijing: 1994. Compendium od Modern Chinese Studies. [Google Scholar]
- Tsesmeli S.N., Seymour P.H.K. Derivational morphology and spelling in dyslexia. Read. Writ. 2006;19:587–625. [Google Scholar]
- Tyler L.K., deMornay-Davies P., Anokhina R., Longworth C., Randall B., Marslen-Wilson W.D. Dissociations in processing past tense morphology: neuropathology and behavioral studies. J. Cogn. Neurosci. 2002;14:79–94. doi: 10.1162/089892902317205348. [DOI] [PubMed] [Google Scholar]
- Tyler L.K., Marslen-Wilson W.D., Stamatakis E.A. Differentiating lexical form, meaning, and structure in the neural language system. Proc. Natl. Acad. Sci. U. S. A. 2005;102:8375–8380. doi: 10.1073/pnas.0408213102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler L.K., Randall B., Marslen-Wilson W.D. Phonology and neuropsychology of the English past tense. Neuropsychologia. 2002;40:1154–1166. doi: 10.1016/s0028-3932(01)00232-9. [DOI] [PubMed] [Google Scholar]
- Tyler L.K., Stamatakis E.A., Post B.E., Randall B., Marslen-Wilson W. Temporal and frontal systems in speech comprehension: an fMRl study of past tense processing. Neuropsychologia. 2005;43:1963–1974. doi: 10.1016/j.neuropsychologia.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Wang X.L., Tao B.P. Shanghai Education Press; Shanghai, China: 1993. Chinese Character Recognition Test Battery and Assessment Scale for Primary School Children. [Google Scholar]
- Wechsler D. The Psychological Corporation; San Antonio, TX: 1974. Wechsler Intelligence Scale for Children-Revised. [Google Scholar]
- Wong S.W.L., Xiao M.X.Y., Chung K.K.H. Issues of culture and language in developmental disorders: the case of dyslexia in Chinese learners. In: Marshall C.R., editor. Current Issues in Developmental Disorders. Psychology Press; London, UK: 2013. pp. 151–170. [Google Scholar]
- Zhang C., Zhang J., Yin R., Zhou J., Chang S. Experimental research on the reading disability of Chinese students. Psychol. Sci. 1996;1996:222–256. [Google Scholar]
- Zhang J.X. A meaning-spelling theory of the Chinese characters: revealing the nature of written Chinese with cognitive psychology. J. South China Normal Univ. 2011:4. [Google Scholar]
- Zhang T., van Heuven W.J., Conklin K. Fast automatic translation and morphological decomposition in Chinese–English bilinguals. Psychol. Sci. 2011;22:1237–1242. doi: 10.1177/0956797611421492. [DOI] [PubMed] [Google Scholar]
- Zhou X., Marslen-Wilson W. Words, morphemes and syllables in the Chinese mental lexicon. Lang. Cog. Proc. 1994;9:393–422. [Google Scholar]
- Zhou X., Marslen-Wilson W. Morphological structure in the Chinese mental lexicon. Lang. Cog. Proc. 1995;10:545–600. [Google Scholar]