Highlights
-
•
Compartments of insular cortex show age-related decreases.
-
•
Age-related increases in future orientation and planning also occur.
-
•
Participants aged 10–22 years were assessed for impulsivity and with MRI.
-
•
We report an inverse relationship for age and nonplanning/anterior insula thickness.
-
•
We also observed a positive relationship between insula thickness and nonplanning.
Keywords: Impulsivity, Development, Adolescence, Insula, Emotion, Planning
Abstract
Insula function has been associated with emotional regulation, adjusting to changing outcomes under risk, reward and loss anticipation, discounting of future rewards, and self-rated impulsivity. The role of the insula in these processes may be fundamentally related to prospective thinking, a trait that increases with age. There is evidence that insular cortical thickness shows age related decreases that parallel age related increases in future orientation and planning. We tested the hypothesis that nonplanning decreases with age and that insula thickness is related to both age and nonplanning impulsivity. Fifty-nine male and female participants, ranging in age from 10 to 22 years old, underwent structural magnetic resonance imaging (MRI) procedures and were assessed using the Barratt Impulsiveness Scale (BIS). We observed that anterior insula thickness and nonplanning impulsivity show an inverse relationship with age and that there is a significant positive linear relationship between anterior insula thickness and nonplanning.
1. Introduction
Several models of neurodevelopment have been advanced to provide a neurobiological schema that clarifies changes in self-control from adolescence to adulthood. Many of the proposed models capitalize on the results of functional neuroimaging studies (fMRI) using decision-making tasks to assess decision under risk, during the evaluation of future versus immediate rewards, and under conditions that require inhibitory control (Casey et al., 2008, Ernst et al., 2008, Galvan et al., 2007, Geier and Luna, 2009, Rubia et al., 2007). These investigations have provided key insights into how decision processes change in relationship to neuromaturation and have primarily focused on the contribution of fronto-striatal and fronto-limbic disconnections to risk taking behavior. Although a network of brain regions is implicated in the development of self-control, the insular cortex, a structure thought to be important in decision-making, has received limited attention in the domain of neurodevelopment.
Studies applying neuroeconomic approaches to assess brain changes associated with anticipation and decision under risk and uncertainty have indicated an important role for the insula (Clark et al., 2008, Venkatraman et al., 2009, Xue et al., 2010). The paradigms used in these studies are thought to be proxies for real-world risky decisions because they often assess preference between smaller less risky or immediate rewards and larger rewards that entail greater risk or increased delays. Delay discounting is an expression of subjective preference for smaller immediate rewards and larger delayed rewards. Individuals that prefer smaller immediate rewards are characterized as being impulsive, whereas those individuals willing to wait for larger delayed rewards are said to be self-controlled. The insula appears to be involved in delay discounting as increased activation of this region has been observed when healthy adult subjects select larger delayed over smaller immediate rewards (Wittmann et al., 2007) and insula activation is greater in response to discounting of future losses than gains (Xu et al., 2009). Insula function has also been associated with self-rated impulsivity. For example, Lee et al. (2008) used the Barratt Impulsiveness Scale (BIS) to assess the relationship between trait impulsivity and brain activity in a group of healthy adult subjects that underwent fMRI scanning while performing a risky decision task. They observed increased activity in several structures, including the insula, among high impulsive, relative to low impulsive, subjects.
In the period from early adolescence to early adulthood, the cortical mantle undergoes extensive refinement (Gogtay et al., 2004). For example, Shaw et al. (2008) found that, in the age range from 5 to 25, cortical thickness for distinct brain regions develop along different trajectories. Findings from this study specifically showed that the anterior insula follows a linear path during early development. Refinement in anterior insula function during development may be reflected in age-related reductions in cortical thickness that putatively reveals experience dependent pruning in this region (Chechik et al., 1998, Huttenlocher and Dabholkar, 1997). This change in cortical composition corresponds to linear changes in levels of self-reported impulsivity and future orientation that occur from adolescence to early adulthood (Steinberg et al., 2008, Steinberg et al., 2009). Thus, during this developmental period, individuals may be gaining a wide range of life experiences that support a refinement in cortical representations broadly and in the insula specifically. It has been argued that the insula is the neural substrate for encoding and retrieval of body states associated with environmental stimuli and that the insula represents the emotional self through time (Craig, 2009b, Damasio, 1999, Naqvi and Bechara, 2010). It has further been proposed that one of the key adaptive functions of the self is to aid in future oriented thought, which may support optimal decision-making (Sedekides and Skowronski, 1997). Thus, an attenuated capacity to imagine possible future states may lead to suboptimal decisions and increased impulsive choice.
In summary, there is evidence that the insula undergoes structural refinement through early development that parallels changes in future orientation, is directly involved in decision-making that requires subjective judgments about value though time, and plays a role in encoding, retrieving, and representing interoceptive states and emotional experience. We hypothesized that anterior insular cortical thickness would show age-related reductions, that we would observe age-related decreases in nonplanning impulsivity, and that insular cortical thickness would be related to changes in self-reported nonplanning impulsiveness. In order to test these hypotheses, we acquired structural brain images from a sample of subjects ranging in age from 10 to 22 years old and also assessed impulsivity using the BIS (Patton et al., 1995).
2. Materials and methods
2.1. Procedure
The University of Utah School of Medicine institutional review board approved the study and all participants read the informed consent and assented to participate in the study. Parents were required to consent for participants less than 18 years of age. On the day of the study visit, MRIs and self-report measures of impulsivity were acquired.
2.2. Subjects
Fifty-nine healthy male and female participants, ranging in age from 10 to 22 years old (female, x = 15.03, SE ± .48 years/male, x = 15.83, SE ± .56), underwent structural magnetic resonance imaging (MRI) procedures and were assessed using the BIS. No significant differences between groups were observed for sex by age distribution χ2 (11, N = 59) = 13.68, p = .25. Participants also completed the Structured Clinical Interview for DSM-IV Patient Version (SCID-I/P) (First et al., 2002) and all participants included in the study sample were free from current major DSM-IV Axis I diagnosis, including substance abuse or dependence. Additional exclusion criteria for all subjects included major sensorimotor disabilities, neurological disease that would impact neurobiology, history of electroconvulsive therapy, and implants that would be contraindicated for MRI.
2.3. MRI acquisition
All acquisitions were performed with a Siemens 3-T Trio magnet using a 12 channel head coil and T-1 weighted 3D MPRAGE sequence: field of view 256 mm, TR 3 ms, TE 3.38 ms, flip angle 8°, 1 mm slice thickness.
2.4. Image analysis
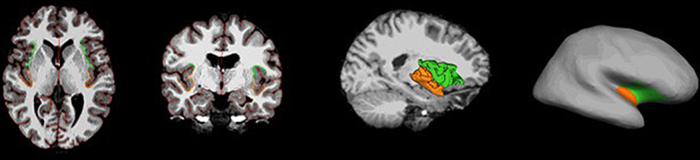
Analyses were completed using the standard FreeSurfer (5.1) processing stream (http://surfer.nmr.mgh.harvard.edu/). The following workflow procedures were used. High-resolution T1 MPRAGE volumes in DICOM format were anonymized and imported into the FreeSurfer image analysis environment. Semi-automated methods employing the default surface-based and volume-based pipelines were used. Processing included registration with the Talairach and Tornoux atlas, intensity normalization, skull stripping, segmentation of white and deep gray matter volumetric structures, gray and white matter boundary determination (Dale et al., 1999, Fischl et al., 2001, Sled et al., 1998). Cortical white matter surfaces were used in a deformation procedure that assigns gray and white matter borders by following intensity gradients to the position at which the maximum shift in intensity designates the transition to the other tissue class (Fischl and Dale, 2000). Several deformation procedures including inflation, spherical registration, and cortical parcellation were also performed (Desikan et al., 2006). An automated registration procedure was used to label each voxel in an MRI volume based on probabilities estimated from a manually labeled training set (Fischl et al., 2002). Standard predefined region of interest (ROI) maps were used in the statistical analysis for the left and right insular cortex (Destrieux et al., 2010; aparc.a2009s.annot). It has been proposed that the function of the insula is somewhat heterogeneous with primary homeostatic representations in the posterior insula integrated into the anterior compartment which is involved in representing ‘global emotional moments’ (Craig, 2009a). There is evidence that the anterior, but not posterior, insula shows age-related linear changes in morphology during early development (Shaw et al., 2008). Taken together with the previous observation concerning the role of the anterior insula in representing emotion, linear morphological changes in the anterior compartment of this structure during early development may be a predictor of emotional development. Consequently, in the present study ROIs for the anterior and superior circular sulcus of the insula, short insular gyrus, and central sulcus of the insula were merged to form a label map of the anterior insula, whereas the insula label map for the posterior insula was formed by merging labels for the inferior circular sulcus of the insula with the long insula gyrus (Fig. 1). These compartments of the anterior and posterior insula are consistent with previously reported delineations (Makris et al., 2006, Rosso et al., 2010).
Fig. 1.
From left to right, axial view, coronal view, midsagital view with 3D reconstruction of the insula, and inflated cortical surface of a single subject with regions of interest (ROIs) in the insula, including the anterior insula in green and the posterior insula in orange.
2.5. Impulsivity instrument and administration
Participants were administered the Barratt Impulsiveness Scale version 11. The BIS is a 30 item self-report questionnaire which measures several dimensions of impulsivity including attention, motor, and nonplanning impulsiveness. These dimensions index acting without thinking, attentional vigilance, and future versus present orientation, respectively (Patton et al., 1995). Subjects were escorted to a private testing room and administered the self-report instrument orally by trained research staff. Scores on each dimension were summed to obtain individual scores for each dimension.
3. Results
3.1. Age and nonplanning impulsivity
Hierarchical multiple regression was used to test the hypothesis that nonplanning decreases with age and determine whether sex was a contributing variable to predicting nonplanning impulsivity. Age was entered in the first step of the analysis and sex was entered in the second step. The results demonstrated a significant effect of age on nonplanning impulsivity F(1, 57) = 4.08, p < .05, with age explaining 7% of the variance in nonplanning impulsivity. However, sex did not provide greater explanation in variance beyond age F(1, 56) = .744, p > .05 (Table 1 and Fig. 2). These results are in agreement with previous research indicating that age, but not sex, contributes to self-reported impulsivity (Steinberg et al., 2008).
Table 1.
Hierarchical regression for nonplanning (age, sex).
| Variable | B | SE B | β | t | ||
|---|---|---|---|---|---|---|
| Step 1 | Age | −.424 | .210 | −.258 | −2.020 | * |
| Step 2 | Age | −.399 | .213 | −.243 | −1.875 | |
| Sex | −1.049 | 1.217 | −.112 | −.862 |
Dependent variable: nonplanning impulsivity. R2 = .07 for Step 1; ΔR2 = .01 for Step 2, n.s.
p < .05.
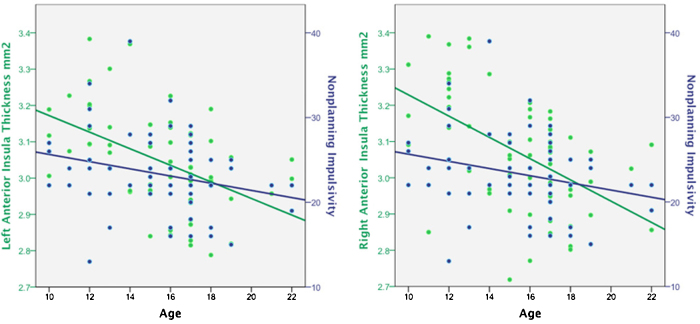
Fig. 2.
Relationships among age, anterior insula thickness, and nonplanning impulsivity.
3.2. Age and insula cortex thickness
Separate hierarchical multiple regressions were used to test the hypothesis that regional insula cortex thicknesses decrease linearly with age and assess whether sex is a contributing variable to predicting insula cortex thickness. Age was entered in the first step and sex was entered in the second step. The results demonstrated a significant effect of age on left anterior, F(1, 57) = 17.61, p < .001 and right anterior, F(1, 57) = 21.20, p < .01, but not left or right posterior (ps > .05) insula cortex. Age explained 24% and 27% of the variance in left and right anterior insula cortex, respectively, whereas sex did not provide greater explanation in variance beyond age, ps > .05 (Table 2). These results are consistent with the proposal that anterior and posterior insula cortex are functionally heterogeneous, and empirical research indicating that anterior, but not posterior, insula cortex changes linearly with age, independent of sex (Shaw et al., 2008).
Table 2.
Hierarchical regression for insula cortex thickness (age, sex).
| Variable | B | SE B | β | t | ||
|---|---|---|---|---|---|---|
| Dependent variable: left anterior insula | ||||||
| Step 1 | Age | −.023 | .005 | −.486 | −4.196 | *** |
| Step 2 | Age | −.022 | .005 | −.468 | −4.008 | *** |
| Sex | −.035 | .031 | −.129 | −1.110 | ||
| Dependent variable: right anterior insula | ||||||
| Step 1 | Age | −.029 | .006 | −.521 | −4.604 | *** |
| Step 2 | Age | −.029 | .006 | −.512 | −4.457 | *** |
| Sex | −.019 | .037 | −.060 | −.526 | ||
| Dependent variable: left posterior insula | ||||||
| Step 1 | Age | −.002 | .009 | −.032 | −.238 | |
| Step 2 | Age | .000 | .009 | .001 | .007 | |
| Sex | −.089 | .050 | −.232 | −1.771 | ||
| Dependent variable: right posterior insula | ||||||
| Step 1 | Age | −.005 | .008 | −.089 | −.673 | |
| Step 2 | Age | −.004 | .008 | −.067 | −.507 | |
| Sex | −.050 | .044 | −.153 | −1.151 | ||
Left anterior insula, R2 = .24 for Step 1; ΔR2 = .02 for Step 2, n.s.; right anterior insula, R2 = .26 for Step 1; ΔR2 < .01 for Step 2, n.s.; left posterior insula, R2 < .01 for Step 1; ΔR2 = .05 for Step 2; right posterior insula, R2 < .01 for Step 1; ΔR2 = .02 for Step 2 n.s.
p < .001.
3.3. Insula cortical thickness and nonplanning impulsivity
Hierarchical multiple regression was used to test the hypothesis that nonplanning decreases with reductions in anterior insular cortical thickness and to further assess whether posterior insula cortical thickness and sex contribute to predicting nonplanning scores over and beyond anterior insula cortex. Left and right anterior insula cortex thicknesses were entered in the first step of the analysis. Posterior insular cortex thicknesses and sex were entered in the second step of the analysis. The results demonstrated a significant effect of anterior insular cortex on nonplanning impulsivity, F(2, 56) = 5.56, p < .001, with anterior insula cortex thickness explaining 17% of the variance in nonplanning impulsivity. However, posterior insula cortex and sex did not provide increased explanation in variance beyond anterior insula cortex thicknesses, F(3, 54) = 1.32, p > .01 (Table 3).
Table 3.
Hierarchical regression for nonplanning (anterior insula, posterior insula).
| Variable | B | SE B | β | t | ||
|---|---|---|---|---|---|---|
| Step 1 | L Ant Ins | 15.543 | 5.739 | .445 | 2.708 | ** |
| R Ant Ins | −1.751 | 4.787 | −.060 | −.366 | ||
| Step 2 | L Ant Ins | 18.132 | 6.136 | .519 | 2.955 | ** |
| R Ant Ins | −.870 | 4.777 | −.030 | −.182 | ||
| L Pos Ins | 1.912 | 3.213 | .078 | .595 | ||
| R Pos Ins | −7.616 | 4.067 | −.266 | −1.872 | ||
| Sex | −.692 | 1.181 | −.074 | −.585 |
Dependent variable: nonplanning impulsivity. L, left; R, right; Ant, anterior; Pos, posterior; Ins, insula. R2 = .17 for Step 1; ΔR2 = .06 for Step 2, n.s.
p < .01.
4. Discussion
Refinement in anterior insula function during development may be reflected in age-related reductions in cortical thickness that reveals experience dependent pruning and synaptic plasticity in this region (Brenhouse and Andersen, 2011, Chechik et al., 1998, Huttenlocher and Dabholkar, 1997). This change in cortical composition corresponds to linear reductions in levels of self-reported impulsivity and future orientation that occur from early adolescence to early adulthood (Steinberg et al., 2008, Steinberg et al., 2009). We hypothesized that, in our cohort of subjects, ranging in age from 10 to 22 years old, insular cortical thickness would decrease with age, that we would observe age-related decreases in nonplanning impulsivity, and that insular cortical thickness would be related to this change. Our results indicate that as age increases anterior insula thickness decreases linearly, as does nonplanning impulsivity, whereas posterior insula does not, consistent with previous observations of linear and nonlinear development of these different insular subregions. Moreover, anterior insula thickness was positively associated with nonplanning impulsivity.
Studies using neuroeconomic approaches to assess brain changes linked to anticipation and decision under risk and uncertainty have indicated an important role for the insula (Clark et al., 2008, Venkatraman et al., 2009, Xue et al., 2010). For example, healthy adult subjects underwent fMRI scans while performing a risky-gains task in which they could select to receive 20, 40, 60 or 80 points. If they chose the 20-point option they received those points for certain, whereas if they selected the 40 or 80 point options they risked losing that amount. However, unknown to the subjects, whatever strategy they selected the final payoff was the same. The results of the study indicated that insula activation was increased for risky compared to safe choices, increased following losses, and was also greater after risky choices that resulted in gains than during risky choices that resulted in losses (Paulus et al., 2003). Self-reported impulsivity and urgency have also been associated with insula activation in fMRI studies where risky decision-making was tested. For example, Lee et al. (2008) assessed subjects using the BIS and categorized individuals as either low or high impulsivity. Subjects were also tested using a risky gains task while undergoing fMRI. The risky gains task involves selecting a certain small reward or an uncertain larger reward that could result in high gains or losses. Increased activity in a network of structures including the orbitofrontal cortex, insula, and parietal cortex was observed in individuals categorized as highly impulsive, in contrast to performance matched low impulsive subjects. Moreover, Xue et al. (2010) tested subjects in a gambling task and found increased insula activation in no-risk trials, compared to risk-win trials, was associated with individual urgency assessed using the urgency subscale of the UPPS Impulsive Behavior Scale.
The insula also appears to play a role in delay discounting. For example, increased activation of this region has been observed when adults select larger future over smaller immediate rewards (Wittmann et al., 2007). It has also been shown that insula activity is related to individual preference for small immediate or larger delay rewards (Marco-Pallares et al., 2010) and is greater in response to discounting of future losses than gains (Xu et al., 2009). One hypothesis regarding delay discounting is that the degree of continuity between an individual's future and present self is related to valuation of delayed rewards. For example, self-continuity is the degree to which an agent believes that their present and future self will be in agreement (Parfit, 1971). Preference for larger delayed rewards in a delay discounting procedure is predicted by self-continuity, as indexed by endorsement of similarity and connectedness to a future self, as well as how much an individual likes and cares about their future self (Ersner-Hershfield et al., 2009).
Damasio (2003) has proposed that feelings and the self have a shared neurobiological basis in the insula. The insula may be specifically involved in conscious awareness of body states that constitutes the necessary substrate for the emotional-self though time (Craig, 2009b). Consistent with this view, as interoceptive awareness increases so does the intensity of experienced emotions (Pollatos et al., 2007). Moreover, interoceptive awareness, as indexed by accuracy on a heartbeat detection task, positively correlates with both insula morphology and functional activity (Critchley et al., 2004). Adolescence marks a period of increased emotional intensity and frequency of emotional change (Larson et al., 1980) and the progression from adolescence to young adulthood also involves the acquisition of competencies that include regulatory skills for responding to emotional change. For example, adolescents, compared to adults, have been shown to have a reduced capacity to identify, assimilate, and understand emotions as assessed by emotional intelligence testing (Mayer et al., 2000). Under emotional challenge, adults show increased activation of the insula compared to adolescents, and magnitude of activation in the insula of adults predicts decreased autonomic response under challenge (Lewis et al., 2008). A critical issue in understanding emotional regulation during adolescence is clarification of neural systems that support reappraisal (Pfeifer and Blakemore, 2012). It has been shown that emotional regulation strategies such as reappraisal can modify risky decision-making (Pitskel et al., 2011) and alter insular activation (Goldin et al., 2008). Thus, insula function may be related to prospective thinking and, ultimately decision-making, through its fundamental role in interoception and emotion (Fig. 3). For example, emotion, emotional regulation such as reappraisal, and traits are thought to interact dynamically and bidirectionally (Lewis, 2005).
Fig. 3.
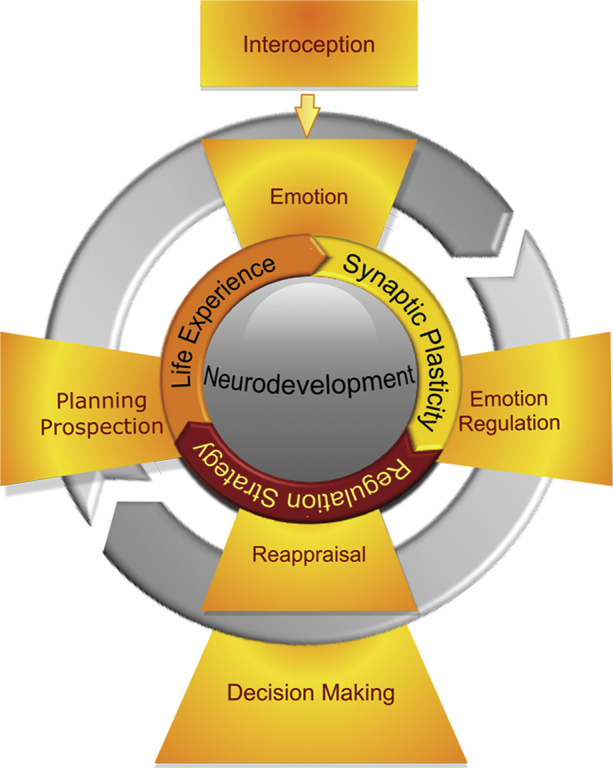
Proposed model with relationships among neurodevelopmental, cognitive, and emotional processes from early adolescence to young adulthood. Experience-dependent synaptic plasticity supports acquiring knowledge about the linkage between interoceptive states, emotion, and emotional regulation strategies, which inform and are informed by prospective thinking that leads to optimal choice during decision-making.
Our data indicate that self-reported nonplanning impulsivity decreases with age as does anterior insula thickness. Further, insula thickness was positively associated with nonplanning impulsivity. We speculate that this set of relationships is a consequence of insula plasticity that supports normal developmental processes from early adolescence to young adulthood that entails acquiring knowledge about the linkage between interoceptive states, emotional experience, and emotional regulation strategies, which inform and are informed by prospective thinking that leads to optimal choice during decision-making.
Conflict of interest statement
The authors do not have any interests that might be interpreted as influencing the research.
Acknowledgments
Research supported by NIH grant 1R01 DA020269-01 and R03DA029180-01 .
References
- Brenhouse H.C., Andersen S.L. Developmental trajectories during adolescence in males and females: a cross-species understanding of underlying brain changes. Neuroscience and Biobehavioral Reviews. 2011;35(8):1687–1703. doi: 10.1016/j.neubiorev.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey B.J., Getz S., Galvan A. The adolescent brain. Developmental Review: DR. 2008;28(1):62–77. doi: 10.1016/j.dr.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chechik G., Meilijson I., Ruppin E. Synaptic pruning in development: a computational account. Neural Computation. 1998;10(7):1759–1777. doi: 10.1162/089976698300017124. [DOI] [PubMed] [Google Scholar]
- Clark L., Bechara A., Damasio H., Aitken M.R., Sahakian B.J., Robbins T.W. Differential effects of insular and ventromedial prefrontal cortex lesions on risky decision-making. Brain: A Journal of Neurology. 2008;131(Pt 5):1311L 1322. doi: 10.1093/brain/awn066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig A.D. Emotional moments across time: a possible neural basis for time perception in the anterior insula. Philosophical Transactions of the Royal Society of London Series B: Biological Sciences. 2009;364(1525):1933–1942. doi: 10.1098/rstb.2009.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig A.D. How do you feel—now? The anterior insula and human awareness. Nature Reviews Neuroscience. 2009;10(1):59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Critchley H.D., Wiens S., Rotshtein P., Ohman A., Dolan R.J. Neural systems supporting interoceptive awareness. Nature Neuroscience. 2004;7(2):189–195. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- Dale A.M., Fischl B., Sereno M.I. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9(2):179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Damasio A. Feelings of emotion and the self. Annals of the New York Academy of Sciences. 2003;1001:253–261. doi: 10.1196/annals.1279.014. [DOI] [PubMed] [Google Scholar]
- Damasio A.R. 1st ed. Harcourt Brace; New York: 1999. The Feeling of What Happens: Body and Emotion in the Making of Consciousness. [Google Scholar]
- Desikan R.S., Segonne F., Fischl B., Quinn B.T., Dickerson B.C., Blacker D. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31(3):968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Destrieux C., Fischl B., Dale A., Halgren E. Automatic parcellation of human cortical gyri and sulci using standard anatomical nomenclature. Neuroimage. 2010;53(1):1–15. doi: 10.1016/j.neuroimage.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M., Romeo R.D., Andersen S.L. Neurobiology of the development of motivated behaviors in adolescence: a window into a neural systems model. Pharmacology Biochemistry and Behavior. 2008;93(3):199–211. doi: 10.1016/j.pbb.2008.12.013. [DOI] [PubMed] [Google Scholar]
- Ersner-Hershfield H., Garton M.T., Ballard K., Samanez-Larkin G.R., Knutson B. Don’t stop thinking about tomorrow: individual differences in future self-continuity account for saving. Judgment and Decision Making. 2009;4(4):280–286. [PMC free article] [PubMed] [Google Scholar]
- First M.B., Spitzer R.L., Gibbon M., Williams J.B. Biometrics Research Department, New York State Psychiatric Institute; New York: 2002. Structured Clinical Interview for DSM-IV-TR Axis I Disorders-Patient Edition. [Google Scholar]
- Fischl B., Dale A.M. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(20):11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B., Liu A., Dale A.M. Automated manifold surgery: constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Transactions on Medical Imaging. 2001;20(1):70–80. doi: 10.1109/42.906426. [DOI] [PubMed] [Google Scholar]
- Fischl B., Salat D.H., Busa E., Albert M., Dieterich M., Haselgrove C. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33(3):341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Galvan A., Hare T., Voss H., Glover G., Casey B.J. Risk-taking and the adolescent brain: who is at risk? Developmental Science. 2007;10(2):F8–F14. doi: 10.1111/j.1467-7687.2006.00579.x. [DOI] [PubMed] [Google Scholar]
- Geier C., Luna B. The maturation of incentive processing and cognitive control. Pharmacology Biochemistry and Behavior. 2009;93(3):212–221. doi: 10.1016/j.pbb.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N., Giedd J.N., Lusk L., Hayashi K.M., Greenstein D., Vaituzis A.C. Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(21):8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin P.R., McRae K., Ramel W., Gross J.J. The neural bases of emotion regulation: reappraisal and suppression of negative emotion. Biological Psychiatry. 2008;63(6):577–586. doi: 10.1016/j.biopsych.2007.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttenlocher P.R., Dabholkar A.S. Regional differences in synaptogenesis in human cerebral cortex. Journal of Comparative Neurology. 1997;387(2):167–178. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Larson R., Csikszentmihalyi M., Graef R. Mood variability and the psychosocial adjustment of adolescents. Journal of Youth and Adolescence. 1980;9(6):469–490. doi: 10.1007/BF02089885. [DOI] [PubMed] [Google Scholar]
- Lee T.M., Chan C.C., Han S.H., Leung A.W., Fox P.T., Gao J.H. An event-related fMRI study on risk taking by healthy individuals of high or low impulsiveness. Neuroscience Letters. 2008;438(2):138–141. doi: 10.1016/j.neulet.2008.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis M.D. Bridging emotion theory and neurobiology through dynamic systems modeling. Behavioral and Brain Sciences. 2005;28(2):169–194. doi: 10.1017/s0140525x0500004x. (discussion 194–245) [DOI] [PubMed] [Google Scholar]
- Lewis M.D., Granic I., Lamm C., Zelazo P.D., Stieben J., Todd R.M. Changes in the neural bases of emotion regulation associated with clinical improvement in children with behavior problems. Development and Psychopathology. 2008;20(3):913–939. doi: 10.1017/S0954579408000448. [DOI] [PubMed] [Google Scholar]
- Makris N., Goldstein J.M., Kennedy D., Hodge S.M., Caviness V.S., Faraone S.V. Decreased volume of left and total anterior insular lobule in schizophrenia. Schizophrenia Research. 2006;83(2–3):155–171. doi: 10.1016/j.schres.2005.11.020. [DOI] [PubMed] [Google Scholar]
- Marco-Pallares J., Mohammadi B., Samii A., Munte T.F. Brain activations reflect individual discount rates in intertemporal choice. Brain Research. 2010;1320:123–129. doi: 10.1016/j.brainres.2010.01.025. [DOI] [PubMed] [Google Scholar]
- Mayer J.D., Caruso D.R., Salovey P. Emotional intelligence meets traditional standards for an intelligence. Intelligence. 2000;27(4):267–298. [Google Scholar]
- Naqvi N.H., Bechara A. The insula and drug addiction: an interoceptive view of pleasure, urges, and decision-making. Brain Structure & Function. 2010;214(5-6):435–450. doi: 10.1007/s00429-010-0268-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parfit D. Personal identity. Philosophical Review. 1971;80(1):3–27. [Google Scholar]
- Patton J.H., Stanford M.S., Barratt E.S. Factor structure of the Barratt impulsiveness scale. Journal of Clinical Psychology. 1995;51(6):768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Paulus M.P., Rogalsky C., Simmons A., Feinstein J.S., Stein M.B. Increased activation in the right insula during risk-taking decision making is related to harm avoidance and neuroticism. Neuroimage. 2003;19(4):1439–1448. doi: 10.1016/s1053-8119(03)00251-9. [DOI] [PubMed] [Google Scholar]
- Pfeifer J.H., Blakemore S.J. Adolescent social cognitive and affective neuroscience: past, present, and future. Social Cognitive and Affective Neuroscience. 2012;7(1):1–10. doi: 10.1093/scan/nsr099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitskel N.B., Bolling D.Z., Kaiser M.D., Crowley M.J., Pelphrey K.A. How grossed out are you? The neural bases of emotion regulation from childhood to adolescence. Developmental Cognitive Neuroscience: A Journal for Cognitive, Affective and Social Developmental Neuroscience. 2011;1(3):324–337. doi: 10.1016/j.dcn.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollatos O., Gramann K., Schandry R. Neural systems connecting interoceptive awareness and feelings. Human Brain Mapping. 2007;28(1):9–18. doi: 10.1002/hbm.20258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosso I.M., Makris N., Britton J.C., Price L.M., Gold A.L., Zai D. Anxiety sensitivity correlates with two indices of right anterior insula structure in specific animal phobia. Depression and Anxiety. 2010;27(12):1104–1110. doi: 10.1002/da.20765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K., Smith A.B., Taylor E., Brammer M. Linear age-correlated functional development of right inferior fronto-striato-cerebellar networks during response inhibition and anterior cingulate during error-related processes. Human Brain Mapping. 2007;28(11):1163–1177. doi: 10.1002/hbm.20347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedekides C., Skowronski J.J. The symbolic self in evolutionary context. Personality and Social Psychology Review: An Official Journal of the Society for Personality and Social Psychology, Inc. 1997;1(1):80–102. doi: 10.1207/s15327957pspr0101_6. [DOI] [PubMed] [Google Scholar]
- Shaw P., Kabani N.J., Lerch J.P., Eckstrand K., Lenroot R., Gogtay N. Neurodevelopmental trajectories of the human cerebral cortex [Research Support, N.I.H., Intramural] Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2008;28(14):3586–3594. doi: 10.1523/JNEUROSCI.5309-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sled J.G., Zijdenbos A.P., Evans A.C. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Transactions on Medical Imaging. 1998;17(1):87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- Steinberg L., Albert D., Cauffman E., Banich M., Graham S., Woolard J. Age differences in sensation seeking and impulsivity as indexed by behavior and self-report: evidence for a dual systems model. Developmental Psychology. 2008;44(6):1764–1778. doi: 10.1037/a0012955. [DOI] [PubMed] [Google Scholar]
- Steinberg L., Graham S., O‘Brien L., Woolard J., Cauffman E., Banich M. Age differences in future orientation and delay discounting. Child Development. 2009;80(1):28–44. doi: 10.1111/j.1467-8624.2008.01244.x. [DOI] [PubMed] [Google Scholar]
- Venkatraman V., Payne J.W., Bettman J.R., Luce M.F., Huettel S.A. Separate neural mechanisms underlie choices and strategic preferences in risky decision making. Neuron. 2009;62(4):593–602. doi: 10.1016/j.neuron.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann M., Leland D.S., Paulus M.P. Time and decision making: differential contribution of the posterior insular cortex and the striatum during a delay discounting task. Experimental Brain Research. 2007;179(4):643–653. doi: 10.1007/s00221-006-0822-y. [DOI] [PubMed] [Google Scholar]
- Xu L., Liang Z.Y., Wang K., Li S., Jiang T. Neural mechanism of intertemporal choice: from discounting future gains to future losses. Brain Research. 2009;1261:65–74. doi: 10.1016/j.brainres.2008.12.061. [DOI] [PubMed] [Google Scholar]
- Xue G., Lu Z., Levin I.P., Bechara A. The impact of prior risk experiences on subsequent risky decision-making: the role of the insula. Neuroimage. 2010;50(2):709–716. doi: 10.1016/j.neuroimage.2009.12.097. [DOI] [PMC free article] [PubMed] [Google Scholar]